Abstract
In a multinational placebo‐controlled phase III clinical trial in 2,185 patients with type 2 diabetes mellitus and stage 4 chronic kidney disease, treatment with the Nrf2 activator bardoxolone methyl increased estimated glomerular filtration rate, a measure of kidney function, but also resulted in increases in serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), and gamma glutamyl transferase. These increases in liver enzyme level(s) were maximal after 4 weeks of treatment and reversible, trending back toward baseline through week 48. Total bilirubin concentrations did not increase, and no cases met Hy’s Law criteria, although two subjects had ALT concentrations that exceeded 10 × the upper limit of the population reference range leading to discontinuation of treatment. Animal and cell culture experiments suggested that the increases in ALT and AST induced by bardoxolone methyl may be related to its pharmacological activity. Bardoxolone methyl significantly induced the mRNA expression of ALT and AST isoforms in cultured cells. Expression of ALT and AST isoforms in liver and kidney also positively correlated with Nrf2 status in mice. Overall, these data suggest that the increases in ALT and AST observed clinically were, at least in part, related to the pharmacological induction of aminotransferases via Nrf2 activation, rather than to any intrinsic form of hepatotoxicity.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
The Bardoxolone Methyl Evaluation in Patients with Chronic Kidney Disease and Type 2 Diabetes (BEACON) trial was a multinational randomized placebo‐controlled phase III clinical trial in 2185 patients with type 2 diabetes mellitus and stage 4 chronic kidney disease. Consistent with prior clinical studies, BEACON participants randomized to bardoxolone methyl experienced significant increases in estimated glomerular filtration rate, as well as increases in hepatic enzymes.
WHAT QUESTION DID THIS STUDY ADDRESS?
This study characterizes the changes in hepatic enzymes induced by bardoxolone methyl.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
The present analyses from BEACON support the contention that bardoxolone methyl‐induced elevations in serum aminotransferase concentrations may be due to, at least in part, a direct transcriptional effect on aminotransferase production rather than to intrinsic hepatotoxicity.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
Our data support that the likelihood that elevations in serum aminotransferases, which are commonly considered to reflect liver injury, may also reflect on‐target effects in the absence of liver injury.
Bardoxolone methyl, an investigational drug, and related analogs are oleanolic acid‐derived synthetic triterpenoid compounds that potently induce the Nrf2‐KEAP1 pathway. 1 , 2 Through interaction with KEAP1, the Nrf2 repressor molecule, bardoxolone methyl, promotes translocation of Nrf2 to the nucleus, where Nrf2 binds to antioxidant response elements in the promoter region of its target genes, leading to induction of antioxidant and cytoprotective enzymes and related proteins. 3 , 4 Bardoxolone methyl is also a potent inhibitor of the NF‐κB inflammatory pathway through both direct (i.e., inhibition of IKKβ kinase activity) and indirect (i.e., detoxification of reactive oxygen species) mechanisms. 5 These dual processes of upregulation of the antioxidant response and suppression of pro‐inflammatory signaling consistently result in protection from oxidative stress, inflammation, and mitochondrial dysfunction in preclinical models. 6 , 7
Improvements in kidney function, assessed using measured inulin clearance, creatinine clearance, and estimated glomerular filtration rate (eGFR), have been consistently observed with bardoxolone methyl treatment in several clinical trials. 8 , 9 , 10 , 11 The largest of these trials was Bardoxolone Methyl Evaluation in Patients with Chronic Kidney Disease and Type 2 Diabetes (BEACON), 10 a multinational, randomized, double‐blind, placebo‐controlled phase III event‐driven trial, which enrolled 2,185 patients with type 2 diabetes mellitus (T2DM) and stage 4 chronic kidney disease (CKD). 12 The BEACON trial was terminated after preliminary analyses showed that patients randomized to bardoxolone methyl experienced a significantly higher rate of heart failure events (a secondary end point) relative to placebo during the first 4 weeks of exposure. At the time of termination, there were 70 coprimary end point events (end‐stage kidney disease or cardiovascular death) reported in 69 patients in both groups. Post hoc analyses identified a history of heart failure and elevated serum concentrations of B‐type natriuretic peptide as risk factors for heart failure events and subsequent clinical trials in other disease states have excluded patients with these clinical characteristics. 13 , 14 In concert with improved kidney function, patients in BEACON randomized to bardoxolone methyl experienced increases in serum concentrations of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and gamma‐glutamyl transferase (GGT). Although increases in hepatic enzymes are commonly considered to reflect liver injury, increases in aminotransferases have been reported in the absence of apparent hepatotoxicity, such as a result of changes in diet in healthy volunteers and in patients with type 1 diabetes mellitus after the first dose of insulin. 15 Moreover, ALT and AST are expressed in other tissues in addition to the liver and play a role in intermediary metabolism and energy homeostasis by catalyzing the conversion of α‐ketoglutarate to glutamate. 16 Preclinical studies have demonstrated that bardoxolone methyl and its analogs protect several animal species against hepatotoxicity in multiple models of hepatic injury (e.g., acetaminophen, concanavalin A, and aflatoxin). 17 , 18 , 19 In this secondary analysis of data from BEACON, we aimed to characterize and determine the cause of changes in hepatic enzymes induced by bardoxolone methyl.
METHODS
Clinical study design
Previous publications have described the BEACON trial design in detail. 10 , 12 Patients with T2DM and CKD stage 4 (eGFR 15 to < 30 mL/min/1.73 m2) were randomized 1:1 to once‐daily administration of oral bardoxolone methyl (20 mg) or placebo. The primary composite end point was end‐stage kidney disease (defined as the provision of maintenance dialysis, kidney transplantation, or death due to kidney disease) and cardiovascular death. Secondary efficacy outcomes included the change in eGFR, hospitalization for heart failure or death due to heart failure, and a cardiovascular composite end point, including nonfatal myocardial infarction, nonfatal stroke, hospitalization for heart failure, or cardiovascular death.
The eGFR was calculated using serum creatinine and the four‐variable Modification of Diet in Renal Disease study equation. The eGFR and clinical chemistries (including ALT, AST, GGT, and total bilirubin) were assessed every 4 weeks through week 12, followed by assessments every 8 weeks thereafter. Patients returned for a post‐treatment visit 4 weeks after the last dose of study drug. Protocol‐specified criteria for management of liver enzyme abnormalities are provided in the Supplementary Methods.
The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki, was approved by Institutional Review Boards at participating study sites, and was registered at ClinicalTrials.gov (NCT01351675). Informed consent in writing was obtained from each patient.
Cell culture and treatment
Human HuH‐7 hepatocellular carcinoma cells were purchased from Health Science Research Resources Bank, Japan. All other cell lines were obtained from American Type Culture Collection, Manassas, VA. Cells were treated with vehicle (DMSO) or bardoxolone methyl for 16–18 hours. Following treatment, cells were lysed, homogenized using QIAShredder columns, and RNA was isolated using RNeasy Plus Mini kits (Qiagen).
Reverse transcription quantitative polymerase chain reaction
For reverse transcription, total RNA was primed with oligo[dT]12‐18. Reverse transcription was performed using Superscript II Reverse Transcriptase (Thermo Fisher Scientific) at 42°C for 1 hour. For quantitative polymerase chain reaction amplification, cDNA was undiluted (HepG2 and mouse tissue samples) or diluted 1:10 in RNase‐free water (all other cell lines) and combined with iQ SYBR Green Supermix (Bio‐Rad) and quantitative polymerase chain reaction primers, which were previously validated for specificity and amplification efficiency. Primer sequences were as follows: mouse ribosomal protein L19 (Rpl19) forward: 5′‐TCAGGCTACAGAAGAGGCTTGC‐3′, reverse: 5′‐ACAGTCACAGGCTTGCGGATG‐3′; mouse glutamic‐pyruvic transaminase 1 (Gpt/Alt1) forward: 5′‐CACGGAGCAGGTCTTCAACG‐3′, reverse: 5′‐AGAATGGTCATCCGGAAATG‐3′; mouse glutamic‐pyruvic transaminase 2 (Gpt/Alt1) forward: 5′‐CGCGGTGCAGGTCAACTACT‐3′, reverse: 5′‐CCTCATCAGCCAGGAGAAAA‐3′; mouse glutamic‐oxaloacetic transaminase 1 (Got1/Ast1) forward: 5′‐GGCTATTCGCTATTTTGTGT‐3′, reverse: 5′‐GACCAGGTGATTCGTACAAT‐3′; mouse glutamic‐oxaloacetic transaminase 2 (Got2/Ast2) forward: 5′‐AGAGTCCTCTTCAGTCATTG‐3′, reverse: 5′‐ATGATTAGAGCAGATGGTGG‐3′; human ribosomal protein S9 (RPS9) forward: 5′‐GATTACATCCTGGGCCTGAA‐3′, reverse: 5′‐GAGCGCAGAGAGAAGTCGAT‐3′; human glutamic‐pyruvic transaminase 1 (GPT/ALT1) forward: 5′‐GGTCTTGGCCCTCTGTGTTA‐3′, reverse: 5′‐CCTGTGGACAGGAAGACGTT‐3′; human glutamic‐pyruvic transaminase 2 (GPT2/ALT2) forward: 5′‐CGCCATCCAGGTGAATTACT‐3′, reverse: 5′‐CCTCATCAGCCAGGAGAAAG‐3′; human glutamic‐oxaloacetic transaminase 1 (GOT1/AST1) forward: 5′‐GGCCATTCGCTATTTTGTGT‐3′, reverse: 5′‐GACCAAGTAATCCGCACGAT‐3′; and human glutamic‐oxaloacetic transaminase 2 (GOT2/AST2) forward: 5′‐CGTGAGAAACAGCACACGTT‐3′, reverse: 5′‐GCATTATTTCCCTTGCTGGA‐3′. RPL19 or RSP9 primers served as controls. The cDNA was amplified using the following cycling conditions: 95°C for 3 minutes, 44 cycles of 95°C for 30 seconds, 60°C for 15 seconds, and 72°C for 15 seconds. A melt curve analysis was performed after the last cycle.
Statistical methods
We analyzed data from the BEACON trial in accordance with the intention‐to‐treat principle. We used longitudinal analyses of ALT, AST, GGT, alkaline phosphatase, and total bilirubin to compare the difference in mean changes for each parameter between the bardoxolone methyl and placebo groups. For each parameter analyzed, mixed‐effects regression used the post‐baseline parameter as the response variable; treatment group, time, the interaction of treatment group with time, interaction of the baseline parameter with time; and continuous covariates (baseline parameter, baseline eGFR, and log‐transformed urinary albumin‐to‐creatinine ratio).
In addition to analyses of mean changes over time, we used shift tables to compare baseline values of ALT and AST to the maximum post‐baseline values while on study drug, and to compare maximum serum concentrations of ALT and AST during study drug administration as compared with the post‐treatment assessment, which occurred 4 weeks after the last dose of study drug. We categorized shifts in ALT and AST using thresholds for lower and upper limits of the population reference range based on the central laboratory (ICON Central Laboratories) reference ranges: 0 and 47 units per liter (U/L) for ALT and 0 and 37 U/L for AST.
Data from cell culture experiments were analyzed by a repeated‐measures one‐way analysis of variance (ANOVA) followed by Dunnett’s post hoc test with significance set at P < 0.05.
RESULTS
Patient population
A total of 2,185 patients were randomly assigned to receive bardoxolone methyl (n = 1,088) or placebo (n = 1,097). Previous publications have detailed the patient demographics and baseline characteristics of the intention‐to‐treat population in BEACON. 12 Table 1 shows demographic factors and baseline serum levels of hepatic enzymes in both treatment arms.
Table 1.
Select demographics and baseline characteristics of BEACON patients
| Intent‐to‐treat population | ||
|---|---|---|
| Placebo (n = 1,097) | Bardoxolone methyl (n = 1,088) | |
| Age, year, mean ± SD | 68.2 ± 9.4 | 68.9 ± 9.7 |
| Female [n, %] | 472 [43] | 462 [42] |
| Race [n, %] | ||
| White | 848 [77] | 846 [78] |
| Black | 176 [16] | 185 [17] |
| Other | 73 [7] | 57 [5] |
| Serum creatinine, mg/dL, mean ± SD | 2.7 ± 0.6 | 2.7 ± 0.6 |
| eGFR, mL/min/1.73 m2, mean ± SD | 22.5 ± 4.6 | 22.4 ± 4.3 |
| UACR, mg/g, geometric mean | 221 | 210 |
| HbA1c, %, mean ± SD | 7.10 ± 1.17 | 7.15 ± 1.27 |
| ALT, U/L, mean ± SD | 18.4 ± 6.9 | 18.7 ± 7.1 |
| AST, U/L, mean ± SD | 19.5 ± 5.3 | 19.9 ± 5.7 |
| Total bilirubin, mg/dL, mean ± SD | 0.32 ± 0.14 | 0.33 ± 0.14 |
Partially reproduced from: Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. De Zeeuw et al. N Engl. J. Med. 369:2495, Copyright © 2013. 10
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BEACON, Bardoxolone Methyl Evaluation in Patients with Chronic Kidney Disease and Type 2 Diabetes; eGFR, estimated glomerular filtration rate; UACR, urinary albumin creatinine ratio; U/L, units per liter.
Bardoxolone methyl transiently increased ALT, AST, GGT, and alkaline phosphatase
Mean baseline ALT and AST values were 18.7 ± 7.1 U/L and 19.9 ± 5.7 U/L in patients randomized to oral bardoxolone methyl 20 mg per day and 18.4 ± 6.9 U/L and 19.5 ± 5.3 U/L in patients randomized to placebo (Table 1 ). Mean changes in ALT and AST were 14.6 U/L (95% confidence interval (CI): 13.2 to 16 U/L) and 5.6 U/L (95% CI: 4.8 to 6.4) in patients randomized to bardoxolone methyl, and 0.3 U/L (95% CI: −0.1 to 1.6 U/L) and −0.1 U/L (95% CI: −0.8 to 0.7 U/L), respectively, in patients randomized to placebo (between group P values < 0.001 and < 0.001, respectively). Figure 1 shows trends in mean ALT and AST over time in the bardoxolone methyl and placebo groups; mean increases in ALT and AST induced by bardoxolone methyl were maximal at week 4 and trended back toward baseline values over time while therapy was continued.
Figure 1.
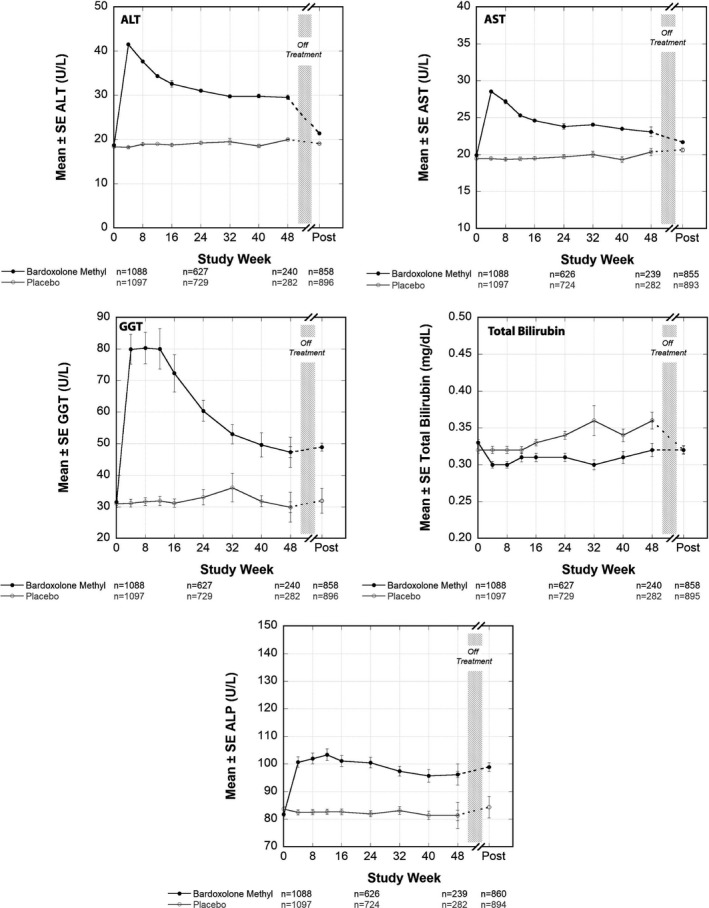
Mean ALT, AST, GGT, alkaline phosphatase and total bilirubin values in Bardoxolone Methyl Evaluation in Patients with Chronic Kidney Disease and Type 2 Diabetes (BEACON). Mean (± SEM) ALT, AST, GGT, alkaline phosphatase, and total bilirubin values (U/L) for patients randomized to bardoxolone methyl (n = 1,088) or placebo (n = 1,097) through 48 weeks of treatment. Post‐treatment values collected 4 weeks after the last dose of study drug was administered are also shown. ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma glutamyl transferase.
As seen in Table 2 , 38% of patients randomized to bardoxolone methyl and 5% of patients randomized to placebo had increases in ALT above the upper limit of the population reference range at any time during the trial. Similarly, 29% of patients randomized to bardoxolone methyl and 6% of patients randomized to placebo had increases in AST at any time during the trial that resulted in values higher than the upper limit of the population reference range (Table 3 ). Increases in aminotransferases were generally mild, with only 31 of 1,088 patients (3%) randomized to bardoxolone methyl experiencing maximum ALT values ≥ 3× the upper limit of the population reference range and 18 of 1,088 (2%) experiencing maximum AST values ≥ 3× the upper limit of the population reference range. A total of 6 of 1,088 patients (0.55%) randomized to bardoxolone methyl discontinued treatment due to aminotransferase elevations.
Table 2.
Shift in ALT (U/L) from baseline to maximum post‐baseline value while on study drug in BEACON
| Treatment group/baseline ALT | Maximum post‐baseline value while on treatment | |||||
|---|---|---|---|---|---|---|
| ≤ULN | >ULN to < 3 × ULN | ≥3 × ULN to < 5 × ULN | ≥5 × ULN to < 8 × ULN | ≥8 × ULN | No post‐baseline value | |
| Placebo [n = 1097] | ||||||
| ALT ≤ ULN [n = 1096; %] | 1,023 [93] | 50 [5] | 2 [<1] | 1 [<1] | 0 | 20 [2] |
| >ULN to < 3 × ULN [n = 1] | 0 | 1 [<1] | 0 | 0 | 0 | 0 |
| ≥3 × ULN to < 5 × ULN [n = 0] | 0 | 0 | 0 | 0 | 0 | 0 |
| ≥5 × ULN to < 8 × ULN [n = 0] | 0 | 0 | 0 | 0 | 0 | 0 |
| ≥8 × ULN [n = 0] | 0 | 0 | 0 | 0 | 0 | 0 |
| Bardoxolone Methyl [n = 1,088] | ||||||
| ALT ≤ ULN [n = 1086; %] | 610 [56] | 380 [35] | 18 [2] | 9 [1] | 2 [< 1] | 67 [6] |
| >ULN to < 3 × ULN [n = 2] | 0 | 0 | 1 [< 1] | 1 [< 1] | 0 | 0 |
| ≥3 × ULN to < 5 × ULN [n = 0] | 0 | 0 | 0 | 0 | 0 | 0 |
| ≥5 × ULN to < 8 × ULN [n = 0] | 0 | 0 | 0 | 0 | 0 | 0 |
| ≥8 × ULN [n = 0] | 0 | 0 | 0 | 0 | 0 | 0 |
Post‐baseline laboratory assessments include those collected on or before a patient’s last dose of study drug. The upper limit of the population reference range (ULN) for ALT is 47 (U/L). Categories listed in the tables are defined by ALT (U/L) values: ≤ 47; > 47 to < 142; ≥ 142 to < 236; ≥ 236 to < 376; and ≥ 376. Denominators include the number of patients within each baseline laboratory assessment category and treatment group with usable assessments at both time points. Highlighted cells indicate no change.
ALT, alanine aminotransferase; BEACON, Bardoxolone Methyl Evaluation in Patients with Chronic Kidney Disease and Type 2 Diabetes; U/L, units per liter; ULN, upper limit of normal.
Table 3.
Shift in AST (U/L) from baseline to maximum post‐baseline value while on study drug in BEACON
| Treatment group/baseline AST | Maximum post‐baseline value while on treatment | |||||
|---|---|---|---|---|---|---|
| ≤ULN | >ULN to < 3 × ULN | ≥ 3 × ULN to < 5 × ULN | ≥ 5 × ULN to < 8 × ULN | ≥ 8 × ULN | No post‐baseline value | |
| Placebo [n = 1,097] | ||||||
| AST ≤ ULN [n = 1096; %] | 1,009 [92] | 65 [6] | 1 [< 1] | 0 | 0 | 21 [2] |
| >ULN to < 3 × ULN [n = 1] | 1 [< 1] | 0 | 0 | 0 | 0 | 0 |
| ≥3 × ULN to < 5 × ULN [n = 0] | 0 | 0 | 0 | 0 | 0 | 0 |
| ≥5 × ULN to < 8 × ULN [n = 0] | 0 | 0 | 0 | 0 | 0 | 0 |
| ≥8 × ULN [n = 0] | 0 | 0 | 0 | 0 | 0 | 0 |
| Bardoxolone Methyl [n = 1088] | ||||||
| AST ≤ ULN [n = 1084; %] | 709 [65] | 292 [27] | 11 [1] | 3 [< 1] | 2 [< 1] | 67 [6] |
| >ULN to < 3 × ULN [n = 4] | 0 | 2 [< 1] | 1 [< 1] | 1 [< 1] | 0 | 0 |
| ≥3 × ULN to < 5 × ULN [n = 0] | 0 | 0 | 0 | 0 | 0 | 0 |
| ≥5 × ULN to < 8 × ULN [n = 0] | 0 | 0 | 0 | 0 | 0 | 0 |
| ≥8 × ULN [n = 0] | 0 | 0 | 0 | 0 | 0 | 0 |
Post‐baseline laboratory assessments include those collected on or before a patient’s last dose of study drug. The upper limit for the population reference range (ULN) for AST is 37 (U/L). Categories listed in the tables are defined by AST (U/L) values: ≤ 37; > 37 to < 112; ≥ 112 to < 186; ≥ 186 to < 296; and ≥ 296. Denominators include the number of patients within each baseline laboratory assessment category and treatment group with usable assessments at both time points. Highlighted cells indicate no change.
Similar to the increases in ALT and AST, patients randomized to bardoxolone methyl had mean increases in serum GGT concentrations relative to placebo; the difference between the two groups was 39.2 U/L (95% CI: 30.3 to 48.1 U/L; P < 0.001). After elevations that peaked between weeks 4 through 12, serum GGT concentrations trended back down toward baseline values (Figure 1 ) in patients randomized to bardoxolone methyl. In patients randomized to placebo, GGT remained unchanged relative to baseline. Patients randomized to bardoxolone methyl also had minimal increases in mean alkaline phosphatase concentrations (Figure 1 ) that remained below the upper limit of the population reference range (135 U/L). In patients randomized to placebo, alkaline phosphatase values remained unchanged relative to baseline.
Patients in the lowest quartile of baseline serum ALT concentrations randomized to bardoxolone methyl (mean (±SE) baseline ALT: 11.4 ± 0.1 U/L) had serum ALT concentrations that followed a similar trajectory to that seen in Figure 1 . Peak elevations were observed at week 4 but decreased to 20.9 ± 0.7 U/L at week 16 and remained nearly flat through week 48 of therapy. In contrast, patients randomized to placebo had unchanged serum ALT concentrations, with mean serum ALT concentrations remaining below 15 U/L throughout 48 weeks of treatment. Patients in the other three quartiles also had serum ALT concentrations that followed a similar pattern and the magnitude of serum ALT increases did not appear related to baseline serum ALT concentrations (Table S1 ). Similar trends were also observed with patients in the lowest quartiles of baseline AST and GGT (Figure S1 ).
Increases in ALT and AST with bardoxolone methyl were reversible
The initial increases in ALT and AST for patients randomized to bardoxolone methyl were not sustained and trended downward over 48 weeks of therapy. They were reversible with mean serum ALT and AST concentrations declining back to baseline values within 4 weeks following the end of treatment (Figure 1 ). As shown in Table 4 , nearly all patients randomized to bardoxolone methyl who experienced maximum ALT elevations above the population reference range and below 3 × the upper limit of the population reference range (290/310 )94%)) had serum ALT concentrations return to below the former at the post‐treatment visit. Twelve patients randomized to bardoxolone methyl experienced ALT values that exceeded 5 × the upper limit of the population reference range, including 2 patients with ALT values that exceeded 10 × the upper limit of the population reference range (whose individual ALT values and other related parameters are presented in Figure S2 ). Of these patients, 10 of 12 (83%) had ALT values that decreased to below the upper limit of the population reference range post‐treatment, 1 patient had ALT values that decreased to ≤ 3 × the upper limit of the population reference range, and 1 patient did not have a post‐treatment ALT value (Table 4 ). Similar trends were also observed for AST (Table S2 ).
Table 4.
Shift in ALT from maximum post‐baseline value while on study drug to post‐treatment visit assessment
| Treatment group/Maximum post‐baseline ALT | Post‐treatment visit value | |||||
|---|---|---|---|---|---|---|
| ≤ ULN | >ULN to < 3 × ULN | ≥3 × ULN to < 5 × ULN | ≥5 × ULN to < 8 × ULN | ≥8 × ULN | No Post‐Treatment Value | |
| Placebo [n = 1097] | ||||||
| No Post‐Baseline Value [n = 20] | 0 | 0 | 0 | 0 | 0 | 20 [2] |
| ALT ≤ ULN [n = 1023] | 841 [77] | 6 | 0 | 1 [<1] | 0 | 175 [16] |
| >ULN to < 3 × ULN [n = 51] | 34 [3] | 4 [<1] | 0 | 0 | 0 | 13 [1] |
| ≥3 × ULN to < 5 × ULN [n = 2] | 2 [<1] | 0 | 0 | 0 | 0 | 0 |
| ≥5 × ULN to < 8 × ULN [n = 1] | 0 | 0 | 0 | 0 | 0 | 1 [<1] |
| ≥8 × ULN [n = 0] | 0 | 0 | 0 | 0 | 0 | 0 |
| Bardoxolone Methyl [n = 1088] | ||||||
| No Post‐Baseline Value [n = 67] | 0 | 0 | 0 | 0 | 0 | 67 [6] |
| ALT ≤ ULN [n = 610] | 483 [44] | 5 [<1] | 0 | 0 | 0 | 122 [11] |
| >ULN to < 3 × ULN [n = 380] | 290 [27] | 20 [2] | 0 | 0 | 0 | 70 [6] |
| ≥3 × ULN to < 5 × ULN [n = 19] | 12 [1] | 1 [<1] | 0 | 0 | 0 | 6 [1] |
| ≥5 × ULN to < 8 × ULN [n = 10] | 8 [<1] | 1 [<1] | 0 | 0 | 0 | 1 [<1] |
| ≥8 × ULN [n = 2] | 2 [<1] | 0 | 0 | 0 | 0 | 0 |
Includes only patients randomized to bardoxolone methyl or placebo with post‐baseline and post‐treatment ALT values. Post treatment values are defined as ALT assessments collected closest to 28 days [but ≥ 14 days and ≤ 84 days] after a patient’s last dose of study drug. The upper limit of the population reference range [ULN] for ALT is 47 [U/L]. Categories listed in the tables are defined by ALT [U/L] values: ≤47; >47 to < 142; ≥142 to < 236; ≥236 to < 376; ≥376. Denominators include the number of patients within each worst post‐baseline laboratory assessment category and treatment group with usable assessments at both time points. Highlighted cells indicate no change.
ALT, alanine aminotransferase; BEACON, Bardoxolone Methyl Evaluation in Patients with Chronic Kidney Disease and Type 2 Diabetes; U/L, units per liter; ULN, upper limit of normal.
Bardoxolone methyl was associated with decreases in serum bilirubin
Patients randomized to bardoxolone methyl experienced mean decreases in serum total bilirubin concentrations relative to baseline and relative to placebo; the difference between the two groups was −0.04 mg/dL (95% CI: −0.05 to 0 mg/dL; P < 0.001). Figure 1 shows the mean changes in total bilirubin over time for patients randomized to bardoxolone methyl or placebo.
As shown in an evaluation of the drug‐induced serious hepatotoxicity plot, one patient in the bardoxolone methyl group experienced an elevation of total bilirubin levels that exceeded 2 × the upper limit of the population reference range and ALT levels that exceeded 3 × the upper limit of the population reference range, which was attributed by the investigator to symptomatic cholelithiasis and therefore did not invoke a true Hy’s Law case as an alternative etiology was felt to be responsible. No other patient randomized to bardoxolone methyl had maximum ALT or total bilirubin values that met potential Hy’s Law criteria (Figure 2 ).
Figure 2.
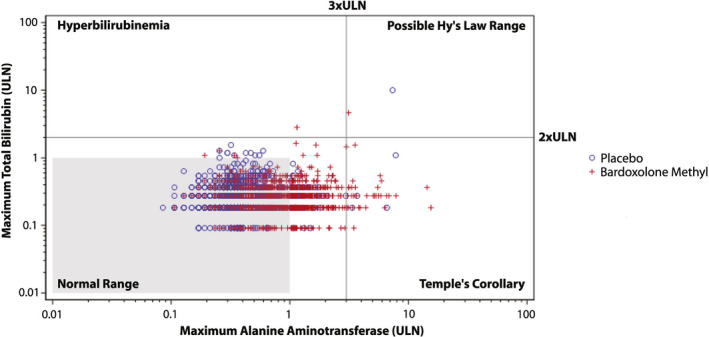
Evaluation of drug‐induced serious hepatotoxicity plot for bardoxolone methyl and placebo patients in BEACON. Maximum post‐baseline total bilirubin and alanine aminotransferase (ALT) values for patients randomized to bardoxolone methyl or placebo in Bardoxolone Methyl Evaluation in Patients with Chronic Kidney Disease and Type 2 Diabetes (BEACON). The upper limit of normal (ULN) for total bilirubin is 1.1 mg/dL and the ULN for ALT is 47 (U/L).
Hepatobiliary adverse events
Hepatobiliary adverse events (Table S3 ) were reported in 31 of 1,088 patients (3%) randomized to bardoxolone methyl and in 16 of 1,097 patients (1%) randomized to placebo. Notably, serious adverse events due to hepatobiliary disorders occurred less frequently in patients randomized to bardoxolone methyl (4/1,088 (0.4%) compared with placebo (8/1,097 (0.7%)).
Studies investigating potential mechanisms underlying serum aminotransferase increases
Although ALT and AST are expressed at high levels in the liver, these aminotransferases are not liver‐specific enzymes and have been reported in several tissues, such as the heart, skeletal muscle, and kidneys. 20 , 21 Furthermore, ALT and AST each have two isoforms encoded by discrete genes and exhibit distinct subcellular localization. 22 , 23 To confirm and expand these results, we assessed the protein levels of the ALT and AST isoforms in human tissues. Several tissues in addition to the liver, such as adipose, kidneys, skeletal muscle, heart, and brain, express high levels of the aminotransferase proteins (Figure S3 ).
Genetic alteration of Nrf2 activity, either by deleting Nrf2 or by knocking down Keap1 to increase Nrf2 activity, in animal models has been shown to affect serum aminotransferase activity and the expression of aminotransferase genes. 17 , 24 In these studies, ALT and AST serum activities were lower in mice that lacked Nrf2 than in mice that had wild‐type or genetically activated Nrf2. To confirm these observations, we assessed ALT serum activity in Nrf2‐null, wild‐type, and Keap1‐knockdown mice. Consistent with these published observations, we found ALT serum activity to trend lower in Nrf2‐null mice and higher in Keap1‐knockdown mice (Figure S4 ). Furthermore, hepatic ALT1 and renal ALT2 mRNA levels were significantly higher in Keap1‐knockdown mice, and hepatic ALT2 and renal ALT1 mRNA levels were significantly lower in Nrf2‐null mice. Taken together, these data suggest that Nrf2 may modulate serum ALT and AST activity by regulating aminotransferase gene expression in hepatic and extrahepatic tissue.
Certain drugs with mechanisms of action that are distinct from that of bardoxolone methyl have also been reported to increase aminotransferase gene expression. 25 , 26 For example, fenofibrate has been reported to selectively increase the expression of ALT1 in cultured hepatocyte cell lines. 25 As a positive control, we assessed the mRNA levels of ALT1, ALT2, AST1, and AST2 in HepG2 hepatocellular carcinoma cells treated with 250 µM fenofibrate. Consistent with previously reported results, 25 only ALT1 was expressed at a higher level (1.4 ± 0.3‐fold vs. vehicle control) in fenofibrate‐treated cells.
We next assessed whether bardoxolone methyl affects the transcription of aminotransferase genes in eight cell lines derived from tissues that abundantly express aminotransferase proteins and/or are targets of bardoxolone methyl (i.e., liver, skeletal muscle, kidney, intestine, and macrophages). Treatment with bardoxolone methyl significantly and dose‐dependently increased aminotransferase gene expression in all cell lines tested (Figure 3 ). The pattern of gene expression differed among the cell lines. For example, ALT1 expression was increased by bardoxolone methyl in HepG2, AML‐12, HT‐29, C2C12, and RAW 264.7 cells, whereas ALT2 expression was increased in SK‐HEP‐1, HuH‐7, HT‐29, C2C12, RAW 264.7, and NRK‐52E cells. Distinct patterns of Nrf2 target gene expression across tissue types has been observed with other Nrf2 activators. 27 , 28 However, expression of the “classical” Nrf2 target gene, NAD(P)H Quinone Dehydrogenase 1 (NQO1), is consistently increased in all tissue types in these studies. Consistent with these observations, bardoxolone methyl increased NQO1 expression in all cell lines under the same conditions that we assessed aminotransferase expression, demonstrating target engagement by the drug (Figure S5 ). Taken together, these results demonstrate that bardoxolone methyl increases the expression of aminotransferase genes in cultured cell lines derived from several tissues, including nonhepatic tissues, at doses that activate Nrf2.
Figure 3.
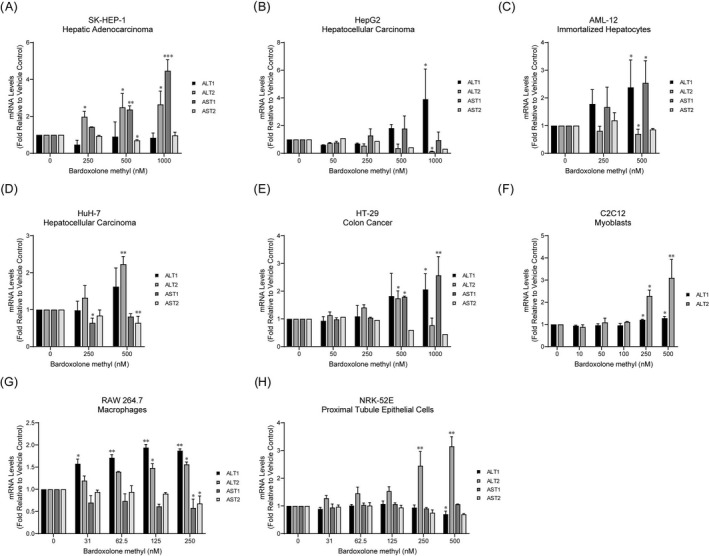
Bardoxolone methyl increases ALT and AST gene expression in several cell types. The indicated cell lines were treated with bardoxolone methyl for 16 to 18 hours. ALT1, ALT2, AST1, and AST2 expression levels were measured by reverse transcription quantitative polymerase chain reaction. Values are expressed as fold‐change relative to vehicle (DMSO) treatment and are the average and standard deviation of at least two independent experiments, with the exception of AST2 expression data in HepG2 and HT‐29 cells, which are from single experiments. Statistical significance vs. vehicle control was determined using repeated measures one‐way analysis of variance and Dunnett’s multiple comparison test. *P < 0.05; **P < 0.01; ***P < 0.001. ALT, alanine aminotransferase; AST, aspartate aminotransferase.
DISCUSSION
In this secondary analysis of data from BEACON, we found that an appreciable percentage of patients treated with bardoxolone methyl experienced increases in serum ALT (38% of patients) and AST (29% of patients) that exceeded the upper limit of the population reference range. Aminotransferase levels peaked following 4 weeks of treatment and trended downward between weeks 4 and 48, while patients remained on the study drug. At the post‐treatment visit (4 weeks after stopping study drug) aminotransferase levels decreased further. Pharmacokinetic studies demonstrate that concentrations of bardoxolone methyl in plasma decrease to subpharmacological levels within 3 to 4 days after stopping treatment. Thus, the observed reversal of these pharmacological effects within 4 weeks of stopping study drug is consistent with not only complete washout of the drug but should also reflect reversal of the induction process, which should be governed by the half‐life of the induced enzymes. A similar pattern in serum GGT levels was also observed. For most patients (≥ 93%) with serum aminotransferase elevations, ALT and AST levels were less than 3 × the upper limit of the population reference range. Although the profile of ALT levels over time, and their apparent reversibility, could potentially be confounded by premature discontinuations, only 6 of 1,088 (0.55%) of patients randomized to bardoxolone methyl discontinued study drug prematurely due to protocol‐specified criteria related to aminotransferase elevations.
The aminotransferase increases were not associated with hepatitis‐like symptoms, increases in total bilirubin, or an increased incidence of other hepatobiliary adverse events. In contrast, total bilirubin actually declined relative to baseline and to placebo, and few patients (< 3%) randomized to bardoxolone methyl experienced hepatobiliary adverse events. The incidence of serious hepatobiliary adverse events was numerically lower in patients receiving bardoxolone methyl than in patients receiving placebo. Moreover, no cases of Hy’s Law, which is considered to be a stronger predictor of significant liver injury risk than ALT elevations alone and is recognized as the most important biomarker for acute drug‐induced liver injury, 29 were reported in BEACON or in any of the other clinical studies involving bardoxolone methyl, which together have enrolled over 2,600 patients. 9 , 10 , 30
Although insufficient pharmacokinetic data from BEACON are available to assess the relationship between exposure to bardoxolone methyl and aminotransferase elevations in this patient population, similar to the observed dose‐dependent increases in eGFR, 9 a prior open‐label, dose‐ranging study of bardoxolone methyl showed that the magnitude of ALT and AST elevations were also dose‐dependent. 31 , 32
A small number of patients randomized to bardoxolone methyl (n = 12) experienced ALT values that exceeded 5 × the upper limit of the population reference range, meeting one of the Drug Induced Liver Injury [DILI] Expert Working Group’s consensus criteria that can be used for identifying potential DILI. 30 However, these ALT increases also were not associated with any hepatitis‐like symptoms or increases in serum bilirubin, and behaved similarly to the mild elevations in terms of their time to occurrence and time to resolution. Although additional data (e.g., liver biopsy) are not available for a full causality assessment for these patients, their ALT levels declined over time in those who continued treatment and, for most patients, fell within the population reference range at the post‐treatment visit. Together, these observations suggest that even these more pronounced ALT increases may also relate to the on‐target transcriptional effects of Nrf2 on aminotransferases rather than being an indication of significant DILI. 29 , 33
Preclinical studies conducted under good laboratory practice conditions in naïve nondiseased cynomolgus monkeys showed that bardoxolone methyl treatment for 28 days or 12 months did not cause adverse histological findings in the liver and resulted in total and direct bilirubin concentrations that were either similar to or lower than those in control animals. We acknowledge, however, that such high elevations were not observed in the animal models or in cell culture experiments, although the correlation to the human condition may not be exact. Likewise, no adverse histopathological findings have been observed in the livers of naïve minipigs treated with bardoxolone methyl for 3 months (unpublished data). Importantly, both monkeys and minipigs obtained systemic exposures to bardoxolone methyl that were higher than those observed clinically in patients. Additionally, bardoxolone methyl and its related analogs have been shown to be protective against hepatotoxicity in multiple models of drug‐induced or chemical‐induced hepatic injury, including acetaminophen, aflatoxin, concanavalin A, or carbon tetrachloride administration. 17 , 18 , 19 , 34 For example, a bardoxolone methyl analog decreased hepatic fibrosis, serum bilirubin, ascites, and hepatocellular carcinoma formation, and increased overall survival in mice with liver injury induced by chronic carbon tetrachloride administration. 34
Although increases in serum ALT and AST were not observed in monkeys or minipigs, this may have been due to the study design, as samples for clinical chemistry were collected only at the start and the end of each study. Therefore, it is possible that the temporal window in which the transient elevations in aminotransferase levels occur in the clinic was not fully captured in the animal studies. Alternatively, transcriptional regulation of aminotransferases in vivo may be influenced by disease status, and all animals used for good laboratory practice toxicity studies were naïve and healthy. Supporting this hypothesis, analogs of bardoxolone methyl mildly increased serum aminotransferase activity while also significantly improving liver histology in an animal model of nonalcoholic steatohepatitis. 35
The profile of transient and reversible increases in aminotransferases with bardoxolone methyl might suggest that a form of possible drug tolerance or adaptation develops, as is often seen with several other classes of drugs, notably the statins. 36 , 37 , 38 It is well‐known that such drug tolerance is seen in a relative minority of patients, typically ranging from 5% to 25%. 36 , 37 Therefore, we speculate that the mechanism by which bardoxolone methyl elevates aminotransferases is related to its pharmacologic actions. In support of this theory, increases in aminotransferases have been observed in clinical studies conducted with other agents that activate Nrf2, including unrelated molecules (dimethyl fumarate) as well as related analogs (omaveloxolone). 39 , 40 In addition, data from our group and others have shown that genetic manipulation of Nrf2, which is activated by bardoxolone methyl, affects the expression of aminotransferase genes and the serum activity of ALT and AST. 17 , 24 Nrf2 is a transcription factor that regulates the expression of genes involved in cellular protection, metabolism, inflammation, and redox status. We have shown that treatment of cultured cell lines derived from several different tissues, including liver, colon, skeletal muscle, kidneys, and macrophages with bardoxolone methyl resulted in dose‐dependent increases in ALT and AST mRNA at doses that increase expression of the “classical” Nrf2 target gene, NQO1. Although bardoxolone methyl increased aminotransferase expression in all cell lines examined, the specific pattern of gene expression varied among them. The phenomenon of tissue‐specific regulation of target genes has been observed with other Nrf2 activators, and suggests that the response to Nrf2 activation is influenced by the function of the particular type of cell, its metabolic profile, and its redox status. 27 , 28
ALT and AST catalyze the reversible transfer of amino groups between alanine or aspartate, respectively, and α‐ketoglutarate to form pyruvate or oxaloacetate and glutamate 15 , 16 and play key roles in several metabolic processes, including amino acid metabolism, glycolysis, glucogenesis, and the tricarboxylic acid cycle. In addition, these enzymes influence redox balance and mitochondrial metabolism by contributing to glutathione production 39 and regulating the NAD+/NADH ratio via the malate‐aspartate shuttle. 40 Nrf2 is known to play critical roles in both glutathione production and mitochondrial metabolism. 41 , 42 Accordingly, changes in aminotransferase levels may reflect metabolic adaptations that take place due to increased Nrf2 activity. 43 Nrf2 also influences the expression of GGT, another enzyme involved in glutathione homeostasis. 44 Oral administration of a bardoxolone methyl analog to rats has been shown to dose‐dependently increase biliary glutathione excretion and increase mRNA expression of GGT without any changes in liver histology. 45 Collectively, the GGT, ALT, and AST increases observed in BEACON are consistent with Nrf2‐mediated increases in glutathione production and changes in cellular and mitochondrial metabolism.
Consistent with these observations, acute increases in serum aminotransferase concentrations have been associated with several conditions that affect cellular metabolism and energy production. For example, increases in aminotransferase activity have been observed following dietary changes in healthy volunteers and in patients with T2DM. 46 Similarly, aminotransferase expression has been shown to increase in the absence of liver injury in rodents fed a methionine choline deficient diet. Transient increases in aminotransferase enzymatic activity have also been observed in severely hyperglycemic patients with type 1 diabetes following their initial administration of insulin therapy. 15 Furthermore, treatment with certain drugs that affect cellular metabolism, including the PPARα agonist fenofibrate and the microsomal triglyceride transfer protein inhibitor (MTPi) BMS‐212122, increase aminotransferase expression at the transcriptional level. 25 , 26 Notably, treatment with BMS‐212122 also increased the release of ALT and AST from the cell in the absence of any cellular toxicity. 26 These data support the notion that an increase in ALT and AST transcription could translate to an increase in ALT and AST serum levels in the absence of hepatic damage. Taken together, these observations are consistent with dynamic regulation of aminotransferase gene expression by factors that influence cellular metabolism and redox status. This underscores the importance of developing more specific markers of hepatocellular damage and suggests that the changes in serum aminotransferase levels in patients treated with drugs that may influence these processes should be carefully interpreted. 40
Patients in the lowest quartile of ALT values at baseline (≤ 13.7 U/L) randomized to bardoxolone methyl had mean increases in ALT that plateaued at 20 U/L between weeks 16 and 48. In contrast, patients in the lowest quartile of ALT randomized to placebo had minimal to unchanged mean ALT values that remained ≤ 15 U/L through 48 weeks of treatment. In the general population, serum concentrations of ALT < 17 U/L are associated with increased all‐cause mortality. 47 , 48 Therefore, increases in ALT induced by bardoxolone methyl, particularly in the subset of patients with the lowest serum ALT concentrations, may reflect restoration of Nrf2 and ALT activity to normal levels and are therefore associated with the improved metabolic and anti‐inflammatory effects of bardoxolone methyl treatment.
In summary, increases in ALT and AST induced by bardoxolone methyl in BEACON were asymptomatic and generally mild, and may be associated with the transcriptional effects of the drug on Nrf2. The reversibility (or failure of progression) of the mild aminotransferases elevations while on treatment suggests a form of drug tolerance or adaptation due to the pharmacologic mechanism of bardoxolone methyl on Nrf2 rather than the induction of any intrinsic hepatotoxicity of the compound that fails to progress. Bardoxolone methyl is currently being studied in a global phase II/III study in patients with CKD due to Alport syndrome (CARDINAL; NCT03019185), in a phase II study in US patients with other rare forms of CKD, including autosomal dominant polycystic kidney disease, type 1 diabetes, IgA nephropathy, and focal segmental glomerulosclerosis (PHOENIX, NCT03366337), and in a phase III study in Japanese patients with T2DM and CKD (AYAME, NCT03550443) in the absence of heart failure risk factors. Liver biochemical tests are being monitored in each of these studies to determine if the presumed adaptive response seen with in patients with T2DM and stage 4 CKD is also seen in association with other kidney diseases. These findings suggest that the role of Nrf2 and other transcriptional factors may have an important impact on future drug development in terms of the risk of hepatotoxicity. As serum aminotransferases serve as nonspecific biomarkers of DILI, the mechanisms by which ALT and AST values may become elevated in clinical trials without apparent liver injury should be fully explored to identify other potential alternative explanations, as appears to be the case here.
Funding
The study sponsor, Reata Pharmaceuticals, played an active role in trial design, analysis, and interpretation of data, in the writing of the report, and in the decision to submit the paper for publication. No author received any funding for authoring the manuscript.
Conflicts of Interest
J.H.L. and G.M.C. are consultants to Reata Pharmaceuticals. M.J. has been a speaker for Abbvie and Merck (both marketing anti‐hepatitis C virus drugs), has participated with Merck Advisory boards, and received an unrestricted educational grant from Merck. He is cochair of Kidney Disease: Improving Global Outcomes. P.E.P. is a consultant for Reata Pharmaceuticals, Akebia, Astra‐Zeneca, Ardelyx, Bayer, Gilead, and TricidaKeryx; as the principal investigator for studies from many pharmaceutical companies, his institution has received research support. G.A.B., M.P.C., D.A.F., A.G., C.J.M., M.O., S.A.R., and C.W. are employees of Reata Pharmaceuticals.
Author Contributions
J.H.L., M.J., S.A.R., P.E.P., G.M.C., G.A.B., M.P.C., D.A.F., A.G., C.J.M., M.O., and C.W. wrote the manuscript. G.M.C., G.A.B., M.P.C., D.A.F., A.G., C.J.M., M.O., and C.W. designed the research. S.A.R., P.E.P., G.M.C., G.A.B., M.P.C., D.A.F., A.G., C.J.M., M.O., and C.W. performed the research. J.H.L, M.J., G.M.C., G.A.B., M.P.C., D.A.F., A.G., C.J.M., M.O., and C.W. analyzed the data.
Supporting information
Fig S1
Fig S2
Fig S3
Fig S4
Fig S5
Table S1
Table S2
Table S3
Supplementary Material
Acknowledgments
The authors acknowledge the supportive role of all BEACON investigators, support staff, and patients. We acknowledge Ron Bumeister, PhD, Isaac Trevino, Rhesa Stidham, and Pritam Kambuj for their expert technical assistance as well as Nicole S. Klee, PhD, and Shobhana Natarajan, PhD of Reata Pharmaceuticals, for assistance in preparation of the manuscript. We also acknowledge the assistance of Dr. Curtis Klaassen of the University of Kansas Medical Center for procurement of tissues from wild‐type, Nrf2‐null, and Keap1‐knockdown mice.
Data Availability Statement
For this study, de‐identified participant data will not be shared for external analysis. The statistical analysis plan and protocol has been published previously and can be found here: https://www.nejm.org/doi/full/10.1056/NEJMoa1306033.10
References
- 1. Yates, M.S. et al Pharmacodynamic characterization of chemopreventive triterpenoids as exceptionally potent inducers of Nrf2‐regulated genes. Mol. Cancer Ther. 6, 154–162 (2007). [DOI] [PubMed] [Google Scholar]
- 2. Rojas‐Rivera, J. , Ortiz, A. & Egido, J. Antioxidants in kidney diseases: the impact of bardoxolone methyl. Int. J. Nephrol. 2012, 321714 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dinkova‐Kostova, A.T. et al Extremely potent triterpenoid inducers of the phase 2 response: correlations of protection against oxidant and inflammatory stress. Proc. Natl. Acad. Sci. USA 102, 4584–4589 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee, D.F. et al KEAP1 E3 ligase‐mediated downregulation of NF‐kappaB signaling by targeting IKKbeta. Mol. Cell 36, 131–140 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Osburn, W.O. & Kensler, T.W. Nrf2 signaling: an adaptive response pathway for protection against environmental toxic insults. Mutat. Res. 659, 31–39 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sporn, M.B. et al New synthetic triterpenoids: potent agents for prevention and treatment of tissue injury caused by inflammatory and oxidative stress. J. Nat. Prod. 74, 537–545 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ruiz, S. et al Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney Int. 83, 1029–1041 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pergola, P. et al Effect of bardoxolone on kidney function in patients with T2D and stage 3b–4 CKD. Am. J. Nephrol. 33, 469–476 (2011). [DOI] [PubMed] [Google Scholar]
- 9. Pergola, P.E. et al Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N. Engl. J. Med. 365, 327–336 (2011). [DOI] [PubMed] [Google Scholar]
- 10. de Zeeuw, D. et al Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N. Engl. J. Med. 369, 2492–2503 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nangaku, M. , Shimazaki, R. & Akizawa, T. Bardoxolone methyl improved GFR measured by standard inulin clearance: the TSUBAKI study. J. Am. Soc. Nephrol. 28, B1 (2017). [Google Scholar]
- 12. de Zeeuw, D. et al Rationale and trial design of bardoxolone methyl evaluation in patients with chronic kidney disease and type 2 diabetes: the occurrence of renal events (BEACON). Am. J. Nephrol. 37, 212–222 (2013). [DOI] [PubMed] [Google Scholar]
- 13. Chin, M.P. et al Mechanisms contributing to adverse cardiovascular events in patients with type 2 diabetes mellitus and stage 4 chronic kidney disease treated with bardoxolone methyl. Am. J. Nephrol. 39, 499–508 (2014). [DOI] [PubMed] [Google Scholar]
- 14. Chin, M.P. et al Risk factors for heart failure in patients with type 2 diabetes mellitus and stage 4 chronic kidney disease treated with bardoxolone methyl. J. Card. Fail. 20, 953–958 (2014). [DOI] [PubMed] [Google Scholar]
- 15. Takaike, H. et al Transient elevation of liver transaminase after starting insulin therapy for diabetic ketosis or ketoacidosis in newly diagnosed type 1 diabetes mellitus. Diabetes Res. Clin. Pract. 64, 27–32 (2004). [DOI] [PubMed] [Google Scholar]
- 16. Felig, P. The glucose‐alanine cycle. Metabolism 22, 179–207 (1973). [DOI] [PubMed] [Google Scholar]
- 17. Osburn, W.O. et al Genetic or pharmacologic amplification of nrf2 signaling inhibits acute inflammatory liver injury in mice. Toxicol. Sci. 104, 218–227 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reisman, S.A. et al CDDO‐Im protects from acetaminophen hepatotoxicity through induction of Nrf2‐dependent genes. Toxicol. Appl. Pharmacol. 236, 109–114 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yates, M.S. et al Potent protection against aflatoxin‐induced tumorigenesis through induction of Nrf2‐regulated pathways by the triterpenoid 1‐[2‐cyano‐3‐,12‐dioxooleana‐1,9(11)‐dien‐28‐oyl]imidazole. Cancer Res. 66, 2488–2494 (2006). [DOI] [PubMed] [Google Scholar]
- 20. Pave‐Preux, M. et al Nucleotide sequence and glucocorticoid regulation of the mRNAs for the isoenzymes of rat aspartate aminotransferase. J. Biol. Chem. 263, 17459–17466 (1988). [PubMed] [Google Scholar]
- 21. Pol, S. et al Nucleotide sequence and tissue distribution of the human mitochondrial aspartate aminotransferase mRNA. Biochem. Biophys. Res. Commun. 157, 1309–1315 (1988). [DOI] [PubMed] [Google Scholar]
- 22. Panteghini, M. Aspartate aminotransferase isoenzymes. Clin. Biochem. 23, 311–319 (1990). [DOI] [PubMed] [Google Scholar]
- 23. Yang, R.Z. et al Alanine aminotransferase isoenzymes: molecular cloning and quantitative analysis of tissue expression in rats and serum elevation in liver toxicity. Hepatology 49, 598–607 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang, Y.K. et al Enhanced expression of Nrf2 in mice attenuates the fatty liver produced by a methionine‐ and choline‐deficient diet. Toxicol. Appl. Pharmacol. 245, 326–334 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thulin, P. et al PPARalpha regulates the hepatotoxic biomarker alanine aminotransferase (ALT1) gene expression in human hepatocytes. Toxicol. Appl. Pharmacol. 231, 1–9 (2008). [DOI] [PubMed] [Google Scholar]
- 26. Josekutty, J. et al Microsomal triglyceride transfer protein inhibition induces endoplasmic reticulum stress and increases gene transcription via Ire1alpha/cJun to enhance plasma ALT/AST. J. Biol. Chem. 288, 14372–14383 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nakajima, H. et al Tissue‐restricted expression of Nrf2 and its target genes in zebrafish with gene‐specific variations in the induction profiles. PLoS One 6, e26884 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brennan, M.S. et al Pharmacodynamics of dimethyl fumarate are tissue specific and involve NRF2‐dependent and ‐independent mechanisms. Antioxid. Redox Signal. 24, 1058–1071 (2016). [DOI] [PubMed] [Google Scholar]
- 29. US Food and Drug Administration (FDA) . Guidance for Industry Drug‐Induced Liver Injury: Premarketing Clinical Evaluation U.S. Department of Health and Human Services (2009). <www.fda.gov >.
- 30. Aithal, G.P. et al Case definition and phenotype standardization in drug‐induced liver injury. Clin. Pharmacol. Ther. 89, 806–815 (2011). [DOI] [PubMed] [Google Scholar]
- 31. Klein, C.N.P. , Mensing, S. , Teuscher, N. , Meyer, C. , Dumas, E. & Awni, W. Exposure‐response analysis of bardoxolone methyl safety and efficacy and clinical trial simulations to inform phase III dose selection, in ERA‐EDTA. 2012: Paris.
- 32. Bakris, G.P.P. , Chin, M. , Potts, M. , Hebbar, S. , Meyer, C. & Audhya, P. Effect of the amorphous SDD formulation of bardoxolone methyl on blood pressure, in ASN. 2012: San Diego.
- 33. Watkins, P.B. et al Hepatotoxic effects of tacrine administration in patients with Alzheimer's disease. JAMA 271, 992–998 (1994). [PubMed] [Google Scholar]
- 34. Getachew, Y. et al The synthetic triterpenoid RTA 405 (CDDO‐EA) halts progression of liver fibrosis and reduces hepatocellular carcinoma size resulting in increased survival in an experimental model of chronic liver injury. Toxicol. Sci. 149, 111–120 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Reisman, S.A.F.D. , Lee, C.I. & Proksch, J.W. Omaveloxolone and TX63682 are hepatoprotective in the STAM mouse model of nonalcoholic steatohepatitis. J. Biochem. Mol. Toxicol. 10.1002/jbt.22526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lewis, J.H. The art and science of diagnosing and managing drug‐induced liver injury in 2015 and beyond. Clin. Gastroenterol. Hepatol. 13, 2173–2189.e8 (2015). [DOI] [PubMed] [Google Scholar]
- 37. Lewis, J.H. The adaptive response (drug tolerance) helps to prevent drug‐induced liver injury. Gastroenterol. Hepatol. (NY) 8, 333–336 (2012). [PMC free article] [PubMed] [Google Scholar]
- 38. Dara, L. , Liu, Z.X. & Kaplowitz, N. Mechanisms of adaptation and progression in idiosyncratic drug induced liver injury, clinical implications. Liver Int. 36, 158–165 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ellinger, J.J. , Lewis, I.A. & Markley, J.L. Role of aminotransferases in glutamate metabolism of human erythrocytes. J. Biomol. NMR 49, 221–229 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McGill, M.R. The past and present of serum aminotransferases and the future of liver injury biomarkers. EXCLI J. 15, 817–828 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hayes, J.D. & Dinkova‐Kostova, A.T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 39, 199–218 (2014). [DOI] [PubMed] [Google Scholar]
- 42. Harvey, C.J. et al Nrf2‐regulated glutathione recycling independent of biosynthesis is critical for cell survival during oxidative stress. Free Radic. Biol. Med. 46, 443–453 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sookoian, S. & Pirola, C.J. Liver enzymes, metabolomics and genome‐wide association studies: from systems biology to the personalized medicine. World J. Gastroenterol. 21, 711–725 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhang, H. et al 4‐Hydroxynonenal induces rat gamma‐glutamyl transpeptidase through mitogen‐activated protein kinase‐mediated electrophile response element/nuclear factor erythroid 2‐related factor 2 signaling. Am. J. Respir. Cell Mol. Biol. 34, 174–181 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Reisman, S.A. et al CDDO‐9,11‐dihydro‐trifluoroethyl amide (CDDO‐dhTFEA) induces hepatic cytoprotective genes and increases bile flow in rats. Xenobiotica 43, 571–578 (2013). [DOI] [PubMed] [Google Scholar]
- 46. Khoo, E.Y. et al Elevation of alanine transaminase and markers of liver fibrosis after a mixed meal challenge in individuals with type 2 diabetes. Dig. Dis. Sci. 57, 3017–3025 (2012). [DOI] [PubMed] [Google Scholar]
- 47. Karaphillis, E. et al Serum alanine aminotransferase levels and all‐cause mortality. Eur. J. Gastroenterol. Hepatol. 29, 284–288 (2017). [DOI] [PubMed] [Google Scholar]
- 48. Oh, C.M. et al Alanine aminotransferase and gamma‐glutamyl transferase have different dose‐response relationships with risk of mortality by age. Liver Int. 36, 126–135 (2016). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Fig S3
Fig S4
Fig S5
Table S1
Table S2
Table S3
Supplementary Material
Data Availability Statement
For this study, de‐identified participant data will not be shared for external analysis. The statistical analysis plan and protocol has been published previously and can be found here: https://www.nejm.org/doi/full/10.1056/NEJMoa1306033.10


