Abstract
Objectives
We studied the determinants of high healthcare costs (highest decile of hospital care and medication costs) and cost trajectories among all community-dwellers with clinically verified Alzheimer’s disease (AD), diagnosed during 2005–2011 in Finland (N = 70,531).
Methods
The analyses were done separately for hospital care costs, medication costs and total healthcare costs that were calculated for each 6-month period from 5 years before to 3 years after AD diagnosis.
Results
Total healthcare costs were driven mainly by hospital care costs. The definition of “high-cost person” was time-dependent as 63% belonged to the highest 10% at some timepoint during the study period and six distinct cost trajectories were identified. Strokes, cardiovascular diseases, fractures and mental and behavioural disorders were most strongly associated with high hospital care costs.
Conclusions
Although persons with AD are often collectively considered as expensive patient group, there is large temporal and inter-individual variation in belonging to the highest decile of hospitalization and/or medication costs. It would be important to assess whether hospitalization rate could be decreased by, e.g., comprehensive outpatient care with more efficient management of comorbidities. In addition, other interventions that could decrease hospitalization rate in persons with dementia should be studied further in this context.
Key messages
Persons with AD had large individual fluctuation in hospital care costs and medication costs over time.
Hospital care costs were considerably larger than medication costs, with fractures, cardiovascular diseases and mental and behavioural disorders being the key predictors.
Antidementia medication was associated with lower hospital care costs.
Keywords: Alzheimer’s disease, costs, hospitalization, medications, trajectory analyses
Introduction
Population aging and consequently increase in the prevalence of Alzheimer’s disease (AD) are creating unmanageable costs for healthcare [1,2]. Regardless of country and healthcare system, persons with AD have higher healthcare costs than those without AD [1,3–9]. However, there is considerable inter-individual variation in the healthcare costs between individuals with AD. Knowledge on the factors associated with high costs could aid in planning and allocating healthcare resources. Currently, there is little data on characteristics associated with high costs or development of different cost trajectories in relation to AD diagnosis. Previous studies have focussed on smaller study populations [6,7,9] or participants of specific insurance schemes and assessed costs after AD/dementia diagnosis [4,6,7,10].
In previous studies from USA, persons with AD/dementia had higher prevalence of diabetes [4,6], congestive heart failure [6,7], cardiovascular and cerebrovascular diseases [4,6,7] or psychiatric disorders [4] and having these comorbidities was also associated with higher costs [1,4,6]. Other studies have assessed indirect costs and whether functional capability, indicated by cognitive status or ability to perform activities of daily living predict high costs among persons who already are diagnosed with AD [10–13].
We assessed the predictors of direct healthcare costs, defined as hospital care and medication costs from 5 years before AD diagnosis to 3 years after. The cohort included all community-dwellers who received a clinical diagnosis of AD in Finland in 2005–2011. The specific aims were to (1) study the predictors of high healthcare costs, (2) compare the predictors of high medication and hospital care costs and (3) identify different cost trajectories.
Methods
Study population
The Medication use and Alzheimer’s disease (MEDALZ) cohort was utilized in this study. It includes all community-dwelling persons who received a clinically verified diagnosis of AD in 2005–2011 (N = 70,719). The cohort and data sources have been previously described in detail [14]. The data sources included national Prescription register (purchased prescribed medications, medication costs), Special Reimbursement register (comorbidities), Care Register for Healthcare (comorbidities, hospitalizations) and socioeconomic data from Statistics Finland. Data retrieval across registers was done by the register maintainers by utilizing Finnish personal identification number, which is appointed to all residents. Data were deidentified before submission to the research team and the study participants were not contacted. Therefore, approval from the ethics committee or a written consent from the cohort participants was not required according to Finnish legislation. The MEDALZ study protocol was approved by the register maintainers.
Diagnoses in the Special Reimbursement register are done according to pre-specified diagnostic protocols monitored by the Social Insurance Institution (SII). The AD diagnosis is done according to the national clinical care guideline [15] and based on the NINCDS-ADRDA [16] and DSM-IV criteria [17]. The verified AD diagnosis includes the exclusion of alternative causes or diagnoses, computed tomography or magnetic resonance imaging scan and confirmation of the diagnosis by a geriatrician or neurologist. As the reimbursement is granted only for persons with mild or moderate AD, all cohort members had either mild or moderate AD at the time of diagnosis.
The Finnish primary healthcare is organized by municipalities. The health care services are implemented with government support according to the legislation and monitored by the Ministry of Social Affairs and Health. The most advanced specialized medical care is organized by five university hospital districts (Helsinki, Turku, Tampere, Oulu and Kuopio). To account for possible regional variation in costs between university hospital districts, these regions were included as a random effect in the models and persons living in Åland archipelago (n = 164) and those with missing region data (n = 24) were excluded from the study. The final sample size was 70,531.
Hospital care costs and medication costs
We investigated the total healthcare costs, consisting from hospitalization and outpatient medication expenses from 5 years before the AD diagnosis until 3 years after. The follow-up ended on end of study period (3 years after AD diagnosis; n = 37,594), end of data linkage (31 December 2012; n = 16,074), death (n = 14,041) or permanent institutionalization (n = 2822). The average follow-up time was 89 months, ranging between 48 and 96 months. The National Health Insurance scheme is part of the Finnish social security system. All permanent residents are covered under this scheme, which covers treatments medically necessary due to illness, pregnancy or childbirth. Insurance covers majority of hospital care costs for patients and does not depend on the received treatment. For medications, the proportion of covered costs varies across medications. Therefore, the costs in our study refer to hospitalization and medication costs payed by society, including the costs reimbursed to patients. The follow-up was divided to 16 six-month periods. Cumulative costs were calculated from each period and transformed to costs per follow-up day. Periods when the person was institutionalized were not included in the analyses (1253 periods for 1152 persons, 254 of whom were later censored due to permanent institutionalization).
Data on hospital stays were obtained from the national Care Register for Healthcare which contains data on all hospitalizations and discharge diagnoses. Hospital costs were calculated based on the length of the hospital stay and the level of the caring unit using the Finnish health care system unit costs from 2006 and 2011 [18]. The most recent version was used for each year. These unit cost estimates were derived specifically for research purposes and they are adjusted for regional price differences. Hospital care costs were calculated from the service provider’s perspective and covered operation and diagnostic costs, and the medication costs during the hospital stay.
The Prescription register contains data on reimbursed drugs dispensed from pharmacies. We utilized the total cost of the medication claim, and costs of all dispensing’s during the study period. All the costs in Euro (€) were then valued at year 2011 price index public expenditure rate [19].
High costs were defined as those belonging to the top 10% percentile of the cost, which was defined at each half-year period. This approach was chosen based on previous studies to determine high-cost persons [4,10,20]. This classification was done separately for hospital care costs, medication costs and total costs.
Predictors of high costs
Comorbidities were chosen on the basis of the previous literature on determinants of cost differences between persons with and without Alzheimer’s disease [1,4,6], and our previous study on the accumulation of hospital stays in different specialties of care in persons with AD [5]. Data on comorbidities were extracted since 1972 and categorized as follows: “none”, “only before follow-up”, “during follow-up” and “both before and during follow-up” and further merged into “none”, “before the follow-up” and “before and during the follow-up” for descriptive analyses, based on the similarity of association of those categories that included comorbidities diagnosed before the follow-up. In mixed models, the covariate status before the period and during the period were taken into account. In latent class growth analyses, latent classes were regressed on covariates measured at baseline.
Data on diabetes (reimbursement code 103), cardiovascular diseases (hypertension, coronary artery disease, familial hypercholesterolemia, heart failure and cardiac arrhythmias; reimbursement codes 201, 205, 206, 207, 213), asthma or chronic obstructive pulmonary disease (203, 210) were obtained from the Special Reimbursement Register [21]. Data on strokes (ICD-10 [22] codes I60-I64), all fractures (S*2 or T02), hip fractures (S72.0–72.2), ischaemic heart diseases (I20–25) and mental and behavioural disorders excluding dementia (F04–F99) were extracted from the national Care Register of Healthcare. Active cancer was defined as cancer treatment with medication, surgery or radiation therapy [23].
Medications (since 1995) were obtained from the Prescription register. Tumour necrosis factor alpha (TNF-α) inhibitors (ATC L04AB), pregabalin (N03AX16), bisphosphonate (M05BA, M05BB), erythropoietin (B03XA01, B03XA02) and antidementia medication (N06D) were chosen due to their high price in the study period and, psychotropic medication antipsychotics N05A, antidepressants N06A, benzodiazepines and related drugs N05BA, N05CD, N05CF) because their use indicates presence of various psychiatric and behavioural symptoms. Data were extracted with the same categorization as comorbidities and based on similarity between categories, merged further to “no” and “yes”, except for psychotropics which were categorized to “Never”, “Before the follow-up”, “During the follow-up” and “Before and during the follow-up”. For the mixed-effect model, the diagnoses and medication were coded to categories “before the follow-up” and “during follow-up”.
Occupational social class was used as a proxy for socioeconomic position. Data on occupational social class were obtained from the censuses maintained by Statistics Finland. The data were collected on 5-year intervals between 1970 and 1990, on 1993, 1995, 2000 and annually from 2004 onwards. The 2010 version of the original classification is available at http://www.stat.fi/meta/luokitukset/ammatti/001-2010/index_en.html, older versions available from authors by request). We investigated the associations between the original categories and AD and collapsed the information into a six-category variable with the following categories were derived “managerial/professional”, “office worker”, “farming/forestry”, “sales/industry/cleaning”, “unknown” and “did not respond”. For each individual, the highest class from 1970 until five years before the AD diagnosis was used.
Statistical analyses
Statistical analyses were conducted utilizing Stata 14.0 (Stata Corp LP, College Station, TX, USA). Cross-tabulation and χ2-tests were used for descriptive analyses and multivariate mixed-effect logistic regression modelling for time-dependent analyses of the predictors of high costs. Nearly all multivariate models converged within 25 iterations. Results of non-convergent models were confirmed with random-effects logit model, which gave similar results to the results observed with the 25th iteration.
Logistic latent class growth analyses were derived with Mplus version 7.4 [24] to identify potential subpopulations with similarities in cost trajectories instead of assuming that a single growth trajectory would adequately approximate the entire population. Number of subpopulations, latent classes, is estimated from the data as well as a set of model parameters (intercept, slopes) for each latent class. In this study, models with linear and quadratic growth factors and with 2–6 latent classes were fitted for the binary indicator of belonging to the highest decile of total costs. Models with more than six classes were determined to be too complex to interpret. Baseline covariates were used to predict latent class (trajectory) membership. The method uses full-information maximum likelihood in estimation with all available data and is valid under assumption of data missing at random. We applied sample-size adjusted Bayesian information criteria (SABIC) to identify the best-fitting model [25]. In addition, Lo-Mendell-Rubin likelihood ratio test (LMR-LRT) was used to compare the fitted n-class model with a n-1-class model with p < .05 indicating that the one additional class significantly improved the model fit [26]. The final model was also required to provide good interpretability and have class sizes over 1% of the total sample size. Multinomial logistic regression models were applied to detect differences in baseline characteristics between latent classes.
Results
Participant characteristics
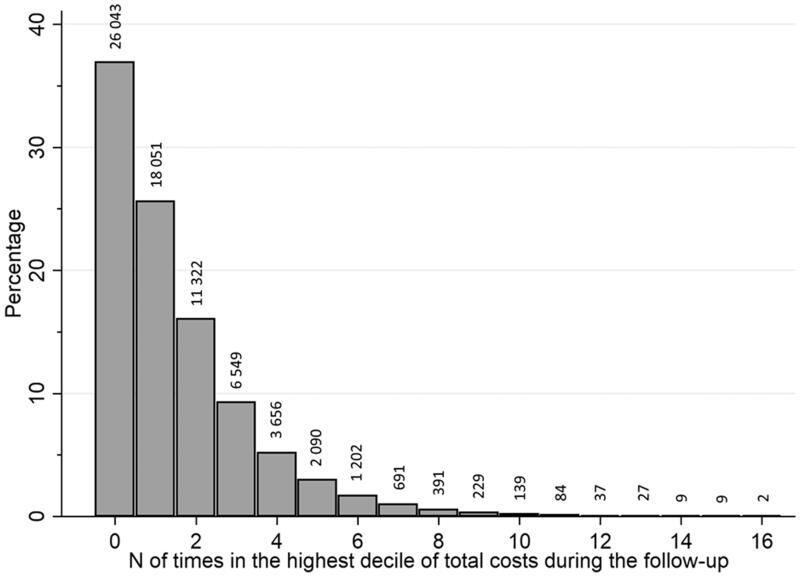
The characteristics of study participants according to whether they belonged to the highest decile of total healthcare cost on any 6-month interval are shown in Table 1. Altogether 44,488 persons (62.9% of the study population) belonged to the highest decile at in at least one of the 16 timepoints, implying that persons belonging in the high-cost decile differed during different time periods. Figure 1 shows the distribution of how many times study group persons belonged to the highest decile during follow-up. All comorbidities were more common among those with the highest total costs (Table 1). Acute events (stroke and fractures), ischaemic heart disease and mental and behavioural disorders during the follow-up were more common in the high-cost group. Use of pregabalin, bisphosphonates, erythropoietin and psychotropic medication were more common, and use of antidementia medication less common among the high-cost persons when total healthcare costs were assessed. On average, two-thirds of the total healthcare costs were due to hospital care costs (median 67.7%, interquartile range 38.0–84.5%).
Table 1.
Characteristics of the study population per the total healthcare costs during the follow-up (5 years before AD diagnosis to 3 years after AD diagnosis; hospital care and medication costs combined).
| Never in highest decile, N = 26,043 | In the highest decile in any time point, N = 44,488 | |
|---|---|---|
| Age (mean and 95% CL)b | 78.50 (78.41–78.59) | 80.97 (80.91–81.03) |
| Sexb | ||
| Men | 8888 (34.1) | 15,638 (35.2) |
| Women | 17,155 (65.9) | 28,850 (64.9) |
| Diabetes Mellitusb | ||
| Never | 23,109 (88.7) | 36,813(82.8) |
| Before and during the follow-up | 2934 (11.3) | 7675 (17.3) |
| Cardiovascular diseasesb | ||
| Never | 14,664 (56.3) | 18,923 (42.5) |
| Before and during the follow-up | 11,379 (43.7) | 25,565 (57.5) |
| Asthma or COPDb | ||
| Never | 24,228 (93.0) | 39,862 (89.6) |
| Before and during the follow-up | 1815 (7.0) | 4626 (10.4) |
| Mental and behavioural disordersb | ||
| Never | 23,815 (91.4) | 35,368 (79.5) |
| Only before the follow-up | 1234 (4.7) | 2399 (5.4) |
| Before and during the follow-up | 994 (3.8) | 6721 (15.1) |
| Any fractureb | ||
| Never | 21,956 (84.3) | 28,888 (64.9) |
| Only before the follow-up | 1498 (5.8) | 2363 (5.3) |
| Before and during the follow-up | 2589 (9.9) | 13,237 (29.8) |
| Ischaemic heart diseaseb | ||
| Never | 20,809 (79.9) | 26,799 (60.2) |
| Only before the follow-up | 2222 (8.5) | 2988 (6.7) |
| Before and during the follow-up | 3012 (11.6) | 14,701 (33.0) |
| Strokeb | ||
| Never | 24,204 (92.9) | 36,462 (82.0) |
| Only before the follow-up | 864 (3.3) | 1895 (4.3) |
| Before and during the follow-up | 975 (3.7) | 6131 (13.8) |
| Hip fracturea | ||
| Never | 25,397 (97.5) | 38,093 (85.6) |
| Only before the follow-up | 234 (0.9) | 750 (1.7) |
| Before and during the follow-up | 412 (1.6) | 5645 (12.7) |
| Active cancerb | ||
| Never | 25,727 (98.8) | 43,263 (97.3) |
| Only before the follow-up | 68 (0.3) | 270 (0.6) |
| Before and during the follow-up | 248 (1.0) | 955 (2.2) |
| Tumour necrosis factor alpha (TNF-α) inhibitorsb | ||
| No | 26,042 (100.0) | 44,468 (99.96) |
| Yes | 1 (<0.001) | 20 (0.04) |
| Pregabalina | ||
| No | 25,015 (96.1) | 40,859 (91.8) |
| Yes | 1028 (4.0) | 3629 (8.2) |
| Bisphosphonatesa | ||
| No | 22,947 (88.1) | 35,156 (79.0) |
| Yes | 3096 (11.9) | 9332 (21.0) |
| Psychotropic drugsa | ||
| Never | 8846 (34.0) | 8 806 (19.8) |
| before the follow-up | 1271 (4.9) | 1551 (3.5) |
| During the follow-up | 8156 (31.3) | 15,149 (34.1) |
| Before and during the follow-up | 7770 (29.8) | 18,982 (42.7) |
| Erythropoietina | ||
| No | 26,026 (99.9) | 44,285 (99.5) |
| Yes | 17 (0.1) | 203 (0.5) |
| Antidementia medicationa | ||
| No | 1368 (5.3) | 4608 (10.4) |
| Yes | 24,675 (94.8) | 39,880 (89.6) |
Data are given as N (%) unless otherwise indicated.
ap < .01.
bp < .001.
Figure 1.
Percentage of study group belonging to the top 10% decile according to amount of different time points.
Characteristics according to high hospital care and medication costs are shown in Supplementary Tables 1 and 2, correspondingly. The associations between characteristics and high hospital care and medication costs were similar to those observed with high total costs. The associations of comorbidities were stronger with hospital than medication costs. The costs in relation to diagnosis of AD and proportions of the highest decile costs are shown in Supplementary Table 3. The costs were concentrated on the top 10% decile, with total costs of persons belonging to this decile accounting for 57.6–70.0% of total costs of the population. Hospital care costs were on average 2–3 times larger than the medication costs.
Predictors of high costs in time-dependent model
Associations between sociodemographic characteristics, comorbidities and high healthcare costs in the multivariate mixed-effect model are shown in Table 2. Older age was associated with higher total healthcare costs and hospital care costs, but with lower medication costs. Men had higher total healthcare costs, and the difference was more evident in medication costs than in hospital care costs. Persons with lower occupational social class were more likely to have high total and hospital care costs than persons in managerial/professional occupations, while persons with higher occupational social class were more likely to have high medication costs.
Table 2.
Associations of demographic characteristics and comorbidities with total, medication, and hospital care costs from 5 years before AD diagnosis to 3 years after AD diagnosis in the multilevel mixed-effects model.
| Total costs |
Medication costs |
Hospital costs |
||||
|---|---|---|---|---|---|---|
| OR | 95% CL | OR | 95% CL | OR | 95% CL | |
| Age | 1.03a | (1.02–1.03) | 0.99a | (0.99–0.99) | 1.03a | (1.03–1.03) |
| Occupational social class | ||||||
| Managerial/professional | 1.00 (reference)a | 1.00 (reference)a | 1.00 (reference)a | |||
| Office | 1.04 | (1.01–1.07) | 0.89 | (0.87–0.91) | 1.06 | (1.03–1.09) |
| Sales/cleaning/industrial | 1.14 | (1.12–1.16) | 0.93 | (0.92–0.95) | 1.16 | (1.14–1.18) |
| Farming/forestry | 1.07 | (1.04–1.09) | 0.89 | (0.88–0.91) | 1.09 | (1.07–1.12) |
| Unknown | 1.31 | (1.28–1.34) | 0.92 | (0.89–0.94) | 1.35 | (1.32–1.38) |
| Did not reply | 1.03 | (0.96–1.12) | 0.61 | (0.60–0.69) | 1.08 | (1.00–1.16) |
| Sex | ||||||
| Women | 1.00 (reference)a | 1.00 (reference)a | 1.00 (reference)a | |||
| Men | 1.07 | (1.06–1.09) | 1.31 | (1.29–1.33) | 1.03 | (1.02–1.05) |
| Diabetes | ||||||
| None | 1.00 (reference)a | 1.00 (reference)a | 1.00 (reference)a | |||
| Before or during follow-up | 1.51 | (1.49–1.54) | 3.23 | (3.18–3.27) | 1.41 | (1.38–1.43) |
| Cardiovascular diseases | ||||||
| None | 1.00 (reference)a | 1.00 (reference)a | 1.00 (reference)a | |||
| Before or during follow-up | 1.49 | (1.47–1.51) | 2.08 | (2.05–2.11) | 1.43 | (1.41-–1.45) |
| Asthma or COPD | ||||||
| None | 1.00 (reference)a,b | 1.00 (reference)a | 1.00 (reference)a | |||
| Before or during follow-up | 1.51 | (1.48–1.54) | 3.56 | (3.50–3.62) | 1.37 | (1.34–1.40) |
| Mental and behavioural disorders | ||||||
| None | 1.00 (reference)a | 1.00 (reference)a | 1.00 (reference)a,b | |||
| Before follow-up | 1.38a | (1.35–1.41) | 1.81a | (1.77–1.84) | 1.3 | (1.28–1.34) |
| During follow-up | 14.76a | (14.21–15.32) | 2.19a | (2.09–2.30) | 14.91 | (14.35–15.48) |
| Any fracture | ||||||
| None | 1.00 (reference)a | 1.00 (reference)a | 1.00 (reference)a,b | |||
| Before follow-up | 1.22 | (1.19–1.24) | 1.23 | (1.20–1.26) | 1.21 | (1.18–1.23) |
| During follow-up | 12.6 | (12.26–12.94) | 1.11 | (1.06–1.16) | 13.07 | (12.72–13.43) |
| Ischaemic heart disease | ||||||
| None | 1.00 (reference)a | 1.00 (reference)a,b | 1.00 (reference)a | |||
| Before follow-up | 1.19 | (1.17–1.21) | 1.95 | (1.91–1.98) | 1.13 | (1.11–1.15) |
| During follow-up | 10.57 | (10.34–10.81) | 2.33 | (2.27–2.40) | 10.53 | (10.29–10.77) |
| Stroke | ||||||
| None | 1.00 (reference)a,b | 1.00 (reference)a,b | 1.00 (reference)a | |||
| Before follow-up | 1.41 | (1.38–1.45) | 1.64 | (1.60–1.68) | 1.37a | (1.34–1.41) |
| During follow-up | 12.74 | (12.23–13.28) | 1.27 | (1.20–1.36) | 13.38 | (12.83–13.95) |
| Hip fracture | ||||||
| None | 1.00 (reference)a | 1.00 (reference)a | 1.00 (reference)a,b | |||
| Before follow-up | 1.61 | (1.54–1.68) | 1.21 | (1.16–1.27) | 1.64 | (1.57–1.71) |
| During follow-up | 37.62 | (35.46–39.91) | 0.72 | (0.66–0.79) | 39.42 | (37.13–41.85) |
| Active cancer | ||||||
| None | 1.00 (reference)a | 1.00(reference)a | 1.00 (reference)a | |||
| Before follow-up | 1.89 | (1.78–2.01) | 2.31 | (2.18–2.44) | 1.68a | (1.58–1.79) |
| During follow-up | 1.99 | (1.89–2.10) | 2.26 | (2.14–2.39) | 1.83a | (1.73–1.93) |
ap < .001.
bResults after 25th iteration, similar results obtained with random-effect logit model.
All comorbidities, regardless of whether they had occurred before or during the follow-up were associated with higher total, hospital and medication costs, except hip fractures during the follow-up, which had an inverse association with high medication costs (Table 2). Hip or other fractures, strokes, mental and behavioural disorders and ischaemic heart diseases were the comorbidities that were most strongly associated with hospital care costs, while diabetes, asthma/COPD, cardiovascular diseases and active cancer were most strongly associated with high medication costs.
Tumour necrosis factor alpha (TNF-α) inhibitors, erythropoietin, pregabalin and psychotropic medications, especially when used during the follow-up, were associated with higher total healthcare costs (Table 3). For erythropoietin and tumour necrosis factor alpha (TNF-α) inhibitors, this was driven by the association with high medication costs. Antidementia medication users were more likely to have higher medication costs, but less likely to have high hospital care costs and consequently, less likely to have high total healthcare costs.
Table 3.
Associations between medications and total, medication, and hospital care costs from 5 years before AD diagnosis to 3 years after AD diagnosis in the multilevel mixed-effects model.
| Total cost |
Medication cost |
Hospital cost |
||||
|---|---|---|---|---|---|---|
| OR | 95% CL | OR | 95% CL | OR | 95% CL | |
| Tumour necrosis factor alpha (TNF-α) inhibitors | ||||||
| None | 1.00 (reference)a | 1.00 (reference)a | 1.00 (reference)a | |||
| Before follow-up | 3.73 | (1.78–7.80) | 7.02 | (3.57–13.82) | 2.76* | (1.25–6.10) |
| During follow-up | 29.83 | (19.82–44.88) | 156.69 | (73.14–335.70) | 2.62 | (1.74–3.97) |
| Pregabalin | ||||||
| None | 1.00 (reference)a | 1.00 (reference)a,b | 1.00 (reference)a,b | |||
| Before follow-up | 1.67 | (1.48–1.89) | 1.97 | (1.75–2.21) | 1.60 | (1.42–1.81) |
| During follow-up | 2.32 | (2.23–2.42) | 5.12 | (4.95–5.30) | 2.07 | (1.98–2.16) |
| Bisphosphonates | ||||||
| None | 1.00 (reference)a | 1.00 (reference)a,b | 1.00 (reference)a | |||
| Before follow-up | 1.40 | (1.35–1.44) | 1.25 | (1.21–1.29) | 1.38 | (1.34–1.42) |
| During follow-up | 1.74 | (1.71–1.78) | 2.93 | (2.88–2.99) | 1.61 | (1.58–1.65) |
| Psychotropic drugs | ||||||
| None | 1.00 (reference)a | 1.00 (reference)a | 1.00 (reference)a,b | |||
| Before follow-up | 1.42 | (1.39–1.45) | 1.46 | (1.43–1.49) | 1.39 | (1.36–1.42) |
| During follow-up | 2.06 | (2.03–2.09) | 2.78 | (2.74–2.82) | 1.93 | (1.90–1.96) |
| Erythropoietin | ||||||
| None | 1.00 (reference)a,b | 1.00 (reference)a | 1.00 (reference)a | |||
| Before follow-up | 5.22 | (3.96–6.87) | 7.76 | (5.95–10.13) | 2.95 | (2.17–4.02) |
| During follow-up | 7.17 | (6.33–8.18) | 36.07 | (30.92–42.08) | 2.84 | (2.46–3.28) |
| Antidementia | ||||||
| None | 1.00 (reference)a | 1.00 (reference)a | 1.00 (reference)a | |||
| medication | 0.76 | (0.75–0.77) | 1.28 | (1.26–1.29) | 0.74 | (0.73–0.76) |
ap < .001.
bResults after 25th iteration, similar results obtained with random-effect logit model.
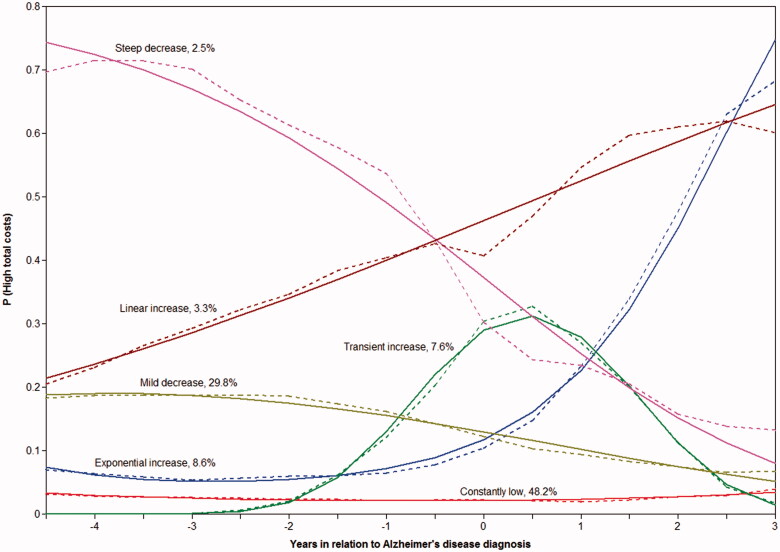
Cost trajectories
A model with linear and quadratic slopes and six latent classes (Figure 2) was the best-fitting model based on the smallest SABIC and statistically significant p value for LMR-LRT (Supplementary Table 4). Trajectory-wise average hospital, medication and total healthcare costs over time are displayed in Supplementary Figure 1.
Figure 2.
Total cost trajectories estimated with six-class conditional latent class growth model. Solid lines present estimated probabilities of being in the highest decile of total costs and dashed lines observed proportions of high-cost persons in each class.
Associations between baseline sociodemographic characteristics, comorbidities and medications at baseline and cost trajectories are shown in Supplementary Table 5. Persons in the “mild decrease”, “linear increase” and “exponential increase” groups were older than those in the “constantly low” group, and persons in the “steep decrease” and “linear increase” groups were more likely to use pregabalin in comparison to reference group “constantly low”. Diabetes was less common among persons belonging to the “steep decrease” group in comparison to the reference group.
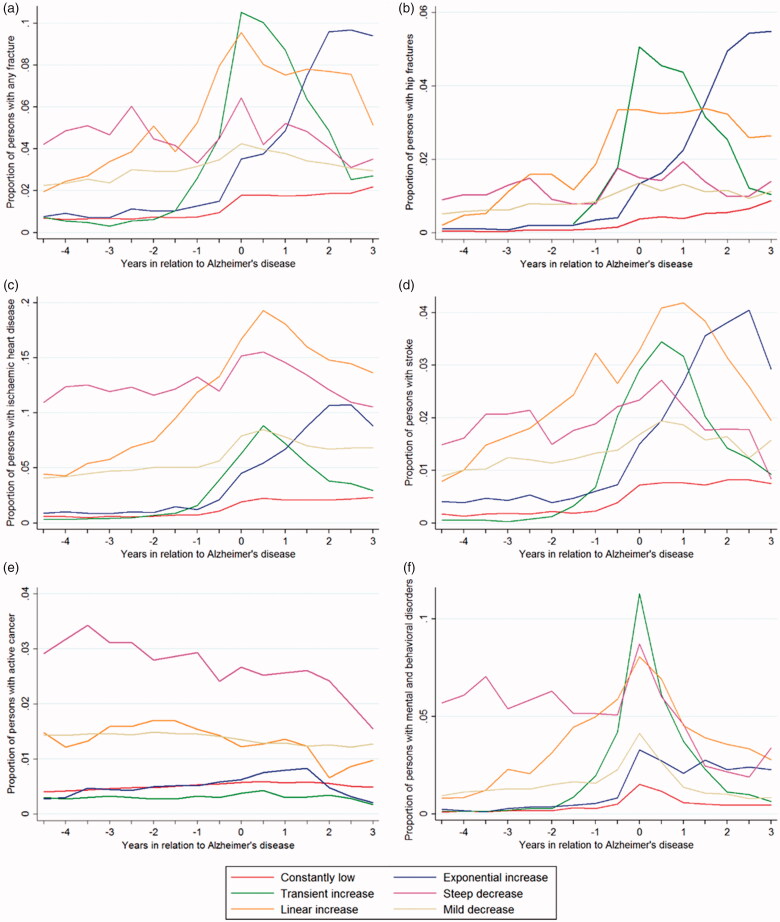
Majority of persons had low probability of belonging to the highest decile, with 48.2% belonging to the “constantly low” trajectory and 29.8% to “mild decrease” trajectory (Figure 2). For 7.6% of persons with AD, the probability of belonging to the highest cost decile was transiently elevated around the AD diagnosis and for 8.6% exponentially increased after AD diagnosis. The temporal fluctuation in the prevalence of comorbidities and drug use during the follow-up was generally consistent with the observed cost trajectories. This was most evident for fractures, strokes and mental and behavioural disorders (Figure 3). Prevalence trajectories of chronic comorbidities extracted from the special reimbursement register had less morphological similarity with the cost trajectories, but the differences in prevalence levels across cost trajectories were generally logical (Supplementary Figure 2), with those in the “constantly low” and “transient increase” trajectories having less cardiovascular diseases, diabetes and asthma/COPD than those in “linear” or “exponential increase” trajectories. On the other hand, these comorbidities were more common in persons in the “mild” and steep decrease trajectory. The use of expensive medications (tumour necrosis factor alpha inhibitors, erythropoietin, bisphosphonates and pregabalin) and psychotropic drugs were also more common among those belonging to “steep decrease” and “linear increase” trajectories (Supplementary Figure 3). Changes in antidementia medication use were similar across trajectories.
Figure 3.
Trajectories of hospital discharge diagnoses (a) any fracture, (b) hip fracture, (c) ischaemic heart disease, (d) stroke, (e) active cancer (f) mental and behavioural disorders according to cost trajectories.
Discussion
The main finding of our study is that the definition of high-cost AD patient varied across time. Over 60% of persons belonged to the highest cost decile at some timepoint between 5 years before the diagnosis and 3 years after the diagnosis, and six distinct trajectories for the probability of belonging to the highest tenth of total costs (including hospital care and medication costs) were identified. Hospital care costs exceeded the medication costs and fractures, especially hip fractures, mental and behavioural disorders, cardiovascular diseases and strokes were the strongest predictors. Nearly all medications were associated with higher costs, with antidementia medications being the only exception.
Our findings are comparable to previous literature on healthcare costs after AD diagnosis. In those studies, the total costs were also driven by hospital costs [3,6]. According to our trajectory analysis, the definition of high-cost person was strongly time-dependent and belonging to the highest cost decile more than five times was rare. Most of the costs were concentrated on the high decile group as the total costs of the top 10% cost decile accounted for 57–70% of total costs. This is in line with previous studies on the concentration of healthcare costs [10,18,20]. The transient increase trajectory and cost trajectories showed an transient increase in total costs around the diagnosis of AD, which is also shown in previous Finnish [8], Danish [9] and US [27] studies. This increase is partially explained by costs of antidementia medication [8], but also hospital [8,9], and outpatient care costs [9] increase in the same time window. Thus, the increase is also likely explained by costs related to diagnostic process, as several laboratory tests as well as imaging are required for excluding alternative diagnoses before granting the reimbursement for antidementia medication. These examinations may also lead to diagnosis and treatment of other comorbidities, which can partially account for the cost peak. Further, follow-up visits after the initiation of antidementia medication is necessary for monitoring possible adverse effects.
Diabetes, cardiovascular diseases, other circulatory diseases, different psychological disorders and symptoms and congestive heart failure have previously been associated with high costs [4,6,7], although in those studies only the costs after AD diagnosis were assessed. In our study, all comorbidities were associated with both high medication and hospitalization costs, with the exception of hip fractures. Their association with lower medication costs may be explained by long hospital stays after hip fracture, when the medications are provided by the hospital and thus not recorded in the prescription register.
Our study complements the previous literature by providing data on the association between medication use and healthcare costs. In general, the associations were weaker than with comorbidities, the only exceptions being erythropoietin and tumour necrosis factor alpha inhibitors, which are expensive medications. Antidementia medications were one of the few characteristics that were associated with smaller probability of belonging to the high-cost group of total or hospital costs. This is in line with previous US study, where lower total costs were observed among those persons with AD who used antidementia medication [6]. In our study, the association between antidementia medication and high medication costs is due to use of original patented medications, which were expensive during the study period. In Finland, costs of antidementia medications have declined since 2012, due to generic substitution and reference price system. Thus, the association with high medication costs may be different with more recent data.
Antidementia medication was used by 91.5% of the study population in at least one time point after the diagnosis. This is relatively high when compared with other countries such as US, UK and Germany where the prevalence of antidementia medication use has varied between 36% and 47% [28–30]. Further studies should be conducted whether more prevalent antidementia medication use can lower hospital care costs or whether our findings reflect differences between antidementia medication users and nonusers, such as better health (lack of contraindications), or different frequency in contact with healthcare personnel.
Our findings with the sociodemographic characteristics are also mostly supported by previous literature. In our study, men had higher probability of having high total costs, which was mainly influenced by high medication costs. This is in line with previous study on medication costs of high-costs patients which consisted of 50% of medication claims done by the adult population in Finland [31]. The observation on persons with lower occupational social class having stronger association with higher total and hospital care costs, but lower risk of high medication costs may imply health inequality, as observed in previous studies where persons with lower socioeconomic position had more comorbidities [32,33].
The study utilized data from Finnish healthcare registers, which have good nationwide coverage and good validity [34]. Our data on persons with AD included all the community-dwelling persons in one country, which enables us to get good results on the backgrounds of high-cost persons with AD. Previous studies have been limited to an insurance scheme or included smaller subgroups in the analysis. The time frame of our study was not limited to only the time after diagnosis of AD and the longitudinal data enabled us to have better information on the costs and development of costs in time, because of the inter-individual changes in cost.
Our study also has some limitations. The data on hospital costs is an estimation utilizing single unit costs, and the Prescription register included data only on the reimbursed drug dispensing. However, the main focus was on expensive medications which are rarely used without reimbursement. We had no data on presence of family caregiver or other support networks, which may impact the need of hospital care. We lacked data on caretaking costs, indirect costs and outpatient care costs. However, in this population, hospitalization costs are the main driver of healthcare costs. In a recent Danish study, the outpatient care costs were approximately 10% of hospital care costs [9].
Further, the severity of AD has previously been associated with high costs [35,36] and a significant increase occurs in hospitalization costs in the advanced stage [37]. Unfortunately, we had no data on clinical assessment of AD severity. However, time since AD diagnosis is a proxy of disease severity, and as this was accounted in our study design, some of the variation in in severity of AD was controlled for. As our study was restricted to community-dwellers, the results are not generalizable to institutionalized persons. However, institutionalized persons have higher healthcare costs [1,35,38] and including them in the analyses would have likely lead to a situation where the highest decile would have consisted of institutionalized persons.
Regardless of these limitations, our study shows that the group of high-cost patients is not constant over time, and the determinants of high costs may differ. Although the severity of AD is related to higher costs [35–37] our findings on the associations between comorbidities and high costs, the number of different likelihood trajectories of being a high-cost person, and the fact that approximately 10% of persons belonged to trajectories where the likelihood of being a high-cost person increased after AD diagnosis imply that factors other than AD progress are also important determinants. Further studies are needed to assess the predictors of high total costs, including other cost types and sources, among persons with AD in representative samples. These studies should also acknowledge the temporal fluctuation of costs in relation to AD diagnosis, as observed by us.
In conclusion, although AD/dementia is the key determinant of healthcare costs, our findings show that there is large inter-individual variation in costs between persons, and over time. It would be important to assess whether any of these hospitalizations would be preventable by e.g. comprehensive outpatient care with more efficient management of comorbidities. In addition, other interventions that could decrease hospitalization rate in persons with dementia should be studied further in this context.
Supplementary Material
Disclosure statement
HT reports grants from Janssen and Eli Lilly, outside the submitted work. AT has participated in research projects funded by Janssen Cilag and Eli Lilly and reports personal fees from Janssen Cilag, all outside the submitted work. JT reports personal fees from EMA (European Medicines Agency), personal fees from Fimea (Finnish Medicines Agency), grants and personal fees from Eli Lilly, grants and personal fees from Janssen-Cilag, personal fees from Lundbeck, personal fees from Otsuka, all outside the submitted work. SH reports personal fees from Swedish Research Council, outside the submitted work. Other authors report no conflicts of interest.
References
- 1.Alzheimer's Association. Alzheimer's disease facts and figures. Alzheimer's Dementia. 2016;12:459–509. [DOI] [PubMed] [Google Scholar]
- 2.Takizawa C, Thompson PL, van Walsem A, et al. Epidemiological and economic burden of Alzheimer's disease: a systematic literature review of data across Europe and the United States of America. J Alzheimer's Dis. 2014;43:1271–1284. [DOI] [PubMed] [Google Scholar]
- 3.Bynum JP, Rabins PV, Weller W, et al. The relationship between a dementia diagnosis, chronic illness, medicare expenditures, and hospital use. J Am Geriatr Soc. 2004;52:187–194. [DOI] [PubMed] [Google Scholar]
- 4.Kuo TC, Zhao Y, Weir S, et al. Implications of comorbidity on costs for patients with Alzheimer disease. Med Care. 2008;46:839–846. [DOI] [PubMed] [Google Scholar]
- 5.Tolppanen A, Taipale H, Purmonen T, et al. Hospital admissions, outpatient visits and healthcare costs of community-dwellers with Alzheimer's disease. Alzheimer's Dementia. 2015;11:955–963. [DOI] [PubMed] [Google Scholar]
- 6.Fillit H, Hill JW, Futterman R.. Health care utilization and costs of Alzheimer's disease: the role of comorbid conditions, disease stage, and pharmacotherapy. Fam Med-Kansas City. 2002;34:528–535. [PubMed] [Google Scholar]
- 7.Gutterman EM, Markowitz JS, Lewis B, et al. Cost of Alzheimer's Disease and related dementia in managed-medicare. J Am Geriatr Soc. 1999;47:1065–1071. [DOI] [PubMed] [Google Scholar]
- 8.Taipale H, Purhonen M, Tolppanen AM, et al. Hospital care and drug costs from five years before until two years after the diagnosis of Alzheimer's disease in a Finnish nationwide cohort. Scand J Public Health. 2016;44:150–158. [DOI] [PubMed] [Google Scholar]
- 9.Sopina E, Spackman E, Martikainen J, et al. Long-term medical costs of alzheimer's disease: matched cohort analysis. Eur J Health Econ. 2019;20:333–342. [DOI] [PubMed] [Google Scholar]
- 10.Lin PJ, Biddle AK, Ganguly R, et al. The concentration and persistence of health care expenditures and prescription drug expenditures in Medicare beneficiaries with Alzheimer disease and related dementias. Med Care. 2009;47:1174–1179. [DOI] [PubMed] [Google Scholar]
- 11.Zhu CW, Leibman C, McLaughlin T, et al. Patient dependence and longitudinal changes in costs of care in Alzheimer's disease. Dement Geriatr Cogn Disord. 2008;26:416–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leicht H, König H, Stuhldreher N, et al. Predictors of costs in dementia in a longitudinal perspective. PLoS One. 2013;8:e70018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reese JP, Heßmann P, Seeberg G, et al. Cost and care of patients with Alzheimer's disease: clinical predictors in German health care settings. J Alzheimer Dis. 2011;27:723–736. [DOI] [PubMed] [Google Scholar]
- 14.Tolppanen AM, Taipale H, Koponen M, et al. Cohort profile: the Finnish Medication and Alzheimer's disease (MEDALZ) study. BMJ Open. 2016;6:12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finnish Medical Society Duodecim . Finnish current care recommendation: memory disorders. Helsinki: 2010. [Google Scholar]
- 16.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease Report of the NINCDS‐ADRDA Work Group* under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939. [DOI] [PubMed] [Google Scholar]
- 17.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 18.Kapiainen S, Visnen A, Haula T. Terveyden-ja sosiaalihuollon yksikkökustannukset Suomessa vuonna Raportti: 2014_003 2014; 2011. [Google Scholar]
- 19.Official Statistics of Finland (OSF). Price index of public expenditure (e-publication). Helsinki: Statistics Finland; 2016. [Google Scholar]
- 20.Cohen SB. The concentration of health care expenditures in the US and predictions of future spending. J Econ Social Meas. 2016;41:167–189. [Google Scholar]
- 21.Social Insurance Institution . Decision on medical criteria required for entitlement to a special reimbursement on 24.11.2011 (in Finnish); 2011. Retrieved from http://www.kela.fi/in/internet/liite.nsf/%28wwwAllDocsById%29/280F01B0C811F242C22579920052E889/$file/ek12-01.pdf
- 22.World Health Organization . International classification of diseases, 10th revision, Finnish version. 1996. Helsinki, Finland: Terveyden ja hyvinvoinnin laitos. [Google Scholar]
- 23.Hamina A, Taipale H, Tanskanen A, et al. Long-term use of opioids among community-dwelling persons with and without Alzheimer's disease. Pain. 2017;158:252–260. [DOI] [PubMed] [Google Scholar]
- 24.Muthén LK, Muthén BO.. Mplus user’s guide. 7th ed. Los Angeles, CA: Muthén & Muthén; 2015. [Google Scholar]
- 25.Schwarz G. Estimating the dimension of a model. Ann Statist. 1978;6:461–464. [Google Scholar]
- 26.Lo Y, Mendell NR, Rubin DB.. Testing the number of components in a normal mixture. Biometrika. 2001;88:767–778. [Google Scholar]
- 27.Lin P, Zhong Y, Fillit HM, et al. Medicare expenditures of individuals with Alzheimer's disease and related dementias or mild cognitive impairment before and after diagnosis. J Am Geriatr Soc. 2016;64:1549–1557. [DOI] [PubMed] [Google Scholar]
- 28.Donegan K, Fox N, Black N, et al. Trends in diagnosis and treatment for people with dementia in the UK from 2005 to 2015: a longitudinal retrospective cohort study. The Lancet Public Health. 2017;2:e149. [DOI] [PubMed] [Google Scholar]
- 29.Gruber‐Baldini AL, Stuart B, Zuckerman IH, et al. Treatment of dementia in community‐dwelling and institutionalized medicare beneficiaries. J Am Geriatr Soc. 2007;55:1508–1516. [DOI] [PubMed] [Google Scholar]
- 30.Bohlken J, Schulz M, Rapp MA, et al. Pharmacotherapy of dementia in Germany: results from a nationwide claims database. Eur Neuropsychopharmacol. 2015;25:2333–2338. [DOI] [PubMed] [Google Scholar]
- 31.Saastamoinen LK, Verho J.. Drug expenditure of high-cost patients and their characteristics in Finland. Eur J Health Econ. 2013;14:495–502. [DOI] [PubMed] [Google Scholar]
- 32.Mielck A, Vogelmann M, Leidl R.. Health-related quality of life and socioeconomic status: inequalities among adults with a chronic disease. Health Qual Life Outcomes. 2014;12:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dalstra JA, Kunst AE, Borrell C, et al. Socioeconomic differences in the prevalence of common chronic diseases: an overview of eight European countries. Int J Epidemiol. 2005;34:316–326. [DOI] [PubMed] [Google Scholar]
- 34.Sund R. Quality of the Finnish Hospital Discharge Register: a systematic review. Scand J Public Health. 2012;40:505–515. [DOI] [PubMed] [Google Scholar]
- 35.Gustavsson A, Brinck P, Bergvall N, et al. Predictors of costs of care in Alzheimer’s disease: a multinational sample of 1222 patients. Alzheimer’s Dementia. 2011;7:318–327. [DOI] [PubMed] [Google Scholar]
- 36.Farré M, Haro JM, Kostov B, et al. Direct and indirect costs and resource use in dementia care: a cross-sectional study in patients living at home. Int J Nurs Stud. 2016;55:39–49. [DOI] [PubMed] [Google Scholar]
- 37.Martikainen J, Viramo P.. Muistisairaudet ja terveystaloustiede. In Erkinjuntti T, Remes A, Rinne J, Soininen H, editors. Muistisairaudet. 2nd ed. Helsinki, Finland: Duodecim. [Google Scholar]
- 38.Duthey B. Background paper 6.11: Alzheimer disease and other dementias. A Public Health Approach to Innovation. Geneva: World Health Organization; 2013. p. 1–74. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.