Abstract
Lipids constitute a significant group of biological metabolites and the building blocks of all cell membranes. The abundance and stoichiometries of different lipid species are known to vary across the lifespan and metabolic state, yet the functional effects of these changes have been challenging to understand. Here we review the potentially powerful intersection of lipid metabolism, which determines membrane composition and aging. We first introduce several key lipid classes that are associated with aging and aging-related disease, where they are found in organisms, and how they act on membrane structure and function. Instead of neutral lipids, which have primary roles in energy storage and homeostasis, we review known functions for polar lipids that control the physicochemical properties of cell membranes. We then focus on aging processes in the central nervous system (CNS), which is enriched in lipids and is highly dependent on membrane structure for function. Recent studies that show how lipids act not just as biomarkers of aging and associated changes in the CNS, but as direct mediators of these processes. As a model system, we explore how fatty acid composition in the retina impact aging and aging-related disease. We propose that the biophysical effects of membrane structure on fundamental eukaryotic processes - mitochondrial respiration and autophagy - provide avenues by which lipid dysregulation can accelerate aging processes. Finally, we lay out ways in which an increased understanding of lipid membrane biology can be applied to studies of aging and lifespan.
Keywords: Lipid composition, membrane structure, polyunsaturated fatty acids, macular degeneration, aging brain, respiratory function
1. Introduction
One of the main challenges in understanding aging is connecting age-related physiological phenotypes to cellular level functions and the molecular players that drive them. In the central nervous system, aging is associated with a host of conditions (e.g., dementia, loss of vision) that are found across animals and age-related neurodegenerative diseases (e.g., Alzheimer’s, Parkinson’s) in humans. Lipids are an especially important and understudied class of biomolecules in the CNS, both because of their abundance and their central role in dictating membrane structure and function. Lipids constitute about half of the dry weight of the human brain (Sastry, 1985), despite it containing very few storage (neutral) fats. The mass of lipids in the CNS reflects an abundance of bilayer membranes in this tissue, a fact that is obvious from volumetric electron microscopy of brain regions across a wide range of metazoans (Briggman and Bock, 2012). Structure and biophysical properties of membranes throughout the body is dictated by the composition of lipid building blocks - including both their chemical structures and stoichiometries. Because the lipidome widely changes during the lifespan, cell membranes and their distinctive properties age as well.
In the brain and CNS, aging is characterized by a progressive deterioration of cognitive functions, a gradual loss of tissue mass, and increased susceptibility to neurodegenerative disease (Yankner et al., 2008). These effects limit the functional lifespan of this tissue and therefore the whole body. The primary drivers of aging are currently thought to be the accumulation of damaging products of metabolism, e.g. reactive oxygen species (ROS), and changes to genetic and epigenetic molecular programs. Both types of perturbations can act directly on lipid species or their metabolic processes and therefore can change membrane structure in aging tissue. Because lipids also play crucial roles in central metabolism, as described in section 6.1, they can also act directly to initiate these processes. Lipid composition has a multifaceted dependence on diet and disease and could thus provide an additional conduit between these conditions and aging processes in cells.
There is a long history of work seeking to identify correlations between the composition of different lipid components and the progression of aging across many tissues. The advent of commercial gas chromatography systems in the 1960s first allowed for robust analyses of fatty acid compositions in brain phospholipids (Fillerup and Mead, 1967). The subsequent rise of membrane biophysics as a field led to the development of hypotheses on lipid-induced changes in membrane structure as a causative agents of aging-related aging phenotypes (Schroeder, 1984). One particularly influential proposal was that changes in plasma membrane permeability act to increase intracellular potassium concentrations (Zs-Nagy, 1979), which would then mediate the efficiency of mRNA translation (Semsei et al., 1982). In the 90s, improved lipidomics techniques led to studies identifying a bulk loss of lipid mass as a hallmark of the aging brain (Svennerholm et al., 1994, 1991). During this time, alleles of APOE, encoding a protein involved in the transport of cholesterol from astrocytes to neurons, were identified as the strongest genetic risk factors for Alzheimer’s disease (reviewed in (Roses, 2006)) bringing attention to lipid transporters as potential modulators of neurodegeneration. Subsequent work characterized the large changes in brain lipid composition associated with Alzheimer’s (SoOderberg et al., 1992) and other degenerative nervous disorders, such as Parkinson’s (Ikenaka et al., 2019).
Studies during the formative years of aging research succeeded in identifying correlations between lipid composition and aging-related processes in the brain. However, they were also quite limited, both in terms of the specificity of where these compositional changes occurred, and how they related to testable molecular functions in cells. More recently, the field has taken two directions that partially addressed these shortcomings. In mammalian systems, a research focus has been on how lipid peroxidation is tied to aging, potentially through its destructive effects on membrane structure as well as through other pathways (Spiteller, 2002). This mirrored to the emergence of oxidative stress as a specific molecular perturbation associated with aging. In this case, the lipids are a secondary player, relaying the chemical signals of oxidative stress to other suspected targets (Cadenas and Davies, 2000; Pacifici and Davies, 1991). A second direction has been driven by vertebrate and invertebrate model organism lifespan studies, where surprising connections between storage lipid homeostasis and aging have been uncovered (Papsdorf and Brunet, 2019).
In this perspective, we seek to re-introduce cellular membranes as functional agents in driving aging-related phenotypes. This effort is motivated by 1) cellular and biophysical experiments in simple systems that have linked membrane composition to the regulation of key molecular functions and 2) clinical and genetic studies in complex systems (e.g. the mammalian retina) on how defects in lipid metabolism drive aging-related processes. We take a broad view of aging that encompasses both phenotypes in natural aging and those in age-associated diseases. At the same time, we focus on a single tissue type - the CNS - as a system where the effects of membrane lipids can be largely isolated from those of neutral lipids involved in energy storage and homeostasis. We introduce an even more specific model system, the mammalian retina, that features distinctive lipid composition, membrane requirements, and aging associated phenotypes.
2. Overview of membrane lipids relevant to aging processes
Organisms can feature hundreds or thousands of distinctive lipid components in homeostatically-maintained levels. Lipids are quite modular in structure and synthesis, so this complexity results from a combinatorial diversity of several key lipid classes and modifications. Different lipid components are not equally mixed within cells or tissues but are instead specifically enriched in specific organelles and compartments. Even single membranes can feature a dramatically different lipidome across its inner and outer leaflet (Lorent et al., 2019).
The distribution of lipid species is driven by their biosynthesis, transport through cell trafficking and membrane contact sites, remodeling, and translocation between leaflets through lipid flippases and floppases. Given the challenges in understanding this complexity, we will briefly review the key bulk lipid structures that have been associated with aging processes (Figure 1).
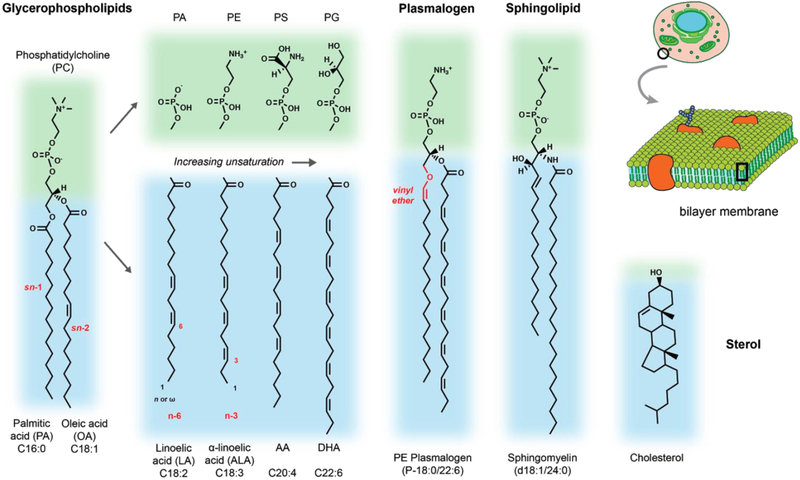
Figure 1:
Overview of lipid structures with relevance to aging processes in the CNS. Blue regions indicate hydrophobic chains or regions and green regions the polar head groups. Representative examples are given for major lipid classes cited in the text as well as the head group and acyl chain diversity generally found in phospholipids. Text in red highlights key structural features, such as the acyl chain position, double bond position, or unique linkage found in plasmalogens.
The fundamental membrane lipid in all cells are phospholipids with two fatty chains. In glycerophospholipids, these chains are straight chain fatty acids connected by ester linkages (acyl chains) to a glycerol-3-phosphate backbone. The phosphate is then esterified to a set of headgroup modifications that define the lipid class (e.g. choline, PC; ethanolamine, PE; glycerol, PG). These lipid classes are enriched in different cellular compartments and even sub-regions of a single membrane. For example, in the plasma membrane (PM) serine lipids (PS) are localized to the inner leaflet, while. PG can be converted into cardiolipin, a four-chain phospholipid that composes > 20% of the inner mitochondrial membrane.
In addition to glycerophospholipids, two other double-chained lipids are highly abundant in mammals. Plasmalogens are a class of phospholipids with ether or vinyl ether linkages at the sn-1 position. They most commonly have ethanolamine or choline headgroups and are enriched in PUFAs at the sn-2 position. Sphingolipids feature a mostly saturated N-acyl linked fatty acid connected to a long-chain sphingosine base, that is either saturated or contains a trans double bond. Sphingolipids are major components of plasma membranes, where they accumulate in the outer leaflet (Lorent et al., 2019). Commonly featuring choline as a headgroup (in sphingomyelin), sphingolipids can also display complex, sugar-containing polar groups, such as in gangliosides. Sphingolipid metabolites, notably sphingosine and ceramide, are also potent signalling molecules.
The fatty chain composition of these lipid classes is key to their effects on membrane structure. If acyl chains of phospholipids are fully saturated, they tightly pack with eachother in the bilayer, which can be progressively reduced by the incorporation of cis double bonds (unsaturations). Unsaturations are added sequentially, with monounsaturated fatty acids (MUFA) being highly abundant across the body. Polyunsaturated fatty acids (PUFAs), with 2–6 double bonds, are also abundant but have tissue-specific patterns. PUFAs fluidize membranes, a concept discussed below, but are also highly prone to peroxidation by ROS. Certain PUFAs, most notably arachidonic acid (AA), can also be converted into a variety of lipid signaling lipids, including eicosanoids, docosanoids and elovanoids. These soluble metabolites bind to cellular receptors, initiating key physiological processes ranging from inflammation to fertility (Bazan, 2018; Mouchlis and Dennis, 2019).
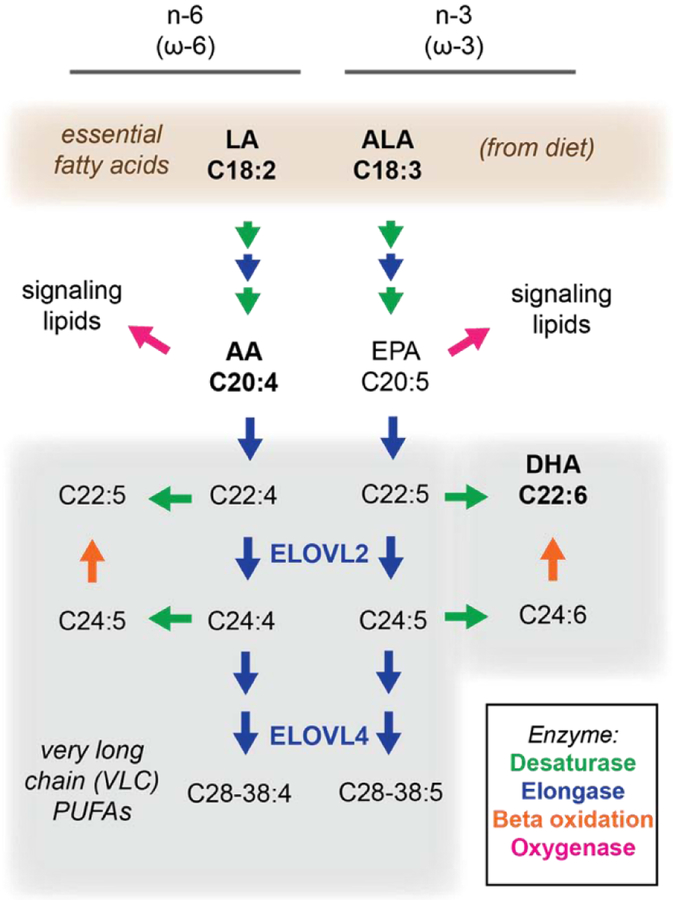
The base PUFAs linoleic acid (LA, C18:2) and alpha-linolenic acid (ALA, C18:3) cannot be synthesized de novo in humans, and thus are essential fatty acids in the diet. However, they can be further elongated and desaturated to longer PUFAs in the liver (Rapoport et al., 2007) and other tissues (Bazan, 2018). Longer species of PUFAs can also be incorporated from the diet, when available. Overall PUFA levels are therefore dictated by a combination of diet, metabolism, and degradation. These pathways are further discussed within the context of retina physiology in section 5.
A typical arrangement for glycerophospholipids is one saturated chain (e.g. palmitic acid, C16:0) at the sn-1 position, with a MUFA or PUFA at the sn-2 position. In contrast, sphingolipids generally feature fully saturated chains that contribute to their packing effects on membranes. Packing is also mediated by cholesterol, an abundant lipid derived from isoprenoids. Cholesterol intercalates between acyl chains in the bilayer, increasing their packing and ordering, but also preventing their crystallization into a gel-like state. Like sphingolipids, cholesterol is enriched in the plasma membrane and rigid cellular compartment (e.g. lysosomes), while being excluded from more dynamic membrane structures in the mitochondria or endoplasmic reticulum.
Although not the focus of this review, it is important to note that two major lipid classes (glycerophospholipids and cholesterol) can be modified via esterification into neutral lipids, which lose their amphillicity. The resulting products, triacylglycerides and sterol esters, accumulate in lipid droplets, serving as energy storage for the cells. The homeostasis of neutral lipids has been implicated in a number of lifespan phenotypes in model organisms, which have recently been reviewed elsewhere (Johnson and Stolzing, 2019).
3. How lipids control membrane structure and properties
One clear function for differences in lipid composition is to optimize the biophysical properties of membranes for different cellular functions (Figure 2). Because changes in lipid composition during the lifespan are expected to directly alter these properties, they can play key roles in aging-related processes. At first approximation, membranes can be modeled as thin sheets of a fluid with a given viscosity (Saffman and Delbrück, 1975) or, inversely, fluidity. Viscosity determines the rate at which lipids or membrane proteins diffuse within a membrane, as well as the permeability coefficient of small molecules across it. Because viscosity is overall much higher in membranes than in solution, it is thought to be an important parameter in determining the rate of membrane-associated reactions (Lauffenburger and Linderman, 1996). Viscosity is determined by the packing of lipids in the bilayer, with membranes enriched in saturated lipids and cholesterol being especially viscous. In contrast, unsaturated and polyunsaturated phospholipids disorder and fluidize membranes. Headgroup composition can also affect packing: PE lipids increase membrane ordering compared to other phospholipids (e.g. PC), which can be an important function for this lipid class in sterol-deficient cell types (Dawaliby et al., 2016).
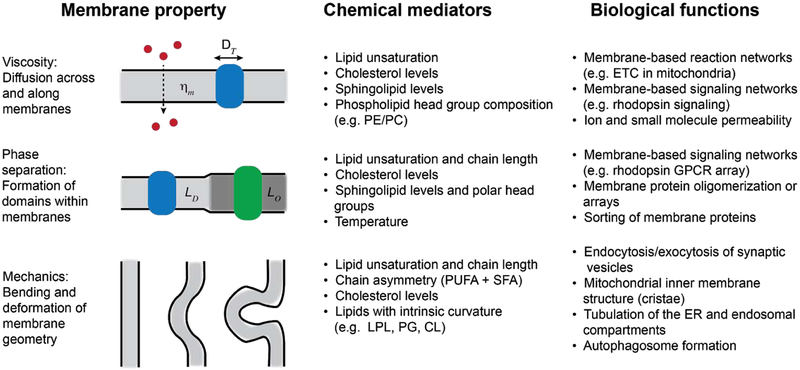
Figure 2:
Lipid-encoded biophysical properties of cell membranes that can mediate aging-related molecular processes. Depending on their chemical composition, membranes have different propensities for diffusion or permeability, domain formation or phase separation, and bending or curvature generation. Identified lipid mediators of these processes are given, as well as potential roles for the properties in cells and aging organisms.
In addition to viscosity, lipid packing controls the lateral pressure profiles of membranes. This concept describes the combination of attractive (generally in the hydrophobic core) and repulsive (in the head groups) forces that have to be balanced in the bilayer structure. Lateral pressure profiles vary depending on lipid composition and act on any transmembrane protein that is inserted within the membrane. In principle, they can thus directly control the equilibrium between different protein conformations (Cantor, 1997). In practice, it has been a challenge to measure this parameter experimentally, especially in cell membranes, and predict how it translates to protein structure.
Complex membranes that feature mixtures of different lipid components are not necessarily homogeneous, but rather can separate into distinct domains. Such lateral heterogeneity can be promoted by the affinity of specific lipids for one another (e.g. sterols and sphingolipids) and allow for distinctive membrane microenvironment within a single continuous bilayer. Foundational work in synthetic vesicle systems has shown that membrane domains can be modeled as 2D phase separations, where a more highly packed and viscous region coexisting alongside (Veatch and Keller, 2003). Coexisting domains have the capability to sort and retain specific membrane proteins based on the transmembrane domains or lipid modifications (e.g. palmitoylation) (Lorent and Levental, 2015). Thus, they could aid in the function of membrane-based signaling and assembly processes by enhancing the co-localization. Domains could also serve as platforms for additional (liquid-liquid) phase separations in neighboring cytoplasm (Snead and Gladfelter, 2019). Despite these wide ranging possibilities, it is still not clear to the extent that in vivo membrane domains follow these models, although lateral heterogeneity at the sub-micron scale has now been well documented (Honigmann et al., 2014).
In many cellular compartments, membranes need to be highly curved for their function. Examples include synaptic vesicle trafficking in neurons or the assembly of high surface area platforms in the photoreceptor discs and the inner mitochondria. Lipids influence the bendability of membranes in two ways. First, lipids with mismatched headgroup and acyl chain areas (“cone-shaped”) can impart a spontaneous curvature that induces membrane deformation. An example is lysophospholipids, which only have one acyl chain but maintain headgroup size (Fuller and Rand, 2001). Secondly, lipid composition mediates the bending modulus of membranes, which determines the energy required to further deform them. Greater lipid unsaturation generally decreases bending energies (Marsh, 2006), while cholesterol has been proposed to have an additional role in relaxing bending energy due to its rapid flip-flop across the bilayer (Bruckner et al., 2009).
4. Lipids in the healthy and aging brain
Membrane structure and properties are especially important in the brain, where information processing depends on a series of membrane functions. These include synaptic vesicle trafficking, neurotransmitter release and reception, signaling by membrane-bound networks, ion channel activation and activity, and action potential propagation. All of these processes are known to depend on the dynamic and mechanical properties of their host membranes. Thus, it is not surprising that the brain and CNS has a unique composition of lipids, which likely servess to optimize these membrane-associated functions. The lipidome of the human brain has been extensively reviewed elsewhere (Naudí et al., 2015), but we will highlight some important features with relevance to physiology and aging.
The defining lipidomic feature of the brain is its very high content of n-3 and n-6 PUFAs that are elongated from essential fatty acids LA and ALA (Figure 3). In particular, docosahexaenoic acid (DHA, C22:6) and AA (C20:4) constitute ~20% of the fatty acids in the brain, with DHA being most abundant (Naudí et al., 2015, 2012). This composition differs between gray matter, white matter and myelin (O’Brien and Sampson, 1965) further adding complexity to our understanding of the role of fatty acids in brain biology. It is important to note that only a small fraction of fatty acids are present as free fatty acids and long-lived triglycerides are also largely absent from the CNS. Fatty acid profiles are therefore indicative of phospholipid acyl chain composition. In phospholipids, PUFAs are generally incorporated in the sn-2 position alongside satatured chains (e.g. palmitic acid, C16:) at the sn-1 position. This acyl chain asymmetry has been proposed to optimize the bendability of membranes, which is especially important for synaptic vesicle trafficking in the CNS (Manni et al., 2018). This biophysical effect could explain why saturated fatty acids are also highly abundant and functional in the brain (Hopiavuori et al., 2018).
Figure 3:
Synthesis of very long chain (VLC) PUFAs, and their metabolites from essential fatty acids in mammals. Pathways for elongation (blue arrows) and desaturation (green arrow) of n-3 or n-6 essential fatty acids, alternatively referred to as ω−3 and ω−6. A common set of desaturases and elongases are used to extend and introduce further unsaturations in these fatty acids. The lipid species discussed in the text are highlighted, as are two key elongases (ELOVL2 and ELOVL4) involved in VLC-PUFA biogenesis.
Age-related changes in both total lipid abundance and region-dependent composition have long been observed (Ledesma et al., 2012). PUFA content has generally been observed to drop during aging in a number of systems (Bourre, 2009; Naudí et al., 2015). In particular, DHA and AA have been shown to decrease in the hippocampus of aged rats. The exact mechanism of this effect is unknown, however it has been suggested to be caused by the altered fatty acid metabolism, including the levels of the enzymes involved in lipid biogenesis (McNamara et al., 2008; Terracina et al., 1992a, 1992b), lower rate of transport of PUFAs from the blood in older animals (de la Torre and Mussivand, 1993; McNamara et al., 2008), and enzymatic and non-enzymatic peroxidation of PUFA pools.
Several epidemiological studies have shown negative correlation of the n-3 PUFA (especially DHA) levels in plasma with reduced cognitive decline and potential protective role of DHA on AD progression (Ajith, 2018; Cole et al., 2009). Therefore, based on epidemiological and research data, multiple clinical trials have been conducted with n-3 fatty acids, notably DHA, for the prevention or treatment of age-related cognitive decline. Interestingly, studies suggest that DHA or fish oil can slow early stages of progression, but these effects may be APOE-genotype specific or affected by the time of administration; larger trials may therefore be required to demonstrate efficacy (Barberger-Gateau et al., 2011; Clemons et al., 2006; Cole and Frautschy, 2010; Yassine and Schneider, 2017).
The most abundant polar group of the brain phospholipids is ethanolamine (PE), which is also most affected by age (Bourre, 2009). We now know that a major portion of brain PE are in the form of PUFA-enriched plasmalogens, whose biosynthetic pathway has only recently been fully elucidated (Gallego-García et al., 2019). In the context of aging, specific plasmalogens have been shown to be markers of neurodegeneration (Su et al., 2019). There are clinical trials testing the ability of dietary plasmalogen supplementation to improve progression of mild Alzheimer’s disease (Fujino et al., 2017). Functionally, plasmalogens have proposed to promote membrane fusion and act as molecular sinks for ROS species (Frooqui and Horrocks, 2001).
However, the primary function of plasmalogen enrichment is still a mystery and is an emerging area of research with strong relevance to aging.
Sphingolipids play key roles in both neurons and as structural components of myelin. Gangliosides, glycosphingolipids with sialic acid-containing headgroups, are especially abundant on the surface of neurons (Ledeen, 1985) and has been proposed to induce membrane domain formation (Yuan et al., 2002) and promote membrane bending (Dasgupta et al., 2018). Sphingomyelin, which features a simple choline headgroup, is a primary component of myelin, for which it is named. It has been also noted that ceramide is accumulated in aging striatum and hippocampus (Jazvinšćak Jembrek et al., 2015; Naudí et al., 2015)
In both neurons and myelin, sphingolipids associate with cholesterol to enhance to increase bilayer order, induce domain formation, and increase the insulation properties of membranes. Cholesterol is also enriched in synaptic vesicles, which underlie neuronal function, and exosomes, which can participate in the transmission of neurodegenerative diseases (Kalani et al., 2014). In rodents, the cholesterol content of synaptosomal membranes increases with age in a diet independent manner, which corresponds with an increase in membrane viscosity (Choi and Yu, 1995; Nagy et al., 1983). These studies, carried out with a combination of lipid analysis and spectroscopic viscosity probes, suggest a model in which aging membrane increase their viscosity due to dysregulation of lipid metabolism. Follow up work showed how dietary restriction and exercise, both of which prolong lifespan, reduce membrane viscosity in older animals (Kim et al., 1996).
In the brain, cholesterol is transported between cells through protein transporters and binding factors, including apolipoproteins. ApoD, ApoE and ApoJ are the most abundantly expressed apolipoproteins in CNS, with distinct spatio-temporal pattern of expression that could indicate specific roles in brain (Elliott et al., 2010). Several neurological disorders have been linked to polymorphism in this molecules. For example, the ε4 allele of APOE is the strongest genetic risk factor for Alzheimer’s disease. Recent work has shown that APOE is important for clearing myelin debris, which is highly enriched in cholesterol, and that this capability is reduced during aging in mice (Cantuti-Castelvetri et al., 2018). Strikingly, inhibition of cholesterol biosynthesis in cell culture and animal models reduces accumulation of β-amyloid peptides (Fassbender et al., 2001). Several clinical trials have thus tested if low cholesterol diets or cholesterol-lowering drugs (statins) could improve progression of the disease, but these have led to conflicting results (Schultz et al., 2018). One potential explanation is that statins act to lower circulating cholesterol carried by lipoprotein, but not necessarily existing cellular pools in the brain. Despite these efforts and the obvious genetic evidence, the functional connection between cholesterol content/transport and Alzheimer’s progression is still largely unexplored.
5. Age-related retinal disorders: diseases of the lipidome?
The retina is a thin layer of neurons that lines the back of the eye and is the site of visual transduction in vertebrates. In embryonic development, the retina and the optic nerve outgrow from the developing brain; the retina is therefore part of the CNS. The lipid composition in the retina is highly unique and plays a critical role in its function and related diseases. The retina is particularly enriched in PUFAs, with DHA accounting for approximately 50% of the total fatty acids in the photoreceptor outer disc membranes (Fliesler and Anderson, 1983). This feature results in a highly fluid disc membranes that permit efficient conformational changes and signaling dynamics for rhodopsin and its associated G-protein during phototransduction (Oates and Watts, 2011). Interestingly, the photoreceptor plasma membrane contains only ~5% DHA (Boesze-Battaglia and Schimmel, 1997), further underlying the specialization of the lipid membranes in outer segment.
Very long chain (VLC) PUFAs (≥C22) are especially suited to build highly curved membranes in photoreceptor outer segment discs. The role of VLC-PUFAs in retina biology has been underlined by the discovery of human mutations in the ELOVL4 gene, which encodes for a key enzyme in the synthesis of VLC-PUFAs. The dominant negative mutation in this gene is associated with Stargardt-like macular dystrophy (STGD3) which shares pathological features with dry age-related macular degeneration (AMD) including macular deposits (Bernstein et al., 2001)(Edwards et al., 2001)(Zhang et al., 2001) but instead occurs in young patients (Agbaga et al., 2008) (Harkewicz et al., 2012). Further studies of VLC-PUFAs in human eyes have reinforced the relationship with VLC-PUFAs and AMD. For example, levels of DHA and other VLC-PUFAs, as well as the ratio of n-3/n-6 VLC-PUFAs, is decreased in the retina and retinal pigment epithelium (RPE)-choroid of human AMD eyes compared to age-matched controls (Liu et al., 2010).
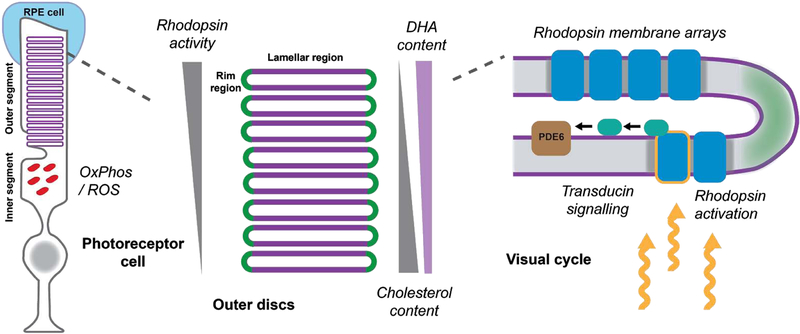
Cholesterol is another lipid present ubiquitously in the retina, especially in the plasma membrane of photoreceptors. It is also present in the disc membranes but its content there is correlated with the position of the disc. Photoreceptor disc membranes are synthesized at the base; therefore, older discs are pushed towards the apex of the photoreceptor over several days. The cholesterol content decreases from the base of the outer segment to the apical pit as cholesterol relocates to the plasma membrane over the lifetime of the disc (reviewed in (Albert et al., 2016). This particularity in cholesterol distribution has profound consequences on the visual cycle (Figure 4). It has been shown that the cholesterol interacts directly and stabilizes rhodopsin (Albert et al., 1996), while DHA has been implicated in rhodopsin regeneration (Bush et al., 1991). Interestingly, the content of DHA and other PUFAs increases towards the apical side of the outer segment (Albert et al., 1998). Thus, the local composition of lipid membranes allows photoreceptors to limit rhodopsin activity to the apical discs.
Figure 4:
Membrane controlled processes in photoreceptor cells. Left - basic layout of rod photoreceptors cells; Center - rhodopsin activity in outer disc stacks is regulated by lipid composition established by opposite gradients of DHA and cholesterol; Right - phototransduction components are localized in lamellar region of disc membranes. Italicized names of processes are potential sites of control by lipid-defined membrane properties.
Several studies have focused on analyzing age-related changes in lipid composition in the retina. For example, the accumulation of cholesterol and neutral lipids has been observed in the Bruch’s membrane, which separates the RPE from the choroid (Curcio et al., 2011). This increase is accompanied with lower levels of n-3 VLC-PUFAs and an altered ratio of n-3/n-6 PUFAs in aging retina (Liu et al., 2010). Beyond these correlations, very little is known about the molecular mechanism of age-related changes in regulation of lipid synthesis or metabolism.
In recent work, we reported that Elongation Of Very Long Chain Fatty Acids-Like 2 (ELOVL2), an enzyme involved in elongation of PUFAs, regulates age-associated functional and anatomical aging in vivo, with direct relevance to age-related eye diseases. We found that an age-related decrease in Elovl2 expression is associated with increased DNA methylation of its promoter in the mouse retina. Mice carrying a point mutation (C234W) that disrupts ELOVL2-specific enzymatic activity have lower levels of LC-PUFAs including DHA and n-3 precursor of VLC-PUFAs (C24:6). Mutant mice display electrophysiological characteristics of premature visual decline, as well as early appearance of autofluorescent deposits, well-established markers of aging in the mouse retina. Finally, we found deposits underneath the RPE in Elovl2 mutant mice, containing components of the complement system and lipid metabolism. Importantly, methylation of the regulatory region of the ELOVL2 gene is one of the most robust biomarkers of human age. Therefore our studies may represent the first example of the DNA methylation clock having direct functional consequences in age-related tissue function, as well as the first molecular link between age-related change in gene expression and membrane function (Chen et al., n.d.).
Multiple epidemiologic studies have suggested that diets rich in n-3 LC-PUFAs are associated with lower rates of age-related macular degeneration, with low dietary intake of n-3 LC-PUFAs associated with higher risk of developing the disease (van Leeuwen et al., 2018) (Chong et al., 2008). In addition, two large early studies demonstrated that high plasma levels of n-3 LC PUFAs were correlated with decreased risk of AMD (Christen et al., 2011) (Merle et al., 2013) Interestingly, in the Age-Related Eye Disease Study (AREDS), a large prospective study investigating factors of progression to advanced AMD, subjects with the highest self-reported intake of foods rich in n-3 LC-PUFAS were 30% less likely to develop central GA and 50% less likely to develop AMD than subjects with the lowest self-reported intake (SanGiovanni et al., 2009). Later, the impact of more defined n-3 LC PUFAs supplementation was investigated by two large prospective studies. The AREDS2 study and the nutritional AMD study (NAT-2) examined the effect of n-3 PUFA supplementation to prevent progression to advanced AMD or wet AMD. Surprisingly, in both studies, there was no significant difference between oral supplementation of PUFAs and placebo in progression to wet AMD (AREDS2 Research Group et al., 2012; Souied et al., 2013) suggesting that other fatty acids or other molecules present in foods rich in n-3 LC-PUFAS may have a preventive role in AMD progression. Future molecular studies focused on specific role of enzymes involved in lipid metabolism will help to understand the results of the clinical findings.
In sum, multiple lines of evidence suggest the important role of lipids in retina function and the role of membrane composition and structure on the tissue homeostasis. Thanks to the particular retina structure and lack of myelination (which interferes with lipidomic analysis of many types of neuronal membranes), aging studies can be performed on multiple levels: tissue, cells, molecular machinery, and membrane structure. This integrated approach has yielded surprising roles for membrane lipids in age-related eye diseases and suggests novel avenues for therapeutics.
6. Aging membranes: what are the underlying molecular mechanisms?
Although still speculative, we hypothesize that there are several direct mechanisms by which changes in lipid composition can promote aging-related processes. We lay these possibilities out not as definitive answers to the phenomenon described above, but avenues for which future mechanistic studies could explore.
6.1. The potential of aging mitochondrial membranes to drive CNS deterioration
One hypothesis is that functions for lipid composition in the electron transport chain (ETC) that are only beginning to be understood could play key roles in reducing energy availability and increasing ROS production during aging. The brain is one of the most metabolically active organs in the human body, as neuronal function depends on energy intensive biochemical machinery at multiple points. As neurons differentiate, they increasingly rely on oxidative phosphorylation for chemical energy (ATP) (Zheng et al., 2016), a process that occurs via the ETC in the inner mitochondrial membrane (IMM). The ETC is the primary site for ROS production, a potential driver of aging. Any inhibition to ETC functions leads to the accumulation of NADH at the beginning of the ETC, which reduces ETC flux (and therefore ATP production) while generating superoxide anion at complex I (Murphy, 2009). ROS species can further damage mitochondrial lipids, proteints, or the mitochondrial genome (which encodes for several ETC complexes), further reducing ETC function. This dynamic can thus lead to a vicious cycle that progressively reduces metabolic functions and increases cellular damage during aging.
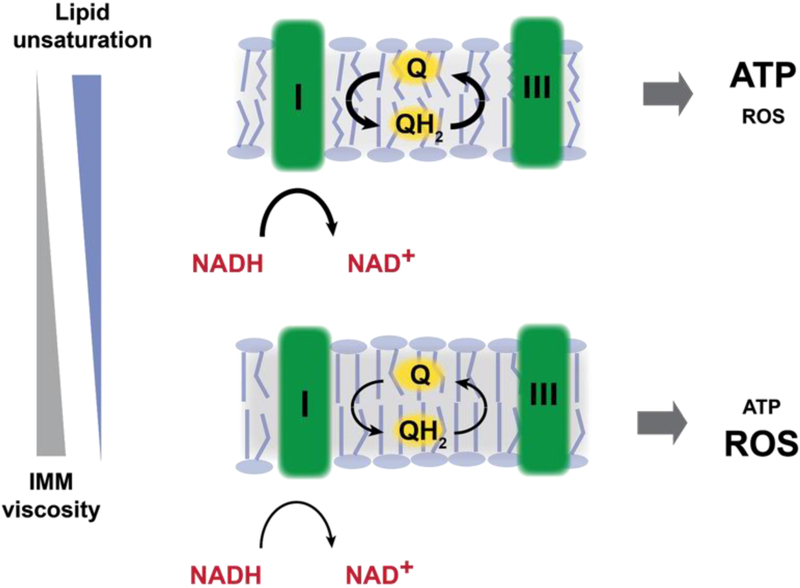
We have recently found that membrane viscosity, as controlled by lipid unsaturation, can have a key role in dictating the rate of ETC flux (Budin et al., 2018). In these experiments, simple model systems (bacteria, yeast) in which unsaturated lipid biosynthesis could be tightly controlled were used to screen for the physiological consequences of increasing membrane viscosity. We found that this parameter tightly controlled respiratory flux. One possibility is this was due to the intrinsic effects that membrane viscosity have on membrane-bound reaction networks. In the ETC, ubiquinone is used as a mobile carrier of electrons between complex I or II and complex III. To carry out this function, ubiquinone - itself a lipid - must diffuse along the membrane, a process that is controlled by membrane viscosity. Modeling of diffusion in the ETC of E. coli showed that measured differences in ubiquinone diffusion rates could wholly explain the lipid control of respiration in this system. As predicted, highly viscous membranes led to the accumulation of fermentation products, indicating a build up of NADH, and expression of oxidative stress response genes. By this mechanism, changes in IMM lipid composition can inhibit ATP production and drive ROS formation (Figure 5).
Figure 5:
Control of ETC function and mitochondrial ROS production by inner membrane lipid composition. Changes in lipid composition that increase membrane viscosity slow down the diffusion of membrane components, such as electron carriers (ubiquinones) in the ETC. The inner mitochondrial membrane in healthy cells is enriched in unsaturated and polyunsaturated lipids (top), which lower membrane viscosity and therefore promote ubiquinone (Q) and ubiquinol (QH2) turnover between ETC enzymes, such as complex I and complex III. Increases in membrane viscosity due to saturated lipid accumulation or lipid peroxidation (bottom) slow down the diffusion of the quinone pool, reducing ATP production of the ETC. Additionally, build up of electron donors (NADH) due to impaired ETC function generates increased ROS, further degrading IMM structure through lipid peroxidation.
A diffusion-based model for lipid ETC control harkens back to foundational work by Charles Hackenbrock and colleagues showing that the mammalian ETC is i) freely mobile in the IMM, ii) is affected by the average distances between membrane protein complexes, and iii) features kinetics consistent with diffusion control (Gupte et al., 1984; Hackenbrock et al., 1986). Since Hackenbrock’s work, in which he posited that membrane diffusion generally controls ETC activity, several new discoveries have modified our view of mammalian ETCs. Most notably, strong biochemical and structural evidence now supports the association of several ETC complexes (e.g. I, III, and IV) into supercomplexes, whose assembly promotes respiratory activity (Lapuente-Brun et al., 2013). The function of these assemblies is still not understood (Milenkovic et al., 2017), but one logical hypothesis is that they reduce the effective distance for electron carriers (ubiquinone and cytochrome c) to diffuse, even in the absence of any substrate channeling.
Supercomplex assembly is thought to require cardiolipin molecules (Zhang et al., 2005), which have been observed in close association with supercomplex interfaces in structural studies (Rathore et al., 2019). The disruption of cardiolipin biosynthesis or its remodeling with PUFA chains leads to reduction in metabolic activity and increase in reactive oxygen species that drive aging processes (Paradies et al., 2010). If mitochondrial dysfunction is a driver of aging, it is likely to be most pronounced in the CNS due to the energy demands for neuronal function and dependence on oxidative phosphorylation. The retina is perhaps an even more extreme example; it is the highest oxygen-consuming organ in the human body (Wong-Riley, 2010). Reduced mitochondrial activity and increased damage could thus explain why this particular tissue is sensitive to age-related deterioration and disease. Notably, oxidative stress is one of the major mechanism affecting survival of retinal ganglion cells in glaucoma, another blinding age-related eye disease (Chrysostomou et al., 2013; Edwards et al., n.d.; Kong et al., 2009).
6.2. Autophagic membranes: a link between lipid supply and cell damage response
When cells become stressed, e.g. due to oxidative damage, a host of response pathways are employed. Lipids have a fundamental role in the formation of the compartments central to these processes: endosomes, exosomes, lysosomes, droplets, and autophagosomes, to name a few. The availability of lipids and their composition can regulate compartment size, abundance, and membrane properties, all of which affect function. Autophagy, a conserved lysosomal degradation pathway essential for cellular homeostasis and adaptation to stress, can be particularly affected by the availability of lipids (de la Ballina et al., 2019). Since autophagy facilitates survival through clearance of damaged molecules and mobilization of storages of nutrients, it is not surprising that many studies show it as a critical regulator of lifespan in many model organisms (reviewed in (Hansen et al., 2018)).
The process of autophagy relies on availability of membranes starting from the formation of the phagophore, a cup-shaped double membrane structure that engulfs the cytoplasmic material to be processed. The mature autophagosome fuses with the lysosome, another membranous vesicle rich in enzymes that will degrade the autophagosome content. Each step of this process depends on the autophagy (ATG) related proteins. Interestingly, some ATG proteins are i) lipid sensing and binding (e.g. vesicle carrying transmembrane protein ATG9), ii) contributing to the direct phospholipid transfer from the ER to the phagophore at contact sites (e.g. ATG2), and iii) membrane-curvature sensing (e.g. ATG3), reviewed in (Osawa et al., 2019). Moreover, members of the LC3/GABARAP protein family, which are key molecules in the autophagosome biogenesis and substrate selection, are modified by lipids for their activation. Their interaction with cargo receptors is dependent on their conjugation to phosphatidylethanolamine (PE) during autophagosome formation (Bento et al., 2016; de la Ballina et al., 2019)
The general mechanism of autophagy depends on lipid availability and an impairment of specific cargo turnover via selective autophagy has been shown to have an impact on aging and age-related diseases. Interestingly, one specific cargo class that undergo selective autophagy are lipids themselves. In this process (lipophagy) the lipid storage vesicles (lipid droplets) are degraded to be used as an energy source in the stressed conditions, especially upon acute starvation. This process can be used in the cell to bring the lipids to rebuild the organelles membranes when needed. However, the effectiveness of this process per se is highly dependent on membranes availability and can be affected by the age-related changes in the cell (Hansen et al., 2018; Singh and Cuervo, 2012). Like for many conserved cellular processes, functional research on autophagy is greatly aided by work in simple model systems. In yeast, micro-lipophagy directs droplets to ordered membrane domains on the yeast lysosome (vacuole) (Seo et al., 2017), a process that is one of the best examples of functional membrane phase separation in cells (Toulmay and Prinz, 2013). In the brain, neurons have few lipid droplets, but this process can still be important for glial cells that support them (such as astrocytes) under stress conditions (Ioannou et al., 2019).
Another example of the process of selective autophagy is the degradation of damaged mitochondria (mitophagy). The accumulation of fragmented mitochondria is one of the hallmarks of aging, likely as a result of the damaging processes described above. Although several mechanisms contribute to this process, the decline in mitophagy is believed to be one of the major factors participating in the process of accumulation of damaged mitochondria. Disruptions in mitophagy has been linked to the pathophysiology of age related retinopathy, Parkinson disease, amyotrophic lateral sclerosis (ALS) and AD (Stavoe and Holzbaur, 2019). More about the role of autophagy in aging has been reviewed extensively recently in (Hansen et al., 2018) and (Leidal et al., 2018).
7. Outlook: how to study the functions and mechanisms of lipids in aging?
We propose that lipids should be a continued and expanding focus for understanding the aging process and the molecular determinants of healthy lifespans. As our knowledge of fundamental lipid and membrane biology increases, we anticipate that mechanistic links between the molecular functions of different lipid species will be tied into aging and aging-associated disease pathologies. In contrast to other well-studied macromolecules, lipids are natural components of the human diet, so functional insights could lead to potential therapeutics to inhibit aging-associated deterioration and disease.
Our understanding of the role of lipids in the tissues health and aging will require further development of interdisciplinary approaches and integrative models. For example, while general trends for changes in lipid composition during aging have been uncovered, we still lack detailed descriptions of the organ, cell-type, and organelle-specific lipidomes during the lifespan. This will require harnessing the advances made in lipidomics and lipid chemistry in collaboration with scientists focusing on aging in complex models. Further biophysical approaches can then focus on identifying how the resulting properties of aging membranes change in vivo. Particular focus should be placed on identifying relevant lipid subdomains in cellular compartments, which is amenable to in vivo imaging with the incredible advancements to these technologies in recent years (Liu et al., 2018).
The next challenge relies on investigating the age-related molecular mechanisms that affect lipid bilayers and therefore the cells and tissues. Recent studies focused on the transcriptomic and epigenetic changes in aging are providing new information regarding changes of expression of enzymes and other molecules involved in lipid synthesis and metabolism. For example, DNA methylation of fatty acid elongating enzyme Elovl2 regulatory region has been shown as the clear biomarker of aging (Garagnani et al., 2012; Hannum et al., 2013). We confirmed this correlation in the retina and shown that ELOVL2 enzymatic activity is indispensable for photoreceptor function. In this way, we uncovered a molecular link between the metabolism of fatty acids and visual function in aging (Chen et al., n.d.).
Fully elucidating lipid function in aging, among other processes, will require better tools for controlling and isolating specific lipid components in living organisms. This necessitates the development of model systems and emerging genetic tools to control their metabolism. In microbial systems, we have shown how synthetic biology-inspired approaches can be used to interrogate basic biochemical functions of lipids and membrane structure (Budin and Keasling, 2019). This same approach can be applied to complex animal systems where aging processes are most relevant. Another consideration is choosing systems in which specific lipid structures can be isolated for lipidomics and imaging. For example, one challenge for studying neuronal membranes in the white matter of brains is the abundance of myelin, which makes up the majority of lipid mass. Non-myelinated tissue, such as the retina, is therefore an attractive model for correlating changes in lipid composition to those in neuronal membrane structure. Invertebrate systems for animal lifespan, such as the fruit fly Drosophila melanogaster, are also not myelinated and feature strong genetic tools amenable for functional interrogation.
Understanding how membrane structure acts through the lifespan could allow us to identify molecular drivers of aging processes and possible therapeutic strategies to mitigate them. Such approaches would start with the dietary supplementation, continue with modulation of key enzymes and transporters, and finish with gene therapy approaches to restore healthy levels of key enzymes in lipid biosynthesis. From the clinical perspective, lipids supplementation is the most attractive therapeutic strategy because of its ease and lack of adverse effects, and therefore much effort has focused on this approach. In has to be noted, however, that without the knowledge of how the dietary lipids are processed and incorporated in the tissues of patients, which is difficult to assay, it is quite challenging to predict or interpret the results of nutritional studies.
8. Conclusion
The aging of cell membranes through changes in their lipid components is potentially relevant to a wide range of aging processes and aging-related disease. Much of these act in the CNS, whose deterioration with age limits the functional lifespan in humans. The aging brain undergoes significant remodelling of lipid composition, including alterations in lipids associated with neuronal membranes (PUFAs and plasmalogens) and myelin sheaths (sterols and sphingomyelin). Because of its centrality to neuronal function, membrane structure could be an important link connecting neurodegeneration with lipid availability from diet and during the lifespan. Interdisciplinary approaches that integrate physiology, genetics, lipid biochemistry, and membrane biophysics will be required to identify the molecular functions underlying these effects and how to effectively treat them.
Highlights.
Age-related changes in lipid metabolism affect the composition of cell membranes
The biophysical properties of membranes change with age and in age-related disease
Biomarkers of neurodegeneration include genes from lipid metabolism and transport
The retina is a model system for understanding lipid drivers of aging
Interdisciplinary approaches are needed to connect molecular changes to physiology
Acknowledgments:
This work was supported by NIH R01 EY02701 and RPB Special Scholar Award (D.S.K.) and NSF MCB-1715681 (I.B.). D.S.K. is a scientific advisor for Visgenx. I.B. declares no competing interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Agbaga M-P, Brush RS, Mandal MNA, Henry K, Elliott MH, Anderson RE, 2008. Role of Stargardt-3 macular dystrophy protein (ELOVL4) in the biosynthesis of very long chain fatty acids. Proc. Natl. Acad. Sci. U. S. A 105, 12843–12848. 10.1073/pnas.0802607105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ajith TA, 2018. A Recent Update on the Effects of Omega-3 Fatty Acids in Alzheimer’s Disease. Curr. Clin. Pharmacol 13, 252–260. 10.2174/1574884713666180807145648 [DOI] [PubMed] [Google Scholar]
- 3.Albert A, Alexander D, Boesze-Battaglia K, 2016. Cholesterol in the rod outer segment: A complex role in a “simple” system. Chem. Phys. Lipids 199, 94–105. 10.1016/j.chemphyslip.2016.04.008 [DOI] [PubMed] [Google Scholar]
- 4.Albert AD, Boesze-Battaglia K, Paw Z, Watts A, Epand RM, 1996. Effect of cholesterol on rhodopsin stability in disk membranes. Biochimica et Biophysica Acta (BBA) - Protein Structure and Molecular Enzymology. 10.1016/0167-4838(96)00102-1 [DOI] [PubMed] [Google Scholar]
- 5.Albert AD, Young JE, Paw Z, 1998. Phospholipid fatty acyl spatial distribution in bovine rod outer segment disk membranes. Biochim. Biophys. Acta 1368, 52–60. 10.1016/s0005-2736(97)00200-9 [DOI] [PubMed] [Google Scholar]
- 6.AREDS2 Research Group, Chew EY, Clemons T, SanGiovanni JP, Danis R, Domalpally A, McBee W, Sperduto R, Ferris FL, 2012. The Age-Related Eye Disease Study 2 (AREDS2): study design and baseline characteristics (AREDS2 report number 1). Ophthalmology 119, 2282–2289. 10.1016/j.ophtha.2012.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barberger-Gateau P, Samieri C, Féart C, Plourde M, 2011. Dietary omega 3 polyunsaturated fatty acids and Alzheimer’s disease: interaction with apolipoprotein E genotype. Curr. Alzheimer Res. 8, 479–491. 10.2174/156720511796391926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bazan NG, 2018. Docosanoids and elovanoids from omega-3 fatty acids are pro-homeostatic modulators of inflammatory responses, cell damage and neuroprotection. Mol. Aspects Med. 64, 18–33. 10.1016/j.mam.2018.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bento CF, Renna M, Ghislat G, Puri C, Ashkenazi A, Vicinanza M, Menzies FM, Rubinsztein DC, 2016. Mammalian Autophagy: How Does It Work? Annu. Rev. Biochem 85, 685–713. 10.1146/annurev-biochem-060815-014556 [DOI] [PubMed] [Google Scholar]
- 10.Bernstein PS, Tammur J, Singh N, Hutchinson A, Dixon M, Pappas CM, Zabriskie NA, Zhang K, Petrukhin K, Leppert M, Allikmets R, 2001. Diverse macular dystrophy phenotype caused by a novel complex mutation in the ELOVL4 gene. Invest. Ophthalmol. Vis. Sci 42, 3331–3336. https://www.ncbi.nlm.nih.gov/pubmed/11726641 [PubMed] [Google Scholar]
- 11.Boesze-Battaglia K, Schimmel R, 1997. Cell membrane lipid composition and distribution: implications for cell function and lessons learned from photoreceptors and platelets. J. Exp. Biol 200, 2927–2936. https://www.ncbi.nlm.nih.gov/pubmed/9359876 [DOI] [PubMed] [Google Scholar]
- 12.Bourre JM, 2009. 12 - Brain lipids and ageing, in: Raats M, de Groot L, van Staveren W (Eds.), Food for the Ageing Population. Woodhead Publishing, pp. 219–251. 10.1533/9781845695484.2.219 [DOI] [Google Scholar]
- 13.Briggman KL, Bock DD, 2012. Volume electron microscopy for neuronal circuit reconstruction. Curr. Opin. Neurobiol 22, 154–161. 10.1016/j.conb.2011.10.022 [DOI] [PubMed] [Google Scholar]
- 14.Bruckner RJ, Mansy SS, Ricardo A, Mahadevan L, Szostak JW, 2009. Flip-flop-induced relaxation of bending energy: implications for membrane remodeling. Biophys. J 97, 3113–3122. 10.1016/j.bpj.2009.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Budin I, de Rond T, Chen Y, Chan LJG, Petzold CJ, Keasling JD, 2018. Viscous control of cellular respiration by membrane lipid composition. Science 362, 1186–1189. 10.1126/science.aat7925 [DOI] [PubMed] [Google Scholar]
- 16.Budin I, Keasling JD, 2019. Synthetic Biology for Fundamental Biochemical Discovery. Biochemistry 58, 1464–1469. 10.1021/acs.biochem.8b00915 [DOI] [PubMed] [Google Scholar]
- 17.Bush RA, Remé CE, Malnoë A, 1991. Light damage in the rat retina: the effect of dietary deprivation of N-3 fatty acids on acute structural alterations. Exp. Eye Res. 53, 741–752. 10.1016/0014-4835(91)90109-r [DOI] [PubMed] [Google Scholar]
- 18.Cadenas E, Davies KJ, 2000. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic. Biol. Med 29, 222–230. 10.1016/s0891-5849(00)00317-8 [DOI] [PubMed] [Google Scholar]
- 19.Cantor RS, 1997. Lateral Pressures in Cell Membranes: A Mechanism for Modulation of Protein Function. J. Phys. Chem. B 101, 1723–1725. 10.1021/jp963911x [DOI] [Google Scholar]
- 20.Cantuti-Castelvetri L, Fitzner D, Bosch-Queralt M, Weil M-T, Su M, Sen P, Ruhwedel T, Mitkovski M, Trendelenburg G, Lütjohann D, Möbius W, Simons M, 2018. Defective cholesterol clearance limits remyelination in the aged central nervous system. Science 359, 684–688. 10.1126/science.aan4183 [DOI] [PubMed] [Google Scholar]
- 21.Chen D, Chao DL, Rocha L, Kolar M, Huu VAN, Krawczyk M, Dasyani M, Wang T, Jafari M, Jabari M, Ross KD, Saghatelian A, Hamilton B, Zhang K, Skowronska-Krawczyk D, n.d. The Lipid Elongation Enzyme ELOVL2 is a molecular regulator of aging in the retina. 10.1101/795559 [DOI] [PMC free article] [PubMed]
- 22.Choi JH, Yu BP, 1995. Brain synaptosomal aging: free radicals and membrane fluidity. Free Radic. Biol. Med 18, 133–139. 10.1016/0891-5849(94)00106-t [DOI] [PubMed] [Google Scholar]
- 23.Chong EW-T, Kreis AJ, Wong TY, Simpson JA, Guymer RH, 2008. Dietary ω−3 Fatty Acid and Fish Intake in the Primary Prevention of Age-Related Macular Degeneration: A Systematic Review and Meta-analysis. Arch. Ophthalmol 126, 826–833. 10.1001/archopht.126.6.826 [DOI] [PubMed] [Google Scholar]
- 24.Christen WG, Schaumberg DA, Glynn RJ, Buring JE, 2011. Dietary ω−3 fatty acid and fish intake and incident age-related macular degeneration in women. Arch. Ophthalmol 129, 921–929. 10.1001/archophthalmol.2011.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chrysostomou V, Rezania F, Trounce IA, Crowston JG, 2013. Oxidative stress and mitochondrial dysfunction in glaucoma. Curr. Opin. Pharmacol 13, 12–15. 10.1016/j.coph.2012.09.008 [DOI] [PubMed] [Google Scholar]
- 26.Clemons TE, Rankin MW, McBee WL, Age-Related Eye Disease Study Research Group, 2006. Cognitive impairment in the Age-Related Eye Disease Study: AREDS report no. 16. Arch. Ophthalmol 124, 537–543. 10.1001/archopht.124.4.537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cole GM, Frautschy SA, 2010. DHA may prevent age-related dementia. J. Nutr 140, 869–874. 10.3945/jn.109.113910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cole GM, Ma Q-L, Frautschy SA, 2009. Omega-3 fatty acids and dementia. Prostaglandins Leukot. Essent. Fatty Acids 81, 213–221. 10.1016/j.plefa.2009.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Curcio CA, Johnson M, Rudolf M, Huang J-D, 2011. The oil spill in ageing Bruch membrane. Br. J. Ophthalmol 95, 1638–1645. 10.1136/bjophthalmol-2011-300344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dasgupta R, Miettinen MS, Fricke N, Lipowsky R, Dimova R, 2018. The glycolipid GM1 reshapes asymmetric biomembranes and giant vesicles by curvature generation. Proc. Natl. Acad. Sci. U. S. A 115, 5756–5761. 10.1073/pnas.1722320115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dawaliby R, Trubbia C, Delporte C, Noyon C, Ruysschaert J-M, Van Antwerpen P, Govaerts C, 2016. Phosphatidylethanolamine Is a Key Regulator of Membrane Fluidity in Eukaryotic Cells. J. Biol. Chem 291, 3658–3667. 10.1074/jbc.M115.706523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de la Ballina LR, Munson MJ, Simonsen A, 2019. Lipids and Lipid-Binding Proteins in Selective Autophagy. J. Mol. Biol 10.1016/j.jmb.2019.05.051 [DOI] [PubMed] [Google Scholar]
- 33.de la Torre JC, Mussivand T, 1993. Can disturbed brain microcirculation cause Alzheimer’s disease? Neurol. Res 15, 146–153. 10.1080/01616412.1993.11740127 [DOI] [PubMed] [Google Scholar]
- 34.Edwards AO, Donoso LA, Ritter R 3rd, 2001. A novel gene for autosomal dominant Stargardt-like macular dystrophy with homology to the SUR4 protein family. Invest. Ophthalmol. Vis. Sci 42, 2652–2663. https://www.ncbi.nlm.nih.gov/pubmed/11581213 [PubMed] [Google Scholar]
- 35.Edwards G, Perkins GA, Kim K-Y, Kong Y, Lee Y, Choi S-H, Skowronska-Krawczyk D, Weinreb RN, Zangwill L, Strack S, Ju W-K, n.d. Loss of AKAP1 triggers Drp1 dephosphorylation-mediated mitochondrial fragmentation and loss in retinal ganglion cells. 10.1101/790139 [DOI] [PMC free article] [PubMed]
- 36.Elliott DA, Weickert CS, Garner B, 2010. Apolipoproteins in the brain: implications for neurological and psychiatric disorders. Clin. Lipidol 51, 555–573. 10.2217/CLP.10.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fassbender K, Simons M, Bergmann C, Stroick M, Lütjohann D, Keller P, Runz H, Kühl S, Bertsch T, von Bergmann K, Hennerici M, Beyreuther K, Hartmann T, 2001. Simvastatin strongly reduces levels of Alzheimer’s disease β-amyloid peptides Aβ42 and Aβ40 in vitro and in vivo. Proc. Natl. Acad. Sci. U. S. A 98, 5856–5861. 10.1073/pnas.081620098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fillerup DL, Mead JF, 1967. The lipids of the aging human brain. Lipids 2, 295–298. 10.1007/BF02532114 [DOI] [PubMed] [Google Scholar]
- 39.Fliesler SJ, Anderson RE, 1983. Chemistry and metabolism of lipids in the vertebrate retina. Prog. Lipid Res. 22, 79–131. 10.1016/0163-7827(83)90004-8 [DOI] [PubMed] [Google Scholar]
- 40.Frooqui AA, Horrocks LA, 2001. Plasmalogens: Workhorse lipids of membranes in normal and injured neurons. Neuroscientist 7, 232–245. 10.1177/107385840100700308 [DOI] [PubMed] [Google Scholar]
- 41.Fujino T, Yamada T, Asada T, Tsuboi Y, Wakana C, Mawatari S, Kono S, 2017. Efficacy and Blood Plasmalogen Changes by Oral Administration of Plasmalogen in Patients with Mild Alzheimer’s Disease and Mild Cognitive Impairment: A Multicenter, Randomized, Double-blind, Placebo-controlled Trial. EBioMedicine. 10.1016/j.ebiom.2017.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fuller N, Rand RP, 2001. The influence of lysolipids on the spontaneous curvature and bending elasticity of phospholipid membranes. Biophys. J 81, 243–254. 10.1016/S0006-3495(01)75695-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gallego-García A, Monera-Girona AJ, Pajares-Martínez E, Bastida-Martínez E, Pérez-Castaño R, Iniesta AA, Fontes M, Padmanabhan S, Elías-Arnanz M, 2019. A bacterial light response reveals an orphan desaturase for human plasmalogen synthesis. Science 366, 128–132. 10.1126/science.aay1436 [DOI] [PubMed] [Google Scholar]
- 44.Garagnani P, Bacalini MG, Pirazzini C, Gori D, Giuliani C, Mari D, Di Blasio AM, Gentilini D, Vitale G, Collino S, Rezzi S, Castellani G, Capri M, Salvioli S, Franceschi C, 2012. Methylation of ELOVL2 gene as a new epigenetic marker of age. Aging Cell 11, 1132–1134. 10.1111/acel.12005 [DOI] [PubMed] [Google Scholar]
- 45.Gupte S, Wu E-S, Hoechli L, Hoechli M, Jacobson K, Sowers AE, Hackenbrock CR, 1984. Relationship between lateral diffusion, collision frequency, and electron transfer of mitochondrial inner membrane oxidation-reduction components. Proceedings of the National Academy of Sciences 81, 2606–2610. 10.1073/pnas.81.9.2606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hackenbrock CR, Chazotte B, Gupte SS, 1986. The random collision model and a critical assessment of diffusion and collision in mitochondrial electron transport. J. Bioenerg. Biomembr 18, 331–368. 10.1007/bf00743010 [DOI] [PubMed] [Google Scholar]
- 47.Hannum G, Guinney J, Zhao L, Zhang L, Hughes G, Sadda S, Klotzle B, Bibikova M, Fan J-B, Gao Y, Deconde R, Chen M, Rajapakse I, Friend S, Ideker T, Zhang K, 2013. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol. Cell 49, 359–367. 10.1016/j.molcel.2012.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hansen M, Rubinsztein DC, Walker DW, 2018. Autophagy as a promoter of longevity: insights from model organisms. Nat. Rev. Mol. Cell Biol. 19, 579–593. 10.1038/s41580-018-0033-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harkewicz R, Du H, Tong Z, Alkuraya H, Bedell M, Sun W, Wang X, Hsu Y-H, Esteve-Rudd J, Hughes G, Su Z, Zhang M, Lopes VS, Molday RS, Williams DS, Dennis EA, Zhang K, 2012. Essential role of ELOVL4 protein in very long chain fatty acid synthesis and retinal function. J. Biol. Chem 287, 11469–11480. 10.1074/jbc.M111.256073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Honigmann A, Mueller V, Ta H, Schoenle A, Sezgin E, Hell SW, Eggeling C, 2014. Scanning STED-FCS reveals spatiotemporal heterogeneity of lipid interaction in the plasma membrane of living cells. Nat. Commun 5, 5412 10.1038/ncomms6412 [DOI] [PubMed] [Google Scholar]
- 51.Hopiavuori BR, Deák F, Wilkerson JL, Brush RS, Rocha-Hopiavuori NA, Hopiavuori AR, Ozan KG, Sullivan MT, Wren JD, Georgescu C, Szweda L, Awasthi V, Towner R, Sherry DM, Anderson RE, Agbaga M-P, 2018. Homozygous Expression of Mutant ELOVL4 Leads to Seizures and Death in a Novel Animal Model of Very Long-Chain Fatty Acid Deficiency. Mol. Neurobiol 55, 1795–1813. 10.1007/s12035-017-0824-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ikenaka K, Suzuki M, Mochizuki H, Nagai Y, 2019. Lipids as Trans-Acting Effectors for α-Synuclein in the Pathogenesis of Parkinson’s Disease. Front. Neurosci 13, 693 10.3389/fnins.2019.00693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ioannou MS, Jackson J, Sheu S-H, Chang C-L, Weigel AV, Liu H, Pasolli HA, Xu CS, Pang S, Matthies D, Hess HF, Lippincott-Schwartz J, Liu Z, 2019. Neuron-Astrocyte Metabolic Coupling Protects against Activity-Induced Fatty Acid Toxicity. Cell 177, 1522–1535.e14. 10.1016/j.cell.2019.04.001 [DOI] [PubMed] [Google Scholar]
- 54.Jazvinšćak Jembrek M, Hof PR, Šimić G, 2015. Ceramides in Alzheimer’s Disease: Key Mediators of Neuronal Apoptosis Induced by Oxidative Stress and Aβ Accumulation. Oxid. Med. Cell. Longev 2015, 346783 10.1155/2015/346783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johnson AA, Stolzing A, 2019. The role of lipid metabolism in aging, lifespan regulation, and age-related disease. Aging Cell 31, 67 10.1111/acel.13048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kalani A, Tyagi A, Tyagi N, 2014. Exosomes: mediators of neurodegeneration, neuroprotection and therapeutics. Mol. Neurobiol 49, 590–600. 10.1007/s12035-013-8544-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim JD, McCarter RJ, Yu BP, 1996. Influence of age, exercise, and dietary restriction on oxidative stress in rats. Aging 8, 123–129. [DOI] [PubMed] [Google Scholar]
- 58.Kong GYX, Van Bergen NJ, Trounce IA, Crowston JG, 2009. Mitochondrial dysfunction and glaucoma. J. Glaucoma 18, 93–100. 10.1097/IJG.0b013e318181284f [DOI] [PubMed] [Google Scholar]
- 59.Lapuente-Brun E, Moreno-Loshuertos R, Acín-Pérez R, Latorre-Pellicer A, Colás C, Balsa E, Perales-Clemente E, Quirós PM, Calvo E, Rodríguez-Hernández MA, Navas P, Cruz R, Carracedo Á, López-Otín C, Pérez-Martos A, Fernández-Silva P, Fernández-Vizarra E, Enríquez JA, 2013. Supercomplex assembly determines electron flux in the mitochondrial electron transport chain. Science 340, 1567–1570. 10.1126/science.1230381 [DOI] [PubMed] [Google Scholar]
- 60.Lauffenburger DA, Linderman JJ, 1996. Receptors: Models for Binding, Trafficking, and Signaling. OUP; USA. [Google Scholar]
- 61.Ledeen R, 1985. Gangliosides of the neuron. Trends Neurosci. 8, 169–174. 10.1016/0166-2236(85)90064-5 [DOI] [Google Scholar]
- 62.Ledesma MD, Martin MG, Dotti CG, 2012. Lipid changes in the aged brain: effect on synaptic function and neuronal survival. Prog. Lipid Res. 51, 23–35. 10.1016/j.plipres.2011.11.004 [DOI] [PubMed] [Google Scholar]
- 63.Leidal AM, Levine B, Debnath J, 2018. Autophagy and the cell biology of age-related disease. Nat. Cell Biol. 20, 1338–1348. 10.1038/s41556-018-0235-8 [DOI] [PubMed] [Google Scholar]
- 64.Liu A, Chang J, Lin Y, Shen Z, Bernstein PS, 2010. Long-chain and very long-chain polyunsaturated fatty acids in ocular aging and age-related macular degeneration. J. Lipid Res. 51, 3217–3229. 10.1194/jlr.M007518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu T-L, Upadhyayula S, Milkie DE, Singh V, Wang K, Swinburne IA, Mosaliganti KR, Collins ZM, Hiscock TW, Shea J, Kohrman AQ, Medwig TN, Dambournet D, Forster R, Cunniff B, Ruan Y, Yashiro H, Scholpp S, Meyerowitz EM, Hockemeyer D, Drubin DG, Martin BL, Matus DQ, Koyama M, Megason SG, Kirchhausen T, Betzig E, 2018. Observing the cell in its native state: Imaging subcellular dynamics in multicellular organisms. Science 360 10.1126/science.aaq1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lorent JH, Ganesan L, Rivera-Longsworth G, Sezgin E, Levental KR, Lyman E, Levental I, 2019. The Molecular and Structural Asymmetry of the Plasma Membrane. bioRxiv. 10.1101/698837 [DOI] [Google Scholar]
- 67.Lorent JH, Levental I, 2015. Structural determinants of protein partitioning into ordered membrane domains and lipid rafts. Chem. Phys. Lipids 192, 23–32. 10.1016/j.chemphyslip.2015.07.022 [DOI] [PubMed] [Google Scholar]
- 68.Manni MM, Tiberti ML, Pagnotta S, Barelli H, Gautier R, Antonny B, 2018. Acyl chain asymmetry and polyunsaturation of brain phospholipids facilitate membrane vesiculation without leakage. Elife 7 10.7554/eLife.34394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Marsh D, 2006. Elastic curvature constants of lipid monolayers and bilayers. Chem. Phys. Lipids 144, 146–159. 10.1016/j.chemphyslip.2006.08.004 [DOI] [PubMed] [Google Scholar]
- 70.McNamara RK, Liu Y, Jandacek R, Rider T, Tso P, 2008. The aging human orbitofrontal cortex: decreasing polyunsaturated fatty acid composition and associated increases in lipogenic gene expression and stearoyl-CoA desaturase activity. Prostaglandins Leukot. Essent. Fatty Acids 78, 293–304. 10.1016/j.plefa.2008.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Merle BMJ, Delyfer M-N, Korobelnik J-F, Rougier M-B, Malet F, Féart C, Le Goff M, Peuchant E, Letenneur L, Dartigues J-F, Colin J, Barberger-Gateau P, Delcourt C, 2013. High concentrations of plasma n3 fatty acids are associated with decreased risk for late age-related macular degeneration. J. Nutr 143, 505–511. 10.3945/jn.112.171033 [DOI] [PubMed] [Google Scholar]
- 72.Milenkovic D, Blaza JN, Larsson N-G, Hirst J, 2017. The Enigma of the Respiratory Chain Supercomplex. Cell Metab. 25, 765–776. 10.1016/j.cmet.2017.03.009 [DOI] [PubMed] [Google Scholar]
- 73.Mouchlis VD, Dennis EA, 2019. Phospholipase A2 catalysis and lipid mediator lipidomics. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1864, 766–771. 10.1016/j.bbalip.2018.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Murphy MP, 2009. How mitochondria produce reactive oxygen species. Biochemical Journal. 10.1042/bj20081386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nagy K, Nagy V, Bertoni-Freddari C, Nagy I, 1983. Alterations of the synaptosomal membrane “microviscosity” in the brain cortex of rats during aging and centrophenoxine treatment. Arch. Gerontol. Geriatr 2, 23–39. 10.1016/0167-4943(83)90014-6 [DOI] [PubMed] [Google Scholar]
- 76.Naudí A, Cabré R, Jové M, Ayala V, Gonzalo H, Portero-Otín M, Ferrer I, Pamplona R, 2015. Lipidomics of human brain aging and Alzheimer’s disease pathology, in: International Review of Neurobiology. Elsevier, pp. 133–189. 10.1016/bs.irn.2015.05.008 [DOI] [PubMed] [Google Scholar]
- 77.Naudí A, Jové M, Ayala V, Ramírez O, Cabré R, Prat J, Portero-Otin M, Ferrer I, Pamplona R, 2012. Region specific vulnerability to lipid peroxidation in the human central nervous system. Intech 437–456. [Google Scholar]
- 78.Oates J, Watts A, 2011. Uncovering the intimate relationship between lipids, cholesterol and GPCR activation. Curr. Opin. Struct. Biol 21, 802–807. 10.1016/j.sbi.2011.09.007 [DOI] [PubMed] [Google Scholar]
- 79.O’Brien JS, Sampson EL, 1965. Lipid composition of the normal human brain: gray matter, white matter, and myelin. J. Lipid Res. 6, 537–544. https://www.ncbi.nlm.nih.gov/pubmed/5865382 [PubMed] [Google Scholar]
- 80.Osawa T, Alam JM, Noda NN, 2019. Membrane-binding domains in autophagy. Chem. Phys. Lipids 218, 1–9. 10.1016/j.chemphyslip.2018.11.001 [DOI] [PubMed] [Google Scholar]
- 81.Pacifici RE, Davies KJ, 1991. Protein, lipid and DNA repair systems in oxidative stress: the free-radical theory of aging revisited. Gerontology 37, 166–180. 10.1159/000213257 [DOI] [PubMed] [Google Scholar]
- 82.Papsdorf K, Brunet A, 2019. Linking Lipid Metabolism to Chromatin Regulation in Aging. Trends Cell Biol. 29, 97–116. 10.1016/j.tcb.2018.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Paradies G, Petrosillo G, Paradies V, Ruggiero FM, 2010. Oxidative stress, mitochondrial bioenergetics, and cardiolipin in aging. Free Radic. Biol. Med 48, 1286–1295. 10.1016/j.freeradbiomed.2010.02.020 [DOI] [PubMed] [Google Scholar]
- 84.Rapoport SI, Rao JS, Igarashi M, 2007. Brain metabolism of nutritionally essential polyunsaturated fatty acids depends on both the diet and the liver. Prostaglandins Leukot. Essent. Fatty Acids 77, 251–261. 10.1016/j.plefa.2007.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rathore S, Berndtsson J, Marin-Buera L, Conrad J, Carroni M, Brzezinski P, Ott M, 2019. Cryo-EM structure of the yeast respiratory supercomplex. Nat. Struct. Mol. Biol 26, 50–57. 10.1038/s41594-018-0169-7 [DOI] [PubMed] [Google Scholar]
- 86.Roses AD, 2006. On the discovery of the genetic association of Apolipoprotein E genotypes and common late-onset Alzheimer disease. J. Alzheimers. Dis 9, 361–366. 10.3233/jad-2006-9s340 [DOI] [PubMed] [Google Scholar]
- 87.Saffman PG, Delbrück M, 1975. Brownian motion in biological membranes. Proc. Natl. Acad. Sci. U. S. A 72, 3111–3113. 10.1073/pnas.72.8.3111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.SanGiovanni JP, Agrón E, Clemons TE, Chew EY, 2009. ω−3 Long-Chain Polyunsaturated Fatty Acid Intake Inversely Associated With 12-Year Progression to Advanced Age-Related Macular Degeneration. Arch. Ophthalmol 127, 109–116. 10.1001/archophthalmol.2008.518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sastry PS, 1985. Lipids of nervous tissue: composition and metabolism. Prog. Lipid Res. 24, 69–176. 10.1016/0163-7827(85)90011-6 [DOI] [PubMed] [Google Scholar]
- 90.Schroeder F, 1984. Role of membrane lipid asymmetry in aging. Neurobiol. Aging 5, 323–333. 10.1016/0197-4580(84)90010-1 [DOI] [PubMed] [Google Scholar]
- 91.Schultz BG, Patten DK, Berlau DJ, 2018. The role of statins in both cognitive impairment and protection against dementia: a tale of two mechanisms. Transl. Neurodegener 7, 5 10.1186/s40035-018-0110-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Semsei I, Szeszák F, Nagy I, 1982. In vivo studies on the age-dependent decrease of the rates of total and mRNA synthesis in the brain cortex of rats. Arch. Gerontol. Geriatr 1, 29–42. 10.1016/0167-4943(82)90004-8 [DOI] [PubMed] [Google Scholar]
- 93.Seo AY, Lau P-W, Feliciano D, Sengupta P, Gros MAL, Cinquin B, Larabell CA, Lippincott-Schwartz J, 2017. AMPK and vacuole-associated Atg14p orchestrate μ-lipophagy for energy production and long-term survival under glucose starvation. Elife 6 10.7554/eLife.21690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Singh R, Cuervo AM, 2012. Lipophagy: connecting autophagy and lipid metabolism. Int. J. Cell Biol. 2012, 282041 10.1155/2012/282041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Snead WT, Gladfelter AS, 2019. The Control Centers of Biomolecular Phase Separation: How Membrane Surfaces, PTMs, and Active Processes Regulate Condensation. Mol. Cell 76, 295–305. 10.1016/j.molcel.2019.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.SoOderberg M, Edlund C, Alafuzoff I, Kristensson K, Dallner G, 1992. Lipid Composition in Different Regions of the Brain in Alzheimer’s Disease/Senile Dementia of Alzheimer’s Type. J. Neurochem 59, 1646–1653. 10.1111/j.1471-4159.1992.tb10994.x [DOI] [PubMed] [Google Scholar]
- 97.Souied EH, Delcourt C, Querques G, Bassols A, Merle B, Zourdani A, Smith T, Benlian P, 2013. Oral Docosahexaenoic Acid in the Prevention of Exudative Age-Related Macular Degeneration. Ophthalmology. 10.1016/j.ophtha.2013.01.005 [DOI] [PubMed] [Google Scholar]
- 98.Spiteller G, 2002. Are changes of the cell membrane structure causally involved in the aging process? Ann. N. Y. Acad. Sci 959, 30–44. 10.1111/j.1749-6632.2002.tb02080.x [DOI] [PubMed] [Google Scholar]
- 99.Stavoe AKH, Holzbaur ELF, 2019. Autophagy in Neurons. Annu. Rev. Cell Dev. Biol 35, 477–500. 10.1146/annurev-cellbio-100818-125242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Su XQ, Wang J, Sinclair AJ, 2019. Plasmalogens and Alzheimer’s disease: a review. Lipids in Health and Disease. 10.1186/s12944-019-1044-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Svennerholm L, Boström K, Helander CG, Jungbjer B, 1991. Membrane lipids in the aging human brain. J. Neurochem 56, 2051–2059. 10.1111/j.1471-4159.1991.tb03466.x [DOI] [PubMed] [Google Scholar]
- 102.Svennerholm L, Boström K, Jungbjer B, Olsson L, 1994. Membrane lipids of adult human brain: lipid composition of frontal and temporal lobe in subjects of age 20 to 100 years. J. Neurochem 63, 1802–1811. 10.1046/j.1471-4159.1994.63051802.x [DOI] [PubMed] [Google Scholar]
- 103.Terracina L, Brunetti M, Avellini L, De Medio GE, Trovarelli G, Gaiti A, 1992a. Arachidonic and palmitic acid utilization in aged rat brain areas. Mol. Cell. Biochem 115, 35–42. 10.1007/bf00229093 [DOI] [PubMed] [Google Scholar]
- 104.Terracina L, Brunetti M, Avellini L, De Medio GE, Trovarelli G, Gaiti A, 1992b. Linoleic acid metabolism in brain cortex of aged rats. Ital. J. Biochem 41, 225–235. https://www.ncbi.nlm.nih.gov/pubmed/1428781 [PubMed] [Google Scholar]
- 105.Toulmay A, Prinz WA, 2013. Direct imaging reveals stable, micrometer-scale lipid domains that segregate proteins in live cells. J. Cell Biol. 202, 35–44. 10.1083/jcb.201301039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.van Leeuwen EM, Emri E, Merle BMJ, Colijn JM, Kersten E, Cougnard-Gregoire A, Dammeier S, Meester-Smoor M, Pool FM, de Jong EK, Delcourt C, Rodrigez-Bocanegra E, Biarnés M, Luthert PJ, Ueffing M, Klaver CCW, Nogoceke E, den Hollander AI, Lengyel I, 2018. A new perspective on lipid research in age-related macular degeneration. Prog. Retin. Eye Res. 67, 56–86. 10.1016/j.preteyeres.2018.04.006 [DOI] [PubMed] [Google Scholar]
- 107.Veatch SL, Keller SL, 2003. Separation of liquid phases in giant vesicles of ternary mixtures of phospholipids and cholesterol. Biophys. J 85, 3074–3083. 10.1016/S0006-3495(03)74726-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wong-Riley MTT, 2010. Energy metabolism of the visual system. Eye Brain 2, 99–116. 10.2147/EB.S9078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yankner BA, Lu T, Loerch P, 2008. The aging brain. Annu. Rev. Pathol 3, 41–66. 10.1146/annurev.pathmechdis.2.010506.092044 [DOI] [PubMed] [Google Scholar]
- 110.Yassine HN, Schneider LS, 2017. Lessons from the Multidomain Alzheimer Preventive Trial. The Lancet Neurology. 10.1016/s1474-4422(17)30227-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yuan C, Furlong J, Burgos P, Johnston LJ, 2002. The size of lipid rafts: an atomic force microscopy study of ganglioside GM1 domains in sphingomyelin/DOPC/cholesterol membranes. Biophys. J 82, 2526–2535. 10.1016/S0006-3495(02)75596-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang K, Kniazeva M, Han M, Li W, Yu Z, Yang Z, Li Y, Metzker ML, Allikmets R, Zack DJ, Kakuk LE, Lagali PS, Wong PW, MacDonald IM, Sieving PA, Figueroa DJ, Austin CP, Gould RJ, Ayyagari R, Petrukhin K, 2001. A 5-bp deletion in ELOVL4 is associated with two related forms of autosomal dominant macular dystrophy. Nat. Genet 27, 89–93. 10.1038/83817 [DOI] [PubMed] [Google Scholar]
- 113.Zhang M, Mileykovskaya E, Dowhan W, 2005. Cardiolipin is essential for organization of complexes III and IV into a supercomplex in intact yeast mitochondria. J. Biol. Chem 280, 29403–29408. 10.1074/jbc.M504955200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zheng X, Boyer L, Jin M, Mertens J, Kim Y, Ma L, Ma L, Hamm M, Gage FH, Hunter T, 2016. Metabolic reprogramming during neuronal differentiation from aerobic glycolysis to neuronal oxidative phosphorylation. eLife. 10.7554/elife.13374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zs-Nagy I, 1979. The role of membrane structure and function in cellular aging: a review. Mech. Ageing Dev. 9, 237–246. 10.1016/0047-6374(79)90102-7 [DOI] [PubMed] [Google Scholar]