Abstract
Background and aims
Creatine kinase (CK), a marker of muscle damage, is potentially associated with a more severe COVID-19. In this systematic review and meta-analysis, we aim to evaluate the association between the elevated CK and severity and mortality in COVID-19.
Methods
We performed a systematic literature search on PubMed, Scopus, and Embase up until January 26, 2020. The main outcome was poor outcome, a composite of mortality and severe COVID-19.
Results
There are 2471 patients from 14 studies included in this systematic review and meta-analysis. The incidence of elevated CK in this pooled analysis was 17% (11%, 22%) and the incidence of poor outcome in this pooled analysis was 27% (19%, 34%). Elevated CK was associated with poor outcome in patients with COVID-19 (OR 3.01 [2.21, 4.10], p < 0.001; I2: 10.2%). The effect estimate did not vary with age (p = 0.610), male (p = 0.449), hypertension (p = 0.490), and diabetes (p = 0.457). Elevated CK has a sensitivity of 0.24 (0.17, 0.32), specificity of 0.91 (0.86, 0.94), PLR of 2.6 (1.9, 3.7), NLR of 0.84 (0.78, 0.90), DOR of 3 (2, 5), and AUC of 0.62 (0.57, 0.66) for predicting poor outcome in patients with COVID-19. In this pooled analysis, elevated CK confers to a 49% probability for poor outcome and a non-elevated CK confers to a 24% probability. Subgroup analysis and univariate meta-regression indicates that the sensitivity and specificity does not vary with age, male, hypertension, and diabetes.
Conclusion
Elevated CK was associated with increased mortality and severity in patients with COVID-19.
PROSPERO
CRD42021233435.
Keywords: Coronavirus, Creatine kinase, Myopathy, Mortality, Severity
1. Introduction
More than a year since the coronavirus disease 2019 (COVID-19) was first discovered in Wuhan (China), the pandemic has infected nearly 100 million people [1]. The clinical features of patients range from asymptomatic to fatal. Those with a severe course of disease may develop acute respiratory syndrome (ARDS) with respiratory failure, heart failure, coagulopathy, sepsis, and multiorgan failure, requiring close care in the intensive care unit and mechanical ventilation [2]. the sorting of patients must be decided carefully given the limitations of human and medical resources.
Several inflammatory markers, including D-dimer, procalcitonin, ferritin, and C-reactive protein, frequently elevated with increasing disease severity [3]. Creatine kinase (CK), which is a marker of muscle damage, was also often found to be elevated in deceased patients and those with severe disease [4,5]. Therefore, higher CK could be used as a prognostic marker to indicate more severe clinical picture of COVID-19. In this systematic review and meta-analysis, we aim to evaluate the association between the elevated CK and severity and mortality in COVID-19.
2. Material and methods
This is a Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines compliant meta-analysis. This study is registered in PROSPERO (ID: CRD42021233435).
2.1. Eligibility criteria
The inclusion criteria were: 1) retrospective and prospective observational studies 2) reporting COVID-19 patients 3) elevated and non-elevated CK and 2) mortality/severe COVID-19/need for intensive unit care (ICU)/invasive mechanical ventilation (IMV).
Studies were excluded if it fulfils one of the following criteria: 1) preprints, 2) conference abstracts, 3) case reports, 4) abstract-only publication, 5) review articles.
2.2. Search strategy and study selection
We performed a systematic literature search on PubMed, Scopus, and Embase with keywords “SARS-CoV-2″ OR “2019-nCoV” OR″COVID-19″ AND “creatine kinase” up until January 26, 2020. Duplicates were then removed and the title/abstracts were screened by two independent authors. Discrepancies in this process were resolved by discussion.
2.3. Data extraction
Data extraction of the included studies were performed by two authors independently. The data of interest includes the author, design of the study, baseline characteristics, cut-off for elevated CK, and the outcome of interest.
The key exposure was elevated CK, defined as elevation of CK beyond cut-off point reported in each studies. The main outcome was poor outcome, a composite of mortality and severe COVID-19. Severe COVID-19 was defined as patients with COVID-19 who fulfill the criteria for severe CAP or the need for ICU care or IMV. The effect estimate was odds ratio (OR). Sensitivity, specificity, positive and negative likelihood ratio (PLR & NLR), diagnostic odds ratio (DOR), and area under curve (AUC) analysis were performed.
2.4. Risk of bias assessment
We performed risk of bias assessment using the Newcastle-Ottawa Scale (NOS), this was performed by two independent authors and discrepancies were resolved by discussion.
2.5. Statistical analysis
Prevalence was pooled using the meta-analysis of proportion. DerSimonian-Laird method was selected to perform random-effects meta-analysis to generate OR and its 95% CI. A p-value <0.05 was considered as significant. I-squared (I2) and Cochran Q test were performed to evaluate heterogeneity, a value of <50% or p < 0.10 indicates substantial heterogeneity. Sensitivity, specificity, PLR, NLR, DOR, and AUC were generated to evaluate the prognostic value. To assess small-study effects and publication bias, deek’s funnel-plot analysis and Deek’s asymmetry test were performed. REML meta-regression was performed for the main outcome, using baseline characteristics as moderator. STATA 16 (Stata Corp) was used to perform meta-analysis.
3. Results
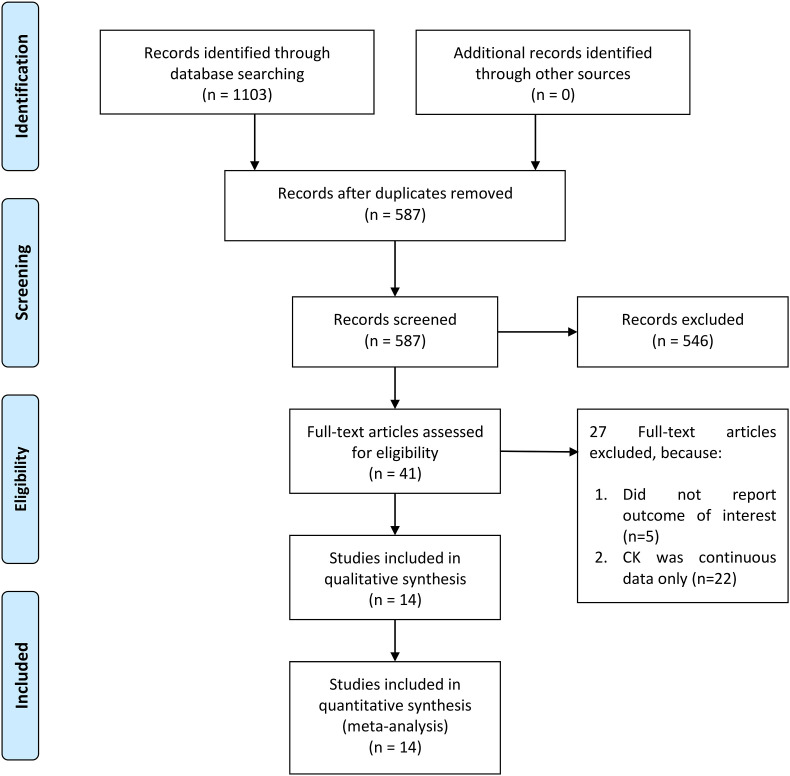
There are 2471 patients from 14 studies included in this systematic review and meta-analysis (Fig. 1 ) [[6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19]]. The baseline characteristics of the included studies can be accessed in Table 1 . The incidence of elevated CK in this pooled analysis was 17% (11%, 22%) and the incidence of poor outcome in this pooled analysis was 27% (19%, 34%).
Fig. 1.
PRISMA flowchart.
Table 1.
Baseline characteristic of the included studies.
| First Author | Design | Cut-off (U/L) | Samples | Age | Male | HT (%) | DM (%) | CKD (%) | CVD (%) | CLD (%) | Outcome | NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Giacomelli 2020 [12] | PC | NR | 104 | NR | 30.9 | NR | NR | NR | NR | NR | Mortality | 7 |
| Guan 2020 [7] | RC | 200 | 657 | 47 | 58.1 | 15 | 7.4 | 0.7 | NR | 1.1 | Severe | 6 |
| Hong 2020 [8] | RC | NR | 98 | 55.4 | 38.8 | 30.6 | 9.2 | NR | 2 | 3.1 | ICU | 6 |
| Huang 2020 [6] | RC | 185 | 40 | 49 | 73 | 15 | 20 | NR | 15 | 2 | ICU | 6 |
| Jang 2020 [19] | RC | 145 | 110 | 56.9 | 43.6 | 33.6 | 26.4 | NR | 9.1 | 3.6 | Severe | 8 |
| Jiang 2020 [18] | RC | 185 | 59 | 64 | 49 | 42 | 15 | NR | 44 | 3 | ICU | 6 |
| Mertoglu 2020 [17] | RC | NR | 377 | 49 | 57.5 | NR | NR | NR | NR | NR | ICU | 8 |
| Wan 2020 [16] | RC | 200 | 135 | 47 | 53.3 | 9.6 | 8.9 | NR | 5.2 | NR | Severe | 6 |
| Wang 2020 [11] | RC | NR | 252 | 49 | 46.5 | 19.6 | 6.2 | 1.5 | NR | NR | Severe | 7 |
| Wei 2020 [10] | RC | 200 | 81 | 51 | 56.2 | 17 | 5.1 | NR | NR | 2.5 | Severe | 7 |
| Zhang 2020 [15] | RC | 200 | 138 | 57 | 50.7 | 30 | 12.1 | 1.4 | NR | 1.4 | Severe | 7 |
| Zhao 2020 [14] | RC | NR | 91 | 46 | 53.8 | 19.8 | 3.3 | 1.1 | NR | 1.1 | Severe | 7 |
| Zheng 2020 [9] | RC | 190 | 161 | 45 | 49.7 | 13.7 | 4.3 | NR | NR | 3.7 | Severe | 6 |
| Zhou 2020 [13] | RC | 185 | 168 | 56 | 62 | 30 | 19 | 1 | NR | 3 | Mortality | 8 |
CKD: Chronic Kidney Disease, CVD: Cardiovascular Disease, CLD: Chronic Lung Disease, HT: Hypertension, RC: Retrospective Cohort, NOS: Newcastle-Ottawa Scale, NR: Not Reported.
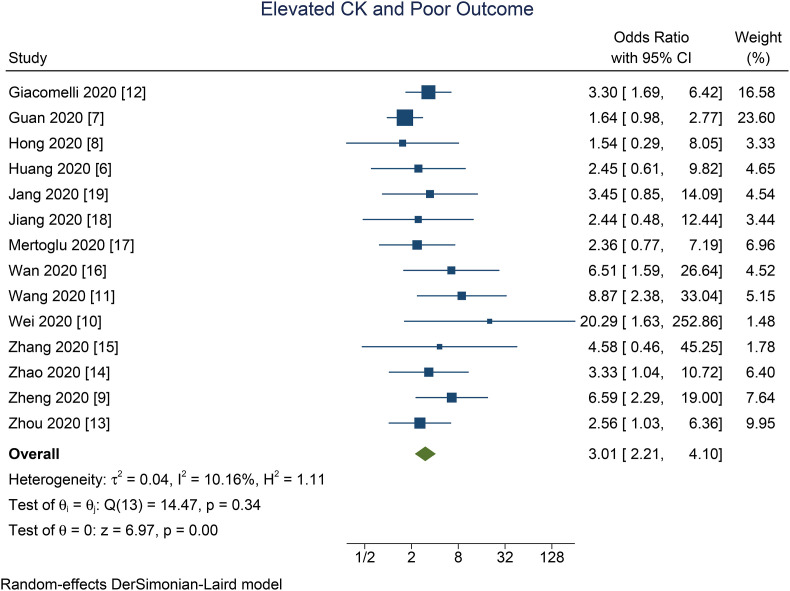
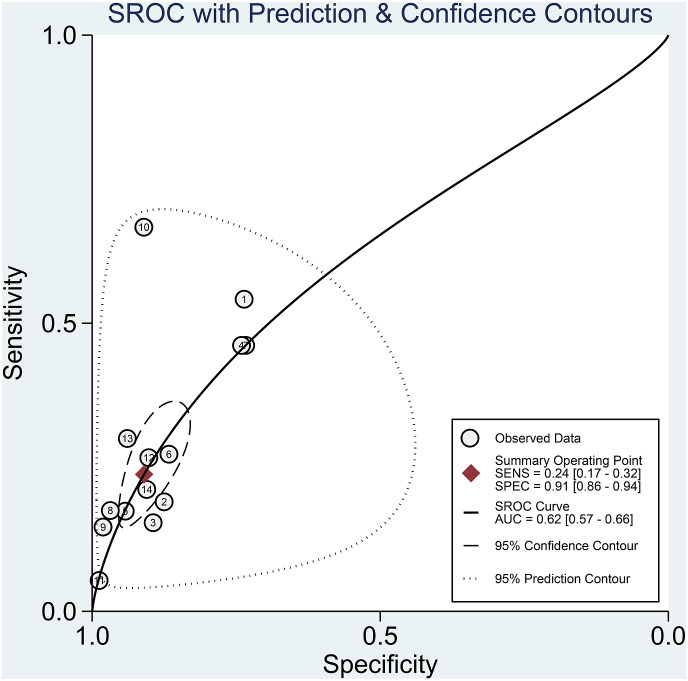
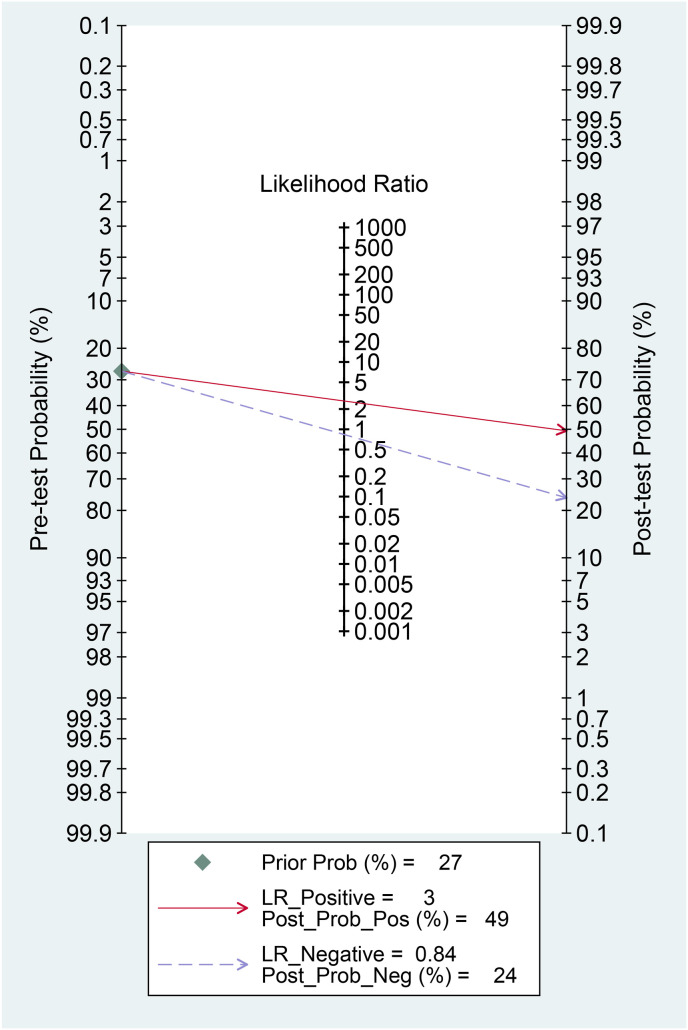
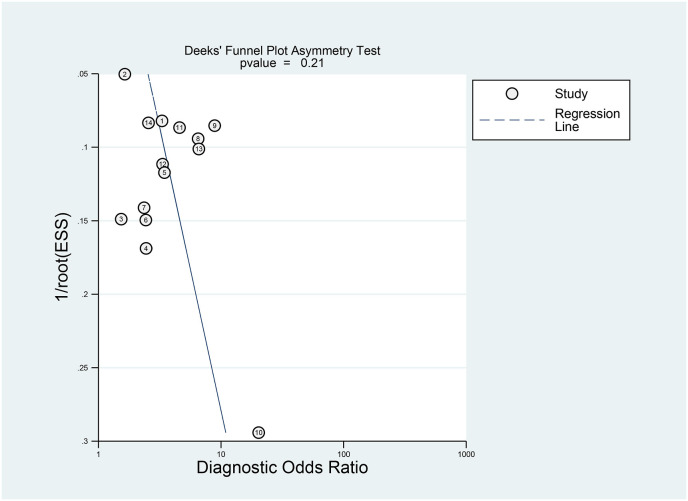
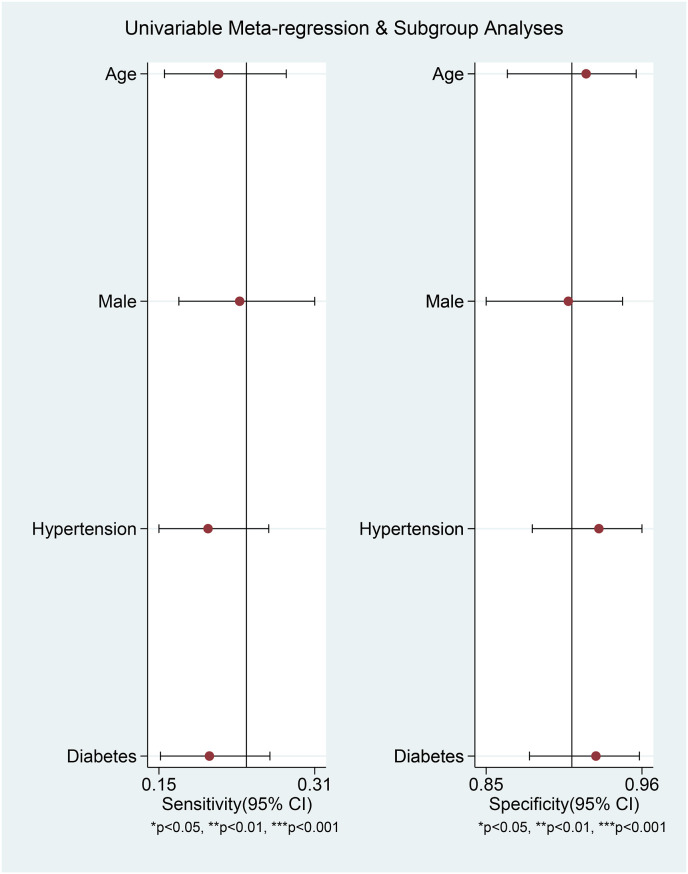
Elevated CK was associated with poor outcome in patients with COVID-19 (OR 3.01 [2.21, 4.10], p < 0.001; I2: 10.2%, p = 0.341) [Fig. 2 ]. The effect estimate did not vary with age (p = 0.610), male (p = 0.449), hypertension (p = 0.490), and diabetes (p = 0.457). Elevated CK has a sensitivity of 0.24 (0.17, 0.32), specificity of 0.91 (0.86, 0.94), PLR of 2.6 (1.9, 3.7), NLR of 0.84 (0.78, 0.90), DOR of 3 (2, 5), and AUC of 0.62 (0.57, 0.66) [Fig. 3 ] for predicting poor outcome in patients with COVID-19. Fagan’s nomogram indicated that in this pooled analysis, elevated CK confers to a 49% post-test probability for poor outcome and a non-elevated CK confers to a 24% post-test probability for poor outcome [Fig. 4 ]. There is no indication of publication bias in the Deek’s funnel plot [Fig. 5 ] and asymmetry test (p = 0.21). Subgroup analysis and univariate meta-regression indicates that the sensitivity and specificity does not vary with age, male, hypertension, and diabetes [Fig. 6 ].
Fig. 2.
Elevated CK and poor outcome. Forest plot demonstrating that elevated CK was associated with poor outcome. CK: Creatine Kinase.
Fig. 3.
Summary receiver operating characteristic of elevated CK. CK: Creatine Kinase.
Fig. 4.
Fagan’s nomogram. Fagan’s nomogram indicated that in this pooled analysis, elevated CK confers to a 49% post-test probability for poor outcome and a non-elevated CK confers to a 24% post-test probability for poor outcome. CK: Creatine Kinase.
Fig. 5.
Deek’s funnel plot for evaluation of publication bias.
Fig. 6.
Univariate meta-regression analysis and subgroup analyses for age, gender, hypertension, and diabetes.
4. Discussion
This meta-analysis indicates that elevated CK was associated with increased poor outcome with a sensitivity of 24% and specificity of 91%. Thus elevated CK is best used as a rule-in test rather than rule-out test.
Several parameters and comorbidities may affect the prognosis in patients with COVID-19 [[20], [21], [22], [23], [24]]. Meta-regression analysis showed that the association between elevated CK and poor outcome was not affected by age, male, hypertension, and diabetes. Although it is important to evaluate chronic kidney disease and cardiovascular disease as variables, there are insufficiently reported to enable meta-regression analysis.
The majority of symptomatic patients present with respiratory tract symptoms or related complications. They tend to present as a viral pneumonia with fever, cough, fatigue, dyspnoea, and sometimes complaint musculoskeletal or neurological symptoms such as headache, muscle pain, joint aches, and smell impairment [4,5]. However, a study [25] reported myalgia as the initial presentation in 22% of cases. One study [25] found that skeletal muscle pain and increased serum CK (>200 U/L) were significantly more common in severe cases [26], while another study [27] reported a higher prevalence of muscle pain in milder cases.
Rhabdomyolysis, a syndrome characterized by muscle weakness, pain, and red coloured urine, is a possible but rare complication in COVID-19 patients. CK and troponin, enzymes located in the muscle tissue, will rise sharply due skeletal muscle damage and necrosis in rhabdomyolysis [28]. Therefore, patients with severe muscle damage may experience multiorgan dysfunction, including more severe kidney and liver abnormalities [26]. Urgent and aggressive fluid challenge and management of electrolyte imbalances are crucial in preventing progression to acute renal failure and severe metabolic abnormalities. However, there are no protocols that recommend extensive fluid resuscitation in the early stage of COVID-19 treatment [5].
The new coronavirus may possibly cause viral myositis, as seen previously in other coronavirus infections, such as severe acute respiratory syndrome (SARS), and other influenza infections (influenza A and B) [29]. It is understood that the novel coronavirus (2019-nCoV or SARS-CoV-2) uses angiotensin converting enzyme 2 (ACE-2) receptor to enter human respiratory cells and cause infection. Due to its presence in various tissues, it is possible that the virus directly invades the skeletal muscle and nervous system via the same pathway. The Immune-mediated pathway might also contribute to cause muscle injury in COVID-19 patients. Moreover, muscle atrophy due to disuse and/or critical illness myopathy and/or polyneuropathy may occur and cause weakness in critically ill patients [4]. In addition, dehydration and hypovolaemia in COVID-19 patients may contribute to renal impairment and subsequent increase in CK levels [30]. It is therefore plausible to suggest CK monitoring in COVID-19 patients, especially when they complain of muscle pain and weakness.
Whether the increase in CK levels is caused by viral myositis, immune hyperactivation, toxic effect of cytokines, or other mechanisms is unclear. Electromyography (EMG), muscle imaging, and muscle histopathology are rarely performed in COVID-19 cases, let alone carrying out a PCR test for detection of SARS-CoV-2 in muscle tissue. A muscle-biopsy specimen of a patient [31] with COVID-19 and myopathy shows evidence of virus-induced type 1 interferonopathy. The specimen revealed mild perivascular inflammatory infiltration in a few vessels without regenerating or necrotic fibers or perifascicular atrophy. Immunohistochemical analysis did not detect SARS-CoV-2 in the muscle, but showcased abnormal expression of major-histocompatibility-complex class I antigen on sarcoplasm and sarcolemma and abnormal presence of myxovirus resistance protein A on muscle fibers and capillaries. This type I interferon-inducible protein is released in response to viral infections, including SARS-CoV-2, and its deposition is an early sign of dermatomyositis, followed by characteristic perifascicular atrophy.
Another report of COVID-19 patient [29] with myalgia, proximal weakness (limb, bulbar, and fascial muscles), increased serum CK, and muscle edema on MRI. Diagnosed with myositis and myasthenia gravis, the muscle biopsy demonstrated perivascular inflammation with endomysial extension, regenerating fibers, and upregulation of HLA Class ABC expression on non-necrotic fibers. Histochemiscally, the enzyme cytochrome oxidase/succinic dehydrogenase were unremarkable. On electron microscopy, vacuolar change and curvilinear bodies indicating hydroxychloroquine were absent.
4.1. Clinical implications
Elevated CK signifies poor prognosis in patients with COVID-19, however, due to low sensitivity; it cannot be used to rule out poor prognosis. Thus, elevated CK is better combined with other variables to form a prediction model rather than used alone.
4.2. Limitations
There is a possibility of publication bias in which only positive studies are being published. There is also slight variation in cut-off points. Some of important variables such as cardiovascular diseases and chronic kidney disease cannot be assessed due to insufficient reporting. Finally, most of the studies were retrospective in nature, and is more prone to bias.
5. Conclusion
Elevated CK was associated with increased mortality and severity in patients with COVID-19.
Declaration of competing interest
None.
Abbreviations
- AUC
Area Under Curve
- CK
Creatine Kinase
- DOR
Diagnostic Odds Ratio
- OR
Odds Ratio
- PLR
Positive Likelihood Ratio
- NLR
Negative Likelihood Ratio
Funding
None.
Ethical approval
Not Applicable.
Informed consent
Not Applicable.
Data availability
Data are available on reasonable request.
Authors contribution
MRA and RP contributes to concept and development of the study. AW and MAL performed data curation and extraction. MAL, MRA, and RP performed data interpretation and analysis. RP performed statistical analysis. AW, MAL, MRA, and RP drafted the manuscript. TAS performed critical revision and editing of the manuscript.
References
- 1.World Health Organization . 2021. Weekly epidemiological update - 5 January 2021. Geneva. [Google Scholar]
- 2.Lim M.A., Pranata R., Huang I., Yonas E., Soeroto A.Y., Supriyadi R. Multiorgan failure with emphasis on acute kidney injury and severity of COVID-19: systematic review and meta-analysis. Can J Kidney Heal Dis. 2020;7 doi: 10.1177/2054358120938573. 2054358120938573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang I., Pranata R., Lim M.A., Oehadian A., Alisjahbana B. C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: a meta-analysis. Ther Adv Respir Dis. 2020;14 doi: 10.1177/1753466620937175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orsucci D. Is creatine kinase associated with outcome in COVID-19? Neuroimmunol Neuroinflammation. 2020 doi: 10.20517/2347-8659.2020.53. 2020:[Online First] [DOI] [Google Scholar]
- 5.Chan K.H., Farouji I., Abu Hanoud A., Slim J. Weakness and elevated creatinine kinase as the initial presentation of coronavirus disease 2019 (COVID-19) Am J Emerg Med. 2020;38:1548. doi: 10.1016/j.ajem.2020.05.015. e1-1548.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/nejmoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hong K.S., Lee K.H., Chung J.H., Shin K.C., Choi E.Y., Jin H.J., et al. Clinical features and outcomes of 98 patients hospitalized with sars-cov-2 infection in daegu, South Korea: a brief descriptive study. Yonsei Med J. 2020;61:431–437. doi: 10.3349/ymj.2020.61.5.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng F., Tang W., Li H., Huang Y.X., Xie Y.L., Zhou Z.G. Clinical characteristics of 161 cases of corona virus disease 2019 (COVID-19) in Changsha. Eur Rev Med Pharmacol Sci. 2020;24:3404–3410. doi: 10.26355/eurrev_202003_20711. [DOI] [PubMed] [Google Scholar]
- 10.Wei Y., Zeng W., Huang X., Li J., Qiu X., Li H., et al. Clinical characteristics of 276 hospitalized patients with coronavirus disease 2019 in Zengdu District, Hubei Province: a single-center descriptive study. BMC Infect Dis. 2020;20:549. doi: 10.1186/s12879-020-05252-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y., Liao B., Guo Y., Li F., Lei C., Zhang F., et al. Clinical characteristics of patients infected with the novel 2019 coronavirus (SARS-Cov-2) in Guangzhou, China. Open Forum Infect Dis. 2020;7:1–7. doi: 10.1093/ofid/ofaa187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giacomelli A., Ridolfo A.L., Milazzo L., Oreni L., Bernacchia D., Siano M., et al. 30-day mortality in patients hospitalized with COVID-19 during the first wave of the Italian epidemic: a prospective cohort study. Pharmacol Res. 2020;158:104931. doi: 10.1016/j.phrs.2020.104931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao X.Y., Xu X.X., Yin H Sen, Hu Q.M., Xiong T., Tang Y.Y., et al. Clinical characteristics of patients with 2019 coronavirus disease in a non-Wuhan area of Hubei Province, China: a retrospective study. BMC Infect Dis. 2020;20:311. doi: 10.1186/s12879-020-05010-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang J jin, Dong X., Cao Y yuan, Yuan Y dong, Yang Y bin, qin Yan Y., et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy Eur J Allergy Clin Immunol. 2020;75:1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 16.Wan S., Xiang Y., Fang W., Zheng Y., Li B., Hu Y., et al. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J Med Virol. 2020;92:797–806. doi: 10.1002/jmv.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mertoglu C., Huyut M.T., Arslan Y., Ceylan Y., Coban T.A. How do routine laboratory tests change in coronavirus disease 2019? Scand J Clin Lab Invest. 2020:1–15. doi: 10.1080/00365513.2020.1855470. 0. [DOI] [PubMed] [Google Scholar]
- 18.Jiang H., Guo W., Shi Z., Jiang H., Zhang M., Wei L., et al. Clinical imaging characteristics of inpatients with coronavirus disease-2019 in Heilongjiang Province, China: a retrospective study. Aging (Albany NY) 2020;12:13860–13868. doi: 10.18632/aging.103633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jang J.G., Hur J., Choi E.Y., Hong K.S., Lee W., Ahn J.H. Prognostic factors for severe coronavirus disease 2019 in daegu, Korea. J Kor Med Sci. 2020;35:1–10. doi: 10.3346/JKMS.2020.35.E209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pranata R., Huang I., Lim M.A., Wahjoepramono E.J., July J. Impact of cerebrovascular and cardiovascular diseases on mortality and severity of COVID-19–systematic review, meta-analysis, and meta-regression. J Stroke Cerebrovasc Dis. 2020;29 doi: 10.1016/j.jstrokecerebrovasdis.2020.104949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pranata R., Henrina J., Lim M.A., Lawrensia S., Yonas E., Vania R., et al. Clinical frailty scale and mortality in COVID-19: a systematic review and dose-response meta-analysis: clinical Frailty Scale in COVID-19. Arch Gerontol Geriatr. 2021;93:104324. doi: 10.1016/j.archger.2020.104324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pranata R., Lim M.A., Yonas E., Vania R., Lukito A.A., Siswanto B.B., et al. Body mass index and outcome in patients with COVID-19: a dose–response meta-analysis. Diabetes Metab. 2020 doi: 10.1016/j.diabet.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pranata R., Supriyadi R., Huang I., Permana H., Lim M.A., Yonas E., et al. The association between chronic kidney disease and new onset renal replacement therapy on the outcome of COVID-19 patients: a meta-analysis. Clin Med Insights Circulatory, Respir Pulm Med. 2020;14 doi: 10.1177/1179548420959165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.July J., Pranata R. Prevalence of dementia and its impact on mortality in patients with coronavirus disease 2019: a systematic review and meta-analysis. Geriatr Gerontol Int. 2020 doi: 10.1111/ggi.14107. ggi.14107. [DOI] [PubMed] [Google Scholar]
- 25.Chen T., Wu D., Chen H., Yan W., Yang D., Chen G., et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lechien J.R., Chiesa-Estomba C.M., Place S., Van Laethem Y., Cabaraux P., Mat Q., et al. Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. J Intern Med. 2020;288:335–344. doi: 10.1111/joim.13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin M., Tong Q. Rhabdomyolysis as potential late complication associated with COVID-19. Emerg Infect Dis. 2020;26:1618–1620. doi: 10.3201/eid2607.200445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang H., Charmchi Z., Seidman R.J., Anziska Y., Velayudhan V., Perk J. COVID-19–associated myositis with severe proximal and bulbar weakness. Muscle Nerve. 2020;62:E57–E60. doi: 10.1002/mus.27003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rivas-García S., Bernal J., Bachiller-Corral J. Rhabdomyolysis as the main manifestation of coronavirus disease 2019. Rheumatology. 2020;59:2174–2176. doi: 10.1093/rheumatology/keaa351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manzano G.S., Woods J.K., Amato A.A. Covid-19–associated myopathy caused by type I interferonopathy. N Engl J Med. 2020;383:2389–2390. doi: 10.1056/nejmc2031085. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on reasonable request.