Abstract
Cenerimod is a sphingosine‐1‐phosphate 1 receptor (S1P1R) modulator in phase II development for treatment of systemic lupus erythematosus. Its pharmacokinetics (PKs), pharmacodynamics (PDs), as well as safety and tolerability were investigated in white and Asian subjects to allow for recruitment of Asian patients in future studies. A randomized, double‐blind, placebo‐controlled parallel‐group study was performed in 20 healthy male subjects (n = 10 per ethnicity). A single, oral dose of 4 mg cenerimod or placebo (ratio 8:2) was administered under fasted conditions. The PKs of cenerimod were similar in white and Asian subjects indicated by geometric mean ratios (90% confidence interval) of 0.99 (0.80–1.21) for maximum plasma concentration, 0.96 (0.75–1.24) for area under the plasma concentration‐time curve from 0 to infinity, and 1.04 (0.86–1.25) for terminal half‐life. Accordingly, the extent and time course of reduction in lymphocyte count (as PD biomarker) were also similar in white and Asian subjects as compared with placebo. As observed for other S1PR modulators, a transient mean (SD) heart rate reduction in white (15.1 (14.8) bpm) and Asian (11.8 (6.16) bpm) subjects was observed following administration of cenerimod. The drug was safe and well‐tolerated indicated by occurrence of a single adverse event of chemical conjunctivitis in a white subject that was not suspected as study drug related. In conclusion, the determined absence of any relevant PK or PD differences supports using the same doses of cenerimod in white and Asian patients in upcoming late‐phase studies.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Cenerimod, a potent selective sphingosine‐1‐phosphate 1 receptor modulator, displaying unique signaling properties, causes a dose‐dependent reduction in lymphocyte count in peripheral blood in humans. It is characterized by a slow elimination leading to built‐in up‐titration and a CYP‐independent metabolism.
WHAT QUESTION DID THIS STUDY ADDRESS?
This study compared the pharmacokinetic (PK), pharmacodynamic (PD), and safety of cenerimod between white and Asian subjects.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
Similar PK and PD profiles were observed in white and Asian subjects following single‐dose administration of cenerimod at the highest phase II dose of 4 mg. Cenerimod was equally well‐tolerated in both ethnicities.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
The same doses may be used in white and Asian patients with systemic lupus erythematosus in future efficacy trials.
Administration of sphingosine‐1‐phosphate 1 receptor (S1P1R) modulators triggers a sustained internalization of this receptor and induces a long‐lasting inhibition of the egress of lymphocytes from lymphoid organs suggesting efficacy in autoimmune disorders. 1
Cenerimod is an orally available, potent, and selective modulator of the S1P1R under clinical development as potential treatment for systemic lupus erythematosus (SLE). 2 , 3 The pharmacokinetics (PKs) of cenerimod have been extensively characterized in healthy white subjects and revealed a time (Tmax) to maximum plasma concentration (Cmax) of 5–6 hours, terminal half‐life (t½) of 7–8 days after single‐dose administration, and dose‐proportional exposure. 3 , 4 Absorption and elimination of cenerimod are slower compared with other S1PR modulators. 5 Cenerimod displays a cytochrome P450 (CYP) enzyme‐independent metabolism and no major metabolites were found in plasma. It is primarily excreted in feces with a single major metabolite formed CYP‐independently by reductive cleavage. 6 In vitro data suggest that cenerimod is a substrate of a single transporter (BCRP) and is not a perpetrator of any CYP or transporter.
Total lymphocyte count measurement is the generally accepted pharmacodynamic (PD) biomarker for S1P1R modulators, including cenerimod based on their mode of action (i.e., inhibition of the egress of T‐lymphocytes and B‐lymphocytes out of lymphoid organs and bone marrow, respectively). In line with its mechanism of action, the PDs of cenerimod revealed a dose‐dependent lymphocyte count reduction in healthy subjects. 3 , 4 , 7 In addition, a proof‐of‐concept study in patients with active SLE 2 was conducted. Here, a similar exposure to cenerimod compared with healthy subjects was measured, a dose‐dependent reduction in total lymphocyte count, and clinical and biological improvement was observed at once‐daily doses of 2 and 4 mg. A second phase II efficacy study is currently ongoing (NCT03742037).
The so‐called “built‐in up‐titration” of cenerimod related to its long t1/2 is expected to mitigate the well‐known class effect of transient decreases in heart rate (HR). 5 In addition, it has been demonstrated that HR returns to baseline upon repeated dosing of an S1P1R modulator 3 , 4 due to desensitization and tolerance development. 8 In contrast to other S1PR modulators (e.g., fingolimod, amiselimod, and siponimod), cenerimod is more selective to S1P1R rendering the cardiovascular system less prone to S1P3‐mediated adverse effects (e.g., bradycardia and atrioventricular block) and it displays unique Ca2+‐mediated S1P1 signaling reducing bronchoconstriction preclinically. 9 Change in HR can serve as a reliable safety biomarker because S1P1Rs are expressed on cardiomyocytes leading to decrease in HR after treatment initiation. This decrease is transient and with repeated dosing, HR returns to baseline values.
In order to allow recruitment of Asian subjects in pivotal registration trials, in the present study, the PK, PD, and safety of cenerimod have been compared head‐to‐head between white and Asian subjects.
METHODS
Study design
The study was conducted at a single center in the United States (Anaheim, CA) in accordance with the Declaration of Helsinki and Good Clinical Practice. All participants provided written informed consent prior to any study‐related procedures. The protocol was provided to the US Food and Drug Administration and approved by an independent review board (Aspire IRB, Santee, CA).
All Asian subjects were of native Japanese descent defined by (i) having parents and grandparents of Japanese descent, (ii) not being away from Japan > 10 years, and (iii) maintaining a Japanese lifestyle (e.g., food habit).
This was a randomized, double‐blind, placebo‐controlled, parallel‐group study evaluating the PK, PD, and safety and tolerability of cenerimod (NCT04052360).
Study population
Twenty healthy male white (n = 10) and Asian (n = 10) subjects aged 18–65 years with body mass index of 18–28 kg/m2 were enrolled. These were matched based on age (± 10 years) and body weight (± 20%). Their healthy status was determined based on the absence of any active or chronic disease, complete physical examination, vital signs, 12‐lead echocardiogram (ECG), and clinical laboratory data. At screening and on day –1, systolic/diastolic blood pressure and HR had to be in the range of 100–145/50–90 mmHg and 55–90 bpm, respectively.
Study conduct
Subjects were screened within 21 days before dosing and admitted to the clinic the day before dosing. On day 1, a single oral dose of 4 mg cenerimod or placebo (ratio 8:2) was administered as a tablet formulation under fasted conditions (i.e., last food intake at least 12 hours prior to dosing). The 4 mg dose was selected because it is the highest dose tested in the ongoing dose‐finding phase II study.
Each subject remained at the study site until discharge at least 48 hours after dosing followed by ambulatory visits on days 6, 9, 12, 15, 18, 21, 28, 35, 42, and 49. The end‐of‐study (EOS) visit was conducted between days 52 and 54 followed by a safety follow‐up telephone call within 30–40 days after the EOS visit.
PK assessments
Blood samples of ~ 3 mL were collected in EDTA tubes predose and at 1, 2, 3, 4, 6, 8, 12, 24, and 48 hours postdose, and once at each ambulatory visit. After centrifugation, plasma was transferred into a polypropylene tube and stored at −21°C (± 5°C) pending analysis. Plasma concentrations of cenerimod were determined using a validated liquid chromatography coupled to tandem mass spectrometry assay with a lower limit of quantification of 0.1 ng/mL, as described earlier. 3 The method was linear in the concentration range 0.1–100 ng/mL. Analysis of quality‐control samples of all runs showed that inter‐batch coefficients of variation (precision) were < 8.9%, whereas the average intra‐batch accuracy was in the range between −4.6 and −1.2%.
Noncompartmental PK analyses were performed using Professional WinNonlin 8.0 software (Pharsight, Mountain View, CA). The variables Cmax and Tmax were directly obtained from the plasma concentration–time profiles. Area under the plasma concentration‐time curve from time point 0 to the end of the dosing interval (AUC0–t) was calculated using the trapezoidal method. 10 AUC from 0 to infinity (AUC0–∞) was calculated by combining AUC0–t and AUCextra. AUCextra represents an extrapolated value obtained by Ct/λz, where Ct is the last plasma concentration measured above the lower limit of quantification, and λz is the elimination rate constant determined by log‐linear regression analysis. The t1/2 was calculated as ln 2/λz.
PK parameters were compared between both ethnic groups based on geometric mean ratios (GMRs) and 90% confidence intervals.
PD assessments
Lymphocyte counts were used as PD biomarker and repeatedly determined in peripheral blood as part of the clinical hematology evaluation predose and postdose at 3, 6, 12, 24, 48, 144, 288, and 432 hours, and at the EOS visit. Blood samples of 2.7 mL were collected into a K3‐EDTA polypropylene tube and analysis was performed using a cell counter.
Safety and tolerability assessments
Safety and tolerability were evaluated based on adverse event (AE), vital signs, 12‐lead ECG (including HR), and clinical laboratory data, as well as physical and neurological examinations. ECG and vital sign assessments were done predose and at 1, 2, 3, 4, 6, 8, 12, 24, and 48 hours postdose and at the EOS visit.
RESULTS
Disposition and demographics
All 20 enrolled subjects completed the study per protocol (n = 10 per ethnicity) and received a single oral dose of 4 mg cenerimod or placebo. Demographic variables were overall similar between white and Asian subjects based on mean (SD) age (41.7 years (9.6) vs. 40.7 years (10.8)) and body mass index (24.9 kg/m2 (1.9) vs. 22.4 kg/m2 (1.9)).
Pharmacokinetics
Plasma concentration vs. time profiles of cenerimod are depicted in Figure 1a and the PK parameters are presented in Table 1 .
Figure 1.
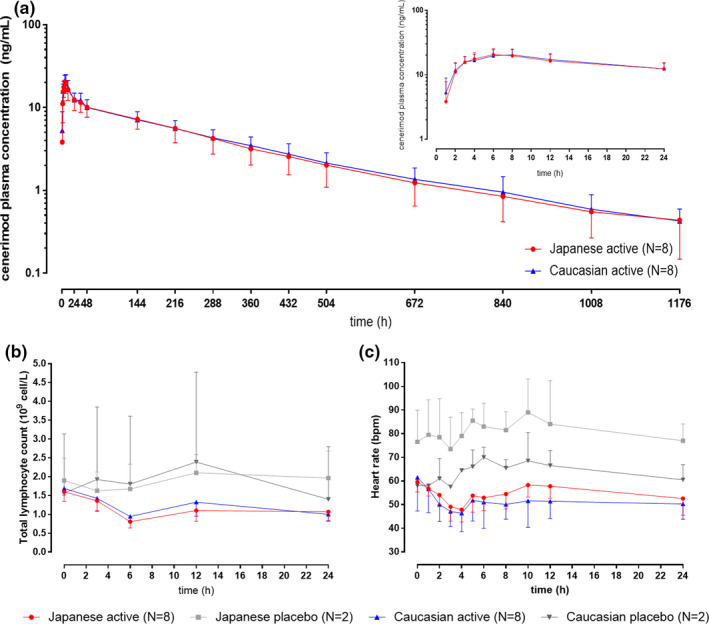
Pharmacokinetics, pharmacodynamics, and heart rate effect of cenerimod in white and Asian subjects. (a) Plasma concentration profile of cenerimod vs. time over the duration of the study on semi‐log scale (over 24 hours in inset). (b) Total lymphocyte count profile vs. time in cenerimod‐treated and placebo‐treated subjects. (c) Heart rate profile vs. time in cenerimod‐treated and placebo‐treated subjects.
Table 1.
Summary of pharmacokinetic variables of cenerimod administered at a single oral dose of 4 mg in white and Asian subjects (n = 8 per ethnicity)
| Cmax, ng/mL | AUC0–t, hour·ng/mL | AUC0–∞, hour·ng/mL | Tmax, hour | t1/2, hour | |
|---|---|---|---|---|---|
| Asian, N = 8a | 20.1 (16.2–24.8) | 3,216 (2,470–4,186) | 3,422 (2,613–4,482) | 6.00 (6.00–8.08) | 299 (244–366) |
| White, N = 8a | 20.4 (17.0–24.4) | 3,357 (2,720–4,143) | 3,548 (2,910–4,327) | 8.00 (6.00–8.05) | 288 (250–332) |
| Ratio of geometric means (90% CI) Asian/whiteb | 0.99 (0.80–1.21) | 0.96 (0.75–1.23) | 0.96 (0.75–1.24) | 1.04 (0.86–1.25) | |
| Median (range) Asian/whiteb | –1.00 (−2.00–0.00) |
AUC0‐∞, area under the plasma concentration‐time curve from zero to infinity; AUC0–t, area under the plasma concentration‐time curve from zero to time of the last measured concentration above the limit of quantification; CI, confidence interval; Cmax, maximum plasma concentration; t1/2, terminal half‐life; Tmax, time to reach maximum plasma concentration.
Data are presented as geometric mean (95% CI) except for Tmax: median (range).
Data are presented as geometric mean ratios (90% CI) except for Tmax: median (range).
Exposure to cenerimod was similar in white and Asian subjects as indicated by GMRs (90% confidence interval) of 0.99 (0.80–1.21) for Cmax and 0.96 (0.75–1.24) for AUC0‐∞. The absorption (i.e., Tmax) and elimination (i.e., t1/2) kinetics were also similar in white and Asian subjects.
Pharmacodynamics
As depicted in Figure 1b , following single‐dose administration of 4 mg cenerimod, a similar time course of total lymphocyte counts was observed in white and Asian subjects. The maximum decrease in total lymphocyte count was observed ~ 6 hours after cenerimod administration in both ethnic groups. The mean (SD) maximum decrease from baseline in total lymphocyte count was also similar in white (−0.74·109 cells/L (0.13)) and Asian subjects (−0.80·109 cells/L (0.18)). Corresponding data in placebo‐treated subjects were −0.40·109 cells/L (0.15) and −0.27·109 cells/L (0.01), in white and Asian subjects, respectively.
Safety and tolerability
In the entire study, a single AE of chemical conjunctivitis was reported in a white subject. This AE was of mild intensity, occurred 6 days after cenerimod administration, and was considered unrelated to the treatment.
After administration of cenerimod, a transient decrease in HR was determined, which is a well‐established class effect of S1PR modulators. As depicted in Figure 1c , the time course and extent of HR reduction in white and Asian subjects were comparable, as reflected by a maximum mean (SD) change of −15.1 bpm (14.8) and −11.8 bpm (6.2) in white and Japanese subjects, respectively. Maximum HR reduction occurred ~ 4 hours after cenerimod administration in both ethnic groups. After placebo administration, there were no relevant decreases in HR.
There were no clinically relevant ECG abnormalities. Other safety parameters were also comparable between treatments (i.e., cenerimod vs. placebo) and ethnic groups (i.e., white vs. Asian subjects).
DISCUSSION
The PKs and PDs of S1PR modulators, such as fingolimod, 11 siponimod, 12 ponesimod, 13 and ozanimod, 14 have been investigated in different ethnicities. Each of these S1PR modulators is generally CYP‐dependently metabolized (e.g., CYP2C9 for siponimod, CYP4F2 for fingolimod, CYP3A4 and CYP2C8 for ozanimod, and unknown enzymes for ponesimod). 5 Although these enzymes show large interindividual variability in terms of activity and expression in different ethnic groups mainly driven by genetic differences, 15 , 16 the PKs and PDs of these drugs were not affected by ethnicity to a clinically meaningful extent after single‐dose administration. Therefore, the same dose was generally recommended in both ethnic groups.
By contrast to the approved S1PR modulators, cenerimod is eliminated CYP independently. 6 Accordingly, the PKs of cenerimod were not affected by ethnicity in the present study, indicated by GMRs close to 1.0 for Cmax, AUCs, and t1/2, as well as a similar Tmax range (Table 1 ). Moreover, exposure parameters of cenerimod including Cmax and AUC were comparable to previous studies indicating study validity. 3 , 4 , 6 Although absorption Tmax was also comparable to previous studies, elimination t1/2 was slightly longer (~ 12 vs. 7–9 days). 3 This may be related to a longer PK sampling duration in the present study (49 vs. 28 days), which allowed to more accurately capture the terminal elimination phase.
In terms of PDs, lymphocyte counts at baseline were similar in both ethnic groups. A single oral dose of 4 mg cenerimod led to a similar decrease from baseline of 0.7–0.8·109 cells/L in white and Japanese subjects corresponding to a reduction from baseline by ~ 40–50% (Figure 1b ). This extent of lymphocyte count reduction is in line with earlier data obtained in healthy white subjects (35 and 61% following a single oral dose of 3 and 10 mg, respectively). 3 These data suggest that the similar PKs in white and Asian subjects also translated into similar PD effects in both ethnic groups in accordance with the well‐established PK/PD relationship of cenerimod. 7
In terms of safety and tolerability, there was only a single AE unrelated to treatment reported, indicating that cenerimod was safe and equally well‐tolerated in both ethnic groups.
In addition, a similar extent of HR decrease was observed in white and Asian subjects in this study. As previously described, 5 first‐dose administration of an S1P1R modulator leads to a transient and reversible decrease in HR. The extent of decrease in this study is comparable to historical data (i.e., −12 bpm after the first dose of cenerimod 4 mg). 3
Although the single‐dose approach may be perceived as a study limitation, previous ethnic sensitivity studies with S1PR modulators have also used this approach. 11 , 12 , 13 , 14 The similar PK, PD, and safety data in white and Japanese subjects warrant further investigations in patients. Long‐term data will be collected in large phase II/III trials with the support of a PK/PD modeling approach. From a PK perspective, the reported dose‐proportional PK of cenerimod allows for extrapolation to a multiple‐dose regimen, 3 which is further supported by absorption not being rate limiting for the PKs of cenerimod and by enzyme‐independent metabolism. From a safety perspective, data collected in healthy subjects and patients with SLE showed that cenerimod is well‐tolerated after single and multiple administration at doses of 0.5–4 mg. The first‐dose HR effect in healthy white and Japanese subjects as well as in patients with SLE was comparable. The dose of 4 mg investigated here is the maximum dose investigated in phase II studies 2 (NCT03742037) and it is not expected that tolerability worsens upon repeated dosing in Asian patients.
In conclusion, the PK, PD, and safety of cenerimod were similar in white and Asian subjects and hence no dose adjustment in Asian subjects is deemed necessary.
Funding
This study was sponsored by Idorsia Pharmaceuticals Ltd., Allschwil, Switzerland.
Conflict of Interest
P.E.J., J.D., and M.U. are employees of Idorsia Pharmaceuticals Ltd., the sponsor of the study, and possess stock options/shares. P.W. was the Principal Investigator and was employed by Anaheim Clinical Trials in the previous 3 years.
Author Contributions
P.E.J., J.D., and M.U. wrote the manuscript and designed the research. P.W. performed the research. P.E.J. analyzed the data.
Acknowledgment
The authors thank Mark Enzler (Swiss BioQuant AG, Reinach, Switzerland) for conducting the bioanalysis, Racheal Rowles for project management, Doreen Mayo for monitoring activities, Denis Boutin for data management, and Anne‐Sophie Guern for statistical analysis of the PK, PD, and safety data.
References
- 1. Thangada, S. et al Cell‐surface residence of sphingosine 1‐phosphate receptor 1 on lymphocytes determines lymphocyte egress kinetics. J. Exp. Med. 207, 1475–1483 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hermann, V. , Batalov, A. , Smakotina, S. , Juif, P.E. & Cornelisse, P. First use of cenerimod, a selective S1P1 receptor modulator, for the treatment of SLE: a double‐blind, randomised, placebo‐controlled, proof‐of‐concept study. Lupus Sci. Med. 6, e000354 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Juif, P.E. et al Pharmacokinetics, pharmacodynamics, tolerability, and food effect of cenerimod, a selective S1P receptor modulator in healthy subjects. Int. J. Mol. Sci. 18, 2636 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Juif, P.E. , Ufer, M. & Dingemanse, J. Cardiodynamic interactions between two S1P1 receptor modulators in an experimental clinical setting: different pharmacokinetic properties as an opportunity to mitigate first‐dose heart rate effects. Int. J. Mol. Sci. 20, 3232 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Juif, P.E. , Kraehenbuehl, S. & Dingemanse, J. Clinical pharmacology, efficacy, and safety aspects of sphingosine‐1‐phosphate receptor modulators. Expert Opin. Drug Metab. Toxicol. 12, 879–895 (2016). [DOI] [PubMed] [Google Scholar]
- 6. Boof, M.L. , van Lier, J.J. , English, S. , Fischer, H. , Ufer, M. & Dingemanse, J. Absorption, distribution, metabolism, and excretion of cenerimod, a selective S1P1 receptor modulator in healthy subjects. Xenobiotica 50, 947–956 (2020). [DOI] [PubMed] [Google Scholar]
- 7. Lott, D. , Juif, P.E. , Dingemanse, J. & Krause, A. Modelling pharmacokinetics and pharmacodynamics of the selective S1P1 receptor modulator cenerimod in healthy subjects and systemic lupus erythematosus patients. Br. J. Clin. Pharmacol. 86, 791–800 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lott, D. , Lehr, T. , Dingemanse, J. & Krause, A. Modeling tolerance development for the effect on heart rate of the selective S1P1 receptor modulator ponesimod. Clin. Pharmacol. Ther. 103, 1083–1092 (2018). [DOI] [PubMed] [Google Scholar]
- 9. Piali, L. et al Cenerimod, a novel selective S1P1 receptor modulator with unique signaling properties. Pharmacol. Res. Perspect. 5, e00370 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gibaldi, M. & Perrier, D. Pharmacokinetics. (Marcel Dekker, New York, NY, 1982). [Google Scholar]
- 11. Kovarik, J.M. et al Ethnic sensitivity study of fingolimod in white and Asian subjects. Int. J. Clin. Pharmacol. Ther. 45, 98–109 (2007). [DOI] [PubMed] [Google Scholar]
- 12. Novartis . Mayzent Prescribing Information. https://www.mayzent.com/index.jsp?utm_source=bing&utm_medium=paid&utm_campaign=Mayzent.com_Brand_Bing_7.2020;S;PH;BR;NER;DTC;BR&utm_term=Brand_Phrase%20%7c%20mayzent&&msclkid=c04f35d2a1211c2f7a056a92a8d5917f&gclid=c04f35d2a1211c2f7a056a92a8d5917f&gclsrc=3p.ds. March 2019.
- 13. Reyes, M. , Hoch, M. , Brossard, P. & Dingemanse, J. Effects of ethnicity and sex on the pharmacokinetics and pharmacodynamics of the selective sphingosine‐1‐phosphate receptor 1 modulator ponesimod: a clinical study in Japanese and Caucasian subjects. Pharmacology 94, 223–229 (2014). [DOI] [PubMed] [Google Scholar]
- 14. Celgene . Zeposia Prescribing Information. https://www.mayzent.com/discover‐mayzent?utm_source=bing&utm_medium=paid&utm_campaign=Mayzent.com_CBrand_Competitive_Bing_7.2020;S;PH;BR;NER;DTC;COM&utm_term=Zeposia_Phrase%20%7c%20zeposia&&msclkid=5e3a7d9011c61b57d85722d01bf36a67&gclid=5e3a7d9011c61b57d85722d01bf36a67&gclsrc=3p.ds. March 2020.
- 15. Mizutani, T. PM frequencies of major CYPs in Asians and Caucasians. Drug Metab. Rev. 35, 99–106 (2003). [DOI] [PubMed] [Google Scholar]
- 16. Van Booven, D. et al Cytochrome P450 2C9‐CYP2C9. Pharmacogenet. Genomics 20, 277–281 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]


