Abstract
Coronavirus disease 2019 (COVID-19), caused by novel enveloped single stranded RNA coronavirus (SARS-CoV-2), is responsible for an ongoing global pandemic. While other countries deployed widespread testing as an early mitigation strategy, the U.S. experienced delays in development and deployment of organism identification assays. As such, there is uncertainty surrounding disease burden and community spread, severely hampering containment efforts. COVID-19 illuminates the need for a tiered diagnostic approach to rapidly identify clinically significant infections and reduce disease spread. Without the ability to efficiently screen patients, hospitals are overwhelmed, potentially delaying treatment for other emergencies. A multi-tiered, diagnostic strategy incorporating a rapid host immune response assay as a screening test, molecular confirmatory testing and rapid IgM/IgG testing to assess benefit from quarantine/further testing and provide information on population exposure/herd immunity would efficiently evaluate potential COVID-19 patients. Triaging patients within minutes with a fingerstick rather than hours/days after an invasive swab is critical to pandemic response as reliance on the existing strategy is limited by assay accuracy, time to results, and testing capacity. Early screening and triage is achievable from the outset of a pandemic with point-of-care host immune response testing which will improve response time to clinical and public health actions.
Key messages
Delayed testing deployment has led to uncertainty surrounding overall disease burden and community spread, severely hampering public health containment and healthcare system preparation efforts.
A multi-tiered testing strategy incorporating rapid, host immune point-of-care tests can be used now and for future pandemic planning by effectively identifying patients at risk of disease thereby facilitating quarantine earlier in the progression of the outbreak during the weeks and months it can take for pathogen specific confirmatory tests to be developed, validated and manufactured in sufficient quantities.
The ability to triage patients at the point of care and support the guidance of medical and therapeutic decisions, for viral isolation or confirmatory testing or for appropriate treatment of COVID-19 and/or bacterial infections, is a critical component to our national pandemic response and there is an urgent need to implement the proposed strategy to combat the current outbreak.
Keywords: COVID-19, Coronavirus, SARS-CoV-2, myxovirus resistance protein A (MxA), C-reactive protein (CRP), molecular diagnostics, serology, rapid diagnostic tests, point-of-care test
Background
Acute respiratory infection (ARI) is responsible for more than 100 million adult ambulatory care visits [1] and 29 million paediatric emergency department (ED) visits annually in the United States [2]. Community-acquired bacterial pneumonia (CABP), which is commonly associated with ARI, is the second most common cause of hospitalization and the most common infectious cause of death in the United States [3]. Viruses cause over 85% of ARIs [1] with seasonal influenza typically affecting 3–8% of the U.S. population each year [4]. Common bacterial infections such as group A streptococcus pharyngitis and CABP comprise the remaining minority of ARI [1]. Coronavirus disease 2019 (COVID-19), caused by novel enveloped single-stranded RNA coronavirus (SARS-CoV-2), has a similar nucleotide identity to other novel coronavirus outbreaks causing ARI in humans, 2003 SARS-CoV and 2012 MERS-CoV [5–7]. SARS-CoV-2 is responsible for a global pandemic that began in the winter of 2019. Although overall COVID-19 mortality rates are low relative to other recent viral epidemics (e.g. SARS, Ebola) [8,9], older adults and those with chronic disease are at substantially increased risk of morbidity and mortality and increasingly, younger adults (<50) are requiring critical care hospital resources [10]. The sudden spike in patients requiring ventilator support overwhelmed local healthcare systems in several areas of the world including Wuhan, China and most recently northern Italy and New York City [11,12]. Per Institute of Healthcare Metrics and Evaluation, the peak of daily COVID-19 deaths was 2,688 on April 15, 2020 and over 55,000 people have died in the U.S. as of April 27, 2020 [13,14].
While other countries deployed widespread testing early during the SARS-CoV-2 pandemic (e.g. South Korea, Germany), the U.S. has experienced significant delays in the development and deployment of organism identification assays. As such, there is uncertainty surrounding overall disease burden and community spread, severely hampering public health containment and healthcare system preparation efforts. COVID-19 illuminates the need for a tiered and coordinated diagnostic approach to rapidly identify clinically significant infections and reduce the spread of disease. Without the ability to efficiently screen patients, healthcare facilities will become overwhelmed, potentially delaying treatment for other emergency conditions (e.g. bacterial sepsis).
Molecular pathogen detection using real-time polymerase chain reaction (PCR) has been established as the gold standard for confirmatory diagnosis of SARS-CoV-2 infections. This technology is designed to be a confirmatory test and is not well suited for large-scale screening efforts. Molecular tests have reported limited sensitivity during the first 7 days of symptom onset (ranging from 67 to 72%), which may be due to low viral loads early in the disease course or collection technique [15,16]. Molecular tests require a nasopharyngeal (NP) swab, an invasive procedure that is uncomfortable for the patient when administered correctly, and specialized training is suggested for those administering the test. After swabbing, the provider mixes the specimen with liquid in an open container which could release a highly contagious pathogen, spread by droplets, into the air. NP swabs may also be considered an aerosol generating procedure (AGP), which may induce a cough and repeatedly expose healthcare workers to droplets transmission. Thus, healthcare workers who perform this testing may need additional protective equipment, which remains in short supply, to safely perform this testing. Inadequate sample collection may also contribute to the reduced sensitivity of molecular tests [17,18].
Increasingly, false negative molecular test results are of concern to clinicians on the frontlines who see patients with clear clinical presentations of COVID-19 (e.g. cough, fever, characteristic chest imaging) and test negative for SARS-CoV-2 on day one but test positive days later [17,18]. Recently released data from the Cleveland Clinic showed a false negative rate of 14.8% of the 239 specimens tested with a rapid PCR test [19]. The risk for false negative results requires repeat molecular testing, potentially adding days to a confirmatory diagnosis. Additionally, as PCR is theoretically capable of detecting small amounts of pathogen, it is yet to be elucidated what a positive result means in terms of infectivity, colonization, and active infection, especially among those who are asymptomatic.
The requirement of ancillary equipment and complex technical operation by trained professionals in certified labs subjects molecular RT-PCR tests to limitations. These include the procurement of expensive materials and equipment. In addition, these assays take 1 to 3 h to perform and total turnaround time can take upwards of 24 hours if the local lab does not have molecular testing capabilities in-house. Considering that specimens often need to be transported to a testing facility, there can be a delay in establishing a diagnosis.
Finally, concerns about testing capacity, including reagent shortages, have resulted in the Centres for Disease Control and Prevention issuing guidelines recommending testing be restricted to only select population (e.g. high risk) [20]. This limits front line providers ability to make rapid triage decisions and further strains the healthcare system. Testing limitations can also negatively impact individual patients via delayed treatment and lack of tailored quarantine recommendations [15].
A new molecular test with testing times reported to be 5 min was approved by the FDA on March 28, 2020 using non-clinical, contrived samples and although reported time to confirmatory diagnosis is shorter, to-date clinical diagnostic performance of this assay has not been validated [19]. Additionally, signs and symptoms of COVID-19 are non-specific and similar to that of bacterial pneumonia. Tests for a single viral pathogen may be negative and cause a bacterial infection to be overlooked leading to a delay in treatment and increase risk of morbidity and mortality. Furthermore, a molecular testing only strategy does not address the possibility that patients have stayed home, as directed, to manage an inciting SARS-CoV-2 infection only to present to a healthcare facility with similar clinical manifestations that are potentially related to a secondary bacterial pneumonia. In this scenario, persistent colonization can generate positive RT-PCR results for as many as 39 days post-active SARS-CoV-2 infection [16,21], leading to misdiagnosis and inappropriate initial treatment.
The most efficient and cost-effective approach to COVID-19 evaluation is a multi-tiered screening and diagnostic strategy. Ideally, any patient requiring evaluation can be (i) quickly triaged as having either viral, bacterial or absent immune response to an infection and then (ii) receive rapid confirmatory testing. Rapid host immune tests can quickly identify the presence of viral or bacterial infection. Accurate ARI characterization is a critical component of optimal therapeutic decisions, antibiotic stewardship, disposition planning, and quarantine procedures.
Available Rapid Technology
Host Immune Response Assay
Myxovirus resistance protein A (MxA) offers advantages as a biomarker for viral infection, including its low basal concentration (less than 15 ng/ml), long half-life (2.3 days) and fast induction (1–2 hours) [22]. Ronni et al. [23] demonstrated that MxA mRNA is detectable in isolated peripheral blood, white blood cells stimulated with interferon (IFN) alpha within 1 to 2 hours of IFN induction, and that MxA protein begins to accumulate shortly thereafter.
Many studies have shown that MxA protein expression in peripheral blood is a sensitive and specific marker of viral infection [24–31]. Higher MxA levels are found in viral infections compared with bacterial infections because MxA protein is induced exclusively by type I IFN and not by IFN-gamma, IL-1, TNF-alpha or any of the other cytokines expressed during bacterial infection [32]. Serum type 1 IFN levels remain within normal limits even in patients with severe bacterial infections [33,34]. Active viral replication later results in hyperproduction type I IFN and influx of neutrophils and macrophages, which are the major sources of pro-inflammatory cytokines. With similar changes in total neutrophils and lymphocytes during COVID-19, SARS-CoV-2 likely induces delayed type I IFN and loss of viral control in an early phase of infection [35].
A dual biomarker point-of-care (POC) lateral flow test (FebriDx, Lumos Diagnostics, Sarasota, FL, USA) capable of rapidly assesses the body’s host immune response to an ARI and helping differentiate viral from bacterial infections is already commercially available in Europe and parts of Asia. The dual biomarker (MxA/CRP) test is a single-use, 10-minute, POC test that (i) identifies host immune response to infection and (ii) aids in the differentiation of viral and bacterial ARI through the simultaneous detection of both Myxovirus resistance protein A (MxA) and C-reactive protein (CRP) directly from a blood sample obtained via fingerstick. When viral pathogens induce a clinically significant host immune response, MxA, a biomarker of the body’s innate response to a viral infection, will elevate. An elevation of MxA with or without an associated elevation in CRP is consistent with a viral infection. MxA with an associated rise in CRP may suggest a more severe underlying viral infection. Furthermore, early data published from the COVID-19 pandemic in China showed that CRP was significantly elevated in patients who progressed to severe illness or death compared to patients who experienced clinical improvement/stabilization (38.9 [14.3, 64.8] mg/L vs. 10.6 [1.9, 33.1] mg/L, U = 1.315, p = .024) [36]. In the context of SARS-CoV-2 screening efforts, MxA can identify if a patient has a host-response to a viral infection while CRP in addition to MxA may provide insight to risk of clinical decompensation. While an elevation in CRP without MxA can help to identify a patient with a true bacterial infection who may benefit from antibiotic therapy.
Two multicenter, U.S. clinical trials, found that MxA elevated in clinically significant viral infections caused by the following pathogens: Adenovirus, Rhinovirus, Influenza A, Influenza B, Metapneumovirus, Parainfluenza Virus 1-4, Respiratory Syncytial Virus, Herpes Simplex Virus, Epstein-Barr Virus, Cytomegalovirus, and Coronavirus (types 229E, OC43, NL63, and HKU1) [37,38]. A prospective multi-center U.S. clinical trial found the dual biomarker test to be 95% sensitive, 94% specific and have a negative predictive value of 99% to exclude a bacterial infection and a positive predictive value of 90% to confirm viral infection in febrile ARI patients [37]. The dual biomarker test has also been shown to influence clinical management in up to 90% of cases and reduce unnecessary use of antibiotics by 80–90% [39,40]. In the context of viruses with similar nucleotide identities, such as MERS-CoV and SARS-CoV, Tynell et al. [41] demonstrated that these coronaviruses can increase MxA expression. As such, the presence of elevated MxA could support a general triage strategy to rapidly triage patients with suspected COVID-19 infection and only refer positive or high risk cases for further confirmatory testing. The proposed paradigm, which uses 10-minute fingerstick screening tests, would result in increased throughput and decrease crowding in clinics, urgent cares, and emergency departments (EDs) and ultimately decrease time to receipt of therapeutics targeting the inciting infection. Additionally, by triaging patients using rapid point-of-care tests, confirmatory molecular testing capacity can be further augmented to alleviate backlogs and reagent shortages while simultaneously reducing costs (i.e. material and labor). In order for this strategy to be successfully implemented on a large scale, manufacturers of host immune point-of-care tests will need to have sufficient production to meet the testing demand.
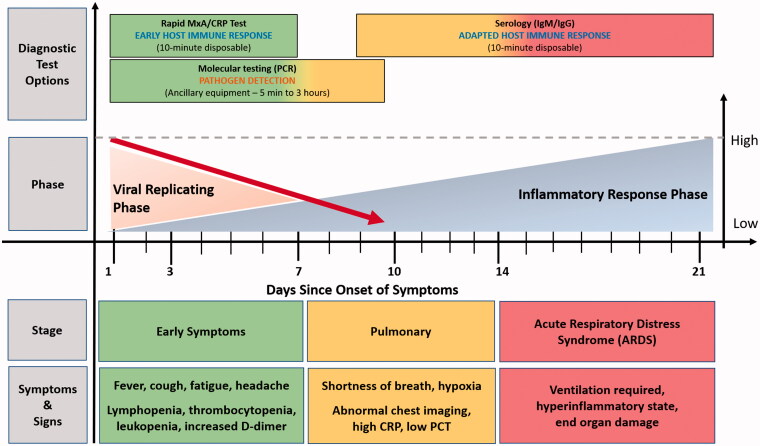
Another rapid POC lateral flow immunoassay detects IgM and IgG antibodies to SARS-CoV-2 virus within 15 minutes [42]. Typically, IgM takes 7–10 days to develop a detectable response and confirms a new infection if present. The detection of both IgM/IgG antibodies suggests a recent infection while IgM negative and IgG positive suggests a previous infection. This testing strategy would be most effective 1–2 weeks after the initial onset of symptoms and would also help to assess herd immunity and the risk of a new infection for those returning from quarantine. The testing sensitivity ranges 28.7% (symptom onset 1–7 days) and increases to 73.3% (symptom onset 8–14 days) and 94.3% by greater than 15 days of symptom onset [16]. Another study reported an overall sensitivity of 88.66% and specificity was 90.63% in clinically suspected SARS-CoV-2 confirmed cases (date of symptom onset not collected) [42]. Figure 1 describes diagnostic tests for (i) triaging (host response), (ii) confirmatory diagnosis (molecular tests) and (iii) disease time course (serology).
Figure 1.
Diagnostic tests categories for detecting SARS-CoV-2 and host response [15,43–46].
Algorithmic Approach
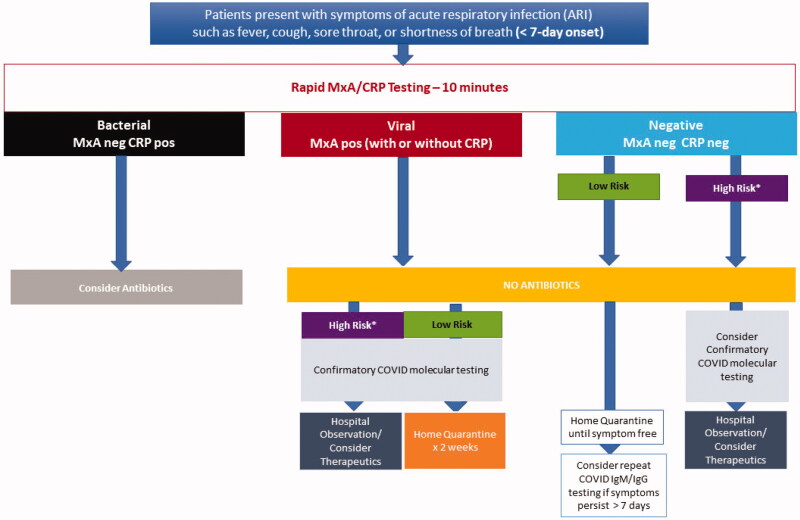
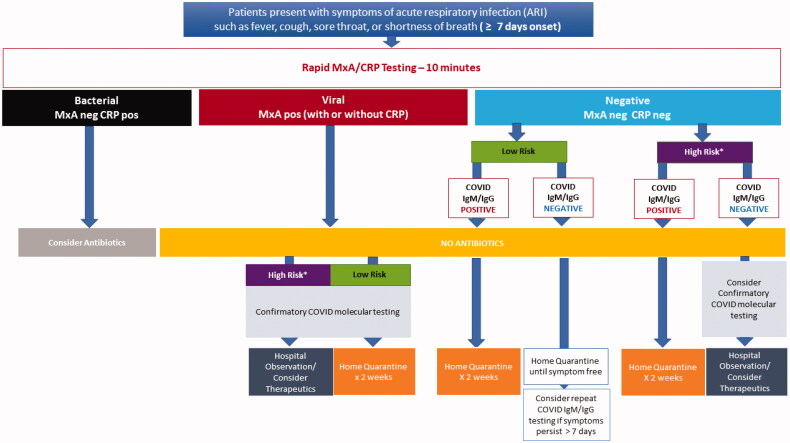
A multi-tiered, rapid diagnostic strategy incorporating (i) a rapid host immune response assay as an initial triage test, (ii) confirmatory molecular testing and (iii) a rapid IgM/IgG serologytest for assessing which patients would benefit from quarantine, further testing, or therapeutics targeting COVID-19 provides both a comprehensive screening and diagnostic testing strategy (Figures 2 and 3). As a first step, the host immune response assay can differentiate the cause of the infection as viral or bacterial. Patients with a viral positive host response would receive pathogen-specific molecular testing. Patients identified as bacterial infection could undergo additional evaluation (e.g. chest imaging) and be started on appropriate antibacterial therapy. Patients with a negative bacterial and viral host response test and less than 7 days of symptoms would be referred for confirmatory molecular testing if defined as high-risk [20] or home quarantine if they do not meet the high-risk criterion (Figure 2). While patients with a negative bacterial and viral host response test and more than 7 days of symptoms would be tested with a rapid COVID-19 IgM/IgG serology test to rule out prior history of COVID-19 (Figure 3).
Figure 2.
A Multi-tiered, rapid diagnostic strategy incorporating rapid host immune response, molecular pathogen detection and serology for patients presenting with less than 7 days of symptoms consistent with COVID-19. *High Risk (patients in long-term care facilities with symptoms, patients 65 years of age and older with symptoms, patients with underlying conditions with symptoms, first responders with symptoms).
Figure 3.
A Multi-tiered, rapid diagnostic strategy incorporating rapid host immune response, molecular pathogen detection and serology for patients presenting with 7 or more days of symptoms consistent with COVID-19. *High Risk (patients in long-term care facilities with symptoms, patients 65 years of age and older, patients with underlying conditions, first responders with symptoms).
The proposed multi-tiered screening and diagnostic strategy is intended to be used as a clinical approach for symptomatic patients, however it should be noted that all diagnostic strategies for COVID-19 are limited by atypical presentations (e.g. gastrointestinal symptoms only), colonization without host immunity, and the known risk of asymptomatic transmission [47,48]. Clinicians should maintain a high degree of suspicion as the absence of ARI symptoms does not rule out SARS-CoV-2 infections. Furthermore, this practical testing approach should be clinically validated prior to widespread deployment. High risk populations, such as immunocompromised patients, should be excluded from the proposed diagnostic screening pathway given the risk of atypical presentation and uncertainty of host response assay performance in this population until validation studies have been completed. Additionally, the testing methodologies proposed in the multi-tiered strategy do not directly address the question of co-infection, defined as the presence of a host response to both a bacterial and viral pathogen, when used independently. PCR and serology-based tests detect RNA/DNA or antibodies of a single pathogen and whole systemic host response tests qualitatively detect a systemic host response to a bacterial or viral infection. Although the prevalence of co-infection in COVID-19 has not been established, bacterial/viral co-infection has been found to be rare in the context of other ARIs [49–51]. Pairing the high specificity of molecular/serology-based tests and high sensitivity and specificity of a dual biomarker (CRP/MxA) host-immune response test, offers clinicians crucial insight into the underlying aetiology causing non-specific clinical manifestations, that are prevalent in non-COVID-19 and COVID-19 viral infections as well as bacterial infections, at the time of presentation. Deploying this rapid, multi-tiered screening strategy, could preserve healthcare resources for those who need them most while containing spread of disease more effectively with targeted quarantine and appropriate therapeutics.
Conclusion
Unless a new diagnostic strategy for viral pandemics is instituted, vulnerabilities will continue to slow identification and containment of these pathogens. In the long-term, novel viral pathogens will continue to emerge which are not detectable by the existing specific molecular tests. As such, there is near certainty that public health emergencies similar to the current COVID-19 dilemma will continue to occur in the future. It can take weeks to months to develop a pathogen specific molecular test each time a new, viral infection emerges. Incorporating a rapid, host immune response assay as the first line of screening/triage, to differentiate viral from bacterial infections, would preserve healthcare resources (e.g. confirmatory testing capacity), improve operational efficiency (e.g. triage, crowding), and aid in public health pandemic control efforts (e.g. case identification, quarantines).
Without an effective way to rapidly triage patients in the community (e.g. border control stations, mobile testing units) or healthcare settings (urgent care, EDs, occupational health services), healthcare resources are being overwhelmed. The ability to quickly and repeatedly test, both symptomatic patients and the worried well, will have a significant impact on public health and resource management. Furthermore, as much needed widespread testing is deployed it is important to also consider how testing methodologies themselves may impact spread of disease. PCR-based testing requires an NP swabbing procedure, specialized training to ensure optimal test performance and enhanced personal protective equipment (PPE). Dual biomarker host immune testing (MxA/CRP) involves a simple to perform fingerstick that does not cause the release of droplets and increased consumption of PPE. Although host immune testing is not intended to replace confirmatory molecular testing, it would streamline patients who benefit from confirmatory testing and thereby reduce droplet exposure to healthcare workers administering screening and diagnostic tests en masse.
Finally, despite the ongoing current COVID-19 outbreak, many patients will continue to develop more common bacterial infections like pharyngitis or pneumonia and the ability to quickly identify these patients and institute appropriate antibiotic therapy will reduce the risk of infection progression (e.g. sepsis). The ability to triage patients at the point of care and support the guidance of medical and therapeutic decisions, for viral isolation or confirmatory testing or for appropriate treatment of COVID-19 and/or bacterial infections, is a critical component to our national pandemic response and there is an urgent need to implement the proposed strategy to combat the current outbreak. Furthermore, a universal strategy incorporating rapid, host immune point-of-care tests can be used now and for future pandemic planning by effectively identifying patients at risk of disease and initiating quarantine earlier in the progression of disease during the weeks and months it can take for pathogen specific confirmatory tests to be developed, validated and manufactured in sufficient quantities. Early screening and triage can be achieved with point-of-care host immune response testing which will improve response time to effective public health mitigation efforts.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Disclosure statement
Financial support was not received. MSP reports grant money to the University of Wisconsin Madison to conduct research conceived and sponsored by Roche Molecular Systems, Inc. and Rapid Pathogen Screening, Inc. (a wholly owned subsidiary of Lumos Diagnostics, Inc.) PCH is a site principle investigator for a Rapid Pathogen Screening, Inc. sponsored clinical trial. RS is the Chief Medical Officer of Lumos Diagnostics. TPO, AS report no conflicts of interest.
References
- 1.Harris AM, Hicks LA, Qaseem A; for the High Value Care Task Force of the American College of Physicians and for the Centers for Disease Control and Prevention . Appropriate antibiotic use for acute respiratory tract infection in adults: advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2016;164:425–434. [DOI] [PubMed] [Google Scholar]
- 2.Poole NM, Shapiro DJ, Fleming-Dutra KE, et al. Antibiotic prescribing for children in United States emergency departments: 2009–2014. Pediatrics. 2019;143:e20181056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pfuntner A, Wier L, Stocks C. Most frequent conditions in US hospitals, 2011: statistical brief #162. 2006. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC) . Key facts about influenza (Flu). [accessed 2020 Mar 15]. Available from: https://www.cdc.gov/flu/about/keyfacts.htm
- 5.Ksiazek TG, Erdman D, Goldsmith CS, et al. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953–1966. [DOI] [PubMed] [Google Scholar]
- 6.Kuiken T, Fouchier RA, Schutten M, et al. Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. Lancet. 2003;362:263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zaki AM, Van Boheemen S, Bestebroer TM, et al. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–1820. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization . Situation report. Ebola virus disease. 10 June 2016. Geneva (Switzerland): WHO; 2016. [Google Scholar]
- 9.Al-Hazmi A. Challenges presented by MERS corona virus, and SARS corona virus to global health. Saudi J Biol Sci. 2016;23:507–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adhanom DT. World Health Organiztion (WHO) News Briefing March 20, 2020. CNN2020. WHO. [Google Scholar]
- 11.Adams JG, Walls RM. Supporting the health care workforce during the COVID-19 global epidemic. JAMA. 2020;323:1439. [DOI] [PubMed] [Google Scholar]
- 12.White DB, Lo B. A framework for rationing ventilators and critical care beds during the COVID-19 pandemic. JAMA. 2020. [DOI] [PubMed] [Google Scholar]
- 13.Institute for Health Metrics and Evaluation (IHME ). COVID-19 Projections: IHME; 2020 [cited 2002 Apr 1]. Available from: https://covid19.healthdata.org/projections
- 14.Johns Hopkins University . COVID-19 U.S. Map. 2020. Available from: https://coronavirus.jhu.edu/data
- 15.Wang M, Wu Q, Xu W, et al. Clinical diagnosis of 8274 samples with 2019-novel coronavirus in Wuhan. medRxiv. 2020. [Google Scholar]
- 16.Zhao J, Yuan Q, Wang H, et al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Omer SB, Malani P, del Rio C. The COVID-19 pandemic in the US: a clinical update. JAMA. 2020. [DOI] [PubMed] [Google Scholar]
- 18.Xie X, Zhong Z, Zhao W, et al. Chest CT for typical 2019-nCoV pneumonia: relationship to negative RT-PCR testing. Radiology. 2020;200343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Public Radio . Study Raises Questions About False Negatives From Quick COVID-19 Test. [accessed 2020 Apr 21]. Available from: https://www.npr.org/sections/health-shots/2020/04/21/838794281/study-raises-questions-about-false-negatives-from-quick-covid-19-test
- 20.Centers for Disease Control and Prevention (CDC) . Evaluating and testing persons for coronavirus disease 2019 (COVID-19): Centers for Disease Control and Prevention ; 2020. [cited 2020 April 1]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-criteria.html
- 21.Marcus AD. Positive covid-19 test results can linger, prevent plasma donation. Wall Street Journal. 2020. [Google Scholar]
- 22.Ronni T, Melen K, Malygin A, et al. Control of IFN-inducible MxA gene expression in human cells. J Immunol. 1993;150:1715–1726. [PubMed] [Google Scholar]
- 23.Ronni T, Matikainen S, Sareneva T, et al. Regulation of IFN-alpha/beta, MxA, 2’, 5’-oligoadenylate synthetase, and HLA gene expression in influenza A-infected human lung epithelial cells. J. Immunol. 1997;158:2363–2374. [PubMed] [Google Scholar]
- 24.Chieux V, Chehadeh W, Hautecoeur P, et al. Increased levels of antiviral MxA protein in peripheral blood of patients with a chronic disease of unknown etiology. J Med Virol. 2001;65:301–308. [DOI] [PubMed] [Google Scholar]
- 25.Chieux V, Hober D, Chehadeh W, et al. MxA protein in capillary blood of children with viral infections. J Med Virol. 1999;59:547–551. [PubMed] [Google Scholar]
- 26.Chieux V, Hober D, Harvey J, et al. The MxA protein levels in whole blood lysates of patients with various viral infections. J Virol Methods. 1998;70:183–191. [DOI] [PubMed] [Google Scholar]
- 27.Forster J, Schweizer M, Schumacher RF, et al. MxA protein in infants and children with respiratory tract infection. Acta Paediatr. 1996;85:163–167. [DOI] [PubMed] [Google Scholar]
- 28.Halminen M, Ilonen J, Julkunen I, et al. Expression of MxA protein in blood lymphocytes discriminates between viral and bacterial infections in febrile children. Pediatr Res. 1997;41:647–650. [DOI] [PubMed] [Google Scholar]
- 29.Nakabayashi M, Adachi Y, Itazawa T, et al. MxA-based recognition of viral illness in febrile children by a whole blood assay. Pediatr Res. 2006;60:770–774. [DOI] [PubMed] [Google Scholar]
- 30.Kawamura M, Kusano A, Furuya A, et al. New sandwich-type enzyme-linked immunosorbent assay for human MxA protein in a whole blood using monoclonal antibodies against GTP-binding domain for recognition of viral infection. J Clin Lab Anal. 2012;26:174–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Engelmann I, Dubos F, Lobert P-E, et al. Diagnosis of viral infections using myxovirus resistance protein A (MxA). Pediatrics. 2015;135:e985–e93. [DOI] [PubMed] [Google Scholar]
- 32.Simon A, Fah J, Haller O, et al. Interferon-regulated Mx genes are not responsive to interleukin-1, tumor necrosis factor, and other cytokines. J Virol. 1991;65:968–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calandra T, Baumgartner JD, Grau GE, et al. ; Swiss-Dutch J5 Immunoglobulin Study Group . Prognostic values of tumor necrosis factor/cachectin, interleukin-1, interferon-alpha, and interferon-gamma in the serum of patients with septic shock. Swiss-Dutch J5 Immunoglobulin Study Group. J Infect Dis. 1990;161:982–987. [DOI] [PubMed] [Google Scholar]
- 34.Girardin E, Grau GE, Dayer JM, et al. ; the J5 Study Group . Tumor necrosis factor and interleukin-1 in the serum of children with severe infectious purpura. N Engl J Med. 1988;319:397–400. [DOI] [PubMed] [Google Scholar]
- 35.Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol. 2020;38:1–9. [DOI] [PubMed] [Google Scholar]
- 36.Liu W, Tao Z-W, Lei W, et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J (Engl). 2020;133:1032–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shapiro NI, Self WH, Rosen J, et al. A prospective, multi-centre US clinical trial to determine accuracy of FebriDx point-of-care testing for acute upper respiratory infections with and without a confirmed fever. Ann Med. 2018;50:420–429. [DOI] [PubMed] [Google Scholar]
- 38.Self WH, Rosen J, Sharp SC, et al. Diagnostic accuracy of FebriDx: a rapid test to detect immune responses to viral and bacterial upper respiratory infections. JCM. 2017;6:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davidson M. FebriDx point-of-care testing to guide antibiotic therapy for acute respiratory tract infection in UK primary care: a retrospective outcome analysis. J Infect Dis Preve Med. 2017;05:165. [Google Scholar]
- 40.Onrubia X, Gonzalez IJ. A pilot evaluation of the FebriDx test in an outpatient pediatric clinic. Clin Med Invest. 2020;5. [Google Scholar]
- 41.Tynell J, Westenius V, Rönkkö E, et al. Middle East respiratory syndrome coronavirus shows poor replication but significant induction of antiviral responses in human monocyte-derived macrophages and dendritic cells. J Gen Virol. 2016;97:344–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Z, Yi Y, Luo X, et al. Development and clinical application of a rapid IgM‐IgG combined antibody test for SARS‐CoV‐2 infection diagnosis. J Med Virol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zou L, Ruan F, Huang M, et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323:1406–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chow EJ, Schwartz NG, Tobolowsky FA, et al. Symptom screening at illness onset of health care personnel with SARS-CoV-2 infection in King County, Washington. JAMA. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsalik EL, Henao R, Nichols M, et al. Host gene expression classifiers diagnose acute respiratory illness etiology. Sci Transl Med. 2016;8:322ra11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.MäKelä MJ, Puhakka T, Ruuskanen O, et al. Viruses and bacteria in the etiology of the common cold. J Clin Microbiol. 1998;36:539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015;373:415–427. [DOI] [PMC free article] [PubMed] [Google Scholar]