Abstract
Background
Oscillometric pulse wave velocity (o-PWV) represents an attractive, non invasive and non operator-dependent method to estimate arterial stiffness. Tonometric carotid-femoral measurements (cf-PWV),are considered the gold-standard for non-invasive aortic stiffness assessment. To date, no studies in the general population comparing the two methods have been performed.
Methods and Results
1162 subjects were analysed. O-PWV and cf-PWV showed a mean difference of −0.31 m/sec(p ≤ 0.001). No significant differences between cf-PWV and o-PWVs were observed in patients without cardiovascular risk factors. The Bland and Altman analysis showed a moderate agreement between 24 h-o-PWV and cf-PWV (mean difference −0.99, LoA 4.23 to −6.22m/s). O-PWVs underestimate and overestimate arterial stiffness under and over 50 years respectively(p ≤ 0.001). Systolic blood pressure (SBP) and age differently impact cf-PWV and in office o-PWV variability (r2 0.35 and 0.88 respectively). In younger subjects a strong relationship between o-PWV and SBP reducing as age increases was found. Analysing the impact of age, an opposite trend was noticed.
Conclusions
Oscillometric PWV estimates provide reliable values in the general population. An o-PWV tendency to underestimate arterial stiffness in younger subjects and in subjects with diseases known to increase arterial stiffness and to overestimate it with increasing age was found, even if scarcely relevant in clinical perspective. Overall the present findings underline an acceptable and satisfactory agreement between oscillometric and tonometric methods for the PWV assessment.
KEY MESSAGES
Oscillometric and tonometric PWV estimates showed a good and satisfactory agreement in the general population, above all in subjects without cardiovascular risk factors or a documented vascular damage.
In comparison with tonometric values, oscillometric PWV estimates showed, however, the tendency to underestimate arterial stiffness in younger subjects and to overestimate it with increasing age, while diverging when diseases known to increase arterial stiffness are present.
The magnitude of differences in PWV estimates between tonometric and oscillometric methods found in the general population appears most likely not to be significant in everyday clinical practice.
Keywords: Arterial stiffness, pulse wave velocity, tonometric method, oscillometric method, vascular ageing, comparative analysis
1. Background
Estimation of pulse wave velocity (PWV) currently represents the best approach to assess arterial stiffness [1], and is recommended for the management of arterial hypertension by the leading scientific societies of cardiology [2–4]. Considering that PWV is widely accepted as an independent risk parameter for the development of cardiovascular events [5–7], the feasibility of its measurement in clinical practice represents a challenge in terms of patients' cardiovascular risk stratification.
To date, the tonometric carotid-femoral PWV measurement (cf-PWV), is considered the gold-standard for the non-invasive assessment of aortic stiffness [8,9], and its association with cardiovascular prognosis in the general population, is well established [10,11].
However, several limiting factors burden the tonometric cf-PWV measurements; the procedure is time-consuming, requires sophisticated equipment, needs trained personnel, could be operator dependent and biases related to patients’ position and calculation of the distance between the two arterial sites could occur [3,11,12]. Recently, non-invasive oscillometric, non-operator-dependent, easy techniques for the assessment of PWV in clinical practice, have become available [13,14]. Using validated algorithms, which combine cuff oscillometry and pulse wave analysis to estimate PWV (o-PWV) on a single oscillometric blood pressure measurement (BP), specific devices such as the widely used Mobil-O-Graph (I.E.M.; IndustrielleEntwicklungMedizintechnik und VertriebsgesellschaftmbH, Stolberg, Germany)have been developed [15]. The role of such devices could become more and more important in the clinical setting, for the advantages, from a physician’s perspective, of simultaneously obtaining a 24 h ambulatory BPmonitoring and a PWV estimate [16]. Furthermore, the o-PWV evaluation is considered an attractive method for its easy approach, the high degree of reproducibility and, as said before, for the possibility to assess arterial stiffness in an operator-independent manner.
Previous validation studies showed a good agreement between o-PWVs provided by Mobil-O-Graph and invasively assessed aortic PWVs [17,18]. Moreover, studies aimed at exploring the agreement between o-PWVs and cf-PWVs, have shown a good inter-method agreement [13,19,20]. Nevertheless, these studies were performed in small selected sample populations and to date comparison studies in the general population are lacking. Furthermore, recently, it has been shown that the Mobil-O-Graph provides unreliable estimations of PWV, delivering significantly lower PWV values than cf-PWV, in a selected population of young patients affected by an early and accelerated vascular aging [21]. Last but not least, in a sub-analysis of the Masked Hypertension Study on 188 patients, it was found that age and systolic blood pressure (SBP) explain about 99% of the PWVs estimated by Mobil-O-Graph, and a scarce correlation between o-PWVs and cf-PWVs [22]. The comparison between the two methods was however limited.
Here, we strive to explore extensively, the tonometric cf-PWV measurements and o-PWV estimates, provided respectively with SphygmoCor and Mobil-O-Graph devices, in a sample of general adult population living in southern Switzerland (Tessin canton).
In the present study we aim to: i) define the agreement between o-PWV estimates and tonometric cf-PWV measurements in the general population ii) explore which one of the o-PWV values provided by the Mobil-O-Graph (in-office, 24 h, day-time and night-time) represents the better estimate of PWV in the general population iii) investigate the method-specific PWV values distribution in the general population iv) identify determinants of PWV values provided by both methods.
2. Methods
2.1. Study population
Analyses were performed in the Ticino epidemiological stiffness (TEST) study; an observational population-based study conducted in adults aged ≥18 years, living in Ticino, the Italian-speaking canton of Switzerland.
Subject’s examinations were performed between June 2017 and July 2018. The study has been detailed elsewhere [23]. Briefly, participants in the TEST study were contacted by mail, in a simple random sampling method, on the basis of a list provided by the Swiss Federal Statistical Department. Among individuals invited, 1202 (response rate of 86%) agreed to participate. Participants underwent an extensive examination including blood and urine analyses, instrumental evaluation including electrocardiogram (ECG), bio-impedance measurement and the compilation of questionnaires regarding lifestyle and health status. Arterial stiffness was assessed by pulse wave velocity with an oscillometric (Mobil-O-Graph) and a tonometric method (SpygmoCor).
The study was carried out in accordance with the Helsinki Declaration and was approved by the local Swiss ethics committee. All participants provided informed written consent. Data and analyses are presented in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) [24].
2.2. Estimation of pulse wave velocity
Tonometric carotid-femoral PWV measurements were performed using a standard operational procedure in a quiet, stable temperature room, with a tonometric method (SphygmoCor, AtCor Medical, Sydney, Australia; ModelMM3, Software version 7.01 S). The assessments, as recommended by the ARTERY Society guidelines, were conducted with a supine patient, after 10 min of rest; measuring cf-PWV on the patient’s dominant side. Participants were instructed to abstain from caffeine and tobacco use for four hours before the examination. Carotid and femoral sites were identified and marked and the path length measured as the distance from the carotid to femoral artery, directly measured using a tape between each artery location and the supra-sternal notch. By entering data into the computer, travelled distance was calculated automatically as the difference between the two distances, that is femoral location-sternal notch minus sternal notch-carotid location. As is known, with the SphygmoCor system cf-PWV measurements take place through two consecutive phases: in the first one the electrocardiogram (ECG) and the carotid pulse wave are simultaneously recorded; in the second, the procedure is repeated on the femoral artery. ECG during pulse wave recording is necessary for carotid and femoral pulse wave time synchronisation. The SphygmoCor uses the “foot” of the pulse wave as an onset point for calculating the time differences between the R wave of the ECG and the pulse waveforms at each site. Wave “foots” are identified using intersecting tangent algorithms. Thus, using this method, PWV is calculated from measurements of pulse transit time and distance travelled by the pulse wave. PWV measurements with a standard deviation less than 10% were used for analysis. Tonometric cf-PWV measurements were performed during the first day of the examination. After cf-PWV measurement, the oscillometric pulse wave velocity estimation was acquired. Each subject, underwent an ambulatory 24 h blood pressure recording with a Mobil-O-Graph device (IEM, Stolberg, Germany), able to perform simultaneously blood pressure and PWV measurements with an inbuilt ARCSolver pulse wave analysis algorithm (AIT Austrian Institute of Technology GmbH, Vienna, Austria), and to integrate age, central systolic blood pressure, and data derived from pulse wave analysis into a mathematical model deriving PWV values. Daytime and night-time periods were a priori pre-defined: 7:00 am to 10:00 pm for daytime and 10:00 pm to 7:00 am for night-time. Blood pressure measurements were recorded using an upper arm BP cuff every 30 min during the day-time and every hour during the night-time period. During monitoring, participants were asked to avoid vigorous physical exercise and to keep their arm relaxed. The first estimation of o-PWV was performed in the office, after cf-PWV acquisition: an appropriately-sized blood pressure cuff was then attached to the participant’s non-dominant arm in a sitting position; the first blood pressure and the in-office o-PWV were then acquired. For each participant, both tonometric and oscillometric PWV assessments were performed on the same day and in the same room. All PWV measurements, in-office and subsequently through the ABPM monitoring, were performed on a weekday. PWV assessment was conducted firstly with the tonometric method, and then by the o-PWV device following the described standardised procedures.
The results of the acquisition of the in-office o-PWV were not immediately available during the procedure, but downloaded into the computer, after the 24 h monitoring. For the comparison all o-PWV estimates provided by the Mobil-O-Graph were taken into account: in office (acquired during the initial examination), 24 h, daytime and night-time mean PWVs.
2.3. Statistical analysis
Statistical analyses were performed using SPSS 20.0 for Windows (SPSS Inc, Chicago, IL, USA) and R Statistical Software (www.r-project.org, version 3.2.0).
Sample size was determined a priori by statistical power calculation based on the statistical procedure for assessing agreement between two methods of clinical measurement [25]. The minimum number of subjects to enrol in this study was calculated to be 800. Considering a 15% dropout rate, 1202 participants were enrolled.
The normality of the distribution was checked, using a Kolmogorovpro–Smirnov test. Descriptive statistics are reported as median (25th–75th percentiles) or as absolute numbers and percentages, as appropriate.
As cf-PWV and o-PWV do not exhibit a normal distribution, median values (25th–75th percentiles) are reported. Differences between cf- and o-PWV values were evaluated in the entire population; across age decades (18–30 years; 30–39 years, 40–49 years, 50–59 years, 60–69 years, and ≥70 years) and across blood pressure categories (normal, i.e. BP < 130/85 mmHg; high-normal, i.e. 130/85≤ BP < 140/90 mmHg; hypertension, i.e. BP ≥140/90 mmHg). The Wilcoxon signed rank-sum test was used to test differences between the two devices.
To assess the correlations between cf-PWV and o-PWV measurements, the Spearman’s correlation coefficient was used. The agreement between PWV measurements was explored based on the analysis described by Bland and Altman [25]. Firstly, data were plotted with the line of identity to evaluate the degree of agreement between methods (regression lines with coefficients of correlation were also reported). Thereafter, the relative differences within each pair of measurements were plotted against the mean of the pair. Given that heteroskedasticity was found, a log-transformation was applied prior to the estimation of the limit of agreement (LoA), and a back-transformation (antilog) was subsequently performed, to allow interpretation of values in relation to the original scale. Both, bias and 95% limits of agreement were reported.
Receiver operating characteristic curve analysis (ROC) was constructed to evaluate the performance of o-PWV in predicting increased arterial stiffness, after categorisation of abnormal cf-PWV (i.e. increased arterial stiffness with values > 10m/s) [26]. The area under the ROC curve (AUC) was computed in order to evaluate the global predictive ability of o-PWV for increased arterial stiffness. Confidence intervals of AUC were also reported. Accordingly, sensitivity and specificity and the optimal cut-off values were calculated for o-PWV, based on the highest Youden index level (sensitivity + specificity − 1). AUC comparisons were carried out in order to analyse the performance of the different o-PWVs collected. AUC comparison was examined using the method proposed by DeLong et al. (a z-statistic, testing the null hypothesis of AUC equivalence) [27].
Multivariate regression analyses were used to estimate the role of systolic and diastolic blood pressures, heart rate and age in affecting cf-PWV measurements and o-PWV estimates. In order to investigate the contribution of selected covariates in determining cf-PWV and o-PWV, univariate linear associations between o-PWVprovided by different methods, and analyses performed in the entire population and per age groups were also performed.
In the linear univariate models only the quadratic term of age was included, considering the nonlinear association between age and PWV and that the models including the quadratic term of age provided better r2values of about 4–6%than those including only the linear term. For all analysis the significance level was set at p < .05.
3. Results
3.1. Study population
We enrolled 1202 subjects. 40 participants were excluded for the following reasons: the presence of cardiac arrhythmia (atrial fibrillation, frequent extrasystoles, n = 8); impalpable arterial pulse at the site of measurement (n = 19); pulse wave not evaluable due to artefacts (n = 8); Mobil-O-Graph registration not performed (n = 5), resulting in a sample of 1162 subjects. The study flow diagram and reasons for exclusion are shown in Figure 1. Median age (IQR) of participants was 52 (43–60) years, and 56.0% (n = 651) of the subjects were women. Anthropometric and hemodynamic data of all participants are shown in Table 1.
Figure 1.
Flow diagram of the study with the exclusion procedure. Abbreviations: PWV: pulse wave velocity, o-PWV: oscillometric pulse wave velocity; cf-PWV: cartoid-femoral pulse wave velocity.
Table 1.
Characteristics of the study population (n: 1162 subjects).
| Clinical characteristics | |
|---|---|
| Gender, females | 651 (56.0%) |
| Age, years | 52 (43–60) |
| Age categories | |
| <30 years | 67 (5.8) |
| 30–39, years | 149 (12.8) |
| 40–49, years | 296 (25.5) |
| 50–59, years | 341 (29.4) |
| 60–69, years | 184 (15.8) |
| ≥70, years | 125(10.7) |
| Smoker | 216 (18.5) |
| Hypertensive | 180 (15.6) |
| Diabetic | 24 (2.1) |
| Hypercolesterolemic | 166 (14.4) |
| BMI, kg/m2 | 24.4 (22.0–27.4) |
| Waist/ Hip, cm | 0.91 (0.86–0.96) |
| SBP in-office, mmHg | 131 (121–143) |
| DBP in-office, mmHg | 81 (74–89) |
| Heart rate in-office, beats/min | 68 (61–75) |
| Pulse wave velocity | |
| cf-PWV , m/sec | 6.9 (6.1–8.1) |
| PWV Mobil-O-Graph in office, m/sec | 7.3 (6.2–8.6) |
| PWV Mobil-O-Graph 24 h, m/sec | 7.0 (6.0–8.2) |
| PWV Mobil-O-Graph daytime, m/sec | 7.1 (6.1–8.3) |
| PWV Mobil-O-Graph night-time, m/sec | 6.7 (5.8–7.9) |
| Ambulatory blood pressure monitoring | |
| SBP 24 h, mmHg | 117 (111–126) |
| DBP 24 h, mmHg | 73 (68–80) |
| SBP daytime, mmHg | 120 (113–129) |
| DBP daytime, mmHg | 76 (70–83) |
| SBP night-time, mmHg | 110 (103–119) |
| DBP night-time, mmHg | 66 (61–73) |
| MBP, mmHg | 90.6 (84.2–97.6) |
| Heart-Rate 24 h, beats/min | 70 (64–75) |
| Pulse Pressure 24 h, mmHg | 44 (39–49) |
| Pulse Pressure daytime, mmHg | 44 (39–50) |
| Pulse Pressure night-time, mmHg | 43 (39–48) |
| Blood pressure categories | |
| Normal | 661 (56.88) |
| High Normal | 187 (16.09) |
| Hypertension | 314 (27.02) |
Data are presented as median (25th–75th percentile) for continuous values, and as number (percentage) for discrete values. Abbreviations: PWV: pluse wave velocity; MBP: mean blood pressure, BMI : body mass index, SBP: systolic blood pressure, DBP: diastolic blood pressure, cf-PWV: carotid-femoral pulse wave velocity.
This population was characterised by normal BP with median (IQR) systolic and diastolic values of 117 (111–126) and 73(68–80)mmHg. Median (IQR) PWV values by methods were: cf-PWV 6.9 (6.1–8.1); in-office o-PWV 7.3(6.2–8.6); 24 h o-PWV 7.0(6.0–8.2); day time o-PWV 7.1(6.1–8.3); night-time o-PWV 6.7(5.8–7.9) m/sec.
3.2. Distribution of pulse wave velocity by methods
Variations in PWV values and differences between methods were investigated and are presented in Table 2. Values of o-PWV (in-office, 24 h, daytime, and night-time) were analysed extensively considering first the entire population, subsequently according to age and BP categories, and then comparing each modality with cf-PWV values.
Table 2.
Variation between methods of pulse wave velocity (m/s), in the entire population and according to age categories and blood pressure levels.
| cf-PWV | o-PWV in-office | Median diff (IQR) | cf-PWV vs o-PWV in office |
o-PWV 24 h |
Median diff (IQR) | cf-PWV vs o-PWV 24 h |
o-PWV daytime |
Median diff (IQR)) | cf-PWV vs o-PWV daytime |
oPWV night-time |
Median diff (IQR) | cf-PWV vs o-PWV night-time |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median (10–90pc) | Median (10–90pc) | p-Value | Median (10–90pc) | p-Value | Median (10–90pc) | p-Value | Median (10–90pc) | p-Value | |||||
| Entire population | 6.9 (5.3–9.6) | 7.3 (5.4–10.4) | −0.2 (−1.3 − 0.75) | <.001* | 7.0 (5.3–9.8) | 0.1 (−0.9–1) | 0.723 | 7.1 (5.3–9.9) | −0.1 (−1.0–0.9) | 0.057 | 6.7 (5.1–9.6) | 0.3 (−0.7–1.2) | <.001* |
| Age class, years | Median (10–90 percentile) | Median (10–90 pc) | p-value | Median (10–90 pc) | p-value | Median (10–90 pc) | p-value | Median (10–90 pc) | p-value | ||||
| <30 | 5.5 (4.6–7.1) | 5.1 (4.6–5.8) | 0.7 (−0.1–1.3) | <.001* | 4.9 (4.5–5.3) | 0.7 (0–1.4) | <.001* | 5.0 (4.6–5.4) | 0.6 (0–1.4) | <.001* | 4.7 (4.3–5.2) | 0.9 (0.35–1.6) | <.001* |
| 30–39 | 6.2 (4.8–8) | 5.7 (5–6.4) | 0.5 (−0.2–1.3) | <.001* | 5.4 (5–5.9) | 0.8 (0.1–1.6) | <.001* | 5.5 (5.0–6.0) | 0.7 (0–1.5) | <.001* | 5.2 (4.7–5.7) | 1 (0.3–1.9) | <.001* |
| 40–49 | 6.7 (5.3–8.5) | 6.5 (5.8–7.3) | 0.2 (−0.6–1) | <.001* | 6.2 (5.7–6.9) | 0.4 (0.2–1.1) | <.001* | 6.4 (5.8–7.0) | 0.3 (−0.3–1.1) | <.001* | 6.0 (5.4–6.8) | 0.7 (0–1.4) | <.001* |
| 50–59 | 7.0 (5.7–9.1) | 7.6 (6.8–8.5) | −0.45 (−1.3–0.4) | <.001* | 7.3 (6.7–8.0) | 0.2 (0.9–0.6) | <.001* | 7.4 (6.8–8.2) | −0.3 (−1.1–0.5) | <.001* | 7.1 (6.4–7.8) | 0 (−0.7–0.9) | .066 |
| 60–69 | 7.9 (6.0–10.1) | 9.1 (7.9–10.4) | −1.25 (−2.3, −0.1) | <.001* | 8.8 (7.9–9.7) | −0.85 (−2.9, −0.1) | <.001* | 8.9 (8.0–9.7) | −0.1 (−2.1–0.1) | <.001* | 8.5 (7.7–9.5) | −0.7 (−1.7–0.4) | <.001* |
| >70 | 8.8 (6.8–12.8) | 11.1 (9.9–12.9) | −2.3 (–3.6, −0.2) | <.001* | 10.6 (9.7–12.3) | −2.05 (−2.9, −0.1) | <.001* | 10.7 (9.8–12.3) | −2.1 (−3, −0.2) | <.001* | 10.5 (9.4–12.1) | −1.8 (−2.8–0.3) | <.001* |
| Blood pressure categories |
Median (10–90pc) | Median (10–90pc) | p-value | Median (10–90pc) | p-value | Median (10–90pc) | p-value | Median (10–90pc) | p-value | ||||
| Normal | 6.6 (5.2–8.7) | 6.8 (5.2–9.4) | −0.1 (−1 − 0.8) | .007* | 6.5 (5.1–8.8) | 0.15 (−0.7–1.0) | .031* | 6.6 (5.1–8.9) | 0.1 (−0.8–0.9) | .796 | 6.3 (4.8–8.6) | 0.4 (−0.5–1.2) | <.001* |
| High Normal | 7.6 (6.0–9.8) | 7.9 (6.4–10.6) | −0.3 (−1.4 − 0.8) | .012* | 7.6 (6.1–11.2) | −0.1 (−1–1.1) | .9151 | 7.7 (6.1–10.3) | −0.1 (−1.1–0.9) | .399 | 7.2 (5.8–9.8) | 0.3 (−0.7 − 1.4) | .012* |
| HYP | 7.7 (5.7–10.6) | 8.1 (5.9–11.8) | −0.6 (−1.7–0.7) | <.001* | 7.7 (5.7–11.2) | −0.2 (−1.4–0.9) | .031* | 7.8 (5.8–11.3) | −0.3 (−1.5–0.7) | .002* | 7.3 (5.4–11.0) | 0.1 (−1.15–1.1) | .914 |
Abbreviations: PWV: pluse wave velocity; cf-PWV: carotid-femoral pulse wave velocity, o-PWV: oscillometric pulse wave velocity, IQR: interquartile range; pc: percentile, HYP: hypertension. Data are presented as median, upper limit of the 10th percentile; lower limit of the 90th percentile, p-values for comparisons between cf-PWV and in office Mobil-O-Graph PWV, cf-PWV and mean 24 h Mobil-O-Graph PWV, cf-PWV and daytime Mobil-O-Graph PWV, cf-PWV and night-time Mobil-O-Graph PWV. *p-value < .05.
Considering the entire population, in office o-PWV showed values significantly higher than cf-PWV (7.3 vs 6.9 m/sec, p < .001); whilst night-time o-PWV was significantly lower than cf-PWV (6.7 vs 6.9 m/sec, p-value < .001). No significant differences comparing cf-PWV with 24 h and daytime o-PWV were found.
Considering age categories, both cf-PWV and o-PWV showed an increasing age-related trend, with the higher PWV values in the oldest age groups (see Table 2 for details). By age categories, o-PWVs were significantly different from cf-PWV values for all age groups and for all o-PWVs considered (in-office, 24 h, daytime and night-time) (p ≤ 0.001). Specifically, cf-PWV values were significantly higher than o-PWV values for the under 50 years age groups and significantly lower than o-PWV values for the over 50 years age groups (p ≤ 0.001 for each comparison).
The analysis of the mean difference between methods revealed a very small difference in younger age classes, with more clinically relevant differences in age classes over 60 and 70 years (respectively −1.3 and −2.3 m/sec).
We also explored PWV values according to BP categories (as mentioned above, classified as normal, high-normal and hypertensive). Both cf-PWV and o-PWV showed higher values with increased BP. For all BP categories, in office o-PWV were significantly higher than cf-PWV. In subjects with normal and high normal BP, daytime o-PWV was not significantly different from cf-PWV. In hypertensive subjects all o-PWV estimates were significantly higher than cf-PWV values (p-value for comparison cf-PWV vsin-office, vs 24 h and vs daytime o-PWVs were respectively: <.001, .031 and .002) (Table 2).
Figure 2 shows the distribution of PWV values related to age (upper part of the panel) and systolic BP (bottom of the panel) by o-PWV and cf-PWV methods. Comparing with cf-PWV, a significant underestimation of o-PWV (in office, 24 h, daytime and night-time) is evident under 50 years, with a switch in trend thereafter, where o-PWV shows an over-estimation of the values.
Figure 2.
Distribution of pulse wave velocity values related to age (upper panels) and systolic blood pressure (bottom panels) according to tonometric and oscillometric approaches. Triangles represent carotid-femoral PWV values measured by tonometric method. Dots represent o-PWV values estimated by oscillometric method. Panel A, E: cf-PWV and in office o-PWV; B, F: cf-PWVand mean 24 h o-PVW; C, G: cf-PWV anddaytime o-PWV; D, H:cf-PWV and night-timeo-PWV. Continuous lines show the relationship between age and PWVs (exponential regression analysis) and between systolic blood pressure and PWVs (linear regression analysis).
In the general population cf-PWV was weakly affected by age (r2 = 0.27, p ≤ 0.001), on the contrary o-PWV was strongly related with age (in office o-PWV r2 = 0.84, p ≤ 0.001; 24 h o-PWV r2 = 0.89, p ≤ 0.001; daytime oPWV r2 = 0.89, p ≤ 0.001; night-time o-PWV r2 = 0.88, p ≤ 0.001).
The analysis of the correlationbetween PWVs by methods and systolic blood pressure showed a weak correlation (cf-PWV r2 = 0.17, p ≤ 0.001; in office o-PWV r2 = 0.22, p ≤ 0.001; 24 h o-PWV r2 = 0.21, p ≤ 0.001; daytime o-PWV r2 = 0.20, p ≤ 0.001; night-time o-PWV r2 = 0.23, p ≤ 0.001).
3.3. Agreement between methods
The agreement between cf-PWV and o-PWV measurements was firstly explored considering the mean differences in the overall population. Both in office o-PWV and night-time o-PWV showed significantly different mean values than cf-PWV, respectively −0.31, p ≤ 0.001 and 0.22, p ≤ 0.001. On the contrary, mean 24 h o-PWV, and daytime o-PWV were not significantly different from cf-PWV (Table 3(A)).
Table 3.
Overall agreement between measurements of pulse wave velocity by the tonometric and oscillometric methods (mean difference, rank correlation, limits of agreement and rank correlation from the Bland & Altman plots) in overall population (a) by PWV values (b) and in subjects at low CV risk (c).
| a | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Method | PVW ± SD | Mean Difference | Standard Error | p-Value | Correlation r2 |
p-Value | Bias Limits of Agreement 95% | Bland& Altman Plot r | p-Value |
| cf-PWV vs o-PWV in office | 7.28 ± 1.79 vs 7.60 ± 1.9 | −0.31 | 0.05 | ≤.001* | 0.532* | ≤.001* | −1.01 (4.12; −6.32) | −0.102 | ≤.001* |
| cf-PWV vs o-PWV mean 24 h | 7.28 ± 1.79 vs 7.29 ± 1.75 | −0.02 | 0.05 | .723 | 0.545* | ≤.001* | −0.99 (4.23; −6.22) | −0.013 | .665 |
| cf-PWV vs o-PWV daytime | 7.28 ± 1.79 vs 7.39 ± 1.75 | −0.11 | 0.04 | .930 | 0.543* | ≤.001* | −0.96 (4.26; −6.18) | −0.025 | .396 |
| cf-PWV vs o-PWV night-time | 7.28 ± 1.79 vs 7.05 ± 1.77 | 0.22 | 0.05 | ≤.001* | 0.534* | ≤.001* | 0.96 (6.11; −4.19) | 0.035 | .253 |
| b | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subjects with PWV < 10 m/sec |
Subjects with PWV ≥ 10 m/sec |
|||||||||||
| Method | PVW ± SD | Mean Difference | Standard Error | p-Value | Correlation r2 |
p-Value | PVW ± SD | Mean Difference | Standard Error | p-Value | Correlation r2 |
p-Value |
| cf-PWV vs o-PWV in office | 6.91 ± 1.27 vs 7.38 ± 1.71 | −0.48 | 0.05 | ≤.001 | 0.470 | ≤.001 | 11.41 ± 1.43 vs 10.02 ± 2.13 | 1.41 | 0.20 | ≤.001 | 0.440 | ≤.001 |
| cf-PWV vs o-PWV mean 24 h | 6.91 ± 1.27 vs 7.09 ± 1.59 | −0.18 | 0.05 | .998 | 0.487 | ≤.001 | 11.41 ± 1.43 vs 9.54 ± 1.97 | 1.90 | 0.20 | ≤.001 | 0.394 | ≤.001 |
| cf-PWV vs o-PWV daytime | 6.91 ± 1.27 vs 7.19 ± 1.59 | −0.28 | 0.05 | 1.000 | 0.485 | ≤.001 | 11.41 ± 1.43 vs 9.64 ± 1.95 | 1.80 | 0.19 | ≤.001 | 0.403 | ≤.001 |
| cf-PWV vs o-PWV night-time | 6.91 ± 1.27 vs 6.83 ± 1.58 | 0.06 | 0.05 | .118 | 0.463 | ≤.001 | 11.41 ± 1.43 vs 9.46 ± 1.98 | 2.03 | 0.21 | ≤.001 | 0.351 | ≤.001 |
| c | ||||||
|---|---|---|---|---|---|---|
| Method | PVW ± SD | Mean Difference | Standard Error | p-value | Correlation r2 |
p-value |
| cf-PWV vs o-PWV in office | 7.02 ± 0.05 vs 7.15 ± 0.05 | −0.14 | 0.05 | 0.971 | 0.481 | ≤0.001 |
| cf-PWV vs o-PWV mean 24 h | 7.02 ± 0.05 vs 6.87 ± 0.048 | 0.14 | 0.05 | 0.019 | 0.500 | ≤0.001 |
| cf-PWV vs o-PWV daytime | 7.02 ± 0.05 vs 6.97 ± 0.048 | 0.04 | 0.05 | 0.278 | 0.499 | ≤0.001 |
| cf-PWV vs o-PWV night-time | 7.02 ± 0.05 vs 6.64 ± 0.02 | 0.37 | 0.05 | ≤0.001 | 0.486 | ≤0.001 |
Abbreviations: 24hBPM, 24 h blood pressure monitoring; SD, standard deviation.
*p value < .05; PWV expressed in m/sec.
Correlations of PWVs measured by the two methods were moderate, with respectively r values of 0.532, 0.545, 0.543 and 0.534 for cf-PWV vs in office,24 h, daytime and night-time o-PWV; p ≤ 0.001 (Table 3(A)). Mean differences and correlation coefficients between methods were explored also in the subgroup of subjects at low CV risk (subjects without a personal history of previous cardiovascular diseases, diabetes and hypertension) (Table 3(B)). In this subgroup of subjects no significant differences between cf-PWV and in office or daytime o-PWV measurements were found (7.02 ± 0.05 vs. 7.15 ± 0.05, p-value .971; 7.02 ± 0.05 vs. 6.97, p-value .278).
The analysis of the subset of patients with confirmed vascular damage (subjects with PWV ≥ 10 m/sec) (Table 3(B and C)), revealed a higher difference in mean values between o-PWV and cf-PWV comparing with the subgroup of subjects with a PWV < 10 m/sec (−0.48 and 1.41 m/sec. respectively). Better r2 correlations between methods were also found in the subgroup of subjects with a PWV <10 m/sec (Table 3(B and C)).
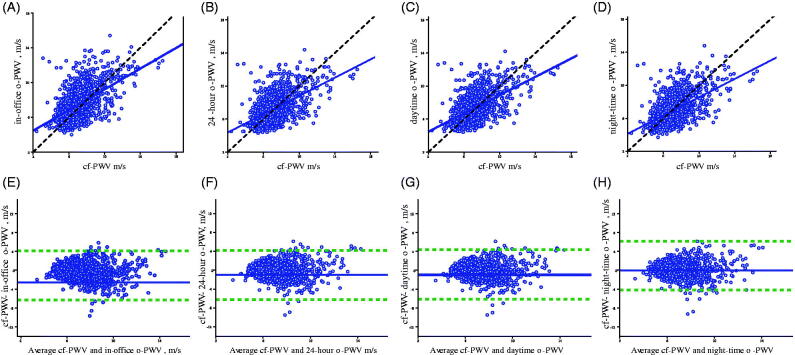
Relationship between measured cf-PWV and o-PWV estimates is shown in Figure 3. In the upper part of Figure 3, scatterplots show the linear correlation between cf-PWV and respectively in-office o-PWV (a), 24 h o-PWV (b); daytime o-PWV (C) and night-time o-PWV (D). Linear regression lines(blue solid lines) and identity lines (black dashed lines) are also shown.
Figure 3.
Relationship between oscillometricPWV estimates and cf-PWV measurements. On the upper part of the panel, scatterplots showthe linear correlation between cf-PWVsmeasured by tonometric method, the non-invasivereference method, and PWVs estimated by oscillometric method. Linear regression lines (blue solid lines) and identity lines (black dashed lines) are also shown. On the bottom part of the panel, the Bland and Altman plots show differences observed between estimatesand average values. A, E: cf-PWV and in office o-PWV; B, F: cf-PWV and 24 hour mean o-PVW; C, G: cf-PWV anddaytime o-PWV; D, H: cf-PWV and night-timeo-PWV. Blue solid lines showthe mean values of the differences, and green dashed lines ±1.96 x SD of differences.
In the bottom of Figure 3, Bland & Altman plots, show differences observed between measurements and average values, between cf-PWVs and respectively in-office o-PWV (E); 24 h o-PWV (F); daytime o-PWV (G) and night-time o-PWV (H).
In the Bland & Altman plots, biases and 95% limits of agreement (LoA) are also shown. On average cf-PWVs show values 1.01 m/sec lower than in-office o-PWVswith wide limits of agreement (4.12 to −6.32) and a significant correlation (r = −0.102, p ≤ 0.001). Compared with the other o-PWV estimates cf-PWVs show lower values in all cases (Table 3). The highest concordance, with lowest biases and narrowest limits of agreement, was found comparing cf-PWV with daytime o-PWV (bias −0.96, LoA 4.26 to −6.18) Table 3, Figure 3(G).
3.4. Relationship of oscillometric PWVs and increased arterial stiffness
Receiver operating characteristic curves were constructed to evaluate the ability to predict increased arterial stiffness with o-PWV (82 cases with cf-PWV ≥ 10 m/sec; 948 o-PWV controls).
The prediction of increased arterial stiffness showed an area under the ROC curve (AUC) of 0.837 for in office o-PWV; 0.837 for 24 h o-PWV; 0.838 for daytime o-PWV and 0.844 for night-time o-PWV (Figure 4). In the same figure, the respective AUCs (95% confidence interval), cut-off points, sensitivity and specificity are shown. All o-PWVs showed moderate sensitivity (in office o-PWV 80.3%, 24 h o-PWV 80.5%; daytime o-PWV 81.9%, night-time o-PWV 74.3%) and low positive predictive (PV+) rates (<3% for all o-PWVs, see Figure 4). Moderate specificity was also found for all o-PWVs: 80.3% for in office o-PWV, 80.5% for 24 h o-PWV; 81.9% for daytime o-PWV and 74.3% for night-time o-PWV); with quite high negative predictive (PV-) rates (>70% for all o-PWVs, see Figure 4). The cut-off values were 8.65 m/s for in office o-PWV, 8.25 m/sec for 24 h o-PWV, 8.45 m/sec for daytime o-PWV and 7.55 for night time o-PWV. Except for night-time o-PWV which was significantly different from daytime and 24 h o-PWV (p 0.698 and 0.490 respectively) all other AUC comparisons documented equivalence (daytime vs. in office and 24 h o-PWV with a p of 0.004 and 0.019 respectively; 24 h vs. in office o-PWV with a p of 0.007).
Figure 4.
Receiver-operating characteristic curves for predicting increased arterial stiffness as defined by cf-PWV ≥ 10 m/s. Panels representing ROC curves for predicting increased arterial stiffness of in office o-PWV (A), 24 hours o-PWV (B); daytime o-PWV (C) and night-time o-PWV (D).
3.5. Determinant factors of PWV values by methods
Determinant factors of PWV values by methods were investigated by multivariate analysis (Table 4). Four models were subsequently constructed, including as predictors heart rate, systolic and diastolic blood pressure, age andage2, and as dependent variable cf-PWV;24 h o-PWV; daytime o-PWV, night-time o-PWV respectively. The analyses showed that cf-PWV was significantly affected by systolic blood pressure and age (β 0.033, p ≤ 0.001; β 0.056, p ≤ 0.001) with a model coefficient r2 of 0.35. Diastolic blood pressure and heart rate did not affect cf-PWV. In office o-PWV was affected by age, 2systolic blood pressure and heart rate (0.114, 0.029; p-values: ≤0.001; and −0.004, p-value 0.024). Multivariate analysis revealed greater r2 for 24 h, daytime and night-time o-PWV with values of 0.91, 0.92 and 0.90 respectively. In a multivariate analysis considering only age2and systolic BP, the coefficient of determination of cf-PWVwas 35.4%according to the formula cf-PWV: age2/1000 + 0.032 SBP (Figure 5). In the same multivariate analysis24 h o-PWV showed a coefficient of determination of 98.4% according to the formula: age2/1000 + 0.033 24 h SBP; daytime o-PWV a coefficient of determination of 98.1% with the formulaage2/1000 + 0.033 daytime SBP; in office o-PWV a coefficient of determination of 90.6% with the formula: age2/1000 + 0.020 in office SBP and night-time o-PWV a coefficient of determination of 97.9% with the formula age2/1000 + 0.034 night-time SBP.
Table 4.
Multiple Regression Analysis with PWVs measured by SphygmoCor and estimated by Mobil-O-Graph (in office, mean 24 h, daytime, and night-time PWVs) as dependent variables.
| Dependent Variable | Independent Variable | Regression Coefficient | SE | Lower 95% CL | Upper 95% CL | Standardised Coefficient | p Value |
|---|---|---|---|---|---|---|---|
| Model 1 | |||||||
| PWV by SphygmoCor (r2model = 0.346) |
Intercept | −0.051 | 0.428 | −0.890 | 0.788 | .905 | |
| Age | 0.056 | 0.003 | 0.049 | 0.062 | 0.424 | <.001 | |
| Systolic BP | 0.033 | 0.005 | 0.024 | 0.042 | 0.294 | <.001 | |
| Diastolic BP | 0.000 | 0.007 | −0.013 | 0.013 | −0.001 | .981 | |
| Heart Rate | 0.003 | 0.004 | −0.006 | 0.011 | 0.016 | .519 | |
| Model 2 | |||||||
| PWV by Mobil-O-Graph-in office (r2model = 0.878) | Intercept | −1.527 | 0.195 | −1.910 | −1.143 | <.001 | |
| Age | 0.116 | 0.002 | 0.113 | 0.119 | 0.835 | <.001 | |
| Systolic BP | 0.029 | 0.002 | 0.025 | 0.034 | 0.251 | <.001 | |
| Diastolic BP | −0.005 | 0.003 | −0.011 | 0.001 | −0.033 | .079 | |
| Heart Rate | −0.004 | 0.002 | −0.008 | −0.001 | −0.024 | .026 | |
| Model 3 | |||||||
| PWV by Mobil-O-Graph 24 h (r2model = 0.919) | Intercept | −0.883 | 0.148 | −1.174 | 0.593 | <.001 | |
| Age | 0.114 | 0.001 | 0.112 | 0.116 | 0.887 | <.001 | |
| Systolic BP | 0.024 | 0.002 | 0.021 | 0.027 | 0.219 | <.001 | |
| Diastolic BP | −0.010 | 0.002 | −0.014 | −0.005 | −0.062 | <.001 | |
| Heart Rate | −0.001 | 0.001 | −0.004 | 0.002 | −0.005 | .565 | |
| Model 4 | |||||||
| PWV by Mobil-O-Graph daytime (r2model = 0.920) |
Intercept | −0.770 | 0.148 | −1.060 | −0.480 | <.001 | |
| Age | 0.114 | 0.001 | 0.112 | 0.117 | 0.886 | <.001 | |
| Systolic BP | 0.023 | 0.002 | 0.020 | 0.027 | 0.215 | <.001 | |
| Diastolic BP | −0.009 | 0.002 | −0.016 | −0.004 | −0.102 | <.001 | |
| Heart Rate | −0.002 | 0.001 | −0.004 | 0.001 | 0.009 | .283 | |
| Model 5 | |||||||
| PWV by Mobil-O-Graph night-time (r2model = 0.903) |
Intercept | −1.084 | 0.167 | −1.412 | −0.755 | <.001 | |
| Age | 0.114 | 0.001 | 0.111 | 0.117 | 0.882 | <.001 | |
| Systolic BP | 0.025 | 0.002 | 0.021 | 0.028 | 0.225 | <.001 | |
| Diastolic BP | −0.012 | 0.003 | −0.017 | −0.007 | −0.112 | .001 | |
| Heart Rate | 0.000 | 0.002 | −0.003 | 0.004 | 0.003 | .782 | |
Figure 5.
Factors affecting pulse wave velocity estimated by oscillometric and measured by tonometric methods. Cf-PWVs measured with tonometric method are weakly associated with age2 and SBP with a r2 of 0.35(A). O-PWVs estimated by oscillometric method are strongly associated with age2 and SBP with r2 of respectively 0.90 for in office o-PWV (B); 0.98 for mean 24 h o-PWV (C); 0.98 for daytime o-PWV (D) and 0.97 fornight-time o-PWV (E).
The fact that cf-PWV was scarcely influenced by age2 and SBP was evident in the univariate analysis (see Table 5 for details), which also showed that o-PWV estimates were largely based on age2 and SBP and that the relationship shows a trend across age. In subjects < 30 years old, o-PWV estimates strongly depend on SBP (r2 0.78, p ≤ 0.001), with a minor effect of age2(r2 0.075, p = .005). Conversely, in subjects ≥ 70 years old, o-PWV estimates largely depends on age2 (r2 0.753 p ≤ 0.001) with a minor effect of SBP(r2 0.166, p ≤ 0.001).
Table 5.
Univariate association between age, systolic blood pressure and PWVs according to methods in the entire population and by age classes.
| Method | Category | Independent Variable | r 2 | β-coefficient | p-value | Independent Variable | r 2 | β-coefficient | p-value |
|---|---|---|---|---|---|---|---|---|---|
| cf-PWV/o-PWVs; office, mean 24 h, daytime night-time | Entire population | Age2 | 0.282/0.876/0.939/0.937/0.927 | 0.531/0.936/0.969/0.968/0.963 | ≤.001** | SBP | 0.186/0.244/0.211/0.199/0.225 | 0.432/0.494/0.459/0.446/0.475 | ≤0.001** |
| cf-PWV/o-PWVs; office, mean 24 h, daytime night-time | <29 | Age2 | 0.114/0.025/0.075/0.079/0.153 | 0.002/0.158/0.299/0.281/0.391 | .005§/≤.001∞ | SBP | 0.125/0.248/0.778/0.756/0.673 | 0.354/0.498/0.882/0.870/0.821 | ≤0.001** |
| cf-PWV/o-PWVs; office, mean 24 h, daytime night-time | 30–39 | Age2 | 0.024/0.092/0.263/0.277/0.184 | 0.001/0.304/0.513/0.526/0.429 | .061§/≤.001∞ | SBP | 0.251/0.301/0.620/0.655/0.629 | 0.501/0.549/0.787/0.810/0.793 | ≤0.001** |
| cf-PWV/o-PWVs; office, mean 24 h, daytime night-time | 40–49 | Age2 | 0.067/0.199/0.346/0.340/0.339 | 0.001/0.446/0.588/0.583/0.582 | ≤.001** | SBP | 0.083/0.339/0.615/0.608/0.598 | 0.288/0.582/0.784/0.780/0.774 | ≤0.001** |
| cf-PWV/o-PWVs; office, mean 24 h, daytime night-time | 50–59 | Age2 | 0.033/0.192/0.399/0.385/0.395 | 0.001/0.438/0.631/0.621/0.629 | ≤.001** | SBP | 0.149/0.288/0.464/0.452/0.509 | 0.386/0.537/0.681/0.672/0.714 | ≤.001** |
| cf-PWV/o-PWVs; office, mean 24 h, daytime night-time | 60–69 | Age2 | 0.021/0.346/0.566/0.556/0.490 | 0.001/0.588/0.752/0.746/0.700 | .049§/≤.001∞ | SBP | 0.177/0.257/0.415/0.425/0.463 | 0.421/0.507/0.644/0.652/0.681 | ≤.001** |
| cf-PWV/o-PWVs; office, mean 24 h, daytime night-time | ≥70 | Age2 | 0.083/0.539/0.753/0.738/0.739 | 0.001/0.734/0.868/0.859/0.859 | ≤.001∞ | SBP | 0.026/0.156/0.166/0.132/0.274 | 0.160/0.395/0.407/0.364/0.524 | ≤.001** |
Abbreviations and symbols: PWV pulse wave velocity, o-PWV: oscillometric pulse wave velocity; cf-PWV: carotid-femoral pulse wave velocity, SBP systolic blood pressure; **p-values for both methods, §p-values for cf-PWVs; ∞p-values for o-PWVs.
4. Discussion
The present study is the first large population-based study comparing pulse wave velocity values provided by two non-invasive methods, the tonometric measurement and the oscillometric estimate.
Here we have striven to elucidate the reliability of the methods, exploring age-related differences and determinants.
Currently, the oscillometric assessment of PWV with Mobil-O-Graph, in the everyday clinical practice is considered an attractive approach, for its easy and operator-independent assessment.
The mean difference in the overall population between oscillometric and tonometric PWV of −0.31 m/sec, in line with findings of previous studies, can be considered non-relevant from a clinical perspective. Moreover, no significant difference was found between tonometric and oscillometric in office PWV values considering the subgroup of patients at low cardiovascular risk.
Analysing age groups, the magnitude of the difference in PWV values provided by the two methods, increases and becomes potentially more relevant for subjects over 60 and even more so over 70 years old (median difference −1.3 and −2.3 m/sec, respectively). The analysis of the subset of subjects with a confirmed vascular damage (subjects with PWV ≥ 10 m/sec), comparing with patients with PWV under the cut off of 10 m/sec, revealed a potentially relevant mean difference (1.4 vs. −0.48 m/sec).
These results are in line with previous findings, in which at higher levels of PWV a reduced agreement in PWV estimates between SphygmoCor and different cuff-based methods was found [28].
Our findings highlight that in the general population, o-PWVs delivered by Mobil-O-Graph show an age-dependent trend, with a peculiar slope, generating a cross-over of the values comparing with the tonometric cf-PWV measurements. Specifically, oscillometric PWV values significantly underestimate arterial stiffness compared with tonometric cf-PWV measurements in subjects under 50 years old and significantly overestimate PWV values in subjects over 50. The same trend is significant and noticeable for all Mobil-O-Graph PWV values (in office, 24 h, daytime and night-time o-PWVs).
The reliability of o-PWVs provided by Mobil-O-Graph in specific subgroups of patients was recently questioned. In certain conditions exposing to early vascular aging, the oscillometric method was found to be inadequate in assessing arterial stiffness [21]. Moreover, a reduced agreement between PWV methods at higher levels of arterial stiffness was highlighted [28]. This aspect also was detected in a small and clinically well-defined population at high risk of accelerated arterial aging [21,28]. Our findings show that in the general population the oscillometric method tested, on one hand, underestimates cf-PWV values in subjects under 50 years, and on the other overestimates the same values with increasing age. However, the magnitude of estimate differences is narrow and most likely not significant in everyday clinical practice. In the absence of other identifiable causes, we can speculate that the underlying reason of the age-related under/overestimation could be the algorithm function used by Mobil-O-Graph for the o-PWV estimation. However, this aspect was not investigated because beyond the aims of this study, and remains a field of hypotheses to further assess.
Moreover, we believe it is important to underline that, even if the disparities in PWV assessment among methods in subgroups of population at high CV risk found here seem in line with results of previous studies [21,28], in the present study they are scarcely relevant from a clinical perspective. Overall, the present results suggest a satisfactory agreement between the PWV assessment methods investigated.
The univariate linear regression analysis by age groups, revealed a significant relationship between o-PWV and age with an increasing trend as age increased (r2 for age2 of 0.08 and 0.75 in subjects under 30 years and over 70 years respectively). On the other hand, a moderate relationship exists between o-PWV estimates and SBP (r2 0.244 in the entire population) which gradually reduces as age increases (r2 0.156 in subjects over 70 years old). The algorithm used to estimate o-PWVs, seems to be essentially based on age and BP, but we could hypothesise that is differently impacted by these explanatory variables, across age groups. This aspect translates into an exponential relationship between arterial stiffness and age, which indeed seems to be unreliable in the general population. It is important to note that the relative contribution on o-PWV of age and SBP found here, refers to the devices we tested and might not be reliable for other oscillometric devices [29–32].
In our study, we also explored factors potentially affecting PWV values by the different methods used, confirming that as expected, in the general population cf-PWV measurements were significantly affected by age and systolic blood pressure. However, the multivariate analysis revealed a weak influence of age2 and SBP on cf-PWV measurements, which accounted for 34% of the model, revealing any significant action of diastolic blood pressure and heart rate.
Our results, indicating a strong dependence of o-PWV estimates on age and SBP (r2 0.88), confirm data of previous studies, in which a comparable correlation was found [21,22,33].
In a recent study, investigating the major determinants of o-PWV, the statistical model predicting o-PWV from age and SBP was also extremely robust (r2 0.97), and a nonlinear effect of age, that was best captured by the addition of a quadratic term, was also found, resulting in an equation with a very high r2 of 0.99 [22]. Moreover, these results are in line with findings of previous studies in which o-PWV was highly associated with age and SBP in an unselected sample of subjects (r2 of respectively 0.98 and 0.99) [31] and in subjects with an accelerated vascular aging process (r2 for age and systolic BP of 0.98) [22].
The oscillometric device tested in our study for the assessment of PWV was an automated arm cuff-based BP monitoring device, able to provide PWV values thanks to an inbuilt technique called ARCSolver (Austrian Institute of Technology, Vienna, Austria). As stated by the producer the algorithm used for the o-PWV estimate is based on three integrated components: age, systolic blood pressure, and data derived from pulse wave analysis [18]. However, considering the derivative nature of o-PWV estimates, which could be the underlying cause of the strong association with age and blood pressure, and despite the close relationship between o-PWV values assessed by Mobil-O-Graph and carotid-femoral PWV measurements, the reliability of o-PWV assessments was recently questioned [22,33,34].
In the present study we also sought to determine the sensitivity and specificity of the oscillometric PWV estimates, and the cut-off values of o-PWVs, that most accurately predict high-risk cf-PWV as measured by the SphygmoCor system. Our results showed that o-PWVs had a moderate predictive value for high-risk arterial stiffness, with an AUC for in office, 24 h, daytime and night-time o-PWV of 0.740, 0.697, 0.694 and 0.689 respectively;all o-PWVs showing a moderate specificity and sensitivity. This aspect should be taken into account in everyday clinical practice, because the possibility of high percentage of false negatives which could result using oscillometric methods for the arterial stiffness estimation, could become relevant and provide unreliable results in specific cases of accelerated arterial ageing. Therefore o-PWVs should be considered prudently, when a subclinical vascular damage, beyond blood pressure and age, and expressed by high pulse wave velocity is suspected.
In the present study, we performed an extensive comparison between cf-PWV measurements and different o-PWV estimates provided by the oscillometric Mobil-o-Graph device (in-office, 24 h, daytime, and night-time respectively). We are however aware, that the only two directly comparable parameters are the in-office o-PWV and the cf-PWV; values referring to the same environmental and temporal circumstances (same time of the day, same office, and same intra-individual conditions). However, considering the limited number of previous studies, which explored the different o-PWV estimates provided during the 24 h, and with the aim of better clarifying which o-PWV measurement could be more accurate in estimating cf-PWV, we decided to perform a complete comparison.
Previous studies have demonstrated that oscillometric PWV measurements (in-office and 24-h) underestimate cf-PWV, with differences more pronounced for the 24 h o-PWV (in-office mean difference 0.3 ± 1.1; 24-h mean difference 0.6 ± 1.3) [17]. In addition recent findings confirmed significantly lower 24-h o-PWV mean values compared with SphygmoCor in-office [21].
The cited studies were however performed in small samples of subjects and no data at the population level exploring the entire 24-h o-PWV estimates have been published until now.
In our study, we compared two non-invasive devices for the assessment of PWV based on two different techniques, a tonometric and an oscillometric one. As discussed above, several studies have previously confirmed a good agreement between the two methods and with the invasive intra-aortic measurements (gold standard) [33,35–38]. It is however important to note that the two techniques use two different methodologies for the assessment of PWV, which can in part explain the difference in the PWV estimates provided [39,40]. The first difference is that the tonometric cf-PWV assessment with SphygmoCor analyzes the pulse wave of the carotid and femoral arteries, estimating the delay with respect to the ECG wave and calculating the PWV. This procedure implies the measurement of two distances: sternal-notch femoral site and sternal-notch carotid site. On the other hand, the oscillometric assessment of PWV with Mobil-o-Graph, is based on the pulsatile pressure changes in the brachial artery, a subsequent analysis of the pulse wave and the extrapolation of the central blood pressure using the ARCSolver algorithm [18], integrated into the Mobil-O-Graph software (HMS). Another important difference between methods is that the two devices assess the arterial stiffness at different arterial sites, which imply different arterial wall characteristics, especially for the elastic properties (predominantly elastic versus muscular in the central versus peripheral arteries) and discrepancies in the progression of arterial ageing (with a more rapid PWV progression in elastic arteries in the elderly) [41–43]. It could be hypothesised that the differences in PWV estimates provided by the two devices could be at least partially explained by the above mentioned device-related peculiarities.
Although our large sample gave us the opportunity to perform a detailed comparative analysis between cf-PWVs and o-PWVs, we have to acknowledge several limitations. This study lacks a comparison to the invasive measurement of PWV, which was obviously not performed in our low-risk outpatient population. A small number of patients in the age group under 30 years of age were enrolled. In-office tonometric and oscillometric assessments, for feasibility, were not performed in a randomised order. Even if all procedures were conducted on a resting patient, we cannot exclude contextual blood pressure changes, which could affect PWV results. Our results were obtained using a Mobil-O-Graph; therefore, their generalisation for other oscillometric devices can only be postulated. Results of previous large epidemiological studies have found good agreement between the tononometric and cuff-based oscillometry methods [16].
In a previous study comparing SpygmoCor and another cuff-based method for PWV assessment it was also found that the o-PWV device tended to give lower measurements than Sphygmocor at the upper end of the PWV range and higher measurements at the lower end of the cf-PWV range [30]. Some differences between devices were also found and a tendency to a fall at higher levels of arterial stiffness was highlighted. Regardless, considering the ease of use and independent prognostic value, the great potential in clinical settings of o-PWV assessment was also emphasised. Further studies are needed to investigate the comparability of these devices, allowing for a wider use in clinical practice.
Some unmeasured factors could have differently influenced cf-PWV values and o-PWVs. Moreover, the analysis of o-PWV estimates, considering the daytime and the nightime period separately, was based on an arbitrarily pre-defined hourly range, which might not adequately reflect the individual subject’s daily activities, partially limiting the reliability of the results.
A complete analysis of all risk factors potentially influencing PWV was not performed because it was beyond the scope of the original study, and lastly, these results should be confirmed by further analyses conducted in different subgroups, specifically including high cardiovascular risk subjects.
The present findings, support the intriguing idea that tonometric cf-PWV measurements provide different information than o-PWV estimates. The predictive power in terms of cardiovascular outcome of cf-PWV measurements was largely demonstrated; to date, however, the clinical significance of o-PWV and its predictive potential still have to be defined. Prospective studies could be helpful in covering the knowledge gap.
5. Conclusions
The results of our study suggest a good agreement between oscillometric and tonometric methods, for all PWV estimates provided by the oscillometric device, especially in subjects without diseases known to increase arterial stiffness or a documented vascular damage. The agreement between tonometric and oscillometric PWV values varied across age groups with a tendency to respectively underestimate and overestimate arterial stiffness in younger and older subjects even if likely to be scarcely relevant in a clinical perspective. This aspect in the present findings could be partially explained by the fact that systolic blood pressure and age represent the major determinants of oscillometric PWV estimates for the methods being investigated.
In conclusion, the oscillometric method used in the present study, provides values of PWV closely correlated with those provided by the carotid-femoral assessment, highlighting a satisfactory agreement between methods in the general population.
Acknowledgements
We would like to thank the participants in the TEST study for their valuable and active contribution and Dr. Giorgio Merlani of the Department of Public Health of the Ticino canton, for his valuable support. We would like also to thank the study nurse Natasa Bettosini for her support in the data collection, Ms. Irene Menghini e her staff for data entry and Salvatore Assenzio for the graphic art assistance.
Glossary
Abbreviations
- PWV
Pulse wave velocity
- cf-PWV
carotid-femoral pulse wave velocity
- o-PWV
oscillometric PWV
- BP
blood pressure
- SBP
systolic blood pressure
- DBP
diastolic blood pressure
- ECG
electrocardiogram
- TEST-study
Ticino epidemiological stiffness study
- STROBE
Strengthening the Reporting of Observational Studies in Epidemiology
- ROC
Receiver operating characteristic curves
- AUC
Area under the ROC curve
Funding Statement
We gratefully acknowledge the financial support Carlo Gianella Foundation for Clinical Research (Locarno, Switzerland). The funding source had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Disclosure statement
The authors declare no conflict of interest.
References
- 1.Reference Values for Arterial Stiffness’ Collaboration . Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values’. Eur Heart J. 2010;31(19):2338–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: the Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018;36(10):1953–2041. [DOI] [PubMed] [Google Scholar]
- 3.Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27(21):2588–2605. [DOI] [PubMed] [Google Scholar]
- 4.Townsend RR, Wilkinson IB, Schiffrin EL, et al. Recommendations for improving and standardizing vascular research on arterial stiffness: a scientific statement from the American Heart Association. Hypertension. 2015;66(3):698–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Safar ME, Henry O, Meaume S.. Aortic pulse wave velocity: an independent marker of cardiovascular risk. Am J Geriatr Cardiol. 2002;11(5):295–298. [DOI] [PubMed] [Google Scholar]
- 6.Laurent S, Boutouyrie P, Asmar R, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37(5):1236–1241. [DOI] [PubMed] [Google Scholar]
- 7.Mattace-Raso FU, van der Cammen TJ, Hofman A, et al. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113(5):657–663. [DOI] [PubMed] [Google Scholar]
- 8.Vlachopoulos C, Aznaouridis K, Stefanadis C.. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55(13):1318–1327. [DOI] [PubMed] [Google Scholar]
- 9.Ben-Shlomo Y, Spears M, Boustred C, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014;63(7):636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ford ML, Tomlinson LA, Chapman TP, et al. Aortic stiffness is independently associated with rate of renal function decline in chronic kidney disease stages 3 and 4. Hypertension. 2010;55(5):1110–1115. [DOI] [PubMed] [Google Scholar]
- 11.Cruickshank K, Riste L, Anderson SG, et al. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function? Circulation. 2002;106(16):2085–2090. [DOI] [PubMed] [Google Scholar]
- 12.Sigrist MK, Chiarelli G, Levin A, et al. Pulse wave velocity measurements are reproducible in multiple trained observers: a short report. Nephron Clin Pract. 2010;116(1):c60–c64. [DOI] [PubMed] [Google Scholar]
- 13.Wassertheurer S, Kropf J, Weber T, et al. A new oscillometric method for pulse wave analysis: comparison with a common tonometric method. J Hum Hypertens. 2010;24(8):498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Leeuwen-Segarceanu EM, Tromp WF, Bos WJ, et al. Comparison of two instruments measuring carotid-femoral pulse wave velocity: Vicorder versus SphygmoCor. J Hypertens. 2010;28(8):1687–1691. [DOI] [PubMed] [Google Scholar]
- 15.Wei W, Tölle M, Zidek W, et al. Validation of the mobil-O-Graph: 24 h-blood pressure measurement device. Blood Press Monit. 2010;15(4):225–228. [DOI] [PubMed] [Google Scholar]
- 16.Milan A, Zocaro G, Leone D, et al. Current assessment of pulse wave velocity: comprehensive review of validation studies. J Hypertens. 2019;37(8):1547–1557. [DOI] [PubMed] [Google Scholar]
- 17.Luzardo L, Lujambio I, Sottolano M, et al. 24-h Ambulatory recording of aortic pulse wave velocity and central systolic augmentation: a feasibility study. Hypertens Res. 2012;35(10):980–987. [DOI] [PubMed] [Google Scholar]
- 18.Hametner B, Wassertheurer S, Kropf J, et al. Oscillometric estimation of aortic pulse wave velocity: comparison with intra-aortic catheter measurements. Blood Press Monit. 2013;18(3):173–176. [DOI] [PubMed] [Google Scholar]
- 19.Weiss W, Gohlisch C, Harsch-Gladisch C, et al. Oscillometric estimation of central blood pressure: validation of the Mobil-O-Graph in comparison with the SphygmoCor device. Blood Press Monit. 2012;17(3):128–131. [DOI] [PubMed] [Google Scholar]
- 20.Berukstis A, Jarasunas J, Daskeviciute A, et al. How to interpret 24-h arterial stiffness markers: comparison of 24-h ambulatory Mobil-O-Graph with SphygmoCor office values. Blood Press Monit. 2019;24(2):93–98. [DOI] [PubMed] [Google Scholar]
- 21.Salvi P, Furlanis G, Grillo A, et al. Unreliable estimation of aortic pulse wave velocity provided by the Mobil-O-Graph algorithm-based system in Marfan syndrome. J Am Heart Assoc. 2019;8(9):e04028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwartz JE, Feig PU, Izzo JL.. Pulse wave velocities derived from cuff ambulatory pulse wave analysis. Hypertension. 2019;74(1):111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Del Giorno R, Gabutti S, Troiani C, et al. Association between HDL cholesterol and QTc interval: a population-based epidemiological study. JCM. 2019;8(10):1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–349. [DOI] [PubMed] [Google Scholar]
- 25.Bland JM, Altman DG.. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;327(8476):307–310. [PubMed] [Google Scholar]
- 26.Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021–3104. [DOI] [PubMed] [Google Scholar]
- 27.DeLong ER, DeLong DM, Clarke-Pearson DL.. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845. [PubMed] [Google Scholar]
- 28.Grillo A, Parati G, Rovina M, et al. Short-term repeatability of noninvasive aortic pulse wave velocity assessment: comparison between methods and devices. Am J Hypertens. 2018;31(1):80–88. [DOI] [PubMed] [Google Scholar]
- 29.Baier D, Teren A, Wirkner K, et al. Parameters of pulse wave velocity: determinants and reference values assessed in the population-based study LIFE-Adult. Clin Res Cardiol. 2018;107(11):1050–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shahin Y, Barakat H, Barnes R, et al. The Vicorder device compared with SphygmoCor in the assessment of carotid-femoral pulse wave velocity in patients with peripheral arterial disease. Hypertens Res. 2013;36(3):208–212. [DOI] [PubMed] [Google Scholar]
- 31.Ellins EA, Smith KE, Lennon LT, et al. Arterial pathophysiology and comparison of two devices for pulse wave velocity assessment in elderly men: the British regional heart study. Open Heart. 2017;4(2):e000645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keehn L, Milne L, McNeill K, et al. Measurement of pulse wave velocity in children: comparison of volumetric and tonometric sensors, brachial-femoral and carotid-femoral pathways. J Hypertens. 2014;32(7):1464–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salvi P, Scalise F, Rovina M, et al. Noninvasive estimation of aortic stiffness through different approaches. Hypertension. 2019;74(1):117–129. [DOI] [PubMed] [Google Scholar]
- 34.Boutouyrie P, Revera M, Parati G.. Obtaining arterial stiffness indices from simple arm cuff measurements: the holy grail? J Hypertension. 2009;27(11):2159–2161. [DOI] [PubMed] [Google Scholar]
- 35.Benas D, Kornelakis M, Triantafyllidi H, et al. Pulse wave analysis using the Mobil-O-Graph, Arteriograph and Complior device: a comparative study. Blood Press. 2019;28(2):107–113. [DOI] [PubMed] [Google Scholar]
- 36.Rajzer MW, Wojciechowska W, Klocek M, et al. Comparison of aortic pulse wave velocity measured by three techniques: Complior, SphygmoCor and Arteriograph. J Hypertens. 2008;26(10):2001–2007. [DOI] [PubMed] [Google Scholar]
- 37.Horváth IG, Németh A, Lenkey Z, et al. Invasive validation of a new oscillometric device (Arteriograph) for measuring augmentation index, central blood pressure and aortic pulse wave velocity. J Hypertens. 2010;28(10):2068–2075. [DOI] [PubMed] [Google Scholar]
- 38.Jatoi NA, Mahmud A, Bennett K, et al. Assessment of arterial stiffness in hypertension: comparison of oscillometric (Arteriograph), piezoelectronic (Complior) and tonometric (SphygmoCor) techniques. J Hypertens. 2009;27(11):2186–2219. [DOI] [PubMed] [Google Scholar]
- 39.Westerhof BE, van den Wijngaard JP, Murgo JP, et al. Location of a reflection site is elusive: consequences for the calculation of aortic pulse wave velocity. Hypertension. 2008;52(3):478–483. [DOI] [PubMed] [Google Scholar]
- 40.Ageenkova OA, Purygina MA.. Central aortic blood pressure, augmentation index, and reflected wave transit time: reproducibility and repeatability of data obtained by oscillometry. Vasc Health Risk Manag. 2011;7:649–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Segers P, Kips J, Trachet B, et al. Limitations and pitfalls of noninvasive measurement of arterial pressure wave reflections and pulse wave velocity. ARTRES. 2009;3(2):79–88. [Google Scholar]
- 42.Majesky MW. Developmental basis of vascular smooth muscle diversity. Arterioscler Thromb Vasc Biol. 2007;27(6):1248–1258. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y, Agnoletti D, Protogerou AD, et al. Characteristics of pulse wave velocity in elastic and muscular arteries: a mismatch beyond age. J Hypertens. 2013;31(3):554–559. discussion 559. [DOI] [PubMed] [Google Scholar]