Abstract
Background: Prediabetes has proven to have many unfavourable impacts on the cardiovascular system.
Methods: The OPERA (Oulu Project Elucidating Risk of Atherosclerosis) study included 1045 middle-aged subjects followed from the years 1990–1993 to 2014. The focus was on peptide hormones.
Results: Plasma resistin levels were higher among prediabetics (p = .001), particularly impaired glucose tolerance (IGT) (p < .001), but not impaired fasting glucose (IFG) patients than among normal glucose tolerance (NGT) or diabetes groups. Diabetics showed lower resistin levels than IGT subjects (p < .001). IGT or diabetes groups showed lower adiponectin and higher leptin levels compared to the NGT group (p < .001). The IFG group had the highest blood pressure and left ventricular mass index, even higher than the diabetic group. Diabetics had the highest, prediabetics (IFG + IGT) intermediate and NGT the lowest risk for CVD events during follow-up (p < .001). Among prediabetics, high plasma ghrelin was an independent predictor of CVD events (p < .05) in the Cox regression analysis although it did not significantly improve either classification or discrimination of the patients.
Conclusions: Among glucose tolerance groups, patients with IGT had the highest resistin, but equally high leptin and low adiponectin levels as diabetics. Among prediabetics, ghrelin seems to predict independently cardiovascular events in the long term.
KEY MESSAGE
Among glucose tolerance groups, patients with IGT had the highest resistin, but equally high leptin and low adiponectin levels as diabetics.
Among prediabetics, ghrelin seems to predict independently cardiovascular events in the long term.
Keywords: Prediabetes, cardiovascular disease, peptide hormones
Introduction
Prediabetes is a state where blood glucose has increased over the normoglycemic level but has not reached the diabetic state. The blood glucose elevation can be seen as an impaired fasting glucose (IFG) but also as impaired glucose tolerance (IGT). Like diabetes, the prediabetic state has become more common worldwide in the past few decades. It is estimated that over 470 million people will suffer from prediabetes in 2030 [1]. IGT is associated with cardiovascular morbidity and mortality in large studies [2], but also IFG has been shown to have association with, for example, coronary artery calcification and cardiovascular diseases (CVDs) [3]. However, a recent report suggested that cardiovascular events were not more common in prediabetics than in those with normal glucose tolerance (NGT) in patients suffering from coronary artery disease [4].
Hypertension as a classical risk factor is 2–3 times more common in prediabetics than in normoglycemics [5]. However, data on the ambulatory blood pressure (ABP) values in prediabetes and their role in cardiovascular risk are almost missing. Earlier studies have reported associations between some obesity-related peptide hormones such as ghrelin [6], adiponectin [7] and resistin and CVDs in non-diabetic population. However, the role of leptin in CVDs remains inconclusive. In addition, the data in prediabetic population are scarce, and our study clarifies this topic.
The aim of this study was to assess these traditional risk markers in both IGT and IFG group, alone and together with obesity-related peptide hormones such as ghrelin, leptin, resistin and adiponectin. More long-term studies considering prediabetes and its connection to CVDs are needed, and our study has one of the longest follow-up times – about 20 years. The biomarkers of obesity may be associated with CVDs already when blood glucose is not yet in the diabetic range. Prediabetes is often an undiagnosed state, meaning that patients are not aware of their cardiovascular risks. Increased information about prediabetes and its risks would lead to decreased morbidity and mortality through prevention among people with a hidden abnormal glycemic condition.
Materials and methods
Study population
OPERA (Oulu Project Elucidating Risk of Atherosclerosis) is a population-based, epidemiological study designed to address the risk factors and disease end points of atherosclerotic CVDs. This study population and selection criteria have been previously described in detail [8]. The subjects were randomly selected, middle-aged drug-treated hypertensives and their age- and sex-matched control subjects who were recruited to the OPERA study between the years 1990 and 1993. The participants were interviewed, examined and tested in our research laboratory. Mortality and hospital events of a total of 1045 subjects were followed up until 2014. The study was conducted according to the principles of the Declaration of Helsinki and approved by the Ethics Committee of the Faculty of Medicine, University of Oulu. Written informed consent was obtained from each participant.
Clinical measurements
The blood pressure measurements were conducted according to the recommendations of the American Society of Hypertension. All the blood pressure measurements were undertaken with an automatic oscillometric blood pressure recorder (Dinamap, Critikon Ltd., Ascot, UK). The resting blood pressure was measured three times at 1-min intervals from the right arm after the patient had been seated for at least 5 min. The mean value of the second and third blood pressure measurements from the sitting patient was used. The lifetime smoking burden was calculated as pack-years (1 pack-year = 20 cigarettes smoked/day in 1 year) and the smoking history was obtained from a questionnaire. Body mass index (BMI) was calculated as weight (kg) divided by height squared (m2). Height was measured to the nearest centimetre without shoes and weight to the nearest 0.1 kg with the subject wearing only light underwear without shoes. All measurements were performed by the same specially trained nurses.
Laboratory analyses
The very-low-density lipoprotein (VLDL) fraction was separated from plasma by ultracentrifugation at 10,500×g for 18 h. The plasma high-density lipoprotein (HDL) cholesterol concentration was measured by mixing 0.5 mL of the VLDL-free fraction with 25 mL of 2.8% (wt/vol) heparin and 25 mL of 2 M manganese chloride and measuring the cholesterol concentration in the supernatant after centrifugation at 1000×g and 4 °C for 30 min. The low-density lipoprotein (LDL) cholesterol concentration was calculated by subtracting the cholesterol concentration in HDL from that in the VLDL-free fraction. The oral glucose tolerance test (OGTT) was performed in the morning after a 12-h fast immediately after fasting blood had been drawn. Normal glucose tolerance, IFG, IGT and type 2 diabetes were determined according to the WHO criteria [9]. Fasting glucose concentrations were measured with the glucose dehydrogenase method (Diagnostica, Merck, Darmstadt, Germany) and the serum insulin levels with a two-site immunoenzymometric assay (AIA-PACK IRI, Tosoh Corp., Tokyo, Japan). These laboratory analyses have been previously described in detail [8]. High-sensitivity C-reactive protein (hsCRP) was analysed using commercially available ELISA kits (Diagnostic Systems Laboratories, Webster, TX) as described before [10].
Fasting plasma leptin concentration was assessed with a commercial double antibody radioimmunoassay (RIA) (Human Leptin RIA Kit; Linco Research, Inc., St. Charles, MO). Plasma adiponectin concentrations were measured with an enzyme-linked immunosorbent assay (ELISA) devised in our laboratory, described previously in detail [11]. Plasma resistin was measured in duplicate using a commercially available enzyme-linked immunoassay kit (Linco Research Inc., Billerica, MA; intra- and interassay coefficients of variation 4.5 and 7.4%, respectively) as described earlier [12]. Quick insulin sensitivity check index, defined as Quick-index, was calculated as 1/[log (fasting insulin)+log (fasting glucose).
Ambulatory blood pressure measurements
ABP measurements were obtained with a non-invasive fully automatic SpaceLabs90207 oscillometric unit (SpaceLabs Inc., Redmond, WA). The measurements were taken every 15 min from 04:00 am to 12:00 pm and every 20 min from 12:00 pm to 04:00 am. The accuracy and reproducibility of blood pressure readings obtained with this device have previously been settled by the British Hypertension Society and the US Association for the Advancement of Medical Instrumentation [13]. The similarity (difference <5 mmHg) between four SpaceLabs blood pressure measurements and four auscultatory readings using a Y-connector was required to ensure the proper positioning of the cuff. The study subjects were instructed to relax their arm during the measurement. Systolic blood pressure values less than 70 or more than 250 mmHg, diastolic blood pressure values less than 40 or more than 150 mmHg, and heart rate less than 40 or more than 150 beats/min were automatically excluded from the analysis. Based on these criteria, less than 3% of the blood pressure measurements were excluded as artefacts [14]. Study subjects with ≥10% decrease in mean systolic blood pressure from daytime to night-time values were considered as dippers and those with a decrease <10% as non-dippers (non-dipping in the tables). The left ventricular mass index (LVMI) was determined by dividing the difference between LVM values by body surface area (BSA) as reported earlier [15].
Outcome classification
Information on causes of death and events leading to hospitalization was obtained from the Finnish Causes-of-Death Register and the Hospital Discharge Register. The diagnoses were classified according to the International Classification of Diseases, Eighth Revision (ICD-8) or Ninth Revision (ICD-9) before 1994 and the Tenth Revision (ICD-10) thereafter. Coronary heart disease (CHD) was defined as diagnoses I20, I21, I22 [ICD-10] and 410, 4110 [ICD-8/9], coronary artery bypass graft or coronary angioplasty or as I20–I25, I46, R96, R98 [ICD-10] and 410–414, 798 (not 7980A) [ICD-8/9] as causes of death. CVD was defined as CHD or stroke that included I61, I63 (not I636), I64 [ICD-10] and 431, 4330A, 4331A, 4339A, 4340A, 4341A, 4349A, 436 [ICD-9] or 431 (excluding 43101, 43191), 433, 434, 436 [ICD-8] according to the FINRISK criteria [16].
Statistical methods
Statistical analyses were calculated using IBM SPSS statistics version 22.0 (IBM Corp, Armonk, NY). Statistical significances between the groups at baseline were tested using analysis of covariance for continuous variables and chi-squared test for categorical variables. Logarithmic transformations were used when variable distributions were not normal (insulin, hs-CRP, triglycerides, leptin). A p value < .05 was considered to be statistically significant.
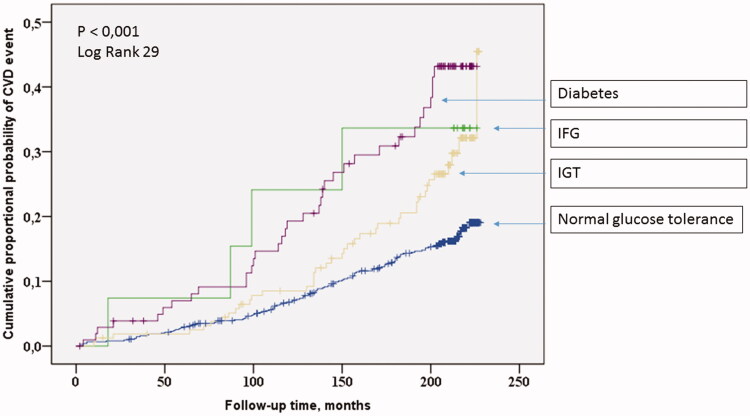
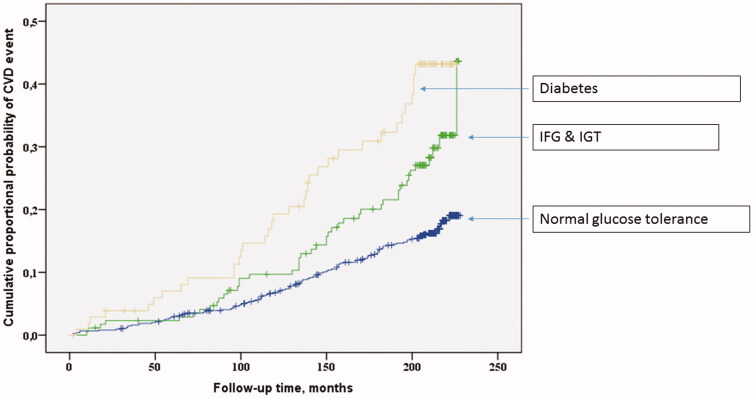
Cardiovascular survival was assessed as cumulative proportional probability of development of CVD events and analysed using the Kaplan–Meier survival curves for glucose tolerance groups (Figures 1 and 2). Statistical significances of the Kaplan–Meier survival curves were calculated using log-rank test.
Figure 1.
The Kaplan–Meier curves for CVD events among normoglycemics, diabetics and separate prediabetic groups.
Figure 2.
The Kaplan–Meier curves for CVD events among normoglycemics, diabetics and combined prediabetic groups.
The association between glucose tolerance groups and risk of development of CVD events during follow-up time was estimated with the Cox proportional hazards models. The models included the following covariates: age, sex, study group (hypertensives/controls), LDL cholesterol levels and smoking in pack-years.
The C-index and integrated discrimination index (IDI) were used to evaluate the discrimination accuracy and net reclassification index (NRI), the reclassification accuracy of the risk markers. Established model included gender, gender, smoking, HDL-cholesterol, triglycerides, leptin, office diastolic blood pressure, heart rate (24 h average) and plasma ghrelin.
Results
Cardiovascular risk markers among subjects with NGT, IFG, IGT and diabetes
When prediabetes groups (IFG and IGT) were considered separately, several baseline characteristics differed significantly (Table 1). Whereas subjects with abnormal glucose tolerance (IFG, IGT or diabetes) belonged more often to the hypertensive study group, most of the NGT subjects were from the control group (p < .001). Subjects with NGT were leaner, had thinner waist, lower fasting triglyceride, glucose and insulin as well as higher quick index values than the other groups (p < .001). Subjects with IFG were more often male (p = .034) and showed higher BMI, waist circumference, fasting glucose and insulin and lower quick index values than subjects with IGT (p < .001). IFG subjects also had lower fasting glucose than diabetics. IGT subjects showed higher total cholesterol levels than subjects with NGT (p = .007). Resistin levels were higher among IGT than NGT or diabetes groups (p < .001). Diabetics showed lower resistin levels than IGT subjects.
Table 1.
Cardiovascular risk markers among subjects with NGT, IFG, IGT and diabetes.
| Glucose tolerance status | NGT (n = 759) | IFG (n = 14) | IGT (n = 162) | Diabetes (n = 106) | p Value |
|---|---|---|---|---|---|
| Male/female (%) | 49/51 | 86/14 | 49/51 | 55/45 | .034 |
| Study group (hypertensives/controls, %) | 43/57 | 71/29 | 62/38 | 78/22 | <.001 |
| Age (years) | 50.7 (6.0) | 49.1 (5.0) | 52.9 (6.0) | 53.0 (5.5) | <.001 (2) |
| BMI (kg/m2) | 26.7 (4.0) | 32.9 (7.9) | 29.7 (5.0) | 31.0 (5.3) | <.001 (1,3) |
| Waist (cm) | 87.9 (12.1) | 106.5 (13.4) | 95.6 (13.0) | 100.0 (13.6) | <.001 (1,3,4) |
| Smoking (pack-years) | 10.2 (13.8) | 17.6 (19.6) | 8.0 (13.6) | 9.8 (17.0) | .064 |
| Fasting glucose (mmol/mL) | 4.3 (0.4) | 5.8 (0.2) | 4.7 (0.6) | 7.6 (3.2) | <.001 (1,3,4,5) |
| Fasting insulin (mU/L) | 11.5 (8.0) | 25.7 (13.6) | 17.5 (15.0) | 22.0 (15.5) | <.001 (1,3,4) |
| Quick-index | 0.64 (0.11) | 0.48 (0.06) | 0.56 (0.09) | 0.48 (0.07) | <.001 (1,3,4) |
| Total cholesterol (mmol/mL) | 5.63 (1.02) | 6.25 (0.91) | 5.88 (0.97) | 5.80 (1.37) | .007 (6) |
| HDL-cholesterol (mmol/mL) | 1.38 (0.39) | 1.32 (0.41) | 1.27 (0.31) | 1.22 (0.34) | <.001(2) |
| LDL-cholesterol (mmol/mL) | 3.49 (0.94) | 3.89 (1.11) | 3.67 (0.83) | 3.50 (1.05) | .074 |
| Triglycerides (mmol/mL) | 1.41 (0.81) | 2.04 (1.12) | 1.87 (0.92) | 2.31 (1.84) | <.001 (1) |
| hsCRP (mg/L) | 3.07 (6.34) | 6.89 (13.21) | 4.93 (7.46) | 6.93 (11.74) | <.001 (2) |
| Leptin (ng/mL) | 9.7 (7.5) | 12.8 (14.1) | 13.2 (9.9) | 12.1 (7.8) | <.001 (2) |
| Plasma ghrelin (pg/mL) | 679.1 (239.7) | 683.2 (225.0) | 661.9 (242.0) | 587.9 (246.2) | .004 (1) |
| Adiponectin (µg/mL) | 16.6 (7.0) | 16.5 (7.9) | 14.2 (6.1) | 13.2 (5.4) | <.001 (2) |
| Resistin, only controls included (ng/mL) | 7.5 (2.3) | 5.9 (1.5) | 8.4 (2.5) | 6.1 (2.2) | <.001 (2,4) |
| Lipid lowering medication (%) | 2 | 0 | 2 | 12 | <.001 |
| Aspirin (%) | 5 | 7 | 6 | 9 | .454 |
Data are percentages (%) or means and standard deviations. p Values obtained from ANOVA or chi-square analyses. In post hoc analyses significance between groups NGT and IFG, IGT or Diabetes (1), NGT and IGT or Diabetes (2), IFG and IGT (3), IGT and Diabetes (4), IFG and Diabetes (5), NGT and IGT (6).
Subjects belonging to the IGT or diabetes groups were older, had lower HDL-cholesterol and adiponectin levels as well as higher hsCRP and leptin values compared to NGT groups (p < .001). Diabetics showed also more abdominal obesity, higher glucose and insulin levels and lower quick index than subjects with IGT (p < .001). Lipid lowering medication was most commonly used among diabetics.
Office and ABP characteristics as well as LVMI among glucose tolerance groups are shown in Table 2. Subjects with NGT had the lowest office systolic and diastolic blood pressure (p < .001). When ABP is considered, the NGT group had the lowest values in the 24-hour, day- and night-time systolic blood pressure (p < .001). IFG subjects had the highest 24-hour (p = .017) and night-time (p = .001) diastolic blood pressure compared to other groups. IFG group also had higher night-time systolic blood pressure than subjects with IGT. Non-dipping was most prevalent among subjects with IFG (p = .042).
Table 2.
Office and ambulatory blood pressure characteristics among subjects with NGT, IFG, IGT and diabetes.
| Glucose tolerance status | NGT | IFG | IGT | Diabetes | p Value |
|---|---|---|---|---|---|
| Systolic BP (mmHg) | 144.6 (21.0) | 163.1 (21.7) | 156.3 (21.8) | 159.6 (22.3) | <.001 (1) |
| Diastolic BP (mmHg) | 87.6 (12.1) | 98.8 (12.7) | 92.1 (11.8) | 94.1 (10.7) | <.001 (1) |
| Ambulatory systolic blood pressure, average (mmHg) | 128.5 (13.2) | 141.7 (16.5) | 133.1 (13.7) | 134.4 (14.2) | <.001 (1) |
| Ambulatory diastolic blood pressure, average (mmHg) | 80.8 (8.4) | 89.0 (12.4) | 81.7 (8.5) | 81.3 (6.9) | .017 (3) |
| Ambulatory systolic blood pressure, daytime average (mmHg) | 133.3 (13.7) | 145.5 (17.3) | 137.6 (14.7) | 137.6 (20.7) | <.001 (1) |
| Ambulatory diastolic blood pressure, daytime average (mmHg) | 84.9 (8.7) | 92.0 (12.7) | 85.7 (9.8) | 85.7 (8.5) | .069 |
| Ambulatory systolic blood pressure, night-time average (mmHg) | 115.2 (13.8) | 132.2 (15.0) | 119.7 (14.0) | 122.3 (15.5) | <.001(1,2) |
| Ambulatory diastolic blood pressure, night-time average (mmHg) | 69.7 (9.3) | 80.2 (11.2) | 71.4 (9.9) | 71.0 (8.1) | .001 (3) |
| Left ventricular mass index (g/m2) | 129.1 (37.5) | 174.4 (42.0) | 133.1 (42.0) | 138.2 (36.3) | <.001 (3) |
| Non-dipping (%) | 24 | 50 | 29 | 35 | .042 |
| Blood pressure medication (%) | 45 | 64 | 64 | 79 | <.001 |
Data are number (%) or means (standard deviation). p Values obtained from ANCOVA or chi-square analyses. In post hoc analyses significance between groups NGT and IFG, IGT or Diabetes (1), NGT and IFG, IFG and IGT or Diabetes, IFG and IGT (2), IFG and NGT, IGT or Diabetes (3).
Left ventricular mass index was higher among subjects with IFG than among subjects belonging to all other groups (p < .001). Diabetics used blood pressure medication most frequently whereas their use among NGT subjects was more rare (Table 2).
Cardiovascular risk markers among subjects with NGT, prediabetes (IFG + IGT combined) and diabetes
In Tables 3 and 4, the results are shown with IFG and IGT combined and considered as the prediabetes group. Subjects in both the prediabetes and diabetes groups were older, had higher BMI, waist circumference, fasting triglyceride, hsCRP, leptin, glucose, and insulin as well as lower quick index, HDL-cholesterol and adiponectin levels than subjects with NGT (p < .001) (Table 3).
Table 3.
Cardiovascular risk markers among subjects with NGT, prediabetes and diabetes.
| Glucose tolerance status | NGT (n = 759) | Prediabetes IFG and IGT (n = 176) | Diabetes (n = 106) | p Value |
|---|---|---|---|---|
| Male/female (%) | 49/51 | 52/48 | 55/45 | .446 |
| Study group (hypertensives/controls, %) | 43/57 | 62.5/37.5 | 78/22 | <.001 |
| Age (years) | 50.7 (6.0) | 52.6 (6.0) | 53.0 (5.5) | <.001 (1) |
| BMI (kg/m2) | 26.7 (4.0) | 30.0 (5.3) | 31.0 (5.3) | <.001 (1) |
| Waist (cm) | 87.9 (12.1) | 96.5 (13.3) | 100.0 (13.6) | <.001 (1) |
| Smoking (pack-years) | 10.2 (13.8) | 8.8 (14.3) | 9.8 (17.0) | .482 |
| Fasting glucose (mmol/mL) | 4.3 (0.4) | 4.8 (0.6) | 7.6 (3.2) | <.001 (1,2) |
| Fasting insulin (mU/L) | 11.5 (8.0) | 18.1 (15.0) | 22.0 (15.5) | <.001 (1,2) |
| Quick-index | 0.64 (0.11) | 0.55 (0.09) | 0.48 (0.07) | <.001 (1,2) |
| Total cholesterol (mmol/mL) | 5.63 (1.02) | 5.91 (0.97) | 5.80 (1.37) | .005 (3) |
| HDL-cholesterol (mmol/mL) | 1.38 (0.39) | 1.27 (0.32) | 1.22 (0.34) | <.001 (1) |
| LDL-cholesterol (mmol/mL) | 3.49 (0.94) | 3.69 (0.85) | 3.50 (1.05) | .044 (3) |
| Triglycerides (mmol/mL) | 1.41 (0.81) | 1.88 (0.93) | 2.31 (1.84) | <.001 (1) |
| hsCRP (mg/L) | 3.07 (6.34) | 5.09 (8.03) | 6.93 (11.74) | <.001 (1) |
| Leptin (ng/mL) | 9.7 (7.5) | 13.2 (10.2) | 12.1 (7.8) | <.001 (1) |
| Plasma ghrelin (pg/mL) | 679.1 (239.7) | 663.6 (240.1) | 587.9 (246.2) | .001 (1,2) |
| Adiponectin (µg/mL) | 16.6 (7.0) | 14.4 (6.3) | 13.2 (5.4) | <.001 (1) |
| Resistin, only controls included (ng/mL) | 7.5 (2.3) | 8.2 (2.5) | 6.1 (2.2) | .001 (1,2) |
| Lipid lowering medication (%) | 2 | 2 | 12 | <.001 |
| Aspirin (%) | 5 | 6 | 9 | .273 |
Data are number (%) or means (standard deviations). p Values obtained from ANCOVA or chi-square. In post hoc analyses significance between groups NGT and prediabetes or diabetes (1), prediabetes and diabetes (2), NGT and prediabetes (3).
Table 4.
Office and ambulatory blood pressure characteristics among subjects with NGT, prediabetes and diabetes.
| Glucose tolerance status | NGT | IFG and IGT | Diabetes | p Value |
|---|---|---|---|---|
| Systolic BP (mmHg) | 144.6 (21.0) | 156.9 (21.8) | 159.6 (22.3) | <.001 (1) |
| Diastolic BP (mmHg) | 87.6 (12.1) | 92.6 (11.9) | 94.1 (10.7) | <.001 (1) |
| Ambulatory systolic blood pressure | 128.5 (13.2) | 133.7 (14.0) | 134.4 (14.2) | <.001 (1) |
| Ambulatory diastolic blood pressure | 80.8 (8.4) | 82.2 (9.0) | 81.3 (6.9) | 0,211 |
| Ambulatory systolic blood pressure, daytime | 133.3 (13.7) | 138.1 (15.0) | 137.6 (20.7) | <.001 (1) |
| Ambulatory diastolic blood pressure, daytime | 84.9 (8.7) | 86.2 (10.1) | 85.7 (8.5) | .293 |
| Ambulatory systolic blood pressure, night-time | 115.2 (13.8) | 120.5 (14.4) | 122.3 (15.5) | <.001 (1) |
| Ambulatory diastolic blood pressure, night-time | 69.7 (9.3) | 72.0 (10.2) | 71.0 (8.1) | .019 (2) |
| Left ventricular mass index (g/m2) | 129.1 (37.5) | 136.3 (43.3) | 138.2 (36.3) | .024 (3) |
| Non-dipping (%) | 24 | 30 | 35 | .050 (3) |
| Blood pressure medication (%) | 45 | 64 | 79 | <.001 |
Data are number (%) or means (standard deviation). p Values obtained from ANCOVA or chi-square analyses. In post hoc analyses significance between groups NGT and prediabetes or diabetes (1), NGT and prediabetes (2). (3) Not significant in post hoc analyses.
Prediabetics had higher total (p = .005) and LDL cholesterol (p = .044) than NGT subjects. In addition, the prediabetic group showed lower fasting glucose, insulin and higher quick index than diabetics (p < .001). Resistin levels were highest among the prediabetes group (p = .001).
Office and ABP characteristics as well as LVMI among subjects with NGT, prediabetes and diabetes are shown in Table 4. Prediabetes and diabetes groups had higher office systolic and diastolic, ambulatory 24-hour mean, and day- and night-time systolic blood pressure. Prediabetics, but not diabetics, had higher night-time diastolic blood pressure than NGT subjects (p = .019).
Left ventricular mass index was higher among both prediabetics and diabetics compared to subjects with NGT (p = .024). Furthermore, the prevalence of non-dipping was almost the same in the prediabetes (30%) and diabetes (35%) groups and higher than in the NGT group (24%) (p = .050).
Glucose tolerance groups and the long-term risk of CVD
The Kaplan–Meier estimate survival analysis. To estimate the cumulative probability of CVD events, the Kaplan–Meier estimate analysis was used. Analysis was performed both by considering the IFG and IGT groups separately (Figure 1) and by combining them (Figure 2).
Subjects with diabetes had the highest and those with IFG or IGT had an intermediate risk of suffering CVD events during the follow-up time compared to subjects with NGT (log-rank p < .001, Figure 1). When the IFG and IGT groups were combined into prediabetes group, diabetics had the highest, prediabetics had intermediate and NGT the lowest risk of CVD events during the follow-up (Figure 2).
The Cox regression model analysis. The long-term risk of developing CVD events was assessed using the Cox proportional hazard survival model analysis. When prediabetes groups were considered separately, diabetes (HR 2.1; 95%CI 1.4–3.2, p < .001) and IGT (HR 1.5; 95%CI 1.0–2.2, p = .027) were independent predictors of CVD when conventional risk factors of CVD (age, sex, study group (hypertensives/controls), LDL cholesterol levels, smoking in pack-years) were added as covariates. IFG (HR 1.1; 95%CI 0.4–3.0, p = .86) was not an independent predictor of CVD.
When IFG and IGT were combined and considered as prediabetes group, diabetes (HR 2.1; 95%CI 1.4–3.2, p < .001) and prediabetes (HR 1.5; 95%CI 1.0–2.1, p = .036) were independent predictors of CVD when conventional risk factors of CVD (age, sex, study group (hypertensives/controls), LDL cholesterol levels, smoking in pack-years) were added as covariates.
The multivariate model analysis exploring predictors of CVD among prediabetics
Table 5 shows the significant predictors of CVD among prediabetics in univariate and multivariate analyses. In the multivariate model, HDL-cholesterol (p = .038), 24-hour heart rate (p = .029) and plasma ghrelin (p = .045) were independent predictors of CVD. HDL-cholesterol (p = .034) and 24-hour heart rate (p = .022) were significantly lower among subjects with cardiovascular event. Plasma ghrelin was higher among the subjects with an occurred event (p = .045).
Table 5.
Predictors of CVD among prediabetics in univariate and multivariate models.
| COX regression analysis | |||||
|---|---|---|---|---|---|
| No CVD (n = 132) | CVD (n = 44) | p Value in univariate analysis | HRa (95% CI) | p Value | |
| Smoking (pack-years) | 7.0 (12.4) | 12.6 (17.4) | .017 | 1.007(0.927–1.028) | .486 |
| HDL-cholesterol (mmol/mL) | 1.3 (0.3) | 1.2 (0.3) | .034 | 0.216 (0.051–0.918) | .038 |
| Triglycerides (mmol/mL) | 1.8 (0.9) | 2.1 (1.0) | .023 | 0.993 (0.708–1.394) | .969 |
| Leptin (ng/mL) | 14.4 (10.5) | 10.5 (9.2) | .018 | 0.989 (0.947–1.034) | .638 |
| Plasma ghrelin (pg/mL) | 634.3 (227.6) | 726.5 (255.9) | .018 | 1.002 (1.000–1.003) | .045 |
| Office diastolic blood pressure | 91.3 (11.6) | 95.6 (12.1) | .024 | 1.015 (0.987–1.044) | .302 |
| Heart rate, 24 hour average | 72.0 (10.2) | 67.6 (10.1) | .022 | 0.967 (0.938–0.997) | .029 |
HR: hazard ratio with 95% confidence intervals (CI) in parenthesis.
The incremental prognostic significance related to plasma ghrelin was assessed by the C-index, the IDI and the continuous NRI. These analyses were based on predicted risks that were calculated with and without plasma ghrelin. Although adding ghrelin to the risk model for CVD increased C-index from 0.700 (95%CI: 0.592–0.806) to 0.723 (95%CI: 0.608–0.838), it did not significantly improve either classification (NRI 0.102; 95%CI 0.149–0.338; p = .239) or discrimination of the patients (IDI 0.035; 95%CI 0.004–0.140; p = .106).
Discussion
This study confirms the hypothesis that the traditional risk factors cluster in prediabetics, leading to increased cardiovascular morbidity. Prediabetics have more often common cardiovascular risk factors, such as higher age, waist circumference and BMI. The typical lipid-profile also stands out: low HDL-cholesterol, high LDL-cholesterol and high triglycerides are seen more commonly in prediabetics compared with normoglycemics. Considering the blood pressure profiles, it seems that prediabetic groups have some specific characteristics: the IFG group had the highest blood pressure values, even higher than diabetic group. This can be seen in office and ambulatory systolic and diastolic blood pressure measurements. In addition, LVMI was higher in the IFG group than in other groups. Also, the non-dipping phenomenon was overrepresented among IFG patients, existing in half of them. The IGT group had also higher ABPM values than normoglycemics, but not as high as the IFG or diabetic group.
When NGT, IFG, IGT, and diabetes groups were compared, the IGT group stood out as significantly different from the other groups considering obesity-related peptide hormone plasma levels. When resistin levels were compared between NGT, prediabetes and diabetes groups, the highest value was seen among prediabetics, especially among IGT subjects. This is an interesting finding, because it seems that the resistin value rises when the glycemic state develops from normal to prediabetic state. After prediabetes has developed into diabetes, the resistin value drops to an even lower level than that observed among normoglycemics. Therefore, resistin, a hormone linked to insulin resistance, behaves like insulin – in prediabetic state, with compensatory increase and, eventually, a drop when beta-cell exhaustion has occurred. Higher resistin concentrations have been reported to be associated with all-cause and cardiovascular mortality among patients with CVD or diabetes [17]. In prediabetics, data are almost lacking. Adiponectin plasma level was remarkably lower and leptin value higher in IGT group than in normoglycemics. In type 2 diabetics, plasma adiponectin levels have been reported to be decreased [18]. In addition, leptin has been suggested to regulate glycemic control in addition to energy balance in both rodent models and clinical settings [19].
In the present study, we also wanted to explore the independent role of obesity-related peptide hormones in addition to traditional risk indicators in the prediction of cardiovascular events in prediabetic population. HDL cholesterol, 24-hour heart rate and plasma ghrelin remained significant factors in the multivariate model. Plasma ghrelin did not differ between prediabetics and those with normoglycemia, supporting the notion that ghrelin is not associated with the development of prediabetic state itself. However, prediabetics who had cardiovascular events had higher plasma ghrelin values compared to prediabetics without events. The role of ghrelin in CVD remains inconclusive. The positive association between ghrelin values and intima-media thickness has previously been reported in men, even after adjustment for the traditional risk factors of atherosclerosis [20]. In addition, ghrelin has been reported to cause vasoconstriction in the coronary vasculature of rat models [21]. Rat models also point out that ghrelin induces adiposity by altering the utilization of fat [22]. Adiposity can lead to inflammation and endothelial cell dysfunction, caused by interleukin-6 and TNF-alpha induced reactive oxygen species (ROS) production [23]. This mechanism of ghrelin can indirectly lead to cardiovascular events. In human studies, plasma ghrelin levels are low in obesity and type 2 diabetes. The higher plasma ghrelin levels in prediabetics with CVD could also be a compensatory phenomenon. Ghrelin has been reported to have many beneficial effects [23]. It has cytoprotective activity on myocardial cells by inhibiting apoptosis of cardiomyocytes and endothelial cells [24]. Ghrelin has also been reported to inhibit proinflammatory cytokine production in human endothelial cells [25], and this anti-inflammatory action can be a protective factor against cardiovascular events. This vascular oxidative stress attenuating feature has also been observed in tests with spontaneously hypertensive rats [26]. Ghrelin has been proven to decrease mean arterial pressure without change in heart rate when given as an intravenous bolus. In addition, stroke volume increase was reported in the same study [27]. In the present study, the measured ghrelin value stands for total, mainly unacylated, ghrelin. However, adding ghrelin to the risk model for CVD did not improve classification or discrimination of the patients.
The increased cardiovascular risk was more clearly seen among prediabetics belonging to the IGT group. It is important to notice the low prevalence of IFG subjects (1.3%). This may have an influence on for example non-significant associations in the Cox multivariate analyses. Patients in the IFG group had a higher risk for cardiac hypertrophy. All in all, some associations were only seen in IGT or IFG group individually, not when combined as a prediabetic group. Therefore, we recommend to use both classifications in defining prediabetes.
In conclusion, high plasma ghrelin level seems to be an independent risk marker for atherosclerosis in prediabetics. IFG state is associated with deleterious blood pressure profile while abnormalities in the obesity-related peptide hormone resistin seem to be specific to subjects with IGT.
Acknowledgements
The authors thank Markku Ikäheimo, Professor, for cardiac ultrasound examinations, and Ms Saija Kortetjärvi, Ms Heidi Häikiö and Ms Leena Ukkola for the excellent technical assistance. Elina Malo, MSc and Meiju Saukko, MSc, are thanked for their co-operation in organizing cardiovascular event and mortality data.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Tabák AG, Herder C, Rathmann W, et al. Prediabetes: a high-risk state for diabetes development. Lancet. 2012;379(9833):2279–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang Y, Cai X, Mai W, et al. Association between prediabetes and risk of cardiovascular disease and all cause mortality: systematic review and meta-analysis. BMJ. 2016;355:i5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eun YM, Kang SG, Song SW. Fasting plasma glucose levels and coronary artery calcification in subjects with impaired fasting glucose. Ann Saudi Med. 2016;36(5):334–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kiviniemi AM, Lepojärvi ES, Tulppo MP, et al. Prediabetes and risk for cardiac death among patients with coronary artery disease: the ARTEMIS study. Diabetes Care. 2019;42(7):1319–1325. [DOI] [PubMed] [Google Scholar]
- 5.Buysschaert M, Medina JL, Bergman M, et al. Prediabetes and associated disorders. Endocrine. 2015;48(2):371–393. [DOI] [PubMed] [Google Scholar]
- 6.Ukkola O. Ghrelin and atherosclerosis. Curr Opin Lipidol. 2015;26(4):288–291. [DOI] [PubMed] [Google Scholar]
- 7.Achari AE, Jain SK. Adiponectin, a therapeutic target for obesity, diabetes, and endothelial dysfunction. Int J Mol Sci. 2017;18(6):1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rantala AO, Kauma H, Lilja M, et al. Prevalence of the metabolic syndrome in drug-treated hypertensive patients and control subjects. J Intern Med. 1999;245(2):163–174. [DOI] [PubMed] [Google Scholar]
- 9.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabetes Med. 1998;15(7):539–553. [DOI] [PubMed] [Google Scholar]
- 10.Hietaniemi M, Pöykkö SM, Ukkola O, et al. IGF-I concentrations are positively associated with carotid artery atherosclerosis in women. Ann Med. 2005;37(5):373–382. [DOI] [PubMed] [Google Scholar]
- 11.Santaniemi M, KesäNiemi YA, Ukkola O. Low plasma adiponectin concentration is an indicator of the metabolic syndrome. Eur J Endocrinol. 2006;155(5):745–750. [DOI] [PubMed] [Google Scholar]
- 12.Kunnari A, Ukkola O, Päivänsalo M, et al. High plasma resistin level is associated with enhanced highly sensitive C-reactive protein and leukocytes. J Clin Endocrinol Metab. 2006;91(7):2755–2760. [DOI] [PubMed] [Google Scholar]
- 13.Verdecchia P. Prognostic value of ambulatory blood pressure: current evidence and clinical implications. Hypertension. 2000;35(3):844–851. [DOI] [PubMed] [Google Scholar]
- 14.Ylitalo A. Cardiovascular autonomic regulation in systemic hypertension. Vol. D 519. Acta Universitatis Ouluensis Medica. Finland: University of Oulu; 1999. p. 37. [Google Scholar]
- 15.Pääkkö TJ, Renko RJ, Perkiömäki JS, et al. Ambulatory pulse pressure predicts the development of left ventricular diastolic dysfunction in over 20 years of follow-up. Am J Hypertens. 2017; 30(10):985–992. [DOI] [PubMed] [Google Scholar]
- 16.Pajunen P, Jousilahti P, Borodulin K, et al. Body fat measured by a near-infrared interactance device as a predictor of cardiovascular events: the FINRISK’92 cohort. Obesity (Silver Spring). 2011;19(4):848–852. [DOI] [PubMed] [Google Scholar]
- 17.Nimptsch K, Konigorski S, Pischon T. Diagnosis of obesity and use of obesity biomarkers in science and clinical medicine. Metabolism. 2019;92:61–70. [DOI] [PubMed] [Google Scholar]
- 18.Liu C, Feng X, Li Q, et al. Adiponectin, TNF-α and inflammatory cytokines and risk of type 2 diabetes: a systematic review and meta-analysis. Cytokine. 2016;86:100–109. [DOI] [PubMed] [Google Scholar]
- 19.Meek TH, Morton GJ. The role of leptin in diabetes: metabolic effects. Diabetologia. 2016;59(5):928–932. [DOI] [PubMed] [Google Scholar]
- 20.Pöykkö SM, Kellokoski E, Ukkola O, et al. Plasma ghrelin concentrations are positively associated with carotid artery atherosclerosis in males. J Intern Med. 2006;260(1):43–52. [DOI] [PubMed] [Google Scholar]
- 21.Pemberton CJ, Tokola H, Bagi Z, et al. Ghrelin induces vasoconstriction in the rat coronary vasculature without altering cardiac peptide secretion. Am J Physiol Heart Circ Physiol. 2004;287(4):H1522–H1529. [DOI] [PubMed] [Google Scholar]
- 22.Tschöp M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407(6806):908–913. [DOI] [PubMed] [Google Scholar]
- 23.Virdis A, Lerman LO, Regoli F, et al. Human ghrelin: a gastric hormone with cardiovascular properties. Curr Pharm Des. 2015;22(1):52–58. [DOI] [PubMed] [Google Scholar]
- 24.Baldanzi G, Filigheddu N, Cutrupi S, et al. Ghrelin and des-acyl ghrelin inhibit cell death in cardiomyocytes and endothelial cells through ERK1/2 and PI 3-kinase/AKT. J Cell Biol. 2002;159(6):1029–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li WG, Gavrila D, Liu X, et al. Ghrelin inhibits proinflammatory responses and nuclear factor-kappaB activation in human endothelial cells. Circulation. 2004;109(18):2221–2226. [DOI] [PubMed] [Google Scholar]
- 26.Kawczynska-Drozdz A, Olszanecki R, Jawien J, et al. Ghrelin inhibits vascular superoxide production in spontaneously hypertensive rats. Am J Hypertens. 2006;19(7):764–767. [DOI] [PubMed] [Google Scholar]
- 27.Nagaya N, Kojima M, Uematsu M, et al. Hemodynamic and hormonal effects of human ghrelin in healthy volunteers. Am J Physiol Regul Integr Comp Physiol. 2001;280(5):R1483–R1487. [DOI] [PubMed] [Google Scholar]