Abstract
Introduction: Partial and advanced interatrial block (IAB) in the electrocardiographic (ECG) represents inter-atrial conduction delay. IAB is associated with atrial fibrillation (AF) and stroke in the general population.
Material and methods: A representative sample of Finnish subjects (n = 6354) aged over 30 years (mean: 52.2 years, standard deviation: 14.6) underwent a health examination including a 12-lead ECG. Five different IAB groups based on automatic measurements were compared to normal P waves using multivariate-adjusted Cox proportional hazard model. Follow-up lasted up to 15 years.
Results: The prevalence of advanced and partial IAB was 1.0% and 9.7%, respectively. In the multivariate model, both advanced (hazard ratio (HR): 1.63 (95% confidence interval (CI): 1.00−2.65)) and partial IAB (HR: 1.39 (1.09−1.77)) were associated with increased risk of AF. Advanced IAB was associated with increased risk of stroke or transient ischaemic attack (TIA) independently of associated AF (HR: 2.22 (1.20−4.13)). Partial IAB was also associated with increased risk of being diagnosed with coronary heart disease (HR: 1.26 (1.01−1.58)).
Discussion: IAB is a rather frequent finding in the general population. IAB is a risk factor for AF and is associated with an increased risk of stroke or TIA independently of associated AF.
Key messages
Both partial and advanced interatrial block are associated with increased risk of atrial fibrillation in the general population.
Advanced interatrial block is an independent risk factor for stroke and transient ischaemic attack.
The clinical significance of interatrial block is dependent on the subtype classification.
Keywords: Interatrial block, ECG, population study, atrial fibrillation, mortality
Introduction
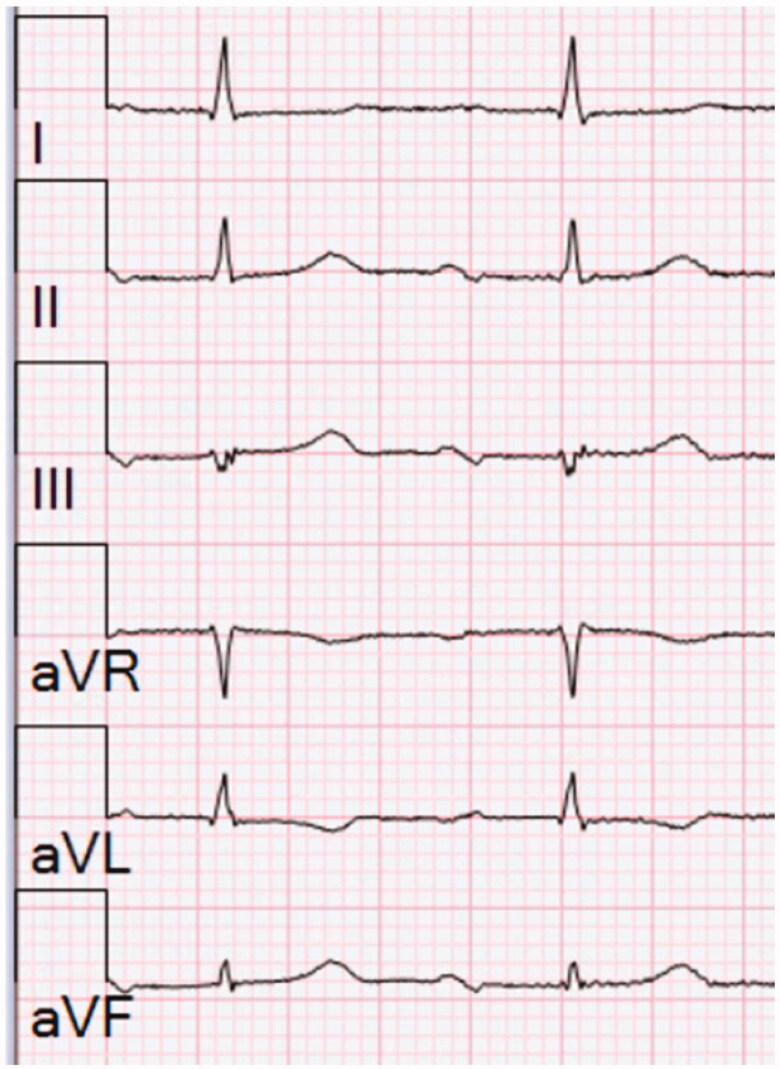
Interatrial block (IAB) is a distinct electrocardiographic (ECG) pattern that has been studied with growing interest since it was first described in 1979 by Bayés de Luna [1]. IAB is caused by conduction delay between the right and left atrium, probably resulting from local fibrosis. When the conduction through the Bachmann’s bundle is blocked, the electrical activation to the left atrium takes an alternative route through the lower parts of the interatrial septum resulting in caudo-cranial activation in the left atrium [2]. This is reflected in the surface ECG as a biphasic morphology of the P wave in the inferior leads (II, III and aVF). This together with a P-wave duration ≥120 ms is considered as advanced IAB (Figure 1). P-wave duration ≥120 ms with normal P-wave morphology is defined as partial IAB and delayed conduction via the interatrial septum through Bachmann’s bundle is considered as the background pathology for this ECG phenomenon [3].
Figure 1.
Limb leads of an ECG with advanced interatrial block. P-wave duration is 140 ms and the P wave is biphasic in the inferior leads (II, III and aVF). The paper speed is 25 mm/s and calibration of 10 mm/mV is used.
Both advanced [4] and partial IAB are risk factors for atrial fibrillation (AF) in the general population [5,6]. The association between IAB and AF is called Bayés’ syndrome [7]. It has also been suggested that IAB is an independent risk factor for ischaemic stroke [8] and cardiovascular and all-cause mortality [5,9]. In some studies, IAB was associated with dementia [10] and coronary heart disease (CHD) [11,12], but data concerning other endpoints than AF is limited. The clinical relevance of IAB lies on the associated risk of AF and the importance of early prevention of stroke by timely anticoagulation therapy. Some authors even suggested anticoagulation therapy based on IAB without a diagnosis of AF [13]. Data on the prevalence, prognostic significance and diseases associated with IAB is sparse.
The primary aims of the study were to explore the prevalence of IAB and the risk for AF and stroke associated with this ECG manifestation in the general population. Secondary aims were to study: (1) the risk of IAB for CHD, dementia and all-cause mortality, (2) the significance of the number of inferior leads with biphasic P waves and (3) the significance of the duration and amplitudes of biphasic inferior P waves.
Materials and methods
Study population
The Health 2000 survey was carried out in 2000–2001. This population-based nationwide study consisted of 8028 individuals aged over 30 years, of whom 79% (6354 individuals) participated in the health examination, which included a structured examination by a physician, health interviews, series of laboratory tests and ECG recordings. The Health 2000 population was designed to cover a nationally representative population sample of the Finnish population. Participants aged 80+ were oversampled with a double sampling fraction. More detailed descriptions of the methods of the Health 2000 survey have been published previously [14]. Ethical approval for the Health 2000 study was obtained from Ethical Committee for Research in Epidemiology and Public Health at the Hospital District of Helsinki and Uusimaa (HUS).
ECG registration and analysis
During the health examination, a standard 12-lead resting ECG in supine position was recorded from each subject with Marquette Hellige MAC 5000 electrocardiographs (Freiburg, Germany and Milwaukee, WI). ECGs were stored electronically and printed at a paper speed of 50 mm/s. The ECG data were sent for further analysis to the Social Insurance Institution’s research centre in Turku, where the ECGs were analyzed with Magellan software (Marquette Electronics Inc, Milwaukee, WI). The Marquette 12SL algorithm uses median complexes of the 10-second ECG tracing and the onset of QRS as the isoelectric line. P-wave durations and amplitudes of different parts of the P wave were automatically measured, the measurement points were checked and corrected if needed. The duration was measured from the earliest onset in any lead to the latest offset in any lead. A wave crossing the baseline level constituting an area of ≥160 µVms represented a separate wave. Two investigators at the Institute of Cardiology, Kaunas Medical Academy, Lithuania, blinded to the clinical data performed the Minnesota coding [15]. The repeatability of the Minnesota Code was ascertained by a repeat analysis of 200 ECGs.
Definition of IAB
Figure 2 shows the different ECG categories. We defined biphasic morphology as follows: the amplitude of the initial part of the P wave ≥20 µV and the amplitude of the terminal part ≤20 µV. We defined advanced IAB as P-wave duration ≥120 ms combined with biphasic P waves in at least two inferior leads (II, III and aVF) and partial IAB as P-wave duration ≥120 ms without biphasic morphology. We categorized subjects with P-wave duration ≥120 ms and two or three leads with biphasic P waves in the inferior leads not fulfilling the above-mentioned amplitude criteria as “minor-aIAB”. In order to establish the significance of the number of biphasic inferior leads, we categorized subjects with one inferior biphasic lead and P-wave duration ≥120 ms as “1 BIF”. To establish the significance of the duration of biphasic P waves, we added a category “2 BIF <120 ms”, which was defined as P-wave duration <120 ms and two or three inferior biphasic leads. ECGs with a P-wave duration <120 ms and maximally one biphasic inferior lead was classified as normal.
Figure 2.
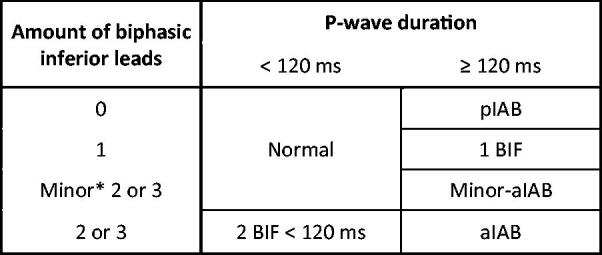
Definitions of different IAB groups in our study. *Biphasic P waves not fulfilling the amplitude criterion (±20 µV) were called “minor”. pIAB: partial interatrial block; BIF: biphasic; aIAB: advanced interatrial block.
To check the validity of the definition of the biphasic morphology, manual comparison blinded to the clinical outcome was performed from 25 randomly selected ECGs defined as advanced IAB, as well as 100 ECGs defined as partial IAB. For this purpose, digitalized ECGs with a zoom of 20 mm/mV and 100 mm/s were used. In the advanced IAB group, in one ECG (4%) the inferior leads were not positive-negative. Among ECGs defined as partial IAB, 11 (11%) ECGs showed positive-negative morphology in one inferior lead, but in none (0%) was the morphology positive-negative in two or more inferior leads.
Study covariates
Trained study personnel performed the health interview and they followed a structural detailed written instruction to gather information about pre-existent diseases. Examining physicians performed another structured interview and physical examination. We included data on prevalent diseases from the Care Register for Health Care (CRHC) maintained by the National Institute for Health and Welfare. CRHC contains data of all inpatient episodes in Finland at the individual level since year 1969 and on outpatients since 1998. The accuracy of the register has been validated previously [16]. Information about medication at baseline were gathered by checking the study participants personal health insurance cards for rights of drug reimbursements and by interviewing the study participants about prescription and non-prescription medicines.
Height, weight and waist circumference were measured and body mass index (BMI) was calculated. Blood pressure was measured from the right arm with a standard mercury manometer (Mercuro 300; Speidel & Keller, Jungingen, Germany). An average of two measurements was used, of which the first one was measured after rest for at least 5 min in sitting position. Arterial hypertension (HTA) was defined as blood pressure ≥140/90. Heart rate was obtained from the ECGs. Smoking was determined as a daily use of cigarettes at the time of the interview. For the diagnosis of chronic obstructive pulmonary disease (COPD), information gathered during the health interview was used. Left ventricular hypertrophy in the ECG (ECG-LVH) was defined by Minnesota code criteria 3-1, 3-3 or 3-4 [15]. Classification of myocardial infarction required either a diagnosis by the examining physician, large Q waves in the resting ECG or a history of myocardial infarction in the CRHC ICD-codes I21-I22 (ICD10) or 410 (ICD8/9).
Serum total cholesterol, high-density lipoprotein cholesterol (HDL), triglyceride and plasma glucose concentrations were determined enzymatically (Roche Diagnostics, GmbH, Mannheim, Germany for HDL and Olympus System Reagent, Hamburg, Germany for total cholesterol, triglycerides and glucose) from venous blood samples with a clinical chemistry analyzer (Olympus, AU400, Hamburg, Germany). Low density lipoprotein cholesterol (LDL) was calculated using the Friedewald formula. The diagnosis of diabetes mellitus (DM) at baseline included fasting serum glucose (fS-Gluc) ≥7 or a history of use of oral glucose lowering agents or insulin injections [17].
Follow-up and study endpoints
The data for mortality and causes of death were gathered from the Causes of Death register maintained by Statistics Finland. It contains 100% of deaths of Finnish citizens in Finland and almost 100% abroad. Information on the incident diseases were obtained from the CRHC. In addition, data on drug purchases since year 1995 and special drug reimbursements since year 1964 were gathered from a separate registry (Statistics on reimbursements for prescription medicines: The Social Insurance Institution of Finland). Databases were linked using a personal identity code. The follow-up lasted until the end of the year 2015. The study endpoints were a new diagnosis of AF, ischaemic stroke or transient ischaemic attack (TIA), dementia, CHD and all-cause mortality. The endpoints were tested separately.
We defined AF as ICD-codes I48 (version 10), 4273 (9) or 42792 (8) in the CRHC and Causes of Death register, right for drug reimbursement for dronedarone or direct oral anticoagulants with diagnose-code (ICD-10) I48 or right for special drug reimbursements for AF. For prevalent and incident stroke and TIA, we included the ICD-10-codes I63-64 and G45 (not I63.6 or G45.4), ICD-9-codes 4330-31A, 4339-41A, 4349A and 435-36, and ICD-8-codes 433-435 in the CRHC and Causes of Death register. Classification of prevalent CHD required at least one of the following: diagnosed angina pectoris, myocardial infarction, percutaneous coronary intervention (PCI) or bypass surgery by examining physician or diagnosed PCI or bypass surgery in the health interview, ICD-codes I20-25 (ICD-10) or 410-14 (ICD-8/9) in the CRHC or the right for drug reimbursements for CHD. For the diagnosis of incident CHD, we also included ICD-codes I21-25, I46, R96, R98 (ICD-10) and 410–414, 798 (not 7980A) (ICD-8/9) in the Causes of Death register. We defined dementia as ICD-10 codes F00-F03, G30, ICD-9-codes 290, 3310, 4378A, and ICD-8-code 290 in the CRHC and in the Causes of Death register, right for drug reimbursements for donepezil, galantamine, memantine, rivastigmine or tacrine or purchases for anti-dementia drugs.
Exclusion criteria
We excluded subjects with missing ECG data (number of participants (n) = 55). Of those, the recording was not successful in 36 participants with entries such as “difficult to move”, “wheelchair”, “denial”, “leg/hand amputated”, “in geriatric chair”, “massive hernia” and “plaster in leg/hand”. In the further process, 19 ECGs were lost (diskette lost (9), coupling error (4), data reading failure (5) and unspecific reason (1)). We also excluded subjects with ECGs showing supraventricular tachyarrhythmias according to Minnesota-code 8-4-1 (n = 6), all prevalent AF and atrial flutter as defined previously (n = 204), paced rhythm (Minnesota-code 6-8, n = 4) and ectopic rhythm defined as totally negative P waves in the inferior leads (II, III and aVF) in computer analysis (n = 24) leaving 6066 individuals in the final study sample. Participants with prior diagnosis of study endpoints (stroke or TIA (n = 143), CHD (n = 435) or dementia (n = 451)) were excluded from analysis considering particular endpoint. In addition, subjects with incident AF (n = 538) were excluded from the analysis when studying IAB as an independent risk factor for stroke and TIA.
Statistical analyses
Comparisons in variables were calculated with either one-way analysis of variance (ANOVA), Kruskal-Wallis, Chi-square or Fisher’s exact test as appropriate. Cox proportional hazard models were constructed separately for different endpoints. AF and stroke and TIA were used as primary endpoints and CHD, dementia and mortality as secondary endpoints. Only the first diagnosis per disease was considered. Advanced IAB and partial IAB were compared to normal P waves (P duration <120 ms with zero or one biphasic inferior P wave) and minor-aIAB, 1 BIF and 2 BIF <120 ms were included as sensitivity analysis. We used the following parameters for multivariate adjusting: age, sex, BMI, HDL, LDL, heart rate, HTA, DM, CHD and ECG-LVH. To establish the prevalence of different IAB groups on the population level, SPSS Complex samples design was used. We tested also the difference between weighted and unweighted Cox proportional hazard models with SPSS Complex samples design, and we found no clinically relevant differences between the two models. All Cox proportional hazard models are presented in unweighted form. All analyses were performed with SPSS 25 (SPSS, Chicago, IL). Statistical significance was based on p < .05.
Results
The final study sample consisted of 6066 participants free of AF at baseline. The mean age of the participants was 52.2 (standard deviation (SD): 14.6) years. Table 1 shows the baseline characteristics of the study population. Table 2 shows the population-corrected prevalence of different IAB groups separately for sinus rhythm and without exclusion criteria. Participants within the different categories of IAB were significantly older than participants with normal P waves and age increased with IAB severity. Participants with IAB were more often men and more often had co-morbidities. The use of all studied medications was more frequent in the subjects with IAB. ECG-LVH was more prevalent in the categories with biphasic morphology.
Table 1.
Baseline characteristics of the Health 2000 Survey participants.
| Variable | aIAB |
Minor-aIAB |
1 BIF |
pIAB |
2 BIF < 120ms |
Normal |
p value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean/ median/N | SD/Q1–Q3/ (%) | Mean/ median/N | SD/Q1–Q3/ (%) | Mean/ median/N | SD/Q1–Q3/ (%) | Mean /median/N | SD/Q1–Q3/ (%) | Mean/ median/N | SD/Q1–Q3/ (%) | Mean/ median/N | SD/Q1–Q3/ (%) | ||
| N | 63 | (1.0%) | 18 | (0.3%) | 264 | (4.4%) | 585 | (9.6%) | 249 | (4.1%) | 4887 | (80.6%) | |
| Age | 67.4 | 12.6 | 64.6 | 12.3 | 57.5 | 14.8 | 55.0 | 14.0 | 58.3 | 15.5 | 51.0 | 14.3 | <.001 |
| Men | 36 | (57.1%) | 9 | (50.0%) | 156 | (59.1%) | 309 | (52.8%) | 96 | (45.0%) | 2123 | (43.4%) | <.001 |
| BMI (kg/m2) | 29.2 | 4.6 | 29.2 | 5.2 | 28.4 | 4.6 | 27.5 | 4.6 | 27.2 | 4.4 | 26.7 | 4.6 | <.001 |
| Waist circumference (cm) | 100.6 | 12.1 | 101.1 | 16.3 | 97.9 | 13.4 | 95.1 | 13.0 | 93.6 | 12.4 | 91.8 | 13.3 | <.001 |
| Heart rate/min | 59.7 | 10.2 | 62.3 | 10.0 | 61.7 | 10.9 | 62.5 | 11.0 | 62.8 | 9.9 | 63.6 | 10.7 | .001 |
| Regular smoking | 9 | (14.3%) | 3 | (16.7%) | 40 | (15.2%) | 125 | (21.4%) | 58 | (23.4%) | 1087 | (22.3%) | .065 |
| COPD | 1 | (1.6%) | 0 | (0.0%) | 5 | (1.9%) | 6 | (1.0%) | 5 | (2.0%) | 58 | (1.2%) | .554 |
| Hypertension | 38 | (60.3%) | 13 | (72.2%) | 144 | (54.5%) | 288 | (49.2%) | 121 | (48.6%) | 1944 | (39.8%) | <.001 |
| Diabetes | 11 | (17.5%) | 2 | (11.1%) | 27 | (10.2%) | 41 | (7.0%) | 35 | (14.1%) | 253 | (5.2%) | <.001 |
| LVH | 15 | (23.8%) | 4 | (22.2%) | 56 | (21.2%) | 93 | (15.9%) | 50 | (20.1%) | 667 | (13.6%) | <.001 |
| CHD | 17 | (27.0%) | 5 | (27.8%) | 32 | (12.1%) | 57 | (9.7%) | 26 | (10.4%) | 298 | (6.1%) | <.001 |
| Myocardial infarction | 8 | (12.7%) | 2 | (11.1%) | 13 | (4.9%) | 23 | (3.9%) | 5 | (2.0%) | 112 | (2.3%) | <.001 |
| Total cholesterol (mmol/L) | 5.8 | 1.4 | 6.1 | 1.2 | 6.1 | 1.2 | 6.0 | 1.0 | 6.2 | 1.1 | 5.9 | 1.1 | .001 |
| HDL (mmol/L) | 1.2 | 0.4 | 1.3 | 0.3 | 1.3 | 0.3 | 1.3 | 0.3 | 1.3 | 0.4 | 1.3 | 0.4 | .001 |
| LDL (mmol/L) | 3.6 | 1.4 | 4.2 | 1.2 | 3.8 | 1.3 | 3.9 | 1.1 | 3.9 | 1.3 | 3.8 | 1.2 | .027 |
| Triglycerides (mmol/L) | 1.4 | 1.1–2.2 | 1.3 | 1.0–2 | 1.5 | 1.1–2.0 | 1.4 | 1.0–1.9 | 1.4 | 1.0–2 | 1.3 | 1.0–1.8 | <.001 |
| CRP (mg/L) | 0.7 | 0.3–2.5 | 1.1 | 0.5–3.6 | 0.9 | 0.4–2.1 | 0.7 | 0.3–1.8 | 1.2 | 0.4–3.1 | 0.7 | 0.3–1.8 | <.001 |
| Uric acid (µmol/L) | 335.4 | 97.2 | 324.7 | 76.3 | 331.2 | 83.5 | 312.4 | 77.7 | 311.9 | 85.6 | 294.8 | 79.0 | <.001 |
| Medication | |||||||||||||
| Beta-blocker | 23 | (36.5%) | 9 | (50.0%) | 68 | (25.8%) | 114 | (19.5%) | 46 | (18.5%) | 556 | (11.4%) | <.001 |
| CCB | 9 | (14.3%) | 6 | (33.3%) | 25 | (9.5%) | 50 | (8.5%) | 19 | (7.6%) | 239 | (4.9%) | <.001 |
| Digitalis | 4 | (6.3%) | 2 | (11.1%) | 4 | (1.5%) | 7 | (1.2%) | 3 | (1.2%) | 29 | (0.6%) | <.001 |
| ACEI/ARB | 11 | (17.5%) | 3 | (16.7%) | 32 | (12.1%) | 58 | (9.9%) | 27 | (10.8%) | 328 | (6.7%) | <.001 |
aIAB: advanced interatrial block; BIF: biphasic; pIAB: partial interatrial block; BMI: body mass index; COPD: chronic obstructive pulmonary disease; LVH: left ventricular hypertrophy; CHD: coronary heart disease; HDL: high-density lipoprotein; LDL: low-density lipoprotein; CCB: calcium channel blocker; ACEI: angiotensin-converting enzyme inhibitor; ARB: angiotensin II receptor antagonist; SD: standard deviation; N: number; Q1–Q3: quartiles.
Table 2.
Prevalence of different IAB groups: weighted Health 2000 population.
| In sinus rhythm* (%) | All (no exclusion criteria) (%) | |
|---|---|---|
| aIAB | 1.0 | 1.0 |
| Minor-aIAB | 0.3 | 0.3 |
| 1 BIF | 4.4 | 4.3 |
| pIAB | 9.7 | 9.9 |
| 2 BIF <120 ms | 4.0 | 4.0 |
*And no previous diagnosis of atrial fibrillation.
aIAB: advanced interatrial block; BIF: biphasic; pIAB: partial interatrial block.
IAB and the risk of adverse events
During a mean follow-up period of 13.42 (SD: 3.74) years, there were 538 incident diagnoses of AF. Table 3 shows the age-adjusted and multi-adjusted hazard ratio (HR) for different endpoints and numbers of incident diseases. Advanced IAB, partial IAB and 1 BIF increased the risk for incident AF in the age-adjusted model. The increase in risk was most evident in the advanced IAB group and similar between the partial IAB and 1 BIF -groups. In a multi-adjusted model, advanced IAB and partial IAB increased the risk for AF. The category 1 BIF lost its statistical significance after multivariate adjustment. Minor-aIAB or 2 BIF <120 ms had no prognostic significance regarding incident AF with HRs close to one.
Table 3.
Cox proportional hazard analysis for different endpoints according to P-wave morphology compared to normal P waves.
| Endpoint (N, %) | Events/1000 person-years | Hazard ratio (95% CI) |
|||
|---|---|---|---|---|---|
| Age adjusted | p value | Multivariate adjusted* | p value | ||
| Atrial fibrillation (538, 8.9%) | 6.6 | ||||
| aIAB | 28.5 | 2.11 (1.30–3.40) | .002 | 1.63 (1.00–2.65) | .048 |
| Minor-aIAB | 13.5 | 1.20 (0.39–3.74) | .754 | 0.99 (0.32–3.10) | .988 |
| 1 BIF | 13.4 | 1.56 (1.14–2.14) | .006 | 1.28 (0.93–1.76) | .129 |
| pIAB | 10.9 | 1.55 (1.22–1.97) | <.001 | 1.39 (1.09–1.77) | .008 |
| 2 BIF <120 ms | 10.3 | 1.16 (0.80–1.66) | .436 | 1.08 (0.75–1.56) | .687 |
| Stroke and TIA (434, 7.3%) | 5.4 | ||||
| aIAB | 30.0 | 2.29 (1.40–3.74) | .001 | 2.09 (1.27–3.44) | .004 |
| Minor-aIAB | 8.8 | 0.84 (0.21–3.35) | .799 | 0.79 (0.20–3.20) | .746 |
| 1 BIF | 7.0 | 0.91 (0.59–1.40) | .658 | 0.84 (0.55–1.29) | .427 |
| pIAB | 6.2 | 0.94 (0.69–1.28) | .686 | 0.93 (0.68–1.27) | .647 |
| 2 BIF <120 ms | 5.8 | 0.70 (0.44–1.13) | .146 | 0.64 (0.40–1.03) | .067 |
| CHD (678, 12.1%) | 8.9 | ||||
| aIAB | 24.5 | 1.20 (0.68–2.14) | .526 | 1.09 (0.61–1.94) | .763 |
| Minor-aIAB | 24.6 | 1.50 (0.56–4.03) | .416 | 1.40 (0.52–3.76) | .508 |
| 1 BIF | 11.7 | 0.93 (0.66–1.31) | .680 | 0.80 (0.57–1.13) | .212 |
| pIAB | 13.0 | 1.30 (1.04–1.62) | .024 | 1.26 (1.01–1.58) | .045 |
| 2 BIF <120 ms | 13.4 | 1.01 (0.73–1.41) | .950 | 0.91 (0.65–1.27) | .572 |
| Dementia (451, 7.5%) | 5.5 | ||||
| aIAB | 20.4 | 1.11 (0.65–1.91) | .694 | 1.11 (0.64–1.93) | .717 |
| Minor-aIAB | 0.0 | No events | No events | ||
| 1 BIF | 10.6 | 1.11 (0.79–1.57) | .557 | 1.13 (0.80–1.60) | .480 |
| pIAB | 6.2 | 0.84 (0.62–1.13) | .253 | 0.85 (0.63–1.15) | .295 |
| 2 BIF <120 ms | 9.2 | 0.95 (0.65–1.38) | .772 | 0.92 (0.63–1.36) | .688 |
| Death (1159, 19.1%) | 13.9 | ||||
| aIAB | 45.4 | 1.09 (0.77–1.54) | .634 | 1.10 (0.78–1.56) | .592 |
| Minor-aIAB | 21.8 | 0.77 (0.32–1.86) | .561 | 0.65 (0.24–1.73) | .388 |
| 1 BIF | 19.2 | 0.86 (0.67–1.10) | .238 | 0.86 (0.67–1.10) | .234 |
| pIAB | 15.6 | 0.87 (0.72–1.05) | .134 | 0.88 (0.73–1.06) | .185 |
| 2 BIF <120 ms | 23.8 | 1.01 (0.80–1.28) | .910 | 1.04 (0.82–1.31) | .770 |
N: number of participants; CI: confidence interval; aIAB: advanced interatrial block; BIF: biphasic; pIAB: partial interatrial block; CHD: coronary heart disease.
*Adjusted for age, sex, body mass index, coronary heart disease, hypertension, diabetes, high-density lipoprotein, low-density lipoprotein, heart rate and ECG left ventricular hypertrophy
Advanced IAB proved to be a significant risk marker for stroke and TIA both in age-adjusted and multi-adjusted models. The other IAB categories were even associated with lower risk than the control group, although the difference was not statistically significant. When participants with incident AF during the follow-up were excluded, advanced IAB increased the risk for stroke and TIA independently in the age-adjusted (HR: 2.39 [95% confidence interval (CI): 1.30−4.42, p = .005]) and in the multi-adjusted model (HR: 2.22 [1.20−4.13, p = .012]). The mean follow-up time for stroke and TIA was 13.56 (SD: 3.62) years.
Partial IAB showed a borderline significance for CHD after multivariate adjustment. In addition, advanced IAB and minor-aIAB seemed to increase the risk for CHD, but the difference was not statistically significant. Despite the increased risk for AF, stroke/TIA and CHD, none of the IAB groups was associated with increased all-cause mortality. In addition, none of the IAB categories predicted dementia.
Discussion
In a population study, where we used weighting adjustment and included individuals aged 30+, without a diagnosis of AF at baseline, the prevalence of advanced IAB was 1.0% and partial IAB was 9.7%, respectively. Both advanced and partial IAB were predictors of incident AF during long-term follow-up and advanced IAB also increased the risk of stroke or TIA after multivariate adjustment independently of associated AF. Partial IAB also seemed to associate with increased risk for CHD, but the association was no longer significant when adjusted for multiple testing. We found no association between IAB and all-cause mortality or dementia. The ECG categories with less advanced signs of IAB had no significant predictive value.
Prevalence of IAB
In the Atherosclerosis Risk in Communities (ARIC) study of 45 to 64 year old male and female subjects (mean age 54 years (±5.8 y)) 0.5% had advanced IAB at baseline [4]. Similar prevalence was found in the Copenhagen ECG study of 152,759 individuals aged 50 to 90 years [5]. In the geriatric population of Ariyarajah et al. [18] the prevalence of advanced IAB was around 6% and the prevalence was even higher in 80 subjects older than 100 years in a study of Martínez-Sellés et al. [10]. They found advanced IAB in 26% and partial IAB in 20%, but they did not exclude participants with pre-existing AF (25%). In the Copenhagen ECG study, subjects with advanced IAB were much older than those without IAB, but there was only a two-year age difference between those with partial IAB and no IAB. We made similar observations: subjects with advanced IAB were more than 16 years older than those without IAB, but the difference between subjects with partial IAB and no IAB was only four years. As atrial fibrosis is considered important in IAB, it is logical that age is a contributing factor in conduction disease development. The mean age in our study (52.2 years (SD 14.6)) was close to the mean age in the ARIC-study, but we noticed a slightly higher prevalence of advanced IAB (1.0%). The difference is likely explained by the different definition of advanced IAB; we included also ECGs with two biphasic inferior leads.
IAB and AF
The association between IAB and supraventricular arrhythmias, mainly AF, the Bayés syndrome, has been demonstrated in many different clinical scenarios [19]. In the present population study, there was a clear association between advanced IAB and incident AF. With age-adjustment, the risk of a new AF diagnosis during long-term follow-up was about twofold, and after multi-variate adjustment, the risk was still more than 1.5-fold. In the ARIC study, there was a threefold increased risk for AF in individuals with advanced IAB [4]. The very large Copenhagen ECG study [5] showed that IAB improves risk prediction of AF when added to a conventional risk model. The highest effect of IAB on the absolute risk of AF was observed in individuals aged 60 to 70 years with baseline cardiovascular disease.
Previous studies are contradictory concerning the increase in risk between advanced and partial forms of IAB. In the REgistre GIroní del COR (REGICOR) population-based (mean age 74.3 years (±7.4)) cohort, a P wave longer than 110 ms increased the risk of AF during 7.12 years of follow-up, but advanced IAB morphology did not provide an additional AF risk beyond that of P-wave duration [20]. However, in the Copenhagen ECG study [5] the risk seemed to increase with the number of affected biphasic inferior leads. In our study also partial IAB was associated with incident AF, but the risk of a new AF diagnosis was lower than for advanced IAB. The multi-variate adjusted HR: 1.39 (1.09−1.77) for partial IAB was close to the corresponding HR of 1.25 (1.19−1.30) in the Copenhagen ECG study [5]. The smaller CIs in the Copenhagen ECG study probably reflect their much larger study population.
IAB and cerebrovascular event
As in the previous population study by Skov et al. [5], we found that advanced, but not partial IAB, was associated with increased stroke risk. Ariyarajah and Spodick [21] hypothesized that progressive worsening of interatrial conduction via the Bachmann Bundle in partial IAB may lead to advanced IAB over time. They found support for their hypothesis in another study, where the progression time from partial to advanced IAB was shorter than from normal P wave to advanced IAB [22]. This offers two potential explanations for the fact that the advanced, but not partial IAB, seems to be associated with risk for stroke or TIA. One possible explanation is that partial IAB is associated with less atrial remodelling and thereby with less risk for thromboembolic substrates. The other potential explanation is simply related to time: if advanced IAB develops later than partial IAB, there is more time for an embolic stroke event to happen.
The pathophysiology between the association of AF and stroke is not completely understood. In patients with AF and no concomitant heart disease or other risk factors, the risk of stroke is similar to the risk in patients without AF [23] and also patients with paroxysmal AF are at risk of stroke even when in sinus rhythm [24]. It has been suggested that chronic atrial injury may lead to increased thrombogenicity of the atria and atrial fibrosis could be a sign of this pathophysiology [25]. Our study seems to support the possibility that mechanisms other than AF-induced thrombus from the left atrium could cause the increased risk, as advanced IAB was associated with stroke or TIA without prevalent and incident AF. It is important to identify diagnostic markers of thrombogenic atria, such as IAB, and target therapies to patients with increased risk of cardioembolic stroke. Some authors have proposed anticoagulation therapy in the elderly with high risk of advanced IAB and AF even before a clinical diagnosis of AF [13]. Propensity score matching and randomized-controlled trials could help to support this hypothesis.
IAB and CHD
Surprisingly in this study, only partial, but not advanced IAB, seemed to associate with incident CHD before accounting for multiple testing in the analyses. It was also noted that subjects with advanced IAB more often had CHD or a history of myocardial infarction at baseline and the proportion of diagnosed CHD increased with the severity of IAB. Alexander et al. [26] found that among patients, who underwent coronary angiography and carotid ultrasonography, the existence of either partial or advanced IAB was associated with more severe CHD and greater carotid intima-media thickness. Álvarez-García et al. [27] demonstrated that patients, who had occlusion of the atrial branches during percutaneous coronary intervention, had three times greater incidence of new-onset IAB and they had also a greater post-procedure increase in P-wave duration than in those without occlusion. In addition, the rate of incident intra-atrial conduction delay was much higher in patients with atrial branch occlusion. According to Apiyasawat et al. [11] IAB appeared more often in patients with CHD during an exercise stress test. Additionally, including IAB to the results of the exercise test improved sensitivity to detect CHD. A previous study of Alexander et al. [12] found an association between diffuse coronary atherosclerosis and IAB and the authors stated that their findings support the concept that IAB may be the result of persistent atrial ischaemia.
The present study is the first to report a possible association between IAB and incident CHD. Due to the number of hypotheses tested, this finding could be a false positive or due to the fact that IAB shares risk factors with CHD, including HTA, diabetes and hypercholesterolaemia [28]. It is also possible that undiagnosed cases of CHD could have influenced the results despite the wide definition of CHD in our study. However, more studies are needed to replicate this finding.
Mortality
We did not find an association between IAB and all-cause mortality in our population study. In a population study of Magnani et al. [9] prolonged P-wave duration was independently associated with cardiovascular mortality. The association with all-cause mortality was not significant when individuals with known cardiovascular disease were excluded. In the Copenhagen ECG study [5], advanced IAB with two or three biphasic inferior leads was associated with increased all-cause mortality. It is possible that our study population was too small to detect the effect on all-cause mortality.
Dementia
AF is independently associated with increased risk of dementia [29], but data of the association between IAB and dementia is limited. Martínes-Sellés et al. [10] found that dementia was more frequent among centenarians with IAB compared to individuals with normal P waves and the association was stronger among participants with advanced than for those with partial IAB. We found no association between IAB and incident dementia. Our analysis included dementia diagnoses in general. In order to study further the association between IAB and incident dementia, restricting analysis only to dementia with signs of vascular changes could give us more information.
Definition of interatrial block
Investigators have used different methods to define biphasic P waves. We decided to use computer-based measurements. Manual analysis of the P-wave morphology may be difficult because of small P-wave amplitudes, wandering baseline and disturbing artefacts. It may also be difficult to get a reliable picture of the end of the P wave because of frequent changes of the PR level. Automatic measurements may help to correct for these factors and the repeatability of automated measurements is excellent. Nonetheless manual ECG measurements may give comparable results, if performed after tracing vertical lines to define the interval between the earliest and the latest detection of atrial depolarization in the frontal leads. After tracing these two vertical lines, the measurement should preferably be performed with callipers or method calculators [2]. Regarding P-wave duration, 120 ms is the established cut-off for IAB [3], although earlier studies also used 110 ms.
There are no established diagnostic criteria for the amplitudes of the initial and terminal parts of biphasic P waves. We chose a cut-off of 20 µV, because changes below this magnitude were not recognized in a reproducible manner on enlarged conventional ECG recordings [30]. In our study in the group with amplitudes below 20 µV (minor-aIAB group), the baseline characteristics were quite similar to those with advanced IAB, but HRs for different endpoints were more comparable with the partial IAB group than the advanced IAB group. Thereby, it seems that the amplitude of the biphasic aspects of the P waves matter. As we found only 18 subjects fulfilling these criteria, it is difficult to draw firm conclusions.
There are only few studies investigating P-wave morphology independently of P-wave duration. Holmqvist et al. [31] found that P-wave morphology was an independent risk factor for FA and non-sudden cardiac death in patients with congestive heart failure and a history of myocardial infarction. However, the P-wave duration in their study population was quite long (unfiltered 145 ± 19 ms). We studied participants with two or three inferior biphasic leads and P-wave duration <120 ms as a separate group, but we did not notice any significant increase in HRs compared to normal P waves. In young patients with high atrial septal aneurysm or septal defect, the electrical stimulus may not be able to cross the upper part of the septum but may depolarize the left atrium with caudo-cranial activation. In these cases, a biphasic morphology may be seen in the inferior lead but the P-wave duration is less than 120 ms [2].
Interestingly, in our study prolonged P-wave duration with one biphasic inferior lead (1 BIF) was not associated with AF and CHD, while prolonged P-wave duration without biphasic morphology (partial IAB) was. In the much larger population reported by Skov et al. [5], IAB with only one biphasic inferior lead was associated with AF and stroke. It should be pointed out that the presence of a biphasic P wave only in lead III is a normal finding [2]. The negative hemifield of lead III starts at +30°, which does not represent caudo-cranial activation of the left atrium. The caudocranial activation occurs when the final part of the biphasic P wave falls in the negative hemifield of aVF. The criteria for advanced IAB used in different studies present slight differences, however in all of them aVF presents final negativity as an expression of caudo-cranial activation of the left atrium, which is the hallmark of the diagnosis of advanced IAB. It is possible that lead specific analysis would have revealed differences in prognosis between different leads [32].
An atypical pattern of advanced IAB has also been described by Bayés de Luna et al. [32]. The criterion includes cases of P-wave duration ≥120 ms with three different morphologies in inferior P waves. In type 1 and 2, there is a biphasic P wave in leads III and aVF and in lead II an isodiphasic (type 1) or biphasic (type 2) final component of the otherwise positive P wave. Type 3 presents biphasic P wave in lead II and is isodiphasic with final negativity in III and aVF. In our study atypical patterns type 1 and 2 were included in the definition of advanced IAB.
Study limitations and strengths
This study was a follow-up study of a large population cohort consisting of individuals aged 30+ years predominant white ethnicity. Our study results may not apply to populations of other ethnicities. Only the baseline ECG was used for analysis, but on the other hand, this represents the clinical situation, where therapeutic decisions have to be made. The long follow-up time up to 15 years resulted in a relatively high number of events. Data of prevalent and incident AF were mainly collected from national registers, but it is possible that some AF paroxysms diagnosed in primary care were not included in our analysis. It is also possible that subclinical paroxysmal AF in the study population may have influenced the results, which was not possible to control for. Also lack of echocardiographic data about the size of atria could be considered as a limitation of the study.
The results of the present study are based on an exploratory analysis with multiple comparisons made between the exposure and outcome variables. However, given the a priori information of the possibly significant association between IAB with AF and cerebrovascular events, it is unlikely our observations are false positives. Supporting this, even after considering a stringent Bonferroni correction for twenty-five independent associations that were tested, the main results would be statistically significant (p < .05).
Conclusion
IAB is a relatively common ECG finding in the general population. We were able to strengthen the results of previous studies showing that IAB is a risk factor for AF in the general population. In addition, advanced IAB proved to be a stronger risk marker than partial IAB. Advanced IAB was associated with increased risk of stroke or TIA independently of associated AF or other cardiovascular risk factors. In contrast, partial IAB did not increase the risk of stroke or TIA.
Finally, our study highlights the importance of the definition of advanced IAB: two (but not one) inferior biphasic leads increased the risk of AF and stroke or TIA, outcome was dependent on the cut-off values of the amplitude of the P-wave deflections and on P-wave duration.
Acknowledgements
The authors thank the investigators, staff and participants of the Health 2000 survey. A. S. Havulinna, V. Salomaa and the National Institute of Health and Welfare for their work on coding endpoints.
Funding Statement
This work was supported by the Doctoral Programme in Medicine and Life Sciences, Faculty of Medicine and Health Technology, Tampere University; the Finnish Medical Foundation; grants from Finska Läkaresällskapet; The Academy of Finland [grant 322098]; Competitive State Research Financing of the Expert Responsibility area of Tampere; Finnish Foundation for Cardiovascular Research; Tampere Tuberculosis Foundation; EU Horizon 2020 [grant 755320 for TAXINOMISIS]; Tampere University Hospital Supporting Foundation and Emil Aaltonen’s Foundation.
Disclosure of interest
No potential conflict of interest was reported by the author(s).
References
- 1.Bayés de Luna AJ. Block at the auricular level. Rev Esp Cardiol. 1979;32:5–10. [PubMed] [Google Scholar]
- 2.Bayés de Luna A, Baranchuk A, Alberto Escobar Robledo L, et al. Diagnosis of interatrial block. J Geriatr Cardiol JGC. 2017;14:161–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bayés de Luna A, Platonov P, Cosio FG, et al. Interatrial blocks. A separate entity from left atrial enlargement: a consensus report. J Electrocardiol. 2012; 45:445–451. [DOI] [PubMed] [Google Scholar]
- 4.O’Neal WT, Zhang Z-M, Loehr LR, et al. Electrocardiographic advanced interatrial block and atrial fibrillation risk in the general population. Am J Cardiol. 2016; 17:1755–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skov MW, Ghouse J, Kühl JT, et al. Risk prediction of atrial fibrillation based on electrocardiographic interatrial block. J Am Heart Assoc. 2018; 7:e008247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lehtonen AO, Langén VL, Puukka PJ, et al. Incidence rates, correlates, and prognosis of electrocardiographic P-wave abnormalities – a nationwide population-based study. J Electrocardiol. 2017; 50:925–932. [DOI] [PubMed] [Google Scholar]
- 7.Bacharova L, Wagner GS. The time for naming the interatrial block syndrome: Bayes syndrome. J Electrocardiol. 2015; 48:133–134. [DOI] [PubMed] [Google Scholar]
- 8.O’Neal WT, Kamel H, Zhang Z-M, et al. Advanced interatrial block and ischemic stroke: the Atherosclerosis Risk in Communities Study. Neurology. 2016;87:352–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Magnani JW, Gorodeski EZ, Johnson VM, et al. P wave duration is associated with cardiovascular and all-cause mortality outcomes: The National Health and Nutrition Examination Survey. Heart Rhythm. 2011; 8:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martínez-Sellés M, Massó-van Roessel A, Álvarez-García J, et al. Interatrial block and atrial arrhythmias in centenarians: prevalence, associations, and clinical implications. Heart Rhythm. 2016;13:645–651. [DOI] [PubMed] [Google Scholar]
- 11.Apiyasawat S, Thomas AJ, Spodick DH. Interatrial block during exercise tolerance tests as an additional parameter for the diagnosis of ischemic heart disease. J Electrocardiol. 2005;8:150–153. [DOI] [PubMed] [Google Scholar]
- 12.Alexander B, MacHaalany J, Lam B, et al. Comparison of the extent of coronary artery disease in patients with versus without interatrial block and implications for new-onset atrial fibrillation. Am J Cardiol. 2017;119:1162–1165. [DOI] [PubMed] [Google Scholar]
- 13.Bayes de Luna A, Martinez-Selles M, Bayes-Genis A, et al. Surface ECG interatrial block-guided treatment for stroke prevention: rationale for an attractive hypothesis. BMC Cardiovasc Disord. 2017;17:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heistaro S. Methodology report: Health 2000 survey. Helsinki, Finland: Publications of the National Public Health Institute B26; 2008. Available from: http://urn.fi/URN:NBN:fi-fe201204193320
- 15.Prineas RJ, Crow RS, Blackburn HW. The Minnesota code manual of electrocardiographic findings. Littleton (MA): John Wright-PSG; 1982. [Google Scholar]
- 16.Sund R. Quality of the Finnish hospital discharge register: a systematic review. Scand J Public Health. 2012;40:505–515. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization [Internet]. International Diabetes Federation. Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia: report of a WHO/IDF consultation. 2006. [cited 2019 Apr 16]. Available from: http://www.who.int/diabetes/publications/diagnosis_diabetes2006/en/
- 18.Ariyarajah V, Puri P, Kranis M, et al. Prevalence of interatrial block in the Program of All-Inclusive Care for the Elderly (PACE). Amer J Geriatric Cardiol. 2006;15:174–177. [DOI] [PubMed] [Google Scholar]
- 19.Baranchuk A, Alexander B, Cinier G, et al. Bayés' syndrome: time to consider early anticoagulation? North Clin Istanb. 2018;5:370–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Massó-van Roessel A, Escobar-Robledo LA, Dégano IR, et al. Analysis of the association between electrocardiographic p-wave characteristics and atrial fibrillation in the REGICOR study. Rev Esp Cardiol Engl Ed. 2017;70:841–847. [DOI] [PubMed] [Google Scholar]
- 21.Ariyarajah V, Spodick DH. Progression of partial to advanced interatrial block. J Electrocardiol. 2006;39:177–179. [DOI] [PubMed] [Google Scholar]
- 22.Ariyarajah V, Kranis M, Apiyasawat S, et al. Potential factors that affect electrocardiographic progression of interatrial block. Ann Noninv Electrocard. 2007;12:21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jahangir A, Lee V, Friedman PA, et al. Long-term progression and outcomes with aging in patients with lone atrial fibrillation: a 30-year follow-up study. Circulation. 2007;115:3050–3056. [DOI] [PubMed] [Google Scholar]
- 24.Disertori M, Franzosi MG, Barlera S, et al. Thromboembolic event rate in paroxysmal and persistent atrial fibrillation: data from the GISSI-AF trial. BMC Cardiovasc Disord. 2013;13:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirsh BJ, Copeland-Halperin RS, Halperin JL. Fibrotic atrial cardiomyopathy, atrial fibrillation, and thromboembolism: mechanistic links and clinical inferences. J Am Coll Cardiol. 2015;65:2239–2251. [DOI] [PubMed] [Google Scholar]
- 26.Alexander B, Baranchuk A, Haseeb S, et al. Interatrial block predicts atrial fibrillation in patients with carotid and coronary artery disease. J Thorac Dis. 2018; 10:4328–4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Álvarez-García J, Vives-Borrás M, Gomis P, et al. Electrophysiological effects of selective atrial coronary artery occlusion in humans. Circulation. 2016;133:2235–2242. [DOI] [PubMed] [Google Scholar]
- 28.Ariyarajah V, Apiyasawat S, Moorthi R, et al. Potential clinical correlates and risk factors for interatrial block. Cardiology. 2006;105:213–218. [DOI] [PubMed] [Google Scholar]
- 29.Santangeli P, Di Biase L, Bai R, et al. Atrial fibrillation and the risk of incident dementia: a meta-analysis. Heart Rhythm. 2012;9:1761–1768.e2. [DOI] [PubMed] [Google Scholar]
- 30.Recommendations for measurement standards in quantitative electrocardiography. The CSE Working Party. Eur Heart J. 1985;6:815–825. [PubMed] [Google Scholar]
- 31.Holmqvist F, Platonov PG, McNitt S, et al. Abnormal P-wave morphology is a predictor of atrial fibrillation development and cardiac death in MADIT II patients. Ann Noninvasive Electrocardiol. 2010;15:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bayés de Luna A, Escobar-Robledo LA, Aristizabal D, et al. Atypical advanced interatrial blocks: dDefinition and electrocardiographic recognition. J Electrocardiol. 2018;51:1091–1093. [DOI] [PubMed] [Google Scholar]