Abstract
Heart failure (HF) is a major cause of morbidity and mortality worldwide, and the burden of HF continues to rise. There has been an interest in sodium-glucose co-transporter-2 (SGLT2) inhibitors for their role in reducing HF hospitalizations in pivotal trials. Since these agents were approved by the Food and Drug Administration for the management of diabetes mellitus, multiple small trials and analyses have tried to explain the underlying beneficial mechanisms in HF . In this review, we discuss different mechanisms by which SGLT2 inhibitors play hemodynamic, metabolic, and cellular roles in different HF phenotypes. We also address issues pertaining to the safety of these relatively newer agents.
KEY MESSAGES
SGLT2 inhibitors are associated with a reduction in HF hospitalizations in both diabetics and non-diabetics.
The beneficial role of SGLT2 inhibitors in reducing HF hospitalization is observed among participants with established cardiovascular disease/HF and at-risk population.
SGLT2 inhibitors pose an important role in renal protection, another mechanism by which these medications can be helpful in HF patients
Keywords: Cardiovascular outcomes, diabetes, heart failure, sodium glucose co-transporter 2 inhibitors
Background
More than 6.5 million adults in the United States have heart failure (HF) and its prevalence is projected to increase by 46% from 2014 to 2030 [1]. HF is associated with increased morbidity and mortality, worse quality of life, and a higher cost of care. Mortality from HF decreased between 2006 and 2009 but has remained unchanged afterwards [1]. While several medical therapies have proven to reduce morbidity and mortality in patients with heart failure and reduced ejection fraction (HFrEF), such as beta-antagonists, neurohormonal antagonists, and mineralocorticoid antagonists, similar therapies have failed to provide similar efficacy among patients with HF with preserved ejection fraction (HFpEF), and the current management relies heavily on the control of risk factors and symptoms [2–5]. Furthermore, the proportion of HFrEF patients who receive guideline-directed medical therapy is suboptimal and their prognosis remains poor. Because of all these factors, HF constitutes a major health problem with significant impact on the health care systems [6,7].
Multiple risk factors exist for HF including hypertension, ischaemic heart disease, valvular heart disease, and diabetes. Diabetes affects > 30 million Americans and is associated with a 2- to 4-fold increase in the risk of HF compared with non-diabetics [8–11]. Diabetes is an important predictor for the development of symptomatic HF in individuals with asymptomatic left ventricular systolic dysfunction [12]. Furthermore, uncontrolled diabetes poses a higher risk for the development of HF. Different reports have described that the incidence of HF increases from 8% to 36% with every 1% increase in HbA1c [11,13–16]. Even though the risk of HF increases with poor control, such risk is not adequately reduced by intensive management of diabetes [17]. Since the Food and Drug Administration (FDA)’s requirement to examine cardiovascular outcomes in trials conducted for the approval of new medications for type 2 diabetes, sodium glucose co-transporter-2 (SGLT2) inhibitors have become a promising class of medications, not only for blood sugar control but also in improving cardiovascular outcomes in patients with diabetes.
Co-transport of glucose and sodium was first proposed by Crane et al [18]. The most widely studied SGLTs are SGLT1 and SGLT2. SGLT1, which has low capacity but high affinity for glucose, is expressed in the gut, heart, and kidneys. SGLT2, which has high capacity and low affinity for glucose, is expressed predominantly in the kidneys [18]. The majority of filtered glucose from nephrons is reabsorbed through SGLT-2 in the initial portion of the proximal convoluted tubules [19]. The remaining filtered glucose is absorbed in later segments of proximal convoluted tubules. In normal individuals, the filtered glucose is absorbed below the concentration of 180 mg/dl. The ability to reabsorb glucose is significantly increased in diabetics compared with non-diabetic individuals [20]. Phlorizin, initially extracted from the root bark of apple trees, is a non-selective SGLT inhibitor [21]. Higher doses of phlorizin required for glycemic control can lead to diarrhoea through SGLT1 in the gastrointestinal tract. A synthetic derivative of phlorizin, T-1095, showed significant improvement in post-prandial glucose and HbA1c levels in animal (mouse) models, but a drug for clinical use was not developed [22]. Washburn and colleagues synthesized phlorizin C-glucoside analogue, dapagliflozin, which showed a significantly higher (1200-times) affinity for SGLT2 when compared with SGLT1 [23]. The discovery of dapagliflozin led to the development of multiple SGLT2 inhibitors since 2008. Currently, the FDA has approved 4 SGLT2 inhibitors for the treatment of diabetes: canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin. This class of oral hypoglycaemics primarily acts by reducing the glucose reabsorption in the kidney through the inhibition of SGLT2 receptors resulting in glucosuria. This effect was found to lower haemoglobin A1c by 0.5-1.0% in multiple studies [24,25].
SGLT2 inhibitors significantly reduce HF hospitalizations in patients with previously diagnosed HF and in participants at risk for HF [17,24–27]. This favourable effect goes beyond the anti-glycemic effect of SGLT2 inhibitors. Multiple studies aimed to examine the mechanism behind the beneficial role of SGLT2 inhibitors in patients with HF; however, the exact mechanism remains unclear. In the current review, we discuss the role of SGLT2 inhibitors in patients with or at risk of HF, as well as the different potential mechanisms involved in the improved outcomes with SGLT2 inhibitors in this high-risk population.
SGLT2 inhibitors and heart failure outcomes in patients with type 2 diabetes
The first major SGLT2 inhibitor trial, EMPA-REG OUTCOMES (Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes) was a randomized, double-blinded placebo-controlled trial that examined the cardiovascular outcomes with empagliflozin versus placebo in patients with type 2 diabetes and established cardiovascular disease [25]. The primary endpoint was the composite of cardiovascular mortality, nonfatal MI, and nonfatal stroke, while HF hospitalizations were evaluated as a secondary endpoint. Among 7020 patients with type 2 diabetes randomized to empagliflozin versus placebo, the primary endpoint occurred in 490 of 4687 patients (10.5%) in the empagliflozin group versus 282 events in 2333 patients (12.1%) in the placebo group (hazard ratio [HR]: 0.86, 95% confidence interval [CI]: 0.74-0.99, p = .04). There was a 35% decrease in HF hospitalizations (HR 0.65, 95% CI: 0.50–0.85) at a median of 3.1 years. This effect was seen among those with a known diagnosis of HF as well as at risk for HF. The favourable outcomes were thought to be driven by improvement in hemodynamics, i.e. increased diuresis and natriuresis as well as a decrease in systolic and diastolic blood pressures with SGLT2 inhibitor use.
Similarly, the CANVAS (Canagliflozin Cardiovascular Assessment Study and Canagliflozin Cardiovascular Assessment Study-Renal) programme randomized 10,142 participants with type 2 diabetes and high cardiovascular risk to canagliflozin versus placebo. The primary outcome was a composite endpoint of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke. The participants were followed for a mean of 188.2 weeks. The CANVAS programme demonstrated a significant reduction in its primary outcome (HR 0.86; 95% CI: 0.75–0.97). Furthermore, canagliflozin was associated with a lower risk of HF hospitalization (HR 0.67, 95% CI: 0.52–0.87) compared with placebo [24]. Again, there was no significant difference in non-fatal MI and stroke between the canagliflozin and placebo arms of the study. Since EMPA-REG Outcomes and CANVAS programme studies showed no significant benefit in non-fatal MI and stroke, but only HF hospitalizations, this paved the way for the inclusion of HF hospitalization as a component in primary endpoint in the next SGLT2 inhibitor trial.
The DECLARE TIMI-58 (Dapagliflozin Effect on Cardiovascular Events‐Thrombolysis in Myocardial Infarction 58) trial included the largest study population (n = 17,160) of type 2 diabetics with, or at risk for, atherosclerotic cardiovascular disease [27]. Unlike the EMPA-REG OUTCOMES and CANVAS, this study focussed on the impact of SGLT2 on HF outcomes, and hence examined the primary composite efficacy endpoint of cardiovascular death or hospitalization for HF with dapagliflozin versus placebo. The trial showed a significant reduction in the primary composite efficacy endpoint of cardiovascular death or hospitalization for HF with dapagliflozin compared with placebo (HR 0.83; 95% CI: 0.73–0.95), mainly driven by a significant reduction in HF hospitalizations (HR 0.73, 95% CI: 0.61–0.88).
While the results of these 3 major trials point to a substantial benefit of SGLT2 inhibitors on HF hospitalization and other cardiovascular outcomes, it is important to highlight that the EMPA-REG OUTCOME trial was the only clinical trial to demonstrate a reduction in both cardiovascular and all-cause mortality. This effect was likely related to the difference in the included population; EMPA-REG OUTCOME included patients with established cardiovascular disease, while CANVAS and DECLARE TIMI 58 included both patients with established and at risk for, atherosclerotic cardiovascular disease (Table 1).
Table 1.
Heart failure outcomes across pivotal SGLT2 inhibitor trials.
| Trial/year of publication | Number of participants | Participants | Duration | Heart failure hospitalization outcome |
|---|---|---|---|---|
| EMPA-REG (2015) | 7020 |
|
3.1 years | (HR 0.73, 95% CI: 0.61–0.88) |
| CANVAS programme (2017) | 10,142 | 1. Secondary prevention
|
188.2 weeks | (HR 0.67, 95% CI: 0.52–0.87) |
| DECLARE TIMI-58 (2019) | 17,160 |
|
4.2 years | (HR 0.73, 95% CI: 0.61–0.88) |
| DAPA-HF (2019) | 4,744 |
|
18.2 months | (HR 0.74, 95% CI: 0.65–0.85) |
SGLT2: sodium glucose co-transporter-2; DM: diabetes mellitus; eGFR: estimated glomerular filtration rate; DMRD: modification of diet in renal disease; BMI: body mass index; HTN: hypertension; LVEF: left ventricular ejection fraction; NYHA: New York Heart Association; NT-pro-BNP: N-terminal-pro brain natriuretic peptide; HF: heart failure; AF: atrial flutter; HR: hazard ratio; CI: confidence interval.
Multiple other observational studies and meta-analyses confirmed the role of SGLT2 inhibitors in improving cardiovascular outcomes in patients with type 2 diabetes. In the CVD-REAL (comparative effectiveness of cardiovascular outcomes in new users of sodium-glucose cotransporter-2 inhibitors), SGLT2 inhibitors were associated with a lower risk of HF hospitalization, all-cause death as well as the composite endpoint of heart failure hospitalization and death (HR 0.61, 0.49 and 0.54, respectively), an effect that persisted despite multiple sensitivity analyses [28].
A meta-analysis of 81 trials, including a total of 37,195 patients, showed lower risk of all-cause mortality (OR 0.72; 95% CI: 0.59–0.86), cardiovascular mortality (OR 0.67; 95% CI: 0.53–0.84), and heart failure (OR 0.67; 95% CI: 0.51–0.87) with SGLT2 inhibitors compared with placebo over a mean follow-up of 89 weeks [29]. A recent meta-analysis of the 3 major trials, EMPA-REG, CANVAS, and DECLARE TIMI 58, included a total of 34,322 patients with type 2 diabetes (11.3% with a history of HF), demonstrated an 11% reduction in major adverse cardiovascular events (defined as a composite of myocardial infarction, stroke, or cardiovascular mortality) (HR 0.89, 95% CI: 0.83–0.96), as well as a 23% reduction in the composite endpoint of cardiovascular mortality or hospitalization for HF (HR 0.77, 95% CI: 0.71–0.84) [30]. It is important to note that the favourable outcomes were mainly observed in patients with known atherosclerotic cardiovascular disease, but not in patients with risk factors only. The favourable effect of SGLT2 inhibitors in reducing heart failure hospitalization was more robust (relative risk reduction of 31%, HR 0.69, 95% CI: 0.61–0.79), and such effect was present across all patients regardless of the presence or absence of atherosclerotic cardiovascular disease or history of HF.
Collectively, there is a plethora of data supporting the beneficial role of SGLT2 inhibitors on cardiovascular outcomes, mainly driven by improvement in HF-related outcomes, in patients with type 2 diabetes.
SGLT2 inhibitors and heart failure outcomes in patients with HFrEF
It is important to note that the afore-mentioned trials included patients with type 2 diabetes, and only ∼10% of the observed cohort with a reported history of HF. However, none of these trials specified the HF phenotype. Despite the favourable role of SGLT2 inhibitors in reducing HF hospitalization in patients with type 2 diabetes in these trials, multiple questions remained unanswered. Whether this observed benefit extends to all HF patients (i.e. reduced and preserved ejection fraction), and to HF patients without diabetes remains unclear.
The first dedicated trial of SGLT2 inhibitors in patients with HFrEF, the DAPA-HF, randomized 4,744 patients with HF NYHA class II-IV and an ejection fraction ≤40%, with or without diabetes, to receive dapagliflozin versus placebo, in addition to optimal medical therapy for HF [26]. Dapagliflozin reduced the primary endpoint of worsening heart failure or cardiovascular mortality (HR: 0.74; 95% CI: 0.65–0.85), as well as the individual end points of worsening heart failure event (HR: 0.70; 95% CI: 0.59–0.83, NNT = 27) and cardiovascular mortality (HR: 0.82; 95% CI: 0.69–0.98) compared with placebo. This effect was independent of diabetes status and observed over the study period of 18.2 months (Table).
SGLT2 inhibitors and heart failure outcomes in patients with HFpEF
Diabetes is a major risk factor for HFpEF and present in up to 45% of those patients [31]. The aetiology of diabetic cardiomyopathy is multifactorial, including hyperinsulinemia, metabolic syndrome, endothelial dysfunction, and renal impairment. Besides adequate blood sugar control, multiple studies have shown a beneficial role of SGLT2 inhibitors on metabolic syndrome through weight reduction, as well as renal protection and modest blood pressure reduction [32–35]. While it is intuitive to observe better outcomes with SGLT2 inhibitors in patients with HFpEF, this remains unclear. Although data regarding left ventricular ejection fraction were lacking in many of the pivotal SGLT2 inhibitors trials, it is likely that a significant proportion of HF hospitalizations was related to HFpEF since these trials included many patients without baseline cardiovascular disease [24,25,36].
A sub-analysis of the CANVAS trial investigated the role of canagliflozin in different subtypes of HF. Due to the lack of information in regards to baseline left ventricular ejection fraction, the investigators examined the impact of canagliflozin on HF hospitalization in patients with HFrEF (n = 122), HFpEF (n = 101), and HF with unknown ejection fraction (n = 61) at the time of their first event. Interestingly, the relative risk reduction was clinically but not statistically significant in both HFrEF (HR: 0.69; 95% CI: 0.48–1.00) and HFpEF (HR: 0.83, 95% CI: 0.55–1.25). When the group of unknown ejection fraction was assumed to be HFrEF, the HR for HFrEF was 0.64 (95% CI: 0.48–0.86), and when it was assumed to be HFpEF, the HR for HFpEF was 0.71 (95% CI: 0.52–0.97). These results suggest the absence of a clear difference in the effect of canagliflozin on patients with HFrEF versus HFpEF [6,37].
SGLT2 inhibitors and heart failure outcomes in patients with renal dysfunction
The recently concluded CARMELINA (Effect of Linagliptin versus Placebo on Major Cardiovascular Events in Adults with Type 2 Diabetes and High Cardiovascular and Renal Risk) trial provided detailed insight into detrimental effects of renal dysfunction on HF hospitalization in diabetics with or without a prior history of HF. The risk of HF hospitalization increased with worsening renal function from 2.7-fold to 4.2-fold in the placebo arm of trial with a decline in eGFR from <60 ml/min/1.73 m2 to <30 ml/min/1.73 m2 [38]. In a meta-analysis of >38,000 participants, SGLT2 inhibitors, when compared with placebo, was not only associated with a decreased risk of acute kidney injury (HR: 0.76, 95% CI: 0.66–0.85), but also decreased the composite of substantial loss of renal function, end-stage renal disease, and mortality due to renal and cardiovascular disease (HR: 0.71, 95% CI: 0.63–0.81). All major trials of SGLT2 inhibitors have also studied HF hospitalizations in participants with eGFR > 30 ml/min/1.73 m2. Beneficial effects of HF hospitalizations have been reported across spectrum in patients with renal disease [39]. Another meta-analysis of the 3 pivotal trials showed SGLT2 inhibitors decrease HF hospitalization across all subgroups eGFR < 60 ml/min/1.73 m2 (HR: 0.67, 95% CI: 0.51–0.89), 60–90 ml/min/1.73 m2 (HR: 0.56, 95% CI: 0.46–0.70), and >90 ml/min/1.73 m2 (HR: 0.44, 95% CI: 0.32–0.59) when compared with placebo [30]. Furthermore, the CREDENCE (Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation) trial enrolled individuals at different stages of renal function (>50% participants with eGFR <60 ml/in/1.73 m2). Notably, there was a significant decrease in HF hospitalization (secondary endpoint) in canagliflozin treated participants when compared with placebo (HR: 0.61, 95% CI: 0.47–0.80) [40].
SGLT2 inhibitors and heart failure outcomes in patients without diabetes
The benefits of SGLT2 inhibitors in HF have been established in the prior mentioned studies amongst diabetics. Additional experimental studies have shown a cardioprotective effect of SGLT2 inhibitors in non-diabetic mice. Non-diabetic mice with reduced left ventricular systolic function had preservation from further decline in systolic function after treatment with empagliflozin [41]. The recently published, DAPA-HF trial, included >50% non-diabetic participants (2605 out of 4744 total participants). A subgroup analysis of the non-diabetic participants showed a significant decrease in HF hospitalization after treatment with dapagliflozin when compared with placebo (HR 0.73, 95% CI: 0.60–0.88) [26]. The reduction in HF hospitalizations was independent of glucose control in patients with diabetes.
Potential mechanisms of SGLT2 inhibitors in improving outcomes among patients with HF
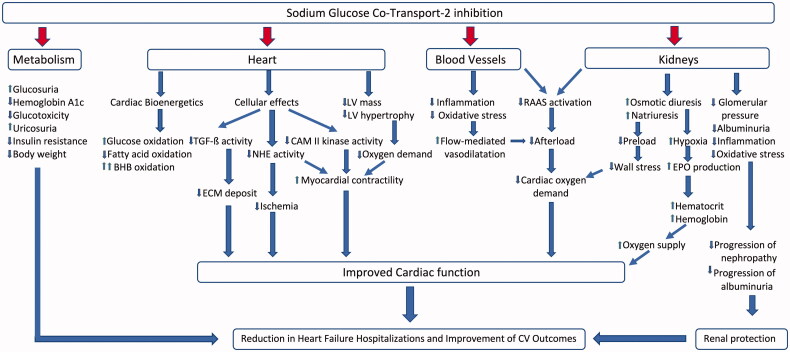
The early and substantial cardiovascular and renal benefits observed with SGLT2 inhibitors in the pivotal trials are unlikely to be solely explained by the favourable effects on haemoglobin HbA1c. Numerous other glucose-independent mechanisms have been under investigation (Figure 1). In this section, we discuss the literature about these proposed mechanisms.
Figure 1.
Mechanism of action of sodium glucose co-transport-2 inhibitors TGF-ß: transforming growth factor-ß; ECM: extracellular matrix; NHE: sodium-proton exchanger; CAM: calcium-calmodulin-dependent kinase II; LV: left ventricular; RAAS: renin–angiotensin–aldosterone system; EPO: erythropoietin.
(A) Effect of SGLT2 inhibitors on plasma volume
The glucosuria induced by SGLT2 inhibitors results in a significant osmotic diuresis and natriuresis with subsequent reduction in plasma volume. A mediation analysis by Inzucchi and colleagues suggested that the markers for plasma volume were the most important mediators for cardiovascular effects of SGLT2 inhibitors [42]. Hallow and colleagues, based on an experimental model, proposed a differential volume hypothesis. Osmotic diuresis with a greater electrolyte-free water clearance leads to increased removal of fluid from the interstitial space rather than from the circulation. This allows greater relief of congestion without significant compromise in arterial filling, perfusion, or excessive activation of the renin–angiotensin–aldosterone-system (RAAS) compared with diuretics [43]. The resultant reduction in preload lowers the ventricular filling pressures. The EMPA-VISION trial assessed left ventricular end-diastolic volume (LVEDV) with cardiac magnetic resonance imaging (CMR) after 6 months of treatment with empagliflozin, and demonstrated a significant reduction in the LVEDV [44].
(B) Effect of SGLT2 inhibitors on metabolic syndrome
Studies showed that there was a 2-fold increase in risk of HF in adults with metabolic syndrome. The risk rises with concomitant increase in the number of components of metabolic syndrome [45]. For example, in the Uppsala Longitudinal Study of Adult Men, the incidence of HF was 3-folds higher in those with metabolic syndrome. This remained significant after adjusting for traditional risk factors for HF [46]. The prevalence of metabolic syndrome in HF patients has been reported at 40% and 68.3% in two different studies [47,48]. Hassan et al. reported a decrease in mortality in HF patients with metabolic syndrome. There was a non-linear decrease in mortality with an increase in the number of components of metabolic syndrome [47]. In a prospective study, there was 24% mortality in participants with HF and metabolic syndrome and 16% in participants with HF without metabolic syndrome at 2.6 years of follow-up [48]. Multiple studies, including pivotal trials, have shown that SGLT2 inhibitors modify various components of the metabolic syndrome including a decrease in systolic blood pressure, decrease in blood sugar, decrease in insulin resistance, decrease in weight and waist circumference, as well as reduced plasma HDL and triglyceride levels [24,25,27,49–52].
(C) Effect of SGLT2 inhibitors on vascular function and blood pressure
SGLT2 inhibitors have shown a modest reduction in systolic blood pressure (4–6 mm Hg), diastolic blood pressure (1–2 mm Hg), mean arterial pressure, and ambulatory blood pressure with improved hemodynamics [53]. This effect not only decreases cardiac work-output but also increases perfusion of kidneys with improved diuresis [51,52]. This effect was observed in both blood pressure dippers as well as non-dippers [50]. Several plausible mechanisms exist behind the favourable effect of SGLT2 inhibitors on blood pressure. The RAAS, an important component of the neurohumoral pathway behind blood pressure regulation as well as HF, appears to be relatively less activated with SGLT2 inhibitors, owing to its natriuretic effect and increased delivery of sodium to distal tubular cells, with subsequent inhibition of renin and angiotensin II release [54,55].
In a recent study, dapagliflozin led to higher electrolyte-free water clearance when compared to bumetanide. Additionally, it resulted in 3-times higher interstitial fluid volume decrease relative to blood volume while bumetanide was associated with only a 66% reduction in interstitial fluid volume relative to blood volume. Renin levels increased with bumetanide, but not with dapagliflozin [43].
The SGLT2 inhibitors appear to play a role at the level of vascular endothelium. In a study by Bosch et al., empagliflozin was associated with lowering of inflammatory markers and improvement in arterial stiffness, resulting in lower central systolic pressure and pulse pressure [56]. In another study, dapagliflozin showed a significant improvement in flow-mediated vasodilation suggestive of improved endothelial function likely from reduced oxidative stress. Flow-mediated vasodilatation occurred independently of improvement in blood pressure in the treated individuals [57].
(D) Effects of SGLT2 inhibitors at the myocardial cellular level
Reduction in left ventricular mass
Left ventricular hypertrophy is seen in up to 70% of diabetic patients and is associated with higher rates of cardiovascular mortality and events [58–60]. The SGLT2 inhibitors can potentially decrease left ventricular hypertrophy by multiple effects i.e. decrease in blood pressure, lesser activation of RAS system, natriuresis and diuresis, and weight loss. In a proof-of-concept randomized, double-blinded, placebo-controlled study, 66 patients with diabetes and left ventricular hypertrophy were treated with dapagliflozin for 10–12 months. Left ventricular mass was measured by CMR. There was a significant decrease in absolute left ventricular mass by −2.82 g (95% CI: −5.13 to −0.51, p = .018) compared with placebo. The mean change in left ventricular mass was higher −3.88 g (95% CI: −7.33 to 0.43, p = .029) when the baseline left ventricular mass index was above the median [61]. In a non-diabetic animal (porcine) model, empagliflozin resulted in lower left ventricular mass when compared with control animals after ligation of the left anterior descending artery to induce HF [62].
Improvement in cardiomyocyte function
Multiple plausible mechanisms by which SGLT2 inhibitors improve the cardiomyocyte function exist. Under normal circumstances, cardiomyocytes utilize free fatty acids and glucose for energy production, however in diabetics, the utilization of glucose is markedly reduced, and free fatty acids become the major fuel for the mitochondrial oxidative metabolism. These changes are associated with reduced cardiac function and an increase in oxidative stress [63]. By increasing the production and reduction of ketone bodies excretion, SGLT2 inhibitors offer a more efficient fuel for the cardiomyocytes, while reducing the oxidative stress, resulting in an improvement in cardiac function. This effect was shown in an animal study, where empagliflozin was associated with an increase in plasma ketone levels and potential improvement in the bioenergetics of cardiac metabolism [64].
Another target for SGLT2 inhibitors is the activity of Na+-H+ Exchanger-1 (NHE1). NHE1 is upregulated in patients with HF and cardiac hypertrophy and is associated with an increase in the intracellular Na+. This stimulates the efflux of Ca2+ out of the mitochondria, with a subsequent decrease in mitochondrial Ca2+ and impairment of cardiomyocyte function [65]. In an animal (rabbit) model, inhibition of NHE1 by a selective NHE1 inhibitor (cariporide) led to reversal of hypertrophy and signs of HF along with ionic and electrophysiologic remodelling [66]. Empagliflozin inhibits the efflux of mitochondrial calcium resulting in a downstream increase in mitochondrial calcium which can potentially explain the benefits in HF patients [67]. The cardioprotective effect of empagliflozin-associated NHE1 inhibition is believed to be present during ischaemia as well (68].
A third potential mechanism of improved cardiomyocyte function with SGLT2 inhibitors was illustrated in a study by Juni et al. [69]. As the cardiomyocyte dysfunction in patients with HFpEF is believed to be initiated by cardiac microvascular endothelial cell (CMEC) dysfunction, the study aimed to examine the role of empagliflozin in the reversal of the CMEC dysfunction and restoration of cardiomyocyte function [70]. When CMEC were incubated with the inflammatory mediator tumour necrosis factor-a (TNF-a) in adult rats, deterioration of cardiomyocyte contractility and relaxation occurred, an effect that was reversed by empagliflozin through reducing the production and accumulation of reactive oxygen species, resulting in an increase in endothelial nitric oxide bioavailability and restoration of cardiomyocyte contractility and relaxation. The investigators concluded that the improvement in HF outcomes with empagliflozin in the EMPA-REG OUTCOME trial could, at least in part, be related to the improvement in the cardiac mechanical implications of microvascular dysfunction and that SGLT2 inhibitors may represent a novel therapy of HF especially in patients with HFpEF.
Overexpression and activation of calcium-calmodulin-dependent kinase II are associated with HF [71–73]. In an experimental study with HF animal model and failing human myocytes, exposure to empagliflozin led to a significant decrease in the activity of calcium-calmodulin-dependent kinase II and calcium-calmodulin-kinase II-dependent phosphorylation of ryanodine receptors [74]. Additionally, in failing human myocytes, empagliflozin reduced the leak of calcium from cytoplasmic reticulum and increased cytoplasmic calcium transient load. Some of the effects of SGLT2 inhibitors in HF may be secondary to improvement in the contractile function from increased load of calcium in the sarcoplasmic reticulum. Assessment of ejection fraction on SGLT2 inhibitors may provide further insight into this potential mechanism.
Finally, the effect of SGLT2 inhibitors on glucagon may play a role. Glucagon has been shown to have direct effects on myocardial contractility in experimental human and animal studies [75,76]. Dapagliflozin led to increased glucagon levels in study participants through inhibition of SGLT2 receptors [77].
Inhibition of myocardial fibrosis
Few studies have suggested a role for SGLT2 inhibitors in preventing myocardial fibrosis, which represents an advanced stage of cardiac remodelling in HF patients. Cardiac fibroblasts were isolated by culture from human atrial tissue during cardiac surgery. Effects of empagliflozin were studied on myofibroblast activity and gene expression. Empagliflozin decreases activation of cardiac myofibroblasts by decreasing transforming growth factor ß-1 leading to myofibroblasts with smaller size and fewer extension. It also modulates cell-mediated extracellular matrix remodelling and modifies expression for multiple profibrotic genes which may be responsible for fibrosis [78].
Another explored mechanism was the effect of SGLT2 inhibitors on the levels of brain natriuretic peptide (BNP). BNP have diuretic, natriuretic and antihypertensive effects. It also has a protective effect against cardiac remodelling and cardiac fibrosis in HF [79]. The use of canagliflozin was associated with a significant decrease in NT-pro-BNP from baseline when compared with placebo in a small trial [80]. In a second study by Nassif and colleagues, this change was not significant from baseline with dapagliflozin versus placebo, however, more participants in the dapagliflozin arm showed a clinically significant change (>20%) from baseline compared with placebo [81].
(E) Effect of SGLT2 inhibitors on uric acid level
Hyperuricaemia is a prevalent finding in patients admitted with HF, and elevated uric acid is associated with increased mortality [82,83]. The exact mechanism of the contribution of uric acid to increased mortality is not well established. The addition of oxypurinol to standard medical therapy in patients with HFrEF was associated with improvement in ejection fraction when compared with placebo [84]. In a normal healthy subject, there was a synergistic effect on diuresis with bumetanide and dapagliflozin with a 20% reduction in serum urate levels as compared with bumetanide alone (4% increase), but less than dapagliflozin alone (40% decrease) [85]. More recently, a mediation analysis reported serum urate level as a significant mediator with an initial beneficial impact on cardiovascular outcomes in canagliflozin treated individuals [86].
(F) Effect of SGLT2 inhibitors on haemoglobin and haematocrit levels
A recent analysis identified multiple mediators which play role in HF with the major effects being on erythrocyte concentration, haemoglobin concentration, and serum urate [86]. Pivotal trials and multiple small studies have shown improvement in haematocrit from osmotic diuresis. Additionally, erythropoietin levels increase within 2–4 weeks after initiation of SGLT2 inhibitor therapy followed by a rise in reticulocyte count, haemoglobin, and haematocrit. Sano and colleagues reported that upregulated glucose uptake in proximal tubules leads to tissue hypoxia with impaired erythropoietin production. Inhibition of glucose uptake by SGLT2 inhibitors reduces oxygen requirement and offsets tubulointerstitial hypoxia with improvement in erythropoietin production [87].
(G) Effect of SGLT2 inhibitors on renal function
HF can lead to renal dysfunction through increased renal venous pressure and poor perfusion from decreased cardiac output. Conversely, chronic renal disease can lead to worsening HF through increased sodium and water retention, inflammation, activation of RAAS, and accelerated atherosclerosis [88]. Pivotal trials of SGLT2 inhibitors and Canagliflozin on Renal and Cardiovascular Outcomes in Participants with Diabetic Nephropathy (CREDENCE) have shown reno-protective effects [40]. Multiple studies have reported different protective mechanisms through hemodynamic modification and metabolic effects. Use of SGLT2 inhibitors results in modest improvement in A1c levels (0.6–1.0%), systolic blood pressure 2–4 mm Hg, and diastolic blood pressure 1–2 mm Hg. These systemic effects may provide some renal protection with the use of SGLT2 inhibitors.
Osmotic diuresis and natriuresis with high tubular fluid sodium lead to inhibition of the juxtaglomerular apparatus with a modest increase in renin secretion. This, in turn, produces relatively lower aldosterone levels and vasoconstriction of afferent and dilation of efferent arterioles causing a decrease in intraglomerular pressure or hydrostatic pressure. All these hemodynamic changes in glomeruli are reno-protective. Preservation of renal function with SGLT2 inhibitors potentially avoids issues of volume overload and diuretic resistance in HF patients especially those with cardiorenal syndrome.
In diabetics, absorption of glucose and sodium is upregulated through SGLT2 with decreased delivery to macula densa cells. This leads to decreased secretion of adenosine causing vasodilatation of afferent arteriole. This increased glomerular pressure causes worsening of renal function. The use of SGLT2 inhibitors restores the distal sodium delivery and increased production of adenosine leading to afferent vasoconstriction and decreased intraglomerular pressure.
Intraglomerular hemodynamic changes with SGLT2 inhibitors result in decreased albuminuria by 30–50% within a few weeks. Furthermore, albuminuria partially returns towards pre-treatment levels within 2–3 weeks of cessation of SGLT2 inhibitor therapy suggesting renal protective effect through hemodynamic modifications rather than alternate mechanisms.
Modification of inflammatory pathways may be responsible for the additional protective role of SGLT2 inhibitors. In experimental studies, diabetes is associated with tissue hypoxia and oxidative stress from the increased energy requirement for sodium-glucose uptake in diabetes. SGLT2 inhibitors reduce oxidative stress by decreasing the absorption of sodium and glucose. They concomitantly improve oxygen delivery and reduce hypoxic insult to kidneys by increasing haematocrit, improving vascular endothelial function, and preserving cardiac systolic function.
Safety of SGLT2 inhibitors
Diabetic ketoacidosis
Despite the metabolic and HF hospitalization benefits, there have been some concerns about the safety of SGLT2 inhibitors. The risk of hypoglycaemia, volume depletion, acute kidney injury, thromboembolic events, and bone fractures were similar in both SGLT2 inhibitors and placebo groups across different trials. There was an increase, even though very small, in the risk of diabetic ketoacidosis with the use of SGLT2 inhibitors versus placebo. The proportional reporting ratio of diabetic ketoacidosis was higher in SGLT2 inhibitors users than non-users (7.9, 95% CI: 7.4–8.5) in the FDA Adverse Event Reporting System. The risk was even higher with off label use in type 1 diabetes mellitus [89]. Multiple mechanisms, including a decrease in the dose of insulin with concern for hypoglycaemia with concurrent use of SGLT2 inhibitors, increased production of ketone bodies, increased renal reabsorption of ketones from urine, reduced secretion of insulin by inhibition of alpha cells by directly by SGLT2, can potentially increase the risk. The FDA has issued a warning with the use of SGLT2 inhibitors and instructions to consider predisposing conditions prior to initiating SGLT2 inhibitors, carefully assess for ketoacidosis with euglycaemia in the presence of symptoms of ketoacidosis, and temporarily discontinue SGLT2 inhibitors in prolonged fasting due to surgery or acute illness [90].
Genital and urinary tract infections
Genital infections, including balanitis and phimosis, are more frequent with the use of SGLT2 inhibitors than placebo. But, the risk of serious infections leading to discontinuation of medication was low in the DECLARE TIMI-58 trial. Excretion of glucose with urine provides a favourable environment for bacterial and fungal growth and infection. Urosepsis was higher in empagliflozin group (0.4% versus 0.1%) when compared with placebo. But rates for urinary tract infection, complicated urinary tract infections, and pyelonephritis were similar in both study arms.
Amputation
The CANVAS programme reported an increased incidence of toe, foot, and leg amputations in the canagliflozin group (6.3 versus 3.4 participants per 1000-participant years, HR 1.97, 95% CI: 1.41–2.75). The risk of amputation was higher in patients with an underlying diagnosis of peripheral arterial disease or prior history of amputations . The risk of fractures was also higher in the canagliflozin group than placebo (15.4 versus 11.9 participants per 1000-participant years, HR 1.26, 95% CI: 1.04–1.52) with similar trends for low-trauma fractures. A subgroup analysis for amputations in EMPA-REG trial did not show an increased risk between treatment and placebo groups [91]. Furthermore, there was no difference in limb amputations in DECLARE-TIMI or DAPA-HF trials between treatment and placebo arms. The exact mechanism of these adverse events is not clear. There is a possibility that dehydration associated with diarrhoea (from SGLT1 inhibition in the gut) may be an underlying mechanism. Analysis of amputations in SGLT2 inhibitors users from the FDA Adverse Event Reporting System found canagliflozin use was associated with a higher risk of amputations than empagliflozin or dapagliflozin [92]. Even though a causal relationship between drug use and amputation cannot be confirmed, the FDA has issued a black box warning for increased amputation risk with the use of canagliflozin without any specific prescription guide [93].
Ongoing trials and future directions
The efficacy and safety of SGLT2 inhibitors have been established in diabetics as well as in HF participants with reduced ejection fraction in large randomized, multicenter trials. The benefits are observed in both diabetic and non-diabetic individuals. Even though both clinical and preclinical studies have demonstrated a benefit with SGLT2 inhibitors use in participants with left ventricular hypertrophy, their role in HFpEF remains to be established. A subgroup analysis of EMPA-REG OUTCOME trial based on 10-point TIMI (Thrombolysis in Myocardial Infarction) Risk for Secondary Prevention showed a reduction in HF hospitalization across different risk groups [94]. Studies are needed to establish the role of SGLT2 inhibitors in non-diabetic patients with asymptomatic left ventricular systolic dysfunction. Both add-on and single therapy of SGLT2 inhibitors showed a reduction in HF hospitalizations, but there were a limited number of patients treated with glucagon-related peptide receptor-1 agonists. Studies with the combined use of SGLT2 inhibitors with glucagon-related peptide receptor-1 agonists would provide insight if there is an additional benefit or any synergistic effects. There were ∼10% of patients on angiotensin-receptor neprilysin inhibitor therapy in the DAPA-HF trial. A subgroup analysis can shed light on outcomes associated with the combined use of these medications.
Conclusion
Sodium glucose cotransporter-2 inhibitors are a great addition to currently available medical therapies for diabetic patients with or at risk for HF. They also provide multisystem benefit through better control of diabetes, renal protection, and all-cause mortality.
Glossary
Abbreviations
- HF
heart failure
- SGLT
sodium glucose co-transporter
Disclosure statement
Authors have no conflict of interest to report.
References
- 1.Benjamin EJ, Virani SS, Callaway CW, et al. ; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation. 2018;137(12):e67–e492. [DOI] [PubMed] [Google Scholar]
- 2.Yusuf S, Pfeffer MA, Swedberg K, et al. ; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362(9386):777–781. [DOI] [PubMed] [Google Scholar]
- 3.McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993–1004. [DOI] [PubMed] [Google Scholar]
- 4.Packer M, Fowler MB, Roecker EB, et al. Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation. 2002;106(17):2194–2199. [DOI] [PubMed] [Google Scholar]
- 5.Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341(10):709–717. [DOI] [PubMed] [Google Scholar]
- 6.Lam CSP, Chandramouli C, Ahooja V, et al. SGLT-2 inhibitors in heart failure: current management, unmet needs, and therapeutic prospects. J Am Heart Assoc. 2019;8(20):e013389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Savarese G, Lund LH. Global public health burden of heart failure. Card Fail Rev. 2017;3(1):7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bahrami H, Bluemke DA, Kronmal R, et al. Novel metabolic risk factors for incident heart failure and their relationship with obesity: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;51(18):1775–1783. [DOI] [PubMed] [Google Scholar]
- 9.Gottdiener JS, Arnold AM, Aurigemma GP, et al. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000;35(6):1628–1637. [DOI] [PubMed] [Google Scholar]
- 10.Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study. JAMA. 1979;241(19):2035–2038. [DOI] [PubMed] [Google Scholar]
- 11.van Melle JP, Bot M, de Jonge P, et al. Diabetes, glycemic control, and new-onset heart failure in patients with stable coronary artery disease: data from the heart and soul study. Diabetes Care. 2010;33(9):2084–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shindler DM, Kostis JB, Yusuf S, et al. Diabetes mellitus, a predictor of morbidity and mortality in the Studies of Left Ventricular Dysfunction (SOLVD) Trials and Registry. Am J Cardiol. 1996;77(11):1017–1020. [DOI] [PubMed] [Google Scholar]
- 13.Iribarren C, Karter AJ, Go AS, et al. Glycemic control and heart failure among adult patients with diabetes. Circulation. 2001;103(22):2668–2673. [DOI] [PubMed] [Google Scholar]
- 14.Matsushita K, Blecker S, Pazin-Filho A, et al. The association of hemoglobin a1c with incident heart failure among people without diabetes: the atherosclerosis risk in communities study. Diabetes. 2010;59(8):2020–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pazin-Filho A, Kottgen A, Bertoni AG, et al. HbA 1c as a risk factor for heart failure in persons with diabetes: the Atherosclerosis Risk in Communities (ARIC) study. Diabetologia. 2008;51(12):2197–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castagno D, Baird-Gunning J, Jhund PS, et al. Intensive glycemic control has no impact on the risk of heart failure in type 2 diabetic patients: evidence from a 37,229 patient meta-analysis. Am Heart J. 2011;162(5):938–948 e2. [DOI] [PubMed] [Google Scholar]
- 18.Crane RK. Intestinal absorption of sugars. Physiol Rev. 1960;40:789–825. [DOI] [PubMed] [Google Scholar]
- 19.Hummel CS, Lu C, Loo DD, et al. Glucose transport by human renal Na+/D-glucose cotransporters SGLT1 and SGLT2. Am J Physiol, Cell Physiol. 2011;300(1):C14–C21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rahmoune H, Thompson PW, Ward JM, et al. Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non-insulin-dependent diabetes. Diabetes. 2005;54(12):3427–3434. [DOI] [PubMed] [Google Scholar]
- 21.Peterson C. Analyse des Phloridzins . Ann Acad Sci Fr. 1835;15:178. [Google Scholar]
- 22.Oku A, Ueta K, Arakawa K, et al. T-1095, an inhibitor of renal Na+-glucose cotransporters, may provide a novel approach to treating diabetes. Diabetes. 1999;48(9):1794–1800. [DOI] [PubMed] [Google Scholar]
- 23.Meng W, Ellsworth BA, Nirschl AA, et al. Discovery of dapagliflozin: a potent, selective renal sodium-dependent glucose cotransporter 2 (SGLT2) inhibitor for the treatment of type 2 diabetes. J Med Chem. 2008;51(5):1145–1149. [DOI] [PubMed] [Google Scholar]
- 24.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–657. [DOI] [PubMed] [Google Scholar]
- 25.Zinman B, Wanner C, Lachin JM, et al. ; EMPA-REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128. [DOI] [PubMed] [Google Scholar]
- 26.McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995–2008. [DOI] [PubMed] [Google Scholar]
- 27.Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–357. [DOI] [PubMed] [Google Scholar]
- 28.Kosiborod M, Cavender MA, Fu AZ, et al. Lower risk of heart failure and death in patients initiated on sodium-glucose cotransporter-2 inhibitors versus other glucose-lowering drugs: the CVD-REAL Study (Comparative Effectiveness of Cardiovascular Outcomes in New Users of Sodium-Glucose Cotransporter-2 Inhibitors). Circulation. 2017;136(3):249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saad M, Mahmoud AN, Elgendy IY, et al. Cardiovascular outcomes with sodium-glucose cotransporter-2 inhibitors in patients with type II diabetes mellitus: a meta-analysis of placebo-controlled randomized trials. Int J Cardiol. 2017;228:352–358. [DOI] [PubMed] [Google Scholar]
- 30.Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393(10166):31–39. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka H, Hirata KI. Potential impact of SGLT2 inhibitors on left ventricular diastolic function in patients with diabetes mellitus. Heart Fail Rev. 2018;23(3):439–444. [DOI] [PubMed] [Google Scholar]
- 32.Ronco C, McCullough P, Anker SD, et al. ; Acute Dialysis Quality Initiative (ADQI) Consensus Group . Cardio-renal syndromes: report from the consensus conference of the acute dialysis quality initiative. Eur Heart J. 2010;31(6):703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosenstock J, Jelaska A, Frappin G, et al. Improved glucose control with weight loss, lower insulin doses, and no increased hypoglycemia with empagliflozin added to titrated multiple daily injections of insulin in obese inadequately controlled type 2 diabetes. Dia Care. 2014;37(7):1815–1823. [DOI] [PubMed] [Google Scholar]
- 34.Wanner C, Inzucchi SE, Zinman B. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(18):1801–1802. [DOI] [PubMed] [Google Scholar]
- 35.Elgendy IY, Pepine CJ. Heart failure with preserved ejection fraction: is ischemia due to coronary microvascular dysfunction a mechanistic factor? Am J Med. 2019;132(6):692–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elgendy IY, Mahtta D, Pepine CJ. Medical therapy for heart failure caused by ischemic heart disease. Circ Res. 2019;124(11):1520–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Figtree GA, Radholm K, Barrett TD, et al. Effects of canagliflozin on heart failure outcomes associated with preserved and reduced ejection fraction in type 2 diabetes mellitus. Circulation. 2019;139(22):2591–2593. [DOI] [PubMed] [Google Scholar]
- 38.Rosenstock J, Perkovic V, Johansen OE, et al. ; for the CARMELINA Investigators . Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA Randomized Clinical Trial. JAMA. 2019;321(1):69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neuen BL, Young T, Heerspink HJL, et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2019;7(11):845–854. [DOI] [PubMed] [Google Scholar]
- 40.Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–2306. [DOI] [PubMed] [Google Scholar]
- 41.Byrne NJ, Parajuli N, Levasseur JL, et al. Empagliflozin prevents worsening of cardiac function in an experimental model of pressure overload-induced heart failure. JACC Basic Transl Sci. 2017;2(4):347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Inzucchi SE, Zinman B, Fitchett D, et al. How does empagliflozin reduce cardiovascular mortality? Insights from a mediation analysis of the EMPA-REG OUTCOME Trial. Diabetes Care. 2018;41(2):356–363. [DOI] [PubMed] [Google Scholar]
- 43.Hallow KM, Helmlinger G, Greasley PJ, et al. Why do SGLT2 inhibitors reduce heart failure hospitalization? A differential volume regulation hypothesis. Diabetes Obes Metab. 2018;20(3):479–487. [DOI] [PubMed] [Google Scholar]
- 44.Cohen ND, Gutman SJ, Briganti EM, et al. Effects of empagliflozin treatment on cardiac function and structure in patients with type 2 diabetes: a cardiac magnetic resonance study. Intern Med J. 2019;49(8):1006–1010. [DOI] [PubMed] [Google Scholar]
- 45.Li C, Ford ES, McGuire LC, et al. Association of metabolic syndrome and insulin resistance with congestive heart failure: findings from the Third National Health and Nutrition Examination Survey. J Epidemiol Community Health. 2007;61(1):67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ingelsson E, Arnlov J, Lind L, et al. Metabolic syndrome and risk for heart failure in middle-aged men. Heart. 2006;92(10):1409–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hassan SA, Deswal A, Bozkurt B, et al. The metabolic syndrome and mortality in an ethnically diverse heart failure population. J Card Fail. 2008;14(7):590–595. [DOI] [PubMed] [Google Scholar]
- 48.Tamariz L, Hassan B, Palacio A, et al. Metabolic syndrome increases mortality in heart failure. Clin Cardiol. 2009;32(6):327–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kashiwagi A, Sakatani T, Nakamura I, et al. Improved cardiometabolic risk factors in Japanese patients with type 2 diabetes treated with ipragliflozin: a pooled analysis of six randomized, placebo-controlled trials. Endocr J. 2018;65(7):693–705. [DOI] [PubMed] [Google Scholar]
- 50.Chilton R, Tikkanen I, Hehnke U, et al. Impact of empagliflozin on blood pressure in dipper and non-dipper patients with type 2 diabetes mellitus and hypertension. Diabetes Obes Metab. 2017;19(11):1620–1624. [DOI] [PubMed] [Google Scholar]
- 51.Ott C, Jumar A, Striepe K, et al. A randomised study of the impact of the SGLT2 inhibitor dapagliflozin on microvascular and macrovascular circulation. Cardiovasc Diabetol. 2017;16(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chilton R, Tikkanen I, Cannon CP, et al. Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes Obes Metab. 2015;17(12):1180–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Striepe K, Jumar A, Ott C, et al. Effects of the selective sodium-glucose cotransporter 2 inhibitor empagliflozin on vascular function and central hemodynamics in patients with type 2 diabetes mellitus. Circulation. 2017;136(12):1167–1169. [DOI] [PubMed] [Google Scholar]
- 54.Burke M, Pabbidi MR, Farley J, et al. Molecular mechanisms of renal blood flow autoregulation. Curr Vasc Pharmacol. 2014;12(6):845–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cherney DZ, Perkins BA. Sodium-glucose cotransporter 2 inhibition in type 1 diabetes: simultaneous glucose lowering and renal protection? Can J Diabetes. 2014;38(5):356–363. [DOI] [PubMed] [Google Scholar]
- 56.Bosch A, Ott C, Jung S, et al. How does empagliflozin improve arterial stiffness in patients with type 2 diabetes mellitus? Sub analysis of a clinical trial. Cardiovasc Diabetol. 2019;18(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Solini A, Giannini L, Seghieri M, et al. Dapagliflozin acutely improves endothelial dysfunction, reduces aortic stiffness and renal resistive index in type 2 diabetic patients: a pilot study. Cardiovasc Diabetol. 2017;16(1):138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cooper RS, Simmons BE, Castaner A, et al. Left ventricular hypertrophy is associated with worse survival independent of ventricular function and number of coronary arteries severely narrowed. Am J Cardiol. 1990;65(7):441–445. [DOI] [PubMed] [Google Scholar]
- 59.Dawson A, Morris AD, Struthers AD. The epidemiology of left ventricular hypertrophy in type 2 diabetes mellitus. Diabetologia. 2005;48(10):1971–1979. [DOI] [PubMed] [Google Scholar]
- 60.Liao Y, Cooper RS, McGee DL, et al. The relative effects of left ventricular hypertrophy, coronary artery disease, and ventricular dysfunction on survival among black adults. JAMA. 1995;273(20):1592–1597. [PubMed] [Google Scholar]
- 61.Brown AG, McCrimmon R, Struthers A, et al. A randomised controlled trial of dapagliflozin on left ventricular hypertrophy in patients with type two diabetes. The DAPA-LVH Trial; 2019;140:A10643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Santos-Gallego CG, Requena-Ibanez JA, San Antonio R, et al. Empagliflozin ameliorates adverse left ventricular remodeling in nondiabetic heart failure by enhancing myocardial energetics. J Am Coll Cardiol. 2019;73(15):1931–1944. [DOI] [PubMed] [Google Scholar]
- 63.Tamargo J. Corrigendum to: sodium-glucose cotransporter 2 inhibitors in heart failure: potential mechanisms of action, adverse effects and future developments . Eur Cardiol. 2019;14(3):201–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Verma S, Rawat S, Ho KL, et al. Empagliflozin increases cardiac energy production in diabetes: novel translational insights into the heart failure benefits of SGLT2 inhibitors. JACC Basic Transl Sci. 2018;3(5):575–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Packer M, Anker SD, Butler J, et al. Effects of sodium-glucose cotransporter 2 inhibitors for the treatment of patients with heart failure: proposal of a novel mechanism of action. JAMA Cardiol. 2017;2(9):1025–1029. [DOI] [PubMed] [Google Scholar]
- 66.Baartscheer A, Hardziyenka M, Schumacher CA, et al. Chronic inhibition of the Na+/H+ – exchanger causes regression of hypertrophy, heart failure, and ionic and electrophysiological remodelling. Br J Pharmacol. 2008;154(6):1266–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baartscheer A, Schumacher CA, Wust RC, et al. Empagliflozin decreases myocardial cytoplasmic Na + through inhibition of the cardiac Na+/H + exchanger in rats and rabbits . Diabetologia. 2017;60(3):568–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Uthman L, Nederlof R, Eerbeek O, et al. Delayed ischaemic contracture onset by empagliflozin associates with NHE1 inhibition and is dependent on insulin in isolated mouse hearts. Cardiovasc Res. 2019;115(10):1533–1545. [DOI] [PubMed] [Google Scholar]
- 69.Juni RP, Kuster DWD, Goebel M, et al. Cardiac microvascular endothelial enhancement of cardiomyocyte function is impaired by inflammation and restored by empagliflozin. JACC Basic Transl Sci. 2019;4(5):575–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62(4):263–271. [DOI] [PubMed] [Google Scholar]
- 71.Morita N, Lee JH, Bapat A, et al. Glycolytic inhibition causes spontaneous ventricular fibrillation in aged hearts. Am J Physiol Heart Circ Physiol. 2011;301(1):H180–H191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wagner S, Ruff HM, Weber SL, et al. Reactive oxygen species-activated Ca/calmodulin kinase IIδ is required for late I(Na) augmentation leading to cellular Na and Ca overload. Circ Res. 2011;108(5):555–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xie LH, Chen F, Karagueuzian HS, et al. Oxidative-stress-induced after depolarizations and calmodulin kinase II signaling. Circ Res. 2009;104(1):79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mustroph J, Wagemann O, Lucht CM, et al. Empagliflozin reduces Ca/calmodulin-dependent kinase II activity in isolated ventricular cardiomyocytes. ESC Heart Fail. 2018;5(4):642–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Farah AE. Glucagon and the circulation. Pharmacol Rev. 1983;35(3):181–217. [PubMed] [Google Scholar]
- 76.Goldstein RE, Skelton CL, Levey GS, et al. Effects of chronic heart failure on the capacity of glucagon to enhance contractility and adenyl cyclase activity of human papillary muscles. Circulation. 1971;44(4):638–648. [DOI] [PubMed] [Google Scholar]
- 77.Bonner C, Kerr-Conte J, Gmyr V, et al. Inhibition of the glucose transporter SGLT2 with dapagliflozin in pancreatic alpha cells triggers glucagon secretion. Nat Med. 2015;21(5):512–517. [DOI] [PubMed] [Google Scholar]
- 78.Kang S, Verma S, Hassanabad AF, et al. Direct effects of empagliflozin on extracellular matrix remodelling in human cardiac myofibroblasts: novel translational clues to explain EMPA-REG OUTCOME results. Can J Cardiol. 2019;36:543–553. [DOI] [PubMed] [Google Scholar]
- 79.Lin X, Hanze J, Heese F, et al. Gene expression of natriuretic peptide receptors in myocardial cells. Circ Res. 1995;77(4):750–758. [DOI] [PubMed] [Google Scholar]
- 80.Kario K, Hoshide S, Okawara Y, et al. Effect of canagliflozin on nocturnal home blood pressure in Japanese patients with type 2 diabetes mellitus: the SHIFT-J study. J Clin Hypertens. 2018;20(10):1527–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nassif ME, Windsor SL, Tang F, et al. Dapagliflozin effects on biomarkers, symptoms, and functional status in patients with heart failure with reduced ejection fraction: the DEFINE-HF Trial. Circulation. 2019;140(18):1463–1476. [DOI] [PubMed] [Google Scholar]
- 82.Thanassoulis G, Brophy JM, Richard H, et al. Gout, allopurinol use, and heart failure outcomes. Arch Intern Med. 2010;170(15):1358–1364. [DOI] [PubMed] [Google Scholar]
- 83.Huang H, Huang B, Li Y, et al. Uric acid and risk of heart failure: a systematic review and meta-analysis. Eur J Heart Fail. 2014;16(1):15–24. [DOI] [PubMed] [Google Scholar]
- 84.Farquharson CA, Butler R, Hill A, et al. Allopurinol improves endothelial dysfunction in chronic heart failure. Circulation. 2002;106(2):221–226. [DOI] [PubMed] [Google Scholar]
- 85.Wilcox CS, Shen W, Boulton DW, et al. Interaction between the sodium-glucose-linked transporter 2 inhibitor dapagliflozin and the loop diuretic bumetanide in normal human subjects. J Am Heart Assoc. 2018;7(4):e007046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li J, Woodward M, Perkovic V, et al. Mediators of the effects of canagliflozin on heart failure in patients with type 2 diabetes. JACC Heart Fail. 2020;8(1):57–66. [DOI] [PubMed] [Google Scholar]
- 87.Sano M, Takei M, Shiraishi Y, et al. Increased hematocrit during sodium-glucose cotransporter 2 inhibitor therapy indicates recovery of tubulointerstitial function in diabetic kidneys. J Clin Med Res. 2016;8(12):844–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mentz RJ, Kelly JP, von Lueder TG, et al. Noncardiac comorbidities in heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol. 2014;64(21):2281–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fadini GP, Bonora BM, Avogaro A. SGLT2 inhibitors and diabetic ketoacidosis: data from the FDA Adverse Event Reporting System. Diabetologia. 2017;60(8):1385–1389. [DOI] [PubMed] [Google Scholar]
- 90.FDA . FDA Drug Safety Communication: FDA revises labels of SGLT2 inhibitors for diabetes to include warnings about too much acid in the blood and serious urinary tract infections. 2018. [Google Scholar]
- 91.Inzucchi SE, Iliev H, Pfarr E, et al. Empagliflozin and assessment of lower-limb amputations in the EMPA-REG OUTCOME Trial. Diabetes Care. 2018;41(1):e4–e5. [DOI] [PubMed] [Google Scholar]
- 92.Fadini GP, Avogaro A. SGLT2 inhibitors and amputations in the US FDA Adverse Event Reporting System. Lancet Diabetes Endocrinol. 2017;5(9):680–681. [DOI] [PubMed] [Google Scholar]
- 93.FDADrug Safety Communication: FDA confirms increased risk of leg and foot amputations with the diabetes medicine canagliflozin [press release]. 2017. [Google Scholar]
- 94.Fitchett D, Inzucchi SE, Cannon CP, et al. Empagliflozin reduced mortality and hospitalization for heart failure across the spectrum of cardiovascular risk in the EMPA-REG OUTCOME Trial. Circulation. 2019;139(11):1384–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]