Abstract
Background and aims: Hepatic resection is the first-line treatment for hepatocellular carcinoma (HCC). Whether to perform anatomical (AR) or non-anatomical resection (NAR) remains controversial. This retrospective study compares the outcomes according to the number and type of circulating tumour cells (CTCs).
Methods: The cohort included 136 patients with HCC treated with R0 resection between 2014 and 2017. CanPatrol CTC-enrichment technique was used to enrich and classify CTCs according to epithelial-to-mesenchymal transition phenotype.
Results: 91.91% of total patients were CTC-positive, with 91.23% in the AR group and 92.41% in the NAR group. Tumour-free survival (TFS) did not differ significantly between the two groups. However, TFS was significantly higher in patients with low CTCs count and mesenchymal- and epithelial/mesenchymal-negative phenotypes. As for the incidence and types of recurrence, high pre-resection CTC count and mesenchymal- and epithelial/mesenchymal-positivity were significantly associated with extrahepatic and multi-intrahepatic recurrence. Higher morbidities for hepatic failure and ascites were observed in patients treated by AR.
Conclusion: AR may be more beneficial than NAR only in patients with low CTC count and mesenchymal- and epithelial/mesenchymal-negative phenotypes. For patients with a high CTC count, the balance between operative risk and prognostic benefit is more important than the resection method performed.
Key messages
Anatomic resection may improve the survival of HCC patients, but only those with low CTC count and negative M- and E/M-CTC phenotypes.
CTC analysis before surgery can be used to better guide the choice of resection method for HCC.
Keywords: Anatomical liver resection, non-anatomical liver resection, hepatocellular carcinoma, circulating tumour cells, epithelial–mesenchymal transition, tumour-free survival
Introduction
Hepatocellular carcinoma (HCC) is a major health problem worldwide, with over 700,000 cases diagnosed annually [1]. Advances in preoperative assessment of HCC and surgical techniques have improved the surgical outcomes and survival of patients who undergo hepatic resection for HCC. However, the recurrence rates for HCC after curative resection are still high. An important decision in any liver resection, particularly for avoiding recurrence, is choosing the amount of parenchyma to be removed. Anatomic resection (AR) usually involves the removal of two or more complete hepatic segments, while non-anatomic resection (NAR) involves resection of the metastases with a margin of uninvolved tissue (segmentectomy) [2]. Portal vein dissemination was once considered to be the main route of intrahepatic metastases, leading to the notion that AR may be preferable for preventing intrahepatic recurrence [3–5]. However, because of the underlying liver diseases of most patients with HCC, such as chronic hepatitis and cirrhosis, NAR is regarded as advantageous for retaining as much liver parenchyma as possible [6]. Some studies suggest that AR confers a survival benefit [7–9], while others show no prognostic difference between AR and non-AR [10–12]. Several studies show that AR benefits only a subset of HCC patients. For example, a Japanese nationwide study demonstrated that AR is only beneficial for HCC lesions of 2–5 cm in size [13]. Another study showed that AR only improves long-term survival in patients with T1–T2 HCC, probably because of the clearance of venous tumour thrombi within the resected domain [14]. Zhao et al. showed that AR is associated with greater tumour-free survival (TFS) in HCC patients without macroscopic vascular invasion [15]. Therefore, comparison of outcomes between AR and NAR is difficult because of the heterogeneities in patient clinical characteristics.
Circulating tumour cells (CTCs) originating from solid tumours are involved in their haematogenous metastatic spread to distant sites. To become metastatic, CTCs must undergo an epithelial-to-mesenchymal transition (EMT). This process allows a polarised epithelial cell, which normally is attached to the basement membrane, to undergo multiple biochemical changes that enable it to assume a mesenchymal cell phenotype, with increased migratory capacity, invasiveness, elevated resistance to apoptosis and greatly increased production of extracellular matrix components [16]. These changes in CTCs promote metastasis and result in poor outcomes [17–19]. The fraction of CTCs with a mesenchymal phenotype increases with increasing tumour stage in multiple types of cancer, including breast cancer [20] and HCC [21]. Studies suggest that extra- and intrahepatic metastasis are facilitated by the flow of tumour blood to distant sites and systemic rehoming of CTCs to the remnant liver, respectively [22,23]. Thus, EMT of CTCs likely underlies HCC recurrence and may differently affect recurrence rates between AR and NAR.
We previously observed that a high total CTC count and high percentage of mesenchymal CTCs are associated with lung metastasis and multiple intrahepatic recurrence in HCC patients with R0 resection [24].Thus, the prognostic outcomes of AR and NAR may differ according to CTC count and CTC-EMT type. CTC phenotypes can be determined using biomarkers specific to epithelial and mesenchymal cells and forms the basis for CTC analysis, an important “liquid biopsy” technique [25] investigated for use in guiding the choice of therapy in several cancer types [17,20]. Using such analysis, the present study compares the prognostic outcomes of AR and NAR with respect to CTC count and EMT type in HCC patients.
Materials and methods
Study population
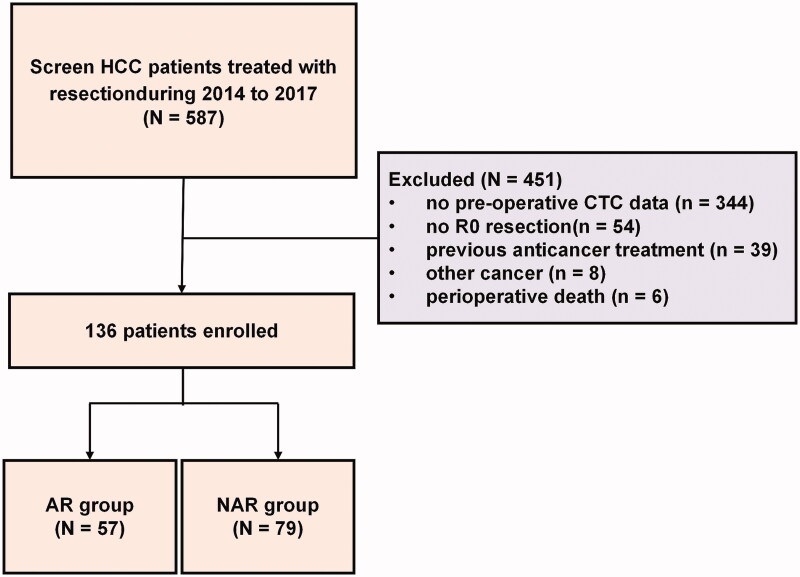
From March 2014 to May 2017, a total of 136 HCC patients treated with R0 resection at the Tumour Hospital of Guangxi Medical University Nanning, Guangxi Province, China were enrolled. Flow chart of patient enrollment was shown in Figure 1. The inclusion criteria are as follows: (1) Treatment with R0 resection, defined as complete macroscopic removal of the tumour, resection margin negative and no detectable intra- or extrahepatic metastatic lesions remaining; (2) Liver function classified as Child–Pugh A stage and PST score of 0–1; (3) CTC examination conducted 1 or 2 days before resection; (4) Definitive pathological diagnosis of HCC based on the World Health Organisation criteria [26]; (5) No previous anticancer treatment. Patients who died from liver failure and those who underwent non-radical resection were excluded.
Figure 1.
Flow chart of patient enrollment.
Clinicopathological features including age, sex, HbsAg status, HBV-DNA level, serum alphafetoprotein (AFP) level, Child–Pugh grade, ICG-R15, BCLC stage, tumour size, tumour number, Edmondson’s grade, resection margin, microvascular invasion (MVI), portal vein tumour thrombus (PVTT), liver cirrhosis and postoperative morbidities were assessed in all patients. Tumour stage was determined according to 2018 Chinese Society of Clinical Oncology (CSCO) staging system, and tumour differentiation was defined according to the Edmondson grading system [27]. MVI was defined as the presence of tumour in the portal vein, hepatic vein or a large capsular vessel of the surrounding hepatic tissue lined by endothelium that was visible only on microscopy. The PVTT type was classified according to the system of Shi and colleagues [28], as follows: type 1, tumour thrombi involving segmental branches at or above the portal vein; type 2, tumour thrombi involving the right/left portal vein; type 3, tumour thrombi involving the main portal vein trunk; type 4, tumour thrombi involving the superior mesenteric vein. In this study, only those patients with type 1 or 2 PVTT received R0 resection. Post-hepatectomy liver failure (PHLF) was defined as a prothrombin time index <50% and serum bilirubin >50 μmol/L (≈2.9 mg/dL) on postoperative day 5 (50–50 criteria) as well as development of massive ascites and/or hepatic encephalopathy [29,30]. The protocol for this study was approved by the ethics committee of the Tumour Hospital of Guangxi Medical University (LW2018033). All patients and healthy volunteers provided written informed consent.
Isolation of CTCs using the CanPatrol system and tri-color RNA-ISH assay
The accuracy and the procedure of the CanPatrol system for isolating CTCs have been described previously [24]. Briefly, blood was collected 1–2 days before resection. Peripheral blood samples (5 mL, anticoagulated with EDTA) were collected after discarding the first 2 mL to avoid potential skin cell contamination from the venipuncture. The filtration system included a filtration tube containing a membrane with 8-μm diameter pores (Sur Exam, Guangzhou, China), a manifold vacuum plate with valve settings (SurExam, Guangzhou, China), an E-Z96 vacuum manifold (Omega, Norcross, GA, USA) and a vacuum pump (Auto Science, Tianjin, China). Before filtration, red blood cell lysis buffer was used to remove erythrocytes, and the cells were resuspended in PBS with 4% formaldehyde (Sigma, St. Louis, MO, USA) for 5 min. The pumping pressure was 0.08 MPa. RNA-ISH was used to detect the following target sequences: CD45 (leukocyte biomarker), EpCAM, CK8/18/19 (epithelial biomarkers) and vimentin and twist (mesenchymal biomarkers). The assay was performed in a 24-well plate (Corning, NY, USA), and the cells on the membrane were treated with a protease (Qiagen, Hilden, Germany) before hybridisation to a capture probe specific for all genes above (Table S1). We used 4′,6-diamidino-2-phenylindole (DAPI) to stain the nuclei, and the cells were analysed using a fluorescence microscope [31].
The R249S mutation in the P53 gene was detected in the DNA of both the primary tumour and CTCs but not in non-tumour liver tissues in our previous study, thus confirming that the CTCs originated from the primary tumour [24].
Surgical procedure
The patients were classified into two groups according to the resection method: AR group (n = 57) and NAR group (n = 79). AR indicates the resection of one or more adjacent hepatic sections along the hepatic vasculature, included segmentectomy, subsegmentectomy, sectoriectomy and hemihepatectomy (left or right hepatectomy). In sectionectomy, the resection line was made along the demarcation on the liver surface after ligation of the sectional pedicle and the trunk of the hepatic vein exposed on the resected surface. NAR is defined as local resection or enucleation regardless of the anatomical segment or section of the lobar anatomy. In NAR, at least 0.5 cm resection margin from the tumour was secured to be as radical as possible, unless the tumour was close to the main hepatic vein or the glissonian pedicle. Liver parenchymal transection was performed by the clamp-crushing method and/or ultrasonic surgical aspirator (CUSA EXcel Plus; USA) or bipolar scissors (Powerstar USA).
Patient follow-up
Patients were followed-up every 1–2 months for the first year and every 3 months thereafter. Postoperative follow-up consisted of ultrasonography, dynamic CT and/or MRI, and laboratory tests, including measurement of the serum AFP. Recurrence was confirmed by the following criteria: (1) A significant AFP level together with one typical imaging modality (contrast-enhanced ultrasonography, CT or MRI); (2) At least two typical imaging modalities reveal the new lesion simultaneously if the AFP level is normal; (3) New lesion biopsy reveals pathological diagnosis of recurrence. The site and time to recurrence were also evaluated. The site of recurrence was divided into intrahepatic and extrahepatic recurrence (i.e. lung metastasis and bone metastasis). Intrahepatic recurrence was also divided into solitary recurrence (including recurrence at the margin, adjacent segment and distant segment), multiple recurrence and PVTT recurrence. The endpoint of follow-up was 31 December 2018.
Statistical analysis
Patient age is presented as the mean and range. All clinical and pathological characteristics, and post-operative complications are summarised as n (%) by group, and the difference between groups was compared using the Pearson Chi-square test or Fisher’s exact test for cell counts less than 5. Kaplan–Meier curve analysis with a Log-rank test was performed to compare the TFS time between groups or levels of CTC count. Results were presented as estimated median TFS time with corresponding 95% confidence intervals (95% CI: lower limit, upper limit) and p. An occurrence rate was also presented as percentage to identify the association of recurrence patterns with considering the surgery type, level of total CTC counts or type of CTC count. Difference between the surgery type, level of total CTC counts or type of CTC count was compared using Pearson Chi-square test or Fisher’s exact test if any cell number less than 5. All statistical assessment were two-tailed and considered significantly as p < .05. All statistical analyses were performed with IBM SPSS statistical software version 22 for Windows (IBM, New York, NY, USA).
Results
A total of 136 HCC patients (112 males and 24 females) with complete data during 2014 to 2017 were enrolled in this retrospective study. The clinical and pathological characteristics of these patients are presented in Table 1. The mean patient age was 45.8 years (range: 20–69 years). All of the clinical and pathological characteristics were similar between the AR group (57 patients) and the NAR group (79 patients) except for the MVI dispersion, with the AR group having a higher MVI positive rate than did the NAR group (77.2% vs. 59.5%; p = .030) (Table 1).
Table 1.
Cohort clinical and disease characteristics (N = 136).
| AR group | NAR group | ||
|---|---|---|---|
| (n = 57) | (n = 79) | p Value | |
| Age (years) | .589 | ||
| ≤45 | 25 (43.9) | 31 (39.2) | |
| >45 | 32 (56.1) | 48 (60.8) | |
| Sex | |||
| Male | 46 (80.7) | 66 (83.5) | .668 |
| Female | 11 (19.3) | 13 (16.5) | |
| HBsAg | |||
| Negative | 9 (15.8) | 6 (7.6) | .132 |
| Positive | 48 (84.2) | 73 (92.4) | |
| HBV-DNA | |||
| <500 | 16 (28.1) | 21 (26.6) | .847 |
| ≥500 | 41 (71.9) | 58 (73.4) | |
| AFP level | |||
| <400 ng/mL | 24 (42.1) | 36 (45.6) | .688 |
| ≥400 ng/mL | 33 (57.9) | 43 (54.4) | |
| Child–Pugh Stage | NA | ||
| Stage A (5–6 points) | 57 (100) | 79 (100) | |
| Stage B (6–9 points) | 0 (0) | 0 (0) | |
| Stage C (10–15 points) | 0 (0) | 0 (0) | |
| ICG-R15 | .948 | ||
| <10% | 50 (87.7) | 69 (87.3) | |
| 10–20% | 7 (12.3) | 10 (12.7) | |
| >20% | 0 (0) | 0 (0) | |
| Tumour sizea | .054 | ||
| <5 cm | 10 (17.5) | 22 (27.8) | |
| 5–10 cm | 19 (33.3) | 34 (43.0) | |
| ≥10 cm | 28 (49.1) | 23 (29.2) | |
| Node number | .867 | ||
| Single | 36 (63.2) | 51 (64.6) | |
| Multi (≥2 nodes) | 21 (36.8) | 28 (35.4) | |
| Edmondson gradea | .832 | ||
| Well differentiated | 6 (10.5) | 6 (7.6) | |
| Moderately differentiated |
32 (56.1) | 45 (57.0) | |
| Poorly differentiated |
19 (33.3) | 28 (35.4) | |
| CSCO stage | .366 | ||
| Stage I (a–b) | 28 (49.1) | 45 (57.0) | |
| Stage II–III | 29 (50.9) | 34 (43.0) | |
| Tumour capsulea | .430 | ||
| incomplete | 24 (42.1) | 28 (35.4) | |
| complete | 33 (57.9) | 51 (64.6) | |
| Resection margin | .821 | ||
| <1 cm | 40 (70.2) | 54 (68.4) | |
| ≥1 cm | 17 (29.8) | 25 (31.6) | |
| MVI | .030* | ||
| Negative | 13 (22.8) | 32 (40.5) | |
| Positive | 44 (77.2) | 47 (59.5) | |
| PVTT/HVTT | .123 | ||
| Negative | 38 (66.7) | 62 (78.5) | |
| Positive | 19 (33.3) | 17 (21.5) | |
| Liver cirrhosis | .794 | ||
| Negative | 3 (5.3) | 5 (6.3) | |
| Positive | 54 (94.7) | 74 (93.7) |
Data are presented as n (%) by group and compared using Pearson Chi-square test or Fisher’s exact test for any cell number less than 5.
HBV: hepatitis B virus; AFP: alpha-fetoprotein; MVI: microvascular invasion; PVTT: portal vein tumour thrombus; LN: lymph node; NA: not assessed.
In cases involving multiple nodes, the largest is indicated.
p < .05 is considered as a significant difference between the AR and NAR groups.
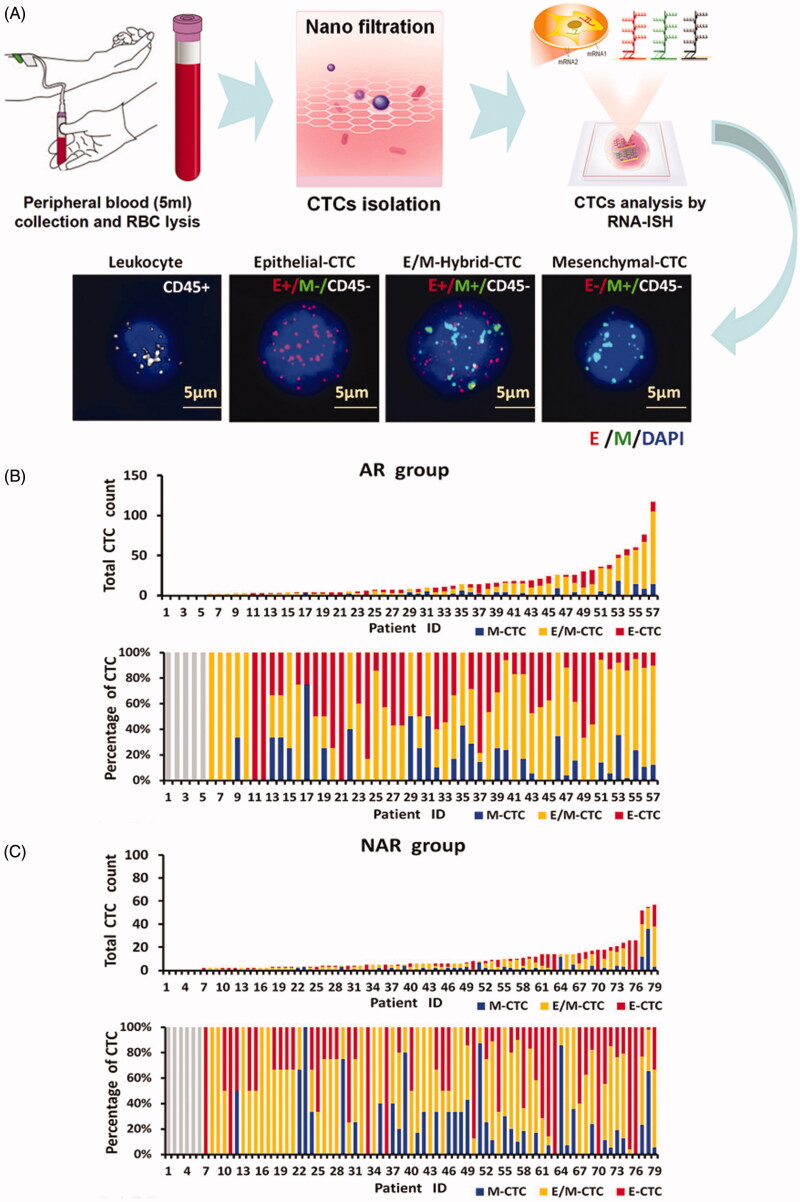
Three CTC subpopulations identified in patient blood
CTCs were detected in blood samples using the CanPatrol CTC-enrichment technique. Red and green fluorescent signals indicate epithelial and mesenchymal cell gene expression, respectively. The white fluorescent signal indicates expression of CD45, a leukocyte marker. Thus, analysis of CTCs by RNA-ISH revealed three subpopulations: (1) epithelial CTCs (E-CTC), (2) epithelial/mesenchymal hybrid CTCs (E/M-CTC) and (3) mesenchymal CTCs (M-CTC) (Figure 2(A)). CTCs were detected in 91.91% (125 of 136) of the total cohort. The preoperative mean total CTC count was 12.04 ± 15.12 (range, 0–117). CTCs were present in 91.23% of the patients treated by AR (52/57) and 92.41% (73/79) of those treated by NAR (Figure 2(B, C)).
Figure 2.
CTCs isolation and RNA-ISH analysis of epithelial–mesenchymal transition (EMT) markers in CTCs from HCC patients. (A) Process of CTC isolation and detection by CanPatrol™ CTC-enrichment and in situ hybridisation (ISH). Leukocytes were stained for CD45 (white fluorescence). CTCs were stained for the epithelial markers EpCAM and CK8/18/19 (red fluorescence) and the mesenchymal markers vimentin and twist (green fluorescence). The cells were analysed using a 100× objective. (B, C) CTC counts and percentage of each CTC type in patients treated by (B) AR (91.23%; 52/57) and (C) NAR (92.41%; 73/79).
TFS with respect to CTC count differs between the AR and NAR groups
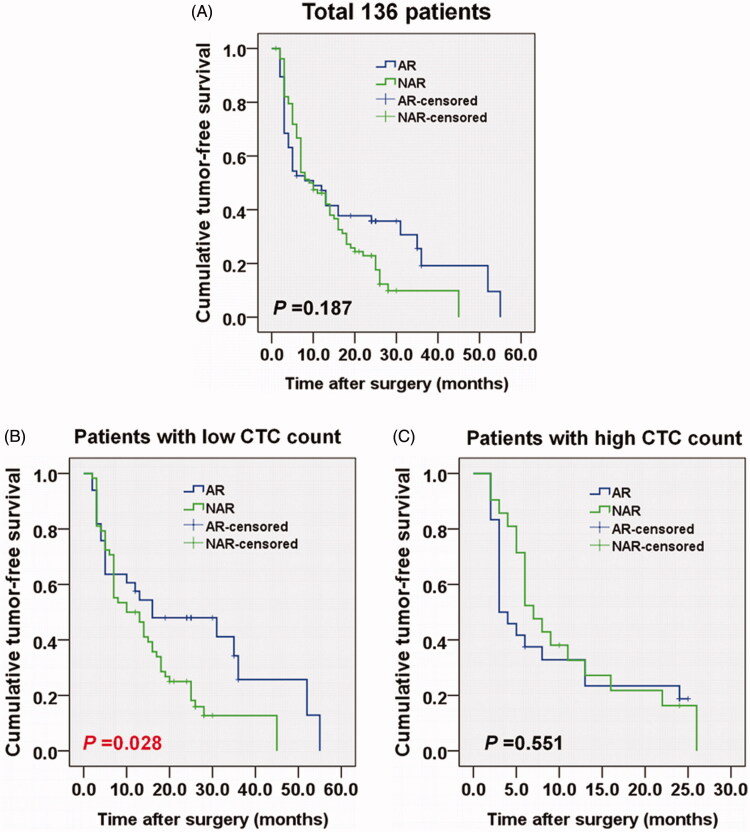
At the end of follow-up, the overall tumour recurrence rate was 80.1% (109/136), including 68 patients in the AR group and 41 in the NAR group. The median TFS was 10 months (95% CI = 4.6–15.4 months) for the AR and 9 months (95% CI = 5.8–12.2 months) for NAR groups. The log-rank test showed that the AR and NAR groups had a similar TFS time during the follow-up (p = .187) (Figure 3(A)).
Figure 3.
Comparison of tumour-free survival (TFS) between patients treated by AR and NAR for (A) all patients, (B) patients with low total CTC count (<12) and (C) patients with high total CTC count (≥12). The Kaplan–Meier curve was determined using the log-rank test.
The 136 patients were further divided into two sub-groups according to CTC count (mean total CTC count, 12), with 91 classified as low total CTC count (<12) and 45 as high CTC count (≥12). Patients with a low total CTC count tended to have higher TFS time than those with high total CTC count (median TFS, 13 months [95% CI = 5–24] vs. 6 months [95% CI = 3–13], p = .0042).
Patients who were negative for E/M- and M-type CTCs had higher TFS than did those who were positive for these types (E/M-type: median TFS, 24.5 [95% CI = 7.5–28] vs. 7 months [95% CI = 3–17.5], p = .0003; M-type: median TFS, 17 months [95% CI = 8–25] vs. 5 months [95% CI = 3–9], p < .0001). In the AR group, the TFS was higher in patients with low total CTC count, negative for E/M-type CTCs, or negative for M-type CTCs (all p ≤ .05). In contrast, the TFS in the NAR group was associated only with the M-type of CTC (median TFS, 15.5 months [95% CI = 8–25] vs. 6 months [95% CI = 3–8], p < .001) (Table 2).
Table 2.
Tumour-free survival (TFS) according to CTC count according to resection type (N = 136).
| Median TFS |
|||
|---|---|---|---|
| Total (n = 136) |
AR group (n = 57) |
NAR group (n = 79) |
|
| Total CTC count | |||
| Low | 13 (5, 24) | 12 (4, 24) | 11 (5, 19.5) |
| High | 6 (3, 13) | 3.5 (3, 13) | 7 (5, 13) |
| p Value | .0042† | .0407† | .2687 |
| E-CTC | |||
| Negative | 8 (3, 25) | 17.5 (3.5, 35.5) | 7 (3, 17) |
| Positive | 10 (4, 22) | 6 (3, 24) | 10.5 (5, 20) |
| p Value | .6745 | .1219 | .3125 |
| E/M-CTC | |||
| Negative | 24.5 (7.5, 28) | 35 (25, 36) | 16 (6, 25) |
| Positive | 7 (3, 17.5) | 5 (3, 17.5) | 7.5 (5, 17.5) |
| p Value | .0003‡ | .0002‡ | .1458 |
| M-CTC | |||
| Negative | 17 (8, 25) | 24 (7, 30.5) | 15.5 (8, 25) |
| Positive | 5 (3, 9) | 3 (3, 12) | 6 (3, 8) |
| p Value | <.0001¶ | <.0001¶ | <.0001¶ |
TFS is reported as the median with corresponding 95% confidence intervals. The log-rank test was performed to compare the TFS between the AR and NAR groups and for different CTC counts.
Significant difference (p < .05) between the number of patients with low and high total CTC count.
Significant difference (p < .05) between the number of patients with negative and positive for E/M-type CTCs.
Significant difference (p < .05) between the number of patients with negative and positive for M-type CTCs.
For patients with low total CTC count, the TFS was higher in the AR group than in the NAR group (median TFS = 12 months [95% CI = 4–24] vs. 11 months [95% CI = 5–19.5], p = .028) (Figure 3(B)). Conversely, the AR group had a lower TFS than the NAR group (median TFS = 3.5 [95% CI = 3–13] vs. 7 months [95% CI = 5–13]), but without statistical significance in patients with high total CTC count (p = .551) (Figure 3(C)).
The data in Table 2 indicate the factor affecting the TFS could be either the total CTC count and/or the CTC type (E/M − or M). We observed that the TFS was higher in the AR group than the NAR group among patients with a low total CTC count and E/M − CTC type (median TFS, 36 months [95% CI = 25.3–46.7] vs. 16 months [95% CI = [0–33.0]), or low total CTC count and M − CTC type (median TFS = 35 months [95% CI = 28.9–41.1] vs. 16 months [95% CI = 10.5–21.5]), respectively (all p ≤ .05) (Table 3). We also performed multivariate analysis to further confirm the conclusion (Supplementary Table 1).
Table 3.
Comparison of tumour-free-survival (TFS) between resection types according to CTC count and type (N = 136).
| Median TFS |
|||||
|---|---|---|---|---|---|
| AR group (n = 57) |
NAR group (n = 39) |
p Value | |||
| Total CTC count | E/M-CTC status | ||||
| Low | Negative | 21 | 36 (25.3, 46.7) | 16 (0, 33.0) | 0.001* |
| High | Negative | 3 | ND | 13 (0.2, 25.8) | NA |
| Low | Positive | 70 | 10 (2.9, 17.1) | 10 (5.6, 14.4) | 0.629 |
| High | Positive | 42 | 3 (1.6, 4.4) | 6 (4.3, 7.7) | 0.542 |
| Total CTC count | M-CTC status | ||||
| Low | Negative | 56 | 35 (28.9, 41.1) | 16 (10.5, 21.5) | 0.010* |
| High | Negative | 14 | 8 (0, 16.4) | 3.9 (8.4, 23.6) | 0.680 |
| Low | Positive | 33 | 5 (2.9, 7.1) | 6 (4.1, 7.9) | 0.326 |
| High | Positive | 27 | 3 (2.4, 3.6) | 6 (5.2, 6.8) | 0.799 |
TFS is reported as the median with corresponding 95% confidence intervals (95% CI: range). A Log-rank test was performed to compare TFS between the AR and NAR groups and counts of different CTC types. Abbreviations: ND, not derived; NA, not assessed.
Significant difference between the AR and NAR groups.
CTC count and type are associated with recurrence type and surgical method
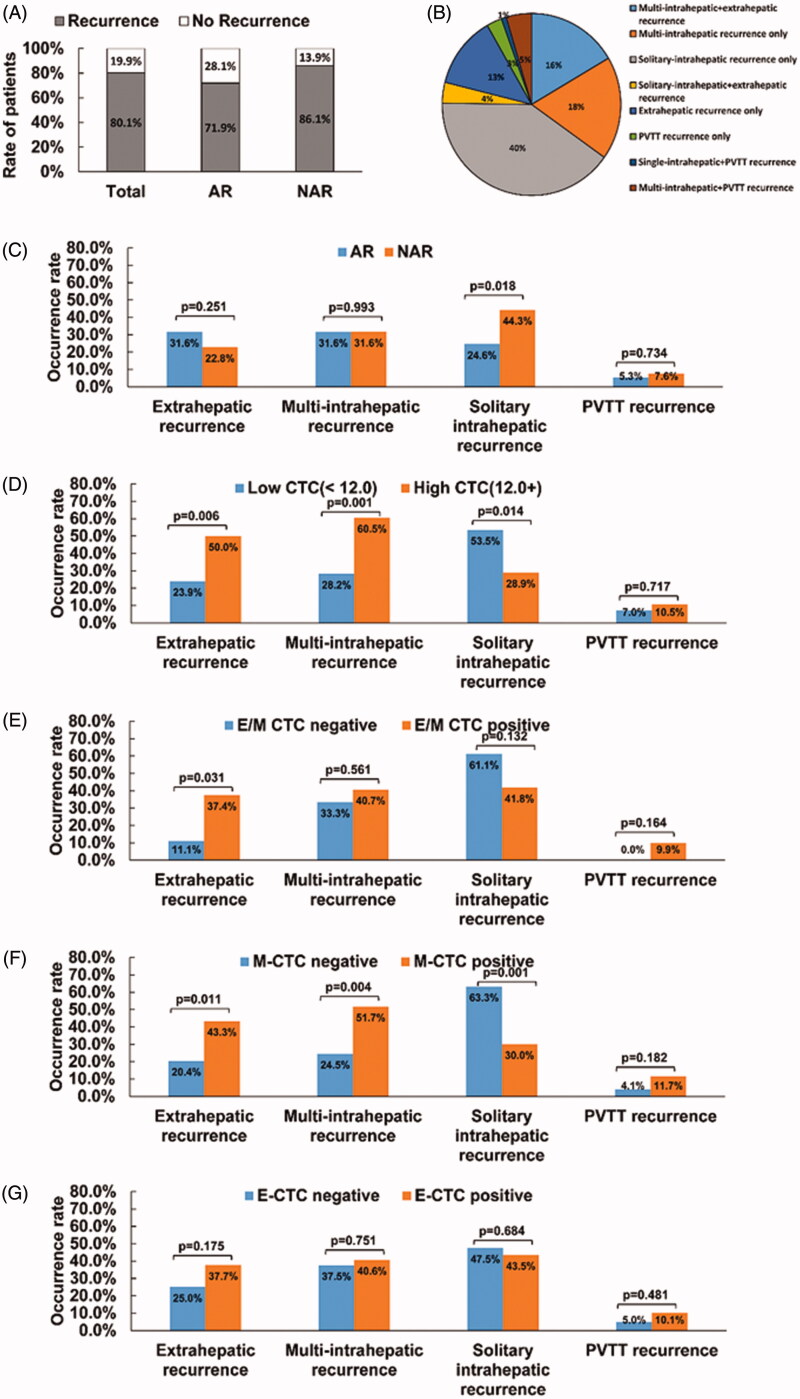
By the end of follow-up, the overall tumour recurrence rate was 80.1% (109/136), with 71.9% in the AR group and 86.1% in the NAR group (p = .051) (Figure 4(A)). The four most common types of recurrence (occurrence rate >10%) were solitary-intrahepatic (40%), multi-intrahepatic (18%), multi-intrahepatic + extrahepatic (16%) and extrahepatic alone (13%). Of the patients with recurrence, 9% had PVTT: 3% exhibiting PVTT alone, 5% with multi-intrahepatic lesions + PVTT and 1% with a single-intrahepatic lesion + PVTT (Figure 4(B)). The AR group had a lower rate of solitary intrahepatic recurrence than did the NAR group (24.6% vs. 44.3%, p = .018) (Figure 4(C)). Patients with a low total CTC count had a lower rate of extrahepatic and multi-intrahepatic recurrence (Extrahepatic recurrence: 23.9% vs. 50.0%, p = .006; multi-intrahepatic recurrence: 28.2% vs. 60.5%, p = .001) and a higher rate of solitary intrahepatic recurrence (53.5% vs. 28.9%, p = .014) (Figure 4(D)). Patients negative for type M CTC had a lower rate of extrahepatic recurrence (20.4% vs. 43.3%; p = .011) and multi-intrahepatic recurrence (24.5% vs. 51.7%; p = .004) and a higher rate of solitary intrahepatic recurrence (63.3% vs. 30%, p < .001) (Figure 4(F)).
Figure 4.
Comparison of recurrence rates between resection types according to recurrence type and CTC status recurrence rates according to (A) resection type, and (B) (C) recurrence type; (D) CTC count, (E) E/M-CTC status (F) M-CTC status, and (G) E-CTC status with respect to the four recurrence patterns (solitary-intrahepatic recurrence, multi-intrahepatic recurrence, extrahepatic recurrence and PVTT recurrence). Results are presented as the rate of occurrence and were compared using the Pearson Chi-square test or Fisher’s exact test. p < .05 was considered statistically significant.
Comparison of postoperative complication rates between the AR and NAR groups
Postoperative complication rates of the AR and NAR groups were summarised in Table 4. The complication rates for PHLF and ascites were significantly higher in the AR group than in the NAR group (PHLF: 14% vs. 2.5%, p = .017; Ascites: 28.1% vs. 5.1%, p < .001) (Table 4).
Table 4.
Comparison of post-operative complications between the AR and NAR groups (N = 136).
| AR group | NAR group | ||
|---|---|---|---|
| Complications | (n = 57) | (n = 79) | p Value |
| PHLF | 8 (14.0) | 2 (2.5) | .017* |
| Ascites | 16 (28.1) | 4 (5.1) | <.001* |
| Hydrothorax | 7 (12.3) | 5 (6.3) | .227 |
| Bleeding | 2 (3.5) | 0 (0) | N/A |
| Biliary fistula | 1 (1.8) | 2 (2.5) | 1.000 |
| Hepatic encephalopathy | 1 (1.8) | 0 (0) | N/A |
| Wound infection | 5 (8.8) | 4 (5.1) | .491 |
Data were summarised as n (%) by group and compared using Pearson Chi-square test or Fisher’s exact test if any cell number less than 5.
PHLF: post-hepatectomy liver failure; N/A: not assessed.
Significant difference between the AR and NAR groups.
Discussion
In this retrospective comparison of outcomes between HCC resection methods, we observed that TFS did not differ significantly between patients treated by AR and NAR. However, TFS was significantly longer in patients with low CTC and no CTCs of the M or E/M phenotype. The incidence and types of HCC recurrence did not differ significantly between AR and NAR resection. High CTC count and M- and E/M-CTC phenotype before resection were significantly associated with extrahepatic and multi-intrahepatic recurrence regardless of resection type. The morbidities for hepatic failure and ascites were higher in patients treated by AR than in those treated by NAR. These findings suggest that AR may be more beneficial than NAR only in patients with low CTC count and M- and E/M-negative CTC phenotypes.
The presence of CTCs is known to be associated with disease progression and poor outcomes [17,20]. In a study of CTCs in HCC patients after liver resection, CTC-positive patients were found to have a significantly higher risk of recurrence and a shorter RFS compared to those who were CTC negative [25]. Our previous study showed that high CTC count before resection was significantly associated with early recurrence of HCC. The present study further confirms that patients with a low CTC count have a longer TFS than do those with a high count. During EMT, CTCs lose epithelial characteristics and gain mesenchymal traits that increase their metastatic potential [18,19]. These findings suggest that high CTC count before surgery may indicate pre-surgical metastasis, with highly aggressive E/M and M CTCs already disseminated in the blood. This hypothesis is consistent with our finding that the TFS does not differ significantly between AR and NAR for patients with a high presurgical CTC count and positivity for M- and E/M-CTCs, as metastasis had begun before resection. Therefore, AR may be considered a better choice only for patients with a low CTC count and no E/M- or M-type CTCs. Large cohort studies should be undertaken to further validate the prognostic significance of CTC subtypes in HCC patients.
HCC recurrence is an important factor in prognosis, although the time to recurrence and mode of recurrence varies between patients [32]. We observed no difference between AR and NAR with respect to extrahepatic or multi-intrahepatic recurrence rates. The significantly higher rates of extrahepatic and multi-intrahepatic recurrence in patients with high CTC count or positive M-CTC and E/M-CTC status suggest that such recurrence may be CTC count/type-induced. Also, there is a type of intrahepatic recurrence after curative therapy that we call “multi-centric recurrence”. Studies indicate that most of these multi-centric recurrences may not be metastasis from the original tumour, but rather de novo cancers arising in a cirrhotic liver or as a result of HBV reactivation [1,33]. Because both extrahepatic and intrahepatic metastasis recurrence may be CTC count/type-induced and may developed earlier than multi-centric recurrence. This is why patients with high CTC count or positive M-CTC and E/M-CTC status, even with AR resection, may also have a greater chance of early extrahepatic and intrahepatic metastasis recurrence. In additions, although no statistically significant, we found that the AR group had a lower rate of single-intrahepatic recurrence than did the NAR group, suggesting that AR may decrease single-intrahepatic recurrence caused by local dissemination in the same hepatic subsegment.
The method and extent of liver resection should be planned for each individual after full consideration of the balance between surgical risk and prognostic benefit, aiming to achieve curative resection while preserving maximal liver function. After major AR resection (half right/left hepatectomy), we observed that hepatic failure and ascites were more frequent in patients with underlying cirrhosis (data not shown). NAR is chosen for the purpose of decreasing postoperative complications, including post-hepatectomy hepatic failure. Therefore, performing NAR for patients with high CTC count, especially those with liver cirrhosis, is a reasonable choice because they may have a poor prognosis to begin with. Although AR should be performed to increase the survival of patients with low CTC count, NAR can be considered if other factors contraindicate AR, such as liver cirrhosis or poor liver function.
This study has several limitations. First, this study is retrospective in design and has a relatively small sample size. Consequently, our cohort was imbalanced with respect to CTC count and subset status. Large cohort studies will be undertaken in our future investigations. In addition, the CanPatrol system used to collect CTCs in this study allows particles <8 μm in diameter to pass through the filter, remaining undetected [34]. To overcome this potential limitation in future studies, multiple CTC detection techniques should be used to improve the detection efficiency.
In conclusion, CTC analysis before surgery can be used to better guide the choice of resection method. AR may improve the survival of patients, but only those with low CTC count and negative M- and E/M-CTC phenotypes. In patients with high CTC count and M- or E/M CTC phenotypes, TFS is not improved by AR, likely due to systemic dissemination of CTCs leading to extrahepatic and multi-intrahepatic recurrence. Thus, in these patients, consideration of the balance between the operative risk and prognostic benefit is more important than the surgical method chosen.
Glossary
Abbreviations
- HCC
hepatocellular carcinoma
- AR
anatomic resection
- NAR
non-anatomical resection
- CTCs
circulating tumour cells
- EMT
epithelial-to-mesenchymal transition
- TFS
tumour-free survival
Funding Statement
This work was supported by the National Natural Science Foundation of China [NSFC 81502533, 81260080, 81160262], and Guangxi Nature Sciences Grants [2013GXNSFBA019196, 2018GXNSFAA138028]. This work was also supported in part by the Guangxi Medical University Training Programme for Distinguished Young Scholars.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- 1.Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut. 2014;63(5):844–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aragon RJ, Solomon NL. Techniques of hepatic resection. J Gastrointest Oncol. 2012;3(1):28–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakashima T, Kojiro M. Pathologic characteristics of hepatocellular carcinoma. Semin Liver Dis. 1986;6(03):259–266. [DOI] [PubMed] [Google Scholar]
- 4.Makuuchi M, Hasegawa H, Yamazaki S. Ultrasonically guided subsegmentectomy. Surg Gynecol Obstet. 1985;161(4):346–350. [PubMed] [Google Scholar]
- 5.Yamamoto M, Takasaki K, Ohtsubo T, et al. Effectiveness of systematized hepatectomy with Glisson's pedicle transection at the hepatic hilus for small nodular hepatocellular carcinoma: retrospective analysis. Surgery. 2001;130(3):443–448. [DOI] [PubMed] [Google Scholar]
- 6.Hsia CY, Lui WY, Chau GY, et al. Perioperative safety and prognosis in hepatocellular carcinoma patients with impaired liver function. J Am Coll Surg. 2000;190(5):574–579. [DOI] [PubMed] [Google Scholar]
- 7.Makuuchi M. Surgical treatment for HCC–special reference to anatomical resection. Int J Surg. 2013;11(Suppl 1):S47–S49. [DOI] [PubMed] [Google Scholar]
- 8.Takasaki K. Glissonean pedicle transection method for hepatic resection: a new concept of liver segmentation. J Hepatobiliary Pancreat Surg. 1998;5(3):286–291. [DOI] [PubMed] [Google Scholar]
- 9.Hasegawa K, Aoki T, Ishizawa T, et al. Comparison of the therapeutic outcomes between surgical resection and percutaneous ablation for small hepatocellular carcinoma. Ann Surg Oncol. 2014;21(S3):348–355. [DOI] [PubMed] [Google Scholar]
- 10.Li SQ, Huang T, Shen SL, et al. Anatomical versus non-anatomical liver resection for hepatocellular carcinoma exceeding Milan criteria. Br J Surg. 2017;104(1):118–127. [DOI] [PubMed] [Google Scholar]
- 11.Marubashi S, Gotoh K, Akita H, et al. Anatomical versus non-anatomical resection for hepatocellular carcinoma. Br J Surg. 2015;102(7):776–784. [DOI] [PubMed] [Google Scholar]
- 12.Okamura Y, Ito T, Sugiura T, et al. Anatomic versus nonanatomic hepatectomy for a solitary hepatocellular carcinoma: a case-controlled study with propensity score matching. J Gastrointest Surg. 2014;18(11):1994–2002. [DOI] [PubMed] [Google Scholar]
- 13.Eguchi S, Kanematsu T, Arii S, et al. Comparison of the outcomes between an anatomical subsegmentectomy and a non-anatomical minor hepatectomy for single hepatocellular carcinomas based on a Japanese nationwide survey. Surgery. 2008;143(4):469–475. [DOI] [PubMed] [Google Scholar]
- 14.Wakai T, Shirai Y, Sakata J, et al. Anatomic resection independently improves long-term survival in patients with T1–T2 hepatocellular carcinoma. Ann Surg Oncol. 2007;14(4):1356–1365. [DOI] [PubMed] [Google Scholar]
- 15.Zhao H, Chen C, Gu S, et al. Anatomical versus non-anatomical resection for solitary hepatocellular carcinoma without macroscopic vascular invasion: a propensity score matching analysis. J Gastroenterol Hepatol. 2017;32(4):870–878. [DOI] [PubMed] [Google Scholar]
- 16.Kalluri R, Neilson EG. Epithelial–mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112(12):1776–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun YF, Xu Y, Yang XR, et al. Circulating stem cell-like epithelial cell adhesion molecule-positive tumor cells indicate poor prognosis of hepatocellular carcinoma after curative resection. Hepatology. 2013;57(4):1458–1468. [DOI] [PubMed] [Google Scholar]
- 18.Guarino M. Epithelial–mesenchymal transition and tumour invasion. Int J Biochem Cell Biol. 2007;39(12):2153–2160. [DOI] [PubMed] [Google Scholar]
- 19.Ksiazkiewicz M, Markiewicz A, Zaczek AJ. Epithelial–mesenchymal transition: a hallmark in metastasis formation linking circulating tumor cells and cancer stem cells. Pathobiology. 2012;79(4):195–208. [DOI] [PubMed] [Google Scholar]
- 20.Yu M, Bardia A, Wittner BS, et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 2013;339(6119):580–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li YM, Xu SC, Li J, et al. Epithelial-mesenchymal transition markers expressed in circulating tumor cells in hepatocellular carcinoma patients with different stages of disease. Cell Death Dis. 2013;4(10):e831–e831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakon M, Ogawa H, Fujita M, et al. Hepatic resection for hepatocellular carcinoma based on tumor hemodynamics. Hepatol Res. 2013;43(2):155–164. [DOI] [PubMed] [Google Scholar]
- 23.Sakon M, Nagano H, Nakamori S, et al. Intrahepatic recurrences of hepatocellular carcinoma after hepatectomy: analysis based on tumor hemodynamics. Arch Surg. 2002;137(1):94–99. [DOI] [PubMed] [Google Scholar]
- 24.Qi LN, Xiang BD, Wu FX, et al. Circulating tumor cells undergoing EMT provide a metric for diagnosis and prognosis of patients with hepatocellular carcinoma. Cancer Res. 2018;78(16):4731–4744. [DOI] [PubMed] [Google Scholar]
- 25.von Felden J, Schulze K, Krech T, et al. Circulating tumor cells as liquid biomarker for high HCC recurrence risk after curative liver resection. Oncotarget. 2017;8(52):89978–89987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cancer TIAfRo: WHO Classification of Tumours of the Digestive System (IARC WHO Classification of Tumours). 4th ed. World Health Organization; 2010. p. 418. [Google Scholar]
- 27.Bruix J, Sherman M. American Association for the Study of Liver D: management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi J, Lai EC, Li N, et al. A new classification for hepatocellular carcinoma with portal vein tumor thrombus. J Hepatobiliary Pancreat Sci. 2011;18(1):74–80. [DOI] [PubMed] [Google Scholar]
- 29.Li SQ, Liang LJ, Peng BG, et al. Outcomes of liver resection for intrahepatic stones: a comparative study of unilateral versus bilateral disease. Ann Surg. 2012;255(5):946–953. [DOI] [PubMed] [Google Scholar]
- 30.Balzan S, Belghiti J, Farges O, et al. The “50–50 criteria” on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg. 2005;242(6):824–828. discussion 828–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu S, Liu S, Liu Z, et al. Classification of circulating tumor cells by epithelial–mesenchymal transition markers. PLoS One. 2015;10(4):e0123976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9(4):274–284. [DOI] [PubMed] [Google Scholar]
- 33.Llovet JM, Bruix J. Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol. 2008;48(Suppl 1):S20–S37. [DOI] [PubMed] [Google Scholar]
- 34.Alix-Panabieres C, Pantel K. Challenges in circulating tumour cell research. Nat Rev Cancer. 2014;14(9):623–631. [DOI] [PubMed] [Google Scholar]