Abstract
Purpose
Emerging data suggest that coronavirus disease 2019 (COVID-19) has extrapulmonary manifestations but its renal manifestations are not clearly defined. We aimed to evaluate renal complications of COVID-19 and their incidence using a systematic meta-analysis.
Design
Observational studies reporting renal complications in COVID-19 patients were sought from MEDLINE, Embase and the Cochrane Library from 2019 to June 2020. The nine-star Newcastle-Ottawa Scale was used to evaluate methodological quality. Incidence with 95% confidence intervals (CIs) were pooled using random-effects models.
Results
We included 22 observational cohort studies comprising of 17,391 COVID-19 patients. Quality scores of studies ranged from 4 to 6. The pooled prevalence of pre-existing chronic kidney disease (CKD) and end-stage kidney disease was 5.2% (2.8–8.1) and 2.3% (1.8–2.8), respectively. The pooled incidence over follow-up of 2–28 days was 12.5% (10.1–15.0) for electrolyte disturbance (e.g. hyperkalaemia), 11.0% (7.4–15.1) for acute kidney injury (AKI) and 6.8% (1.0–17.0) for renal replacement therapy (RRT). In subgroup analyses, there was a higher incidence of AKI in US populations and groups with higher prevalence of pre-existing CKD.
Conclusions
Frequent renal complications reported among hospitalized COVID-19 patients are electrolyte disturbance, AKI and RRT. Aggressive monitoring and management of these renal complications may help in the prediction of favourable outcomes.
Systematic review registration: PROSPERO 2020: CRD42020186873
KEY MESSAGES
COVID-19 affects multiple organs apart from the respiratory system; however, its renal manifestations are not clearly defined.
In this systematic meta-analysis of 22 observational cohort studies, the prevalence of pre-existing chronic kidney disease (CKD) in COVID-19 patients was 5.2%.
The most frequent renal complication was electrolyte disturbance (particularly hyperkalaemia) with an incidence of 12.5% followed by acute kidney injury (AKI) with an incidence of 11.0%; US populations and groups with higher prevalence of CKD had higher incidence of AKI.
Keywords: Renal complications, acute kidney injury, COVID-19, meta-analysis
Introduction
Coronavirus disease 2019 (COVID-19), which is caused by severe acute respiratory syndrome coronavirus 2 (SARS CoV-2) was declared a global public health emergency on 30 January 2020. The COVID-19 pandemic has caused substantial morbidity and mortality worldwide [1] and poses the most significant modern-day public health challenge since the Spanish flu of 1918. Coronavirus disease 2019 predominantly affects the respiratory system, typically manifesting as acute respiratory distress syndrome (ARDS) and severe pneumonia in a few, whereas the majority of patients are asymptomatic or present with mild symptoms [2]. The most common symptoms of COVID-19 are fever, cough, myalgia or fatigue [3]. Older patients, males and those with pre-existing comorbidities such as cardiovascular disease (CVD), hypertension, chronic kidney disease (CKD), chronic liver disease and diabetes are reported to be more likely to be infected with SARS CoV-2 [4] and are at highest risk for severe illness or death [5,6]. Emerging data suggest that COVID-19 also affects multiple organs, leading to organ failure and eventually death [7]. Common cardiovascular complications reported to be associated with COVID-19 include myocardial injury and heart failure [8], which have been shown to correlate with the severity of or mortality from COVID-19 [9]. Furthermore, emerging data also suggest COVID-19 contributes to adverse renal manifestations such as acute kidney injury (AKI), which is also associated with severe COVID-19 or mortality [10]. Given the sparse data, the renal manifestations of COVID-19 are not clearly defined. Despite the rapidly growing knowledge base on the clinical course of the disease, no effective therapeutic agents have been identified. Further data on the clinical course of the disease could help in the development of effective treatment strategies. Understanding the interplay between COVID-19 and its renal manifestations could assist in the management of patients. In this context, we sought to address the following questions using a first systematic review and meta-analysis of published evidence: (i) what are the renal complications associated with COVID-19? (ii) what is the incidence of these complications? and (iii) are patients with pre-existing renal conditions more susceptible to these renal complications?
Materials and methods
Data sources and search strategy
The review was based on a predefined protocol which was registered in the PROSPERO International prospective register of systematic reviews (CRD42020186873) and it was conducted in accordance with PRISMA and MOOSE guidelines [11,12] (Supplementary materials 1–2). MEDLINE, Embase and The Cochrane library were searched from 2019 to 13 June 2020 for published studies reporting on renal complications in patients with COVID-19. We combined search terms and key words related to the population (e.g. “COVID-19”, “SARS-CoV-2”) and outcomes (e.g. “kidney”, “renal”, “acute kidney injury”, “renal transplant therapy”, “albuminuria”) in humans, which was limited to only reports published in the English language given the potential for duplicate reporting using the same study participants [13]. The detailed search strategy can be found in Supplementary material 3. Titles and abstracts were screened for potential eligible studies following retrieval of citations. Following initial screening, full texts of potentially eligible studies were acquired for detailed evaluation. Manual scanning of key articles and review papers was conducted to identify additional articles missed by the search strategy.
Study selection and eligibility criteria
The protocol was pre-specified to include observational studies (prospective and retrospective, nested case-control and case-control designs), non-randomized clinical studies and randomized controlled trials (RCTs) which reported renal complications in patients with COVID-19. Our protocol was pre-specified to include all renal complications anticipated to be reported by studies such as AKI, proteinuria, haematuria, albuminuria, electrolyte disturbance, renal acidosis and alkalosis, need for renal replacement therapy (RRT) and kidney transplant among others. We also sought for studies reporting information on any pre-existing renal conditions (e.g. CKD, end-stage kidney disease); however, they were not included if they did not report on any renal complication. Patients must have had a period of follow-up prior to developing complications.
Data extraction and quality assessment
Using a pre-designed data extraction form, the following data were extracted from the eligible studies: author and year of publication; study characteristics (design, location and date of data collection); patient characteristics (average age, sex, percentage of males, total number of patients, pre-existing renal conditions and their counts and follow-up duration or hospital stay); and renal complications and their counts. Data were extracted and analyzed as reported. However, hyperkalaemia was reported by one study and was classified as an electrolyte disturbance to enhance consistency and enable pooling. To minimize over- and under-reporting and maintain consistency, extraction of prevalence and incidence data employed an intention-to-treat principle. Methodological quality of studies was assessed using the nine-star Newcastle-Ottawa Scale (NOS) [14], a tool which has been validated for assessing the quality of non-randomized studies.
Statistical analysis
Pooled prevalence of pre-existing renal conditions (e.g. CKD) with 95% confidence intervals (CIs) was calculated from the number of pre-existing renal conditions/total number of patients with COVID-19 in the study. Incidence of renal complications with 95% CIs was estimated from the number of patients experiencing the specific renal complication within period of follow-up (hospital stay)/total number of patients with COVID-19. Given that the data were binary with some low counts, the Freeman–Tukey variance stabilizing double arcsine transformation [15] was used in calculating prevalence and incidence estimates as done in previous reports [16–18]. Heterogeneity was assessed and quantified using Cochrane χ2 and I2 statistics [19]. We also estimated 95% prediction intervals to determine the degree of heterogeneity, as they provide a region in which about 95% of the true effects of a new study are expected to be found [20,21]. Pre-defined study-level characteristics such as location, age and comorbidities which may explain heterogeneity were explored using stratified analysis and random effects meta-regression. STATA release MP 16 (StataCorp LP, College Station, TX, USA) was used for all statistical analyses.
Results
Study identification and selection
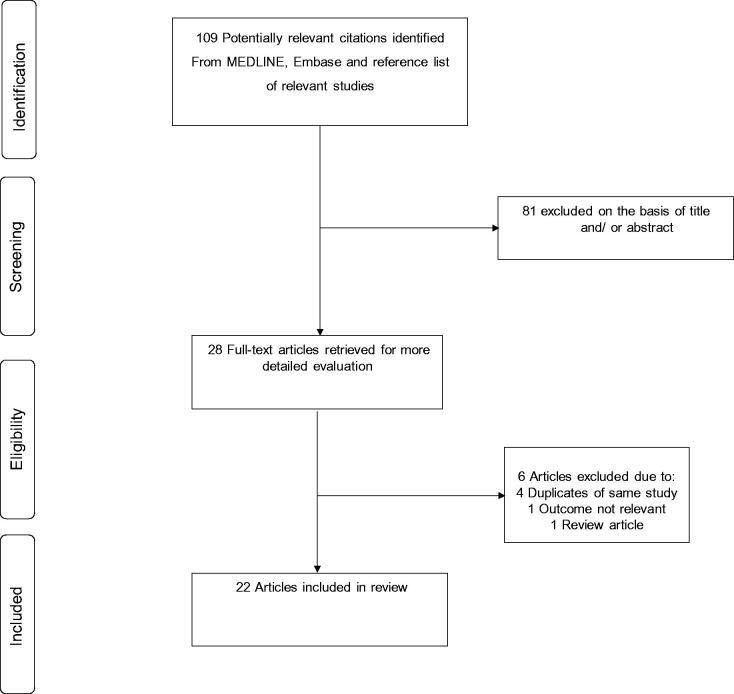
The process of study selection is presented in Figure 1. Overall, a total of 109 articles were identified from the search of major databases and manual scanning of reference lists of relevant articles. After initial screening based on titles and abstracts, full texts of 28 articles were retrieved for further evaluation. Six articles were excluded on the basis of (i) duplicates of the same study (n = 4); (ii) outcome not relevant (n = 1) and (iii) review article (n = 1). This left a total of 22 articles for inclusion in the review [1,3,9,22–40].
Figure 1.
Selection of studies included in the meta-analysis.
Study characteristics and quality
All 22 studies were based on observational cohort designs (21 retrospective cohorts and 1 prospective cohort), altogether comprising of 17,391 patients with COVID-19 (Table 1). Sixteen studies were based in China and six based in the United States. The average age at baseline ranged from 46 to 71 years, with a weighted mean (standard deviation, SD) of 60 (5) years. All studies enrolled both male and female patients. Hospital stay or follow-up duration ranged from 2 to 28 days with a weighted mean (SD) of 7.0 (4.0) days. The overall NOS methodological quality scores of studies ranged from 4 to 6.
Table 1.
Characteristics of included studies.
| Author, year of publication | Source of data | Country | Date of data collection | Mean/median age (years) | Males, % | Hospital stay/ follow-up (days) | No. of patients | AKI cases | NOS score |
|---|---|---|---|---|---|---|---|---|---|
| Aggarwal, 2020 | UnityPoint Clinic | USA | March–April 2020 | 67.0 | 75 | 2.0 | 16 | 11 | 4 |
| Arentz, 2020 | Evergreen Hospital in Kirkland, Washington | USA | Feb–March 2020 | 70.0 | 52 | 5.2 | 21 | 4 | 4 |
| Cao, 2020 | Zhongnan Hospital of Wuhan University | China | Jan–Feb 2020 | 54.0 | 52 | 11.0 | 102 | 20 | 4 |
| Chen, 2020 | Tongji Hospital in Wuhan | China | Jan–Feb 2020 | 62.0 | 62 | 13.0 | 274 | 29 | 4 |
| Cheng, 2020 | Tertiary teaching hospital | China | NR | 63.0 | 52.4 | 10.0 | 701 | 36 | 6 |
| Guan, 2020 | National Health Commission | China | Dec–Jan 2020 | 47.0 | 58.1 | 12.0 | 1099 | 6 | 4 |
| Guo, 2020 | Seventh Hospital of Wuhan City | China | Jan–Feb 2020 | 58.5 | 48.7 | 16.3 | 187 | 18 | 5 |
| Huang, 2020 | Jin Yintan Hospital, Wuhan | China | Dec–Jan 2020 | 49.0 | 73 | 7.0 | 41 | 3 | 4 |
| Liu, 2020 | Shenzhen Third People’s hospital | China | Dec–Jan 2020 | NR | 66.7 | 8.6 | 12 | 2 | 4 |
| Phipps, 2020 | New York-Presbyterian network | USA | March–April 2020 | 65.0 | 57.0 | 6.0 | 2,273 | NR | 6 |
| Price-Haywood, 2020 (W) | Ochsner Health in Louisiana | USA | March–April 2020 | 55.5 | 45.7 | 7.0 | 1,030 | 34 | 6 |
| Price-Haywood, 2020 (B) | Ochsner Health in Louisiana | USA | March–April 2020 | 53.6 | 37.7 | 6.0 | 2,451 | 163 | 6 |
| Richardson, 2020 | 12 Hospitals in New York-Northwell Health system | USA | March–April 2020 | 63.0 | 60.3 | 4.5 | 5700 | 523 | 4 |
| Ruan, 2020 | Jin Yin-tan Hospital and Tongji Hospital | China | NR | 57.7 | 68 | 10.1 | 150 | 23 | 4 |
| Shi, 2020 | Renmin Hospital of Wuhan University | China | Jan–Feb 2020 | 64.0 | 49.3 | NR | 416 | 8 | 6 |
| Wang, 2020 | Zhongnan Hospital of Wuhan University | China | Jan, 2020 | 56.0 | 54.3 | 7.0 | 138 | 5 | 4 |
| Wang, 2020b | Renmin Hospital of Wuhan University | China | Jan–Feb 2020 | 71.0 | 49 | 28.0 | 339 | 27 | 4 |
| Wang, 2020c | Zhongnan Hospital of Wuhan University and Xishui People’s Hospital | China | Up to Feb, 2020 | 51.0 | 53.3 | 11.0 | 107 | 14 | 5 |
| Yang, 2020 | Wuhan Jin Yin-tan hospital | China | Dec–Jan 2020 | 59.7 | 67 | 10.0 | 52 | 15 | 4 |
| Zhao, 2020 | Jingzhou Central Hospital | China | Jan–Feb 2020 | 46.0 | 53.8 | NR | 91 | 5 | 4 |
| Zhao, 2020b | Shouyi and East districts of Renmin Hospital of Wuhan University | China | Jan–Feb 2020 | 61.0 | 46.6 | 7.0 | 1,000 | 29 | 4 |
| Zhou, 2020 | Jinyintan Hospital & Wuhan Pulmonary Hospital | China | Dec–Jan 2020 | 56.0 | 62 | 11.0 | 191 | 28 | 5 |
AKI: acute kidney injury; NOS: Newcastle-Ottawa Scale; NR: not reported.
Prevalence of pre-existing renal conditions
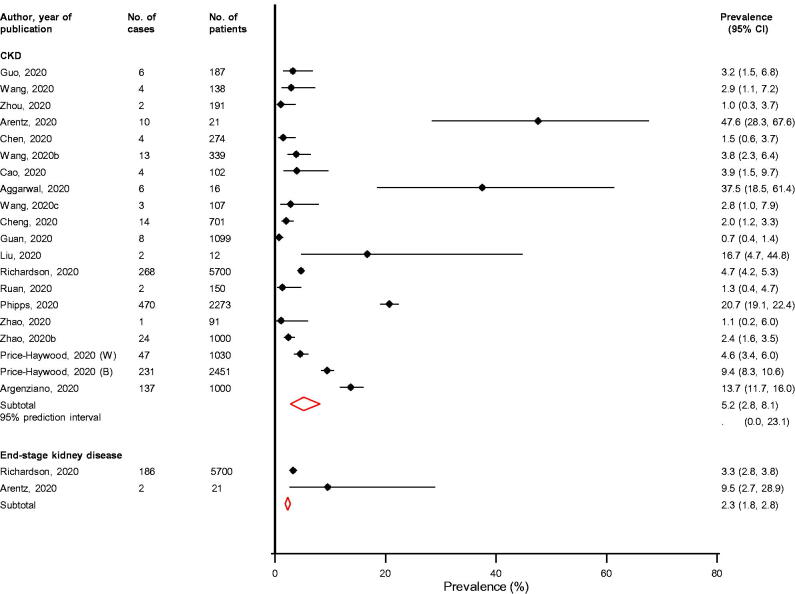
Across 20 studies providing relevant data, the prevalence of pre-existing CKD in COVID-19 patients ranged from 0.7% to 47.6%, with a pooled random effects prevalence (95% CI) of 5.2% (2.8–8.1; I2 = 98%; 95% CI 97, 98%; p for heterogeneity < .01) (Figure 2). The 95% prediction interval for the summary prevalence was 0.0–23.1%, suggesting that the true prevalence of pre-existing CKD for any single new study will usually fall within this range. The prevalence of pre-existing end-stage kidney disease based on pooled analysis of two studies was 2.3% (1.8–2.8) (Figure 2).
Figure 2.
Prevalence of pre-existing renal conditions in COVID-19 patients. B: black; CI: confidence interval (bars); CKD: chronic kidney disease; W: white.
Incidence of renal complications
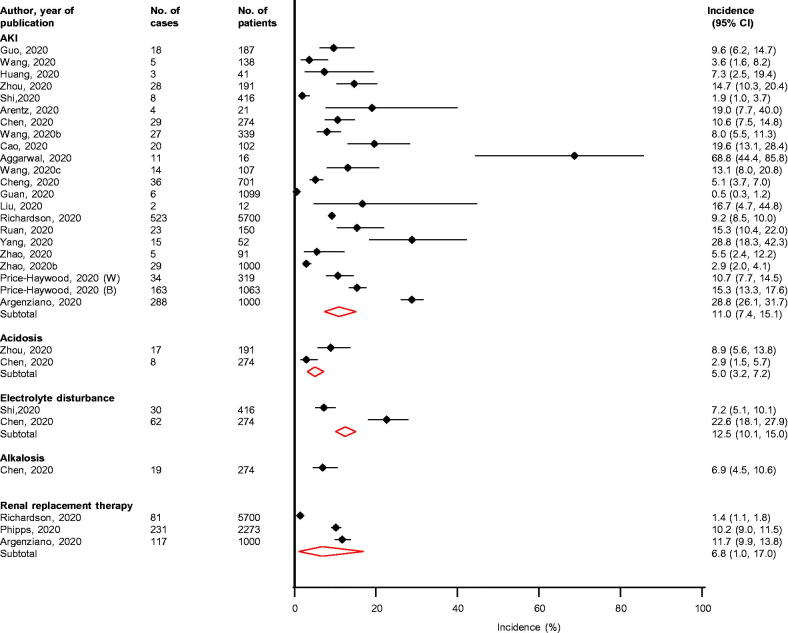
Overhospital stays ranging from 2 to 28 days following admission, the pooled incidence for AKI (n = 22 studies) was 11.0% (7.4–15.1; I2 = 97%; 95% CI 97, 98%; p for heterogeneity < .01) (Figure 3). For 14 studies reporting data on the definition of AKI, 12 defined AKI according to Kidney Disease Improving Global Outcomes (KDIGO) criteria (Supplementary Appendix 4) [41]. The incidence of electrolyte disturbance (n = 2 studies), need for RRT (n = 3 studies) and acidosis (n = 2 studies) were 12.5% (10.1–15.0), 6.8% (1.0–17.0) and 5.0 (3.2–7.2), respectively (Figure 3). Based on the report by a single study, the incidence of alkalosis was 6.9 (4.5–10.6).
Figure 3.
Incidence of renal complications in COVID-19 patients. AKI: acute kidney injury; CI: confidence interval (bars).
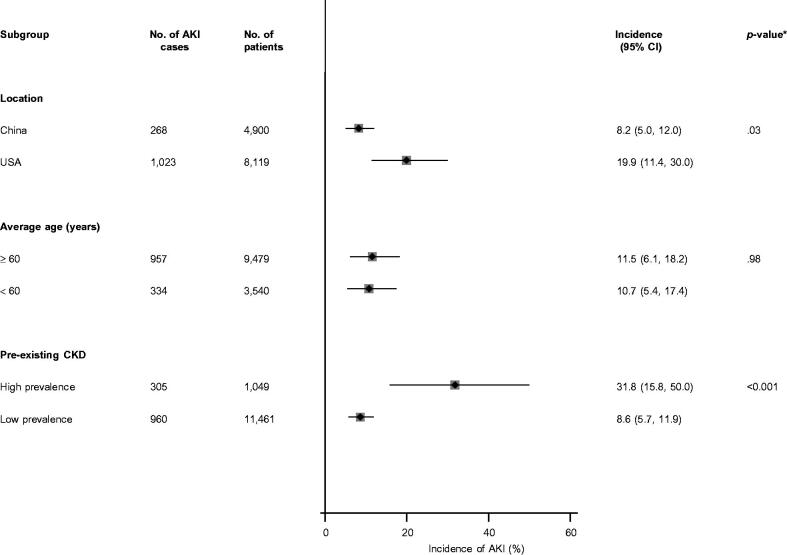
Given AKI was the outcome commonly reported by studies (22 studies) and with the substantial heterogeneity between contributing studies, we explored for potential sources of heterogeneity using stratified analysis and random effects meta-regression. There was statistically significant evidence of effect modification on the incidence of AKI by location (p value for meta-regression = 0.03) and pre-existing CKD (p value for meta-regression < .001). There was no evidence of effect modification by age (Figure 4).
Figure 4.
Incidence of acute kidney injury in COVID-19 patients, by clinically relevant characteristics. AKI: acute kidney injury; CI: confidence interval (bars); CKD: chronic kidney disease; *p value for meta-regression.
Discussion
In hospitalized patients with renal manifestations of COVID-19, the prevalence of pre-existing CKD was 5.2% and that for end-stage kidney disease was 2.3%. Over hospital stays ranging from 2 to 28 days, AKI was the common outcome reported by studies; howeverelectrolyte disturbance (hyperkalaemia) was the most frequent renal complication with an incidence of 12.5% followed by AKI and RRT at 11.0% and 6.8%, respectively. Other reported complications included acidosis and alkalosis. Subgroup analyses suggested evidence of effect modification by location and pre-existing history of CKD on the incidence of AKI; the incidence of AKI was higher in the US population (than the Chinese population) and among groups with higher prevalence of pre-existing CKD (than those with lower prevalence). However, AKI incidence was comparable in younger (<60 years) and older (≥60 years) individuals.
Although COVID-19 predominantly affects the respiratory system, there is involvement of multiple organs, such as the gastrointestinal system, the cardiovascular system, the liver as well as the kidneys. These multiple organ disturbances may then interact with each other, which correlates with the severity of the disease. The virus SARS-CoV-2 is known to enter human lung cells by binding to angiotensin-converting enzyme 2 (ACE2) [42]. The multiorgan involvement of SARS-CoV-2 has been linked to the wide distribution of ACE2 in the body; the highest expression of ACE2 is found in the ileum and kidneys [43,44]. In the kidney, ACE2 is expressed on several cells including mesangial cells, podocytes, parietal epithelium of the Bowman’s Capsule, and the collecting ducts [45]. Although the mechanisms for the renal manifestations of COVID-19 are still elusive, a complex multifactorial pathway has been proposed and it includes the following: (i) direct viral involvement and replication in the kidneys leading to dysfunction [46]; (ii) local disruption in renin–angiotensin–aldosterone system (RAAS) homeostasis [44]; (iii) lung protective fluid management strategy during treatment of ARDS [40] and (iv) as a result of a systemic inflammatory response “cytokine storm” [44].
COVID-19 represents a great medical challenge and appears to have multisystem effects which include renal manifestations. The current data based on up-to-date evidence suggest that AKI is commonly reported as a complication among patients with COVID-19. In addition to pre-existing CKD being associated with severe illness or death in COVID-19 [5], it is also an independent risk factor for AKI [47]. Consistently, our study findings showed that groups with higher prevalence of pre-existing CKD might have higher incidence of AKI. Emerging evidence also suggests that renal manifestations of COVID-19 are also associated with increased risk of severe COVID-19 and fatal outcomes [10,30]. Monitoring of markers of kidney function during hospitalization for COVID-19 could help in the identification of patients who at high risk for worse outcomes, to enable early and more aggressive intervention. The current evidence provides better insight on the extent of kidney damage by COVID-19. However, more work is needed to help us better understand the pathophysiology underlying renal manifestations of COVID-19, to help in the identification of effective management strategies.
We have provided up-to-date data on the different renal manifestations of COVID-19 as well as their incidence rates. In addition, prevalence estimates of common renal comorbidities have also been presented. Other strengths of this study include ability to synthesize the data quantitatively as well as exploration for sources of heterogeneity. There were some limitations which were mostly inherent and included (i) inability to generalize the findings and the possibility of patient overlap, given that the majority of studies were based in China; (ii) the definition of CKD and classification into stages were not reported by included studies; (iii) a number of studies did not report on the definition for AKI; however, the majority defined AKI according to KDIGO criteria; (iv) studies reporting on the complications of acidosis and alkalosis did not distinguish whether these outcomes were of renal or lung origin; (v) one study reported an outcome of electrolyte disturbance, but did not provide a definition of this; (vi) the potential for differences in the timing during hospitalisation with regards to assessment of complications; and (vii) the low sample sizes of some of the studies.
Conclusion
Aggregate up-to-date synthesis of the existing literature suggests that the most frequent renal complications among patients hospitalized with COVID-19 are electrolyte disturbance (particularly hyperkalaemia), AKI and the need for RRT. Aggressive monitoring and management of these renal complications may help in the prediction of more favourable outcomes.
Supplementary Material
Funding Statement
Dr. Kunutsor acknowledges support from the NIHR Biomedical Research Centre at University Hospitals Bristol NHS Foundation Trust and the University of Bristol. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health and Social Care. These sources had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Zhou F, Yu T, Du R, et al. . Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Z, McGoogan JM.. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239. [DOI] [PubMed] [Google Scholar]
- 3.Huang C, Wang Y, Li X, et al. . Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng YY, Ma YT, Zhang JY, et al. . COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17(5):259–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weiss P, Murdoch DR.. Clinical course and mortality risk of severe COVID-19. Lancet. 2020;395(10229):1014–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kunutsor SK, Laukkanen JA.. Markers of liver injury and clinical outcomes in COVID-19 patients: a systematic review and meta-analysis. J Infect. [cited 2020 May 28]. DOI: 10.1016/j.jinf.2020.05.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Q, Guan X, Wu P, et al. . Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):1199–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kunutsor SK, Laukkanen JA.. Cardiovascular complications in COVID-19: a systematic review and meta-analysis. J Infect. [cited 2020 Jun 3]. DOI: 10.1016/j.jinf.2020.05.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo T, Fan Y, Chen M, et al. . Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020:;e201017. DOI: 10.1001/jamacardio.2020.1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang X, Fang X, Cai Z, et al. . Comorbid chronic diseases and acute organ injuries are strongly correlated with disease severity and mortality among COVID-19 patients: a systemic review and meta-analysis. Research (Wash D C). 2020;2020:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stroup DF, Berlin JA, Morton SC, et al. . Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283(15):2008–2012. [DOI] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, PRISMA Group, et al.. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bauchner H, Golub RM, Zylke J.. Editorial concern-possible reporting of the same patients with COVID-19 in different reports. JAMA. 2020;323(13):1256. [DOI] [PubMed] [Google Scholar]
- 14.Wells GA, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses; 2011. [cited 2020 Jul 3]. www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 15.Freeman MF, Tukey JW.. Transformations related to the angular and the square root. Ann Math Statist. 1950;21(4):607–611. [Google Scholar]
- 16.Kunutsor SK, Whitehouse MR, Blom AW, Inform Team, et al.. Re-infection outcomes following one- and two-stage surgical revision of infected hip prosthesis: a systematic review and meta-analysis. PLoS One. 2015;10(9):e0139166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kunutsor SK, Whitehouse MR, Lenguerrand E, Inform Team, et al.. Re-infection outcomes following one- and two-stage surgical revision of infected knee prosthesis: a systematic review and meta-analysis. PLoS One. 2016;11(3):e0151537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kunutsor SK, Beswick AD, Whitehouse MR, et al. . Debridement, antibiotics and implant retention for periprosthetic joint infections: a systematic review and meta-analysis of treatment outcomes. J Infect. 2018;77(6):479–488. [DOI] [PubMed] [Google Scholar]
- 19.Higgins JP, Thompson SG, Deeks JJ, et al. . Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riley RD, Higgins JP, Deeks JJ.. Interpretation of random effects meta-analyses. BMJ. 2011;342:d549. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JP, Thompson SG, Spiegelhalter DJ.. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc. 2009;172(1):137–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aggarwal S, Garcia-Telles N, Aggarwal G, et al. . Clinical features, laboratory characteristics, and outcomes of patients hospitalized with coronavirus disease 2019 (COVID-19): early report from the United States. Diagnosis (Berl). 2020;7(2):91–96. [DOI] [PubMed] [Google Scholar]
- 23.Chen T, Wu D, Chen H, et al. . Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang D, Hu B, Hu C, et al. . Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang D, Yin Y, Hu C, et al. . Clinical course and outcome of 107 patients infected with the novel coronavirus, SARS-CoV-2, discharged from two hospitals in Wuhan, China. Crit Care. 2020;24(1):188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang L, He W, Yu X, et al. . Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. J Infect. 2020;80(6):639–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi S, Qin M, Shen B, et al. . Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;e200950. DOI: 10.1001/jamacardio.2020.0950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arentz M, Yim E, Klaff L, et al. . Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020;323(16):1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao J, Tu WJ, Cheng W, et al. . Clinical features and short-term outcomes of 102 patients with corona virus disease 2019 in Wuhan, China. Clin Infect Dis. 2020:ciaa243. DOI: 10.1093/cid/ciaa243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng Y, Luo R, Wang K, et al. . Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97(5):829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guan WJ, Ni ZY, Hu Y, et al. . Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Y, Yang Y, Zhang C, et al. . Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63(3):364–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richardson S, Hirsch JS, Narasimhan M, et al. . Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020;323(20):2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ruan Q, Yang K, Wang W, et al. . Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(6):1294–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang X, Yu Y, Xu J, et al. . Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475-481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phipps MM, Barraza LH, LaSota ED, et al. . Acute liver injury in COVID-19: prevalence and association with clinical outcomes in a large US cohort. Hepatology. [cited 2020 May 30].. DOI: 10.1002/hep.31404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao M, Wang M, Zhang J, et al. . Comparison of clinical characteristics and outcomes of patients with coronavirus disease 2019 at different ages. Aging (Albany, NY). 2020;12(11):10070–10086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao XY, Xu XX, Yin HS, Hu QM, et al. . Clinical characteristics of patients with 2019 coronavirus disease in a non-Wuhan area of Hubei Province, China: a retrospective study. BMC Infect Dis. 2020;20(1):311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Price-Haywood EG, Burton J, Fort D, et al. . Hospitalization and mortality among Black patients and White patients with Covid-19. N Engl J Med. 2020;382(26):2534–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Argenziano MG, Bruce SL, Slater CL, et al. . Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020;369:m1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138. [Google Scholar]
- 42.Yan R, Zhang Y, Li Y, et al. . Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367(6485):1444–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zou X, Chen K, Zou J, et al. . Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14(2):185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martinez-Rojas MA, Vega-Vega O, Bobadilla NA.. Is the kidney a target of SARS-CoV-2? Am J Physiol Renal Physiol. 2020;318(6):F1454-F1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hamming I, Timens W, Bulthuis ML, et al. . Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Diao B, Wang C, Wang R, et al. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. medRxiv. 2020:2020.03.04.20031120. [Google Scholar]
- 47.Chawla LS, Eggers PW, Star RA, et al. . Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med. 2014;371(1):58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.