Abstract
Cardiac troponins (cTn) are currently the standard of care for the diagnosis of acute coronary syndromes (ACS) in patients presenting to the emergency department (ED) with chest pain (CP). However, their plasma kinetics necessitate a prolonged ED stay or overnight hospital admission, especially in those presenting early after CP onset. Moreover, ruling out ACS in low-risk patients requires prolonged ED observation or overnight hospital admission to allow serial measurements of c-Tn, adding cost. Heart-type fatty acid-binding protein (H-FABP) is a novel marker of myocardial injury with putative advantages over cTn. Being present in abundance in the myocellular cytoplasm, it is released rapidly (<1 h) after the onset of myocardial injury and could potentially play an important role in both earlier diagnosis of high-risk patients presenting early after CP onset, as well as in risk-stratifying low-risk patients rapidly. Like cTn, H-FABP also has a potential role as a prognostic marker in other conditions where the myocardial injury occurs, such as acute congestive heart failure (CHF) and acute pulmonary embolism (PE). This review provides an overview of the evidence examining the role of H-FABP in early diagnosis and risk stratification of patients with CP and in non-ACS conditions associated with myocardial injury.
Key messages
Heart-type fatty acid-binding protein is a biomarker that is elevated early in myocardial injury
The routine use in the emergency department complements the use of troponins in ruling out acute coronary syndromes in patients presenting early with chest pain
It also is useful in risk stratifying patients with other conditions such as heart failure and acute pulmonary embolism.
Keywords: HFABP, heart-type fatty acid-binding protein, review, acute coronary syndrome, troponin, congestive heart failure, prognosis
Introduction
Chest pain (CP) is a common presenting complaint in emergency departments (ED), accounting for >5% of all visits, >7.5 million ED encounters/year in the US alone [1]. The most pressing concern in patients with CP is identifying acute coronary syndrome (ACS), i.e. patients with acute myocardial infarction (AMI) or unstable angina (UA), for a rapid institution of guideline-based therapy. Significant improvements in this regard over the last two decades have led to rates of missed AMI of less than 1–2% [2–4]. Conversely, among all-comers with CP, over half have “non-specific” CP, with 30% being admitted to the hospital and barely5% eventually diagnosed with ACS, costing billions of dollars in unnecessary diagnostic testing and hospital stays [5].
Risk assessment of CP centers on history, electrocardiogram (EKG), and biomarkers. Aspartate transaminase (AST) was the first biomarker used in defining AMI in 1959 [6]. Since then several legacy biomarkers, including lactate dehydrogenase (LDH), myoglobin, creatine-kinase (CK), its cardiac-specific iso-enzyme CK-MB, were used, but they have been superseded by cardiac troponins (cTn) [7]. Though proven to be the most sensitive and specific biomarker, cTn still leaves important gaps. First, there is a 4–6 hours delay from symptom-onset to first appearance of measurable cTn in plasma. This often necessitates overnight stay for many patients to allow serial measurements before AMI can be reliably ruled out, thus increasing hospitalisations and health care costs [8]. The use of high-sensitivity cardiac troponin (hs-Tn), does offset this delay to a certain degree, but at the cost of high false-positives. Second, prolonged elevation of plasma cTn (7–10 days) after an AMI complicates utility as a marker of early re-infarction. To address these gaps, a host of novel biomarkers-including structural proteins, enzymes of energy metabolism, inflammatory markers, cell-adhesion molecules, and extracellular matrix proteins have been investigated. More prominent among these include heart-type fatty acid-binding protein (H-FABP), glycogen phosphorylase isoenzyme-BB (GPBB), copeptin, and ischaemia-modified albumin, among others [9,10]. Among these, (H-FABP) is oldest known, and hence perhaps the most well-studied.
The current review aims to: (i) put in perspective the current literature comparing H-FABP to cTn and hs-Tn, (ii) offer insights as to whether H-FABP still has a role as a marker of myocardial injury in the current era of cTn, and if so, the population most suited for it, and (iii) briefly review some emerging, non-ACS indications for H-FABP use.
Tissue distribution and plasma kinetics of H-FABP
Fatty acid-binding proteins (FABP) are members of the lipid-binding proteins superfamily. They are both membrane-bound – aiding cellular long-chain fatty acid (FA) uptake – and cytoplasmic, being crucial to intracellular transport of FAs to sites of metabolic conversion. Hence, FABPs are ubiquitous, though especially abundant in tissues with an active FA metabolism, including heart, kidneys, brain, and mammary glands, among others [11]. Among nine tissue-specific cytoplasmic FABPs identified so far, FABP-3 is predominantly distributed in cardiac myocytes and is also named heart-type fatty acid-binding protein (H-FABP) [12]. However, the myocardial tissue-specificity of H-FABP is not absolute, significant amounts being present in skeletal muscle, kidneys, mammary glands, testes, lungs and stomach [13,14].
Plasma kinetics of H-FABP reflects small size (15 kDa), and abundant existence in freely soluble form in the cardiomyocytecytoplasm, in contrast to cTn, which is largely bound to the contractile elements of the cardiomyocyte. Hence, significant myocardial injury or even necrosis has to occur before cTn is released into the plasma in quantities detectable by standard assays. The abundance and freely soluble cytoplasmic location of H-FABP are evidenced by the fact that plasma H-FABP concentrations in response to myocardial injury rise to >100 times the plasma concentration of cTn, hence the normal cut-off of 5–7 ng/ml versus ≈0.05 for the latter (Tables 1 and 2). Whilst CK-MB and cTn are undetectable for around 4–6 h after symptom-onset, peak at around 12 h, and return to baseline at 24–72 h and 7–10 days, respectively [39], plasma H-FABP levels start rising within one hour, peak at 4–6 h, and return to baseline around 24 h after myocardial injury, owing to rapid renal clearance [40,41]. The distinct plasma kinetic profile offers two theoretical advantages, i.e. (i) enhanced utility as an earlier biomarker of AMI, and (ii) utility as a marker of re-infarction. Moreover, given the predominant presence in soluble form, even minor myocardial ischaemia and injury should cause detectable plasma elevations of H-FABP. Hence, beyond aiding early diagnosis of AMI, H-FABP may help identify troponin-negative high-risk patients with CP, and hence refine risk-stratification of such patients.
Table 1.
Comparative sensitivity and specificity of HFABP versus troponin by time of symptom onset in evaluation of acute chest pain.
| First author, year. (End-point) | Population (N) | Plasma cut-off (ng/ml) |
Time to s/s onset | HFABP |
Tn |
HFABP + Tn |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sens. (NPV) % | Spec. (PPV)% | AUC | Sens. (NPV)% | Spec. (PPV)% | AUC | Sens. (NPV) % | Spec (PPV) % | |||||
| Tn | HFABP | |||||||||||
| Haastrup, 2000 [15] | Typical CP (130) | 0.5 | 8 | 2.3 (0–6) h | 76 (95) | 83 (46) | NR | 33 (88) | 96 (64) | NR | NR | NR |
| 1.0 | 12 | 76 (95) | 96 (80) | 19 (86) | 98 (67) | |||||||
| NSTEMI = 12.3% | 2.0 | 15 | 62 (93) | 97 (81) | 19 (86) | 99 (80) | ||||||
| Seino, 2003 [16] (AMI) | CP + Non-diagnostic EKG (371) | NR (TnT) | 6.2 | <2 h | 89 (80) | 52 (69) | 0.72 | 22 (50) | 94 (80) | NR | NR | NR |
| AMI= 49% | 2–4 h | 96 (91) | 45 (68) | 0.84 | 57 (65) | 70 (91) | NR | |||||
| 4–6 h | 100 (100) | 40 (57) | 0.96 | 67 (71) | 66 (61) | |||||||
| 6–12 h | 97 (97) | 55 (55) | NR | 94 (95) | 68 (62) | |||||||
| 12–24 h | 95 (90) | 53 (70) | NR | 95 (92) | 65 (76) | |||||||
| Seino, 2004 [17] (AMI) | CP+↑ST or ↑Tn (129) | NR (TnT) | 6.2 | <3 h | 100 (100) | 63 (44.4) | NR | 50 (86.7) | 96.3 (80) | NR | NR | NR |
| AMI = 24% | 3–6 h | 75 (93.8) | 93.8 (75) | 0 (78.9) | 93.8 (0) | NR | ||||||
| 6–12 h | 100 (100) | 72.7 (62.5) | 60 (84.6) | 100 (100) | ||||||||
| >12 h | 100 (100) | 75 (62.5) | 100 (100) | 87.5 (76) | ||||||||
| Ruzgar, 2006 [18] (ACS) | Patients with ACS (40) | 0.01 (TnT) | 6.2 | <6 h | 95.2 | 100 | NR | 38.1 | 100 | NR | NR | NR |
| STEMI = 52% | 6–24 h | 91 | 100 | 100 | 100 | NR | ||||||
| NSTEMI= 30% | ||||||||||||
| Cavus, 2006 [19] (ACS) | Typical CP < 1 hour (67) | 0.1 (TnT) | 7 | <1 h | 97.6 | 38.5 | NR | 100 | 23.1 | NR | NR | NR |
| STEMI = 27% NSTEMI = 10% |
4 h | 97.6 | 88.5 | 100 | 88.5 | NR | ||||||
| McCann, 2008 [20] (AMI) | Ischaemic CP (415) | 0.03 (TnT) | 5 | <4 h | 73 (73) | 71 (71) | 0.77 | 55 (68) | 95 (92) | 0.78 | 85 (83) | 69 (73) |
| STEMI = 18% NSTEMI = 30% |
≥4 h | 78 (75) | 56 (61) | 0.74 (all) | 88 (90) | 94 (92) | 0.88 (all) | 98 (97) | 55 (66) | |||
| Valle, 2008 [21] (AMI + ACS) | Suspected ACS (419) | NR (TnT) | 7 | 74 ± 51 min | 60 (80) | 88 (72) | NR | 19 (69) | 99 (97) | NR | NR | NR |
| AMI = 35% | ||||||||||||
| Orak, 2010 [22] (ACS) | Sudden CP + dyspnea/syncope/nausea / vomiting <6 h duration (83) | 0.01 (TnI) | 2 | <3 h | 100 | 75 | 0.967 (<6h) | 100 | 20 | 0.556 | NR | NR |
| 3–6 h | 97 | 68 | 75 | 21 | (<6 h) | NR | ||||||
| STEMI = 58% NSTEMI = 6% |
<6 h (all) | 98 | 71 | 77 | 20 | |||||||
| Haltern, 2010 [23] (AMI) | Ischaemic-type CP (94) | 0.03 (TnT) | 7.3 | <4 h | 86 (92) | 66 (50) | 0.76 (<4h) | 42 (81) | 100 (100) | 0.71(<4h) | 93 (96) | 66 (52) |
| STEMI = 12% | >4 h | 59 (72) | 64 (50) | 0.71 (all) | 100 (100) | 100 (100) | 0.87(all) | 100 (100) | 64 (63) | |||
| NSTEMI = 16% | NR | |||||||||||
| Kim, 2010 [24] (AMI) | CP suggesting AMI (117) | 0.1 (TnT) | 19 | <2 h | 60 | 77.4 (all) | 0.78 (all) | 20.2 | 98.1 (all) | 0.82 (all) | 33.3 | NR |
| 2–4 h | 64.7 | 17.7 | 82.4 | NR | ||||||||
| AMI-55% | 4–6 h | 91.7 | 58.3 | 91.7 | ||||||||
| 6–12 h | 88.9 | 66.7 | 80 | |||||||||
| McMahon, 2012 [25] (AMI) | CP considered cardiac (1128) | 0.37 (TnI) | 5.24 | <3 h | 64.3 (93) | 84.2 (43) | 0.84 | 50 (92) | 93.3 (60) | 0.76 | 71.4 (94) | |
| AMI = 10% | 3–6 h | 85.3 (97) | 88.7 (55) | 0.89 | 67.6 (95) | 94.3 (66) | 0.85 | 88.2 (98) | ||||
| 6–12 h | 89.9 (98) | 93.5 (70) | 0.94 | 81 (97) | 94.2 (70) | 0.90 | 92.4 (99) | |||||
| 12–24 h | 90.1 (99) | 91.4 (56) | 0.97 | 95.8 (99) | 94.3 (71) | 0.98 | NR (100) | |||||
| Garcia-Valdecasas, 2011 [26] (AMI) | Ischaemic CP within 6 h (165) | 0.6 (TnI) | 6.2 | <3 h | 81 (82) | 53 (52) | 0.73 | 6 (61) | 98 (67) | 0.66 | NR | NR |
| AMI = 40% | <6 h | 81 (80) | 50 (52) | 0.72 | 25 (65) | 91 (64) | 0.66 | NR | ||||
| Aldous, 2012 [27] (AMI) | CP s/o AMI w/o STEMI | 0.028 (TnI) | 60 | ≤4 h | 50 (90.7) | 89.8 (47.6) | NR | 85 (95.1) | 94.7 (81) | NR | 86.7 (97.2) | 87 (55.3) |
| NSTEMI = 15.6% | ||||||||||||
| Gerede, 2015 [28] (AMI) | Ischaemic-type CP >30 min duration (48) | 0.04 (TnI) | 7 | <3 h | 89 (86) | 100 (100) | NR | 33 (50) | 100 (100) | NR | NR | NR |
| NSTEMI = 50% | 3–6 h | 70 (73) | 89(88) | 70 (67) | 67 (70) | NR | ||||||
| >6 h | 100 (100) | 89 (83) | 100 (100) | 89 (83) | ||||||||
| Vupputuri, 2015 [29] (AMI) | CP suggestive of ischaemia (77) | 0.14 (TnI) | 6.4 | ≤6 h | 100 (100) | 85.7 (88.2) | NR | 46.1 (63.6) | 100 (100) | NR | NR | NR |
| AMI > 50% | >6 h | 78.6 (62.5) | 45.5 (64.7) | 78.6 (78.6) | 100 (100) | |||||||
Time to symptom onset reported as Mean (SD) or Median (IQR), as applicable. NPV and PPV were calculated in case of Seino 2003 using published raw data.
AM: Acute myocardial infarction; ACS: acute coronary syndrome; CP: Chest pain; NSTEMI: non ST-elevation myocardial infarction; s/o-symptoms of; STEMI: ST-elevation myocardial infarction; NR: not reported.
Table 2.
Test characteristics of H-FABP versus hs-Tn in those presenting to ED with CP, stratified by time to symptom onset.
| First author, Year | Population (N), prevalence of AMI | Plasma cut-off (ng/ml) |
Time to s/s | HFABP |
hs-Tn |
hs-Tn + H-FABP |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sens. (NPV) | Spec. (PPV) | AUC | Sens. (NPV) | Spec.(PPV) | AUC | Sens. (NPV) | Spec. (PPV) | |||||
| hs-Tn | HFABP | |||||||||||
| Inoue, 2011 [30] (ACS) | CP > 20 min (432) | 0.014 | 6.2 | <2 h | 79 (42) | 66 (87) | 0.70 | 72 (40) | 57 (84) | 0.68 | NR | NR |
| STEMI = 52% | (hs-TnT) | <3 h | 73 (41) | 61 (87) | 0.69 | 77 (38) | 48 (83) | 0.67 | ||||
| NSTEMI = 9% | <4 h | 76 (43) | 73 (93) | 0.74 | 83 (44) | 52 (86) | 0.72 | |||||
| <6 h | 80 (49) | 65 (88) | 0.74 | 85 (52) | 57 (86) | 0.72 | ||||||
| Eggers, 2012 [31] (AMI) | CP + no STEMI (360) | 0.014 | 5.8 | <4 h | 28.6 | 73.5 | ||||||
| NSTEMI = 35.6% | (hs-TnT) | <8 h | 39.1 (73.8) | 94.8 (94.8) | 0.80 | 78.9 (86.5) | 74.6 (63.1) | 0.84 | 79.7 (86.9) | 74.6 (63.4) | ||
| Aldous, []2012 [27] (AMI) | CP s/o AMI, no STEMI (384) | 0.014 | 6 | <4 h | 50 (90.7) | 89.8 (47.6) | NR | 90 (97.7) | 79.6 (45) | NR | 90 (97.5) | 73.5 (38.6) |
| NSTEMI = 15.6% | (hs-TnT) | |||||||||||
| Kitamura, 2013 [32] (AMI) | S/S suggestive of AMI (85) | 0.014 | 6.2 | <2 h | 38 (57) | 93 (86) | 0.70 | 25 (40) | 57 (40) | 0.48 | NR | NR |
| NSTEMI = 12% | (hs-TnT) | 2–4 h | 88 (67) | 75 (92) | 0.95 | 100(100) | 75 (93) | 0.94 | ||||
| STEMI = 43% | >4 h | 50 (84) | 100 (100) | 0.81 | 100 (100) | 81 (67) | 0.96 | |||||
| Shortt, 2013 [33] (AMI) | Possible ACS s/s < 6 h (163) | 0.014 | 5.2 | <6 h | 43 (90) | 80 (24) | NR | 86 (97) | 63 (25) | NR | 86 (96) | 54 (21) |
| AMI = 8.6% | (hs-TnI) | |||||||||||
| Shoenenberger, 2014 [34] (AMI) | CP s/o AMI (105) AMI = 32.4% | (hs-TnT) | 5.76 | <1 h | 58.8 (83.3) | 98.6 (95.2) | 0.84 | 70.6 (85.9) | 85.9 (70.6) | 0.88 | NR | NR |
| Bank, 2015 [35] (ACS) | CP s/o ACS, no STEMI (453) | 0.014 (hs-TnT) | 7 | <3 h | 47 (76) | 85 (61) | 0.73 | 63 (83) | 92 (81) | 0.86 | 69 (84) | 83 (68) |
| 3–6 h | 68 (89) | 79 (50) | 0.78 | 64 (89) | 89 (64) | 0.86 | 71(86) | 79 (59) | ||||
| NSTEMI = 23% | >6 h | 59 (77) | 77 (59) | 0.73 | 87(92) | 92 (80) | 0.91 | 87 (91) | 72 (64) | |||
| Gami, 2015 [36] (AMI) | CP <6 h (88) | 0.014 (hs-TnT) | 5 | <6 h | 85 (90) | 88 (82) | 0.89 | 94 (94) | 62 (61) | 0.8 | 100 (100) | 88.9 (85) |
| AMI = 38.6% | ||||||||||||
| Kellens, 2016 [37] (AMI) | Typical CP (152) | (hs-TnT) | 5.3 | 158 min | 54 (52) | 84 (85) | 0.79 | 72 (61) | 73 (82) | 0.83 | 82 (70) | 71(83) |
| STEMI = 33% | (median) | |||||||||||
| NSTEMI = 30% | ||||||||||||
| Agnello, 2017 [38] | CP < 1-h duration + normal cTn (n = 28 CP + 28 controls) | (hs-TnI) | 6.1 | <1 hour | 55.5 (67) | 89.2 (83) | 0.65 | 34 (60.4) | 100 (100) | 0.80 | NR | NR |
ACS: Acute Coronary Syndrome; AMI: Acute myocardial infarction; CP: Chest pain; NSTEMI: Non ST-Elevation Myocardial Infarction; S/o: Symptoms of; STEMI: ST-Elevation Myocardial Infarction; NR: Not Reported.
Data from Inoue et al. was extracted from graphs using the online graph reader tool (www.graphreader.com).
H-FABP versus cTn as biomarker of AMI
H-FABP versus cTn: sensitivity, specificity and accuracy
First recognised as a potential marker of myocardial damage in the late 1980s–early 1990s [40,42,43], the following decade saw H-FABP easily surpassing the legacy markers (CK-MB and myoglobin), especially early after symptom onset [44,45]. However, the rapid development of cTn assays in the late 1990s, after demonstration of excellent sensitivity and specificity in the eventual diagnosis of AMI, led to the adoption of cTn in the universal definition of AMI in 2000, and relegated H-FABP to the background.
Early comparisons between H-FABP and cTn, before the latter became part of universal definition of AMI, revealed H-FABP far exceeding the sensitivity of cTn, especially in those presenting ≤3-hours of symptom-onset in both high-risk and low-risk cohorts [15–17]. Notably, using cTn to define AMI led to a decrease in H-FABP’s sensitivity and an increase in that of cTn, though the former remained significantly better [16]. As noted in Table 1, there is significant heterogeneity in findings across studies, likely due to small sample sizes, different cut-off values, specific cTn and H-FABP assays used, population characteristics, definition of end-points (AMI versus ACS), and time to symptom-onset, among others. Nevertheless, there is certainly consistency regarding the superior sensitivity of H-FABP over cTn, especially early after symptom-onset, with cTn catching up or exceeding H-FABP after about hour 4–6. On the other hand, cTn remains more specific at all times. Consolidating the evidence, several meta-analyses have confirmed a higher sensitivity for H-FABP in early diagnosis of AMI, though at the cost of a lower specificity [46–48]. Combining the two markers improved sensitivity, albeit at the cost of even lower specificity [47,48]. The better sensitivity and lower specificity of H-FABP translates to a similar or only marginally better overall test accuracy over cTn in most reports. Of note, almost all investigations after 2004 use cTn elevation to define AMI, making it difficult and perhaps impossible for a novel marker to exceed cTn in test accuracy, and more importantly, likely underestimating the true sensitivity of H-FABP.
H-FABP versus cTn: predictive values and role of population characteristics
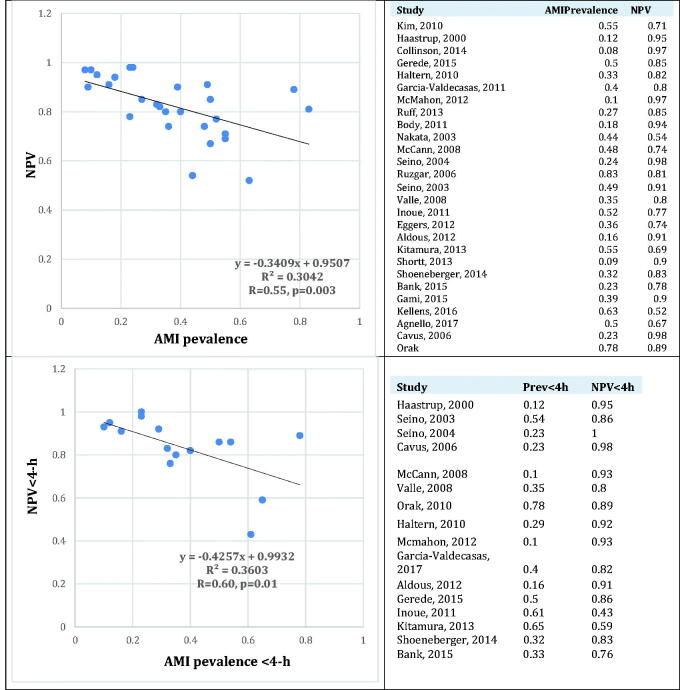
Though sensitivity, specificity, and receiver operating characteristics-area under the curve (ROC-AUC) are useful measures of a test’s fundamental credentials when compared to a “gold standard”, they are little help in guiding clinical decisions. To that end, negative and positive predictive values (NPV and PPV respectively) are much more relevant, since they directly depict the probability of a negative or positive test indicating the absence or presence of a disease, respectively [49]. In the report by McCann et al., for example, H-FABP had a NPV of 73% in 415 patients presenting within 4-hours of symptom-onset, given an AMI prevalence of 50% [20]. Changing prevalence to a more realistic 5%, while keeping sensitivity and specificity unchanged, the NPV of H-FABP increases to 98.5%. Even a conservative 10% prevalence yields an NPV of 95.5%.
To demonstrate this more clearly, we extracted raw data of AMI prevalence, time to symptom-onset, and H-FABP test characteristics from reports in Tables 1 and 2. To study whether, and to what extent the first two could explain the observed heterogeneity in NPV among these reports, we regressed AMI prevalence (independent variable) against overall NPV of H-FABP(dependent variable) using the Analyze-it Tool Pak in Microsoft Excel 2016. As expected, there was a moderate correlation (R=–0.55, p = .0003) between AMI prevalence and NPV (Figure 1, upper panel), with prevalence accounting for ≈1/3 (R2=0.3042) of the variability in NPV. Obviously, given the rapid rise and decline of H-FABP, time from symptom-onset should also influence NPV. To isolate the effects of time and AMI prevalence, Figure 1 (lower panel) displays the relation between prevalence of AMI and NPV only among early presenters (<3–4 h), this time revealing an even stronger correlation between the two (R= −0.60, p = .01), with prevalence accounting for over one-third the variability in reported NPVs (R2=0.36).
Figure 1.
Correlation between AMI prevalence and negative predictive value overall (upper panel), and among those presenting <4-h after symptom-onset (lower panel).
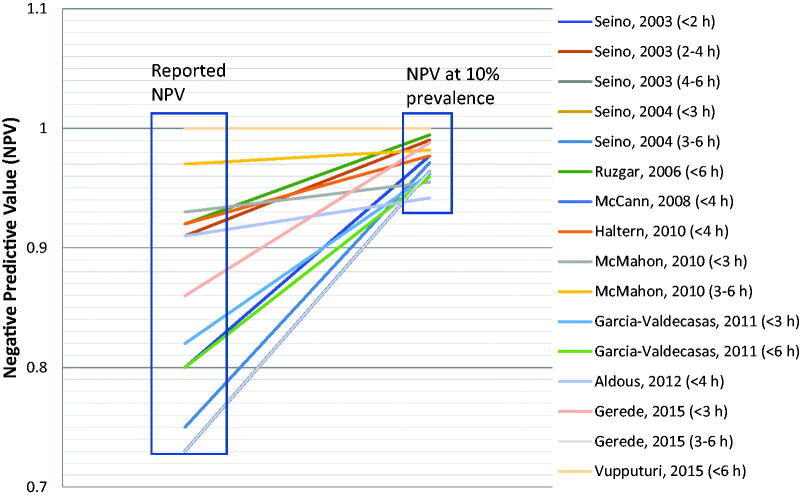
It should be noted that since most reports did not report AMI prevalence in each time from symptom onset sub-group, we assumed that AMI prevalence in each sub-group was not significantly different from the overall prevalence. Though a possible source of error, we believe that the prevalence of AMI among early presenters should indeed be higher than late presenters. Hence our assumption, even if erroneous, should result in an error on the conservative side, i.e. underestimate the correlation between the two variables rather than overestimate it. Put another way, using the same raw data, Figure 2 depicts the effect of changing AMI prevalence to 10% on NPV among early presenters, keeping sensitivity and specificity unchanged. We again assumed a similar AMI prevalence between early presenters and the entire. As expected, NPV uniformly increases markedly in each case. Much more importantly, heterogeneity among these reports almost disappears, revealing an NPV of >95% consistently across reports.
Figure 2.
Reported NPV and NPV if prevalence of AMI in each report were to be 10%. NPV at 10% prevalence calculated using the formula: NPV= Raw data of AMI prevalence and NPV were obtained from studies in Table 1.
H-FABP is most suited to rule out AMI in low-risk early presenters
The clear association between AMI prevalence and NPV, as well as the impact of a “real-world” prevalence of AMI on NPV of H-FABP, as suggested by Figures 1 and 2, respectively, offer clues regarding causes of heterogeneity in the literature, while aiding clinical application of H-FABP. Indeed, H-FABP may be best suited for ruling out AMI in low-risk patients presenting early after CP onset (<4 h).
Directly supporting this, a few large cohorts with AMI prevalence ≤10% have consistently found very high NPVs for H-FABP [25,50]. McMahon et al. reported an NPV of 93% for H-FABP versus 92% for cTn in a large cohort with an AMI prevalence of 10% among those presenting within 3-hours of symptom-onset [25]. In the RATPAC trial, a low-risk (8% ACS prevalence) cohort of 850 patients presenting to the ED at a median 220 min after symptom onset, admission H-FABP had an NPV of 97% versus 98% for cTn [50]. However, NPV for the early presenter (≤3 h) cohort was not reported by the RATPAC authors, neither was raw data available to allow calculation of NPV for this sub-group.
Hence, when applied to a more real-world population and early presenters, H-FABP does indeed seem to consistently show a very high NPV, providing a potential tool to “rule out” AMI during the early window of cTn-negativity. To put this in further perspective, a clinically acceptable marker should not exceed the current “accepted” rate of missed AMI, i.e. 1–2%, dictating the need for an NPV ≥98% [51]. In this regard, Body et al. reported an NPV of 98.8% when H-FABP and cTn were combined in clinically low-risk patients, enabling AMI to be ruled out at presentation in ≈45% of all patients, at the cost 6 AMIs missed per 1000 patients, a miss rate of 0.6% [52].
Finally, given the staggered time course of the two markers’ rise in plasma, combining them may aid in better assessing the onset time of ischaemia. Hence, patients with an uncertain time of symptom-onset, a positive H-FABP with a normal cTn will likely mean duration of symptoms of 0–4 h, whereas a normal H-FABP with elevated cTn would indicate the ischaemic event having occurred >24 h previously. Such knowledge/assessment could have important implications on individualising treatment strategies.
On the other hand, the specificity and PPV of H-FABP is consistently lower than cTn, regardless of time from symptom-onset. Given the high prevalence of AMI in populations studied, PPV can only be expected to be much lower in a real-world population, making it largely unsuited, or at the very least inferior to c-Tn for confirming AMI, patients deemed high risk based on history and/or EKG. This low specificity is likely multi-factorial, including known elevations of H-FABP in those with renal disease, skeletal muscle disorders/trauma, and myocardial injury of diverse aetiologies like heart failure, pulmonary embolism (see later), etc.
Finally, two other factors need to be considered when interpreting extant evidence. Firstly, using the comparator biomarker (cTn) itself as the diagnostic gold standard for the outcome (AMI) being studied, as has been the case in the majority of reports in the last two decades is flawed. Intuitively, test characteristics of cTn will have a direct influence on the test characteristics of H-FABP, independent of all other factors. In particular, low specificity of cTn will impact the sensitivity of H-FABP, since some false-positives (due to low specificity) on cTn-testing will be erroneously deemed false-negatives (hence lower sensitivity)on H-FABP-testing. Indeed, Seino et al. found a decline of H-FABP’s sensitivity by about 5–10% for all time points after symptom-onset [16], and a recent meta-analysis reported H-FABP having a sensitivity of 76% when cTn was sued to diagnose AMI, versus 91% otherwise [46].
The second issue pertains to the relative assay quality of the two markers. Given that cTn has become the standard of care, and in fact now defines AMI, there is obviously a greater commercial interest in advancing cTn assays, rather than a novel test that bears significantly greater burden of evidence to bring. As a result, cTn assays have constantly improved in sensitivity and precision, while H-FABP assays have seen little change [53]. Indeed, most studies use point-of-care, semi-quantitative H-FABP assays, which detect either normal or elevated H-FABP above a cut-off set at ≈6ng/ml. This is problematic since such tests may suffer inert-observer variability in result interpretation (usually colour development), as well as the inherent inability to distinguish moderate from high levels of the marker. Even with these early generation assays, H-FABP has proven to be equally sensitive as even the latest generation hs-Tn. Hopefully, novel, highly sensitive and precise H-FFABP assays will aid this marker to realise its full promise.
H-FABP vs cTn diagnosis and prognosis of unstable angina (UA)
UA and NSTEMI represent a continuum, with the boundary between them constantly changing as precision and sensitivity of biomarkers has advanced. In essence, many patients who were classified as UA in the era of CK/CK-MB, are now classified as NSTEMI, due to the much higher sensitivity of cTn. Biomarker release likely begins even with minor myocardial injury, but may not cross the assay threshold or diagnostic cut-off. A soluble marker like H-FABP rises early and to a greater degree than a structurally bound marker like cTn, the latter requiring significant necrosis before release. Given the continuum of severity and amount of myocardial injury in patients with UA, some patients will have enough biomarker leak to cross the detection threshold of an assay, while some may not. This would especially be true of semi-quantitative assays as have mostly been used for H-FABP.
Seino et al. found admission H-FABP elevated in 24/51 patients with UA (14/51 had elevated cTn), whereas Cavus et al. found admission and 4-hour H-FABP elevated in only 1/42 patients with UA [16,19]. Compared to hs-Tn, Eggers et al. found mean admission H-FABP levels not significantly different from those without ACS, whereas mean hs-Tn levels were, though even the latter were elevated in only 18/68 patients with UA [31]. Besides being generally small in size, each of these reports had differing definitions of UA, and variable proportions of patients with confounding illnesses, like renal failure, heart failure, tachy-arrhythmias, etc. Valle et al. found the sensitivity/NPV of H-FABP to fall from 60%/80% to 47%/56%, respectively, when ACS (AMI + UA) was used as outcomes versus AMI [21]. Only 24.4% of patients with UA had elevated H-FABP versus 13.2% for cTn. However, this is controversial, as according to the universal definition of AMI, any significant rise in cardiac enzymes would class the definition as NSTEMI rather than UA.
Regardless of these barriers, H-FABP has repeatedly been demonstrated to have prognostic value incrementally to-and indeed independent of-cTn among patients presenting to the ED with ACS (see below). Though lacking direct evidence of its role in UA, largely due to inherent issues with the clinical entity itself, the enhanced prognostic ability of H-FABP likely stems from its ability to identify patients with minor myocardial injury. Definitive evidence of this would require large prospective studies excluding patients with any co-existent confounding co-morbidities, like heart failure, renal disease, tachy-arrhythmias, myo-pericarditis, etc. Indeed, maybe exquisitely sensitive markers like H-FABP, or for that matter hs-Tn, could be used to define UA when cTn is normal. Again, large scale studies to determine cut-offs for normality, and the development of high-precision assays are both fundamental to achieving this.
H-FABP in the era of high-sensitivity troponin (hs-Tn)
The last decade has seen significant improvements in cTn assays, with current generation “highly-sensitive” troponin (hs-Tn) assays able to detect very low concentrations of cTn in the plasma. In general, these assays have <10% coefficient of variation at plasma concentrations that are an order of magnitude lower than conventional cTn assays. Although largely structurally bound to the myocyte contractile elements, about 5% of cTn is present in free form in the cytoplasm [54]. Akin to H-FABP, this cytoplasmic cTn is released early after onset of ischaemic injury, but falls below the detection limit of conventional assays. Hence, hs-Tn assays, by detecting this miniscule rise early after symptom-onset have proven more sensitive than cTn, and displayed very high NPVs [55–57]. Obviously, this increased sensitivity comes at the cost of poorer specificity. Intuitively, it follows that the major improvement of hs-Tn over cTn lies largely in rapidly “ruling out”, rather than “ruling in” AMI. Therefore, hs-Tn has a role very analogous to H-FABP, i.e. shortening the window of cTn-negativity.
In one of the earliest reports of hs-Tn, H-FABP was noted to be the only biomarker among several to have equivalent diagnostic accuracy as hs-Tn in those with CP onset <3-hours, and superior to hs-Tn in those with onset <2 h [56]. Several investigations since have compared the two markers, especially early after symptom onset (Table 2). Importantly, the hs-Tn assay itself has evolved rapidly since being introduced, making it difficult to compare earlier reports to more recent ones. As with the c-Tn studies, most reports have a very high AMI prevalence. Nevertheless, and especially in more recent reports, using the latest-generation assays, hs-Tn has generally demonstrated superior sensitivity and NPV compared to H-FABP, even early after symptom onset. This increased sensitivity, however, comes at a cost of lower specificity. Overall test accuracy, as measured by the ROC-AUC, seems largely equivalent between the two markers.
In the largest cohort to date, the APACE study enrolled 1074 consecutive patients with CP suggestive of AMI [58]. H-FABP had a lower ROC-AUC than hs-Tn in the overall cohort (0.84 vs 0.94), and in those presenting <3-hours from symptom-onset (0.85 vs 0.92). Combining the two markers yielded an even lower accuracy than hs-Tn alone (ROC-AUC 0.88 vs 0.92). However, both H-FABP and hs-Tn had very high NPV (94% vs 98%, respectively), and poor PPV (41% vs 42%) with an AMI prevalence of 20%. Similar findings were reported by Collinson et al. in another large cohort of 850 low-risk patients presenting early to the ED with CP [50]. Meta-analyses seem to confirm the higher sensitivity of hs-Tn, and the relative lack of improvement with H-FABP [59,60]. However, there was significant heterogeneity among studies, and early presenters (<3–4 h from symptom-onset) – the real target population for both markers – were largely missing in both analyses, still leaving questions about the utility of H-FABP in the era of hs-Tn.
To help put these findings in a clinical context, a novel risk scoring system, the Manchester Acute Coronary Syndromes Rule (MACS), incorporating both hs-Tn and H-FABP levels, along with clinical features and EKG was developed and validated [61,62]. A recent pilot RCT found that the MACS rule enabled 26% patients to be successfully discharged from the ED within 4 h with no incident AMI in 30 days among those discharged [63]. Similarly, an analysis of a single-center arm of the multi-center ADAPT study found that when combined with EKG, H-FABP orhs-Tn alone had unacceptably low sensitivity [64]. However, in combination H-FABP + hs-Tn + EKG changes maximised rule-outs (≈41% testing negative) while maintaining >99% sensitivity. Other authors have also demonstrated the benefits of this combination approach [65].
To summarise, H-FABP may yet prove to be an important adjunct to hs-Tn, enabling an optimal balance between sensitivity (and NPV) and specificity (and PPV). Current evidence suggests that hs-Tn + H-FABP combination strategy would maximize safe discharges while minimising missed AMIs. Obviously, adequately powered RCTs examining the optimised cut-off values and timing in relation to symptom-onset are needed.
Prognostic value of H-FABP in patients with ACS
Since H-FABP may be released into the plasma following myocardial injury even without myocardial necrosis, the prognostic value of H-FABP in those with suspected ACS has been extensively studied, to identify cTn-negative patients who may be high risk, and hence warrant observation or diagnostic workup.
Ishii et al. first reported that in 328 consecutive patients with ACS (47% STEMI, 26.5% UA/NSTEMI), serum H-FABP > 9.8 ng/mL in first 6 h after CP onset, but not elevated cTn, was a strong predictor of cardiac death and non-fatal AMI within 6 months [66]. Several subsequent reports have consistently demonstrated superiority of H-FABP over c-Tn, and indeed other biomarkers in this regard (Table 3). Viswanathan et al. tested the prognostic value of H-FABP against hs-Tn for the first time, and in a lower risk population than prior studies (AMI prevalence 20.8%, STEMI excluded) [72]. Of note, both were measured in the plasma > 12 h after symptom onset. Even so, H-FABP predicted death or AMI within 18 months independent of hs-Tn levels across the entire cohort. More importantly, H-FABP >6.48 ng/ml strongly predicted 18-month death and AMI in hs-Tn-negative patients, proving generalizability of previous findings to a more “real world” cohort of unselected patients. Hence, H-FABP levels during the first hours after symptom onset have consistently been proven to identify a high-risk population, regardless of cTn (or indeed hs-Tn) levels. RCTs comparing outcomes using treatment/diagnostic algorithms based on H-FABP, either alone or as part of a multiple biomarker strategy, are needed to facilitate adoption in clinical practice.
Table 3.
Prognostic utility of H-FABP in patients with ACS.
| First Author, Year | N | Population (Time of blood sampling) | Follow up | Primary outcome | Biomarkers | Findings |
|---|---|---|---|---|---|---|
| Ishii, 2005 [66] | 328 | ACS (<6-h after s/s onset) | 6-mo | Cardiac death Cardiac events |
H-FABP c-Tn |
H-FABP predicted 6-month cardiac events (RR 8.92, 1.15–69.4) but not cTn. |
| Suzuki, 2005 [67] | 90 | ACS (Admission) | 30-d | All-cause death Cardiac event |
H-FABP Troponin T CK-MB |
HFABP predicted cardiac events at 30-days (RR 44.98, 1.48–1364.88) but not cTn or CK-MB |
| O'Donoghue, 2006 [68] | 2,287 | ACS (41 ± 20 h after s/s onset) |
10-mo | All-cause death Nonfatal AMI New/worsening CHF |
H-FABP cTn BNP Myoglobin |
H-FABP predicteddeath (HR 4.1, 2.6–6.5), CHF (HR 4.5, 2.6–7.8), MI (HR 1.6, 1.0–2.5), or all (HR 2.6, 1.9–3.5)10 months independent of cTn/BNP. H-FABP incremental to cTn/BNP for prognosis. |
| Kilcullen, 2007 [69] | 1,448 | ACS (12-24 h after s/s) | 12-mo | All-cause death | H-FABP cTn |
H-FABP ≥ 5.8 ng/ml predicted 1-yr mortality (HR 11.35, 2–64.34) in cTn-negative (UA) patients, as well in those with ↑ cTn (NSTEMI) (HR 3.11, 1.45–6.7). |
| Ilva, 2009 [70] | 293 | Suspected ACS (Median 4.7 h after s/s onset) | 6-mo | All-cause death Recurrent MI |
cTnI H-FABP |
cTnI independently predicted 6-mo death + AMI (RR 3.02, 1.62–5.63) but not H-FABP. |
| McCann, 2009 [71] | 550 | Suspected ACS (Median 6 h after s/s onset) | 12-mo | All-cause death Recurrent AMI |
H-FABP cTn NT-pro-BNP hs-CRP MPO MMP-9 others |
AmissionH-FABP(OR 2.7, 1.1–6.4), admission NT-pro-BNP (OR 2.7, 1.4-5.2), and peak cTn(OR 3.6, 1.4–9.0)independently predicted 1-yr mortality. |
| H-FABP, cTn, NT-pro-BNP had incremental prognostic value | ||||||
| Viswanathan, 2010 [72] | 955 | hs-Tn-negative suspected ACS (NR) | 18-mo | All-cause death Recurrent AMI |
H-FABP hs-Tn |
H-FABP predicted 18-month death/MI in hs-Tn (-) patients incrementally when stratified by degree of H-FABP elevation. |
| Garcia-Valdecasas, 2011 [26] | 165 | Chest pain (<6 h after s/s onset) | 6-mo | All-cause death Recurrent ACS/AMI Other cardiac events |
cTnI H-FABP CK-MB |
Increased H-FABP (HR 2.50, 1.31–4.80) and cTnI(2.53, 1.19–5.38) independently predicted 6 month outcomes. |
| Reiter, 2013 [58] | 955 | Chest pain suggestive of MI (<12-h after onset/peak of symptoms) | 12-mo | All-cause death | H-FABP Copeptin hs-Tn |
H-FABP predicted 1-yr mortalityirrespective of hs-Tn |
H-FABP in non-ACS disorders
Given the prognostic ability of H-FABP in ACS (Table 3), its utility to identify high-risk patients in other non-ACS disorders known to cause myocardial strain, and perhaps injury in severe cases, has attracted attention.
H-FABP in congestive heart failure (CHF)
Cardiac biomarkers have become integral to the management of CHF. Established and widely available biomarkers including brain natriuretic peptide (BNP) and N-terminal-pro-brain natriuretic peptide (NT-pro-BNP), are useful in diagnosis (to rule out heart failure) [73,74], ascertain prognosis [75,76], predict mortality or re-hospitalization [77–79], and possibly guide early lifestyle and pharmacologic interventions in asymptomatic at-risk patients [80,81].
There seems to be little added value or improvement with H-FABP over natriuretic peptides in confirming the diagnosis of CHF. A post hoc analysis of the MANPRO cohort reported that H-FABP levels correlated with CHF clinical severity, and with echocardiographic indices of systolic and diastolic dysfunction [82]. However, though H-FABP plus NT-proBNPhad a significant improvement in PPV compared to NT-proBNP alone (58% vs 45%, p < 0.0001), it was well short of being recommended for clinical use. There was no improvement in NPV over NT-proBNP alone. Importantly, almost 25% patients in the “no acute CHF” group had a history of chronic CHF, making it difficult to interpret findings since H-FABP and NT-proBNP are known to be raised in those with chronic stable CHF, each to a variable degree. More recently, Lichtenauer et al., in a cohort of 124 patients with systolic CHF (ischaemic and non-ischaemic), showed H-FABP as having the highest AUC (0.80, 95% CI = 0.74–0.86) among several novel inflammatory markers, though no comparison to natriuretic peptides was performed [83].
Majority of the studies investigating H-FABP in CHF have focussed on prognostic utility, both in acute decompensated and chronic stable CHF (Table 4). Notably, these reports span widely varying populations in terms of the degree of systolic dysfunction, aetiology of CHF, and clinical severity as assessed by New York Heart Association Classification (NYHA). As noted in Table 4, reports have consistently found H-FABP to be the only, or at the very least, best predictor of outcomes in those with chronic stable CHF, when compared to other commonly used biomarkers [84,90–92]. In the only prognostic study restricted to HFpEF, Kutsuzawa et al. found H-FABP to be the sole predictor of CV events among a host of clinical (age, NYHA class, hypertension, diabetes, renal function) and biochemical markers (BNP, cTn, hs-CRP) [91]. Physiologically, it may be that since BNP is a surrogate for myocardial “stretch” engendered by pressure/volume overload, while H-FABP directly depicts myocardial injury, the latter is a better predictor of adverse outcomes by virtue of indicating ongoing myocardial damage. In a small study comparing hs-Tn, H-FABP and NT-proBNP between 49 patients with HFpEF, 51 patients with asymptomatic diastolic dysfunction, and 30 controls with normal diastolic function, all three markers were elevated in HFpEF, only hs-Tn and H-FABP were elevated in asymptomatic diastolic dysfunction, indicating that subtle myocardial injury precedes the development of CHF [96].
Table 4.
Studies investigating prognostic value of H-FABP in acute decompensated CHF and chronic stable CHF.
| First Author, Year | Population (n) | Primary outcome | Follow up | Biomarker | Risk estimate, 95% CI |
|---|---|---|---|---|---|
| Setsuta, 2002 [84] | Stable chronic CHF (56) | All-cause death CHF readmission |
16 ± 12-mo | H-FABP | HR 2.6, 1.1–6.5 per 3.86ng/ml increase |
| cTn | HR 7, 1.1–44 | ||||
| BNP | NS | ||||
| ANP | NS | ||||
| CK-MB | NS | ||||
| Arimoto, 2005 [85] | Acute CHF (179) | Cardiac death CHF readmission |
20-mo | H-FABP | HR 7.39, p = 0.0065 |
| LDH | NS | ||||
| CK | NS | ||||
| Niizaki, 2005 [86] | Acute CHF (186) | Cardiac death CHF readmission |
534 ± 350 days | H-FABP | HR 5.42, 2.20–13.32 |
| BNP | HR 2.41, 1.02–5.73 | ||||
| Komamura, 2006 [87] | Chronic stable non-ischaemic DCM (92) | Cardiac death Heart transplant LV assist device |
48 months | H-FABP | RR 7.5, 0.7–36.1 |
| BNP | RR 10.9, 3.5–35.3 | ||||
| cTn | NS | ||||
| Niizeki, 2007 [88] | Acute CHF (126) | Cardiac death CHF readmission |
474 ± 328-days | H-FABP | HR 15.7, 3.8–64.5 |
| BNP | HR 2.6, 0.87–7.8 | ||||
| cTn | NS | ||||
| Niizek, 2008 [89] | Acute CHF (113) (Admission + discharge) |
Cardiac death CHF readmission |
624 ± 299 days | H-FABP (at discharge) | HR 5.7, 2–9.5 |
| BNP (at discharge) | OR 4.62, 1.49–14.33a | ||||
| Setsuta, 2008 [90] | Chronic stable CHF (103) | All-cause death CHF readmission |
28 ± 26 mo | H-FABP | HR 2.24, 1.21–4.14 |
| cTn | HR 1.95, 1.02-3.71 | ||||
| Kutsuzawa, 2012 [91] | Chronic CHF with preserved EF (151) | Cardiac death CHF readmission |
694 (29-2000) days | H-FABP | HR 1.165, 1.034–1.314 |
| cTn | NS | ||||
| BNP | NS | ||||
| hs-CRP | NS | ||||
| Hoffmann, 2015 [82] | Acute CHF (122) | All-cause death CHF readmission |
5-yrs | H-FABP | ACM-NS CHF readmit-HR 1.07, 1.02-1.13 |
| cTn | CHF readmit /ACM-NS | ||||
| NT-proBNP | CHF readmit/ACM-NS | ||||
| Otaki, 2014 [92] | Chronic stable CHF ± AF (402) | All-cause death Cardiac death CHF readmission |
643 days-AF 488 days-SR |
H-FABP-AF HFABP-SR | HR 1.57, 1.2–2 HR 1.28, 1.04–1.58 |
| cTn-AF cTn-SR |
HR 1.4, 1.13, 1.74 NS |
||||
| BNP-AF/SR | NS | ||||
| Shirakabe, 2015 [93] | CHF ± AKI admitted to ICU (NYHA III/IV) | All-cause death CHF readmission |
90-days | H-FABP | HR 5.1, 1.86–14.17 |
| hs-Tn | NS | ||||
| BNP | NS | ||||
| Kadowaki, 2017 [94] | Acute CHF (322) | Cardiac death CHF readmission |
534 (203-1014) days | H-FABP | HR 1.745, 1.088–2.7903 |
| BNP | NS | ||||
| Kazimierczyk, 2018 [95] | Acute NYHA III/IV CHF (71) Admission + discharge |
CV death CHF readmission |
9.2 ± 7.3-mo | H-FABP (at discharge) | (OR 1.3, 1.06–1.68) |
| BNP | NS |
Unless specified, risk estimates in last column are for composite end-point, and are those achieved after multi-variate analyses, including co-markers checked in each study.
aOR for Niizeki, 2008 calculated from reported raw data.
In acute decompensated CHF (Table 4), H-FABP has again been found to exceed BNP and NT-proBNP as a predictor of mortality and readmissions [89]. In fact, among admission and discharge BNP and H-FABP, only discharge H-FABP was found to predict cardiac death and CHF readmission [95]. Hence, the admission H-FABP, as well as the H-FABP response to therapy in acute CHF assessed at the time of discharge, akin to similar findings with BNP, may indeed identify candidates for aggressive therapy and close follow-up.
In summary, H-FABP may be a robust prognostic marker, both in chronic stable CHF, in acute decompensated CHF and also HFpEF, and indeed seems superior to BNP, NT-proBNP, and cTn.
Low and intermediate risk pulmonary embolism
In-hospital and early mortality in acute PE varies widely depending on the severity at presentation [97]. Those with severe or “massive” PE, as indicated by hemodynamic instability, are clearly recommended to receive immediate mechanical or chemical thrombolysis [98]. However, optimal management of non-high-risk, hemodynamically stable patients has been somewhat challenging, since this sub-group itself varies widely in terms of risk of adverse outcomes. In particular, identifying those with subclinical right ventricular strain or myocardial injury, a group with an intermediate mortality risk, has attracted much focus [99,100].
Recent guidelines recommend using a combination of clinical assessment, imaging evidence of right ventricular dysfunction (RVD), and biomarkers (cTn) to further stratify this group into low, intermediate-low and intermediate-high risk, with different management strategies for each group, i.e. home discharge with anti-coagulation, inpatient observation, or close monitoring and rescue reperfusion if needed, respectively [98]. The guidelines also opine that “the optimal, clinically most relevant combination (and cut-off levels) of clinical and biochemical predictors of early PE-related death remain to be determined, particularly about identifying possible candidates for reperfusion treatment among patients with intermediate-risk PE”, hence the ongoing search for novel markers.
The role of H-FABP in PE was first demonstrated by Kaczynska et al. in 2006, in a prospective cohort of 77 patients, including 9 with massive, 43 with sub-massive, and 25 with non-massive PE [101]. Compared to cTn, NT-pro-BNP, and myoglobin, H-FABP emerged as the only predictor of 30-day PE-related as well as all-cause mortality. These findings were quickly replicated by Puls et al. the following year, in a cohort of 107 patients [102]. Again, H-FABP was superior to cTn or NT-proBNP even when 24-hour peak levels of the latter were considered, and had additional prognostic ability over echocardiographic assessment of RV dysfunction (Table 5) summarizes subsequent reports investigating the prognostic value of H-FABP alone and against other markers in sub-massive/normotensive PE, whereby H-FABP appears to be a strong marker of adverse clinical outcomes in this population. A meta-analysis of 9 studies including 1680 patients found that elevated H-FABP levels were associated with an increased risk of RVD (OR 2.57; 95% CI, 1.05–6.33), complicated clinical course (OR 17.67; 95% CI, 6.02–51.89), and30-day PE-related mortality (OR, 32.94; 95% CI, 8.80–123.21) [110]. Compared to hs-Tn, brain natriuretic peptide (BNP), and N-terminal-pro-BNP (NT-pro-BNP), H-FABP was the strongest predictor of short-term PE-related and all-cause mortality, and had the lowest negative likelihood ratio for mortality.
Table 5.
Prognostic performance of H-FABP in low-intermediate risk acute PE.
| First Author, Year | Sample size | Primary outcome | Biomarkers | Findings (Risk estimate, 95%CI) |
|---|---|---|---|---|
| Boscheri, 2010 [103] | 101 | All-cause mortality at 6-mo | H-FABP Troponin I |
H-FABP alone predicted 30-day PE-related mortality (OR 37, 5–266). |
| Dellas, 2010 [104] | 126 | All-cause mortality at 30-days CPR Endotracheal intubation Catecholamine use |
H-FABP cTnT NT-proBNP |
H-FABP alone predicted 30-day composite outcome (OR 36.6, 4.3–308) H-FABP alone predicted long term (median 499 days) mortality (HR 4.5, 2.0–9.8) |
| Gul, 2012 [105] | 61 | All-cause mortality at 30-days | H-FABP Troponin I CK-MB |
H-FABP alone predicted 30-day mortality (OR 7.27, 1.78–29.7) |
| Lankeit, 2013 [106] | 257 | 30-day adverse outcome (death, catecholamine use, endotracheal intubation, CPR) | H-FABP cTn NY-pro-BNP |
H-FABP (OR 6.79, 2.4–19.26) stronger predictor of 30-day adverse outcomes than cTn (OR 3.47, 1.21–9.90), or NT-proBNP (OR 3.79, 1.20–11.95) |
| Gul, 2014 [107] | 80 | IHM and 30-day mortality | H-FABP cTn |
H-FABP alone predicted in–hospital (HR 6.63, 1.33–33.34) & 30-day mortality (HR 7.81, 1.59–38.34) Thrombolysis in patients with ↑ H-FABP did not improve outcomes. |
| Langer, 2016 [108] | 161 | All-cause mortality at 30-days | H-FABP CK-MB Troponin I |
HFABP (OR 27.1, 2.1–352.3) stronger predictor of 30-day mortality than CK-MB (OR 5.3, 1.3–23.3). cTn did not predict outcomes after adjusting for other variables. |
| Dellas, 2018 [109] | 716 | Death, Resuscitation, Intubation or Catecholamine use in 30 days | H-FABP sPESI Multidetector CT |
H-FABP had incremental prognostic value in low risk (sPESI = 0) and intermediate-risk (sPESI ≥1 or RVD on MDCT) patients. |
ACM: all cause mortality; CK-MB: creatinine kinase MB; IHM: in-hospital mortality; RVD: right ventricular dysfunction; sPESI: simplified pulmonary embolism severity index; MDCT: multidetector computed tomography; NT-proBNP: N-terminal pro b-type natriuretic protein.
H-FABP was tested as part of the European Society of Cardiology (ESC) guidelines algorithm for risk-stratifying patients with acute PE [109]. In 271 patients assessed to be low-risk by the simplified PE severity index (sPESI), 30-day complication rate (death, catecholamine use, mechanical ventilation, resuscitation) was 1.1%; however, those with an elevated H-FABP had a 4.3% complication rate, compared with 0.4% for those with normal H-FABP, thereby achieving significantly enhanced precision over clinical assessment alone. Hence, H-FABP seems to be a promising biomarker for risk-stratifying low-intermediate risk patients with acute PE. From the standpoint of triaging patients for thrombolysis, one small observational study did not find a difference in 30-day mortality between H-FABP-positive patients who received thrombolysis versus those who did not [107], although interventional RCTs are awaited.
Other conditions
In patients undergoing coronary artery bypass grafting (CABG), the slow rise and fall of traditional biomarkers like CK-MB and cTn makes them unsuitable to discriminate between early graft failure, on the one hand, and the expected myocardial injury resulting from the surgery itself or ischaemia-reperfusion injury on the other. Consistent with its presence in freely soluble form in the cardiac myocellular cytoplasm, H-FABP has been repeatedly found to be the earliest marker (as early as 60–90 min post-operatively) to increase post-operatively after CABG, even excluding patients with post-operative AMI [111–113]. Not surprisingly, in a prospective cohort of 1298 patients undergoing CABG, H-FABP peaked earlier, and was superior to c-Tn and CK-MB as a predictor of long-term mortality and ventricular dysfunction [114]. Hence, H-FABP may be a marker of high-risk patients and predict the requirement of closer post-operative monitoring, or more aggressive application of strategies to reduce ischaemia-reperfusion injury.
Given known tissue distribution patterns, H-FABP was also thought to have potential value in diagnosis and prognostication in neurologic disorders, most prominently ischaemic stroke and traumatic brain injury (TBI). In the context of ischaemic stroke, a small pilot study in 2004 indicated H-FABP may be significantly more sensitive and specific than the hitherto most extensively studied markers, neuron-specific enolase and S100B [115]. H-FABP seems to peak ≈3 h after symptom onset and remain elevated for up to 5 days, and more importantly, peak H-FABP values correlated with the severity of neurological deficit at 10–12 days (r2=0.49), and functional outcomes at 90 days (r2=0.56) [116]. However, the relation between H-FABP levels and infarct volume was non-linear, with markedly elevated levels of H-FABP restricted to those with an infarct volume of >150 ml [116]. Subsequent small cohorts, as well as a very recent meta-analysis indicate that though H-FABP as a single marker has modest diagnostic and prognostic value in acute ischaemic stroke, it falls short of clinical applicability, though it may add value as part of a biomarker panel [117–119]. Whether H-FABP alone, or as part of a biomarker panel, has value identifying late-presenting stroke patients who might benefit from thrombolysis remains to be investigated.
The major role of biomarkers in traumatic brain injury (TBI) pertains to their role in improving initial triage in those with clinically mild TBI in order to reduce the need for costly and potentially harmful (radiation exposure) neuroimaging. This is especially important since the incidence of clinically significant imaging abnormalities in this sub-group is quite low, and computed tomography (CT) imaging is overused in this context [120,121]. Hence, the ideal biomarker in this case should have a very high NPV, in order to reliably mitigate the need of CT imaging in clinically mild, low-risk TBI. A screening study examining 87 biomarkers in 110 patients with clinically mild TBI found a predictive model with 6 of the markers including H-FABP to have an NPV of 98.6%, though a PPV of just 60% [122]. In a larger cohort of 261 patients with mild TBI, both H-FABP and S100B displayed high sensitivity and NPV, but the former had a higher specificity (6% vs 29% with sensitivity set at 100%), though the improvement in specificity hardly made H-FABP a clinically usable positive predictor of CT findings [123]. Most recently a panel of 8 biomarkers in TBI of all severities identified H-FABP combined with two other markers to constitute the best biomarker panel in terms of sensitivity to predict CT abnormalities [124]. Sensitivity, specificity, and predictive values of all markers individually were found sub-par for clinical use. Hence, H-FABP’s role in predicting CT-negativity in those with mild TBI seems best suited as part of a panel of biomarkers, a field that remains rapidly evolving, given the large number of potential markers being investigated.
More recently, myocardial injury, as defined by an elevated cTn, has been found to predict severe coronavirus disease 2019 (COVID-19) in some reports, raising speculation as to the high incidence of myocarditis in these patients [125,126]. Intriguingly, though hardly surprising, a recent small cohort reported significantly higher H-FABP levels in those with severe versus non-severe COVID-19 infection [127].
Conclusion
To summarise, H-FABP remains a biomarker of high interest, even in the era of highly sensitive troponin assays, particularly in the context of ruling out AMI in low-risk early presenters, allowing earlier discharge of such patients from the ED and reducing cost. Lack of specificity, as has been seen with other biomarkers obviously makes it unsuitable for confirming AMI, especially as the sole marker. Lack of data, lack of progress in improving assay kits, iii) paucity of studies incorporating H-FABP with or without cTn or hs-Tn as part of clinical decision pathways.
By virtue of the fact, it is elevated in many cardiovascular conditions, helps identify patients at higher risk of complications, similar to the hs-cTn. Enough evidence now exists to directly investigate outcome-oriented clinical decision-making algorithms incorporating H-FABP, for example, whether these patients warrant further inpatient observation or testing. Its role in other non-cardiac conditions are also being appreciated. Improvement in assays and more studies would help make clinicians more aware of its utility and perhaps it would find its rightful place in management algorithms.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Centers for Disease Control and Prevention, Ambulatory and Hospital Care Statistics Branch . National Hospital Ambulatory Medical CareSurvey: 2016 emergency department summary tables. Available from: https://www.cdc.gov/nchs/data/nhamcs/web_tables/2016_ed_web_tables.pdf.
- 2.Schull MJ, Vermeulen MJ, Stukel TA.. The risk of missed diagnosis of acute myocardial infarction associated with emergency department volume. Ann Emerg Med. 2006;48(6):647–655. [DOI] [PubMed] [Google Scholar]
- 3.Moy E, Barrett M, Coffey R, et al. Missed diagnoses of acute myocardial infarction in the emergency department: variation by patient and facility characteristics. Diagnosis (Berl)). 2015;2(1):29–40. [DOI] [PubMed] [Google Scholar]
- 4.Pope HJ, Aufderheide TP, Ruthazer R, et al. Missed diagnoses of acute cardiac ischemia in the emergency department. N Engl J Med. 2000;342(16):1163–1170. [DOI] [PubMed] [Google Scholar]
- 5.Hsia RY, Hale Z, Tabas JA.. A national study of the prevalence of life-threatening diagnoses in patients with chest pain. JAMA Intern Med. 2016;176(7):1029–1032. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Technical report series no. 168. Hypertension and coronary heart disease: Classification and criteria for epidemiological studies. [cited 2020 May 25]. Availabel from: http://whqlibdoc.who.int/ trs/WHO_TRS_168.pdf. Published 1959. [PubMed]
- 7.Alpert JS, Thygesen K, Antman E, et al. Myocardial infarction redefined – a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee f or the redefinition of myocardial infarction. J Am Coll Cardiol. 2000;36(3):959–969. [DOI] [PubMed] [Google Scholar]
- 8.Tucker JF, Collins RA, Anderson AJ, et al. Early diagnostic efficiency of cardiac troponin I and troponin T for acute myocardial infarction. Acad Emerg Med. 1997;4(1):13–21. [DOI] [PubMed] [Google Scholar]
- 9.Aydin S, Ugur K, Aydin S, et al. Biomarkers in acute myocardial infarction: current perspectives. Vasc Health Risk Manag. 2019;15:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J, Tan GJ, Han LN, et al. Novel biomarkers for cardiovascular risk prediction. J Geriatr Cardiol. 2017;14(2):135–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glatz JFC, Van Nieuwenhoven FA, Luiken JJFP, et al. Role of membrane-associated and cytoplasmic fatty acid-binding proteins in cellular fatty acid metabolism. Prostaglandins Leukot Essent Fatty Acids. 1997;57(4–5):373–378. [DOI] [PubMed] [Google Scholar]
- 12.Schaap FG, Van der Vusse GJ, Glatz JFC.. Fatty acid-binding proteins in the heart. Mol Cell Biochem. 1998;180(1-2):43–51. [PubMed] [Google Scholar]
- 13.Watanabe K, Wakabayashi H, Veerkamp JH, et al. Immunohistochemical distribution of heart-type fatty acid-binding protein immunoreactivity in normal human tissues and in acute myocardial infarct. J Pathol. 1993;170(1):59–65. [DOI] [PubMed] [Google Scholar]
- 14.Zschiesche-Appie Kleine WH, Spitzer Jacques Veerkamp-Jan FC, Glatz EH, et al. Histochemical localization of heart-type fatty-acid binding protein in human and murine tissues. Histochem Cell Biol. 1995;103(2):147–156. [DOI] [PubMed] [Google Scholar]
- 15.Haastrup B, Gill S, Risom Kristensen S, et al. Biochemical markers of ischaemia for the early identification of acute myocardial infarction without ST segment elevation. Vol 94.; 2000. Available from: www.karger.com. [DOI] [PubMed] [Google Scholar]
- 16.Seino Y, Ogata K. i, Takano T, et al. Use of a whole blood rapid panel test for heart-type fatty acid-binding protein in patients with acute chest pain: Comparison with rapid troponin T and myoglobin tests. Am J Med. 2003;115(3):185–190. [DOI] [PubMed] [Google Scholar]
- 17.Seino Y, Tomita Y, Takano T, et al. Office Cardiologists Cooperative Study on whole blood rapid panel tests in patients with suspicious acute myocardial infarction: comparison between heart-type fatty acid-binding protein and troponin T tests. Circ J. 2004;68(2):144–148. [DOI] [PubMed] [Google Scholar]
- 18.Ruzgar O, Bilge AK, Bugra Z, et al. The use of human heart-type fatty acid-binding protein as an early diagnostic biochemical marker of myocardial necrosis in patients with acute coronary syndrome, and its comparison with troponin-T and creatine kinase-myocardial band. Heart Vessels. 2006;21(5):309–314. [DOI] [PubMed] [Google Scholar]
- 19.Cavus U, Coskun F, Yavuz B, et al. Heart-type, fatty-acid binding protein can be a diagnostic marker in acute coronary syndromes. J Natl Med Assoc. 2006;98(7):1067–1070. [PMC free article] [PubMed] [Google Scholar]
- 20.McCann CJ, Glover BM, Menown IBA, et al. Novel biomarkers in early diagnosis of acute myocardial infarction compared with cardiac troponin T. Eur Heart J. 2008;29(23):2843–2850. [DOI] [PubMed] [Google Scholar]
- 21.Valle HA, Riesgo LGC, Bel MS, et al. Clinical assessment of heart-type fatty acid binding protein in early diagnosis of acute coronary syndrome. Eur J Emerg Med. 2008;15(3):140–144. [DOI] [PubMed] [Google Scholar]
- 22.Orak M, Ustündağ M, Güloğlu C, et al. The role of the heart-type fatty acid binding protein in the early diagnosis of acute coronary syndrome and its comparison with troponin i and creatine kinase-MB isoform. Am J Emerg Med. 2010;28(8):891–896. [DOI] [PubMed] [Google Scholar]
- 23.Haltern G, Peiniger S, Bufe A, et al. Comparison of usefulness of heart-type fatty acid binding protein versus cardiac troponin T for diagnosis of acute myocardial infarction. Am J Cardiol. 2010;105(1):1–9. [DOI] [PubMed] [Google Scholar]
- 24.Kim Y, Kim H, Kim SY, et al. Automated heart-type fatty acid-binding protein assay for the early diagnosis of acute myocardial infarction. Am J Clin Pathol. 2010;134(1):157–162. [DOI] [PubMed] [Google Scholar]
- 25.McMahon CG, Lamont JV, Curtin E, et al. Diagnostic accuracy of heart-type fatty acid-binding protein for the early diagnosis of acute myocardial infarction. Am J Emerg Med. 2012;30(2):267–274. [DOI] [PubMed] [Google Scholar]
- 26.Garcia-Valdecasas S, Ruiz-Alvarez MJ, De Tena JG, et al. Diagnostic and prognostic value of heart-type fatty acid-binding protein in the early hours of acute myocardial infarction. Acta Cardiol. 2011;66(3):315–321. [DOI] [PubMed] [Google Scholar]
- 27.Aldous S, Pemberton C, Troughton R, et al. Heart fatty acid binding protein and myoglobin do not improve early rule out of acute myocardial infarction when highly sensitive troponin assays are used. Resuscitation. 2012;83(2):e27–e28. [DOI] [PubMed] [Google Scholar]
- 28.Gerede DM, Gülec S, Kiliçkap M, et al. Comparison of a qualitative measurement of heart-type fatty acid-binding protein with other cardiac markers as an early diagnostic marker in the diagnosis of non-ST-segment elevation myocardial infarction. CVJA. 2015;26(6):204–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vupputuri A, Sekhar S, Krishnan S, et al. Heart-type fatty acid-binding protein (H-FABP) as an early diagnostic biomarker in patients with acute chest pain. Indian Heart J. 2015;67(6):538–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inoue K, Suwa S, Ohta H, et al. Heart fatty acid-binding protein offers similar diagnostic performance to high-sensitivity troponin T in emergency room patients presenting with chest pain. Circ J. 2011;75(12):2813–2820. [DOI] [PubMed] [Google Scholar]
- 31.Eggers KM, Venge P, Lindahl B.. High-sensitive cardiac troponin T outperforms novel diagnostic biomarkers in patients with acute chest pain. Clin Chim Acta. 2012;413(13-14):1135–1140. [DOI] [PubMed] [Google Scholar]
- 32.Kitamura M, Hata N, Takayama T, et al. High-sensitivity cardiac troponin T for earlier diagnosis of acute myocardial infarction in patients with initially negative troponin T test-comparison between cardiac markers. J Cardiol. 2013;62(6):336–342. [DOI] [PubMed] [Google Scholar]
- 33.Shortt CR, Worster A, Hill SA, et al. Comparison of hs-cTnI, hs-cTnT, hFABP and GPBB for identifying early adverse cardiac events in patients presenting within six hours of chest pain-onset. Clin Chim Acta. 2013;419:39–41. [DOI] [PubMed] [Google Scholar]
- 34.Schoenenberger AW, Stallone F, Walz B, et al. Incremental value of heart-type fatty acid-binding protein in suspected acute myocardial infarction early after symptom onset. Eur Heart J Acute Cardiovasc Care. 2016;5(2):185–192. [DOI] [PubMed] [Google Scholar]
- 35.Bank IE, Dekker MS, Hoes AW, et al. Suspected acute coronary syndrome in the emergency room: Limited added value of heart type fatty acid binding protein point of care or ELISA tests: The FAME-ER (Fatty Acid binding protein in Myocardial infarction Evaluation in the Emergency Room) study. Eur Hear Journal Acute Cardiovasc Care. 2016;5(4):364–374. [DOI] [PubMed] [Google Scholar]
- 36.Gami BN, Patel DS, Haridas N, et al. Utility of heart-type fatty acid binding protein as a new biochemical marker for the early diagnosis of acute coronary syndrome. J Clin Diagn Res. 2015;9(1):BC22–BC24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kellens S, Verbrugge FH, Vanmechelen M, et al. Point-of-care heart-type fatty acid binding protein versus high-sensitivity troponin T testing in emergency patients at high risk for acute coronary syndrome. Eur Heart J Acute Cardiovasc Care. 2016;5(2):177–184. [DOI] [PubMed] [Google Scholar]
- 38.Agnello L, Bivona G, Novo G, et al. Heart-type fatty acid binding protein is a sensitive biomarker for early AMI detection in troponin negative patients: a pilot study. Scand J Clin Lab Invest. 2017;77(6):428–432. [DOI] [PubMed] [Google Scholar]
- 39.Bertinchant JP, Larue C, Pernel I, et al. Release kinetics of serum cardiac troponin I in ischemic myocardial injury. Clin Biochem. 1996;29(6):587–594. [DOI] [PubMed] [Google Scholar]
- 40.Kleine AH, Glatz JFC, Van Nieuwenhoven FA, et al. Release of heart fatty acid-binding protein into plasma after acute myocardial infarction in man. Mol Cell Biochem. 1992;116(1–2):155–162. [DOI] [PubMed] [Google Scholar]
- 41.Glatz JFC, Renneberg R.. Added value of H-FABP as plasma biomarker for the early evaluation of suspected acute coronary syndrome. Clin Lipidol. 2014;9(2):205–220. [Google Scholar]
- 42.Glatz JFC, van Bilsen M, Paulussen RJA, et al. Release of fatty acid-binding protein from isolated rat heart subjected to ischemia and reperfusion or to the calcium paradox. Biochim Biophys Acta (BBA)/Lipids Lipid Metab. 1988;961(1):148–152. [DOI] [PubMed] [Google Scholar]
- 43.Tanaka T, Hirota Y, Sohmiya K-I, et al. Serum and urinary human heart fatty acid-binding protein in acute myocardial infarction. Clin Biochem. 1991;24(2):195–201. [DOI] [PubMed] [Google Scholar]
- 44.Tsuji R, Tanaka T, Sohmiya K, et al. Human heart-type cytoplasmic fatty acid-binding protein in serum and urine during hyperacute myocardial infarction. Int J Cardiol. 1993;41(3):209–217. [DOI] [PubMed] [Google Scholar]
- 45.Glatz JFC, Van Der Vusse GJ, Simoons ML, et al. Fatty acid-binding protein and the early detection of acute myocardial infarction. Clin Chim Acta. 1998;272(1):87–92. [DOI] [PubMed] [Google Scholar]
- 46.Bruins Slot MHE, Reitsma JB, Rutten FH, et al. Heart-type fatty acid-binding protein in the early diagnosis of acute myocardial infarction: a systematic review and meta-analysis. Heart. 2010;96(24):1957–1963. [DOI] [PubMed] [Google Scholar]
- 47.Carroll C, Khalaf MA, Stevens JW, et al. Heart-type fatty acid binding protein as an early marker for myocardial infarction: systematic review and meta-analysis. Emerg Med J. 2013;30(4):280–286. [DOI] [PubMed] [Google Scholar]
- 48.Lippi G, Mattiuzzi C, Cervellin G.. Critical review and meta-analysis on the combination of heart-type fatty acid binding protein (H-FABP) and troponin for early diagnosis of acute myocardial infarction. Clin Biochem. 2013;46(1–2):26–30. [DOI] [PubMed] [Google Scholar]
- 49.Trevethan R. Sensitivity, specificity, and predictive values: foundations, pliabilities, and pitfalls in research and practice. Front Public Heal. 2017; 5(November):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Collinson P, Gaze D, Goodacre S.. Comparison of contemporary troponin assays with the novel biomarkers, heart fatty acid binding protein and copeptin, for the early confirmation or exclusion of myocardial infarction in patients presenting to the emergency department with chest pain. Heart. 2014;100(2):140–145. [DOI] [PubMed] [Google Scholar]
- 51.MacGougan CK, Christenson JM, Innes GD, et al. Emergency physicians’ attitudes toward a clinical prediction rule for the identification and earlydischarge of low risk patients with chest discomfort. Can J Emerg Med. 2001;3(02):89–94. [DOI] [PubMed] [Google Scholar]
- 52.Body R, McDowell G, Carley S, et al. A FABP-ulous 'rule out' strategy? Heart fatty acid binding protein and troponin for rapid exclusion of acute myocardial infarction. Resuscitation. 2011;82(8):1041–1046. [DOI] [PubMed] [Google Scholar]
- 53.Pelsers MMAL, Hermens WT, Glatz JFC.. Fatty acid-binding proteins as plasma markers of tissue injury. Clin Chim Acta. 2005;352(1–2):15–35. [DOI] [PubMed] [Google Scholar]
- 54.Wu AHB. Release of cardiac troponin from healthy and damaged myocardium. Front Lab Med. 2017;1(3):144–150 [Google Scholar]
- 55.Carlton E, Greenslade J, Cullen L, et al. Evaluation of high-sensitivity cardiac troponin I levels in patients with suspected acute coronary syndrome. JAMA Cardiol. 2016;1(4):405–412. [DOI] [PubMed] [Google Scholar]
- 56.Keller T, Zeller T, Ojeda F, et al. Serial changes in highly sensitive troponin I assay and early diagnosis of myocardial infarction. JAMA. 2011;306(24):2684–2693. [DOI] [PubMed] [Google Scholar]
- 57.Rubini Giménez M, Hoeller R, Reichlin T, et al. Rapid rule out of acute myocardial infarction using undetectable levels of high-sensitivity cardiac troponin. Int J Cardiol. 2013;168(4):3896–3901. [DOI] [PubMed] [Google Scholar]
- 58.Reiter M, Twerenbold R, Reichlin T, et al. Heart-type fatty acid-binding protein in the early diagnosis of acute myocardial infarction. Heart. 2013;99(10):708–714. [DOI] [PubMed] [Google Scholar]
- 59.Liou K, Ho S, Ooi SY.. Heart-type fatty acid binding protein in early diagnosis of myocardial infarction in the era of high-sensitivity troponin: a systematic review and meta-analysis. Ann Clin Biochem. 2015;52(3):370–381. [DOI] [PubMed] [Google Scholar]
- 60.Xu LQ, Yang YM, Tong H, et al. Early diagnostic performance of heart-type fatty acid binding protein in suspected acute myocardial infarction: evidence from a meta-analysis of contemporary studies. Hear Lung Circ. 2018;27(4):503–512. [DOI] [PubMed] [Google Scholar]
- 61.Body R, Carley S, McDowell G, et al. The Manchester Acute Coronary Syndromes (MACS) decision rule for suspected cardiac chest pain: derivation and external validation. Heart. 2014;100(18):1462–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carlton E, Body R, Greaves K.. External validation of the Manchester Acute Coronary Syndromes decision rule. Acad Emerg Med. 2016;23(2):136–143. [DOI] [PubMed] [Google Scholar]
- 63.Body R, Boachie C, McConnachie A, et al. Feasibility of the Manchester Acute Coronary Syndromes (MACS) decision rule to safely reduce unnecessary hospital admissions: A pilot randomised controlled trial. Emerg Med J. 2017;34(9):586–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Young JM, Pickering JW, George PM, et al. Heart fatty acid binding protein and cardiac troponin: development of an optimal rule-out strategy for acute myocardial infarction. BMC Emerg Med. 2016;16(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Van Hise CB, Greenslade JH, Parsonage W, et al. External validation of heart-type fatty acid binding protein, high-sensitivity cardiac troponin, and electrocardiography as rule-out for acute myocardial infarction. Clin Biochem. 2018;52:161–163. [DOI] [PubMed] [Google Scholar]
- 66.Ishii J, Ozaki Y, Lu J, et al. Prognostic value of serum concentration of heart-type fatty acid-binding protein relative to cardiac troponin T on admission in the early hours of acute coronary syndrome. Clin Chem. 2005;51(8):1397–1404. [DOI] [PubMed] [Google Scholar]
- 67.Suzuki M, Hori S, Noma S, et al. Prognostic value of a qualitative test for heart-type fatty acid-binding protein in patients with acute coronary syndrome. Int Heart J. 2005;46(4):601–606. [DOI] [PubMed] [Google Scholar]
- 68.O'Donoghue M, de Lemos JA, Morrow DA, et al. Prognostic utility of heart-type fatty acid binding protein in patients with acute coronary syndromes. Circulation. 2006;114(6):550–557. [DOI] [PubMed] [Google Scholar]
- 69.Kilcullen N, Viswanathan K, Das R, et al. Heart-type fatty acid-binding protein predicts long-term mortality after acute coronary syndrome and identifies high-risk patients across the range of troponin values. J Am Coll Cardiol. 2007;50(21):2061–2067. [DOI] [PubMed] [Google Scholar]
- 70.Ilva T, Lund J, Porela P, et al. Early markers of myocardial injury: cTnI is enough. Clin Chim Acta. 2009;400(1-2):82–85. [DOI] [PubMed] [Google Scholar]
- 71.McCann CJ, Glover BM, Menown IBA, et al. Prognostic value of a multimarker approach for patients presenting to hospital with acute chest pain. Am J Cardiol. 2009;103(1):22–28. [DOI] [PubMed] [Google Scholar]
- 72.Viswanathan K, Kilcullen N, Morrell C, et al. Heart-type fatty acid-binding protein predicts long-term mortality and re-infarction in consecutive patients with suspected acute coronary syndrome who are troponin-negative. J Am Coll Cardiol. 2010;55(23):2590–2598. [DOI] [PubMed] [Google Scholar]
- 73.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016. ;37(27):2129–2200. [DOI] [PubMed] [Google Scholar]
- 74.Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70(6):776–803. [DOI] [PubMed] [Google Scholar]
- 75.Anand IS, Fisher LD, Chiang YT, et al. Changes in brain natriuretic peptide and norepinephrine over time and mortality and morbidity in the Valsartan Heart Failure Trial (Val-HeFT). Circulation. 2003;107(9):1278–1283. [DOI] [PubMed] [Google Scholar]
- 76.Berger R, Huelsman M, Strecker K, et al. B-type natriuretic peptide predicts sudden death in patients with chronic heart failure. Circulation. 2002;105(20):2392–2397. [DOI] [PubMed] [Google Scholar]
- 77.Kociol RD, Horton JR, Fonarow GC, et al. Admission, discharge, or change in B-type natriuretic peptide and long-term outcomes: data from Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) linked to Medicare claims. Circ Heart Fail. 2011;4(5):628–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Logeart D, Thabut G, Jourdain P, et al. Predischarge B-type natriuretic peptide assay for identifying patients at high risk of re-admission after decompensated heart failure. J Am Coll Cardiol. 2004;43(4):635–641. [DOI] [PubMed] [Google Scholar]
- 79.Verdiani V, Ognibene A, Rutili MS, et al. NT-ProBNP reduction percentage during hospital stay predicts long-term mortality and readmission in heart failure patients. J Cardiovasc Med. 2008;9:694–699. [DOI] [PubMed] [Google Scholar]
- 80.Huelsmann M, Neuhold S, Resl M, et al. PONTIAC (NT-proBNP selected prevention of cardiac eveNts in a population of diabetic patients without a history of cardiac disease): a prospective randomized controlled trial. J Am Coll Cardiol. 2013;62(15):1365–1372. [DOI] [PubMed] [Google Scholar]
- 81.Ledwidge M, Gallagher J, Conlon C, et al. Natriuretic peptide–based screening and collaborative care for heart failure: the STOP-HF randomized trial. Vol 310; 2013. Available from: https://jamanetwork.com/. [DOI] [PubMed]
- 82.Hoffmann U, Espeter F, Weiß C, et al. Ischemic biomarker heart-type fatty acid binding protein (hFABP) in acute heart failure – diagnostic and prognostic insights compared to NT-proBNP and troponin I. BMC Cardiovasc Disord. 2015;15(1):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lichtenauer M, Jirak P, Wernly B, et al. A comparative analysis of novel cardiovascular biomarkers in patients with chronic heart failure. Eur J Intern Med. 2017;44:31–38. [DOI] [PubMed] [Google Scholar]
- 84.Setsuta K, Seino Y, Ogawa T, et al. Use of cytosolic and myofibril markers in the detection of ongoing myocardial damage in patients with chronic heart failure. Am Jourmal Med. 2002;113(9):717–722. [DOI] [PubMed] [Google Scholar]
- 85.Arimoto T, Takeishi Y, Shiga R, et al. Prognostic value of elevated circulating heart-type fatty acid binding protein in patients with congestive heart failure. J Card Fail. 2005;11(1):56–60. [DOI] [PubMed] [Google Scholar]
- 86.Niizeki T, Takeishi Y, Arimoto T, et al. Combination of heart-type fatty acid binding protein and brain natriuretic peptide can reliably risk stratify patients hospitalized for chronic heart failure. Circ J. 2005;69:922–927. [DOI] [PubMed] [Google Scholar]
- 87.Komamura K, Sasaki T, Hanatani A, et al. Heart-type fatty acid binding protein is a novel prognostic marker in patients with non-ischaemic dilated cardiomyopathy. Heart. 2006;92(5):615–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Niizeki T, Takeishi Y, Arimoto T, et al. Heart-type fatty acid-binding protein is more sensitive than troponin t to detect the ongoing myocardial damage in chronic heart failure patients. J Card Fail. 2007;13(2):120–127. [DOI] [PubMed] [Google Scholar]
- 89.Niizeki T, Takeishi Y, Arimoto T, et al. Persistently increased serum concentration of heart-type fatty acid-binding protein predicts adverse clinical outcomes in patients with chronic heart failure. Circ J. 2008;72(1):109–114. [DOI] [PubMed] [Google Scholar]
- 90.Setsuta K, Seino Y, Kitahara Y, et al. Elevated levels of both cardiomyocyte membrane and myofibril damage markers predict adverse outcomes in patients with chronic heart failure. Circ J. 2007;72(4):569–574. [DOI] [PubMed] [Google Scholar]
- 91.Kutsuzawa D, Arimoto T, Watanabe T, et al. Ongoing myocardial damage in patients with heart failure and preserved ejection fraction. J Cardiol. 2012;60(6):454–461. [DOI] [PubMed] [Google Scholar]
- 92.Otaki Y, Arimoto T, Takahashi H, et al. Prognostic value of myocardial damage markers in patients with chronic heart failure with atrial fibrillation. Intern Med. 2014;53(7):661–668. [DOI] [PubMed] [Google Scholar]
- 93.Shirakabe A, Hata N, Kobayashi N, et al. Serum heart-type fatty acid-binding protein level can be used to detect acute kidney injury on admission and predict an adverse outcome in patients with acute heart failure. Circ J. 2015;79(1):119–128. [DOI] [PubMed] [Google Scholar]
- 94.Kadowaki S, Watanabe T, Otaki Y, et al. Combined assessment of myocardial damage and electrical disturbance in chronic heart failure. World J Cardiol. 2017;9(5):396–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kazimierczyk E, Kazimierczyk R, Harasim-Symbor E, et al. Persistently elevated plasma heart-type fatty acid binding protein concentration is related with poor outcome in acute decompensated heart failure patients. Clin Chim Acta. 2018;487:48–53. [DOI] [PubMed] [Google Scholar]
- 96.Dinh W, Nickl W, Füth R, et al. High sensitive troponin T and heart fatty acid binding protein: Novel biomarker in heart failure with normal ejection fraction?: a cross-sectional study. BMC Cardiovasc Disord. 2011;11(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kasper W, Konstantinides S, Geibel A, et al. Management strategies and determinants of outcome in acute major pulmonary embolism: results of a multicenter registry J Am Coll Cardiol 1997;30:165–171. [DOI] [PubMed] [Google Scholar]
- 98.Konstantinides SV, Meyer G, Becattini C, et al. ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41(4):543-603. [DOI] [PubMed] [Google Scholar]
- 99.Barco S, Mahmoudpor SH, Planquette B, et al. Assessing the severity of acute pulmonary embolism: back to the future?. Eur Heart J. 2019;40(11):911–913. [DOI] [PubMed] [Google Scholar]
- 100.Kucher N, Rossi E, De Rosa M, et al. Prognostic role of echocardiography among patients with acute pulmonary embolism and a systolic arterial pressure of 90 Mm Hg or higher. Arch Intern Med. 2005;165:1777–1781. [DOI] [PubMed] [Google Scholar]
- 101.Kaczyñska A, Pelsers MMAL, Bochowicz A, et al. Plasma heart-type fatty acid binding protein is superior to troponin and myoglobin for rapid risk stratification in acute pulmonary embolism. Clin Chim Acta. 2006;371(1–2):117–123. [DOI] [PubMed] [Google Scholar]
- 102.Puls M, Dellas C, Lankeit M, et al. Heart-type fatty acid-binding proteins (H-FABP): A reliable tool for initial risk stratification of pulmonary embolism?. Eur Heart J. 2007;28(2):146–147. [DOI] [PubMed] [Google Scholar]
- 103.Boscheri A, Wunderlich C, Langer M, et al. Correlation of heart-type fatty acid-binding protein with mortality and echocardiographic data in patients with pulmonary embolism at intermediate risk. Am Heart J. 2010;160(2):294–300. [DOI] [PubMed] [Google Scholar]
- 104.Dellas C, Puls M, Lankeit M, et al. Elevated heart-type fatty acid-binding protein levels on admission predict an adverse outcome in normotensive patients with acute pulmonary embolism. J Am Coll Cardiol. 2010;55(19):2150–2157. [DOI] [PubMed] [Google Scholar]
- 105.Gül EE, Can I, Güler I, et al. Association of pulmonary artery obstruction index with elevated heart-type fatty acid binding protein and short-term mortality in patients with pulmonary embolism at intermediate risk. Diagn Interv Radiol. 2012;18(6):531–536. [DOI] [PubMed] [Google Scholar]
- 106.Lankeit M, Dellas C, Benz V, et al. The predictive value of heart-type fatty acid-binding protein is independent from symptom duration in normotensive patients with pulmonary embolism. Thromb Res. 2013;132(5):543–547. [DOI] [PubMed] [Google Scholar]
- 107.Gul EE, Can I, Kayrak M, et al. Thrombolysis in patients with pulmonary embolism and elevated heart-type fatty acid-binding protein levels. J Thromb Thrombolysis. 2014;37(4):483–489. [DOI] [PubMed] [Google Scholar]
- 108.Langer M, Forkmann M, Richter U, et al. Heart-type fatty acid-binding protein and myocardial creatine kinase enable rapid risk stratification in normotensive patients with pulmonary embolism. J Crit Care. 2016;35:174–179. [DOI] [PubMed] [Google Scholar]
- 109.Dellas C, Lobo JL, Rivas A, et al. Risk stratification of acute pulmonary embolism based on clinical parameters, H-FABP and multidetector CT. Int J Cardiol. 2018;265:223–228. [DOI] [PubMed] [Google Scholar]
- 110.Bajaj A, Rathor P, Sehgal V, et al. Risk stratification in acute pulmonary embolism with heart-type fatty acid-binding protein: a meta-analysis. J Crit Care. 2015;30(5):1151.e1–e7. [DOI] [PubMed] [Google Scholar]
- 111.Fransen EJ, Maessen JG, Hermens WT, et al. Demonstration of ischemia-reperfusion injury separate from postoperative infarction in coronary artery bypass graft patients. Ann Thorac Surg. 1998;65:48–53. [DOI] [PubMed] [Google Scholar]
- 112.Thielmann M, Pasa S, Holst T, et al. Heart-type fatty acid binding protein and ischemia-modified albumin for detection of myocardial infarction after coronary artery bypass graft surgery. Ann Thorac Surg. 2017;104(1):130–137. [DOI] [PubMed] [Google Scholar]
- 113.Hayashida N, Chihara S, Akasu K, et al. Plasma and urinary levels of heart fatty acid-binding protein in patients undergoing cardiac surgery. Jpn Circ J. 2000;64(1):18–20. [DOI] [PubMed] [Google Scholar]
- 114.Muehlschlegel JD, Perry TE, Liu KY, et al. Heart-type fatty acid binding protein is an independent predictor of death and ventricular dysfunction after coronary artery bypass graft surgery. Anesth Analg. 2010;111(5):1101–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zimmermann-Ivol CG, Burkhard PR, Le Floch-Rohr J, et al. Fatty acid binding protein as a serum marker for the early diagnosis of stroke: a pilot study. Mol Cell Proteomics. 2004;3(1):66–72. [DOI] [PubMed] [Google Scholar]
- 116.Wunderlich MT, Hanhoff T, Goertler M, et al. Release of brain-type and heart-type fatty acid-binding proteins in serum after acute ischaemic stroke. J Neurol. 2005;252(6):718–724. [DOI] [PubMed] [Google Scholar]
- 117.Park SY, Kim MH, Kim OJ, et al. Plasma heart-type fatty acid binding protein level in acute ischemic stroke: Comparative analysis with plasma S100B level for diagnosis of stroke and prediction of long-term clinical outcome. Clin Neurol Neurosurg. 2013;115(4):405–410. [DOI] [PubMed] [Google Scholar]
- 118.Park SY, Kim J, Kim OJ, et al. Predictive value of circulating interleukin-6 and heart-type fatty acid binding protein for three months clinical outcome in acute cerebral infarction: multiple blood markers profiling study. Crit Care. 2013;17(2):R45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dolmans LS, Rutten FH, Koenen NCT, et al. Candidate biomarkers for the diagnosis of transient ischemic attack: a systematic review. Cerebrovasc Dis. 2019;47(5–6):207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Borg J, Holm L, Cassidy JD, et al. Diagnostic procedures in mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med Suppl. 2004; 36:61–75. [DOI] [PubMed] [Google Scholar]
- 121.Melnick ER, Szlezak CM, Bentley SK, et al. CT overuse for mild traumatic brain injury. Jt Comm J Qual Patient Saf. 2012;38(11):483–489. [DOI] [PubMed] [Google Scholar]
- 122.Sharma R, Rosenberg A, Bennett ER, et al. A blood-based biomarker panel to risk-stratify mild traumatic brain injury. PLOS One. 2017;12(3):e0173798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lagerstedt L, Egea-Guerrero JJ, Bustamante A, et al. H-FABP: a new biomarker to differentiate between CT-positive and CT-negative patients with mild traumatic brain injury. PLOS One. 2017;12(4):e0175572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Posti JP, Takala RSK, Lagerstedt L, et al. Correlation of blood biomarkers and biomarker panels with traumatic findings on computed tomography after traumatic brain injury. J Neurotrauma. 2019;36(14):2178–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Du RH, Liang LR, Yang CQ, et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARSCoV-2: a prospective cohort study. Eur Respir J. 2020;55(5):2000524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan. JAMA Cardiol. 2020;5(7):802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yin L, Mou H, Shao J, et al. Correlation between heart fatty acid binding protein and severe COVID-19: a case-control study. PLOS One. 2020;15(4):e0231687. [DOI] [PMC free article] [PubMed] [Google Scholar]