Abstract
Microparticles are a distinctive group of small vesicles, without nucleus, which are involved as significant modulators in several physiological and pathophysiological mechanisms.
Plasma microparticles from various cellular lines have been subject of research. Data suggest that they are key players in development and manifestation of cardiovascular diseases and their presence, in high levels, is associated with chronic inflammation, endothelial damage and thrombosis. The strong correlation of microparticle levels with several outcomes in cardiovascular diseases has led to their utilization as biomarkers. Despite the limited clinical application at present, their significance emerges, mainly because their detection and enumeration methods are improving.
This review article summarizes the evidence derived from research, related with the genesis and the function of microparticles in the presence of various cardiovascular risk factors and conditions. The current data provide a substrate for several theories of how microparticles influence various cellular mechanisms by transferring biological information.
Keywords: Microparticles, cardiovascular, inflammation, thrombosis, atherosclerosis, heart failure
1. Introduction
Both eukaryotic and prokaryotic cells, under the influence of several external or internal factors, can produce small vesicles. These vesicles are enclosed in a biphospholipid layer and contain part of the paternal cytosol [1]. Part of this heterogeneous population of cell-derived vesicles are the microparticles which serve as a disseminated storage pool of active biological molecules [2]. For long time after their discovery, microparticles were considered as cell debris, result of biological processes without any significant meaning. However, technological advantages of their detection and characterization stimulated research for investigation of their roles in various clinical situations [3].
The current evidence suggests that microparticles formation from different types of cells, in normal circumstances, is not just a passive process following apoptosis, necrosis or cellular dysfunction but is a balanced mechanism that promotes communication between various cellular types. Microparticles influence vital physiological functions such as inflammation, coagulation, apoptosis and cell differentiation and may trigger pathophysiological mechanisms which contribute to the genesis of atherosclerosis and thrombosis, the cornerstones for the development of cardiovascular disorders [4]. Apart from the current role as reflectors/biomarkers of certain cardiovascular and other diseases, microparticles have been proposed as potential targets in order to regulate various conditions with auto-immune or thrombotic causality [5].
This review article summarizes the evidence derived from research, related with the genesis and the function of microparticles in the presence of various cardiovascular risk factors and conditions.
2. Definition and nomenclature
Knowledge on extracellular vesicles has significantly expanded over the last two decades. Even at the last meeting of International Society of Extracellular Vesicles in 2018, there was no definitive consensus about the definition of microparticles [6].
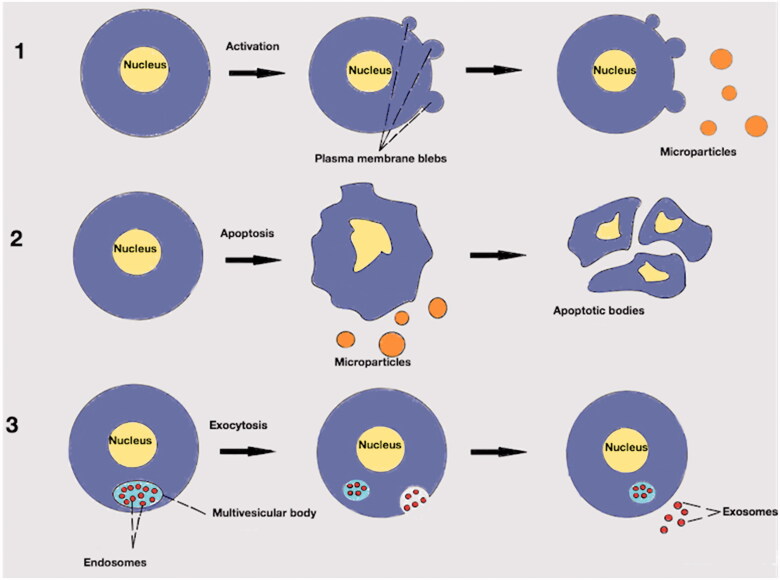
The broad term to describe particles released from cells by natural process such that their cytosol is enclosed by a lipid bilayer and lacking synthetic capacity, is “extracellular vesicles”. The term “microparticles” has been used for a variety of extracellular vesicles in the past. The distinction between exosomes and ectosomes (Figure 1) is important in order to approach the nature of microparticles. Exosomes are formed after an inward bleeding of the plasma membrane and stored into a bigger intracellular vesicle, the multivesicular body. Later, exosomes can be released in the extracellular environment by exocytosis. On the contrary, ectosomes are vesicles which directly released from the paternal cell by outwards rearrangement of the cellular membrane [7]. Of note, there is a significant overlap regarding size (diameter), membrane protein composition and cellular origin of the extracellular vesicles which at present makes difficult their categorization [8]. Even their characterization as ectosomes or exosomes is generally not advisable unless particle biogenesis is documented by a live imaging technique [6].
Figure 1.
Extracellular vesicles. (1): Production of microparticles after stimulation of paternal cell. Microparticles are released from activated cell after outwards rearrangement of the cellular membrane. (2): Production of microparticles during apoptotic process. Microparticles are released before the formation of apoptotic bodies. (3): Endosomes in multivesicular body. After exocytosis of the endosomes into the extracellular environment may be called exosomes.
Other types of extracellular vesicles are the apoptotic bodies which have usually a diameter between 1 and 5 µm. Formation of apoptotic bodies is exclusively linked to the latest stages of apoptosis where there is cellular shrinkage and nuclear fragmentation. Nuclear material, cell organelles and a permeable membrane are distinguishable characteristics of apoptotic bodies [9]. In general, ectosomes are larger than exosomes (30–100 nm) and smaller than apoptotic bodies (Figure 1). Additionally, the content may be different. For example, exosomes contain some membrane specific markers which are related with their formation process, such as lysosomal-associated membrane protein 1 and the membrane protein CD63. For microparticles, externalization of the negatively charged phospholipid phosphatidylserine is the rule but for exosomes it is a rare structural condition. Also, exosomes might contain cytosolic RNA but not like microparticles nuclear material [10]. The above “rules” have exceptions to the degree that specific identification criteria based on size and/or markers seems to be causing more confusion than consensus [11].
In this review, we have included research articles related with extracellular vesicles which their diameter is between 100 and 1 µm and they have at least one marker to describe their membrane biochemical composition. Furthermore, the cell(s) of origin are usually known to be related either with the biomarker or with the experimental process and the analysis method. In the vast majority of the literature, these vesicles are called “microparticles” and thus, we kept that term.
3. Biology
3.1. Inducers and mechanisms of formation
Potentially, any cell of an eukaryotic organism can produce microparticles [12]. In the blood, the most common (70–90%) of the circulating microparticles are platelet-derived microparticles. The rest of the blood containing microparticles is from endothelial, granulocyte, erythrocyte and smooth-muscle cells [13]. Microparticles from epithelial, tumour cells, fibroblasts and other cellular origin have been also isolated [14,15].
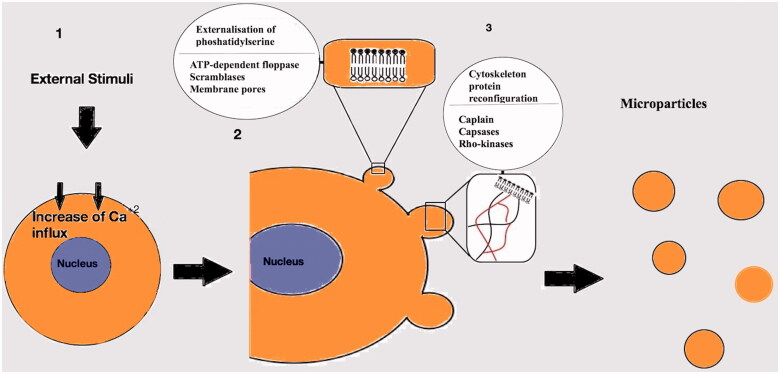
Apart from the formation of the microparticles under normal circumstances, which is mainly linked with growth, differentiation and apoptosis [16], there are other non-physiological conditions that promote microparticle production. These conditions include hypoxia [17], shear stress [18], inflammation [19] and a variety of prothrombotic or proapoptotic factors [20] (Table 1). Usually, the first result after exposure of the cell to these factors, is an increase of Ca+2 influx [22] (Figure 2). High concentration of the intracellular Ca+2 induces molecular processes resulting in the release of the microparticles in the extracellular media [22].
Table 1.
| Type of cell | Endothelial cell | Platelet | Neutrophil | T-cell | Monocyte and macrophage | Smooth cell |
|---|---|---|---|---|---|---|
| Stimuli | ||||||
| Modified LDL | Flow conditions | Pro-inflammatory cytokines | Pro-inflammatory cytokines | Cigarette extract | Modified LDL | |
| HDL cholesterol | Thrombin | Phytohemagglutinin | Pro-inflammatory cytokines | HDL cholesterol | ||
| Uremic toxin | Collagen | Phorbol myristate acetate | Staurosporin | Calcimycin | ||
| Flow conditions | Homocysteine | Etoposide | Flow conditions | |||
| Thrombin | Pro-inflammatory cytokines | Anti-neutrophil cytoplasmic antibodies | Actinomycin D | Lipopolysaccharides | Activated protein C | |
| Homocysteine | Calcimycin | Bacterial infection | Phorbol myristate acetate | Etoposide | Pro-inflammatory cytokines | |
| Activated protein C | collagen | N-formyl-methionyl-leucyl-phenylalanine | Fas Ligand | Fas-ligand | ||
| Plasminogen activator inhibitor | Lipopolysaccharides | – | – | – | Platelet derived growth factor | |
| Pro-inflammatory cytokinesa | Shiga toxin | – | – | – | ||
| Oxidative stress | sCD40 Ligand | – | – | – | – | |
| High glucose | Erythropoietin | – | – | – | – | |
| Uremic toxins | Noradrenaline | – | – | – | – | |
| Lipopolysaccharides | acid phosphatase 5 | – | – | – | – | |
| Camptothecin | Reactive oxygen species | – | – | – | – | |
| Angiotensin II | – | – | – | – | – | |
| Plasminogen Activator Inhibitor 1 | – | – | – | – | – | |
| C Reactive Protein | – | – | – | – | – |
Tumour necrosis factor a, interleukin 1b, interleukin 6.
Figure 2.
Mechanisms involved in the generation of the microparticles. (1): After activation of the cell an increased Ca+2 influx follows. (2): Externalisation of phosphatidylserine mediated by ATP-dependent floppases, scramblases and membrane pores. (3): Cytoskeleton Protein reconfiguration in order to produce outward membrane blebs. Capsases, caplains and Rho kinases are involved in the process.
The general assumption is that loss of the phospholipid asymmetrical set up of the plasma membrane, which is present during cellular relaxation, leads to the production of the microparticles [23]. Externalization of phosphatidylserine, a negatively charged phospholipid, primarily located on the inner surface of the plasma membrane of the non-activated cell, results in the membrane asymmetry [24]. Several phospholipid transporters regulate the inwards (flip) or outwards (flop) translocation of the plasma membrane lipids. An ATP-dependent “floppase” is responsible for the outwards translocation of the phosphatidylserine with simultaneous inhibition of flippase(s) [25]. Non-specific, bidirectional lipid transporters, the “scramblases” [26] along with the formation of transient membrane pores constitute another pathway for the membrane remodelling [27].
The intracellular influx of Ca+2 is also involved in the shaping of the plasma membrane protrusions which results in the formation of microparticles [23]. This process begins with degradation and reconfiguration of the cytoskeleton proteins [28]. The proteolysis of specific part of the cytoskeleton network, by calpain activation, causes separation of the membrane protrusion from the parental cell as independent vesicle into the extracellular media [29]. Another group of proteases, the caspsases, is also associated with cytoskeleton ingredient lysis like talin, filamin and gelsolin [30]. Caspsases are involved in the actin-myosin cytoskeletal network reorganization by interacting with various Rho-kinases isoforms [31,32]. Rho-kinases mediated microparticle shedding, with possible cellular nucleic acid redistribution, appears to be involved in apoptotic processes [33].
Phosphatidylserine exposure is commonly involved in microparticle formation from activated or apoptotic cells [34]. However, there are less controlled situations where stress or injury induces cellular necrosis and loss of membrane integrity with production of microparticles [35]. Also, there are populations of microparticles that are phosphatidylserine – (i.e. they do not bind Annexin-V), suggesting alternative formation processes [36]. Further evidence of other ion channel involvement in the microparticle formation cascade, apart from Ca+2, support the presence of unknown mechanisms associated with plasma membrane shedding [21,37] .
3.2. Structure and content
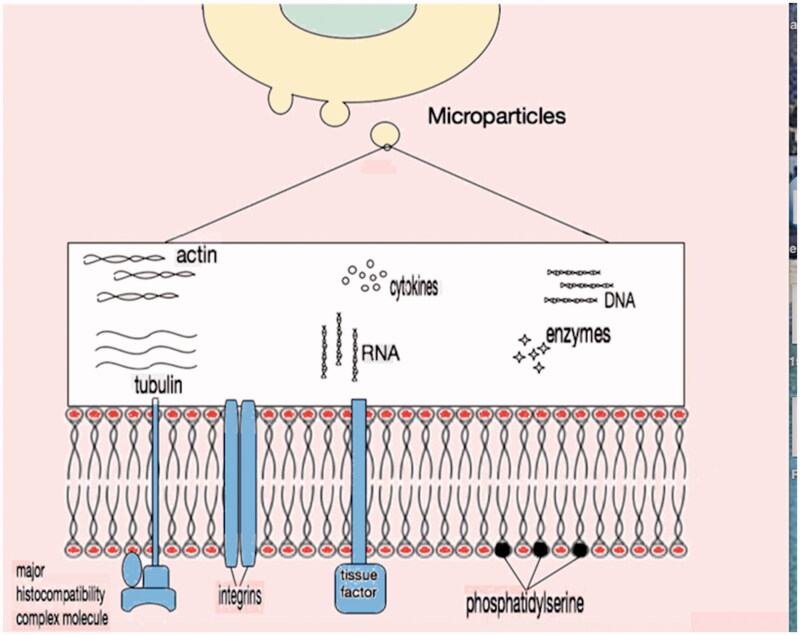
Microparticles contain a wide variety of biological molecules as part of their phospholipid membrane or within the cytosol that they enclose (Figure 3). These molecules are proteins (signal proteins, receptors and effector proteins), lipids and nucleic acids [38–40]. Various techniques have been tried in order to characterize the components of the microparticles [41,42]. Irrespective of the origin of microparticles, the plasma membrane is negatively charged due to translocation of phospholipids such as phosphatidylserine and phosphatidylcholine from internal to external surface [43,44]. Other phospholipids of the membrane include lysophosphatidylcholine, sphingomyelin, lysophosphatidylethanolamine, phosphatidylethanolamine, lysophosphatidylserine and phosphatidylinositol [45]. It appears that the bi-lipid layer of the microparticles affects the attached protein activities and the general properties of the vesicles [46].
Figure 3.
Microparticle content. External surface of plasma membrane in general contains negatively charged phosphatidylserine along with membrane proteins like major histocompatibility complex molecules, integrins and tissue factor. In the cytosol, there is no organised nucleus but apart from cytoskeleton proteins and enzymes, nucleic acid remnants (DNA or RNA) are present.
The origin of the microparticles influences their composition. For example, platelet-derived microparticles are enrich in various membrane proteins that are important in the coagulation process such as GPIb, GPII-IIIb, P-selectin, integrins [47–49]. Similarly, microparticles from endothelial cells carry characteristic endothelial proteins (vascular endothelium cadherin, E-selectin) [50] and leucocyte-derived microparticles are enriched in metalloproteinases and other proteolytic enzymes [51] involved in inflammation process. Antigenic clusters of differentiation (CD31, CD105, etc.) which derive directly from the parental cell, are present in the plasma membrane of the microparticles [52] (Table 2).
Table 2.
Main antigen markers used for MP cell origin determination.
| Cell | Cluster of differentiation (CD) |
|---|---|
| Endothelial | CD31 [53] |
| – | CD51[54] |
| – | CD105 [55] |
| – | CD62E [55] |
| – | CD144 [56] |
| – | CD34 [57] |
| Platelet | CD41 [58] |
| – | CD42a [59] |
| – | CD42b [60] |
| – | CD61 [57] |
| Red cell | CD235 [61] |
| Leukocyte | CD45 [61] |
| Monocyte | CD14 [62] |
| Neutrophil | CD66b [58] |
| T cell | CD4 [63] |
| – | CD8 [64] |
CD31, CD51, CD105 are not specific for endothelial cells. CD31 is also expressed on platelets, CD51 on platelets and macrophages and CD105 in activated monocytes/macrophages. For MP detection, markers are usually combined to discriminate this population from other MPs. For example, platelet MPs are CD31 positive/CD42b positive whereas endothelial MPs are CD31 positive/CD42b negative.
The stimuli that trigger the formation of the microparticles regulate the ratio and the composition of the expressed membrane proteins. For example, monocytes have been stimulated by various substances in vitro (lipopolysaccharide, soluble P-selectin chimera, phosphate-buffered saline) and the produced microparticles expressing different membrane proteins. Similar findings were reported for microparticles derived from other cellular lines such as T cells [65], endothelial cells [52] and leucocytes [66]. However, all microparticles shared some common molecules [67].
The nucleic acids contained into the microparticles are usually resulting in apoptotic process [68]. Different types of RNA (ribosomal, micro and messenger) and DNA are enclosed into membrane vesicles which are protected from nuclease exposure and might be activated into the target cells. RNA packaging is influenced by the variation of the stimuli that trigger microparticle formation [69]. This selective translocation of nucleic acids contributes to intercellular communication [53,70].
4. Functions related with cardiovascular physiology
4.1. Transfer of biological information
Several biological functions of microparticles can be summarized with the title “factors of intercellular communication and information exchange”. In principle, there are two ways microparticles may contribute to intercellular signalling [54–64,71]. The first is mediated by activation of receptors on the plasma membrane of the target cell by presentation of molecules which result in alteration of the cellular function. The second way of interaction is by direct transfer to the target cell bioactive components such as proteins, lipids and nucleic acids (Table 3). The target cell can utilize these molecules by affecting its biological function by activation of certain pathways or by phenotypic modification [48,77,98]. Phenotypic modification is achieved usually by transferring membrane receptors to the recipient cell. These receptors interfere with stimuli that before transfer did not influence cellular activity at all or not by the same way [72].
Table 3.
Bioactive molecules of microparticles.
| Molecule | Type of cell producing microparticles | Target cell or environment |
|---|---|---|
| Receptor/membrane molecule | ||
| Chemokine receptor CCR5 [72] | Peripheral blood mononuclear cells | Various cells |
| CXCR4 receptor [73,74] | Platelets | Various cells |
| Glycoprotein IIb/IIIa receptors [75] | Platelets | Neutrophils |
| Oncogenic receptor EGFRvIII (epidermal growth factor receptor variant III) [76] | Tumour cells | Various cells |
| Major histocompatibility complex (MHC) class II [77] | Immune cells | Immune cells |
| Tissue factor [48] | Monocytes | Platelets |
| Peroxisome proliferator-activated receptor gamma [78] | Platelets | Monocytes |
| Cytokines | ||
| Interleukin-1beta [79–82] | Various cells | Various cells |
| Chemokine (C-C motif) ligand 5[83] | Platelets | Endothelial cells |
| Growth factors | ||
| Vascular endothelial growth factor [84–85] | Platelets/tumour cells | Endothelial cells |
| Basic fibroblast growth factor [84,86] | Platelets/tumour cells | Endothelial cells |
| Platelet-derived growth factor [86] | Platelets | Endothelial cells |
| Lysis enzymes | ||
| Matrix metalloproteinases [85] | Tumour cells | Extracellular matrix |
| Extracellular matrix metalloproteinase inducer [87] | Tumour cells | Extracellular matrix |
| Caspase 1 [88] | Monocyte | Smooth muscle cell |
| Lipids | ||
| Arachidonic acid [89–91] | Platelets | Various cells |
| Platelet activated factor [92,93] | Various cells | Platelets |
| Ribonucleic acid (RNA) | ||
| Messenger RNA [38,94] | Stem cells | Various cells |
| Micro RNA [95–97] | Stem cells | Various cells |
Proteins can also be carriers of biological signal. Apart from membrane proteins, microparticles might have proteins in their cytosol in various forms. After incorporation of the microparticles into the target cell by phagocytosis, the proteomic load can be in an activated form or can be cleaved and activated by proteolytic enzymes into the target cell [80,81].
Reverse transcription polymerase chain reaction and microarray analysis demonstrated that microparticles carry a specific subset of messenger RNA or microRNA from the origin cell [80]. By this manner, microparticles transfer to the target cell transcriptional information, analogous to the stimulating factor. The recipient cell will promote several processes, such as differentiation, proliferation and apoptosis by expressing different gene [99,100].
Lipids are not only components of the plasma membrane of the microparticles but actively determine the role of the ectosomes and their interaction with other cells [89]. This function is mediated by the surface provided by microparticle membrane and from bioactive lipids such as arachidonic acid, cyclooxygenase 2 and prostacyclin [90,101].
4.2. Coagulation
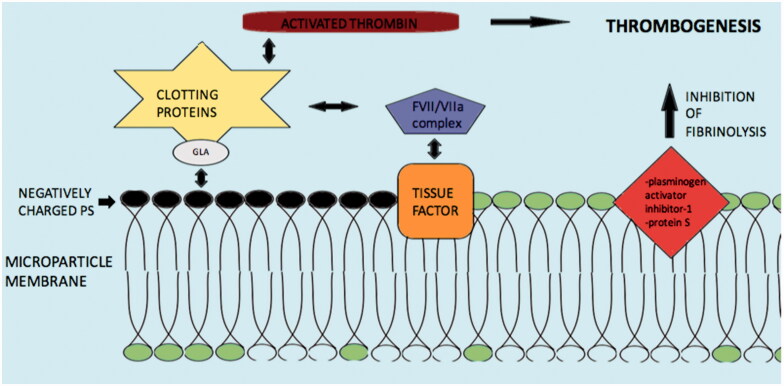
The involvement of microparticles in the coagulation process was apparent from the time of their discovery [102]. The strongest procoagulant activity is mainly related to the negatively charged, external surface of their plasma membrane due to the presence of phosphatidylserine. The phosphatidylserine electrostatically attracts the positively charged segment of clotting proteins such as factors VII, IX and X, and prothrombin. The presence of γ-carboxyglutamic acid (GLA) domains creates the cationic features of these clotting factors [103] (Figure 4).
Figure 4.
Mechanisms and molecules related with Microparticle induced coagulation. Abbreviations: PS: phospatildylserine; GLA: γ-carboxyglutamic acid; clotting proteins factors VII, IX, X and prothrombin. Negatively charged PS electrostatically attract the positively charged segment of clotting proteins/GLA complex and induce thrombogenesis. Tissue factor may also activate the coagulation cascade via the FVII/VIIa complex. Additionally, inhibition of fibrinolysis by microparticle membrane proteins such as plasminogen activator inhibitor-1 and protein S may augment thrombogenesis.
Additionally, tissue factor as plasma membrane protein of the microparticles appears to play key role in the coagulation process. Tissue factor is an integral protein of the coagulation cascade and acts as a receptor of the FVII/VIIa complex, which activates both factors IX and X to initiate thrombin formation. Tissue factor positive microparticles may derive from monocytes, neutrophils, endothelial cells and platelets as response to various pathological conditions [104]. P-selectin, a cell adhesion receptor, interacts with tissue factor positive microparticles, through P-selectin glycoprotein ligand 1 (PSGL-1) on monocytes and causes further tissue factor positive microparticles generation which carry PSGL-1. These microparticles bind to activated platelets on the site of vascular injury and contribute further to thrombus expansion [105].
Finally, another possible mechanism of microparticles prothrombotic actions is inhibition of the fibrinolytic process. Expression of proteins on the plasma membrane of microparticles like plasminogen activator inhibitor-1 and protein S, leads to amplification of thrombogenesis by suppression of fibrinolysis [106,107].
4.3. Inflammation and immune regulation
Part of intercellular communication features of microparticles has been related with the immune regulation. Immune and non-immune cells may produce microparticles which carry antigens. In this context, microparticles can influence immune responses to foreign [108] or self-antigens [109]. All immune cell types under certain stimuli can generate microparticles but the most effective, with regard to the regulation of immune response, are the “professional” antigen-presenting cells, such as dendritic cells, macrophages and B cells [110]. This is achieved by binding the antigen to the cell surface or by phagocytosis [111,112].
Microparticles have pro-inflammatory effects mainly by inducing the production of cytokines and chemokines and by the activation of inflammatory cells [21] (Table 4). This recruitment of inflammatory mediators can be done without the presence of micro-organisms [120]. Administration of endothelial microparticles to rats is associated with release of pro-inflammatory cytokines IL-1β and TNF-α, acute lung injury and histological damage as evidence from the neutrophil infiltration into the perivascular space [113].
Table 4.
Inflammation and microparticles (MPs).
| Type of cell producing MPs | Target cell | Molecules involved in the pathogenesis of inflammation mediated by MPs |
|---|---|---|
| Cytokines | ||
| Endothelial | Various inflammatory cells | IL-1β and TNF-α [113] |
| Leucocytes | Endothelial | IL-6 and MCP-1 [114] |
| T cells | Monocytes | IL-8, TNF-a and IL-1β [43,115] |
| Adhesion molecules | ||
| Monocytes Platelet |
Endothelial | Intercellular adhesion molecule-1, vascular cell adhesion molecule-1 E-selectin [91,116] |
| – | – | Lipids |
| Platelet | Endothelial | Thromboxane A2 and cyclooxygenase [89] |
| Other | ||
| Polymorphonuclear | Macrophage | transforming growth factor beta1 [117] |
| – | – | Annexin V [118] |
| Monocytes | Macrophage | peroxisome proliferator-activated receptor gamma protein [119] |
| Polymorphonuclear | Various cells | complement proteins (C1q) [51] |
IL: interleukin; TNF: tumour necrosis factor; MCP-1: monocyte chemoattractant protein-1.
In vitro studies reported release of cytokines IL-6 and monocyte chemotactic protein from endothelial cells after stimulation by neutrophil microparticles [114]. Furthermore, pro-inflammatory cytokine secretion, such as IL-8, TNF-a and IL-1β, have been described from other cellular cultures after exposure to microparticles [43,115].
In addition to chemotactic factors, microparticles are involved in the production of specific cellular membrane proteins which promote adhesion of inflammatory cells to endothelium. Examples are the intercellular adhesion molecule-1, vascular cell adhesion molecule-1 and E-selectin [91,116]. Bioactive lipids produced by platelet-derived microparticles, like thromboxane A2 and cyclooxygenase, may act as mediators of inflammation. The target tissue is usually the endothelium [89].
Cases have been described where microparticles from polymorphonuclear leukocytes antagonize parallel pro-inflammatory stimuli by releasing cytokines like transforming growth factor beta 1. Polymorphonuclear-derived microparticles also contain Annexin-1, a protein with anti-inflammatory properties. Annexin-1 and transforming growth factor beta 1 inhibit macrophages. This action usually occurs during the early stages of the inflammatory process [117,118]. Microparticles from monocytes were found to induce macrophages and monocytes expression of peroxisome proliferator-activated receptor gamma (PPAR-γ) protein with anti-inflammatory action. In the same study, monocyte microparticles promoted inflammatory (reactive oxygen species [ROS], cytokines) or anti-inflammatory (PPAR-γ) molecules in dose dependant manner [119]. Another mechanism which associated with polymorphonuclear-derived microparticles mediated inflammatory process is activation of complement. Purified complement proteins like C1q from serum were found to be carried by polymorphonuclear-derived microparticles [51].
4.4. Angiogenesis
The platelet-derived microparticles were the first group of microvesicles demonstrated angiogenetic properties in vitro. Platelets are known carriers of neovascularization factors. Bioactive lipids from platelet-derived microparticles (Table 5) are the main inducers of proliferation and tube formation in human umbilical vein endothelial cells [84,121]. Experiments in rat models reported angiogenesis in ischaemic myocardium after injection of platelet-derived microparticles. The process was facilitated by vascular endothelial growth factor, basic fibroblast growth factor and inhibition of platelet factor-4 [86]. Also, tissue factor positive microparticles can induce endothelial cell proliferation through a beta 1-integrin and extracellular signal regulated kinase activation [122].
Table 5.
Angiogenesis and microparticles (MPs).
| Type of cell producing MPs | Target cell or environment | Molecules involved in the angiogenesis mediated by MPs |
|---|---|---|
| Promotion of neovascularisation | ||
| Platelets | Human umbilical vein endothelial cells | Bioactive lipids [84,121] |
| Platelets | Ischaemic myocardium | Vascular endothelial growth factor (VEGF), basic fibroblast growth factor (BFGF), inhibition of platelet factor-4 [86] |
| Various cells expressing membrane tissue factor | Endothelial | beta1-integrin, extracellular signal regulated kinase [122] |
| T-cells | Endothelial | Activation of morphogen Sonic Hedgehog (Shh) pathway which promotes synthesis of adhesion molecules and proangiogenic factors [123,124] |
| Endothelial | Endothelial | Plasmin activating factors [125] |
| Endothelial | Matrix | Metalloproteinases [126] |
| Endothelial | endothelial | Messenger RNA [127] |
| Adipose cells | Human umbilical vein endothelial cells | Leptin, fibroblast growth factor alpha (FGFa), Tumour necrosis factor a, matrix metalloprotease (MMP)-2 and MMP-9 mediated activation [128] |
| Inhibition of neovascularisation | ||
| Lymphocyte | Endothelial | CD36 antiangiogenic receptor [129] |
CD: cluster of differentiation; RNA: ribonucleic acid.
Another microparticle related neovascularization mechanism is possibly associated with the morphogen Sonic Hedgehog pathway. T-cell derived microparticles, harbouring the Sonic Hedgehog antigen, promote in vitro and in vivo formation of new vascular network by regulating the nitric oxide pathway and stimulate genes coding expression of adhesion molecules and proangiogenic factors [123,124].
Endothelial-derived microparticles have also angiogenetic properties. Plasmin formation on the surface of the endothelial-derived microparticles might activate proteolytic pathways and generation of factors which promote tube formation from endothelial cells [125]. In addition, matrix metalloproteinases (MMPs) carried by endothelial-derived microparticles contribute to matrix-degrading proteolytic activity necessary for the angiogenetic events [126]. Neovascularization programming by endothelial-derived microparticles appears to be conducted in relation with direct transfer of mRNA to target endothelial cells which codify the formation of capillary-like structures [38]. In vivo demonstration of the angiogenetic features of microparticles was reported by Leroyer et al. [127]. Microparticles derived from ischaemic muscle injected into ischaemic legs in a rat model resulted in enhanced neovascularization.
Adipose cell-derived microparticles from rats were also found to be carriers of angiogenetic molecules, such as leptin, fibroblast growth factor alpha (FGFa) and TNFa and in collaboration with tissue MMP-2 and MMP-9 may promote neovascularization [128].
A combined signal transmission from microparticles that regulates the functions of angiogenesis, apoptosis, differentiation and migration might contribute to tissue regeneration and remodelling [130]. After permanent middle cerebral artery occlusion in rats, administration of platelet-derived microparticles increased neurogenetic and angiogenetic activity, followed by behavioural improvement but no changes in infracted volumes [131].
The role of microparticles in angiogenesis is not always promotive but may be inhibitory. There are reports about prevention of neovascularization by microparticles. For example, human umbilical vein endothelial cells vascular network proliferation was inhibited by the presence of endothelial-derived microparticles [132]. Similarly, lymphocyte-derived microparticles were found to cause overexpression of the CD36 anti-angiogenic receptor while significantly downregulated protein levels related with angiogenesis [129]. The generation of ROS such as hyperoxide is likely to be involved in the inhibitory process. The balance between inhibition and promotion of neovascularization, for different types of microparticles, appears to be affected by their concentration along with other potential unknown factors [126,129,133].
4.5. Regulation of vascular tone
Several studies have established a link between endothelial activity, expressed by modification of vascular tone and microparticles. Microparticles may affect the regulation of nitric oxide synthetase resulting in impaired production of nitric oxide in vivo [108,134]. Endothelial-derived microparticle may also impair vasorelaxation. This was demonstrated in patients with end stage renal failure and type 2 diabetes, where different sonographic indices of arterial function have been assessed [55,135]. Furthermore, endothelial relaxation was impaired in aortic rings after exposure to endothelial-derived microparticles from patients with recent myocardial infarction, in contrast with endothelial-derived microparticles from non-ischaemic patients [136].
Lymphocyte-derived microparticles also affect the nitric oxide synthetase activity [137,138]. The mechanism driven the synthetase downregulation is related with the phosphorylation of the extracellular signal-regulated kinase 1/2 via phosphatidylinositol-3-kinase and nuclear factor κ-light-chain-enhancer of activated B cell pathways [137]. Additionally, lymphocyte-derived microparticles may induce endothelial overexpression of the integral membrane protein caveolin-1 which inhibits the nitric oxide synthetase [138].
Endothelial and platelet-derived microparticles are carriers of endothelial nitric oxide synthase and in patients with cardiovascular risk factors the isolated microparticles found to have significantly less levels of the synthase compared with healthy subjects [139]. Platelet-derived microparticles can also influence the vascular tone as they are involved in the production of vasoactive molecules such as prostacyclin (vasodilator) [90] or thromboxane (vasoconstrictor) [140]. In experimental models, microparticles can modify cyclo-oxygenase metabolites levels through the Fas antigen and its natural ligand FasL pathway [141].
4.6. Apoptosis
Microparticle production can be the result from an apoptotic process along with the formation of apoptotic bodies. Additionally, microparticles can also induce programmed cellular death of remote cells [142,143]. Anti-apoptotic stimulation showed a reduction in the cell “blebbing” and microparticle formation in Human tonsil germinal centre B cells in vitro. B cell blebs appear to have chemo-attractive properties to macrophages which carry out the apoptotic cell removal [144]. Endothelial-derived microparticles can initiate apoptosis in angiogenic cells. The rich in arachidonic acid microparticles are phagocytosed by the angiogenic cells and signalize apoptosis. This action, as other functional roles of the microparticles, is concentration dependant [145]. Apoptosis mediated by the lipid synthesis of the microparticle is also possible, even without involvement of phagocytosis. PtdIns [3,5] BP is a specific inhibitor of the acid sphingomyelinase which can inhibit the apoptosis pathway via upregulation of the capsase-8. Inhibition of the extracellular signal regulated kinase 1 prevents the apoptosis of macrophages in vitro. Extracellular signal regulated kinase 1 as microparticle membrane protein activates target cell membrane phospholipases and contributes to formation of arachidonic acid from phospholipids [146]. Capsase-3 protein is also involved in apoptosis [147] and has been identified as membrane protein in platelet-derived microparticles and endothelial-derived microparticles [148,149].
The signal for programmed cellular death to remote cells can be transmitted by microparticles through the Fas antigen and its natural ligand FasL. This mechanism was demonstrated in human tumour cells in vitro [150]. Additionally, tumour-derived microparticles contain matrix metalloproteinases responsible for matrix degradation but also adhesion molecules and receptors like the CX3CL1/fractalkine system which regulates migration and apoptosis [15].
4.7. Oxidative stress
Oxidative stress is caused when there is an imbalance between production of ROS and the antioxidant defence mechanisms [151]. Controlled production of ROS is important as contributes to cell growth, adhesion, differentiation and apoptosis [152]. Brodsky et al. [134] reported an active role of endothelial-derived microparticles in the formation of superoxide. Also, the p22(phox) subunit of NADPH oxidase has been detected in endothelial-derived microparticles. Also, microparticles from other cellular origin, such as lymphocytes and monocytes, may lead to the production of ROS [129,153]. Monocyte-derived microparticles can induce nitrosative stress in endothelial cells in vitro. This occurs by increasing the nitration of several proteins in endothelial cells after regulating calveolin-1 expression or activation of phosphatidylinositide-3 kinase and other extracellular signal-regulated kinases [154].
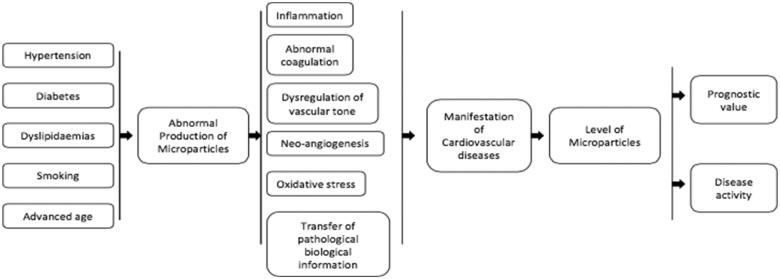
5. Microparticles and cardiovascular risk factors
5.1. Essential hypertension
Mechanisms, such as excessive amounts of ROS and oxidative stress, which are linked with microparticles, have been described to be involved in the pathogenesis of endothelial dysfunction due to hypertension [155,156].
The Renin-angiotensin system is known to have a fundamental role in the regulation of arterial hypertension [157]. Angiotensin II, a potent vasoconstrictive hormone can induce the formation of microparticles from monocytes in vitro. The derived microparticles expressed tissue factor on their membrane and demonstrated procoagulant activity [158]. Procoagulant features of microparticles reported by Preston et al. [159] in patients with severe hypertension. The hypertensive cohort had increased levels of platelet and endothelial-derived microparticles with strong positive correlation between two types circulating microparticles (CD31+ endothelial-derived microparticles and CD62P + platelet-derived microparticles) and absolute levels of systolic and diastolic blood pressure. Tissue factor expression on endothelial-derived microparticle and platelet factor 3 on platelet-derived microparticles might explain the procoagulant features of microparticles and thrombogenity of hypertension.
High levels of circulating endothelial-derived microparticles with synchronous reduction of endothelial progenitor cells (EPC) as expressed by increased ratio of endothelial-derived microparticles/EPC were detected in hypertensive patients with reduced glomerular filtration rate and microalbuminuria. The EPC is considered to promote endothelial integrity and vascular repair. On the other hand, the CD31/Annexin V + apoptotic microparticles were related with atherosclerotic disease and further deterioration of the renal function [160,161].
5.2. Diabetes mellitus
Endothelial-derived microparticles (CD62E+) are higher in a pre-diabetic cohort along with elevated biomarkers of endothelial dysfunction [162] (Table 6), suggesting an involvement of microvesicles in the pathogenesis of the disease. In patients with established diabetes, the absolute number of microparticles was also found to be elevated. Kurtzman et al. [163] reported increased number of several microparticle phenotypes in diabetics compared with healthy controls [163]. Similar findings were demonstrated by a metanalysis of 48 studies involving 2460 patients with Type 2 diabetes [175].
Table 6.
Studies with microparticles (MPs) in diabetic populations or high glucose concentration conditions.
| Type of MPs | Conclusion | References |
|---|---|---|
| Endothelial-derived microparticles CD62E positive, CD62P positive, CD142 positive, CD45 positive circulating MPs, their apoptotic (AnnexinV positive) fractions and miRNA-126 expression | CD62E positive MPs level and miR-126-3p content in MPs are abnormal in subjects with pre-diabetes | Giannella et al. [162] |
| CD3 positive T- Lymphocyte MPs, CD105 positive EMPs, Annexin V positive MPs, CD31 positive MPs, CD41a positive and Annexin V/CD31/ CD41a positive | Increased number of MPs in diabetic patients compared with healthy control group | Kurtzman et al. [163] |
| PMPs | Association with vascular changes in T2DM/ endothelial dysfunction and activated platelets/PMPs | Nomura et al. [164] |
| Μonocytes-derived MPs Annexin V/CD14 positive, PMPs GPI positive | higher in diabetic patients of with related vascular complications | Omoto et al. [165] |
| AnnexinV positive, PMP CD31/CD42 positive, LMP CD45positive , CD31positive/CD42negative EMP, CD51positive EMP | MPs increased in patients with T2DM. EMPs levels are associated with vascular dysfunction. | Feng et al. [55] |
| CD36 positive | CD 36 positive MPs in DM patients were from erythrocyte origin compare with healthy subjects, originated from endothelial cells. | Alkhatatbeh et al. [166] |
| PMPs CD41 positive, EMPs CD51 positive, leukocytes-derived MPs CD45 positive, neutrophil-derived MPs CD66b positive, monocyte-derived MPs CD14 positive and total Annexin V-positive MPs. | Different phenotypes identified between T1, T2 DM and healthy subjects. Differences in properties and particularly the procoagulant activities. | Sabatier et al. [59] |
| Annexin V positive, MPs from non- activated platelet (CD41positive), MPs from activated platelets (CD62p positive), EMPs (CD144 positive) | MPs properties and type (composition, content and cellular origin) are related with the type of vascular complications due to DM. | Tsimerman et al. [167] |
| MPs from Human umbilical vein endothelial cells | Raised glucose levels is a potent stimulus for MP formation that affects their molecular composition and may cause endothelial injury | Burger et al. [168] |
| PMPs CD41 positive, Annexin V MPs, MPs expressing tissue factor (CD142) | DM is associated with high levels circulating MPs with procoagulant features | Tripodi et al. [169] |
| EMPs CD144 positive, PMPs CD42b positive, monocyte-derived MPs CD14 positive | MPs from T1DM patients promoted platelet/endothelial cell interaction with an intensity correlated with the degree of the associated vascular complications | Terrisse et al. [61] |
| Lymphocyte and plasma MPs (Surface markers: CD3, CD11a, GP1b, CD31) | Lymphocyte-derived MPs from diabetic patients or in vivo circulating MPs from diabetic patients reduced endothelial NO synthase expression | Martin [138] |
| Surface markers: CD41a, CD64, CD144, CD144/CD31, Annexin V, CD144/annexin V and CD144/CD31/annexin V. | Apoptotic endothelial cell-derived were significantly increased in diabetic patients and associated with asymptomatic atherosclerosis | Berezin et al. [170] |
| Human coronary endothelial cells-derived MPs | High glucose environment increases NADPH oxidase activity in EMPs and promotes endothelial dysfunction | Jansen et al. [171] |
| EMPs (surface markers: CD31, CD42b, Annexin V, and CD62E) | EMP levels are associated with different risk of diabetic vascular complications | Jung et al. [54] |
| PMPs CD41 (GPIIb) positive, EMPs CD144 (VE-Cadherin) positive | Unstable coronary artery plaques in diabetics are associated with elevated EMPs | Bernard et al. [57] |
| PMPs (surface marker: antiplatelet GPIX monoclonal antibody) | Plasma PMPs are significantly higher in patient with DM compare with normal controls. Antiplatelet therapy reduces the level of PMPs. | Omoto et al. [172] |
| EMPs CD 144 positive, monocyte-derived MPs CD 14 positive, PMPs tissue factor and CD 41 positive | Normalisation of glycaemic control in DM patients after bariatric surgery leads to reduction of the MPs levels | Cheng et al. [63] |
| PMPs, T-lymphocytes-MPs and leukocyte-derived MPs (surface markers: quadruple-stained with Annexin V, CD61, anti-TF, and CD15 (ligand for P-selectin), CD66e (granulocytic marker), or CD62P (P-selectin), or with CD4 (T-lymphocytes), anti-TF, and CD11b (leukocyte marker) | TF on MPs from DM patients may be involved in processes other than coagulation such as angiogenesis | Diamant et al. [64] |
| TF bearing MPs from human renal mesangial cells and human dermal microvascular endothelial cells | MPs expressing TF might be a mediator to neovascularisation due to elevated glucose levels | Ettelaie et al. [173] |
| MPs from human umbilical vein endothelial cells | Reduction of miR-126 from plasma vesicles might explain the impaired neo-angiogenesis in DM patients | Zampetaki et al. [174] |
CD: cluster of differentiation; EMP: endothelial-derived MP; PMP: platelet-derived MP; T2DM; Type 2 diabetes mellitus; GP: glycoprotein; LMP: leukocyte-derived MP; TF: tissue factor; miR: micro Ribonucleic acid; NADPH: nicotinamide adenine dinucleotide phosphate; NO: nitric monoxide.
Different types of microparticles, such as endothelial, platelet, erythrocyte and monocyte-derived microparticles have also been found to be elevated in diabetic populations [55,164–166]. Monocyte, endothelial and platelet-derived microparticles are significantly higher in diabetic patients with related vascular complications such as nephropathy, retinopathy or neuropathy compared with diabetic patients without complications [57,165,172,176].
Patients with type 1 diabetes mellitus have not only higher but also different types of circulating microparticles compared with patients with type 2 diabetes mellitus and healthy people [59]. Platelet, endothelial and apoptotic cell (Annexin V+) derived microparticles were significantly elevated in type 1 diabetes. The different microparticle phenotypes between the two conditions reflect differences in their functional and particularly their procoagulant properties. For type 2 diabetes patients, these microparticles have limited procoagulant action but for type 1 diabetes their prothrombotic properties were positively correlated with the glycaemic control [HbA1c] [59]. The relation between glycaemic control and microparticles has been demonstrated in a study of overweight subjects with type 2 diabetes. The levels of the circulating microparticles have been reduced after bariatric surgery with synchronous normalization of glycaemic control [63].
The pathophysiology of hypercoagulopathy in diabetes has been linked with the presence of microparticles [59,168,177]. Abnormal production of ROS mediated by microparticles along with inappropriate protein expression on the microparticle membrane which relates to coagulation and immune pathways have been demonstrated in vitro [168]. Tissue factor antigens on the membrane of circulating microparticles in patients with type 2 diabetes were reported by Cimmino et al. [177] and are likely to be involved in the prothrombotic activity along with other athero-inflammatory processes observed in diabetic populations. Microparticle coagulability (expressed by the density of membrane tissue factor and membrane tissue factor pathway inhibitor ratio) was found to be high in diabetic patients with severe foot ulcers and manifestations of coronary artery disease [167]. Similar findings regarding hypercoagulability of plasma microparticles in diabetic patients reported by Tripodi et al. [169] as measured and correlated by conventional coagulation tests, such as antithrombin and protein C activity and levels of factors II and VIII.
Diabetic vascular complications are associated with vascular inflammation and endothelial dysfunction [178]. Endocytosis of platelet-derived microparticles from endothelial cells in vitro induced expression of von Willebrand factor on the plasma membrane of the endothelial cells, which promoted adhesion of platelets and excessive production of ROS leading to inflammation [61]. Microparticles mediated ROS production in diabetics is involved in downregulation of NO activity affecting the vascular tone and contribute to leukocytes chemotaxis to endothelium by expression of surface antigens [138,171]. In clinical studies, type 2 diabetes, is associated with high levels of endothelial/apoptotic cell derived microparticles and asymptomatic coronary atherosclerotic disease [170].
Microparticles have been demonstrated to be involved in impaired neovascularization process in diabetic populations. Incubation of human umbilical vein endothelial cells with extracellular microvesicles from patients with diabetic foot or diabetic retinopathy induced the formation of tube networks, suggesting an important role of the vesicles in the process of angiogenesis [167]. Tissue factor positive microparticles in well controlled diabetic patients are not always involved in coagulation process and potentially have a role in signal transmission, including angiogenesis [64,173]. MiR-126, a type of RNA, which is contained in microparticles was reported to play important role in endothelial integrity and angiogenesis [179]. Reduced expression of miR-126 in endothelial-derived microparticles from diabetic patients might contribute to endothelial injury, abnormal vascular remodelling and impaired angiogenesis [174,180].
Various medications were investigated regarding their effects in the levels of circulating microparticles in diabetes. Apart from the antidiabetic drugs, anti-hypertensives, statins and anti-platelets have been demonstrated to decrease the microparticles in various populations with diabetes [181] (Table 7).
Table 7.
List of medications induced reduction of microparticle (MP) levels in diabetic populations.
| Medication category | Pharmacological name | Type of MPs investigated | Other co-morbidities apart from DM |
|---|---|---|---|
| Antiplatelet | Cilostazol [182] | PMPs GPIX positive | |
| – | Ticlopidine [183–185] | PMPs GPIX positive, Monocyte-derived MPs CD14 positive | Hyperlipidemia |
| – | Sarpogrelate [186] | PMPs | – |
| – | Aspirin & Clopidogrel [187] | PMPs CD151 positive, Monocyte-derived MPs CD14 positive | TIA |
| Antihypertensive | Efonidipine [188] | PMPs GPIX positive, Monocyte-derived MPs CD14 positive | HTN |
| – | Nifedipine [189,190] | PMPs GPIX positive, Monocyte-derived MPs CD14/Annexin V positive, EMPs CD51/Annexin V positive | HTN |
| – | Losartan [191,192] | PMPs GPIX positive, Monocyte-derived MPs CD14/ Annexin V positive, EMPs CD51/ Annexin V positive | HTN |
| – | Valsartan [193] | Monocyte-derived MPs CD14/ Annexin V positive | HTN |
| Anti-lipidaemic | Pitavastatin [194] | PMPs CD42b and CD42a (glycoprotein Ib and IX) positive | Hyperlipidemia |
| – | Pravastatin [71] | MPs derived from: platelets (CD61 positive), T-helper cells (CD4 positive), T-suppressor cells (CD8 positive), monocytes (CD14 positive), B cells (CD20 positive), endothelial cells (CD62e positive), erythrocytes (glyco-A positive) and granulocytes (CD66b positive), annexin V and TF positive MPs | Hyperlipidemia |
| – | Simvastatin [191,192] | PMPs GPIX positive, Monocyte-derived MPs CD14/ Annexin V positive, EMPs CD51/ Annexin V positive | Hyperlipidemia, HTN |
| – | Atorvastatin [195] | PMPs, surface markers CD42a (Glycoprotein IX), together with either CD61 (GPIIIa), CD62P (P-selectin) or CD142 (TF) | Hyperlipidemia |
| – | Bezafibrate [196] | PMPs CD42b positive | Hyperlipidemia |
| – | Probucol [183] | PMPs GPIX positive , Monocyte-derived MPs CD14 positive | Hyperlipidemia |
| – | Eicosapentaenoic acid [194,197,198] | PMPs CD42b and CD42a (glycoprotein Ib and IX) positive | Hyperlipidemia |
| – | Vitamin C [199] | EMPs CD31 positive, PMPs GPIb positive | Acute myocardial infarction |
| Antidiabetic | Acarbose [200] | PMPs CD42b and CD42a (glycoprotein Ib and IX) positive | – |
| – | Miglitol [201] | PMPs CD42b and CD42a (glycoprotein Ib and IX) positive | – |
| – | Pioglitazone [202] | EMPs CD31 positive | – |
CD: cluster of differentiation; HTN: hypertension; EMP: endothelial-derived MP; PMP: platelet-derived MP; GP: glycoprotein; TF: tissue factor; DM: diabetes mellitus.
5.3. Smoking
Smokers have significantly higher levels of plasma tissue factor concentrations [203]. Human monocytes and macrophages produce microparticles and demonstrated apoptotic activity in vitro, after exposure to tobacco smoking extract. These microparticles were found to be tissue factor positive, reflecting their procoagulant properties [204].
Smokers with normal spirometry but reduced diffusing capacity of the lung for carbon monoxide have also elevated levels of endothelial-derived microparticles, likely derived from apoptotic endothelial capillary cells [205]. Another mechanism which appears to be induced by tobacco smoke inhalation is microparticle gelatinolytic and collagenolytic activities. After exposure to tobacco smoking extract in vitro, human macrophages produce microparticles with transmembrane MMP14. These microparticles may mediate extracellular matrix destruction leading to inflammation, atherosclerotic plaque vulnerability and tissue necrosis [206]. Elevation of endothelial-derived microparticles was also observed in passive smokers along with endothelial dysfunction, as assessed by flow-mediated dilation using ultrasound. In the same study, the endothelial progenitor chemotaxis towards vascular endothelial growth factor was impaired resulting in reduction of nitric oxide production and endothelial dysfunction [207].
5.4. Dyslipidaemias
Endothelial-derived microparticles were found to be elevated in patients with uncomplicated type 2 diabetes mellitus after consumption of high-fat meals. This elevation was correlated with other dysmetabolic changes, such as high levels of glucose, insulin, and triglycerides and low levels of high-density lipoprotein [208]. Hypercholesterolaemia along with endothelial-derived microparticles has inhibitory activity in cardiac angiogenetic mechanisms through imbalance of the endothelial nitric oxide synthetase regulation [209]. Hypercholesterolaemic conditions may induce endothelial damage associated with microparticles but there is evidence that can also promote generation of prothrombotic vesicles. Aggregated low-density lipoprotein was found to induce release of tissue factor positive microparticles from human vascular smooth muscle cells [210]. Similarly, monocytes enriched by cholesterol in vitro, appear to expose phosphatidylserine on their cellular membrane along with induction of apoptosis and release increased levels of tissue factor positive microparticles [46]. Additionally, phosphatidylserine expression mediated by oxidative low-density lipoprotein in diabetic population was associated with elevated levels of platelet-derived microparticles [211].
The relation between the endothelial protective role of high-density lipoprotein and microparticles has been investigated [43,212]. High-density lipoprotein was reported to inhibit the binding of the T cell-derived microparticles to monocytes and sequelae monocyte activation. Furthermore, high-density lipoprotein partially inhibited production of pro-inflammatory cytokines from monocytes.
In several studies which recruited patients with type 2 diabetes and hyperlipidaemias (Table 5) there was a reduction of the circulating microparticles after treatment with statins. For example, in patients with type 1 diabetes mellitus and hyperlipidaemia, treatment with atorvastatin reduced GpIIIa, P-selectin- and tissue factor-containing microparticles [195]. For patients with type 2 diabetes and hyperlipidaemia, treatment with pravastatin for 8 weeks did not alter significantly the blood cholesterol concentrations but reduced the GpIIb/IIIa membrane receptor in the circulating platelet-derived microparticles. GpIIb/IIIa is an important receptor for fibrinogen involved in thrombus formation. Downregulation of this receptor which is possibly induced by changes to platelets and microparticles lipids membrane composition might contribute to less thrombotic risk [71]. Fluvastatin reduced microparticles in vitro from cultured human coronary artery endothelial cells by inhibition of the Rho kinase pathway which is responsible for alteration of cytoskeleton [213].
High triglycerides levels are a component of metabolic syndrome among with hyperinsulineamia, hypertension and low high-density lipoprotein cholesterol levels [214]. Elevation of endothelial [215], platelet [216] and leukocytes-derived microparticles [217] in patients with metabolic syndrome contribute to vascular inflammation and hypercoagulant status which promote atherosclerosis and thrombosis [141]. In an observational study [218] participating patients with metabolic syndrome, dyslipidaemia was associated with higher levels of endothelial-derived microparticle (CD144+) and erythrocyte microparticle (CD235a+) compared with healthy control group. Obesity influenced the levels of platelet (GpIIb/IIIa+) and endothelial-derived microparticles and hypertension only the endothelial-derived microparticles. Levels of Annexin V + microparticles were affected by each of the different components of metabolic syndrome.
6. Microparticles and cardiovascular diseases
6.1. Atherosclerosis
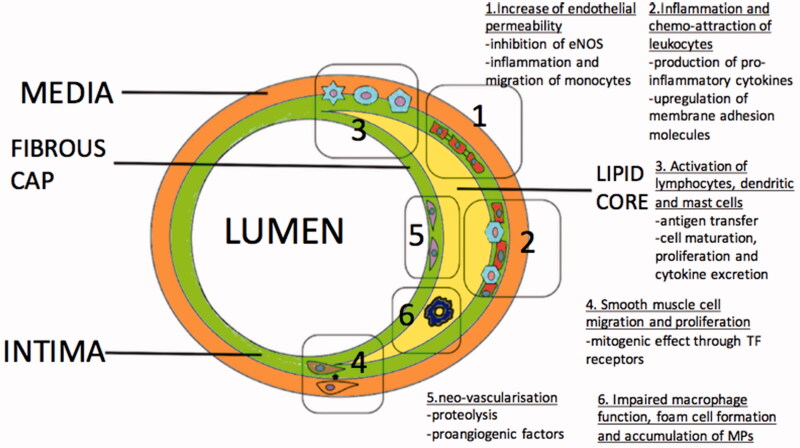
Various mechanisms contributing to initiation, progression and clinical manifestation of atherosclerotic disease are associated with the presence and formation of microparticles (Figure 5). The disruption of the normal levels and composition of the microparticles might represent one of the initial steps of the atherosclerotic disease. The link between apoptosis and microparticle production is well-established [142]. Reduced laminar shear stress is also reported as a signal for endothelial apoptosis [219] which potentially contributes to microparticle production and imbalance of the normal endothelial features [220].
Figure 5.
Mechanisms associated with initiation and progression of atherosclerosis mediated by Microparticles (MPs).
Endothelial permeability occurs during the first phase of the atherosclerotic process [221]. Findings suggest a link between endothelial permeability and microparticles. Injection of endothelial-derived microparticles in rats significantly increases the pulmonary capillary permeability and causes acute lung injury. This action is likely mediated through inhibition of nitric oxide generation and sequelae impaired vasodilation [222]. Abnormal endothelial homeostasis related with nitric oxide production has been reported with endothelial-derived microparticles [136] and T lymphocyte-derived microparticles in vitro [138]. Another mechanism, mediated by CD54, might play a role in atherogenesis. It was described in patients with multiple sclerosis where endothelial-derived microparticle CD54+ induces inflammation and increasing migration of monocytes through the endothelium [223]. Additionally, platelet-derived microparticles can affect the endothelial cell barrier integrity, in a manner related with their size and protein composition [224].
Chemo-attraction of leukocytes to the inflamed endothelial segment is essential for the progression of atherosclerosis [225]. Microparticles from different cellular origins may trigger production of pro-inflammatory cytokines from the endothelium, such as IL-6 and IL-8, which attract and activate leukocytes [91,226]. Another suggested mechanism which contributes to the progression of the atheroma is linked to microparticles-induced expression of adhesion molecules on the endothelial cells. An example is platelet-derived microparticles mediated upregulation of intercellular adhesion molecule-1 on the endothelial cell membrane [91,227]. Plaque microparticles isolated from endarterectomy specimens could transfer ICAM-1 to endothelium [228]. Also microparticles induce integrin expression on the surface of the leukocytes, such as CD11a and CD11b, which interact with intercellular adhesion molecule-1[91]. Chemokines delivered from microparticles to inflamed or atherosclerotic endothelium promote further leukocyte recruitment. Mause et al. described a platelet-derived microparticle-associated delivery of the chemokine regulated on activation, normal T cell expressed and secreted (RANTES) to human microvascular endothelial cells which promotes monocyte adhesion [83].
Microparticles concentrations are 200 times higher in atherosclerotic plaques than in blood [229]. Microparticles derived from leukocytes have higher levels in plaques; 29% found to be from macrophages, 15% from lymphocytes and 8% from neutrophils. Other significant populations of microparticles concentration delivered from erythrocytes (27%), smooth muscle (13%) and endothelial cells (8%) [229]. Microparticle origin in atherosclerotic plaques was not found to be affected by symptoms of ischaemia [229]. All plaque microparticles, regardless their origin, possess pro-coagulant activity as they express tissue factor on their external plasma membrane surface [230].
Furthermore, atherosclerotic plaques microparticles were described that contain immunoglobulins. The immunoglobulins they expressed were found to be different from the plasma circulating microparticles [231]. Co-labelling of IgG and CD14 demonstrated that the vast majority of microparticles (93 ± 7%) containing IgG were CD14+, revealing their macrophage origin [231]. High macrophage infiltration was observed also in raptured atherosclerotic lesions with concurrent macrophage apoptosis [232]. Microparticles are involved in macrophage apoptosis but are unknown if this mechanism contributes to rapture or characterize vulnerability of the fibrous cap of the atheromatous plaques [146]. Additionally, as macrophages have been involved in the clearance of microparticles, defective phagocytosis due to increased macrophage apoptosis may lead to accumulation of microparticles [233–235]. A point towards this hypothesis is the acceleration of atherosclerotic lesions in mice which lack lactadherin activity [236]. Lactadherin is essential protein for the removal of the microparticles [237].
Atherosclerosis is a complex immune-inflammatory disease which several types of cells are involved, including lymphocytes (T, B, Natural killer T), dendritic cells and mast cells [238]. In vitro, endothelial-derived microparticles induced dendritic cell maturation and secretion of pro-inflammatory cytokines, contributing to CD4 T cells activation and proliferation [239]. In the same study, microparticles from activated T cells or platelets failed to stimulate dendritic cell maturation [239]. Polymorphonuclear neutrophil-derived microparticles were reported to interact with human monocyte-derived dendritic cells and to promote morphologic changes which reduce monocyte phagocytic activity and increase cytokines excretion [240]. Additionally, microparticles from activated dendritic cells can interfere with resting dendritic cells and transfer antigens to them [98]. These antigens can be presented to lymphocytes leading to their activation and proliferation [98]. Microparticles from T cells may also stimulate mast cells. This action can take place by transfer of membrane biomolecules via microparticles instead of cellular contact [241].
Smooth muscle cell proliferation and migration from the media to intima is essential for the formation of the atheroma [225]. Platelet-derived microparticles were found to have mitogenic effect on smooth muscle cell in vitro, with no chemotactic contribution [242]. The mitogenic effect increased synergically if platelet-derived microparticles were combined with serotonin or thromboxane A2 [243]. The chemotaxis for smooth muscle cell migration is strongly mediated by tissue factor receptors [244,245]. Microparticles isolated from atherosclerotic plaques express tissue factor in their surface [230] and might act as a chemo-attractive factor for smooth muscle cell migration and proliferation.
The intraplaque neovessel formation due to hypoxia and inflammation influences the stability of the atherosclerotic lesion [246,247]. Microparticles might play a significant role in the neovascularization. They can carry proteolytic enzymes on their membrane and they have ability to induce the production of metalloproteases from other cells, so the endothelial tissue would be able to penetrate the surrounding interstitial matrix [248–250]. Furthermore, plaque microparticles mainly of macrophage origin can induce endothelial cell proliferation in vivo [251]. On the contrary, in this study circulating microparticles were unable to induce endothelial cell proliferation [251]. CD40/CD40 ligand system appears to play an important role in the microparticles endothelial cell interaction [251]. Patients with symptoms of cardiac ischaemia expressed more CD40L than asymptomatic patients and their microparticles were more potent endothelial proliferation inducers in vitro [251].
Studies of patients with early atherosclerosis and chronic coronary artery disease showed increased levels of CD144+/CD31+ endothelial-derived microparticles expressing T-cadherin compared with healthy volunteers. T-cadherin was found to reflect endothelial dysfunction as measured by reactive hyperaemia following brief peripheral flow occlusion [252]. Plasma levels of CD144+/CD42b-endothelial-derived microparticles predicted the presence of coronary artery disease in asymptomatic diabetic patients [253].
6.1.1. Acute coronary syndromes
In the setting of acute coronary syndromes, the most studied microparticles are platelet-derived microparticles and endothelial-derived microparticles. Observational studies showed increased levels of CD146+ and CD31+ endothelial-derived microparticles (Table 8) in patients with acute coronary syndrome compared to patients with stable angina or with no coronary artery disease [254,255]. Acute coronary syndromes are associated with raised thrombotic activity. Morel et al. described that patients with ST elevation myocardial infarction and unstable angina had higher levels of procoagulant microparticles compared with patients with stable angina. The principal population of procoagulant circulating microparticles were platelet-derived microparticles GPIb + and endothelial-derived microparticles CD31 + [256]. The majority of endothelial-derived microparticles expressed the pro-atherogenic adhesion molecule, vascular cell adhesion molecule-1 (VCAM-1) [257].
Table 8.
Studies with microparticles (MPs) in acute coronary syndrome populations (ACS).
| Type of MPs | Conclusion | References |
|---|---|---|
| Surface markers: Anexin V, anti-CD3, anti-CD11a, anti-CD31, anti-CD146 and anti-GP Ib | Higher levels of procoagulant EMPs in Patients with ACS compare with patients with no CAD or SA | Mallat et al. [254] |
| Combination of CD31 (PECAM-1) or CD51 (aV□3, vitronectin receptor) with CD42. EMPs and PMPs (PMPs CD42 positive) | EMPs were elevated in patients with CAD compared with control subjects, CD31□EMPs higher in patients with ACS compare with SA, among patients with a first MI, CD31 EMPs released in ACS and CD51 released in SA. | Bernal-Mizrachi et al. [255] |
| Leukocyte-derived MP CD11a positive , EMPs CD31 positive, PMPs GPIb positive, Anexin V positive | Early decrease of procoagulant MPs in patients with STEMI treated with PPCI and abciximab compare with patients treated only with PPCI. | Morel et al. [256] |
| EMPs CD146 and CD 106 (VCAM-1) positive | High density of VCAM-1 expressed on EMPs from patients with ACS | Radecke et al. [257] |
| Annexin V-binding MPs, MPs CD42b positive, EMPs CD144 positive and monocyte-derived MPs CD14 positive | In NSTEMI, EMP and monocyte-derived MPs were independently predictive for future admissions related to heart failure and PMPs for major bleedings | Montoro-García et al. [258] |
| EMPs CD144 positive, PMPs CD41 positive and leukocyte-derived MPs CD45 positive | Variation of levels of different origin MPs associated with time in patients before and after PPCI | Zhou et al. [259] |
| Surface markers: anti- CD11a (leukocytes), anti-CD31 (endothelial cells), anti-CD42b (endothelial cells), and anti-CD146 (platelets), Anexin V for apoptotic MPs | Increased levels of MPs in culprit coronary arteries after STEMI and significant reduction after successful PCI | Min et al. [260] |
| Lymphocyte-derived MPs CD45/CD3 positive, monocyte-derived MPs CD14 positive, EMPs CD146 positive or CD62e positive, granulocytes-derived MPs CD66b positive, other surface markers: CD142 (TF), Anexin V, CD31 | Increased levels of MPs in culprit coronary arteries after STEMI, levels of MPs positively correlated to time of revascularisation post STEMI | Suades et al. [261] |
| EMPs CD31 positive/CD42 negative, PMPs CD31 positive/CD42 positive, anexin V positive | ACS are associated with higher levels of circulating MPs compare with SA patients. In SA the degree of atherosclerotic plaque is not related with the levels of plasma MPs | Biasucci et al. [262] |
| TF positive MPs | Thrombolysis failure in acute MI is associated with higher levels of procoagulant MPs | Huisse et al. [263] |
| TF positive MPs | MPs were lower as the severity of the stable angina/ACS was increasing (stable CAD vs UA vs MI) | Maly et al. [264] |
| PMPs CD42 positive, Leukocyte-derived MPs CD45 positive, Monocyte-derived MPs CD14 positive, EMPs CD31, CD51/61, CD34 positive and activated tissue factor positive (TF) MPs | Increased levels of MPs in ACS and positive correlation of MP levels with platelet activation markers | Stępień et al. [58] |
| EMPs CD31 positive/ CD42negative and CD144 positive, PMPs CD31/CD42 positive | EMPs and PMPs reflect the size of myocardium at risk in patients with STEMI | Jung et al. [265] |
CD: cluster of differentiation; EMP: endothelial MP: STEMI: ST elevation myocardial infarction: PPCI: primary percutaneous coronary intervention; TF: tissue factor; PMP: platelet-derived MP; CAD: coronary artery disease; NSTEMI: non-ST elevation myocardial infarction; SA: stable angina; VCAM-1: vascular cell adhesion protein 1; PECAM-1: platelet endothelial cell adhesion molecule-1.
Revascularization affects the levels of the circulating microparticles as reduces the endothelial injury. In a cross-sectional study, a significant reduction in Annexin V-binding and endothelial microparticles observed after primary percutaneous coronary intervention (PCI) in ST elevation myocardial infarction patients, compared to patients with stable coronary artery disease or non-ST elevation myocardial infarction before PCI [258]. Zhou et al. [259] investigated the levels of three groups of microparticles (endothelial-derived microparticles CD144+, platelet-derived microparticles CD41+ and leukocyte-derived microparticles CD45+) during the acute phase of ST elevation myocardial infarction/primary PCI and 48 h later. Platelet-derived microparticles increased immediately post PCI and reached the maximum levels 48 h later. Endothelial-derived microparticles and CD45+ microparticles decreased immediately post PCI and gradually increased up to 48 h later [259]. Abciximab, an GPIIb-IIIa antagonist, in combination with primary coronary angioplasty reduces the level of platelet-derived microparticles compared with patients that had only percutaneous angioplasty [256]. Failure of revascularization strategy, such as thrombolysis, induced higher levels of procoagulant microparticles (tissue factor + microparticles) [263].
Differences were also detected in the levels of circulating Annexin V + and tissue factor + microparticles as regard the vascular level of origin. Microparticles were elevated in the culprit artery compared with the peripheral blood in patients with ST elevation myocardial infarction after primary PCI, suggesting local vascular damage [260,261].
Biasucci et al. [262] demonstrated a relation between high-sensitivity C-reactive protein (hs-CRP) endothelial and platelet-derived microparticles levels in patients with stable coronary artery disease and acute coronary syndrome. The Annexin V + microparticles were related to the troponin T levels and the degree of myocardial injury. No significant differences were detected regarding type of acute coronary syndrome (Non-ST elevation myocardial infarction vs. ST elevation myocardial infarction) and the levels of circulating microparticles [262]. Endothelial-derived microparticles and platelet-derived microparticles were also found to be related with the extension of the MI [265].
6.1.2. Peripheral vascular disease and ischaemic stroke
Endothelial-derived microparticles were found to be elevated in patients with peripheral arterial disease and particularly microparticles expressing the monomeric CRP molecule on their membrane, suggesting the inflammatory nature of the condition [266]. High levels of leukocyte-derived microparticles (CD11a+) were also found in patients with non-symptomatic atherosclerotic disease demonstrated by ultrasound examination of carotid, abdominal aorta and femoral arteries [217]. Leukocyte (CD11a+) along with endothelial-derived microparticles (CD105+) observed to be associated with higher carotid intima-media thickness in patients before atherosclerotic disease is evident [267]. Platelet-derived microparticles (CD63+) were also raised in patients with peripheral arterial disease with or without myocardial infraction [268] and after bypass grafting, reflecting the thrombotic nature of the disease. Certain cytokines, such as IL-6, G-CSF and thrombopoietin were also detected to be high in patients with peripheral arterial disease and may related with microparticle formation from platelets [269].
Patients with recent ischaemic stroke were found to have higher levels of CD62E + endothelial-derived microparticles. More severe strokes, classified by National Institute of Health Stroke Scale score, were associated with higher CD62E + endothelial-derived microparticle levels, reflecting the severity of the endothelial damage and the degree of endothelial activation. From the same study, patients without stroke but with significant risk factors for atherogenesis and extracranial arterial disease had higher CD62E + endothelial-derived microparticle levels, in contrast to patients with intracranial arterial stenosis where the CD31+/CD42b − and CD31+/Annexin V + endothelial-derived microparticle subpopulation levels were raised [270]. Simak et al. [271] investigated the levels of endothelial-derived microparticles in acute stroke patients using flow cytometry. Endoglin-positive endothelial-derived microparticles (CD105+/CD41a−/CD45−), endothelial-derived microparticles expressing VE-cadherin and endoglin (CD105+/CD144+), phosphatidylserine(CD105+/phosphatidylserine+/CD41a−) and Intercellular Adhesion Molecule-1(CD105+/CD54+/CD45−) were analysed. Only phosphatidylserine + endothelial-derived microparticles were significantly higher in the acute stroke group compared with the control. All the endothelial-derived microparticles subtypes were elevated in patients suffering moderate to severe stroke (according to National Institutes of Health Stroke Scale). Significantly elevated Endogline and Intercellular Adhesion Molecule-1 positive endothelial-derived microparticles on admission were associated with worse prognosis. Apart from endothelial-derived microparticles, patients with acute ischaemic stroke were found to have increased levels of platelet-derived microparticles (GpIIIa+) during the acute phase and up to 6 months later, compared with healthy controls [272], suggesting that thrombotic tendency might last more than the acute phase of the infarct.
6.2. Heart failure
For patients with non-ST elevation myocardial infarction, elevated endothelial-derived microparticles and monocyte-derived microparticles were associated with higher readmission rates from heart failure (Table 9) [258]. Different factors may affect the levels and the origin of microparticles in heart failure patients. Endothelial-derived microparticles (CD31+/Annexin V+) were higher in patients with 3 vessel coronary artery disease and Heart failure with reduced ejection fraction compared with patients with same degree of coronary artery disease but preserved Left Ventricular systolic function [274]. The same type of microparticles was higher in patients with heart failure with reduced left ventricular ejection fraction and increased body mass index (BMI > 25 kg/m2) compared with other patients with lower BMI [275]. Apoptotic Annexin V + microparticles were found to be elevated in heart failure patients with worse functional status (dyspnoea class III–IV of New York Heart Association scale) [273].
Table 9.
Studies with microparticles (MPs) in heart failure.
| Type of MPs | Conclusion | References |
|---|---|---|
| Annexin V-binding MPs, MPs CD42b positive, EMPs CD144 positive and monocyte-derived MPs CD14 positive | In NSTEMI, EMP and monocyte-derived MPs were independently predictive for future admissions related to heart failure | Montoro-García et al. [258] |
| Annexin V-binding MPs, MPs CD42b positive, EMPs CD144 positive | Annexin V-MPs levels appear to reflect accurately the acute decompensated phase in systolic heart failure | Montoro-García et al. [273] |
| CD 31/Annexin V positive | EMPs was increased in patients with LV dysfunction compared with those with normal or preserved LV function. | Bulut et al. [274] |
| Surface markers for EMPs: CD31 (platelet endothelial cell adhesion molecule [PECAM]-1), CD144 (vascular endothelial [VE]-cadherin), CD62E (E-selectin) and annexin V | EMPs levels are higher in patients with HFrEF and increased Body Mass Index (BMI > 25kg/m2) compare with other patients with lower BMI. | Berezin et al. [275] |
| Surface markers for EMPs: CD31, CD62E, CD105, Annexin V | Adding CD31/annexin V positive EMPs to CD14/ CD309 positive cell ratio to other variables predict the risk of HFpEF. | Berezin et al. [56] |
| EMPs CD31/CD62E positive | EMPs in post-transplant patients are consistent with increased apoptotic activity | Garcia et al. [276] |
| Red blood cell-derived MPs (CD235 positive), leukocyte MPs (CD45 positive), platelet (CD31/CD41positive) and EMPs (CD31 positive/CD41 negative, CD62e positive, CD144 positive) | All types of MPs were elevated in patients with LVAD compare with patients with CAD and healthy subjects due to haemolysis and disturbed endothelial integrity | Sansone et al. [62] |
| EMPs | LVAD do not cause vascular damage 3 months after implantation. | Ivak et al. [277] |
| Surface markers for EMPs: CD31, CD41a, CD64, CD105, CD144, CD62E and Annexin V. PMPs CD41a positive | Patients with HF and metabolic syndrome have higher levels of CD31/Annexin V positive EMPs in addition with lower levels of CD62E positive EMPs compare with healthy subjects. | Berezin et al. [60] |
| Surface markers: CD31, CD144, CD62E, CD14, CD45, Annexin V, CD62P, CD42b | Patients with PPCM appears to have elevated levels of activated EMPs (CD144 positive) and PMPs (CD42b positive) compare with patients with ischaemic cardiomyopathy | Walenta et al. [278] |
CD: cluster of differentiation; EMP: endothelial-derived MP: NSTEMI: non-ST elevation myocardial infarction; PMP: platelet-derived MP; HFrEF; heart failure with reduced ejection fraction; HFpEF: heart failure with preserved ejection fraction; LV: left ventricle; LVAD: LV assisting device; CAD; coronary artery disease; BMI: body mass index; PPCM: postpartum cardiomyopathy.
Subjects with heart failure and preserved left ventricular ejection fraction were found to have lower ratio of CD31+/Annexin V + endothelial-derived microparticles to EPC (as expressed by CD14+/CD309+ and CD14+/CD309+/Tie-2+) in comparison with Heart failure with reduced ejection fraction patients. That ratio in combination with pro-BNP levels was demonstrated to have good discriminatory value between heart failure with reduced and preserved ejection fraction [56].
Few studies were conducted to assess the differences of various microparticles levels among patients with end stage heart failure requiring left ventricular assisting devices or heart transplantation. From an observational study [276], heart transplant patients were compared with heart failure patients and healthy controls regarding the levels of endothelial-derived microparticles (CD62 E + and CD31+). Heart failure patients had higher numbers of CD62+ endothelial-derived microparticles compared with healthy and post-transplant patients. Apoptotic microparticles, based by the ratio of CD62E/CD31 (apoptosis = low ratio), were higher in transplant patients. More microparticle populations investigated in a study that enrolled heart failure patients, patients with left ventricular assisting devices and patients post heart transplant. Left ventricular assisting devices were associated with elevated levels of all microparticles types suggesting erythrocyte destruction and endothelial activation. In patients with heart transplant post left ventricular assisting devices, all microparticles levels were decreased [62]. The endothelial damage, as expressed with phosphatidylserine + microparticles, 3 months post left ventricular assisting device implantation, demonstrated to be lower compared to pre-implant period in heart failure patients, regardless the aetiology of heart failure [277].
Patients with congestive heart failure and metabolic syndrome were demonstrated to have higher levels of CD31+/Annexin V + endothelial-derived microparticles in addition with lower levels of CD62E + endothelial-derived microparticles compared with healthy subjects. The ratio of CD62E + to CD31+/Annexin V + endothelial-derived microparticles was significantly lower among the patients with heart failure and metabolic syndrome in contrast to patients with metabolic syndrome but without heart failure and healthy subjects [60].
6.3. Atrial fibrillation
Patients with atrial fibrillation have increased risk of ischaemic stroke and were found to have higher levels of platelet-derived microparticles (CD42b+/CD61+) in contrast with healthy subjects. There was no difference in the levels of circulating platelet-derived microparticles between patients with permanent or paroxysmal atrial fibrillation or between patients on treatment with aspirin or warfarin. In the same study, there were no significant differences in platelet-derived microparticles for patients in atrial fibrillation and patients with established coronary artery disease and sinus rhythm [279]. In another observational study, Annexin V + microparticle levels were higher in atrial fibrillation patients compare with healthy controls and with patients with sinus rhythm but cardiovascular risk factors (disease control). On the contrary, platelet-derived microparticles (anti-glycoprotein Ib+) and endothelial-derived microparticles (CD31+) were similar in patients with AF and disease control group but higher compared with healthy control subjects [280].
In patients with chronic AF due to mitral stenosis CD41+ platelet-derived microparticles levels were higher compared to healthy controls and there was a significant direct relationship between the mitral valve area and the levels of circulating platelet-derived microparticles [281], suggesting higher risk of thromboembolic events with increasing severity of mitral stenosis.
Induction of AF during electrophysiology studies increased the P-selectin + microparticles compared with chronic AF and control patients which might reflect different procoagulant mechanism between chronic and paroxysmal AF [282].
6.4. Pulmonary hypertension
Diehl et al. [283] reported increased levels platelet (CD31+/CD61+), Leukocyte (CD11b+) and endothelial-derived microparticles(CD62E+) in pulmonary hypertensive patients compared with healthy subjects, suggesting that the mechanisms which are involved in the progression of the disease are associated with platelet activation, inflammation and endothelial dysfunction [284]. In another study [284], several types of microparticles [platelet (CD31+/CD41+), endothelial (CD62E+, CD144+, CD31+/CD41−), leukocyte (CD45+) and apoptotic (Annexin V +) microparticles] were analysed from patients with precapillary pulmonary hypertension before any treatment with endothelium-active vasodilator therapy. Endothelial and leukocytes-derived microparticles levels were higher among pulmonary hypertension patients compared to healthy control group. CD31+ and CD144+ microparticles levels were strongly associated with the haemodynamic severity of the disease.
Results from an observational study [285] with paediatric patients and Eisenmenger syndrome suggested that CD144+ endothelial-derived microparticles along with the thickness of pulmonary artery intima media and pulmonary stiffness can be used as non-invasive alternative tests to monitor the progression of the disease. Lin et al. [286] also reported elevated endothelial-derived microparticles (CD31+/CD42b−) in adult patients with pulmonary hypertension secondary to atrial or ventricular septal defect.
A possible link between endothelial-derived microparticles and increased phosphorylation of the P38 mitogen-activated protein kinases, was demonstrated in a rat model, which can induce inflammatory process along with impaired function of endothelial NO synthetase. Endothelial-derived microparticles (endoglin+) from rats with advanced pulmonary hypertension can induce expression of Intercellular Adhesion Molecule-1 in pulmonary artery endothelial cells and Intercellular Adhesion Molecule-1 can promote inflammation [287]. Endoglin + microparticles were also found to be elevated in patients with pulmonary hypertension due to chronic thromboembolic disease compared to healthy control subjects. This might represent a protective/reactive mechanism as in vitro endoglin promoted angiogenesis and increased the life of cultured endothelial cells [288].
7. Prognostic value of microparticles
Several studies have demonstrated an association between microparticles and outcomes in patients with cardiovascular diseases (Table 10). Coronary artery disease is the most studied condition. From an observational study that recruited patients with cardiovascular risk factors and stable coronary artery disease, CD31+/Annexin V + microparticles (apoptotic endothelial and platelet-derived microparticles) levels were correlated with higher risk of major adverse cardiovascular and cerebrovascular events and the need for revascularization [289]. In patients with ST elevation myocardial infarction post primary angioplasty, high levels of monocyte-derived microparticles were strongly related with poor long term survival [290]. Additionally, post angioplasty levels of erythrocyte-derived microparticles in ST elevation myocardial infarction patients were higher compared to healthy individuals and associated with higher risk of major adverse cardiovascular and cerebrovascular events [291]. Increased tissue factor + microparticle levels were also related with poor outcomes in patients with acute coronary syndrome (unstable angina or acute MI) [292].
Table 10.
Studies where microparticle levels are associated with outcomes.
| Type of MPs | Prognostic value | References |
|---|---|---|
| Annexin V-binding MPs, MPs CD42b positive, EMPs CD144 positive and monocyte-derived MPs CD14 positive | In NSTEMI, EMP and monocyte-derived MPs were independently predictive for future admissions related to heart failure | Montoro-García et al. [258] |
| EMPs apoptotic CD31/Annexin V positive | CD31/Annexin V positive MPs is an independent predictor of MACCE in stable CAD patients | Sinning et al. [289] |
| Monocyte-derived MPs (surface markers: CD14+, CD14/CD11b and CD14/CD142) | Monocyte-derived MPs levels assessed in the acute phase of STEMI are related to the prognosis of long term CV death | Chiva-Blanch et al. [290] |
| Annexin V positive, CD41 positive PMPs, CD235a positive erythrocyte derive MPs | CD235a positive erythrocyte derive MPs concentrations appear to be positively associated with MACCE in patients with STEMI and PPCI | Giannopoulos et al. [291] |
| Surface markers: Annexin V, TF , CD41, CD66b | In the plasma of patients with ACS, the TF activity is a consequence of circulating MPs and is an independent predictor for MACCE | Steppich et al. [292] |
| EMPs CD146, CD31 | Higher EMP levels had a higher risk of MACCE in ACS patients. | Fan et al. [293] |
| EMPs CD31/Annexin V positive | EMPs levels were associated with increased mortality and recurrent hospitalization due to CHF | Berezin et al. [294] |
| Apoptotic MPs Annexin V positive | elevation of apoptotic MP levels in LVAD-supported patients are associated with increased risk for adverse events. | Nascimbene et al. [295] |
| EMPs CD62e positive, CD144 positive, CD31 positive (+)/CD41 negative leukocytes-derived MPs CD45 positive | Elevated levels of CD62e positive EMPs in PHTN patients prior to treatment are associated with adverse clinical events | Amabile et al. [296] |
MACCE: major adverse cardiovascular and cerebral event; CD: cluster of differentiation; EMP: endothelial-derived MP; NSTEMI: non-ST elevation myocardial infarction; CAD: coronary artery disease; STEMI: ST elevation myocardial infarction; CV: cardiovascular; TF: tissue factor; ACS: acute coronary syndrome; LVAD: left ventricular assisting device; PHTN: pulmonary hypertension.
Montoro-Garcia et al. [258] reported that in patients with non-ST elevation myocardial infarction, levels of endothelial and monocyte-derived microparticles were associated with a higher risk of future admissions due to left ventricular failure and levels of platelet-derived microparticles with a higher risk of major haemorrhagic events [258]. Higher counts of endothelial-derived microparticles were also associated with worse outcomes in patients who presented with cardiac sounding chest pain and underwent coronary angiography for further evaluation [293].
Apart from coronary artery disease, several studies reported association between microparticles and outcomes for patients with heart failure. In acute decompensated heart failure due to coronary artery disease the levels of endothelial-derived microparticles (CD31+/Annexin V+) were related with increased mortality within the 3-year follow up period [294]. Bulut et al. [274] reported similar findings in patients with ischaemic cardiomyopathy. The ratio of EPC to endothelial-derived microparticles (CD31+/Annexin V+) was decreased in the cardiomyopathy group compared with patients with Table 3 vessel coronary artery disease and preserved left ventricular systolic function, suggesting that apart from endothelial dysfunction there is impaired vascular repair capacity. Worse outcomes were also described in patients with left ventricular assisting devices and elevated phosphatidylserine/Annexin V + microparticles [295]. Finally, patients with pre-capillary pulmonary hypertension prior to treatment, high levels of CD62E + endothelial-derived microparticles were associated with higher risk of death and hospitalization due to right heart failure [296].
8. Conclusion
Microparticles appear to be key players in important biological functions and to have fundamental role in many cardiovascular pathophysiological mechanisms. Abnormal production of microparticles as result of different risk factors leads to inflammation, thrombosis and endothelial damage (Figure 6). The consequences of these pathological processes are the manifestation of cardiovascular disorders. Alternative, microparticles may be generated due to various cardiovascular conditions and their levels reflect disease activity and severity. As the knowledge of their functions expands, their role as biomarker is rising. Research data suggest that levels of microparticles also indicate prognosis. An important issue, however, is that there is no standardized analysis technique for microparticles which results in a large diversity of data. This makes difficult to compare results from different analyses and each study should be interpreted separately. Beyond that limitation, microparticles seem to move slowly from research towards clinical level. However, further research is needed in order to guide clinicians how to use microparticles as biomarkers in several cardiovascular diseases and tailor treatments according to individual response and prognosis.
Figure 6.
Cardiovascular diseases and microparticles. Flow chart which summarises the role of microparticles in the genesis (related with several risk factors) and manifestation of cardiovascular diseases. Utilisation as biomarkers to assess disease activity/severity, prognosis and treatment guidance is emerging as detection and enumeration methods for microparticles are improving.
Disclosure statement
CV and ES: None declared.
GYHL: Consultant for Bayer/Janssen, BMS/Pfizer, Biotronik, Medtronic, Boehringer Ingelheim, Novartis, Verseon and Daiichi-Sankyo. Speaker for Bayer, BMS/Pfizer, Medtronic, Boehringer Ingelheim, and Daiichi-Sankyo. No fees are directly received personally.
References
- 1.Conde-Vancells J, Rodriguez-Suarez E, Embade N, et al. . Characterization and comprehensive proteome profiling of exosomes secreted by hepatocytes. J Proteome Res. 2008;7:5157–5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Pol E, Böing AN, Harrison P, et al. . Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev. 2012;64:676–705. [DOI] [PubMed] [Google Scholar]
- 3.Wu ZH, Ji CL, Li H, et al. . Membrane microparticles and diseases. Eur Rev Med Pharmacol Sci. 2013;17:2420–2427. [PubMed] [Google Scholar]
- 4.Shantsila E, Kamphuisen PW, Lip G. Circulating microparticles in cardiovascular disease: implications for atherogenesis and atherothrombosis. J Thromb Haemost. 2010;8:2358–2368. [DOI] [PubMed] [Google Scholar]
- 5.Dinkla S, Brock R, Joosten I, et al. . Gateway to understanding microparticles: standardized isolation and identification of plasma membrane-derived vesicles. Nanomedicine (Lond). 2013;8:1657–1668. [DOI] [PubMed] [Google Scholar]
- 6.Théry C, Witwer KW, Aikawa E, et al. . Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pilzer D, Gasser O, Moskovich O, et al. . Emission of membrane vesicles: roles in complement resistance, immunity and cancer. Springer Semin Immun. 2005;27:375–387. [DOI] [PubMed] [Google Scholar]
- 8.The fourth international meeting of ISEV, ISEV2015. J Extracell vesicles. 2015;4:27783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elmore S Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteomics. 2010;73:1907–1920. [DOI] [PubMed] [Google Scholar]
- 11.van der Pol E, Böing AN, Gool EL, et al. . Recent developments in the nomenclature, presence, isolation, detection and clinical impact of extracellular vesicles. J Thromb Haemost. 2016;14(1):48–56. [DOI] [PubMed] [Google Scholar]
- 12.Beyer C, Pisetsky DS. The role of microparticles in the pathogenesis of rheumatic diseases. Nat Rev Rheumatol. 2010;6:21–29. [DOI] [PubMed] [Google Scholar]
- 13.Flaumenhaft R, Dilks JR, Richardson J, et al. . Megakaryocyte-derived microparticles: direct visualization and distinction from platelet-derived microparticles. Blood. 2008;113:1112–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moulin VJ, Mayrand D, Messier H, et al. . Shedding of microparticles by myofibroblasts as mediator of cellular cross-talk during normal wound healing. J Cell Physiol. 2010;225:734–740. [DOI] [PubMed] [Google Scholar]
- 15.Castellana D, Zobairi F, Martinez MC, et al. . Membrane microvesicles as actors in the establishment of a favorable prostatic tumoral niche: a role for activated fibroblasts and CX3CL1-CX3CR1 axis. Cancer Res. 2009;69:785–793. [DOI] [PubMed] [Google Scholar]
- 16.Beaudoin AR, Grondin G. Shedding of vesicular material from the cell surface of eukaryotic cells: different cellular phenomena. Biochim Biophys Acta. 1991;1071:203–219. [DOI] [PubMed] [Google Scholar]
- 17.Zhang HC, Liu XB, Huang S, et al. . Microvesicles derived from human umbilical cord mesenchymal stem cells stimulated by hypoxia promote angiogenesis both in vitro and in vivo. Stem Cells Dev. 2012;21:3289–3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen YW, Chen YC, Wang JS. Absolute hypoxic exercise training enhances in vitro thrombin generation by increasing procoagulant platelet-derived microparticles under high shear stress in sedentary men. Clin Sci. 2013;124:639–649. [DOI] [PubMed] [Google Scholar]
- 19.Lee SK, Yang SH, Kwon I, et al. . Role of tumour necrosis factor receptor-1 and nuclear factor-κB in production of TNF-α-induced pro-inflammatory microparticles in endothelial cells. Thromb Haemost. 2014;112:580–588. [DOI] [PubMed] [Google Scholar]
- 20.Rautou PE, Vion AC, Amabile N, et al. . Microparticles, vascular function, and atherothrombosis. Circ Res. 2011;109:593–606. [DOI] [PubMed] [Google Scholar]
- 21.Burger D, Schock S, Thompson CS, et al. . Microparticles: biomarkers and beyond. Clin Sci. 2013;124:423–441. [DOI] [PubMed] [Google Scholar]
- 22.Ratajczak J, Wysoczynski M, Hayek F, et al. . Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia. 2006;20:1487–1495. [DOI] [PubMed] [Google Scholar]
- 23.Freyssinet JM, Toti F. Formation of procoagulant microparticles and properties. Thromb Res. 2010;125:S46–S48. [DOI] [PubMed] [Google Scholar]
- 24.Manno S, Takakuwa Y, Mohandas N. Identification of a functional role for lipid asymmetry in biological membranes: phosphatidylserine-skeletal protein interactions modulate membrane stability. Proc Natl Acad Sci USA. 2002;99:1943–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daleke DL Regulation of transbilayer plasma membrane phospholipid asymmetry. J Lipid Res. 2003;44:233–242. [DOI] [PubMed] [Google Scholar]
- 26.Bevers EM, Williamson PL. Phospholipid scramblase: an update. FEBS Lett. 2010;584:2724–2730. [DOI] [PubMed] [Google Scholar]
- 27.Raphael RM, Waugh RE, Svetina S, et al. . Fractional occurrence of defects in membranes and mechanically driven interleaflet phospholipid transport. Phys Rev E Stat Nonlin Soft Matter Phys. 2001;64:051913. [DOI] [PubMed] [Google Scholar]
- 28.Yan R, Wang Z, Yuan Y, et al. . Role of cAMP-dependent protein kinase in the regulation of platelet procoagulant activity. Arch Biochem Biophys. 2009;485:41–48. [DOI] [PubMed] [Google Scholar]
- 29.Miyoshi H, Umeshita K, Sakon M, et al. . Calpain activation in plasma membrane bleb formation during tert-butyl hydroperoxide-induced rat hepatocyte injury. Gastroenterology. 1996;110:1897–1904. [DOI] [PubMed] [Google Scholar]
- 30.Cauwenberghs S, Feijge MAH, Harper AGS, et al. . Shedding of procoagulant microparticles from unstimulated platelets by integrin-mediated destabilization of actin cytoskeleton. FEBS Lett. 2006;580:5313–5320. [DOI] [PubMed] [Google Scholar]
- 31.Sebbagh M, Renvoizé C, Hamelin J, et al. . Caspase-3-mediated cleavage of ROCK I induces MLC phosphorylation and apoptotic membrane blebbing. Nat Cell Biol. 2001;3:346–352. [DOI] [PubMed] [Google Scholar]
- 32.Sapet C, Simoncini S, Loriod B, et al. . Thrombin-induced endothelial microparticle generation: identification of a novel pathway involving ROCK-II activation by caspase-2. Blood. 2006;108:1868–1876. [DOI] [PubMed] [Google Scholar]
- 33.Coleman ML, Sahai EA, Yeo M, et al. . Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat Cell Biol. 2001;3:339–345. [DOI] [PubMed] [Google Scholar]
- 34.Zwaal RFA, Comfurius P, Bevers EM. Surface exposure of phosphatidylserine in pathological cells. Cell Mol Life Sci. 2005;62:971–988. [DOI] [PubMed] [Google Scholar]
- 35.Hugel B, Martínez MC, Kunzelmann C, et al. . Membrane microparticles: two sides of the coin. Physiology (Bethesda). 2005;20:22–27. [DOI] [PubMed] [Google Scholar]
- 36.Hargett LA, Bauer NN. On the origin of microparticles: from “platelet dust” to mediators of intercellular communication. Pulm Circ. 2013;3:329–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bucki R, Pastore JJ, Giraud F, et al. . Involvement of the Na+/H + exchanger in membrane phosphatidylserine exposure during human platelet activation. Biochim Biophys Acta. 2006;1761:195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deregibus MC, Cantaluppi V, Calogero R, et al. . Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood. 2007;110:2440–2448. [DOI] [PubMed] [Google Scholar]
- 39.Fox JE, Austin CD, Boyles JK, et al. . Role of the membrane skeleton in preventing the shedding of procoagulant-rich microvesicles from the platelet plasma membrane. J Cell Biol. 1990;111:483–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Biró E, Akkerman JWN, Hoek FJ, et al. . The phospholipid composition and cholesterol content of platelet-derived microparticles: a comparison with platelet membrane fractions. J Thromb Haemost. 2005;3:2754–2763. [DOI] [PubMed] [Google Scholar]
- 41.György B, Szabó TG, Pásztói M, et al. . Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci. 2011;68:2667–2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raimondo F, Morosi L, Chinello C, et al. . Advances in membranous vesicle and exosome proteomics improving biological understanding and biomarker discovery. Proteomics. 2011;11:709–720. [DOI] [PubMed] [Google Scholar]
- 43.Scanu A, Molnarfi N, Brandt KJ, et al. . Stimulated T cells generate microparticles, which mimic cellular contact activation of human monocytes: differential regulation of pro- and anti-inflammatory cytokine production by high-density lipoproteins. J Leukoc Biol. 2008;83:921–927. [DOI] [PubMed] [Google Scholar]
- 44.Bratton DL, Fadok VA, Richter DA, et al. . Appearance of phosphatidylserine on apoptotic cells requires calcium-mediated nonspecific flip-flop and is enhanced by loss of the aminophospholipid translocase. J Biol Chem. 1997;272:26159–26165. [DOI] [PubMed] [Google Scholar]
- 45.Weerheim AM, Kolb AM, Sturk A, et al. . Phospholipid composition of cell-derived microparticles determined by one-dimensional high-performance thin-layer chromatography. Anal Biochem. 2002;302:191–198. [DOI] [PubMed] [Google Scholar]
- 46.Liu ML, Reilly MP, Casasanto P, et al. . Cholesterol enrichment of human monocyte/macrophages induces surface exposure of phosphatidylserine and the release of biologically-active tissue factor-positive microvesicles. Arterioscler Thromb Vasc Biol. 2007;27:430–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heijnen HF, Schiel AE, Fijnheer R, et al. . Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood. 1999;94:3791–3799. [PubMed] [Google Scholar]
- 48.Del Conde I, Shrimpton CN, Thiagarajan P, et al. . Tissue-factor-bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood. 2005;106:1604–1611. [DOI] [PubMed] [Google Scholar]
- 49.Sims PJ, Faioni EM, Wiedmer T, et al. . Complement proteins C5b-9 cause release of membrane vesicles from the platelet surface that are enriched in the membrane receptor for coagulation factor Va and express prothrombinase activity. J Biol Chem. 1988;263:18205–18212. [PubMed] [Google Scholar]
- 50.Horstman LL, Jy W, Jimenez JJ, et al. . New horizons in the analysis of circulating cell-derived microparticles. Keio J Med. 2004;53:210–230. [DOI] [PubMed] [Google Scholar]
- 51.Gasser O, Hess C, Miot S, et al. . Characterisation and properties of ectosomes released by human polymorphonuclear neutrophils. Exp Cell Res. 2003;285:243–257. [DOI] [PubMed] [Google Scholar]
- 52.Jimenez JJ, Jy W, Mauro LM, et al. . Endothelial cells release phenotypically and quantitatively distinct microparticles in activation and apoptosis. Thromb Res. 2003;109:175–180. [DOI] [PubMed] [Google Scholar]
- 53.Teruel R, Corral J, Pérez-Andreu V, et al. . Potential role of miRNAs in developmental haemostasis. PLoS One. 2011;6:e17648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jung KH, Chu K, Lee ST, et al. . Risk of macrovascular complications in type 2 diabetes mellitus: endothelial microparticle profiles. Cerebrovasc Dis. 2011;31:485–493. [DOI] [PubMed] [Google Scholar]
- 55.Feng B, Chen Y, Luo Y, et al. . Circulating level of microparticles and their correlation with arterial elasticity and endothelium-dependent dilation in patients with type 2 diabetes mellitus. Atherosclerosis. 2010;208:264–269. [DOI] [PubMed] [Google Scholar]
- 56.Berezin AE, Kremzer AA, Martovitskaya YV, et al. . Pattern of endothelial progenitor cells and apoptotic endothelial cell-derived microparticles in chronic heart failure patients with preserved and reduced left ventricular ejection fraction. EBioMedicine. 2016;4:86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bernard S, Loffroy R, Sérusclat A, et al. . Increased levels of endothelial microparticles CD144 (VE-Cadherin) positives in type 2 diabetic patients with coronary noncalcified plaques evaluated by multidetector computed tomography (MDCT). Atherosclerosis. 2009;203:429–435. [DOI] [PubMed] [Google Scholar]
- 58.Stępień E, Stankiewicz E, Zalewski J, et al. . Number of microparticles generated during acute myocardial infarction and stable angina correlates with platelet activation. Arch Med Res. 2012;43:31–35. [DOI] [PubMed] [Google Scholar]
- 59.Sabatier F, Darmon P, Hugel B, et al. . Type 1 and type 2 diabetic patients display different patterns of cellular microparticles. Diabetes. 2002;51:2840–2845. [DOI] [PubMed] [Google Scholar]
- 60.Berezin AE, Kremzer AA, Berezina TA, et al. . Pattern of circulating microparticles in chronic heart failure patients with metabolic syndrome: relevance to neurohumoral and inflammatory activation. BBA Clin. 2015;4:69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Terrisse A, Puech N, Allart S, et al. . Internalization of microparticles by endothelial cells promotes platelet/endothelial cell interaction under flow. J Thromb Haemost. 2010;8:2810–2819. [DOI] [PubMed] [Google Scholar]
- 62.Sansone R, Stanske B, Keymel S, et al. . Macrovascular and microvascular function after implantation of left ventricular assist devices in end-stage heart failure: role of microparticles. J Heart Lung Transplant. 2015;34:921–932. [DOI] [PubMed] [Google Scholar]
- 63.Cheng V, Kashyap SR, Schauer PR, et al. . Restoration of glycemic control in patients with type 2 diabetes mellitus after bariatric surgery is associated with reduction in microparticles. Surg Obes Relat Dis. 2013;9:207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Diamant M, Nieuwland R, Pablo RF, et al. . Elevated numbers of tissue-factor exposing microparticles correlate with components of the metabolic syndrome in uncomplicated type 2 diabetes mellitus. Circulation. 2002;106:2442–2447. [DOI] [PubMed] [Google Scholar]
- 65.Martínez MC, Larbret F, Zobairi F, et al. . Transfer of differentiation signal by membrane microvesicles harboring hedgehog morphogens. Blood. 2006;108:3012–3020. [DOI] [PubMed] [Google Scholar]
- 66.Pluskota E, Woody NM, Szpak D, et al. . Expression, activation, and function of integrin alphaMbeta2 (Mac-1) on neutrophil-derived microparticles. Blood. 2008;112:2327–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bernimoulin M, Waters EK, Foy M, et al. . Differential stimulation of monocytic cells results in distinct populations of microparticles. J Thromb Haemost. 2009;7:1019–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reich CF, Pisetsky DS. The content of DNA and RNA in microparticles released by Jurkat and HL-60 cells undergoing in vitro apoptosis. Exp Cell Res. 2009;315:760–768. [DOI] [PubMed] [Google Scholar]
- 69.Diehl P, Fricke A, Sander L, et al. . Microparticles: major transport vehicles for distinct microRNAs in circulation. Cardiovasc Res. 2012;93:633–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Risitano A, Beaulieu LM, Vitseva O, et al. . Platelets and platelet-like particles mediate intercellular RNA transfer. Blood. 2012;119:6288–6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sommeijer D, Joop K, Leyte A, et al. . Pravastatin reduces fibrinogen receptor gpIIIa on platelet-derived microparticles in patients with type 2 diabetes. J Thromb Haemost. 2005;3:1168–1171. [DOI] [PubMed] [Google Scholar]
- 72.Mack M, Kleinschmidt A, Brühl H, et al. . Transfer of the chemokine receptor CCR5 between cells by membrane-derived microparticles: a mechanism for cellular human immunodeficiency virus 1 infection. Nat Med. 2000;6:769–775. [DOI] [PubMed] [Google Scholar]
- 73.Rozmyslowicz T, Majka M, Kijowski J, et al. . Platelet- and megakaryocyte-derived microparticles transfer CXCR4 receptor to CXCR4-null cells and make them susceptible to infection by X4-HIV. AIDS. 2003;17:33–42. [DOI] [PubMed] [Google Scholar]
- 74.Mause SF, Ritzel E, Liehn EA, et al. . Platelet microparticles enhance the vasoregenerative potential of angiogenic early outgrowth cells after vascular injury. Circulation. 2010;122:495–506. [DOI] [PubMed] [Google Scholar]
- 75.Salanova B, Choi M, Rolle S, et al. . Beta2-integrins and acquired glycoprotein IIb/IIIa (GPIIb/IIIa) receptors cooperate in NF-kappaB activation of human neutrophils. J Biol Chem. 2007;282:27960–27969. [DOI] [PubMed] [Google Scholar]
- 76.Al-Nedawi K, Meehan B, Micallef J, et al. . Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol. 2008;10:619–624. [DOI] [PubMed] [Google Scholar]
- 77.Whale TA, Wilson HL, Tikoo SK, et al. . Passively acquired membrane proteins alter the functional capacity of bovine polymorphonuclear cells. J Leukoc Biol. 2006;80:481–491. [DOI] [PubMed] [Google Scholar]
- 78.Ray DM, Spinelli SL, Pollock SJ, et al. . Peroxisome proliferator-activated receptor gamma and retinoid X receptor transcription factors are released from activated human platelets and shed in microparticles. Thromb Haemost. 2008;99:86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.MacKenzie A, Wilson HL, Kiss-Toth E, et al. . Rapid secretion of interleukin-1beta by microvesicle shedding. Immunity. 2001. ;15:825–835. [DOI] [PubMed] [Google Scholar]
- 80.Bianco F, Pravettoni E, Colombo A, et al. . Astrocyte-derived ATP induces vesicle shedding and IL-1 beta release from microglia. J Immunol. 2005;174:7268–7277. [DOI] [PubMed] [Google Scholar]
- 81.Pizzirani C, Ferrari D, Chiozzi P, et al. . Stimulation of P2 receptors causes release of IL-1beta-loaded microvesicles from human dendritic cells. Blood. 2007;109:3856–3864. [DOI] [PubMed] [Google Scholar]
- 82.Lindemann S, Tolley ND, Dixon DA, et al. . Activated platelets mediate inflammatory signaling by regulated interleukin 1beta synthesis. J Cell Biol. 2001;154:485–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mause SF, von Hundelshausen P, Zernecke A, et al. . Platelet microparticles: a transcellular delivery system for RANTES promoting monocyte recruitment on endothelium. Arterioscler Thromb Vasc Biol. 2005;25:1512–1518. [DOI] [PubMed] [Google Scholar]
- 84.Kim HK, Song KS, Chung JH, et al. . Platelet microparticles induce angiogenesis in vitro. Br J Haematol. 2004;124:376–384. [DOI] [PubMed] [Google Scholar]
- 85.Taraboletti G, D’Ascenzo S, Giusti I, et al. . Bioavailability of VEGF in tumor-shed vesicles depends on vesicle burst induced by acidic pH. Neoplasia. 2006;8:96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Brill A, Dashevsky O, Rivo J, et al. . Platelet-derived microparticles induce angiogenesis and stimulate post-ischemic revascularization. Cardiovasc Res. 2005;67:30–38. [DOI] [PubMed] [Google Scholar]
- 87.Sidhu SS, Mengistab AT, Tauscher AN, et al. . The microvesicle as a vehicle for EMMPRIN in tumor-stromal interactions. Oncogene. 2004;23:956–963. [DOI] [PubMed] [Google Scholar]
- 88.Sarkar A, Mitra S, Mehta S, et al. . Monocyte derived microvesicles deliver a cell death message via encapsulated caspase-1 Unutmaz D, editor. PLoS One. 2009;4:e7140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Barry OP, Pratico D, Lawson JA, et al. . Transcellular activation of platelets and endothelial cells by bioactive lipids in platelet microparticles. J Clin Invest. 1997;99:2118–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Barry OP, Kazanietz MG, Praticò D, et al. . Arachidonic acid in platelet microparticles up-regulates cyclooxygenase-2-dependent prostaglandin formation via a protein kinase C/mitogen-activated protein kinase-dependent pathway. J Biol Chem. 1999;274:7545–7556. [DOI] [PubMed] [Google Scholar]
- 91.Barry OP, Praticò D, Savani RC, et al. . Modulation of monocyte-endothelial cell interactions by platelet microparticles. J Clin Invest. 1998;102:136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lorant DE, Zimmerman GA, McIntyre TM, et al. . Platelet-activating factor mediates procoagulant activity on the surface of endothelial cells by promoting leukocyte adhesion. Semin Cell Biol. 1995;6:295–303. [DOI] [PubMed] [Google Scholar]
- 93.Watanabe J, Marathe GK, Neilsen PO, et al. . Endotoxins stimulate neutrophil adhesion followed by synthesis and release of platelet-activating factor in microparticles. J Biol Chem. 2003;278:33161–33168. [DOI] [PubMed] [Google Scholar]
- 94.Ratajczak J, Miekus K, Kucia M, et al. . Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006;20:847–856. [DOI] [PubMed] [Google Scholar]
- 95.Hunter MP, Ismail N, Zhang X, et al. . Detection of microRNA expression in human peripheral blood microvesicles. PLoS One. 2008;3:e3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Collino F, Deregibus MC, Bruno S, et al. . Microvesicles derived from adult human bone marrow and tissue specific mesenchymal stem cells shuttle selected pattern of miRNAs. PLoS One. 2010;5:e11803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yuan A, Farber EL, Rapoport AL, et al. . Transfer of microRNAs by embryonic stem cell microvesicles. PLoS One. 2009;4:e4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Obregon C, Rothen-Rutishauser B, Gitahi SK, et al. . Exovesicles from human activated dendritic cells fuse with resting dendritic cells, allowing them to present alloantigens. Am J Pathol. 2006;169:2127–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen CZ, Li L, Lodish HF, et al. . MicroRNAs modulate hematopoietic lineage differentiation. Science. 2004;303:83–86. [DOI] [PubMed] [Google Scholar]
- 100.Aliotta JM, Pereira M, Johnson KW, et al. . Microvesicle entry into marrow cells mediates tissue-specific changes in mRNA by direct delivery of mRNA and induction of transcription. Exp Hematol. 2010;38:233–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.English D, Garcia JG, Brindley DN. Platelet-released phospholipids link haemostasis and angiogenesis. Cardiovasc Res. 2001;49:588–599. [DOI] [PubMed] [Google Scholar]
- 102.Chargaff E, West R. The biological significance of the thromboplastic protein of blood. J Biol Chem. 1946;166:189–197. [PubMed] [Google Scholar]
- 103.Owens AP, Mackman N. Microparticles in hemostasis and thrombosis. Circ Res. 2011;108:1284–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mackman N, Tilley RE, Key NS. Role of the extrinsic pathway of blood coagulation in hemostasis and thrombosis. Arterioscler Thromb Vasc Biol. 2007;27:1687–1693. [DOI] [PubMed] [Google Scholar]
- 105.Polgar J, Matuskova J, Wagner DD. The P-selectin, tissue factor, coagulation triad. J Thromb Haemost. 2005;3:1590–1596. [DOI] [PubMed] [Google Scholar]
- 106.Somajo S, Koshiar RL, Norström E, et al. . Protein S and factor V in regulation of coagulation on platelet microparticles by activated protein C. Thromb Res. 2014;134:144–152. [DOI] [PubMed] [Google Scholar]
- 107.Alexandru N, Andrei E, Dragan E, et al. . Interaction of platelets with endothelial progenitor cells in the experimental atherosclerosis: role of transplanted endothelial progenitor cells and platelet microparticles. Biol Cell. 2015;107:189–204. [DOI] [PubMed] [Google Scholar]
- 108.Tual-Chalot S, Guibert C, Muller B, et al. . Circulating microparticles from pulmonary hypertensive rats induce endothelial dysfunction. Am J Respir Crit Care Med. 2010;182:261–268. [DOI] [PubMed] [Google Scholar]
- 109.Turiák L, Misják P, Szabó TG, et al. . Proteomic characterization of thymocyte-derived microvesicles and apoptotic bodies in BALB/c mice. J Proteomics. 2011;74:2025–2033. [DOI] [PubMed] [Google Scholar]
- 110.Robbins PD, Dorronsoro A, Booker CN. Regulation of chronic inflammatory and immune processes by extracellular vesicles. J Clin Invest. 2016;126:1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mohning MP, Thomas SM, Barthel L, et al. . Phagocytosis of microparticles by alveolar macrophages during acute lung injury requires MerTK. Am J Physiol Cell Mol Physiol. 2018;314:L69–L82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ma J, Wei K, Zhang H, et al. . Mechanisms by which dendritic cells present tumor microparticle antigens to CD8 + T cells. Cancer Immunol Res. 2018;6:1057–1068. [DOI] [PubMed] [Google Scholar]
- 113.Buesing KL, Densmore JC, Kaul S, et al. . Endothelial microparticles induce inflammation in acute lung injury. J Surg Res. 2011;166:32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mesri M, Altieri DC. Leukocyte microparticles stimulate endothelial cell cytokine release and tissue factor induction in a JNK1 signaling pathway. J Biol Chem. 1999;274:23111–23118. [DOI] [PubMed] [Google Scholar]
- 115.Neri T, Armani C, Pegoli A, et al. . Role of NF-kappaB and PPAR-gamma in lung inflammation induced by monocyte-derived microparticles. Eur Respir J. 2011;37:1494–1502. [DOI] [PubMed] [Google Scholar]
- 116.Wang JG, Williams JC, Davis BK, et al. . Monocytic microparticles activate endothelial cells in an IL-1β-dependent manner. Blood. 2011;118:2366–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gasser O, Schifferli JA. Activated polymorphonuclear neutrophils disseminate anti-inflammatory microparticles by ectocytosis. Blood. 2004;104:2543–2548. [DOI] [PubMed] [Google Scholar]
- 118.Dalli J, Norling LV, Renshaw D, et al. . Annexin 1 mediates the rapid anti-inflammatory effects of neutrophil-derived microparticles. Blood. 2008;112:2512–2519. [DOI] [PubMed] [Google Scholar]
- 119.Bardelli C, Amoruso A, Federici Canova D, et al. . Autocrine activation of human monocyte/macrophages by monocyte-derived microparticles and modulation by PPARγ ligands. Br J Pharmacol. 2012;165:716–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chen GY, Nuñez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Radziwon-Balicka A, Moncada de la Rosa C, Jurasz P. Platelet-associated angiogenesis regulating factors: a pharmacological perspective. Can J Physiol Pharmacol. 2012;90:679–688. [DOI] [PubMed] [Google Scholar]
- 122.Collier MEW, Ettelaie C. Induction of endothelial cell proliferation by recombinant and microparticle-tissue factor involves 1-integrin and extracellular signal regulated kinase activation. Arterioscler Thromb Vasc Biol. 2010;30:1810–1817. [DOI] [PubMed] [Google Scholar]
- 123.Benameur T, Soleti R, Porro C, et al. . Microparticles carrying Sonic hedgehog favor neovascularization through the activation of nitric oxide pathway in mice. PLoS One. 2010;5:e12688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Soleti R, Benameur T, Porro C, et al. . Microparticles harboring Sonic Hedgehog promote angiogenesis through the upregulation of adhesion proteins and proangiogenic factors. Carcinogenesis. 2009;30:580–588. [DOI] [PubMed] [Google Scholar]
- 125.Lacroix R, Sabatier F, Mialhe A, et al. . Activation of plasminogen into plasmin at the surface of endothelial microparticles: a mechanism that modulates angiogenic properties of endothelial progenitor cells in vitro. Blood. 2007;110:2432–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Taraboletti G, D'Ascenzo S, Borsotti P, et al. . Shedding of the matrix metalloproteinases MMP-2, MMP-9, and MT1-MMP as membrane vesicle-associated components by endothelial cells. Am J Pathol. 2002;160:673–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Leroyer AS, Ebrahimian TG, Cochain C, et al. . Microparticles from ischemic muscle promotes postnatal vasculogenesis. Circulation. 2009;119:2808–2817. [DOI] [PubMed] [Google Scholar]
- 128.Aoki N, Yokoyama R, Asai N, et al. . Adipocyte-derived microvesicles are associated with multiple angiogenic factors and induce angiogenesis in vivo and in vitro. Endocrinology. 2010;151:2567–2576. [DOI] [PubMed] [Google Scholar]
- 129.Yang C, Mwaikambo BR, Zhu T, et al. . Lymphocytic microparticles inhibit angiogenesis by stimulating oxidative stress and negatively regulating VEGF-induced pathways. Am J Physiol Regul Integr Comp Physiol. 2008;294:R467–R476. [DOI] [PubMed] [Google Scholar]
- 130.Shai E, Varon D. Development, cell differentiation, angiogenesis—microparticles and their roles in angiogenesis. Arterioscler Thromb Vasc Biol. 2010;31:10–14. [DOI] [PubMed] [Google Scholar]
- 131.Varon D, Haion Y, Brill A, et al. . Cell-driven angiogenesis and neurogenesis after stroke is regulated by platelet’s microparticles. Blood. 2015;112:1899. [Google Scholar]
- 132.Mezentsev A, Merks RMH, O’Riordan E, et al. . Endothelial microparticles affect angiogenesis in vitro: role of oxidative stress. Am J Physiol Heart Circ Physiol. 2005;289:H1106–H1114. [DOI] [PubMed] [Google Scholar]
- 133.Klinkner DB, Densmore JC, Kaul S, et al. . Endothelium-derived microparticles inhibit human cardiac valve endothelial cell function. Shock. 2006;25:575–580. [DOI] [PubMed] [Google Scholar]
- 134.Brodsky SV, Zhang F, Nasjletti A, et al. . Endothelium-derived microparticles impair endothelial function in vitro. Am J Physiol Hear Circ Physiol. 2004;286:H1910–H1915. [DOI] [PubMed] [Google Scholar]
- 135.Amabile N, Guérin AP, Leroyer A, et al. . Circulating endothelial microparticles are associated with vascular dysfunction in patients with end-stage renal failure. J Am Soc Nephrol. 2005;16:3381–3388. [DOI] [PubMed] [Google Scholar]
- 136.Boulanger CM, Scoazec A, Ebrahimian T, et al. . Circulating microparticles from patients with myocardial infarction cause endothelial dysfunction. Circulation. 2001;104:2649–2652. [DOI] [PubMed] [Google Scholar]
- 137.Mostefai HA, Agouni A, Carusio N, et al. . Phosphatidylinositol 3-kinase and xanthine oxidase regulate nitric oxide and reactive oxygen species productions by apoptotic lymphocyte microparticles in endothelial cells. J Immunol. 2008;180:5028–5035. [DOI] [PubMed] [Google Scholar]
- 138.Martin S, Tesse A, Hugel B, et al. . Shed membrane particles from T lymphocytes impair endothelial function and regulate endothelial protein expression. Circulation. 2004;109:1653–1659. [DOI] [PubMed] [Google Scholar]
- 139.Horn P, Cortese-Krott MM, Amabile N, et al. . Circulating microparticles carry a functional endothelial nitric oxide synthase that is decreased in patients with endothelial dysfunction. J Am Heart Assoc. 2012;2:e003764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pfister SL Role of platelet microparticles in the production of thromboxane by rabbit pulmonary artery. Hypertension. 2004;43:428–433. [DOI] [PubMed] [Google Scholar]
- 141.Agouni A, Ducluzeau P-H, Benameur T, et al. . Microparticles from patients with metabolic syndrome induce vascular hypo-reactivity via fas/fas-ligand pathway in mice. PLoS One. 2011;6:e27809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gregory CD, Pound JD. Cell death in the neighbourhood: direct microenvironmental effects of apoptosis in normal and neoplastic tissues. J Pathol. 2011;223:178–195. [DOI] [PubMed] [Google Scholar]
- 143.Aupeix K, Hugel B, Martin T, et al. . The significance of shed membrane particles during programmed cell death in vitro, and in vivo, in HIV-1 infection. J Clin Invest. 1997;99:1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Segundo C, Medina F, Rodríguez C, et al. . Surface molecule loss and bleb formation by human germinal center B cells undergoing apoptosis: role of apoptotic blebs in monocyte chemotaxis. Blood. 1999;94:1012–1020. [PubMed] [Google Scholar]
- 145.Distler JHW, Akhmetshina A, Dees C, et al. . Induction of apoptosis in circulating angiogenic cells by microparticles. Arthritis Rheum. 2011;63:2067–2077. [DOI] [PubMed] [Google Scholar]
- 146.Huber LC, Jüngel A, Distler JHW, et al. . The role of membrane lipids in the induction of macrophage apoptosis by microparticles. Apoptosis. 2007;12:363–374. [DOI] [PubMed] [Google Scholar]
- 147.Abid Hussein MN, Böing AN, Sturk A, et al. . Inhibition of microparticle release triggers endothelial cell apoptosis and detachment. Thromb Haemost. 2007;98:1096–1107. [DOI] [PubMed] [Google Scholar]
- 148.Abid Hussein MN, Nieuwland R, Hau CM, et al. . Cell-derived microparticles contain caspase 3 in vitro and in vivo. J Thromb Haemost. 2005;3:888–896. [DOI] [PubMed] [Google Scholar]
- 149.Böing AN, Hau CM, Sturk A, et al. . Platelet microparticles contain active caspase 3. Platelets. 2008;19:96–103. [DOI] [PubMed] [Google Scholar]
- 150.Albanese J, Meterissian S, Kontogiannea M, et al. . Biologically active FAS antigen and its cognate ligand are expressed on plasma membrane-derived extracellular vesicles. Blood. 1998;91:3862–3874. [PubMed] [Google Scholar]
- 151.Juránek I, Bezek S. Controversy of free radical hypothesis: reactive oxygen species-cause or consequence of tissue injury? Gen Physiol Biophys. 2005;24:263–278. [PubMed] [Google Scholar]
- 152.Dröge W Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. [DOI] [PubMed] [Google Scholar]
- 153.Essayagh S, Xuereb JM, Terrisse AD, et al. . Microparticles from apoptotic monocytes induce transient platelet recruitment and tissue factor expression by cultured human vascular endothelial cells via a redox-sensitive mechanism. Thromb Haemost. 2007;98:831–837. [PubMed] [Google Scholar]
- 154.Mastronardi ML, Mostefai HA, Soleti R, et al. . Microparticles from apoptotic monocytes enhance nitrosative stress in human endothelial cells. Fundam Clin Pharmacol. 2011;25:653–660. [DOI] [PubMed] [Google Scholar]
- 155.Touyz RM Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension: what is the clinical significance? Hypertension. 2004;44:248–252. [DOI] [PubMed] [Google Scholar]
- 156.Rodrigo R, Prat H, Passalacqua W, et al. . Relationship between oxidative stress and essential hypertension. Hypertens Res. 2007;30:1159–1167. [DOI] [PubMed] [Google Scholar]
- 157.Unger T The role of the renin-angiotensin system in the development of cardiovascular disease. Am J Cardiol. 2002;89:3–9A. [DOI] [PubMed] [Google Scholar]
- 158.Cordazzo C, Neri T, Petrini S, et al. . Angiotensin II induces the generation of procoagulant microparticles by human mononuclear cells via an angiotensin type 2 receptor-mediated pathway. Thromb Res. 2013;131:e168–e174. [DOI] [PubMed] [Google Scholar]
- 159.Preston RA, Jy W, Jimenez JJ, et al. . Effects of severe hypertension on endothelial and platelet microparticles. Hypertens. 2003;41:211–217. [DOI] [PubMed] [Google Scholar]
- 160.Hsu CY, Huang PH, Chiang CH, et al. . Increased circulating endothelial apoptotic microparticle to endothelial progenitor cell ratio is associated with subsequent decline in glomerular filtration rate in hypertensive patients. PLoS One. 2013;8:e68644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Huang PH, Huang SS, Chen YH, et al. . Increased circulating CD31+/annexin V + apoptotic microparticles and decreased circulating endothelial progenitor cell levels in hypertensive patients with microalbuminuria. J Hypertens. 2010;28:1655–1665. [DOI] [PubMed] [Google Scholar]
- 162.Giannella A, Radu CM, Franco L, et al. . Circulating levels and characterization of microparticles in patients with different degrees of glucose tolerance. Cardiovasc Diabetol. 2017;16:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kurtzman N, Zhang L, French B, et al. . Personalized cytomic assessment of vascular health: evaluation of the vascular health profile in diabetes mellitus. Cytometry. 2013;84B:255–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Nomura S, Shouzu A, Omoto S, et al. . 5-HT2A receptor antagonist increases circulating adiponectin in patients with type 2 diabetes. Blood Coagul Fibrinolysis. 2005;16:423–428. [DOI] [PubMed] [Google Scholar]
- 165.Omoto S, Nomura S, Shouzu A, et al. . Detection of monocyte-derived microparticles in patients with Type II diabetes mellitus. Diabetologia. 2002;45:550–555. [DOI] [PubMed] [Google Scholar]
- 166.Alkhatatbeh MJ, Enjeti AK, Acharya S, et al. . The origin of circulating CD36 in type 2 diabetes. Nutr Diabetes. 2013;3:e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Tsimerman G, Roguin A, Bachar A, et al. . Involvement of microparticles in diabetic vascular complications. Thromb Haemost. 2011;106:310–321. [DOI] [PubMed] [Google Scholar]
- 168.Burger D, Turner M, Xiao F, et al. . High glucose increases the formation and pro-oxidative activity of endothelial microparticles. Diabetologia. 2017;60:1791–1800. [DOI] [PubMed] [Google Scholar]
- 169.Tripodi A, Branchi A, Chantarangkul V, et al. . Hypercoagulability in patients with type 2 diabetes mellitus detected by a thrombin generation assay. J Thromb Thrombolysis. 2011;31:165–172. [DOI] [PubMed] [Google Scholar]
- 170.Berezin AE, Kremzer AA, Berezina TA, et al. . The pattern of circulating microparticles in patients with diabetes mellitus with asymptomatic atherosclerosis. Acta Clin Belg. 2016;71:38–45. [DOI] [PubMed] [Google Scholar]
- 171.Jansen F, Yang X, Franklin BS, et al. . High glucose condition increases NADPH oxidase activity in endothelial microparticles that promote vascular inflammation. Cardiovasc Res. 2013;98:94–106. [DOI] [PubMed] [Google Scholar]
- 172.Omoto S, Nomura S, Shouzu A, et al. . Significance of platelet-derived microparticles and activated platelets in diabetic nephropathy. Nephron. 1999;81:271–277. [DOI] [PubMed] [Google Scholar]
- 173.Ettelaie C, Su S, Li C, et al. . Tissue factor-containing microparticles released from mesangial cells in response to high glucose and AGE induce tube formation in microvascular cells. Microvasc Res. 2008;76:152–160. [DOI] [PubMed] [Google Scholar]
- 174.Zampetaki A, Kiechl S, Drozdov I, et al. . Plasma microRNA profiling reveals loss of endothelial MiR-126 and other microRNAs in type 2 diabetes. Circ Res. 2010;107:810–817. [DOI] [PubMed] [Google Scholar]
- 175.Li S, Wei J, Zhang C, et al. . Cell-derived microparticles in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Cell Physiol Biochem. 2016;39:2439–2450. [DOI] [PubMed] [Google Scholar]
- 176.Rodrigues KF, Pietrani NT, Fernandes AP, et al. . Circulating microparticles levels are increased in patients with diabetic kidney disease: a case-control research. Clin Chim Acta. 2018;479:48–55. [DOI] [PubMed] [Google Scholar]
- 177.Cimmino G, D’Amico C, Vaccaro V, et al. . The missing link between atherosclerosis, inflammation and thrombosis: is it tissue factor? Expert Rev Cardiovasc Ther. 2011;9:517–523. [DOI] [PubMed] [Google Scholar]
- 178.Domingueti CP, Dusse LMS, Carvalho MDG, et al. . Diabetes mellitus: the linkage between oxidative stress, inflammation, hypercoagulability and vascular complications. J Diabetes Complications. 2016;30:738–745. [DOI] [PubMed] [Google Scholar]
- 179.Fish JE, Santoro MM, Morton SU, et al. . miR-126 regulates angiogenic signaling and vascular integrity. Dev Cell. 2008;15:272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Jansen F, Stumpf T, Proebsting S, et al. . Intercellular transfer of miR-126-3p by endothelial microparticles reduces vascular smooth muscle cell proliferation and limits neointima formation by inhibiting LRP6. J Mol Cell Cardiol. 2017;104:43–52. [DOI] [PubMed] [Google Scholar]
- 181.Wang Y, Chen L, Liu M. Microvesicles and diabetic complications – novel mediators, potential biomarkers and therapeutic targets. Acta Pharmacol Sin. 2014;35:433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Nomura S, Shouzu A, Omoto S, et al. . Effect of cilostazol on soluble adhesion molecules and platelet-derived microparticles in patients with diabetes. Thromb Haemost. 1998;80:388–392. [PubMed] [Google Scholar]
- 183.Nomura S, Takahashi N, Inami N, et al. . Probucol and ticlopidine: effect on platelet and monocyte activation markers in hyperlipidemic patients with and without type 2 diabetes. Atherosclerosis. 2004;174:329–335. [DOI] [PubMed] [Google Scholar]
- 184.Nomura S, Shouzu A, Omoto S, et al. . Significance of chemokines and activated platelets in patients with diabetes. Clin Exp Immunol. 2000;121:437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Shouzu A, Nomura S, Omoto S, et al. . Effect of ticlopidine on monocyte-derived microparticles and activated platelet markers in diabetes mellitus. Clin Appl Thromb Hemost. 2004;10:167–173. [DOI] [PubMed] [Google Scholar]
- 186.Shouzu A, Nomura S, Hayakawa T, et al. . Effect of sarpogrelate hydrochloride on platelet-derived microparticles and various soluble adhesion molecules in diabetes mellitus. Clin Appl Thromb Hemost. 2000;6:139–143. [DOI] [PubMed] [Google Scholar]
- 187.Serebruany VL, Malinin AI, Pokov AN, et al. . Antiplatelet profiles of the fixed-dose combination of extended-release dipyridamole and low-dose aspirin compared with clopidogrel with or without aspirin in patients with type 2 diabetes and a history of transient ischemic attack: a randomized, single-blind, 30-day trial. Clin Ther. 2008;30:249–259. [DOI] [PubMed] [Google Scholar]
- 188.Nomura S, Kanazawa S, Fukuhara S. Effects of efonidipine on platelet and monocyte activation markers in hypertensive patients with and without type 2 diabetes mellitus. J Hum Hypertens. 2002;16:539–547. [DOI] [PubMed] [Google Scholar]
- 189.Nomura S, Inami N, Kimura Y, et al. . Effect of nifedipine on adiponectin in hypertensive patients with type 2 diabetes mellitus. J Hum Hypertens. 2007;21:38–44. [DOI] [PubMed] [Google Scholar]
- 190.Nomura S, Shouzu A, Omoto S, et al. . Long-term treatment with nifedipine modulates procoagulant marker and C-C chemokine in hypertensive patients with type 2 diabetes mellitus. Thromb Res. 2005;115:277–285. [DOI] [PubMed] [Google Scholar]
- 191.Nomura S, Shouzu A, Omoto S, et al. . Losartan and simvastatin inhibit platelet activation in hypertensive patients. J Thromb Thrombolysis. 2004;18:177–185. [DOI] [PubMed] [Google Scholar]
- 192.Nomura S, Shouzu A, Omoto S, et al. . Effects of losartan and simvastatin on monocyte-derived microparticles in hypertensive patients with and without type 2 diabetes mellitus. Clin Appl Thromb Hemost. 2004;10:133–141. [DOI] [PubMed] [Google Scholar]
- 193.Nomura S, Shouzu A, Omoto S, et al. . Effect of valsartan on monocyte/endothelial cell activation markers and adiponectin in hypertensive patients with type 2 diabetes mellitus. Thromb Res. 2006;117:385–392. [DOI] [PubMed] [Google Scholar]
- 194.Nomura S, Inami N, Shouzu A, et al. . The effects of pitavastatin, eicosapentaenoic acid and combined therapy on platelet-derived microparticles and adiponectin in hyperlipidemic, diabetic patients. Platelets. 2009;20:16–22. [DOI] [PubMed] [Google Scholar]
- 195.Tehrani S, Mobarrez F, Antovic A, et al. . Atorvastatin has antithrombotic effects in patients with type 1 diabetes and dyslipidemia. Thromb Res. 2010;126:e225–e231. [DOI] [PubMed] [Google Scholar]
- 196.Koga H, Sugiyama S, Kugiyama K, et al. . Elevated levels of remnant lipoproteins are associated with plasma platelet microparticles in patients with type-2 diabetes mellitus without obstructive coronary artery disease. Eur Heart J. 2006;27:817–823. [DOI] [PubMed] [Google Scholar]
- 197.Nomura S, Kanazawa S, Fukuhara S. Effects of eicosapentaenoic acid on platelet activation markers and cell adhesion molecules in hyperlipidemic patients with Type 2 diabetes mellitus. J Diabetes Complications. 2003;17:153–159. [DOI] [PubMed] [Google Scholar]
- 198.Nomura S, Shouzu A, Omoto S, et al. . Effects of eicosapentaenoic acid on endothelial cell-derived microparticles, angiopoietins and adiponectin in patients with type 2 diabetes. J Atheroscler Thromb. 2009;16:83–90. [DOI] [PubMed] [Google Scholar]
- 199.Morel O, Jesel L, Hugel B, et al. . Protective effects of vitamin C on endothelium damage and platelet activation during myocardial infarction in patients with sustained generation of circulating microparticles. J Thromb Haemost. 2003;1:171–177. [DOI] [PubMed] [Google Scholar]
- 200.Shimazu T, Inami N, Satoh D, et al. . Effect of acarbose on platelet-derived microparticles, soluble selectins, and adiponectin in diabetic patients. J Thromb Thrombolysis. 2009;28:429–435. [DOI] [PubMed] [Google Scholar]
- 201.Nomura S, Omoto S, Yokoi T, et al. . Effects of miglitol in platelet-derived microparticle, adiponectin, and selectin level in patients with type 2 diabetes mellitus. Int J Gen Med. 2011;4:539–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Esposito K, Maiorino MI, Di Palo C, et al. . Effects of pioglitazone versus metformin on circulating endothelial microparticles and progenitor cells in patients with newly diagnosed type 2 diabetes–a randomized controlled trial. Diabetes Obes Metab. 2011;13:439–445. [DOI] [PubMed] [Google Scholar]
- 203.Sambola A, Osende J, Hathcock J, et al. . Role of risk factors in the modulation of tissue factor activity and blood thrombogenicity. Circulation. 2003;107:973–977. [DOI] [PubMed] [Google Scholar]
- 204.Li M, Yu D, Williams KJ, et al. . Tobacco smoke induces the generation of procoagulant microvesicles from human monocytes/macrophages. Arterioscler Thromb Vasc Biol. 2010;30:1818–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Gordon C, Gudi K, Krause A, et al. . Circulating endothelial microparticles as a measure of early lung destruction in cigarette smokers. Am J Respir Crit Care Med. 2011;184:224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Li CJ, Liu Y, Chen Y, et al. . Novel proteolytic microvesicles released from human macrophages after exposure to tobacco smoke. Am J Pathol. 2013;182:1552–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Heiss C, Amabile N, Lee AC, et al. . Brief secondhand smoke exposure depresses endothelial progenitor cells activity and endothelial function. J Am Coll Cardiol. 2008;51:1760–1771. [DOI] [PubMed] [Google Scholar]
- 208.Tushuizen ME, Nieuwland R, Rustemeijer C, et al. . Elevated endothelial microparticles following consecutive meals are associated with vascular endothelial dysfunction in type 2 diabetes. Diabetes Care. 2007;30:728–730. [DOI] [PubMed] [Google Scholar]
- 209.Ou ZJ, Chang FJ, Luo D, et al. . Endothelium-derived microparticles inhibit angiogenesis in the heart and enhance the inhibitory effects of hypercholesterolemia on angiogenesis. Am J Physiol Endocrinol Metab. 2011;300:E661–E668. [DOI] [PubMed] [Google Scholar]
- 210.Llorente-Cortes V Aggregated low-density lipoprotein uptake induces membrane tissue factor procoagulant activity and microparticle release in human vascular smooth muscle cells. Circulation. 2004;110:452–459. [DOI] [PubMed] [Google Scholar]
- 211.Nomura S, Shouzu A, Omoto S, et al. . Activated platelet and oxidized LDL induce endothelial membrane vesiculation: clinical significance of endothelial cell-derived microparticles in patients with type 2 diabetes. Clin Appl Thromb Hemost. 2004;10:205–215. [DOI] [PubMed] [Google Scholar]
- 212.Carpintero R, Gruaz L, Brandt KJ, et al. . HDL interfere with the binding of T cell microparticles to human monocytes to inhibit pro-inflammatory cytokine production. PLoS One. 2010;5:e11869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Tramontano AF, O’Leary J, Black AD, et al. . Statin decreases endothelial microparticle release from human coronary artery endothelial cells: implication for the Rho-kinase pathway. Biochem Biophys Res Commun. 2004;320:34–38. [DOI] [PubMed] [Google Scholar]
- 214.Galassi A, Reynolds K, He J. Metabolic syndrome and risk of cardiovascular disease: a meta-analysis. Am J Med. 2006;119:812–819. [DOI] [PubMed] [Google Scholar]
- 215.Arteaga RB, Chirinos JA, Soriano AO, et al. . Endothelial microparticles and platelet and leukocyte activation in patients with the metabolic syndrome. Am J Cardiol. 2006;98(1):70–74. [DOI] [PubMed] [Google Scholar]
- 216.Ueba T, Haze T, Sugiyama M, et al. . Level, distribution and correlates of platelet-derived microparticles in healthy individuals with special reference to the metabolic syndrome. Thromb Haemost. 2008;100:280–285. [PubMed] [Google Scholar]
- 217.Chironi G, Simon A, Hugel B, et al. . Circulating leukocyte-derived microparticles predict subclinical atherosclerosis burden in asymptomatic subjects. Arterioscler Thromb Vasc Biol. 2006;26:2775–2780. [DOI] [PubMed] [Google Scholar]
- 218.Helal O, Defoort C, Robert S, et al. . Increased levels of microparticles originating from endothelial cells, platelets and erythrocytes in subjects with metabolic syndrome: relationship with oxidative stress. Nutr Metab Cardiovasc Dis. 2011;21:665–671. [DOI] [PubMed] [Google Scholar]
- 219.Tricot O, Mallat Z, Heymes C, et al. . Relation between endothelial cell apoptosis and blood flow direction in human atherosclerotic plaques. Circulation. 2000;101:2450–2453. [DOI] [PubMed] [Google Scholar]
- 220.Boulanger CM, Amabile N, Guérin AP, et al. . In vivo shear stress determines circulating levels of endothelial microparticles in end-stage renal disease. Hypertension. 2007;49:902–908. [DOI] [PubMed] [Google Scholar]
- 221.Mundi S, Massaro M, Scoditti E, et al. . Endothelial permeability, LDL deposition, and cardiovascular risk factors-a review. Cardiovasc Res. 2018;114:35–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Densmore JC, Signorino PR, Ou J, et al. . Endothelium-derived microparticles induce endothelial dysfunction and acute lung injury. Shock. 2006;26:464–471. [DOI] [PubMed] [Google Scholar]
- 223.Jy W, Minagar A, Jimenez JJ, et al. . Endothelial microparticles (EMP) bind and activate monocytes: elevated EMP-monocyte conjugates in multiple sclerosis. Front Biosci. 2004;9:3137–3144. [DOI] [PubMed] [Google Scholar]
- 224.Dean WL, Lee MJ, Cummins TD, et al. . Proteomic and functional characterization of platelet microparticle size classes. Thromb Haemost. 2009;102:711–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Lusis AJ Atherosclerosis. Nature. 2000;407:233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Mesri M, Altieri DC. Endothelial cell activation by leukocyte microparticles. J Immunol. 1998;161:4382–4387. [PubMed] [Google Scholar]
- 227.Curtis A, Wilkinson P, Gui M, et al. . p38 mitogen-activated protein kinase targets the production of proinflammatory endothelial microparticles. J Thromb Haemost. 2009;7:701–709. [DOI] [PubMed] [Google Scholar]
- 228.Rautou PE, Leroyer AS, Ramkhelawon B, et al. . Microparticles from human atherosclerotic plaques promote endothelial ICAM-1-dependent monocyte adhesion and transendothelial migration. Circ Res. 2011;108:335–343. [DOI] [PubMed] [Google Scholar]
- 229.Leroyer AS, Isobe H, Lesèche G, et al. . Cellular origins and thrombogenic activity of microparticles isolated from human atherosclerotic plaques. J Am Coll Cardiol. 2007;49:772–777. [DOI] [PubMed] [Google Scholar]
- 230.Mallat Z, Hugel B, Ohan J, et al. . Shed membrane microparticles with procoagulant potential in human atherosclerotic plaques: a role for apoptosis in plaque thrombogenicity. Circulation. 1999;99:348–353. [DOI] [PubMed] [Google Scholar]
- 231.Mayr M, Grainger D, Mayr U, et al. . Proteomics, metabolomics, and immunomics on microparticles derived from human atherosclerotic plaques. Circ Cardiovasc Genet. 2009;2:379–388. [DOI] [PubMed] [Google Scholar]
- 232.Kolodgie FD, Narula J, Burke AP, et al. . Localization of apoptotic macrophages at the site of plaque rupture in sudden coronary death. Am J Pathol. 2000;157:1259–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Tabas I Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis: the importance of lesion stage and phagocytic efficiency. Arterioscler Thromb Vasc Biol. 2005;25:2255–2264. [DOI] [PubMed] [Google Scholar]
- 234.Antwi-Baffour S, Kholia S, Aryee YD, et al. . Human plasma membrane-derived vesicles inhibit the phagocytosis of apoptotic cells – possible role in SLE. Biochem Biophys Res Commun. 2010;398:278–283. [DOI] [PubMed] [Google Scholar]
- 235.Hanayama R, Tanaka M, Miwa K, et al. . Identification of a factor that links apoptotic cells to phagocytes. Nature. 2002;417:182–187. [DOI] [PubMed] [Google Scholar]
- 236.Ait-Oufella H, Kinugawa K, Zoll J, et al. . Lactadherin deficiency leads to apoptotic cell accumulation and accelerated atherosclerosis in mice. Circulation. 2007;115:2168–2177. [DOI] [PubMed] [Google Scholar]
- 237.Dasgupta SK, Abdel-Monem H, Niravath P, et al. . Lactadherin and clearance of platelet-derived microvesicles. Blood. 2009;113:1332–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Tedgui A, Mallat Z. Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol Rev. 2006;86:515–581. [DOI] [PubMed] [Google Scholar]
- 239.Angelot F, Seilles E, Biichle S, et al. . Endothelial cell-derived microparticles induce plasmacytoid dendritic cell maturation: potential implications in inflammatory diseases. Haematologica. 2009;94:1502–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Eken C, Gasser O, Zenhaeusern G, et al. . Polymorphonuclear neutrophil-derived ectosomes interfere with the maturation of monocyte-derived dendritic cells. J Immunol. 2008;180:817–824. [DOI] [PubMed] [Google Scholar]
- 241.Shefler I, Salamon P, Reshef T, et al. . T cell-induced mast cell activation: a role for microparticles released from activated T cells. J Immunol. 2010;185:4206–4212. [DOI] [PubMed] [Google Scholar]
- 242.Weber A, Köppen HO, Schrör K. Platelet-derived microparticles stimulate coronary artery smooth muscle cell mitogenesis by a PDGF-independent mechanism. Thromb Res. 2000;98:461–466. [DOI] [PubMed] [Google Scholar]
- 243.Pakala R Serotonin and thromboxane A2 stimulate platelet-derived microparticle-induced smooth muscle cell proliferation. Cardiovasc Radiat Med. 2004;5:20–26. [DOI] [PubMed] [Google Scholar]
- 244.Sato Y, Asada Y, Marutsuka K, et al. . Tissue factor induces migration of cultured aortic smooth muscle cells. Thromb Haemost. 1996;75:389–392. [PubMed] [Google Scholar]
- 245.Marutsuka K, Hatakeyama K, Sato Y, et al. . Protease-activated receptor 2 (PAR2) mediates vascular smooth muscle cell migration induced by tissue factor/factor VIIa complex. Thromb Res. 2002;107:271–276. [DOI] [PubMed] [Google Scholar]
- 246.Moreno PR, Purushothaman KR, Zias E, et al. . Neovascularization in human atherosclerosis. Curr Mol Med. 2006;6:457–477. [DOI] [PubMed] [Google Scholar]
- 247.Kolodgie FD, Gold HK, Burke AP, et al. . Intraplaque hemorrhage and progression of coronary atheroma. N Engl J Med. 2003;349:2316–2325. [DOI] [PubMed] [Google Scholar]
- 248.Canault M, Leroyer AS, Peiretti F, et al. . Microparticles of human atherosclerotic plaques enhance the shedding of the tumor necrosis factor-alpha converting enzyme/ADAM17 substrates, tumor necrosis factor and tumor necrosis factor receptor-1. Am J Pathol. 2007;171:1713–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Distler JHW, Jungel A, Huber LC, et al. . The induction of matrix metalloproteinase and cytokine expression in synovial fibroblasts stimulated with immune cell microparticles. Proc Natl Acad Sci. 2005;102:2892–2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Dashevsky O, Varon D, Brill A. Platelet-derived microparticles promote invasiveness of prostate cancer cells via upregulation of MMP-2 production. Int J Cancer. 2009;124:1773–1777. [DOI] [PubMed] [Google Scholar]
- 251.Leroyer AS, Rautou PE, Silvestre JS, et al. . CD40 ligand + microparticles from human atherosclerotic plaques stimulate endothelial proliferation and angiogenesis. J Am Coll Cardiol. 2008;52:1302–1311. [DOI] [PubMed] [Google Scholar]
- 252.Philippova M, Suter Y, Toggweiler S, et al. . T-cadherin is present on endothelial microparticles and is elevated in plasma in early atherosclerosis. Eur Heart J. 2011;32:760–771. [DOI] [PubMed] [Google Scholar]
- 253.Koga H, Sugiyama S, Kugiyama K, et al. . Elevated levels of VE-cadherin-positive endothelial microparticles in patients with type 2 diabetes mellitus and coronary artery disease. J Am Coll Cardiol. 2005;45:1622–1630. [DOI] [PubMed] [Google Scholar]
- 254.Mallat Z, Benamer H, Hugel B, et al. . Elevated levels of shed membrane microparticles with pocoagulant potential in the peripheral circulating blood of patients with acute coronary syndromes. Circulation. 2000;101:841–843. [DOI] [PubMed] [Google Scholar]
- 255.Bernal-Mizrachi L, Jy W, Jimenez JJ, et al. . High levels of circulating endothelial microparticles in patients with acute coronary syndromes. Am Heart J. 2003;145:962–970. [DOI] [PubMed] [Google Scholar]
- 256.Morel O, Hugel B, Jesel L, et al. . Circulating procoagulant microparticles and soluble GPV in myocardial infarction treated by primary percutaneous transluminal coronary angioplasty. A possible role for GPIIb-IIIa antagonists. J Thromb Haemost. 2004;2:1118–1126. [DOI] [PubMed] [Google Scholar]
- 257.Radecke CE, Warrick AE, Singh GD, et al. . Coronary artery endothelial cells and microparticles increase expression of VCAM-1 in myocardial infarction. Thromb Haemost. 2015;113:605–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Montoro-García S, Shantsila E, Tapp LD, et al. . Small-size circulating microparticles in acute coronary syndromes: relevance to fibrinolytic status, reparative markers and outcomes. Atherosclerosis. 2013;227:313–322. [DOI] [PubMed] [Google Scholar]
- 259.Zhou B, Li J, Chen S, et al. . Time course of various cell origin circulating microparticles in ST-segment elevation myocardial infarction patients undergoing percutaneous transluminal coronary intervention. Exp Ther Med. 2016;11:1481–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Min PK, Kim JY, Chung KH, et al. . Local increase in microparticles from the aspirate of culprit coronary arteries in patients with ST-segment elevation myocardial infarction. Atherosclerosis. 2013;227:323–328. [DOI] [PubMed] [Google Scholar]
- 261.Suades R, Padró T, Crespo J, et al. . Circulating microparticle signature in coronary and peripheral blood of ST elevation myocardial infarction patients in relation to pain-to-PCI elapsed time. Int J Cardiol. 2016;202:378–387. [DOI] [PubMed] [Google Scholar]
- 262.Biasucci LM, Porto I, Di Vito L, et al. . Differences in microparticle release in patients with acute coronary syndrome and stable angina. Circ J. 2012;76:2174–2182. [DOI] [PubMed] [Google Scholar]
- 263.Huisse MG, Ajzenberg N, Feldman L, et al. . Microparticle-linked tissue factor activity and increased thrombin activity play a potential role in fibrinolysis failure in ST-segment elevation myocardial infarction. Thromb Haemost. 2009;101:734–740. [PubMed] [Google Scholar]
- 264.Maly M, Hrachovinova I, Tomasov P, et al. . Patients with acute coronary syndromes have low tissue factor activity and microparticle count, but normal concentration of tissue factor antigen in platelet free plasma: a pilot study. Eur J Haematol. 2009;82:148–153. [DOI] [PubMed] [Google Scholar]
- 265.Jung C, Sörensson P, Saleh N, et al. . Circulating endothelial and platelet derived microparticles reflect the size of myocardium at risk in patients with ST-elevation myocardial infarction. Atherosclerosis. 2012;221:226–231. [DOI] [PubMed] [Google Scholar]
- 266.Crawford JR, Trial J, Nambi V, et al. . Plasma levels of endothelial microparticles bearing monomeric C-reactive protein are increased in peripheral artery disease. J Cardiovasc Trans Res. 2016;9:184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Chironi GN, Simon A, Boulanger CM, et al. . Circulating microparticles may influence early carotid artery remodeling. J Hypertens. 2010;28:789–796. [DOI] [PubMed] [Google Scholar]
- 268.van der Zee PM, Biró E, Ko Y, et al. . P-Selectin- and CD63-exposing platelet microparticles reflect platelet activation in peripheral arterial disease and myocardial infarction. Clin Chem. 2006;52:657–664. [DOI] [PubMed] [Google Scholar]
- 269.Nomura S, Imamura A, Okuno M, et al. . Platelet-derived microparticles in patients with arteriosclerosis obliterans: enhancement of high shear-induced microparticle generation by cytokines. Thromb Res. 2000;98:257–268. [DOI] [PubMed] [Google Scholar]
- 270.Jung KH, Chu K, Lee ST, et al. . Circulating endothelial microparticles as a marker of cerebrovascular disease. Ann Neurol. 2009;66:191–199. [DOI] [PubMed] [Google Scholar]
- 271.Simak J, Gelderman MP, Yu H, et al. . Circulating endothelial microparticles in acute ischemic stroke: a link to severity, lesion volume and outcome. J Thromb Haemost. 2006;4:1296–1302. [DOI] [PubMed] [Google Scholar]
- 272.Cherian P, Hankey GJ, Eikelboom JW, et al. . Endothelial and platelet activation in acute ischemic stroke and its etiological subtypes. Stroke. 2003;34:2132–2137. [DOI] [PubMed] [Google Scholar]
- 273.Montoro-García S, Shantsila E, Wrigley BJ, et al. . Small-size microparticles as indicators of acute decompensated state in ischemic heart failure. Rev Española Cardiol (Engl Ed). 2015;68:951–958. [DOI] [PubMed] [Google Scholar]
- 274.Bulut D, Maier K, Bulut-Streich N, et al. . Circulating endothelial microparticles correlate inversely with endothelial function in patients with ischemic left ventricular dysfunction. J Card Fail. 2008;14:336–340. [DOI] [PubMed] [Google Scholar]
- 275.Berezin AE, Kremzer AA. Impaired phenotype of circulating endothelial microparticles in chronic heart failure patients: relevance to body mass index. Diabetes Metab Syndr Clin Res Rev. 2015;9:230–236. [DOI] [PubMed] [Google Scholar]
- 276.Garcia S, Chirinos J, Jimenez J, et al. . Phenotypic assessment of endothelial microparticles in patients with heart failure and after heart transplantation: switch from cell activation to apoptosis. J Hear Lung Transplant. 2005;24:2184–2189. [DOI] [PubMed] [Google Scholar]
- 277.Ivak P, Pitha J, Wohlfahrt P, et al. . Endothelial dysfunction expressed as endothelial microparticles in patients with end-stage heart failure. Physiol Res. 2014;63:S369–S373. [DOI] [PubMed] [Google Scholar]
- 278.Walenta K, Schwarz V, Schirmer SH, et al. . Circulating microparticles as indicators of peripartum cardiomyopathy. Eur Heart J. 2012;33:1469–1479. [DOI] [PubMed] [Google Scholar]
- 279.Choudhury A, Chung I, Blann AD, et al. . Elevated platelet microparticle levels in nonvalvular atrial fibrillation: relationship to p-selectin and antithrombotic therapy. Chest. 2007;131:809–815. [DOI] [PubMed] [Google Scholar]
- 280.Ederhy S, Di Angelantonio E, Mallat Z, et al. . Levels of circulating procoagulant microparticles in nonvalvular atrial fibrillation. Am J Cardiol. 2007;100:989–994. [DOI] [PubMed] [Google Scholar]
- 281.Azzam H, Zagloul M. Elevated platelet microparticle levels in valvular atrial fibrillation. Hematology. 2009;14:357–360. [DOI] [PubMed] [Google Scholar]
- 282.Hayashi M, Takeshita K, Inden Y, et al. . Platelet activation and induction of tissue factor in acute and chronic atrial fibrillation: involvement of mononuclear cell-platelet interaction. Thromb Res. 2011;128:e113–e118. [DOI] [PubMed] [Google Scholar]
- 283.Diehl P, Aleker M, Helbing T, et al. . Increased platelet, leukocyte and endothelial microparticles predict enhanced coagulation and vascular inflammation in pulmonary hypertension. J Thromb Thrombolysis. 2011;31:173–179. [DOI] [PubMed] [Google Scholar]
- 284.Amabile N, Heiss C, Real WM, et al. . Circulating endothelial microparticle levels predict hemodynamic severity of pulmonary hypertension. Am J Respir Crit Care Med. 2008;177:1268–1275. [DOI] [PubMed] [Google Scholar]
- 285.Narin N, Yilmaz E, Pamukcu O, et al. . Are endothelial microparticles early markers of pulmonary hypertension? Biomarkers. 2014;19:319–325. [DOI] [PubMed] [Google Scholar]
- 286.Lin ZB, Ci HB, Li Y, et al. . Endothelial microparticles are increased in congenital heart diseases and contribute to endothelial dysfunction. J Transl Med. 2017;15:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Blair LA, Haven AK, Bauer NN. Circulating microparticles in severe pulmonary arterial hypertension increase intercellular adhesion molecule-1 expression selectively in pulmonary artery endothelium. Respir Res. 2016;17:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Belik D, Tsang H, Wharton J, et al. . Endothelium-derived microparticles from chronically thromboembolic pulmonary hypertensive patients facilitate endothelial angiogenesis. J Biomed Sci. 2016;23:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Sinning JM, Losch J, Walenta K, et al. . Circulating CD31+/Annexin V + microparticles correlate with cardiovascular outcomes. Eur Heart J. 2011;32:2034. [DOI] [PubMed] [Google Scholar]
- 290.Chiva-Blanch G, Bratseth V, Ritschel V, et al. . Monocyte-derived circulating microparticles (CD14+, CD14+/CD11b + and CD14+/CD142+) are related to long-term prognosis for cardiovascular mortality in STEMI patients. Int J Cardiol. 2017;227:876–881. [DOI] [PubMed] [Google Scholar]
- 291.Giannopoulos G, Oudatzis G, Paterakis G, et al. . Red blood cell and platelet microparticles in myocardial infarction patients treated with primary angioplasty. Int J Cardiol. 2014;176:145–150. [DOI] [PubMed] [Google Scholar]
- 292.Steppich BA, Braun SL, Stein A, et al. . Plasma TF activity predicts cardiovascular mortality in patients with acute myocardial infarction. Thrombosis J. 2009;7:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Fan Y, Wang L, Li Y, et al. . Quantification of endothelial microparticles on modified cytometric bead assay and prognosis in chest pain patients. Circ J. 2014;78:206–214. [DOI] [PubMed] [Google Scholar]
- 294.Berezin AE, Kremzer AA, Samura TA, et al. . Circulating endothelial-derived apoptotic microparticles in the patients with ischemic symptomatic chronic heart failure: relevance of pro-inflammatory activation and outcomes. Int Cardiovasc Res J. 2014;8:116–123. [PMC free article] [PubMed] [Google Scholar]
- 295.Nascimbene A, Hernandez R, George JK, et al. . Association between cell-derived microparticles and adverse events in patients with nonpulsatile left ventricular assist devices. J Heart Lung Transplant. 2014;33:470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Amabile N, Heiss C, Chang V, et al. . Increased CD62e + endothelial microparticle levels predict poor outcome in pulmonary hypertension patients. J Hear Lung Transplant. 2009;28:1081–1086. [DOI] [PubMed] [Google Scholar]