Abstract
Postpericardiotomy syndrome (PPS) is a well-known complication after cardiac surgery. The syndrome results in prolonged hospital stay, readmissions, and invasive interventions. Previous studies have reported inconsistent results concerning the incidence and risk factors for PPS due to the differences in the applied diagnostic criteria, study designs, patient populations, and procedure types. In recent prospective studies the reported incidences have been between 21 and 29% in adult cardiac surgery patients. However, it has been stated that most of the included diagnoses in the aforementioned studies would be clinically irrelevant. This challenges the specificity and usability of the currently recommended diagnostic criteria for PPS. Moreover, recent evidence suggests that PPS requiring invasive intervention such as the evacuation of pleural and/or pericardial effusion is associated with increased mortality. In the present review, we summarise the existing literature concerning the incidence, clinical features, diagnostic criteria, risk factors, management, and prognosis of PPS. We also propose novel approaches regarding to the definition and diagnosis of PPS.
Key messages:
Current diagnostic criteria of PPS should be reconsidered, and the analyses should be divided into subgroups according to the severity of the syndrome to achieve more clinically applicable and meaningful results in the future studies.
In contrast with the previous presumption, severe PPS – defined as PPS requiring invasive interventions – was recently found to be associated with higher all-cause mortality during the first two years after cardiac surgery. The association with an increased mortality supports the use of relatively aggressive prophylactic methods to prevent PPS.
The risk factors clearly increasing the occurrence of PPS are younger age, pleural incision, and valve and ascending aortic procedures when compared to CABG.
Keywords: Postpericardiotomy syndrome, pericardium, cardiac surgery, epidemiology, clinical features, diagnostic criteria, risk factors, treatment, adverse events, prognosis
Introduction
Postpericardiotomy syndrome (PPS) is a common complication after cardiac surgery. The syndrome is a subgroup of post-cardiac injury syndromes (PCIS) together with postmyocardial infarction syndrome (Dressler’s syndrome) and posttraumatic pericarditis [1]. The typical clinical picture consists of pleuritic chest pain and fever appearing few days to several weeks after cardiac surgery [2]. Although the syndrome was first described in the 1950s [3,4], the aetiology of the syndrome has remained obscure. Currently, PPS is presumed to be an immune-mediated process initiated by pericardial and/or pleural damage and pericardial bleeding but the role other possible acquired factors is not well understood [2].
In previous studies, the incidence of PPS has mostly varied between 10 and 30% [3–13]. In recent prospective randomised trials the reported incidences have been between 21 and 29% [7,8]. The large variability between the studies has evolved mostly from the differences in diagnostic criteria, patient populations, and procedure types [2,14]. Standardised criteria for the syndrome were first described in 2015 in the European Society of Cardiology Guidelines with an objective to reduce the variability between studies [15]. The recommended criteria have, however, already received criticism due to their weak correlation with the clinical picture, overflowing sensitivity, and non-specificity [14]. The prevailing practice of diagnosing PPS with maximal sensitivity has reflected in the recent prospective studies that have included PPS patients with a broad spectrum resulting in a high apparent incidence of the syndrome. However, the clinical relevance of the diagnoses is uncertain [12].
The current guidelines recommend the use of non-steroidal anti-inflammatory drugs (NSAIDs) combined with colchicine in more severe cases in the treatment of the syndrome [15]. Even though PPS occasionally requires invasive interventions, such as the evacuation of pleural and/or pericardial effusion, as well as longer hospital stays and readmissions [5,12], the prognosis of the syndrome has been previously considered to be benign [1,2,16]. However, this perception has been mainly based on clinical experience rather than appropriate epidemiological studies concentrating on the long-term prognosis of the syndrome until the last years.
The objective of this review is to (a) summarise the existing literature concerning the incidence, clinical features, diagnostic criteria, risk factors, management, and prognosis of PPS and (b) propose new scientific approaches regarding to the diagnosing of PPS.
Epidemiology of PPS
The first descriptions of PPS date back to 1950s, shortly after the establishment of the first surgical cardiac valve procedures, but before the introduction of cardiopulmonary bypass (CPB). In the year 1952, Janton and colleagues reported the results of their first 100 consecutive commissurotomies for mitral stenosis [3]. The authors observed unpredictable pleuropericardial pain unresponsive to salicylates, antibiotics, and ordinary doses of narcotics in about 30 percent of the patients. The symptoms occurred typically during the second postoperative week. The episodes were considered to be possible manifestations of smouldering rheumatic activity, though only one sixth of all patients had microscopic evidence of active rheumatic infection in the left auricular appendage. Soloff and colleagues investigated the syndrome further and observed an incidence of 24% after consecutive mitral commissurotomies [4]. Because of the misunderstood causality, the syndrome was named “postcommissurotomy syndrome” until 1958, when an identical state was detected following cardiac surgery in patients without rheumatic heart disease [17]. The syndrome was only observed when the pericardial cavity was entered, and consequently it was renamed “postpericardiotomy syndrome”.
The reported incidences of the syndrome have varied largely. During the first years after the discovery of the syndrome the incidence varied from 4% to 63% after mitral commissurotomy with an average of 17% [3,4,18–27]. Besides the differences in diagnostic criteria, which were not reported in most of the studies, this exceptionally large variety illustrates the lack of effective differential diagnostic equipment, especially the echocardiography. After the inclusion of different procedure types, the syndrome was detected in 27–31% of patients [11,28]. Studies requiring good response to steroid therapy for the diagnosis reported incidences of 6–7% [29,30].
Engle concluded that the incidence of PPS, according to their quite comprehensive prospective studies, was 27% in children and 18% in adults aged 21 years or older [31]. After the age of 70 years the incidence decreased to 10%. Furthermore, patients aged under 2 years presented very few cases of PPS (3.5%) and infants under the age of six months did not seem to present with the syndrome at all. Later, consistent with the aforementioned study, a large prospective epidemiological study by Miller et al. observed an incidence of 24% in patients under 54 years, 18% in patients 55–64 years, and 11% in patients over 65 years after careful differential diagnostic procedures [9].
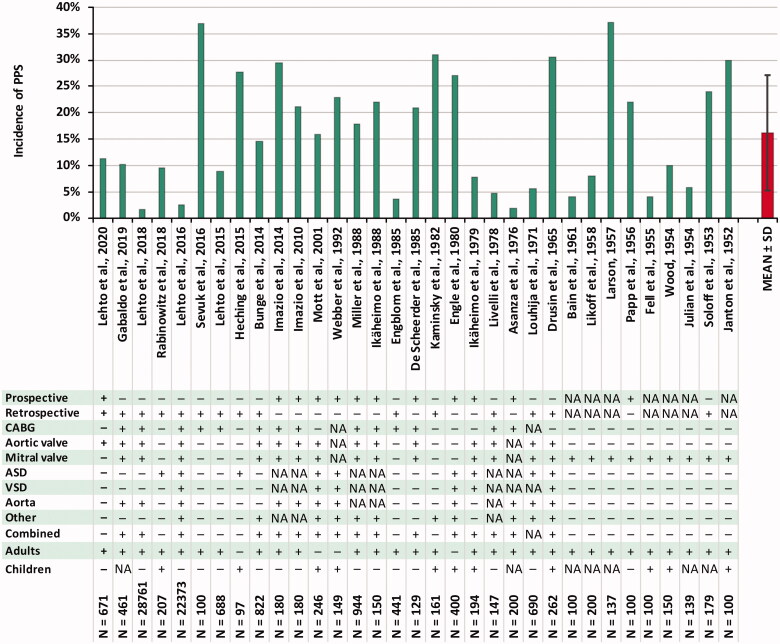
In the major PPS studies during the last decades, the reported incidences have been the following: 10–28% in children [32–36] and 9–21% in adults [8,12,13,37,38] with a median of 16% in adult patients [14]. In the recent Colchicine for Prevention of Postpericardiotomy Syndrome and Postoperative Atrial Fibrillation (COPPS-2) trial [7], the incidence in adult patients was as high as 29% in elderly patients, although the time limit of fever was abandoned resulting in a markedly higher occurrence during the first postoperative days compared to the Colchicine for the Prevention of the Post-pericardiotomy Syndrome (COPPS) trial [8]. Lehto and colleagues reported an incidence of 8.9% after coronary artery bypass grafting (CABG) in a retrospective analysis only including PPS cases requiring medical attention, referring to hospital stay prolongation, readmission, or medical therapy for its treatment [12]. This finding suggests that majority of the PPS diagnoses included in the previous prospective studies are clinically irrelevant. The result has been recently replicated also in an isolated aortic valve replacement (AVR) population [13]. The reported incidences of PPS and details of major PPS studies are presented in Figure 1.
Figure 1.
Reported incidences of PPS and study specifications in major previous studies. ASD: atrial septal defect; CABG: coronary artery bypass grafting; PPS: postpericardiotomy syndrome; SD: standard deviation; VSD: ventricular septal defect.
After pacemaker implantation, the incidence of PPS has been reported to be 1.8%, with a higher risk after the insertion of epicardial leads (2.5%) compared to transvenous leads (1.0%) [39], although markedly lower incidences (<0.2%) have also been reported [40]. It appears that the incidence is roughly 2–5% after the implantation of active-fixation leads and notably lower after passive-fixation leads [41]. After radiofrequency ablation complicated by cardiac perforation, the incidence is 28.6%, in other words similar to that after cardiac procedures [42].
Overall, the observed incidence of PPS is highly dependent on the applied diagnostic criteria as well as study design, patient population, and operation type. The recent prospective studies seem to have achieved a great sensitivity in the diagnosing of PPS. However, most of the diagnoses are clinically irrelevant, reflecting the problematic nature of the currently recommended diagnostic criteria.
Clinical features of PPS
PPS typically occurs within one month after the surgery, and an initial onset after six months is rare [5,12,13,26,43–47]. In children, the onset is slightly earlier, typically within 1 to 2 weeks [11,33,35]. The median duration of the syndrome is 2–3 weeks [28,35,46], and possible relapses tend to occur within 2–11 weeks after the initial onset [44].
The prevalence of typical symptoms and clinical findings are detailed in Table 1. The most characteristic symptom of PPS is pleuritic or pericarditic chest pain, referring to a stabbing pain often radiating to precordial region, neck, back, shoulders, arms, lower chest, and abdomen, made worse by coughing, deep breathing, swallowing, or any movement, and in severe cases leading to a fast and shallow respiration easily confused with the dyspnoea of congestive heart failure [23,26,46,48–50]. The reported incidences of the symptom have varied largely, but according to a recent prospective study, pleuritic chest pain occurs in over a half of the PPS episodes [5]. An intermittent, low grade fever is another common feature of PPS, and it occurs in approximately a half of the PPS cases [5,12,43]. The fever is usually the first manifestation. It may merge with the early postoperative temperature elevations so that the patient has a prolonged febrile course, but more often the fever recurs as a delayed reaction after a distinct afebrile period [55]. Another characteristic clinical finding is pericardial friction rub detected in the heart auscultation. The reported incidences of the friction rub have varied tremendously. According to recent studies it is detected in 20 to 30% of patients [5,47], although it has been suggested that it could probably be heard at some time in all patients but due to the transient nature a serial auscultation strategy is necessary [55]. The start of medical treatment, especially corticosteroids, offers a prompt relief of symptoms, typically within 24 to 48 h [10].
Table 1.
Postpericardiotomy syndrome (PPS) findings.
| Finding | Percentage, % |
|---|---|
| Symptoms: | |
| Pleuritic or pericarditic chest pain | >50 |
| Intermittent, low grade fever | ∼50 |
| Clinical findings: | |
| Pericardial friction rub | 20–30 |
| Elevated C-reactive protein (CRP) | 80–90 |
| Elevated erythrocyte sedimentation rate (ESR) | 80–90 |
| Leukocytosis | 80–90 |
| Electrocardiogram (ECG): low voltage of the QRS, T-wave inversion, ST-elevation or depression | ∼50 |
| Imaging: | |
| Chest X-ray: pleural effusion | >90 |
| Heart echocardiography: pericardial effusion | ∼90 |
| Mild (< 10 mm) | ∼75* |
| Moderate (10–20 mm) | ∼10* |
| Large (>20 mm) | ∼5* |
| Pleuropericardial involvement | >80 |
C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) are elevated in most PPS patients [4,12,17,22,43,46,51–53], although their specificity is poor during the first weeks after cardiac surgery [56,57]. Other typical laboratory findings include neutrophilic leukocytosis [4,17,49,50,52,53]. If pericardiocentesis is performed, the pericardial effusion is usually clear, straw-coloured fluid or serosanguineous fluid and sterile on culture [17,23,52,55,58–61]. The fluid is exudate containing high counts of lymphocytes and red blood cells and relatively high counts of granulocytes [59–61]. The specifications of pleural effusions are similar to the ones in pericardial fluid [23,62].
The thoracic X-ray reveals unilateral (usually left) or bilateral pleural effusion in most of the patients with PPS [5,12,17,43,44,46,50,51,62]. The separation of the pleuritic effusions from the normal surgery-related effusions is however complicated, as >80% of patients have at least minor effusions during the postoperative period [38]. Electrocardiogram (ECG) findings, including low voltage of the QRS, T-wave inversion, or ST-segment elevation or depression, can be detected in approximately half of the patients [12,23,46,49,51,52,54]. However, these findings as well seem to be relatively non-specific and thus only marginally helpful [9], and ECG changes suggestive of pericarditis appear only in <¼ of cases [5].
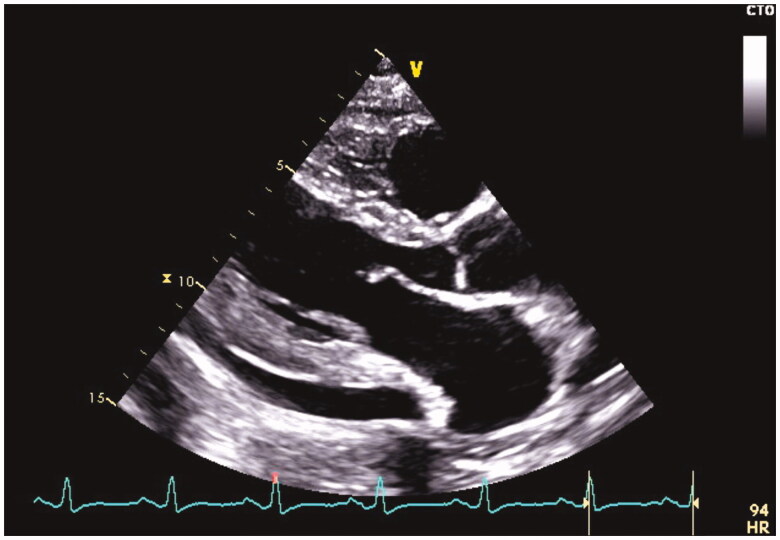
The pericardial effusion can often be detected on chest x-ray as an enlargement of the cardiac silhouette. However, it is often difficult to tell whether this enlargement is cardiac or pericardial or both [10,46,55]. According to recent studies, pericardial effusion can be detected as often as in 88–93% of the episodes [5,12,47]. The effusion is typically mild (<10 mm), and moderate (10–20 mm) and large (>20 mm) effusions are detected in 13% and 4% of the patients with detectable pericardial effusion, respectively [5]. Most patients (>80%) have combined pleuropericardial involvement [5,12]. The typical echocardiographic finding is presented in Figure 2.
Figure 2.
An echocardiographic image taken in the parasternal long axis view demonstrates moderate pericardial effusion in the posterior pericardium.
Prior and current diagnostic criteria – where should the line be drawn?
PPS is a diagnosis of exclusion. Since there are no specific tests with which to establish the diagnosis, it is made by excluding other diseases potentially producing similar symptoms. The main diagnoses to exclude are detailed in Table 2. The evaluation of a patient with a suspected PPS includes a focussed physical examination to detect pleural or pericardial rubs, laboratory tests (blood count and markers of inflammation and myocardial necrosis), ECG, chest X-ray, and transthoracic echocardiography to detect the presence, size, and hemodynamic importance of the pericardial effusion [1,15]. Although the aforementioned diagnostic procedures usually exclude the other possible postoperative states and diseases with sufficient accuracy, the empirical application of antibiotics, NSAIDs, or colchicine is often required to confirm the diagnosis of PPS.
Table 2.
Differential diagnosis of postpericardiotomy syndrome.
| Surgery-related: |
| Incisional pain |
| Atelectasis |
| Haemorrhagic pleural and pericardial effusions |
| Benign postoperative pleural and pericardial effusions |
| Chylothorax |
| Inflammatory: |
| Pneumonia |
| Wound infection |
| Mediastinitis |
| Sepsis |
| Bacterial endocarditis |
| Cardiovascular: |
| Congestive heart failure |
| Myocardial infarction |
| Pulmonary embolism |
| Aortic dissection |
Since the first description of PPS, numerous different diagnostic criteria have been used in research with a striking inconsistency between one another [14]. Engle et al. were the first to specify the applied diagnostic criteria for PPS [63]. Patients were diagnosed with PPS when there was either the persistence or appearance of the following combination of findings beyond the first postoperative week: fever not otherwise explained, together with signs of pericardial reaction on physical examination and serially obtained electrocardiograms and X-rays. At that time, the one week boundary was in general use, as it is usually safe to assume that symptoms occurring before the seventh day are part of the normal postoperative course [64]. In fact, fever early in the first postoperative week is virtually universal [65]. It is usually caused by complement activation by CPB, early stasis atelectasis, and sensitivity reactions to drugs, transfusions, etc. [66]. Also, benign, surgery-related pleural effusion appear in 50% of the patients without PPS during the first 3 postoperative days [10]. The effusion may result from a variety of reasons related to the postoperative state, including atelectasis following diaphragm dysfunction, haemorrhagic effusion following internal mammary artery harvesting, congestive heart failure, decreased chest wall compliance, pulmonary embolism, and pneumonia [67]. Furthermore, small pericardial effusions during the time of discharge are also a common finding in patients without PPS [35]. Besides fever and the effusions, benign, surgery-related leukocytosis, pericardial friction rub, and electrographic changes of pericarditis also diminish after the 1st postoperative week enabling the diagnosing of PPS [68].
In 2015, European Society of Cardiology (ESC) published the first guideline of diagnostic criteria for PPS, which are the following: 1) fever without alternative causes, 2) pericarditic or pleuritic chest pain, 3) pericardial or pleural rubs, 4) evidence of pericardial effusion and/or 5) pleural effusion with elevated CRP (Table 3). Two of the criteria should be fulfilled for the diagnosis, and the demonstration of inflammatory activity should be essential to establish the diagnosis [15]. Neither the one-week or a 24-to-72-hour time limit for fever often used in the earlier studies nor the “new or worsening” term of pericardial or pleural effusion included in the review concerning the diagnostic criteria [69] were included in the criteria for a reason not specified. The criteria have been recently criticised accordingly [14], although any sort of universal recommendations should reduce the variety of employed diagnostic criteria in future PPS studies.
Table 3.
Definition and diagnosis of postpericardiotomy syndrome (PPS) according to the European Society of Cardiology Guideline [15].
| 1. Fever without alternative causes |
| 2. Pericarditic or pleuritic chest pain |
| 3. Pericardial or pleural rubs |
| 4. Evidence of pericardial effusion |
| 5. Pleural effusion with elevated C-reactive protein (CRP) |
At least 2 of 5 criteria should be fulfilled.
In prospective trials, the detection of the benign, surgery-related findings is extremely sensitive because the follow-ups are mostly performed by PPS researchers. If the aforementioned diagnostic criteria of the ESC guideline [15] are interpreted without criticism, this will result in an erroneously high incidence of PPS. Although the criteria have been slightly different compared to the ones currently recommended, it is likely that a large part of the PPS diagnoses included in recent prospective trials are a result of the process ahead. This process also allows the diagnosing of asymptomatic pericardial and pleural effusions as PPS. This is possible even if the demonstration of inflammatory activity is required for the diagnosis because of the high frequency of fever and inflammatory marker rise during the postoperative era. It is questionable, whether a completely asymptomatic patient fulfils the classic definition of a postpericardiotomy “syndrome”. In the clinical practice, only the “clinically relevant” cases are detected, and therefore, these should be of interest also in the future prospective trials. This can be achieved either by revising the recommended diagnostic criteria or by separating the PPS diagnoses into subgroups according to their severity. The possible solutions are discussed below.
Although not included in the ESC guidelines, exclusive criteria for children have been also proposed by Heching and colleagues [35]. The proposed criteria include the presence of a pericardial effusion in echocardiography, along with two or more of the following: 1) fever >38 °C more than 72 h postoperatively, 2) pericardial friction rub, 3) patient irritability, and/or 4) pleuritic chest pain. The authors also suggested that the elevation of inflammatory markers could be considered as a supplementary sign of PPS.
Can we predict who gets PPS?
Patient-dependent factors
Numerous previous studies have evaluated the risk factors for PPS to both clarify the cause of the syndrome and to identify the patients with the highest risk of developing the disease. One of the few risk factors several studies have agreed upon is female sex [5,34,70,71]. It has been previously suggested that the female predominance might reflect the different predisposition to autoimmune pathogenesis, as it is known that women are more susceptible to a variety of autoimmune diseases [72] possibly due to the effects of sex hormones on the immune system, genetic factors, and sex-specific behaviours and exposures [73]. However, it is noteworthy that the two largest epidemiological PPS studies reported conflicting results regarding the effect of sex [71,74]. As the studies had otherwise identical design, the exclusion of complex combination procedures in the latter study [74] could possibly account for the discrepancy. A previous prospective study identifying female sex as an independent risk factor for PPS also included patients undergoing cardiac surgery for any reason [5]. It is therefore possible that the effect of female sex is mediated by differences in procedure types, possibly by complex combination procedures in female aged 40–70 years [71]. Overall, the effect of sex still remains somewhat unclear and needs further clarification.
Another factor most certainly influencing the occurrence of PPS is age. The incidence of PPS decreases above the age of thirty [28], is minimal in children under two years, and is the highest immediately after that, at the age of two years [11]. The absence of the syndrome in infants has been hypothesised to be related to the short period of experience with exposure to viruses, so that these patients do not react immunologically to the new stress by developing PPS [31]. It needs to be taken into consideration, however, that infants are generally unable to express themselves concerning chest pain and other subjective findings. The incidence of PPS seems to decrease steadily from early adulthood to old age [71,74], and the incidence in patients aged 70 years falls to 10% [31,57]. While the overall incidence of autoimmune diseases increases with age [73], different autoimmune diseases can affect a specific age group predominantly [75] which could possibly explain the finding. It has been also proposed that the elderly could be relatively spared because of the acquired protection against viral illnesses and the less frequent exposure to viral infections because of the alterations in the mode of life [31]. Furthermore, with aging, possibly comes some immunosenescence, such as the activity reduction of T and B cells, that could reduce the capability to mount an immune response against the intrapericardial trauma and possible exposure to viruses in the hospital [31,76]. Negative results concerning the association of PPS and age has also been reported [5,13,35,50,77–81], although typically in cohorts with a highly delineated age distribution. Moreover, a nonsignificant trend towards higher PPS occurrence in younger patients is detectable in most of the studies.
During the first decades after the description of PPS, the research payed attention to seasonal changes in PPS occurrence. McGuinness and colleagues found a summer peak of incidence [44]. Drusin et al. suggested that the incidence of PPS was higher during the consecutive six-month period from February through July [28]. These findings were later confirmed in a large prospective epidemiological study, as summer months (May, June, and July) presented a significant seasonal peak the lowest incidence occurring during early fall months (August, September, and October) [9]. This seasonal peak suggested the possibility that a pre-existing virus is activated by the operation and produces the syndrome which led to a substantial amount of research concerning the virology of PPS. These studies are not discussed in the present review. Nevertheless, a recent large epidemiological study found no significant annual changes in PPS occurrence [71] and the seasonal variation is therefore questionable.
Besides the younger age, summer months, and the operation type discussed below, a large prospective epidemiological study performed in adult population by Miller and colleagues also identified the following as independent risk factors for PPS: lower preoperative platelet count, history of prednisone treatment (past or present), history of pericarditis, lower weight, and halothane anaesthesia [9]. Preoperative prednisone was given to patients for variety of reasons, but in general, the drug was used to suppress an inflammatory response. The fact that PPS patients more often had a history of pericarditis suggests that the trauma to the pericardium might trigger a recurrence or that these patients are prone to pericarditis. Also, despite the number of subjects in this group was low, blood type B– represented notably higher PPS occurrence. The authors suggested that even though PPS was represented in patients with all blood types, certain blood types may predispose to developing PPS.
In the Finland Postpericardiotomy Syndrome (FIN-PPS) study including 688 patients undergoing isolated CABG procedure, diabetes was identified as an independent protective factor against PPS [12]. Metformin appeared to be the protective factor for PPS, although all the other medications also had a nonsignificant trend towards lower PPS frequency. The authors discussed that the immunomodulatory effect of metformin could account for the lesser PPS frequency within diabetes patients. Other reports concerning the effect of diabetes have been inconsistent. Contrary to the results of Lehto et al., Imazio et al. reported a nonsignificant trend towards higher diabetes rate within PPS patients (28% vs. 23%, p = 0.390). However, the data consisted of the patients of the COPPS trial that includes cardiac surgeries for any reason. Diabetes is a common comorbidity for numerous cardiac diseases, especially coronary artery disease [82]. Therefore, the evaluation of the influence of diabetes is complicated in settings including a wide variety of different procedure types. In a study by van Osch et al., a nonsignificant trend towards lower diabetes rate in PPS patients was noticeable (10.9% vs. 14.2%, p = 0.79) [77]. The study only included patients undergoing valve procedure with or without concomitant CABG, resulting in a more restricted patient population compared to the COPPS trial. Similar results were also reported by Lehto et al. in a study only including isolated AVR patients (10.7% vs. 15.0%, p = 0.42) [13]. Overall, diabetes may have a protective influence against PPS, but this needs to be further clarified in appropriately delimited patient populations.
Another recent retrospective study by van Osch and colleagues including 822 patients undergoing valve surgery found higher BMI to be an independent protective factor for PPS [77]. Treatment for pulmonary disease without corticosteroids was associated with a higher risk of PPS. The authors suggested that the protective effect of higher weight, also identified by Miller et al. [9], could be related to the immunomodulatory effects of obesity, referring to the higher levels of anti-inflammatory interleukins (IL-4 and IL-13) [83]. However, negative results concerning the association between BMI and PPS has also been reported [12,13], and Lehto et al. found a significant association between higher BMI and PPS recurrences [12]. The higher incidence of PPS in patients with pulmonary disease without corticosteroid treatment was presumed to be related to the vulnerability of these patients to develop systemic and pericardial inflammation.
To conclude, the only patient-dependent factor clearly increasing the occurrence of PPS is younger age. The effect of female sex is possibly mediated by different procedure types and needs further clarification. Other possible risk factors include history of prednisone treatment (past or present), history of pericarditis, treatment for pulmonary disease without corticosteroids, lower BMI, and blood type B–. In addition, diabetes may have a protective effect against PPS, possibly by the immunomodulatory effects of metformin.
Operation type
The type and/or extent of the procedure influence the incidence of PPS. Engle et al. found PPS occurring at a higher frequency in procedures for the tetralogy of Fallot and ventricular septal defect (VSD) accompanied by some other anomaly compared those entailing less damage to the cardiac muscle (atrial septal defect (ASD), isolated VSD, transposition of the great arteries (TGA), pulmonary stenosis) [11]. De Scheerder et al. also found that PPS was more common after valve replacement operation compared to CABG [84,85]. In the study by Miller et al., AVR patients were at greater risk for PPS and mitral valve replacement patients appeared to have a reduced risk [9]. Other procedures presented relatively high incidence. In a recent epidemiological study by Lehto et al., the occurrence of PPS was significantly higher after AVR, MVR, and ascending aortic surgery when compared to CABG [74]. The occurrence was equally higher in those undergoing AVR and MVR procedures when compared to those undergoing CABG, but clearly the highest occurrence appeared after aortic surgery. Urgent or emergency procedures were also associated with higher PPS occurrence.
More traumatic procedures seem to be related to the higher incidence of PPS. The effect is presumably mediated by more extensive pericardial trauma rather than myocardial trauma, as the degree of myocardial injury, at least assessed by circulating cardiac enzymes and sarcomeric proteins, seems to be unrelated to the occurrence of PPS [86]. These findings support the previously presumed mechanism of direct pericardial trauma acting as a trigger for the immune-mediated process leading to PPS. That said, it needs to be kept in mind that the syndrome can appear also after exploratory pericardiotomies in whom no further surgical procedures are attempted [17]. According to previous literature, isolated valve procedures present lower postoperative bleeding within the first 12 h after the operation compared to CABG procedure [205]. Therefore, pericardial bleeding alone is unlikely to be responsible for the higher occurrence of PPS after valve procedures, although it may still play a role in the pathophysiological mechanism of PPS. Besides the more extensive pericardial trauma, a more complex procedure usually means longer time in CPB [87] and longer pericardial exposure to air and other foreign materials.
Other triggers of PPS
Although PPS most commonly occurs after cardiac surgeries and especially frequently after the most extensive procedures, numerous case reports concerning more unusual triggers of PPS have been previously published. A picture similar to PPS has followed penetrating injuries of the chest [52,88,89], gunshots of the thoracic area [52,90,91], nonpenetrating trauma to the chest [92–95], left ventricular puncture [96,97], pulmonary embolism [98], pacemaker implantation with epicardial leads [99–101], transvenous pacemaker implantation [40,41,102–114], coronary artery perforation during balloon angioplasty [115], uncomplicated coronary angioplasty and stenting [116–124], percutaneous mitral balloon valvuloplasty [125], atrial radiofrequency ablation [126–131], diagnostic transdiaphragmatic pericardial window [132], thymectomy [133–135], accidental opening of the pericardium during lung lobectomy [136], and Nuss procedure [206,207].
In the case reports of nonpenetrating traumas to the chest, the pericardiocentesis and/or thoracocentesis revealed either blood in the pleural or pericardial cavities during the initial trauma or serosanguineous fluid during the pleuropericarditis by rule. Therefore, according to these reports, the only required trigger for the syndrome seems to be the presence of pericardial haematoma and/or a compressing injury of the heart during a car accident, although laceration or contusion of the pericardium cannot be ruled out. Atrial or ventricular wall perforation with bleeding into the pericardial sac occurring without recognition is a possible complication of transvenous pacemaker implantation, and it has been discussed to be the possible aetiology of the relatively rare cases resulting to PPS [41]. This hypothesis is strengthened by the observations that only leads placed on the lateral or anterolateral wall seem to provoke acute pericarditis [137] and that active-fixation leads seem crucial for the development of the disease [40,41]. Direct irritation of the pericardium by slightly protruding electrodes might be another possible trigger for the inflammatory response [40,41,138]. Hemopericardium is also a rare but possible complication of percutaneous mitral balloon valvuloplasty [139]. An unrecognised perforation of a coronary artery causing small bleeding into the pericardial space is a potential complication of percutaneous coronary intervention [117,119,123], although also prolonged endothelial trauma [118] combined with negative remodelling due to the myocardial damage [124] has been suggested to be the inducing factors. In one of the percutaneous coronary intervention cases, PPS required pericardiocentesis, and erythrocytes were identified in the evacuated fluid [119]. In the seven cases of atrial radiofrequency ablation, the authors suggested either the extensive transmural atrial or pericardial injury [126,131] or a possible cardiac perforation [127,129] to be the triggering factors for the syndrome. In contrast to the typical onset of PPS, the symptoms generally appeared either immediately after the procedure or during the first postoperative day. The outstandingly early onset was also presented in a study assessing the incidence of PCIS after radiofrequency ablation complicated by cardiac perforation [42], supporting the perforation-based aetiology in the aforementioned cases. A study containing 303 patients undergoing Nuss procedure, pericarditis occurred in 7 (2.3%) patients [140]. However, no new PPS cases were detected after the substitution of the introducer into a new model allowing gentle dissection of the pericardium away from the underside of the sternum.
Overall, according to the previous case reports concerning PPS-like states after different procedures and injuries, penetrating pericardial incision or injury does not seem to be necessary for the development of PPS. Instead, PPS appears to require pericardial haemorrhage and/or intensive pericardial manipulation as a minimum trigger.
Peri- and postoperative characteristics
Besides the operation type discussed above, many other factors related to the peri- and postoperative period have been associated with PPS. Halothane anaesthesia is associated with a twofold increase in the incidence of PPS [9]. The authors presumed this to be mediated by lymphocyte stimulation which has been described before in patients with hepatitis [141].
In a substudy of the COPPS trial assessing the risk factors for PPS, besides female sex, pleural incision also was found to be an independent predisposing factor for the syndrome [5]. In cardiac surgery, pleural incision may be either part of the surgical plan or accidental. If the pleural cavity is entered, it is advised to insert a chest tube accordingly [142] which causes pleural irritation. The intended or unintended opening of the pleura may also lead to the drainage of pericardial fluid through pleural drains via pleural cavity possibly triggering pleuritis and/or PPS which might offer an additional explanation for the finding.
In the FIN-PPS study, patients receiving red blood cells during the periprocedural period were found to be at a higher risk for PPS [12]. Only two previous studies have investigated the frequency of blood product transfusions in PPS patients [50,77]. In line with the FIN-PPS study, in both studies, patients with PPS received more blood transfusions during the periprocedural period, although not reaching statistical significance in the study by van Osch et al. (transfusion of ≥ 2 units packed red cells 23.1% vs. 17.0%, p = 0.09). The effect of blood products may be associated either to the immunological aspects related to blood transfusions themselves, such as IL-8 expression [81,143,144], or to the greater amount of perioperative bleeding. The study by Lehto et al. found a nonsignificant trend towards more postoperative bleeding in patients with PPS (980 ml vs. 830 ml, p = 0.099) supporting the latter mechanism. However, there is also a possibility of the two aforementioned mechanisms having a combined influence on the development of PPS.
Recently, Lehto et al. found the patients developing postoperative pneumonia during the index hospital stay to have higher risk for PPS [13]. The study combined both retrospective and prospective data and included overall 671 patients undergoing isolated AVR. The authors suggested that either some of the pneumonia were actually early stages of PPS, PPS is exposing patients to pneumonia via hypoventilation, or pneumonia causes an immunological activation exposing the patients to the development of PPS. Overall, the finding needs to be confirmed and clarified in future studies.
No laboratory test has been proven to reliably predict the onset of PPS during the perioperative period. However, some associations have been detected. Miller et al. found that PPS patients had significantly lower mean platelet count preoperatively compared to patients not developing PPS [9]. Also, higher mean % lymphocyte cell count was associated with PPS after adjustment for age and sex although it did not reach statistical significance in the overall multivariable model. A retrospective study by Sevuk et al. investigated pre- and postoperative laboratory data in 72 patients with PPS and 100 control patients who had underwent isolated CABG [78]. There were no differences in the preoperative white blood cell (WBC) count, neutrophil count, lymphocyte count, or neutrophil-to-lymphocyte ratio (NLR). In the postoperative values measured in the first postoperative day, patients with PPS had significantly higher WBC as well as neutrophil count with no changes in the lymphocyte count leading to higher NLR. The results were also significant in the multivariable model. The authors suggested the higher postoperative neutrophil count to be a consequence of the IL-8-mediated chemotaxis which has been associated with PPS [81,144]. The Receiver Operating Characteristics (ROC) analysis observed a sensitivity of 60% and specificity of 59% for NLR when using a cut-off value of 8.34. The studies investigating more specific immunological markers and virology of PPS are not discussed in the present review.
Heching and colleagues investigated the incidence and risk factors for PPS in paediatric patients following surgical closure of secundum ASD [35]. Overall 27 (27.8%) of the included 97 patients developed PPS. A small pericardial effusion during the time of discharge was identified in 17 (63.0%) PPS patients, whereas only 19 (27.1%) of patients in the non-PPS group presented the effusions (p = 0.001). The median length of hospital stay was 4 days. Therefore, although some of the patients could have developed an active PPS before the discharge, it seems that echocardiogram at the time of discharge could have predictive value for the syndrome.
A systematic review published by van Osch and colleagues investigated the risk factors for PPS (14). The review included all major studies concerning the risk factors and representing adequate quality. A total of 7 studies met the quality requirements. The discrepancy of identified risk factors between different studies was striking. Authors presumed this to be due to different definitions of PPS as well as the evolution of surgical techniques during the past 50–60 years which make it rather impossible to compare the findings of all the studies available. Overall, the authors concluded that both the inflammatory response and perioperative bleeding and coagulation may play a role in the development of PPS, suggesting a multifactorial aetiology of the syndrome. The variability in the observed risk factors led to the same conclusions also decades earlier, as Kirsh et al. suggested that the syndrome may be a symptom-complex with multiple aetiological factors [145].
Retained blood syndrome
The concept of retained blood syndrome (RBS) was recently described [146]. RBS covers a spectrum of mechanical and inflammatory complications related to drainage system failure to adequately evacuate blood after cardiac surgery. The diagnostic criteria for the syndrome are specified as at least one of the following: (1) pleural effusion/hemothorax requiring drainage, (2) pericardial effusion requiring drainage, (3) re-exploration for washout of blood, (4) interventions for postoperative pericardial constriction, and (5) interventions for postoperative fibrothorax [146]. Therefore, there is an obvious overlap within the entities of PPS and RBS. The authors went on to state that RBS is the root cause of PPS via the acute, subacute, and chronic inflammatory response the retained blood triggers. It was suggested that once the retained blood clots within the pericardium or pleural space, thrombin and fibrin are generated. These products are powerful chemoattractants of inflammatory cells that can stimulate the mesothelial cells of the pericardium and pleura to release inflammatory cytokines. As stated above, it seems that PPS requires pericardial haemorrhage and/or intensive pericardial manipulation as a minimum trigger. Therefore, the hypothesis of Boyle and colleagues seems logical, although the inadequate blood evacuation does not seem necessary for the onset of PPS, as no previous studies have reported excessive pericardial blood collections to be in association with PPS. Instead, it seems that even minimal amount of pericardial blood can serve as a trigger for the syndrome, and even though the active techniques to enhance the surgical drainage appear to reduce the development of postoperative new-onset AF [147], it is unlikely that they would eliminate the entity of PPS. Still, the pathophysiological mechanism suggested by the authors seems conceivable. Also, according to previous studies, it seems plausible that the extended chest tube drainage as well as posterior pericardiotomy could reduce late tamponade related to PPS [148,149].
Management of PPS
Medical treatment
The investigations concerning possible methods for the treatment and prevention of PPS started rapidly after the first description of the syndrome. The value of corticosteroids in the treatment of PPS was shortly observed [4,18]. Also NSAIDs were found to be efficacious [51], and later they were recommended as the first-line treatment for the milder forms of PPS [46,49,150–152], whereas corticosteroids were recommended for the most severe clinical presentations [151,152].
Later, both NSAIDs and corticosteroids, in addition to colchicine, have been investigated in double-blind, placebo-controlled randomised clinical trials. In a trial including overall 149 PPS patients, either ibuprofen or indomethacin administered for 10 days relieved symptoms and shortened the duration of the illness without considerable side effects [37]. Another trial including 21 patients with PPS found that a two-week corticosteroid treatment increased the one-week remission rates and fastened the time to symptom relief [32]. However, the use of corticosteroids in the treatment of pericarditis is no longer recommended as it has been associated with higher recurrence rate [15,153–157]. As stated before, however, evidence against their use in postsurgical pericardial syndromes are scarce [158]. In most of the aforementioned studies, the number of patients using corticosteroids were small, the drug was only administered in case of aspirin contraindications or failure, and the durations of the treatments were relatively short. Moreover, the notably effective response during the treatment leading to too rapid tapering is a typical pitfall of corticosteroid use, and rather than abandoning the drug it should be used with awareness and slow tapering, and routine administration during the first episode should be avoided [15,159]. Colchicine has been found to be effective in the most persistent cases of PPS [160], and it has been also associated with a decreased need for invasive interventions [47]. The ICAP trial focussed on the benefits of first-line use of colchicine in the treatment of acute pericarditis including overall 48 (20.0%) PCIS patients found a significant reduction in the rate of symptom persistence at 72 h (19.2% vs. 40.0%, p = 0.001), the number of recurrences (16.7% vs. 37.5%, p < 0.001), and the hospitalisation rate (5.0% vs. 58.3%, p < 0.001) and increased remission rate at one week (85.0% vs. 58.3%, p < 0.001) [157]. Multiple meta-analyses have confirmed the efficacy of colchicine both in the first-line treatment of pericarditis as well as in the secondary prevention of recurrent episodes [161–167].
According to the ESC guidelines, the treatment of PPS is based on the use of NSAIDs and colchicine, with the occasional addition of corticosteroids. Although symptomatic effusions require medical treatment, the management of asymptomatic effusions is controversial. Recommended doses for uncomplicated cases include aspirin 750–1000 mg or ibuprofen 600 mg every 8 h with a duration of 1–2 weeks followed by tapering. Colchicine should only be used in the presence of systemic inflammation, and the recommended dose is 0.5 mg once (<70 kg) or twice (≥70 kg) daily for 3 months with an optional halving of the dose in the last weeks of the treatment. Corticosteroids should be considered as a second option in patients with contraindications and the failure of NSAIDs with low to moderate doses (i.e. prednisone 0.2–0.5 mg/kg/day or equivalent) [15].
There are anecdotal case reports on the use of methotrexate [168], azathioprine [169,170], as well as high-dose intravenous immunoglobulin [171–174], in the treatment of steroid-dependent recurrent PPS. Moreover, in chronic recurrent pericarditis, anakinra, a recombinant IL-1β receptor antagonist [175–178] as well as cyclophosphamide [170] have been successfully used. Furthermore, intrapericardial triamcinolone has been used in the treatment of chronic autoreactive pericardial effusion [179], and it is also mentioned in the ESC guideline as well as azathioprine, intravenous immunoglobulin, and anakinra [15].
Interventions for PPS
If the patient has evidence of clinical pre-tamponade or tamponade or if the symptoms persist regardless of the medical treatment and an alternative diagnosis is suspected, the evacuation of pericardial effusion is required [15,68]. In the presence of tamponade, the needle pericardiocentesis is preferred, whereas surgical approach might be preferred when the pericardial fluid is not free, it is located in a lateral or posterior position, or the effusion is mild (<10 mm) [15]. The pericardiocentesis should be performed under guidance either by fluoroscopy or echocardiography and under local anaesthesia. The ideal entry site in the echocardiography-guided procedure is the point where the effusion is closest to the transducer and the fluid collection is maximal and the liver or pleura are not on the way, whereas in the fluoroscopy-guided procedure the subxiphoid route is more common. If there is a need for pericardial biopsy for the differential diagnostics, the surgical approach is the gold standard, classically through a subxiphoid incision. In this procedure, a small drain is left in place to evacuate any remaining effusion [15]. In the presence of extensive pleural effusions, a pleural drainage or thoracocentesis may be required, and they should be preferably performed on ultrasound-guidance. The drain should be inserted in the “triangle of safety”, referring to the area bordered by the lateral edge of the latissimus dorsi, the lateral border of the pectoralis major muscle and superior to the horizontal level of the fifth intercostal space [180].
In the most persistent cases of pericardial effusion, pericardiectomy may be considered [15]. Fortunately, this is needed only in extremely rare cases [68]. Other possible interventional treatments include prolonged pericardial drainage, the aforementioned intrapericardial triamcinolone treatment, and pericardial window [15]. The pericardial window can be performed either by conventional heart surgery, by thoracoscopy, or by balloon pericardiotomy by inserting a deflated single catheter or double balloon catheters into the pericardial space [181]. If the surgery is performed, pericardiectomy is the procedure of choice, because pericardial window may not relieve loculated pericardial fluid or may close soon after being performed [15,59]. If the persistent PPS manifests as large, symptomatic pleural effusions continuing despite several thoracocenteses, thoracoscopy should be considered. At thoracoscopy, any fibrous tissue coating the visceral pleura should be removed and either the parietal pleura should be abraded or talc should be used to create a pleurodesis [182,183]. In addition, a single case report has been published of the use of indwelling pleural catheter for the recurrent pleural effusions secondary to PPS [184].
A retrospective analysis by Alraies et al. assessed the predictors of PPS leading to procedural intervention due to the pericardial effusion or pericardial constriction [47]. Independent predictors of invasive interventions included younger age, early onset of PPS, and constrictive physiology when compared to PPS patients not requiring invasive interventions. However, the results have been challenged, as the older patients also received a somewhat more aggressive medical treatment for the disease [185]. No other studies have assessed the risk factors for PPS leading to procedural intervention.
Prevention
The first study concerning PPS prophylaxis was published in 1956 [18]. A prophylactic 3–8 weeks cortisone treatment postponed the development of PPS and resulted in a less stormy immediate postoperative course, although the incidence of PPS was similar after drug withdrawal (29 vs. 31%). Later, a double-blind, placebo-controlled randomised clinical trial including 246 children found no effect of short-term prophylactic intravenous methylprednisolone (1 mg/kg before CPB plus four additional doses within 24 h) on the incidence of PPS [33]. Instead, a greater proportion of the treatment group developed PPS leading to hospital admission (6.3% vs. 0.8%). A substudy of the Dexamethasone for Cardiac Surgery (DECS) trial investigated the effect of intraoperative dose of 1 mg/kg dexamethasone in 822 adults and found no benefits in either the occurrence of PPS or complicated PPS [38]. On the other hand, a retrospective study including overall 200 adult patients reported significant PPS reduction in patients receiving a single intraoperative dose of 1 mg/kg methylprednisolone (22.0% vs. 37.0%, p < 0.026) [79]. It has been suggested that the positive result conflicting with previous studies could be due to the strict exclusion criteria used [186]. Further large-scale studies are needed to assess the possible value of corticosteroids in the prevention of PPS maintaining special focus on the exact timing and route of the drug administration [186].
A prospective study evaluating the effect of one week aspirin prophylaxis (60 mg/kg/day) in children found no significant reduction in the size of postoperative pericardial effusions [187]. This finding has been supported by later studies in children and young adults [36,188]. By contrast, a retrospective study evaluating the effect of diclofenac treatment for postoperative analgesia during the hospital admission in 100 adult patients found a significant reduction in PPS occurrence when compared to patients not administered to NSAIDs (20.0% vs. 43.0%, p = 0.001) [80]. However, no prospective trials have yet confirmed this finding.
The first trial reporting the possible value of colchicine for the prevention of PPS was published in 2002 [6]. After that, two randomised, placebo-controlled trials have been published accordingly. The COPPS trial included overall 360 adult cardiac surgery patients, from which 180 received placebo and 180 received colchicine for a month postoperatively. Colchicine significantly reduced the incidence of PPS (8.9% vs. 21.1%, p = 0.002, NNT = 8) and the combination endpoint of PPS-related hospitalisation, cardiac tamponade, constrictive pericarditis, and relapses (0.6% vs. 5.0%, p = 0.024). A few years later the COPPS-2 trial included another 360 adult cardiac surgery patients, from which 180 received colchicine starting two to three days preoperatively and continuing until 1 month after the surgery. Again, the administration of colchicine resulted in a significant reduction of PPS (19.4% vs. 29.4%, NNT = 10). The effect was significantly higher in patients with systemic inflammation, specified as an elevation of C-reactive protein (CRP) during the hospital admission. This time, no significant reduction in the PPS-related secondary endpoints was detected. The three aforementioned studies have been also analysed using meta-analytic pooling that has shown a relative risk reduction of 44–52% in PPS incidence [164,165,167,189]. Therefore, colchicine seems effective in the prevention of PPS, although its use is limited by the gastrointestinal side effects. According to previous studies, colchicine causes an increment of 8–10 percentage points in gastrointestinal side effects compared to placebo but serious side effects have not been observed [6,8,153,157,161–167]. The effect is worsened if the drug is started preoperatively [7].
Currently, because of the relatively good outcome of PPS patients and the adverse effects of colchicine, instead of the primary prevention, clinicians are advised to consider early recognition and treatment of the syndrome [15,190]. However, according to the ESC guidelines, a one-month colchicine prophylaxis should be considered after cardiac surgery for the prevention of PPS if there is evidence of systemic inflammation, there are no contraindications, and the drug is tolerated (class IIa level A recommendation).
Prognosis after PPS
According to previous studies, PPS is associated with a prolonged hospital stay [5,50,77,191] and readmissions [5,33,38,71,74]. The epidemiological studies have reported that PPS leads to hospital admission in 1.7–2.5% of all patients after cardiac surgery [71,74]. The reported recurrence rates have varied between 4% and 38% and typical timing of the repeating episodes is from 1 to 3 months after the first episode [5,12,13,28,46]. In the FIN-PPS study, obesity and shorter in-hospital stay after the index surgery were found to be associated with higher recurrence rate [12]. No other studies have assessed the risk factors for adverse outcomes and higher recurrence rate of PPS.
The incidence of PPS-related cardiac tamponade is 0.1–1.0% of all cardiac surgery patients after the first postoperative week, making PPS the most common aetiology of delayed cardiac tamponade [12,13,58,59]. The tamponade may occur anywhere between 7 and 180 days after the operation. In a recent study, PPS was associated with an over 15-fold risk of reoperation for tamponade [77].
Apart from the aforementioned complications, the previous knowledge of PPS-related adverse events is limited. A study including 100 patients undergoing mitral valvotomy found no association between PPS and new-onset AF [23]. Another retrospective study including 60 patients developing postoperative atrial fibrillation (POAF) and 142 patients without POAF after CABG found a significant association between POAF and PPS (incidence of PPS after POAF (61.7%) vs. no-POAF (45.8%), OR 1.9, 95% CI 1.03–3.5, p = 0.04) [192]. However, there were multiple baseline differences between the study groups, and the incidence of PPS was strikingly high (overall incidence 51%) reflecting either selection bias or problems with the differential diagnostics of PPS. In the recent study by Lehto et al., PPS patients had a 1.7-fold risk of new-onset AF after hospital discharge [13]. The difference appeared within one month after the surgery. Overall, it seems that the pericardial irritation caused by PPS may provoke AF paroxysms during the first months after the surgery. However, the effect is most likely transient, and therefore, it should not affect the overall prognosis of the patients.
Constrictive pericarditis has been suggested to be a complication of PPS appearing during long-term follow-up [2]. An analysis including 37 patients identified as having constrictive pericarditis patients after cardiac surgery with adequate clinical information, PPS was observed in 23 (62.2%) patients [193]. Similar results have been reported in several studies [194–196]. According to the aforementioned studies, constrictive pericarditis occurs in a broad time frame of 1 month to 17 years postoperatively, typically within 24 months, with an overall incidence of 0.2–0.3%. Assuming 60% of constrictive pericarditis would be of PPS-related aetiology, approximately 0.5% of the patients with PPS would develop constrictive pericarditis during long-term follow-up. This incidence is similar to idiopathic/viral pericarditis in the general population [197]. However, markedly higher incidences of constrictive pericarditis have also been reported [47], although possibly reflecting the selection bias of the original patient selection as stated also by the authors themselves. Only one prospective study has evaluated the rate of constrictive pericarditis after PCIS [197]. However, even though PCIS was combined with connective tissue diseases, the number of patients was insufficient (n = 36) to draw any conclusions. Another study including 119 PPS patients with a follow-up of one year found no constrictive pericarditis cases [77]. Therefore, the question of whether constrictive pericarditis is associated with the development of PPS or to a common pathophysiological trigger, e.g. surgery related hemopericardium, is controversial. Either way, the presence of PPS should serve as a warning signal to clinicians, as a theoretical risk of constriction exists [15].
Symptomatic treatment (analgesics) of PPS has been associated with a higher incidence of venous graft occlusion when compared to patients receiving NSAIDs in combination with prednisolone for a time period of 6 weeks [53]. The patients were observed to have diffuse adhesions between the pericardium and epicardium, often obliterating the pericardial space. The venous grafts had microscopical fibrinoid degeneration and infiltration by chronic inflammatory cells suggesting external compression rather than intimal hyperplasia as the cause of the stenosis or occlusion. However, similar results have not been reported since apart from a single case report of a patient developing rapid coronary artery occlusion after PPS [198]. Other reported possible complications include the extrusion of the pulse generator from the pacemaker pocket [100] and the thickening and scarring of autogenous pericardial baffle [199].
Van Osch et al. were the first to publish a study concentrating on the prognosis of PPS [77]. This retrospective subanalysis of the DECS trial included 822 patients undergoing valve surgery followed up to a year postoperatively. Overall 119 (14%) patients developed PPS. Patients with PPS presented markedly more reoperations for tamponade at one year (20.9% vs. 2.5%, OR 15.49, 95% CI 7.14–33.58, p < 0.001) with the difference appearing within two months after the surgery in addition to longer hospital stay (13 vs. 11 days, p = 0.001). However, the one-year prognosis was excellent: 4/119 (4.2%) of PPS patients and 37/703 (5.5%) of patients without PPS died during the first year after the surgery (OR 0.63, 95% CI 0.22–1.79, p = 0.497). Also, no significant associations were observed between PPS and reoperation for surgical bleeding, pleural puncture, or postoperative AF (preoperative AF not excluded) within one month, or stroke, myocardial infarction, or readmissions within one year postoperatively.
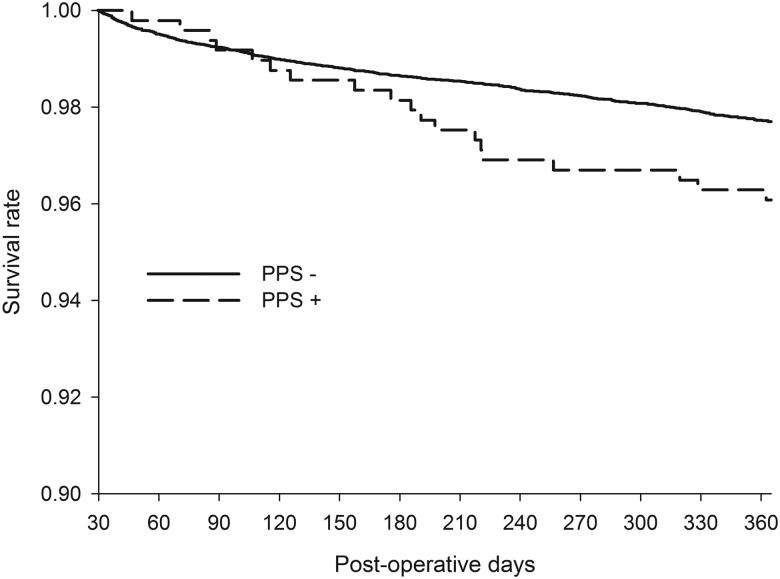
In contrast, a recent epidemiological study including overall 493 PPS patients, found a significant association between PPS and mortality [74]. PPS was associated with a 78% increase in the risk of death within the first year after the surgery. Moreover, of the PPS patients who died within the first year after the surgery, 10.5% had the diagnosis included in the death certificate. The typical delay between the initial PPS admission and death was 2 to 6 months (Figure 3). None of the causes of death was overrepresented in patients with PPS who died within the first year after the surgery. Instead, the patients had an equal rise in all causes of death which from ischaemic heart disease was the most common. Although the reasons for the higher mortality remained to be elucidated, the authors suggested that it could be related to the underlying immunological changes caused by or resulting in PPS. Lehto et al. investigated the reasons further and found no associations between PPS requiring medical attention and cerebrovascular events or major bleeds during long-term follow-up [13]. However, severe PPS, referring to PPS requiring invasive interventions such as pericardial or pleural drainage, resulted in a two-fold mortality risk. The difference in mortality appeared within the first 24 months after the surgery and the median time from the diagnosis to death was 460 (83–1300) days. The underlying cause of death appearing within 24 months after severe PPS was often registered as atherosclerotic heart disease or aortic valve stenosis. Pneumonia was registered as the immediate cause of death in half of the deaths. The authors suggested that the higher mortality is caused by the incremental disease burden of PPS and its sequelae, and especially the required interventions. Therefore, PPS is likely to complicate previously weak and vulnerable patients, thus leading to higher mortality with a delay. The authors stated that because of the higher mortality, the patients with severe PPS are in the need of more intensive follow-up and treatment.
Figure 3.
PPS is associated with a 1.8-fold increase in all-cause mortality. Severe PPS, referring to PPS requiring invasive interventions, seems to be the subgroup where the higher mortality of PPS patients is originated from. The incremental deaths appear within 24 months after the surgery and a typical delay between PPS admission and death is 5 months. From the original publication by Lehto et al. (74).
Previously, despite the limited published data, the prognosis of PPS has been considered to be benign [1,15,16]. However, the aforementioned studies by Lehto et al. challenge this point of view that has prevailed for over half a century without adequate evidence. Further studies are needed to evaluate the possible treatment solutions to prevent the incremental deaths caused by severe PPS. However, the finding supports the use of relatively aggressive prophylactic methods to prevent PPS. Therefore, for example, the increased risk of gastrointestinal adverse effects of colchicine should not be interpreted as an insuperable obstacle for the preventive use of the drug. Considering these results, the one-month colchicine prophylaxis, also recommended for consideration in the ESC Guideline (15) (class IIa level A recommendation), should be adopted in the current clinical practice. If necessary, the preventive treatment could be targeted to the patients in the highest risk of developing PPS, especially to the ones undergoing more extensive procedures and to younger patients. It needs to be taken into consideration, however, that the evidence of whether the preventive therapies diminish the risk of mortality is lacking [7]. Nevertheless, no studies have been powered to investigate the possible mortality benefits of the treatments in either the preventive use or the management of PPS.
Future aspects
Major challenge in prior PPS studies has undoubtedly been the large variability between the included PPS diagnoses due to inconsistent diagnostic criteria. This has resulted in largely conflicting results and only few of the studies have been able to confirm the results between one another [14]. The challenge is that if one evaluates patients critically, most patients with cardiac trauma will meet the criteria for PPS. There is probably a continuum of responses to post-cardiac trauma ranging from mild fever and pericardial effusion to a complete syndrome with intermittent fever, notable pericardial and/or pleural effusion, and pleuritic chest pain.
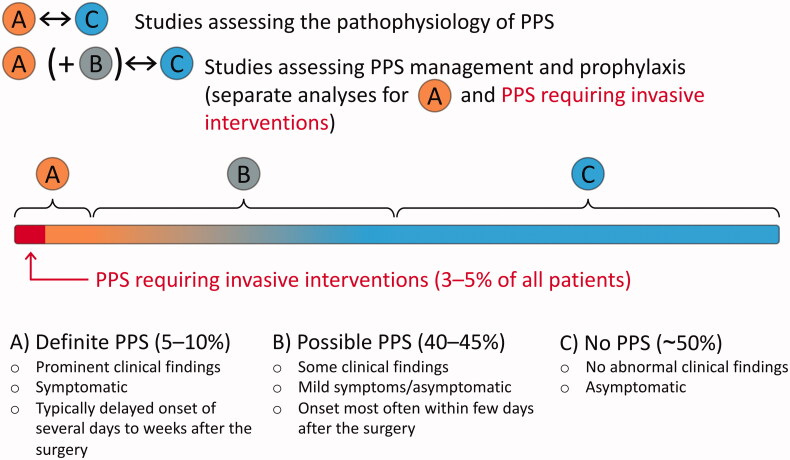
During the first decade after the discovering of PPS, patients were considered to have the disease if they were toxic and extremely enervated [48]. In the recent decades, however, the inclination of research has intentionally focussed more and more on the sensitivity of the diagnosing of PPS [2], also reflecting in the current guideline-recommended diagnostic criteria. It would presumably be more objective and specific to use the criteria introduced by Bunge et al.: at least two of the following: 1) new significant (>10 mm) pericardial effusion on echocardiography, 2) new significant (above the highest level of the diaphragm) pleural effusion on chest x-ray, 3) unexplained fever >72 h postoperatively, 4) pleuritic chest pain, and/or 5) the presence of pericardial or pleural rubbing on physical examination [38]. This would likely discard the early post-traumatic effusions and enable the separation of the patients with the “classic” immune-mediated PPS. An even more sophisticated approach would be the one introduced by De Scheerder et al. [84,85,200,201], including the concepts of “complete PPS” and “incomplete PPS”. The integration of the two aforementioned methods in addition to careful differential diagnostic procedures would allow the comparison of the most certain positive PPS patients with the most certain negative PPS patients. This is particularly important in the studies assessing the pathophysiological mechanisms of PPS, as it is uncertain whether the borderline PPS cases, especially the ones within the first few days after the surgery, share the same pathophysiological mechanism with the delayed, more certain cases. The proposed concept is illustrated in Figure 4. The hypothesis of two separate mechanisms is strengthened by the fact that although colchicine is effective in the prevention of PPS, it does not seem to be effective in the treatment of early postoperative pericardial effusions [202,203].
Figure 4.
The proposed concept for future postpericardiotomy syndrome (PPS) studies. The comparison of definite PPS (A) and no PPS (C) groups leads to more consistent results between different studies and allows more specific exploration of the certain, most likely immune-mediated PPS cases. Moreover, the future clinical trials should include definite PPS (A) as well as PPS requiring invasive interventions as separate endpoints to better evaluate the effect of the management options and prophylactic methods on patients’ health and the adverse events of PPS.
Conclusions
In conclusion, the previous reports of PPS have been outstandingly inconsistent due to the problematic nature of the definition of PPS. The incidence of the syndrome is highly dependent on the applied diagnostic criteria as well as study design, patient population, and operation type. In the recent prospective trials, most of the PPS diagnoses have been clinically irrelevant. Therefore, the recommended diagnostic criteria should be reconsidered, and the analyses should be divided into subgroups according to the severity of the syndrome to achieve more clinically applicable results in the future studies. The typical clinical features of PPS include pleuritic or pericarditic chest pain, intermittent, low grade fever, elevated inflammatory markers, pleural effusion in the chest X-ray, and usually mild pericardial effusion in the heart echocardiography. However, none of these findings are specific for the disease during the early postoperative period, and consequently, a careful differential diagnostic procedure is required for the diagnosis. The only risk factors clearly increasing the occurrence of PPS are younger age, pleural incision, and valve and ascending aortic procedures when compared to CABG. The effect of female sex is possibly mediated by different procedure types and needs further clarification. According to the current guidelines, the treatment of PPS is based on the use of NSAIDs and colchicine, with the occasional addition of corticosteroids. Currently, because of the relatively good outcome of PPS patients and the adverse effects of colchicine, instead of the primary prevention, clinicians are advised to consider early recognition and treatment of the syndrome. However, severe PPS has been recently found to be associated with higher overall mortality during the first two years after cardiac surgery. Therefore, in contrast with the previous presumption, PPS is not just a benign complication after cardiac surgery, but a state affecting the prognosis during the patient recovery. In that case, the increased risk of gastrointestinal adverse effects of colchicine should not be interpreted as an insuperable obstacle for the preventive use of the drug.
Disclosure statement
Joonas Lehto received research grants from Orion Research Foundation, the Finnish Foundation for Cardiovascular Research, the Finnish Cultural Foundation, Turku University Foundation, and Emil Aaltonen Foundation.
Tuomas O. Kiviniemi received lecture fees from Bayer, Boehringer Ingelheim, MSD, Astra Zeneca, St Jude Medical, and Bristol-Myers-Squibb-Pfizer, and research grants from the Finnish Medical Foundation, the Finnish Foundation for Cardiovascular Research, Clinical Research Fund (EVO) of Turku University Hospital, Turku, Finland, Finnish Cardiac Society, the Emil Aaltonen Foundation, the Maud Kuistila Foundation, and an unrestricted grant from Bristol-Myers Squibb-Pfizer. Tuomas O. Kiviniemi is a member of the advisory board of Boehringer-Ingelheim, and MSD.
References
- 1.Imazio M, Hoit BD.. Post-cardiac injury syndromes. An emerging cause of pericardial diseases. Int J Cardiol. 2013;168(2):648–652. [DOI] [PubMed] [Google Scholar]
- 2.Imazio M. The post-pericardiotomy syndrome. Curr Opin Pulm Med. 2012;18:366–374. [DOI] [PubMed] [Google Scholar]
- 3.Janton HO, Glover Robert P, O’Neill TJE, et al. Results of the surgical treatment for mitral stenosis; analysis of one hundred consecutive cases. Circulation. 1952;6(3):321–333. [DOI] [PubMed] [Google Scholar]
- 4.Soloff LA, Zatuchni J, Janton OH, et al. Reactivation of rheumatic fever following mitral commissurotomy. Circulation. 1953;8(4):481–493. [DOI] [PubMed] [Google Scholar]
- 5.Imazio M, Brucato A, Rovere ME, et al. Contemporary features, risk factors, and prognosis of the post-pericardiotomy syndrome. Am J Cardiol. 2011;108(8):1183–1187. [DOI] [PubMed] [Google Scholar]
- 6.Finkelstein Y, Shemesh J, Mahlab K, et al. Colchicine for the prevention of postpericardiotomy syndrome. Herz. 2002;27(8):791–794. [DOI] [PubMed] [Google Scholar]
- 7.Imazio M, Brucato A, Ferrazzi P, et al. Colchicine for prevention of postpericardiotomy syndrome and postoperative atrial fibrillation. The COPPS-2 randomized clinical trial. J Am Med Assoc. 2014;312(10):1016–1023. [DOI] [PubMed] [Google Scholar]
- 8.Imazio M, Trinchero R, Brucato A, et al. ; on behalf of the COPPS Investigators. Colchicine for the Prevention of the Post-pericardiotomy Syndrome (COPPS): a multicentre, randomized, double-blind, placebo-controlled trial. Eur Heart J. 2010;31(22):2749–2754. [DOI] [PubMed] [Google Scholar]
- 9.Miller RH, Horneffer PJ, Gardner TJ, et al. The epidemiology of the postpericardiotomy syndrome: A common complication of cardiac surgery. Am Heart J. 1988;116(5):1323–1329. [DOI] [PubMed] [Google Scholar]
- 10.Kaminsky ME, Rodan BA, Osborne DR, et al. Postpericardiotomy syndrome. Am J Roentgenol. 1982;138(3):503–508. [DOI] [PubMed] [Google Scholar]
- 11.Engle MA, Zabriskie JB, Senterfit LB, et al. Viral illness and the postpericardiotomy syndrome. A prospective study in children. Circulation. 1980;62(6):1151–1158. [DOI] [PubMed] [Google Scholar]
- 12.Lehto J, Gunn J, Karjalainen P, et al. Incidence and risk factors of postpericardiotomy syndrome requiring medical attention: the Finland postpericardiotomy syndrome study. J Thorac Cardiovasc Surg. 2015;149(5):1324–1329. [DOI] [PubMed] [Google Scholar]
- 13.Lehto J, Gunn J, Björn R, et al. Adverse events and survival with postpericardiotomy syndrome after surgical aortic valve replacement. J Thorac Cardiovasc Surg. [cited 2020 Jan 28]. DOI:10.1016/j.jtcvs.2019.12.114 [DOI] [PubMed] [Google Scholar]
- 14.van Osch D, Nathoe HM, Jacob KA, et al. Determinants of the postpericardiotomy syndrome: a systematic review. Eur J Clin Invest. 2017;47(6):456–467. [DOI] [PubMed] [Google Scholar]
- 15.Adler Y, Charron P, Imazio M, et al. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases. Eur Heart J. 2015;36(42):2921–2964. [DOI] [PubMed] [Google Scholar]
- 16.Bucekova E, Simkova I, Hulman M.. Postpericardiotomy syndrome – post-cardiac injury syndrome. BLL. 2012;113(08):481–485. [DOI] [PubMed] [Google Scholar]
- 17.Ito T, Engle MA, Goldberg HP.. Postpericardiotomy syndrome following surgery for nonrheumatic heart disease. Circulation. 1958;17(4):549–556. [DOI] [PubMed] [Google Scholar]
- 18.Dresdale DT, Ripstein CB, Guzman SV, et al. Postcardiotomy syndrome in patients with rheumatic heart disease; cortisone as a prophylactic and therapeutic agent. Am J Med. 1956;21(1):57–74. [DOI] [PubMed] [Google Scholar]
- 19.January LE, Bedell GN, Batemant RD.. Problem of mitral valve disease. JAMA. 1954;155(3):231–234. [DOI] [PubMed] [Google Scholar]
- 20.Wood P. An appreciation of mitral stenosis: part II. Investigations and results. Br Med J. 1954;1(4871):1113–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Julian OC, Dye WS, Grove WJ.. Selection of patients for mitral commissurotomy in relation to clinical results. Arch Surg. 1954;69(3):273–281. [DOI] [PubMed] [Google Scholar]
- 22.Fell EH, Helmen RT.. Reactivation of rheumatic fever following mitral commissurotomy. Arch Surg. 1955;71(4):512–517. [DOI] [PubMed] [Google Scholar]
- 23.Papp C, Zion MM.. The postcommissurotomy syndrome. Br Heart J. 1956;18(2):153–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hall P, Biörck G.. The natural history of rheumatic valvular heart disease and its bearing upon the results of surgery for mitral stenosis: paper read at the sixth scandinavian Congress for rheumatology in lund, 1956. Acta Rheumatol Scand. 1958;4(1-4):70–78. [DOI] [PubMed] [Google Scholar]
- 25.Likoff W, Uricchio JF.. Results of mitral commissurotomy; clinical status of two hundred patients five to eight years after operation. JAMA. 1958;166(7):737–740. [DOI] [PubMed] [Google Scholar]
- 26.Lisan P, Reale A, Likoff W.. The postmitral commissurotomy syndrome: a four-year clinical, pathologic and serologic study, and its relation to restenosis. Ann Intern Med. 1959;50(6):1352–1358. [DOI] [PubMed] [Google Scholar]
- 27.Bain WH, Thomson RM, Mackey WA.. Mitral valvotomy: operative procedure and immediate post-operative course. Scott Med J. 1961;6(3):108–111. [DOI] [PubMed] [Google Scholar]
- 28.Drusin LM, Engle MA, Hagstrom JWC, et al. The postpericardiotomy syndrome - A six-year epidemiologic study. N Engl J Med. 1965;272(12):597–602. [DOI] [PubMed] [Google Scholar]
- 29.Louhija A, Kaihilahti J, Halonen PI.. Postcardiotomy syndrome - an infectious disease? Am Heart J. 1971;82(2):283. [DOI] [PubMed] [Google Scholar]
- 30.Ikäheimo MJ, Huikuri HV, Airaksinen KEJ, et al. Pericardial effusion after cardiac surgery: incidence, relation to the type of surgery, antithrombotic therapy, and early coronary bypass graft patency. Am Heart J. 1988;116(1):97–102. [DOI] [PubMed] [Google Scholar]
- 31.Engle MA. Humoral immunity and heart disease: postpericardiotomy syndrome. Adv Exp Med Biol. 1982;3:155–157. [DOI] [PubMed] [Google Scholar]
- 32.Wilson NJ, Webber SA, Patterson MWH, et al. Double-blind placebo-controlled trial of corticosteroids in children with postpericardiotomy syndrome. Pediatr Cardiol. 1994;15(2):62–65. [DOI] [PubMed] [Google Scholar]
- 33.Mott AR, Fraser CD, Kusnoor AV, et al. The effect of short-term prophylactic methylprednisolone on the incidence and severity of postpericardiotomy syndrome in children undergoing cardiac surgery with cardiopulmonary bypass. J Am Coll Cardiol. 2001;37(6):1700–1706. [DOI] [PubMed] [Google Scholar]
- 34.Webber SA, Wilson NJ, Junker AK, et al. Postpericardiotomy syndrome: no evidence for a viral etiology. Cardiol Young. 2001;11(1):67–74. [DOI] [PubMed] [Google Scholar]
- 35.Heching HJ, Bacha EA, Liberman L.. Post-pericardiotomy syndrome in pediatric patients following surgical closure of secundum atrial septal defects: incidence and risk factors. Pediatr Cardiol. 2015;36(3):498–502. [DOI] [PubMed] [Google Scholar]
- 36.Rabinowitz EJ, Meyer DB, Kholwadwala P, et al. Does prophylactic ibuprofen after surgical atrial septal defect repair decrease the rate of post-pericardiotomy syndrome? Pediatr Cardiol. 2018;39(8):1535–1539. [DOI] [PubMed] [Google Scholar]
- 37.Horneffer PJ, Miller RH, Pearson TA, et al. The effective treatment of postpericardiotomy syndrome after cardiac operations. A randomized placebo-controlled trial. J Thorac Cardiovasc Surg. 1990;100(2):292–296. [PubMed] [Google Scholar]
- 38.Bunge JJH, van Osch D, Dieleman JM, et al. Dexamethasone for the prevention of postpericardiotomy syndrome: a dexamethasone for cardiac surgery substudy. Am Heart J. 2014;168(1):126–131.e1. [DOI] [PubMed] [Google Scholar]
- 39.Zeltser I, Rhodes LA, Tanel RE, et al. Postpericardiotomy syndrome after permanent pacemaker implantation in children and young adults. Ann Thorac Surg. 2004;78(5):1684–1687. [DOI] [PubMed] [Google Scholar]
- 40.Gatzoulis K, Archontakis S, Tsiachris D, et al. Post-cardiac injury syndrome after permanent electronic cardiac device implantation. Incidence, presentation, management and long-term prognosis. Int J Cardiol. 2014;174(1):163–164. [DOI] [PubMed] [Google Scholar]
- 41.Sedaghat-Hamedani F, Zitron E, Kayvanpour E, et al. Post cardiac injury syndrome after initially uncomplicated CRT-D implantation: a case report and a systematic review. Clin Res Cardiol. 2014;103(10):781–789. [DOI] [PubMed] [Google Scholar]
- 42.Liu Y, Wang C, Zhao R, et al. Incidence and clinical characteristics of postcardiac injury syndrome complicating cardiac perforation caused by radiofrequency catheter ablation for cardiac arrhythmias. Int J Cardiol. 2013;168(4):3224–3229. [DOI] [PubMed] [Google Scholar]
- 43.Gabaldo K, Sutlić Ž, Mišković D, et al. Postpericardiotomy syndrome incidence, diagnostic and treatment strategies: experience AT two collaborative centers. Acta Clin Croat. 2019;58(1):57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McGuinness JB, Taussig HB.. The postpericardiotomy syndrome. Circulation. 1962;26(4):500–507. [Google Scholar]
- 45.Ikäheimo M, Takkunen J.. Postpericardiotomy syndrome diagnosed by Echocardiography. Scand J Thorac Cardiovasc Surg. 1979;13(3):305–308. [DOI] [PubMed] [Google Scholar]
- 46.Nishimura RA, Fuster V, Burgert SL, et al. Clinical features and long-term natural history of the postpericardiotomy syndrome. Int J Cardiol. 1983;4(4):443–454. [DOI] [PubMed] [Google Scholar]
- 47.Alraies MC, Al Jaroudi W, Shabrang C, et al. Clinical features associated with adverse events in patients with post-pericardiotomy syndrome following cardiac surgery. Am J Cardiol. 2014;114(9):1426–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gore M. The post-commissurotomy syndrome. Q Bull Northwest Univ Med Sch. 1960;34:202–205. [PMC free article] [PubMed] [Google Scholar]
- 49.Uricchio JF. The postcommissurotomy (Postpericardiotomy) syndrome. Am J Cardiol. 1963;12(4):436–438. [DOI] [PubMed] [Google Scholar]
- 50.Maisch B, Berg PA, Kochsiek K.. Clinical significance of immunopathological findings in patients with post-pericardiotomy syndrome. I. Relevance of antibody pattern. Clin. Exp. Immunol. 1979;38(2):189–197. [PMC free article] [PubMed] [Google Scholar]
- 51.Elster SK, Wood HF, Seely RD.. Clinical and laboratory manifestations of the postcommissurotomy syndrome. Am J Med. 1954;17(6):826–838. [DOI] [PubMed] [Google Scholar]
- 52.Tabatznik B, Isaacs JP.. Postpericardiotomy syndrome following traumatic hemopericardium. Am J Cardiol. 1961;7(1):83–96. [DOI] [PubMed] [Google Scholar]
- 53.Urschel HC, Razzuk MA, Gardner M.. Coronary artery bypass occlusion secondary to postcardiotomy syndrome. Ann Thorac Surg. 1976;22(6):528–531. [DOI] [PubMed] [Google Scholar]
- 54.Johnson JL. Postpericardiotomy syndrome in congenital heart deformities. Am Heart J. 1959;57(5):643–653. [DOI] [PubMed] [Google Scholar]
- 55.Engle MA, Ito T.. The postpericardiotomy syndrome. Am J Cardiol. 1961;7(1):73–82. [DOI] [PubMed] [Google Scholar]
- 56.Bartels C, Hönig R, Burger G, et al. The significance of anticardiolipin antibodies and anti-heart muscle antibodies for the diagnosis of postpericardiotomy syndrome. Eur Heart J. 1994;15(11):1494–1499. [DOI] [PubMed] [Google Scholar]
- 57.Köhler I, Saraiva PJ, Wender OB, et al. Behavior of inflammatory markers of myocardial injury in cardiac surgery. Laboratory correlation with the clinical picture of postpericardiotomy syndrome. Arq Bras Cardiol. 2003;81(3):279–290. [PubMed] [Google Scholar]
- 58.Bortolotti U, Livi U, Frugoni C, et al. Delayed cardiac tamponade following open heart surgery analysis of 12 patients. Thorac Cardiovasc Surg. 1981;29(04):233–236. [DOI] [PubMed] [Google Scholar]
- 59.Ofori-Krakye SK, Tyberg TI, Geha AS, et al. Late cardiac tamponade after open heart surgery: incidence, role of anticoagulants in its pathogenesis and its relationship to the postpericardiotomy syndrome. Circulation. 1981;63(6):1323–1328. [DOI] [PubMed] [Google Scholar]
- 60.Meyers DG, Meyers RE, Prendergast TW.. The usefulness of diagnostic tests on pericardial fluid. Chest. 1997;111(5):1213–1221. [DOI] [PubMed] [Google Scholar]
- 61.Karatolios K, Pankuweit S, Maisch B.. Diagnostic value of biochemical biomarkers in malignant and non-malignant pericardial effusion. Heart Fail Rev. 2013;18(3):337–344. [DOI] [PubMed] [Google Scholar]
- 62.Bielsa S, Corral E, Bagüeste P, et al. Characteristics of pleural effusions in acute idiopathic pericarditis and post-cardiac injury syndrome. Ann Am Thorac Soc. 2016;13(2):298–300. [DOI] [PubMed] [Google Scholar]
- 63.Engle MA, McCabe JC, Ebert PA, et al. The postpericardiotomy syndrome and antiheart antibodies. Circulation. 1974;49(3):401–406. [DOI] [PubMed] [Google Scholar]
- 64.Lessof MH. Postcardiotomy syndrome: pathogenesis and management. Hosp Pract. 1976;11(9):81–86. [DOI] [PubMed] [Google Scholar]
- 65.Livelli FD, Johnson RA, McEnany MT, et al. Unexplained in-hospital fever following cardiac surgery. Natural history, relationship to postpericardiotomy syndrome, and a prospective study of therapy with indomethacin versus placebo. Circulation. 1978;57(5):968–975. [DOI] [PubMed] [Google Scholar]
- 66.Gay WA. Postpericardiotomy syndrome. In: Alex GA, editor. Complications in cardiothoracic surgery: avoidance and treatment. 1st ed.; 2004. [Google Scholar]
- 67.Heidecker J, Sahn SA.. The spectrum of pleural effusions after coronary artery Bypass grafting surgery. Clin Chest Med. 2006;27(2):267–283. [DOI] [PubMed] [Google Scholar]
- 68.Engle MA, Gay WA, Kaminsky ME, et al. The postpericardiotomy syndrome then and now. Curr Probl Cardiol. 1978;3(2):1–40. [DOI] [PubMed] [Google Scholar]
- 69.Imazio M, Brucato A, Ferrazzi P, et al. Postpericardiotomy syndrome: a proposal for diagnostic criteria. J Cardiovasc Med. 2013;14(5):351–353. [DOI] [PubMed] [Google Scholar]
- 70.Larson DL. Relation of the postcommissurotomy syndrome to the rheumatic state. Circulation. 1957;15(2):203–209. [DOI] [PubMed] [Google Scholar]
- 71.Lehto J, Kiviniemi TO, Gunn J, et al. Occurrence of postpericardiotomy syndrome admissions: A population-based registry study. Ann Med. 2016;48(1-2):28–33. [DOI] [PubMed] [Google Scholar]
- 72.Voskuhl R. Sex differences in autoimmune diseases. Biol Sex Dif. 2011;2(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gubbels Bupp MR. Sex, the aging immune system, and chronic disease. Cell Immunol. 2015;294(2):102–110. [DOI] [PubMed] [Google Scholar]
- 74.Lehto J, Kiviniemi T, Gunn J, et al. Occurrence of postpericardiotomy syndrome: association with operation type and postoperative mortality after open‐heart operations. J Am Heart Assoc. 2018;7(22):e010269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ramos-Casals M, Brito-Zerón P, Kostov B, et al. Google-driven search for big data in autoimmune geoepidemiology: analysis of 394,827 patients with systemic autoimmune diseases. Autoimmun Rev. 2015;14(8):670–679. [DOI] [PubMed] [Google Scholar]
- 76.Simon AK, Hollander GA, McMichael A.. Evolution of the immune system in humans from infancy to old age. Proc R Soc B. 2015;282(1821):20143085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van Osch D, Dieleman JM, Bunge JJ, et al. Risk factors and prognosis of postpericardiotomy syndrome in patients undergoing valve surgery. J Thorac Cardiovasc Surg. 2017;153(4):878–885.e1. [DOI] [PubMed] [Google Scholar]
- 78.Sevuk U, Bilgic A, Altindag R, et al. Value of the neutrophil-to-lymphocyte ratio in predicting post-pericardiotomy syndrome after cardiac surgery. Eur Rev Med Pharmacol Sci. 2016;20:906–911. [PubMed] [Google Scholar]
- 79.Sevuk U, Baysal E, Altindag R, et al. Role of methylprednisolone in the prevention of postpericardiotomy syndrome after cardiac surgery. Eur Rev Med Pharmacol Sci. 2016;20:514–519. [PubMed] [Google Scholar]
- 80.Sevuk U, Baysal E, Altindag R, et al. Role of diclofenac in the prevention of postpericardiotomy syndrome after cardiac surgery. Vasc Health Risk Manag. 2015;11:373–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jaworska-Wilczyńska M, Magalska A, Piwocka K, et al. Low interleukin - 8 Level predicts the occurrence of the postpericardiotomy syndrome. Pizzi C, editor. PLoS One. 2014;9(10):e108822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Oktay AA, Akturk HK, Esenboğa K, Javed F, et al. Pathophysiology and prevention of heart disease in diabetes mellitus. Curr Probl Cardiol. 2018;43(3):68–110. [DOI] [PubMed] [Google Scholar]
- 83.Zampieri FG, Jacob V, Barbeiro HV, et al. Influence of body mass index on inflammatory profile at admission in critically ill septic patients. Int J Inflam. 2015;2015:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.De Scheerder I, Wulfrank D, Van Renterghem L, et al. Association of anti-heart antibodies and circulating immune complexes in the post-pericardiotomy syndrome. Clin. Exp. Immunol. 1984;57(2):423–428. [PMC free article] [PubMed] [Google Scholar]
- 85.De Scheerder IK, De Buyzere M, Delanghe J, et al. Humoral immune response against contractile proteins (actin and myosin) during cardiovascular disease. Eur Heart J. 1991;12(suppl D):88–94. [DOI] [PubMed] [Google Scholar]
- 86.Nomura Y, Yoshinaga M, Haraguchi T, et al. Relationship between the degree of injury at operation and the change in antimyosin antibody titer in the postpericardiotomy syndrome. Pediatr Cardiol. 1994;15(3):116–120. [DOI] [PubMed] [Google Scholar]
- 87.Salis S, Mazzanti VV, Merli G, et al. Cardiopulmonary bypass duration is an independent predictor of morbidity and mortality after cardiac surgery. J Cardiothorac Vasc Anesth. 2008;22(6):814–822. [DOI] [PubMed] [Google Scholar]
- 88.Segal F, Tabatznik B.. Postpericardiotomy syndrome following penetrating stab wounds of the chest: Comparison with the postcommissurotomy syndrome. Am Heart J. 1960;59(2):175–183. [DOI] [PubMed] [Google Scholar]
- 89.Loughlin V, Murphy A, Russell C.. The post-pericardiotomy syndrome and penetrating injury of the chest. Injury. 1987;18(6):412–414. [DOI] [PubMed] [Google Scholar]
- 90.Zeft HJ, McIntosh HD.. Postpericardiotomy syndrome in a patient with a retained foreign body. Am J Cardiol. 1965;16(4):593–597. [DOI] [PubMed] [Google Scholar]
- 91.Akdemir R, Gunduz H, Erbilen E, et al. Recurrent pericardial effusion due to retained cardiac pellets: a case report and review of the literature. Heart Vessels. 2003;18(1):57–59. [DOI] [PubMed] [Google Scholar]
- 92.Goodkind MJ, Bloomer WE, Goodyer A.. Recurrent pericardial effusion after nonpenetrating chest trauma: report of two cases treated with adrenocortical steroids. N Engl J Med. 1960;263(18):874–881. [DOI] [PubMed] [Google Scholar]
- 93.Gunnar RM, Gunnar HP, Teplitz R.. The syndrome of post-traumatic pericarditis. A case report. Am Heart J. 1962;64(4):543–546. [DOI] [PubMed] [Google Scholar]
- 94.Sikes CR. Posttraumatic hemorrhagic pericardial effusion. A case treated successfully with adrenocortical steroids. Am J Cardiol. 1963;11(1):115–118. [DOI] [PubMed] [Google Scholar]
- 95.Goldstein S, Yu PN.. Constrictive pericarditis after blunt chest trauma. Am Heart J. 1965;69(4):544–550. [PubMed] [Google Scholar]
- 96.Stewart JT, McKenna DH, Danforth WH.. Postpericardiotomy syndrome following left heart catheterization. Am J Cardiol. 1962;9(5):810–812. [DOI] [PubMed] [Google Scholar]
- 97.Peter RH, Whalen RE, Orgain ES, et al. Postpericardiotomy syndrome as a complication of percutaneous left ventricular puncture. Am J Cardiol. 1966;17(1):86–90. [DOI] [PubMed] [Google Scholar]
- 98.Jerjes-Sanchez C, Ibarra-Perez C, Ramirez-Rivera A, et al. Dressler-like syndrome after pulmonary embolism and infarction. Chest. 1987;92(1):115–117. [DOI] [PubMed] [Google Scholar]
- 99.Dressler W. Postcardiotomy syndrome after implantation of a pacemaker. Am Heart J. 1962;63(6):757–759. [DOI] [PubMed] [Google Scholar]
- 100.Wang RYC, Mok CK.. Erosion of an epicardial pacemaker secondary to postpericardiotomy syndrome. Pacing Clin Electro. 1983;6(1):33–34. [DOI] [PubMed] [Google Scholar]
- 101.Terada Y, Mitsui T, Kaminishi Y, et al. Postpericardiotomy syndrome after pacemaker implantation. Ann Thorac Surg. 1995;59(5):1272–1273. [DOI] [PubMed] [Google Scholar]
- 102.Kaye D, Frankl W, Arditi LI.. Probable postcardiotomy syndrome following implantation of a transvenous pacemaker: report of the first case. Am Heart J. 1975;90(5):627–630. [DOI] [PubMed] [Google Scholar]
- 103.Snow ME, Agatston AS, Kramer HC, et al. The postcardiotomy syndrome following transvenous pacemaker insertion. Pacing Clin Electro. 1987;10(4):934–936. [DOI] [PubMed] [Google Scholar]
- 104.Lau C-P, Fong P-C, Tai Y-T, et al. Postpericardiotomy syndrome complicating transvenous dual-chamber rate-adaptive pacing: diagnosis aided by transesophageal echocardiography. Am Heart J. 1992;123(5):1388–1390. [DOI] [PubMed] [Google Scholar]
- 105.Hargreaves M, Bashir Y.. Postcardiotomy syndrome following transvenous pacemaker insertion. Eur Heart J. 1994;15(7):1005–1007. [DOI] [PubMed] [Google Scholar]
- 106.Miller GL, Coccio EB, Sharma SC.. Postpericardiotomy syndrome and cardiac tamponade following transvenous pacemaker placement. Clin Cardiol. 1996;19(3):255–256. [DOI] [PubMed] [Google Scholar]
- 107.Bajaj BPS, Evans KE, Thomas P.. Postpericardiotomy syndrome following temporary and permanent transvenous pacing. Postgrad Med J. 1999;75(884):357–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Spindler M, Burrows G, Kowallik P, et al. Postpericardiotomy syndrome and cardiac tamponade as a late complication after pacemaker implantation. Pacing Clin Electrophysiol. 2001;24(9):1433–1434. [DOI] [PubMed] [Google Scholar]
- 109.Sasaki A, Kobayashi H, Okubo T, et al. Repeated Postpericardiotomy Syndrome Following a Temporary Transvenous Pacemaker Insertion, a Permanent Transvenous Pacemaker Insertion and Surgical Pericardiotomy. Jpn Circ J. 2001;65(4):343–344. [DOI] [PubMed] [Google Scholar]
- 110.Turkie W, Khattar RS.. Right ventricular failure due to postpericardiotomy syndrome following transvenous dual chamber permanent pacemaker implantation. Int J Cardiol. 2005;99(3):465–466. [DOI] [PubMed] [Google Scholar]
- 111.Cevik C, Wilborn T, Corona R, et al. Post-cardiac injury syndrome following transvenous pacemaker insertion: a case report and review of the literature. Hear Lung Circ. 2009;18(6):379–383. [DOI] [PubMed] [Google Scholar]
- 112.Tsai W-C, Liou C-T, Cheng C-C, et al. Post-cardiac injury syndrome after permanent pacemaker implantation. ACTA Cardiol Sin. 2012;28:53–55. [Google Scholar]
- 113.Salih M, Ayan M, Gashouta M, et al. Pacemaker induced post cardiac injury syndrome. MOJ Clin Med Case Rep. 2015;2:00028. [Google Scholar]
- 114.Kumar S, Madanieh A, Patel H, et al. Large unilateral pleural effusion with pacemaker-associated post-cardiac injury syndrome. Cureus. 2018;10(7):e2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Escaned J, Ahmad RAS, Shiu MF.. Pleural effusion following coronary perforation during balloon angioplasty: an unusual presentation of the postpericardiotomy syndrome. Eur Heart J. 1992;13(5):716–717. [DOI] [PubMed] [Google Scholar]
- 116.Hearne C, Forjuoh SN.. Postcardiac injury syndrome after coronary angioplasty and stenting. J Am Board Fam Med. 2003;16(1):73–74. [DOI] [PubMed] [Google Scholar]
- 117.Setoyama T, Furukawa Y, Abe M, et al. Acute pleuropericarditis after coronary stenting. Circ J. 2006;70(3):358–361. [DOI] [PubMed] [Google Scholar]
- 118.Gungor B, Ucer E, Erdinler İC.. Uncommon presentation of postcardiac injury syndrome: Acute pericarditis after percutaneous coronary intervention. Int J Cardiol. 2008;128(1):e19–21. [DOI] [PubMed] [Google Scholar]
- 119.Park J-S, Kim D-H, Choi W-G, et al. Postcardiac injury syndrome after percutaneous coronary intervention. Yonsei Med J. 2010;51(2):284–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kawase Y, Ota H, Okubo M, et al. Post-cardiac injury syndrome after a simple coronary stenting. Cardiovasc Interv and Ther. 2015;30(3):287–292. 92. [DOI] [PubMed] [Google Scholar]
- 121.Gurunathan S, Parsons G, Young G, et al. Postcardiac Injury Syndrome: A Rare Complication of Elective Coronary Angioplasty. Am J Med. 2016;129(5):e13–4. [DOI] [PubMed] [Google Scholar]
- 122.Paiardi S, Cannata F, Ciccarelli M, et al. Post-cardiac injury syndrome: an atypical case following percutaneous coronary intervention. Am J Emerg Med. 2017;35(12):1985.e1–1985.e2. [DOI] [PubMed] [Google Scholar]
- 123.Elbaz-Greener G, Wijeysundera HC.. A presentation of postcardiac injury syndrome after successful chronic total occlusion percutaneous coronary intervention using dissection re-entry techniques. Clin Case Rep. 2017;5(6):855–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gavrielatos G, Michas G, Grigoriou K, et al. Acute post cardiac injury syndrome occurring immediately after a demanding percutaneous coronary intervention. Hosp Chronicles. 2018;13:27–31. [Google Scholar]
- 125.Demirtas M, Birand A, San M, et al. Dressler-like syndrome after percutaneous mitral balloon valvuloplasty pericarditis? Cathet Cardiovasc Diagn. 1998;44(1):103–103. [DOI] [PubMed] [Google Scholar]
- 126.Wood MA, Ellenbogen KA, Hall J, et al. Post-pericardiotomy syndrome following linear left atrial radiofrequency ablation. J Interv Card Electrophysiol. 2003;9(1):55–57. [DOI] [PubMed] [Google Scholar]
- 127.Koller ML, Maier SKG, Bauer WR, et al. Postcardiac injury syndrome following radiofrequeny ablation of atrial flutter. Z Kardiol. 2004;93(7):560–565. [DOI] [PubMed] [Google Scholar]
- 128.Tang R, Liu X, Dong J, et al. Postcardiac injury syndrome complicating circumferential pulmonary vein radiofrequency ablation for atrial fibrillation. Chin Med J. 2007;120(21):1940–1942. [PubMed] [Google Scholar]
- 129.Turitto G, Abordo MG, Mandawat MK, et al. Radiofrequency ablation for cardiac arrhythmias causing poscardiac injury syndrome. Am J Cardiol. 1998;81(3):369–370. [DOI] [PubMed] [Google Scholar]
- 130.Zheng LR, Hu X, Xia S, et al. Postcardiac injury syndrome following radiofrequency ablation of idiopathic left ventricular tachycardia. J Interv Card Electrophysiol. 2007;18(3):269–271. [DOI] [PubMed] [Google Scholar]
- 131.Yukumi S, Ichiki H, Funada J, et al. Postcardiac injury syndrome following vascular interventional radiofrequency ablation for paroxysmal atrial fibrillation. Respir Med Case Reports. 2015;15:89–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.King DR, Vlahakes GJ, Johri AM, et al. Postpericardiotomy syndrome from transdiaphragmatic pericardial window following trauma: first description and review of the literature. J Cardiovasc Med. 2009;10:806–809. [DOI] [PubMed] [Google Scholar]
- 133.Santos CC, Sanders DB.. Postpericardiotomy syndrome following thymectomy. Clin Pediatr (Phila)). 1992;31(5):311–312. [DOI] [PubMed] [Google Scholar]
- 134.Yukumi S, Suzuki H, Kashu Y, et al. Postpericardiotomy syndrome after thymothymectomy: report of two cases. Gen Thorac Cardiovasc Surg. 2012;60(7):462–464. [DOI] [PubMed] [Google Scholar]
- 135.Nishimura M, Goda N, Hatazawa K, et al. Delayed diagnosis of postcardiac injury syndrome. BMJ Case Rep. 2019;12(2):e228877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Eguchi T, Yoshida K, Hamanaka K, et al. Colchicine as an effective treatment for postpericardiotomy syndrome following a lung lobectomy. Interact Cardiovasc Thorac Surg. 2010;11(6):869–871. [DOI] [PubMed] [Google Scholar]
- 137.Greene TO, Portnow AS, Huang SK.. Acute pericarditis resulting from an endocardial active fixation screw-in atrial lead. Pacing Clin Electro. 1994;17(1):21–25. [DOI] [PubMed] [Google Scholar]
- 138.Wolk B, Dandes E, Martinez F, et al. Postcardiac injury syndrome following transvenous pacer or defibrillator insertion: CT imaging and review of the literature. Curr Probl Diagn Radiol. 2013;42(4):141–148. [DOI] [PubMed] [Google Scholar]
- 139.Tuzcu EM, Block PC, Griffin BP, et al. Immediate and long-term outcome of percutaneous mitral valvotomy in patients 65 years and older. Circulation. 1992;85(3):963–971. [DOI] [PubMed] [Google Scholar]
- 140.Croitoru DP, Kelly RE, Goretsky MJ, et al. Experience and modification update for the minimally invasive Nuss technique for pectus excavatum repair in 303 patients. J Pediatr Surg. 2002;37(3):437–445. [DOI] [PubMed] [Google Scholar]
- 141.Paronetto F, Popper H.. Lymphocyte stimulation induced by halothane in patients with hepatitis following exposure to halothane. N Engl J Med. 1970;283(6):277–280. [DOI] [PubMed] [Google Scholar]
- 142.Sellke FW, del Nido PJ, Swanson SJ.. Sabiston & Spencer Surgery of the Chest. -8th ed. 2010. [DOI] [PubMed] [Google Scholar]
- 143.Escobar GA, Cheng AM, Moore EE, et al. Stored packed red blood cell transfusion up-regulates inflammatory gene expression in circulating leukocytes. Ann Surg. 2007;246:129–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Snefjellå N, Lappegård KT.. Development of post-pericardiotomy syndrome is preceded by an increase in pro-inflammatory and a decrease in anti-inflammatory serological markers. J Cardiothorac Surg. 2012;7(1):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kirsh MM, McIntosh K, Kahn DR, Sloan H.. Postpericardiotomy syndromes. Ann Thorac Surg. 1970;9(2):158–179. [DOI] [PubMed] [Google Scholar]
- 146.Boyle EM, Gillinov AM, Cohn WE, et al. Retained blood syndrome after cardiac surgery. Innovations (Phila). 2015;10(5):296–303. [DOI] [PubMed] [Google Scholar]
- 147.Sirch J, Ledwon M, Püski T, et al. Active clearance of chest drainage catheters reduces retained blood. J Thorac Cardiovasc Surg. 2016;151(3):832–838.e2. [DOI] [PubMed] [Google Scholar]
- 148.Khan J, Khan N, Mennander A.. Lower incidence of late tamponade after cardiac surgery by extended chest tube drainage. Scand Cardiovasc J. 2019;53(2):104–109. [DOI] [PubMed] [Google Scholar]
- 149.Gozdek M, Pawliszak W, Hagner W, et al. Systematic review and meta-analysis of randomized controlled trials assessing safety and efficacy of posterior pericardial drainage in patients undergoing heart surgery. J Thorac Cardiovasc Surg. 2017;153(4):865–875. [DOI] [PubMed] [Google Scholar]
- 150.Engle MA. Early and late manifestations of the postpericardiotomy syndrome. Int J Cardiol. 1983;4(4):451–454. [Google Scholar]
- 151.McClendon CE, Leff RD, Clark EB.. Postpericardiotomy syndrome. Drug Intell Clin Pharm. 1986;20(1):20–23. [DOI] [PubMed] [Google Scholar]
- 152.Engle MA, O'Loughlin JE.. Complications of cardiac surgery in children. Pediatr Rev. 1987;9(5):147–154. [DOI] [PubMed] [Google Scholar]
- 153.Imazio M, Bobbio M, Cecchi E, et al. Colchicine in addition to conventional therapy for acute pericarditis: results of the colchicine for acute pericarditis (COPE) trial. Circulation. 2005;112(13):2012–2016. [DOI] [PubMed] [Google Scholar]
- 154.Imazio M, Bobbio M, Cecchi E, et al. Colchicine as first-choice therapy for recurrent pericarditis. Arch Intern Med. 2005;165(17):1987–1991. [DOI] [PubMed] [Google Scholar]
- 155.Imazio M, Demichelis B, Parrini I, et al. Management, risk factors, and outcomes in recurrent pericarditis. Am J Cardiol. 2005;96(5):736–739. [DOI] [PubMed] [Google Scholar]
- 156.Artom G, Koren-Morag N, Spodick DH, et al. Pretreatment with corticosteroids attenuates the efficacy of colchicine in preventing recurrent pericarditis: a multi-centre all-case analysis. Eur Heart J. 2005;26(7):723–727. [DOI] [PubMed] [Google Scholar]
- 157.Imazio M, Brucato A, Cemin R, et al. A randomized trial of colchicine for acute pericarditis. N Engl J Med. 2013;369(16):1522–1528. [DOI] [PubMed] [Google Scholar]
- 158.Cantinotti M, Spadoni I, Assanta N, et al. Controversies in the prophylaxis and treatment of postsurgical pericardial syndromes: a critical review with a special emphasis on paediatric age. J Cardiovasc Med. 2014;15(12):847–854. [DOI] [PubMed] [Google Scholar]
- 159.Imazio M, Lazaros G.. Corticosteroids for pericarditis: a warning but don’t throw the baby out with the bathwater. Hell J Cardiol. 2019;60(6):364–365. [DOI] [PubMed] [Google Scholar]
- 160.Tenenbaum A, Koren-Morag N, Spodick DH, et al. The efficacy of colchicine in the treatment of recurrent pericarditis related to postcardiac injury (Postpericardiotomy and Postinfarcted) syndrome: a multicenter analysis. Hear Drug. 2004;4(3):141–144. [Google Scholar]
- 161.Alam M, Kayani WT, Bandeali SJ, et al. Impact of colchicine on pericardial inflammatory syndromes — An analysis of randomized clinical trials. Int J Cardiol. 2012;161(1):59–62. [DOI] [PubMed] [Google Scholar]
- 162.Imazio M, Brucato A, Forno D, et al. Efficacy and safety of colchicine for pericarditis prevention. Systematic review and meta-analysis. Heart. 2012;98(14):1078–1082. [DOI] [PubMed] [Google Scholar]
- 163.Imazio M, Brucato A, Belli R, et al. Colchicine for the prevention of pericarditis: what we know and what we do not know in 2014 – systematic review and meta-analysis. J Cardiovasc Med. 2014;15(12):840–846. [DOI] [PubMed] [Google Scholar]
- 164.Agarwal SK, Vallurupalli S, Uretsky BF, et al. Effectiveness of colchicine for the prevention of recurrent pericarditis and post-pericardiotomy syndrome: an updated meta-analysis of randomized clinical data. Eur Heart J Cardiovasc Pharmacother. 2015;1(2):117–125. [DOI] [PubMed] [Google Scholar]
- 165.Wang M, Deng X, Mu B-Y, et al. Effect of colchicine in prevention of pericardial effusion and atrial fibrillation: a meta-analysis. Intern Emerg Med. 2016;11(6):867–876. [DOI] [PubMed] [Google Scholar]
- 166.Li Y-L, Qiao S-B, Wang J-Y, et al. Colchicine in addition to conventional therapy for pericarditis recurrence. An update meta-analysis. Herz. 2016;41(7):630–638. [DOI] [PubMed] [Google Scholar]
- 167.Papageorgiou N, Briasoulis A, Lazaros G, et al. Colchicine for prevention and treatment of cardiac diseases: A meta-analysis. Cardiovasc Ther. 2017;35(1):10–18. [DOI] [PubMed] [Google Scholar]
- 168.Zucker N, Levitas A, Zalzstein E.. Methotrexate in recurrent postpericardiotomy syndrome. Cardiol Young. 2003;13(2):206–208. [DOI] [PubMed] [Google Scholar]
- 169.Vianello F, Cinetto F, Cavraro M, et al. Azathioprine in isolated recurrent pericarditis: a single centre experience. Int J Cardiol. 2011;147(3):477–478. [DOI] [PubMed] [Google Scholar]
- 170.Marcolongo R, Russo R, Laveder F, et al. Immunosuppressive therapy prevents recurrent pericarditis. J Am Coll Cardiol. 1995;26(5):1276–1279. [DOI] [PubMed] [Google Scholar]
- 171.Wendelin G, Fandl A, Beitzke A.. High-dose intravenous immunoglobulin in recurrent postpericardiotomy syndrome. Pediatr Cardiol. 2008;29(2):463–464. [DOI] [PubMed] [Google Scholar]
- 172.del Fresno MR, Peralta JE, Granados MA, et al. Intravenous immunoglobulin therapy for refractory recurrent pericarditis. Pediatrics. 2014;134(5):e1441–6. [DOI] [PubMed] [Google Scholar]
- 173.Moretti M, Buiatti A, Merlo M, et al. Usefulness of high-dose intravenous human immunoglobulins treatment for refractory recurrent pericarditis. Am J Cardiol. 2013;112(9):1493–1498. [DOI] [PubMed] [Google Scholar]
- 174.Imazio M, Lazaros G, Picardi E, et al. Intravenous human immunoglobulins for refractory recurrent pericarditis: a systematic review of all published cases. J Cardiovasc Med (Hagerstown). 2016;17(4):263–269. [DOI] [PubMed] [Google Scholar]
- 175.Lazaros G, Vasileiou P, Koutsianas C, et al. Anakinra for the management of resistant idiopathic recurrent pericarditis. Initial experience in 10 adult cases. Ann Rheum Dis. 2014;73(12):2215–2217. [DOI] [PubMed] [Google Scholar]
- 176.Lazaros G, Imazio M, Brucato A, et al. Anakinra: an emerging option for refractory idiopathic recurrent pericarditis: a systematic review of published evidence. J Cardiovasc Med (Hagerstown). 2016;17(4):256–262. [DOI] [PubMed] [Google Scholar]
- 177.Brucato A, Imazio M, Gattorno M, et al. Effect of anakinra on recurrent pericarditis among patients with colchicine resistance and corticosteroid dependence: the AIRTRIP randomized clinical trial. JAMA. 2016;316(18):1906–1912. [DOI] [PubMed] [Google Scholar]
- 178.Imazio M, Andreis A, De Ferrari GM, et al. Anakinra for corticosteroid-dependent and colchicine-resistant pericarditis: the IRAP (International Registry of Anakinra for Pericarditis) study. Eur J Prev Cardiolog. [cited 2019 Oct 15]. DOI:10.1177/2047487319879534. [DOI] [PubMed] [Google Scholar]
- 179.Maisch B, Ristić AD, Pankuweit S.. Intrapericardial treatment of autoreactive pericardial effusion with triamcinolone: the way to avoid side effects of systemic corticosteroid therapy. Eur Heart J. 2002;23(19):1503–1508. [DOI] [PubMed] [Google Scholar]
- 180.Havelock T, Teoh R, Laws D, et al. ; on behalf of the BTS Pleural Disease Guideline Group . BTS Pleural Disease Guideline Group. Pleural procedures and thoracic ultrasound: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65 (Suppl 2):ii61–ii76. [DOI] [PubMed] [Google Scholar]
- 181.Imazio M, Gaido L, Battaglia A, et al. Contemporary management of pericardial effusion: practical aspects for clinical practice. Postgrad Med. 2017;129(2):178–186. [DOI] [PubMed] [Google Scholar]
- 182.Light RW. Pleural effusions after coronary artery bypass graft surgery. Curr Opin Pulm Med. 2002;8(4):308–311. [DOI] [PubMed] [Google Scholar]
- 183.Paull DE, Delahanty TJ, Weber FJ, et al. Thoracoscopic talc pleurodesis for recurrent, symptomatic pleural effusion following cardiac operations. Surg Laparosc Endosc Percutan Tech. 2003;13(5):339–344. [DOI] [PubMed] [Google Scholar]
- 184.Bujarski S, Guy E.. Use of indwelling pleural catheter for recurrent pleural effusion due to postpericardiotomy syndrome. J Bronchology Interv Pulmonol. 2016;23(2):160–162. [DOI] [PubMed] [Google Scholar]
- 185.Chhabra L, Kowlgi NG, Spodick DH.. Age as a factor to predict postpericardiotomy syndrome. Am J Cardiol. 2015;115(4):554–555. [DOI] [PubMed] [Google Scholar]
- 186.Wamboldt R, Bisleri G, Glover B, et al. Primary prevention of post-pericardiotomy syndrome using corticosteroids: a systematic review. Expert Rev Cardiovasc Ther. 2018;16(6):405–412. [DOI] [PubMed] [Google Scholar]
- 187.Béland MJ, Paquet M, Gibbons JE, et al. Pericardial effusion after cardiac surgery in children and effects of aspirin for prevention. Am J Cardiol. 1990;65(18):1238–1241. [DOI] [PubMed] [Google Scholar]
- 188.Gill PJ, Forbes K, Coe JY.. The effect of short-term prophylactic acetylsalicylic acid on the incidence of postpericardiotomy syndrome after surgical closure of atrial septal defects. Pediatr Cardiol. 2009;30(8):1061–1067. [DOI] [PubMed] [Google Scholar]
- 189.Briasoulis A, Afonso L.. Prevention of pericarditis with colchicine. J Cardiovasc Med. 2015;16(2):144–147. [DOI] [PubMed] [Google Scholar]
- 190.Imazio M. Colchicine for pericarditis. Trends Cardiovasc Med. 2015;25(2):129–136. [DOI] [PubMed] [Google Scholar]
- 191.Engle MA, Zabriskie JB, Senterfit LB, et al. Immunologic and virologic studies in the postpericardiotomy syndrome. J Pediatr. 1975;87(6):1103–1108. [DOI] [PubMed] [Google Scholar]
- 192.Sevuk U, Ayaz F, Köse K, et al. Relationship between early post-pericardiotomy syndrome and atrial fibrillation after cardiac surgery. Kosuyolu Hear J. 2016;19(2):85–90. [Google Scholar]
- 193.Killian DM, Furiasse JG, Scanlon PJ, et al. Constrictive pericarditis after cardiac surgery. Am Heart J. 1989;118(3):563–568. [DOI] [PubMed] [Google Scholar]
- 194.Miller JI, Mansour KA, Hatcher CR.. Pericardiectomy: current indications, concepts, and results in a university center. Ann Thorac Surg. 1982;34(1):40–45. [DOI] [PubMed] [Google Scholar]
- 195.Rice PL, Pifarré R, Montoya A.. Constrictive pericarditis following cardiac surgery. Ann Thorac Surg. 1981;31(5):450–453. [DOI] [PubMed] [Google Scholar]
- 196.Kutcher MA, King SB, Alimurung BN, et al. Constrictive pericarditis as a complication of cardiac surgery: recognition of an entity. Am J Cardiol. 1982;50(4):742–748. [DOI] [PubMed] [Google Scholar]
- 197.Imazio M, Brucato A, Maestroni S, et al. Risk of constrictive pericarditis after acute pericarditis. Circulation. 2011;124(11):1270–1275. [DOI] [PubMed] [Google Scholar]
- 198.De Scheerder I, De Buyzere M, Clement D.. Association between post-pericardiotomy syndrome and coronary occlusion after aortic valve replacement. Br Heart J. 1985;54(4):445–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Luken JA, Monson DO, Weinberg M.. Post-pericardiotomy syndrome as a cause of intracardiac pericardial baffle obstruction. Pediatr Cardiol. 1986;7(1):58–59. [DOI] [PubMed] [Google Scholar]
- 200.De Scheerder I, De Buyzere M, Robbrecht J, et al. Postoperative immunological response against contractile proteins after coronary bypass surgery. Br Heart J. 1986;56(5):440–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.De Scheerder I, Vandekerckhove J, Robbrecht J, et al. Post-cardiac injury syndrome and an increased humoral immune response against the major contractile proteins (actin and myosin). Am J Cardiol. 1985;56(10):631–633. [DOI] [PubMed] [Google Scholar]
- 202.Izadi Amoli A, Bozorgi A, HajHossein Talasaz A, et al. Efficacy of colchicine versus placebo for the treatment of pericardial effusion after open-heart surgery: a randomized, placebo-controlled trial. Am Heart J. 2015;170(6):1195–1201. [DOI] [PubMed] [Google Scholar]
- 203.Meurin P, Lelay-Kubas S, Pierre B, et al. Colchicine for postoperative pericardial effusion: a multicentre, double-blind, randomised controlled trial. Heart. 2015;101(21):1711–1716. [DOI] [PubMed] [Google Scholar]
- 204.Engblom E, Arstila M, Inberg MV, et al. Early results and complications of coronary artery bypass surgery: a consecutive series of 441 patients. Scand J Thorac Cardiovasc Surg. 1985;19(1):21–27. 7. [DOI] [PubMed] [Google Scholar]
- 205.Ranucci M, Castelvecchio S, Romitti F, et al. Living without aprotinin: the results of a 5-year blood saving program in cardiac surgery. Acta Anaesthesiol Scand. 2009;53(5):573–580. [DOI] [PubMed] [Google Scholar]
- 206.Muensterer OJ, Schenk DS, Praun M, et al. Postpericardiotomy syndrome after minimally invasive pectus excavatum repair unresponsive to nonsteroidal anti-inflammatory treatment. Eur J Pediatr Surg. 2003;13(3):206–208. [DOI] [PubMed] [Google Scholar]
- 207.Berberich T, Haecker F-M, Kehrer B, et al. Postpericardiotomy syndrome after minimally invasive repair of pectus excavatum. J Pediatr Surg. 2004;39(11):e1–3. [DOI] [PubMed] [Google Scholar]