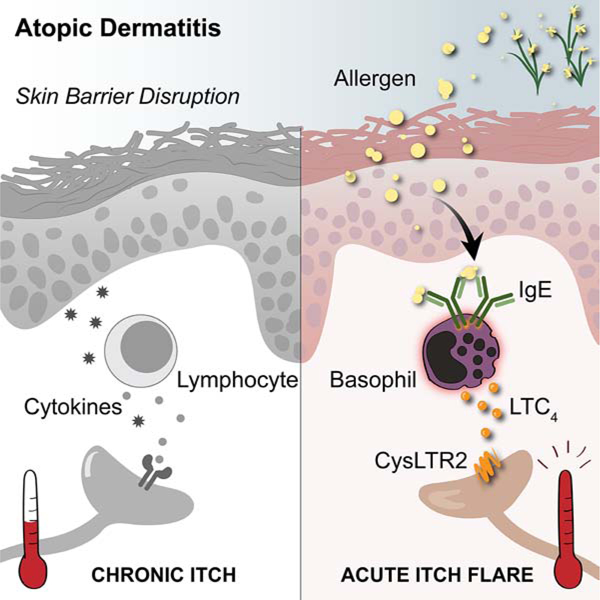
SUMMARY
Itch is an evolutionarily conserved sensation that facilitates expulsion of pathogens and noxious stimuli from the skin. However, in organ failure, cancer, and chronic inflammatory disorders like atopic dermatitis (AD), itch becomes chronic, intractable, and debilitating. In addition to chronic itch, patients often experience intense acute itch exacerbations. Recent discoveries have unearthed the neuroimmune circuitry of itch, leading to the development of anti-itch treatments. However, mechanisms underlying acute itch exacerbations remain overlooked. Herein, we identify that a large proportion of patients with AD harbor allergen-specific IgE and exhibit a propensity for acute itch flares. In mice, while allergen-provoked acute itch is mediated by the mast cell-histamine axis in steady-state, AD-associated inflammation renders this pathway dispensable. Instead, a previously unrecognized basophil-leukotriene axis emerges as critical for acute itch flares. By probing fundamental itch mechanisms, our study highlights a basophil-neuronal circuit that may underlie a variety of neuroimmune processes.
Graphical Abstract
In Brief
Atopic dermatitis-associated inflammation promotes a basophil-leukotriene neuroimmune axis to evoke acute itch flares.
INTRODUCTION
Itch (i.e. pruritus) is defined as an uncomfortable sensation on the skin that causes a desire to scratch. When acute, itch is a protective mechanism to rapidly expel noxious environmental stimuli. However, itch can become chronic and pathologic in nature, underlying a variety of medical conditions that range from inflammatory skin disorders to chronic kidney disease and cancer (Larson et al., 2019; Sommer et al., 2007). Chronic itch is clinically defined in humans as itch that lasts for greater than six weeks (Kim et al., 2019a; Stander et al., 2017). Notably, chronic itch often persists for years due to the lack of effective therapeutics and can profoundly affect quality of life of patients (Altinok Ersoy and Akyar, 2019; Kini et al., 2011). In recent years, the morbidity of chronic itch has been increasingly recognized and clinical trials now routinely measure itch as a key endpoint (Fishbane et al., 2020; Kim et al., 2019b; Kim et al., 2020; Silverberg et al., 2020; Stander et al., 2020). However, current assessments quantify itch severity as an aggregate score over time (Erickson and Kim, 2019), despite the fact that patients with chronic itch often experience acute itch flares, i.e. rapid and intense exacerbations of itch (Fourzali et al., 2020; Langan et al., 2006). This results in studies failing to assess the dynamic nature of itch in chronic conditions. Although substantial progress has been made in the identification of itch-specific neural pathways (Cevikbas et al., 2014; Liu et al., 2016; Liu et al., 2009; Mishra and Hoon, 2013; Oetjen et al., 2017; Sun and Chen, 2007; Wilson et al., 2013), the neuroimmune mechanisms that modulate itch acuity remain poorly defined.
Atopic dermatitis (AD) is a pruritic inflammatory skin disease with a chronic but relapsing course. Although chronic itch is a well-defined feature of AD, patients also report experiencing sudden itch flares, which remains poorly characterized (Chang et al., 2016; Fourzali et al., 2020; Wassmann-Otto et al., 2018). Recent advances in neuroimmunology have demonstrated that effector cytokines associated with AD like IL-4, IL-13, and IL-31 can directly stimulate sensory neurons to promote chronic itch (Cevikbas et al., 2014; Oetjen et al., 2017). Whether acute itch flares simply represent enhanced signaling of these known pathways or employ other molecular circuits remains unknown.
Characterized by scaly, leaky, and oozing skin, patients with AD become epicutaneously sensitized to environmental allergens and thus harbor allergen-specific IgE (Spergel and Paller, 2003; Weidinger et al., 2018). Although the acquisition of allergen-specific IgE predisposes patients with AD to develop other atopic disorders like asthma and food allergy (Brough et al., 2015; Celakovska et al., 2015; Flohr et al., 2014; Gustafsson et al., 2000), the role of IgE in AD pathogenesis has remained surprisingly elusive (Ogawa et al., 2016). Even in murine models of AD that are induced by model allergens, cutaneous inflammation occurs independently of IgE (Spergel et al., 1999). Further, anti-IgE therapy has produced mixed results and has not advanced beyond phase 2 clinical trials in human AD, for which trial endpoints are primarily focused on skin inflammation and not itch (Deleanu and Nedelea, 2019; Heil et al., 2010). Notwithstanding this, several studies have demonstrated that patients with AD have seasonal variation of itch symptoms (Kim et al., 2017; Vocks et al., 2001) and exhibit enhanced itch following allergen exposure (Jaworek et al., 2019; Kramer et al., 2005; Werfel et al., 2015). Therefore, we hypothesized that IgE may represent a key mechanism that drives acute itch flares in response to allergens in the context of AD.
Residing in close proximity to sensory nerve fibers at barrier surfaces (Egan et al., 1998; Letourneau et al., 1996; Stead et al., 1987; Undem et al., 1995), mast cells are poised to rapidly respond to a variety of stimuli to orchestrate a multitude of physiologic processes (Benoist and Mathis, 2002; Galli and Tsai, 2012; Gupta and Harvima, 2018; Marshall, 2004; Voehringer, 2013). The most well-studied mechanism of mast cell activation is IgE-mediated degranulation. Allergen recognition by IgE bound to the high-affinity receptor FcεRI results in IgE crosslinking and triggers the release of a variety of effector molecules such as histamine and serotonin (Benditt et al., 1955; Ishizaka et al., 1970). These mediators in turn can activate sensory neurons to provoke neuroinflammation and itch sensation (Wang et al., 2020). Indeed, histamine was one of the first factors identified to elicit itch through its direct stimulation of sensory neurons (i.e. act as a pruritogen) and is now recognized as a canonical mediator of acute itch (Dale and Laidlaw, 1910; Weisshaar et al., 1997). However, antihistamines have demonstrated poor efficacy in most chronic itch disorders including AD (He et al., 2018; Rajagopalan et al., 2017).
In the current study, we examined clinical itch datasets from phase 3 clinical trials for moderate-to-severe AD and found that a large proportion of patients exhibit acute itch flares that would not be captured in traditional analyses. Further, we identified that patients with allergen-specific IgE have a higher likelihood of exhibiting acute itch flares than those without, provoking the hypothesis that allergen exposure is a factor that drives acute itch flares. To investigate this, we generated a murine model of AD-like disease in which challenge with a model allergen elicits acute itch flares. Although dependent on IgE, acute itch flares surprisingly occurred independently of tissue-resident mast cells, but instead, were critically dependent on basophils. Strikingly, chemogenetic activation of basophils alone was sufficient to provoke itch-induced scratching behavior in mice. Intravital imaging further demonstrated basophil-sensory neuron interactions following cutaneous allergen exposure. Moreover, allergen-stimulated basophils exhibited enhanced production of leukotriene C4 (LTC4). The presence of CysLTR2 on neurons, a receptor for LTC4, was critically required for acute itch flares in AD-associated inflammation. Collectively, our study unveils a form of acute itch flare that emerges in the context of chronic skin inflammation to activate a non-canonical basophil-neuronal circuit. Importantly, we highlight how itch manifests heterogeneously even within one disease.
RESULTS
Patients with AD Exhibit Acute Itch Flare Patterns That Are Associated with Allergen-Specific IgE
Although patients suffering from chronic itch can experience acute periods of intense itch exacerbation (Fourzali et al., 2020; Langan et al., 2006), this phenomenon remains poorly characterized. To study AD-associated acute itch flares, we performed a post-hoc analysis on two phase 3 clinical trials for moderate-to-severe AD (Simpson et al., 2016). We assessed the daily numerical rating scale (NRS) itch scores from placebo-treated patients (N = 159) over a two-month period (Figure 1A). The NRS itch score is a single-item self-assessment where patients rate their severity of itch from 0 (“no itch”) to 10 (“worst imaginable itch”) over the prior 24 hours (Phan et al., 2012). While some patients exhibited consistent itch severity over the assessment period, others exhibited a fluctuating itch pattern with rapid and frequent spikes of itch (Figure 1B). We classified patients as having an acute itch flare if there was an elevation in their daily NRS itch score of ≥ 2 points relative to their baseline (day 0) within a 3 day period (Langan et al., 2006). During the first month, 26.4% of the patients (42/159) exhibited acute itch flares (Figure 1C). In the second month, interestingly, N = 5 patients (5/159, 3.1%) lost their acute itch phenotype while N = 32 patients (32/159, 20.1%), who previously exhibited a non-flare pattern, developed acute itch flares (Figure 1D). Overall, 46.5% of patients with AD (74/159) presented with acute itch flares during the course of the two-month period (Figures 1C and 1D). Collectively, our data demonstrate that acute itch flares are exhibited by a large proportion of patients with AD. However, what factors drive these periodic itch exacerbations remain unclear.
Figure 1. Acute Itch Flares Are Associated with Allergen-Specific IgE in Human AD and Mast Cell-Independent in Murine AD-Like Disease.
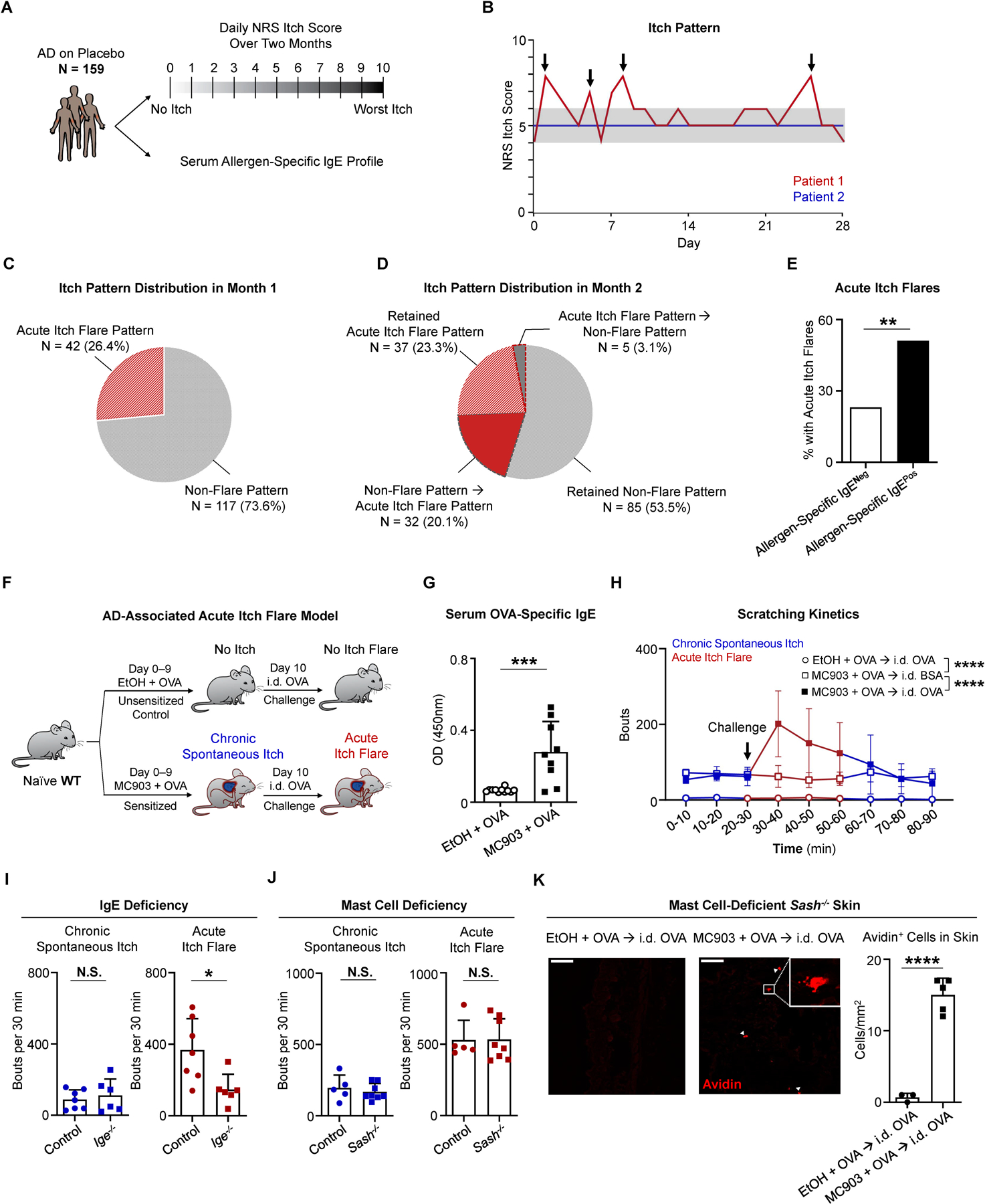
(A) Schematic of post hoc analysis of phase 3 clinical trial data from a cohort of placebo-treated patients with atopic dermatitis (AD, N = 159). For each patient, the daily numerical rating scale (NRS) itch scores over a two-month period and the serum allergen-specific IgE repertoire were assessed.
(B) Itch patterns from two representative individuals. Patient 1 (red line) has an acute itch flare pattern due to the presence of at least one acute itch flare (indicated by arrows). Patient 2 (blue line) has a non-flare itch pattern due to a lack of apparent itch flares. Gray shading highlights 2-point threshold above baseline for patient 1.
(C) Pie chart depicting the percentage (%) of patients with an acute itch flare or non-flare pattern in the first month.
(D) Pie chart depicting the percentage (%) of patients with various itch patterns in month 2. Wedges outlined by dotted lines represent patients whose itch pattern changed from month 1 to month 2.
(E) Frequency of patients who exhibited acute itch flares over the 2-month observation period out of all patients that tested positive for allergen-specific IgE (black bar, N = 68/133) and out of all patients that tested negative (White bar, N = 6/26). **p < 0.01 by Chi-square test.
(F) Schematic of the AD-associated acute itch flare model. Calcipotriol (MC903) + ovalbumin (OVA)-treated (sensitized; from day 0 to day 9) or ethanol (EtOH) + OVA-treated (unsensitized control; from day 0 to day 9) wild-type (WT) mice received an intradermal (i.d.) injection of OVA into adjacent non-lesional cheek skin on day 10. Prior to and following i.d. OVA challenge, chronic spontaneous itch and acute itch flares were recorded, respectively.
(G) ELISA quantification of OVA-specific IgE in the serum of MC903 + OVA-treated and EtOH + OVA-treated WT mice on day 10 of the AD-associated acute itch flare model. n = 9–11 mice per group. ***p < 0.001 by unpaired Student’s t-test.
(H) Number of scratching bouts in 10-minute (min) intervals prior to and following i.d. allergen (OVA or bovine serum albumin [BSA]) challenge on day 10 of the AD-associated acute itch flare model. Unsensitized (EtOH + OVA) mice were challenged with i.d. OVA (open circle) and sensitized (MC903 + OVA) mice were challenged with i.d. OVA (closed square) or i.d. BSA (open square). Blue line indicates chronic spontaneous itch and red line indicates acute itch flares. n = 7 mice per group. ****p < 0.0001 by Two-way ANOVA test.
(I) Number of scratching bouts in littermate control and Ige−/− mice prior to i.d. OVA challenge (chronic spontaneous itch; left) and following i.d. OVA challenge (acute itch flares; right) on day 10 of the AD-associated acute itch flare model. n = 6–7 mice per group. N.S., not significant, *p < 0.05 by unpaired Student’s t-test.
(J) Number of scratching bouts in littermate control and mast cell-deficient Sash−/− mice prior to i.d. OVA challenge (chronic spontaneous itch; left) and following i.d. OVA challenge (acute itch flares; right) on day 10 of the AD-associated acute itch flare model. n = 5–8 mice per group. N.S., not significant by unpaired Student’s t-test.
(K) Representative images of i.d. OVA-challenged skin sections stained with avidin-Texas Red (TRITC) in unsensitized (EtOH + OVA) or sensitized (MC903 + OVA) mast cell-deficient Sash−/− mice and the number of avidin positive cells quantified per square millimeter from each treatment group. White arrows indicate positively stained cells. White square indicates zoomed view of an avidin positive cell. Scale bar, 50µm. n = 3–5 mice per group. ****p < 0.0001 by unpaired Student’s t-test.
Data are represented as mean ± SD.
See also Figure S1.
Prior studies have found that allergens provoke itch in AD (Jaworek et al., 2019; Kramer et al., 2005; Werfel et al., 2015). Further, the majority of patients with AD harbor allergen-specific IgE (Flohr et al., 2004). To investigate whether reactivity to allergens is linked to acute itch flares, we retrospectively profiled the serum allergen-specific IgE repertoire from our cohort of N = 159 patients (Figure 1A). We separated patients into those who harbor allergen-specific IgE (N = 133 patients; 83.6%) and those who do not (N = 26 patients; 16.4%). Strikingly, a higher frequency of patients with allergen-specific IgE (68/133, 51.1%) experienced acute itch flares than those without (6/26, 23.1%) (Figure 1E). Taken together, these findings indicate that the presence of allergen-specific IgE is likely associated with acute itch flares in AD, provoking the hypothesis that allergen exposure might be a factor that drives acute itch flares.
Acute Itch Flares Are Mast Cell-Independent in a Murine Model of AD-Like Disease
To test whether allergen recognition by IgE promotes acute itch flares in the setting of AD-associated inflammation, we generated a murine model of acute itch flares. We induced AD-like disease by topically treating mice on both ears with the irritant calcipotriol (MC903) daily for 10 days. Concurrently with MC903, the model allergen ovalbumin (OVA) was also topically applied onto the ear skin in order to mirror epicutaneous allergen sensitization (Figures 1F and S1A) that occurs in patients (Han et al., 2017; Noti et al., 2013). After 10 days, MC903 + OVA-treated wild-type (WT) mice developed robust AD-like skin inflammation (Figures S1B and S1C), allergen (OVA)-specific IgE (Figure 1G), and chronic spontaneous itch (~60 bouts/10 minutes [min]) (Figure 1H, blue line, open and closed squares). This was in contrast to WT mice that received the control treatment of ethanol vehicle and OVA (EtOH + OVA), which lacked skin inflammation (Figures S1B and S1C), OVA-specific IgE (Figure 1G), and chronic spontaneous itch behavior (~5 bouts/10 min) over the same interval (Figure 1H, blue line, open circle). Upon intradermal (i.d.) injection of OVA into the adjacent non-lesional cheek skin of mice with AD-associated inflammation (Figure 1F), an acute itch flare was immediately observed (~200 bouts/10 min) and resolved over the subsequent 30 min (Figure 1H, red line, closed square). However, this acute itch flare phenomenon was absent in EtOH + OVA-treated control mice i.d. challenged with OVA (Figure 1H, red line, open circle) and in mice with AD-associated inflammation that were i.d. challenged with an irrelevant allergen, bovine serum albumin (BSA) (Figure 1H, red line, open square). As expected, IgE was required for acute itch flares as IgE-deficient (Ige−/−) mice failed to generate a response to i.d. OVA challenge, while IgE was dispensable for chronic spontaneous itch (Figure 1I). These studies demonstrate that acute itch flares triggered by allergen exposure are dependent on IgE in the context of AD.
Mast cell degranulation induced by IgE-crosslinking is well known to trigger acute itch sensation (Gould and Sutton, 2008; Meixiong et al., 2019). Indeed, when naïve WT were passively sensitized by intravenous (i.v.) transfer of exogenous anti-OVA IgE and then challenged with i.d. OVA (Figure S1D), they experienced robust acute itch (Figure S1E) that was dependent on both mast cells (Figure S1F) and histamine (Figure S1G). Surprisingly, however, in our AD-associated acute itch flare model, both chronic spontaneous itch and acute itch flares were not significantly different between mast cell-deficient Sash−/− and littermate control mice (Figure 1J). To corroborate these observations using an alternative method of sensitization, we transferred anti-OVA IgE into MC903-treated WT mice or mast cell-deficient Sash−/− mice and subsequently challenged them with i.d. OVA to elicit acute itch flares (Figure S1H). Again, Sash−/− mice had comparable acute itch flares to their littermate controls in this setting (Figures S1I and S1J). Taken together, these findings demonstrate that allergen-provoked acute itch, while mast cell-dependent and histaminergic in the steady-state, occurs independently of mast cells in the setting of AD-like disease. Thus, it appears that AD-associated inflammation activates an alternative IgE-dependent cellular circuit to evoke acute itch flares. In support of this, cells with a distinctly degranulated morphology as identified by avidin staining (Bergstresser et al., 1984; Mukai et al., 2017) were evident in MC903 + OVA-treated mast cell-deficient Sash−/− mice at the site of i.d OVA challenge (Figure 1K), indicating the presence of an alternative cell type.
Circulating Basophils Exhibit a Distinct Phenotype in AD-Associated Inflammation in Mice and Humans
Basophils, like tissue-resident mast cells, also express the high-affinity IgE receptor FcεRI, and release similar effector molecules like histamine, serotonin, tryptase, and leukotrienes (LTs) following IgE crosslinking (Voehringer, 2013). Despite their importance in driving cutaneous inflammation and functional similarities to mast cells (Borriello et al., 2014; Ito et al., 2011; Kim et al., 2014a; Mashiko et al., 2017; Mukai et al., 2005), the role of basophils in itch remains unknown. Given that basophils are not skin-resident, but rather circulate in the blood and enter the skin upon stimulation, we sought to investigate whether AD-associated inflammation systemically alters basophils.
To test this, we performed flow cytometry on the blood of patients with AD (N = 12) and healthy control subjects (N = 12) (Figure 2A; Table S1). Strikingly, AD-associated blood basophils, although unchanged in frequency (Figure 2B), exhibited elevated expression of the human basophil marker CD203c (Figure 2C). Cytokines like IL-3 upregulate CD203c expression on human basophils and enhance IgE-mediated responses (Brunner et al., 1993). In support of the possibility that AD-associated basophils may be more reactive to IgE stimulation, blood basophils from patients with AD demonstrated significantly enhanced expression of FcεRIα compared to basophils from control subjects (Figure 2D). Similarly, mice with AD-like disease (Figures 2E and S2A) did not display differences in the frequency of circulating basophils (Figure 2F). Instead, they exhibited rapid and sustained upregulation of the murine basophil activation marker CD200R (Figures 2G and S2B) and FcεRIα compared to control mice (Figures 2H and S2C). These findings provoke the hypothesis that basophils are more responsive to IgE-mediated stimulation, which may enable their ability to promote itch.
Figure 2. Circulating Basophils Exhibit a Distinct Phenotype in AD-Associated Inflammation in Mice and Humans.
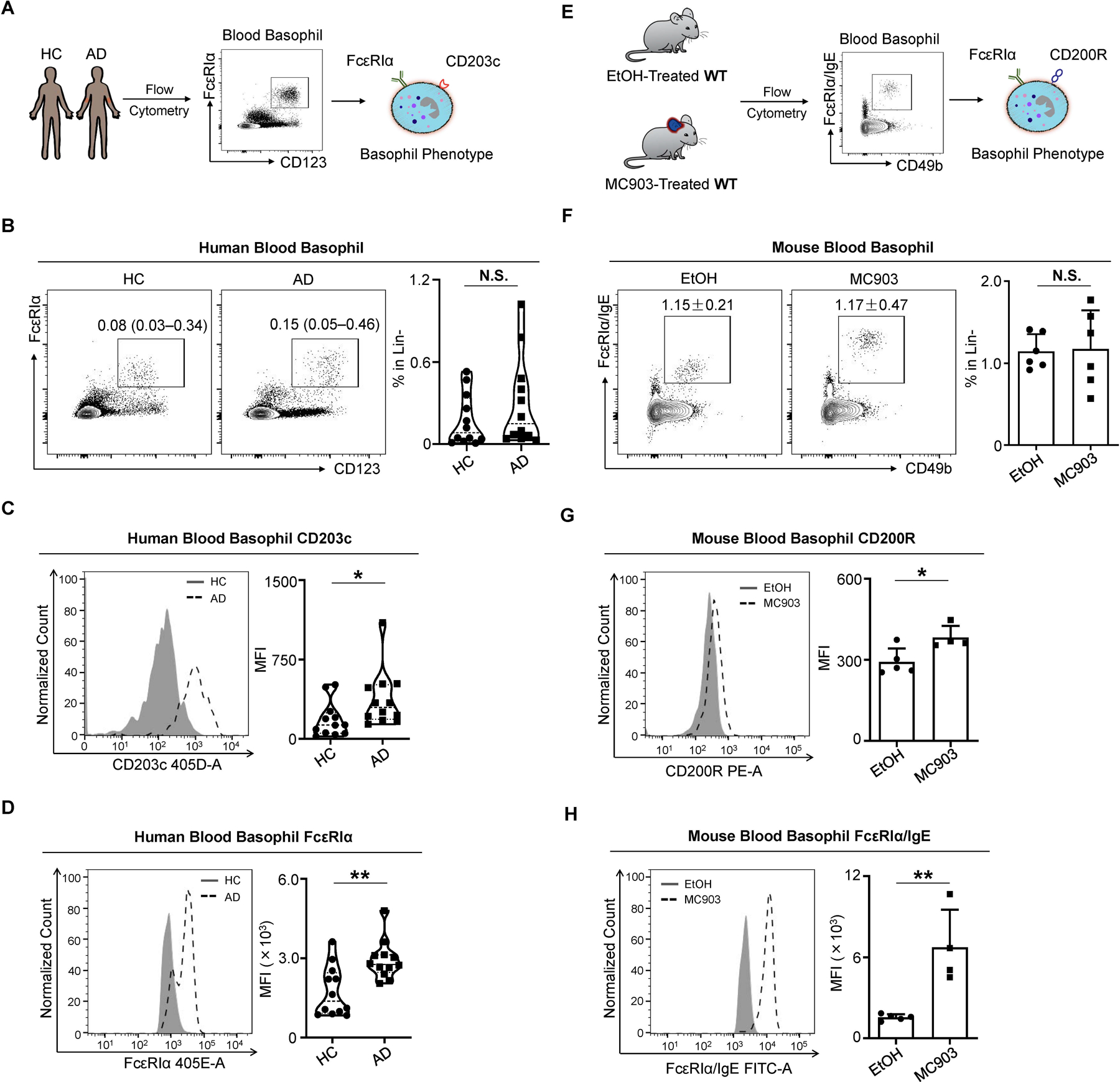
(A) Schematic of human blood basophil analysis by flow cytometry from healthy controls (HC) and patients with AD.
(B) Representative flow cytometry plots and frequency of lineage negative (Lin-) (CD3, CD4, CD19, CD14, CD34, CD56, c-Kit) CD123+ FcεRIα+ blood basophils from HCs and patients with AD. N = 12 subjects per group. N.S., not significant by Wilcoxon–Mann–Whitney nonparametric test.
(C and D) CD203c (C) and FcεRIα (D) expression measured by mean fluorescence intensity (MFI) on blood basophils from HCs and patients with AD. N = 12 subjects per group. *p < 0.05, **p < 0.01 by Wilcoxon–Mann–Whitney nonparametric test.
(E) Schematic of murine blood basophil analysis by flow cytometry. Vehicle EtOH or MC903 was topically applied on the ear skin of WT mice from day 0 to day 9 to induce AD-like disease.
(F) Representative flow cytometry plots and frequency of Lin- (CD3e, CD5, CD11c, CD19, NK1.1) CD49b+ FcεRIα/IgE+ blood basophils in EtOH- or MC903-treated WT mice on day 10 of the AD-like disease model. n = 6 mice per group. N.S., not significant by unpaired Student’s t-test.
(G and H) CD200R (G) and FcεRIα/IgE (H) expression measured by MFI on blood basophils in EtOH- or MC903-treated WT mice on day 10 of the AD-like disease model. n = 4–5 mice per group. *p < 0.05, **p < 0.01 by unpaired Student’s t-test.
Data are represented as median (interquartile range) in (B–D) and mean ± SD in (F–H).
Chemogenetic Activation of Basophils Is Sufficient to Elicit Itch
We next asked whether direct activation of basophils alone is sufficient to induce acute itch. To test this, we employed a chemogenetic approach by crossing the basophil-specific Mcpt8-Cre-YFP mouse with the Rosa26-LSL-Gq-DREADD line. The resulting Mcpt8-Gq mice have a targeted insertion of the artificial G protein-coupled receptor (GPCR) hM3Dq into basophils allowing for selective activation upon administration of an otherwise inert compound, clozapine-N-oxide (CNO) (Figure 3A). To confirm this, we sort-purified blood basophils from Mctp8-Gq mice and littermate controls and stimulated them ex vivo with CNO. As expected, basophils from Mctp8-Gq mice displayed enhanced degranulation compared to littermate controls (Figures 3B–3D). We then assessed itch behavior following systemic intraperitoneal (i.p.) administration of CNO to Mcpt8-Gq mice. Strikingly, Mcpt8-Gq mice exhibited markedly enhanced scratching following CNO injection compared to littermate control mice (Figure 3E). These findings demonstrate that basophil activation alone is sufficient to evoke itch behavior.
Figure 3. Chemogenetic Activation of Basophils Is Sufficient to Elicit Itch.
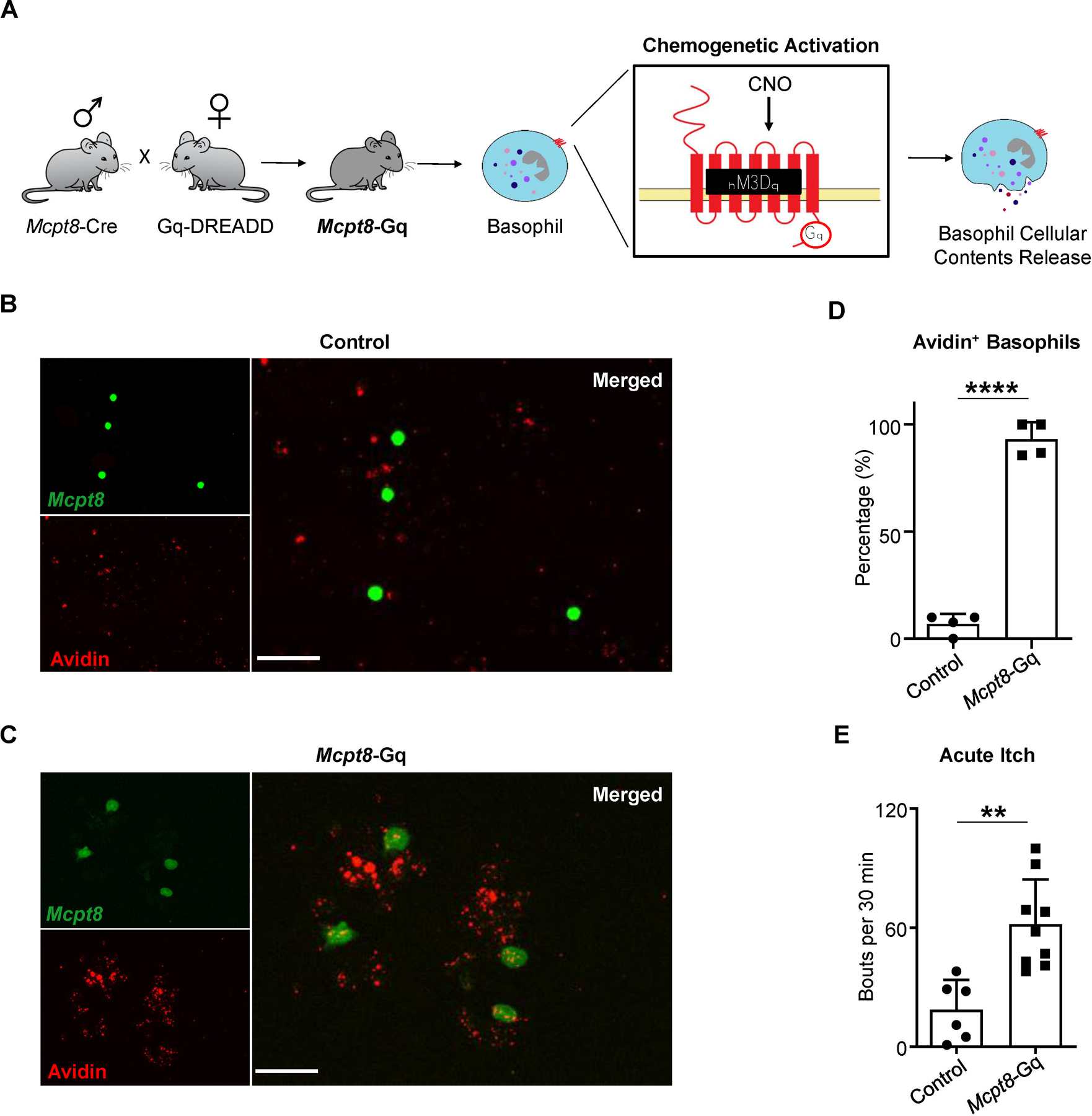
(A) Schematic of the Mcpt8-Gq mouse line. Generated by crossing the Cre-dependent Gq-DREADD line with the Mcpt8-Cre-YFP line, Mcpt8-Gq mice specifically express hM3Dq in basophils allowing for selective chemogenetic activation of basophils upon clozapine-N-oxide (CNO) administration.
(B and C) Representative images of basophils isolated from the blood of (B) control (Mcpt8-Cre) or (C) Mcpt8-Gq mice stimulated ex vivo with CNO. Identified based on their expression of the YFP reporter, basophils (FITC, green) were additionally stained with avidin-Texas Red (TRITC, red). Scale bar, 50µm.
(D) Frequency of blood basophils isolated from control (Mcpt8-Cre) and Mcpt8-Gq mice that were avidin-positive following ex vivo stimulation with CNO. n = 4 mice per group. ****p < 0.0001 by unpaired Student’s t-test.
(E) Number of scratching bouts following intraperitoneal (i.p.) injection of CNO in control (Mcpt8-Cre) and Mcpt8-Gq mice. n = 6–9 mice per group. **p < 0.01 by unpaired Student’s t-test.
Data are represented as mean ± SD.
Basophils Are Required for Acute Itch Flares in AD-Associated Inflammation
Next, we tested whether basophils are required to mediate acute itch flares in the context of AD-associated inflammation. We depleted basophils with systemic anti-CD200R3 monoclonal antibody (mAb) administration on day 7 and day 9 of the AD-associated acute itch flare model (Figure 4A). Basophil depletion (Figure 4B) significantly decreased the acute itch flare response to i.d. OVA challenge (Figure 4C). We next employed an alternative approach by utilizing Bas-TRECK mice, which exclusively express the diphtheria toxin (DT) receptor on basophils (Figure 4D) (Noti et al., 2013). Genetic depletion of basophils with i.p. DT administration on day 8 and day 9 of the AD-associated acute itch flare model (Figure 4E) resulted in reduced scratching in response to i.d. OVA challenge (Figure 4F). Moreover, neither endogenous OVA-specific IgE production nor the underlying chronic spontaneous itch behavior was affected by basophil depletion with either anti-CD200R3 mAb treatment (Figure S3A) or in Bas-TRECK mice (Figure S3B). Therefore, our data demonstrate that basophils are critically required in promoting acute itch flares in the setting of AD-associated inflammation. Further, to test the specificity of the contribution of basophils to acute itch flares, we employed an alternative method of OVA sensitization whereby mice were systemically sensitized by i.p. injection of OVA along with the adjuvant alum (Figure S3C) (Huang et al., 2016; Meixiong et al., 2019). In the context of systemic sensitization, acute itch was indeed observed in WT mice following i.d. OVA challenge (Figure S3D). However, in this context, acute itch was attenuated in mast cell-deficient Sash−/− mice (Figure S3E) but not basophil-depleted Bas-TRECK mice (Figure S3F). Strikingly, upon induction of AD-like disease by MC903 along with systemic sensitization with i.p. OVA + alum (Figure S3G), allergen-provoked acute itch flares were unaffected in mast cell-deficient Sash−/− mice (Figure S3H), but significantly attenuated in basophil-depleted Bas-TRECK mice (Figure S3I). Taken together, these results indicate that the contribution of basophils to acute itch flares arises in the context of AD-like inflammation rather than other forms of sensitization (i.e. OVA + alum).
Figure 4. Basophils Are Required for Acute Itch Flares in AD-Associated Inflammation.
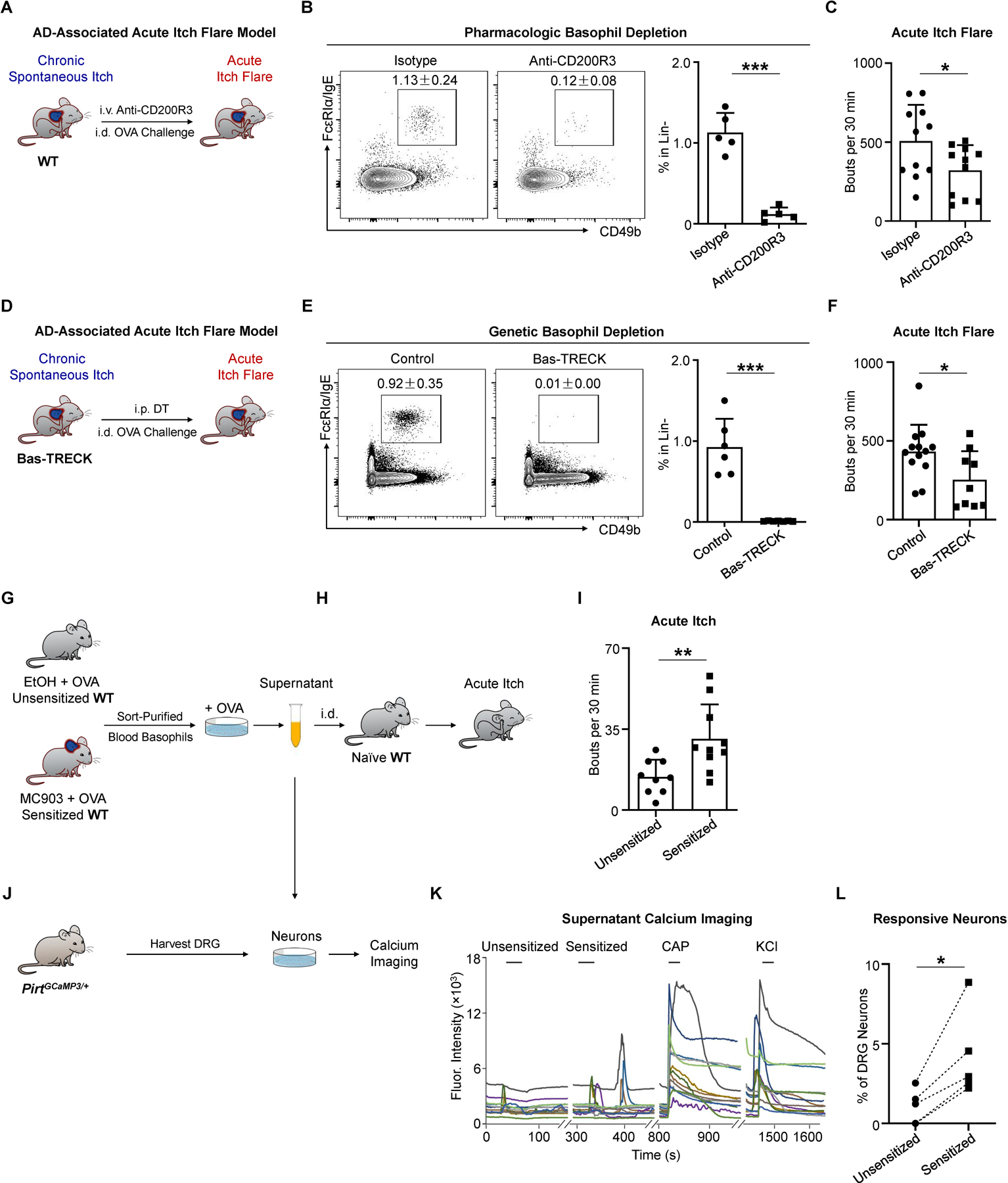
(A) Schematic of pharmacologic basophil depletion. WT mice received intravenous (i.v.) injection of isotype control or anti-CD200R3 monoclonal antibody (mAb) on day 7 and day 9 of the AD-associated acute itch flare model (MC903 + OVA).
(B) Representative flow cytometry plots and frequency of Lin- (CD3e, CD5, CD11c, CD19, NK1.1) FcεRIα/IgE+ CD49b+ basophils from the blood of isotype-treated and anti-CD200R3 mAb-treated WT mice prior to i.d. OVA challenge on day 10 of the AD-associated acute itch flare model. n = 5 mice per group. ***p < 0.001 by unpaired Student’s t-test.
(C) Number of scratching bouts following i.d. OVA challenge on day 10 of the AD-associated acute itch flare model in isotype-treated WT mice and basophil-depleted (anti-CD200R3 mAb-treated) WT mice. n = 11 mice per group. *p < 0.05 by unpaired Student’s t-test.
(D) Schematic of conditional basophil depletion by i.p. injection of diphtheria toxin (DT) into Bas-TRECK mice on day 8 and day 9 of the AD-associated acute itch flare model.
(E) Representative flow cytometry plots and frequency of Lin- (CD3e, CD5, CD11c, CD19, NK1.1) FcεRIα/IgE+ CD49b+ basophils from the blood of littermate control and Bas-TRECK mice prior to i.d. OVA challenge on day 10 of the AD-associated acute itch flare model. n = 6–7 mice per group. ***p < 0.001 by unpaired Student’s t-test.
(F) Number of scratching bouts following i.d. OVA challenge on day 10 of the AD-associated acute itch flare model in littermate control and basophil-depleted Bas-TRECK mice. n = 9–13 mice per group. *p < 0.05 by unpaired Student’s t-test.
(G) Schematic of sort purification and culture of circulating basophils from unsensitized (EtOH + OVA) or sensitized (MC903 + OVA) WT mice. Supernatants were collected one hour following ex vivo stimulation with OVA.
(H) Schematic of i.d. injection of supernatants from OVA-stimulated basophils (from Figure 4G) into naïve WT mice to test acute itch responses.
(I) Number of scratching bouts in naïve WT recipient mice following i.d. injection of basophil-derived supernatants from unsensitized (EtOH + OVA) or sensitized (MC903 + OVA) WT mice. n = 9–10 recipient mice per group. **p < 0.01 by unpaired Student’s t-test.
(J) Schematic of dorsal root ganglia (DRG) neurons isolated from PirtGCaMP3/+ calcium reporter mice being stimulated with supernatants from OVA-stimulated basophils (from Figure 4G) to test calcium responses using calcium imaging.
(K) Representative calcium traces of mouse DRG responses to supernatant stimulation. Calcium responses were measured by fluorescence (Fluor.) intensity (488 nm). Neurons isolated from PirtGCaMP3/+ mice were sequentially stimulated with supernatants from unsensitized (EtOH + OVA) mice, supernatants from sensitized (MC903 + OVA) mice, capsaicin (CAP, 500 nM), and KCl (50 mM). Each color trace represents one neuron.
(L) Percentage (%) of supernatant-responsive neurons out of all KCl-responsive neurons. Each data point represents the percentage of supernatant-responsive neurons from one individual PirtGCaMP3/+ mouse. n = 5 mice (> 200 neurons each). *p < 0.05 by paired Student’s t-test.
Data are represented as mean ± SD.
See also Figure S3.
Mast cells release a number of mediators which directly stimulate sensory neurons to modify sensory behavior (Voehringer, 2013). We thus hypothesized that factors derived from allergen-stimulated basophils are sufficient to provoke acute itch behavior. To test this, we first purified blood basophils from sensitized (MC903 + OVA) and unsensitized (EtOH + OVA) WT mice and stimulated them ex vivo with OVA (Figure 4G). We then i.d. injected supernatants into naïve WT recipient mice to determine their ability to induce acute itch (Figure 4H). Strikingly, basophil-derived factors from sensitized mice induced robust scratching not observed with supernatants from unsensitized mice (Figure 4I). We next sought to test whether basophil-derived factors can directly stimulate primary sensory neurons. We isolated dorsal root ganglia (DRG) from PirtGCaMP3/+ calcium reporter mice (Kim et al., 2014b) and tested if different factors could activate primary sensory neurons by calcium imaging. We first validated that sensory neurons were not responsive to OVA stimulation alone (Figures S3J and S3K). We then added basophil-derived supernatants from both unsensitized (EtOH + OVA) and sensitized (MC903 + OVA) WT mice to DRG neurons (Figure 4J). Notably, enhanced calcium responses were detected in sensory neurons stimulated with supernatants from allergen-sensitized basophils compared to supernatants from unsensitized basophils (Figures 4K and 4L). Additionally, the subpopulation of sensory neurons responsive to basophil-derived factors was also activated by capsaicin (Figure 4K), a common feature of many itch-sensory or pruriceptive neurons (Dong and Dong, 2018). Overall, these findings demonstrate that factors derived from basophils can induce acute itch and activate sensory neurons.
Acute Itch Flares Require Basophil-Intrinsic Leukotriene Pathways
Next, we investigated the specific effector mechanisms by which basophils promote acute itch flares in AD-like disease. The most well-defined pruritogens associated with mast cells include histamine, serotonin, tryptase, and LTs (Akiyama et al., 2010; Andoh and Kuraishi, 1998; Meixiong et al., 2019; Solinski et al., 2019). To determine if any of these pathways are enriched in basophils, we analyzed publicly available microarray datasets to specifically compare murine blood basophils to skin-resident mast cells (Benoist et al., 2012; Dwyer et al., 2016). Skin mast cells expressed higher levels of Hdc (histidine decarboxylase, the primary enzyme catalyzing histamine synthesis), Tph1 (tryptophan hydroxylase [TPH] 1, which controls peripheral serotonin synthesis), and Tpsab1 (tryptase) compared to blood basophils (Figures 5A–5C). In contrast, blood basophils expressed higher levels of Alox5ap (Figure 5D) which encodes 5-lipoxygenase-activating protein (FLAP) (Mancini et al., 1993). LT biosynthesis is critically controlled by the key enzyme 5-lipoxygenase (5-LOX), which requires FLAP for activation (Hedi and Norbert, 2004). Therefore, we hypothesized that LT biosynthesis is a key pathway underlying acute itch flares. In support of this, antihistamines (Figure 5E), an inhibitor of serotonin production (Figure 5F), and an antagonist of the tryptase receptor protease-activated receptor 2 (PAR2) (Figure 5G) were all ineffective in attenuating allergen-provoked acute itch flares during AD-associated inflammation. In contrast, pharmacologic inhibition of 5-LOX by zileuton or genetic deficiency of 5-LOX (Alox5−/− mice) markedly and significantly reduced acute itch flares (Figure 5H). Additionally, disruption of the 5-LOX pathway did not affect the quantity of allergen-specific IgE (Figure S4A) nor the levels of chronic spontaneous itch (Figure S4B). Collectively, these results indicate that LTs may mediate acute itch flares.
Figure 5. Acute Itch Flares Require Basophil-Intrinsic Leukotriene Pathways.
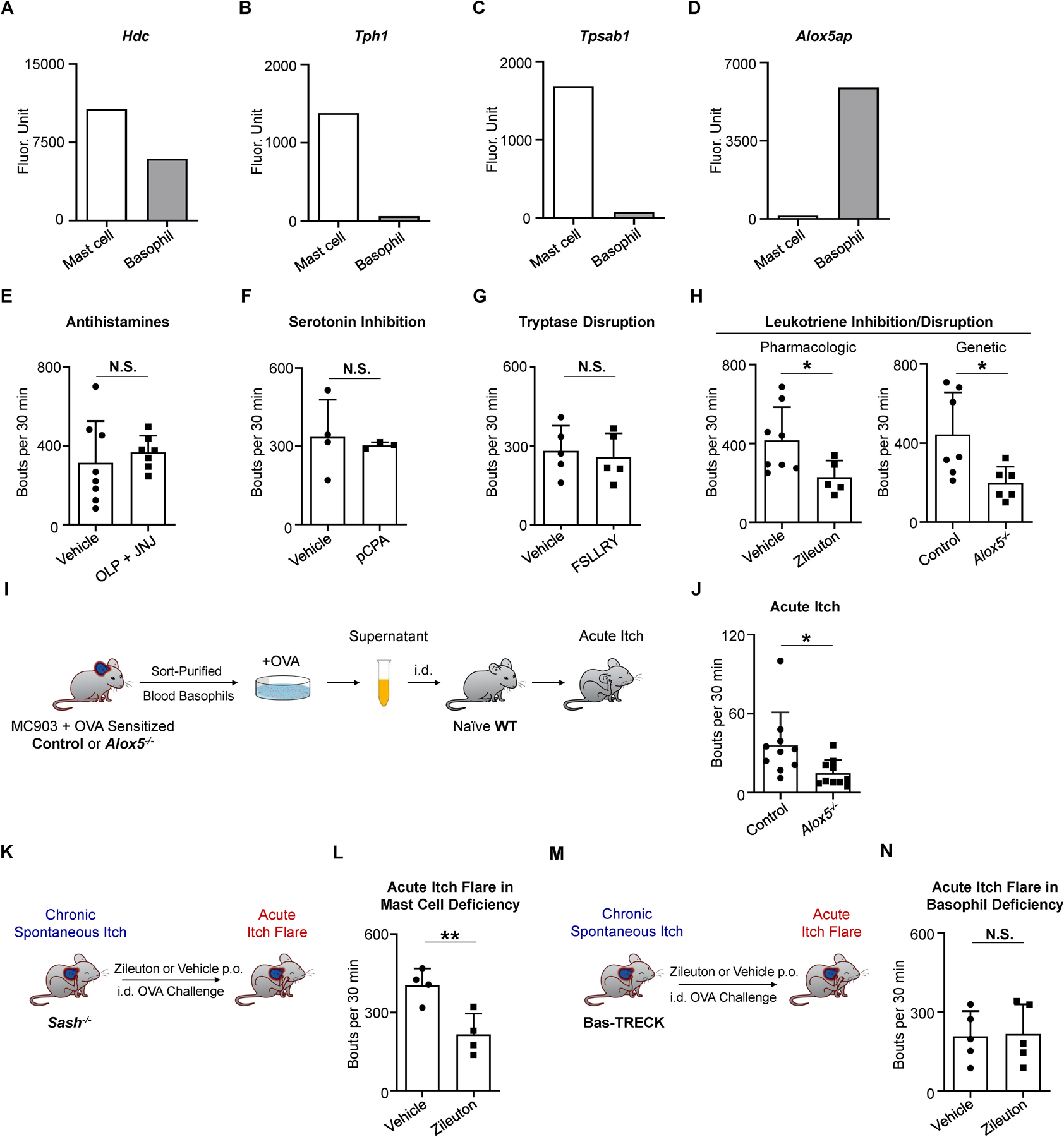
(A–D) RNA expression of (A) Hdc, (B) Tph1, (C) Tpsab1, and (D) Alox5ap in murine skin mast cells and blood basophils. Raw data from www.immgen.org.
(E) Number of scratching bouts following i.d. OVA challenge on day 10 of the AD-associated acute itch flare model in WT mice that were pre-administered vehicle or antihistamines olopatadine (OLP, 3 mg/kg; i.p.) and JNJ7777120 (JNJ, 20 mg/kg; subcutaneous injection into the nape) 30 minutes prior to i.d. OVA challenge. n = 7–8 mice per group. N.S., not significant by unpaired Student’s t-test.
(F) Number of scratching bouts following i.d. OVA challenge on day 10 of the AD-associated acute itch flare model in WT mice that were pre-administered vehicle or tryptophan hydroxylase inhibitor p-Chlorophenylalanine (pCPA, 150 mg/kg; i.p.) on day 7, day 8, and day 9. n = 3–4 mice per group. N.S., not significant by unpaired Student’s t-test.
(G) Number of scratching bouts following i.d. OVA challenge on day 10 of the AD-associated acute itch flare model in WT mice that were pre-administered vehicle or protease-activated receptor 2 (PAR2; the tryptase receptor) antagonist FSLLRY-NH2 (FSLLRY, 7.5 mg/kg; i.p.) 30 minutes prior to i.d. OVA challenge. n = 5 mice per group. N.S., not significant by unpaired Student’s t-test.
(H) Number of scratching bouts following i.d. OVA challenge on day 10 of the AD-associated acute itch flare model in WT mice that were pre-administered vehicle or zileuton (a 5-lipoxygenase [5-LOX] inhibitor, 50 mg/kg; gavage) 60 minutes prior to i.d. OVA challenge (left). Number of scratching bouts following i.d. OVA challenge on day 10 of the AD-associated acute itch flare model in littermate control and Alox5−/− mice (right). n = 5–8 mice per group. *p < 0.05 by unpaired Student’s t-test.
(I) Schematic for testing the pruritogenic properties of circulating basophils from sensitized (MC903 + OVA) littermate control or Alox5−/− mice stimulated ex vivo with OVA. Supernatants suspended from stimulated basophils are i.d. injected into naïve WT mice to provoke acute itch responses.
(J) Number of scratching bouts in naïve WT recipient mice i.d. injected with basophil-derived supernatants from sensitized littermate control or sensitized Alox5−/− mice. n = 10 recipient mice per group. *p < 0.05 by unpaired Student’s t-test.
(K) Schematic for testing the 5-LOX pathway in mast cell-deficient Sash−/− mice pre-administered vehicle or zileuton prior to i.d. OVA challenge on day 10 of the AD-associated acute itch flare model.
(L) Number of scratching bouts following i.d. OVA challenge on day 10 of the AD-associated acute itch flare model in mast cell-deficient Sash−/− mice that were pre-administered vehicle or zileuton (50 mg/kg; gavage). n = 4 mice per group. **p < 0.01 by unpaired Student’s t-test.
(M) Schematic for testing 5-LOX pathway in basophil-depleted Bas-TRECK mice pre-administered vehicle or zileuton prior to i.d. OVA challenge on day 10 of the AD-associated acute itch flare model.
(N) Number of scratching bouts following i.d. OVA challenge on day 10 of the AD-associated acute itch flare model in basophil-depleted Bas-TRECK mice that were pre-administered vehicle or zileuton (50 mg/kg; gavage). n = 5 mice per group. N.S., not significant by unpaired Student’s t-test.
Data are represented as mean ± SD.
See also Figure S4.
We then tested whether LTs released from basophils mediate allergen-provoked acute itch flares. We sort-purified basophils from sensitized (MC903 + OVA) Alox5−/− or littermate control mice (Figure 5I), stimulated them ex vivo with OVA and then i.d. injected the respective basophil-derived supernatants into naïve WT recipient mice. Indeed, supernatants derived from Alox5−/− basophils did not evoke acute itch behavior like supernatants from littermate control basophils (Figure 5J). To exclude the role of LTs released from mast cells, we topically treated mast cell-deficient Sash−/− mice with MC903 + OVA and then gave them an oral gavage of the 5-LOX inhibitor zileuton or vehicle control prior to i.d. OVA challenge (Figure 5K). Pharmacologic inhibition of 5-LOX attenuated acute itch flares (Figure 5L), indicating that the effect of LT inhibition is independent of mast cells. In contrast, zileuton treatment of basophil-deficient Bas-TRECK mice was not able to additionally suppress acute itch flares (Figures 5M and 5N), demonstrating that basophils selectively mediate the induction of acute itch flares via LTs. Taken together, our data implicate basophil-derived LTs as key inducers of allergen-provoked acute itch flares in AD-associated inflammation.
Basophils Interact with Sensory Neurons in the Skin upon Allergen Challenge
Due to the close proximity of mast cells to nerves in the skin and their capacity to evoke histaminergic itch, the mast cell-nerve unit has classically represented a central neuroimmune axis in itch (Gupta and Harvima, 2018; Wang et al., 2020). Having found that factors derived from allergen-activated basophils can directly activate sensory neurons in vitro, we next sought to visualize whether basophils interact with cutaneous sensory neurons in vivo. Although rare in circulation, basophils can rapidly migrate in response to various stimuli like cytokines and IgE stimulation (Hirsch and Kalbfleisch, 1980; Lett-Brown et al., 1981; Suzukawa et al., 2005), and thereby infiltrate tissues (Egawa et al., 2013; Hellman et al., 2017; Ito et al., 2011; Min et al., 2004; Voehringer, 2017; Wardlaw et al., 1994). Therefore, we hypothesized that stimulation of basophils with allergen in the skin may result in unique basophil-neuronal interactions that could support their role in itch.
To test this, we employed Mcpt8-Cre-YFP mice, in which YFP is specifically expressed on basophils but not mast cells (Sullivan et al., 2011; Voehringer, 2013). We subjected Mcpt8-Cre-YFP reporter mice to our AD-associated acute itch flare model and performed real-time intravital two-photon imaging to visualize allergen-challenged skin (Figure 6A). In unsensitized (EtOH + OVA) control mice, no basophils were observed in the skin before or after i.d. OVA challenge (Figure 6B). However, in the skin of sensitized (MC903 + OVA) mice, basophils were detected in the dermis, but were primarily non-motile with a round morphology (Figure 6B; Movie S1A). Strikingly, after i.d. OVA challenge, the majority of basophils developed an enlarged, elongated morphology, and began to crawl through the dermis (Figure 6B; Movie S1B). Collectively, these findings demonstrate that in the setting of AD-associated inflammation, basophils alter their shape and increase their patrolling behavior in response to allergen challenge.
Figure 6. Basophils Interact with Sensory Neurons in the Skin upon Allergen Challenge.
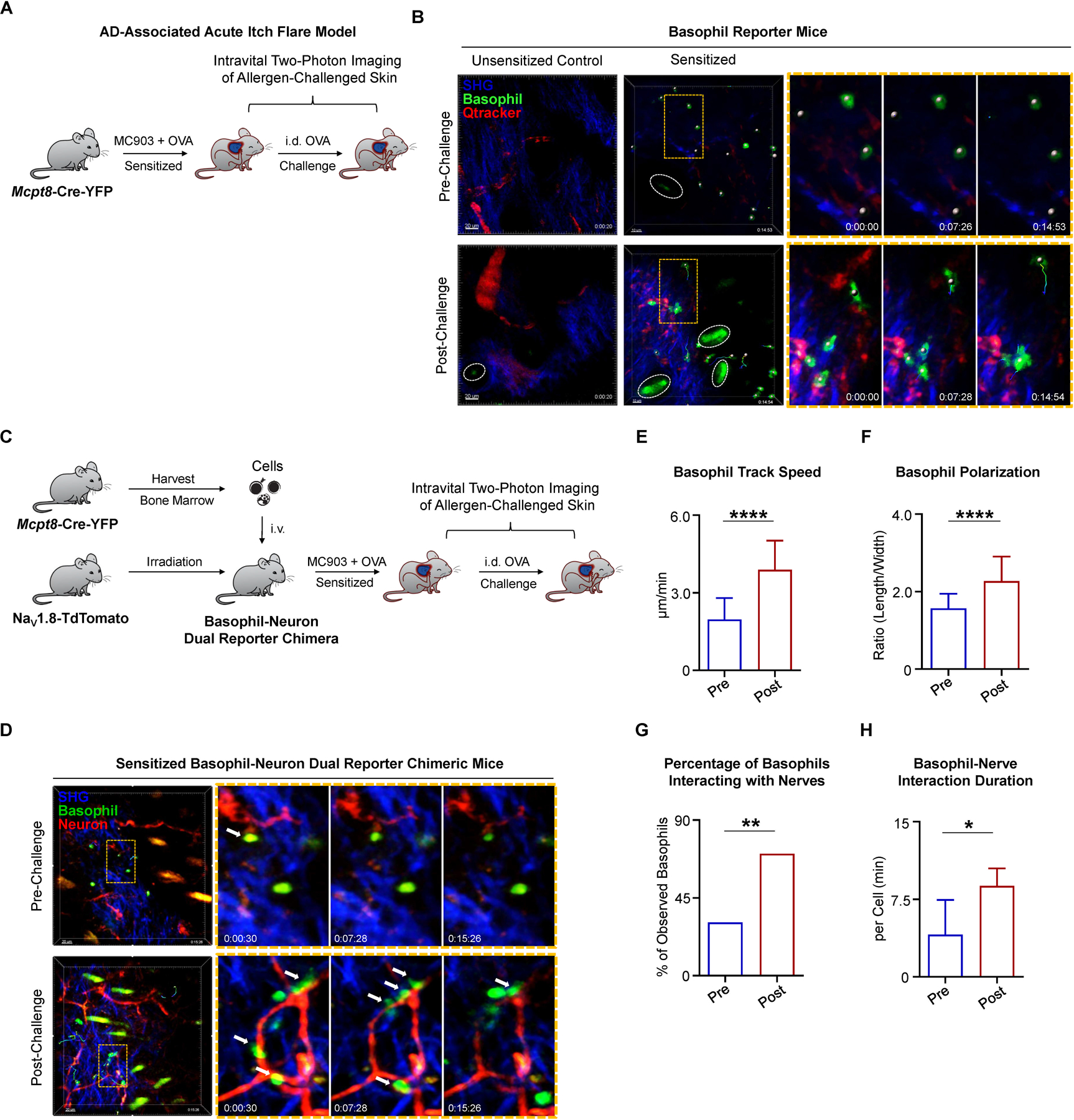
(A) Schematic of intravital two-photon imaging of cheek skin (injection site) pre- and post-challenge with i.d. OVA in Mcpt8-Cre-YFP mice on day 10 of the AD-associated acute itch flare model.
(B) Time-lapse intravital two-photon imaging of basophil behavior. Representative images were taken of the cheek skin from unsensitized (EtOH + OVA) control and sensitized (MC903 + OVA) Mcpt8-Cre-YFP mice, pre- and post-challenge with i.d. OVA. Tracked basophils (green) are indicated by white dots (center of mass). Autofluorescent hairs are identified by white ellipses. Basophil motility is indicated by time encoded colored tracks. Zoomed views are taken from the regions outlined by orange rectangles. Blood vessels (red) were labeled by i.v. injection of Qtracker 655 vascular label 15–30 minutes prior to imaging. Collagen (blue) is imaged by collecting the second harmonic generation signal (SGH).
(C) Schematic of basophil-neuron dual reporter chimera generation and intravital two-photon imaging of the cheek skin (injection site) in sensitized (MC903 + OVA) dual reporter mice pre- and post-challenge with i.d. OVA on day 10 of the AD-associated acute itch flare model. Bone marrow cells harvested from Mcpt8-Cre-YFP donors were i.v. injected into NaV1.8-TdTomato recipients after X-ray irradiation to generate dual reporter mice. Mice were rested 8 weeks before initiation of AD-associated acute itch flare model.
(D) Time-lapse intravital two-photon imaging of basophil-neuron interactions in the cheek skin of basophil-neuron dual reporter chimeric mice, pre- and post-challenge with i.d. OVA on day 10 of the AD-associated acute itch flare model. Representative images show sensory nerve fibers (red) and basophils (green, white dots). Tracked cell motility is shown as time-encoded colored tracks. Autofluorescent hairs appear yellow or green and collagen appears blue due to the SHG signal. Zoomed views are taken from the regions outlined by orange rectangles. White arrows show examples of basophils making apparent contacts with sensory nerves in the skin.
(E) Basophil track speed in the cheek skin of basophil-neuron dual reporter chimeric mice, pre- and post-challenge with i.d. OVA on day 10 of the AD-associated acute itch flare model. n > 20 basophils per group. Data are represented as median (interquartile range). ****p < 0.0001 by Wilcoxon–Mann–Whitney nonparametric test. n = 3 mice.
(F) Basophil polarization (length/width ratio) in the cheek skin of basophil-neuron dual reporter chimeric mice, pre- and post-challenge with i.d. OVA on day 10 of the AD-associated acute itch flare model. n > 20 basophils per group. ****p < 0.0001 by unpaired Student’s t-test. n = 3 mice.
(G) Percentage (%) of basophils interacting with nerve fibers in the cheek skin of basophil-neuron dual reporter chimeric mice, pre- and post-challenge with i.d. OVA on day 10 of the AD-associated acute itch flare model. n > 20 basophils per group. **p < 0.01 by Chi-square test. n = 3 mice.
(H) Basophil-nerve interaction durations normalized to cell number of basophils in the skin of basophil-neuron dual reporter chimeric mice, pre- and post-challenge with i.d. OVA on day 10 of the AD-associated acute itch flare model. n > 20 observed basophils per group. *p < 0.05 by unpaired Student’s t-test. n = 3 mice.
Data are represented as mean ± SD in (F and H).
To examine whether basophils can acquire the ability to interact with sensory neurons in vivo, we generated bone marrow chimeric mice by transferring bone marrow cells from Mcpt8-Cre-YFP donors into irradiated sensory neuron reporter (NaV1.8-TdTomato) mice (Figure 6C). Intravital two-photon imaging confirmed the presence of round and immotile basophils in the skin of sensitized (MC903 + OVA) chimeric mice prior to allergen challenge (Figure 6D; Movie S3A). As observed previously, allergen (OVA) challenge rapidly induced basophil motility with track speeds ranging from 2–6 µm/min (Figure 6E; Movie S2) and polarized morphology (Figure 6F) consistent with a migratory phenotype (Rappel and Edelstein-Keshet, 2017). Strikingly, the motile basophils were seen migrating through the dermis and making frequent and extended apparent contacts with sensory nerve fibers (Figures 6D, 6G, and 6H; Movie S3B). Taken together, these data demonstrate that basophils are capable of directly interacting with sensory nerve fibers in the skin.
The LTC4-CysLTR2 Neuroimmune Axis Underlies Acute Itch Flares
Given that basophils interact with sensory neurons and are a significant source of LTs, we sought to investigate the neuronal mechanisms by which LTs promote acute itch flares. Generated from arachidonic acid via the 5-LOX pathway, LTs are a diverse family comprised of LTA4, LTB4, LTC4, LTD4, and LTE4 (Luster and Tager, 2004; Schauberger et al., 2016). LTB4 and LTC4 have previously been shown to function as pruritogens in mice (Andoh and Kuraishi, 1998; Fernandes et al., 2013; Solinski et al., 2019). To determine which LT likely mediates allergen-provoked acute itch flares, we measured serum LTB4 and LTC4 levels in sensitized (MC903 + OVA) WT mice challenged with i.d. OVA or BSA. Strikingly, in contrast to LTB4 (Figure 7A), higher levels of LTC4 were detected in mice challenged with i.d. OVA compared to mice challenged with i.d. BSA (Figure 7B). In addition, when we sort-purified basophils from sensitized (MC903 + OVA) and unsensitized (EtOH + OVA) WT mice and stimulated them ex vivo with OVA, we found elevated LTC4 production from sensitized basophils compared to unsensitized basophils (Figure 7C). More importantly, basophil-depleted Bas-TRECK mice exhibited lower serum LTC4 levels compared to littermate controls following i.d. OVA challenge in the AD-associated acute itch flare model (Figure 7D). Collectively, these data suggest that LTC4 upregulation is dependent on basophils and could be a key pruritogen in allergen-evoked acute itch flares.
Figure 7. The LTC4-CysLTR2 Axis Underlies Acute Itch Flares.

(A) ELISA quantification of serum leukotriene (LT) B4 levels in sensitized (MC903 + OVA) WT mice challenged with i.d. BSA or OVA on day 10 of the AD-associated acute itch flare model. n = 7 mice per group. N.S., not significant by unpaired Student’s t-test.
(B) ELISA quantification of serum LTC4 levels in sensitized (MC903 + OVA) WT mice challenged with i.d. BSA or OVA on day 10 of the AD-associated acute itch flare model. n = 5–6 mice per group. *p < 0.05 by unpaired Student’s t-test.
(C) ELISA quantification of LTC4 levels in supernatants collected from basophils stimulated ex vivo with OVA from unsensitized (EtOH + OVA) or sensitized (MC903 + OVA) WT mice (as in Figure 4G). n = 5–10 mice per group. *p < 0.05 by unpaired Student’s t-test.
(D) ELISA quantification of serum LTC4 levels in sensitized (MC903 + OVA) littermate control and basophil-depleted Bas-TRECK mice challenged with i.d. OVA on day 10 of the AD-associated acute itch flare model. n = 8 mice per group. ***p < 0.001 by unpaired Student’s t-test.
(E) Number of scratching bouts following i.d. injection of saline or N-methyl LTC4 (N-met LTC4, 0.75 µg) in WT mice. n = 6 mice per group. ***p < 0.001 by unpaired Student’s t-test.
(F) Dose-response curves of scratching bouts quantified in WT mice following i.d. challenge with increasing doses of histamine (0–10,000 µg) or N-met LTC4 (0–1.5 µg). n ≥ 3 mice per dosage in each group. ****p < 0.0001 by Two-way ANOVA test.
(G) Schematic for pharmacologic inhibition of CysLTR2 in WT mice pre-administered vehicle or HAMI3379 prior to i.d. OVA challenge on day 10 of the AD-associated acute itch flare model.
(H) Number of scratching bouts following i.d. OVA challenge on day 10 of the AD-associated acute itch flare model in WT mice that were pre-administered vehicle or CysLTR2 antagonist HAMI3379 (HAMI, 0.4 mg/kg; i.p.) on day 9 (two doses) and on day 10 (one dose, 60 minutes prior to challenge). n = 5–8 mice per group. *p < 0.05 by unpaired Student’s t-test.
(I) Schematic for neuronal in vivo CysLTR2 inhibition in WT mice pretreated with daily intracisternal (i.c.) injection of control siRNA or CysLTR2 siRNA from day 7 to day 9 followed by i.d. OVA challenge on day 10 of the AD-associated acute itch flare model.
(J) Representative images of the trigeminal ganglion stained with PGP9.5 (TRITC, red) and CysLTR2 (FITC, green) and the percentage (%) of CysLTR2 positive cells out of PGP9.5 positive neurons in sensitized (MC903 + OVA) WT mice that were pretreated with i.c. control siRNA or CysLTR2 siRNA. Scale bar, 50µm. n = 3 mice per group. **p < 0.01 by unpaired Student’s t-test.
(K) Number of scratching bouts following i.d. OVA challenge on day 10 of the AD-associated acute itch flare model in WT mice that were pretreated with i.c. control siRNA or CysLTR2 siRNA. n = 5 mice per group. **p < 0.01 by unpaired Student’s t-test.
(L) Representative calcium traces of mouse sensory neuron responses to N-met LTC4 stimulation. Calcium levels were measured as a ratio of 340/380 nm fluorescence over time. DRG neurons isolated from WT mice were sequentially stimulated with N-met LTC4 (100 nM), allyl isothiocyanate (AITC, 100 µM), capsaicin (CAP, 500 nM), and KCl (50 mM). Each trace represents one neuron.
(M) Representative Venn diagram depicting the overlapping responses of DRG neuron subsets to N-met LTC4 (100 nM), AITC (100 µM), and CAP (500 nM). n > 200 neurons from a WT mouse.
(N) Percentage (%) of N-met LTC4-responsive neurons out of all KCl-responsive neurons. DRG neurons were isolated from WT, Trpv1−/−, Trpa1−/−, or compound Trpv1−/− Trpa1−/− mice. Each data point represents the percentage of neurons that were responsive to N-met LTC4 in an individual mouse. n = 3 mice (> 200 neurons each) per group. N.S., not significant, **p < 0.01 by unpaired Student’s t-test.
(O) Number of scratching bouts following i.d. injection of N-met LTC4 in WT (control) and compound Trpv1−/− Trpa1−/− mice. n = 4–5 mice per group. *p < 0.05 by unpaired Student’s t-test.
(P) Number of scratching bouts following i.d. OVA challenge on day 10 of the AD-associated acute itch flare model in WT (control) and compound Trpv1−/− Trpa1−/− mice. n = 5–7 mice per group. ***p < 0.001 by unpaired Student’s t-test.
Data are represented as mean ± SD.
See also Figures S5–S7.
Because LTC4 can be converted to LTD4 and metabolized, we used a non-metabolizable form of LTC4, N-methyl LTC4 (N-met LTC4) and confirmed that i.d. injection induces robust scratching behavior in naïve WT mice (Figure 7E). Indeed, dose-response analysis comparing N-met LTC4 to histamine indicates that N-met LTC4 is a more potent pruritogen (Figure 7F). LTC4 has two receptors: CysLTR1 and CysLTR2, both of which are broadly expressed in multiple tissues. However, CysLTR2 is also expressed on sensory neurons (Sasaki and Yokomizo, 2019). To explore which sensory neurons may be responsive to LTC4, we mined a single cell RNA-sequencing (scRNA-seq) database of mouse DRG (Usoskin et al., 2015). Three putative clusters of itch-sensory neurons have been codified by transcriptional profiling: NP1, NP2, and NP3. CysLTR2 is predominantly and selectively expressed by NP3 neurons (Figure S5A) (Solinski et al., 2019; Usoskin et al., 2015). By performing calcium imaging, we found that approximately 12% of DRG neurons responded to N-met LTC4, which almost exclusively overlapped with serotonin (5-HT)-responsive NP3 neurons, but not β-alanine-responsive NP1 or chloroquine-responsive NP2 neurons (Figures S5B and S5C). Therefore, we sought to investigate whether blockade of CysLTR2 signaling could ameliorate acute itch flares in AD-like disease. Indeed, systemic CysLTR2 blockade with the antagonist HAMI3379 (Figure 7G) significantly reduced allergen-provoked itch flares in sensitized (MC903 + OVA) WT mice (Figures 7H and S6A) while systemic disruption of the LTB4 pathway or CysLTR1 pathway had no effect (Figures S6B and S6C). Further, we sought to test whether disruption of CysLTR2 on neurons would be sufficient to reduce acute itch flares. We performed siRNA in vivo delivery via intracisternal injection to knockdown the expression of CysLTR2 at the neuronal level (Figure 7I) (Li et al., 2019; Liu et al., 2011). Compared to control siRNA, delivery of CysLTR2 siRNA resulted in notable knockdown of CysLTR2 protein as determined by immunofluorescence of sensory trigeminal ganglia (Figure 7J). Importantly, siRNA knockdown of CysLTR2 also significantly inhibited acute itch flares (Figures 7K and S6D). These results indicate that the LTC4-CysLTR2 axis is critical for the pathogenesis of acute itch flares.
The downstream signaling events of CysLTR2 on sensory neurons remain poorly understood. The majority of newly identified itch receptors are GPCRs which depend on various downstream transient receptor potential (TRP) cation channels for their function (Sun and Dong, 2016; Veldhuis et al., 2015). Thus, we hypothesized that CysLTR2, also a GPCR (Evans, 2002), would require TRP signaling for its function in sensory neurons. Using calcium imaging, we found that the N-met LTC4-responsive neurons almost entirely overlap with neurons that respond to both the TRPV1 agonist capsaicin and the TRPA1 agonist allyl isothiocyanate (AITC) (Figures 7L and 7M). However, genetic deletion of Trpv1 or Trpa1 alone was not sufficient to block N-met LTC4-mediated calcium responses (Figure 7N). We then employed compound Trpv1 and Trpa1 double-deficient mice (Feng et al., 2017) and found that dual deletion completely abolished calcium responses in sensory neurons (Figure 7N). These findings mirror our in vivo results that N-met LTC4-induced scratching behavior was unaffected in either Trpv1−/− or Trpa1−/− mice compared to their respective littermate controls (Figures S7A and S7B). In contrast, Trpv1−/− Trpa1−/− mice exhibited significant reduction in itch behavior in response to N-met LTC4 (Figure 7O). Similar to N-met LTC4 injection, Trpv1−/− Trpa1−/− mice exhibited a reduction in OVA-mediated acute itch flares (Figure 7P) while having no effect on the underlying chronic spontaneous itch (Figure S7C). Collectively, we demonstrate that acute itch flares in the inflammatory state employ the LTC4-CysLTR2 pathway which can utilize either TRPV1 or TRPA1 to promote itch signaling in sensory neurons.
DISCUSSION
Atopy is defined as a predisposition to allergen hypersensitivity and enhanced IgE production (Coca and Cooke, 1923; Justiz Vaillant and Jan, 2020). The family of atopic diseases include AD, allergic rhinitis, asthma, and food allergy. Despite being a canonical atopic disorder, the role of IgE in AD remains surprisingly unclear. Indeed, clinical trials with anti-IgE mAb (omalizumab) have been met with mixed results in AD (Deleanu and Nedelea, 2019). Our study suggests, however, that IgE is critical, not for chronic itch development, but for promoting acute itch flares. Thus, the failures of these clinical trials may have been due to an inability to capture the rapid and dynamic changes in itch by routine clinical assessments. Our high-resolution analysis of phase 3 clinical trial data uncovered that a larger proportion of patients with AD exhibit acute itch flares than previously recognized. Further, we found that the presence of allergen-specific IgE in patients with AD may predispose them to the development of acute itch flares. Thus, our findings demonstrate that heterogeneous forms of itch with unique underlying mechanisms can co-exist within one chronic condition.
Basophils have been suggested to induce pruritus due to their expression of a multitude of pruritogens such as histamine and type 2 cytokines (Steinhoff et al., 2018). However, this has remained unstudied. Thus, we undertook a chemogenetic gain-of-function approach to demonstrate that selective activation of basophils alone is sufficient to induce itch in mice. Although basophils critically promote AD-like skin inflammation (Imai et al., 2019; Kim et al., 2014a; Walsh et al., 2019), surprisingly, we found that they do not mediate chronic spontaneous itch. Furthermore, our study reveals that while mast cells and histamine are critical for acute itch in the steady-state, basophils and LTC4 mediate acute itch flares in AD-like disease. Thus, our findings provide insight into the long-standing controversy about the role of mast cells and histamine in atopy and unveil a role for basophils in mediating itch.
Although our study shows that both mast cells and basophils can induce itch in response to the same allergen, in the setting of AD-associated inflammation, basophils emerge as the key effector cell. It is well-known that cytokines like IL-3 can prime basophils to become more reactive to IgE stimulation (Brunner et al., 1993). Indeed, we found that both murine and human basophils upregulate FcERIα in the setting of AD-associated inflammation. Taken together, these findings suggest that AD-associated inflammation enhances the capacity of basophils to mediate allergen-induced itch. While our current study focused on IgE-dependent itch, we speculate that basophils may employ other mechanisms as well to promote itch in other settings. Indeed, in mast cells, two distinct itch-promoting pathways have recently emerged: 1) histaminergic, IgE-dependent itch and 2) non-histaminergic, Mas-related G protein-coupled receptor (Mrgpr) B2-dependent itch (Meixiong et al., 2019). Recent studies have shown that the human ortholog of MrgprB2, MRGPRX2, is expressed on human basophils (Wedi et al., 2020). Thus, future studies will be required to understand the precise role of MRGPRs and other GPCRs on basophils and their potential role in itch.
A striking observation in our study was that LTC4 almost exclusively activated a subpopulation of sensory neurons referred to as NP3. Interestingly, the receptor for IL-31 (Il31ra) is exclusively expressed on the NP3 population (Usoskin et al., 2015). The first cytokine to be identified as a pruritogen (Cevikbas et al., 2014), therapies targeting IL-31 like nemolizumab are rapidly advancing in clinical trials for AD and other chronic itch disorders (Kabashima et al., 2018; Ruzicka et al., 2017; Stander et al., 2020). Recent studies suggest that human basophils may be a cellular source of IL-31 (Raap et al., 2017). Whether basophil-derived IL-31 may synergize with LTC4 to amplify acute itch flares in AD remains to be determined. Collectively, these findings demonstrate that basophils activate a subpopulation of sensory neurons that is strongly associated with inflammatory itch.
It is widely appreciated that mast cells are tissue-resident in nature and mediate a variety of homeostatic neuroimmune processes such as vasoregulation, neuroinflammation, and sensation due to their close proximity with neurons (Gupta and Harvima, 2018; Voehringer, 2013). In contrast, basophils are circulating and generally not present in tissues in the steady-state. However, under pathologic conditions, basophils can be rapidly recruited into inflamed tissue (Miyake and Karasuyama, 2017). Thus, we speculate that basophils are more likely to be proinflammatory in nature and underlie maladaptive neuroimmune processes. Our study provides additional evidence of evolutionarily distinct roles between mast cells and basophils in both health and disease.
STAR ★ METHODS
RESOURCE AVAILABILITY
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Brian S. Kim (briankim@wustl.edu).
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability
This study did not generate any unique datasets or code. All other data supporting the findings of this study are available in the manuscript or the supplementary materials and available upon request to the lead contact author.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Human subjects
For analysis of itch pattern and allergen-specific IgE related to atopic dermatitis (AD), numerical rating scale (NRS) itch scores and serum allergen-specific IgE repertoire were respectively reviewed on N = 159 subjects from the placebo group of the SOLO1 and SOLO2 phase 3 clinical trials (Simpson et al., 2016). Patient serum allergen-specific IgE repertoires were measured using the ImmunoCAP system (Phadia AB, Uppsala, Sweden) for a panel of 12 allergens/antigens including Dermatophagoides pteronyssinus, Dermatophagoides farinae, Canadida albicans, Pityrosporum, cat dander, mountain juniper, white oak, olive tree, Staphylococcal enterotoxin A, Staphylococcal enterotoxin B, Japanese cedar, and wall pellitory. Positive levels for each allergen were defined as an allergen-specific IgE concentration ≥ 0.35 kU/L (McGowan et al., 2015).
In order to examine human basophil phenotypes, 12 adult patients with AD, who visited the Dermatology Clinic at the Perelman School of Medicine at the University of Pennsylvania from October 2018 to June 2019, and 12 age- and sex-matched healthy controls were recruited. Patients were diagnosed with AD according to the U.K. Working Party’s Diagnostic Criteria (Williams et al., 1994). The disease severity was assessed according to Patient Oriented Eczema Measure (POEM) (Charman et al., 2013). After informed consent was obtained, peripheral blood was collected and peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll density gradient purification and frozen at −80˚C until assayed. These studies were conducted in accordance with the provisions of the Declaration of Helsinki and International Conference on Harmonisation Good Clinical Practice guidelines. All the patients provided written informed consent before participation in the studies. The local institutional review board or ethics committee at each study center oversaw study conduct and documentation.
Research animals
Wild-type (WT) C56Bl/6J, C57BL/6-KitW-sh/W-sh (also known as Sash−/−), Mcpt8-Cre, R26-LSL-Gq-DREADD, Ai9, and Alox5−/− mice were purchased from Jackson Laboratories. Bas-TRECK and Ige−/− mice were donated by Dr. Masato Kubo (RIKEN, Yokohama Institute, Japan). Trpv1−/−, Trpa1−/−, and compound Trpv1−/− Trpa1−/− double-deficient mice were donated by Dr. Hongzhen Hu. NaV1.8-Cre mice were provided by Dr. Rohini Kuner (Heidelberg University). PirtGCamp3/+ mice were donated by Dr. Qin Liu and Dr. Xinzhong Dong. Mcpt8-Cre:R26-LSL-Gq-DREADD mice were obtained by crossing Mcpt8-Cre animals with R26-LSL-Gq-DREADD animals. NaV1.8-TdTomato mice were generated by crossing NaV1.8-Cre mice with Ai9 mice. All experiments were conducted with the approval of the Washington University Institutional Animal Care and Use Committee. Animals were housed on a standard 12:12 hour light-dark cycle with free access to food and water. For dorsal root ganglia (DRG) neuron isolation, 4–6-week-old mice were used. For other experiments, 8–12-week-old mice were used. Experiments were performed on independent cohorts of male and female mice and no differences between sexes were observed.
METHOD DETAILS
Mouse model
The right cheeks of mice were shaved 2 days prior to any treatments (day −2). To induce AD-associated acute itch flares, bilateral ear skin were topically treated with 0.5 nmol of MC903 (calcipotriol, Tocris Bioscience) in 15 µL of 100% ethanol and then with 20 µL of 5 mg/mL ovalbumin (OVA, Sigma-Aldrich) in phosphate-buffered saline [PBS] daily for 10 days (day 0 to day 9) to sensitize mice. Unsensitized control mice were treated with 15 µL of 100% ethanol (vehicle control) followed by 20 µL of 5 mg/mL OVA in PBS. On day 10, mice were given intradermal (i.d.) injection of 20 µL of 2.5 mg/mL OVA in 0.9% saline in their right cheek in order to provoke acute itch responses. To test the specificity of the allergen response, sensitized (MC903 + OVA) mice were i.d. challenged with 20 µL of an irrelevant allergen bovine serum albumin (BSA, 3.7 mg/mL in 0.9% saline; Sigma-Aldrich). As an alternative to this model, we passively sensitized mice with OVA in the setting of AD-like disease. The AD-like disease was induced by topical MC903 (0.5 nmol in 15 µL of 100% ethanol) administration on bilateral ear skin of mice daily from day 0 to day 9. On day 8 and day 9, mice received an intravenous (i.v.) injection of 100 µL of 20 µg/mL anti-OVA IgE antibody (Bio-Rad) in PBS to induce passive sensitization. Control unsensitized mice received an i.v injection with 100 µL PBS alone. On day 10, an acute itch flare was evoked by i.d. injection of 20 µL of 2.5 mg/mL OVA in 0.9% saline into the right cheek. To assess allergen-mediated acute itch in the steady-state, mice were passively sensitized to OVA via i.v. injection of 100 µL of 20 µg/mL anti-OVA IgE antibody in PBS on day 0 and day 1. Control unsensitized mice received an i.v. injection of 100 µL PBS alone. On day 2, mice were challenged i.d. with 20 µL of 2.5 mg/mL OVA in 0.9% saline in their right cheek to provoke acute itch. The systemic sensitization model was induced as previously described (Huang et al., 2016). In brief, OVA (0.2 mg/mL in PBS; Sigma-Aldrich) was prepared fresh each time and was emulsified with an equal volume of Imject Alum (alum; Thermo Scientific). Mice received intraperitoneal (i.p.) injection of 200 µl of OVA + alum mixture or control PBS + alum mixture on day 0 and day 9. On day 13, mice were challenged i.d. with 20 µL of 2.5 mg/mL OVA in 0.9% saline in their right cheek. To test acute itch flares in the setting of systemic sensitization along with AD-like disease, we sensitized mice with i.p. injection of 200 µL of OVA + alum mixture (prepared as above) on day 0 and day 9. Concurrently, bilateral ear skin were treated topically with MC903 (0.5 nmol in 15 µL of 100% ethanol) from day 3 to day 12. To provoke acute itch flares, mice were challenged with i.d. injection of 20 µL of 2.5 mg/mL OVA in 0.9% saline in the right cheek on day 13. For all models, scratching behavior was recorded for 60 minutes before i.d. allergen (OVA or BSA) challenge and for 70 minutes after challenge.
Skin inflammation assessment and histopathology
To assess mouse ear skin inflammation induced by MC903 + OVA, ear thickness was measured daily with dial calipers as previously described (Kim et al., 2013; Kim et al., 2014a). Prior to i.d. OVA challenge on experimental day 10 of the AD-associated acute itch flare model, mice were euthanized and tissues were harvested for analysis. The ear skin were fixed in 4% paraformaldehyde (PFA; Thermo Scientific) and embedded in paraffin before sectioning and staining with Hematoxylin & Eosin (H&E). Slides were imaged using the NanoZoomer 2.0-HT System (Hamamatsu).
Pharmacologic administration in mouse models
In our passive sensitization model, mice were treated with an i.p. injection of 100 µL the H1R antagonist olopatadine (3 mg/kg in PBS; Sigma-Aldrich) and subcutaneous (s.c.) injection (nape) with 100 µL of the H4R antagonist JNJ7777120 (20 mg/kg in PBS; Sigma-Aldrich) 30 minutes prior to i.d. OVA challenge.
In the AD-associated acute itch flare model, mice were treated 30 minutes prior to i.d. OVA challenge (day 10) with i.p. injection of 100 µL of the H1R antagonist olopatadine (3 mg/kg in of PBS; Sigma-Aldrich) and s.c. injection (nape) of 100 µL of the H4R antagonist JNJ7777120 (20 mg/kg in of PBS; Sigma-Aldrich). Protease-activated receptor 2 (PAR2) was inhibited by the administration of 100 µL of FSLLRY-NH2 (7.5 mg/kg in 0.9% saline; Sigma-Aldrich) i.p. 30 minutes prior to i.d. OVA challenge (day 10). To interrupt serotonin synthesis, 200 µL of p-Chlorophenylalanine (pCPA, 150 mg/kg in 0.9% saline; Tocris Bioscience) was i.p. injected on day 7, day 8, and day 9 of the 10-day sensitization process. Mice were gavaged with 100 µL of the leukotriene (LT) pathway inhibitor zileuton (50 mg/kg in 0.5% cellulose; Sigma-Aldrich) 60 minutes prior to assessment of chronic spontaneous itch (day 10) or 60 minutes prior to i.d. OVA challenge when assessing acute itch flare behavior (day 10). The LTB4 receptor antagonist CP-105,696 (100 µL of 3 mg/kg CP-105,696 in 0.5% cellulose; Sigma-Aldrich) was administered by oral gavage 60 minutes prior to i.d. OVA challenge. Oral gavage of 100 µL of the CysLTR1 antagonist zafirlukast (10 mg/kg in PBS; Sigma-Aldrich) was given twice on day 9 in addition to once on day 10, 60 minutes prior to i.d. OVA challenge. Mice were given two 100 µL i.p. injections of the CysLTR2 antagonist HAMI3379 (0.4 mg/kg in of PBS; Cayman) on day 9, and once on day 10, 60 minutes prior to assessment of chronic spontaneous itch (day 10) or 60 minutes prior to i.d. OVA challenge for the assessment of acute itch flare behavior (day 10).
Behavioral tests
All applicable behavioral tests were performed and analyzed with the experimenter blinded to genotype with the exception of the Sash−/− strain given the genotypic differences in coat color. Itch behavior experiments were performed between 8 a.m. and 12 p.m CST. Two days prior to recording, animals were habituated in the test chamber for 90 minutes and underwent a series of three mock i.d. injections where a capped needle was pressed against the shaved cheek of the experimental mouse. On the day of behavioral test, animals were allowed to acclimatize to the test chamber for 10 minutes prior to video recording. Video recordings were manually scored to assess the number of scratching bouts during a 30-minute period. A bout of scratching was defined as an instance of hind paw directed continuous scratching of the back, cheeks, or ears that ended when the mouse placed their hind paw in their mouth or to the chamber floor. To test the pruritogenic capability of N-methyl (N-met) LTC4, histamine and basophil-derived factors, 20 µL of solution was i.d. injected into the shaved right cheeks of mice before immediate assessment of itch behavior. Only scratching bouts directed toward the site of injection were scored. For N-met LTC4 (Cayman) and histamine (Sigma-Aldrich), a range of doses were tested, 0–10,000 µg and 0–1.5 µg respectively. For basophil-derived factors, we collected supernatants from OVA-stimulated basophils. Briefly, 6 × 104–8 × 104 basophils, sorted from mouse blood and cultured overnight, were incubated with 2.5 mg/ml OVA in 0.9% saline (200 µL) at 37ºC and 5% CO2 for 60 minutes. Collected supernatants was delivered i.d. undiluted (20 µL).
ELISA
To measure mouse serum OVA-specific IgE levels, 0.5–1 mL blood samples were collected into 1.5 mL microcentrifuge tubes and allowed to clot for 60 minutes at room temperature. Tubes were then centrifuged for 10 minutes at 1,000g at 4˚C. Sera were collected and stored at −80˚C until OVA-specific IgE was quantified using the mouse OVA-IgE ELISA kit (Biolegend) according to manufacturer’s instructions. To measure mouse serum LTB4 and LTC4 levels, 0.5–1 mL blood samples were collected into 1.5 mL microcentrifuge tubes 30 minutes after allergen challenge and were allowed to clot for 60 minutes at room temperature. Tubes were then centrifuged for 15 minutes at 1,000g at 4˚C. Serum was collected and stored at −80˚C until LTB4 and LTC4 were respectively quantified using the mouse LTB4 ELISA kit (Biomatik) and mouse LTC4 ELISA kit (LS Bio) according to manufacturer’s instructions.
To test supernatant levels of LTC4 derived from basophils, sort-purified basophils (6 × 104– 8 × 104 cells) were stimulated with 0.25 mg/mL OVA in 200 µL of saline at 37ºC and 5% CO2 in a 96 plate well for 60 minutes. After centrifuge for 20 minutes at 1,000g at 4˚C, supernatants were collected and stored at −80˚C until LTC4 was quantified using the mouse LTC4 ELISA kit (LS Bio) according to manufacturer’s instructions.
Flow cytometry
For human studies, thawed PBMCs were stained with Zombie UV viability dye (1:500; Biolegend) at room temperature for 20 minutes, washed and then stained with primary antibodies on ice for 30 minutes before being acquired on a BD Fortessa X-20 (BD Biosciences). Human Basophils were defined as live CD123+ FcεRIα+ cells that lacked expression of c-Kit and lineage (Lin) markers CD3, CD4, CD19, CD14, CD34, and CD56. arker CD203c was included in the primary antibodies to reveal the physiological state of human basophils. For animal studies, 50–100 µL of blood was collected into EDTA coated tubes, followed by RBC lysis using RBC lysis buffer (Sigma-Aldrich) at room temperature for 5 minutes twice and washed by PBS once. All cells were stained with Zombie UV viability dye (1:500; Biolegend) for viability at room temperature for 20 minutes, followed by primary antibodies on ice for 30 minutes prior to data acquisition on a BD Fortessa X-20 (BD Biosciences). Basophils were defined as live CD49b+ FcεRIα/IgE+ cells that were negative for expression of c-Kit and Lin markers CD3e, CD5, CD11c, CD19, and NK1.1. The mouse basophil canonical activation marker CD200R was also stained. All flow cytometry data were analyzed with Flowjo v10 software (Tree Star).
Immunofluorescence staining
Immunofluorescence imaging was performed as previously described (Huang et al., 2018). For in vivo immunofluorescence staining of basophil degranulation in our AD-associated acute itch flare model, the right cheek skin (OVA challenge site) of Sash−/− mice were harvested 30 minutes following i.d. OVA challenge. Then, samples were fixed in 4% PFA (Thermo Scientific) for 4–6 hours at 4ºC and incubated in 30% sucrose overnight. Tissues were embedded in optimal cutting temperature (OCT) medium (Sakura) and sectioned at 12 µm on a cryotome (Leica). Sections were dried and stained with 200 µL avidin-Texas Red (5 µg/mL; Invitrogen) in room temperature for 30 minutes before imaging on a Nikon Al Confocal Laser Microscope with NIS-Elements imaging software (Nikon Instruments). To detect basophil degranulation ex vivo, 0.5–1 mL of blood from naïve Mcpt8-Cre:R26-LSL-Gq-DREADD and littermate control Mcpt8-Cre-YFP mice was collected into EDTA-coated tubes. Samples were then treated with RBC lysis buffer (Sigma-Aldrich) at room temperature for 5 minutes twice and washed by PBS once. Following negative selection for CD4, CD8a, CD11c, and B220 using a Mouse Streptavidin RapidSpheres Isolation Kit (Stemcell), purified leukocytes from each mouse were incubated with 200 µL clozapine-N-oxide (CNO, 400 µM in PBS; hello bio) and 200 µL avidin-Texas Red (5 µg/mL; Invitrogen) simultaneously for 30 minutes at room temperature before imaging on a Nikon Al Confocal Laser Microscope with NIS-Elements imaging software (Nikon Instruments). To examine the knockdown effect of in vivo siRNA treatment, WT mice that received intracisternal injection of siRNA were harvested on day 10 of the AD-associated acute itch flare model. Then mice trigeminal ganglia were dissected and fixed in 4% PFA (Thermo Scientific) for 4–6 hours at 4ºC followed by incubation in 30% sucrose overnight. Tissues were embedded in OCT medium (Sakura) and sectioned at 12 µm on a cryotome (Leica). For further staining, sectioned tissues were blocked with 10% goat serum (Abcam) in UltraCruz blocking reagent (Santa Cruz Biotechnology) for 30 minutes and incubated with primary antibodies at 4 °C overnight. After rinsing, sections were incubated with secondary antibodies for 1 hour at room temperature. Images were taken and analyzed using a Nikon Al Confocal Laser Microscope with NIS-Elements imaging software (Nikon Instruments). Primary antibodies used: anti-CysLTR2 receptor monoclonal antibody (B-7; Santa Cruz Biotechnology; 1:100) and guinea pig anti-PGP9.5 polyclonal antibody (abcam; 1:500). Secondary antibodies used: m-IgGκ BP-FITC (Santa Cruz Biotechnology; 1:100) and Cy3 AffiniPure donkey anti-guinea pig IgG (H+L) (Jackson ImmunoResearch; 1:500).
Basophil in vivo chemogenetic activation
To chemogenetically activate basophils in vivo, Mcpt8-Cre:R26-LSL-Gq-DREADD mice received a i.p. injection of 50 µL of 1 mg/kg CNO (hello bio) in PBS. The scratching behavior was video recorded for 60 minutes after CNO injection and the itch behavior was quantified by manually counting the number of scratching bouts from 10 minutes to 40 minutes after CNO injection.
Basophil in vivo depletion
Basophil depletion was performed as previously described (Noti et al., 2013). Briefly, for pharmacologic depletion of basophils, WT mice received i.v. injections of 100 µL of purified anti-mouse CD200R3 antibody (1.0 mg/mL; Biolegend) or Rat IgG2a, κ isotype control (Biolegend) on day 7 and day 9 of the AD-associated acute itch flare model. For basophil genetic depletion, Bas-TRECK and littermate control mice were given an i.p. injection of 500 ng diphtheria toxin (DT; Sigma-Aldrich) diluted in 100 µL PBS daily on two consecutive days prior to the last day of the disease model. Specifically, in the AD-associated acute itch flare model (MC903 + OVA), mice were treated with DT i.p. on experimental day 8 and day 9. In the mouse model sensitized with i.p. OVA + alum, DT was i.p. injected on day 11 and day 12. The effect of depletion was confirmed by assessing blood basophil levels using flow cytometry prior to i.d. OVA challenge on the last day of each model.
Basophil sorting and cultures
Basophil sorting and cultures were performed as previously described (Hussain et al., 2018). Briefly, 0.5–1 mL mouse blood was collected into EDTA coated tubes. Red blood cells were lysed using RBC lysis buffer (Sigma-Aldrich). All live, CD45+ Lin- CD49b+ FcεRIα/IgE+ basophils from donor mice were sort-purified on an Aria II (BD Biosciences). Isolated basophils (6 × 104 – 8 × 104 cells /mL) were then cultured in Mast Cell Medium (RPMI 1640, 15% fetal bovine serum [FBS; Sigma-Aldrich], 100 U/mL penicillin [GIBCO], 100 µg/mL streptomycin [GIBCO], 2.9 mg/mL glutamine [Corning], 50 mM 2-mercaptoethanol [GIBCO], 1 mM sodium pyruvate [Corning], 1 × nonessential amino acids [Corning], 10 mM HEPES) overnight at 37ºC and 5% CO2 before further stimulation.
DRG neuronal cultures
Mouse DRG neurons were extracted, dissociated, and cultured as previously described (Kim et al., 2014b; Oetjen et al., 2017). Briefly, 4–6 week-old mice were euthanized by CO2 inhalation. The DRGs were dissected and enzymatically dissociated with 1 mL Ca2+/Mg2+-free HBSS containing collagenase type I (342 U/mL; GIBCO) and dispase II (3.8 U/mL; GIBCO) for 30 minutes at 37ºC. After digestion, neurons were gently triturated, pelleted, and then resuspended in Neurobasal-A culture medium (GIBCO) containing 2% B-27 supplement (GIBCO), 100 U/mL penicillin plus 100 mg/mL streptomycin (Sigma-Aldrich), 100 ng/mL nerve growth factor (NGF, Sigma-Aldrich), 20 ng/mL glial cell-derived neurotrophic factor (GDNF; Sigma-Aldrich), and 10% FBS (Sigma-Aldrich). Neurons were then plated on 8 mm glass cover slips pre-coated with poly-D-lysine (PDL, 20 µg/mL) and laminin (20 µg/mL). Cells were cultured overnight at 37ºC and 5% CO2 before use in calcium imaging studies.
Calcium imaging of mouse DRG neurons
All reagents were applied to DRG neurons by perfusion except for basophil-derived factors, which were manually loaded by gently pipetting the culture supernatant into the recording chamber. Only sensory neurons that were responsive to a final challenge of KCl (50 mM) were used in downstream analyses. For cultured DRG neurons isolated from calcium reporter PirtGCaMP3/+ mice, fluorescence was recorded at 488 nm excitation wavelength using an inverted Nikon Ti-S microscope (Nikon Instruments) with CoolSNAP HQ2 CCD camera (Photometrics) and NIS-elements software (Nikon Instruments). Cells were considered responsive to a particular stimulus if they exhibited at least a 15% increase in fluorescent intensity to baseline. For non-reporter mice (WT, Trpv1−/−, Trpa1−/−, and compound Trpv1-/Trpa1−/− mice), cultured DRG neurons were first loaded with the calcium indicator dye Fura-2 AM (4 µM; Invitrogen) in DRG culture medium for 30 minutes at 37ºC. Before use, cells were washed and incubated in calcium imaging buffer for at least 15 minutes at room temperature as previously described (Oetjen et al., 2017). Images were acquired with alternating 340 nm and 380 nm excitation wavelengths (F340, F380) using an inverted Nikon Ti-S microscope (Nikon Instruments) with CoolSNAP HQ2 CCD camera (Photometrics) and NIS-elements software (Nikon Instruments). Cells were considered responsive if they demonstrated a change in fluorescence ratio (340/380) > 15% to baseline. Basophil-derived factors were collected from OVA-stimulated cultures. Briefly, sort-purified and cultured basophils (6,000–8,000) were centrifuged for 5 minutes at 400g at 4 ˚C. Then, supernatants were carefully removed and the remaining basophils were stimulated with 0.25 mg/mL OVA in 200 µL of calcium imaging buffer at 37ºC and 5% CO2 for one hour. After centrifugation for 20 minutes at 1000g at 4 ˚C, supernatants were collected and applied to cultured DRG neurons (100 µL per culture slide) to test for neuronal activation as indicated by an intracellular calcium influx response. OVA solution (0.25 mg/mL in calcium imaging buffer) or N-met LTC4 (100 nM; Cayman) was applied by perfusion onto cultured DRG neurons to test for calcium responses. For additional characterization, responsiveness to the following reagents was also assessed: the TRPV1 agonist capsaicin (500 nM; Sigma-Aldrich); the TRPA1 agonist allyl isothiocyanate (AITC; 100 µM; Sigma-Aldrich); serotonin (5-HT) (200 µM; Sigma-Aldrich); β-alanine (1 mM; Sigma-Aldrich); and chloroquine (2 mM; Sigma-Aldrich).
Bone marrow transplant
NaV1.8-TdTomato recipients were sublethally X-ray irradiated (950 cGy) using X-RAD 320 (Precision X-Ray). 10 × 106 bone marrow cells from Mcpt8-Cre-YFP mice were i.v. injected into the recipient mice within 24 hours after radiation. Sulfatrim (sulfamethoxazole/trimethoprim) was added to the drinking water of the irradiated animals (5mL/200mL) from 1 day prior to radiation until 1 week post-radiation and reconstitution. Irradiated mice were used for experimentation 8 weeks following bone marrow transplantation, allowing for full immune reconstitution. Chimerism was tested 4 weeks after reconstitution by measuring the presence of YFP+ cells in the peripheral blood by flow cytometry.
Two-photon microscopy
In vivo two-photon imaging of cell trafficking was performed as previously described (Wang et al., 2012). Briefly, the right cheeks of mice were shaved the day prior to imaging. On day 10 of the AD-associated acute itch model, mice were anesthetized with isoflurane, placed on a warming pad and their right cheeks were secured to the imaging chamber using VetBond (cat #1469SB, 3 M). Time-lapse imaging was performed pre- and post-challenge with i.d. OVA by using a custom-built dual-laser video-rate two-photon microscope. To visualize blood vessels, 20 µL of non-targeted Qtracker 655 vascular labels (Invitrogen) were diluted in 100 µL of PBS and i.v. injected 15–30 minutes before imaging. Qtracker 655-labeled blood vessels and YFP-labeled basophils were excited by a Chameleon Vision II Ti:Sapphire laser (Coherent) tuned to 920 nm in Mcpt8-Cre-YFP mice. Fluorescence emission was detected as red (> 560 nm), green (495–560 nm), and blue (< 495 nm). To assess potential basophil-neuron interactions, YFP-labeled basophils and TdTomato-labeled nerves in bone marrow chimeric mice (Mcpt8-Cre × NaV1.8-TdTomato) were excited by a Chameleon Vision II Ti:Sapphire laser (Coherent) tuned to 950 nm. Fluorescence emission was detected as red (> 540 nm), green (495–540 nm) and blue (< 495 nm). Each plane represents an image of 300 µm by 300 µm at 0.585 µm/pixel. Z-stacks were acquired by taking 31 sequential steps at 2 µm spacing. Image reconstruction and multidimensional rendering were performed with Imaris (Bitplane). Cell track datasets were generated in Imaris using automated spot tracking and analyzed in MotilityLab (2Ptrack.net). In cases where motion artifacts induced z-plane tracking errors, cells were manually tracked using an orthogonal perspective in Imaris. To identify interactions between basophils and nerves, we inferred cell-cell contacts first by looking for evidence that the cells made apparent contacts with nerves in 3D rendered images. We then digitally zoomed into apparent contact regions and rotated them in 3D to confirm that green pixels (basophils) and red pixels (nerves) were in direct apposition. This was performed frame by frame in the videos to measure basophil-nerve interaction durations. The caveat to this approach is that we can only infer cell-cell interactions due to the limits of our image resolution, which is 0.585 µm in X and Y, and 2 µm in the Z-dimension. The interaction duration was then normalized to cell number by dividing the sum of apparent contact durations by the number of cells in each image.
Preparation and intracisternal injection of siRNA
We performed CysLTR2 siRNA knockdown in vivo by delivering siRNA using intracisternal (i.c.) injection as previously described (Li et al., 2019; Liu et al., 2011). The napes of the mice were shaved 2 days prior to the i.c. treatment. RVG-9R trifluoroacetate salt (BECHEM) was dissolved with 10% glucose (Fisher Scientific) to reach the concentration of 1.45 µg/µL. Meanwhile, siGENOME non-targeting siRNA control pools (horizon) or siGENOME mouse CysLTR2 siRNA (horizon) was dissolved with nuclease free water (Thermo Scientific) to reach the final concentration of 1 µg/µL. Before injection, 5 µL of RVG-9R (1.45 µg/µL in 10% glucose) was mixed with 5 µL of siRNA (1 µg/µL in nuclease free water) and incubated at room temperature for 15 minutes. To deliver the RVG-9R + siRNA mixture, adult WT mice were anesthetized with 2% isoflurane and then the mixture of RVG-9R and siRNA (10 µL) was injected slowly into the cisterna magna daily from day 7 to day 9 of the AD-associated acute itch flare model. On experimental day 10, trigeminal ganglia were dissected and stained for CysLTR2 to validate the knockdown effect.
QUANTIFICATION AND STATISTICAL ANALYSIS
Normally distributed group data were expressed as mean ± standard deviation (SD) and statistical significance was determined using the two-tailed Student’s t-test. If data was not normally distributed, median (interquartile range) was used and statistical significance was determined using the Wilcoxon–Mann–Whitney nonparametric test. For tests with multiple comparisons, the Two-way ANOVA test was used. Differences of incidence rate between two groups were compared by Chi-square test or Fisher’s exact test when total N assessed in the contingency tables was less than 40 or when the expected frequency of one or more cells was less than 5. Data from independent experiments were pooled when possible or represent at least two independent replicates. Sample sizes were chosen based on pilot experiments to accurately detect statistical significance as well as considering technical feasibility, resource availability, and ethical animal and sample use. No samples or animals subjected to successful procedures and/or treatments were excluded from analysis. Statistical details can be found in figure legends. All statistical tests were conducted with GraphPad Prism 8. Significance is labeled as: ****p < 0.0001, ***p < 0.001, **p < 0.01, *p < 0.05, N.S., Not Significant.
Supplementary Material
(A) Schematic of allergen sensitization in the atopic dermatitis (AD)-associated acute itch flare model. Wild-type (WT) mice received daily topical treatment with calcipotriol (MC903) + ovalbumin (OVA) to bilateral ears from day 0 to day 9.
(B) Ear thickness (percent change from baseline) of vehicle ethanol (EtOH) control + OVA-treated and MC903 + OVA-treated WT mice from day 0 to day 10. n = 12–13 mice per group. ****p < 0.0001 by two-way ANOVA test.
(C)Representative clinical images (left) and H&E histopathology images (right) of lesional ear skin from EtOH control + OVA-treated and MC903 + OVA-treated WT mice on day 10 of the AD-like disease model. Scale bar = 50 µm.
(D) Schematic of naïve mice that received intravenous (i.v.) injection of anti-OVA IgE antibody (passive sensitization) on day 0 and day 1 followed by intradermal (i.d.) OVA challenge into cheek and assessment of scratching behavior on day 2.
(E) Number of scratching bouts following i.d. OVA challenge in WT mice that were pretreated with i.v. phosphate-buffered saline (PBS) control or i.v. anti-OVA IgE antibody. n = 5–6 mice per group. **p < 0.01 by unpaired Student’s t-test.
(F) Number of scratching bouts following i.d. OVA challenge in sensitized (i.v. anti-OVA IgE antibody) littermate control and mast cell-deficient Sash−/− mice. n = 5–6 mice per group. ****p < 0.0001 by unpaired Student’s t-test.
(G) Number of scratching bouts following i.d. OVA challenge in sensitized (i.v. anti-OVA IgE antibody) WT mice that were pretreated with vehicle or antihistamines olopatadine (OLP, 3 mg/kg; intraperitoneal [i.p.]) and JNJ7777120 (JNJ, 20 mg/kg; subcutaneous injection into the nape) 30 minutes prior to i.d. OVA challenge. n = 4–5 mice per group. *p < 0.05 by unpaired Student’s t-test.
(H) Schematic of acute itch flares in the passive sensitization model in AD-like disease. Naïve mice were topically treated with MC903 on the ear skin from day 0 to day 9 and received i.v. injection of PBS control or anti-OVA IgE antibody on day 8 and day 9. On day 10, i.d. injection of OVA was administered into non-lesional cheek skin of mice. Chronic spontaneous itch and acute itch flares were recorded prior to and following i.d. OVA challenge on day 10, respectively.
(I) Number of scratching bouts prior to i.d. OVA challenge (chronic spontaneous itch; left) and following i.d. OVA challenge (acute itch flares; right) in unsensitized (i.v. PBS) and sensitized (i.v. anti-OVA IgE antibody) WT mice with AD-like disease. n = 4–5 mice per group. N.S., not significant, **p < 0.01 by unpaired Student’s t-test.
(J) Number of scratching bouts prior to i.d. OVA challenge (chronic spontaneous itch; left) and following i.d. OVA challenge (acute itch flares; right) in sensitized (i.v. anti-OVA IgE antibody) littermate control and mast cell-deficient Sash−/− mice with AD-like disease. n = 6 mice per group. N.S., not significant by unpaired Student’s t-test.
Data are represented as mean ± SD.
(A) Schematic of the AD-like disease model. Topical vehicle EtOH control or MC903 was applied to bilateral ear skin of WT mice from day 0 to day 9. Blood basophils from WT mice were assessed for phenotypic alterations on day 2, day 4, and day 6 of the AD-like disease model.
(B and C) CD200R (B) and FcεRIα/IgE (C) expression measured by mean fluorescence intensity (MFI) on blood basophils in EtOH- or MC903-treated WT mice on day 2, day 4, and day 6 of the AD-like disease model. n = 3–5 mice per group. Data are represented as mean ± SD. N.S., not significant, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 by unpaired Student’s t-test.
(A) ELISA quantification of serum OVA-specific IgE (left) and assessment of chronic spontaneous itch (right) in isotype- or anti-CD200R3 monoclonal antibody-administered WT mice on day 10 of the AD-associated acute itch flare model. Scratching behavior was recorded prior to i.d. OVA challenge for chronic spontaneous itch assessment. n = 8–10 mice per group. N.S., not significant by unpaired Student’s t-test.
(B) ELISA quantification of serum OVA-specific IgE (left) and assessment of chronic spontaneous itch (right) in littermate control and basophil-depleted Bas-TRECK mice on day 10 of the AD-associated acute itch flare model. To quantify chronic spontaneous itch bouts, scratching behavior was recorded prior to i.d. OVA challenge. n = 9–10 mice per group. N.S., not significant by unpaired Student’s t-test.
(C) Schematic of Imject Alum (alum) and OVA systemic sensitization model. Naïve WT mice received intraperitoneal (i.p.) injection of adjuvant alum and OVA mixture on day 0 and day 9 followed by i.d. OVA challenge into cheek skin and assessment of scratching behavior on day 13.
(D) Number of scratching bouts following i.d. OVA challenge in unsensitized (i.p. alum + PBS) or sensitized (i.p. alum + OVA) WT mice. n = 3–4 mice per group. ****p < 0.0001 by unpaired Student’s t-test.
(E) Number of scratching bouts following i.d. OVA challenge in sensitized (i.p. alum + OVA) littermate control and mast cell-deficient Sash−/− mice. n = 4–6 mice per group. ****p < 0.0001 by unpaired Student’s t-test.
(F) Number of scratching bouts following i.d. OVA challenge in sensitized (i.p. alum + OVA) littermate control and basophil-depleted Bas-TRECK mice. n = 4–7 mice per group. N.S., not significant by unpaired Student’s t-test.
(G) Schematic of acute itch flares in the i.p. alum + OVA systemic sensitization model in the context of AD-like disease. Naïve mice received i.p. alum + OVA on day 0 and day 9. From day 3 to day 12, mice were topically treated with MC903 on the ear skin followed by an i.d. injection of OVA into non-lesional cheek skin on day 13. Chronic spontaneous itch and acute itch flares were recorded prior to and following i.d. OVA challenge on day 13, respectively.
(H) Number of scratching bouts prior to i.d. OVA challenge (chronic spontaneous itch; left) and following i.d. OVA challenge (acute itch flares; right) in littermate control and mast cell-deficient Sash−/− mice sensitized with i.p. alum + OVA in the context of AD-like disease on day 13. n = 3–4 mice per group. N.S., not significant by unpaired Student’s t-test.
(I) Number of scratching bouts prior to i.d. OVA challenge (chronic spontaneous itch; left) and following i.d. OVA challenge (acute itch flares; right) in littermate control and basophil-depleted Bas-TRECK mice sensitized with i.p. alum + OVA in the context of AD-like disease on day 13. n = 4–10 mice per group. N.S., not significant, *p < 0.05 by unpaired Student’s t-test.
(J) Representative calcium traces of mouse dorsal root ganglia (DRG) responses to OVA. Calcium responses were measured by fluorescence (Fluor.) intensity (488 nm). Neurons isolated from PirtGCaMP3/+ mice were sequentially stimulated with OVA (0.25 mg/mL in calcium imaging buffer), capsaicin (CAP, 500 nM), and KCl (50 mM). Each color trace represents one neuron.
(K) Percentage (%) of OVA-responsive and capsaicin-responsive neurons out of all KCl-responsive neurons. Each data point represents the percentage of responsive neurons from one individual PirtGCaMP3/+ mouse. n = 4 mice (> 200 neurons each). ****p < 0.0001 by paired Student’s t-test.
Data are represented as mean ± SD.
(A) ELISA quantification of serum OVA-specific IgE levels in littermate control and Alox5−/− mice on day 10 of the AD-associated acute itch flare model. n = 5–7 mice per group.
(B) Assessment of chronic spontaneous itch prior to i.d. OVA challenge on day 10 of the AD-associated acute itch flare model in WT mice that were pre-administered vehicle or zileuton (50 mg/kg; gavage) 60 minutes prior to chronic spontaneous itch assessment (left).
Assessment of chronic spontaneous itch prior to i.d. OVA challenge on day 10 of the AD-associated acute itch flare model in littermate control and Alox5−/− mice (right). n = 4–8 mice per group.
Data are represented as mean ± SD. N.S., not significant by unpaired Student’s t-test.
(A) Expression of selected genes in mouse DRG neuron populations NP1, NP2, NP3, PEP1 and PEP2 based on single cell RNA-sequencing data. Pruriceptor populations are considered to be NP1, NP2, and NP3, while nociceptors are considered to primarily be in PEP1 and PEP2 population. Full database is available in Usoskin et al. (2015).
(B) Percentage (%) of mouse DRG neurons isolated from PirtGCaMP3/+ calcium reporter mice that are responsive to N-methyl leukotriene C4 (N-met LTC4, 100 nM), β-alanine (1 mM), chloroquine (CQ, 2 mM), and serotonin (5-HT, 200 µM) out of all KCl-responsive neurons. Each data point represents the percentage of neurons that were responsive to a stimulant in an individual mouse. n = 3 mice (> 200 neurons each). Data are represented as mean ± SD.
(C) Representative Venn diagram depicting the overlapping responses of mouse DRG neurons to stimulation with N-met LTC4 (100 nM), β-alanine (1 mM), CQ (2 mM), 5-HT (200 µM), and capsaicin (CAP, 500nM). n > 200 neurons for each test from a PirtGCaMP3/+ mouse.
(A) Assessment of chronic spontaneous itch prior to i.d. OVA challenge on day 10 of the AD-associated acute itch flare model in WT mice that were pre-administered vehicle or the CysLTR2 antagonist HAMI3379 (HAMI, 0.4 mg/kg; i.p.) on day 9 (two doses) and day 10 (1 dose, 60 minutes prior to chronic spontaneous itch assessment). n = 5–6 mice per group.
(B) Number of scratching bouts following i.d. OVA challenge on day 10 of the AD-associated acute itch flare model in WT mice that were pre-administered vehicle or the LTB4 receptor inhibitor CP-105,696 (3 mg/kg; gavage) 60 minutes prior to i.d. OVA challenge. n = 4–5 mice per group.
(C) Number of scratching bouts following i.d. OVA challenge on day 10 of the AD-associated acute itch flare model in WT mice that were pre-administered vehicle or the CysLTR1 antagonist zafirlukast (10 mg/kg; gavage) on day 9 (two doses) and day 10 (1 dose, 60 minutes prior to i.d. OVA challenge). n =4–5 mice per group.
(D) Assessment of chronic spontaneous itch prior to i.d. OVA challenge on day 10 of the AD-associated acute itch flare model in WT mice that were pretreated with i.c. injections of control siRNA or CysLTR2 siRNA. n = 5 mice per group.
Data are represented as mean ± SD. N.S., not significant by unpaired Student’s t-test.
(A) Number of scratching bouts following i.d. injection of N-met LTC4 (0.75 µg) in naïve littermate control and Trpv1−/− mice. n = 6 mice per group.
(B)Number of scratching bouts following i.d. injection of N-met LTC4 (0.75 µg) in naïve littermate control mice and Trpa1−/− mice. n = 4–5 mice per group.
(C)Assessment of chronic spontaneous itch prior to i.d. OVA challenge on day 10 of the AD-associated acute itch flare model in WT and compound Trpv1−/− Trpa1−/− mice. n = 5–7 mice per group.
Data are represented as mean ± SD. N.S., not significant by unpaired Student’s t-test.
(A) Intravital two-photon time-lapse movie capturing basophil motility prior to (A) and following
(B) i.d. OVA challenge on day 10 of the AD-associated acute itch flare model in sensitized (MC903 + OVA) basophil-reporter (Mcpt8-Cre-YFP) mice. Basophils are green (reporter) while blood vessels are red, labeled by i.v. injection of Qtracker 655 vascular labels 15–30 minutes prior to imaging. Collagen (blue) is imaged by collecting the second harmonic generation signal. Relative time is displayed in min:sec:msec.
Synthesized 2D movie showing tracks of basophil movement prior to (blue dots) and following (red dots) i.d. OVA challenge on day 10 of the AD-associated acute itch flare model in sensitized (MC903 + OVA) basophil-neuron dual reporter chimeric (Mcpt8-Cre × NaV1.8-TdTomato) mice.
Intravital two-photon time-lapse movie of interactions between basophils (green) and sensory nerves fibers (red) prior to (A) and following (B) i.d. OVA challenge on day 10 of the AD-associated acute itch flare model in the sensitized (MC903 + OVA) basophil-neuron dual reporter chimeric (Mcpt8-Cre × NaV1.8-TdTomato) mice. Collagen (blue) is imaged by collecting the second harmonic generation signal. Relative time is displayed in min:sec:msec.
Highlights.
Heterogeneous forms of itch underlie atopic dermatitis
Basophils promote a mast cell-independent form of IgE-mediated itch
Allergen-stimulated basophils release leukotriene C4 and interact with sensory nerves
Leukotriene C4-CysLTR2 neuronal signaling mediates acute itch flares
ACKNOWLEDGMENTS
We thank Diane Bender and the Immunomonitoring Lab (IML) at the Burksy Center for Human Immunology & Immunotherapy Programs (ChiiPs). We also thank the In Vivo Imaging Core (IVIC) at the Washington University School of Medicine for their support of the intravital two-photon imaging studies. The Kim lab is supported by NIAMS grants (K08-AR065577 and R01-AR070116), the American Skin Association, Doris Duke Charitable Foundation (to B.S.K.), and LEO Pharma. D.J.M. is supported by NIAMS grants (R01-AR060962 and R01-AR070873). M.J.M is supported by NIAID (R01-AI077600). A.M.T and M.R.M were supported by NIH NIAID training grant T32-AI716339. A.M.T. was also supported by the NIH NIAID F30 AI154912–01. T.B.Y. was supported by the Dermatology Foundation Dermatologist Investigator Research Fellowship (DIRF). The IML is a shared resource of the Alvin J. Siteman Cancer Center and is supported by the Andrew M. and Jane M. Bursky Center for Human Immunology and Immunotherapy Programs (ChiiPs) and NCI Cancer Center Support Grant P30CA91842.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLERATION OF INTERESTS
B.S.K. has served as a consultant for AbbVie, ABRAX Japan, Almirall, AstraZeneca, Cara Therapeutics, Daewoong Pharmaceutical, Incyte Corporation, LEO Pharma, Lilly, Maruho, Menlo Therapeutics, OM Pharma, Pfizer, and Third Rock Ventures. He has also participated on the advisory board for Almirall, Boehringer Ingelheim, Cara Therapeutics, Kiniksa Pharmaceuticals, Menlo Therapeutics, Regeneron Pharmaceuticals, Sanofi Genzyme, and Trevi Therapeutics. He is stockholder of Locus Biosciences. All other authors declare that they have no relevant conflicts of interest.
REFERENCES
- Akiyama T, Carstens MI, and Carstens E (2010). Facial injections of pruritogens and algogens excite partly overlapping populations of primary and second-order trigeminal neurons in mice. Journal of neurophysiology 104, 2442–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altinok Ersoy N, and Akyar I (2019). Multidimensional pruritus assessment in hemodialysis patients. BMC nephrology 20, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andoh T, and Kuraishi Y (1998). Intradermal leukotriene B-4, but not prostaglandin E-2, induces itch-associated responses in mice. Eur J Pharmacol 353, 93–96. [DOI] [PubMed] [Google Scholar]
- Benditt EP, Wong RL, Arase M, and Roeper E (1955). 5-Hydroxytryptamine in mast cells. Proc Soc Exp Biol Med 90, 303–304. [DOI] [PubMed] [Google Scholar]
- Benoist C, Lanier L, Merad M, Mathis D, and Immunological Genome P (2012). Consortium biology in immunology: the perspective from the Immunological Genome Project. Nature reviews Immunology 12, 734–740. [DOI] [PubMed] [Google Scholar]
- Benoist C, and Mathis D (2002). Mast cells in autoimmune disease. Nature 420, 875–878. [DOI] [PubMed] [Google Scholar]
- Bergstresser PR, Tigelaar RE, and Tharp MD (1984). Conjugated Avidin Identifies Cutaneous Rodent and Human Mast-Cells. Journal of Investigative Dermatology 83, 214–218. [DOI] [PubMed] [Google Scholar]
- Borriello F, Granata F, and Marone G (2014). Basophils and skin disorders. The Journal of investigative dermatology 134, 1202–1210. [DOI] [PubMed] [Google Scholar]
- Brough HA, Liu AH, Sicherer S, Makinson K, Douiri A, Brown SJ, Stephens AC, Irwin McLean WH, Turcanu V, Wood RA, et al. (2015). Atopic dermatitis increases the effect of exposure to peanut antigen in dust on peanut sensitization and likely peanut allergy. The Journal of allergy and clinical immunology 135, 164–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner T, Heusser CH, and Dahinden CA (1993). Human peripheral blood basophils primed by interleukin 3 (IL-3) produce IL-4 in response to immunoglobulin E receptor stimulation. The Journal of experimental medicine 177, 605–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celakovska J, Ettlerova K, Ettler K, Vaneckova J, and Bukac J (2015). Sensitization to aeroallergens in atopic dermatitis patients: association with concomitant allergic diseases. Journal of the European Academy of Dermatology and Venereology : JEADV 29, 1500–1505. [DOI] [PubMed] [Google Scholar]
- Cevikbas F, Wang X, Akiyama T, Kempkes C, Savinko T, Antal A, Kukova G, Buhl T, Ikoma A, Buddenkotte J, et al. (2014). A sensory neuron-expressed IL-31 receptor mediates T helper cell-dependent itch: Involvement of TRPV1 and TRPA1. The Journal of allergy and clinical immunology 133, 448–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A, Robison R, Cai M, and Singh AM (2016). Natural History of Food-Triggered Atopic Dermatitis and Development of Immediate Reactions in Children. The journal of allergy and clinical immunology In practice 4, 229–236 e221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charman CR, Venn AJ, Ravenscroft JC, and Williams HC (2013). Translating Patient-Oriented Eczema Measure (POEM) scores into clinical practice by suggesting severity strata derived using anchor-based methods. Br J Dermatol 169, 1326–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coca AF, and Cooke RA (1923). On the classification of the phenomena of hypersensitiveness. Journal of immunology 8, 163–182. [Google Scholar]
- Dale HH, and Laidlaw PP (1910). The physiological action of beta-iminazolylethylamine. The Journal of physiology 41, 318–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deleanu D, and Nedelea I (2019). Biological therapies for atopic dermatitis: An update. Experimental and therapeutic medicine 17, 1061–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, and Dong X (2018). Peripheral and Central Mechanisms of Itch. Neuron 98, 482–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer DF, Barrett NA, Austen KF, and Immunological Genome Project C (2016). Expression profiling of constitutive mast cells reveals a unique identity within the immune system. Nature immunology 17, 878–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan CL, Viglione-Schneck MJ, Walsh LJ, Green B, Trojanowski JQ, Whitaker-Menezes D, and Murphy GF (1998). Characterization of unmyelinated axons uniting epidermal and dermal immune cells in primate and murine skin. J Cutan Pathol 25, 20–29. [DOI] [PubMed] [Google Scholar]
- Egawa M, Mukai K, Yoshikawa S, Iki M, Mukaida N, Kawano Y, Minegishi Y, and Karasuyama H (2013). Inflammatory monocytes recruited to allergic skin acquire an anti-inflammatory M2 phenotype via basophil-derived interleukin-4. Immunity 38, 570–580. [DOI] [PubMed] [Google Scholar]
- Erickson S, and Kim BS (2019). Research Techniques Made Simple: Itch Measurement in Clinical Trials. Journal of Investigative Dermatology 139, 264-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JF (2002). Cysteinyl leukotriene receptors. Prostaglandins & other lipid mediators 68–69, 587–597. [DOI] [PubMed] [Google Scholar]
- Feng J, Yang P, Mack MR, Dryn D, Luo J, Gong X, Liu S, Oetjen LK, Zholos AV, Mei Z, et al. (2017). Sensory TRP channels contribute differentially to skin inflammation and persistent itch. Nature communications 8, 980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes ES, Vong CT, Quek S, Cheong J, Awal S, Gentry C, Aubdool AA, Liang L, Bodkin JV, Bevan S, et al. (2013). Superoxide generation and leukocyte accumulation: key elements in the mediation of leukotriene B(4)-induced itch by transient receptor potential ankyrin 1 and transient receptor potential vanilloid 1. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 27, 1664–1673. [DOI] [PubMed] [Google Scholar]
- Fishbane S, Jamal A, Munera C, Wen W, Menzaghi F, and Investigators K-T (2020). A Phase 3 Trial of Difelikefalin in Hemodialysis Patients with Pruritus. The New England journal of medicine 382, 222–232. [DOI] [PubMed] [Google Scholar]
- Flohr C, Johansson SG, Wahlgren CF, and Williams H (2004). How atopic is atopic dermatitis? The Journal of allergy and clinical immunology 114, 150–158. [DOI] [PubMed] [Google Scholar]
- Flohr C, Perkin M, Logan K, Marrs T, Radulovic S, Campbell LE, MacCallum SF, McLean WHI, and Lack G (2014). Atopic dermatitis and disease severity are the main risk factors for food sensitization in exclusively breastfed infants. The Journal of investigative dermatology 134, 345–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourzali KM, Golpanian RS, Chan YH, and Yosipovitch G (2020). Average daily itch vs. worst daily itch in chronic itch evaluation. Br J Dermatol. [DOI] [PubMed] [Google Scholar]
- Galli SJ, and Tsai M (2012). IgE and mast cells in allergic disease. Nat Med 18, 693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould HJ, and Sutton BJ (2008). IgE in allergy and asthma today. Nature reviews Immunology 8, 205–217. [DOI] [PubMed] [Google Scholar]
- Gupta K, and Harvima IT (2018). Mast cell-neural interactions contribute to pain and itch. Immunological reviews 282, 168–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson D, Sjoberg O, and Foucard T (2000). Development of allergies and asthma in infants and young children with atopic dermatitis--a prospective follow-up to 7 years of age. Allergy 55, 240–245. [DOI] [PubMed] [Google Scholar]
- Han H, Roan F, and Ziegler SF (2017). The atopic march: current insights into skin barrier dysfunction and epithelial cell-derived cytokines. Immunological reviews 278, 116–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He A, Feldman SR, and Fleischer AB (2018). An assessment of the use of antihistamines in the management of atopic dermatitis. Journal of the American Academy of Dermatology 79, 92–96. [DOI] [PubMed] [Google Scholar]
- Hedi H, and Norbert G (2004). 5-Lipoxygenase Pathway, Dendritic Cells, and Adaptive Immunity. J Biomed Biotechnol 2004, 99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil PM, Maurer D, Klein B, Hultsch T, and Stingl G (2010). Omalizumab therapy in atopic dermatitis: depletion of IgE does not improve the clinical course - a randomized, placebo-controlled and double blind pilot study. J Dtsch Dermatol Ges 8, 990–998. [DOI] [PubMed] [Google Scholar]
- Hellman LT, Akula S, Thorpe M, and Fu Z (2017). Tracing the Origins of IgE, Mast Cells, and Allergies by Studies of Wild Animals. Frontiers in immunology 8, 1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch SR, and Kalbfleisch JH (1980). Existence of basophil chemotaxis in subjects with hay fever. The Journal of allergy and clinical immunology 65, 274–277. [DOI] [PubMed] [Google Scholar]
- Huang CC, Kim YS, Olson WP, Li F, Guo C, Luo W, Huang AJW, and Liu Q (2016). A histamine-independent itch pathway is required for allergic ocular itch. The Journal of allergy and clinical immunology 137, 1267–1270 e1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CC, Yang W, Guo C, Jiang H, Li F, Xiao M, Davidson S, Yu G, Duan B, Huang T, et al. (2018). Anatomical and functional dichotomy of ocular itch and pain. Nat Med 24, 1268–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain M, Borcard L, Walsh KP, Pena Rodriguez M, Mueller C, Kim BS, Kubo M, Artis D, and Noti M (2018). Basophil-derived IL-4 promotes epicutaneous antigen sensitization concomitant with the development of food allergy. The Journal of allergy and clinical immunology 141, 223–234 e225. [DOI] [PubMed] [Google Scholar]
- Imai Y, Yasuda K, Nagai M, Kusakabe M, Kubo M, Nakanishi K, and Yamanishi K (2019). IL-33-Induced Atopic Dermatitis-Like Inflammation in Mice Is Mediated by Group 2 Innate Lymphoid Cells in Concert with Basophils. The Journal of investigative dermatology 139, 2185–2194 e2183. [DOI] [PubMed] [Google Scholar]
- Ishizaka K, Tomioka H, and Ishizaka T (1970). Mechanisms of passive sensitization. I. Presence of IgE and IgG molecules on human leukocytes. Journal of immunology 105, 1459–1467. [PubMed] [Google Scholar]
- Ito Y, Satoh T, Takayama K, Miyagishi C, Walls AF, and Yokozeki H (2011). Basophil recruitment and activation in inflammatory skin diseases. Allergy 66, 1107–1113. [DOI] [PubMed] [Google Scholar]
- Jaworek AK, Szafraniec K, Jaworek M, Doniec Z, Zalewski A, Kurzawa R, Wojas-Pelc A, and Pokorski M (2019). Cat Allergy as a Source Intensification of Atopic Dermatitis in Adult Patients. Adv Exp Med Biol [DOI] [PubMed] [Google Scholar]
- Justiz Vaillant AA, and Jan A (2020). Atopy. In StatPearls (Treasure Island; (FL)). [Google Scholar]
- Kabashima K, Furue M, Hanifin JM, Pulka G, Wollenberg A, Galus R, Etoh T, Mihara R, Nakano M, and Ruzicka T (2018). Nemolizumab in patients with moderate-to-severe atopic dermatitis: Randomized, phase II, long-term extension study. The Journal of allergy and clinical immunology 142, 1121–1130.e1127. [DOI] [PubMed] [Google Scholar]
- Kim BS, Berger TG, and Yosipovitch G (2019a). Chronic pruritus of unknown origin (CPUO): Uniform nomenclature and diagnosis as a pathway to standardized understanding and treatment. Journal of the American Academy of Dermatology 81, 1223–1224. [DOI] [PubMed] [Google Scholar]
- Kim BS, Howell MD, Sun K, Papp K, Nasir A, Kuligowski ME, and Investigators IS (2019b). Treatment of atopic dermatitis with ruxolitinib cream (JAK1/JAK2 inhibitor) or triamcinolone cream. The Journal of allergy and clinical immunology. [DOI] [PubMed] [Google Scholar]
- Kim BS, Siracusa MC, Saenz SA, Noti M, Monticelli LA, Sonnenberg GF, Hepworth MR, Van Voorhees AS, Comeau MR, and Artis D (2013). TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Science translational medicine 5, 170ra116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BS, Sun K, Papp K, Venturanza M, Nasir A, and Kuligowski ME (2020). Effects of Ruxolitinib Cream on Pruritus and Quality of Life in Atopic Dermatitis: Results From a Phase 2, Randomized, Dose-Ranging, Vehicle- and Active-Controlled Study. Journal of the American Academy of Dermatology. [DOI] [PubMed] [Google Scholar]
- Kim BS, Wang K, Siracusa MC, Saenz SA, Brestoff JR, Monticelli LA, Noti M, Tait Wojno ED, Fung TC, Kubo M, et al. (2014a). Basophils promote innate lymphoid cell responses in inflamed skin. Journal of immunology 193, 3717–3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Kim YM, Lee JY, Yang HK, Kim H, Cho J, Ahn K, and Kim J (2017). Seasonal variation and monthly patterns of skin symptoms in Korean children with atopic eczema/dermatitis syndrome. Allergy and asthma proceedings 38, 294–299. [DOI] [PubMed] [Google Scholar]
- Kim YS, Chu Y, Han L, Li M, Li Z, LaVinka PC, Sun S, Tang Z, Park K, Caterina MJ, et al. (2014b). Central terminal sensitization of TRPV1 by descending serotonergic facilitation modulates chronic pain. Neuron 81, 873–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kini SP, DeLong LK, Veledar E, McKenzie-Brown AM, Schaufele M, and Chen SC (2011). The impact of pruritus on quality of life: the skin equivalent of pain. Archives of dermatology 147, 1153–1156. [DOI] [PubMed] [Google Scholar]
- Kramer U, Weidinger S, Darsow U, Mohrenschlager M, Ring J, and Behrendt H (2005). Seasonality in symptom severity influenced by temperature or grass pollen: results of a panel study in children with eczema. The Journal of investigative dermatology 124, 514–523. [DOI] [PubMed] [Google Scholar]
- Langan SM, Thomas KS, and Williams HC (2006). What is meant by a “flare” in atopic dermatitis? A systematic review and proposal. Archives of dermatology 142, 1190–1196. [DOI] [PubMed] [Google Scholar]
- Larson VA, Tang O, Stander S, Kang S, and Kwatra SG (2019). Association between itch and cancer in 16,925 patients with pruritus: Experience at a tertiary care center. Journal of the American Academy of Dermatology 80, 931–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneau R, Pang X, Sant GR, and Theoharides TC (1996). Intragranular activation of bladder mast cells and their association with nerve processes in interstitial cystitis. Brit J Urol 77, 41–54. [DOI] [PubMed] [Google Scholar]
- Lett-Brown MA, Aelvoet M, Hooks JJ, Georgiades JA, Thueson DO, and Grant JA (1981). Enhancement of basophil chemotaxis in vitro by virus-induced interferon. J Clin Invest 67, 547–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Yang W, Jiang H, Guo C, Huang AJW, Hu H, and Liu Q (2019). TRPV1 activity and substance P release are required for corneal cold nociception. Nature communications 10, 5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Hener P, Zhang Z, Kato S, Metzger D, and Chambon P (2006). Topical vitamin D3 and low-calcemic analogs induce thymic stromal lymphopoietin in mouse keratinocytes and trigger an atopic dermatitis. Proc Natl Acad Sci U S A 103, 11736–11741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Hener P, Zhang ZK, Ganti KP, Metzger D, and Chambon P (2009). Induction of Thymic Stromal Lymphopoietin Expression in Keratinocytes Is Necessary for Generating an Atopic Dermatitis upon Application of the Active Vitamin D3 Analogue MC903 on Mouse Skin. Journal of Investigative Dermatology 129, 498–502. [DOI] [PubMed] [Google Scholar]
- Liu B, Tai Y, Achanta S, Kaelberer MM, Caceres AI, Shao X, Fang J, and Jordt SE (2016). IL-33/ST2 signaling excites sensory neurons and mediates itch response in a mouse model of poison ivy contact allergy. Proc Natl Acad Sci U S A 113, E7572–E7579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, Ru F, Guan Y, Weng HJ, Geng Y, et al. (2009). Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell 139, 1353–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XY, Liu ZC, Sun YG, Ross M, Kim S, Tsai FF, Li QF, Jeffry J, Kim JY, Loh HH, et al. (2011). Unidirectional cross-activation of GRPR by MOR1D uncouples itch and analgesia induced by opioids. Cell 147, 447–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luster AD, and Tager AM (2004). T-CELL trafficking in asthma: Lipid mediators grease the way. Nature Reviews Immunology 4, 711–724. [DOI] [PubMed] [Google Scholar]
- Mancini JA, Abramovitz M, Cox ME, Wong E, Charleson S, Perrier H, Wang Z, Prasit P, and Vickers PJ (1993). 5-lipoxygenase-activating protein is an arachidonate binding protein. Febs Lett 318, 277–281. [DOI] [PubMed] [Google Scholar]
- Marshall JS (2004). Mast-cell responses to pathogens. Nature reviews Immunology 4, 787–799. [DOI] [PubMed] [Google Scholar]
- Mashiko S, Mehta H, Bissonnette R, and Sarfati M (2017). Increased frequencies of basophils, type 2 innate lymphoid cells and Th2 cells in skin of patients with atopic dermatitis but not psoriasis. J Dermatol Sci 88, 167–174. [DOI] [PubMed] [Google Scholar]
- McGowan EC, Bloomberg GR, Gergen PJ, Visness CM, Jaffee KF, Sandel M, O’Connor G, Kattan M, Gern J, and Wood RA (2015). Influence of early-life exposures on food sensitization and food allergy in an inner-city birth cohort. Journal of Allergy and Clinical Immunology 135, 171–U261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meixiong J, Anderson M, Limjunyawong N, Sabbagh MF, Hu E, Mack MR, Oetjen LK, Wang F, Kim BS, and Dong X (2019). Activation of Mast-Cell-Expressed Mas-Related G-Protein-Coupled Receptors Drives Non-histaminergic Itch. Immunity 50, 1163–1171 e1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min B, Prout M, Hu-Li J, Zhu J, Jankovic D, Morgan ES, Urban JF Jr., Dvorak AM, Finkelman FD, LeGros G, et al. (2004). Basophils produce IL-4 and accumulate in tissues after infection with a Th2-inducing parasite. The Journal of experimental medicine 200, 507–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra SK, and Hoon MA (2013). The cells and circuitry for itch responses in mice. Science 340, 968–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra SK, Wheeler JJ, Pitake S, Ding H, Jiang C, Fukuyama T, Paps JS, Ralph P, Coyne J, Parkington M, et al. (2020). Periostin Activation of Integrin Receptors on Sensory Neurons Induces Allergic Itch. Cell reports 31, 107472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake K, and Karasuyama H (2017). Emerging roles of basophils in allergic inflammation. Allergology international : official journal of the Japanese Society of Allergology 66, 382–391. [DOI] [PubMed] [Google Scholar]
- Mukai K, Chinthrajah RS, Nadeau KC, Tsai M, Gaudenzio N, and Galli SJ (2017). A new fluorescent-avidin-based method for quantifying basophil activation in whole blood. Journal of Allergy and Clinical Immunology 140, 1202-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai K, Matsuoka K, Taya C, Suzuki H, Yokozeki H, Nishioka K, Hirokawa K, Etori M, Yamashita M, Kubota T, et al. (2005). Basophils play a critical role in the development of IgE-mediated chronic allergic inflammation independently of T cells and mast cells. Immunity 23, 191–202. [DOI] [PubMed] [Google Scholar]
- Noti M, Wojno ED, Kim BS, Siracusa MC, Giacomin PR, Nair MG, Benitez AJ, Ruymann KR, Muir AB, Hill DA, et al. (2013). Thymic stromal lymphopoietin-elicited basophil responses promote eosinophilic esophagitis. Nat Med 19, 1005–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oetjen LK, Mack MR, Feng J, Whelan TM, Niu H, Guo CJ, Chen S, Trier AM, Xu AZ, Tripathi SV, et al. (2017). Sensory Neurons Co-opt Classical Immune Signaling Pathways to Mediate Chronic Itch. Cell 171, 217–228 e213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa Y, Kono M, Tsujikawa M, Tsujiuchi H, and Akiyama M (2016). IgE-independent pathophysiology of severe atopic dermatitis demonstrated in an IgE-deficient patient. J Dermatol Sci 82, 139–141. [DOI] [PubMed] [Google Scholar]
- Phan NQ, Blome C, Fritz F, Gerss J, Reich A, Ebata T, Augustin M, Szepietowski JC, and Stander S (2012). Assessment of pruritus intensity: prospective study on validity and reliability of the visual analogue scale, numerical rating scale and verbal rating scale in 471 patients with chronic pruritus. Acta dermato-venereologica 92, 502–507. [DOI] [PubMed] [Google Scholar]
- Raap U, Gehring M, Kleiner S, Rudrich U, Eiz-Vesper B, Haas H, Kapp A, and Gibbs BF (2017). Human basophils are a source of - and are differentially activated by - IL-31. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology 47, 499–508. [DOI] [PubMed] [Google Scholar]
- Rajagopalan M, Saraswat A, Godse K, Shankar DS, Kandhari S, Shenoi SD, Tahiliani S, and Zawar VV (2017). Diagnosis and Management of Chronic Pruritus: An Expert Consensus Review. Indian J Dermatol 62, 7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappel WJ, and Edelstein-Keshet L (2017). Mechanisms of Cell Polarization. Curr Opin Syst Biol 3, 43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzicka T, Hanifin JM, Furue M, Pulka G, Mlynarczyk I, Wollenberg A, Galus R, Etoh T, Mihara R, Yoshida H, et al. (2017). Anti-Interleukin-31 Receptor A Antibody for Atopic Dermatitis. N Engl J Med 376, 826–835. [DOI] [PubMed] [Google Scholar]
- Sasaki F, and Yokomizo T (2019). The leukotriene receptors as therapeutic targets of inflammatory diseases. International immunology 31, 607–615. [DOI] [PubMed] [Google Scholar]
- Schauberger E, Peinhaupt M, Cazares T, and Lindsley AW (2016). Lipid Mediators of Allergic Disease: Pathways, Treatments, and Emerging Therapeutic Targets. Curr Allergy Asthma Rep 16, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverberg JI, Pinter A, Pulka G, Poulin Y, Bouaziz JD, Wollenberg A, Murrell DF, Alexis A, Lindsey L, Ahmad F, et al. (2020). Phase 2B randomized study of nemolizumab in adults with moderate-to-severe atopic dermatitis and severe pruritus. The Journal of allergy and clinical immunology 145, 173–182. [DOI] [PubMed] [Google Scholar]
- Simpson EL, Bieber T, Guttman-Yassky E, Beck LA, Blauvelt A, Cork MJ, Silverberg JI, Deleuran M, Kataoka Y, Lacour JP, et al. (2016). Two Phase 3 Trials of Dupilumab versus Placebo in Atopic Dermatitis. The New England journal of medicine 375, 2335–2348. [DOI] [PubMed] [Google Scholar]
- Solinski HJ, Kriegbaum MC, Tseng PY, Earnest TW, Gu X, Barik A, Chesler AT, and Hoon MA (2019). Nppb Neurons Are Sensors of Mast Cell-Induced Itch. Cell reports 26, 3561–3573 e3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer F, Hensen P, Bockenholt B, Metze D, Luger TA, and Stander S (2007). Underlying diseases and co-factors in patients with severe chronic pruritus: a 3-year retrospective study. Acta dermato-venereologica 87, 510–516. [DOI] [PubMed] [Google Scholar]
- Spergel JM, Mizoguchi E, Oettgen H, Bhan AK, and Geha RS (1999). Roles of TH1 and TH2 cytokines in a murine model of allergic dermatitis. J Clin Invest 103, 1103–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spergel JM, and Paller AS (2003). Atopic dermatitis and the atopic march. The Journal of allergy and clinical immunology 112, S118–127. [DOI] [PubMed] [Google Scholar]
- Stander S, Blome C, Anastasiadou Z, Zeidler C, Jung KA, Tsianakas A, Neufang G, and Augustin M (2017). Dynamic Pruritus Score: Evaluation of the Validity and Reliability of a New Instrument to Assess the Course of Pruritus. Acta dermato-venereologica 97, 230–234. [DOI] [PubMed] [Google Scholar]
- Stander S, Yosipovitch G, Legat FJ, Lacour JP, Paul C, Narbutt J, Bieber T, Misery L, Wollenberg A, Reich A, et al. (2020). Trial of Nemolizumab in Moderate-to-Severe Prurigo Nodularis. N Engl J Med 382, 706–716. [DOI] [PubMed] [Google Scholar]
- Stead RH, Tomioka M, Quinonez G, Simon GT, Felten SY, and Bienenstock J (1987). Intestinal Mucosal Mast-Cells in Normal and Nematode-Infected Rat Intestines Are in Intimate Contact with Peptidergic Nerves. P Natl Acad Sci USA 84, 2975–2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhoff M, Buddenkotte J, and Lerner EA (2018). Role of mast cells and basophils in pruritus. Immunological reviews 282, 248–264. [DOI] [PubMed] [Google Scholar]
- Sullivan BM, Liang HE, Bando JK, Wu D, Cheng LE, McKerrow JK, Allen CD, and Locksley RM (2011). Genetic analysis of basophil function in vivo. Nature immunology 12, 527–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S, and Dong X (2016). Trp channels and itch. Seminars in immunopathology 38, 293–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YG, and Chen ZF (2007). A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature 448, 700–703. [DOI] [PubMed] [Google Scholar]
- Suzukawa M, Hirai K, Iikura M, Nagase H, Komiya A, Yoshimura-Uchiyama C, Yamada H, Ra C, Ohta K, Yamamoto K, et al. (2005). IgE- and FcepsilonRI-mediated migration of human basophils. International immunology 17, 1249–1255. [DOI] [PubMed] [Google Scholar]
- Undem BJ, Riccio MM, Weinreich D, Ellis JL, and Myers AC (1995). Neurophysiology of Mast Cell-Nerve Interactions in the Airways. International archives of allergy and immunology 107, 199–201. [DOI] [PubMed] [Google Scholar]
- Usoskin D, Furlan A, Islam S, Abdo H, Lonnerberg P, Lou D, Hjerling-Leffler J, Haeggstrom J, Kharchenko O, Kharchenko PV, et al. (2015). Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nature neuroscience 18, 145–153. [DOI] [PubMed] [Google Scholar]
- Veldhuis NA, Poole DP, Grace M, McIntyre P, and Bunnett NW (2015). The G protein-coupled receptor-transient receptor potential channel axis: molecular insights for targeting disorders of sensation and inflammation. Pharmacological reviews 67, 36–73. [DOI] [PubMed] [Google Scholar]
- Vocks E, Busch R, Frohlich C, Borelli S, Mayer H, and Ring J (2001). Influence of weather and climate on subjective symptom intensity in atopic eczema. International journal of biometeorology 45, 27–33. [DOI] [PubMed] [Google Scholar]
- Voehringer D (2013). Protective and pathological roles of mast cells and basophils. Nature reviews Immunology 13, 362–375. [DOI] [PubMed] [Google Scholar]
- Voehringer D (2017). Recent advances in understanding basophil functions in vivo. F1000Research 6, 1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh CM, Hill RZ, Schwendinger-Schreck J, Deguine J, Brock EC, Kucirek N, Rifi Z, Wei J, Gronert K, Brem RB, et al. (2019). Neutrophils promote CXCR3-dependent itch in the development of atopic dermatitis. eLife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang BM, Zinselmeyer BH, Runnels HA, LaBranche TP, Morton PA, Kreisel D, Mack M, Nickerson-Nutter C, Allen PM, and Miller MJ (2012). In vivo imaging implicates CCR2(+) monocytes as regulators of neutrophil recruitment during arthritis. Cell Immunol 278, 103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Yang TB, and Kim BS (2020). The Return of the Mast Cell: New Roles in Neuroimmune Itch Biology. The Journal of investigative dermatology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw AJ, Walsh GM, and Symon FA (1994). Mechanisms of eosinophil and basophil migration. Allergy 49, 797–807. [DOI] [PubMed] [Google Scholar]
- Wassmann-Otto A, Heratizadeh A, Wichmann K, and Werfel T (2018). Birch pollen-related foods can cause late eczematous reactions in patients with atopic dermatitis. Allergy 73, 2046–2054. [DOI] [PubMed] [Google Scholar]
- Wedi B, Gehring M, and Kapp A (2020). The pseudoallergen receptor MRGPRX2 on peripheral blood basophils and eosinophils: Expression and function. Allergy. [DOI] [PubMed] [Google Scholar]
- Weidinger S, Beck LA, Bieber T, Kabashima K, and Irvine AD (2018). Atopic dermatitis. Nature reviews Disease primers 4, 1. [DOI] [PubMed] [Google Scholar]
- Weisshaar E, Ziethen B, and Gollnick H (1997). Can a serotonin type 3 (5-HT3) receptor antagonist reduce experimentally-induced itch? Inflamm Res 46, 412–416. [DOI] [PubMed] [Google Scholar]
- Werfel T, Heratizadeh A, Niebuhr M, Kapp A, Roesner LM, Karch A, Erpenbeck VJ, Losche C, Jung T, Krug N, et al. (2015). Exacerbation of atopic dermatitis on grass pollen exposure in an environmental challenge chamber. The Journal of allergy and clinical immunology 136, 96–103 e109. [DOI] [PubMed] [Google Scholar]
- Williams HC, Burney PG, Hay RJ, Archer CB, Shipley MJ, Hunter JJ, Bingham EA, Finlay AY, Pembroke AC, Graham-Brown RA, et al. (1994). The U.K. Working Party’s Diagnostic Criteria for Atopic Dermatitis. I. Derivation of a minimum set of discriminators for atopic dermatitis. Br J Dermatol 131, 383–396. [DOI] [PubMed] [Google Scholar]
- Wilson SR, The L, Batia LM, Beattie K, Katibah GE, McClain SP, Pellegrino M, Estandian DM, and Bautista DM (2013). The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell 155, 285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Schematic of allergen sensitization in the atopic dermatitis (AD)-associated acute itch flare model. Wild-type (WT) mice received daily topical treatment with calcipotriol (MC903) + ovalbumin (OVA) to bilateral ears from day 0 to day 9.
(B) Ear thickness (percent change from baseline) of vehicle ethanol (EtOH) control + OVA-treated and MC903 + OVA-treated WT mice from day 0 to day 10. n = 12–13 mice per group. ****p < 0.0001 by two-way ANOVA test.
(C)Representative clinical images (left) and H&E histopathology images (right) of lesional ear skin from EtOH control + OVA-treated and MC903 + OVA-treated WT mice on day 10 of the AD-like disease model. Scale bar = 50 µm.
(D) Schematic of naïve mice that received intravenous (i.v.) injection of anti-OVA IgE antibody (passive sensitization) on day 0 and day 1 followed by intradermal (i.d.) OVA challenge into cheek and assessment of scratching behavior on day 2.
(E) Number of scratching bouts following i.d. OVA challenge in WT mice that were pretreated with i.v. phosphate-buffered saline (PBS) control or i.v. anti-OVA IgE antibody. n = 5–6 mice per group. **p < 0.01 by unpaired Student’s t-test.
(F) Number of scratching bouts following i.d. OVA challenge in sensitized (i.v. anti-OVA IgE antibody) littermate control and mast cell-deficient Sash−/− mice. n = 5–6 mice per group. ****p < 0.0001 by unpaired Student’s t-test.
(G) Number of scratching bouts following i.d. OVA challenge in sensitized (i.v. anti-OVA IgE antibody) WT mice that were pretreated with vehicle or antihistamines olopatadine (OLP, 3 mg/kg; intraperitoneal [i.p.]) and JNJ7777120 (JNJ, 20 mg/kg; subcutaneous injection into the nape) 30 minutes prior to i.d. OVA challenge. n = 4–5 mice per group. *p < 0.05 by unpaired Student’s t-test.
(H) Schematic of acute itch flares in the passive sensitization model in AD-like disease. Naïve mice were topically treated with MC903 on the ear skin from day 0 to day 9 and received i.v. injection of PBS control or anti-OVA IgE antibody on day 8 and day 9. On day 10, i.d. injection of OVA was administered into non-lesional cheek skin of mice. Chronic spontaneous itch and acute itch flares were recorded prior to and following i.d. OVA challenge on day 10, respectively.
(I) Number of scratching bouts prior to i.d. OVA challenge (chronic spontaneous itch; left) and following i.d. OVA challenge (acute itch flares; right) in unsensitized (i.v. PBS) and sensitized (i.v. anti-OVA IgE antibody) WT mice with AD-like disease. n = 4–5 mice per group. N.S., not significant, **p < 0.01 by unpaired Student’s t-test.
(J) Number of scratching bouts prior to i.d. OVA challenge (chronic spontaneous itch; left) and following i.d. OVA challenge (acute itch flares; right) in sensitized (i.v. anti-OVA IgE antibody) littermate control and mast cell-deficient Sash−/− mice with AD-like disease. n = 6 mice per group. N.S., not significant by unpaired Student’s t-test.
Data are represented as mean ± SD.
(A) Schematic of the AD-like disease model. Topical vehicle EtOH control or MC903 was applied to bilateral ear skin of WT mice from day 0 to day 9. Blood basophils from WT mice were assessed for phenotypic alterations on day 2, day 4, and day 6 of the AD-like disease model.
(B and C) CD200R (B) and FcεRIα/IgE (C) expression measured by mean fluorescence intensity (MFI) on blood basophils in EtOH- or MC903-treated WT mice on day 2, day 4, and day 6 of the AD-like disease model. n = 3–5 mice per group. Data are represented as mean ± SD. N.S., not significant, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 by unpaired Student’s t-test.
(A) ELISA quantification of serum OVA-specific IgE (left) and assessment of chronic spontaneous itch (right) in isotype- or anti-CD200R3 monoclonal antibody-administered WT mice on day 10 of the AD-associated acute itch flare model. Scratching behavior was recorded prior to i.d. OVA challenge for chronic spontaneous itch assessment. n = 8–10 mice per group. N.S., not significant by unpaired Student’s t-test.
(B) ELISA quantification of serum OVA-specific IgE (left) and assessment of chronic spontaneous itch (right) in littermate control and basophil-depleted Bas-TRECK mice on day 10 of the AD-associated acute itch flare model. To quantify chronic spontaneous itch bouts, scratching behavior was recorded prior to i.d. OVA challenge. n = 9–10 mice per group. N.S., not significant by unpaired Student’s t-test.
(C) Schematic of Imject Alum (alum) and OVA systemic sensitization model. Naïve WT mice received intraperitoneal (i.p.) injection of adjuvant alum and OVA mixture on day 0 and day 9 followed by i.d. OVA challenge into cheek skin and assessment of scratching behavior on day 13.
(D) Number of scratching bouts following i.d. OVA challenge in unsensitized (i.p. alum + PBS) or sensitized (i.p. alum + OVA) WT mice. n = 3–4 mice per group. ****p < 0.0001 by unpaired Student’s t-test.
(E) Number of scratching bouts following i.d. OVA challenge in sensitized (i.p. alum + OVA) littermate control and mast cell-deficient Sash−/− mice. n = 4–6 mice per group. ****p < 0.0001 by unpaired Student’s t-test.
(F) Number of scratching bouts following i.d. OVA challenge in sensitized (i.p. alum + OVA) littermate control and basophil-depleted Bas-TRECK mice. n = 4–7 mice per group. N.S., not significant by unpaired Student’s t-test.
(G) Schematic of acute itch flares in the i.p. alum + OVA systemic sensitization model in the context of AD-like disease. Naïve mice received i.p. alum + OVA on day 0 and day 9. From day 3 to day 12, mice were topically treated with MC903 on the ear skin followed by an i.d. injection of OVA into non-lesional cheek skin on day 13. Chronic spontaneous itch and acute itch flares were recorded prior to and following i.d. OVA challenge on day 13, respectively.
(H) Number of scratching bouts prior to i.d. OVA challenge (chronic spontaneous itch; left) and following i.d. OVA challenge (acute itch flares; right) in littermate control and mast cell-deficient Sash−/− mice sensitized with i.p. alum + OVA in the context of AD-like disease on day 13. n = 3–4 mice per group. N.S., not significant by unpaired Student’s t-test.
(I) Number of scratching bouts prior to i.d. OVA challenge (chronic spontaneous itch; left) and following i.d. OVA challenge (acute itch flares; right) in littermate control and basophil-depleted Bas-TRECK mice sensitized with i.p. alum + OVA in the context of AD-like disease on day 13. n = 4–10 mice per group. N.S., not significant, *p < 0.05 by unpaired Student’s t-test.
(J) Representative calcium traces of mouse dorsal root ganglia (DRG) responses to OVA. Calcium responses were measured by fluorescence (Fluor.) intensity (488 nm). Neurons isolated from PirtGCaMP3/+ mice were sequentially stimulated with OVA (0.25 mg/mL in calcium imaging buffer), capsaicin (CAP, 500 nM), and KCl (50 mM). Each color trace represents one neuron.
(K) Percentage (%) of OVA-responsive and capsaicin-responsive neurons out of all KCl-responsive neurons. Each data point represents the percentage of responsive neurons from one individual PirtGCaMP3/+ mouse. n = 4 mice (> 200 neurons each). ****p < 0.0001 by paired Student’s t-test.
Data are represented as mean ± SD.
(A) ELISA quantification of serum OVA-specific IgE levels in littermate control and Alox5−/− mice on day 10 of the AD-associated acute itch flare model. n = 5–7 mice per group.
(B) Assessment of chronic spontaneous itch prior to i.d. OVA challenge on day 10 of the AD-associated acute itch flare model in WT mice that were pre-administered vehicle or zileuton (50 mg/kg; gavage) 60 minutes prior to chronic spontaneous itch assessment (left).
Assessment of chronic spontaneous itch prior to i.d. OVA challenge on day 10 of the AD-associated acute itch flare model in littermate control and Alox5−/− mice (right). n = 4–8 mice per group.
Data are represented as mean ± SD. N.S., not significant by unpaired Student’s t-test.
(A) Expression of selected genes in mouse DRG neuron populations NP1, NP2, NP3, PEP1 and PEP2 based on single cell RNA-sequencing data. Pruriceptor populations are considered to be NP1, NP2, and NP3, while nociceptors are considered to primarily be in PEP1 and PEP2 population. Full database is available in Usoskin et al. (2015).
(B) Percentage (%) of mouse DRG neurons isolated from PirtGCaMP3/+ calcium reporter mice that are responsive to N-methyl leukotriene C4 (N-met LTC4, 100 nM), β-alanine (1 mM), chloroquine (CQ, 2 mM), and serotonin (5-HT, 200 µM) out of all KCl-responsive neurons. Each data point represents the percentage of neurons that were responsive to a stimulant in an individual mouse. n = 3 mice (> 200 neurons each). Data are represented as mean ± SD.
(C) Representative Venn diagram depicting the overlapping responses of mouse DRG neurons to stimulation with N-met LTC4 (100 nM), β-alanine (1 mM), CQ (2 mM), 5-HT (200 µM), and capsaicin (CAP, 500nM). n > 200 neurons for each test from a PirtGCaMP3/+ mouse.
(A) Assessment of chronic spontaneous itch prior to i.d. OVA challenge on day 10 of the AD-associated acute itch flare model in WT mice that were pre-administered vehicle or the CysLTR2 antagonist HAMI3379 (HAMI, 0.4 mg/kg; i.p.) on day 9 (two doses) and day 10 (1 dose, 60 minutes prior to chronic spontaneous itch assessment). n = 5–6 mice per group.
(B) Number of scratching bouts following i.d. OVA challenge on day 10 of the AD-associated acute itch flare model in WT mice that were pre-administered vehicle or the LTB4 receptor inhibitor CP-105,696 (3 mg/kg; gavage) 60 minutes prior to i.d. OVA challenge. n = 4–5 mice per group.
(C) Number of scratching bouts following i.d. OVA challenge on day 10 of the AD-associated acute itch flare model in WT mice that were pre-administered vehicle or the CysLTR1 antagonist zafirlukast (10 mg/kg; gavage) on day 9 (two doses) and day 10 (1 dose, 60 minutes prior to i.d. OVA challenge). n =4–5 mice per group.
(D) Assessment of chronic spontaneous itch prior to i.d. OVA challenge on day 10 of the AD-associated acute itch flare model in WT mice that were pretreated with i.c. injections of control siRNA or CysLTR2 siRNA. n = 5 mice per group.
Data are represented as mean ± SD. N.S., not significant by unpaired Student’s t-test.
(A) Number of scratching bouts following i.d. injection of N-met LTC4 (0.75 µg) in naïve littermate control and Trpv1−/− mice. n = 6 mice per group.
(B)Number of scratching bouts following i.d. injection of N-met LTC4 (0.75 µg) in naïve littermate control mice and Trpa1−/− mice. n = 4–5 mice per group.
(C)Assessment of chronic spontaneous itch prior to i.d. OVA challenge on day 10 of the AD-associated acute itch flare model in WT and compound Trpv1−/− Trpa1−/− mice. n = 5–7 mice per group.
Data are represented as mean ± SD. N.S., not significant by unpaired Student’s t-test.
(A) Intravital two-photon time-lapse movie capturing basophil motility prior to (A) and following
(B) i.d. OVA challenge on day 10 of the AD-associated acute itch flare model in sensitized (MC903 + OVA) basophil-reporter (Mcpt8-Cre-YFP) mice. Basophils are green (reporter) while blood vessels are red, labeled by i.v. injection of Qtracker 655 vascular labels 15–30 minutes prior to imaging. Collagen (blue) is imaged by collecting the second harmonic generation signal. Relative time is displayed in min:sec:msec.
Synthesized 2D movie showing tracks of basophil movement prior to (blue dots) and following (red dots) i.d. OVA challenge on day 10 of the AD-associated acute itch flare model in sensitized (MC903 + OVA) basophil-neuron dual reporter chimeric (Mcpt8-Cre × NaV1.8-TdTomato) mice.
Intravital two-photon time-lapse movie of interactions between basophils (green) and sensory nerves fibers (red) prior to (A) and following (B) i.d. OVA challenge on day 10 of the AD-associated acute itch flare model in the sensitized (MC903 + OVA) basophil-neuron dual reporter chimeric (Mcpt8-Cre × NaV1.8-TdTomato) mice. Collagen (blue) is imaged by collecting the second harmonic generation signal. Relative time is displayed in min:sec:msec.
Data Availability Statement
This study did not generate any unique datasets or code. All other data supporting the findings of this study are available in the manuscript or the supplementary materials and available upon request to the lead contact author.


