Abstract
Coproporphyrin‐I (CP‐I) in plasma is a sensitive and specific endogenous probe for phenotyping organic anion transporting polypeptides 1B (OATP1B, encoded by SLCO1B). A few small‐scale studies suggested that plasma CP‐I concentration is affected by OATP1B1 polymorphism, but detailed studies are lacking. In this large‐scale study, we measured plasma CP‐I concentrations in 391 subjects from the Japanese general population, and evaluated the relationship between plasma CP‐I concentrations and OATP1B1 polymorphisms to further assess the utility of plasma CP‐I concentrations as an endogenous OATP1B probe. Plasma CP‐I concentrations were 0.45 ± 0.12, 0.47 ± 0.16, 0.47 ± 0.20, 0.50 ± 0.15, 0.54 ± 0.14, and 0.74 ± 0.31 ng/mL in participants with OATP1B1*1b/*1b (n = 103), *1a/*1b (n = 122), *1a/*1a (n = 40), *1b/*15 (n = 74), *1a/*15 (n = 41), and *15/*15 (n = 11), respectively, showing an ascending rank order with significant difference (P < 0.0001). Post hoc analysis revealed significant increases in plasma CP‐I concentration in OATP1B1*1b/*15 (P = 0.036), *1a/*15 (P = 0.0005), and *15/*15 (P = 0.0003) groups compared with the OATP1B1*1b/*1b group. There was no significant difference among OATP1B genotypes in plasma concentration of 3‐carboxy‐4‐methyl‐5‐propyl‐2‐furanpropanoic acid, a uremic toxin reported to decrease OATP1B activity in vivo. These findings confirm the utility of plasma CP‐I concentrations as an endogenous biomarker for phenotyping of OATP1B activity. Plasma CP‐I concentration is potentially useful for the study of drug‐drug interactions via OATP1B or individual dose adjustment of OATP1B substrates.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Phenotyping of organic anion transporting polypeptides 1B (OATP1B) seems to be a useful tool for quantitative assessment of in vivo OATP1B activity. Several endogenous biomarkers for OATP1B phenotyping have been reported and coproporphyrin‐I (CP‐I) is especially focused as a sensitive and specific probe.
WHAT QUESTION DID THIS STUDY ADDRESS?
Does OATP1B1*15 allele, which decreases the transporting function of OATP1B1, affect plasma CP‐I concentrations in Japanese general population?
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
Plasma CP‐I concentrations showed an ascending rank order in subjects with OATP1B1*1b/*1b, *1a/*1b, *1a/*1a, *1b/*15, *1a/*15, and *15/*15, with significant difference. Post hoc analysis revealed significant increases in plasma CP‐I concentrations in OATP1B1*1b/*15, *1a/*15, and *15/*15 groups compared with the OATP1B1*1b/*1b group. Especially, substantial increase was observed in OATP1B1*15/*15.
HOW MIGHT THIS CHANGE CLINICAL PHARMA‐COLOGY OR TRANSLATIONAL SCIENCE?
The results further substantiated the utility of plasma CP‐I concentrations as an endogenous biomarker for phenotyping OATP1B activity. Plasma CP‐I concentrations can be utilized in studies of drug‐drug interactions via OATP1B and individual dose adjustment of OATP1B substrates.
Drug transporters are localized at cell membranes and involved in drug transport in various tissues such as the liver, kidneys, gastrointestinal tract, and brain endothelium. Organic anion transporting polypeptides 1B (OATP1B, encoded by SLCO1B) is a hepatic uptake transporter that substantially affect pharmacokinetics of some drugs, such as hydroxymethylglutaryl (HMG)‐CoA reductase inhibitors and some anti‐hepatitis C virus drugs. 1 , 2 , 3 , 4 , 5 , 6 In vivo OATP1B activity has large interindividual variability and is affected by environmental, physiologic, and genetic factors. For example, OATP inhibitors, such as rifampicin and cyclosporin A, inhibit OATP1B activity and increase plasma concentrations of OATP1B substrates. 7 , 8 Chronic kidney failure has been reported to decrease OATP1B activity, and 3‐carboxy‐4‐methyl‐5‐propyl‐2‐furanpropanoic acid (CMPF) is suggested as a causative substance. 9 , 10 , 11 As genetic factors, SLCO1B1 exhibits two major single nucleotide polymorphisms: A388G and T521C. These polymorphisms form four haplotypes: OATP1B1*1a (c.388A‐c.521T), OATP1B1*1b (c.388G‐c.521T), OATP1B1*5 (c.388A‐c.521C), and OATP1B1*15 (c.388G‐c.521C). 1 , 12 Several studies have revealed that OATP1B1*5 and OATP1B1*15 are associated with decreased transporting activities of OATP1B1. 12 , 13 , 14 , 15 , 16 In the Japanese population, c.521C shows strong linkage disequilibrium with c.388G (r 2 = 0.0708, D' = 0.9999). 1 Thus, OATP1B1*15 is an important polymorphism that affects individual OATP1B activity in vivo.
Phenotyping of OATP1B seems to be a useful tool for quantitative assessment of in vivo OATP1B activity. Several probes for phenotyping of OATP1B have been reported, such as probe drugs, including HMG‐CoA reductase inhibitors, and endogenous probes, including total and direct bilirubin, chenodeoxycholate 24‐glucuronide, glycochenodeoxycholate‐3‐sulfate, and coproporphyrins. 17 , 18 Especially coproporphyrin‐I (CP‐I) and CP‐III in plasma are sensitive and specific endogenous probes for phenotyping OATP1B. 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 Rifampicin increases plasma CP‐I and CP‐III concentrations to a greater extent than other endogenous compounds and rosuvastatin. 22 CP‐I has been reported to be superior to CP‐III as an endogenous OATP1B probe, because CP‐I is a selective substrate for OATP1B1 and OATP1B3, whereas CP‐III is also a substrate for OATP2B1. 19 , 20 Thus, quantification of plasma CP‐I concentration is a potentially useful tool for dose individualization of OATP1B substrates and assessment of OATP1B‐mediated drug‐drug interactions.
A few previous small‐scale studies suggested that plasma CP‐I concentration is affected by OATP1B1 polymorphism, but detailed studies are lacking. Shen et al. 25 reported a significant decrease in plasma CP‐I concentration in healthy volunteers with SLCO1B1 c.388AG compared with SLCO1B1 c.388AA, but there were no data on haplotypes of SLCO1B1 T521C. Yee et al. 27 measured plasma CP‐I concentrations in 16 healthy volunteers and found significant difference among 3 groups: SLCO1B1 c.521TT (n = 8), c.521TC (n = 6), and c.521CC (n = 2), but the number of participants were inadequate and data on haplotypes of SLCO1B1 A388G were unknown. The report by Mori et al. 28 showed that plasma CP‐I concentrations increased in Japanese participants with OATP1B1*15/*15 compared with those with OATP1B1*1b/*1b or OATP1B1*1b/*15, but only 2 participants with OATP1B1*15/*15 were included in the study and statistical analysis was not performed. In the present large‐scale study, we measured plasma CP‐I concentrations in 391 subjects from the Japanese general population, and evaluated the relationship between plasma CP‐I concentrations and OATP1B1 polymorphisms to further assess the utility of plasma CP‐I concentration as an endogenous OATP1B probe.
METHODS
Study participants
We analyzed the data of participants recruited in the Japan Multi‐Institutional Collaborative Cohort (J‐MICC) Study. The J‐MICC study recruited subjects from the general Japanese population, and detected and confirmed gene–environment interactions related to lifestyle‐associated diseases using genetic and clinical data. 29 All participants in the J‐MICC study gave written informed consent, and the study protocol was approved by the ethics committees at institutions participating in the J‐MICC study. We analyzed the data of 500 randomly selected subjects who received a health check in Kyoto Prefectural University of Medicine, and selected 391 participants who met the following inclusion criteria: body mass index lower than 30 kg/m2, estimated glomerular filtration rate (eGFR) higher than 60 mL/min/1.73 m2, total bilirubin lower than 1.5 mg/dL, and alanine aminotransaminase lower than 100 IU/L. The eGFR was calculated by the Chronic Kidney Disease Epidemiology Collaboration (CKD‐EPI) equation for Japanese. 30 All plasma samples for quantification of CP‐I and CMPF were stored in deep freeze (−80°C) and light‐resistant condition. This study was approved by the ethics committees of Kyoto Prefectural University of Medicine (approval number: ERB‐C‐1384) and Meiji Pharmaceutical University (approval number: 3023).
Genotyping
The 14,539 study participants in the J‐MICC study from 12 areas of Japan were genotyped at RIKEN Center for Integrative Medicine using a Human OmniExpressExome‐8 version 1.2 BeadChip array (Illumina, San Diego, CA, USA). 31 Quality control filtering of samples and single nucleotide polymorphisms (SNPs) have been reported previously. 31 Briefly, for quality control filtering, samples with inconsistent sex information, one of each pair of samples found to be closely related by descent, and samples with ancestry estimated to be outside the Japanese population were excluded. SNPs with a call rate < 0.98 or a Hardy Weinberg equilibrium P value < 1 × 10−6 were also filtered out. Such quality control filtering resulted in the selection of 14,091 subjects and 873,254 SNPs, from whom we selected the above‐mentioned 391 participants and their data. Among the SNPs that passed quality control, we identified rs2306283 (c.A388G, p.N130D, and exon 5) and rs4149056 (c.T521C, p.V174A, and exon 5) from genomewide association study data, and formed four haplotypes: OATP1B1*1a (c.388A‐c.521T), OATP1B1*1b (c.388G‐c.521T), OATP1B1*5 (c.388A‐c.521C), and OATP1B1*15 (c.388G‐c.521C). Finally, the participants were stratified into six polymorphism groups: OATP1B1*1b/*1b, OATP1B1*1a/*1b, OATP1B1*1a/*1a, OATP1B1*1b/*15, OATP1B1*1a/*15, and OATP1B1*15/*15 (Table 1 ). CP‐1 may also be transported by other OATPs, such as OATP2B1 (encoded by SLCO2B1) and OATP1B3 (encoded by SLCO1B3), and also by other types of transporters, such as MRP2 (encoded by ABCC2). 19 , 32 We therefore evaluated the association of plasma CP‐1 concentration with the following SNPs: rs717620 (ABCC2 variant; c.C‐24T and 5′UTR), 33 rs12422149 (SLCO2B1 variant; c.G935A, p.R312Q, and exon 7), 34 rs4149117 (SLCO1B3 variant; c.T334G, p.S112A, and exon 33), 35 and rs7311358 (SLCO1B3 variant; c.G699A, p.M233I, and exon 6). 35
Table 1.
OATP1B1 polymorphisms according to the haplotypes
| T521C | |||
|---|---|---|---|
| T/T | T/C | C/C | |
| A388G | |||
| A/A | *1a/*1a | ‐ | ‐ |
| A/G | *1a/*1b | *1a/*15 | ‐ |
| G/G | *1b/*1b | *1b/*15 | *15/*15 |
OATP, organic anion transporting polypeptide.
Measurement of plasma concentrations of CP‐I and CMPF
Plasma concentrations of CP‐I and CMPF were measured simultaneously using an ultra‐high performance liquid chromatography coupled to tandem mass spectrometry according to the procedures that we reported previously. 36 Inter‐assay and intra‐assay accuracy and precision were < 7.6% for CP‐I and < 4.1% for CMPF.
Data analysis and statistics
Data are expressed as mean ± SD. The genotype distributions were tested for Hardy–Weinberg equilibrium by χ2 test. Data were tested for normal distribution using Shapiro–Wilk test. Values in some genotype groups were found to be not normally distributed. Clinical characteristics of participants and plasma CMPF concentrations among groups were compared by Kruskal–Wallis test or χ2 test. Plasma CP‐I concentrations between groups were compared using Kruskal–Wallis test with Dunn’s post hoc test. Correlation between plasma CP‐I and CMPF concentrations was assessed by Spearman’s rank correlation coefficient. A P value < 0.05 was considered statistically significant. Statistical analyses were performed using Graph Pad Prism 7 (GraphPad Software, La Jolla, CA, USA).
RESULTS
Participants background
Table 2 shows the laboratory data of the 391 study participants. The genotype distributions of these 2 SNPs conformed to Hardy–Weinberg equilibrium with P values > 0.05 (rs2306283: χ2 = 0.281 and rs4149056: χ2 = 0.123). The participants were divided according to OATP1B1 polymorphism into OATP1B1*1b/*1b (n = 103), *1a/*1b (n = 122), *1a/*1a (n = 40), *1b/*15 (n = 74), *1a/*15 (n = 41), and *15/*15 (n = 11). There were no significant differences in demographic and laboratory variables among genotypes. The eGFR was within the normal range in all groups, with no significant difference among groups.
Table 2.
Characteristics of participants divided into OATP1B1 genotypes
| Characteristic | OATP1B1*1b/*1b | OATP1B1*1a/*1b | OATP1B1*1a/*1a | OATP1B1*1b/*15 | OATP1B1*1a/*15 | OATP1B1*15/*15 | P value |
|---|---|---|---|---|---|---|---|
| No. of participants | 103 | 122 | 40 | 74 | 41 | 11 | |
| Males/ females | 32/71 | 37/85 | 12/28 | 17/57 | 10/31 | 4/7 | NS |
| Age, year | 55.9 ± 11.0 [40–74] | 55.3 ± 10.0 [39–74] | 56.9 ± 9.1 [39–72] | 56.0 ± 9.3 [40–73] | 58.2 ± 8.7 [39–74] | 56.6 ± 10.2 [41–68] | NS |
| BMI, kg/m2 | 21.9 ± 2.8 [15.4–29.9] | 22.3 ± 3.0 [14.5–29.8] | 22.1 ± 2.9 [17.4–28.0] | 21.7 ± 3.4 [15.8–29.7] | 21.4 ± 2.4 [17.6–29.5] | 21.9 ± 3.6 [16.2–27.1] | NS |
| eGFR, mL/min/1.73 m2 | 77.3 ± 8.4 [60.0–96.3] | 78.6 ± 9.1 [60.7–94.9] | 78.9 ± 8.4 [60.4–‐92.7] | 79.9 ± 8.0 [60.5–94.7] | 78.8 ± 7.6 [60.7–90.6] | 80.1 ± 6.5 [74.0–94.2] | NS |
| Total bilirubin, mg/dL | 0.79 ± 0.24 [0.4–1.4] | 0.81 ± 0.24 [0.4–1.4] | 0.70 ± 0.19 [0.4–1.0] | 0.74 ± 0.22 [0.3–1.3] | 0.78 ± 0.22 [0.3–1.3] | 0.77 ± 0.28 [0.4–1.2] | NS |
| ALT, IU/L | 17.5 ± 9.4 [5–59] | 17.5 ± 9.7 [6–53] | 20.5 ± 10.1 [10–53] | 19.7 ± 12.4 [7–99] | 16.0 ± 6.7 [8–49] | 26.1 ± 18.3 [9–61] | NS |
| Plasma CP‐I concentrations, ng/mL | 0.45 ± 0.12 [0.21–0.88] | 0.47 ± 0.16 [0.13–1.22] | 0.47 ± 0.20 [0.19–1.41] | 0.50 ± 0.15 [0.21–0.95] | 0.54 ± 0.14 [0.25–0.84] | 0.74 ± 0.31 [0.44–1.37] | p < 0.0001 |
Data are expressed as numbers, or mean ± SD [range].
ALT, alanine aminotransaminase; BMI, body mass index; CP‐I, coproporphyrin‐I; eGFR, estimated glomerular filtration rate; NA, not significant; OATP1B1, organic anion transporting polypeptides 1B1.
Plasma CP‐I concentrations in six OATP1B1 genotypes
Plasma CP‐I concentration (mean ± SD) in all participants was 0.48 ± 0.17 (range 0.13–1.41) ng/mL, showing large individual variability. When stratified by OATP1B1 genotype, plasma CP‐I concentrations were 0.45 ± 0.12, 0.47 ± 0.16, 0.47 ± 0.20, 0.50 ± 0.15, 0.54 ± 0.14, and 0.74 ± 0.31 ng/mL in participants with OATP1B1*1b/*1b, *1a/*1b, *1a/*1a, *1b/*15, *1a/*15, and *15/*15, respectively, showing an ascending rank order with a significant difference detected by Kruskal–Wallis test (P < 0.0001; Figure 1 ). Post hoc analysis revealed significant increases in plasma CP‐I concentrations in OATP1B1*1b/*15, *1a/*15, and *15/*15 groups compared with OATP1B1*1b/*1b group (Figure 1 ).
Figure 1.
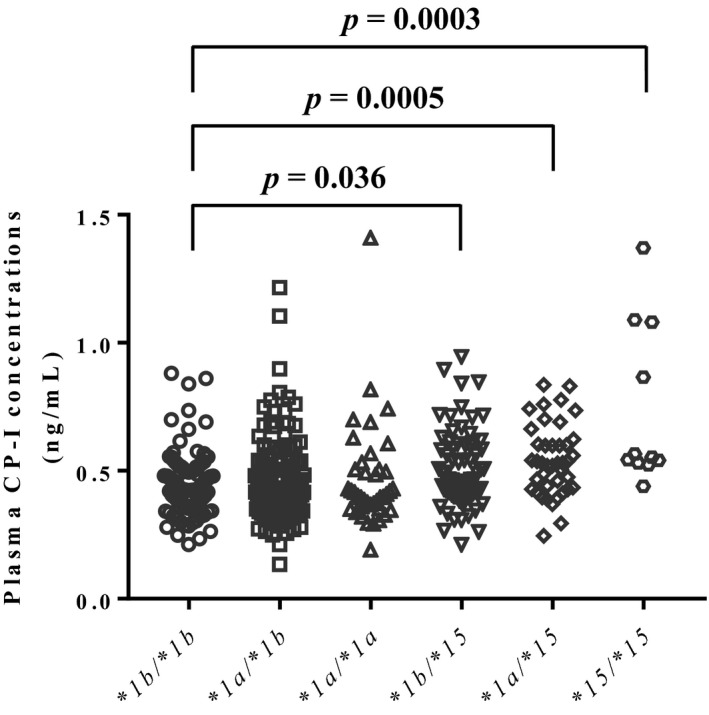
Plasma coproporphyrin‐I (CP‐I) concentrations in participants with organic anion transporting polypeptide (OATP)1B1*1b/*1b, *1a/*1b, *1a/*1a, *1b/*15, *1a/*15, and *15/*15. Data were analyzed by Kruskal–Wallis test (P < 0.0001) followed by Dunn’s post hoc test.
Plasma CP‐I concentrations in additional transporter genotypes
Figure 2 shows the association of plasma CP‐I concentrations with genotypes of four additional transporter genes. There was no significant difference among three genotypes in rs717620 (MRP2), rs12422149 (OATP2B1), rs4149117 (OATP1B3), and rs7311358 (OATP1B3).
Figure 2.
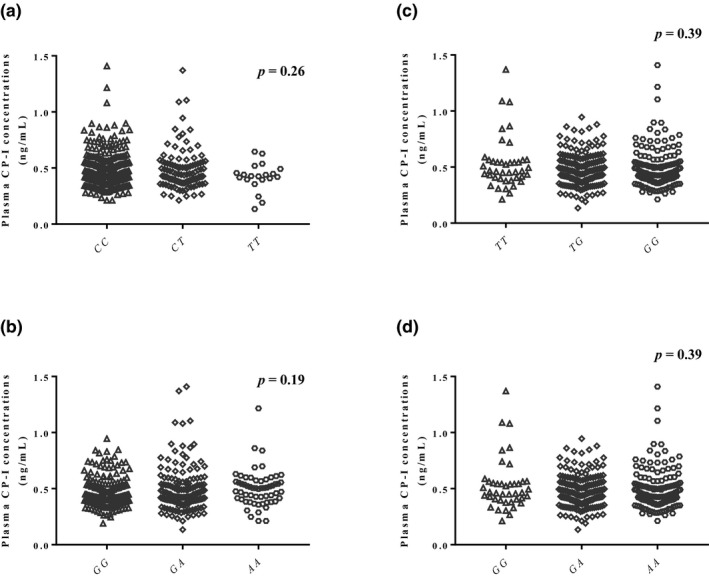
Relationship between plasma coproporphyrin‐I (CP‐I) concentration and genotypes of rs717620 (a), rs12422149 (b), rs4149117 (c), and rs7311358 (d). The rs4149117 c and rs7311358 d demonstrate complete linkage disequilibrium.
Relationship between plasma CP‐I and CMPF concentrations
Plasma CMPF concentrations were 2,739 ± 1,591, 2,583 ± 1,384, 2,684 ± 1,582, 2,508 ± 1,210, 2,445 ± 1,745, and 2,660 ± 1,461 ng/mL in participants with OATP1B1*1b/*1b, *1a/*1b, *1a/*1a, *1b/*15, *1a/*15, and *15/*15, respectively, with no significant difference (Figure 3 ). Figure 4 shows the relationship between plasma CP‐I and CMPF concentrations for each OATP1B1 genotype. No significant correlation was observed for all genotypes.
Figure 3.
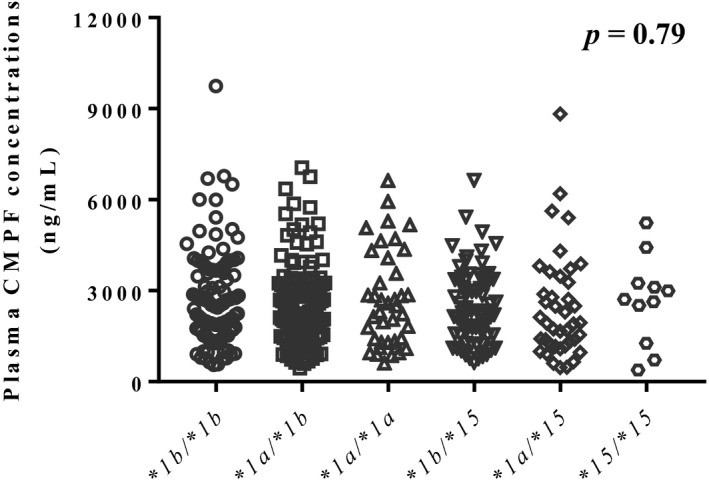
Plasma 3‐carboxy‐4‐methyl‐5‐propyl‐2‐furanpropanoic acid (CMPF) concentrations in participants with organic anion transporting polypeptide (OATP)1B1*1b/*1b, *1a/*1b, *1a/*1a, *1b/*15, *1a/*15, and *15/*15. Data were analyzed by Kruskal–Wallis test.
Figure 4.
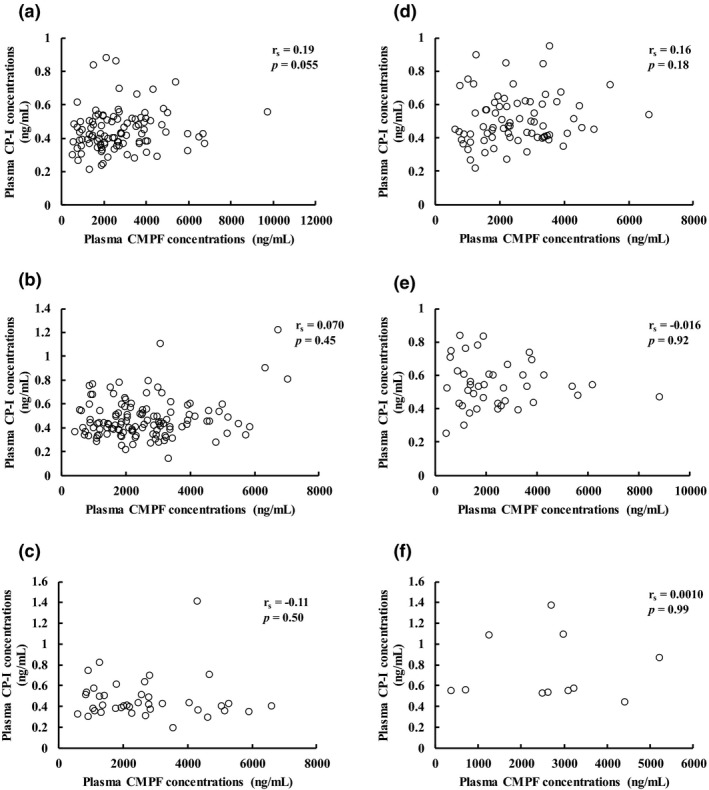
Correlation between plasma coproporphyrin‐I (CP‐I) and 3‐carboxy‐4‐methyl‐5‐propyl‐2‐furanpropanoic acid (CMPF) concentrations for each organic anion transporting polypeptide (OATP)1B1 genotype.
DISCUSSION
In 2016, CP‐I was reported for the first time as a useful endogenous biomarker for phenotyping of OATP1B activity. 19 , 20 , 21 Currently, CP‐I is assumed to be the most sensitive and specific biomarker for phenotyping of OATP1B activity compared with the other endogenous biomarkers and probe drugs, such as HMG‐CoA reductase inhibitors. 22 In fact, CP‐I was used for OATP1B activity phenotyping in clinical drug‐drug interaction studies 24 , 37 , 38 , 39 and in model‐based analysis of drug‐drug interactions. 23 , 40 , 41 , 42 Three previous studies reported the relationship between plasma CP‐I concentrations and OATP1B1 polymorphism, but the numbers of participants in all studies were insufficient to detect significant differences among the genotypes. 25 , 27 , 28 In the present study of a large sample from the Japanese general population, we evaluated the differences in plasma CP‐I concentrations in participants with OATP1B1*1b/*1b, *1a/*1b, *1a/*1a, *1b/*15, *1a/*15, or *15/*15 to evaluate the utility of plasma CP‐I concentrations.
Hepatic failure decreased mRNA levels of OATPs in liver biopsy specimens in a previous study. 43 Chronic kidney disease was also reported to decrease OATP1B activity in patients. 9 , 10 , 11 Furthermore, polycystic kidney disease decreased the protein expression of OATPs in rats. 44 In our study, all of the 391 participants had normal values of eGFR, total bilirubin, and alanine aminotransaminase, suggesting that plasma CP‐I concentrations in this study were not affected by disease states, such as hepatic and renal failure, but were influenced mainly by OATP1B1 polymorphisms. We evaluated four haplotypes: OATP1B1*1a, OATP1B1*1b, OATP1B1*5, and OATP1B1*15. SLCO1B1 c.521C shows strong linkage disequilibrium with SLCO1B1 c.388G in the Japanese population (r 2 = 0.0708, D' = 0.9999). 1 Therefore, we evaluated the influence of 6 types of polymorphism; OATP1B1*1b/*1b, *1a/*1b, *1a/*1a, *1b/*15, *1a/*15, and *15/*15, on plasma CP‐I concentrations. The allele frequency of OATP1B1*15 in the Japanese population was reported to be 0.11–0.16, 45 which is consistent with the finding in this study. Our study population included 11 participants with OATP1B1*15/*15, and this is the largest number compared with previous studies that evaluated OATP1B1 polymorphism and plasma CP‐I concentrations. 25 , 27 , 28 The Japanese population has higher allele frequency of OATP1B1*15 compared with white, African American, and other Asian populations. 45 , 46 Thus, the Japanese population seems to be suitable for the purpose of this study.
We obtained several important findings in this study. First, significant increases in plasma CP‐I concentration were observed in OATP1B1*1b/*15, *1a/*15, and *15/*15 groups compared with the OATP1B1*1b/*1b group. Especially, substantial increase was observed in OATP1B1*15/*15. These findings suggest that plasma CP‐I concentrations reflect the individual variation of OATP1B activity caused by OATP1B1 polymorphism. Second, we observed an ascending rank order of plasma CP‐I concentrations in subjects with OATP1B1*1b/*1b, *1a/*1b, *1a/*1a, *1b/*15, *1a/*15, and *15/*15. This trend is rational considering previous reports showing that the OATP1B1*1b allele increases the transportation function of OATP1B1, 47 whereas the OATP1B1*15 allele decreases transportation function compared with wild type (OATP1B1*1a). 12 , 13 , 14 , 15 , 16 On the other hand, Choi et al. 48 studied the effect of OATP1B1 polymorphisms on rosuvastatin pharmacokinetics, and reported that mean areas under the curve of rosuvastatin in OATP1B1*1b/*1b, *1a/*1b, *1a/*1a, *1b/*15, *1a/*15, and *15/*15 were 116, 126, 87.4, 105, 167, and 191 ng h/mL, respectively, which showed no consistent pattern, unlike the ascending trend found in the present study. These results suggest that plasma CP‐I concentrations may have better specificity than rosuvastatin as an OATP1B probe. Third, polymorphisms of MRP2, OATP2B1, and OATP1B3 were not associated with plasma CP‐I concentrations in this study. This suggests that CP‐I is a more sensitive endogenous probe for OATP1B1 than other transporters. Finally, there was no significant difference in plasma CMPF concentrations among the OATP1B1 genotypes, and no significant correlation between plasma CMPF and CP‐I concentrations in all genotypes, suggesting that the variation in CP‐I concentration was not due to recruitment of subjects with chronic kidney disease. CMPF has been reported to decrease OATP1B activity in a concentration–dependent manner, both in vitro 9 and in clinical studies, 10 , 36 which further support the absence of effect of CMPF in our study and that the effect of OATP1B1 polymorphism on plasma CP‐I concentrations was evaluated appropriately. These findings further demonstrate the utility of plasma CP‐I concentration as an endogenous biomarker for phenotyping of OATP1B activity. Plasma CP‐I concentrations is useful for studies of drug‐drug interaction via OATP1B and individual dose adjustment of OATP1B substrates.
In this large‐scale study in the Japanese general population, the mean plasma CP‐I concentration was 0.48 ng/mL. This value was slightly lower compared with previous studies in the white population (mean concentration: 0.60–0.71 ng/mL). 49 The difference might be due to genetic differences, such as OATP1B1 polymorphism, but the detail mechanism is unknown. Further studies are needed to reveal the racial differences of plasma CP‐I concentrations.
There was a limitation in this study. We measured plasma CP‐I concentrations in this study, but did not measure other endogenous probes, such as direct bilirubin, chenodeoxycholate 24‐glucuronide, and glycochenodeoxycholate‐3‐sulfate. Further studies are needed to evaluate the relationship of OATP1B1 genotypes with these probes.
In conclusion, significant differences in plasma CP‐I concentrations were observed among OATP1B1 genotypes, and the concentrations were significantly increased in OATP1B1*1b/*15, *1a/*15 and *15/*15 compared with OATP1B1*1b/*1b. These findings further substantiate the utility of plasma CP‐I concentrations as an endogenous biomarker for phenotyping of OATP1B activity.
Funding
This work was supported in part by Grant‐in‐Aid for Japan Research Foundation for Clinical Pharmacology and Grant‐in‐Aid for JSPS KAKENHI Grant Number JP19K16455.
Conflicts of Interest
The authors declared no competing interests for this work.
Author Contributions
Y.Su., T.K., M.N., and K.O. wrote the manuscript. Y.Su., T.K., R.U., and K.O. designed the research. Y.Su., Y.Sa., T.K., C.Y., M.N., M.K., and Y.M. performed the research. Y.Su., Y.Sa., and C.Y. analyzed the data.
References
- 1. Nishizato, Y. et al Polymorphisms of OATP‐C (SLC21A6) and OAT3 (SLC22A8) genes: consequences for pravastatin pharmacokinetics. Clin. Pharmacol. Ther. 73, 554–565 (2003). [DOI] [PubMed] [Google Scholar]
- 2. Chung, J.Y. et al Effect of OATP1B1 (SLCO1B1) variant alleles on the pharmacokinetics of pitavastatin in healthy volunteers. Clin. Pharmacol. Ther. 78, 342–350 (2005). [DOI] [PubMed] [Google Scholar]
- 3. Lee, E. et al Rosuvastatin pharmacokinetics and pharmacogenetics in white and Asian subjects residing in the same environment. Clin. Pharmacol. Ther. 78, 330–341 (2005). [DOI] [PubMed] [Google Scholar]
- 4. Pasanen, M.K. , Neuvonen, M. , Neuvonen, P.J. & Niemi, M. SLCO1B1 polymorphism markedly affects the pharmacokinetics of simvastatin acid. Pharmacogenet. Genomics 16, 873–879 (2006). [DOI] [PubMed] [Google Scholar]
- 5. Pasanen, M.K. , Fredrikson, H. , Neuvonen, P.J. & Niemi, M. Different effects of SLCO1B1 polymorphism on the pharmacokinetics of atorvastatin and rosuvastatin. Clin. Pharmacol. Ther. 82, 726–733 (2007). [DOI] [PubMed] [Google Scholar]
- 6. Furihata, T. et al Different interaction profiles of direct‐acting anti‐hepatitis C virus agents with human organic anion transporting polypeptides. Antimicrob. Agents Chemother. 58, 4555–4564 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lemahieu, W.P. et al Combined therapy with atorvastatin and calcineurin inhibitors: no interactions with tacrolimus. Am. J. Transplant. 5, 2236–2243 (2005). [DOI] [PubMed] [Google Scholar]
- 8. Maeda, K. et al Identification of the rate‐determining process in the hepatic clearance of atorvastatin in a clinical cassette microdosing study. Clin. Pharmacol. Ther. 90, 575–581 (2011). [DOI] [PubMed] [Google Scholar]
- 9. Fujita, K. et al Direct inhibition and down‐regulation by uremic plasma components of hepatic uptake transporter for SN‐38, an active metabolite of irinotecan, in humans. Pharm. Res. 31, 204–215 (2014). [DOI] [PubMed] [Google Scholar]
- 10. Fujita, K. et al Increased plasma concentrations of unbound SN‐38, the active metabolite of irinotecan, in cancer patients with severe renal failure. Pharm. Res. 33, 269–282 (2016). [DOI] [PubMed] [Google Scholar]
- 11. Suzuki, Y. et al Recovery of OATP1B activity after living kidney transplantation in patients with end‐stage renal disease. Pharm. Res. 36, 59 (2019). [DOI] [PubMed] [Google Scholar]
- 12. Iwai, M. , Suzuki, H. , Ieiri, I. , Otsubo, K. & Sugiyama, Y. Functional analysis of single nucleotide polymorphisms of hepatic organic anion transporter OATP1B1 (OATP‐C). Pharmacogenetics 14, 749–757 (2004). [DOI] [PubMed] [Google Scholar]
- 13. Tirona, R.G. , Leake, B.F. , Merino, G. & Kim, R.B. Polymorphisms in OATP‐C: identification of multiple allelic variants associated with altered transport activity among European‐ and African‐Americans. J. Biol. Chem. 276, 35669–35675 (2001). [DOI] [PubMed] [Google Scholar]
- 14. Kameyama, Y. , Yamashita, K. , Kobayashi, K. , Hosokawa, M. & Chiba, K. Functional characterization of SLCO1B1 (OATP‐C) variants, SLCO1B1*5, SLCO1B1*15 and SLCO1B1*15+C1007G, by using transient expression systems of HeLa and HEK293 cells. Pharmacogenet. Genomics 15, 513–522 (2005). [DOI] [PubMed] [Google Scholar]
- 15. Ho, R.H. et al Drug and bile acid transporters in rosuvastatin hepatic uptake: function, expression, and pharmacogenetics. Gastroenterology 130, 1793–1806 (2006). [DOI] [PubMed] [Google Scholar]
- 16. Zhang, W. et al Effect of SLCO1B1 genetic polymorphism on the pharmacokinetics of nateglinide. Br. J. Clin. Pharmacol. 62, 567–572 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Takehara, I. et al Investigation of glycochenodeoxycholate sulfate and chenodeoxycholate glucuronide as surrogate endogenous probes for drug interaction studies of OATP1B1 and OATP1B3 in healthy Japanese volunteers. Pharm. Res. 34, 1601–1614 (2017). [DOI] [PubMed] [Google Scholar]
- 18. Takehara, I. et al Comparative study of the dose‐dependence of OATP1B inhibition by rifampicin using probe drugs and endogenous substrates in healthy volunteers. Pharm. Res. 35, 138 (2018). [DOI] [PubMed] [Google Scholar]
- 19. Bednarczyk, D. & Boiselle, C. Organic anion transporting polypeptide (OATP)‐mediated transport of coproporphyrins I and III. Xenobiotica 46, 457–466 (2016). [DOI] [PubMed] [Google Scholar]
- 20. Lai, Y. et al Coproporphyrins in plasma and urine can be appropriate clinical biomarkers to recapitulate drug‐drug interactions mediated by organic anion transporting polypeptide inhibition. J. Pharmacol. Exp. Ther. 358, 397–404 (2016). [DOI] [PubMed] [Google Scholar]
- 21. Shen, H. et al Coproporphyrins I and III as functional markers of OATP1B activity. In vitro and in vivo evaluation in preclinical species. J. Pharmacol. Exp. Ther. 357, 382–393 (2016). [DOI] [PubMed] [Google Scholar]
- 22. Shen, H. et al Comparative evaluation of plasma bile acids, dehydroepiandrosterone sulfate, hexadecanedioate, and tetradecanedioate with coproporphyrins I and III as markers of OATP inhibition in healthy subjects. Drug Metab. Dispos. 45, 908–919 (2017). [DOI] [PubMed] [Google Scholar]
- 23. Barnett, S. et al Gaining mechanistic insight into coproporphyrin I as endogenous biomarker for OATP1B‐mediated drug‐drug interactions using population pharmacokinetic modeling and simulation. Clin. Pharmacol. Ther. 104, 564–574 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kunze, A. , Ediage, E.N. , Dillen, L. , Monshouwer, M. & Snoeys, J. Clinical investigation of coproporphyrins as sensitive biomarkers to predict mild to strong OATP1B‐mediated drug‐drug interactions. Clin. Pharmacokinet. 57, 1559–1570 (2018). [DOI] [PubMed] [Google Scholar]
- 25. Shen, H. et al Further studies to support the use of coproporphyrin I and III as novel clinical biomarkers for evaluating the potential for organic anion transporting polypeptide 1B1 and OATP1B3 inhibition. Drug Metab. Dispos. 46, 1075–1082 (2018). [DOI] [PubMed] [Google Scholar]
- 26. Mori, D. et al Dose‐dependent inhibition of OATP1B by rifampicin in healthy volunteers: comprehensive evaluation of candidate biomarkers and OATP1B probe drugs. Clin. Pharmacol. Ther. 107, 1004–1013 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yee, S.W. et al Organic anion transporter polypeptide 1B1 polymorphism modulates the extent of drug‐drug interaction and associated biomarker levels in healthy volunteers. Clin. Transl. Sci. 12, 388–399 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mori, D. et al Effect of OATP1B1 genotypes on plasma concentrations of endogenous OATP1B1 substrates and drugs, and their association in healthy volunteers. Drug Metab. Pharmacokinet. 34, 78–86 (2019). [DOI] [PubMed] [Google Scholar]
- 29. Hamajima, N. The Japan Multi‐Institutional Collaborative Cohort Study (J‐MICC Study) to detect gene‐environment interactions for cancer. Asian Pac. J. Cancer Prev. 8, 317–323 (2007). [PubMed] [Google Scholar]
- 30. Horio, M. , Imai, E. , Yasuda, Y. , Watanabe, T. & Matsuo, S. Modification of the CKD epidemiology collaboration (CKD‐EPI) equation for Japanese: accuracy and use for population estimates. Am. J. Kidney Dis. 56, 32–38 (2010). [DOI] [PubMed] [Google Scholar]
- 31. Koyama, T. et al Genetic variants of RAMP2 and CLR are associated with stroke. J. Atheroscler. Thromb. 24, 1267–1281 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gilibili, R.R. et al Coproporphyrin‐I: a fluorescent, endogenous optimal probe substrate for ABCC2 (MRP2) suitable for vesicle‐based MRP2 inhibition assay. Drug Metab. Dispos. 45, 604–611 (2017). [DOI] [PubMed] [Google Scholar]
- 33. Laechelt, S. , Turrini, E. , Ruehmkorf, A. , Siegmund, W. , Cascorbi, I. & Haenisch, S. Impact of ABCC2 haplotypes on transcriptional and posttranscriptional gene regulation and function. Pharmacogenomics J. 11, 25–34 (2011). [DOI] [PubMed] [Google Scholar]
- 34. Mougey, E.B. , Feng, H. , Castro, M. , Irvin, C.G. & Lima, J.J. Absorption of montelukast is transporter mediated: a common variant of OATP2B1 is associated with reduced plasma concentrations and poor response. Pharmacogenet. Genomics 19, 129–138 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tague, L.K. et al Impact of SLCO1B3 polymorphisms on clinical outcomes in lung allograft recipients receiving mycophenolic acid. Pharmacogenomics J. 20, 69–79 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Suzuki, Y. et al Simultaneous quantification of coproporphyrin‐I and 3‐carboxy‐4‐methyl‐5‐propyl‐2‐furanpropanoic acid in human plasma using ultra‐high performance liquid chromatography coupled to tandem mass spectrometry. J. Pharm. Biomed. Anal. 184, 113202 (2020). [DOI] [PubMed] [Google Scholar]
- 37. King‐Ahmad, A. et al A fully automated and validated human plasma LC‐MS/MS assay for endogenous OATP biomarkers coproporphyrin‐I and coproporphyrin‐III. Bioanalysis 10, 691–701 (2018). [DOI] [PubMed] [Google Scholar]
- 38. Cheung, K.W.K. et al GDC‐0810 pharmacokinetics and transporter‐mediated drug interaction evaluation with an endogenous biomarker in the first‐in‐human. Dose Escalation Study. Drug Metab. Dispos. 47, 966–973 (2019). [DOI] [PubMed] [Google Scholar]
- 39. Jones, N.S. , Yoshida, K. , Salphati, L. , Kenny, J.R. , Durk, M.R. & Chinn, L.W. Complex DDI by fenebrutinib and the use of transporter endogenous biomarkers to elucidate the mechanism of DDI. Clin. Pharmacol. Ther. 107, 269–277 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yoshida, K. , Guo, C. & Sane, R. Quantitative prediction of OATP‐mediated drug‐drug interactions with model‐based analysis of endogenous biomarker kinetics. CPT Pharmacometrics Syst. Pharmacol. 7, 517–524 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yoshikado, T. et al PBPK modeling of coproporphyrin I as an endogenous biomarker for drug interactions involving inhibition of hepatic OATP1B1 and OATP1B3. CPT Pharmacometrics Syst. Pharmacol. 7, 739–747 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Asaumi, R. et al Expanded physiologically‐based pharmacokinetic model of rifampicin for predicting interactions with drugs and an endogenous biomarker via complex mechanisms including organic anion transporting polypeptide 1B induction. CPT Pharmacometrics Syst. Pharmacol. 8, 845–857 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nakai, K. et al Decreased expression of cytochromes P450 1A2, 2E1, and 3A4 and drug transporters Na+‐taurocholate‐cotransporting polypeptide, organic cation transporter 1, and organic anion‐transporting peptide‐C correlates with the progression of liver fibrosis in chronic hepatitis C patients. Drug Metab. Dispos. 36, 1786–1793 (2008). [DOI] [PubMed] [Google Scholar]
- 44. Bezencon, J. et al Altered expression and function of hepatic transporters in a rodent model of polycystic kidney disease. Drug Metab. Dispos. 47, 899–906 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ieiri, I. , Takane, H. , Hirota, T. , Otsubo, K. & Higuchi, S. Genetic polymorphisms of drug transporters: pharmacokinetic and pharmacodynamic consequences in pharmacotherapy. Expert Opin. Drug Metab. Toxicol. 2, 651–764 (2006). [DOI] [PubMed] [Google Scholar]
- 46. Jada, S.R. et al Pharmacogenetics of SLCO1B1: haplotypes, htSNPs and hepatic expression in three distinct Asian populations. Eur. J. Clin. Pharmacol. 63, 555–563 (2007). [DOI] [PubMed] [Google Scholar]
- 47. Maeda, K. et al Effects of organic anion transporting polypeptide 1B1 haplotype on pharmacokinetics of pravastatin, valsartan, and temocapril. Clin. Pharmacol. Ther. 79, 427–439 (2006). [DOI] [PubMed] [Google Scholar]
- 48. Choi, J.H. , Lee, M.G. , Cho, J.Y. , Lee, J.E. , Kim, K.H. & Park, K. Influence of OATP1B1 genotype on the pharmacokinetics of rosuvastatin in Koreans. Clin. Pharmacol. Ther. 83, 251–257 (2008). [DOI] [PubMed] [Google Scholar]
- 49. Njumbe Ediage, E. et al Development of an LC‐MS method to quantify coproporphyrin I and III as endogenous biomarkers for drug transporter‐mediated drug‐drug interactions. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 1073, 80–89 (2018). [DOI] [PubMed] [Google Scholar]


