Abstract
Purpose of Review
The surface of the HIV-1 Env glycoprotein, the target of neutralizing antibodies, is extensively covered by N-linked glycans that create a glycan shield. Broadly neutralizing antibodies (bNAbs), the primary targets of HIV-1 vaccine design, have to negotiate this glycan shield. Here, we review the barriers and opportunities that the HIV-1 glycan shield presents for vaccine induction of bNAbs.
Recent findings
Glycan shields can impact the nature of the antibody response and influence the development of neutralization breadth in HIV-1 infections. The architecture of the glycan shield arising from glycan interactions and dynamics have been modeled, and its fine structure, i.e. the site-wise glycan heterogeneity, have been determined for some isolates. While the extent of glycan shielding is conserved, the precise number, location, and processing of glycans, however, is strain dependent. New insights continue to reveal how such differences can impact bNAb activity and development. Novel approaches have exploited the glycan shield for designing immunogens that bind the germline precursors of bNAbs, a critical roadblock for vaccine-induction of bNAbs.
Summary
The HIV-1 glycan shield can significantly impact the induction and maturation of bNAbs, and a better understanding of how to manipulate it will improve immunogen design.
Keywords: Glycan shield, HIV-1 vaccines, broadly neutralizing antibodies
Introduction
The HIV-1 envelope glycoprotein (Env) is the most heavily glycosylated of viral glycoproteins [1]. Through their position and dynamics, glycans form a “glycan shield” that protects most of the Env surface against humoral responses, as glycans are recognized as self. The immense genetic diversity of HIV-1 world-wide requires successful vaccines to induce cross-reactive responses [2]. Thus, vaccines that can induce broadly neutralizing antibodies (bNAbs) with great cross-reactive potential are highly sought after. All known bNAbs have to negotiate the glycan shield, and the glycan shield properties of Env immunogens can dramatically alter antibody sensitivity [3*,4] and determine the targeted epitopes [5*]. Here, we review recent advances in our understanding of the HIV-1 glycan shield and its impact on bNAb interactions, as well as rational vaccine designs that exploit the Env glycan shield to induce bNAbs.
Architecture and conservation of HIV-1 Env glycan shield.
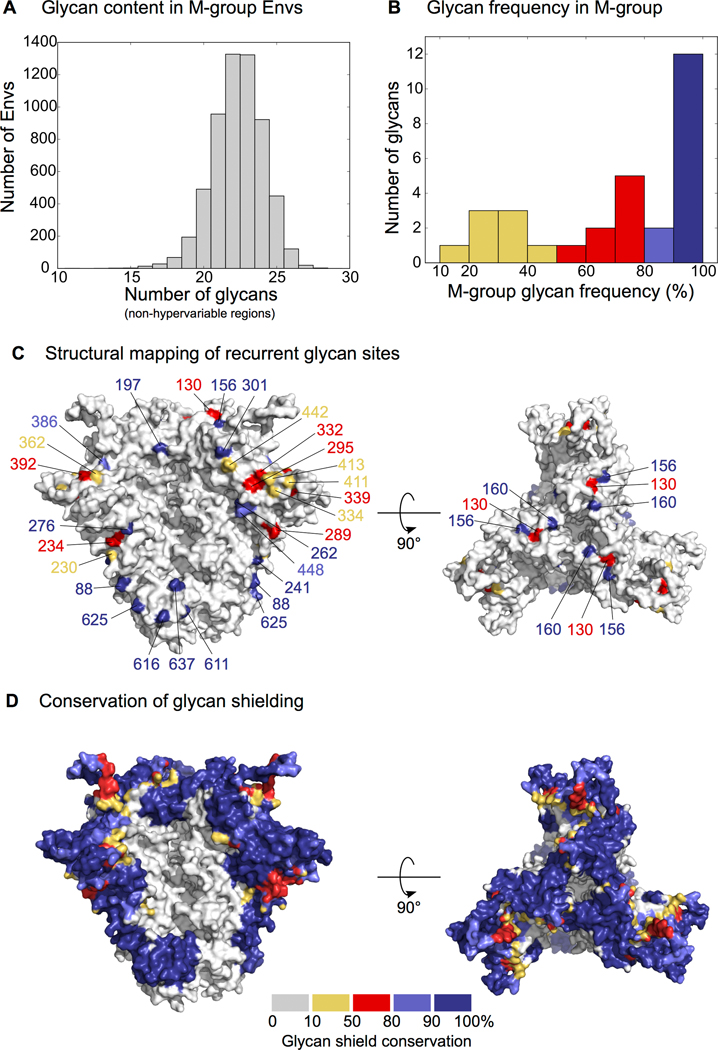
Envs of the global M-group strains have a median of 30 potential N-linked glycan sites (PNGSs) per protomer, with an inter-quartile range (IQR) of 28 to 31. These account for roughly half of the molecular weight of the Env glycoprotein. Glycans protrude out from the protein surface and are highly flexible, which provides a highly dynamic glycan shield [6,7*]. In addition, glycans are densely packed and form inter-glycan interactions, some of which can stabilize their dynamics [1,8*]. Until recently, glycan dynamics could not be directly observed, but were only accessible through molecular dynamics simulations. However, recent advances in cryoelectron microscopy (cryo-EM) now allow a direct, albeit low-resolution, visualization of the extent of glycan dynamics [9**]. We have developed a high throughput strategy of glycan shield mapping that accurately predicted glycan shield properties relevant for neutralizing antibody responses [5*].
Of the total number of Env glycans per protomer, a median of 7 (IQR=6–8) are distributed across the four hypervariable loops (typically 0–4 per hypervariable loop depending on the strain [4]). The hypervariable regions are largely unconstrained in their structure and exhibit immense sequence and length variation, even among viral quasispecies members in longitudinally followed individuals [10,11]. In fact, the length diversity precludes meaningful alignments both within and between infections, and thus, the precise placement of the glycan is strain-dependent. A median of 22 glycans (IQR = 21–24) are in regions outside the hypervariable loops (Fig. 1A). Amongst these, the majority are highly conserved, with 12 glycans present in greater than 90% and 19 glycans present in greater than 70% of M-group viruses (Fig. 1B–C). The remaining glycans are found at modest or low frequency. We previously have shown that a radius of 10Å around each potential N-linked glycosylation site (PNGS) provides a good approximation of the impact of the glycan shield on the protein surface [5*]. Using this strategy, we mapped the glycan shield in ~4,500 M-group Envs, showing that despite the variation in PNGS locations, most of the outer regions of the Env trimer are shielded in the vast majority of M-group Envs (Fig. 1D). In particular, the region around the site 332 (“oligomannose patch”) contains PNGSs whose positions are variable among M-group Envs, and yet, this region is glycan shielded in greater than 90% of viruses. This suggests that the presence or absence of PNGSs is not random, and while certain Envs may not encode particular glycans, they contain nearby glycans that compensate for the glycan shielding. An important example is N332, whose absence can be compensated by N295, N334 and N413. This glycan is typically required for neutralization by a potent class of bNAbs (V3 glycan bNAbs) and its shift to N334 can confer resistance, despite leaving the glycan shield intact [3*,12,13].
Figure 1.
Conservation of glycans and the glycan shield in M-group Envs.
(A) Distribution of unique (i.e. per protomer) glycans in an alignment of ~4,800 M-group Envs (2018 Filtered Web alignment from the Los Alamos HIV Database, www.hiv.lanl.gov). Hypervariable loop glycans are excluded. (B) Distribution of glycan frequency in M-group Envs. Hypervariable loop glycans and glycans with <10% frequency are excluded. (C) Structural mapping of glycan sites in (B). PDB: 5FYJ [1]. (D) Conservation of glycan shielding in M-group using the calculations from Wagh et al. [5*].
Original.
Glycans are added to the Env protein co-translationally and are processed post-folding by multiple enzymes that can add or remove sugar moieties in a substrate-specific manner [14]. As a result, not all PNGSs are fully glycosylated and can differ in the fine structure of their sugar content. N-linked glycans are broadly classified as oligomannose, complex, or hybrid (containing oligomannose and complex sugar subunits). Early glycan precursors are oligomannose (Man9), and sites that undergo less processing result in oligomannose glycans (Man9 to Man5), while hybrid and complex glycans require a higher degree of processing. Mass-spectrometry based approaches have been used together with enzymatic cleavage to identify the level of glycosylation and class of glycans present in Env derived glycopeptides. The most detailed view of glycan heterogeneity was reported by Behrens et al., who reported that while an HIV-1 Env trimeric protein (BG505 SOSIP.664) contains a large fraction of oligomannose glycans (~68%), there is substantial variation in the glycan content between sites [15]. Some sites in the glycan-rich region near the V3 loop were predominantly oligomannose, while other sites, particularly in gp41, were dominated by complex glycans. Most sites showed several glycan species, including some that had both oligomannose and complex sugars, thus indicating different levels of processing. The high density of glycans may hinder access to glycan processing enzymes, which is likely the reason for oligomannose dominated glycans in the V3 patch [16]. Moreover, recent studies reported site-wise heterogeneity comparing Envs from infectious virions with soluble trimeric Envs, as well as Env context-dependent variability in glycan occupancy and composition [17**,18**]; however, some glycan shield features such as the oligomannose V3 patch and predominantly complex glycans in gp41 appear to be conserved [17**,19–21].
Glycan holes and development of neutralization breadth.
Although glycan shielding is a conserved protective strategy, some Envs lack particular glycans and thus contain “glycan holes.” Previous studies have shown that when such Envs are used as trimeric immunogens, neutralizing antibody (NAb) responses preferentially target the glycan holes [22–24]. There are two reasons for why the absence rather than the presence of a glycan elicits such immunodominant responses. First, HIV-1 glycans are host-cell derived and B cells targeting such glycans are likely regulated by tolerance mechanisms [25]. Second, glycans themselves are difficult targets due to their compositional heterogeneity and dynamics. Thus, exposed protein surfaces in glycan holes offer a relatively easier target. The NAb responses that target such rare glycan holes can neutralize only immunogen-matched Env, or mutant heterologous Envs with shared holes, but not the heterologous wildtype strains because the glycan holes are usually strain specific (Fig. 1D). For example, JR-FL and BG505 immunogens lack glycans at positions 197 and 241, respectively, which are present in 97–98% M-group Envs.
Several recent studies have since confirmed and extended these concepts. First, a significant relationship between autologous neutralizing activity and the size of glycan holes was found by Zhou et al., who designed immunogens that lacked 4 glycans surrounding the CD4 binding site. Testing immunized guinea pigs and rhesus macaques, they found autologous NAb titers in the millions [26*]. Adding these same glycans back into the immunogen reduced NAb titers exponentially. Moreover, no cross-reactivity with wildtype glycosylated heterologous viruses was found. Second, by filling an existing glycan hole in their BG505 SOSIP immunogen, while removing other glycans, Ringe et al. were able to redirect the autologous NAb responses to the new glycan holes [27**], indicating that glycan hole immunogenicity is not location dependent, but a general phenomenon. Third, Cottrell et al. found that BG505 SOSIP immunized rhesus macaques developed antibodies that could only neutralize the autologous virus with the N611 glycan knocked-out, with structural studies showing that this PNGS was unoccupied in the immunogen [28*]. Thus, in addition to absence of PNGS, glycan holes can emerge due to no or low glycan occupancy. Fourth, Yang et al. showed that despite two separate trimeric immunogens having partially overlaping glycan holes, the autologous NAb responses did not cross-react due to amino acid differences in the glycan hole epitopes, and thus highlighted a further roadblock to breadth for glycan hole targeting NAbs [29]. Finally, soluble trimeric SOSIP immunogens have artificially truncated ectodomains that expose protein surface on the base, which are extreme examples of glycan holes. Such surfaces are exquisitely immunogenic, and the elicited antibodies are non-neutralizing because native virions lack such exposed surfaces [30]. Strategies have been explored to divert such undesirable responses using glycan masking (i.e. introducing artificial PNGS) or through presentation of trimers multiplexed to nanoparticles [31*,32]. Furthermore, glycan masking has been used to focus the NAb response to desired epitopes [33*].
The fact that glycan holes generate immunodominant strain-specific NAb responses in the context of immunizations prompted us to explore their relevance for the development of breadth in HIV-1 infected individuals [5*]. Studying 12 longitudinally followed patients, we found that glycan holes in the transmitted-founder (TF) Envs elicited similar off-target glycan hole targeting NAb responses. Furthermore, neutralization breadth development was significantly correlated with the completeness of the TF glycan shield. Hole-targeting NAbs drove viral escape to acquire glycans to fill the glycan holes, and this was temporally correlated with the onset of plasma neutralization breadth. Thus, these findings together with those from the immunization studies suggest that immunogens with complete glycan shields may be beneficial for steering NAb responses away from unwanted strain-specific glycan holes, and towards M-group conserved glycan shield epitopes.
Broadly neutralizing antibody interactions with the glycan shield.
There are seven epitope clusters on the native Env trimer that are commonly targeted by bNAbs: the V2 apex, CD4 binding site (CD4bs), V3 high mannose patch, gp120/gp41 interface, fusion peptide, glycosylated silent face and membrane proximal external region (MPER) [34]. Given their breadth, all bNAbs have to accommodate the conserved HIV-1 glycan shield. A wealth of knowledge has accumulated about the detailed interactions between bNAbs and glycans, and we refer the reader to previous reviews and manuscripts [12,34–42]. Here, we focus on the principles governing the multiple ways glycans can interact with bNAbs.
First, certain bNAb classes, like the V2 apex and V3 glycan classes, form extensive contacts with glycans and rely on such glycans for activity. Notable examples are N160 and N332, which are typically required for activity of V2 apex and V3 glycan bNAbs, respectively [3*,10,12,13,40,43]. Other examples include 2G12 and a new silent face targeting bNAb, both of which form predominant contacts with glycans [14,39,44]. Second, glycan dynamics can hinder access to certain bNAb epitopes, and thus, removal of such glycans enhances bNAb sensitivity. Interaction of N276 with CD4bs bNAbs is one example, since removal of this glycan can lead to improved sensitivity [26*]. Consistent with this, structural studies have shown that the CD4 bNAb VRC01 pushes this glycan away to access the protein epitope [1,26*]. MPER, gp120-gp41 interface and fusion peptide bNAbs also exhibit similar interactions [38,42].
Third, the presence or absence of next-to-nearest glycans or even distal glycans can also subtly influence bNAb sensitivity. Usually, specific mutants are used to identify amino acids and glycans that are important for bNAb activity. However, we have searched for potential allosteric (i.e. long-range or indirect) interactions and identified statistically and phylogenetically robust associations (“signatures”) between Env sequence variants and bNAb neutralization activity [3*]. These analyses revealed several glycan signatures were associated with bNAb sensitivity or resistance, most of them in bNAb epitopes. However, next-to-nearest contacts, such as N234 next to N276, or more distant interactions, such as the association of N332 with resistance to V2 apex bNAbs or of N616 with sensitivity to CD4bs bNAbs, were also identified. Such associations could arise due to the extensive inter-glycan interactions that are facilitated by the high density of HIV-1 glycan shield [1,8*,9**]. Moreover, the processing of key glycans could be modulated by the presence/absence of neighboring glycans, since such processing is determined by glycan density [16]. For example, the N332 signature of V2 apex bNAbs has a significant impact on glycan processing of the BG505 SOSIP trimer [15]. Fourth, hypervariable region glycan content can significantly impact bNAb potency. Hypervariable loops are relatively unconstrained, and show high diversity, both in their length and protein sequence. They are embedded in, or are proximal to, the core V2 apex, V3 glycan and CD4bs bNAb epitopes [2]. Since these regions cannot be meaningfully aligned, we characterized them by alignment-free metrics such as length, net charge and number of glycans to test for associations with bNAb sensitivity. Several such characteristics exhibited highly significant associations, including a negative correlation between the number of glycans in V5 as well as V1 and V2 loops combined with CD4bs and V3 bNAb sensitivity, respectively [3*]. Finally, in addition to presence/absence of glycans, the nature of glycoforms can also impact bNAb activity. Crooks et al. systematically analyzed the sensitivity of bNAbs to pseudoviruses subjected to drastically different glycan processing, and found that certain bNAb classes show preference for certain glycoforms, e.g. V2 apex bNAbs typically prefer sialylated glycans and V3 bNAbs prefer high mannose, while CD4bs bNAbs do not show any preference [45*].
Glycan modified immunogens for inducing broadly neutralizing antibodies.
The development of bNAbs in infection and the vaccine approaches designed to induce such bNAbs has been reviewed previously [36,40,46*,47]. A key roadblock is the rarity of B-cell precursors and their lack of cross-reactivity to most HIV-1 strains. Thus, high affinity immunogens capable of activating bNAb germline precursors have to be specifically designed (reviewed in [48]). A common feature is that certain roadblock glycans need to be removed for enhanced access. For example, N276 inhibits the germline targeting of CD4bs bNAbs [49–51], N130 inhibits the germline targeting of V2 apex bNAbs [40,52], and N156 and hypervariable V1 loop glycans inhibit the germline targeting of V3 glycan bNAbs [53–55*]. However, removal of these glycans can also expose underlying protein surfaces, which can induce strain-specific NAb responses akin to glycan holes discussed above, especially since the respective glycans are highly conserved (Fig. 1). Furthermore, absence of barrier glycans may induce NAbs approaching in orientations incompatible with the presence of glycans on most heterologous strains. To resolve these issues, re-introduction of glycans in boosting immunogens towards a more intact glycan shield has recently been tried, and met with some success [56*].
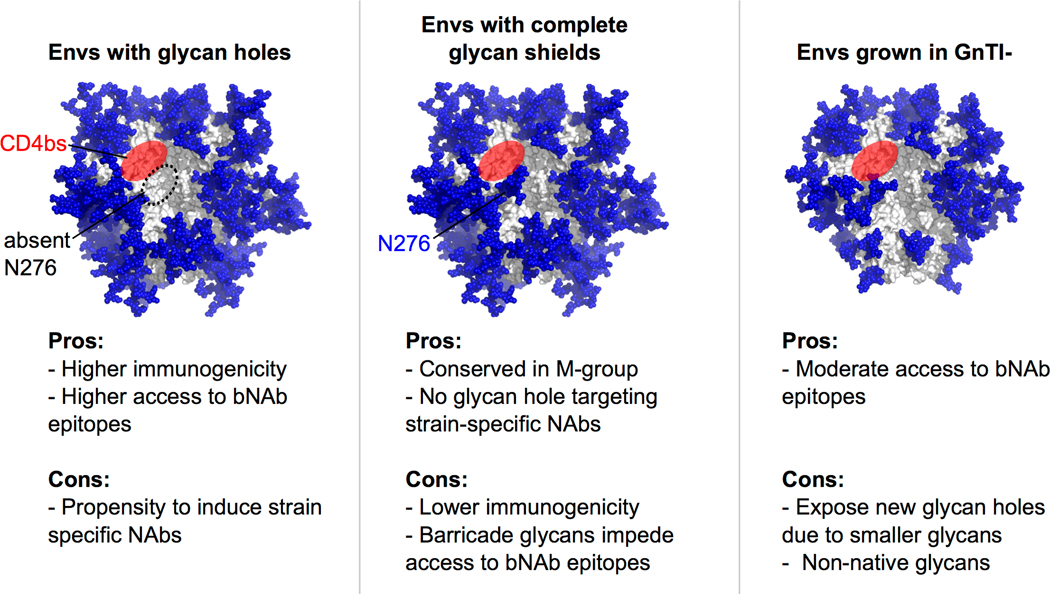
Germline targeting immunogens with intact glycan shields that maintain sufficient affinity to bNAb precursors may be most desirable. Most bNAbs make high affinity contacts with protein, and thus designing amino acid variants that improve such interactions could be one way of achieving this; however, this has not been achieved yet. In the meanwhile, a recent study provides a reasonable compromise. LaBranche et al. found that by growing pseudoviruses in N-acetylglucosaminyltransferase I (GnTI) knockout cells, with two rationally designed amino acid mutations, could confer neutralization by a CD4bs precursor [57**]. Absence of GnTI arrests glycan processing to oligomannose glycans (Man9 to Man5), leading to replacement of the larger complex glycans by the much smaller Man5 glycans [14]. The glycan shielding on such Envs could be a compromise between opening potentially undesirable glycan holes promoting germline targeting and intact glycan shields preventing off-target responses (Fig. 2). Such smaller glycans exist naturally in some Envs (on immunogens [15,58] as well as virions [17**,18**]), and could explain the apparent paradox as to why Envs with intact glycan shields seem to be correlated with broader responses in infected individuals, despite typically having no affinity to precursors of known bNAbs. Crooks et al. also found that GnTI- grown trimers could bind some bNAb precursors [45*]. It should be noted that such approaches would not work for bNAbs that require complex glycans such as the V2 apex bNAbs, but could work for those depending on oligomannose glycans such as V3 glycan bNAbs.
Figure 2.
Pros and cons of immunogens with varying glycan shields.
Left: An Env with a glycan hole at N276 is shown, similar to CD4bs germline targeting immunogens. Middle: An Env with complete glycan shield is shown. Right: An Env with all conserved glycans, but grown in GnTI knockout cell-lines is shown. The pros and cons for each immunogen type are listed based on discussion in the text. Glycans were modeled on BG505 trimer structure PDB: 5FYL [1] using GlyProt (www.glycosciences.de) with default oligomannose or complex glycans, or Man5 for GnTI-. Missing glycans in BG505 at sites 130, 241 and 289 were artificially introduced. For left and middle panels, information from Cao et al. [17**] was used on whether a site is predominantly oligomannose or complex. For the right panel, all complex glycans in the middle panel were replaced by Man5 to approximate GnTI- activity.
Original
Regardless of whether glycan deletion for induction of germline bNAbs is needed or not, glycan masking of off-target epitopes is likely to be beneficial. Such epitopes exist on subunit proteins that are being used for vaccines that expose non-native surfaces, such as the soluble trimer base [31]. They are also found on epitope-focused immunogens that have other glycan holes [53] or other exposed protein surfaces [33*]. In each of these studies, glycan masking improved on-target responses.
Conclusion
In summary, Env glycan shield properties, both local (i.e. specific PNGS) and global (such as the glycan network, glycoform processing variants, etc.), have profound impact on the sensitivity to bNAbs and their precursors, and on vaccine induced NAb responses. Thus, further exploration of such properties is warranted, and careful consideration of the glycan shield in immunogen design will be needed for successful induction of bNAbs.
Key points.
HIV-1 Env glycan shield is an immune evasion strategy that needs to be accommodated by bNAbs.
Glycan shields play an important role in modulating bNAb interactions and shaping NAb responses.
Individual glycan sites as well as properties of the entire glycan shield need to be considered in vaccine design.
Acknowledgements
We acknowledge our several collaborators on previous and ongoing studies.
Financial support and sponsorship
This work was funded by grants from the NIH (R01 AI 159590, R01 AI 050529, UM1 AI 144371) and LANL (LDRD ER 20190441ER (KW, BK)).
Funding: National Institutes of Health (NIH); Los Alamos National Laboratory Lab-directed R&D (LANL LDRD).
Abbreviations
- PNGS
Potential N-linked glycosylation sites
- bNAb
Broadly neutralizing antibody
- NAb
Neutralizing antibody
- CD4bs
CD4 binding site
- MPER
membrane-proximal external region
- GnTI
N- acetylglucosaminyltransferase I
Footnotes
Conflicts of interests
None.
References and recommended reading
- [1].Stewart-Jones GBE, Soto C, Lemmin T, Chuang G-Y, Druz A, Kong R, et al. Trimeric HIV-1-Env Structures Define Glycan Shields from Clades A, B, and G. Cell 2016;165:813–26. 10.1016/j.cell.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Korber B, Hraber P, Wagh K, Hahn BH. Polyvalent vaccine approaches to combat HIV-1 diversity. Immunological Reviews 2017;275:230–44. 10.1111/imr.12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bricault CA, Yusim K, Seaman MS, Yoon H, Theiler J, Giorgi EE, et al. HIV-1 Neutralizing Antibody Signatures and Application to Epitope-Targeted Vaccine Design. Cell Host & Microbe 2019;25:59–72.e8. 10.1016/j.chom.2018.12.001.* In this study, we identified a comprehensive set of sequence (amino acid, glycans and hypervariable region characteristics) signatures associated with activity of multiple bNAbs from each major bNAb-class.
- [4].Stephenson KE, Wagh K, Korber B, Barouch DH. Vaccines and Broadly Neutralizing Antibodies for HIV-1 Prevention. Annual Review of Immunology 2020;38:673–703. 10.1146/annurev-immunol-080219-023629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wagh K, Kreider EF, Li Y, Barbian HJ, Learn GH, Giorgi E, et al. Completeness of HIV-1 Envelope Glycan Shield at Transmission Determines Neutralization Breadth. Cell Reports 2018;25:893–908.e7. 10.1016/j.celrep.2018.09.087.* In this study, we developed an approximate but accurate strategy to map glycan shields for a given Env sequence, and used this to explore the impact of glycan shield evolution on bNAb development.
- [6].Tian J, López CA, Derdeyn CA, Jones MS, Pinter A, Korber B, et al. Effect of Glycosylation on an Immunodominant Region in the V1V2 Variable Domain of the HIV-1 Envelope gp120 Protein. PLoS Comput Biol 2016;12 10.1371/journal.pcbi.1005094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lemmin T, Soto C, Stuckey J, Kwong PD. Microsecond Dynamics and Network Analysis of the HIV-1 SOSIP Env Trimer Reveal Collective Behavior and Conserved Microdomains of the Glycan Shield. Structure 2017;25:1631–1639.e2. 10.1016/j.str.2017.07.018.* This study used molecular dynamics simulations to highlight the extensive glycan dynamics underlying the glycan shield.
- [8].Chakraborty S, Berndsen ZT, Hengartner NW, Korber BT, Ward AB, Gnanakaran S. Quantification of the Resilience and Vulnerability of HIV-1 Native Glycan Shield at Atomistic Detail. BioRxiv 2020:846071. 10.1101/846071.* This study highlighted the extensive inter-glycan interaction networks by using high-throughput glycan dynamics modeling and network theory.
- [9].Berndsen ZT, Chakraborty S, Wang X, Cotrell CA, Torres JL, Diedrich JK, et al. Visualization of the HIV-1 Env Glycan Shield Across Scales. BioRxiv 2019:839217. 10.1101/839217.** This study reported an innovative approach to analyzing cryo-EM data to visualize the extent of glycan dynamics.
- [10].Bonsignori M, Kreider EF, Fera D, Meyerhoff RR, Bradley T, Wiehe K, et al. Staged induction of HIV-1 glycan-dependent broadly neutralizing antibodies. Sci Transl Med 2017;9 10.1126/scitranslmed.aai7514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gao F, Bonsignori M, Liao H-X, Kumar A, Xia S-M, Lu X, et al. Cooperation of B Cell Lineages in Induction of HIV-1-Broadly Neutralizing Antibodies. Cell 2014;158:481–91. 10.1016/j.cell.2014.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Garces F, Sok D, Kong L, McBride R, Kim HJ, Saye-Francisco KF, et al. Structural Evolution of Glycan Recognition by a Family of Potent HIV Antibodies. Cell 2014;159:69–79. 10.1016/j.cell.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Caskey M, Schoofs T, Gruell H, Settler A, Karagounis T, Kreider EF, et al. Antibody 10–1074 suppresses viremia in HIV-1-infected individuals. Nat Med 2017;23:185–91. 10.1038/nm.4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Seabright GE, Doores KJ, Burton DR, Crispin M. Protein and Glycan Mimicry in HIV Vaccine Design. Journal of Molecular Biology 2019;431:2223–47. 10.1016/j.jmb.2019.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Behrens A-J, Vasiljevic S, Pritchard LK, Harvey DJ, Andev RS, Krumm SA, et al. Composition and Antigenic Effects of Individual Glycan Sites of a Trimeric HIV-1 Envelope Glycoprotein. Cell Reports 2016;14:2695–706. 10.1016/j.celrep.2016.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Pritchard LK, Spencer DIR, Royle L, Bonomelli C, Seabright GE, Behrens A-J, et al. Glycan clustering stabilizes the mannose patch of HIV-1 and preserves vulnerability to broadly neutralizing antibodies. Nature Communications 2015;6:7479 10.1038/ncomms8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cao L, Pauthner M, Andrabi R, Rantalainen K, Berndsen Z, Diedrich JK, et al. Differential processing of HIV envelope glycans on the virus and soluble recombinant trimer. Nature Communications 2018;9:3693 10.1038/s41467-018-06121-4.** see below.
- [18].Struwe WB, Chertova E, Allen JD, Seabright GE, Watanabe Y, Harvey DJ, et al. Site-Specific Glycosylation of Virion-Derived HIV-1 Env Is Mimicked by a Soluble Trimeric Immunogen. Cell Reports 2018;24:1958–1966.e5. 10.1016/j.celrep.2018.07.080.** These two studies have analyzed site-wise glycan heterogeneity for virion-derived Env and compared these data to those from soluble Env trimers.
- [19].Go EP, Ding H, Zhang S, Ringe RP, Nicely N, Hua D, et al. Glycosylation Benchmark Profile for HIV-1 Envelope Glycoprotein Production Based on Eleven Env Trimers. J Virol 2017;91 10.1128/JVI.02428-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Yu W-H, Su D, Torabi J, Fennessey CM, Shiakolas A, Lynch R, et al. Predicting the broadly neutralizing antibody susceptibility of the HIV reservoir. JCI Insight n.d;4 10.1172/jci.insight.130153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cao L, Diedrich JK, Kulp DW, Pauthner M, He L, Park S-KR, et al. Global site-specific N-glycosylation analysis of HIV envelope glycoprotein. Nature Communications 2017;8:14954. 10.1038/ncomms14954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Crooks ET, Tong T, Chakrabarti B, Narayan K, Georgiev IS, Menis S, et al. Vaccine-Elicited Tier 2 HIV-1 Neutralizing Antibodies Bind to Quaternary Epitopes Involving Glycan-Deficient Patches Proximal to the CD4 Binding Site. PLoS Pathog 2015;11 10.1371/journal.ppat.1004932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Klasse PJ, LaBranche CC, Ketas TJ, Ozorowski G, Cupo A, Pugach P, et al. Sequential and Simultaneous Immunization of Rabbits with HIV-1 Envelope Glycoprotein SOSIP.664 Trimers from Clades A, B and C. PLoS Pathog 2016;12 10.1371/journal.ppat.1005864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].McCoy LE, van Gils MJ, Ozorowski G, Messmer T, Briney B, Voss JE, et al. Holes in the Glycan Shield of the Native HIV Envelope Are a Target of Trimer-Elicited Neutralizing Antibodies. Cell Reports 2016;16:2327–38. 10.1016/j.celrep.2016.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Scanlan CN, Offer J, Zitzmann N, Dwek RA. Exploiting the defensive sugars of HIV-1 for drug and vaccine design. Nature 2007;446:1038–45. 10.1038/nature05818. [DOI] [PubMed] [Google Scholar]
- [26].Zhou T, Doria-Rose NA, Cheng C, Stewart-Jones GBE, Chuang G-Y, Chambers M, et al. Quantification of the Impact of the HIV-1-Glycan Shield on Antibody Elicitation. Cell Reports 2017;19:719–32. 10.1016/j.celrep.2017.04.013.* This study revealed the exponential relationship between neutralizing titers and glycan hole area.
- [27].Ringe RP, Pugach P, Cottrell CA, LaBranche CC, Seabright GE, Ketas TJ, et al. Closing and Opening Holes in the Glycan Shield of HIV-1 Envelope Glycoprotein SOSIP Trimers Can Redirect the Neutralizing Antibody Response to the Newly Unmasked Epitopes. Journal of Virology 2019;93 10.1128/JVI.01656-18.** This study showed that autologous NAb responses could be redirected to novel glycan holes created in the backbone of the same trimeric immunogen.
- [28].Cottrell CA, van Schooten J, Bowman CA, Yuan M, Oyen D, Shin M, et al. Mapping the immunogenic landscape of near-native HIV-1 envelope trimers in non-human primates. BioRxiv 2020:2020.02.05.936096. 10.1101/2020.02.05.936096.* This study showed that an under-occupied glycan site could lead to an immunogenic glycan hole.
- [29].Yang YR, McCoy LE, van Gils MJ, Andrabi R, Turner HL, Yuan M, et al. Autologous Antibody Responses to an HIV Envelope Glycan Hole Are Not Easily Broadened in Rabbits. Journal of Virology 2020;94 10.1128/JVI.01861-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bianchi M, Turner HL, Nogal B, Cottrell CA, Oyen D, Pauthner M, et al. Electron-Microscopy-Based Epitope Mapping Defines Specificities of Polyclonal Antibodies Elicited during HIV-1 BG505 Envelope Trimer Immunization. Immunity 2018;49:288–300.e8. 10.1016/j.immuni.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kulp DW, Steichen JM, Pauthner M, Hu X, Schiffner T, Liguori A, et al. Structure-based design of native-like HIV-1 envelope trimers to silence non-neutralizing epitopes and eliminate CD4 binding. Nature Communications 2017;8:1655 10.1038/s41467-017-01549-6.* This study used glycan masking to hinder the off-target responses to the base of soluble trimeric immunogens.
- [32].Brouwer PJM, Antanasijevic A, Berndsen Z, Yasmeen A, Fiala B, Bijl TPL, et al. Enhancing and shaping the immunogenicity of native-like HIV-1 envelope trimers with a two-component protein nanoparticle. Nature Communications 2019;10:4272 10.1038/s41467-019-12080-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Duan H, Chen X, Boyington JC, Cheng C, Zhang Y, Jafari AJ, et al. Glycan Masking Focuses Immune Responses to the HIV-1 CD4-Binding Site and Enhances Elicitation of VRC01- Class Precursor Antibodies. Immunity 2018;49:301–311.e5. 10.1016/j.immuni.2018.07.005.* This study showed that glycan masking of irrelevant epitopes on a promising germline targeting immunogen could improve on-target responses.
- [34].Sok D, Burton DR. Recent progress in broadly neutralizing antibodies to HIV. Nature Immunology 2018;19:1179–88. 10.1038/s41590-018-0235-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Crispin M, Ward AB, Wilson IA. Structure and Immune Recognition of the HIV Glycan Shield. Annual Review of Biophysics 2018;47:499–523. 10.1146/annurev-biophys-060414-034156.** An informative review on the nature and structure of the HIV glycan shield.
- [36].Pancera M, Changela A, Kwong PD. How HIV-1 entry mechanism and broadly neutralizing antibodies guide structure-based vaccine design. Curr Opin HIV AIDS 2017;12:229–40. 10.1097/COH.0000000000000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Gristick HB, von Boehmer L, West AP, Schamber M, Gazumyan A, Golijanin J, et al. Natively glycosylated HIV-1 Env structure reveals new mode for antibody recognition of the CD4-binding site. Nat Struct Mol Biol 2016;23:906–15. 10.1038/nsmb.3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lee JH, Ozorowski G, Ward AB. CryoEM structure of a native, fully glycosylated and cleaved HIV-1 envelope trimer. Science 2016;351:1043–8. 10.1126/science.aad2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Zhou T, Zheng A, Baxa U, Chuang G-Y, Georgiev IS, Kong R, et al. A Neutralizing Antibody Recognizing Primarily N-Linked Glycan Targets the Silent Face of the HIV Envelope. Immunity 2018;48:500–513.e6. 10.1016/j.immuni.2018.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Andrabi R, Voss JE, Liang C-H, Briney B, McCoy LE, Wu C-Y, et al. Identification of Common Features in Prototype Broadly Neutralizing Antibodies to HIV Envelope V2 Apex to Facilitate Vaccine Design. Immunity 2015;43:959–73. 10.1016/j.immuni.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Gorman J, Chuang G-Y, Lai Y-T, Shen C-H, Boyington JC, Druz A, et al. Structure of Super-Potent Antibody CAP256-VRC26.25 in Complex with HIV-1 Envelope Reveals a Combined Mode of Trimer-Apex Recognition. Cell Reports 2020;31:107488. 10.1016/j.celrep.2020.03.052. [DOI] [PubMed] [Google Scholar]
- [42].Kong R, Duan H, Sheng Z, Xu K, Acharya P, Chen X, et al. Antibody Lineages with Vaccine-Induced Antigen-Binding Hotspots Develop Broad HIV Neutralization. Cell 2019;178:567–584.e19. 10.1016/j.cell.2019.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Gorman J, Soto C, Yang MM, Davenport TM, Guttman M, Bailer RT, et al. Structures of HIV-1 Env V1V2 with broadly neutralizing antibodies reveal commonalities that enable vaccine design. Nat Struct Mol Biol 2016;23:81–90. 10.1038/nsmb.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Sanders RW, Venturi M, Schiffner L, Kalyanaraman R, Katinger H, Lloyd KO, et al. The mannose-dependent epitope for neutralizing antibody 2G12 on human immunodeficiency virus type 1 glycoprotein gp120. J Virol 2002;76:7293–305. 10.1128/jvi.76.14.7293-7305.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Crooks ET, Grimley SL, Cully M, Osawa K, Dekkers G, Saunders K, et al. Glycoengineering HIV-1 Env creates ‘supercharged’ and ‘hybrid’ glycans to increase neutralizing antibody potency, breadth and saturation. PLOS Pathogens 2018;14:e1007024. 10.1371/journal.ppat.1007024.* This study uncovered the glycoform specificity of different bNAbs and their precursors by global alterations in the Env glycan processing/maturation.
- [46].Bonsignori M, Liao H-X, Gao F, Williams WB, Alam SM, Montefiori DC, et al. Antibody-virus co-evolution in HIV infection: paths for HIV vaccine development. Immunological Reviews 2017;275:145–60. 10.1111/imr.12509.* An informative review on the antibody-virus coevolution that leads to the development of bNAbs in HIV-1 infected individuals.
- [47].Haynes BF, Burton DR, Mascola JR. Multiple roles for HIV broadly neutralizing antibodies. Science Translational Medicine 2019;11:eaaz2686. 10.1126/scitranslmed.aaz2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Stamatatos L, Pancera M, McGuire AT. Germline-targeting immunogens. Immunological Reviews 2017;275:203–16. 10.1111/imr.12483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Medina-Ramírez M, Garces F, Escolano A, Skog P, de Taeye SW, Del Moral-Sanchez I, et al. Design and crystal structure of a native-like HIV-1 envelope trimer that engages multiple broadly neutralizing antibody precursors in vivo. J Exp Med 2017;214:2573–90. 10.1084/jem.20161160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Jardine JG, Ota T, Sok D, Pauthner M, Kulp DW, Kalyuzhniy O, et al. Priming a broadly neutralizing antibody response to HIV-1 using a germline-targeting immunogen. Science 2015;349:156–61. 10.1126/science.aac5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].McGuire AT, Dreyer AM, Carbonetti S, Lippy A, Glenn J, Scheid JF, et al. Antigen modification regulates competition of broad and narrow neutralizing HIV antibodies. Science 2014;346:1380–3. 10.1126/science.1259206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Voss JE, Andrabi R, McCoy LE, de Val N, Fuller RP, Messmer T, et al. Elicitation of Neutralizing Antibodies Targeting the V2 Apex of the HIV Envelope Trimer in a Wild-Type Animal Model. Cell Rep 2017;21:222–35. 10.1016/j.celrep.2017.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Escolano A, Gristick HB, Abernathy ME, Merkenschlager J, Gautam R, Oliveira TY, et al. Immunization expands HIV-1 V3-glycan specific B-cells in mice and macaques. Nature 2019;570:468–73. 10.1038/s41586-019-1250-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Steichen JM, Kulp DW, Tokatlian T, Escolano A, Dosenovic P, Stanfield RL, et al. HIV Vaccine Design to Target Germline Precursors of Glycan-Dependent Broadly Neutralizing Antibodies. Immunity 2016;45:483–96. 10.1016/j.immuni.2016.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Saunders KO, Wiehe K, Tian M, Acharya P, Bradley T, Alam SM, et al. Targeted selection of HIV-specific antibody mutations by engineering B cell maturation. Science 2019;366 10.1126/science.aay7199.* This study used the glycan modified Env trimers from ref. 57 to initiate antibody lineages that mimicked early development of a CD4bs bNAb lineage.
- [56].Dubrovskaya V, Tran K, Ozorowski G, Guenaga J, Wilson R, Bale S, et al. Vaccination with Glycan-Modified HIV NFL Envelope Trimer-Liposomes Elicits Broadly Neutralizing Antibodies to Multiple Sites of Vulnerability. Immunity 2019;51:915–929.e7. 10.1016/j.immuni.2019.10.008.* This study showed that priming with glycan hole immunogens, and boosting with immunogens with progressively more intact glycan shields could induce monoclonal bNAbs.
- [57].LaBranche CC, Henderson R, Hsu A, Behrens S, Chen X, Zhou T, et al. Neutralization-guided design of HIV-1 envelope trimers with high affinity for the unmutated common ancestor of CH235 lineage CD4bs broadly neutralizing antibodies. PLOS Pathogens 2019;15:e1008026. 10.1371/journal.ppat.1008026.** This study showed that a specific pseudovirus grown in GnTI- cells could be neutralized by the germline precursor of a CD4bs bNAb, and used this to produce a glycan-modified germline-targeting trimeric immunogen.
- [58].Borst AJ, Weidle CE, Gray MD, Frenz B, Snijder J, Joyce MG, et al. Germline VRC01 antibody recognition of a modified clade C HIV-1 envelope trimer and a glycosylated HIV-1 gp120 core. ELife 2018;7:e37688. 10.7554/eLife.37688. [DOI] [PMC free article] [PubMed] [Google Scholar]