Abstract
Observational and interventional studies have unequivocally demonstrated that “present”, i.e. single-occasion, blood pressure is one of the key determinants of cardiovascular disease risk. Over the past two decades, however, numerous publications have suggested that longitudinal blood pressure data and assessment of long-term blood pressure exposure provide incremental prognostic value over present blood pressure. These studies have used several different indices to quantify the overall exposure to blood pressure, such as time-averaged blood pressure, cumulative blood pressure, blood pressure trajectory patterns, and age of hypertension onset. This review summarises existing research on the association between these indices and hard cardiovascular outcomes, outlines the strengths and weaknesses of these indices, and provides an overview of how longitudinal blood pressure changes can be measured and used to improve cardiovascular disease risk prediction.
KEY MESSAGES
Numerous recent publications have examined the relation between cardiovascular disease and long-term blood pressure (BP) exposure, quantified using indices such as time-averaged BP, cumulative BP, BP trajectory patterns, and age of hypertension onset.
This review summarises existing research on the association between these indices and hard cardiovascular outcomes, outlines the strengths and weaknesses of these indices, and provides an overview of how longitudinal BP changes can be measured and used to improve cardiovascular disease risk prediction.
Although longitudinal BP indices seem to predict cardiovascular outcomes better than present BP, there are considerable differences in the clinical feasibility of these indices along with a limited number of prospective data.
Keywords: Blood pressure, time-averaged blood pressure, cumulative blood pressure, blood pressure trajectories, age of hypertension onset, blood pressure exposure, risk factors, cardiovascular disease
Introduction
Already in the 1920s, the data collected by the Actuarial Society of America demonstrated that elevated blood pressure (BP) was associated with an increased risk of death from myocardial infarction or stroke in insurance applicants [1]. Thereafter, numerous observational and interventional studies have confirmed the role of elevated BP as a key factor underlying cardiovascular disease (CVD) [2,3]. In most of these prior studies, BP was quantified using relatively simple methods. Until the turn of the millennium, virtually all evidence on the harmful effects of hypertension was based on BP readings measured on a single occasion at the clinic using a mercury sphygmomanometer. BP quantification therefore lacked the granularity and precision needed to fully capture the CVD risk resulting from hypertension.
Over the past 20 years, numerous attempts have been made to improve the quality and quantity of data derived from BP measurements. One major advance has been the adoption of out-of-office BP measurements as the method of choice for diagnosing hypertension by virtually all major hypertension guidelines [4,5]. This relatively rapid transition from office to out-of-office measurements was based on the improved prognostic accuracy that home and ambulatory BP measurement offer over conventional office measurements due to a greater number of readings and the lack of the white coat effect [4,5]. A second major improvement in BP quantification has been the use of longitudinal BP data for CVD risk prediction in lieu of the conventionally used single BP measurements that provide only a single snapshot of BP in time.
In recent years, numerous publications have suggested that long-term BP data provide incremental prognostic value over the “present” BP level measured on a single occasion. Several indices have been developed to more effectively quantify BP experienced earlier in life or the changes in BP levels over time. The goal of this review is to summarise the existing research on this topic and to provide an understanding of how longitudinal BP changes can be measured and used for improving CVD risk prediction.
Normal lifetime BP progression
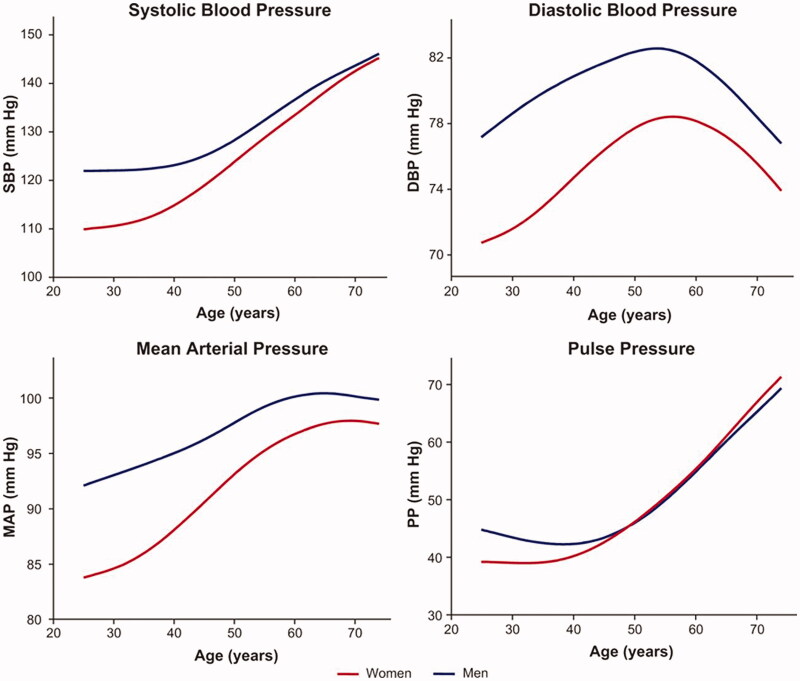
Although the progression of BP with increasing age is a well-known phenomenon, the patterns of BP progression vary by population [6–8]. Systolic blood pressure (SBP) and pulse pressure normally rise consistently over the whole lifecourse (Figure 1) [8]. In contrast, diastolic blood pressure (DBP) initially follows a pattern similar to SBP and pulse pressure but reaches an apex around the fifth decade whereafter it decreases. As a result of the changes in SBP and DBP, mean arterial pressure maintains a plateau level after midlife (Figure 1). The trends of BP progression are largely similar in both sexes. However, BP tends to be higher in young men, but this difference between the sexes gradually diminishes with age.
Figure 1.
Average lifetime progression of systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial blood pressure (MAP), and pulse pressure (PP) by age in participants of the Framingham Heart Study. From Cheng et al. Hypertension. 2012;60:1393–1399. Published with the permission of Wolters Kluwer Health.
The evidence on the hemodynamic correlates of lifetime BP progression is somewhat controversial. The age-related increase in arterial stiffness, commonly referred to as vascular aging, is believed to explain the majority of the BP changes during life. Increased arterial stiffness is mainly a result of decreases in elasticity and compliance of the large arteries, which in turn lead to reduced cardiac output adaptability and an increased pulse pressure [9,10]. Conversely, childhood BP has been demonstrated to predict increased arterial stiffness in adulthood, whereas arterial stiffness seems to predict BP at old age [11–13]. These findings indicate a two-way association between vascular remodelling, hemodynamic determinants, and BP. In contrast to arterial stiffening, factors such as vascular resistance and observed wave reflection are likely to be merely secondary factors in the observed BP increases over the life course [7,9,13].
The age-related increase in BP is observed in virtually all developed and developing countries [6,14,15]. Global population growth and aging have resulted in a shift of BP trends and an increased burden of elevated BP. Despite genetic factors being an important underlying cause of hypertension, evidence suggests that the majority of the changes in global BP trajectories are explained by lifestyle factors, such as diet, physical activity, and smoking [16–18]. In addition, some correlates of BP tracking, such as sex, race, weight gain, family history of hypertension, socioeconomic status, and birth weight, may be observed already in childhood [19–23]. However, a lack of strong evidence on the determinants of BP tracking still remains, as the previously mentioned factors have been shown to explain only a relatively small proportion of BP trajectories at the individual level [21,24].
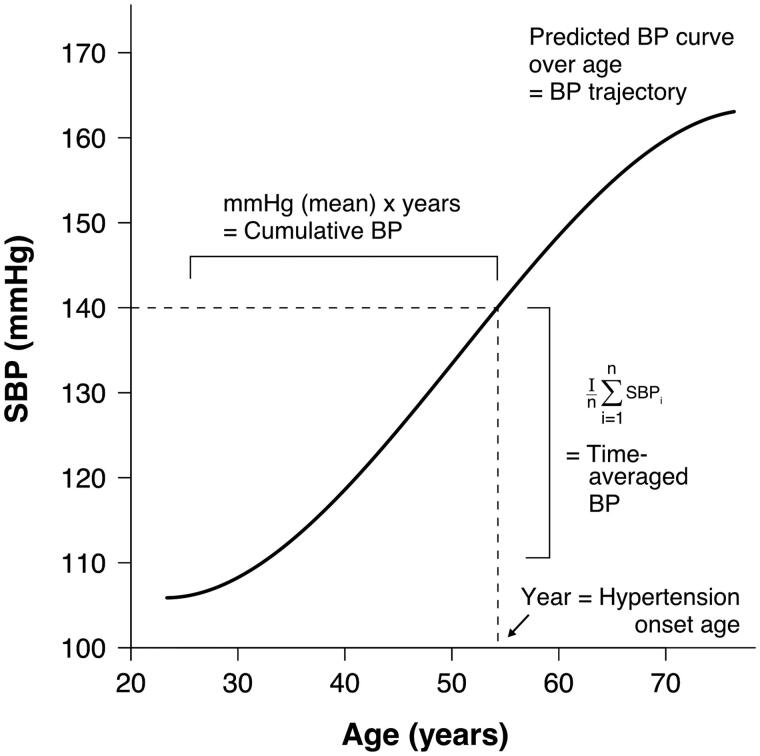
Although BP increases in most with aging, individual-level BP trajectories can vary considerably [25]. Differences in these trajectories also seem to associate with CVD risk, independent of the “present” BP [26]. Prior studies have made numerous attempts to improve risk prediction by examining the impact of long-term exposure to high BP, as compared with single-occasion BP measurements, on CVD risk [25,27–29]. These studies have employed several different indices to define the overall exposure to BP, such as time-averaged BP, cumulative BP, BP trajectory patterns, and age of hypertension onset (Figure 2). In principle, all these indices aim to assess the area under curve effect of BP over time using different approaches. We will next review the features of these indices and compare their associations with CVD outcomes.
Figure 2.
Graphic demonstration of different methods used for assessing overall long-term exposure to high blood pressure. SBP, systolic blood pressure; BP, blood pressure.
Time-averaged blood pressure
One simple index used for assessing long-term BP levels is time-averaged BP which is calculated by averaging BP measures taken over time. Already in 1991, Lauer et al. reported that an average of SBP readings over a 30-year period was superior in predicting left ventricular hypertrophy compared to a single SBP measurement, and several analogous studies with hard CVD outcomes have corroborated these results thereafter (Table 1) [28].
Table 1.
The association of time-averaged blood pressure with cardiovascular events.
| Exposure variable | Baseline age, yr | Estimate (95% CI) |
||||
|---|---|---|---|---|---|---|
| Study | N | Outcome | Systolic BP | Diastolic BP | ||
| Sasai et al. [30] | 46,484 | BP 5 years prior baseline | CVDa death |
62 | 1.11 (1.05–1.16) | 1.11 (1.06–1.15) |
| Baseline BP | 1.13 (1.07–1.18) | 1.12 (1.07–1.16) | ||||
| Time-averaged BP | 1.17 (1.10–1.24) | 1.17 (1.11–1.23) | ||||
| Vasan et al. [31] | 2,313 | Recent antecedent BP |
CVD eventb | 60 | W: 1.73 (1.13–2.64); M: 1.20 (0.81–1.79) | W: 2.03 (1.36–3.02); M: 1.19 (0.82–1.73) |
| 70 | W: 1.40 (1.09–1.81); M: 1.30 (0.98–1.74) | W: 1.33 (1.03–1.72); M: 1.18 (0.89–1.57) | ||||
| 80 | W: 2.16 (1.55–3.01); M: 2.04 (1.14–3.65) | W: 1.37 (0.97–1.92); M: 1.06 (0.61–1.84) | ||||
| Remote antecedent BP | 60 | W: 1.31 (0.97–1.77); M: 0.97 (0.72–1.31) | W: 1.48 (1.10–1.99); M: 1.10 (0.84–1.43) | |||
| 70 | W: 1.18 (0.98–1.45); M: 1.24 (1.00–1.55) | W: 1.21 (0.99–1.47); M: 1.23 (0.99–1.51) | ||||
| 80 | W: 1.48 (1.19–1.84); M: 1.84 (1.30–2.59) | W: 1.27 (1.01–1.61); M: 1.75 (1.18–2.59) | ||||
| Seshadri et al. [32] | 5,197 | Recent antecedent BP | Ischaemic strokec | 60 | W: 1.68 (1.25–2.25); M: 1.92 (1.39–2.66) | W: 1.78 (1.33–2.38); M: 1.73 (1.26–2.38) |
| 70 | W: 1.66 (1.28–2.14); M: 1.30 (0.97–1.75) | W: 1.44 (1.11–1.88); M: 1.14 (0.84–1.54) | ||||
| 80 | W: 1.19 (0.84–1.70); M: 1.25 (0.76–2.04) | W: 1.21 (0.86–1.70); M: 1.32 (0.79–2.21) | ||||
| Remote antecedent BP | 60 | W: 1.48 (1.07–2.07); M: 1.54 (0.96–2.45) | W: 1.57 (1.13–2.17); M: 1.30 (0.88–1.91) | |||
| 70 | W: 1.41 (1.17–1.69); M: 1.45 (1.14–1.86) | W: 1.47 (1.23–1.75); M: 1.42 (1.13–1.80) | ||||
| 80 | W: 1.05 (0.79–1.42); M: 1.25 (0.81–1.93) | W: 1.14 (0.86–1.51); M: 1.20 (0.80–1.79) | ||||
| Lee et al. [33] | 3,362 | Recent antecedent BP | Heart failured | 62 | 1.31 (1.11–1.55) | 1.02 (0.88–1.19) |
| Remote antecedent BP | 1.17 (1.04–1.31) | 1.05 (0.93–1.18) | ||||
| Bonifonte et al. [34] | 3,344 | Antecedent SBP | CVD evente | 48 | 1.18 (1.09 − 1.27) | – |
| Ayala Solares et al. [35] | 80,964 | Time-averaged SBP | CVD eventf | 50 | 1.40 (1.27–1.52) | – |
Abbreviations: BP: blood pressure; CVD: cardiovascular disease; M: men; SBP: systolic blood pressure; W: women; yr: years.
aHazard ratio per 10 mmHg increase, adjusted for sex, age, body mass index, total and HDL-cholesterol, triglycerides, glucose, antihypertensive and lipid-lowering medications, diabetes, smoking, alcohol consumption, and fasting status.
bHazard ratio per 1-SD increment, adjusted for current BP, smoking, body mass index, diabetes, cholesterol, and antihypertensive treatment. CVD event defined as CVD death, coronary heart disease, stroke or transient ischaemic attack, peripheral vascular disease (intermittent claudication), or congestive heart failure.
cRelative risk per 1-SD increment, adjusted for current BP levels, diabetes, and smoking.
dHazard ratio per 1-SD increment. Age-stratified multivariable models adjusted for sex, current BP, serum cholesterol, hypertension treatment, diabetes, smoking, valve disease, and previous myocardial infarction and for incidence of an interim myocardial infarction on follow-up. Models evaluating systolic BP variables were adjusted for baseline diastolic BP, and models examining diastolic BP variables were adjusted for baseline systolic BP.
eHazard ratio per 10 mmHg increase. The model included age, sex, current BP, smoking, diabetes, total cholesterol, HDL-cholesterol, and antihypertensive medication as covariates. CVD event defined as CVD death, myocardial infarction, coronary insufficiency, cerebrovascular disease, intermittent claudication, or congestive heart failure.
fHazard ratio per 20 mmHg increase, adjusted for calendar year of study entry, sex, smoking, deprivation index, diabetes mellitus, body mass index, total cholesterol, low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, and current systolic BP.
Three studies from the Framingham Heart Study Original Cohort with a uniform statistical approach have compared the predictive value of antecedent BP (i.e. BP measured before baseline) levels to current BP levels (Table 1) [31–33]. In these studies, antecedent BP levels were categorised as recent antecedent BP (average of all available BP readings during the decade preceding the current BP) and remote antecedent BP (average of all available BP readings obtained 11–20 years before the current BP). First, the association between time-averaged BP levels and CVD events during a 10-year follow-up was studied by Vasan et al. using data from 2,313 participants who had been examined at least 4 times in each of the two previous decades [31]. The results from this study indicated that antecedent time-averaged SBP predicted the incidence of CVD events even after adjusting for baseline SBP and conventional risk factors. This finding was evident for both recent and remote antecedent BP, consistent among men and women and across all age groups and held for both SBP and DBP. Second, a report by Seshadri et al. addressed the relationship between long-term BP levels and the risk of ischaemic stroke in 3,761 participants using a similar study design as the previously described work [32]. The principal findings of this study showed that antecedent BP levels were an important determinant of future risk for stroke beyond a single BP measure in both sexes and the finding was similar for all BP components. In a third study, Lee et al. demonstrated that antecedent SBP levels were associated with the future risk of heart failure even after adjustment for current SBP levels and other confounding factors [33]. Similarly, Bonifonte et al. examined the determinants of CVD risk comparing the effect of antecedent and current BP levels among 3,334 participants emanating from the Framingham Heart Study Offspring Cohort (Table 1) [34]. Participants had been examined three times, and an average of BP measurements obtained 12 and 4 years before current BP was used to represent antecedent BP. The authors reported that in a traditional risk factor model which included both antecedent and current BP, the former predicted CVD (HR 1.18 [95% CI 1.09–1.27] per 10 mmHg increase) while the latter did not (HR 1.01 [95% CI 0.95–1.08] per 10 mmHg increase). These results from the Framingham Heart Study indicate that BP levels in the past are highly associated with the subsequent risk for CVD and should be taken into consideration when possible.
Apart from the Framingham Heart Study, the usefulness of time-averaged BP levels for predicting CVD mortality has been examined by Sasai et al. among 46,848 Japanese participants aged from 40–79 years (Table 1) [30]. An average of two BP measurements obtained 5 years apart was used to represent long-term BP levels and was then compared to both available single measurements. The results showed that time-averaged BP was associated with a higher hazard ratio for CVD death than a single BP measurement at baseline or 5-year follow-up. Another recent study by Ayala Solares et al. examined whether the use of long-term BP measurements could enhance the accuracy of CVD risk prediction over a single BP measurement (Table 1) [35]. This study included over 80,000 participants with an average age of 50 years at baseline (70.1% women) and was based on data obtained from electronic health records representing “real life measurements”. In line with the previous studies, the authors concluded that time-averaged BP was more strongly associated with incident CVD than current BP alone. However, using information on long-term BP levels improved only slightly CVD risk prediction beyond current BP levels in multivariable models.
All in all, the results from these studies indicate that exposure to higher BP levels over time increases CVD risk and suggest that effective prevention of CVD might be best achieved by adequate control of BP starting already early in life. Nevertheless, the possible additional prognostic value of time-averaged BP beyond single BP measurements in clinical practice remains ambiguous and further research is warranted.
Cumulative blood pressure
Cumulative exposure is calculated as the product of the intensity and the duration of a certain exposure. In medical research, quantifying cumulative exposures has been widely used since the 1950s when the association of cumulative exposure to smoking and lung cancer was observed [36]. Cumulative BP exposure is usually calculated as first averaging BP values at each time point between consecutive visits, then multiplying by number of years between visits and finally summing these values together. The difference between cumulative BP and time-averaged BP is that the calculation of time-averaged BP values disregards the amount of exposure time to a given BP level. Several earlier studies have examined the association of cumulative BP with subclinical markers of CVD. However, research on the impact of cumulative BP levels on risk of hard incident CVD outcomes is scarce.
To this date, only three prior studies have examined the association of cumulative BP levels and clinical CVD events. First, a recent study from the Lifetime Risk Pooling Project examined whether inclusion of cumulative SBP in the American College of Cardiology/American Heart Association 10-year atherosclerotic CVD risk equation would enhance CVD risk prediction over single SBP measurements. The study included data from three American cohort studies [37] and comprised 11,767 participants (58% women) with an average age of 59.1 years at baseline. A composite end point of clinical CVD events including coronary heart disease death, nonfatal myocardial infarction, and fatal or nonfatal stroke was used as a study outcome, and a total of 1,877 events occurred during the follow-up period. When 5-year and 10-year cumulative SBP levels were used in the risk prediction instead of current SBP levels, the resulting C-statistic values were materially similar between the models (ranging from 0.67 to 0.68) and no significant improvement in the prediction was observed. Nevertheless, C-statistic is relatively insensitive to change if risk factors with strong associations with the outcome are already included in the initial model. Modest improvements were observed in the net reclassification index (0.04 for men and 0.03 for women) and the relative integrated discrimination index (0.12 for men and 0.10 for women), suggesting that use of cumulative SBP could slightly enhance the accuracy of CVD risk prediction models. Second, Wang et al. studied the predictive value of cumulative BP on cardiovascular events (myocardial infarction or stroke) in a prospective cohort study comprising 52,385 Chinese participants (76.6% men) who had attended three medical examinations and were followed for approximately 3 years for CVD events [38]. The results of this study demonstrate that cumulative SBP/DBP (HR 1.018/1.017 per 10/5 mmHg x year increase [95% CI 1.010–1.027/1.010–1.024]) predicted CVD events even after adjustments for numerous confounding risk factors, including baseline BP. However, baseline SBP was the strongest predictor of subsequent myocardial infarction. Furthermore, repeated BP measurements that were used to calculate cumulative BP levels were obtained after baseline, limiting their application to clinical decision making in real life. Third, the previously mentioned study by Ayala Solares et al. also used cumulative BP as an exposure variable. In this case, the authors reported a multivariable-adjusted hazard ratio of 1.32 for each 20 mmHg x year increment. Altogether, further research on the association of cumulative BP and hard clinical endpoints is needed.
In addition to studies on hard clinical outcomes, three recent reports from the CARDIA study have evaluated the association between cumulative BP and surrogate markers of CVD [39–41]. A study by Kishi et al. demonstrated that chronic exposure to higher BP, even within the considered normal range, is independently related to diastolic left ventricular dysfunction 25 years later. Furthermore, results from Vasconcellos et al. showed that cumulative BP levels are independently associated with adverse remodelling of the left atrium as assessed by three-dimensional echocardiography. The results from both of these studies underscore the fact that long-term exposure to higher cumulative BP levels throughout early adulthood is harmful to cardiac structure and function. In addition, the effects of cumulative SBP on urine albumin-to-creatinine ratio, a risk marker for CVD and kidney disease [42], has been studied by Kramer et al.[39] The results show that higher exposure to cumulative SBP was associated with higher albumin-to-creatinine ratios that persisted even after adjusting for concurrent SBP and multiple other confounders. A similar association was also seen in a report originating from the Multi-Ethnic Study of Atherosclerosis cohort [29].
In summary, these studies demonstrate that the cumulative BP load plays a major role in determining the risk for CVD. However, more research is needed concerning the relationship between cumulative BP exposure and overt CVD.
Blood pressure trajectories
Long-term BP trajectories reflect changes in an individual’s BP levels over time, taking into consideration multiple aspects of lifetime patterns such as starting levels, slope, and cumulative exposure. In practice, BP trajectories are usually computed using latent class models that identify subgroups of individuals sharing a similar underlying BP trajectory [43]. The computational process is complex and requires statistical expertise. Moreover, calculations during the modelling are dependent on user-specified parameters of number and shape of the trajectories, which could affect the results and their interpretation. However, BP trajectories may have additional value for predicting CVD compared to approaches such as the time-averaged BP, because trajectories capture both the level and longitudinal changes of BP.
To this date, three studies have addressed the relationship between long-term BP trajectories and subsequent CVD mortality (Table 2) [26,44,45]. First, Tielemans et al. studied two cohorts of men aged 50 years originating from the Minnesota Business and Professional Men Study and the Zutphen Study [44]. BP was measured annually over a 10-year time period, after which most of the participants had complete or near-complete data on BP levels. Afterwards, these two cohorts were followed to practically extinction, as all the Minnesota participants had died and only 12 participants of the Zutphen study were alive at the end of the follow-up. The authors found that CVD mortality was higher in those groups with greater increase in SBP levels over time, and that SBP trajectories were also associated with life-years lost and all-cause mortality. In the Minnesota Business and Professional Men Study population, SBP trajectories were a stronger predictor of mortality than time-averaged SBP and single SBP measurements. In contrast, time-averaged SBP was the strongest predictor of mortality in the Zutphen Study cohort. A plausible explanation for this finding could be that the BP trajectories observed in the Zutphen Study were nearly linear. The information contained by the trajectories could have therefore been captured by the time-averaged BP.
Table 2.
The associations of long-term blood pressure trajectories with cardiovascular mortality.
| Study | N | Systolic BP trajectory (exposure) | Outcome | Estimate (95% CI) | ||
|---|---|---|---|---|---|---|
| Tielemans et al. [44] | 261 in Minnesota | 632 in Zutphen | 1 (lowest risk) | CVD death | Ref. (Minnesota)a | Ref. (Zutphen)a |
| 2 | 1.82 (1.25–2.66) | 1.34 (1.00–1.81) | ||||
| 3 | 3.80 (2.18–6.64) | 2.05 (1.47–2.87) | ||||
| 4 (highest risk) | 3.95 (1.17–13.38) | 3.05 (1.74–5.33) | ||||
| Tielemans et al. [45] | 762 | 1 (lowest risk) | CVD death | Ref.b | ||
| 2 | 0.91 (0.46 − 1.80) | |||||
| 3 | 1.72 (1.06 − 2.74) | |||||
| 4 (highest risk) | 3.34 (1.39 − 7.99) | |||||
| Petruski-Ivleva et al. [26] | 9,845 | 1 (lowest risk) | CVD death | 19 (14 − 27)c | ||
| 2 | 25 (21 − 30) | |||||
| 3 | 36 (31 − 43) | |||||
| 4 | 58 (40 − 85) | |||||
| 5 | 56 (43 − 72) | |||||
| 6 (highest risk) | 85 (55 − 132) | |||||
| Portegies et al. [46] | 6,745 | 1 (lowest risk) | Stroke | 15 (13–17)d | ||
| 2 | 19 (6–40) | |||||
| 3 | 24 (17–32) | |||||
| 4 (highest risk) | 29 (21–37) | |||||
| Li et al. [47] | 79,308 | 1 (lowest risk) | Stroke | Ref.e | ||
| 2 | 1.64 (1.26–2.14) | |||||
| 3 | 2.56 (1.79–3.65) | |||||
| 4 | 2.78 (2.03–3.81) | |||||
| 5 (highest risk) | 3.77 (2.57–5.51) | |||||
Abbreviations: CVD: cardiovascular disease; Minnesota: The Minnesota Business and Professional Men Study; SBP: systolic blood pressure; Zutphen: The Zutphen Study.
aHazard ratio, adjusted for age, cholesterol, smoking status, and diabetes.
bHazard ratio, adjusted for age, sex, cholesterol, smoking, antihypertensive medication, and diabetes.
cIncidence rates per 10 000 person years, adjusted for age, sex, race, study centre, obesity, diabetes, smoking, antihypertensive medication, and systolic blood pressure at last visit.
dCumulative incidence of stroke by age 90, adjusted for sex, and use of antihypertensive medication.
eHazard ratio, adjusted for age, sex, smoking status, alcohol intake, education, physical activity, income, salt intake, use of hypoglycaemic, antihypertensive, lipid-lowering agents and aspirin, average body mass index, estimated glomerular filtration rate, serum triglycerides, high-density lipoprotein cholesterol, total cholesterol, fasting blood glucose, high-sensitive C-reactive protein, and baseline systolic blood pressure.
In a second study based on The Atherosclerosis Risk in Communities Study data, the relationships of six distinct SBP patterns with stroke, heart failure, CHD, and CVD mortality were investigated. In this study, a total of 9,845 participants, with an average age of 53.7 years at baseline, were examined four times during a 9-year follow-up period (Table 2) [26]. Increasing gradients of coronary heart disease, heart failure and stroke incidence across SBP patterns were observed, but these differences were attenuated after adjustment for demographic characteristics, comorbidities, and SBP level at last visit. However, observed differences in CVD mortality were not attenuated even after adjustment for covariates. Moreover, participants belonging to the trajectory group with highest SBP levels had the highest risk of CVD mortality. In order to estimate the effect of cumulative SBP, participants who had similar SBP levels at last visit but who had gradually reached that BP either from the initial mean of approximately 130 mmHg (i.e. lower average long-term BP levels) or 150 mmHg (i.e. higher average long-term BP levels) were compared. The study revealed that those with a longer exposure to SBP above 140 mmHg had a higher risk of CVD death and heart failure. Similarly, participants whose SBP had increased steeply from prehypertensive levels to above 140 mmHg had a lower CVD mortality rate compared with participants whose SBP had remained above 140 mm Hg constantly. Finally, participants with different SBP trajectory patterns (i.e. different SBP levels at baseline and at last visit) but similar cumulative SBP exposure had similar rates for all events. These findings underscore the importance of cumulative SBP load during midlife, as opposed to the direction of longitudinal change of that accumulation.
In a third publication, the association between four SBP trajectories and subsequent CVD mortality were investigated in the Rancho Bernardo Study (Table 2) [45]. Over 15 years of follow-up, a maximum of five BP measurements were obtained from 762 participants (mean age 65.7 years at baseline, 67% women) who were followed up for 12 years for CVD outcomes. The authors observed that SBP trajectories were significant predictors of CVD mortality, and a three-fold risk of CVD death was observed for the two highest trajectory groups as compared with the lowest trajectory group. Moreover, SBP trajectories were associated with all-cause mortality. However, SBP trajectories did not demonstrate any incremental prognostic value over time-averaged SBP in CVD risk prediction. In addition, two previous reports from the Rotterdam Study and the Kailuan Study have shown that SBP trajectories are associated with an increased risk of subsequent stroke (Table 2) [46,47].
In addition, the use of long-term BP trajectories for CVD risk prediction has also been studied in younger populations. As clinical CVD events are rare in younger age groups, surrogate measures for CVD risk have to be used instead. In the CARDIA study, five BP trajectories were characterised over a 25-year period among 4,681 participants aged 18–30 years at baseline [25]. Data was collected on eight time points, and all participants had at least three BP measurements. Presence of coronary calcification greater or equal to Agatston score of 100 Hounsfield units, a strong predictor of incident coronary heart disease, was used as a marker of subclinical atherosclerosis [48]. The results indicated that participants with elevated BP levels from youth throughout middle age and those who had steep increases in BP levels over time had the greatest odds of having subclinical CVD. These associations remained significant after adjusting for conventional CVD risk factors and even after further adjustments for baseline and 25-year follow-up BP level. These findings demonstrate that also in younger populations, long-term BP levels enhance the prognostication of future coronary artery calcification beyond the consideration of single BP measurements. In addition, BP trajectories have also been associated with other forms of target-organ damage such as left ventricular hypertrophy and subclinical renal damage [49–52].
In summary, all of these studies suggest that defining long-term BP trajectories could provide additional value to cardiovascular risk prediction. However, it is still controversial whether the direction of longitudinal change in BP levels yields additional prognostic value over the mere cumulative SBP load in the prediction of clinical CVD events. As computing long-term trajectories requires complicated statistical modelling, other approaches might prove more practical and thus better suited for clinical work.
Age of hypertension onset
Evidence on the association of hypertension onset age and CVD risk is still limited. Only two case-control studies have assessed the relation between age of hypertension onset and clinical CVD end-points (Table 3). The first results on this domain were published in 1987 from a cohort of 10,313 primary care patients [27]. In that study, the participants were divided at baseline into groups according to newly diagnosed hypertension, defined as diastolic BP ≥ 90 mmHg on two consecutive visits. The participants were then followed up for a period of five years for incident CVD events, and the event rates were then compared with normotensive individuals of similar age. The authors observed that the risk of incident CVD was significantly lower if age of onset was at 69 years, as compared with 40 years. However, no confidence intervals were reported for the point estimates, and the results were not adjusted for other CVD risk factors [27].
Table 3.
The association between hypertension onset age and cardiovascular disease.
| Study | N | Age of hypertension onset (exposure) | Outcome | Odds ratio (95% CI) | p-value |
|---|---|---|---|---|---|
| Buck et al. [27] | 10,313 | 40–49 years | CVD eventa | 5.2 (n/a) | n/a |
| 50–59 years | 1.8 (n/a) | n/a | |||
| 60–65 years | 1.2 (n/a) | n/a | |||
| No hypertension | Ref. | ||||
| Niiranen et al. [53] | 3,614 | <45 years | CVD death | 2.19 (1.77–2.70) | p < .001 |
| 45–54 years | 2.10 (1.67–2.63) | n/a | |||
| 55–64 years | 1.86 (1.48–2.34) | n/a | |||
| ≥65 years | 1.47 (1.16–1.87) | p = .001 | |||
| No hypertension | Ref. | ||||
| Niiranen et al. [53] | 3,614 | <45 years | CHD death | 2.26 (1.75–2.93) | p < .001 |
| 45–54 years | 2.18 (1.64–2.90) | n/a | |||
| 55–64 years | 1.71 (1.26–2.32) | n/a | |||
| ≥65 years | 1.36 (0.98–1.87) | p = .07 | |||
| No hypertension | Ref. |
Abbreviations: CVD: cardiovascular disease; CHD: coronary heart disease; n/a: not applicable.
aMyocardial infarction, stroke, congestive heart failure, or renal failure.
Similar results were recently reported from the Framingham Heart Study Original Cohort (Table 3) [53]. In that study, onset of hypertension was defined as SBP ≥140/90 mm Hg or use of antihypertensive drugs on at least two consecutively attended examinations during a follow-up of up to 60 years. The authors reported an increased trend in odds of CVD mortality versus non-CVD mortality with decreasing age of hypertension onset (p < .001 in all multivariable-adjusted analyses). Apart from hard CVD endpoints, the relation between hypertension onset age and hypertensive target end-organ damage was determined in 2,680 middle-aged participants of the CARDIA cohort study [54]. In that study, the analyses were adjusted for conventional CVD risk factors, including present SBP. Hypertension onset at <35 years of age was associated with increased odds of left ventricular hypertrophy, coronary calcification, and diastolic dysfunction (odds ratio 2.29, 2.94 and 2.06, respectively; p < .05 for all). In contrast, hypertension onset at ≥45 years of age was not associated with any of the organ damage markers (p > .05 for all). Individuals with early onset hypertension also had the highest risk of having damage in two or more organs. These findings provide some data on the potential pathways of how early onset hypertension increases CVD risk, as organ damage is a well-known harbinger of CVD morbidity and mortality [55–58].
Interestingly, findings from both the Johns Hopkins Precursor Study and the Framingham Heart Study suggest that particularly early onset hypertension might also be a strongly heritable trait [53,59]. In these studies, individuals whose parents had developed hypertension at an early age had more than three-fold odds of developing hypertension themselves, compared to those with normotensive parents. The same phenomenon was observed to carry over generations from grandparents to grandchildren, though less robustly [60]. In addition to observed heritability patterns in population studies, specific genetic determinants have been identified for early onset hypertension in several studies [61–65].
Overall, in all studies on this domain, the highest odds of CVD outcomes were observed in the subgroups with early hypertension onset age (Table 3). However, the research in this domain is still limited and based on observational case-control studies. More research in this field is therefore warranted. However, considering the previous findings on time-averaged and cumulative BP, it is unclear whether the observed increase in CVD risk is solely caused by the increased long-term exposure to high blood pressure, or if early onset hypertension should be considered a separate entity.
Conclusions
Our goal was to review the existing research on how longitudinal BP changes can be measured and used for improving CVD risk prediction. The most common indices used for characterising overall lifetime exposure to BP have been time-averaged BP, cumulative BP, BP trajectory patterns, and age of hypertension onset.
All of the indices that characterise long-term BP exposure seem to offer incremental predictive value over “present” BP (Tables 1–4). However, the level of evidence is stronger for some indices than for others. Namely, numerous studies have demonstrated that long-term, time-averaged BP is more strongly associated with CVD prognosis than single-occasion BP (Table 1). However, it remains unclear which of these novel indices is the most accurate predictor of incident CVD, as head-to-head data from outcome studies are lacking. In addition to longitudinal BP indices covered by this review, BP measurements taken during exercise and antihypertensive therapy could also provide incremental predictive value over conventional “present” BP [66–68]. Nevertheless, until additional data become available, more emphasis should be put on assessing the clinical feasibility of these BP indices.
Table 4.
Strengths and weaknesses of various methods used for quantifying blood pressure patterns over time.
| Characteristic | Present (single-occasion) BP | Time-averaged BP | Cumulative BP | BP trajectory patterns | Age of hypertension onset |
|---|---|---|---|---|---|
| Association with CVD prognosis | + | ++ | ++ | ++ | ++ |
| Feasibility | +++ | ++ | ++ | + | +++ |
| Heritability | + | ? | ? | ? | ++ |
| Reproducibility | + | ++ | ++ | + | ++ |
| Level of evidence | +++ | ++ | + | + | + |
Abbreviations: BP: blood pressure; CVD: cardiovascular disease.
Most of the previous studies on BP change patterns and risk of CVD have been performed using research-grade data from established epidemiological studies. Understandably, these data, nor the time needed to calculate time-averaged or cumulative BP, are not usually available during normal patient contacts, reducing the clinical relevance of these indices (Table 4). In addition, defining a patient’s past BP trajectory accurately may prove overwhelming because individual-level BP trajectories could have poor reproducibility and might not always be in line with those reported in the literature. However, either self-reported or objectively defined age of hypertension onset could provide a feasible and rapid method for assessing an individual’s cumulative BP load more accurately than by just using single-occasion BP measurements. In addition to its feasible assessment, age of hypertension onset has also been shown to be a highly heritable trait and a strong predictor of incident hypertension in offspring (Table 4) [53]. Early onset hypertension could be therefore used both as a familial trait when assessing an individual’s risk for hypertension, and as a specific type of BP trait when estimating risk for CVD outcomes.
Although numerous studies have examined the association between longitudinal BP changes and CVD outcomes, the use of long-term BP exposure for CVD risk assessment in clinical practice has remained non-existent. More studies are needed to examine the independent predictive power of less-studied BP indices, such as cumulative BP, BP trajectories, and age of hypertension onset. Moreover, additional studies are needed to rank the predictive power of the different BP indices used for quantifying long-term BP exposure. Finally, randomised clinical trials should be conducted in the future to assess the impact of antihypertensive therapy on individuals with varying levels of long-term BP exposure.
Funding Statement
J. Nuotio was funded by Finnish Foundation for Cardiovascular Research, Juho Vainio Foundation, Turku University Foundation, and Yrjö Jahnsson Foundation. K. Suvila was supported by grants from the Aarne Koskelo Foundation and Finnish Foundation for Cardiovascular Research. V. Langén was supported by a grant from the State Research Funding of the Turku University Hospital expert responsibility area. T.J. Niiranen was funded by the Academy of Finland [grant n:o 321351], the Urmas Pekkala Foundation, the Paavo Nurmi Foundation, the Finnish Medical Foundation, and the Emil Aaltonen Foundation. S. Cheng was supported by National Institute of Health grants R01-HL134168, R01-HL131532, R01-HL143227, and R01-HL142983.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Joint Committee of the Actuarial Society of America and the Association of Life Insurance Medical Directors. Blood Pressure: Report. 1925:1–54.
- 2.Lewington S, Clarke R, Qizilbash N, et al. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–1913. [DOI] [PubMed] [Google Scholar]
- 3.Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. The Lancet. 2016;387(10022):957–967. [DOI] [PubMed] [Google Scholar]
- 4.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American heart association task force on clinical practice guidelines. Circulation. 2018;138(17):e426–83. [DOI] [PubMed] [Google Scholar]
- 5.Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021–3104. [DOI] [PubMed] [Google Scholar]
- 6.Wolf-Maier K, Cooper RS, Banegas JR, et al. Hypertension prevalence and blood pressure levels in 6 European countries, Canada, and the United States. JAMA. 2003;289(18):2363–2369. [DOI] [PubMed] [Google Scholar]
- 7.Franklin SS, Gustin W, 4th, Wong ND, et al. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation. 1997;96(1):308–315. [DOI] [PubMed] [Google Scholar]
- 8.Cheng S, Xanthakis V, Sullivan LM, et al. Blood pressure tracking over the adult life course: patterns and correlates in the Framingham heart study. Hypertension. 2012;60(6):1393–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitchell GF, Wang N, Palmisano JN, et al. Hemodynamic correlates of blood pressure across the adult age spectrum. Circulation. 2010;122(14):1379–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Safar ME. Arterial aging—hemodynamic changes and therapeutic options. Nat Rev Cardiol. 2010;7(8):442–449. [DOI] [PubMed] [Google Scholar]
- 11.Li S, Chen W, Srinivasan SR, et al. Childhood blood pressure as a predictor of arterial stiffness in young adults. Hypertension. 2004;43(3):541–546. [DOI] [PubMed] [Google Scholar]
- 12.Juonala M, Järvisalo MJ, Mäki-Torkko N, et al. Risk factors identified in childhood and decreased carotid artery elasticity in adulthood. Circulation. 2005;112((10):1486–1493. [DOI] [PubMed] [Google Scholar]
- 13.Kaess BM, Rong J, Larson MG, et al. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA. 2012;308(9):875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.NCD Risk Factor Collaboration (NCD-RisC) . Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population-based measurement studies with 19·1 million participants. The Lancet. 2017;389(10064):37–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forouzanfar MH, Liu P, Roth GA, et al. Global burden of hypertension and systolic blood pressure of at Least 110–115 mm Hg, 1990–2015. JAMA. 2017;317(2):165–182. [DOI] [PubMed] [Google Scholar]
- 16.Surendran P, CHARGE-Heart Failure Consortium, Drenos F, Young R, et al. Trans-ancestry meta-analyses identify rare and common variants associated with blood pressure and hypertension. Nat Genet. 2016;48(10):1151–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conen D, Cheng S, Steiner LL, et al. Association of 77 polymorphisms in 52 candidate genes with blood pressure progression and incident hypertension: the Women’s Genome Health Study. J Hypertens. 2009;27(3):476–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gurven M, Blackwell AD, Rodríguez DE, et al. Does blood pressure inevitably rise with age?: Longitudinal evidence among forager-horticulturalists. Hypertension. 2012;60(1):25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Juhola J, Magnussen CG, Viikari JSA, et al. Tracking of serum lipid levels, blood pressure, and body mass index from childhood to adulthood: the cardiovascular risk in Young Finns study. J Pediatr. 2011;159(4):584–590. [DOI] [PubMed] [Google Scholar]
- 20.Bao W, Threefoot SA, Srinivasan SR, et al. Essential hypertension predicted by tracking of elevated blood pressure from childhood to adulthood: the Bogalusa heart study. Am J Hypertens. 1995;8(7):657–665. [DOI] [PubMed] [Google Scholar]
- 21.Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood. Circulation. 2008;117(25):3171–3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burke V, Beilin LJ, Dunbar D. Tracking of blood pressure in Australian children. J Hypertens. 2001;19(7):1185–1192. [DOI] [PubMed] [Google Scholar]
- 23.Conen D, Glynn RJ, Ridker PM, et al. Socioeconomic status, blood pressure progression, and incident hypertension in a prospective cohort of female health professionals. Eur Heart J. 2009;30(11):1378–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tielemans S, Susanne MA, Geleijnse JM, et al. Ten‐year blood pressure trajectories, cardiovascular mortality, and life years lost in 2 extinction cohorts: the minnesota business and professional men study and the zutphen study. JAHA. 2015;4(3):4. e001378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allen NB, Siddique J, Wilkins JT, et al. Blood pressure trajectories in early adulthood and subclinical atherosclerosis in middle age. JAMA. 2014;311(5):490–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petruski-Ivleva N, Viera AJ, Shimbo D, et al. Longitudinal patterns of change in systolic blood pressure and incidence of cardiovascular disease: the atherosclerosis risk in communities study. Hypertension. 2016;67(6):1150–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buck C, Baker P, Bass M, et al. The prognosis of hypertension according to age at onset. Hypertension. 1987;9(2):204–208. [DOI] [PubMed] [Google Scholar]
- 28.Lauer MS, Anderson KM, Levy D. Influence of contemporary versus 30-year blood pressure levels on left ventricular mass and geometry: the Framingham heart study. J Am Coll Cardiol. 1991;18(5):1287–1294. [DOI] [PubMed] [Google Scholar]
- 29.Zemaitis P, Liu K, Jacobs DR, Jr, et al. Cumulative systolic BP and changes in urine albumin-to-creatinine ratios in nondiabetic participants of the multi-ethnic study of atherosclerosis. Clin J Am Soc Nephrol . 2014;9(11):1922–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sasai H, Sairenchi T, Irie F, et al. Long-term exposure to elevated blood pressure and mortality from cardiovascular disease in a Japanese population: the Ibaraki Prefectural health study. Hypertens Res. 2011;34(1):139–144. [DOI] [PubMed] [Google Scholar]
- 31.Vasan RS, Massaro JM, Wilson PWF, et al. Antecedent blood pressure and risk of cardiovascular disease: the Framingham heart study. Circulation. 2002;105(1):48–53. [DOI] [PubMed] [Google Scholar]
- 32.Seshadri S, Wolf PA, Beiser A, et al. Elevated midlife blood pressure increases stroke risk in elderly persons: the Framingham study. Arch Intern Med. 2001;161(19):2343–2350. [DOI] [PubMed] [Google Scholar]
- 33.Lee DS, Massaro JM, Wang TJ, et al. Antecedent blood pressure, body mass index, and the risk of incident heart failure in later life. Hypertension. 2007;50(5):869–876. [DOI] [PubMed] [Google Scholar]
- 34.Bonifonte A, Ayer T, Veledar E, et al. Antecedent blood pressure as a predictor of cardiovascular disease. J Am Soc Hypertens. 2015;9(9):690–696.e1. [DOI] [PubMed] [Google Scholar]
- 35.Ayala Solares JR, Canoy D, Raimondi FED, et al. Long-term exposure to elevated systolic blood pressure in predicting incident cardiovascular disease: evidence from large-scale routine electronic health records. J Am Heart Assoc. 2019;8(12):e012129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Doll R, Hill AB. A study of the aetiology of carcinoma of the lung. BMJ. 1952;2(4797):1271–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pool LR, Ning H, Wilkins J, et al. Use of long-term cumulative blood pressure in cardiovascular risk prediction models. JAMA Cardiol. 2018;3(11):1096–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang YX, Song L, Xing AJ, et al. Predictive value of cumulative blood pressure for all-cause mortality and cardiovascular events. Sci Rep. 2017;7(1):41969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kramer H, Colangelo L, Lewis CE, et al. Cumulative exposure to systolic blood pressure during young adulthood through midlife and the urine albumin-to-creatinine ratio at midlife. Am J Hypertens. 2017;30(5):502–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vasconcellos HD, Moreira HT, Ciuffo L, et al. Cumulative blood pressure from early adulthood to middle age is associated with left atrial remodelling and subclinical dysfunction assessed by three-dimensional echocardiography: a prospective post hoc analysis from the coronary artery risk development in young adults study. Eur Heart J. 2018;19(9):977–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kishi S, Teixido-Tura G, Ning H, et al. Cumulative blood pressure in early adulthood and cardiac dysfunction in middle age: the Cardia study. J Am Coll Cardiol. 2015;65(25):2679–2687. [DOI] [PubMed] [Google Scholar]
- 42.Gerstein HC, Mann JF, Yi Q, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286(4):421–426. [DOI] [PubMed] [Google Scholar]
- 43.Jones BL, Nagin DS. Advances in group-based trajectory modeling and an sas procedure for estimating them. Sociol Methods Res. 2007;35(4):542–571. [Google Scholar]
- 44.Tielemans S, Geleijnse JM, Menotti A, et al. Ten-year blood pressure trajectories, cardiovascular mortality, and life years lost in 2 extinction cohorts: the Minnesota business and professional men study and the Zutphen Study. J Am Heart Assoc. 2015;4(3):e001378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tielemans S, Geleijnse JM, Laughlin GA, et al. Blood pressure trajectories in relation to cardiovascular mortality: the Rancho Bernardo Study. J Hum Hypertens. 2017;31(8):515–519. [DOI] [PubMed] [Google Scholar]
- 46.Portegies MLP, Mirza SS, Verlinden VJA, et al. Mid- to late-life trajectories of blood pressure and the risk of stroke: the Rotterdam study. Hypertension. 2016;67(6):1126–1132. [DOI] [PubMed] [Google Scholar]
- 47.Li W, Jin C, Vaidya A, et al. Blood pressure trajectories and the risk of intracerebral hemorrhage and cerebral infarction: a prospective study. Hypertension. 2017;70(3):508–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358(13):1336–1345. [DOI] [PubMed] [Google Scholar]
- 49.Zhang T, Li S, Bazzano L, et al. Trajectories of childhood blood pressure and adult left ventricular hypertrophy: the Bogalusa heart study. Hypertension. 2018;72(1):93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zheng W, Mu J, Chu C, et al. Association of blood pressure trajectories in early life with subclinical renal damage in middle age. J Am Soc Nephrol. 2018;29(12):2835–2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hao G, Wang X, Treiber FA, et al. Blood pressure trajectories from childhood to young adulthood associated with cardiovascular risk: results from the 23-year longitudinal Georgia stress and heart study. Hypertension. 2017;69(3):435–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ku E, Vittinghoff E, Jacobs DR, Jr, et al. Changes in blood pressure during young adulthood and subsequent kidney function decline: findings from the coronary artery risk development in young adulthood (Cardia) study. Am J Kidney Dis. 2018;72(2):243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Niiranen TJ, McCabe EL, Larson MG, et al. Heritability and risks associated with early onset hypertension: multigenerational, prospective analysis in the Framingham heart study. BMJ. 2017;357:j949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suvila K, McCabe EL, Lehtonen A, et al. Early onset hypertension is associated with hypertensive end-organ damage already by MidLife. Hypertension. 2019;74(2):305–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Viazzi F, Leoncini G, Conti N, et al. Combined effect of albuminuria and estimated glomerular filtration rate on cardiovascular events and all-cause mortality in uncomplicated hypertensive patients. J Hypertens. 2010;28(4):848–855. [DOI] [PubMed] [Google Scholar]
- 56.Redfield MM, Jacobsen SJ, Burnett JC, et al. Burden of systolic and diastolic ventricular dysfunction in the community. JAMA. 2003;289(2):194–202. [DOI] [PubMed] [Google Scholar]
- 57.Levy D, Garrison RJ, Savage DD, et al. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham heart study. N Engl J Med. 1990;322(22):1561–1566. [DOI] [PubMed] [Google Scholar]
- 58.Vernooij JWP, van der Graaf Y, Nathoe HM, et al. Hypertensive target organ damage and the risk for vascular events and all-cause mortality in patients with vascular disease. J Hypertens. 2013;31(3):492–499. [DOI] [PubMed] [Google Scholar]
- 59.Wang N-Y, Young JH, Meoni LA, et al. Blood pressure change and risk of hypertension associated with parental hypertension: the Johns Hopkins precursors study. Arch Intern Med. 2008;168(6):643–648. [DOI] [PubMed] [Google Scholar]
- 60.Niiranen TJ, McCabe EL, Larson MG, et al. Risk for hypertension crosses generations in the community: a multi-generational cohort study. Eur Heart J. 2017;38(29):2300–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wilk JB, Djousse L, Arnett DK, et al. Genome-wide linkage analyses for age at diagnosis of hypertension and early-onset hypertension in the HyperGEN study. Am J Hypertens. 2004;17(9):839–844. [DOI] [PubMed] [Google Scholar]
- 62.Chiang K-M, Yang H-C, Liang Y-J, et al. A three-stage genome-wide association study combining multilocus test and gene expression analysis for young-onset hypertension in Taiwan Han Chinese. Am J Hypertens. 2014;27(6):819–827. [DOI] [PubMed] [Google Scholar]
- 63.Shahin DS, Irshaid YM, Saleh AA. TheA1166Cpolymorphism of the AT1R gene is associated with an early onset of hypertension and high waist circumference in Jordanian males attending the Jordan University Hospital. Clin Exp Hypertens. 2014;36(5):333–339. [DOI] [PubMed] [Google Scholar]
- 64.von WF, von Wowern F. A genome wide scan for early onset primary hypertension in Scandinavians. Hum Mol Genet. 2003;12(16):2077–2081. [DOI] [PubMed] [Google Scholar]
- 65.Chang T-J, Wang W-C, Hsiung CA, et al. Genetic variation in the human Sorbs1 gene is associated with blood pressure regulation and age at onset of hypertension: a Sapphire cohort study. Medicine. 2016;95(10):e2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mariampillai JE, Liestøl K, Kjeldsen SE, et al. Exercise systolic blood pressure at moderate workload is linearly associated with Coronary disease risk in healthy men. Hypertension. 2020;75(1):44–50. [DOI] [PubMed] [Google Scholar]
- 67.Okin PM, Hille DA, Larstorp ACK, et al. Effect of lower on-treatment systolic blood pressure on the risk of atrial fibrillation in hypertensive patients. Hypertension. 2015;66(2):368–373. [DOI] [PubMed] [Google Scholar]
- 68.Okin PM, Wachtell K, Devereux RB, et al. Regression of Electrocardiographic left ventricular hypertrophy and decreased incidence of new-onset atrial fibrillation in patients with hypertension. JAMA. 2006;296(10):1242–1248. [DOI] [PubMed] [Google Scholar]