Abstract
This review considers current advances in tools to investigate the functional biology of Giardia, it’s coding and non-coding genes, features and cellular and molecular biology. We consider major gaps in current knowledge of the parasite and discuss the present state-of-the-art in its in vivo and in vitro cultivation. Advances in in silico tools, including for the modelling non-coding RNAs and genomic elements, as well as detailed exploration of coding genes through inferred homology to model organisms, have provided significant, primary level insight. Improved methods to model the three-dimensional structure of proteins offer new insights into their function, and binding interactions with ligands, other proteins or precursor drugs, and offer substantial opportunities to prioritise proteins for further study and experimentation. These approaches can be supplemented by the growing and highly accessible arsenal of systems-based methods now being applied to Giardia, led by genomic, transcriptomic and proteomic methods, but rapidly incorporating advanced tools for detection of real-time transcription, evaluation of chromatin states and direct measurement of macromolecular complexes. Methods to directly interrogate and perturb gene function have made major leaps in recent years, with CRISPr-interference now available. These approaches, coupled with protein over-expression, fluorescent labelling and in vitro and in vivo imaging, are set to revolutionize the field and herald an exciting time during which the field may finally realise Giardia’s long proposed potential as a model parasite and eukaryote.
1. Introduction
Undertaking comprehensive functional studies has remained a persistent obstacle in parasitological research, outside of a small number of apicomplexans, such as species of Plasmodium and Toxoplasma gondii (Limenitakis and Soldati-Favre, 2011; Meissner et al., 2007). Primary obstacles to genetically tractable parasites include an inability to readily culture them outside of a host, a lack of knowledge of the genetic and regulatory systems of parasites or the unavailability of tools to apply to them. Giardia intestinalis has been in vitro culturable for several decades in complex media, principally Keister’s modified TYI-S-33 (Davids and Gillin, 2011) and its regulatory genetics and gene composition have been explored through the publication of reference genomes (Franzen et al., 2009; Jerlstrom-Hultqvist et al., 2010; Morrison et al., 2007) and other “omics” driven research over the past decade (e.g., Ansell et al., 2015a, 2017; Emery et al., 2018; Franzén et al., 2013; Ma’ayeh et al., 2018; Spycher et al., 2013), albeit with much still to be done. The complex binucleate and multiploidal (2N, 4N and 8N at various stages of the life cycle; Bernander et al., 2001) cellular biology of Giardia has proven a persistent obstacle to functional research, and for much of its post-genomic period efforts to develop functional tools for Giardia have met with limited success (Luján and Svärd, 2011). Because of its importance as a parasite, its scalable culturability in cell-free media, and its deep-branching position within the eukaryotic tree of life (Morrison et al., 2007), Giardia has long been proposed as an intriguing and potentially impactful model organism (Luján and Svärd, 2011), but its recalcitrance to genetic manipulation has proven a persistent road-block to realising this potential.
Here, we review advances in functional research of Giardia, drawing on recent publications in the field, as well as novel advances in other fields that may be applicable for Giardia. Our hope is that this review will act not only as a summary of the progress in research in this field over the last few years, but also as stimulus for renewed thinking on functional research in Giardia and on the potential to exploit these technologies for novel anti-giardial therapies, but to harness this fascinating protist as a model for eukaryotic biology.
2. Advances in in vitro cultivation
The inability to culture parasites in a laboratory setting, either in vitro or in vivo, presents the primary obstacle to advancing functional research. Fortunately, this obstacle has been overcome for Giardia for several decades. In vitro cultivation of G. intestinalis is readily undertaken in TYI-S-33 media (Davids and Gillin, 2011), with or without nitrogen-sparging for culture-adapted strains isolates. Giardia intestinalis trophozoites (at least of the assemblage A) can be triggered into in vitro cyst formation in encystation media, which is typically modified “high-bile” version of TYI-S-33 (Davids and Gillin, 2011).
Although G. intestinalis is a micro-aerophile and TYI-S-33 can be made anaerobic through nitrogen-sparging (i.e., gassing) or, in small volumes, using anaerobic pouches in a sealed container (Ansell et al., 2016), it is common practice to simply make media up in an aerobic environment, inoculate the culture with Giardia trophozoites and then seal the culture flask with a non-vented lid (Davids and Gillin, 2011). In this non-sparged approach, Giardia trophozoites may show a 24–48h lag-phase before exponential growth. Ansell et al. (2015a) showed that although trophozoites grow in the early 24–48h period, they transcribe many regulators of the oxidative stress response even up to 60h after inoculation and appear largely reliant on arginine metabolism for ATP. After ~60h in culture, these trophozoites dramatically shift their transcriptional behaviour, down-regulating their stress responses and up-regulating oxygen-sensitive glycolytic ATP production. Companion experiments in fully anaerobic media are needed, but these data suggest that culture conditions may have a significant effect on the cellular behaviour of this oxygen-sensitive parasite and are worth considering in functional studies.
2.1. Systems for studies of Giardia-host cell interactions in vitro and in vivo
Giardia infects the upper small intestine and primarily interacts with intestinal epithelial cells (IECs) during host-cell adherence via its adhesive disc. However, it is difficult to grow primary IECs, and this has led to the use of intestinal cell lines as models in most in vitro studies of the small intestine (Dosh et al., 2018). Several in vitro models of host parasite-interactions during giardiasis have been developed using IECs in co-incubations with axenic Giardia parasites. The best described and most used cell line in studies of IECs is Caco-2, an adenocarcinomal colonic epithelial cell line that is restricted in propagation by cell-to-cell contact and can be induced to express small intestinal features (such as apical to basolateral polarization, formation of tight junctions and the appearance of microvilli) by growth post-confluence for around 20 days (Sambuy et al., 2005). Proteomic analyses have shown similar expression profiles between Caco-2 cells and cells derived from scrapings of the human intestinal epithelium (Lenaerts et al., 2007).
The Caco-2 cell line in its differentiated state is also the most used IEC in studies of Giardia-IEC interactions in vitro (Fisher et al., 2013; Kraft et al., 2017; Ma’ayeh et al., 2017, 2018; Roxstrom-Lindquist et al., 2005). The first studies were published in the early 1990s by Favennec et al. (1990, 1991) and mainly studied attachment and drug treatment, something that has been followed up in several other studies (Muller et al., 2006). The role of nitric oxide, studies of the intestinal barrier and several gene expression studies have been performed later (Eckmann et al., 2000; Ma’ayeh et al., 2018; Teoh et al., 2000). The human HT-29 cell line has also been used in Giardia-IEC interaction studies. It is more heterogeneous than the Caco-2 cell line, consisting mainly of un-differentiated cells and a smaller mucus producing subpopulation (3–5%) (Huet et al., 1987; Maoret et al., 1989). It can be differentiated into an enterocyte-like cell by glucose starvation, and several mucus-secreting clones have been established (Chastre et al., 1985; Lievin-Le Moal, 2013).
Other cell-lines for Giardia interactions include the HCT-8 cell line, which is derived from a human ileocecal adenocarcinoma and has been used in at least three studies of Giardia-host cell interactions (Koh et al., 2013; Panaro et al., 2007). The rat intestinal epithelial cells, IEC-6 have been used in several Giardia interaction studies and continuous co-culture, with purified giardial proteins and analysed from these interactions (Cabrera-Licona et al., 2017; Ma’ayeh and Brook-Carter, 2012; McCabe et al., 1991; Ortega-Pierres et al., 2018). A few studies of Giardia-host cell interactions have used the SCBN cell line, as it was initially described as a “small intestine epithelial cell line of human origin”, but later studies showed that this cell line is of canine origin (Buret and Lin, 2008). However, the SCBN cell line does show different responses to Giardia parasites from different assemblages, and Giardia parasites are capable of both tight junction alteration and apoptotic induction and resistance in SCBN cells (Buret et al., 2002; Scott et al., 2002; Teoh et al., 2000).
The breadth of datasets from in vitro IEC-Giardia interaction models has highlighted several important processes during early pathogenesis (Einarsson et al., 2016c). Identification of key molecular mechanisms and pathways indicate multiple levels of crosstalk in the context of virulence biology in the parasite, and immunology of the host. The in vitro data the community has generated to date, using simple in vitro systems, can lead to directed experiments in more complex human models like human small intestinal enteroids (Chen et al., 2017; Zachos et al., 2016). It should also be possible to use human biopsies from giardiasis patients in studies of specific factors and mechanisms.
In vivo model systems described below will also be important to verify the role of factors identified in vitro and to identify new factors and mechanism important during Giardia infections. Giardia muris has been used as a model for understanding the pathogenesis and immunological responses of the host during establishment of infection, beginning in the 1960s (Friend, 1966). The availability of knock-out mice and other host-related resources makes G. muris a powerful alternative model to study the induction of pathogenesis during Giardia infections, which cannot be accomplished by studying natural human infections or using human cell lines (Fink and Singer, 2017). The life cycle and infective process of G. muris is a close reflection of the infection by G. intestinalis (Dann et al., 2018). Several major findings of Giardia biology (flagellar and disc function and cellular differentiation) (Holberton, 1973; Schaefer et al., 1984) and immunity (IgA and Th17) responses, defensins and post-infectious colitis (Dann et al., 2018; Dreesen et al., 2014; Langford et al., 2002; Manko et al., 2017)have been pioneered in G. muris, and later been shown to be transferable to human G. intestinalis infections (Saghaug et al., 2016). Unfortunately, the research on G. muris has been constrained by the absolute lack of genome information and gene expression data, however, with a draft genome of G. muris is now available in public databases (NCBI and GiardiaDB), this should stimulate wider uptake of G. muris as an in vivo model.
3. Major knowledge gaps in functional biology in Giardia
Draft genomes have been available for Giardia intestinalis assemblage A (WB: Morrison et al., 2007), B (GS: Franzen et al., 2009) and E (P15: Jerlstrom-Hultqvist et al., 2010) for a number of years. These assemblies have provided the basis for predicting Giardia’s ~5500 coding gene models. Recent efforts (see current release in GiardiaDB) to curate these models have significantly improved their functional annotation; yet, ~60% of these are defined as “conserved” or “hypothetical” proteins. Additional work on completing the G. intestinalis genomes and refining the annotation of these coding genes (including through transcriptomic and proteomic support) are needed.
Regulatory regions of the Giardia genome are even less defined. Among coding models, neither promoters nor 5′ or 3′ untranslated regions (UTRs) are well characterized. To date, studies indicate that most coding G. intestinalis genes have little to no 5′ UTR and a greatly reduced 3′ UTR (Adam, 2000; Morrison et al., 2007), but this is not confirmed through broad-scale direct transcript sequencing (e.g., using PacBIO or Nanopore technology). There are few annotated transcription factors in the G. intestinalis gene set (Franzen et al., 2009; Jerlstrom-Hultqvist et al., 2010; Morrison et al., 2007), and their binding sites and the genes they regulate are largely unknown. To our knowledge, there has been no comprehensive study of enhancer regions in Giardia. Lastly, although Giardia shows significant variation in karyotype and chromatin condensation between nuclei (Tůmová et al., 2007) and during the cell-cycle (Tůmová et al., 2015) and histone methyltransferases appear to be important in encystation (Carranza et al., 2016; Salusso et al., 2017; Sonda et al., 2010), antigenic variation (Carranza et al., 2016) and drug resistance (Ansell et al., 2015b), to date there has been no extensive study of chromatin level regulation in this parasite.
Post-transcriptional and post-translational regulation plays an important but largely unexplored role in Giardia biology. Giardia intestinalis was one of the first eukaryotes in which DICER was structurally resolved (MacRae et al., 2006). Consistent with this, Giardia has functional RNA interference systems (Prucca et al., 2008), but these have, as yet, been relatively unexplored. Small RNA sequencing has been undertaken in G. intestinalis WB trophozoites at confluence in TYI-S-33 media (Chen et al., 2009). This work characterised a large number of potential small-interfering and microRNAs, but did not define the genes regulated by them. To date, the only confirmed role for RNAi systems in Giardia is in regulation of variant-surface protein expression (Gargantini et al., 2016; Prucca et al., 2008; Saraiya et al., 2014). Studies of other post-transcriptional and post-translation regulatory systems are limited. Williams and Elmendorf (2011) showed evidence for Pumilio-domain (Puf) proteins in Giardia, which are RNA-binding proteins that play important post-transcriptional regulatory roles in a variety of eukaryotes (Gerber et al., 2004; Spassov and Jurecic, 2003). These have not been further explored.
Giardia has a reduced core kinome (Manning et al., 2011) as well as chromatin modifying enzymes including acetyltransferases, deacetyltransferase and methyltransferases (Iyer et al., 2008). In Giardia protein post-translational modifications of serine, threonine and tyrosine phosphorylationhas been demonstrated by Manning et al. (2011), ubiquitin by Niño et al. (2013) and SUMOylation by Vranych et al. (2014). Emery et al. (2018) provided evidence for widespread post-translational modifications (e.g., methylation) in metronidazole resistance, and Carranza et al. (2016) for roles of acetyllysine and methyllysine histone marks in Giardia, but broad-scale characterization of these modifications, particularly at site-specific levels, has not been undertaken.
4. Computational and “omic” approaches to functional biology research in Giardia
It is clear that the next major breakthroughs in our understanding of the molecular biology of Giardia must make full use of advances in functional research. Conceptually, these advances can be categorized into three major and complimentary themes: (i) improved in silico inference, (ii) direct empirical assessment of function through “omics”, labelling and other approaches and (iii) direct empirical assessment of function through targeted genetic manipulation.
4.1. In silico inference of function
Given its history as a promising but ultimately genetically intractable (or at best challenging) organism, much of what we currently understand about Giardia’s functional biology is based on in silico inference through comparative analyses with model organisms and other parasitic protists. In large part, this has restricted our understanding to the function of coding genes and is based primarily on BLAST homology with existing curated protein databases, including the Kyoto Encyclopaedia of Genes and Genomes (KEGG: Kanehisa and Goto, 2000) and Uniprot (Consortium, 2014). However, although Giardia’s deep-branching origins in eukaryotes (Morrison et al., 2007) makes it an intriguing organism to study, the evolutionary distance between this parasite and other, more well characterized, eukaryotes is severely problematic. As noted above, nearly two-thirds of G. intestinalis coding genes lack an identifiable homologue in a genetically tractable organism.
Protein-domain modelling can assist in extracting additional functional information from these coding genes. Interproscan is the most well-known and widely used of these approaches (Jones et al., 2014). This method identifies conserved features of known protein domains from across eukaryotic and prokaryotic life, stored in a number of curated protein family databases, including InterPro (Mitchell et al., 2018), PANTHER (Mi et al., 2016) and pfam (El-Gebali et al., 2018). These conserved domain data can be used in combination to infer each protein’s involvement in common biological functions and provided further functional context using the Gene Ontology Hierarchy (Consortium, 2016) or PANTHER (Mi et al., 2016). Hidden Markov Modelling (e.g., using HMMER: Potter et al., 2018) and other weighted matrix search strategies (e.g., Position Specific Information (PSI)-BLAST: Altschul et al., 1997) have added value for identifying homologous sequences for protein families or domains not covered in the InterProScan analysis. Whether searching curated or custom databases, in essence, these approaches rely on identifying proteins features that are widely conserved across evolutionary time. For the most part, these features are so conserved because of their importance in forming and maintaining the three-dimensional shape of the protein domain, which is crucial to its overall function.
Protein structure, which largely imparts function, generally evolves more slowly than protein sequence and can be determined by a small number of essential amino acid residues not readily identifiable through comparative sequence alignments. Noting this, protein structural homology can be used as a powerful, in silico method to infer protein function, even across large evolutionary distances. These approaches rely on modelling protein structure from the underlying sequence and assessing the similarity of that model in three-dimensional space to solved structures of known proteins, for example, stored in curated repositories, such as the Protein Databank (Berman et al., 2000). Many programs are available for protein structural modelling and inference of function. These programs rely on two major approaches including (1) ab initio prediction based on amino acid sequence [e.g., ROBETTA (Kim et al., 2004) and EVfold (Sheridan et al., 2015)] and (2) a guided (Bayesian) approach threading the protein sequence across known solved structures based on underlying sequence similarity [e.g., SPARKS-X (Yang et al., 2011)] and considering the amino acid sequence. Programs such as Swiss Model (Waterhouse et al., 2018), LOMETS (Wu and Zhang, 2007) and MODELLER (Eswar et al., 2007) support inference of protein homology based on comparisons between the predicted protein structure and solved structures for known proteins in curated databases. Software packages including HHpred (Söding et al., 2005), RaptorX (Källberg et al., 2012) and Phyre2 (Kelley et al., 2015) combine threading and homology-based inference in one package. Finally, complex suites of prediction methods, most notably packaged together in the I-Tasser suite (Iterative-threading assembly refinement: Roy et al., 2010), combine ab initio prediction with iterative refinement through guided threading methods, followed finally by homology based inference and predicted functional annotation. Protein models generated from these predictions can be rendered and visualised in a variety of software packages, including Jmol (Hanson, 2010) and Chimera (Yang et al., 2012).
Accuracy and computational time varies dramatically among these approaches, with complex methods, not surprisingly, being the most computationally intensive and robust. The Critical Assessment of Methods of Protein Structural Prediction (CASP: Moult, 2005) provides an independent benchmarking of these methods, in which programmers are given 24 months (the time between CASP rounds) to provide their best prediction of the structure of ~50–100 unsolved proteins while structural biologists attempt to solve the structure using empirical approaches (e.g., X-RAY crystallography, NMR and Cryo-EM). Programs are scored on a variety of criteria, including the accuracy of the protein backbone, of comparative alignments among the predicted models and similar solved structures, and of ab initio modelling of protein subdomains present in the query protein and absent from related solved structures. Since 2006, I-Tasser has been rated the, or among the, best performing packages for protein structural homology prediction (Roy et al., 2010). The package is limited to proteins under 1500 amino acids and typically performs best for proteins under 750 amino acids. Larger proteins (up to ~3500 amino acids) can be modelled using Phyre2 (Kelley et al., 2015), but only for components of the protein with close homology to known structures (i.e., no ab initio folding).
In an effort to expand on the functional annotation of G. intestinalis WB genes, Ansell et al. (2019) undertook genome-wide protein structural homology prediction for most conserved and hypothetical proteins encoded in the G. intestinalis WB reference genome, as well as a subset of several hundred proteins with an existing function annotation. These predictions and their underlying meta-data are available for individual or bulk download through http://www.predictein.org/giardia_intestinalis or accessible via individual gene-pages in GiardiaDB. Structural prediction of already functionally annotated proteins can assist in testing and refining these annotations, which for Giardia and other non-model organisms are often based on sequence homology only. These predictions can help in identifying ligand or co-factor binding sites; I-Tasser has an in-built function to predict ligand interactions (Roy et al., 2010), or support subsequent in silico docking experiments (Śledź and Caflisch, 2018), for prospective chemical inhibitor development.
Structural homology programs, including I-Tasser, produce a number of quality metrics that can be used to assist researchers in interpreting the outcome and separating higher and lower confidence predictions (Roy et al., 2010). Root mean standard deviation (RMSD) is a common metric, measured in angstroms, to assess the physical distance between the predicted 3D structure of the query protein and its structural homologue among solved proteins in the PDB. This metric is calculated by measuring the distance between each amino acid in the query protein and its nearest structurally equivalent amino acid in the PDB homologue and then computing the overall variance among these distances across the entire predicted model. In general terms, the lower the RMSD value, the more structurally similar are the two proteins in 3D space. In our experience, an RMSD ~<5Å is generally usable for functional inference and, according to Roy et al. (2010), an RMSD <2–3 is of sufficiently resolved to support subsequent in silico docking and protein-protein interaction modelling. However, this will vary among predictions.
It is important to note that RMSD can only be calculated for the portion of the query model for which a structural homologue is available among the solved PDB proteins. Due to the challenges associated with expressing and crystallising proteins for empirical structural analysis, it is a common occurrence for PDB models to represent only a critical subdomain of a given protein. It is also common for I-Tasser to identify a conserved structural fold in an otherwise diverged protein. It is important to consider the proportion of the query and target models that overlap in the structural homology prediction (calculated as “cov” or coverage by I-Tasser) when inferring a functional annotation from these data. The ab initio software incorporated in the I-Tasser suite is still able to predict a putative structure for the non-overlapping regions of the query protein, but of course these will not contribute to the structural homology prediction. In addition to simple metrics to assess the similarity of the query to a structural homologue, these software packages also produce several composite “confidence” scores. In the case of I-Tasser, this includes a TM-score metric (computed by TM-align), which provides a confidence value based on the RMSD, amino acid similarity and coverage and other metrics, producing a normalized value between 0 (low) and 1. Per Roy et al. (2010), a TM-score about 0.5 is considered to represent a level of structural similarity between the predicted protein and its nearest solved structural homologue that is beyond chance (i.e., biologically informative).
The specific metrics provided by I-Tasser are useful for assessing the quality and reliability of the predicted structure, as well as the functional information that can be derived from its nearest structural homologue. Although Roy et al. (2010) provide advice on quality thresholds for these metrics based on extensive modelling of human proteins, parasites are often highly diverged from model organisms and particularly humans, especially for the deep-branching G. intestinalis, and the suitability of human-benchmarked quality thresholds is unclear. Using a machine learning algorithm (Random Forest Classifier), Ansell et al. (2019) sought to develop an organism in-dependent method to assign a confidence score to protein structural homology predictions. In this approach, Ansell et al. (2019) selected Giardia proteins with identifiable conserved protein domains based on Interproscan analysis. The Random Forest Classifier was provided each I-Tasser output metric (e.g., RMSD, TM-Score, % amino acid similarity and % coverage) and assessed these for their agreement with the domain comparisons, producing a weighted score that could split into “high” (those query and target proteins that had good domain agreement) and “low” (those that did not) confidence predictions. The Random Forest Classifier (RFC) then applied this weighted scoring system to Giardia proteins for which no a priori protein domain data were identifiable, separating these predictions in “high-confidence like” and “low-confidence like” categories.
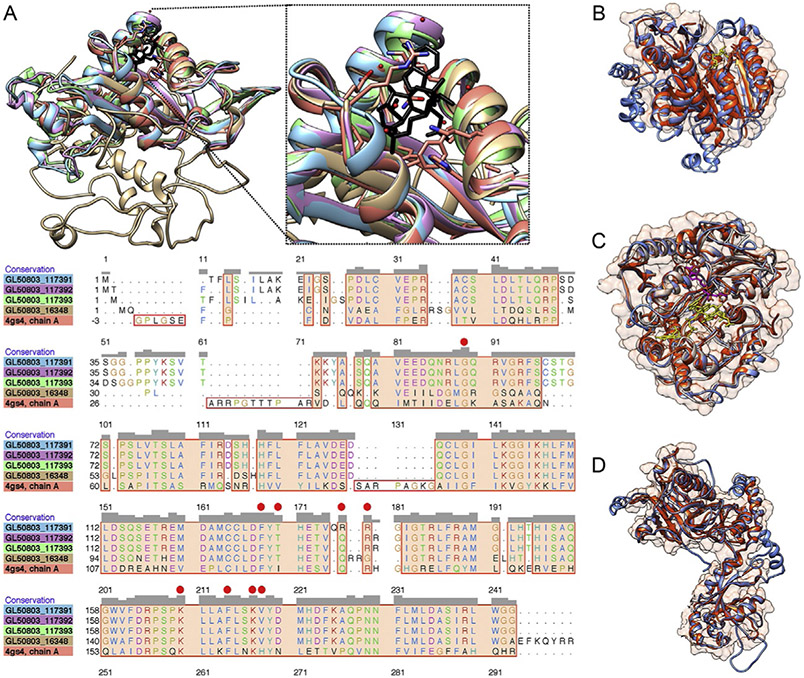
Using the RFC approach, high-confidence functional annotations were assigned to hundreds of hypothetical proteins for which no function data had previously be available (Ansell et al., 2019). Some of these novel annotations include what appear to be key players in critical redox stress systems within the parasite (Ansell et al., 2019), including a novel ortholog of Entamoeba histolytica NADPH-dependent oxidoreductase 1, which in the latter species is implicated in metronidazole activation (Jeelani et al., 2010). Others include putative, novel epigenetic modifiers, that may be important in post-transcriptional or post-translation regulation (Fig. 1). Importantly, I-Tasser functions based on the knowledge that proteins with similar 3D structure tend to have similar functions. However, “function” can include a protein’s molecular (i.e., as a kinase, transporter or structural protein) or biological function (e.g., involvement in a specific metabolic or signalling pathway). I-Tasser assigns Gene Ontology classification data based on shared molecular and biological functions of the nearest structural homologues for each prediction. However, in our experience, predicting biological function from structural data alone is unreliable and should be undertaken with caution. As we discuss below, supplementing structural homology prediction data with additional evidence of biological function (e.g., using transcriptomic, proteomic or protein localization data) is advisable.
Fig. 1.
Novel functional annotations for Giardia proteins based on 3D structural homology. Examples of high-confidence models with potential and novel roles in post-transcriptional and post-translation regulation in Giardia include (A) Several homologues of human α-tubulin acetyltransferase 1 (PDB ID: 4GS4; red). Protein model includes overlay of G. intestinalis WB C6 (GL50803) coding genes GL50803_16348 (gold), GL50803_117391 (blue), GL50803_117392 (purple) and GL50803_117393 (green). Inset (dotted border) shows expanded view of the α-tubulin binding pocket containing an acetyl-CoA (black). Sequence alignment shows conserved residues among all models. Red circles in the alignment depict core catalytic residues required by human αTAT1 for acetylating α-tubulin. (B) A Giardia homologue [GL50803_22338 (blue)] of human mRNA cap guanine N7-methyltransferase [5′ m7g MTase; PDB ID: 3BGV (red)], showing S-Adenysl-l-Homocysteine in the RNA-binding pocket; (C) Two homologues [GL50803_100887 (blue) and GL50803_103058 (grey)] of yeast cap methyltransferase 1 (red), showing S-adenosyl-l-methionine (purple) and 7N-Methyl-8-Hydroguanosine-5′-Triphosphate (yellow) in RNA-binding pocket, and (D) one homologue [GL50803_17308; (blue)] of yeast Cet1 mRNA guanine-N7 capping guanylyltransferase [PDB ID: 3KYH (red)]. Data from Ansell, B.R.E., Pope, B.J., Georgeson, P., Emery-Corbin, S.J., Jex, A.R., 2019. Annotation of the Giardia proteome through structure-based homology and machine learning. Gigascience 8, giy150.
In silico prediction can be used also to classify and infer functions for non-coding space. RepeatModeller (Tarailo-Graovac and Chen, 2009) and Tandem Repeats Finder (Benson, 1999) are widely used to identify transposable elements and other repetitive sequences. Programs such miRanda (Enright et al., 2003) and PITA (Kertesz et al., 2007) can predict the coding genes regulated by a specific miRNA. PromoterInspector (Scherf et al., 2000) and TRED (Zhao et al., 2005) can model promoter regions and sequence motif modellers, such as DREME (Bailey, 2011), can be used to identify potential transcription factor binding sites. However, most of these in silico predictive methods, aside from repeat finding, have high false discovery rates and should be used with caution or supplemented with additional data. Largely, researchers interested in non-coding regulatory features of the Giardia genome are likely better served by “omics” applications, which are discussed below.
4.2. Applying systems biology to function studies
In silico methods provide substantial guidance for researchers interested in Giardia molecular biology, but are a starting point to guide empirical experiments. This empirical work includes target-based (e.g., through gene silencing or knockout, or protein over-expression and localization) and systems-based (e.g., through transcriptomic, proteomic or other, similar and broad-spectrum methods) interrogation. This section will focus on systems-based functional study.
To date, transcriptomics and proteomics are the most broadly accessed systems-based approaches for Giardia. Franzén et al. (2013) provided the first RNAseq data for G. intestinalis, including the only available data to date for an assemblage B (GS) or E (P15) isolate. This work indicated significant sense and antisense transcription in Giardia and the authors postulated that Giardia’s transcription may be quite “leaky”. This observation, coupled with the few transcription factors identified in the genome, suggests Giardia exerts little transcriptional level. Despite this, G. intestinalis mounts specific transcriptional responses to external stimuli, including ER and starvation stress (Spycher et al., 2013), heat, oxidative-stress and sublethal metronidazole exposure (Ansell et al., 2016) and NO-induced, oxidative-stress and other interactions with host-cells (Ma’ayeh et al., 2015, 2017, 2018).
Not only do transcriptomic data indicate Giardia is capable of mounting stress-specific responses (Spycher et al., 2013) and differentiating physiological versus xenobiotic sources of these stress stimuli (Ansell et al., 2016), transcription responses differ among Giardia isolates. A recent study (Ansell et al., 2017) followed the transcriptional changes of G. intestinalis WB, 713 and 106, preceding and upon development of metronidazole resistance, identifying dynamic balancing between oxidative stress responses and metabolic activity, but also finding major differences among isolates. Metronidazole resistance in G. intestinalis WB and 713 appears to centre around up-regulation of oxidative stress response coupled with downregulation of the glycolytic system and reliance on arginine metabolism. This shift seems to come at a substantial fitness cost that manifests, particularly in G. intestinalis WB, as a reduced in vitro growth rate. In contrast, the transcriptional response to metronidazole resistance by G. intestinalis 106 appears focused on changes in nitroreductase activity, including a shift from metronidazole activating, nitroreductase-1 (NR1) transcription, to metronidazole deactivating, nitroreductase-2 (NR2) transcription. Proteomic data for these isolates show that transcriptional changes lead to dynamic responses at the protein level (Emery et al., 2018). Emery et al. (2018) also found that proteomic changes associated with G. intestinalis 106 were stable after 24 weeks of passage in in vitro in the absence of metronidazole. In contrast, most likely owing to an increased fitness cost, metronidazole resistance rapidly decayed of the same in vitro selection experiments when either G. intestinalis WB or G. intestinalis 713 isolate was tested, as has been described for these isolates elsewhere (Ansell et al., 2015b).
Transcriptomic and proteomic datasets supports Giardia’s coding gene models and improves mapping of gene boundaries, UTRs, splice junctions and other regulatory features. Numerous “omics” based approaches are available to expand on this knowledge, refining our understanding of the overall genomic and epigenetic regulation in Giardia, and supporting identification of novel players in important regulatory, signalling, stress response, metabolic and myriad other essential cellular functions. Many of these methods are available, but yet to be applied to Giardia. For example, further studies employing the targeted immunoprecipitation of AGO and other RNA-interacting proteins (e.g., per DICER: Saraiya et al., 2014) could greatly improve understanding of the importance of small RNAs in Giardia biology and identify their specific target genes. Chromatin-immunoprecipitation (ChIP) sequencing (Park, 2009) can be used to better understand the role of specific histone modifications in chromatin-level regulation in the cell cycle, during encystation/excystation and antigen switching, and following drug exposure or in vitro selected resistance.
Clearly, there are a variety of circumstances in which transcriptomic and proteomic approaches can be further employed to dissect parasite behaviour. Similarly, although several studies have indicated an important role for protein methylation, phosphorylation and acetylation (e.g., in encystation or drug resistance; reviewed in Ansell et al., 2015b), there are as yet no broad-scale studies of these modifications in Giardia. Metabolomics presents a considerable and as yet almost entirely untapped method to explore functional biology of Giardia, and would further open up more quantitative proteomic technologies (e.g., SILAC). However, metabolomics research is most powerful in its application in quantifying the rate of change (i.e., flux) in metabolites over time. This requires a defined media that can be altered to include radioactively labelled carbon, nitrogen or phosphorous, which is currently not available for Giardia.
As “omics” resources are developed for Giardia, it becomes possible to leverage their combined value in integrative analyses either combining datasets of the same (e.g., transcriptomics) or multiple origins (e.g., transcriptomics, proteomics and metabolomics data). R-language scripts, such as voom (Law et al., 2014), support identification and removal of systematic biases or batch effects between studies (this is essential for accurate analysis). Methods such as weighted-gene co-expression network analysis (WGCNA: Langfelder and Horvath, 2008) support identification of genes that strongly correlate in their transcriptional or expressional behaviour across a variety of complex conditions (e.g., stage differentiation, stress responses, cell division, etc.). Such tightly correlated genes are often co-regulated and tend to have related biological functions. These approaches can be used to infer novel functions for genes even with no a priori knowledge of their biology, for example, based on their co-regulation with multiple well annotated genes.
In-direct methods provide powerful, systems-level tools to explore the molecular biology of non-model organisms, such as Giardia intestinalis. They are, of course, a starting point for detailed functional research and can greatly assist development of specific, direct experiments for further exploration. Advances in tools for direct empirical study of gene function in Giardia are discussed in the next section.
5. Direct, targeted-based assessment of gene function
As most parasites are unculturable and genetically intractable, much of the functional biology is understood through in-direct inference by in silico modelling and, more recently, “omics” and systems-based research. These are powerful tools, but are most valuable when they are used to guide empirical interrogation of gene function through direct, target-based studies. For many years, Giardia was proposed as a model parasite because of its ready in vitro cultivation, but significant challenges in its genetic manipulation confounded this potential and stymied critical efforts to better understand the parasite. Recent major advances in the application of functional genetic tools to Giardia will open up exciting pathways for research in the coming years. These advances are discussed here.
5.1. Transfection vectors in Giardia and their use in studies of Giardia biology and pathogenesis
The first report of transient DNA transfection of Giardia was published in 1995 by Yee and Nash (1995). The plasmids were of bacterial origin and based on the pGEM (Promega) backbone containing the luciferase (luc) gene driven by 82bp upstream sequence of the glutamate dehydrogenase (gdh) gene, the first 18 gdh codons fused to luc and 129bp of downstream sequence of gdh, including the polyadenylation signal (Yee and Nash, 1995). Expression of luciferase required 50 μg of pure plasmid DNA in the transfection, and optimal expression was seen after 6h, decreasing to 13% after 24h (Yee and Nash, 1995). This system was further developed to a stable transfection system by insertion of a gdh regulated puromycin-N-acetyltransferase gene (pac) in to the transient transfection vector (Singer et al., 1998). Stable transfectants could be obtained after selection in 100μM puromycin (current standard concentrations for stable transfection of Giardia using pac containing plasmids is 50 μg/ml) and the plasmids were shown to be episomal (Singer et al., 1998). Later studies showed that the plasmid localized randomly to one of the two Giardia nuclei for up to 1 year during selection in puromycin (Yu et al., 2002). An integration vector was constructed using fragments of the triose phosphate isomerase (tpi) gene and integration was shown to be more effective in the assemblage B isolate GS than in the assemblage A isolate WB (Singer et al., 1998). This study also showed that the green fluorescent protein can be used as a reporter gene in Giardia (Singer et al., 1998). Another study showing stable transfection of Giardia using bacterial neomycin phosphotransferase (neo) as selectable marker in the pGEM plasmid was published by Sun et al. (1998) at the same time. The neo gene was driven by regulatory fragments from the giardial RAN gene and 150 μg/mL G418 was used in the selection. An increase of the amount of neomycin in the medium from 150 to 1200 μg/mL G418 increased the copy number of the plasmid, and it was shown that the plasmid remained in the cells for up to 50 days without selection (Sun et al., 1998).
The replication of external DNA fragments without selection has been seen in several later publications (Ebneter et al., 2016; McInally et al., 2019). The pac-gdh cassette was transferred to the pBlueScriptII KS(+)(Stratagene) vector and a deletion study was performed on the encystation-specific promoter of glucosamine-6-phosphate isomerase (Gln6PI-B) using luciferase as reporter (Knodler et al., 1999). This showed that only 50bp upstream of the start codon of Gln6PI-B is needed for developmental regulation, with this region later shown to bind the encystation-specific transcription factor Myb2 (Sun et al., 2002). The RAN-neo cassette was moved into the pBlueBcript vector by Hehl et al. and they could show that cyst wall protein 1 (CWP1) is regulated by a 110bp upstream sequence (Hehl et al., 2000). Importantly, they also showed, by using CWP1-GFP fusions, the mechanism by which cyst-wall material is transported in encystation-specific vesicles (ESVs) out to the emerging cyst-wall in encysting cells (Hehl et al., 2000). Thus, gene regulatory fragments and protein localization signals can be studied in Giardia since year 2000. Other inducible systems in Giardia including use of promoters of CWP-1 and −2 have later been used (Ebneter et al., 2016) and a tetracycline controlled gene expression system (Sun and Tai, 2000). Nonetheless, there is a need for new inducible gene expression systems in Giardia since both the tetracycline system is not very tightly controlled, and the CWP-system dependent on encystation induction. A new system for rapid triple-hemagglutinin (3HA) tagging and integration of Giardia genes into their endogenous regions was developed by Gourguechon and Cande (2011) and it was shown that blasticidin (75 μg/mL) can be used as a third selectable marker in Giardia by using a blasticidin resistance gene from the plasmid pBOSH2BGFP (Clontech).
The need to over-express and purify specific Giardia proteins for further characterization in vitro, as well as purify protein complexes, sparked the development of a suite of cassette-based expression vectors (Jerlstrom-Hultqvist et al., 2012). These vectors contain N- and C-terminal streptavidin binding peptide-glutathione S-transferase (SBP-GST) tags for production of recombinant proteins and N- and C-terminal Strep II-FLAG-tandem affinity tags for tandem affinity (TAP) purification of protein complexes (Jerlstrom-Hultqvist et al., 2012). The Giardia virulence factor arginine deiminase (ADI) and the proteasome complex were purified as proofs of concept. This suite of vectors has multiple cloning sites for cloning of genes of interest and have been modified to contain 3HA, 6 × His and the tetracycline regulatory system (Einarsson et al., 2016a).
In addition to over-expression, targeted gene repression or knockout is a major step in understanding protein function. However, Giardia is a tetraploid organism with two diploid nuclei (Bernander et al., 2001; Yu et al., 2002). This makes it difficult to genetically deplete proteins for functional analyses. The development of molecular genetic tools for gene knockout in Giardia has lagged behind that of other parasitic protists (DiCarlo et al., 2013; Hwang et al., 2013; Mali et al., 2013; Wang et al., 2013) or other polyploid organisms (Bedell et al., 2012; Clark et al., 2011). A rudimentary understanding of transcription factors and promoters (Davis-Hayman and Nash, 2002) and a limited number of selectable markers (Bedell et al., 2012; Clark et al., 2011; Gourguechon and Cande, 2011) have contributed to the slow pace of tool development. The giardial Cre/loxP system was used by Ebneter et al. (2016) to generate the first true knock-out mutants of Giardia lacking one to four copies of the CWP-1 gene. Knocking out one or two of the four copies did not create any detectable phenotypes, but trophozoites with one or no CWP-1 gene copy did not produce viable cysts and had impaired transport of CWP-2 in ESVs (Ebneter et al., 2016). Disc and flagellar disassembly and nuclear division still occurred, generating pseudocysts (Ebneter et al., 2016). The generation of the first knock-out mutants was major step in Giardia research, but this approach is onerous in that it has to be done sequentially with recycling of selectable markers, and it takes a long time to generate parasites with all four alleles knocked-out. Strategies beyond gene knockout are critical when assessing the functions of unknown proteins (Qi et al., 2013). Knockouts of genes in essential metabolic pathways or processes such as cell division can be lethal (Hardin et al., 2017), and require alternative strategies such transcriptional repression or translational blocking are also useful tools for determining protein function.
The Giardia genome contains conserved components of the RNA interference (RNAi) machinery, yet RNAi, a powerful tool for gene silencing in many organisms, is inefficient in Giardia (Krtkova and Paredez, 2017; Marcial-Quino et al., 2017). Other transcriptional repression methods, such as the overexpression of long double-stranded RNAs or hammerhead ribozymes (Chen et al., 2007; Dan et al., 2000), also have been used in Giardia with limited success and reproducibility. Morpholino oligonucleotides, which act by blocking translation, have been used most extensively for gene knockdown (Dawson et al., 2007; Hoeng et al., 2008; House et al., 2011; Paredez et al., 2011; Woessner and Dawson, 2012). However, although morpholino knockdowns can be robust in Giardia, morpholinos are expensive, and are thus less applicable for genome-wide functional screens (Krtkova and Paredez, 2017). Morpholinos can also lack complete penetrance and are transient, lasting less than 48h after electroporation. These limitations make them less than optimal for the characterization of infection dynamics in animal hosts (see below and Barash et al., 2017).
5.2. Creating robust and stable gene knockdown strains with CRISPR interference
CRISPR/Cas9 gene editing provides a potentially transformative technology for Giardia research. However, the use of CRISPR/Cas9 in Giardia was stalled by the parasites lack of a non-homologous end-joining (NHEJ) pathway (Morrison et al., 2007) and a defined sexual cycle (Poxleitner et al., 2008), as well as problems with nuclear localization of Cas9 using standard nuclear localization signals (SV40 NLS: Ebneter et al., 2016). Nonetheless, CRISPR/Cas technology has revolutionized genome editing in eukaryotes, and CRISPR/Cas-mediated knockout strategies have recently been implemented for several parasitic protists (Ren and Gupta, 2017). Beyond their utility for gene knockout, Cas proteins have been exploited for their ability to bind to target nucleic acid sequences and recruit a variety of effector proteins, including transcriptional repressors and activators, epigenetic modifiers and fluorophores for imaging (Larson et al., 2013; Pickar-Oliver and Gersbach, 2019). In both eukaryotic and bacterial model systems, CRISPR interference (CRISPRi) has been shown to be a robust alternative to RNAi-mediated gene silencing for knockdown of gene expression (Kampmann, 2018; Larson et al., 2013).
CRISPRi is a modification of the CRISPR/Cas system using a catalytically inactive, or “dead”, Cas protein (dCas) to promote stable, inducible, or reversible gene knockdown in human cell lines and yeast (Larson et al., 2013) and diverse bacteria (Kaczmarzyk et al., 2018; Larson et al., 2013; Liu et al., 2017; Tao et al., 2017; Zhang et al., 2016; Zuberi et al., 2017). Like Cas proteins, inactive dCas proteins are directed to precise genomic targets using a complementary guide RNA (gRNA). Rather than catalysing double-stranded breaks in DNA, the inactive dCas/gRNA complex prevents transcription initiation and/or elongation upon binding (Larson et al., 2013). CRISPRi is as effective as RNAi in transcriptional silencing, and fewer off-target effects have been reported (Larson et al., 2013). Thus CRISPRi directly and stably inhibits transcription, and offers significant advantages over RNAi or morpholino knockdown (Larson et al., 2013). CRISPRi does not interfere with endogenous microRNA expression or function (Larson et al., 2013), and because CRISPRi acts at the DNA level, Giardia noncoding RNAs (Saraiya and Wang, 2008), microRNAs (Li et al., 2011; Zhang et al., 2009), antisense transcripts (Elmendorf et al., 2001), and polymerase III transcripts (Hudson et al., 2012) could be targeted for repression.
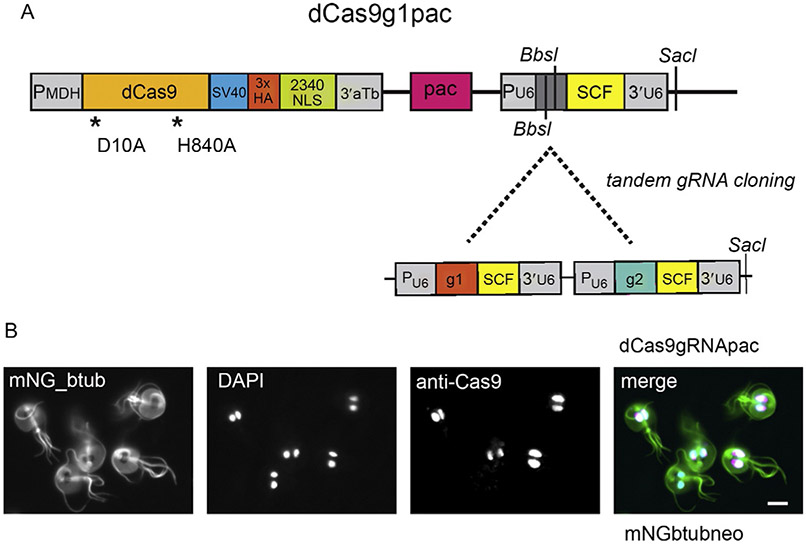
CRISPRi was recently adapted to repress both exogenous and endogenous genes (McInally et al., 2019) in Giardia. CRISPRi does not require Giardia host or viral factors as is required for antisense (Rivero et al., 2010b) or hammerhead ribozyme-mediated transcriptional repression (Chen et al., 2007; Dan et al., 2000). In Giardia, targeting of the Streptococcus pyogenes Cas9 or dCas9/gRNA DNA recognition complex to both nuclei required the addition of a native Giardia nuclear localizing sequence (NLS) (McInally et al., 2019). A CRISPRi episomal vector (dCas9g1pac) was then created that includes cassettes for the expression of dCas9 and a gRNA, as well as a marker for puromycin resistance for positive selection in Giardia (Fig. 2). Giardia gRNAs are designed using the CRISPR Guide RNA design tool at Benchling [Biology Software] (Benchling, 2019) or the Eukaryotic Pathogen CRISPR guide RNA/DNA Design Tool (EuPaGDT) (Peng and Tarleton, 2015) at GiardiaDB (giardiadb.org), and are chosen based on predictions of gRNA efficiency and the results of on- and off-target analyses. Complementary gRNA oligonucleotides are then annealed and cloned into the CRISPRi vector using a one-step digestion/ligation reaction. gRNA design and cloning can be accomplished in a week, and CRISPRi strains are obtained 10–14 days after electroporation of constructs into Giardia trophozoites.
Fig. 2.
The Giardia CRISPRi plasmid includes a Giardia-specific nuclear localization signal (NLS) to localize dCas9 to both nuclei. The schematic of the Giardia CRISPRi vector dCas9g1pac indicates the catalytically inactive dCas9 with a C-terminal Giardia-specific 2340NLS and a 3XHA epitope tag (A). dCas9 expression is driven by the Giardia malate dehydrogenase promoter (PMDH), and a puromycin resistance marker (pac) allows positive selection in Giardia. The dCas9g1pac plasmid also includes inverted BbsI restriction sites for cloning of specific gRNA target sequences (g1 or g2) upstream of the gRNA scaffold sequence (SCF). The Giardia U6 spliceosomal RNA pol III promoter is used to express the gRNA cassette (g1+SCF); a non-specific gRNA is expressed unless the sequence between the BbsI sites is replaced with annealed oligomers targeting a specific genomic region. The SacI site can be used to add additional gRNA cassettes in tandem. Anti-Cas9 immunostaining of a Giardia strain carrying dCas9g1pac shows that a Giardia-specific native 34-amino acid C-terminal NLS from the Giardia protein GL50803_2340 (2340NLS) is necessary for the localization of 2340GFP to both nuclei (B). Over 50% of cells express dCas9 in both nuclei. In this strain, the microtubule cytoskeleton is visualized by expression of an integrated N-terminal mNeonGreen-tagged beta-tubulin gene selected with neomycin (mNGbtubneo). Scale bars = 5 μm.
Stable, precise and robust CRISPRi-based gene regulation of single or multiple genes will transform the study of molecular and cellular biology and pathogenesis in this widespread intestinal parasite. The modular design of the Giardia gRNA expression cassette allows for the concatenation of more than one gRNA to target multiple sites in one target gene, or more than one Giardia gene or gene family. Once imported into the nuclei, the dCas9/gRNA complex is targeted to a specific genomic locus where it sterically interferes with RNA polymerase or transcription factor binding or with transcriptional elongation, as has been shown in bacteria or other eukaryotes (Larson et al., 2013). dCas9 expression and overall penetrance is quantified through the use of anti-Cas9 antibodies to score dCas9-positive trophozoites. The use of FACS and other methods to enrich for trophozoites that strongly express dCas9 could increase knockdown penetrance, as has been seen in other CRISPRi systems (Gilbert et al., 2013).
In every CRISPRi system (Larson et al., 2013), the choice of gRNA target site has been shown to impact the degree of transcriptional repression. In contrast to human cells (Gilbert et al., 2013; Qi et al., 2013), targeting gRNAs close to the transcriptional start site (TSS) results in stronger repression in some bacterial systems. To determine how gRNA positioning influenced the magnitude of transcriptional repression in Giardia, 11 different gRNAs were systematically designed to target the transcriptional start site and the coding region of the bioluminescent NanoLuc (NLuc) gene in 50-bp increments (McInally et al., 2019). There was no correlation between gRNA position and the magnitude of repression; however, in general, more repression was observed with gRNAs that targeted the first 200bp of the coding region. Because gRNAs targeted the coding region rather than the promoter sequence used for NLuc expression, it is likely that efficient transcriptional repression occurred via the inhibition of transcriptional elongation, rather than inhibition of transcriptional initiation (Larson et al., 2013). For successful gene repression in Giardia, several gRNAs should be tested for each target, as has been suggested for CRISPRi in human cells (Gilbert et al., 2013). The ability to direct dCas9 to both nuclei in Giardia could enable the modulation of transcriptional networks not only by repression, but also by differential expression or overexpression through the fusion of dCas9 to Giardia-specific transcription factors, enhancers, or other native transcriptional elements (Kampmann, 2018).
Giardia’s dynamic microtubule cytoskeleton is of critical importance throughout both stages of its life cycle (Dawson, 2010; Nosala and Dawson, 2015). CRISPRi-mediated stable transcriptional repression was also used recently to knock down the expression of three endogenous cytoskeletal proteins, highlighting the versatility of CRISPRi to interrogate Giardia microtubule functioning in flagellar motility and disc-mediated attachment (McInally et al., 2019). Specifically, 2 of the 24 Giardia kinesin motor proteins (kinesin-2a and kinesin-13) and one ventral disc protein (DAP16343) were targeted for CRISPRi knockdown. The heterotrimeric kinesin-2 motor is required for assembly of external regions of axonemes (Dawson et al., 2007; Hoeng et al., 2008). Kinesin-13 regulates flagellar length in all Giardia axonemes through the promotion of microtubule disassembly at the distal flagellar tips (Dawson et al., 2007). Overexpression of dominant negative kinesin-2 or kinesin-13 thus results in aberrant flagellar lengths of all of the eight flagella (Dawson et al., 2007; Hoeng et al., 2008). Similar length defects were observed with the CRISPRi-based knockdowns of kinesin-2a and kinesin-13, supporting the essential roles of these two kinesins in flagellar length regulation in Giardia.
Over 90 proteins compose Giardia’s ventral disc. Prior transient morpholino-based knockdown of the disc-associated protein DAP16343 (median body protein, or MBP) resulted in discs with an open and flattened conformation and significant attachment defects (Woessner and Dawson, 2012). Stable CRISPRi-mediated knockdown of MBP resulted in the same “open” and “flat” disc phenotype observed with morpholinos (Woessner and Dawson, 2012), again confirming the use of CRISPRi as a genetic tool to affect disc structure and attachment. In contrast to morpholino-based knockdowns, the MBP knockdown strain is stable over time and phenotypes are 60–100% penetrant in Cas9+ cells. As noted previously, the degree of CRISPRi knockdown of cytoskeletal genes was contingent upon the targeting position of the gRNA and the degree of expression of dCas9 in the nuclei. More severe phenotypes were associated with dCas9 positive cells, with overall population-level transcriptional knockdowns ranging from 10 to 60%. Rather than a deficit, the variations in knockdown levels highlight the overall tunability of this system for knockdown of endogenous genes in contrast to transient morpholino knockdown. The ability to examine cells with varying degrees of knockdown and to reduce the severity of phenotypes by selecting gRNAs with less than complete transcriptional repression are critical toward the evaluation of essential Giardia genes. Furthermore, this work demonstrated the ability to knock down two different genes simultaneously with the concatenation of gRNAs targeting both kinesin-13 and MBP. This is a key technology for evaluation of the redundant functions of genes in a gene family or pathway.
5.3. Using CRISPRi to study basic and pathogenic aspects of Giardia biology
CRISPRi will rapidly change how we study basic cell biology, development, and pathogenesis of Giardia. CRISPRi would be an inexpensive and fruitful strategy to identify essential genes associated with key aspects of Giardia’s life cycle, such as attachment (Nosala et al., 2018), motility (Dawson and House, 2010), or encystation (Einarsson et al., 2016c; Pham et al., 2017). The ability to rapidly create stable knockdown strains will facilitate the functional identification of genes involved in pathogenicity (e.g., motility or attachment) that can then be screened to identify druggable targets. Genomes and genetic tools are available for the assemblage A strain WBC6 (Morrison et al., 2007) and the assemblage B strain GS (Davis-Hayman and Nash, 2002); however, no genetic tools have been developed for other sequenced assemblage A strains such as DH (Adam et al., 2013), or for other human clinical A and B assemblage isolates (Hanevik et al., 2015). Thus, ongoing work should also aim to develop genetic tools in other Giardia strains of clinical relevance.
5.4. In vivo bioluminescent imaging of Giardia infection dynamics in small animal models
The molecular and cellular mechanisms underlying Giardia’s colonization and differentiation into cysts in the gastrointestinal tract remain unclear. Mammalian hosts ingest Giardia cysts that excyst and release motile trophozoites as they transit into the gastrointestinal tract (Einarsson et al., 2016b). The excysted trophozoites then attach and extracellularly colonize the epithelium of the small intestine. Later, trophozoites detach and encyst and these cysts are disseminated in faeces (Adam, 2001). Most studies of Giardia’s pathogenesis have relied on in vitro models of giardiasis that may not be adequate proxies for infection within the host or may not accurately reflect in vivo parasite physiology. In vivo studies of giardiasis have been limited by the inaccessibility of the intestinal tract; thus, indirect quantification methods have been used to assess in vivo parasite burden, differentiation, and physiology, including the isolation of cysts from faeces or the isolation and enumeration of parasites from the intestines of euthanized study animals (Bartelt et al., 2013; Solaymani-Mohammadi and Singer, 2011). The inaccessibility of the site of Giardia colonization in the gut has restricted tracking temporal infection dynamics in a single animal throughout the course of infection, and instead necessitated the use of cohorts of animals for each experimental time point for adequate experimental rigour and reproducibility (Barash et al., 2017). Furthermore, Giardia colonization of the small intestine overlaps with ecological niches inhabited by commensal microbiota, yet in vivo Giardia-microbiome interactions have largely been ignored in models of pathogenesis (Bartelt and Sartor, 2015).
The adhesive disc of Giardia is a unique cytoskeletal structure containing more than 100 proteins (Hagen et al., 2011). In order to study putative adhesive disc proteins, identified by sub-cellular fractionation and proteomic analyses, the Dawson lab developed a ligation-independent high-throughput cloning method (Gateway cloning) for C-terminal GFP or mNeon green (NG) tagging of Giardia proteins (Hagen et al., 2011). This system has been used to determine the subcellular localization of selected Giardia proteins that lack homology or have limited similarity to proteins in other organisms (www.Giardiadb.org). The ORFs plus 200bp of upstream sequence containing the native promoter were PCR-amplified from genomic Giardia DNA to create Gateway entry clones and subsequently cloned into the Gateway expression vector pcGFP1F.pac (Hagen et al., 2011) to create C-terminal GFP fusion strains. Until now ~600 proteins have been localized in Giardia using this system and the data is available at Giardia DB.
Recently developed methods for bioluminescent imaging (BLI) using integrated luciferase bioreporter strains now allow real-time quantitative visualization of the temporal and spatial dynamics of Giardia infections in the gastrointestinal tracts of small animal (Barash et al., 2017). BLI has been used previously to monitor parasitic infection dynamics for malaria, leishmaniasis, trypanosomiasis, and toxoplasmosis (D’Archivio et al., 2013; Reimao et al., 2013; Saeij et al., 2005), as well as bacterial colonization of the intestine (Hutchens and Luker, 2007). In general, BLI enables sensitive quantification and real-time reporting of metabolic activity via imaging of the transcriptional activity of promoter-luciferase fusions or imaging of protein expression through the expression of native proteins fused to luminescent proteins such as NanoLuc (Luo et al., 2006; Weissleder and Ntziachristos, 2003; Welsh and Kay, 2005). Non-invasive in vivo BLI relies on the external detection of light produced internally, and signal intensity may be limited by the overall level of luciferase expression, the oxygen tension within relevant tissues, pigmentation of organs and skin, or any background signal from the animal (Andreu et al., 2011). However, the gut is sufficiently oxygenated to permit signal detection. Importantly, although animal tissues exhibit relatively high background levels of autofluorescence, they have nearly nonexistent levels of autoluminescence, which facilitates bioluminescent signal detection even at low signal strength (Andreu et al., 2011; Foucault et al., 2010; Rhee et al., 2011).
In vivo models of infection are needed to help define the complex interactions between Giardia and the gastrointestinal tract of the mammalian host. Zoonotic Giardia strains have varied physiologies and assemblage A (strains WBC6 and DH) and assemblage B (strains GS and HS) (Ankarklev et al., 2015; Sprong et al., 2009) are the only assemblages identified in human infections. Animal models of giardiasis have used adult (Byrd et al., 1994; Singer, 2016) or suckling mice (Mayrhofer et al., 1992) or adult gerbils (Rivero et al., 2010a) infected with either human isolates of Giardia assemblages A (strain WBC6) or B (strains GS or H3), or murine G. muris isolates (Aggarwal and Nash, 1987). Assemblage B strain H3 cysts have been used to establish animal infections (Bartelt et al., 2013), yet strain H3 currently lacks a genome sequence, and cannot be transfected or otherwise genetically manipulated. Variation in the degree or timing of experimental infections have been noted with the use of isolates from different assemblages or the use of different animal models, and mirrors variability in human giardiasis (Watkins and Eckmann, 2014). Such variation can confound the interpretation of infection dynamics from experimental cohorts and underscores the need for additional methods of evaluating Giardia infections in animal models.
In vivo BLI offers both real-time and longitudinal monitoring of infection dynamics in small animals such as mice or gerbils. Infection with recombinant Giardia strains containing integrated bioluminescent proteins such as firefly luciferase fused to physiological or encystation-specific promoters or genes (commonly termed “bioreporters”) has permitted the temporal and spatial imaging and quantification of parasite colonization, metabolism and differentiation (Barash et al., 2017). As the WBC6 (assemblage A) strain is genetically tractable, three transcriptional bioreporter strains have been created for in vivo and ex vivo BLI by integrating firefly luciferase driven by either endogenous metabolic or encystation-specific promoters (Barash et al., 2017). Infections with the integrated constitutive glutaraldehyde dehydrogenase (GDH) bioreporter (PGDH-FLuc) strain allow the quantification of in vivo infection dynamics including parasite density and metabolism. Optical imaging with this constitutive strain directly correlates with qPCR-based measures of parasite density (Barash et al., 2017). Luciferase expression from the GDH promoter continues at significant levels for at least 24h after the PGDH-FLuc strain is transferred to encystation medium; therefore, BLI of constitutive metabolic genes could be used as a proxy for in vivo Giardia abundance even during encystation. BLI of infections with the constitutively expressed PGDH-FLuc strain confirm that maximal infection occurs at approximately 7 days, consistent with prior studies of experimental Giardia infections in mice (Byrd et al., 1994). In vivo Giardia physiology and encystation in cohorts of mice or gerbils have been monitored non-invasively for at least 21 days. Longitudinal BLI of the same study animal provided a robust method to estimate variance within infections, which is essential for determining statistically informative study animal numbers. Additional in vivo bioreporter strains could include those associated with parasite stress response, physiology, or excystation. In vivo parasite protein expression could also be monitored if Giardia proteins were fused to bioluminescent proteins such as NanoLuc (Stacer et al., 2013), which could be particularly informative for the in vivo quantitation of secreted Giardia proteins.
Ex vivo BLI of tagged Giardia strains has enabled the spatial quantification of colonization and parasite differentiation in the GI tract of mice and gerbil animal models. After live imaging, study animals are euthanized, and the GI tract is excised and imaged again with BLI to provide greater spatial resolution. Quantitative ex vivo BLI of the constitutive bioreporter PGDH-FLuc in both mice and gerbils showed that rather than uniformly colonizing throughout a region of the GI tract, Giardia colonizes the proximal small intestine non-uniformly in discrete high-density foci. Such BLI-based anatomical sampling strategies facilitate the precise identification of regions of the gastrointestinal tract associated with high or low densities of colonizing or encysting parasites. In this way, ex vivo spatial localization of bioreporter signals can be used to correlate discrete areas of Giardia abundance and encystation in the gastrointestinal tracts of study animals with spatially defined histological analyses or transcriptional profiling of the same discrete Giardia foci (Pham et al., 2017).
Trophozoites are believed to be induced to encyst after receiving biochemical cues from a specific gastrointestinal anatomical site (Gillin et al., 1987; Lujan et al., 1997). Encystation can be induced in vitro by lowering the pH or adjusting the concentrations of other components of the medium, particularly bile (Gillin et al., 1988; Lujan et al., 1996). Parasite commitment to encystation and excystation are key highly regulated transitions in Giardia’s life cycle (Einarsson et al., 2016c). Hallmarks of the developmental commitment to encystation include the significant transcriptional upregulation of genes encoding the cyst wall proteins CWP1 and CWP2, and the appearance of encystation specific vesicles (ESVs) that transport the cyst wall proteins (e.g., CWP1 and CWP2) to build the cyst wall (Hehl et al., 2000; Lujan et al., 1995).
To monitor the temporal and spatial dynamics of in vivo encystation with BLI, two integrated encystation-specific bioreporters (PCWP1-FLuc and PCWP2-FLuc) have also been developed (see Fig. 3). By imaging encystation-specific bioreporters, it was observed that encystation initiates shortly after inoculation and continues throughout the entire duration of infection. Using ex vivo BLI, encystation initiation was reported to occur in discrete foci within the proximal and distal small intestine, rather than uniformly throughout a particular region of the gut. Thus Giardia BLI has uncovered that parasites colonize and encyst in the gastrointestinal tract of both mice and gerbils with a localized or “patchy” distribution (Barash et al., 2017), as has been observed for many other pathogens of the gastrointestinal tract or other organs (Contag et al., 1995; D’Archivio et al., 2013; Foucault et al., 2010; Reimao et al., 2013; Saeij et al., 2005). To discriminate between encysting and non-encysting parasites in ex vivo samples, dual reporter bioluminescent Giardia strains using two spectrally different bioluminescent tags (such as firefly luciferase and red-shifted firefly luciferase) could be used to simultaneously image and quantify metabolically active (GDH-FLuc) and encysting (CWP1-RLuc) parasites (Branchini et al., 2007; Cevenini et al., 2014; Maguire et al., 2013).
Fig. 3.
Bioluminescent imaging enables quantitative evaluation of temporal and spatial infection dynamics in the gastrointestinal tracts of small animals. Transcriptional upregulation of the Giardia cyst wall protein 2 (CWP2) is a genetic indicator of the initiation of encystation. Representative in vivo (A) and ex vivo (B) bioluminescent images are shown for three mice infected with the PCWP2-FLuc strain and euthanized at 7 days post-infection (A). High bioluminescent signal is indicated for the distal small intestine (dsi) in animal 1 and for the proximal small intestine (psi) in animals 2 and 3. Photon flux or radiance (p/s/cm2/sr) for each intestinal segment is shown and has been normalized to the maximal ex vivo bioluminescence signal on the radiance scale, yielding the percent total signal per segment. These values are represented graphically on the grey scale maps below each ex vivo image (clear = 0–10% and black = 75–100%, with values between 10% and 75% indicated as shades of grey). The regions of the gastrointestinal tract (psi = proximal small intestine, dsi = distal small intestine, cec = cecum, and li = large intestine) are noted on the ex vivo images (B). The stomach (stm = stomach) is shown for orientation but lacks bioluminescence.
Ultimately, the use of single or dual spectra bioreporters of additional metabolic genes will provide a more comprehensive understanding of the in vivo interactions of Giardia with the host or with the microbiome. Giardia trophozoites produce no known toxin, and colonization does not elicit a robust inflammatory reaction (Bartelt and Sartor, 2015; Cotton et al., 2015). Although it has been speculated that Giardia infection results in intestinal epithelial damage, changes in the ultrastructure of the small intestine that are sufficient to cause clinical symptoms have not been consistently observed (Robertson et al., 2010). With BLI-directed ex vivo sampling of foci of colonization, one can precisely correlate trophozoite abundance and metabolism with host pathology in the same anatomical region.
The ability to create stable CRISPRi knockdowns in the luciferase bioreporter strains used for bioluminescent imaging (BLI) will be essential toward understanding the cellular roles of Giardia proteins during colonization and encystation in the host (Barash et al., 2017). Combined with BLI, CRISPRi will enable the examination of fitness, colonization, or encystation differences in knockdown Giardia strains. BLI has also been validated for the analysis of anti-Giardia drugs by the demonstration that metronidazole, the standard of care anti-Giardia drug that targets parasite metabolic activity (Tejman-Yarden and Eckmann, 2011), reduced in vivo bioluminescence of the constitutively-expressing PGDH-FLuc bioreporter strain. As it has been used extensively to evaluate the efficacy of anti-parasite or anti-bacterial drugs (Xu et al., 2016), BLI will be valuable as an alternative and real-time method to evaluate anti-Giardia drugs in relevant animal models of giardiasis.
6. Concluding remarks
Giardia has proven a tantalizingly close but frustratingly distant model eukaryote and genetically tractable parasitic species. Its ready culturability and early discoveries in its regulatory biology, as well as its status as an early diverging branch of the eukaryotic tree and cause of significant disease in humans and animals, has piqued interest in the species for many years. However, its binucleate cell, tetraploid genome and limited acceptance of seemingly attractive genetic tools, such as RNA interference and conventional gene knockout, have stymied research and limited opportunities for researchers to slake their interest. These limitations appear largely over-come and Giardia is poised to take its place as a model system that can be studied both as a significant infectious organism and as an early glimpse into evolution of eukaryotic biology, metabolism and regulatory systems. Advances, particularly in the demonstration of CRISPr-based methods for genetic manipulation, will no doubt shape the field for many years to come. Some challenges still remain, particularly in the ability to generate stable gene knockouts and the need for a defined culture media to support label-based proteomic and metabolomic studies. Yet, the primary tools are now in place, the genomic and systems-based resources established, and the limitations to its exploration now appear readily surmountable. The field is poised to enter a new and exciting era of biology discovery, in which we, and no doubt our many colleagues, are eager to take part.
Acknowledgements
A.J. acknowledges funding support from the Australian National Health and Medical Research Council (Career Development Fellowship APP1126395), Melbourne Water and the Victorian State Government Operational Infrastructure Support and Australian Government National Health and Medical Research Council Independent Research Institute Infrastructure Support Scheme.
References
- Adam RD, 2000. The Giardia lamblia genome. Int. J. Parasitol 30, 475–484. [DOI] [PubMed] [Google Scholar]
- Adam RD, 2001. Biology of Giardia lamblia. Clin. Microbiol. Rev 14, 447–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam RD, Dahlstrom EW, Martens CA, Bruno DP, Barbian KD, Ricklefs SM, Hernandez MM, Narla NP, Patel RB, Porcella SF, Nash TE, 2013. Genome sequencing of Giardia lamblia genotypes A2 and B isolates (DH and GS) and comparative analysis with the genomes of genotypes A1 and E (WB and Pig). Genome Biol. Evol 5, 2498–2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal A, Nash TE, 1987. Comparison of two antigenically distinct Giardia lamblia isolates in gerbils. Am. J. Trop. Med. Hyg 36, 325–332. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ, 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25, 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreu N, Zelmer A, Wiles S, 2011. Noninvasive biophotonic imaging for studies of infectious disease. FEMS Microbiol. Rev 35, 360–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankarklev J, Franzen O, Peirasmaki D, Jerlstrom-Hultqvist J, Lebbad M, Andersson J, Andersson B, Svard SG, 2015. Comparative genomic analyses of freshly isolated Giardia intestinalis assemblage a isolates. BMC Genomics 16, 697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansell BR, McConville MJ, Baker L, Korhonen PK, Young ND, Hall RS, Rojas CA, Svard SG, Gasser RB, Jex AR, 2015a. Time-dependent transcriptional changes in axenic Giardia duodenalis trophozoites. PLoS Negl. Trop. Dis 9 e0004261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansell BR, McConville MJ, Ma’ayeh SY, Dagley MJ, Gasser RB, Svard SG, Jex AR, 2015b. Drug resistance in Giardia duodenalis. Biotechnol. Adv 33, 888–901. [DOI] [PubMed] [Google Scholar]
- Ansell BR, McConville MJ, Baker L, Korhonen PK, Emery SJ, Svard SG, Gasser RB, Jex AR, 2016. Divergent transcriptional responses to physiological and xenobiotic stress in Giardia duodenalis. Antimicrob. Agents Chemother 60, 6034–6045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansell BR, Baker L, Emery SJ, McConville MJ, Svard SG, Gasser RB, Jex AR, 2017. Transcriptomics indicates active and passive metronidazole resistance mechanisms in three seminal Giardia lines. Front. Microbiol 8, 398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansell BRE, Pope BJ, Georgeson P, Emery-Corbin SJ, Jex AR, 2019. Annotation of the Giardia proteome through structure-based homology and machine learning. Gigascience 8, giy150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey TL, 2011. DREME: motif discovery in transcription factor ChIP-seq data. Bioinformatics 27, 1653–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barash N, Nosala C, Pham JK, McInally SG, Gourguechon S, McCarthy-Sinclair B, Dawson SC, 2017. Giardia colonizes and encysts in high density foci in the murine small intestine. mSphere 2, e00316–e00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartelt LA, Sartor RB, 2015. Advances in understanding Giardia: determinants and mechanisms of chronic sequelae. F1000Prime Rep. 7, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartelt LA, Roche J, Kolling G, Bolick D, Noronha F, Naylor C, Hoffman P, Warren C, Singer S, Guerrant R, 2013. Persistent G. lamblia impairs growth in a murine malnutrition model. J. Clin. Invest 123, 2672–2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedell VM, Wang Y, Campbell JM, Poshusta TL, Starker CG, Krug RG 2nd, Tan W, Penheiter SG, Ma AC, Leung AY, Fahrenkrug SC, Carlson DF, Voytas DF, Clark KJ, Essner JJ, Ekker SC, 2012. In vivo genome editing using a high-efficiency TALEN system. Nature 491, 114–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benchling, 2019. Biology Software. Retrieved from, https://benchling.com. [Google Scholar]
- Benson G, 1999. Tandem repeats finder: a program to analyze DNA sequences. Nucleic Acids Res. 27, 573–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE, 2000. The protein data bank. Nucleic Acids Res. 28, 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernander R, Palm JE, Svard SG, 2001. Genome ploidy in different stages of the Giardia lamblia life cycle. Cell. Microbiol 3, 55–62. [DOI] [PubMed] [Google Scholar]
- Branchini BR, Ablamsky DM, Murtiashaw MH, Uzasci L, Fraga H, Southworth TL, 2007. Thermostable red and green light-producing firefly luciferase mutants for bioluminescent reporter applications. Anal. Biochem 361, 253–262. [DOI] [PubMed] [Google Scholar]
- Buret A, Lin YC, 2008. Genotypic characterization of an epithelial cell line for the study of parasite-epithelial interactions. J. Parasitol 94, 545–548. [DOI] [PubMed] [Google Scholar]
- Buret AG, Mitchell K, Muench DG, Scott KG, 2002. Giardia lamblia disrupts tight junctional ZO-1 and increases permeability in non-transformed human small intestinal epithelial monolayers: effects of epidermal growth factor. Parasitology 125, 11–19. [DOI] [PubMed] [Google Scholar]
- Byrd LG, Conrad JT, Nash TE, 1994. Giardia lamblia infections in adult mice. Infect. Immun 62, 3583–3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera-Licona A, Solano-Gonzalez E, Fonseca-Linan R, Bazan-Tejeda ML, Raul A-G, Bermudez-Cruz RM, Ortega-Pierres G, 2017. Expression and secretion of the Giardia duodenalis variant surface protein 9B10A by transfected trophozoites causes damage to epithelial cell monolayers mediated by protease activity. Exp. Parasitol 179, 49–64. [DOI] [PubMed] [Google Scholar]
- Carranza PG, Gargantini PR, Prucca CG, Torri A, Saura A, Svard S, Lujan HD, 2016. Specific histone modifications play critical roles in the control of encystation and antigenic variation in the early-branching eukaryote Giardia lamblia. Int. J. Biochem. Cell Biol 81, 32–43. [DOI] [PubMed] [Google Scholar]
- Cevenini L, Camarda G, Michelini E, Siciliano G, Calabretta MM, Bona R, Kumar TR, Cara A, Branchini BR, Fidock DA, Roda A, Alano P, 2014. Multicolor bioluminescence boosts malaria research: quantitative dual-color assay and single-cell imaging in Plasmodium falciparum parasites. Anal. Chem 86, 8814–8821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chastre E, Emami S, Rosselin G, Gespach C, 1985. Vasoactive intestinal peptide receptor activity and specificity during enterocyte-like differentiation and retrodifferentiation of the human colonic cancerous subclone HT29-18. FEBS Lett. 188, 197–204. [DOI] [PubMed] [Google Scholar]
- Chen L, Li J, Zhang X, Liu Q, Yin J, Yao L, Zhao Y, Cao L, 2007. Inhibition of krr1 gene expression in Giardia canis by a virus-mediated hammerhead ribozyme. Vet. Parasitol 143, 14–20. [DOI] [PubMed] [Google Scholar]
- Chen XS, Collins LJ, Biggs PJ, Penny D, 2009. High throughput genome-wide survey of small RNAs from the parasitic protists Giardia intestinalis and Trichomonas vaginalis. Genome Biol. Evol 1, 165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, et al. , 2017. In vitro enteroid-derived three-dimensional tissue model of human small intestinal epithelium with innate immune responses. PLoS One 12 (11), e0187880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark KJ, Voytas DF, Ekker SC,2011. A TALE of two nucleases: gene targeting for the masses? Zebrafish 8, 147–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contag CH, Contag PR, Mullins JI, Spilman SD, Stevenson DK, Benaron DA, 1995. Photonic detection of bacterial pathogens in living hosts. Mol. Microbiol 18, 593–603. [DOI] [PubMed] [Google Scholar]
- Cotton JA, Amat CB, Buret AG, 2015. Disruptions of host immunity and inflammation by Giardia duodenalis: potential consequences for co-infections in the gastrointestinal tract. Pathogens 4, 764–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan M, Wang AL, Wang CC, 2000. Inhibition of pyruvate-ferredoxin oxidoreductase gene expression in Giardia lamblia by a virus-mediated hammerhead ribozyme. Mol. Microbiol 36, 447–456. [DOI] [PubMed] [Google Scholar]
- Dann SM, Le CHY, Hanson EM, Ross MC, Eckmann L, 2018. Giardia infection of the small intestine induces chronic colitis in genetically susceptible hosts. J. Immunol 201, 548–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Archivio S, Cosson A, Medina M, Lang T, Minoprio P, Goyard S, 2013. Non-invasive in vivo study of the Trypanosoma vivax infectious process consolidates the brain commitment in late infections. PLoS Negl. Trop. Dis 7, e1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davids BJ, Gillin FD, 2011. Methods for Giardia culture, cryopreservation, encystation, and excystation in vitro In: Svärd S, Lujan HD (Eds.), Giardia - A Model Organism. Springer, pp. 381–394. [Google Scholar]
- Davis-Hayman SR, Nash TE, 2002. Genetic manipulation of Giardia lamblia. Mol. Biochem. Parasitol 122, 1–7. [DOI] [PubMed] [Google Scholar]
- Dawson SC, 2010. An insider’s guide to the microtubule cytoskeleton of Giardia. Cell. Microbiol 12, 588–598. [DOI] [PubMed] [Google Scholar]
- Dawson SC, House SA, 2010. Life with eight flagella: flagellar assembly and division in Giardia. Curr. Opin. Microbiol 13, 480–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson SC, Sagolla MS, Mancuso JJ, Woessner DJ, House SA, Fritz-Laylin L, Cande WZ, 2007. Kinesin-13 regulates flagellar, interphase, and mitotic microtubule dynamics in Giardia intestinalis. Eukaryot. Cell 6, 2354–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCarlo JE, Norville JE, Mali P, Rios X, Aach J, Church GM, 2013. Genome engineering in Saccharomyces cerevisiae using CRISPR-Cas systems. Nucleic Acids Res. 41, 4336–4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosh RH, Jordan-Mahy N, Sammon C, Le Maitre CL, 2018. Tissue engineering laboratory models of the small intestine. Tissue Eng. Part B Rev 24, 98–111. [DOI] [PubMed] [Google Scholar]
- Dreesen L, De Bosscher K, Grit G, Staels B, Lubberts E, Bauge E, Geldhof P, 2014. Giardia muris infection in mice is associated with a protective interleukin 17A response and induction of peroxisome proliferator-activated receptor alpha. Infect. Immun 82, 3333–3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebneter JA, Heusser SD, Schraner EM, Hehl AB, Faso C, 2016. Cyst-Wall-Protein-1 is fundamental for Golgi-like organelle neogenesis and cyst-wall biosynthesis in Giardia lamblia. Nat. Commun 7, 13859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckmann L, Laurent F, Langford TD, Hetsko ML, Smith JR, Kagnoff MF, Gillin FD, 2000. Nitric oxide production by human intestinal epithelial cells and competition for arginine as potential determinants of host defense against the lumen-dwelling pathogen Giardia lamblia. J. Immunol 164, 1478–1487. [DOI] [PubMed] [Google Scholar]
- Einarsson E, Astvaldsson A, Hultenby K, Andersson JO, Svard SG, Jerlstrom-Hultqvist J, 2016a. Comparative cell biology and evolution of annexins in diplomonads. mSphere 1, e00032–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einarsson E, Ma’ayeh S, Svard SG, 2016b. An up-date on Giardia and giardiasis. Curr. Opin. Microbiol 34, 47–52. [DOI] [PubMed] [Google Scholar]
- Einarsson E, Troell K, Hoeppner MP, Grabherr M, Ribacke U, Svard SG, 2016c. Coordinated changes in gene expression throughout encystation of Giardia intestinalis. PLoS Negl. Trop. Dis 10, e0004571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Gebali S, Mistry J, Bateman A, Eddy SR, Luciani A, Potter SC, Qureshi M, Richardson LJ, Salazar GA, Smart A, 2018. The Pfam protein families database in 2019. Nucleic Acids Res. 47, D427–D432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmendorf HG, Singer SM, Nash TE, 2001. The abundance of sterile transcripts in Giardia lamblia. Nucleic Acids Res. 29, 4674–4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery SJ, Baker L, Ansell BR, Mirzaei M, Haynes PA, McConville MJ, Svärd SG, Jex AR, 2018. Differential protein expression and post-translational modifications in Metronidazole-resistant Giardia duodenalis. Gigascience 7, giy024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enright AJ, John B, Gaul U, Tuschl T, Sander C, Marks DS, 2003. MicroRNA targets in Drosophila. Genome Biol. 5, R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eswar N, Webb B, Marti-Renom MA, Madhusudhan M, Eramian D, Shen MY, Pieper U, Sali A, 2007. Comparative protein structure modeling using modeller. Curr. Protoc. Protein Sci 50, 2.9.1–2.9.31. [DOI] [PubMed] [Google Scholar]
- Favennec L, Chochillon C, Meillet D, Magne D, Savel J, Raichvarg D, Gobert JG, 1990. Adherence and multiplication of Giardia intestinalis on human enterocyte-like differentiated cells in vitro. Parasitol. Res 76, 581–584. [DOI] [PubMed] [Google Scholar]
- Favennec L, Magne D, Gobert JG, 1991. Cytopathogenic effect of Giardia intestinalis, in vitro. Parasitol. Today 7, 141. [DOI] [PubMed] [Google Scholar]
- Fink MY, Singer SM, 2017. The intersection of immune responses, microbiota, and pathogenesis in giardiasis. Trends Parasitol. 33, 901–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher BS, Estrano CE, Cole JA, 2013. Modeling long-term host cell-Giardia lamblia interactions in an in vitro co-culture system. PLoS One 8, e81104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foucault ML, Thomas L, Goussard S, Branchini BR, Grillot-Courvalin C, 2010. In vivo bioluminescence imaging for the study of intestinal colonization by Escherichia coli in mice. Appl. Environ. Microbiol 76, 264–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzen O, Jerlström-Hultqvist J, Castro E, Sherwood E, Ankarklev J, Reiner DS, Palm D, Andersson JO, Andersson B, Svärd SG, 2009. Draft genome sequencing of Giardia intestinalis assemblage B isolate GS: is human giardiasis caused by two different species? PLoS Pathog. 5, e1000560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzén O, Jerlström-Hultqvist J, Einarsson E, Ankarklev J, Ferella M, Andersson B, Svärd SG, 2013. Transcriptome profiling of Giardia intestinalis using strand-specific RNA-seq. PLoS Comput. Biol 9, e1003000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend DS, 1966. The fine structure of Giardia muris. J. Cell Biol 29, 317–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargantini PR, del Carmen Serradell M, Ríos DN, Tenaglia AH, Luján HD, 2016. Antigenic variation in the intestinal parasite Giardia lamblia. Curr. Opin. Microbiol 32, 52–58. [DOI] [PubMed] [Google Scholar]
- Gerber AP, Herschlag D, Brown PO, 2004. Extensive association of functionally and cytotopically related mRNAs with Puf family RNA-binding proteins in yeast. PLoS Biol. 2, e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert LA, Larson MH, Morsut L, Liu Z, Brar GA, Torres SE, Stern-Ginossar N, Brandman O, Whitehead EH, Doudna JA, Lim WA, Weissman JS, Qi LS, 2013. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell 154, 442–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillin FD, Boucher SE, Reiner DS, 1987. Stimulation of in-vitro encystation of Giardia lamblia by small intestinal conditions. Clin. Res 35, 475A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillin FD, Reiner DS, Boucher SE, 1988. Small-intestinal factors promote encystation of Giardia lamblia in vitro. Infect. Immun 56, 705–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourguechon S, Cande WZ, 2011. Rapid tagging and integration of genes in Giardia intestinalis. Eukaryot. Cell 10, 142–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen KD, Hirakawa MP, House SA, Schwartz CL, Pham JK, Cipriano MJ, De La Torre MJ, Sek AC, Du G, Forsythe BM, Dawson SC, 2011. Novel structural components of the ventral disc and lateral crest in Giardia intestinalis. PLoS Negl. Trop. Dis 5, e1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanevik K, Bakken R, Brattbakk HR, Saghaug CS, Langeland N, 2015. Whole genome sequencing of clinical isolates of Giardia lamblia. Clin. Microbiol. Infect 21, 192.e1–192.e3. [DOI] [PubMed] [Google Scholar]
- Hanson RM, 2010. Jmol—a paradigm shift in crystallographic visualization. J. Appl. Cryst 43, 1250–1260. [Google Scholar]
- Hardin WR, Li R, Xu J, Shelton AM, Alas GCM, Minin VN, Paredez AR, 2017. Myosin-independent cytokinesis in Giardia utilizes flagella to coordinate force generation and direct membrane trafficking. Proc. Natl. Acad. Sci. U. S. A 114, E5854–E5863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hehl AB, Marti M, Kohler P, 2000. Stage-specific expression and targeting of cyst wall protein-green fluorescent protein chimeras in Giardia. Mol. Biol. Cell 11, 1789–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeng JC, Dawson SC, House SA, Sagolla MS, Pham JK, Mancuso JJ, Lowe J, Cande WZ, 2008. High-resolution crystal structure and in vivo function ofa kinesin-2homologue in Giardia intestinalis. Mol. Biol. Cell 19, 3124–3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holberton DV, 1973. Fine structure of the ventral disk apparatus and the mechanism of attachment in the flagellate Giardia muris. J. Cell Sci 13, 11–41. [DOI] [PubMed] [Google Scholar]
- House SA, Richter DJ, Pham JK, Dawson SC, 2011. Giardia flagellar motility is not directly required to maintain attachment to surfaces. PLoS Pathog. 7, e1002167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson AJ, Moore AN, Elniski D, Joseph J, Yee J, Russell AG, 2012. Evolutionarily divergent spliceosomal snRNAs and a conserved non-coding RNA processing motif in Giardia lamblia. Nucleic Acids Res. 40, 10995–11008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huet C, Sahuquillo-Merino C, Coudrier E, Louvard D, 1987. Absorptive and mucus-secreting subclones isolated from a multipotent intestinal cell line (HT-29) provide new models for cell polarity and terminal differentiation. J. Cell Biol 105, 345–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchens M, Luker GD, 2007. Applications of bioluminescence imaging to the study of infectious diseases. Cell. Microbiol 9, 2315–2322. [DOI] [PubMed] [Google Scholar]
- Hwang WY, Fu Y, Reyon D, Maeder ML, Tsai SQ, Sander JD, Peterson RT, Yeh JR, Joung JK, 2013. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat. Biotechnol 31, 227–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer LM, Anantharaman V, Wolf MY, Aravind L, 2008. Comparative genomics of transcription factors and chromatin proteins in parasitic protists and other eukaryotes. Int. J. Parasitol 38, 1–31. [DOI] [PubMed] [Google Scholar]
- Jeelani G, Husain A, Sato D, Ali V, Suematsu M, Soga T, Nozaki T, 2010. Two atypical L-cysteine-regulated NADPH-dependent oxidoreductases involved in redox maintenance, L-cysteine and iron reduction, and metronidazole activation in the enteric protozoan Entamoeba histolytica. J. Biol. Chem 285, 26889–26899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerlstrom-Hultqvist J, Franzen O, Ankarklev J, Xu F, Nohynkova E, Andersson JO, Svard SG, Andersson B, 2010. Genome analysis and comparative genomics of a Giardia intestinalis assemblage E isolate. BMC Genomics 11, 543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerlstrom-Hultqvist J, Stadelmann B, Birkestedt S, Hellman U, Svard SG, 2012. Plasmid vectors for proteomic analyses in Giardia: purification of virulence factors and analysis of the proteasome. Eukaryot. Cell 11, 864–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P, Binns D, Chang H-Y, Fraser M, Li W, McAnulla C, McWilliam H, Maslen J, Mitchell A, Nuka G, 2014. InterProScan 5: genome-scale protein function classification. Bioinformatics 30, 1236–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarzyk D, Cengic I, Yao L, Hudson EP, 2018. Diversion of the long-chain acyl-ACP pool in Synechocystis to fatty alcohols through CRISPRi repression ofthe essential phosphate acyltransferase PlsX. Metab. Eng 45, 59–66. [DOI] [PubMed] [Google Scholar]
- Källberg M, Wang H, Wang S, Peng J, Wang Z, Lu H, Xu J, 2012. Template-based protein structure modeling using the RaptorX web server. Nat. Protoc 7, 1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampmann M, 2018. CRISPRi and CRISPRa screens in mammalian cells for precision biology and medicine. ACS Chem. Biol 13, 406–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M, Goto S, 2000. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley LA, Mezulis S, Yates CM,Wass MN, Sternberg MJ,2015. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc 10, 845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz M, Iovino N, Unnerstall U, Gaul U, Segal E, 2007. The role of site accessibility in microRNA target recognition. Nat. Genet 39, 1278. [DOI] [PubMed] [Google Scholar]
- Kim DE, Chivian D, Baker D, 2004. Protein structure prediction and analysis using the Robetta server. Nucleic Acids Res. 32, W526–W531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knodler LA, Svard SG, Silberman JD, Davids BJ, Gillin FD, 1999. Developmental gene regulation in Giardia lamblia: first evidence for an encystation-specific promoter and differential 5’ mRNA processing. Mol. Microbiol 34, 327–340. [DOI] [PubMed] [Google Scholar]
- Koh WH, Geurden T, Paget T, O’Handley R, Steuart RF, Thompson RC, Buret AG, 2013. Giardia duodenalis assemblage-specific induction of apoptosis and tight junction disruption in human intestinal epithelial cells: effects of mixed infections. J. Parasitol 99, 353–358. [DOI] [PubMed] [Google Scholar]
- Kraft MR, Klotz C, Bucker R, Schulzke JD, Aebischer T, 2017. Giardia’s epithelial cell interaction in vitro: mimicking asymptomatic infection? Front. Cell. Infect. Microbiol 7, 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krtkova J, Paredez AR, 2017. Use of translation blocking morpholinos for gene knockdown in Giardia lamblia. Methods Mol. Biol 1565, 123–140. [DOI] [PubMed] [Google Scholar]
- Langfelder P, Horvath S, 2008. WGCNA: anR package for weighted correlation network analysis. BMC Bioinf. 9, 559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford TD, Housley MP, Boes M, Chen J, Kagnoff MF, Gillin FD, Eckmann L, 2002. Central importance of immunoglobulin A in host defense against Giardia spp. Infect. Immun 70, 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson MH, Gilbert LA, Wang X, Lim WA, Weissman JS, Qi LS, 2013. CRISPR interference (CRISPRi) for sequence-specific control of gene expression. Nat. Protoc 8, 2180–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law CW, Chen Y, Shi W, Smyth GK, 2014. Voom: precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol. 15, R29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenaerts K, Bouwman FG, Lamers WH, Renes J, Mariman EC, 2007. Comparative proteomic analysis of cell lines and scrapings of the human intestinal epithelium. BMC Genomics 8, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Saraiya AA, Wang CC, 2011. Gene regulation in Giardia lambia involves a putative microRNA derived from a small nucleolar RNA. PLoS Negl. Trop. Dis 5, e1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lievin-Le Moal V, 2013. Dysfunctions at human intestinal barrier by water-borne protozoan parasites: lessons from cultured human fully differentiated colon cancer cell lines. Cell. Microbiol 15, 860–869. [DOI] [PubMed] [Google Scholar]
- Limenitakis J, Soldati-Favre D, 2011. Functional genetics in Apicomplexa: potentials and limits. FEBS Lett. 585, 1579–1588. [DOI] [PubMed] [Google Scholar]
- Liu X, Gallay C, Kjos M, Domenech A, Slager J, van Kessel SP, Knoops K, Sorg RA, Zhang JR, Veening JW, 2017. High-throughput CRISPRi phenotyping identifies new essential genes in Streptococcus pneumoniae. Mol. Syst. Biol 13, 931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luján HD, Svärd S, 2011. Giardia: A Model Organism. Springer Science & Business Media. [Google Scholar]
- Lujan HD, Mowatt MR, Conrad JT, Bowers B, Nash TE, 1995. Identification of a novel Giardia lamblia cyst wall protein with leucine-rich repeats. Implications for secretory granule formation and protein assembly into the cyst wall. J. Biol. Chem 270, 29307–29313. [DOI] [PubMed] [Google Scholar]
- Lujan HD, Mowatt MR, Byrd LG, Nash TE, 1996. Cholesterol starvation induces differentiation of the intestinal parasite Giardia lamblia. Proc. Natl. Acad. Sci. U. S. A 93, 7628–7633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan HD, Mowatt MR, Nash TE, 1997. Mechanisms of Giardia lamblia differentiation into cysts. Microbiol. Mol. Biol. Rev 61, 294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Lin AH, Masliah E, Wyss-Coray T, 2006. Bioluminescence imaging of Smad signaling in living mice shows correlation with excitotoxic neurodegeneration. Proc. Natl. Acad. Sci. U. S. A 103, 18326–18331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma’ayeh SY, Brook-Carter PT, 2012. Representational difference analysis identifies specific genes in the interaction of Giardia duodenalis with the murine intestinal epithelial cell line, IEC-6. Int. J. Parasitol 42, 501–509. [DOI] [PubMed] [Google Scholar]
- Ma’ayeh SY, Knorr L, Svard SG, 2015. Transcriptional profiling of Giardia intestinalis in response to oxidative stress. Int. J. Parasitol 45, 925–938. [DOI] [PubMed] [Google Scholar]
- Ma’ayeh SY, Liu J, Peirasmaki D, Hornaeus K, Bergstrom Lind S, Grabherr M, Bergquist J, Svard SG, 2017. Characterization of the Giardia intestinalis secretome during interaction with human intestinal epithelial cells: the impact on host cells. PLoS Negl. Trop. Dis 11, e0006120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma’ayeh SY, Knorr L, Skold K, Garnham A, Ansell BRE, Jex AR, Svard SG, 2018. Responses of the differentiated intestinal epithelial cell line Caco-2 to infection with the Giardia intestinalis GS isolate. Front. Cell. Infect. Microbiol 8, 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRae IJ, Zhou K, Li F, Repic A, Brooks AN, Cande WZ, Adams PD, Doudna JA, 2006. Structural basis for double-stranded RNA processing by Dicer. Science 311, 195–198. [DOI] [PubMed] [Google Scholar]
- Maguire CA, Bovenberg MS, Crommentuijn MH, Niers JM, Kerami M, Teng J, Sena-Esteves M, Badr CE, Tannous BA, 2013. Triple bioluminescence imaging for in vivo monitoring of cellular processes. Mol. Ther. Nucleic Acids 2, e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE, Norville JE, Church GM, 2013. RNA-guidedhuman genome engineering via Cas9. Science 339, 823–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manko A, Motta JP, Cotton JA, Feener T, Oyeyemi A, Vallance BA, Wallace JL, Buret AG, 2017. Giardia co-infection promotes the secretion of antimicrobial peptides beta-defensin 2 and trefoil factor 3 and attenuates attaching and effacing bacteria-induced intestinal disease. PLoS One 12, e0178647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning G, Reiner DS, Lauwaet T, Dacre M, Smith A, Zhai Y, Svard S, Gillin FD, 2011. The minimal kinome of Giardia lamblia illuminates early kinase evolution and unique parasite biology. Genome Biol. 12, R66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maoret JJ, Font J, Augeron C, Codogno P, Bauvy C, Aubery M, Laboisse CL, 1989. A mucus-secreting human colonic cancer cell line. Purification and partial characterization of the secreted mucins. Biochem. J 258, 793–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcial-Quino J, Gomez-Manzo S, Fierro F, Rufino-Gonzalez Y, Ortega-Cuellar D, Sierra-Palacios E, Vanoye-Carlo A, Gonzalez-Valdez A, Torres-Arroyo A, Oria-Hernandez J, Reyes-Vivas H, 2017. RNAi-mediated specific gene silencing as a tool for the discovery of new drug targets in Giardia lamblia; evaluation using the NADH oxidase gene. Genes (Basel) 8, 303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayrhofer G, Andrews RH, Ey PL, Albert MJ, Grimmond TR, Merry DJ, 1992. The use of suckling mice to isolate and grow Giardia from mammalian faecal specimens for genetic analysis. Parasitology 105 (Pt. 2), 255–263. [DOI] [PubMed] [Google Scholar]
- McCabe RE, Yu GS, Conteas C, Morrill PR, McMorrow B, 1991. In vitro model of attachment of Giardia intestinalis trophozoites to IEC-6 cells, an intestinal cell line. Antimicrob. Agents Chemother 35, 29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInally SG, Hagen KD, Nosala C, Williams J, Nguyen K, Booker J, Jones K, Dawson SC, 2019. Robust and stable transcriptional repression in Giardia using CRISPRi. Mol. Biol. Cell 30, 119–130. mbc. E18-09-0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner M, Breinich MS, Gilson PR, Crabb BS, 2007. Molecular genetic tools in Toxoplasma and Plasmodium: achievements and future needs. Curr. Opin. Microbiol 10, 349–356. [DOI] [PubMed] [Google Scholar]
- Mi H, Huang X, Muruganujan A, Tang H, Mills C, Kang D, Thomas PD, 2016. PANTHER version 11: expanded annotation data from gene ontology and Reactome pathways, and data analysis tool enhancements. Nucleic Acids Res. 45, D183–D189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AL, Attwood TK, Babbitt PC, Blum M, Bork P, Bridge A, Brown SD, Chang H-Y, El-Gebali S, Fraser MI, 2018. InterPro in 2019: improving coverage, classification and access to protein sequence annotations. Nucleic Acids Res. 47, D351–D360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison HG, McArthur AG, Gillin FD, Aley SB, Adam RD, Olsen GJ, Best AA, Cande WZ, Chen F, Cipriano MJ, 2007. Genomic minimalism in the early diverging intestinal parasite Giardia lamblia. Science 317, 1921–1926. [DOI] [PubMed] [Google Scholar]
- Moult J, 2005. A decade of CASP: progress, bottlenecks and prognosis in protein structure prediction. Curr. Opin. Struct. Biol 15, 285–289. [DOI] [PubMed] [Google Scholar]
- Muller J, Ruhle G, Muller N, Rossignol JF, Hemphill A, 2006. In vitro effects of thiazolides on Giardia lamblia WB clone C6 cultured axenically and in coculture with Caco2 cells. Antimicrob. Agents Chemother 50, 162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niño CA, Chaparro J, Soffientini P, Polo S, Wasserman M, 2013. Ubiquitination dynamics in the early-branching eukaryote Giardia intestinalis. Microbiologyopen 2, 525–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosala C, Dawson SC, 2015. The critical role of the cytoskeleton in the pathogenesis of Giardia. Curr Clin Microbiol Rep 2, 155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosala C, Hagen KD, Dawson SC, 2018. Disc-o-Fever’: getting down with Giardia’s groovy microtubule organelle. Trends Cell Biol. 28, 99–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-Pierres G, Arguello-Garcia R, Laredo-Cisneros MS, Fonseca-Linan R, Gomez-Mondragon M, Inzunza-Arroyo R, Flores-Benitez D, Raya-Sandino A, Chavez-Munguia B, Ventura-Gallegos JL, Zentella-Dehesa A, Bermudez-Cruz RM, Gonzalez-Mariscal L, 2018. Giardipain-1, a protease secreted by Giardia duodenalis trophozoites, causes junctional, barrier and apoptotic damage in epithelial cell monolayers. Int. J. Parasitol 48, 621–639. [DOI] [PubMed] [Google Scholar]
- Panaro MA, Cianciulli A, Mitolo V, Mitolo CI, Acquafredda A, Brandonisio O, Cavallo P, 2007. Caspase-dependent apoptosis of the HCT-8 epithelial cell line induced by the parasite Giardia intestinalis. FEMS Immunol. Med. Microbiol 51, 302–309. [DOI] [PubMed] [Google Scholar]
- Paredez AR, Assaf ZJ, Sept D, Timofejeva L, Dawson SC, Wang CJ, Cande WZ, 2011. An actin cytoskeleton with evolutionarily conserved functions in the absence of canonical actin-binding proteins. Proc. Natl. Acad. Sci. U. S. A 108, 6151–6156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park PJ, 2009. ChIP–seq: advantages and challenges of a maturing technology. Nat. Rev. Genet 10, 669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng D, Tarleton R, 2015. EuPaGDT: a web tool tailored to design CRISPR guide RNAs for eukaryotic pathogens. Microb. Genom 1, e000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham JK, Nosala C, Scott EY, Nguyen KF, Hagen KD, Starcevich HN, Dawson SC, 2017. Transcriptomic profiling ofhigh-density Giardia foci encysting in the murine proximal intestine. Front. Cell. Infect. Microbiol 7, 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickar-Oliver A, Gersbach CA, 2019. The next generation of CRISPR-Cas technologies and applications. Nat. Rev. Mol. Cell Biol 20, 490–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter SC, Luciani A, Eddy SR, Park Y, Lopez R, Finn RD, 2018. HMMER web server: 2018 update. Nucleic Acids Res. 46, W200–W204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poxleitner MK, Carpenter ML, Mancuso JJ, Wang CJ, Dawson SC, Cande WZ, 2008. Evidence for karyogamy and exchange of genetic material in the binucleate intestinal parasite Giardia intestinalis. Science 319, 1530–1533. [DOI] [PubMed] [Google Scholar]
- Prucca CG, Slavin I, Quiroga R, Elías EV, Rivero FD, Saura A, Carranza PG, Luján HD, 2008. Antigenic variation in Giardia lamblia is regulated by RNA interference. Nature 456, 750. [DOI] [PubMed] [Google Scholar]
- Qi LS, Larson MH, Gilbert LA, Doudna JA, Weissman JS, Arkin AP, Lim WA, 2013. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell 152, 1173–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimao JQ, Trinconi CT, Yokoyama-Yasunaka JK, Miguel DC, Kalil SP, Uliana SR, 2013. Parasite burden in Leishmania (Leishmania) amazonensis-infected mice: validation of luciferase as a quantitative tool. J. Microbiol. Methods 93, 95–101. [DOI] [PubMed] [Google Scholar]
- Ren B, Gupta N, 2017. Taming parasites by tailoring them. Front. Cell. Infect. Microbiol 7, 292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee KJ, Cheng H, Harris A, Morin C, Kaper JB, Hecht G, 2011. Determination of spatial and temporal colonization of enteropathogenic E. coli and enterohemorrhagic E. coli in mice using bioluminescent in vivo imaging. Gut Microbes 2, 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivero FD, Saura A, Prucca CG, Carranza PG, Torri A, Lujan HD, 2010a. Disruption of antigenic variation is crucial for effective parasite vaccine. Nat. Med 16, 551–557. [DOI] [PubMed] [Google Scholar]
- Rivero MR, Kulakova L, Touz MC, 2010b. Long double-stranded RNA produces specific gene downregulation in Giardia lamblia. J. Parasitol 96, 815–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson LJ, Hanevik K, Escobedo AA, Mørch K, Langeland N, 2010. Giardiasis–why do the symptoms sometimes never stop? Trends Parasitol. 26, 75–82. [DOI] [PubMed] [Google Scholar]
- Roxstrom-Lindquist K, Ringqvist E, Palm D, Svard S, 2005. Giardia lamblia-induced changes in gene expression in differentiated Caco-2 human intestinal epithelial cells. Infect. Immun 73, 8204–8208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, Kucukural A, Zhang Y, 2010. I-TASSER: a unified platform for automated protein structure and function prediction. Nat. Protoc 5, 725–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeij JP, Boyle JP, Grigg ME, Arrizabalaga G, Boothroyd JC, 2005. Bioluminescence imaging of Toxoplasma gondii infection in living mice reveals dramatic differences between strains. Infect. Immun 73, 695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghaug CS, Sornes S, Peirasmaki D, Svard S, Langeland N, Hanevik K, 2016. Human memory CD4+ T cell immune responses against Giardia lamblia. Clin. Vaccine Immunol 23, 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salusso A, Zlocowski N, Mayol GF, Zamponi N, Rópolo AS, 2017. Histone methyltransferase 1 regulates the encystation process in the parasite Giardia lamblia. FEBS J. 284, 2396–2409. [DOI] [PubMed] [Google Scholar]
- Sambuy Y, De Angelis I, Ranaldi G, Scarino ML, Stammati A, Zucco F, 2005. The Caco-2 cell line as a model of the intestinal barrier: influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol. Toxicol 21, 1–26. [DOI] [PubMed] [Google Scholar]
- Saraiya AA, Wang CC, 2008. snoRNA, a novel precursor of microRNA in Giardia lamblia. PLoS Pathog. 4, e1000224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraiya AA, Li W, Wu J, Chang CH, Wang CC, 2014. The microRNAs in an ancient protist repress the variant-specific surface protein expression by targeting the entire coding sequence. PLoS Pathog. 10, e1003791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer FW 3rd, Rice EW, Hoff JC, 1984. Factors promoting in vitro excystation of Giardia muris cysts. Trans. R. Soc. Trop. Med. Hyg 78, 795–800. [DOI] [PubMed] [Google Scholar]
- Scherf M, Klingenhoff A, Werner T, 2000. Highly specific localization of promoter regions in large genomic sequences by Promoter Inspector: a novel context analysis approach. J. Mol. Biol 297, 599–606. [DOI] [PubMed] [Google Scholar]
- Scott KG, Meddings JB, Kirk DR, Lees-Miller SP, Buret AG, 2002. Intestinal infection with Giardia spp. reduces epithelial barrier function in a myosin light chain kinase-dependent fashion. Gastroenterology 123, 1179–1190. [DOI] [PubMed] [Google Scholar]
- Sheridan R, Fieldhouse RJ, Hayat S, Sun Y, Antipin Y, Yang L, Hopf T, Marks DS, Sander C, 2015. Evfold. org: evolutionary couplings and protein 3d structure prediction. biorxiv, 021022. [Google Scholar]
- Singer SM, 2016. Control of giardiasis by interleukin-17 in humans and mice—are the questions all answered? Clin. Vaccine Immunol 23, 2–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer SM, Yee J, Nash TE, 1998. Episomal and integrated maintenance of foreign DNA in Giardia lamblia. Mol. Biochem. Parasitol 92, 59–69. [DOI] [PubMed] [Google Scholar]
- Śledź P, Caflisch A,2018. Protein structure-based drug design: from docking to molecular dynamics. Curr. Opin. Struct. Biol 48, 93–102. [DOI] [PubMed] [Google Scholar]
- Söding J, Biegert A, Lupas AN, 2005. The HHpred interactive server for protein homology detection and structure prediction. Nucleic Acids Res. 33, W244–W248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solaymani-Mohammadi S, Singer SM, 2011. Host immunity and pathogen strain contribute to intestinal disaccharidase impairment following gut infection. J. Immunol 187, 3769–3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonda S, Morf L, Bottova I, Baetschmann H, Rehrauer H, Caflisch A, Hakimi MA, Hehl AB, 2010. Epigenetic mechanisms regulate stage differentiation in the minimized protozoan Giardia lamblia. Mol. Microbiol 76, 48–67. [DOI] [PubMed] [Google Scholar]
- Spassov D, Jurecic R, 2003. The PUF family of RNA-binding proteins: does evolutionary conserved structure equal conserved function? IUBMB Life 55, 359–366. [DOI] [PubMed] [Google Scholar]
- Sprong H, Caccio SM, van der Giessen JW, ZOOPNET Network and Partners, 2009. Identification of zoonotic genotypes of Giardia duodenalis. PLoS Negl. Trop. Dis 3, e558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spycher C, Herman EK, Morf L, Qi W, Rehrauer H, Aquino Fournier C, Dacks JB, Hehl AB, 2013. An ER-directed transcriptional response to unfolded protein stress in the absence of conserved sensor-transducer proteins in Giardia lamblia. Mol. Microbiol 88, 754–771. [DOI] [PubMed] [Google Scholar]
- Stacer AC, Nyati S, Moudgil P, Iyengar R, Luker KE, Rehemtulla A, Luker GD, 2013. NanoLuc reporter for dual luciferase imaging in living animals. Mol. Imaging 12, 1–13. [PMC free article] [PubMed] [Google Scholar]
- Sun CH, Tai JH, 2000. Development of a tetracycline controlled gene expression system in the parasitic protozoan Giardia lamblia. Mol. Biochem. Parasitol 105, 51–60. [DOI] [PubMed] [Google Scholar]
- Sun CH, Chou CF, Tai JH, 1998. Stable DNA transfection of the primitive protozoan pathogen Giardia lamblia. Mol. Biochem. Parasitol 92, 123–132. [DOI] [PubMed] [Google Scholar]
- Sun CH, Palm D, McArthur AG, Svard SG, Gillin FD, 2002. A novel Myb-related protein involved in transcriptional activation of encystation genes in Giardia lamblia. Mol. Microbiol 46, 971–984. [DOI] [PubMed] [Google Scholar]
- Tao W, Lv L, Chen GQ, 2017. Engineering Halomonas species TD01 for enhanced polyhydroxyalkanoates synthesis via CRISPRi. Microb. Cell Fact 16, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarailo-Graovac M, Chen N, 2009. Using RepeatMasker to identify repetitive elements in genomic sequences. Curr. Protoc. Bioinformatics 25, 4.10. 1–4.10. 14. [DOI] [PubMed] [Google Scholar]
- Tejman-Yarden N, Eckmann L, 2011. New approaches to the treatment of giardiasis. Curr. Opin. Infect. Dis 24, 451–456. [DOI] [PubMed] [Google Scholar]
- Teoh DA, Kamieniecki D, Pang G, Buret AG, 2000. Giardia lamblia rearranges F-actin and alpha-actinin in human colonic and duodenal monolayers and reduces transepithelial electrical resistance. J. Parasitol 86, 800–806. [DOI] [PubMed] [Google Scholar]
- The Gene Ontology Consortium, 2016. Expansion of the Gene Ontology knowledgebase and resources. Nucleic Acids Res. 45, D331–D338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tůmová P, Hofśtetrová K, Nohýnková E, Hovorka O, Král J, 2007. Cytogenetic evidence for diversity of two nuclei within a single diplomonad cell of Giardia. Chromosoma 116, 65–78. [DOI] [PubMed] [Google Scholar]
- Tůmová P, Uzlíková M, Wanner G, Nohýnková E, 2015. Structural organization of very small chromosomes: study on a single-celled evolutionary distant eukaryote Giardia intestinalis. Chromosoma 124, 81–94. [DOI] [PubMed] [Google Scholar]
- UniProt Consortium, 2014. UniProt: a hub for protein information. Nucleic Acids Res. 43, D204–D212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vranych CV, Rivero MR, Merino MC, Mayol GF, Zamponi N, Maletto BA, Pistoresi-Palencia MC, Touz MC, Rópolo AS, 2014. SUMOylation and deimination of proteins: two epigenetic modifications involved in Giardia encystation. Biochim. Biophys. Acta 1843, 1805–1817. [DOI] [PubMed] [Google Scholar]
- Wang H, Yang H, Shivalila CS, Dawlaty MM, Cheng AW, Zhang F, Jaenisch R, 2013. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell 153, 910–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse A, Bertoni M, Bienert S, Studer G, Tauriello G, Gumienny R, Heer FT, de Beer TAP, Rempfer C, Bordoli L, 2018. SWISS-MODEL:homology modelling of protein structures and complexes. Nucleic Acids Res. 46, W296–W303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins RR, Eckmann L, 2014. Treatment of giardiasis: current status and future directions. Curr. Infect. Dis. Rep 16, 396. [DOI] [PubMed] [Google Scholar]
- Weissleder R, Ntziachristos V, 2003. Shedding light onto live molecular targets. Nat. Med 9, 123–128. [DOI] [PubMed] [Google Scholar]
- Welsh DK, Kay SA, 2005. Bioluminescence imaging in living organisms. Curr. Opin. Biotechnol 16, 73–78. [DOI] [PubMed] [Google Scholar]
- Williams CW, Elmendorf HG, 2011. Identification and analysis of the RNA degrading complexes and machinery of Giardia lamblia using an in silico approach. BMC Genomics 12, 586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woessner DJ, Dawson SC, 2012. The Giardia median body protein is a ventral disc protein that is critical for maintaining a domed disc conformation during attachment. Eukaryot. Cell 11, 292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Zhang Y, 2007. LOMETS: a local meta-threading-server for protein structure prediction. Nucleic Acids Res. 35, 3375–3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Close D, Handagama W, Marr E, Sayler G, Ripp S, 2016. The expanding toolbox of in vivo bioluminescent imaging. Front. Oncol 6, 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Faraggi E, Zhao H, Zhou Y, 2011. Improving protein fold recognition and template-based modeling by employing probabilistic-based matching between predicted one-dimensional structural properties of query and corresponding native properties of templates. Bioinformatics 27, 2076–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Lasker K, Schneidman-Duhovny D, Webb B, Huang CC, Pettersen EF, Goddard TD, Meng EC, Sali A, Ferrin TE, 2012. UCSF Chimera, MODELLER, and IMP: an integrated modeling system. J. Struct. Biol 179, 269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee J, Nash TE, 1995. Transient transfection and expression of firefly luciferase in Giardia lamblia. Proc. Natl. Acad. Sci. U. S. A 92, 5615–5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu LZ, Birky CW Jr., Adam RD, 2002. The two nuclei of Giardia each have complete copies of the genome and are partitioned equationally at cytokinesis. Eukaryot. Cell 1, 191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachos NC, et al. , 2016. Human enteroids/colonoids and intestinal organoids functionally recapitulate normal intestinal physiology and pathophysiology. J. Biol. Chem 291 (8), 3759–3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YQ, Chen DL, Tian HF, Zhang BH, Wen JF, 2009. Genome-wide computational identification of microRNAs and their targets in the deep-branching eukaryote Giardia lamblia. Comput. Biol. Chem 33, 391–396. [DOI] [PubMed] [Google Scholar]
- Zhang B, Liu ZQ, Liu C, Zheng YG, 2016. Application of CRISPRi in Corynebacterium glutamicum for shikimic acid production. Biotechnol. Lett 38, 2153–2161. [DOI] [PubMed] [Google Scholar]
- Zhao F, Xuan Z, Liu L, Zhang MQ, 2005. TRED: a transcriptional regulatory element database and a platform for in silico gene regulation studies. Nucleic Acids Res. 33, D103–D107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuberi A, Misba L, Khan AU, 2017. CRISPR interference (CRISPRi) inhibition of luxS gene expression in E. coli: an approach to inhibit biofilm. Front. Cell. Infect. Microbiol 7, 214. [DOI] [PMC free article] [PubMed] [Google Scholar]