Abstract
Dry eye disease (DED) signs and symptoms are causally associated with increased ocular surface (OS) inflammation. Modulation of key regulators of aberrant OS inflammation is of interest for clinical management. We investigated the status and the potential to harness key endogenous protective factors, such as cystic fibrosis transmembrane conductance regulator (CFTR) and vitamin D receptor (VDR) in hyperosmotic stress‐associated inflammation in patients with DED and in vitro. Conjunctival impression cytology samples from control subjects (n = 11) and patients with DED (n = 15) were used to determine the status of hyperosmotic stress (TonEBP/NFAT5), inflammation (IL‐6, IL‐8, IL‐17A/F, TNFα, MMP9, and MCP1), VDR, and intracellular chloride ion (GLRX5) by quantitative polymerase chain reaction and/or immunofluorescence. Human corneal epithelial cells (HCECs) were used to study the effect of CFTR activator (genistein) and vitamin D (calcitriol) in hyperosmotic stress (HOs)‐induced response in vitro. Western blotting was used to determine the expression of these proteins, along with p‐p38. Significantly, higher expression of inflammatory factors, TonEBP, GLRX5, and reduced VDR were observed in patients with DED and in HOs‐induced HCECs in vitro. Expression of TonEBP positively correlated with expression of inflammatory genes in DED. Increased TonEBP and GLRX5 provides confirmation of osmotic stress and chloride ion imbalance in OS epithelium in DED. These along with reduced VDR suggests dysregulated OS homeostasis in DED. Combination of genistein and calcitriol reduced HOs‐induced TonEBP, inflammatory gene expression, and p‐p38, and abated VDR degradation in HCECs. Henceforth, this combination should be further explored for its relevance in the management of DED.
Dry eye disease (DED) is a chronic multifactorial condition of the ocular surface (OS), often associated with pain, discomfort and blurring of vision. 1 DED is characterized by tear‐film instability, hyperosmolarity, OS inflammation and damage, and neurosensory abnormalities. 1 DED has evolved into one of the common public health concerns with a worldwide prevalence ranging up to 50%. 2 Abnormal or exaggerated nociception or pain is the major morbidity associated with DED 3 and strategies to manage DED 4 are dependent on its severity grade, which is structured based on signs and symptoms. 1 However, there are a group of patients who have severe symptoms but with no observable signs of DED or in whom the symptoms continue to persist despite resolution of clinical signs. 5 , 6 This group presents a major challenge as they do not always respond favorably with the current management strategies and pose a significant psychological and economic burden. Hence, the lacunae in treatment modalities for such patients suggests a need for additional disease mechanism‐specific strategies to alleviate morbidity associated with DED, irrespective of the type of etiopathological factors, such as aging, autoimmune disease, contact lens use, refractive surgery, medication, nutritional status, occupation and environment. 7 , 8 DED is characterized by aberrant OS inflammation in patients 9 and increased tear osmolarity is associated with signs and symptoms of DED and OS inflammation. 10 In vivo or in vitro studies have shown the induction of inflammatory factors (e.g., TNFα and MMP9), cellular stress, and death following exposure to hyperosmotic stress. 11 , 12 , 13 Hyperosmotic stress‐induced inflammation has been shown to be mediated via an osmo‐responsive factor, TonEBP/NFAT5 (Tonicity‐responsive enhancer binding protein / nuclear factor of activated T cells‐5) in OS cells. 14
Electrolyte imbalance in tears in DED, and sodium chloride in particular, underlies the hyperosmotic stress associated with OS inflammation. 15 , 16 It is known that active ion transport by the corneal and conjunctival epithelium also contributes to basal tear production. Transport of chloride (Cl‐), a dominant anion in the tears, in OS cells is key in establishing high osmotic gradient for fluid to enter the tear film. Cystic fibrosis transmembrane conductance regulator (CFTR) is a major channel protein that regulates Cl‐ transport and balance both in the lacrimal gland and OS, 17 , 18 thus aiding in the maintenance of OS integrity. Intracellular accumulation of Cl‐ have been associated with inflammation and abnormal nociception. 19 , 20 Further, patients with cystic fibrosis (with defective CFTR) exhibit dry eye signs and symptoms along with increased OS inflammation. 21 , 22 Hence, determining Cl‐ transport status and the restoring Cl‐ balance would be beneficial in the management of DED.
The role of vitamin D—a multifunctional and naturally synthesized steroid hormone—in regulating and dampening inflammation, calcium homeostasis and cell fate, is well known. 23 Thus, vitamin D serves as an endogenous regulator of inflammation in various cells and tissues. Vitamin D brings about its effects, such as inflammation modulation and protection of barrier function, by binding to vitamin D receptor (VDR) that is present on many cell types including OS cells. 24 , 25 A number of studies report the association of vitamin D levels, including tear vitamin D and DED along with the restoration of tear film osmolarity following vitamin D supplementation. 9 , 26 , 27 , 28 However, there has also been contradicting studies regarding the relationship between serum vitamin D levels and DED signs and symptoms. 29 , 30 These studies suggest an unexplored possibility of dysregulated VDR expression or function, in addition to lower vitamin D levels, in DED. Therefore, the current study investigated the status of VDR and Cl‐ channel function on the OS of patients with DED and human corneal epithelial cells exposed to hyperosmotic stress. In addition, the study also explored the potential therapeutic effects of CFTR and VDR activation in mitigating hyperosmotic stress‐associated response in human corneal epithelial cells.
METHODS
Study design and cohort details
The cross‐sectional study was approved by Narayana Nethralaya Institutional Review Board. Subject recruitment and sample collection procedures were conducted as per institutional ethics board guidelines and in accordance with the tenets of the Declaration of Helsinki. Written informed consent was obtained prior to subject recruitment. DED diagnosis and classification was based on TFOS DEWSII. 1 DED investigations, including Schirmer’s test 1, tear film break‐up time, and corneal and conjunctival fluorescein staining were conducted and observations were recorded. Patients presenting with signs and symptoms of dry eye were included in the DED group (n = 15). The control group included volunteer subjects (n = 11) with no signs and symptoms of OS conditions. The average age of the control cohort was 44 ± 3.7 years (M/F ‐ 7/4) and the DED cohort was 48.4 ± 3.3 years (M/F ‐ 5/10). Exclusion criteria include contact lens use, allergy, ongoing ocular or systemic diseases with ocular manifestations, subjects with lacrimal gland or lid disorders, subjects who have recently undergone ocular surgery including those for refractive correction, and subjects on any form of topical medication.
Conjunctival impression cytology
Conjunctival impression cytology (CIC) samples of the lower bulbar conjunctival epithelial cells from study subjects (controls, n = 11; DED, n = 15) were obtained using EYEPRIM device (Opia Technologies, S.A.S, France) as per manufacturer’s instructions. CIC samples from nine subjects with DED were randomly allocated for RNA extraction and subsequent mRNA expression measurements of inflammatory genes by quantitative PCR (qPCR). The remaining CIC samples from six subjects with DED were randomly allocated to study the protein expression of TonEBP and VDR by immunofluorescence.
Human corneal epithelial cell culture and hyperosmotic stress induction
SV40–Immortalized human corneal epithelial cells (HCECs) were cultured using DMEM/F‐12 media (Gibco, Baltimore, MD) with antibiotics–antimycotic (Gibco) and 10% fetal bovine serum (Gibco). Hyperosmotic stress in HCECs was induced in vitro using specific media composition. Briefly, HCECs were cultured in DMEM/F‐12 media without serum supplementation for 12 hours followed by the replacement of serum free hyperosmotic stress media for another 6 or 24 hours. Hyperosmotic media was prepared by increasing NaCl concentration in serum free DMEM/F‐12 media by addition of 25, 50, or 100 mM of NaCl to obtain culture media with increased osmolarity of +50 mOsm, +100 mOsm, or +200 mOsm, respectively. CFTR activator—genistein (50 μM), CFTR inhibitor—CFTRinh‐172 (5 μM) and/or 1,25‐dihydroxyvitamin D3/calcitriol (40 nM) were added to the HCECs 2 hours prior to addition of hyperosmotic media.
Cell morphology and viability assessments
Morphology of HCECs in normal and hyperosmotic stress conditions were visualized by light microscopy using a bright field microscope (EVOS FL cell imaging system; ThermoFisher Scientific, Waltham, MA). The number of viable cells in various treatment conditions at the end of 24 hours were measured by cell counting using the trypan blue dye exclusion method and/or crystal violet staining.
RNA isolation, complementary DNA synthesis, and qPCR
The mRNA expressions of IL‐6, IL‐8, IL‐17A, IL‐17F, TNFα, MMP9, MCP1, TonEBP/NFAT5, VDR, GLRX5, GAPDH, and Actin were measured in CIC samples and HCECs by qPCR. RNA extraction was performed using TRIZOL reagent according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA). Complementary DNA (cDNA) was synthesize using iScript cDNA synthesis kit (Bio‐Rad, Philadelphia, PA). Quantitative real‐time PCR was performed using SYBR Green dye and CFX Connect real time PCR detection system (Bio‐Rad). Primer sequence details are provided in Table S1 .
Immunofluorescence and immunocytochemistry
Cells from CIC samples were transferred to charged slides and fixed using cold methanol followed by permeabilization and blocking by 5% fetal bovine serum with 0.1% Triton X‐100 for 1 hour. The cells were then incubated with either anti‐TonEBP (Abcam, Cambridge, UK) or anti‐VDR (Santa Cruz Biotechnology, Dallas, TX) primary antibody over night at 4°C followed by washing. The cells were incubated with the relevant fluorescence conjugated secondary anti‐rabbit IgG antibodies, such as CY3 (Jackson ImmunoResearch Laboratory, West Grove, PA) for TonEBP staining and Alexa Fluor 488 (Abcam) for VDR staining for 1 hour at room temperature. Images were captured (40×) using fluorescent microscope (CKX53 Inverted microscope; Olympus, Tokyo, Japan). Immunofluorescence staining of CFTR performed in human formalin‐fixed and paraffin‐embedded whole corneal tissues was obtained from Narayana Nethralaya pathology repository. The 4 μm thick sections on charged glass slides were dewaxed at 60°C, rehydrated, and antigen retrieval (heated sodium citrate buffer, pH 6) was done. Blocking was done by using 3% bovine serum albumin and 0.1% Triton X‐100 for 1 hour at room temperature. Sections were incubated with anti‐CFTR primary antibody (Abcam) overnight at 4°C and washed followed by incubation with Alexa Flour 488 conjugated anti‐mouse IgG secondary antibody (Abcam) for 1 hour at room temperature. Images were captured (20×) after mounting with Fluoroshield with DAPI (F6057‐20ML; Sigma‐Aldrich, Darmstadt, Germany) using fluorescent microscope (EVOS FL cell imaging system, ThermoFisher). CFTR protein expression was studied in HCECs using immunocytochemistry. Cells were fixed using 4% paraformaldehyde for 10 minutes at room temperature and permeabilized using 0.1% Triton X‐100 in 1% bovine serum albumin for 15 minutes, followed by washing. Peroxidase block and staining step was performed as per instructions (Dako EnVision + System‐HRP kit; Dako North America, Carpinteria, CA). The cells were washed and incubated with anti‐CFTR primary antibody (Cystic Fibrosis Foundation, University of North Carolina, Raleigh, NC) overnight at 4°C. The cells were then washed and incubated with anti‐mouse secondary antibody (horseradish peroxidase (HRP)) according to manufacturer’s instructions. Cells were counterstained using hematoxylin for 3 minutes after the final substrate reaction. Images were captured using a light microscope (Leica Microsystems, Wetzlar, Germany) and image acquisition software (DM 3000; LAS version 3.1.0).
Cell surface protein expression assessment by flow cytometry
Cell surface expression of CFTR was determined using a flow cytometer (BD FACSCanto II; BD Biosciences). Briefly, HCECs were blocked using 1% fetal bovine serum for 30 minutes and then incubated with anti‐CFTR antibody (Abcam) for 1 hour at room temperature. The cells were washed and incubation with fluorescent conjugated anti‐mouse IgG secondary antibody, Alexa Fluor 647 (Abcam) incubation for 1 hour at room temperature. Washed cells were resuspended in 1× phosphate‐buffered saline and acquired (10,000 events) by a flow Cytometer using FACSDiva software (BD Biosciences). The raw data were then analyzed using FCS Express 6 Research Edition (DeNovo software, Pasadena, CA).
Western blotting
The cells were lysed on ice using RIPA buffer for 30 minutes by intermittent vortexing and incubation on ice during this period. Protein concentration of whole cell lysates was measured using the Bradford assay (Bio‐Rad). Whole cell lysates (20 μg) for each sample was separated on 8% sodium dodecyl sulfate polyacrylamide gel electrophoresis gel. The separated proteins were transferred onto a polyvinylidene difluoride membrane followed by blocking at room temperature using 5% non‐fat milk diluted in 0.1% TRIS‐buffered saline‐Tween‐20 for an hour. This was followed by an overnight incubation with either anti‐TonEBP (Abcam), anti‐VDR (Santa Cruz), anti‐total p38 (Cell Signaling Technology, Danvers, MA), anti‐phosphorylated p38 (Cell Signaling Technology), anti‐CFTR (Abcam), anti‐tubulin (Cell Signaling Technology), or anti‐beta actin (Santa Cruz) primary antibodies at 4°C. The membranes were washed and incubated with the relevant secondary antibodies conjugated with HRP (anti‐mouse and anti‐rabbit; BosterBio, Pleasanton, CA) for an hour at room temperature. The membranes were washed and incubated with Clarity ECL Western blotting substrate (Bio‐Rad) and resulting chemiluminescence was imaged using Image quant (GE Image Quant LAS 500; GE Healthcare, Chicago, IL). Densitometry analysis was done using ImageJ software (version 6, NIH, Bethesda, MD).
Statistical analysis
The distribution status of data set was determined by Shapiro–Wilk normality test. Mann–Whitney U test, unpaired t‐test, one‐way analysis of variance with Tukey’s multiple comparisons test and Spearman rank correlation coefficient analysis were used to analyze data sets. P < 0.05 was considered as statistically significant. Statistical analyses were performed with either GraphPad Prism 6.0 (GraphPad Software, La Jolla, CA) or MedCalc version 12.5 (MedCalc Software bvba, Ostend, Belgium).
RESULTS
A significant increase in OS discomfort or pain as indicated by raised Ocular Surface Disease Index scores along with a significant reduction in the Schirmer’s test 1 and tear break up time was observed in patients with DED compared to controls (Table S2 ). CIC samples from these patients revealed the presence of significantly higher mRNA expression of IL‐6, IL‐8, IL‐17A, IL‐17F, TNFα, MCP1, and MMP9 compared to controls (Figure 1 ). Further, increased osmolarity on the OS was determined by measuring the expression of TonEBP, an osmosensitive protein that is induced in cells exposed to hyperosmotic stress. A significant increase in the mRNA expression of TonEBP (Figure 2a ) and a distinguishable increase in the protein level of TonEBP (Figure 2b ) was observed in the OS cells of patients with DED compared to controls. In addition, a positive association was observed between TonEBP and most of the inflammatory genes in patients with DED, but not in controls (Table 1 ). This suggests a functional relationship between hyperosmotic stress and inflammation in DED. This was validated in vitro using NaCl‐induced hyperosmotic stress model in HCECs. A dose‐dependent increase in the induction of TonEBP expression was observed in HCECs at the end of 24 hours (Figure 2c ). A marked increase in TonEBP and phosphorylated p38 expression was only observed in higher dose of hyperosmolarity (i.e., +200 mOsm; Figure 2c ). A significant increase in the mRNA expression of IL‐6, IL‐8, IL‐17A, IL‐17F, TNFα, MCP1, and MMP9 was also observed in the HCECs exposed to hyperosmotic stress (Figure 2d ). A marked change in the cellular morphology and a significant reduction in cell viability was also observed in HCECs exposed to a higher dose of hyperosmotic stress (Figure S1 ).
Figure 1.
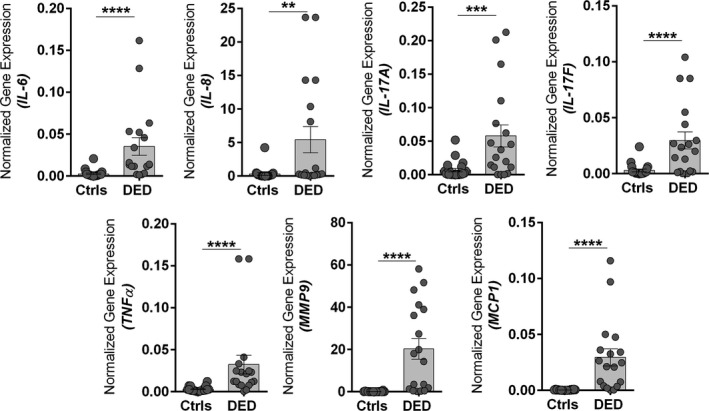
Increased ocular surface inflammation in patients with dry eye disease (DED). The graphs indicate mRNA expression levels of IL‐6, IL‐8, IL‐17A, IL‐17F, TNFα, MMP9, and MCP1 normalized to expression of GAPDH (housekeeping gene) in conjunctival impression cytology samples of control (Ctrl) subjects (n = 11) and patients with DED (n = 9). Scatter plot with bar indicates mean ± SEM and data points from two technical replicates for each subject. **P < 0.01, ***P < 0.001, ****P < 0.0001, Mann–Whitney U test.
Figure 2.
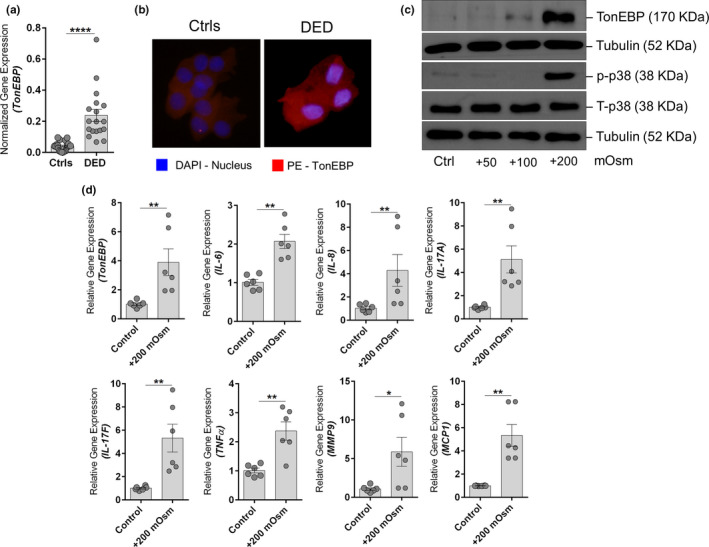
Hyperosmotic stress status and effects in patients with dry eye disease (DED) and human corneal epithelial cells (HCECs). (a) Graphs indicate the mRNA expression level of TonEBP normalized to expression of GAPDH (housekeeping gene) in conjunctival impression cytology (CIC) samples of control (Ctrl) subjects (n = 11) and patients with DED (n = 9). Scatter plot with bar indicates mean ± SEM and data points from two technical replicates for each subject. ****P < 0.0001, Mann–Whitney U test. (b) The panels exhibit the protein expression TonEBP in cells obtained by CIC from control subjects (n = 3) and patients with DED (n = 3) using immunofluorescence (40× magnification; DAPI – nuclear stain; PE – TonEBP). Images shown are representative of three different fields from three subjects in each group. Note: Sufficient cells for immunofluorescence imaging from CIC samples could only be obtained from three of the six CIC samples collected from DED subjects. (c) Panels shows the protein level of TonEBP, phosphorylated p38, total p38, and tubulin in HCECs following exposure to different doses of hyperosmotic stress (+50 mOsm, +100 mOsm, and +200 mOsm) for 24 hours in vitro. Tubulin was used as protein loading controls. The blots shown are representative images of three independent experiments. (d) Graphs indicate mean mRNA expression of TonEBP, IL‐6, IL‐8, IL‐17A, IL‐17F, TNFα, MMP9, and MCP1 normalized to expression of β‐Actin (housekeeping gene) in human HCECs in vitro following exposure to hyperosmotic stress (+200 mOsm) for 6 hours. The categories include untreated cells (Ctrl), cells under hyperosmotic stress (+200 mOsm). Scatter plot with bar indicates mean ± SEM and data points from two technical replicates for each of the three independent experiments. *P < 0.05, **P < 0.01, Mann–Whitney U test. The graphs shown in panel (d) are controls and + 200 mOsm groups from experiments shown in Figure S4 b.
Table 1.
Association between the expression of TonEBP and inflammatory factors in patients with DED
| TonEBP | ||||
|---|---|---|---|---|
| Controls | DED | |||
| R | P value | r | P value | |
| IL‐6 | 0.400 | 0.223 | 0.776 | 0.0002 |
| IL‐8 | 0.445 | 0.169 | 0.075 | 0.766 |
| IL‐17A | −0.055 | 0.873 | 0.639 | 0.025 |
| IL‐17F | −0.555 | 0.076 | 0.561 | 0.015 |
| TNF‐α | −0.200 | 0.555 | 0.424 | 0.080 |
| MMP9 | 0.552 | 0.098 | 0.456 | 0.057 |
| MCP1 | 0.091 | 0.790 | 0.598 | 0.009 |
DED, dry eye disease.
Control – n = 11; DED – n = 9.
P < 0.05 is statistically significant; r, Spearman correlation coefficient. Bold indicates statistically significant correlation.
Vitamin D mediates its immunomodulatory and anti‐inflammatory effects via the interaction of its activated form with VDR. The mRNA expression of VDR was observed to be increased in both CIC samples of patients with DED (Figure 3a ) and in HCECs under hyperosmotic stress (Figure 3b ). However, a discernible decrease in the protein levels of VDR was observed in patients with DED compared to controls (Figure 3c ). Similarly, hyperosmolarity dose‐dependent decrease in VDR protein expression was also observed in HCECs in vitro (Figure 3 d,e). These observations suggest abnormally enhanced degradation for VDR protein in OS cells under hyperosmotic stress, as in DED. Hence, the rescuing and stabilizing VDR protein from undergoing degradation due to hyperosmotic stress would be beneficial.
Figure 3.
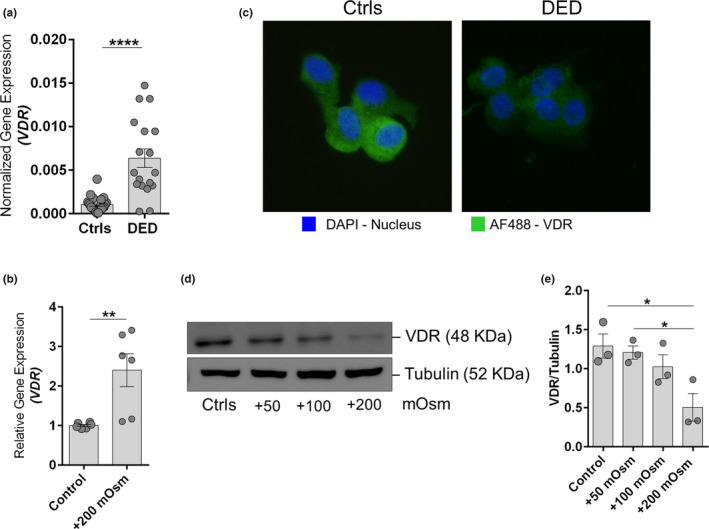
Decreased vitamin D receptor expression in patients with dry eye disease (DED) and in human corneal epithelial cells (HCECs) under hyperosmotic stress. (a) The graph indicates mRNA expression level of vitamin D receptor (VDR) normalized to expression of GAPDH (housekeeping gene) in conjunctival impression cytology (CIC) samples of control (Ctrl) subjects (n = 11) and patients with DED (n = 9). Scatter plot with bar indicates mean ± SEM and data points from two technical replicates for each subject. ****P < 0.0001; Mann–Whitney U test. (b) Graph indicates mean mRNA expression of VDR normalized to expression of β‐Actin (housekeeping gene) in HCECs in vitro following exposure to hyperosmotic stress (+200 mOsm) for 6 hours. Scatter plot with bar indicates mean ± SEM and data points from two technical replicates for each of the three independent experiments. **P < 0.01; Mann–Whitney U test. (c) The panels exhibit the protein expression VDR in cells obtained by CIC from control subjects (n = 3) and patients with DED (n = 3) using immunofluorescence (40× magnification; DAPI – nuclear stain; AF488 – VDR). Images shown are representative of three different fields from three subjects in each group. Note: Sufficient cells for immunofluorescence imaging from CIC samples could only be obtained from three of the six CIC samples collected from DED subjects. (d) Panels shows the protein level of VDR and tubulin in HCECs following exposure to different doses of hyperosmotic stress (+50 mOsm, +100 mOsm, and +200 mOsm) for 24 hours in vitro. Tubulin was used as protein loading controls. The blots shown are representative images of three independent experiments. (e) Graph indicates quantification of protein expression of the immunoblot by densitometry analysis. The expression of the VDR protein is indicated as ratio of respective protein to tubulin (indicated in the Y‐axis). Scatter plot with bar indicates mean ± SEM and data point from each of the three independent experiments. *P < 0.05, one‐way analysis of variance (ANOVA) with Tukey’s multiple comparisons test. The categories include untreated cells (Control), cells under hyperosmotic stress (+200 mOsm).
Imbalances in intracellular ions, such as Cl‐ act as second messengers to modulate the expression of variety of genes, including those associated with inflammation. Hence, the status of intracellular accumulation of Cl‐ was determined by measuring the mRNA expression of GLRX5, a chloride‐dependent gene that is increased in the presence of intracellular Cl‐. 31 A marked increase in the expression of GLRX5 was observed in OS cells of patients with DED compared to controls (Figure 4a ) and in HCECs under hyperosmotic stress (Figure 4b ). Because these observations suggested enhanced levels of intracellular Cl‐, we investigated the status of Cl‐ specific channel, CFTR. The CFTR was observed to be expressed in human corneal epithelium and HCECs (Figure S2 ). However, the whole cell and cell surface protein expression of CFTR remained unaltered in cells under hyperosmotic stress (Figure S3 ).
Figure 4.
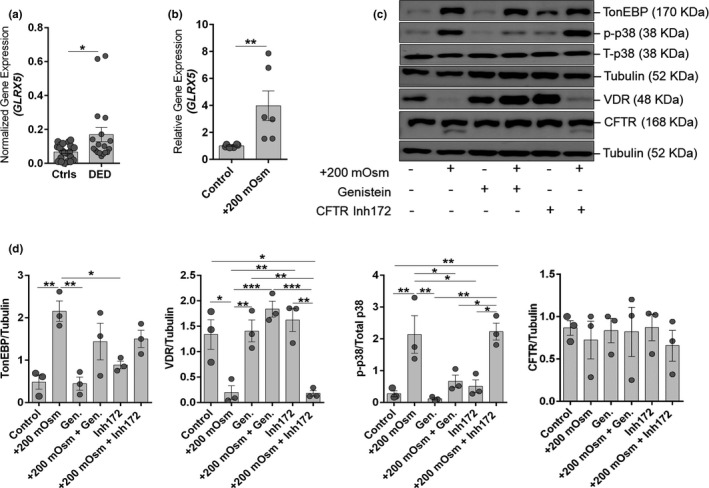
Increased intracellular chloride ion responsive gene expression in patients with dry eye disease (DED) and in human corneal epithelial cells (HCECs) undergoing hyperosmotic stress and activation of cystic fibrosis transmembrane conductance regulator (CFTR) alleviates hyperosmotic stress induced effects in human corneal epithelial cells. (a) Graph indicate the mRNA expression level of GLRX5 normalized to expression of GAPDH (housekeeping gene) in conjunctival impression cytology samples of control subjects (n = 11) and patients with DED (n = 9). Scatter plot with bar indicates mean ± SEM and data points from two technical replicates for each subject. *P < 0.05, Mann–Whitney U test. (b) Graph indicates mRNA expression of GLRX5 normalized to expression of β‐Actin (housekeeping gene) in human HCECs in vitro following exposure to hyperosmotic stress (+200 mOsm) for 6 hours. Scatter plot with bar indicates mean ± SEM and data points from two technical replicates for each of the three independent experiments. **P < 0.01, Mann–Whitney U test. (c) Panel shows the protein level of TonEBP, vitamin D receptor (VDR), phosphorylated p38, total p38, CFTR, and tubulin in HCECs following exposure to hyperosmotic stress (+200 mOsm) with or without genistein (CFTR activator) or CFTR inh172 (CFTR inhibitor) for 24 hours in vitro. Tubulin was used as protein loading control. The blots shown are representative images of three independent experiments. (d) Panels exhibits quantification of protein expression of the immunoblot by densitometry analysis. The expression of the protein is indicated as ratio of respective protein to Tubulin (indicated in the Y‐axis). The expression of p38 was quantified by normalizing its level to total p38 expression (indicated in the Y‐axis). Scatter plot with bar indicates mean ± SEM and data point from each of the three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001, one‐way analysis of variance (ANOVA) with Tukey’s multiple comparisons test.
Genistein, an isoflavone, is a known activator of CFTR and has also been reported to improve stability of VDR. 32 , 33 Here, we demonstrate that genistein rescues hyperosmotic stress‐induced VDR degradation and reduces hyperosmotic stress‐induced phosphorylation of p38, without affecting hyperosmotic stress‐induced TonEBP expression in HCECs in vitro (Figure 4 c,d). CFTR inhibitor (CFTR inh‐172) on the other hand did not rescue hyperosmotic stress‐induced VDR degradation. On the contrary, CFTR inh‐172 enhanced hyperosmotic stress‐induced phosphorylation of p38 and induced the expression of TonEBP (Figure 4 c,d). Genistein prevented hyperosmotic stress‐induced changes in cellular morphology and density, whereas CFTR inh‐172 was observed to abet further deterioration induced by hyperosmotic stress in HCECs (Figure S4 a). Furthermore, genistein reduced hyperosmotic stress‐induced GLRX5 and inflammatory gene expression in HCECs (Figure S4 b). The observed effect of genistein was brought about without altering the expression of CFTR (Figure 4 c,d). These observations suggest that genistein may be beneficial for managing hyperosmotic stress‐induced changes in the OS.
Calcitriol, the activated form of vitamin D, in addition to the induction of VDR, was also observed to induce the mRNA, protein, and cell surface expression of CFTR in HCECs (Figure 5 ). Genistein’s ability to rescue stress‐induced VDR degradation (Figure 4 c,d) and the calcitriol’s property to induce the expression of CFTR, suggests a plausible beneficial effect when used in combination. Therefore, the beneficial effects of the combination of genistein and calcitriol on hyperosmotic stress‐induced responses was studied in HCECs. Combination treatment of genistein and calcitriol exhibited rescue of hyperosmotic stress‐induced VDR degradation and facilitated induction of VDR (P < 0.01), compared to lone use of either genistein or calcitriol (Figure 6 a,b). Combination treatment of genistein and calcitriol was able reduce hyperosmotic stress‐induced TonEBP expression (P < 0.05), compared to lone use of either genistein or calcitriol (Figure 6 a,b). It is interesting to note that combination treatment of genistein and calcitriol, and lone use of either genistein or calcitriol were able to significantly reduce hyperosmotic stress‐induced phosphorylation of p38 (Figure 6 a,b). Combination treatment of genistein and calcitriol rescued hyperosmotic stress‐induced decrease in cell viability by 34% (P < 0.01), whereas the lone use of genistein and calcitriol were able rescue by 16% and 3%, respectively (Figure 6c ). Additionally, the combination treatment of genistein and calcitriol exhibited substantial reduction in the expression of inflammatory genes induced by hyperosmotic stress in HCECs (Figure S5 a). Further, the combination treatment of genistein and calcitriol helped retain normal cellular morphology in hyperosmotic stress‐induced HCECs (Figure S5 b). Collectively, the observations indicate the benefit of genistein and calcitriol combination in mitigating the deleterious effects of hyperosmotic stress on HCECs.
Figure 5.
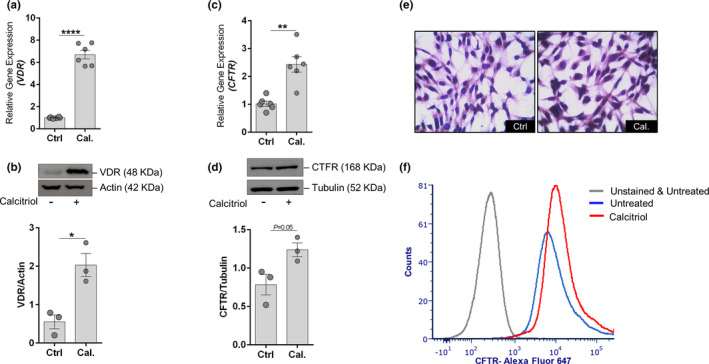
Calcitriol induces the expression of cystic fibrosis transmembrane conductance regulator (CFTR) in human corneal epithelial cells (HCECs). (a) Graph shows the mRNA expression level of vitamin D receptor (VDR) in HCECs treated with calcitriol for 6 hours. Scatter plot with bar indicates mean ± SEM and data points from two technical replicates for each of the three independent experiments. ****P < 0.0001, Mann–Whitney U test. (b) Panels shows the protein level of VDR in HCECs following calcitriol treatment for 24 hours in vitro. The bar graph represents the densitometry quantification of VDR protein normalized to its loading control beta‐actin. Scatter plot with bar indicates mean ± SEM and data point from each of the three independent experiments. *P < 0.05, unpaired t test with Welch’s correction. (c) Graph shows the mRNA expression level of CFTR in HCECs treated with calcitriol for 6 hours. Scatter plot with bar indicates mean ± SEM and data points from two technical replicates for each of the three independent experiments. **P < 0.01, Mann–Whitney U test. (d) Panels shows the protein level of CFTR in HCECs following calcitriol treatment for 24 hours in vitro. The bar graph represents the densitometry quantification of CFTR protein normalized to its loading control beta‐actin. Scatter plot with bar indicates mean ± SEM and data point from each of the three independent experiments. P = 0.05, unpaired t test with Welch’s correction. (e) Panels shows light microscopy images (40× magnification) of HCECs immunostained for CFTR following calcitriol treatment for 24 hours in vitro. Images shown are representative of three different fields from three independent experiments. (f) Histogram shows cell surface expression of CFTR using flow cytometry in HCECs following calcitriol treatment for 24 hours in vitro. Histogram shown is a representative of three independent experiments.
Figure 6.
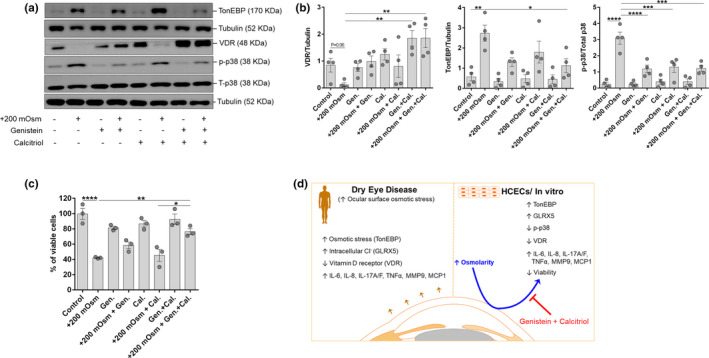
Combined effect of genistein (Gen.) and calcitriol (Cal.) mitigates hyperosmotic stress‐induced effects in human corneal epithelial cells. (a) Panel shows the protein level of TonEBP, vitramin D receptor (VDR), phosphorylated p38, total p38 and Tubulin in human corneal epithelial cells (HCECs) following exposure to hyperosmotic stress (+200 mOsm) with or without genistein and/or calcitriol for 24 hours in vitro. Tubulin was used as protein loading control. The blots shown are representative images of four independent experiments. (b) Panels exhibits quantification of protein expression of the immunoblot by densitometry analysis. The expression of the protein is indicated as ratio of respective protein to Tubulin (indicated in the Y‐axis). The expression of p38 was quantified by normalizing its level to total p38 expression (indicated in the Y‐axis). Scatter plot with bar indicates mean ± SEM and data point from each of the four independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, one‐way analysis of variance (ANOVA) with Tukey’s multiple comparisons test. (c) The graph indicates percentage of viable cells following exposure of HCECs under hyperosmotic stress (+200 mOsm) with or without genistein and/or calcitriol for 24 hours in vitro. The viability of cells was determined by crystal violet assay and scatter plot with bar indicates mean ± SEM and data point from each of the three independent experiments. *P < 0.05, **P < 0.01, ****P < 0.0001, one‐way ANOVA with Tukey’s multiple comparisons test. (d) Schema summarizes the observations made in the study. Briefly, increased osmotic stress, intracellular chloride ion and inflammatory factors, and reduced VDR in patients with DED and in HCECs exposed to hyperosmotic stress. Combined use of genistein and calcitriol mitigated hyperosmotic stress induced effects in human corneal epithelial cells, indicating the potential for its use in the management of dry eye disease.
DISCUSSION
Aberrant OS inflammation has emerged as a key driver of DED pathogenesis, signs, and symptoms. Therefore, regulating the underlying inflammation has emerged as one of the key targets in the management of DED with the use of cyclosporine and Lifitegrast. 34 Mechanistic studies have linked increased osmolarity as a primary contributor to OS inflammation. 16 , 35 Due to the variability observed in tear osmolarity measurements, 36 there has been a dearth of objective associations between increased tear osmolarity and OS inflammation. The variability was due to device‐related issues, and inter‐day and intra‐day variability in the absolute tear osmolarity status measured on the OS by the current tear osmolarity measurement systems. Hence, in the current study, we tested a molecular marker (TonEBP) that provides a measure of the cellular response to a plausible increase in osmolarity. It is well known that TonEBP is an osmo‐responsive factor that is induced when the cells are exposed to increase osmolarity. 37 However, TonEBP is also known to induce the expression of inflammatory genes TNFα, IL‐6, IL‐8, and MCP1 through TonEBP, NFκB, and MAPK pathways in many cell types leading to disease pathogenesis. 13 In addition, hyperosmotic stress have been shown to induce OS inflammation and cell death. 11 , 12 , 38
In the current study, we have observed that the levels of TonEBP were significantly higher in patients with DED (at both protein and mRNA level) compared to controls, and further, it was also seen to have positive correlation with inflammatory factor expression in patients with DED and HCECs exposed to hyperosmotic stress. This is a critical molecular evidence associating increase in tear osmolarity and inflammatory status of the ocular surface in patients with DED. This observation was corroborated in vitro using the HCEC hyperosmolar stress model, which also demonstrated the activation of the stress‐associated kinase, p38. Studies have reported the therapeutic benefits of TonEBP modulation in the management of hyperosmotic stress‐induced inflammation and associated effects in OS disease models. 39 , 40 This suggests that agents modulating the TonEBP levels in cells exposed to hyperosmotic stress would be beneficial.
The aqueous part of the tear contains soluble electrolytes secreted by the lacrimal gland, meibomian gland, and the apical cells of the OS. Maintaining the osmotic balance of electrolyte between the tear film and OS epithelial is very important for regulating cell function and homeostasis. Any ionic imbalance of electrolytes leads to pathologies like dry eye disease. 16 Tear film hyperosmolarity due to increase in electrolytes, such as sodium chloride, is regarded as a key mechanism leading to OS damage and inflammation. 15 Various functional ion channels, including the Cl‐ channels, are expressed on OS epithelial cells to balance the electrolyte imbalance. Cell surface chloride channels play a major role in maintaining cellular homeostasis. Among all chloride channels expressed, CFTR, a major chloride channel in epithelial cells are also expressed on the apical side of the OS cells to efflux the chloride ions. 18 , 41 In airway epithelial cells, increase in intracellular chloride ions is known to increase inflammation by directly activating SGK1 and NFκB. 19 Loss of either expression or function of CFTR leads to increased inflammation. 42 , 43 Furthermore, defect in CFTR channels leads to dry eye like symptoms in human as well as in animal models. 17 , 21 , 22 In the current study, we observed an increase in the accumulation of intracellular Cl‐ as seen with an increase in GLRX5 expression in both patients with DED and HCECs exposed to hyperosmotic stress. Although increase in intracellular Cl‐ has been observed in hyperosmotic stress conditions, the effect of hyperosmotic stress on the CFTR expression remains inconclusive. 44 The total CFTR protein expression and cell surface expression remained unaffected by hyperosmotic stress in HCECs in our current study. This suggested the need for CFTR potentiators to reduce the intracellular Cl‐ by facilitating its efflux. Activation of CFTR by various agents have been shown to improve DED phenotype in experimental preclinical models. 45
Vitamin D plays a critical role in regulating inflammatory response; hence, it has been studied with reference to a variety of diseases, including DED. Although a discordance in the association between reduced serum vitamin D levels and DED signs and symptoms was reported, 29 , 30 , 46 we had shown that the tear film levels rather than serum vitamin D levels exhibited stronger association with DED signs and symptoms. 9 To further the concept of investigating the local status of vitamin D homeostasis, we investigated the levels of VDR in patients with DED. VDR mediates the cellular response induced by vitamin D and absence of VDR increased basal level of inflammation, rendering cells more susceptible to stress. 47 Reports suggest that vitamin D can reduce hyperosmotic stress‐induced inflammation, 47 however, the effect of the former on VDR protein expression has not been explored. In the current study, we report that the protein expression of VDR was markedly reduced in the patients with DED and in HCECs exposed to hyperosmotic stress, albeit with increased mRNA expression of VDR. This suggests reduced VDR stability in hyperosmotic conditions and it could be one of the critical reasons behind increased inflammatory state or the reduced efficacy of vitamin D supplementation in DED. Hence, agents that could stabilize VDR would be crucial in mediating vitamin D effects in DED.
Genistein is a well‐known CFTR activator, which increases the CFTR channel opening time and helps in improving chloride transport. 48 In addition, genistein also increased the VDR half‐life by stabilizing the VDR protein. 32 , 33 Hence, genistein was used to mitigate hyperosmotic stress‐associated response in the current study, as demonstrated by the decrease in GLRX5, phospho‐p38, and inflammatory factors along with VDR stabilization. On the other hand, vitamin D is shown to increase CFTR expression, regulate chloride channel, and reduce inflammation in the absence of CFTR. 49 , 50 Hence, a combined treatment of genistein and vitamin D (calcitriol) was used to mitigate hyperosmotic stress‐induced response through their complimenting roles. Interestingly, the combination treatment exhibited a statistically significant reduction in the expression of hyperosmotic stress‐induced TonEBP, compared to lone treatment of either genistein or calcitriol. The combination treatment also improved the cell viability and cell morphology of cells under hyperosmotic stress. Genistein and vitamin D can be considered for their unique therapeutic potential in DED management as both these agents render their beneficial effects by enhancing the cells’ endogenous stress adaption and anti‐inflammatory properties, as summarized in schematic Figure 6d . Despite various reports indicating the tolerable use of genistein and vitamin D combination in humans, the combination is yet to be studied for its direct use on the eye as an ophthalmic drug, such as topical eye drops, in humans. Therefore, an appropriately designed study should be conducted to determine the safe dose and regimen of this combination for direct ophthalmic use prior to its exploration for the management of DED.
Funding
This work was supported by Early Career Research Award, DST‐SERB, Government of India to S.S. VGST, Govt. of Karnataka to A.G. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest
The authors declare no competing interests for this work.
Author Contributions
T.P., S.S., A.S.G., and E.J.R.N. wrote the manuscript. S.S., A.S.G., R.S., and E.J.R.N. designed the research. T.P., A.P.N., and S.D. performed research. T.P., S.S., and A.G. analyzed data. E.J.R.N., S.S., and A.S.G. contributed new reagents/analytical tools.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Dry eye disease (DED) is a public health issue. Ocular surface (OS) discomfort is the major morbidity and a subset of patients do not respond to available treatments. Aberrant OS inflammation and osmolarity have emerged as key drivers of DED.
WHAT QUESTION DID THIS STUDY ADDRESS?
Identification of additional disease‐specific mechanisms for novel and effective therapeutic options; molecular biomarker for OS osmolarity status.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
TonEBP can serve as a biomarker for OS osmolarity, particularly in the absence of a reliable tool to assess the latter. Genistein‐calcitriol can be used to mitigate hyperosmotic stress‐mediated inflammation and re‐establish homeostatic status of intracellular chloride ion balance and vitamin D receptor in DED.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
The potential to harness key endogenous protective factors or modulate disease‐specific mechanisms in the management of DED.
Supporting information
Fig S1
Fig S2
Fig S3
Fig S4
Fig S5
Table S1‐S2
References
- 1. Craig, J.P. et al TFOS DEWS II definition and classification report. Ocul. Surf. 15, 276–283 (2017). [DOI] [PubMed] [Google Scholar]
- 2. Stapleton, F. et al TFOS DEWS II epidemiology report. Ocul. Surf. 15, 334–365 (2017). [DOI] [PubMed] [Google Scholar]
- 3. Belmonte, C. et al TFOS DEWS II pain and sensation report. Ocul. Surf. 15, 404–437 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jones, L. et al TFOS DEWS II management and therapy report. Ocul. Surf. 15, 575–628 (2017). [DOI] [PubMed] [Google Scholar]
- 5. Rosenthal, P. , Baran, I. & Jacobs, D.S. Corneal pain without stain: is it real? Ocul. Surf. 7, 28–40 (2009). [DOI] [PubMed] [Google Scholar]
- 6. Ong, E.S. et al Epidemiology of discordance between symptoms and signs of dry eye. Br. J. Ophthalmol. 102, 674–679 (2018). [DOI] [PubMed] [Google Scholar]
- 7. Clayton, J.A. Dry eye. N. Engl. J. Med. 379, e19 (2018). [DOI] [PubMed] [Google Scholar]
- 8. Galor, A. , Gardener, H. , Pouyeh, B. , Feuer, W. & Florez, H. Effect of a Mediterranean dietary pattern and vitamin D levels on dry eye syndrome. Cornea 33, 437–441 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Khamar, P. et al Dysregulated tear fluid nociception‐associated factors, corneal dendritic cell density, and vitamin D levels in evaporative dry eye. Invest Ophthalmol. Vis. Sci. 60, 2532–2542 (2019). [DOI] [PubMed] [Google Scholar]
- 10. Bron, A.J. et al TFOS DEWS II pathophysiology report. Ocul. Surf. 15, 438–510 (2017). [DOI] [PubMed] [Google Scholar]
- 11. Luo, L. , Li, D.Q. , Corrales, R.M. & Pflugfelder, S.C. Hyperosmolar saline is a proinflammatory stress on the mouse ocular surface. Eye Contact Lens 31, 186–193 (2005). [DOI] [PubMed] [Google Scholar]
- 12. Luo, L. , Li, D.Q. & Pflugfelder, S.C. Hyperosmolarity‐induced apoptosis in human corneal epithelial cells is mediated by cytochrome c and MAPK pathways. Cornea 26, 452–460 (2007). [DOI] [PubMed] [Google Scholar]
- 13. Choi, S.Y. , Lee‐Kwon, W. & Kwon, H.M. The evolving role of TonEBP as an immunometabolic stress protein. Nat. Rev. Nephrol 16, 352–364 (2020). [DOI] [PubMed] [Google Scholar]
- 14. Warcoin, E. et al Hyperosmolarity and benzalkonium chloride differently stimulate inflammatory markers in conjunctiva‐derived epithelial cells in vitro. Ophthalmic Res. 58, 40–48 (2017). [DOI] [PubMed] [Google Scholar]
- 15. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul. Surf. 5, 75–92 (2007). [DOI] [PubMed] [Google Scholar]
- 16. Woodward, A.M. , Senchyna, M. & Argueso, P. Differential contribution of hypertonic electrolytes to corneal epithelial dysfunction. Exp. Eye Res. 100, 98–100 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Berczeli, O. et al Novel insight into the role of CFTR in lacrimal gland duct function in mice. Invest. Ophthalmol. Vis. Sci. 59, 54–62 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cao, L. , Zhang, X.D. , Liu, X. , Chen, T.Y. & Zhao, M. Chloride channels and transporters in human corneal epithelium. Exp. Eye Res. 90, 771–779 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang, Y.L. et al Increased intracellular Cl(‐) concentration promotes ongoing inflammation in airway epithelium. Mucosal Immunol. 11, 1149–1157 (2018). [DOI] [PubMed] [Google Scholar]
- 20. Price, T.J. , Cervero, F. , Gold, M.S. , Hammond, D.L. & Prescott, S.A. Chloride regulation in the pain pathway. Brain Res. Rev. 60, 149–170 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mrugacz, M. CCL4/MIP‐1beta levels in tear fluid and serum of patients with cystic fibrosis. J. Interferon Cytokine Res. 30, 509–512 (2010). [DOI] [PubMed] [Google Scholar]
- 22. Mrugacz, M. et al IL‐8 and IFN‐gamma in tear fluid of patients with cystic fibrosis. J. Interferon Cytokine Res. 26, 71–75 (2006). [DOI] [PubMed] [Google Scholar]
- 23. Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 357, 266–281 (2007). [DOI] [PubMed] [Google Scholar]
- 24. Lu, X. & Watsky, M.A. Effects of vitamin D receptor knockout on cornea epithelium gap junctions. Invest. Ophthalmol. Vis. Sci. 55, 2975–2982 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lu, X. , Chen, Z. , Vick, S. & Watsky, M.A. Vitamin D receptor and metabolite effects on corneal epithelial cell gap junction proteins. Exp. Eye Res. 187, 107776 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bae, S.H. et al Vitamin D supplementation for patients with dry eye syndrome refractory to conventional treatment. Sci. Rep. 6, 33083 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yang, C.H. , Albietz, J. , Harkin, D.G. , Kimlin, M.G. & Schmid, K.L. Impact of oral vitamin D supplementation on the ocular surface in people with dry eye and/or low serum vitamin D. Contact Lens Anterior Eye 41, 69–76 (2018). [DOI] [PubMed] [Google Scholar]
- 28. Kizilgul, M. et al Vitamin D replacement improves tear osmolarity in patients with vitamin D deficiency. Semin. Ophthalmol. 33, 589–594 (2017). [DOI] [PubMed] [Google Scholar]
- 29. Jeon, D.H. et al Are serum vitamin D levels associated with dry eye disease? Results from the study group for environmental eye disease. J. Prevent. Med. Public Health 50, 369–376 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim, M.J. et al Association between serum 25‐Hydroxyvitamin D levels and dry eye in Korean adults: a study based on Korean National Health and Nutrition Examination Survey, 2010–2011. Korean J. Family Med. 38, 81–85 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Valdivieso, A.G. , Clauzure, M. , Massip‐Copiz, M. & Santa‐Coloma, T.A. The chloride anion acts as a second messenger in mammalian cells ‐ modifying the expression of specific genes. Cell Physiol. Biochem. 38, 49–64 (2016). [DOI] [PubMed] [Google Scholar]
- 32. Rao, A. et al Vitamin D receptor and p21/WAF1 are targets of genistein and 1,25‐dihydroxyvitamin D3 in human prostate cancer cells. Cancer Res. 64, 2143–2147 (2004). [DOI] [PubMed] [Google Scholar]
- 33. Ye, C.F. , Pan, Y.M. & Zhou, H. Regulation of vitamin D receptor and genistein on bone metabolism in mouse osteoblasts and the molecular mechanism of osteoporosis. J. Biol. Regul. Homeost. Agents 32, 497–505 (2018). [PubMed] [Google Scholar]
- 34. Periman, L.M. , Perez, V.L. , Saban, D.R. , Lin, M.C. & Neri, P. The immunological basis of dry eye disease and current topical treatment options. J. Ocul. Pharmacol. Ther. 36, 137–146 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Deng, R. et al Osmoprotectants suppress the production and activity of matrix metalloproteinases induced by hyperosmolarity in primary human corneal epithelial cells. Mol. Vis. 20, 1243–1252 (2014). [PMC free article] [PubMed] [Google Scholar]
- 36. Tashbayev, B. et al Utility of tear osmolarity measurement in diagnosis of dry eye disease. Sci. Rep. 10, 5542 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Halterman, J.A. , Kwon, H.M. & Wamhoff, B.R. Tonicity‐independent regulation of the osmosensitive transcription factor TonEBP (NFAT5). Am. J. Physiol. Cell Physiol. 302, C1–C8 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li, D.‐Q. , Chen, Z. , Song, X. , Farley, W. & Pflugfelder, S. Hyperosmolarity stimulates production of MMP‐9, IL‐1ß and TNF‐ by human corneal epithelial cells via a c‐Jun NH2‐terminal kinase pathway. Invest. Ophthalmol. Vis. Sci. 43, 1981 (2002). [Google Scholar]
- 39. Warcoin, E. , Baudouin, C. , Gard, C. & Brignole‐Baudouin, F. In vitro inhibition of NFAT5‐mediated induction of CCL2 in hyperosmotic conditions by cyclosporine and dexamethasone on human HeLa‐modified conjunctiva‐derived cells. PLoS One. 11, e0159983 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Corrales, R.M. , Luo, L. , Chang, E.Y. & Pflugfelder, S.C. Effects of osmoprotectants on hyperosmolar stress in cultured human corneal epithelial cells. Cornea 27, 574–579 (2008). [DOI] [PubMed] [Google Scholar]
- 41. Levin, M.H. & Verkman, A.S. CFTR‐regulated chloride transport at the ocular surface in living mice measured by potential differences. Invest. Ophthalmol. Vis. Sci. 46, 1428–1434 (2005). [DOI] [PubMed] [Google Scholar]
- 42. Dong, Z.W. et al CFTR‐regulated MAPK/NF‐kappaB signaling in pulmonary inflammation in thermal inhalation injury. Sci. Rep. 5, 15946 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Crites, K.S. et al CFTR knockdown induces proinflammatory changes in intestinal epithelial cells. J. Inflamm. (Lond.) 12, 62 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lassance‐Soares, R.M. et al The hypertonic environment differentially regulates wild‐type CFTR and TNR‐CFTR chloride channels. Cell. Physiol. Biochem. 26, 577–586 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chen, X. et al Nanomolar potency aminophenyltriazine CFTR activator reverses corneal epithelial injury in a mouse model of dry eye. J. Ocul. Pharmacol. Ther. 36, 147–153 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Meng, Y.F. , Lu, J. , Xing, Q. , Tao, J.J. & Xiao, P. Lower serum vitamin D level was associated with risk of dry eye syndrome. Med. Sci. Monit. 23, 2211–2216 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhang, J. , Dai, Y. , Wu, D. & Xu, J. Calcitriol, the active metabolite of vitamin D3, inhibits dry eye related corneal inflammation in vivo and in vitro. Ocul. Immunol. Inflamm. 27, 257–265 (2019). [DOI] [PubMed] [Google Scholar]
- 48. Deachapunya, C. & Poonyachoti, S. Activation of chloride secretion by isoflavone genistein in endometrial epithelial cells. Cell Physiol. Biochem. 32, 1473–1486 (2013). [DOI] [PubMed] [Google Scholar]
- 49. DiFranco, K.M. , Mulligan, J.K. , Sumal, A.S. & Diamond, G. Induction of CFTR gene expression by 1,25(OH)2 vitamin D3, 25OH vitamin D3, and vitamin D3 in cultured human airway epithelial cells and in mouse airways. J. Steroid Biochem. Mol. Biol. 173, 323–332 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Morin, G. , Orlando, V. , St‐Martin Crites, K. , Patey, N. & Mailhot, G. Vitamin D attenuates inflammation in CFTR knockdown intestinal epithelial cells but has no effect in cells with intact CFTR. Am. J. Physiol. Gastrointest. Liver Physiol. 310, G539–G549 (2016). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Fig S3
Fig S4
Fig S5
Table S1‐S2


