Abstract
Atrial fibrillation (AF) is the most common heart arrhythmia and is associated with poor outcomes. The adverse effects of AF are mediated through multiple pathways, including endothelial dysfunction, as measured by flow-mediated dilatation. Flow-mediated dilatation has demonstrated endothelial dysfunction in several conditions and is associated with poor outcomes including mortality, yet can be improved with medical therapy. It is thus a useful tool in assessing endothelial function in patients. Endothelial dysfunction is present in patients with AF and is associated with poor outcomes. These patients are generally older and have other co-morbidities such as hypertension, hypercholesterolaemia and diabetes. The precise process by which AF is affiliated with endothelial damage/dysfunction remains elusive. This review explores the endothelial structure, its physiology and how it is affected in patients with AF. It also assesses the utility of flow mediated dilatation as a technique to assess endothelial function in patients with AF.
Key Messages
Endothelial function is affected in patients with atrial fibrillation as with other cardiovascular conditions.
Endothelial dysfunction is associated with poor outcomes such as stroke, myocardial infarction and death, yet is a reversible condition.
Flow-mediated dilatation is a reliable tool to assess endothelial function in patients with atrial fibrillation.
Patients with atrial fibrillation should be considered for endothelial function assessment and attempts made to reverse this condition.
Keywords: Atrial fibrillation, endothelial function, vascular function, flow-mediated dilatation
Introduction
Atrial fibrillation (AF), as the most common cardiac dysrhythmia is associated with poor outcomes, including stroke [1]. The incidence of stroke attributable to AF increases from 1.5% at age 50–59 years to 23.5% at age 80–89 years [2]. Approximately, 15 million people worldwide have a stroke each year, of which at least 15% have been directly attributed to clinically diagnosed AF [1,3]. Compared to people without AF, people with AF have distinctly reduced survival rates, with risk factor-adjusted odds ratio for death of 1.5 in men and 1.9 in women [4]. The adverse effects of AF are perceived to be due to haemodynamic changes with multiple factors leading to a prothrombotic state in vivo [5]. These include abnormal haemostasis including inappropriate platelet activation, intra-atrial blood stasis, structural heart disease and endothelial dysfunction/damage causing predisposition to thrombogensis [5]. In short, the commonly known Virchow’s triad of factors, hypercoagulability, flow disturbance and endothelial dysfunction, which are necessary for the development of thrombosis are all present in patients with AF.
The precise process by which AF is affiliated with endothelial damage/dysfunction remains elusive, given that indices of endothelial dysfunction such as von Willebrand factor (vWF) are abnormal even in lone AF [6]. Flow abnormalities may occur in AF due to heart failure (a commonly associated condition), valvular heart disease or the irregularity of the heart rate itself. This results in turbulent flow both in the left atrium and systemically. Loss of shear stress, as occurs in conditions of turbulent flow is related to reduced expression of endothelial nitric oxide (NO) synthase (eNOS), a key regulator of endothelial function. This review will explore the endothelial structure, its physiology, the role of NO and how endothelial function is affected in patients with AF.
Endothelial structure
Endothelium refers to cells that line the interior surface of blood vessels, forming an interface between circulating blood in the lumen and the vessel wall. It is a single layer of simple squamous cells lining the entire circulatory system, from the heart to the smallest capillaries [7]. Endothelium is mesodermal in origin. In a straight section of a blood vessel, vascular endothelial cells typically align and extend in the direction of fluid flow. Endothelial cells are able to alter their structure and phenotype depending on the vessel type. For example, endothelial cells lining the artery tend to be thicker than those in capillaries, which are fenestrated and thinner to allow for exchange of gases, nutrients and metabolites. Furthermore, endothelial cells can respond differently to stimulation in different vascular beds and even in different sections of the same vascular bed [8–10].
Endothelial physiology
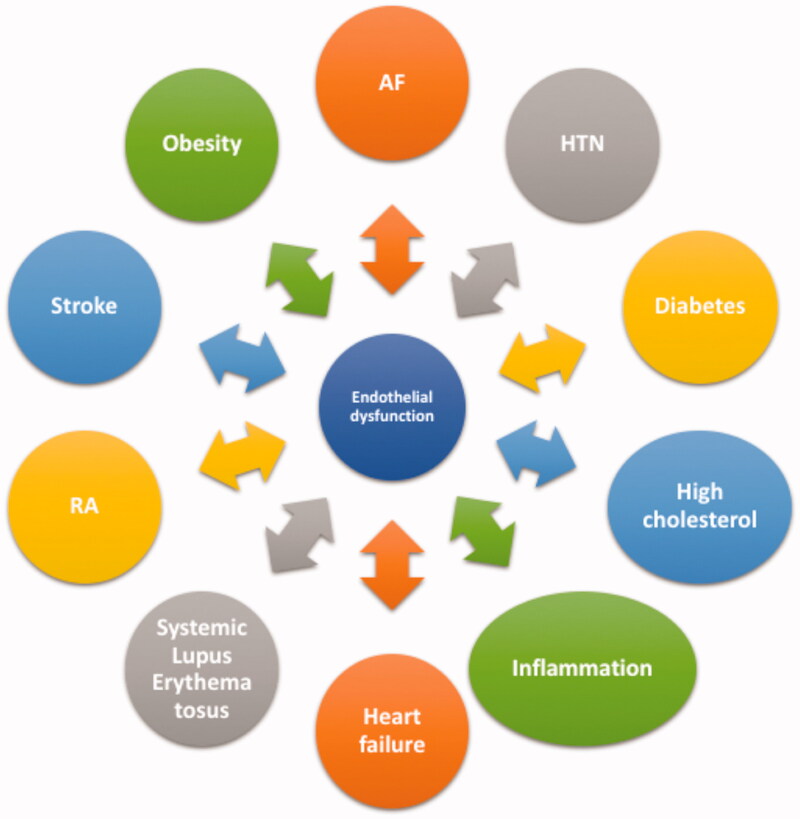
The endothelium is considered a dynamic organ [7]. It constitutes several unique functions in vascular biology by responding to various hormones, neurotransmitters, vasoactive factors and processes which then affect blood vessel tone (vasomotion) and haemostasis (thrombosis, platelet aggregation and inflammation) [7]. The endothelium releases several vasoactive factors, which are either vasodilatory such as NO, prostacyclin (PGI2) and endothelium-derived hyperpolarizing factor (EDHF) or vasoconstrictive factors such as thromboxane (TXA2) and endothelin-1 (ET-1) [11]. The balanced production of these vasoactive factors is atheroprotective, whereas disrupted production of these factors leads to endothelial dysfunction. Endothelial dysfunction is a hallmark of vascular diseases and has been shown to predict future adverse cardiovascular events such as cardiac death, myocardial infarction, unstable angina and stroke. It is also present in inflammatory disease processes, such as rheumatoid arthritis or systemic lupus erythematosus (Figure 1) [12]. Endothelial dysfunction presents a final common pathway or “barometer” of the combined impact of traditional atherosclerotic risk factors; thus assessment of endothelial dysfunction in humans presents an attractive option for determination of risk associated with thrombogenesis and combined risk factor impact on atherogenesis [13].
Figure 1.
Endothelial dysfunction as a link to several conditions. AF: atrial fibrillation; HTN: hypertension; RA: rheumatoid arthritis.
Nitric oxide
Nitric oxide (NO) has been recognized as a key component in regulation of vascular tone and in mediating the prothrombotic state in AF. It is synthesized by NO synthase (NOS) enzyme, which converts amino acid L-arginine to NO [14]. Three isoforms of NOS exist, including neuronal isoform (nNOS), which produces NO that act as a neuronal messenger in the synapses; inducible isoform (iNOS), expressed in cells exposed to inflammatory mediators or injurious stimulus and; endothelial NOS (eNOS), which produces NO in the vasculature [15–17]. The property of blood vessel to dilate is heavily dependent on the activity of eNOS [11].
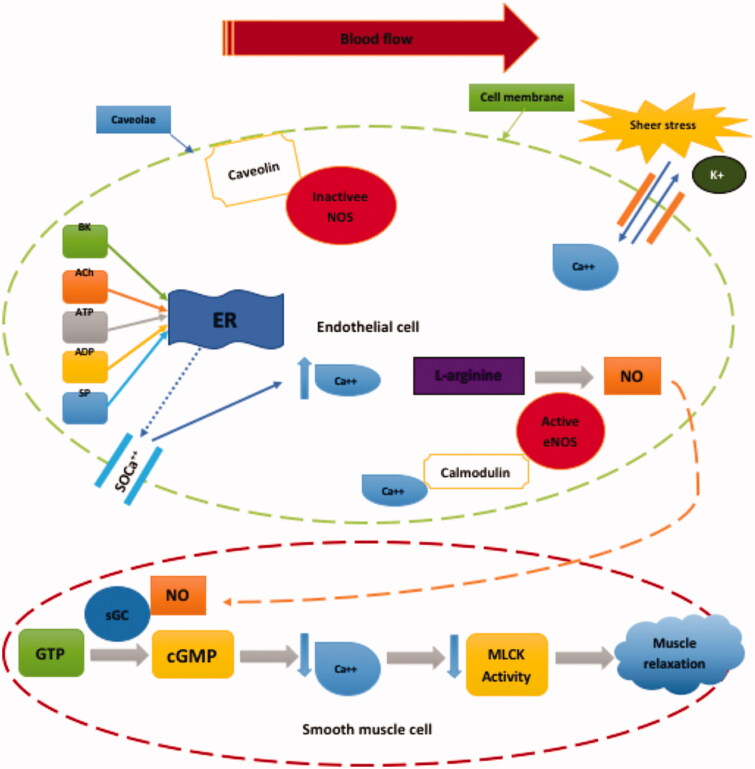
Inactive eNOS is bound to a protein called caveolin and is situated in small openings within the cell membrane called caveolae (Figure 2) [18]. eNOS detaches from caveolin and is activated when intracellular levels of calcium increase [18]. The detachment of eNOS can be influenced by NO agonists such as bradykinin (BK), acetylcholine (ACh), adenosine tri-phosphate (ATP), adenosine di-phosphate (ADP), substance P and thrombin by releasing calcium from the endoplasmic reticulum [19,20]. Once intracellular calcium stores are depleted, a signal is sent to the membrane receptors to open calcium channels allowing extracellular calcium release into the cell [21,22]. Calcium attaches to cytoplasmic calmodulin, and undergoes structural changes which allow it to bind to eNOS [23]. eNOS then converts L-arginine into NO [14].
Figure 2.
Endothelial nitric oxide production and its action on vascular smooth muscle cell. Ach: acetylcholine; BK: bradykinin; ATP: adenosine triphosphate; ADP: adenosine diphosphate; SP: substance P; SOCa2+: store-operated Ca channel; ER: endoplasmic reticulum; NO: nitric oxide; eNOS: endothelial nitric oxide synthase; GTP: guanosine 5′-triphosphate; sGC: soluble guanylyl cyclase; cGMP: cyclic guanosine-3′, 5-monophosphate; MLCK: myosin light chain kinase. *When Ca2+ stores of the endoplasmic reticulum are depleted, a signal is sent to SOCa2+ channel which allows extracellular Ca2+ into the endothelial cell.
Once synthesised, NO diffuses across the endothelial cells into the adjacent smooth muscle, where it binds to the soluble guanylyl cyclase (sGC) enzyme [24]. The activated sGC enzyme escalates the conversion rate of guanosine triphosphate (GTP) to cGMP, which decreases smooth muscle tension of blood vessels [25]. Furthermore, cGMP reduces calcium release from the sarcoplasmic reticulum in the smooth muscle cell [26]. Both actions lead to relaxation of smooth muscle cells as shown in Figure 2. These processes are continuously active in producing NO to maintain basal vasodilator tone.
Endothelial function in AF
It has been established that eNOS expression is regulated by a variety of stimuli [27,28]. One of the key physiologically important stimuli is laminar shear stress, the tangential force exerted by flow over the surface of the endothelium. Areas of the vascular system exposed to high shear are protected from the development of atherosclerosis, while areas exposed to low shear are prone to atherosclerotic lesion development [29]. It is thought that increases in eNOS caused by shear may contribute to this phenomenon as NO has antithrombotic properties as described earlier. Thus a healthy vessel has good concentration of eNOS.
Shear stress results from increased blood flow in the vessel. Flow-mediated shear stress regulates the expression of NOS, and is therefore, down-regulated at sites with low flow velocity [30–32]. Since AF leads to a loss of organised atrial contraction and predisposes to low blood flow in the left atrium, AF is associated with a marked decrease in eNOS expression and NO bioavailability [33]. Reduced NO bioavailability and resulting endothelial function impairment may explain AF as a cause of endothelial dysfunction. In an animal model, laminar blood flow in sinus rhythm and the cyclic stretch of atrial endocardial cells acted as a stimulus to maintain normal endocardial expression and function of NOS [33].
NO has displayed strong antithrombotic effects when released from activated platelets in the arterial endothelium [34]. It prevents platelet recruitment to the developing thrombus, while also impeding PAI-1 activity [35]. An animal model of AF has shown reduced NO bioavailability and an increase in PAI-1 expression due to diminished expression of NOS in the left atrium. This is possibly as a result of impaired atrial contraction and a subsequent decrease in shear stress [33]. The concentrations of NO are also reduced in the left atrial appendage (LAA) compared with control animals, providing further evidence to the concept that atrial thrombus is a common occurrence in the LAA [33]. Although it is important to bear in mind that the method of induction of AF in this animal model was significantly different compared to development of AF seen in humans. The delay between the onset of AF and hypercoagulability as discussed earlier is consistent with the observation that AF induces endocardial dysfunction, since downregulation of NOS and its subsequent effects take time to develop [33]. Additionally, patients with AF are generally older and have other co-morbidities such as hypertension, hypercholesterolaemia and diabetes, all of which exert adverse effects on the endothelial function.
Mechanical stimuli have been shown to influence various aspects of endothelial function [33]. The mechanisms linking mechanical forces to gene expression remain poorly defined [30]. Shear stress is known to activate numerous intracellular signalling molecules, including tyrosine kinases (in particular c-Src), G-proteins, PI-3 kinase, c-Jun N-terminal kinase (JNK), protein kinase C and mitogen activated protein kinases (MAPK) extracellular-related kinases [36,37]. There is further evidence that shear stress may enhance the activity of the eNOS protein promoter through transcriptional regulation of the eNOS gene [38]. The commonly accepted theory is that constant shear stress produced by unidirectional flow maintains normal eNOS expression by activation of the tyrosine kinase c-Src, which then leads to deviating pathways modulating both eNOS transcription rate and also its messenger RNA (mRNA) stability [30]. Shear stress also acutely increases NO bioavailability in the endothelium by stimulating eNOS phosphorylation [39,40]. The constitutive eNOS importantly regulates vascular haemostasis [31]. This allows the endothelium to respond to the stress and dilate accordingly to compensate for increase in blood flow.
In addition to shear stress causing endothelial dysfunction in AF, there are other mechanisms linking AF with endothelial dysfunction. Dimethylarginines, including asymmetric dimethylarginine (ADMA) and symmetric dimethylarginine (SDMA) are thought to play a part in the endothelial dysfunction seen in AF. ADMA and SDMA are both endogenous methylated analogues of L-arginine, the precursor of NO. Elevated levels of ADMA inhibit NO synthase, thus leading to endothelial dysfunction, oxidative stress and inflammation in cardiovascular diseases [41,42]. SDMA does not inhibit NOS directly but interferes with the cellular uptake of L-arginine [43]. Elevated levels of ADMA and SDMA have been found in patients with AF and this may point towards another mechanism of endothelial dysfunction seen in patients with AF [44–47].
Furthermore, AF induces atrial inflammation and elevation of c-reactive protein and cytokines, exerting a proinflammatory effect on endothelial cells which can potentially lead to endothelial dysfunction [48]. The Renin–Angiotensin system (RAS) may also play a part in endothelial dysfunction seen in patient with AF. Angiotensin II increases atrial cell death leading to AF. Moreover, RAS contributes to the myocardial and vascular oxidative stress in AF [48]. This is in addition to RAS effect on increasing blood pressure and leading to endothelial dysfunction.
Several studies have shown that endothelial dysfunction in terms of reduced NO activity is one of the earliest markers in patients with atherogenic risk factors [7,49–51]. Endothelial dysfunction, as demonstrated by endothelial-dependent flow-mediated dilatation (FMD) has also been shown to be present in AF [52–57]. Plasma von Willebrand factor (vWF), a marker of endothelial damage/dysfunction is consistently elevated in AF and has been associated with adverse outcomes [58]. Multiple studies have shown that restoration of sinus rhythm in patients with AF leads to improvement of endothelial function [59,60]. Thus, further providing evidence that AF is perhaps a cause of endothelial dysfunction and that endothelial dysfunction is a reversible condition.
Role of flow-mediated dilatation (FMD) in assessing vascular function
Assessment of endothelial function is a good predictor of future cardiac events in groups at risk of cardiovascular disease or those with established cardiovascular disease [61,62]. Such assessment at an individual level (unlike group level) is in fact poor in predicting outcomes. Nevertheless, endothelial dysfunction is common in individuals with cardiovascular risk factors [63–65]. Changes in NO have been quantified by measurement of nitrate/nitrite product in the plasma (NOx), but also indirectly by the validated non-invasive technique of FMD [66–68]. This technique measures a vasodilatory response attributed to endothelial NO production in response to situations of elevated shear stress [69,70]. Reduced vasodilatory response following an increase in shear forces is representative of impaired NO bioavailability [71]. Thus, FMD is a good surrogate marker of NO bioavailability.
FMD has been widely used to demonstrate endothelial dysfunction in both cardiovascular disease and its risk factors (Table 1) [74–76,82,83,86,94,96]. FMD provides independent prognostic information that may exceed that available from traditional risk factors measurement based on the concept of direct assessment of the function of the vascular system [13]. Several studies have shown that endothelial function can be improved by treatment of cardiovascular disease or its risk factors [7,77,78,97]. Indeed, FMD has been established as a reliable and reproducible technique for assessment of endothelial dysfunction [96,98].
Table 1.
Use of FMD in cardiovascular and associated risk factor conditions.
| Author(s) | Year | Condition studied | Number of patients | Summary |
|---|---|---|---|---|
| Anderson et al. [72] | 1995 | Coronary artery disease (CAD) | 50 | Patients with CAD had worse FMD than those with normal coronary arteries (4.5 ± 4.6% versus 9.7 ± 8.1%, p<.02) |
| Anderson et al. [73] | 2000 | CAD | 80 | Quinapril associated with significant improvement in FMD (1.8 ± 1%, p<.02). No change observed with Losartan (0.8 ± 1.1%, p=.57), Amlodipine (0.3 ± 0.9%, p=.97) or Enalapril (−0.2 ± 0.8%, p = .84) |
| Borschel et al. [53] | 2019 | Atrial fibrillation (AF) | 466 AF versus 14,330 non-AF | Decreased FMD in patients with AF observed but this was not statistically significant |
| Celermajer et al. [68] | 1992 | Atherosclerosis [Smoking, familial hypercholesterolaemia (FH), CAD] | 50 controls versus 20 cigarette smokers versus 10 FH children versus 20 CAD | FMD was reduced or absent in smokers (4%), FH children (0%) and adults with CAD (0%) when compared with controls (11%) (p<.001) |
| Celermajer et al. [74] | 1993 | Atherosclerosis (smoking) | 80 controls versus 80 current smokers versus 40 ex-smokers | FMD was impaired or absent in smokers compared to controls (4 ± 3.9% versus 10 ± 3.3%, p<.0001). FMD was inversely related to lifetime dose smoked |
| Chambers et al. [75] | 1999 | Hyperhomocysteinemia | 17 | Inverse linear relationship between homocysteine concentration and FMD (p<.001) |
| Clarkson et al. [76] | 1997 | Family history of CAD | 50 first-degree relatives versus 50 controls | FMD was impaired in family history group compared to control group (4.9 ± 4.6% versus 8.3 ± 3.5%, p<.005) |
| Dupuis et al. [77] | 1999 | CAD, Hypercholesterolaemia | 30 (Pravastatin) versus 30 (placebo) | FMD increased with Pravastatin (4.93 ± 0.81% to 7.0 ± 0.79%, p=.02). FMD was unchanged with placebo (5.43 ± 0.74% to 5.84 ± 0.81%) |
| Felmeden et al. [78] | 2003 | Hypertension (HTN) | 76 HTN versus 48 controls | FMD lower in HTN patients compared with control (4.8 ± 1.3% versus 8.6 ± 2.2%, p<.001). After intensified hypertensive treatment, FMD improved (4.8 ± 1.3% (baseline) to 7.3 ± 1.7%, p<.001) |
| Freestone et al. [52] | 2008 | AF | 40 AF versus 26 NSR | Worse FMD in AF patients than NSR patients (0.0 versus 8.9%, p<.0001) |
| Gerhard et al. [79] | 1998 | Post menopause, hypercholesterolaemia | 17 | Oestradiol therapy improved FMD compared with placebo (11.1 ± 1.0% versus 4.7 ± 0.6%, p<.001). Modest decrease in total and LDL cholesterol with oestradiol |
| Gokce et al. [80] | 2002 | CAD | 187 | 45 patients with cardiovascular event. FMD independent predictor of events (4.9 ± 3.1% versus 7.3 ± 5%; p<.001) |
| Gokce et al. [61] | 2003 | Peripheral arterial disease (PAD) | 199 | Worse FMD in patients ending up having a cardiovascular event (cardiac death, myocardial infarction, unstable angina, stroke) (4.4 ± 2.8% versus 7.0 ± 4.9%, p<.0001) |
| Hornig et al. [81] | 1998 | Heart failure | 30 | Quinaprilat improved FMD by 40% (10.2 ± 0.6% versus 6.9 ± 0.6%; p<.01) whereas enalaprilat had no effect |
| Iiyama et al. [82] | 1996 | Hypertension | 13 HTN versus 13 controls | FMD found to be less in patients with HTN than controls (13.1 ± 1.6% versus 18.5 ± 1.9%, p<.05) |
| Komatsu et al. [54] | 2018 | AF | 184 paroxysmal AF (PAF) versus 53 chronic AF versus 79 sinus rhythm controls | FMD was 5.4 ± 2.6% in PAF patients versus 4.3 ± 2.1% in chronic AF versus 6.5 ± 3.5% in controls, which was significant (all, p<.05) |
| Lekakis et al. [83] | 1997 | Diabetes mellitus | 26 insulin-dependent diabetes mellitus (IDDM) without microalbuminuria versus 5 IDDM with microalbuminuria versus 26 controls | FMD was lower in patients with IDDM with and without microalbuminuria compared with controls (0.75 ± 2.5% versus 5.8 ± 7% versus 11 ± 7%, p=.003 and .01, respectively) |
| Lieberman et al. [84] | 1994 | Post menopause | 13 | FMD greater in patients receiving Oestradiol than placebo (13.5 versus 6.8%, p<.05) |
| Mazaris et al. [55] | 2014 | AF | 35 PAF versus 117 permanent AF | Patients with permanent AF had impaired FMD compared to PAF (4.09 ± 1.67% versus 6.83 ± 1.38%, p<.001). Endothelial dysfunction associated with atrial remodelling in patients with AF and implicated in the progression from paroxysmal to permanent AF |
| Modena et al. [85] | 2002 | Hypertension | 400 | 47 patients with cardiovascular event. Majority of these had poor FMD response (7.1 ± 2.5% versus 13.9 ± 2.6%). FMD can be improved following 6 months of antihypertensive treatment |
| Neunteufl et al. [86] | 1997 | CAD | 44 (CAD) versus 30 (angina pectoris – non CAD) versus 14 controls | CAD patients showed markedly impaired FMD compared to non-CAD group and to control (5.7 ± 4.8% versus 12.6 ± 6.7% versus 15.7 ± 3.9%, p<.00001) |
| Neunteufl et al. [87] | 2000 | CAD | 73 | 27 patients with cardiovascular event. FMD < 10% predictive of events |
| O’Neal et al. [88] | 2014 | AF | 2936 | Smaller brachial FMD values associated with higher rates of AF. Each 1SD increase in %FMD values (SD, 2.8%) associated with less incident AF (hazard ratio 0.84; 95% CI 0.70–0.99) |
| Perri et al. [89] | 2015 | AF | 514 | Patients who experienced a cardiovascular event showed significantly reduced FMD compared to those who did not (3.06% [IQR 0.00–6.00] versus 4.67% [IQR 1.58–8.22], p=.027) |
| Polovina et al. [56] | 2013 | AF | 38 AF versus 28 controls | Median FMD significantly lower in AF patients compared to control (5.0% [IQR 2.87–7.50%] versus 8.85% [IQR 5.80–12.50%], p<.001) |
| Rossi et al. [62] | 2008 | Post menopause | 2264 | FMD ≤ 4.5% associated with a greater cardiovascular event rate compared with FMD > 4.5% |
| Schachinger et al. [90] | 2000 | CAD | 147 | 28 patients with cardiovascular event. FMD independent predictor of events |
| Shaikh et al. [91] | 2016 | AF | 3921 | Lower FMD associated with an increased risk of incident AF (hazard ratio: 0.79, 95% CI 0.63–0.99, p=.04) |
| Shaposhnikova et al. [92] | 2017 | AF | 29 PAF versus 32 persistent AF versus 35 permanent AF | Progressive deterioration of FMD observed from PAF (7.96 ± 1.22%) to persistent AF (6.35 ± 1.18%) to permanent AF (4.81 ± 1.15%) (p = .001). Inverse correlation between permanent AF and FMD (r= −0.061, 95% CI 0.032–0.081) even after adjustment for comorbid diseases |
| Siasos et al. [93] | 2015 | AF | 65 (30 PAF and 35 Permanent AF) | Duration of AF inversely associated with FMD (rho= −0.058, p=.006). This was even after adjustment for confounders |
| Simons et al. [94] | 1998 | Hypercholesterolaemia | 32 | Median FMD improved compared to baseline with atorvastatin (2.2% → 5.5%) and simvastatin + cholestyramine therapy (1.8% → 4.5%) (p<.01 for both). FMD at baseline correlated with HDL cholesterol (r = 0.49, p<.01). Change in FMD was inversely correlated with baseline FMD (r= −0.54, p<.001) |
| Ulgen et al. [57] | 2014 | AF | 40 PAF versus 40 controls | FMD in AF group was significantly lower relative to control group (5.27 versus 6.65,p = .001) |
| Woo et al. [95] | 2002 | Hyperhomocysteinemia | 17 | Folic acid supplementation significantly improved FMD compared to placebo (7.4 ± 2% versus 8.9 ± 1.5%, p<.0001) |
The FMD technique has a widely accepted standardised protocol, which allows accurate comparison between heterogenous groups and serially over time [96]. Due to accessibility and characteristics of the vessel, brachial artery is typically used for this technique. FMD protocol involves a baseline period of 1–2 min of scanning of the brachial artery followed by a 5-min period of cuff inflation on the study forearm (distal to brachial artery) to induce tissue ischaemia and dilatation of downstream resistance vessels via autoregulatory mechanisms. The cuff placement close to the wrist is dependent on NO, while on the upper arm is only partially mediated by NO [13,70]. Furthermore, upper arm cuff placement leads to initiation of local ischaemia due to complete blood flow occlusion. Five minutes of limb occlusion is adequate to evoke endothelium-dependent dilatation, as longer cuff durations have shown a non-NO response [99]. Upon cuff release, an expected sudden increase in blood flow (reactive hyperaemia) through the artery fills the dilated vessels and in doing so exerts shear stress on the endothelial cells [96]. The resulting dilatation, which usually peaks at 60–90 s post-cuff release is dependent on NO activity [69].
FMD is expressed as the maximum percentage change in vessel diameter after cuff release relative to baseline vessel diameter; a low percentage suggesting poor endothelial function [100]. FMD responses can be affected by external factors such as sleep deprivation, hyperhomocysteinemia, caffeine, smoking, antioxidant therapy, menstrual cycle and time of day [75,101–106]. Thus it is important to control these factors to prevent bias.
Conclusion
The endothelium is vital in maintaining vascular haemostasis and its functional integrity is a fundamental element for vascular health. However, disruption in its role can lead to endothelial dysfunction. Endothelial dysfunction is present in patients with AF. These patients are generally older and have other co-morbidities such as hypertension, hypercholesterolaemia and diabetes, all of which exert adverse effects on the endothelial function. Thus adding further complexity to the assessment of endothelial function and its management. Consequently, endothelial dysfunction in patients with AF is associated with poor outcomes.
Modulation of endothelial function is possible suggesting that endothelial dysfunction is a reversible condition as shown by several studies who have looked at FMD pre and post-intervention and shown improvement in FMD post-intervention [71,74,75,77,78,85,95,97]. FMD reflects dynamic vascular haemostasis and thus raises the possibility of adopting a treat-to-target approach, where FMD can be monitored intermittently with the goal of normalising of enhancing vascular health [13]. It is not yet known whether endothelial dysfunction is simply a biomarker of AF or an intermediate step in a causal pathway in developing AF, perhaps it is both. However, improved endothelial function is a clinical marker of atherogenic risk factor modification [107].
There are several non-invasive methods available to assess vascular function including FMD. Patients with AF should be considered for vascular function assessment and attempts made to modify their vascular function. These are at present limited to the traditional risk factor modifications such as exercise, weight reduction, smoking control, better diabetes control, antithrombotic and antihypertensive treatments.
Disclosure statement
AAK has no financial interest in the subject matter or materials discussed. GNT has no financial interest in the subject matter or materials discussed. GYHL has no financial interest in the subject matter or materials discussed. AS has no financial interest in the subject matter or materials discussed. GYHL has served as a consultant for Bayer/Janssen, BMS/Pfizer, Biotronik, Medtronic, Boehringer Ingelheim, Microlife and Daiichi-Sankyo; and a speaker for Bayer, BMS/Pfizer, Medtronic, Boehringer Ingelheim, Microlife, Roche and Daiichi-Sankyo. No personal fees received.
References
- 1.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–988. [DOI] [PubMed] [Google Scholar]
- 2.Wolf PA, Mitchell JB, Baker CS, et al. Impact of atrial fibrillation on mortality, stroke, and medical costs. Arch Intern Med. 1998;158:229–234. [DOI] [PubMed] [Google Scholar]
- 3.Mackay J, Mensah G. The atlas of heart disease and stroke. Geneva: World Health Organisation; 2004. Global burden of stroke. [Google Scholar]
- 4.Benjamin EJ, Wolf PA, D’Agostino RB, et al. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–952. [DOI] [PubMed] [Google Scholar]
- 5.Lip GY. Does atrial fibrillation confer a hypercoagulable state? Lancet. 1995;346:1313–1314. [DOI] [PubMed] [Google Scholar]
- 6.Li-Saw-Hee FL, Blann AD, et al. A cross-sectional and diurnal study of thrombogenesis among patients with chronic atrial fibrillation. J Am Coll Cardiol. 2000;35:1926–1931. [DOI] [PubMed] [Google Scholar]
- 7.Galley HF, Webster NR. Physiology of the endothelium. Br J Anaesth. 2004;93:105–113. [DOI] [PubMed] [Google Scholar]
- 8.Ferrer M, Encabo A, Conde MV, et al. Heterogeneity of endothelium-dependent mechanisms in different rabbit arteries. J Vasc Res. 1995;32:339–346. [DOI] [PubMed] [Google Scholar]
- 9.Thorin E, Shatos MA, Shreeve SM, et al. Human vascular endothelium heterogeneity. A comparative study of cerebral and peripheral cultured vascular endothelial cells. Stroke. 1997;28:375–381. [DOI] [PubMed] [Google Scholar]
- 10.Hill CE, Phillips JK, Sandow SL. Heterogeneous control of blood flow amongst different vascular beds. Med Res Rev. 2001;21:1–60. [DOI] [PubMed] [Google Scholar]
- 11.Sandoo A, van Zanten J, Metsios GS, et al. The endothelium and its role in regulating vascular tone. Open Cardiovasc Med J. 2010;4:302–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steyers CM, 3rd, Miller FJ. Jr.. Endothelial dysfunction in chronic inflammatory diseases. Int J Mol Sci. 2014;15:11324–11349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green DJ, Jones H, Thijssen D, et al. Flow-mediated dilation and cardiovascular event prediction: does nitric oxide matter? Hypertension. 2011;57:363–369. [DOI] [PubMed] [Google Scholar]
- 14.Palmer RM, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333:664–666. [DOI] [PubMed] [Google Scholar]
- 15.Prast H, Philippu A. Nitric oxide as modulator of neuronal function. Prog Neurobiol. 2001;64:51–68. [DOI] [PubMed] [Google Scholar]
- 16.Michel T, Feron O. Nitric oxide synthases: which, where, how, and why? J Clin Invest. 1997;100:2146–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamas S, Marsden PA, Li GK, et al. Endothelial nitric oxide synthase: molecular cloning and characterization of a distinct constitutive enzyme isoform. Proc Natl Acad Sci U S A. 1992;89:6348–6352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bucci M, Gratton JP, Rudic RD, et al. In vivo delivery of the caveolin-1 scaffolding domain inhibits nitric oxide synthesis and reduces inflammation. Nat Med. 2000;6:1362–1367. [DOI] [PubMed] [Google Scholar]
- 19.Moncada S, Higgs EA. The discovery of nitric oxide and its role in vascular biology. Br J Pharmacol. 2009;147:S193–S201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bae SW, Kim HS, Cha YN, et al. Rapid increase in endothelial nitric oxide production by bradykinin is mediated by protein kinase A signaling pathway. Biochem Biophys Res Commun. 2003;306:981–987. [DOI] [PubMed] [Google Scholar]
- 21.Schilling WP, Cabello OA, Rajan L. Depletion of the inositol 1,4,5-trisphosphate-sensitive intracellular Ca2+ store in vascular endothelial cells activates the agonist-sensitive Ca(2+)-influx pathway. Biochem J. 1992;284 :521–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schilling WP, Elliott SJ. Ca2+ signaling mechanisms of vascular endothelial cells and their role in oxidant-induced endothelial cell dysfunction. Am J Physiol. 1992;262:H1617–30. [DOI] [PubMed] [Google Scholar]
- 23.Fleming I, Busse R. Signal transduction of eNOS activation. Cardiovasc Res. 1999;43:532–541. [DOI] [PubMed] [Google Scholar]
- 24.Ignarro LJ, Harbison RG, Wood KS, et al. Activation of purified soluble guanylate cyclase by endothelium-derived relaxing factor from intrapulmonary artery and vein: stimulation by acetylcholine, bradykinin and arachidonic acid. J Pharmacol Exp Ther. 1986;237:893–900. [PubMed] [Google Scholar]
- 25.Jones KA, Wong GY, Jankowski CJ, et al. cGMP modulation of Ca2+ sensitivity in airway smooth muscle. Am J Physiol. 1999;276:L35–40. [DOI] [PubMed] [Google Scholar]
- 26.Collins P, Griffith TM, Henderson AH, et al. Endothelium-derived relaxing factor alters calcium fluxes in rabbit aorta: a cyclic guanosine monophosphate-mediated effect. J Physiol. 1986;381:427–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harrison DG, Sayegh H, Ohara Y, et al. Regulation of expression of the endothelial cell nitric oxide synthase. Clin Exp Pharmacol Physiol. 1996;23:251–255. [DOI] [PubMed] [Google Scholar]
- 28.Harrison DG, Venema RC, Arnal JF, et al. The endothelial cell nitric oxide synthase: is it really constitutively expressed? Agents Actions Suppl. 1995;45:107–117. [DOI] [PubMed] [Google Scholar]
- 29.Ku DN, Giddens DP, Zarins CK, et al. Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low oscillating shear stress. Arteriosclerosis. 1985;5:293–302. [DOI] [PubMed] [Google Scholar]
- 30.Davis ME, Cai H, Drummond GR, et al. Shear stress regulates endothelial nitric oxide synthase expression through c-Src by divergent signaling pathways. Circ Res. 2001;89:1073–1080. [DOI] [PubMed] [Google Scholar]
- 31.Nishida K, Harrison DG, Navas JP, et al. Molecular cloning and characterization of the constitutive bovine aortic endothelial cell nitric oxide synthase. J Clin Invest. 1992;90:2092–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uematsu M, Ohara Y, Navas JP, et al. Regulation of endothelial cell nitric oxide synthase mRNA expression by shear stress. Am J Physiol. 1995;269:C1371–C1378. [DOI] [PubMed] [Google Scholar]
- 33.Cai H, Li Z, Goette A, et al. Downregulation of endocardial nitric oxide synthase expression and nitric oxide production in atrial fibrillation: potential mechanisms for atrial thrombosis and stroke. Circulation. 2002;106:2854–2858. [DOI] [PubMed] [Google Scholar]
- 34.Freedman JE, Loscalzo J, Barnard MR, et al. Nitric oxide released from activated platelets inhibits platelet recruitment. J Clin Invest. 1997;100:350–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swiatkowska M, Cierniewska-Cieslak A, Pawlowska Z, et al. Dual regulatory effects of nitric oxide on plasminogen activator inhibitor type 1 expression in endothelial cells. Eur J Biochem. 2000;267:1001–1007. [DOI] [PubMed] [Google Scholar]
- 36.Traub O, Berk BC. Laminar shear stress: mechanisms by which endothelial cells transduce an atheroprotective force. Arterioscler Thromb Vasc Biol. 1998;18:677–685. [DOI] [PubMed] [Google Scholar]
- 37.Ishida T, Takahashi M, Corson MA, et al. Fluid shear stress-mediated signal transduction: how do endothelial cells transduce mechanical force into biological responses? Ann NY Acad Sci. 1997;811:12–23. [DOI] [PubMed] [Google Scholar]
- 38.Malek AM, Jiang L, Lee I, et al. Induction of nitric oxide synthase mRNA by shear stress requires intracellular calcium and G-protein signals and is modulated by PI 3 kinase. Biochem Biophys Res Commun. 1999;254:231–242. [DOI] [PubMed] [Google Scholar]
- 39.Corson MA, James NL, Latta SE, et al. Phosphorylation of endothelial nitric oxide synthase in response to fluid shear stress. Circ Res. 1996;79:984–991. [DOI] [PubMed] [Google Scholar]
- 40.Fisslthaler B, Dimmeler S, Hermann C, et al. Phosphorylation and activation of the endothelial nitric oxide synthase by fluid shear stress. Acta Physiol Scand. 2000;168:81–88. [DOI] [PubMed] [Google Scholar]
- 41.Böger RH. Asymmetric dimethylarginine (ADMA): a novel risk marker in cardiovascular medicine and beyond. Ann Med. 2006;38:126–136. [DOI] [PubMed] [Google Scholar]
- 42.Sibal L, Agarwal SC, Home PD, et al. The role of asymmetric dimethylarginine (ADMA) in endothelial dysfunction and cardiovascular disease. CCR. 2010;6:82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Closs EI, Basha FZ, Habermeier A, et al. Interference of L-arginine analogues with L-arginine transport mediated by the y + carrier hCAT-2B. Nitric Oxide. 1997;1:65–73. [DOI] [PubMed] [Google Scholar]
- 44.Cengel A, Sahinarslan A, Biberoğlu G, et al. Asymmetrical dimethylarginine level in atrial fibrillation. Acta Cardiol. 2008;63:33–37. [DOI] [PubMed] [Google Scholar]
- 45.Goette A, Hammwohner M, Bukowska A, et al. The impact of rapid atrial pacing on ADMA and endothelial NOS. Int J Cardiol. 2012;154:141–146. [DOI] [PubMed] [Google Scholar]
- 46.Schulze F, Carter AM, Schwedhelm E, et al. Symmetric dimethylarginine predicts all-cause mortality following ischemic stroke. Atherosclerosis. 2010;208:518–523. [DOI] [PubMed] [Google Scholar]
- 47.Stamboul K, Lorin J, Lorgis L, et al. Atrial fibrillation is associated with a marker of endothelial function and oxidative stress in patients with acute myocardial infarction. PLoS One. 2015;10:e0131439–e0131439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guazzi M, Arena R. Endothelial dysfunction and pathophysiological correlates in atrial fibrillation. Heart. 2009;95:102–106. [DOI] [PubMed] [Google Scholar]
- 49.Vita JA, Treasure CB, Nabel EG, et al. Coronary vasomotor response to acetylcholine relates to risk factors for coronary artery disease. Circulation. 1990;81:491–497. [DOI] [PubMed] [Google Scholar]
- 50.Zeiher AM, Drexler H, Saurbier B, et al. Endothelium-mediated coronary blood flow modulation in humans. Effects of age, atherosclerosis, hypercholesterolemia, and hypertension. J Clin Invest. 1993;92:652–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nitenberg A, Valensi P, Sachs R, et al. Impairment of coronary vascular reserve and ACh-induced coronary vasodilation in diabetic patients with angiographically normal coronary arteries and normal left ventricular systolic function. Diabetes. 1993;42:1017–1025. [DOI] [PubMed] [Google Scholar]
- 52.Freestone B, Chong AY, Nuttall S, et al. Impaired flow mediated dilatation as evidence of endothelial dysfunction in chronic atrial fibrillation: relationship to plasma von Willebrand factor and soluble E-selectin levels. Thromb Res. 2008;122:85–90. [DOI] [PubMed] [Google Scholar]
- 53.Borschel CS, Rubsamen N, Ojeda FM, et al. Noninvasive peripheral vascular function and atrial fibrillation in the general population. J Hypertens. 2019;37:928–934. [DOI] [PubMed] [Google Scholar]
- 54.Komatsu T, Kunugita F, Ozawa M, et al. Relationship between impairment of the vascular endothelial function and the CHA2DS2-VASc score in patients with sinus rhythm and non-valvular atrial fibrillation. Intern Med. 2018;57:2131–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mazaris ST, Siasos G, Zisimos K, et al. The role of endothelial function on paroxysmal and chronic atrial fibrillation. Circ J. 2014;130:A17526. [Google Scholar]
- 56.Polovina M, Potpara T, Giga V, et al. Impaired endothelial function in lone atrial fibrillation. Vojnosanit Pregl. 2013;70:908–914. [DOI] [PubMed] [Google Scholar]
- 57.Ulgen AK, Canpolat U, Sunman H, et al. Evaluation of the association between endothelial function, and intra-interatrial conduction in patients with lone paroxysmal atrial fibrillation. Anatol J Cardiol. 2014;14:63. [Google Scholar]
- 58.Conway DS, Pearce LA, Chin BS, et al. Prognostic value of plasma von Willebrand factor and soluble P-selectin as indices of endothelial damage and platelet activation in 994 patients with nonvalvular atrial fibrillation. Circulation. 2003;107:3141–3145. [DOI] [PubMed] [Google Scholar]
- 59.Okawa K, Miyoshi T, Tsukuda S, et al. Differences in endothelial dysfunction induced by paroxysmal and persistent atrial fibrillation: insights from restoration of sinus rhythm by catheter ablation. Int J Cardiol. 2017;244:180–185. [DOI] [PubMed] [Google Scholar]
- 60.Yoshino S, Yoshikawa A, Hamasaki S, et al. Atrial fibrillation-induced endothelial dysfunction improves after restoration of sinus rhythm. Int J Cardiol. 2013;168:1280–1285. [DOI] [PubMed] [Google Scholar]
- 61.Gokce N, Keaney JF Jr, Hunter LM, et al. Predictive value of noninvasively determined endothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular disease. J Am Coll Cardiol. 2003;41:1769–1775. [DOI] [PubMed] [Google Scholar]
- 62.Rossi R, Nuzzo A, Origliani G, et al. Prognostic role of flow-mediated dilation and cardiac risk factors in post-menopausal women. J Am Coll Cardiol. 2008;51:997–1002. [DOI] [PubMed] [Google Scholar]
- 63.Widlansky ME, Gokce N, Keaney JF Jr, et al. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–1160. [DOI] [PubMed] [Google Scholar]
- 64.Thomas GN, Chook P, Yip TW, et al. Smoking without exception adversely affects vascular structure and function in apparently healthy Chinese: implications in global atherosclerosis prevention. Int J Cardiol. 2008;128:172–177. [DOI] [PubMed] [Google Scholar]
- 65.Thomas GN, Chook P, Qiao M, et al. Deleterious impact of “high normal” glucose levels and other metabolic syndrome components on arterial endothelial function and intima-media thickness in apparently healthy Chinese subjects: the CATHAY study. ATVB. 2004;24:739–743. [DOI] [PubMed] [Google Scholar]
- 66.Rhodes PM, Leone AM, Francis PL, et al. The L-arginine: nitric oxide pathway is the major source of plasma nitrite in fasted humans. Biochem Biophys Res Commun. 1995;209:590–596. [DOI] [PubMed] [Google Scholar]
- 67.Moshage H, Kok B, Huizenga JR, et al. Nitrite and nitrate determinations in plasma: a critical evaluation. Clin Chem. 1995;41:892–896. [PubMed] [Google Scholar]
- 68.Celermajer DS, Sorensen KE, Gooch VM, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–1115. [DOI] [PubMed] [Google Scholar]
- 69.Joannides R, Haefeli WE, Linder L, et al. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation. 1995;91:1314–1319. [DOI] [PubMed] [Google Scholar]
- 70.Doshi SN, Naka KK, Payne N, et al. Flow-mediated dilatation following wrist and upper arm occlusion in humans: the contribution of nitric oxide. Clin Sci. 2001;101:629–635. [PubMed] [Google Scholar]
- 71.Celermajer DS. Endothelial dysfunction: does it matter? Is it reversible? J Am Coll Cardiol. 1997;30:325–333. [DOI] [PubMed] [Google Scholar]
- 72.Anderson TJ, Uehata A, Gerhard MD, et al. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol. 1995;26:1235–1241. [DOI] [PubMed] [Google Scholar]
- 73.Anderson TJ, Elstein E, Haber H, et al. Comparative study of ACE-inhibition, angiotensin II antagonism, and calcium channel blockade on flow-mediated vasodilation in patients with coronary disease (BANFF study). J Am Coll Cardiol. 2000;35:60–66. [DOI] [PubMed] [Google Scholar]
- 74.Celermajer DS, Sorensen KE, Georgakopoulos D, et al. Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation. 1993;88:2149–2155. [DOI] [PubMed] [Google Scholar]
- 75.Chambers JC, McGregor A, Jean-Marie J, et al. Demonstration of rapid onset vascular endothelial dysfunction after hyperhomocysteinemia. Circulation. 1999;99:1156–1160. [DOI] [PubMed] [Google Scholar]
- 76.Clarkson P, Celermajer DS, Powe AJ, et al. Endothelium-dependent dilatation is impaired in young healthy subjects with a family history of premature coronary disease. Circulation. 1997;96:3378–3383. [DOI] [PubMed] [Google Scholar]
- 77.Dupuis J, Tardif J-C, Cernacek P, et al. Cholesterol reduction rapidly improves endothelial function after acute coronary syndromes. Circulation. 1999;99:3227–3233. [DOI] [PubMed] [Google Scholar]
- 78.Felmeden DC, Spencer CGC, Chung NAY, et al. Relation of thrombogenesis in systemic hypertension to angiogenesis and endothelial damage/dysfunction (a Substudy of the Anglo-Scandinavian Cardiac Outcomes Trial [ASCOT]). Am J Cardiol. 2003;92:400–405. [DOI] [PubMed] [Google Scholar]
- 79.Gerhard M, Walsh BW, Tawakol A, et al. Estradiol therapy combined with progesterone and endothelium-dependent vasodilation in postmenopausal women. Circulation. 1998;98:1158–1163. [DOI] [PubMed] [Google Scholar]
- 80.Gokce N, Keaney JF, Jr., Hunter LM, et al. Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function: a prospective study. Circulation. 2002;105:1567–1572. [DOI] [PubMed] [Google Scholar]
- 81.Hornig B, Arakawa N, Haussmann D, et al. Differential effects of quinaprilat and enalaprilat on endothelial function of conduit arteries in patients with chronic heart failure. Circulation. 1998;98:2842–2848. [DOI] [PubMed] [Google Scholar]
- 82.Iiyama K, Nagano M, Yo Y, et al. Impaired endothelial function with essential hypertension assessed by ultrasonography. Am Heart J. 1996;132:779–782. [DOI] [PubMed] [Google Scholar]
- 83.Lekakis J, Papamichael C, Anastasiou H, et al. Endothelial dysfunction of conduit arteries in insulin-dependent diabetes mellitus without microalbuminuria. Cardiovasc Res. 1997;34:164–168. [DOI] [PubMed] [Google Scholar]
- 84.Lieberman EH, Gerhard MD, Uehata A, et al. Estrogen improves endothelium-dependent, flow-mediated vasodilation in postmenopausal women. Ann Intern Med. 1994;121:936–941. [DOI] [PubMed] [Google Scholar]
- 85.Modena MG, Bonetti L, Coppi F, et al. Prognostic role of reversible endothelial dysfunction in hypertensive postmenopausal women. J Am Coll Cardiol. 2002;40:505–510. [DOI] [PubMed] [Google Scholar]
- 86.Neunteufl T, Katzenschlager R, Hassan A, et al. Systemic endothelial dysfunction is related to the extent and severity of coronary artery disease. Atherosclerosis. 1997;129:111–118. [DOI] [PubMed] [Google Scholar]
- 87.Neunteufl T, Heher S, Katzenschlager R, et al. Late prognostic value of flow-mediated dilation in the brachial artery of patients with chest pain. Am J Cardiol. 2000;86:207–210. [DOI] [PubMed] [Google Scholar]
- 88.O'Neal WT, Efird JT, Yeboah J, et al. Brachial flow-mediated dilation and incident atrial fibrillation: the multi-ethnic study of atherosclerosis. Arterioscler Thromb Vasc Biol. 2014;34:2717–2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Perri L, Pastori D, Pignatelli P, et al. Flow-mediated dilation is associated with cardiovascular events in non-valvular atrial fibrillation patients. Int J Cardiol. 2015;179:139–143. [DOI] [PubMed] [Google Scholar]
- 90.Hemphill JC, 3rd, Greenberg SM, Anderson CS, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46:2032–2060. [DOI] [PubMed] [Google Scholar]
- 91.Shaikh AY, Wang N, Yin X, et al. Relations of arterial stiffness and brachial flow-mediated dilation with new-onset atrial fibrillation: the Framingham Heart Study. Hypertension. 2016;68:590–596. [DOI] [PubMed] [Google Scholar]
- 92.Shaposhnikova Y II, Bobronnikova L. Peculiarities of endothelial dysfunction impairment in different forms of atrial fibrillation. J Interv Card Electrophysiol. 2017;48:S100. [Google Scholar]
- 93.Siasos G, Mazaris S, Zisimos K, et al. The impact of atrial fibrillation on endothelial dysfunction. J Am Coll Cardiol. 2015;65:A477. [Google Scholar]
- 94.Simons LA, Sullivan D, Simons J, Celermajer DS. Effects of atorvastatin monotherapy and simvastatin plus cholestyramine on arterial endothelial function in patients with severe primary hypercholesterolaemia. Atherosclerosis. 1998;137:197–203. [DOI] [PubMed] [Google Scholar]
- 95.Woo KS, Chook P, Chan LL, et al. Long-term improvement in homocysteine levels and arterial endothelial function after 1-year folic acid supplementation. Am J Med. 2002;112:535–539. [DOI] [PubMed] [Google Scholar]
- 96.Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery. A report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–265. [DOI] [PubMed] [Google Scholar]
- 97.Mancini GBJ, Henry GC, Macaya C, et al. Angiotensin-converting enzyme inhibition with quinapril improves endothelial vasomotor dysfunction in patients with coronary artery disease. Circulation. 1996;94:258–265. [DOI] [PubMed] [Google Scholar]
- 98.Thijssen DHJ, Bruno RM, van Mil A, et al. Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. Eur Heart J. 2019;40:2534–2547. [DOI] [PubMed] [Google Scholar]
- 99.Mullen MJ, Kharbanda RK, Cross J, et al. Heterogenous nature of flow-mediated dilatation in human conduit arteries in vivo: relevance to endothelial dysfunction in hypercholesterolemia. Circ Res. 2001;88:145–151. [DOI] [PubMed] [Google Scholar]
- 100.Deanfield J, Donald A, Ferri C, et al. Endothelial function and dysfunction. Part I: methodological issues for assessment in the different vascular beds: a statement by the Working Group on Endothelin and Endothelial Factors of the European Society of Hypertension. J Hypertens. 2005;23:7–17. [DOI] [PubMed] [Google Scholar]
- 101.Takase B, Akima T, Uehata A, et al. Effect of chronic stress and sleep deprivation on both flow-mediated dilation in the brachial artery and the intracellular magnesium level in humans. Clin Cardiol. 2004;27:223–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Papamichael CM, Aznaouridis KA, Karatzis EN, et al. Effect of coffee on endothelial function in healthy subjects: the role of caffeine. Clin Sci (Lond). 2005;109:55–60. [DOI] [PubMed] [Google Scholar]
- 103.Lekakis J, Papamichael C, Vemmos C, et al. Effect of acute cigarette smoking on endothelium-dependent brachial artery dilatation in healthy individuals. Am J Cardiol. 1997;79:529–531. [DOI] [PubMed] [Google Scholar]
- 104.Engler MM, Engler MB, Malloy MJ, et al. Antioxidant vitamins C and E improve endothelial function in children with hyperlipidemia: endothelial assessment of risk from lipids in youth (EARLY) trial. Circulation. 2003;108:1059–1063. [DOI] [PubMed] [Google Scholar]
- 105.Hashimoto M, Akishita M, Eto M, et al. Modulation of endothelium-dependent flow-mediated dilatation of the brachial artery by sex and menstrual cycle. Circulation. 1995;92:3431–3435. [DOI] [PubMed] [Google Scholar]
- 106.Etsuda H, Takase B, Uehata A, et al. Morning attenuation of endothelium-dependent, flow-mediated dilation in healthy young men: possible connection to morning peak of cardiac events? Clin Cardiol. 1999;22:417–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Park KH, Park WJ. Endothelial dysfunction: clinical implications in cardiovascular disease and therapeutic approaches. J Korean Med Sci. 2015;30:1213–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]