Abstract
Background
Pneumonitis related to immune checkpoint blockade is uncommon but can be severe, fatal or chronic. Steroids are first-line treatment, however, some patients are refractory or become resistant to steroids. Like many immune-related adverse events, little is known regarding the outcomes and optimal management of patients in whom steroids are ineffective.
Methods
We performed a single-center retrospective cohort study at a high-volume tertiary cancer center to evaluate the clinical course, management strategies and outcomes of patients treated for immune checkpoint pneumonitis with immune modulatory medications in addition to systemic steroids. Pharmacy records were queried for patients treated with both immune checkpoint blockade and receipt of additional immune modulators. Records were then manually reviewed to identify patients who received the additional immune modulators for immune checkpoint pneumonitis.
Results
From 2013 to 2020, we identified 26 patients treated for immune checkpoint pneumonitis with additional immune modulators in addition to steroids. Twelve patients (46%) were steroid-refractory and 14 (54%) were steroid-resistant. Pneumonitis severity included grade 2 (42%) or grade 3–4 (58%). Additional immune modulation consisted of tumor necrosis factor-alpha inhibitor (77%) and/or mycophenolate (23%). Durable improvement in pneumonitis following initiation of additional immune modulators occurred in 10 patients (38%), including three patients (12%) in whom pneumonitis resolved and all immunosuppressants ceased. The rate of 90-day all-cause mortality/hospice referral was 50%. At last follow-up, mortality attributable to pneumonitis was 23%. In addition to mortality from pneumonitis and cancer, 3 patients (12%) died due to infections possibly associated with immunosuppression.
Conclusions
Steroid-refractory or -resistant immune checkpoint pneumonitis is uncommon but associated with significant morbidity and mortality. Additional immunomodulators can yield durable improvement, attained in over one third of patients. An improved understanding of the underlying biology of immune-related pneumonitis will be crucial to guide more precise and effective treatment strategies in the future.
Keywords: immunotherapy, inflammation
Introduction
Immune checkpoint blockade is a well-established treatment for a range of malignancies. While immune checkpoint inhibitors (ICIs) are generally well tolerated, they are associated with a variety of immune-related adverse events (irAEs) which can affect any organ system.1 Severe irAEs are uncommon but can be fatal.2 Steroids are the most common initial treatment for most non-endocrine irAEs, but some patients are refractory or become resistant to steroids. When steroids are not effective, there are limited data to guide management, particularly in the context of pneumonitis.
Immune checkpoint pneumonitis occurs in approximately 5% of patients treated with programmed cell death protein-1 (PD-1) or programmed cell death ligand-1(PD-L1) blockade and perhaps higher rates in those treated with combination therapy.3 4 Steroid-refractory and chronic pneumonitis have been described in a small number of case reports and case series, often with high mortality rates.5–7 For patients in whom steroids are ineffective, treatment guidelines suggest several additional immune modulatory options including infliximab, mycophenolate mofetil, intravenous immunoglobulin (IG) and cyclophosphamide. Current use of these agents is largely extrapolated from experience with other irAEs and other inflammatory lung diseases and is subject to local expertise and practice patterns. Prospective comparative data are lacking and the exact role for these agents remains unclear.8
We describe our single-center experience in the use of alternate immune modulators in addition to steroids in patients with steroid-refractory or -resistant ICI-related pneumonitis.
Methods
After institutional review board approval, all patients at Memorial Sloan Kettering Cancer Center treated with ICIs from 2013 through February 2020 were queried for receipt of a non-steroid immune modulator (eg, tumor necrosis factor (TNF)-alpha antagonists, mycophenolate mofetil, cyclophosphamide, intravenous IG; online supplemental table S1). A query was also performed for pneumonitis in all patients exposed to ICIs. Records of patients who received ICIs as well as an additional immune modulator were manually reviewed to identify patients who received such therapy, in addition to systemic steroids, for management of pneumonitis which appeared to be related to ICI. Patients with a clear alternative etiology for their respiratory illness including malignant lung infiltration, active infection, alternate systemic pneumotoxic agent or radiation pneumonitis, were excluded. For all patients, the following data were collected retrospectively: patient demographics, prior and/or concomitant oncological therapy, clinical features of pneumonitis and pneumonitis treatments. Common terminology criteria for adverse events V.5.0 was used to grade severity of pneumonitis.9
jitc-2020-001884supp001.pdf (210.9KB, pdf)
Patients were classified in to two subgroups based on the indication for additional immune modulator: (1) ‘steroid-refractory’—patients with no improvement or worsening of pneumonitis with initial treatment with systemic steroids; (2) ‘steroid-resistant’—patients who initially responded to steroids but subsequently developed recurrent pneumonitis in the context of steroid tapering, in the absence of immune checkpoint rechallenge.
For the assessment of pneumonitis disease course, pneumonitis improvement was defined as existence of a preponderance of evidence in the medical record (dates of disease course, notes, vital signs, oxygen requirement, level of care, laboratory values, medication changes and radiological information when available) that the patient was steadily improving toward recovery from pneumonitis. We stratified pneumonitis course into ad hoc clinical categories as follows: (1) durable improvement, with follow-up of 8 weeks or more past initial dosing of additional immune modulator; (2) transient improvement: initial benefit followed by pneumonitis relapse or inadequate follow-up—for example, unrelated cause of death, referral to hospice; or (3) no improvement. We also defined pneumonitis resolution as cessation of all immune modulators including steroids with sustained resolution of pneumonitis on imaging for 8 weeks or more.
Statistical analysis
Data were summarized with descriptive statistics. Fisher’s exact test was used for comparisons of groups using categorical variables. Mann-Whitney U test was used for comparison of groups using continuous variables. Kaplan-Meier method was used to characterize and estimate overall survival.
Results
Baseline demographic and clinical features
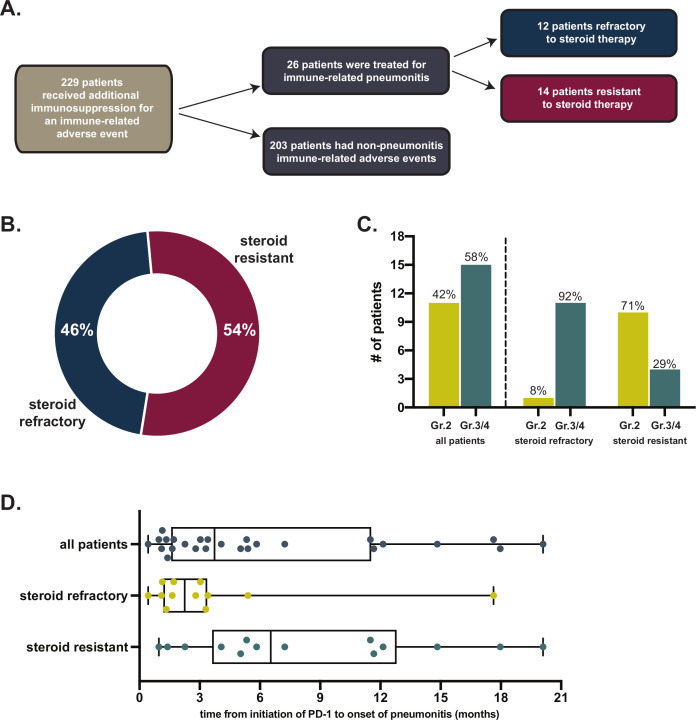
Our data query and manual analysis identified 26 patients who received steroids as well as additional immune modulators for treatment of ICI-related pneumonitis (figure 1A). Among these 26 patients, 8 had non-small cell lung cancer (31%), 4 had malignant melanoma (15%), 4 had renal cell carcinoma (15%) and 10 patients had other cancers (table 1). All patients had advanced stage malignancy. Most patients were former/current smokers (85%), although only a minority (27%) had pre-existing pulmonary conditions (eg, pulmonary embolism, chronic obstructive pulmonary disease, obstructive sleep apnea, interstitial lung disease) prior to beginning ICI. Most patients were treated with PD-1/PD-L1 antibody monotherapy (19, 73%), while 23% had received a combination of PD-1 and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) blocking antibodies. Nine (35%) had received chest radiation therapy prior to initial pneumonitis (median time between radiation and pneumonitis onset was 4.9 months, range 3.0–21.3 months).
Figure 1.
(A) Flow diagram of retrospective study design. (B) Proportion of patients with steroid-refractory or -resistant pneumonitis. (C) CTCAE severity grades of pneumonitis; (D) Timeline of ICI initiation to onset of pneumonitis. CTCAE, common terminology criteria for adverse events; ICI, immune checkpoint inhibitor.
Table 1.
Baseline characteristics of patients with steroid-refractory or -resistant pneumonitis
| No (%) | |
| No of patients | 26 |
| Median age, years (range) | 67 (52–79) |
| Female sex | 9 (34.6) |
| BMI, median (range) | 26.1 (21.3–−38.5) |
| Smoking status | |
| Former | 22 (84.6) |
| Never | 4 (15.4) |
| Pulmonary history* | |
| No | 19 (73.1) |
| Yes | 7 (26.9) |
| Primary malignancy | |
| NSCLC | 8 (30.8) |
| Malignant melanoma | 4 (15.4) |
| Renal cell carcinoma | 4 (15.4) |
| Sarcoma | 2 (7.7) |
| Head and neck squamous cell cancer | 2 (7.7) |
| Bladder carcinoma | 1 (3.8) |
| Colorectal carcinoma | 1 (3.8) |
| Esophageal squamous cell cancer | 1 (3.8) |
| Multiple myeloma | 1 (3.8) |
| Prostate cancer | 1 (3.8) |
| SCLC | 1 (3.8) |
| Line of therapy | |
| 1 | 9 (34.6) |
| 2 | 9 (34.6) |
| ≥3 | 8 (30.8) |
| Prior chest radiation | |
| No | 17 (65.4) |
| Yes | 9 (34.6) |
| Causative checkpoint inhibitor agent | |
| PD1 | 16 (61.5) |
| PDL1 | 3 (11.5) |
| CTLA4 | 1 (3.8) |
| Combination | 6 (23.1) |
*Asthma, COPD, OSA, bronchiectasis, ILD, history of pneumonitis, pulmonary embolism, pleural effusion.
BMI, body mass index; COPD, chronic obstructive pulmonary disease; ILD, interstitial lung diseas; NSCLC, non-small cell lung cancer; OSA, obstructive sleep apnea.
Clinical features and management of steroid-refractory pneumonitis
Of patients treated with additional immune modulators for immune checkpoint pneumonitis, 12 (46%) had steroid-refractory pneumonitis while 14 patients (54%) had steroid-resistant pneumonitis. Time to onset of initial pneumonitis was longer in resistant cases in comparison to refractory cases (steroid-refractory median 68 days vs resistant median 182 days; p=0.02) (figure 1D). The median time from initial treatment with steroids to treatment with additional immune modulator in steroid-refractory patients was 7 days (range 2 to 23 days; 10/12 patients treated in <14 days). In patients with resistant pneumonitis the median time from initial treatment with first steroid course to treatment with additional immune modulator was 2.9 months (range 22 days to 10.7 months) (figure 1B).
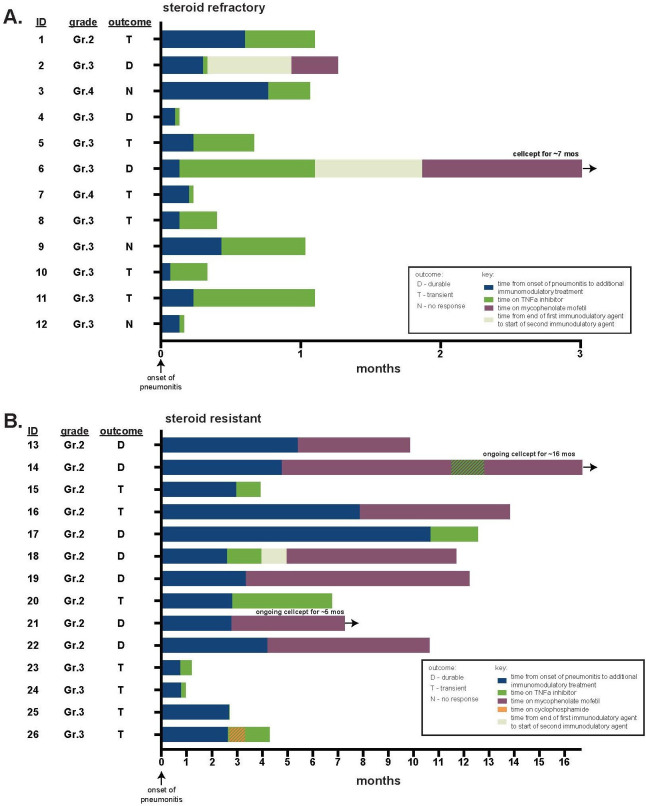
At the time of initiation of additional immune modulator, 2 patients had grade 4 pneumonitis (8%), 13 had grade 3 (50%) and 11 had grade 2 (42%) pneumonitis. As expected, severity was greater in patients with steroid-refractory compared with steroid-resistant pneumonitis (grade 3%–4 100% vs 29%, respectively, p=0.0002) (figure 1C). The majority of patients were hospitalized (16, 62%) and all patients were on oral or intravenous steroids at the time of initiation of additional immune modulator agent (median dose 100 mg prednisone equivalent, range 30–1250 mg) (figure 2, table 2). Among all patients, the first-choice additional immune modulators included TNF-alpha blockade (20, 77% of patients; 19 treated with infliximab;1 treated with adalimumab) or mycophenolate mofetil (6, 23% of patients). The number of TNF-alpha blockade doses used varied between 1 and 7 doses with 76% receiving more than one dose. In four patients treated initially with TNF-alpha blockade, mycophenolate (n=3) or cyclophosphamide (n=1) were also tried. In one patient first treated with mycophenolate, TNF-alpha blockade was subsequently added.
Figure 2.
Swimmer plots of management of steroid-refractory/resistant pneumonitis. (A) Refractory patients; (B) resistant patients. TNF, tumor necrosis factor.
Table 2.
Management and outcomes
| No (%) | |
| CTCAE grade | |
| 2 | 11 (42.3) |
| 3 | 13 (50.0) |
| 4 | 2 (7.7) |
| Refractory/resistant | |
| Steroid refractory | 12 (46.2) |
| Steroid resistant | 14 (53.8) |
| Maximum steroid dose, prednisone equivalent | |
| Range (median) | 30–1250 mg (100 mg) |
| ≥60 mg* | 21 (80.7) |
| Additional Immune modulator | |
| TNF antagonist | 21 (80.8) |
| Mycophenolate mofetil | 9 (34.6) |
| Cyclophosphamide | 1 (3.8) |
| >1 | 5 (19.2) |
| Response to additional immune modulator | |
| Durable improvement | 10 (38.5) |
| Transient improvement | 13 (50.0) |
| No improvement | 3 (11.5) |
| Outcomes | |
| Mortality due to pneumonitis | 6 (23.1) |
| Mortality due to infection | 3 (11.5) |
*All patients with steroid refractory pneumonitis were treated with ≥60 mg.
CTCAE, common terminology criteria for adverse events; TNF, tumor necrosis factor.
Twelve patients (46%) underwent minimally invasive procedures as part of their diagnostic evaluation including 10 bronchoscopies (7 of whom underwent transbronchial lung biopsies) and 2 percutaneous lung biopsies. Pathological patterns included cellular interstitial pneumonia, granulomatous inflammation, diffuse alveolar damage/acute lung injury and organizing pneumonia (online supplemental table S3). Bronchoalveolar lavage(BAL) cell differentials were available from 6 of the bronchoscopies and included increased neutrophilic and lymphocytic predominant profiles.
Outcomes with additional immune modulators for steroid-refractory pneumonitis
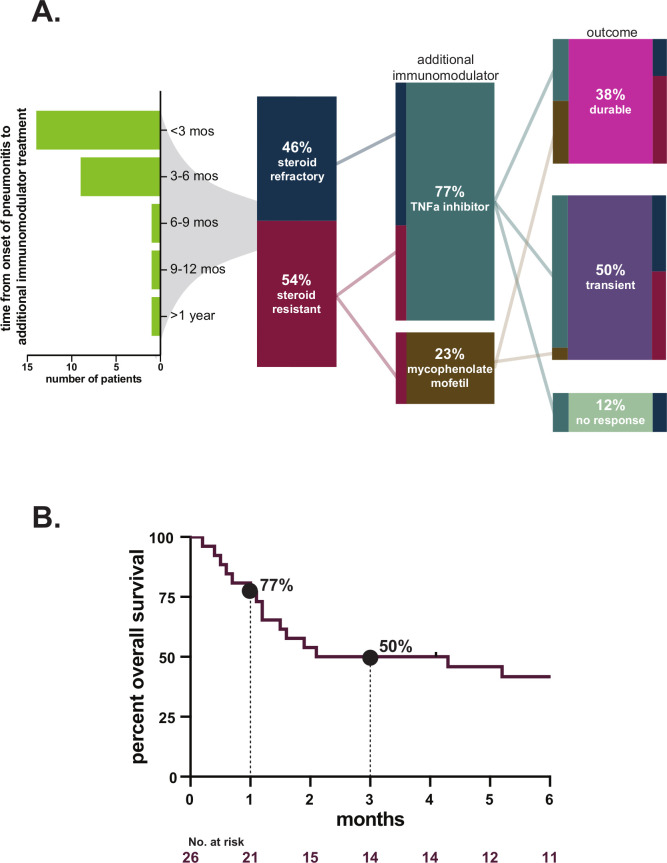
Among all 26 patients, durable improvement of pneumonitis occurred in 10 patients (38%). Among these patients with durable improvement, there were 3 (12%) in whom pneumonitis completely resolved and all immunosuppressants could be stopped. Of the patients who experienced resolution of pneumonitis, two were treated for steroid-refractory pneumonitis initially with infliximab followed by mycophenolate, the third was treated for resistance with mycophenolate (figure 3A). Time from initiation of additional immune modulator to resolution of pneumonitis was 2.3 and 8.4 months in the steroid-refractory patients; in the resistant patient, resolution occurred after 6.1 months (10.5 months after initial pneumonitis). In the seven other patients with durable improvement, at the time of last available follow-up, two had died while still on immunosuppression (one was on 10 mg of prednisone alone, one was on mycophenolate alone), one was referred to hospice off immunosuppressants with grade 1 pneumonitis, and four patients were alive and remained on immune modulators (one was on 5 mg prednisone, one was on 10 mg of prednisone as well as mycophenolate, one was on mycophenolate and 20 mg prednisone and one was on mycophenolate alone).
Figure 3.
(A) Flow diagram/alluvial figure of patient outcomes; (B) Kaplan-Meier curve of 30-day and 90-day mortality estimates (time from initiation of additional immune modulator). TNF, tumor necrosis factor.
Transient pneumonitis improvement occurred in 13 patients (50%). In five of these patients, pneumonitis recurred (including three who died from pneumonitis), five had inadequate follow-up due to unrelated death or hospice referral and three had infections leading to death.
Three patients (12%) had no benefit from additional immune modulator and died due to pneumonitis.
Among all patients, 90-day all-cause mortality/hospice referral was 50% (figure 3B). With last available follow-up, mortality attributable directly to pneumonitis was 23%, and due to infection was 12% (table 2). As expected, the rate of durable pneumonitis improvement and 90-day survival differed between steroid-refractory and resistant pneumonitis (durable response: 25% vs 50%, p=0.25; 90-day survival: 25% vs 71%, p=0.047). The rate of durable pneumonitis improvement with infliximab as the initial immunomodulator was 20% (4/20; 90-day survival: 7/20, 35%) and with mycophenolate was 83% (5/6, 83%; 90-day survival: 6/6, 100%), but it should be noted that infliximab was favored for use in the most severe cases. Features of individual patients in this report are detailed in online supplemental table S3.
Regarding complications during treatment with additional immune modulators, four patients (15%) had severe infections (three were bacterial, one was viral) requiring hospitalization and leading to death in three (12%) patients (online supplemental table S2). No patients in this cohort were rechallenged with ICI therapy. There were no evident cases of loss of anti-cancer response from ICI therapy associated with the use of additional immunomodulation.
Discussion
With an ever-increasing number of patients with cancer eligible for immune checkpoint blockade therapy, the absolute burden of ICI-related pneumonitis will continue to increase, including patients in whom treatment with steroids is insufficient.10 Treatment guidelines provide lists of suggested additional immune modulators, however evidence for the use of these agents in steroid-refractory ICI pneumonitis and data to guide expectations for patients and providers is limited.5–7 11–15 Here, we describe the largest reported series of ICI pneumonitis patients treated with additional immune modulators along with steroids, with a majority of patients managed with TNF blockade. This experience enhances knowledge of clinical course, prognosis, and complications of therapy in these high-risk patients.
Overall, we observed generally poor response to treatment and significant morbidity and mortality. However, survival with durable pneumonitis improvement and without complication did occur in about a third of cases. We identified two clinical phenotypes of patients treated with additional immune modulators for ICI pneumonitis: primary steroid-refractory and steroid-resistant. Steroid-refractory patients had earlier onset, more severe pneumonitis, with less likelihood of durable response in comparison to resistant patients. Among patients categorized as steroid-refractory, there was heterogeneity in time between onset of pneumonitis and dosing of additional immune modulators (range up to 23 days). The time to initiation of additional immune modulator varied as did the clinical course of each patient, since the dynamic of this entity and response pattern to steroids is often individualized. It is possible that routine early use of additional immune modulators on an initial signal of refractory response could be a strategy for improving outcomes in the future.
Beyond poor outcomes directly related to the pneumonitis and the underlying advanced malignancy, infectious complications of immune suppression were a notable conclusion from this study. Similar to previous reports of patients treated for irAEs who developed severe infections,16 it is cautionary that four severe infections occurred, and that these infections included opportunistic infections (eg, fungemia, disseminated herpes simplex virus-HSV). In addition to opportunistic infections, another theoretical risk of use of immunosuppressant is the loss of antitumor immunity. One report has described poorer outcomes for patients treated with anti-TNF agents±steroids in comparison to steroids alone,17 although this has not been described in most other cases nor obviously noted in this report.18 In fact, preclinical data demonstrates that with anti-TNF therapy toxicity management can be uncoupled from efficacy and that TNF blockade might even have positive effects on anticancer control.19 Overall, continued work is needed to clarify how immune-mediated toxicities can be optimally managed without sacrificing antitumor immune control.
There are several limitations to our retrospective, single-center study. Only a few immunomodulators are includes in this report. For example, immune modulatory agents such as intravenous IG and interleukin-6 inhibitors were not used. Overall, the sample size here remains modest and precludes comparison of efficacy of individual immunomodulators, or conclusions related to likelihood of response based on baseline features. Of note, we did not capture patients who may have had steroid-refractory disease who died of pneumonitis without initiation of additional immune modulators. Additionally, and inherent to any analysis of immune-related pneumonitis, is the challenge of making the definitive diagnosis. Here, we reviewed the preponderance of evidence in the medical chart by experts in this entity and erred on the side of caution in excluding patients with other potential etiologies. Relatedly, it is noteworthy that many but not all patients in our series underwent bronchoscopy as part of their evaluation. While the safety of performing bronchoscopy in patients with severe acute respiratory disease is a perpetual concern, ideally, bronchoscopy should be considered a standard of care diagnostic procedure for patients thought to have immune checkpoint pneumonitis that is clinically severe or that responds poorly to steroids.
Despite poor outcomes overall in this vulnerable population, we conclude that durable pneumonitis improvement is possible with both TNF blockade and with mycophenolate, although resolution with ability to discontinue all immune suppression is rare.
In those patients who show initial improvement with pneumonitis treatment with additional immune modulators, careful clinical awareness is needed for the risks of recurrent worsening of pneumonitis as well as new infections. This experience also highlights the slow recovery and chronicity among survivors. The protracted pneumonitis course that occurred in many of the patients in our cohort is consistent with previous reports of chronic immune checkpoint pneumonitis.20 Our data further highlight the critical need to address the many knowledge gaps that remain in ICI pneumonitis.8 For example, a better biologic understanding of immune-related pneumonitis is needed to guide better and more personalized approaches to more successful outcomes in the future. Additionally, high-quality prospective clinical data is needed to understand key clinical elements such as risk factors for, early signs of and optimal management of ICI pneumonitis. Furthermore, with regard to steroid-refractory ICI pneumonitis, the rarity of this syndrome is such that multicentre trials and registries may prove useful; one such trial is currently underway (NCT04438382).
Footnotes
Twitter: @Jia_Luo
Contributors: JB, HR, PF, JL and MDH: conceived and designed the analysis. JB, HR, PF and JL: collected the data. JB, HR, PF, I-HL and MDH: contributed data or analysis tools. JB, MDH: wrote the paper. AS, MP, MC, MHV, NJS, ABW, MC and MDH: contributed to conception and provided critical revision of the article
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: JB, HR, PF, AS, I-HL, NJS, MC report no COI. JL reports honoraria from Targeted Oncology. MP reports consulting fees from BMS, Merck, Array BioPharma, Novartis, Incyte, NewLink Genetics, Aduro, Eisai; honoraria from BMS and Merck; institutional support from RGenix, Infinity, BMS, Merck, Array BioPharma, Novartis, AstraZeneca. MC reports institutional research support and employment of a family member by Bristol-Myers Squibb; consulting, advisory, or speaking compensation for: AstraZeneca/MedImmune, Incyte, Moderna, Immunocore and Merck. MHV reports receiving commercial research grants from Bristol-Myers Squibb, Pfizer; honoraria from BMS, travel/accommodation from Astra Zeneca, Eisai; consultant/advisory board member for Corvus Pharmaceuticals, Exelixis, Eisai, Merck, Onquality Pharmaceuticals and Pfizer as well as institutional support from Astra Zeneca, BMS, Corvus, Medimmune, Merck, Pfizer. ABW reports consulting fees from Nanobiotix; honorarium from LG Chem Life Sciences, Novartis; advisory board member of Lovance. MDH reports research support from Bristol-Myers Squibb; consulting fees from Merck, Bristol-Myers Squibb, AstraZeneca, Genentech/Roche, Nektar, Syndax, Mirati, Shattuck Labs, Immunai, Blueprint Medicines, Achilles and Arcus; travel support/honoraria from AstraZeneca, Eli Lilly and Bristol-Myers Squibb; options from Shattuck Labs, Immunai and Arcus; patent filed by his institution related to the use of tumor mutation burden to predict response to immunotherapy (PCT/US2015/062208), which has received licensing fees from PGDx.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available on reasonable request. Deidentified patient data available on reasonable request.
References
- 1.Postow MA, Sidlow R, Hellmann MD. Immune-Related adverse events associated with immune checkpoint blockade. N Engl J Med 2018;378:158–68. 10.1056/NEJMra1703481 [DOI] [PubMed] [Google Scholar]
- 2.Wang DY, Salem J-E, Cohen JV, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol 2018;4:1721–8. 10.1001/jamaoncol.2018.3923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khunger M, Rakshit S, Pasupuleti V, et al. Incidence of Pneumonitis With Use of Programmed Death 1 and Programmed Death-Ligand 1 Inhibitors in Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis of Trials. Chest 2017;152:271–81. 10.1016/j.chest.2017.04.177 [DOI] [PubMed] [Google Scholar]
- 4.Su Q, Zhu EC, Wu J-B, et al. Risk of pneumonitis and pneumonia associated with immune checkpoint inhibitors for solid tumors: a systematic review and meta-analysis. Front Immunol 2019;10:108. 10.3389/fimmu.2019.00108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naidoo J, Wang X, Woo KM, et al. Pneumonitis in patients treated with Anti-Programmed Death-1/Programmed death ligand 1 therapy. J Clin Oncol 2017;35:709–17. 10.1200/JCO.2016.68.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suresh K, Voong KR, Shankar B, et al. Pneumonitis in non-small cell lung cancer patients receiving immune checkpoint immunotherapy: incidence and risk factors. J Thorac Oncol 2018;13:1930–9. 10.1016/j.jtho.2018.08.2035 [DOI] [PubMed] [Google Scholar]
- 7.Koyauchi T, Inui N, Karayama M, et al. Clinical outcomes of Anti-programmed death-1 Antibody–Related pneumonitis in patients with non-small cell lung cancer. SN Compr Clin Med 2020;2:570–8. 10.1007/s42399-020-00259-3 [DOI] [Google Scholar]
- 8.Sears CR, Peikert T, Possick JD, et al. Knowledge gaps and research priorities in immune checkpoint Inhibitor-related pneumonitis. An official American thoracic Society research statement. Am J Respir Crit Care Med 2019;200:e31–43. 10.1164/rccm.201906-1202ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Cancer Institute, National Institutes of Health, U.S. Department of Health and Human Services Common terminology criteria for adverse events (CTCAE) version 5.0, 2017. Available: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50
- 10.Haslam A, Prasad V. Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw Open 2019;2:e192535. 10.1001/jamanetworkopen.2019.2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson JA, Schneider BJ, Brahmer J, et al. Management of Immunotherapy-Related toxicities, version 1.2019. J Natl Compr Canc Netw 2019;17:255–89. 10.6004/jnccn.2019.0013 [DOI] [PubMed] [Google Scholar]
- 12.Puzanov I, Diab A, Abdallah K, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for immunotherapy of cancer (SITC) toxicity management Working group. J Immunother Cancer 2017;5:95. 10.1186/s40425-017-0300-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brahmer JR, Lacchetti C, Thompson JA. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of clinical oncology clinical practice guideline summary. J Oncol Pract 2018;14:247–9. 10.1200/JOP.18.00005 [DOI] [PubMed] [Google Scholar]
- 14.Haanen JBAG, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv119–42. 10.1093/annonc/mdx225 [DOI] [PubMed] [Google Scholar]
- 15.Petri CR, Patell R, Batalini F, et al. Severe pulmonary toxicity from immune checkpoint inhibitor treated successfully with intravenous immunoglobulin: case report and review of the literature. Respir Med Case Rep 2019;27:100834. 10.1016/j.rmcr.2019.100834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kyi C, Hellmann MD, Wolchok JD, et al. Opportunistic infections in patients treated with immunotherapy for cancer. J Immunother Cancer 2014;2:19. 10.1186/2051-1426-2-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verheijden RJ, May AM, Blank CU, et al. Association of anti-TNF with decreased survival in steroid refractory ipilimumab and Anti-PD1-Treated patients in the Dutch melanoma treatment registry. Clin Cancer Res 2020;26:2268–74. 10.1158/1078-0432.CCR-19-3322 [DOI] [PubMed] [Google Scholar]
- 18.Schadendorf D, Wolchok JD, Hodi FS, et al. Efficacy and safety outcomes in patients with advanced melanoma who discontinued treatment with nivolumab and ipilimumab because of adverse events: a pooled analysis of randomized phase II and III trials. J Clin Oncol 2017;35:3807–14. 10.1200/JCO.2017.73.2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perez-Ruiz E, Minute L, Otano I, et al. Prophylactic TNF blockade uncouples efficacy and toxicity in dual CTLA-4 and PD-1 immunotherapy. Nature 2019;569:428–32. 10.1038/s41586-019-1162-y [DOI] [PubMed] [Google Scholar]
- 20.Naidoo J, Cottrell TR, Lipson EJ, et al. Chronic immune checkpoint inhibitor pneumonitis. J Immunother Cancer 2020;8:e000840. 10.1136/jitc-2020-000840 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2020-001884supp001.pdf (210.9KB, pdf)