Abstract
Many academic institutions are collecting blood samples from patients seeking treatment for coronavirus disease 2019 (COVID‐19) to build research biorepositories. It may be feasible to extract pharmacogenomic (PGx) information from biorepositories for clinical use. We sought to characterize the potential value of multigene PGx testing among individuals hospitalized with COVID‐19 in the United States. We performed a cross‐sectional analysis of electronic health records from consecutive individuals hospitalized with COVID‐19 at a large, urban academic health system. We characterized medication orders, focusing on medications with actionable PGx guidance related to 14 commonly assayed genes (CYP2C19, CYP2C9, CYP2D6, CYP3A5, DPYD, G6PD, HLA‐A, HLA‐B, IFNL3, NUDT15, SLCO1B1, TPMT, UGT1A1, and VKORC1). A simulation analysis combined medication data with population phenotype frequencies to estimate how many treatment modifications would be enabled if multigene PGx results were available. Sixty‐four unique medications with PGx guidance were ordered at least once in the cohort (n = 1,852, mean age 60.1 years). Nearly nine in 10 individuals (89.7%) had at least one order for a medication with PGx guidance and 427 patients (23.1%) had orders for 4 or more actionable medications. Using a simulation, we estimated that 17 treatment modifications per 100 patients would be enabled if PGx results were available. The genes CYP2D6 and CYP2C19 were responsible for the majority of treatment modifications, and the medications most often affected were ondansetron, oxycodone, and clopidogrel. PGx results would be relevant for nearly all individuals hospitalized with COVID‐19 and would provide the opportunity to improve clinical care.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Patients hospitalized with coronavirus disease 2019 (COVID‐19) are typically older and have comorbidities. Most people in the general population possess actionable pharmacogenomic (PGx) variants.
WHAT QUESTION DID THIS STUDY ADDRESS?
How often would a multigene PGx panel provide relevant information for patients treated for COVID‐19?
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
Nine in 10 patients hospitalized for COVID‐19 had an order for a medication with PGx guidance. These medications included those intended to treat COVID‐19 symptoms and for chronic conditions. PGx medication use and actionable results often co‐occur, and using a multigene PGx panel would yield 17 opportunities for modifying treatment per 100 patients tested.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
If institutions used pre‐emptive PGx testing or extracted PGx information from research biorepositories, these results would be highly relevant during COVID‐19 hospitalization.
Pharmacogenomic (PGx) testing can aid healthcare providers in selecting appropriate treatment and dosing. More than 200 medications have PGx information included in their US Food and Drug Administration (FDA)‐approved drug labeling. 1 Despite this, PGx is not routinely used in most clinical practice areas due to barriers related to testing platforms, provider education, and reimbursement. In clinical settings where PGx is used, a reactive testing module has typically been deployed, in which a single gene test is ordered after a patient is prescribed a drug with PGx guidance. However, there are potential economic and logistical advantages to a multigene, pre‐emptive model in which broader testing of many genes is performed in anticipation of future clinical utility. 2 Multigene assays provide more PGx data than single gene tests at similar cost, 3 and a pre‐emptive testing model allows for PGx information to be applied before the patient receives a single dose of a potentially harmful or ineffective medication.
The value of pre‐emptive PGx testing is dependent on the frequency of actionable PGx variants and medication use patterns in the population of interest. Although some specific PGx variants are rare, in the context of multigene testing, 99% of individuals carry an actionable PGx variant. 4 Given the ubiquity of actionable PGx variants, the utility of pre‐emptive multigene PGx testing primarily depends on medication use patterns. Intuitively, pre‐emptive PGx information will only be useful if medications with actionable PGx guidance are being used or considered in the patient population. Thus, pre‐emptive PGx testing in patients with new diagnoses likely to require medication treatment (e.g., newly diagnosed depression) or with multiple comorbidities may yield more actionable PGx information in the short‐term than testing relatively young, healthy patients.
As of September 2020, there have been > 7.1 million cases of the novel coronavirus disease 2019 (COVID‐19) in the United States, associated with > 204,000 deaths. 5 Older patients and patients with higher comorbidity burden are more likely to require hospitalization for COVID‐19. 6 We hypothesize that patients hospitalized with COVID‐19 require medications that are affected by PGx for treatment of acute symptoms and chronic conditions. Although prior investigations have characterized the use of PGx‐guided medications in primary care, 7 perioperative, and cardiovascular disease cohorts, 8 , 9 , 10 we are not aware of prior assessments of the potential value of PGx testing among individuals with COVID‐19.
The COVID‐19 pandemic presents an opportunity for multigene PGx testing from a logistical standpoint. Many academic healthcare systems are collecting patient DNA for COVID‐19 research biorepositories. This effort is primarily intended to fuel research initiatives examining the genetics of disease susceptibility, severity, and treatment response. 11 However, institutions could return PGx results from these assays to the electronic health record (EHR) for clinical use, as is done with actionable secondary findings in medical genetics, 12 and, in some cases, PGx results. 13 This process could be rapid at institutions that have assayed a critical mass of samples and have existing governance for returning genomic data in the EHR. 14
We used data from a large, urban, tertiary health system in order to determine the potential value of multigene PGx testing among individuals hospitalized with COVID‐19. We characterized the frequency of medication orders, focusing on medications with guidelines or FDA recommendations for tailoring treatment using PGx results. We then used population phenotype frequencies in conjunction with medication use data to simulate how often PGx testing would lead to clinically actionable treatment recommendations.
METHODS
Data source and study population
We conducted a cross‐sectional analysis using data from JH‐CROWN: The COVID‐19 Precision Medicine Analytic Platform registry. The registry collects EHR data on patients with COVID‐19 from five hospitals affiliated with Johns Hopkins Health System. Our study population included patients 18 years or older hospitalized with confirmed COVID‐19 between March 12, 2020, and June 26, 2020. COVID‐19 diagnosis was established using either a positive polymerase chain reaction test for severe acute respiratory syndrome‐coronavirus 2 or an International Classification of Disease‐10th edition Clinical Modification code of U07.1 or a confirmed COVID‐19 infection flag in the EHR. Data were queried using Clarity (Expanded PrEP Implementation in Communities (EPIC) structured query language (SQL) reporting database). Sociodemographic variables, baseline clinical characteristics, clinical outcomes (ventilation and mortality), and medication orders were extracted from the index hospital admission. Elixhauser comorbidities were used to describe patient comorbidities. Our local institutional review board determined that this study was exempt from review.
PGx medications of interest
We defined medications with actionable PGx guidance by combining information from the FDA Table of Pharmacogenetic Associations 15 and the Clinical Pharmacogenetics Implementation Consortium (CPIC). 16 Figure 1 is the flow diagram for selection of medications with actionable recommendations. We considered a drug/gene pair “actionable” if the FDA designated the data “support therapeutic management recommendations” or “indicate a potential impact on safety or response.” We also considered medications to have actionable PGx guidance if they were CPIC level A (genetic information should be used to change prescribing of affected drug) or level B (genetic information could be used to change prescribing of the affected drug because alternative therapies/dosing are extremely likely to be as effective and as safe as nongenetically based dosing). 16 , 17 We further restricted our sample to those with actionable recommendations for 14 genes commonly assayed on commercially available multigene PGx assays (CYP2C19, CYP2C9, CYP2D6, CYP3A5, DPYD, G6PD, HLA‐A, HLA‐B, IFNL3, NUDT15, SLCO1B1, TPMT, UGT1A1, and VKORC1). 18 For each drug‐gene pair, we identified the genetically defined phenotypes that had recommendations for dose modification or alternative therapy according to the FDA Table of Pharmacogenetic Associations 15 or CPIC Guidelines. 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 The CPIC guideline supplements and the Pharmacogenomics Knowledge Base (PharmGKB) 35 were the primary sources of phenotype frequencies. Phenotype frequencies for “Latino” populations were used for Hispanic Americans in our calculations. When CPIC guidelines and appendices did not provide population phenotype frequencies by ethnicity, we calculated estimates using the Hardy–Weinberg equation and published allele frequencies 35 , 36 or identified external literature with allele/phenotype frequencies by ethnicity. 37 , 38 , 39 , 40 Warfarin dosage requirement is highly dependent on the combination of CYP2C9 and VKORC1, so we defined the actionable phenotype based on the combination of CYP2C9 and VKORC1 diplotype, consistent with the FDA‐approved drug labeling. 41 “Sensitive and highly sensitive” responders had any of the following combinations of diplotypes: VKORC1 −1639 A/A regardless of CYP2C9 diplotype; VKORC1 −1639 G/A with CYP2C9 *1/*2, *1/*3, *2/*2, *2/*3, or *3/*3; or VKORC1 −1639 G/G with CYP2C9 *1/*3, *2/*2, *2/*3, or *3/*3. One drug (risperidone) was considered actionable by CPIC criteria but did not have treatment recommendations from the CPIC or the FDA. For this drug‐gene pair, we used the Dutch Pharmacogenetics Working Group (DPWG) guidelines (May 2020 update) to define the actionable phenotype. 42
Figure 1.
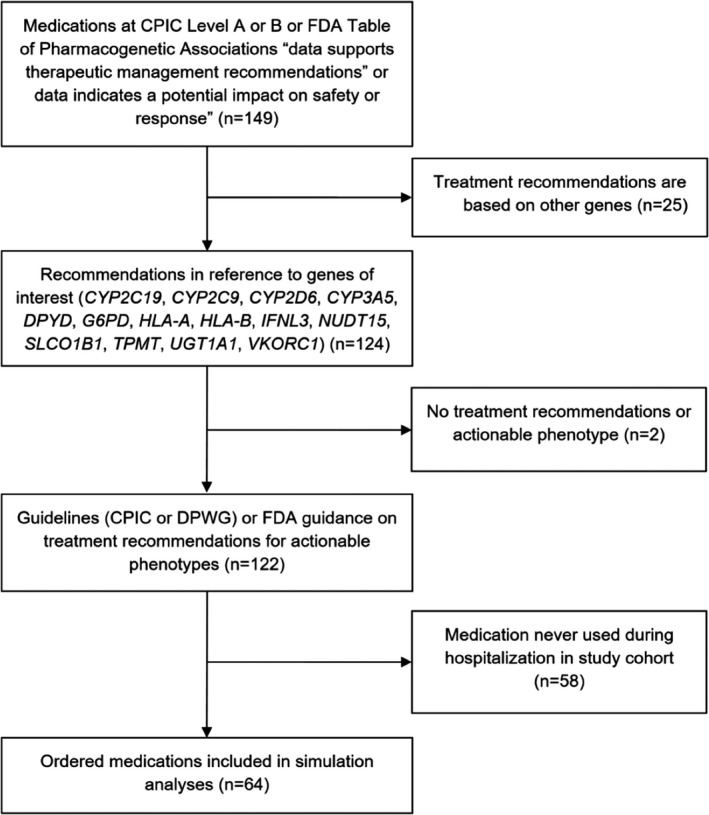
Flow Diagram for Selection of Medications with Actionable Recommendations. CPIC, Clinical Pharmacogenetics Implementation Consortium; DPWG, Dutch Pharmacogenetics Working Group; FDA, US Food and Drug Administration.
Statistical analysis
We used descriptive statistics to describe patient characteristics and medication order frequencies. Using patient‐level data, we quantified the proportion of patients who had orders of 0, 1, 2, 3, or 4 or more medications with actionable PGx guidance. We then performed a simulation analysis to estimate how often orders for each actionable medication would occur in patients with the actionable phenotype. Population phenotype frequency data was extracted from CPIC guideline appendices to estimate the percentage of patients with the actionable phenotype for each drug/gene pair (e.g., CYP2C19 poor or intermediate metabolizer for clopidogrel). 24 Because phenotype frequencies vary based on ancestry, we estimated phenotype frequencies for our total study population according to the proportion of individuals self‐identified as white non‐Hispanic, black non‐Hispanic, or Hispanic. We then calculated the percentage of patients projected to have an actionable PGx intervention for each medication by multiplying the percentage of patients treated with the medication by the percentage of patients estimated to have the actionable phenotype. After completing this process for each drug/gene pair, we summed the interventions for each drug‐gene pair to estimate the total number of PGx interventions that would be enabled by multigene testing for our study population, and also reported this number per 100 patients. Our results are presented with and without hydroxychloroquine orders, given that this medication was commonly used during the study period but was no longer recommended for routine use at the time of manuscript submission. 43
RESULTS
A total of 1,852 patients met inclusion criteria. The mean age was 60.1 years (SD 18.8), mean body mass index was 29.7 kg/m2 (SD 10.0), and 53.3% were men. Patients primarily identified as Black non‐Hispanic (n = 653, 35.3%), white non‐Hispanic (n = 541, 29.2%), or Hispanic (n = 530, 28.6%), with other race groups reported for 128 patients (6.9%). The most common comorbidities were hypertension (55.0%), diabetes (31.7%), anemia (21.2%), and neurological disorders (21.0%). The mean length of stay was 8.6 days (SD 9.8), 19.7% required mechanical ventilation during the index hospitalization, and 14.4% died during the index hospitalization.
Of 122 medications with actionable PGx guidance, 64 were ordered at least once in our patient cohort and 58 were not ordered for any patients. The PGx medications ordered in the highest percentage of patients are described in Figure 2 . The vast majority of patients had at least one order for a medication with actionable PGx guidance (n = 1,662, 89.7%), and 427 patients (23.1%) had orders for 4 or more actionable medications (Figure 3 ). Excluding hydroxychloroquine, 87.9% of patients had at least one order for a medication with PGx guidance. Medications affected by CYP2D6 were most common, followed by CYP2C19 representing 48.6 and 25.4% of actionable PGx medication orders, respectively.
Figure 2.
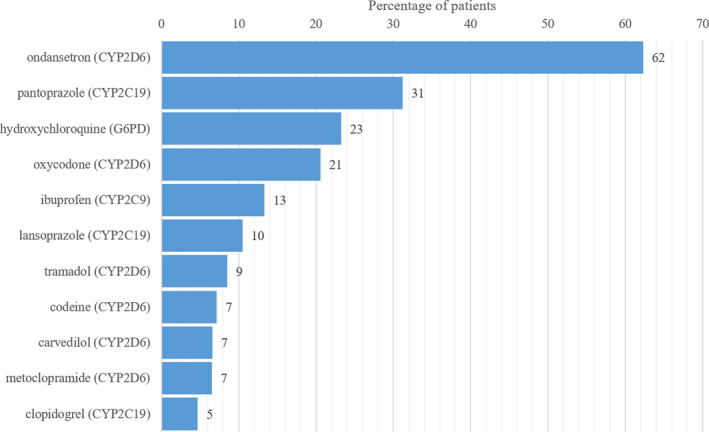
Most commonly ordered actionable pharmacogenomic medications in patients hospitalized with coronavirus disease 2019 (COVID‐19).
Figure 3.
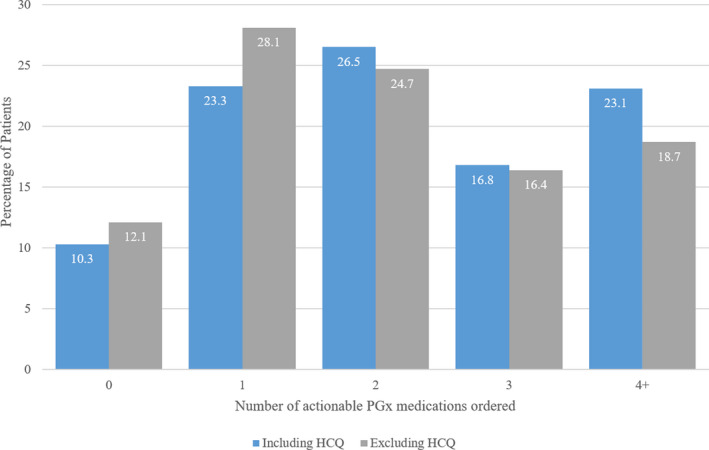
Number of actionable PGx medications ordered per patient. Blue bars show the distribution when considering all included medications, grey bars display the distribution when ignoring orders for HCQ. HCQ, hydrochloroquine; PGx, pharmacogenomics.
In our simulation analysis, we estimated that a total of 315 opportunities for genotype‐guided treatment modifications would be enabled by PGx testing in our 1,852 patient cohort (17 per 100 patients). The medications most often associated with these opportunities were ondansetron, oxycodone, and clopidogrel. On the gene level, CYP2D6 and CYP2C19 were most often driving opportunities for treatment modification. Table 1 presents the medication order frequencies and simulation analysis for medications affected by CYP2D6 and CYP2C19, and Table 2 presents the simulation analysis for medications affected by other genes.
Table 1.
Simulation estimating PGx interventions enabled by CYP2D6 and CYP2C19 testing
| Medication | Actionable phenotype | Consequence | Supporting evidencea | Unique patients w/ order | Actionable phenotype frequencyb | Projected # of PGx interventionsd | |||
|---|---|---|---|---|---|---|---|---|---|
| Black | White | Hispanic | Study populationc | ||||||
| CYP2D6 | |||||||||
| Ondansetron | UM | Decreased efficacy | CPIC | 1154 | 4.45% | 3.08% | 4.44% | 4.02% | 46 |
| Oxycodone | UM or PM | Adverse reaction or decreased efficacy | CPIC | 381 | 6.78% | 9.54% | 7.56% | 7.89% | 30 |
| Tramadol | UM or PM | Adverse reaction or decreased efficacy | CPIC | 158 | 6.78% | 9.54% | 7.56% | 7.89% | 12 |
| Codeine | UM or PM | Adverse reaction or decreased efficacy | CPIC | 133 | 6.78% | 9.54% | 7.56% | 7.89% | 10 |
| Carvedilol | PM | Adverse reaction | FDA | 122 | 2.33% | 6.47% | 3.12% | 3.87% | 5 |
| Metoclopramide | PM | Adverse reaction | FDA | 121 | 2.33% | 6.47% | 3.12% | 3.87% | 5 |
| Risperidone | UM or PM | Adverse reaction or decreased efficacy | DPWG | 43 | 6.78% | 9.54% | 7.56% | 7.89% | 3 |
| Venlafaxine | PM | Adverse reaction | FDA | 18 | 2.33% | 6.47% | 3.12% | 3.87% | 1 |
| Paroxetine | UM or PM | Adverse reaction or decreased efficacy | CPIC | 12 | 6.78% | 9.54% | 7.56% | 7.89% | 1 |
| Meclizine | UM, IM, or PM | Adverse reaction or decreased efficacy | FDA | 11 | 42.99% | 48.49% | 36.64% | 42.76% | 5 |
| Aripiprazole | PM | Adverse reaction | FDA | 11 | 2.33% | 6.47% | 3.12% | 3.87% | 0 |
| Tolterodine | PM | Adverse reaction | FDA | 11 | 2.33% | 6.47% | 3.12% | 3.87% | 0 |
| Nortriptyline | UM, IM, or PM | Adverse reaction | CPIC | 5 | 37.57% | 38.95% | 33.24% | 36.67% | 2 |
| Clozapine | PM | Adverse reaction | FDA | 3 | 2.33% | 6.47% | 3.12% | 3.87% | 0 |
| Tamoxifen | IM or PM | Decreased efficacy | CPIC | 3 | 38.53% | 45.41% | 32.20% | 38.75% | 1 |
| Vortioxetine | PM | Adverse reaction | FDA | 2 | 2.33% | 6.47% | 3.12% | 3.87% | 0 |
| Fluvoxamine | PM | Adverse reaction | CPIC | 2 | 2.33% | 6.47% | 3.12% | 3.87% | 0 |
| Brexpiprazole | PM | Adverse reaction | FDA | 2 | 2.33% | 6.47% | 3.12% | 3.87% | 0 |
| Amphetamine | PM | Adverse reaction | FDA | 2 | 2.33% | 6.47% | 3.12% | 3.87% | 0 |
| Perphenazine | PM | Adverse reaction | FDA | 1 | 2.33% | 6.47% | 3.12% | 3.87% | 0 |
| Tetrabenazine | PM | Adverse reaction | FDA | 1 | 2.33% | 6.47% | 3.12% | 3.87% | 0 |
| Total (n = 1,852) | 123 | ||||||||
| Per 100 patients | 7 | ||||||||
| CYP2C19 | |||||||||
| Pantoprazole | UM | Decreased efficacy | CPIC | 578 | 4.29% | 4.68% | 2.77% | 3.95% | 23 |
| Lansoprazole | UM | Decreased efficacy | CPIC | 194 | 4.29% | 4.68% | 2.77% | 3.95% | 8 |
| Clopidogrel | IM or PM | Decreased efficacy | CPIC | 87 | 38.94% | 28.54% | 20.59% | 30.04% | 26 |
| Sertraline | PM | Adverse reaction | CPIC | 76 | 4.76% | 2.39% | 1.19% | 2.92% | 2 |
| Citalopram | UM, RM, or PM | Adverse reaction or decreased efficacy | CPIC | 75 | 32.79% | 34.29% | 28.10% | 31.82% | 24 |
| Omeprazole | UM | Decreased efficacy | CPIC | 53 | 4.29% | 4.68% | 2.77% | 3.95% | 2 |
| Escitalopram | UM, RM, or PM | Adverse reaction or decreased efficacy | CPIC | 52 | 32.79% | 34.29% | 28.10% | 31.82% | 17 |
| Voriconazole | UM, RM, or PM | Adverse reaction or decreased efficacy | CPIC | 16 | 32.79% | 34.29% | 28.10% | 31.82% | 5 |
| Esomeprazole | UM | Decreased efficacy | CPIC | 7 | 4.29% | 4.68% | 2.77% | 3.95% | 0 |
| Total (n = 1,852) | 107 | ||||||||
| Per 100 patients | 6 | ||||||||
| CYP2D6 + CYP2C19 | |||||||||
| Amitriptyline | CYP2D6 UM, IM, or PM; CYP2D6 NMs who are CYP2C19 UMs, RMs, or PMs | Adverse reaction or decreased efficacy | CPIC | 8 | 55.82% | 56.45% | 49.88% | 54.27% | 4 |
| Doxepin | CYP2D6 UM, IM, or PM; CYP2D6 NMs who are CYP2C19 UMs, RMs, or PMs | Adverse reaction or decreased efficacy | CPIC | 3 | 55.82% | 56.45% | 49.88% | 54.27% | 2 |
| Clomipramine | CYP2D6 UM, IM, or PM; CYP2D6 NMs who are CYP2C19 UMs, RMs, or PMs | Adverse reaction or decreased efficacy | CPIC | 2 | 55.82% | 56.45% | 49.88% | 54.27% | 1 |
| Imipramine | CYP2D6 UM, IM, or PM; CYP2D6 NMs who are CYP2C19 UMs, RMs, or PMs | Adverse reaction or decreased efficacy | CPIC | 1 | 55.82% | 56.45% | 49.88% | 54.27% | 1 |
| Total (n = 1,852) | 8 | ||||||||
| Per 100 patients | 0 | ||||||||
CPIC, Clinical Pharmacogenetics Implementation Consortium; FDA, US Food and Drug Administration; DPWG, Dutch Pharmacogenetics Working Group; IM, intermediate metabolizer; NM, normal metabolizer; PGx, pharmacogenomics; PM, poor metabolizer; RM, rapid metabolizer; UM, ultra‐rapid metabolizer.
DPWG guidelines were used to define actionable phenotype for CPIC A/B drugs that did not have a CPIC guideline and were not listed on the FDA Table of Pharmacogenetic Associations.
Actionable phenotype population frequencies by ancestry extracted from CPIC guideline appendices.
Study population frequency based is based on proportion of Black, White, and Hispanic values of self‐reported race (excluding unknown and other races): 38% Black, 31% White, and 31% Hispanic.
Projected PGx interventions = Unique patients with order * Study population actionable phenotype frequency.
Table 2.
Simulation estimating PGx interventions enabled by other genes
| Medication | Actionable phenotype | Consequence | Supporting evidence | Unique patients w/ order | Actionable phenotype frequencya | Projected # of PGx interventionsc | |||
|---|---|---|---|---|---|---|---|---|---|
| Black | White | Hispanic | Study populationb | ||||||
| G6PD | |||||||||
| Hydroxychloroquine | G6PD deficient | Adverse reaction | CPIC | 431 | 9.50% | 0.40% | 1.50% | 4.18% | 18 |
| Glipizide | G6PD deficient | Adverse reaction | CPIC | 62 | 9.50% | 0.40% | 1.50% | 4.18% | 3 |
| Trimethoprim/sulfamethoxazole | G6PD deficient | Adverse reaction | CPIC | 46 | 9.50% | 0.40% | 1.50% | 4.18% | 2 |
| Levofloxacin | G6PD deficient | Adverse reaction | CPIC | 43 | 9.50% | 0.40% | 1.50% | 4.18% | 2 |
| Ciprofloxacin | G6PD deficient | Adverse reaction | CPIC | 40 | 9.50% | 0.40% | 1.50% | 4.18% | 2 |
| Moxifloxacin | G6PD deficient | Adverse reaction | CPIC | 30 | 9.50% | 0.40% | 1.50% | 4.18% | 1 |
| Nitrofurantoin | G6PD deficient | Adverse reaction | CPIC | 15 | 9.50% | 0.40% | 1.50% | 4.18% | 1 |
| Erythromycin | G6PD deficient | Adverse reaction | CPIC | 13 | 9.50% | 0.40% | 1.50% | 4.18% | 1 |
| Phenazopyridine | G6PD deficient | Adverse reaction | CPIC | 8 | 9.50% | 0.40% | 1.50% | 4.18% | 0 |
| Sulfadiazine | G6PD deficient | Adverse reaction | CPIC | 8 | 9.50% | 0.40% | 1.50% | 4.18% | 0 |
| Glimepiride | G6PD deficient | Adverse reaction | CPIC | 7 | 9.50% | 0.40% | 1.50% | 4.18% | 0 |
| Dapsone | G6PD deficient | Adverse reaction | CPIC | 3 | 9.50% | 0.40% | 1.50% | 4.18% | 0 |
| Quinidine | G6PD deficient | Adverse reaction | CPIC | 2 | 9.50% | 0.40% | 1.50% | 4.18% | 0 |
| Sulfasalazine | G6PD deficient | Adverse reaction | CPIC | 2 | 9.50% | 0.40% | 1.50% | 4.18% | 0 |
| Total (n = 1,852) | 30 | ||||||||
| Per 100 patients | 2 | ||||||||
| CYP2C9 | |||||||||
| Ibuprofen | PM | Adverse reaction | CPIC | 247 | 0.52% | 2.56% | 0.95% | 1.29% | 3 |
| Warfarin | VKORC1/CYP2C9 warfarin sensitive/highly sensitive | Adverse reaction | CPIC | 63 | 3.29% | 37.72% | 26.20% | 21.14% | 13 |
| Meloxicam | IM (AS = 1) or PM | Adverse reaction | CPIC | 12 | 5.49% | 16.32% | 8.97% | 9.96% | 1 |
| Dronabinol | IM or PM | Adverse reaction | FDA | 11 | 24.13% | 37.08% | 25.42% | 28.59% | 3 |
| Celecoxib | PM | Adverse reaction | CPIC | 8 | 0.52% | 2.56% | 0.95% | 1.29% | 0 |
| Phenytoin | IM or PM or HLA‐B*15:02 carrier | Adverse reaction | CPIC | 6 | 24.33% | 37.16% | 25.48% | 28.71% | 2 |
| Total (n = 1,852) | 23 | ||||||||
| Per 100 patients | 1 | ||||||||
| HLA‐A or HLA‐B | |||||||||
| Allopurinol | HLA‐B*58:01 carrier | Adverse reaction | CPIC | 42 | 7.63% | 2.62% | 1.79% | 4.26% | 2 |
| Carbamazepine | HLA‐B*15:02 or HLA‐A*31:01 carriers | Adverse reaction | CPIC | 7 | 2.15% | 5.68% | 10.40% | 5.79% | 0 |
| Oxcarbazepine | HLA‐B*15:02 or HLA‐A*31:01 carriers | Adverse reaction | CPIC | 6 | 2.15% | 5.68% | 10.40% | 5.79% | 0 |
| Abacavir | HLA‐B*57:01 carriers | Adverse reaction | CPIC | 5 | 0.20% | 6.36% | 2.52% | 2.85% | 0 |
| Total (n = 1,852) | 3 | ||||||||
| Per 100 patients | 0 | ||||||||
| Others | |||||||||
| Simvastatin | SLCO1B1 intermediate or low function | Adverse reaction | CPIC | 45 | 8.80% | 34.07% | 29.61% | 23.12% | 10 |
| Tacrolimus | CYP3A5 EM or IM | Decreased efficacy | CPIC | 27 | 70.06% | 14.27% | 31.57% | 40.72% | 11 |
| Azathioprine | TPMT IM or PM or NUDT15 IM or PM | Adverse reaction | CPIC | 5 | 7.32% | 9.34% | 18.15% | 11.28% | 1 |
| Atazanavir | UGT1A1 PM | Adverse reaction | CPIC | 1 | 36.67% | 19.92% | 30.66% | 29.57% | 0 |
| Irinotecan | UGT1A1 PM | Adverse reaction | FDA | 1 | 36.67% | 19.92% | 30.66% | 29.57% | 0 |
| Total (n = 1,852) | 23 | ||||||||
| Per 100 patients | 1 | ||||||||
AS, activity score; CPIC, Clinical Pharmacogenetics Implementation Consortium; EM, extensive metabolizer; FDA, US Food and Drug Administration; IM, intermediate metabolizer; NM, normal metabolizer; PGx, pharmacogenomics; PM, poor metabolizer; RM, rapid metabolizer; UM, ultra‐rapid metabolizer.
Actionable phenotype population frequencies by ancestry extracted from CPIC guideline appendices; G6PD phenotype frequencies extracted from PMID 31860324; warfarin sensitive phenotype based on CYP2C9 and VKORC1, phenotype frequencies extracted from PMID 28689179 and PMID 21185752.
Study population frequency based is based on proportion of Black, White, and Hispanic values of self‐reported race (excluding unknown and other races): 38% Black, 31% White, and 31% Hispanic.
Projected PGx interventions = Unique patients with order * Study population actionable phenotype frequency.
DISCUSSION
At an urban, tertiary care academic medical system in the United States, we found that medications affected by PGx are ordered in most individuals hospitalized with COVID‐19. Multigene PGx results would present opportunities for treatment optimization, primarily for medications metabolized by CYP2D6 and CYP2C19. These findings compliment assessments of the value of multigene PGx in other medically complex patients, such as those undergoing percutaneous coronary intervention (PCI). CYP2C19 genotype is used to guide antiplatelet medication selection after PCI at some institutions. 44 Analyses have examined the potential of the CYP2C19 result to be used for other treatment decisions and whether a multigene panel would provide more useful PGx information. 8 , 9 , 45 One study found that 71.6% of patients received a medication with PGx guidance other than the antiplatelet medication in the year following PCI. 9 A separate simulation estimated that a multigene PGx panel would enable 17.5 interventions per 100 patients tested in the year following PCI. 8 Despite the differences in patient population and follow‐up time, these findings are similar to ours. We found a higher percentage of patients (89.7%) were prescribed an actionable medication, and testing 100 patients would enable 17 treatment modifications during the index hospitalization. To a degree, it is unsurprising that multigene PGx testing could be useful in complex patients requiring multiple medications, given that almost all individuals carry an actionable PGx variant in commonly tested pharmacogenes. 4
Anti‐emetic and analgesic medications were commonly prescribed in our study, and would present numerous opportunities for treatment optimization if PGx results were available according to our simulation. Ondansetron was the most commonly ordered medication with PGx guidance, and prior work has demonstrated that CYP2D6 ultra‐rapid metabolizers treated with ondansetron have significantly higher incidence of vomiting than patients with other phenotypes. 46 , 47 This represents an opportunity for treatment optimization in the ~ 4% of the population that are CYP2D6 ultra‐rapid metabolizers, given that alternative anti‐emetics are widely available—including granisetron, a 5‐HT3 antagonist unaffected by CYP2D6 phenotype. Additionally, a large proportion of our study population was treated with opioid analgesics and nonsteroidal anti‐inflammatory drugs. CYP2D6 bioactivates prodrug opioids, such as codeine, tramadol, hydrocodone, and oxycodone. CPIC has published a guideline for modifying opioid treatment based upon CYP2D6 phenotype, 30 and evidence demonstrating the clinical benefit of genotype‐guided opioid analgesia is emerging. 48 CYP2C9 genotype or phenotype is a predictor of gastrointestinal bleeding during treatment with nonsteroidal anti‐inflammatory drugs. 49 , 50 Lower starting doses or alternative agents unaffected by CYP2C9 phenotype (e.g., naproxen) can be pursued in patients at higher risk of bleed due to CYP2C9. 26
Our findings may underestimate the long‐term utility of multigene PGx testing among individuals with COVID‐19, because our analysis focused exclusively on inpatient orders during the index hospitalization. PGx results have lifelong utility, so new actionable medications—both for consequences of COVID‐19 and unrelated illnesses—are likely to be prescribed months or years after the COVID‐19 hospitalization. This “downstream” utility highlights the need for PGx results to be integrated into the EHR with clinical decision support, and ideally to be portable for use at other healthcare systems. 44 On the other hand, the presented simulation may overestimate the utility of multigene PGx during the index hospitalization. Many orders were treatments for chronic conditions, and if patients were previously titrated to an appropriate dose and/or tolerating the medication prior to index hospitalization, it is unlikely that clinicians would act on the PGx results.
We recognize several limitations to our study. Our simulation is based upon expected phenotype frequencies instead of directly assaying patient biospecimens. However, these phenotype frequencies are well‐described in literature, and we adjusted expected phenotype frequencies based on the racial demographics of our cohort. Expected population phenotype frequencies are dependent on population ancestry. 24 The demographics of our population differ from many institutions in the United States and abroad. Thus, a simulation analysis using the same medication data would yield different results in cohorts with different racial characteristics.
We did not measure, nor attempt to estimate, the frequency of suboptimal medication outcomes, such as lack of efficacy or adverse drug reactions, for the medications of interest. Such an approach was not feasible given the dozens of medications of interest and short study period that was limited to the index hospitalization. In addition, medication use patterns will change as patient demographics (e.g., age) and treatment strategies shift over time. For example, hydroxychloroquine was used in 25% of the patients in our cohort, but this treatment is now recommended against the treatment of COVID‐19 outside the context of a clinical trial. 43 Regardless, our conclusion that multigene PGx testing would often present opportunities for treatment optimization in patients with COVID‐19 is independent of hydroxychloroquine use.
However, our study also has several strengths. Our urban, tertiary care health system allowed for analysis of a large and diverse population of patients. The shared EHR infrastructure allowed for the timely creation of a patient registry with detailed medication data. We presented our data both with and without including hydroxychloroquine orders—a major way that routine treatment has changed between the study period and current practice. The level of detail provided in our tables allows readers to consider how our estimated clinical implications would change if a more limited PGx panel was used or if this analysis was applied to a population with different racial characteristics or with certain medications off‐formulary.
In conclusion, individuals hospitalized with COVID‐19 are treated with multiple medications for acute illness as well as common chronic comorbidities. Many of these medications have actionable PGx guidance, and nearly 9 in 10 patients have at least one such drug ordered. We estimated that multigene PGx testing would present 17 opportunities for genotype‐guided treatment modification per 100 patients tested. Future studies are needed to determine how often these treatment modifications would be pursued by prescribers and the subsequent impact on clinical outcomes. Nonetheless, our findings suggest that multi‐gene PGx testing would present opportunities for treatment optimization during hospitalization, and intuitively for future outpatient care as well.
Funding
No funding was received for this work.
Conflict of Interest
Dr. Alexander is past Chair of the FDA’s Peripheral and Central Nervous System Advisory Committee; has served as a paid advisor to IQVIA; is a co‐founding Principal and equity holder in Monument Analytics, a health care consultancy whose clients include the life sciences industry as well as plaintiffs in opioid litigation; and is a member of OptumRx’s National P&T Committee. These arrangements have been reviewed and approved by Johns Hopkins University in accordance with its conflict of interest policies. All other authors declared no competing interests for this work.
Author Contributions
J.M.S., G.C.A., and H.B.M. wrote the manuscript. J.M.S. and G.C.A. designed the research. J.M.S., N.P., and H.B.M. performed the research. J.M.S. and N.P. analyzed data.
Acknowledgments
The data utilized for this publication were part of the JH‐CROWN: COVID PMAP Registry, which is based on the contribution of many patients and clinicians. JH‐CROWN received funding from Hopkins inHealth, the Johns Hopkins Precision Medicine Program. The authors gratefully acknowledge Diana Gumas, Jacob Fiksel, Stuart Ray, and Bonnie Woods for their contributions to the JH‐CROWN and our analytic approach.
References
- 1. United States Food and Drug Administration . Table of pharmacogenomic biomarkers in drug labeling <http://www.fda.gov/drugs/scienceresearch/researchareas/pharmacogenetics/ucm083378.htm> (2020). Accessed May 5, 2020.
- 2. Dunnenberger, H.M. et al Preemptive clinical pharmacogenetics implementation: current programs in five US medical centers. Annu. Rev. Pharmacol. Toxicol. 55, 89–106 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dong, O.M. et al Cost‐effectiveness of multigene pharmacogenetic testing in patients with acute coronary syndrome after percutaneous coronary intervention. Value Health 23, 61–73 (2020). [DOI] [PubMed] [Google Scholar]
- 4. Chanfreau‐Coffinier, C. et al Projected prevalence of actionable pharmacogenetic variants and level A drugs prescribed among US Veterans Health Administration Pharmacy users. JAMA Netw. Open 2, e195345 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention . COVIDView: A Weekly Surveillance Summary of U.S. COVID‐19 Activity <https://www.cdc.gov/coronavirus/2019‐ncov/covid‐data/covidview/index.html> (2020). Accessed September 30, 2020.
- 6. Price‐Haywood, E.G. , Price‐Haywood, E.G. , Burton, J. , Fort, D. & Seoane, L. Hospitalization and mortality among black patients and white patients with COVID‐19. N. Engl. J. Med. 382, 2534–2543 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grice, G.R. , Seaton, T.L. , Woodland, A.M. & McLeod, H.L. Defining the opportunity for pharmacogenetic intervention in primary care. Pharmacogenomics 7, 61–65 (2006). [DOI] [PubMed] [Google Scholar]
- 8. Black, R.M. et al Projected impact of pharmacogenomic testing on medications beyond antiplatelet therapy in percutaneous coronary intervention patients. Pharmacogenomics 21, 431–441 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rouby, N.E. et al Clinical utility of pharmacogene panel‐based testing in patients undergoing percutaneous coronary intervention. Clin. Transl. Sci. 13, 473–481 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Smith, D.M. et al Pharmacogenetics in practice: estimating the clinical actionability of pharmacogenetic testing in perioperative and ambulatory settings. Clin. Transl. Sci. 13, 618–627 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Murray, M.F. et al COVID‐19 outcomes and the human genome. Genet. Med. 22, 1175–1177 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Manickam, K. et al Exome sequencing‐based screening for BRCA1/2 expected pathogenic variants among adult biobank participants. JAMA Netw. Open 1, e182140 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aquilante, C.L. et al Clinical implementation of pharmacogenomics via a health system‐wide research biobank: the University of Colorado experience. Pharmacogenomics 21, 375–386 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stergachis, A.B. , Weiss, S.T. & Green, R.C. Biobanks could identify medically actionable findings relevant for COVID‐19 clinical care. Nat. Med. 26, 991 (2020). [DOI] [PubMed] [Google Scholar]
- 15. United States Food and Drug Administration . Table of Pharmacogenetic Associations <https://www.fda.gov/medical‐devices/precision‐medicine/table‐pharmacogenetic‐associations> (2020). Accessed June 15, 2020.
- 16. Caudle, K.E. et al Evidence and resources to implement pharmacogenetic knowledge for precision medicine. Am. J. Health Pharm. 73, 1977–1985 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Clinical Pharmacogenetics Implementation Consortium . Genes‐Drugs ‐ CPIC <https://cpicpgx.org/genes‐drugs/>. Accessed June 15, 2020.
- 18. Haga, S.B. & Kantor, A. Horizon scan of clinical laboratories offering pharmacogenetic testing. Health Aff. 37, 717–723 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lima, J.J. et al Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for CYP2C19 and proton pump inhibitor dosing. Clin. Pharmacol. Ther. https://doi.org/ 10.1002/cpt.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hicks, J.K. et al Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for CYP2D6 and CYP2C19 genotypes and dosing of selective serotonin reuptake inhibitors. Clin. Pharmacol. Ther. 98, 127–134 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brown, J.T. et al Clinical Pharmacogenetics Implementation Consortium guideline for cytochrome P450 (CYP)2D6 genotype and atomoxetine therapy. Clin. Pharmacol. Ther. 106, 94–102 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Phillips, E.J. et al Clinical Pharmacogenetics Implementation Consortium guideline for HLA genotype and use of carbamazepine and oxcarbazepine: 2017 update. Clin. Pharmacol. Ther. 103, 574–581 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Relling, M.V. et al Clinical pharmacogenetics implementation consortium guideline for thiopurine dosing based on TPMT and NUDT15 genotypes: 2018 update. Clin. Pharmacol. Ther. 105, 1095–1105 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Scott, S.A. et al Clinical pharmacogenetics implementation consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin. Pharmacol. Ther. 94, 317–323 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Caudle, K.E. et al Clinical pharmacogenetics implementation consortium guidelines for CYP2C9 and HLA‐B genotypes and phenytoin dosing. Clin. Pharmacol. Ther. 96, 542–548 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Theken, K.N. et al Clinical Pharmacogenetics Implementation Consortium Guideline (CPIC) for CYP2C9 and nonsteroidal anti‐inflammatory drugs. Clin. Pharmacol. Ther. 108, 191–200 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goetz, M.P. et al Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2D6 and tamoxifen therapy. Clin. Pharmacol. Ther. 103, 770–777 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bell, G.C. et al Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for CYP2D6 genotype and use of ondansetron and tropisetron. Clin. Pharmacol. Ther. 102, 213–218 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gammal, R.S. et al Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for UGT1A1 and atazanavir prescribing. Clin. Pharmacol. Ther. 99, 363–369 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Crews, K.R. et al Clinical pharmacogenetics implementation consortium (CPIC) guidelines for codeine therapy in the context of cytochrome P450 2D6 (CYP2D6) genotype. Clin. Pharmacol. Ther. 91, 321–326 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moriyama, B. et al Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for CYP2C19 and voriconazole therapy. Clin. Pharmacol. Ther. 102, 45–51 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Birdwell, K.A. et al Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for CYP3A5 genotype and tacrolimus dosing. Clin. Pharmacol. Ther. 98, 19–24 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Saito, Y. et al CPIC: Clinical Pharmacogenetics Implementation Consortium of the pharmacogenomics research network. Clin. Pharmacol. Ther. 99, 36–37 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hicks, J.K. et al Clinical pharmacogenetics implementation consortium guideline (CPIC) for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants: 2016 update. Clin. Pharmacol. Ther. 102, 37–44 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Whirl‐Carrillo, M. et al Pharmacogenomics knowledge for personalized medicine. Clin. Pharmacol. Ther. 92, 414–417 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gonzalez‐Galarza, F.F. et al Allele frequency net database (AFND) 2020 update: gold‐standard data classification, open access genotype data and new query tools. Nucleic Acids Res. 49. D783–D788 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cavallari, L.H. et al Pharmacogenomics of Warfarin dose requirements in Hispanics. Blood Cells Mol. Dis. 46, 147–150 (2011). [DOI] [PubMed] [Google Scholar]
- 38. Vandell, A.G. et al Genetics and clinical response to warfarin and edoxaban in patients with venous thromboembolism. Heart 103, 1800–1805 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee, J. & Poitras, B.T. Prevalence of glucose‐6‐phosphate dehydrogenase deficiency, U.S. Armed Forces, May 2004–September 2018. Med. Surveill. Mon. Rep. 26, 14–17 (2019). [PubMed] [Google Scholar]
- 40. Endo, S. et al Association study of genetic polymorphisms of drug transporters, SLCO1B1, SLCO1B3 and ABCC2, in African‐Americans, Hispanics and Caucasians and olmesartan exposure. J. Hum. Genet. 57, 531–544 (2012). [DOI] [PubMed] [Google Scholar]
- 41. U.S. National Library of Medicine . DailyMed ‐ COUMADIN ‐ warfarin sodium tablet <https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d91934a0‐902e‐c26c‐23ca‐d5accc4151b6> (2020). Accessed May 5, 2020.
- 42. PharmGKB . Annotation of DPWG Guideline for risperidone and CYP2D6 <https://www.pharmgkb.org/guidelineAnnotation/PA166104943> (2020). Accessed May 5, 2020.
- 43. COVID‐19 Treatment Guidelines Panel . Coronavirus Disease 2019 (COVID‐19) Treatment Guidelines. National Institutes of Health; <https://www.covid19treatmentguidelines.nih.gov> (2020). Accessed June 15, 2020. [PubMed] [Google Scholar]
- 44. Empey, P.E. et al Multisite investigation of strategies for the implementation of CYP2C19 genotype‐guided antiplatelet therapy. Clin. Pharmacol. Ther. 104, 664–674 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Beitelshees, A.L. et al Evaluating the extent of reusability of CYP2C19 genotype data among patients genotyped for antiplatelet therapy selection. Genet. Med. 22, 1898–1902 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kaiser, R. et al Patient‐tailored antiemetic treatment with 5‐hydroxytryptamine type 3 receptor antagonists according to cytochrome P‐450 2D6 genotypes. J. Clin. Oncol. 20, 2805–2811 (2002). [DOI] [PubMed] [Google Scholar]
- 47. Candiotti, K.A. et al The impact of pharmacogenomics on postoperative nausea and vomiting: Do CYP2D6 allele copy number and polymorphisms affect the success or failure of ondansetron prophylaxis? Anesthesiology 102, 543–549 (2005). [DOI] [PubMed] [Google Scholar]
- 48. Cavallari, L.H. et al Multi‐site investigation of strategies for the clinical implementation of CYP2D6 genotyping to guide drug prescribing. Genet. Med. 21, 2255–2263 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Figueiras, A. et al CYP2C9 variants as a risk modifier of NSAID‐related gastrointestinal bleeding: a case‐control study. Pharmacogenet. Genomics 26, 66–73 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Macías, Y. , Gómez Tabales, J. , García‐Martín, E. & Agúndez, J.A.G. An update on the pharmacogenomics of NSAID metabolism and the risk of gastrointestinal bleeding. Expert Opin. Drug Metab. Toxicol. 16, 319–332 (2020). [DOI] [PubMed] [Google Scholar]


