Abstract
Nonalcoholic fatty liver disease (NAFLD) is a highly prevalent, dynamic disease that occurs across the age spectrum and can lead to cirrhosis and hepatocellular carcinoma. There are currently no US Food and Drug Administration (FDA) approved treatments for NAFLD; however, this is a field of active research. This review summarizes emerging pharmacotherapies for the treatment of adult and pediatric NAFLD. Investigated pharmacotherapies predominantly target bile acid signaling, insulin resistance, and lipid handling within the liver. Three drugs have gone on to phase III trials for which results are available. Of those, obeticholic acid is the single agent that demonstrates promise according to the interim analyses of the REGENERATE trial. Obeticholic acid showed reduction of fibrosis in adults with nonalcoholic steatohepatitis (NASH) taking 25 mg daily for 18 months (n = 931, reduction in fibrosis in 25% vs. 12% placebo, P < 0.01). Ongoing phase III trials include REGENERATE and MAESTRO‐NASH, which investigates thyroid hormone receptor‐β agonist MGL‐3196. Outcomes of promising phase II trials in adults with NASH are also available and those have investigated agents, including the fibroblast growth factor (FGF)19 analogue NGM282, the GLP1 agonist liraglutide, the FGF21 analogue Pegbelfermin, the sodium glucose co‐transporter 2 inhibitor Empagliflozin, the ketohexokinase inhibitor PF‐06835919, the acetyl‐coenzyme A carboxylase inhibitor GS‐0976, and the chemokine receptor antagonist Cenicriviroc. Completed and ongoing clinical trials emphasize the need for a more nuanced understanding of the phenotypes of subgroups within NAFLD that may respond to an individualized approach to pharmacotherapy.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THIS TOPIC?
NAFLD affects up to one billion individuals globally and is the most common cause of elevated liver enzymes in children. Mechanisms contributing to hepatic steatosis and fibrosis include increased dietary fat intake, alterations in lipid generation and transport, and the influences of insulin resistance, inflammation, and oxidative stress. Despite prior clinical trials targeting these mechanisms, consistently effective pharmacotherapy remains lacking.
WHAT QUESTION DID THIS STUDY ADDRESS?
This study addresses the question of what novel pharmacotherapies for NAFLD in stage 2 or 3 clinical trials exist and investigates their strengths and limitations.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
This study shows that novel pharmacotherapies for NAFLD are limited and not yet ready for clinical use. The only phase 3 trial to date with encouraging results is REGENERATE; this study shows the potential for obeticholic acid over placebo to improve hepatic fibrosis but is limited by its small effect in only one quarter of subjects.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
This study shows that novel pharmacotherapy for NAFLD is still an active and highly‐needed area of ongoing investigation. This study suggests that in the future, patients with NAFLD will likely require combination therapy in order to address the multiple and potentially individualized mechanisms of their hepatic steatosis and fibrosis.
Nonalcoholic fatty liver disease (NAFLD) affects up to 1 billion individuals worldwide 1 and is the most common cause of elevated liver enzymes in children. End‐stage liver disease and/or hepatocellular carcinoma secondary to NAFLD are among the leading causes of liver failure in adults in the United States. Effective and affordable treatments to reduce the medical and societal burden of this increasingly prevalent condition are urgently needed. 2 , 3 NAFLD represents a spectrum of diseases ranging from hepatocellular fat deposition (nonalcoholic fatty liver) to steatohepatitis (nonalcoholic steatohepatitis (NASH) with or without fibrosis) to cirrhosis. NAFLD is a dynamic disease that can progress to cirrhosis, but in a small percent of patients it may also spontaneously regress. 4 With the exception of cirrhosis, the histologic findings associated with NAFLD/NASH are generally reversible with treatment. The most important treatment is lifestyle modification leading to weight loss and enhanced physical activity. 5 Lifestyle interventions, however, have proven challenging both to implement and to sustain over time; this underscores the need for effective pharmacotherapy. 6 The objective of this review is to summarize the current medical literature on emerging pharmacotherapy for the treatment of both adult and pediatric patients with NAFLD.
PATHOPHYSIOLOGY
NAFLD is the result of multiple, often concurrent, hits to the liver that originate from dietary overnutrition, impaired hormonal signaling (particularly insulin), defects in cellular metabolism that regulate hepatocellular lipid handling, changes in the intestinal microbiome in the context of a compromised intestinal barrier, and augmented proinflammatory/profibrotic processes. The pathogenesis of NAFLD has been extensively reviewed elsewhere. 7 Briefly, steatosis occurs when there is an imbalance between the mechanisms that regulate lipid handling within the liver. Sources of hepatic fat include dietary intake, fatty acid flux to the liver from adipose tissue, as well as hepatic de novo lipogenesis (e.g., conversion of carbohydrates into fatty acids by the hepatocyte). 8 Insulin resistance is an important driver of lipogenesis in the context of NAFLD. 9 Mechanisms to attenuate hepatic steatosis include increased mitochondrial fatty acid oxidation, decreased hepatic lipogenesis, and enhanced lipid export from the hepatocytes in the form of very low‐density lipoproteins. When there is an imbalance between input/synthesis vs. export/oxidation of hepatocellular fat, hepatic steatosis develops. Furthermore, the accumulation of toxic lipid species can trigger the development of lipotoxicity, oxidative stress, as well as immune cell and stellate cell activation, ultimately leading to the development of hepatic inflammation and fibrosis. 10 Gene expression regulated by PNPLA3, and TM6SF2, bile acid signaling, AMPK signaling, insulin, and adipokine signaling modulates hepatocellular lipid handling. 11 , 12 Recent data suggest that intestinal health and particular gut barrier integrity is crucial to prevent microbial signals (e.g., endotoxemia and/or bacterially derived ethanol) from contributing to the pathogenesis of NAFLD. 13 , 14 Treatment approaches investigated to date have targeted many of these mechanisms (Figure 1 ).
Figure 1.
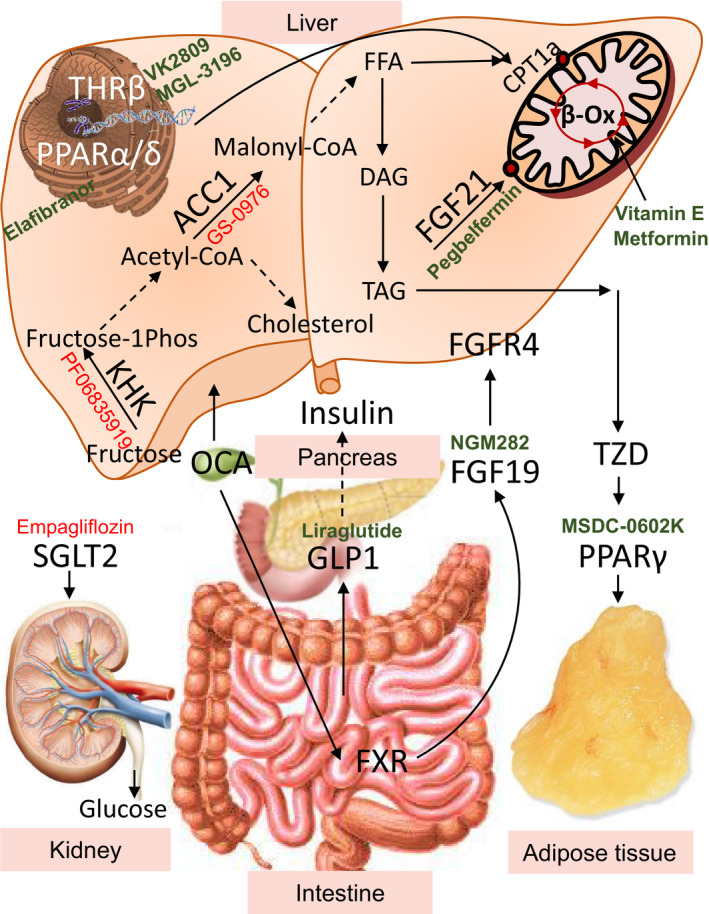
Tissue specific pathways targeted by the novel pharmacotherapies. Novel therapeutics under investigation for treatment of nonalcoholic fatty liver disease target metabolic pathways in the liver and other tissue. Fructose metabolized in the liver supports de novo lipogenesis. PF‐06835919 targets ketohexokinase (KHK), the rate‐limiting enzyme of fructose metabolism. Conversion of acetyl‐CoA to malonly‐CoA by acetyl‐CoA carboxylase (ACC) is the first committed step in lipogenesis. Malonyl‐CoA is an inhibitor of carnitine palmitoyltransferase 1 alpha, the rate‐limiting enzyme of fatty acid metabolism. GS‐0976 is an inhibitor of ACC, thus it prevents lipogenesis and increases fatty acid oxidation. However, increased acetyl‐CoA pool can serve as a substrate for cholesterol synthesis. Once fat is made in the liver, it can be oxidized in the mitochondria to generate energy. Elafibranor is a peroxisome proliferator‐activated receptors alpha/delta (PPARα/δ) agonist that increases fatty acid oxidation. MGL‐3196 and VK2809 are thyroid hormone receptor beta (THR‐β) agonists that increase the transcription of genes to upregulate mitochondrial fatty acid oxidation. Fibroblast growth factor 19 (FGF19) analogue NGM282 and Pegbelfermin, as well as PEGylated human FGF21, work in the liver and other tissues via fibroblast growth factor receptor 4 (FGFR4) to increase glucose utilization and fatty acid oxidation. Whereas FGF21 is made in the liver, FGF19 is made in the intestine through FXR activation. Obeticholic acid (OCA) is a semisynthetic bile acid that is a highly potent FXR agonist in the liver and the intestine. Activation of FXR in the liver can decrease conversion of cholesterol to bile acids, so a common side effect of OCA is increased serum cholesterol. Lipids made in the liver, but not oxidized, can be excreted from the liver to be utilized or stored in other organs, such as adipose tissue. Thiazolidinediones (TZDs) are PPAR gamma (PPARγ) agonists that increase the storage of fatty acids in adipocytes. As such, they create a sink for excess fat from the liver to be deposited in the adipose tissue; thus, they can also cause weight gain. MSDC‐0602K is a second generation PPARγ agonist that has fewer systemic side effects. Liraglutide is an analogue of the gut‐secreted hormone glucagon‐like peptide 1 (GLP‐1), which increases glucose‐stimulated insulin secretion. It also decreases gastric emptying leading to decreased appetite and weight loss. Empagliflozin is a sodium‐glucose transport protein 2 (SGLT‐2) inhibitor that works on the kidney to prevent glucose reabsorption, leading to calorie loss via glucosuria. Agonists are in GREEN color whereas antagonists are in RED.
SUMMARY OF PRIOR TRIALS INVESTIGATING MEDICAL OPTIONS FOR THE TREATMENT OF NAFLD
Various treatments have been investigated for the management of both adult and pediatric NAFLD.
Metformin: Metformin has been studied in both adults and children with NAFLD. In adults, a wealth of data have shown that metformin can improve serum aminotransferase elevations and ameliorate insulin resistance; however, it does not have a meaningful impact on liver histology. 15 , 16 Similarly, in children, metformin does not alter histological outcomes. 17
Thiazolidinediones (TZD): Pioglitazone has been studied in adults with NASH (with and without type 2 diabetes mellitus (T2DM)) and is superior to placebo in resolving NASH, reducing (necro)inflammation, and fibrosis. 18 Its use is associated with weight gain, however, a significant concern for patients with NAFLD, as well as osteopenia and increased risk of fractures in older adults.
Vitamin E: The antioxidant vitamin E has been studied in both nondiabetic adults and children with NAFLD. In adults, vitamin E is superior to placebo in improving histologic components of NASH. However, due to the lack of data from patients with diabetes, its use is not recommended in diabetics with NASH. 18 In children, vitamin E is also successful at improving histology (e.g., NASH resolution) in a proportion of patients. 17
Other treatments: Various other treatments have been studied for the treatment of NAFLD/NASH. These include probiotics/prebiotics/synbiotics and omega‐3 polyunsaturated fatty acids, among others. Probiotics and prebiotics have a beneficial impact on serum aminotransferase levels and other markers of metabolic dysregulation but have not been studied adequately in well‐designed trials with histologic end points. 19 Omega‐3 fatty acids have been extensively studied, predominantly in adults, and although they are beneficial from a steatosis reduction stand point, they do not impact key histological outcomes such as inflammation and fibrosis. 20
NOVEL THERAPIES
There are multiple novel pharmacotherapies in various phases of investigation (Figure 2 ). The drugs that have gone through phase III investigations are summarized in Table 1 . Herein, we discuss the most notable medications studied grouped by mechanism of action.
Figure 2.
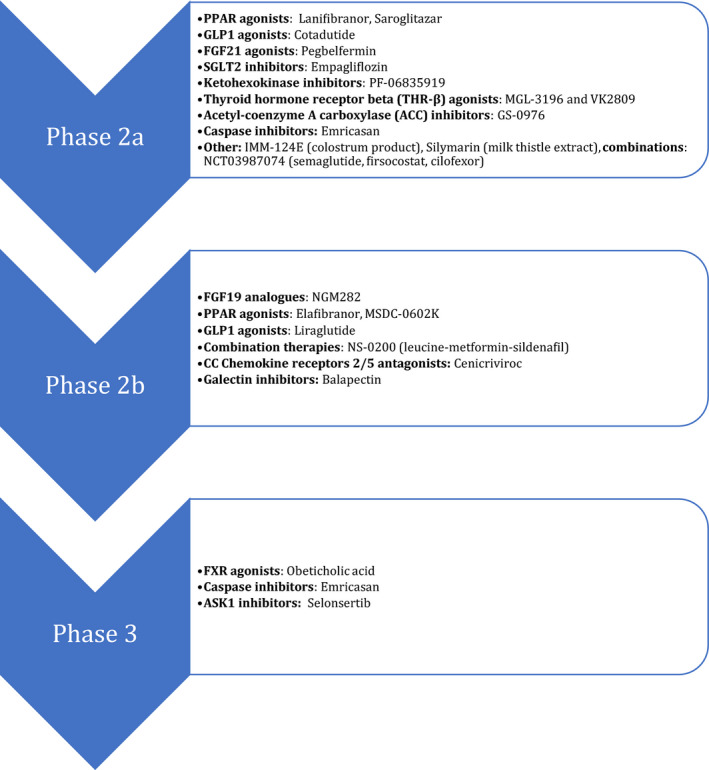
Summary of novel medications at the various stages of investigation (active or completed).
Table 1.
Phase III trials for the treatment of NAFLD/NASH
| Study/funding | Population | Intervention/duration | Comparison | Primary outcome | Results |
|---|---|---|---|---|---|
|
Regenerate Younossi et al. Lancet 2019 Intercept Pharmaceuticals |
NASH with stages 1–3 fibrosis (n = 931, results of interim analysis) |
Obeticholic acid:
×18 months |
Placebo |
|
25 mg obeticholic acid superior to placebo at improving fibrosis (seen in 25% vs. 12% on placebo, P < 0.01) |
|
Stellar‐3 and 4 Harrison et al. J Hepatol 2020 Gilead |
NASH and bridging fibrosis (3) (n = 802) NASH and compensated cirrhosis (4) (n = 877) |
Selonsertib:
×48 weeks |
Placebo |
|
Selonsertib not superior to placebo |
|
ENCORE‐PH Garcia‐Tsao et al. J Hepatol 2019 Conatus Pharmaceuticals |
NASH‐related cirrhosis with severe portal hypertension (n = 263) |
Emricasan:
× 24 weeks |
Placebo |
|
Emricasan not superior to placebo |
NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis.
Bile acid signaling
Obeticholic acid
Obeticholic acid (OCA) is an farnesoid X receptor (FXR) agonist that has been extensively investigated for the treatment of NAFLD. FXR agonism has been studied because FXR is a bile acid receptor that exerts multiple beneficial metabolic effects. FXR contributes to glucose regulation at both the hepatic (regulating glycogenolysis and gluconeogenesis) and the peripheral level (modulating insulin sensitivity in the muscle and the adipose tissue). It facilitates intrahepatic lipid handling (balancing de novo lipogenesis and fatty acid oxidation) and also exerts anti‐inflammatory effects. 12 Following the encouraging results of the “FLINT” trial, a phase IIb randomized controlled trial (RCT), which showed superiority of 25 mg of OCA vs. placebo at reducing NAFLD activity score (NAS) by 2 points without fibrosis worsening at 72 weeks of treatment in adults with NASH, 21 a phase III trial followed. The latter, called “REGENERATE,” is currently underway; the results of its interim analysis recently became available. 22 In REGENERATE, 10 mg of OCA daily were compared against 25 mg of OCA daily vs. placebo. Of the 1,968 patients enrolled, 931 with stages 2 and 3 fibrosis were included in the interim analysis, which was performed at 18 months of treatment. The primary outcomes were either fibrosis improvement (≥ 1 stage) without NASH worsening or NASH resolution without fibrosis worsening. OCA at 25 mg daily was superior to placebo at improving fibrosis (outcome met in 25% of patients vs. 12% on placebo, P < 0.01); however, OCA did not meet the other primary outcome of NASH resolution. The most common side effect was mild to moderate pruritus, which affected 51% of those treated with 25 mg OCA vs. 19% of those receiving placebo.
In post hoc analyses of the phase IIb FLINT trial, of the 200 patients with paired biopsies in the beginning and at the end of the 72 week intervention, there was a trend toward increased likelihood of weight loss with the use of OCA (44% of OCA vs. 32% of placebo‐treated patients lost at least 2% of their baseline weight, P = 0.08). This was an added benefit to OCA’s therapeutic impact, in addition to reduced serum aminotransferases and improved liver histology (reduction in NAS). 23 However, OCA was associated with an increase in serum alkaline phosphatase, low‐density lipoprotein‐C (LDL‐C), and hemoglobin A1c levels. Combination treatment of OCA with a statin (e.g., 25 mg OCA + 10 mg atorvastatin) has subsequently proven efficacious in preventing the LDL‐C increase seen with OCA monotherapy. 24
NGM282
NGM282 is a humanized fibroblast growth factor (FGF)19 analogue, which acts on the same pathway as intestinal FXR agonists. Specifically, FGF19 is released following the activation of intestinal FXR, with similar downstream effects as those noted following FXR activation. 12 Following beneficial effects of treatment with FGF19 noted using magnetic resonance imaging‐proton density fat fraction (MRI‐PDFF), 25 Harrison et al. reported the results of a 12‐week open label multicenter trial of NGM282 for the treatment of NASH. 26 Patients receiving subcutaneous NGM282 at either 1 or 3 mg with paired biopsies 12 weeks apart were included in the analyses (n = 43). NGM282 at 3 mg decreased fibrosis by ≥ 1 stage without NASH worsening in 42% of patients and improved NAS by ≥ 2 points without fibrosis worsening in 63% of subjects. A 24‐week phase IIb trial of NGM282 for the treatment of NASH is currently under way (NCT03912532).
Treatments that improve insulin resistance/metabolic dysregulation
Peroxisome proliferator‐activated receptor agonists
Peroxisome proliferator‐activated receptor (PPAR) isoforms alpha, delta, and gamma are nuclear hormone receptors with the potential to ameliorate NAFLD owing to their effects on lipid metabolism (e.g., increased mitochondrial fatty acid oxidation), insulin sensitivity, and inflammation. 27 Both PPAR alpha and delta receptors facilitate fat metabolism (e.g., fatty acid oxidation), whereas PPAR delta also has anti‐inflammatory effects. 27 PPAR gamma helps regulate glucose metabolism as well as fat cell differentiation and fatty acid storage. 27 Ratziu et al. evaluated the combined PPAR α/δ agonist elafibranor in a 52 week phase II RCT that compared 120 mg vs. 80 mg of elafibranor vs. placebo in adults with NASH (n = 91–93 per arm). 28 The primary outcome was reversal of NASH (score 0 in at least 1 of the components of NAS, namely steatosis, lobular inflammation, ballooning) without fibrosis worsening. Per original protocol definition, there was no difference for any dose of elafibranor on primary outcome. However, in a post hoc analysis, a modified definition of the primary outcome was introduced showing that reversal of NASH occurred when ballooning resolved in the context of no or minimal lobular inflammation. Per this analysis, the 120 mg dose led to improvement in 19% vs. 12% in those receiving placebo (P = 0.045). There was no difference in patients receiving the 80 mg dose. Adverse outcomes were similar among intervention and control arms. Secondary effects included lower LDL‐C and triglycerides with higher high‐density lipoprotein in the intervention groups vs. control. Ongoing clinical trials include NCT03008070 assessing efficacy and safety of pan‐PPAR agonist IVA337/lanifibranor at 800 mg and 1,200 mg daily vs. placebo for 24 weeks in adult patients with moderate to severe NASH without cirrhosis. Another ongoing trial is NCT03639623, a phase IIA, single center, open‐label, single‐arm, 24‐week study to evaluate the safety, tolerability, and efficacy of PPAR alpha/gamma agonist Saroglitazar magnesium 4 mg in liver transplant recipients with NAFLD (EVIDENCES VIII).
MSDC‐0602K is a second‐generation TZD, a PPAR gamma agonist. Second generation TZDs, like MSDC‐0602K, do not have the typical TZD side effects of edema, fractures, and hypoglycemia due to their mode of action. In the EMMINENCE study, Harrison et al. investigated MSDC‐0602K in a phase IIb trial in adults with NASH (defined as NAS ≥ 4; with a score ≥ 1 in each NAS component) and fibrosis stages 1–3. 29 After 52 weeks of daily oral MSDC‐0620K at 62.5 mg vs. 125 mg vs. 250 mg vs. placebo given to n = 99 to 101 participants per arm, they found no difference in their primary outcome of improved NAS.
Glucagon‐like peptide‐1 agonists
Glucagon‐like peptide‐1 (GLP1) agonists improve glucose homeostasis by enhancing glucose‐dependent insulin secretion and inhibiting the release of glucagon from the pancreas. They also delay gastric emptying and, when used to treat T2DM, contribute to weight loss. These effects are ideal for patients with NAFLD, as they are typically overweight or obese and have insulin resistance and/or diabetes. The GLP1 agonist liraglutide was investigated in a 42‐week phase II RCT (1.8 mg subcutaneous liraglutide daily vs. placebo) for the treatment of NASH. 30 The primary outcome was definite NASH resolution without worsening of fibrosis. The relative risk of NASH resolution was 4.3 (P = 0.019) with the use of liraglutide, which also led to weight loss and was overall well‐tolerated. Cotadutide, a dual receptor agonist for GLP1 and glucagon, was recently shown to be superior to liraglutide in reducing weight and serum aminotransferases in an adult cohort of patients with diabetes. 31 These encouraging results are now being investigated further in a phase II trial specific to patients with NASH (NCT04019561).
Fibroblast growth factor 21 agonists
FGFs are hormones key to cell growth and differentiation. 32 FGF21 is transcriptionally regulated, in part, by PPAR alpha and upregulates fatty acid oxidation. FGF21 can also attenuate proinflammatory signals. Sanyal et al. investigated the FGF21 analogue pegbelfermin (BMS‐986036) given via subcutaneous injection in a 16‐week phase IIa trial. 33 The trial recruited patients with NASH, stages 1–3 fibrosis, and ≥ 10% hepatic fat fraction by MRI‐PDFF and compared 10 mg daily, vs. 20 mg weekly pegbelfermin vs. placebo (24–26 adults in each group). 33 Both daily and weekly treatments were superior to placebo in achieving the primary outcome of PDFF reduction (10 mg daily: −6.8%, P = 0.0004 vs. 20 mg weekly: −5.2, P = 0.008 vs. placebo −1.30%) with no difference for those with or without T2DM. Anti‐pegbelfermin and anti‐FGF21 antibodies were detected in the majority of patients, decreased in most by the end of treatment, and did not affect outcome. Notable adverse outcomes were diarrhea and nausea.
Sodium glucose co‐transporter 2 inhibitors
Sodium glucose co‐transporter 2 (SGLT2) inhibitors act by inhibiting reabsorption of glucose in the kidney and can also inhibit de novo lipogenesis in the liver. 34 Due to their action, they contribute to weight loss and improvements in systolic blood pressure. 35 SGTL2 inhibitors have been used for the treatment of T2DM and more recently have been investigated for the treatment of NAFLD, particularly in patients with diabetes, in small studies. Empagliflozin (10 mg daily ×20 weeks) was compared against standard treatment for diabetes in a cohort of adults with diabetes with NAFLD with a primary outcome of change in MRI‐PDFF. 36 Although empagliflozin was superior to the standard of care at reducing the fat fraction, the reduction was modest (only 5% from baseline), and the difference from the control group at 20 weeks was only 4%. No significant adverse events were noted in this study. The effect of SGLT2 inhibitors on liver histology remains to be investigated.
Fructose metabolism
Dietary fructose strongly increases hepatic de novo lipogenesis 37 , 38 and decreases hepatic fatty acid oxidation, 39 thus predisposing to development of NAFLD. The rate‐limiting enzyme of fructose metabolism is ketohexokinase (KHK), which is increased in adult subjects with biopsy‐proven NAFLD 40 and in obese adolescents with more severe forms of NAFLD. 38 In animal studies, knockdown of KHK improves NAFLD and insulin resistance. 38 , 41 Small molecule inhibitors of KHK have been of interest to pharmaceutical industry for a long time, but initial KHK inhibitors lacked specificity. 42 , 43 More recently, Pfizer developed a potent reversible inhibitor of human KHK. 44 In a phase II study of 53 subjects with NAFLD (> 6% liver fat assessed by MRI‐PDFF), subjects received either placebo or the KHK inhibitor PF‐06835919 at 75 or 300 mg of once daily dosing for 6 weeks (NCT03256526). Participants treated with 300 mg of KHK inhibitor showed a 26.5% reduction in PDFF (P = 0.039) from baseline as compared with the placebo group. A dose‐dependent trend in decrease from baseline was also observed for liver enzymes (alanine aminotransferase (ALT), aspartate aminotransferase, and gamma glutamyltransferase), in a homeostasis model of assessment of insulin resistance, and in markers of systemic inflammation (hs‐CRP, IL‐6, and increased adiponectin) in the 300 mg group relative to placebo. 45 Fructose may also directly and indirectly induce hepatic insulin resistance 46 leading to hyperinsulinemia; this further stimulates hepatic lipogenesis. 47 Due to the effect of fructose on hyperinsulinemia, the encouraging initial results of PF‐06835919 are being further investigated in a phase II study of 150 participants with MRI‐PDFF liver fat of > 8% and T2DM treated with placebo or PF‐06835919 at 150 mg or 300 mg once daily for 16 weeks (NCT03969719).
Thyroid hormone receptor
Thyroid hormone regulates glucose and lipid metabolism, food intake, and the oxidation of fatty acids. 48 Systemic thyroid hormone replacement therapy, however, is not indicated for the treatment of obesity and NAFLD due to adverse cardiac and bone effects. Recently, a more selective thyroid hormone receptor beta (THR‐β) agonist has been developed to optimize beneficial effects on the liver while minimizing the actions on heart and bone that are mainly mediated by THR alpha (THR‐α). Two THR‐β agonists, MGL‐3196 and VK2809, are currently in clinical development.
In a phase II study of 125 adults with biopsy‐confirmed NASH, MGL‐3196 at 80 mg daily dose with ± 20 mg dose adjustment resulted in 32.9% relative reduction of hepatic fat as assessed by change in MRI‐PDFF compared with 10.4% reduction in placebo‐treated patients after 12 weeks (NCT02912260). After 36 weeks of treatment, MGL‐3196 resulted in a sustained 37.3% reduction in liver fat compared with 8.5% reduction in the placebo treated group. In spite of reduced liver fat at 12 weeks, there was no difference in serum ALT; but with longer exposure at 36 weeks, a significant reduction in ALT was observed. NASH resolution, defined as ballooning score of 0, inflammation score of 0 or 1, with ≥ 2‐point reduction in NAS on liver biopsy, was a secondary end point in the study. NASH resolution was observed in 27% of patients treated with MGL‐3196 compared with 6% in those receiving placebo (P = 0.018) at 36 weeks. Similarly, NASH resolution was significantly greater (P = 0.017) in the MGL‐3196 group (46%) compared with placebo (19%) in patients who lost < 5% of body weight. Interestingly, overall improved outcomes in subjects treated with MGL‐3196 occurred with no change in total body weight, indicating that improved liver metabolism is sufficient to induce NASH resolution. Whereas several other classes of medication under development for the treatment of NASH result in dyslipidemia, treatment with MGL‐3196 resulted in reductions in multiple atherogenic lipids including LDL‐C, triglycerides, apolipoprotein B, and CIII. Side effects of MGL‐3196 therapy were minimal and mainly included transient diarrhea. These promising findings are being further explored in MAESTRO‐NASH, a phase III clinical trial in patients with NASH and stage F2–F3 fibrosis (NCT03900429).
A second THR‐β selective agonist VK2809 is being evaluated for treatment of NASH in a phase IIa clinical study (NCT02927184). In this study, 45 patients with liver fat content ≥ 8% as assessed by MRI‐PDFF, LDL‐C ≥ 110 mg/dL, and triglycerides ≥ 120 mg/dL were randomized to receive either oral VK2809 doses of 5 mg daily, 10 mg every other day, 10 mg daily, or placebo for 12 weeks. At the end of the study, median relative change from baseline in liver fat content was 53.8% for 5 mg daily (P = 0.0001), 56.5% for 10 mg every other day (P = 0.0018), and 59.7% for 10 mg daily (P = 0.0004), vs. 9.4% for placebo. 49 Surprisingly, ALT levels increased transiently at the onset of treatment, but after 12 weeks of administration, they were not different in subjects that received VK2809 or placebo. 50 This study is being followed up by VOYAGE, a phase IIb study of 337 subjects with biopsy proven NASH (NAS ≥ 4) and MRI‐PDFF liver fat fraction ≥ 8% (NCT04173065). This is a 5‐treatment arm study of placebo and VK2809 at 1.0, 2.5, 5.0, or 10 mg daily administered for 52 weeks.
Other
Acetyl‐coenzyme A carboxylase inhibitors
Acetyl‐coenzyme A carboxylase (ACC) inhibitors impede de novo lipogenesis. GS‐0976 is an ACC1 and ACC2 inhibitor investigated by Loomba et al. in a phase II clinical trial. 51 Patients with NASH and stages 1–3 fibrosis or MRI‐PDFF ≥ 8% and liver stiffness ≥ 2.5 kPa were randomized to receive 20 mg GS‐0976 (n = 49), 5 mg GS‐0976 (n = 51), or placebo (n = 26) orally once daily for 12 weeks. The primary end point of this trial was safety. The 20 mg dose was superior to placebo at reducing the MRI‐PDFF (median change in hepatic fat fraction with 20 mg: −29%, P = 0.0002; vs. 5 mg: −13%, P = 0.43; vs. placebo: −8%). Adverse outcomes were nausea, abdominal pain, diarrhea, and headache. Hypertriglyceridemia was seen in both intervention groups and was concentrated especially in participants with TG ≥ 250 mg/dL at baseline. This resolved spontaneously or with treatment by week 12.
Combination therapies
L‐leucine alters lipid and energy metabolism and insulin sensitivity through activation of the AmPK/Srtiun 1 pathway. L‐leucine in combination with metformin and sildenafil has an additive impact on the Srtiun 1pathway through endothelial nitric oxide synthase activation. NS‐0200 is a combination of leucine‐metformin‐sildenafil recently investigated by Chalasani et al. in a small phase IIb clinical trial. 52 They randomized adults (n = 24–32 per arm) with NAFLD and MRI‐PFF ≥ 15% to a 16 week course of twice daily placebo, low‐dose (1.1 g leucine/0.5 g metformin/0.5 mg sildenafil), or high‐dose NS‐0200 (1.1 g leucine/0.5 g metformin/1.0 mg sildenafil). The primary outcome of change in PDFF did not differ between treatment groups and placebo; however, dose‐dependent decreases in HgbA1c were noted in the NS‐0200 arms compared with placebo (placebo adjusted decreases of 0.21%, P = 0.002, and 0.17%, P = 0.016) for low‐dose and high‐dose, respectively). Adverse effects included blurry vision, diarrhea, nausea, and erosive gastritis.
Other ongoing clinical trials of combination therapies include phase II investigation of semaglutide, firsocostat, and cilofexor combination therapy (NCT03987074). Semaglutide is a GLP1 agonist, firsocostat is an acetyl‐CoA carboxylase inhibitor, and cilofexor is a bile acid FXR agonist.
Anti‐apoptosis/anti‐inflammatory agents
Emricasan
Emricasan is a pan‐caspase inhibitor and given the involvement of caspases in inflammation, apoptosis, and necrosis, emricasan has the potential to reduce portal pressure and fibrosis. Garcia‐Tsao et al. investigated emricasan in a phase III clinical trial of NASH‐related cirrhosis with severe portal hypertension. 53 Adults with NASH‐related compensated and decompensated (25% of participants) cirrhosis were randomized to 5, 25, or 50 mg vs. placebo orally twice daily for 24 weeks (n = 65–67 per arm). Emricasan failed to achieve the primary outcome of change in hepatic venous pressure gradient (HVPG); however, there was a modest trend toward improved HVPG in the compensated patients with higher baseline HVPG receiving any dose of Emricasan. There were no notable adverse events.
A more recent phase II clinical trial of emricasan assessed the effect of 5 mg or 50 mg twice daily vs. placebo for 72 weeks in adults (n = 105–107 per arm) with histologically‐confirmed NASH and stage F1–F3 fibrosis. 54 There was no difference in the primary outcome of improvement in fibrosis without worsening of NASH at 72 weeks.
IMM‐124E
IMM‐124E is a product from colostrum that concentrates anti‐E. coli lipopolysaccharide IgG. IMM‐124E’s proposed mechanism for NAFLD treatment is to reduce inflammation by binding bacterial endotoxins, and it has shown promise in murine models of colitis. 55 Due to the potential role of endotoxins in the pathogenesis of NAFLD, IMM‐124E is the intervention of interest in a completed but not yet published phase II RCT by Immuron Ltd. (NCT02316717).
Silymarin
Silymarin is an antioxidant extract of milk thistle (Silybum marianum) with potential in reducing lipid peroxidation and hepatic free radical injury. Navarro et al. investigated a proprietary silymarin preparation (Legalon) in a small phase II trial (SyNCH study). 56 Adults with a NAS ≥ 4 without cirrhosis were randomized to 420 mg, 700 mg, or placebo orally 3 times daily for 48–54 weeks (n = 25–27 per arm). Legalon was not superior to placebo in reaching the primary outcome of reduction in NAS by ≥ 2 points. Adverse events occurred in the majority of study participants receiving Legalon (420 mg: 65.4%, 700 mg: 55.6% vs. 48% placebo participants). The most common reported adverse events were classified as gastrointestinal or cardiac complaints (not further specified) with no serious adverse events deemed related to the study drug.
Antifibrotic medications
C‐C chemokine receptor 2 and 5 inhibitors
C‐C chemokine receptors 2 and 5 and their ligands contribute to the development of fibrosis in the context of NASH rendering inhibition of this pathway as an attractive treatment target. CENTAUR is a large phase IIb trial that investigated the effectiveness of cenicriviroc (CVC), a dual C‐C chemokine receptors 2 and 5 antagonist, for the treatment of NASH with fibrosis. In this 2‐year study, adults with NASH and fibrosis stages 1–3 were enrolled and randomized to receive 150 mg of CVC or placebo once daily for 1 year. Groups A (CVC) and C (placebo) continued with the same treatment during year 2, but group B (placebo) crossed over to CVC during year 2. At the end of year 1, twice as many patients (20% on CVC vs. 10% on placebo, P = 0.02) achieved reduction of ≥ 1 stage of fibrosis without NASH worsening. 57 At the end of year 2, the response in terms of fibrosis reduction was similar between groups A and C. However, twice as many patients who had responded to CVC during year 1 had a sustained response during year 2 compared with those receiving placebo (60% on CVC vs. 30% on placebo). No significant adverse events were noted. In summary, a small number of patients responded to the antifibrotic effect of CVC. The long‐term impact of CVC on fibrosis, a slow evolving process, remains to be determined.
Galectin inhibitors
Galectins are a group of cytosolic proteins that are upregulated in and contribute to inflammation and fibrosis. Galectin‐3 is secreted specifically by macrophages and contributes to macrophage function including in apoptosis, adhesion, and angiogenesis. Chalassani et al. performed a phase IIb double‐blinded RCT of galectin‐3 inhibitor belapectin in adults with cirrhosis and portal hypertension secondary to NASH. 58 After biweekly infusions of 2 or 8 mg/kg belapectin vs. placebo for 52 weeks (n = 54 in each arm), they found no significant differences in the primary outcome of reduction in HVPG. In subgroup analyses, patients without varices receiving 2 mg/kg belapectin experienced a reduction in HVPG compared with placebo. Adverse events were similar across all arms, including placebo, and there were no differences in complications of cirrhosis between groups.
Apoptosis signal‐regulating kinase 1 inhibitors
Apoptosis signal‐regulating kinase 1 (ASK1) inhibition is another target of interest in NASH, as ASK1 activation leads to hepatocyte apoptosis, hepatic inflammation, and fibrosis. Selonsertib (GS‐4997) is an ASK1 inhibitor. Simtuzumab, an antibody against lysyl oxidase‐like molecule 2 blocks collagen and elastin cross‐linkage contributing to fibrosis. In a proof of concept study, Loomba et al. investigated 24 weeks of selonsertib 6 or 18 mg daily orally with and without weekly 125 mg simtuzumab injections vs. simtuzumab alone in a phase II randomized open‐label trial in adults with histologically diagnosed NASH with stage 2 or 3 fibrosis without cirrhosis (n = 10–32 per arm). 59 They observed trends to reduction in one or more stages of liver fibrosis and reduction in progression to cirrhosis at 24 weeks in patients receiving 18 mg selonsertib. Simtuzumab had no effect on histology. This had also been shown in another study previously which was terminated early due to simtuzumab’s lack of efficacy 60 . Adverse effects with selonsertib were mild.
Phase III companion trials STELLAR‐3 and STELLAR‐4 investigated 6 or 18 mg daily of oral selonsertib vs. placebo for 48 weeks in adults with NASH and bridging fibrosis and NASH with compensated cirrhosis, respectively. 61 These studies had > 300 participants in each intervention arm and showed no difference with treatment in NASH resolution or progression to cirrhosis. There was a slight increase in adverse effects in the intervention groups.
SUMMARY AND CONCLUSIONS
In summary, NAFLD is an area of intense investigations for the identification of the optimal medications that, alone or in combination, can prevent disease progression and/or reverse advanced liver disease. In spite of promising data from phase II trials, the only phase III trial that has generated encouraging results to date is REGENERATE. Although obeticholic acid has so far been proven superior to placebo in improving fibrosis, it is only effective in one quarter of patients. This suggests that additional effective medications are still urgently needed, and that NAFLD may have subgroups responding differently to various medication pathways. For that reason, the results of the currently enrolling phase III trials are eagerly awaited. It is likely that in the future, patients with NAFLD will require a combination of medications with each addressing a different aspect in the pathogenesis of their particular NAFLD phenotype.
Financial Disclosure
The authors have no relevant financial disclosures.
Funding
No funding was received for this work.
Conflict of Interest
The authors declared no competing interests for this work.
References
- 1. Younossi, Z.M. et al Global epidemiology of nonalcoholic fatty liver disease‐meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology 64, 73–84 (2016). [DOI] [PubMed] [Google Scholar]
- 2. Younossi, Z. et al Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 15, 11–20 (2018). [DOI] [PubMed] [Google Scholar]
- 3. Doycheva, I. et al Nonalcoholic steatohepatitis is the most rapidly increasing indication for liver transplantation in young adults in the United States. J. Clin. Gastroenterol. 52, 339–346 (2018). [DOI] [PubMed] [Google Scholar]
- 4. Anstee, Q.M. et al From NASH to HCC: current concepts and future challenges. Nat. Rev. Gastroenterol. Hepatol. 16, 411–428 (2019). [DOI] [PubMed] [Google Scholar]
- 5. Vilar‐Gomez, E. et al Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology 149, 367–378.e5 (2015). [DOI] [PubMed] [Google Scholar]
- 6. Franz, M.J. et al Weight‐loss outcomes: a systematic review and meta‐analysis of weight‐loss clinical trials with a minimum 1‐year follow‐up. J. Am. Diet Assoc. 107, 1755–1767 (2007). [DOI] [PubMed] [Google Scholar]
- 7. Friedman, S.L. et al Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 24, 908–922 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Donnelly, K.L. et al Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Invest. 115, 1343–1351 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Smith, G.I. et al Insulin resistance drives hepatic de novo lipogenesis in nonalcoholic fatty liver disease. J. Clin. Invest. 130, 1453–1460 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marra, F. & Svegliati‐Baroni, G. Lipotoxicity and the gut‐liver axis in NASH pathogenesis. J. Hepatol. 68, 280–295 (2018). [DOI] [PubMed] [Google Scholar]
- 11. Mann, J.P. & Anstee, Q.M. NAFLD: PNPLA3 and obesity: a synergistic relationship in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 14, 506–507 (2017). [DOI] [PubMed] [Google Scholar]
- 12. Arab, J.P. et al Bile acids and nonalcoholic fatty liver disease: molecular insights and therapeutic perspectives. Hepatology 65, 350–362 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mouries, J. et al Microbiota‐driven gut vascular barrier disruption is a prerequisite for non‐alcoholic steatohepatitis development. J. Hepatol. 71, 1216–1228 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yuan, J. et al Fatty liver disease caused by high‐alcohol‐producing Klebsiella pneumoniae. Cell Metab. 30, 1172 (2019). [DOI] [PubMed] [Google Scholar]
- 15. Li, Y. et al Metformin in non‐alcoholic fatty liver disease: a systematic review and meta‐analysis. Biomed. Rep. 1, 57–64 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Musso, G. et al A meta‐analysis of randomized trials for the treatment of nonalcoholic fatty liver disease. Hepatology 52, 79–104 (2010). [DOI] [PubMed] [Google Scholar]
- 17. Lavine, J.E. et al Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA 305, 1659–1668 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chalasani, N. et al The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 67, 328–357 (2018). [DOI] [PubMed] [Google Scholar]
- 19. Loman, B.R. et al Prebiotic and probiotic treatment of nonalcoholic fatty liver disease: a systematic review and meta‐analysis. Nutr. Rev. 76, 822–839 (2018). [DOI] [PubMed] [Google Scholar]
- 20. Spooner, M.H. & Jump, D.B. Omega‐3 fatty acids and nonalcoholic fatty liver disease in adults and children: where do we stand? Curr. Opin. Clin. Nutr. Metab. Care 22, 103–110 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Neuschwander‐Tetri, B.A. et al Farnesoid X nuclear receptor ligand obeticholic acid for non‐cirrhotic, non‐alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo‐controlled trial. Lancet 385, 956–965 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Younossi, Z.M. et al Obeticholic acid for the treatment of non‐alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo‐controlled phase 3 trial. Lancet 394, 2184–2196 (2019). [DOI] [PubMed] [Google Scholar]
- 23. Hameed, B. et al Clinical and metabolic effects associated with weight changes and obeticholic acid in non‐alcoholic steatohepatitis. Aliment. Pharmacol. Ther. 47, 645–656 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pockros, P.J. et al CONTROL: a randomized phase 2 study of obeticholic acid and atorvastatin on lipoproteins in nonalcoholic steatohepatitis patients. Liver Int. 39, 2082–2093 (2019). [DOI] [PubMed] [Google Scholar]
- 25. Harrison, S.A. et al NGM282 for treatment of non‐alcoholic steatohepatitis: a multicentre, randomised, double‐blind, placebo‐controlled, phase 2 trial. Lancet 391, 1174–1185 (2018). [DOI] [PubMed] [Google Scholar]
- 26. Harrison, S.A. et al NGM282 improves liver fibrosis and histology in 12 weeks in patients with nonalcoholic steatohepatitis. Hepatology 71, 1198–1212 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tyagi, S. et al The peroxisome proliferator‐activated receptor: a family of nuclear receptors role in various diseases. J. Adv. Pharm. Technol. Res. 2, 236–240 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ratziu, V. et al Elafibranor, an agonist of the peroxisome proliferator‐activated receptor‐alpha and ‐delta, induces resolution of nonalcoholic steatohepatitis without fibrosis worsening. Gastroenterology 150, 1147 – 1159, e5 (2016). [DOI] [PubMed] [Google Scholar]
- 29. Harrison, S.A. et al Insulin sensitizer MSDC‐0602K in non‐alcoholic steatohepatitis: a randomized, double‐blind, placebo‐controlled phase IIb study. J. Hepatol. 72, 613–626 (2020). [DOI] [PubMed] [Google Scholar]
- 30. Armstrong, M.J. et al Liraglutide safety and efficacy in patients with non‐alcoholic steatohepatitis (LEAN): a multicentre, double‐blind, randomised, placebo‐controlled phase 2 study. Lancet 387, 679–690 (2016). [DOI] [PubMed] [Google Scholar]
- 31. Nahra, T. et al Effects of cotadutide (Medi0382) on biomarkers of nonalcoholic Steatohepatitis (NASH) in overweight or obese subjects with type 2 diabetes mellitus (T2DM): a 26‐week analysis of a randomized phase 2b study. Hepatology 70, 24A (2019). [Google Scholar]
- 32. Maratos‐Flier, E. Fatty liver and FGF21 physiology. Exp. Cell Res. 360, 2–5 (2017). [DOI] [PubMed] [Google Scholar]
- 33. Sanyal, A. et al Pegbelfermin (BMS‐986036), a PEGylated fibroblast growth factor 21 analogue, in patients with non‐alcoholic steatohepatitis: a randomised, double‐blind, placebo‐controlled, phase 2a trial. Lancet 392, 2705–2717 (2019). [DOI] [PubMed] [Google Scholar]
- 34. Aso, Y. et al Impact of dapagliflozin, an SGLT2 inhibitor, on serum levels of soluble dipeptidyl peptidase‐4 in patients with type 2 diabetes and non‐alcoholic fatty liver disease. Int. J. Clin. Pract. 73, e13335 (2019). [DOI] [PubMed] [Google Scholar]
- 35. Hsia, D.S. , Grove, O. & Cefalu, W.T. An update on sodium‐glucose co‐transporter‐2 inhibitors for the treatment of diabetes mellitus. Curr. Opin. Endocrinol. Diabetes Obes. 24, 73–79 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kuchay, M.S. et al Effect of empagliflozin on liver fat in patients with type 2 diabetes and nonalcoholic fatty liver disease: a randomized controlled trial (E‐LIFT Trial). Diabetes Care 41, 1801–1808 (2018). [DOI] [PubMed] [Google Scholar]
- 37. Softic, S. , Cohen, D.E. & Kahn, C.R. Role of dietary fructose and hepatic de novo lipogenesis in fatty liver disease. Dig. Dis. Sci. 61, 1282–1293 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Softic, S. et al Divergent effects of glucose and fructose on hepatic lipogenesis and insulin signaling. J. Clin. Invest. 127, 4059–4074 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Softic, S. et al Dietary sugars alter hepatic fatty acid oxidation via transcriptional and post‐translational modifications of mitochondrial proteins. Cell Metab. 30, 735–753.e4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ouyang, X. et al Fructose consumption as a risk factor for non‐alcoholic fatty liver disease. J. Hepatol. 48, 993–999 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ishimoto, T. et al High‐fat and high‐sucrose (western) diet induces steatohepatitis that is dependent on fructokinase. Hepatology 58, 1632–1643 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Maryanoff, B.E. et al Inhibitors of ketohexokinase: discovery of pyrimidinopyrimidines with specific substitution that complements the ATP‐binding site. ACS Med. Chem. Lett. 2, 538–543 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Maryanoff, B.E. et al Pyrimidinopyrimidine inhibitors of ketohexokinase: exploring the ring C2 group that interacts with Asp‐27B in the ligand binding pocket. Bioorg. Med. Chem. Lett. 22, 5326–5329 (2012). [DOI] [PubMed] [Google Scholar]
- 44. Huard, K. et al Discovery of fragment‐derived small molecules for in vivo inhibition of ketohexokinase (KHK). J. Med. Chem. 60, 7835–7849 (2017). [DOI] [PubMed] [Google Scholar]
- 45. Calle, R. , Bergman, A. , Somayaji, V. , Chidsey, K. & Kazierad, D. PS‐110‐Ketohexokinase inhibitor PF‐06835919 administered for 6 weeks reduces whole liver fat as measured by magnetic resonance imaging‐proton density fat fraction in subjects with non‐alcoholic fatty liver disease. J. Hepatol. 70, e69–e70 (2019). [Google Scholar]
- 46. Softic, S. et al Fructose and hepatic insulin resistance. Crit. Rev. Clin. Lab. Sci. 10.1080/10408363.2019.1711360. [epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Softic, S. et al Insulin concentration modulates hepatic lipid accumulation in mice in part via transcriptional regulation of fatty acid transport proteins. PLoS One 7, e38952 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Reinehr, T. Obesity and thyroid function. Mol. Cell. Endocrinol. 316, 165–171 (2010). [DOI] [PubMed] [Google Scholar]
- 49. Rohit Loomba, J.N. et al LBP‐20‐VK2809, a novel liver‐directed thyroid receptor beta agonist, significantly reduces liver fat with both low and high doses in patients with non‐alcoholic fatty liver disease: a phase 2 randomized, placebo‐controlled trial. J. Hepatol. 70, e150–e151 (2019). [Google Scholar]
- 50. Esler, W.P. & Bence, K.K. Metabolic targets in nonalcoholic fatty liver disease. Cell. Mol. Gastroenterol. Hepatol. 8, 247–267 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Loomba, R. et al GS‐0976 reduces hepatic steatosis and fibrosis markers in patients with nonalcoholic fatty liver disease. Gastroenterology 155, 1463–1473.e6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chalasani, N. et al Randomised clinical trial: a leucine‐metformin‐sildenafil combination (NS‐0200) vs placebo in patients with non‐alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 47, 1639–1651 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Garcia‐Tsao, G. et al Randomized placebo‐controlled trial of emricasan for non‐alcoholic steatohepatitis‐related cirrhosis with severe portal hypertension. J. Hepatol. 72, 885–895 (2019). [DOI] [PubMed] [Google Scholar]
- 54. Harrison, S.A. et al A randomized, placebo‐controlled trial of emricasan in patients with NASH and F1–F3 fibrosis. J. Hepatol. 72, 816–827 (2019). [DOI] [PubMed] [Google Scholar]
- 55. Spalinger, M.R. et al Administration of the hyper‐immune bovine colostrum extract IMM‐124E ameliorates experimental murine colitis. J. Crohns Colitis 13, 785–797 (2019). [DOI] [PubMed] [Google Scholar]
- 56. Navarro, V.J. et al Silymarin in non‐cirrhotics with non‐alcoholic steatohepatitis: A randomized, double‐blind, placebo controlled trial. PLoS One 14, e0221683 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ratziu, V. et al Cenicriviroc treatment for adults with nonalcoholic steatohepatitis and fibrosis: final analysis of the phase 2b CENTAUR study. Hepatology. 10.1002/hep.31108. [epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 58. Chalasani, N. et al Effects of belapectin, an inhibitor of galectin‐3, in patients with nonalcoholic steatohepatitis with cirrhosis and portal hypertension. Gastroenterology 158, 1334–1345 (2020). [DOI] [PubMed] [Google Scholar]
- 59. Loomba, R. et al The ASK1 inhibitor selonsertib in patients with nonalcoholic steatohepatitis: A randomized, phase 2 trial. Hepatology 67, 549–559 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Harrison, S.A. et al Simtuzumab is ineffective for patients with bridging fibrosis or compensated cirrhosis caused by nonalcoholic steatohepatitis. Gastroenterology 155, 1140–1153 (2018). [DOI] [PubMed] [Google Scholar]
- 61. Harrison, S.A. et al Selonsertib for patients with bridging fibrosis or compensated cirrhosis due to NASH: results from randomized Ph III STELLAR trials. J. Hepatol. 73, 26–39 (2020). [DOI] [PubMed] [Google Scholar]


