Abstract
Early life stress (ELS), such as childhood maltreatment, is a well-known etiological factor in psychopathology including psychosis. Exposure to ELS disrupts the neurodevelopment of widespread brain systems including key components of the Hypothalamic-Pituitary-Adrenal (HPA) axis stress response, such as the amygdala, hippocampus, and medial prefrontal cortex, as well as key components of the brains reward system, such as the nucleus accumbens and orbitofrontal cortex. These disruptions have a considerable impact on the function of emotion and reward circuitry, which play a central role in the emergence and severity of psychosis. While this overlap may provide insight into the pathophysiology of psychosis, it also provides unique opportunities to elucidate neurobiological substrates that may promote resilience to psychosis. In this review, we discuss the HPA axis stress response, the disruption in the neurodevelopment of emotion and reward processing associated with early stress exposures, and examine how this circuitry may contribute to resilience to psychotic disorders.
Keywords: early life stress, stress response, psychosis, HPA axis, emotion, reward
Stress has long been recognized as an etiological factor for the emergence of psychopathology (1) and the impact of stress exposures on the neurodevelopmental processes underlying the risk for mental health disorders is becoming increasingly evident. While stress is an adaptive response to a threat to an organism’s homeostasis, the resulting neurobiological cascade can negatively impact mental health through dysregulation of the stress systems and/or restructuring of the underlying neurobiology. Human brain development is protracted, beginning prenatally and continuing well into adulthood (2), providing necessary opportunities for the emergence of context-dependent, species-appropriate behavior (3). However, this protracted development also provides opportunities for stress exposures during childhood to alter neurodevelopmental trajectories and can have rippling effects across the life span. Thus, exposure to stressors during childhood can have profound consequences not just during childhood but also during adulthood; contributing to the emergence of a range of psychiatric illnesses including psychotic disorders. Indeed, a recent meta-analysis examining the impact of various type of childhood stress exposures on risk for psychotic disorders estimated that 33% of cases worldwide are attributable to such exposures (4).
To date, the vast majority of work examining the impact of stress on the later development of psychotic disorders has focused on broadly defined stress exposures during childhood and early adolescence, which we collectively refer to in the present review as early life stress (ELS). It should be noted however, that recent efforts seeking to understand the impact of childhood stress exposures on development have begun to call for more refined definitions that parse different categories of stressors. For example, one approach has sought to examine the unique effects of experiences of deprivation (i.e., the absence of expected environmental inputs and complexity) and experiences of threat (i.e., the presence of experiences that represent a threat to one’s physical integrity) on neurodevelopment (5-9). Although such approaches are beginning to reveal more nuanced relationships between early stress exposures and psychopathological outcomes, data specific to psychotic outcomes are very limited. Thus, we have opted to use a more global definition of ELS, which encompasses a range of childhood stress exposures including abuse, neglect, institutionalization, poverty, parental psychopathology and family dysfunction, which has contributed significantly to our understanding of psychosis risk.
Moreover, there are multiple pathways through which ELS exerts enduring adverse effects on neurodevelopment that may contribute to the later emergence of psychiatric illnesses such as psychotic disorders (see (10) for a comprehensive review). In the current review we first focus primarily on the Hypothalamic-Pituitary-Adrenal (HPA) axis stress response and its impact on core stress reactive regions, such as the hippocampus, amygdala, and the medial prefrontal cortex (MPFC). We then discuss the impact of ELS on these core stress reactive regions, the role that these regions play in aversive processing, emotion regulation, and affective behavior, and ELS-related alterations of these regions in psychosis specifically. Next we review ELS-related alterations in reward circuitry and the central role of reward processes in risk for psychosis. Finally, we take a dimensional approach to psychosis and consider the role of ELS-related neurodevelopmental alterations across the spectrum of psychosis severity. Critically, we review the brain circuits involved in the response to ELS and highlight unique insights that this research may provide into resilience to psychotic disorders.
Brain Circuitry Involved in the Stress Response
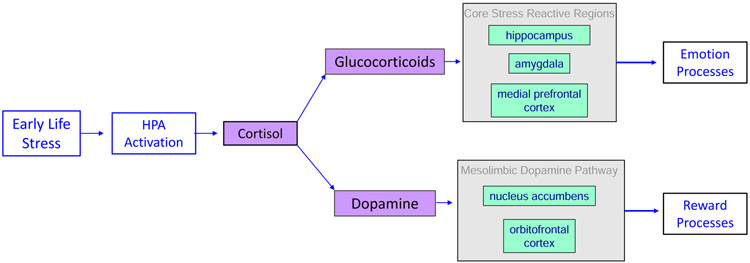
Upon detection of a stressor, the Hypothalamic-Pituitary-Adrenal (HPA) axis is activated to release a series of hormones from the Para-Ventricular Nucleus of the hypothalamus and the pituitary gland, which signal cortisol (the major glucocorticoid in humans) to be released from the cortex of the adrenal glands. Cortisol has widespread impacts on neurotransmission across the brain including at glutamatergic synapses in the hippocampus, amygdala, and the prefrontal cortex (PFC) (11). Among other functions, these regions play a central role in emotional responses to stress which includes interpreting, learning from, and coping with stressful stimuli (12). Additionally, cortisol modulates the effects of dopamine release in the mesolimbic dopamine pathway, particularly in the nucleus accumbens (NAcc) (13), dampening functional connectivity between the NAcc and orbitofrontal cortex (OFC) (14). These regions are critical to a host of basic learning processes including reward processing (15) and decision-making (16). Thus, as shown in Figure 1, stress exposures directly impact circuitry critical to both emotion- and reward-based processes
Figure 1.
The impact of Early Life Stress (ELS) on brain circuitry and potential outcomes contributing to psychopathology. Core stress reactive regions are shown in green. Key noncore regions that are also affected by stress are shown in purple. While each region may represent a unique pathway through which ELS contributes to psychopathology, it is important to note that ELS may also affect the neurodevelopment of connections between these unique pathways.
The HPA axis is self-regulatory, leading to an adaptive reduction of the stress response and a decrease in cortisol levels after a threat is alleviated (17). HPA axis dysregulation, however, leads to increased basal cortisol levels under resting conditions, a reduced response to acute stressors, and widespread disruptions in neurotransmission (11, 18). According to the “glucocorticoid cascade hypothesis”, hippocampal atrophy results from HPA axis dysregulation of cortisol levels under conditions of chronic stress (19). Although hippocampal volume may be affected by genetic or neurodevelopmental predisposing factors, it is also a critical target for the effects of chronic stress. The amygdala and PFC, however, also display considerable restructuring under chronic stress conditions (20-22). For example, while dendritic retraction occurs within the hippocampus, dendritic growth occurs within the amygdala in response to long-term restraint stress in rodents (23). Such structural alterations may underlie some of the behavioral changes associated with psychosis (23, 24).
Early Life Stress Induces Developmental Alterations in Stress-Related Neurobiology
One of the first studies to investigate the impact of ELS on the brain found that institutionally-reared children had a significant reduction in glucose metabolism in MPFC, OFC and infralimbic regions, as well as in the amygdala and hippocampus (25). Since that initial study, the consequences of ELS on the structure of the brain have been extensively studied with several early studies reporting global structural changes across multiple brain regions. These included reduced volume in temporal, frontal, parietal, and occipital regions, and in overall cortical gray and white matter volume (26-31) with at least one study finding that an earlier age of onset and longer duration of childhood stress exposure predicted greater reductions in brain volumes (32). Given the role of limbic regions in the stress response, however, HPA axis regions such as the hippocampus and amygdala, have been more extensively studied in relation to ELS.
Consistent with the “glucocorticoid cascade hypothesis”, early work in rodents found that elevating stress-related hormone levels for an extended period reduced the number of neurons in the hippocampus (33) and this effect is particularly pronounced during early postnatal development (34, 35). In humans, evidence for the relation between ELS and decreased hippocampal volumes has been found in patients with a range of psychiatric disorders including psychotic disorders (36-38), depression (39-43), and post-traumatic stress disorder (PTSD) (44-46). Hippocampal reduction, however, is not a sufficient hallmark of psychiatric disorders as it has also been observed in non-patient samples with a history of ELS (47-49). Notably, hippocampal changes have generally not been observed in children immediately following trauma exposure, even in those with PTSD (26, 29, 32, 50, 51), suggesting that the effect of ELS on the hippocampus is likely protracted. This is supported by findings demonstrating that hippocampal changes have been observed in children with PTSD 12–18 months following trauma exposure (52).
While stress contributes to atrophy in the hippocampus, it has been associated with growth of the amygdala. In humans, several notable studies have found larger amygdala volumes in those with a history of ELS (53-55); although as reviewed previously (56), these effects have not been consistently observed. Like results in the hippocampus, when effects are observed, they are generally not observed in children immediately following trauma exposure, even in those with PTSD (26, 27, 50), suggesting that that the impact of ELS on the amygdala may also be protracted. Notably, many psychiatric disorders linked to exposure to ELS including psychotic disorders (57), depression (58) and PTSD (59) typically find reduced, rather than enlarged, amygdala volumes. Thus, exposure to ELS may act to sensitize the amygdala to subsequent stressors leading to volume reductions later in life that may contribute to risk for psychiatric illness (60).
Another HPA axis region highly sensitive to the effects of stress is the PFC. In contrast to the hippocampus and amygdala, however, widespread changes in the PFC have been observed in children soon after exposure to ELS (61). PFC development underlies a number of higher-order functions including regulatory emotional and cognitive functions that contribute to goal-directed and prosocial behavior (3), which are often disrupted in children exposed to ELS. Variation in MPFC volume, a region central to emotion regulation and fear extinction, has consistently been associated with exposure to ELS. In rodent models, the MPFC is highly sensitive to the effects of chronic stress in childhood (62, 63) and in human studies, decreased MPFC volume is commonly associated with ELS (64-67). ELS-related MPFC abnormalities are found in nearly every major psychiatric disorder (68) and the MPFC is a common neural substrate for most forms of mental illness (69). Similarly, reduced volume and thickness of the adjacent OFC (28, 29, 70-73), a region central to reward-based learning and emotion regulation (74), is also found in children exposed to ELS. The OFC has increasingly been recognized as playing a central role in the pathophysiology of nearly all psychiatric disorders (75) and may contribute to disrupted motivational processes, which are a common transdiagnostic feature of psychiatric illness (74).
Early Life Stress Induces Alterations in Cognitive Function
Global Cognitive Function:
It has been argued that vulnerability to mental health problems following exposure to ELS may be due to alterations or calibrations in neurocognitive systems in response to early stressful environments that, over time, can become maladaptive (76, 77). Due to the disrupted neurodevelopment of these systems, however, ELS may more profoundly affect cognitive functioning than stress exposures later in life. This is supported by findings indicating that ELS impacts intellectual functioning at a global level. For example, neglected children score significantly lower on intelligence quotient (IQ) than controls (78). Moreover, institutionalized children have decreased intellectual performance relative to neverinstitutionalized children (79-81) and a recent meta-analysis including over 3,800 children found an IQ difference of nearly 20 points (82). Such global cognitive deficits are commonly associated with adult psychopathology (83).
Emotion Processing:
In addition to these global cognitive effects, ELS also affects affective processing more specifically (84). For example, a recent meta-analysis of over 11,000 (85) children and adolescents found that exposure to ELS was associated with poor emotion regulation as well as increased avoidance, emotional suppression, and expression of negative emotions in response to stress. Altered capacity to regulate emotional responses is a common feature of many psychiatric disorders and thus, studying the impact of ELS on emotion processing circuitry may improve understanding of how ELS contributes to the later emergence of adult psychiatric disorders.
A recent meta-analysis of 162 MRI studies of emotion found that, in addition to activations seen in the amygdala, ventral striatum, thalamus, hypothalamus, and periaqueductal gray, regions within the medial, orbital, and inferior lateral frontal cortices were also consistently activated during emotion-based tasks (86). Moreover, the generation of emotional responses involves both bottom-up (i.e. amygdala-MPFC) and top-down (i.e. MPFCamygdala) activity, which are critically shaped by developmental stage and context (3). Given this complexity, it is not surprising that ELS effects on emotion processing have been linked to widespread alterations in brain function. However, given the hierarchical nature of brain development, it is difficult to discriminate the direct effects of ELS on stress-sensitive regions from their effects on downstream developmental targets (87).
Reward Processing:
Recently, increased attention has also been directed towards understanding the impact of ELS on reward-based processing. Unfortunately, few behavioral studies have directly examined the impact of ELS on reward-related behavior in children. However, data derived from younger (88) and older adults (89) have found associations between ELS and impairments in reward learning. Generally, the neural circuitry involved in reward processing includes a central core: the ventral striatum/NAcc and the ventral tegmental area (VTA) of the midbrain, which contribute to detecting rewarding stimuli. Information related to reward value is processed across glutamatergic projections from the OFC and anterior cingulate cortex (ACC), as well as dopaminergic projections from the VTA onto the ventral striatum. Information from multiple components of this network are then integrated in the PFC where it is translated into action. Several other structures including the amygdala, hippocampus, lateral habenular nucleus and regions of the raphe, may modulate this circuitry (90, 91).
Over the last several years, exposure to ELS has commonly been associated with variation in reward circuitry. For example, a history of exposure to ELS has been associated with reductions in size of the striatum (48, 92, 93), alterations in the developmental trajectory of NAcc volume (94), and reduced volume, thickness or connectivity of the OFC (28, 29, 70-72). Moreover, data derived from task-based fMRI studies find that a history of ELS is consistently associated with variation in striatal response to anticipation and/or receipt of reward (60). Finally, relative to children in the lowest quartile of childhood stress exposure, those exposed to high levels of stress evidence altered brain activation within the reward network to a monetary incentive delay task 10 years later and this altered activation is associated with real-world reward-seeking behavior (88). Notably, a recent review has concluded that alterations in reward behavior and circuitry may be among the most prominent transdiagnostic feature of psychiatric disorders (95) and thus, may be critical for understanding how ELS contributes to the later emergence of adult psychopathology.
Overlap in Stress-Related Circuitry Underlying Risk and Resilience to Psychotic Disorders
Although early adversity may increase risk for the later emergence of psychiatric illness, it is also important to note that there is considerable variability in the outcomes associated with ELS. For example, data from the Isle of Wight study, a large longitudinal epidemiological study, demonstrated that of the ~10% of individuals who reported repeated or severe abuse in childhood, ~44% evidenced no psychopathology over a period of 30 years. Moreover, in those exposed to repeated or severe abuse, rates of personality functioning difficulties, relationship instability, crime and poor self-reported health were significantly lower in individuals without vs. those with adult psychopathology (96). Thus, although exposure to ELS may impact neurodevelopment, these impacts do not always lead to poor outcomes.
Perhaps not surprisingly, stress-related neural circuitry that contributes to resilience to adult psychopathology overlaps considerably with the circuitry implicated in risk for later psychiatric disorders. For example, a recent review of 48 MRI studies (56) examining adult outcomes associated with childhood maltreatment found that resilient adults evidenced increased hippocampal volume and increased resting state functional connectivity of limbic regions as well as a greater ability to regulate emotion relative to those who were not resilient. Although it is not yet clear whether such effects represent pre-existing, adaptive or compensatory processes in the underlying neurobiology, the overlap in neural circuitry of both risk and resilience to adult psychopathology holds considerable promise for advancing our understanding of the pathophysiology of chronic mental illnesses such as psychosis.
In addition to the meta-analytic data suggesting that roughly 33% of psychotic disorder cases are attributable to ELS exposures (4), other forms of childhood adversity including parental mental illness, poverty and urbanicity (97) have consistently been implicated in risk for psychotic disorders. Thus, capitalizing on what is known about the effects of ELS on the brain to further our understanding of the neurodevelopmental mechanisms that may increase risk for psychosis, is an intuitive approach. However, elucidating the overlap between the circuitry impacted by ELS and the circuitry contributing to the later emergence of psychosis, also provides unique opportunities to understand resilience to psychotic disorders. Recent years have seen a dramatic increase in work seeking to apply the concept of resilience to the study of psychosis. Indeed, in the last 5 years, the number of publications on this topic (n=190) have more than doubled from that of the entire period from 1980-2014 (n=75) (PubMed keywords; psychosis + resilience, 5/4/2020). This is in line with the growing recognition that psychosis should be viewed as a dimensional, rather than a categorical construct.
Dimensional approaches to the study of psychosis have been proposed in multiple forms throughout the history of psychiatry (98) but have only recently begun to attract widespread attention. The dimensional approach encompasses a full range of psychotic symptom expressions from subclinical or prodromal manifestations, typically observed in non-psychiatric populations or high-risk samples, to the clinically significant symptoms typically observed in patients with psychotic disorders. This approach, which allows for the examination of individuals who exhibit subclinical psychotic symptoms but who do not meet criteria for a psychiatric disorder, offers novel opportunities for elucidating risk and resilience to psychotic disorders (99). To date, most of the studies exploring the psychosis dimension have aimed to identify characteristics (clinical, neurocognitive, imaging) that facilitate the prediction of transition to a clinical state. However, such studies also provide insight into mechanisms of resilience, which reduce the risk for transition and contribute to better long-term outcomes.
Perhaps one of the most notable findings linking stress-related neurocircuitry to resilience in psychotic disorders is that reductions in hippocampal and amygdala volumes are not present in the high-risk state but are present in patients with psychotic disorders (100). Thus, it seems possible that alterations in the developmental trajectory of these stress sensitive regions either contribute to, or protect against, the development of illness. Another striking finding, is that the pituitary, a core HPA axis region, is enlarged in those who transition to a clinical syndrome, with greater enlargement associated with a decreased time to transition (101, 102). Notably, basal cortisol levels have also been found to be elevated in high-risk individuals and may be predictive of transition (103, 104). Collectively, these data suggest that variation in the stress response systems, longitudinally, may contribute, at least in part, to both risk and resilience psychotic disorders.
Not surprisingly, much of the work examining transition from a subclinical to a clinical syndrome suggest that emotion and reward processes are a central feature of resilience to poor outcomes. Emotion and reward processing deficits are consistently implicated in risk for the development of psychotic disorders and significantly contribute to the poor outcomes associated with it. Such impairments are also present in those exhibiting subclinical symptoms. For example, patients with psychotic disorders typically use more maladaptive emotion regulation strategies and fewer adaptive strategies than healthy controls (105, 106). Such maladaptive emotion regulation is found across the entire spectrum of psychotic symptoms (107) including chronic patients (106, 108), first-episode patients (109), high-risk or prodromal (110) and general population samples (111, 112). Moreover, poor outcomes in individuals with psychotic disorders are strongly associated with a range of social cognitive processes encompassing emotion processing (113) and its underlying circuitry contributes to poor outcomes even in those with subclinical symptoms. In a high-risk sample, low levels of overall functioning at a 12-month follow-up visit was associated with baseline alterations in the recognition of anger and relative to those with high levels of overall functioning at 12-month follow-up, those with low levels of functioning evidenced more negative associations between anger recognition and left hippocampal volume as well as between fear recognition and left MPFC volume (114).
Similarly, behavioral studies have consistently demonstrated that deficits in reward learning (115-117), reward valuation (118-120) and effort-based decision making (121-124) are a core feature of psychotic disorders and such deficits have also been observed across the psychosis spectrum (125-130). These findings are not surprising given the plethora of data implicating dopaminergic function in both psychosis and reward-processing (131). Relative to those who do not transition, high-risk individuals who do transition have elevated baseline striatal dopamine (132) suggesting that progressive changes in dopaminergic function contribute to the emergence of illness. Thus, stable dopaminergic function may protect against transition; facilitating resilience. Indeed, it has been argued that the ability to maintain properly functioning reward pathways, which are dependent on dopamine, may be critical to resilience more generally. Specifically, resilient individuals may have a reward system that is hypersensitive to reward or more resistant to change (133). However, in this context, resilience may represent adaptive changes to reward circuitry rather than an absence of change (134). Consistent with this view, Richter et al. (135) recently demonstrated that healthy young adults who had high levels of childhood adversity showed reduced bottom-up activation in the VS, VTA and hippocampus. However, in those with a high adversity load, high trait resilience was related to increased activation in the VTA and hippocampus and less negative functional interactions between these regions, suggesting a compensatory or protective mechanism counteracting the effects of adversity.
Longitudinal Structural Changes Predicting Risk and Resilience
In addition to regions directly impacted by the stress response, imaging studies of resilience to psychotic disorders implicate many structures and functions that contribute to emotion and reward processes, but may be down-stream targets of HPA axis regions. One of the earliest studies to examine differences in the brains of individuals at high-risk for psychotic disorders who later transitioned to a clinical syndrome vs. those who did not found that those who did develop a psychotic disorder demonstrated significant longitudinal reductions of gray matter in the left parahippocampal gyrus, fusiform gyrus, OFC and cerebellum as well as in the cingulate (136). More recent data suggest that such changes are not just predictive of transition of a clinical syndrome but may also relate to the worsening of symptoms. For example, Tognin and colleagues (137) found that regions that provided the greatest contribution to prediction of symptom progression in a high-risk sample were the right temporal fusiform cortex, right temporal pole, right parahippocampal gyrus, inferior temporal gyrus, and left insular cortex, regions that are more peripherally involved in emotion regulation and reward (138-140). Notably, other findings have suggested that compensatory increases in activity in some of these regions, including inferior frontal, anterior cingulate, and parahippocampal regions, are associated with clinical improvement in high-risk samples longitudinally (100).
Alterations in brain structure contribute not just to the risk of transitioning to a clinical state, but also to both short- and long-term outcomes in high risk samples. For example, in a high-risk sample studied over a 52-week follow-up period, those who did not transition to a clinical disorder showed a significant improvement in subclinical symptoms at follow-up that correlated with increased volume (141) and structural connectivity (142) of the corpus callosum, which has been shown to contribute to emotion processing deficits in psychotic disorders (143). In another study, Cropley et al. (144) examined baseline differences in gray matter volume in high-risk samples who did or did not transition after approximately 7 years of follow-up and found that, in those who continued to exhibit symptoms, gray matter volume was reduced in the middle frontal gyrus, right precuneus/posterior cingulate, left inferior temporal gyrus, and a broad posterior region encompassing amygdala, superior occipital cortex, and middle frontal pre- and post-central gyrus. Similarly, de Wit et al (145) examined a high-risk sample over a 6-year follow-up and found that the trajectory of cingulate gyrus change differentiated between those who had poor outcomes relative to those who improved at follow-up. Specifically, resilient individuals had less tissue loss, which they suggested might reflect a compensatory mechanism. More recent work by the same group (146) used Support Vector Regression analysis to predict long-term functional and clinical outcome from baseline MRI measures and found strong correlations between subcortical volume across regions encompassing the caudate nucleus, thalamus, pallidum, and amygdala as well as corpus callosum, cerebellum and third and lateral ventricle and long-term level of functioning.
Future Directions
Collectively, these data highlight the considerable overlap between the circuitry impacted by exposure to ELS and the circuitry associated with risk and resilience to psychotic disorders. We have previously argued that ELS-related insults to brain circuitry may be functioning to “add insult to injury” (147) such that the very same circuitry that is being affected by these exposures overlaps with the circuitry that underlies psychosis. However, it also seems likely that this circuitry, when preserved, contributes to resilience to psychotic disorder outcomes. Thus, early intervention strategies for children exposed to significant levels of stress may be a critical step in bolstering resilience to psychotic disorders and their associated poor outcomes.
McLaughlin et al. (148) recently proposed several intervention strategies that could be implemented in children exposed to ELS to interrupt the emergence of dysfunctional emotion- and reward-related processing. Moreover, because maternal regulation of a child’s emotional responses to stress may scaffold emotional regulation (149) and reward directed responding (135), interventions directed towards parenting of children exposed to high levels of stress may be beneficial. This is supported by recent findings that a mother’s behavioral regulation of her 3-year-old child is associated with alterations in emotion processing circuitry, including amygdala connectivity to frontal and parietal regions, and reward circuitry, including VS connectivity to the OFC and inferior frontal gyrus, 7-8 years later (150). Thus, early interventions aimed at improving emotion and reward-related processing long before the onset of psychopathology may hold considerable promise for bolstering resilience to psychotic disorders. Despite targeting individuals who are already exhibiting attenuated psychotic symptoms, early intervention strategies in high-risk psychosis samples have been successful in delaying the onset of the illness and improving outcomes (151). Interventions applied sooner, specifically targeted at processes that are known to contribute to risk for the disorder, would likely have a much greater impact.
Conclusions
Despite substantial progress in understanding how ELS may contribute to risk and resilience to psychotic disorders, there are still many unanswered questions regarding the neurodevelopmental impact of ELS. First, different types of ELS (e.g. poverty, deprivation, abuse) and variation in the severity of exposure may have differing influences on brain circuitry. Second, the developmental stage at which stress exposure occurs and whether that stress exposure is chronic or transient are also important factors that are likely to influence neurodevelopment. Finally, individual differences in the underlying neurobiology may predispose some individuals toward resilience in the presence of ELS. Therefore, while it is clear that ELS has a profound impact on neurodevelopment underlying risk and resilience to psychosis, more research is needed to understand the ELS-related mechanisms, timing, and factors contributing to neurodevelopmental changes that underlie psychosis.
Acknowledgements:
This work was supported in part by grants from the National Institute of Mental Health to Dr. DeRosse (MH086756) and Dr. Barber (MH122886).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Dr.’s DeRosse and Barber report no biomedical financial interests or potential conflicts of interest.
References
- 1.Roth N (1958): Psychopathology and stress. American journal of psychotherapy. 12:127–144. [DOI] [PubMed] [Google Scholar]
- 2.Lebel C, Beaulieu C (2011): Longitudinal development of human brain wiring continues from childhood into adulthood. Journal of Neuroscience. 31:10937–10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tottenham N (2020): Early adversity and the neotenous human brain. Biological Psychiatry. 87:350–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Varese F, Smeets F, Drukker M, Lieverse R, Lataster T, Viechtbauer W, et al. (2012): Childhood adversities increase the risk of psychosis: a meta-analysis of patient-control, prospective-and cross-sectional cohort studies. Schizophrenia bulletin. 38:661–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sheridan MA, Peverill M, Finn AS, McLaughlin KA (2017): Dimensions of childhood adversity have distinct associations with neural systems underlying executive functioning. Development and psychopathology. 29:1777–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dennison MJ, Rosen ML, Sambrook KA, Jenness JL, Sheridan MA, McLaughlin KA (2019): Differential associations of distinct forms of childhood adversity with neurobehavioral measures of reward processing: A developmental pathway to depression. Child development. 90:e96–e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sheridan MA, McLaughlin KA (2014): Dimensions of early experience and neural development: deprivation and threat. Trends in cognitive sciences. 18:580–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lambert HK, King KM, Monahan KC, McLaughlin KA (2017): Differential associations of threat and deprivation with emotion regulation and cognitive control in adolescence. Development and psychopathology. 29:929–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller AB, Sheridan MA, Hanson JL, McLaughlin KA, Bates JE, Lansford JE, et al. (2018): Dimensions of deprivation and threat, psychopathology, and potential mediators: A multi-year longitudinal analysis. Journal of Abnormal Psychology. 127:160–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agorastos A, Pervanidou P, Chrousos GP, Baker DG (2019): Developmental Trajectories of Early Life Stress and Trauma: A Narrative Review on Neurobiological Aspects Beyond Stress System Dysregulation. Frontiers in psychiatry. 10:118–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Popoli M, Yan Z, McEwen BS, Sanacora G (2011): The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci. 13:22–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maren S, Phan KL, Liberzon I (2013): The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci. 14:417–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oswald LM, Wong DF, McCaul M, Zhou Y, Kuwabara H, Choi L, et al. (2005): Relationships among ventral striatal dopamine release, cortisol secretion, and subjective responses to amphetamine. Neuropsychopharmacology. 30:821–832. [DOI] [PubMed] [Google Scholar]
- 14.Chun J-W, Choi J, Cho H, Choi M-R, Ahn K-J, Choi J-S, et al. (2018): Role of Frontostriatal Connectivity in Adolescents With Excessive Smartphone Use. Frontiers in psychiatry. 9:437–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spicer J, Galvan A, Hare TA, Voss H, Glover G, Casey B (2007): Sensitivity of the nucleus accumbens to violations in expectation of reward. Neuroimage. 34:455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyer HC, Bucci DJ (2016): Imbalanced activity in the orbitofrontal cortex and nucleus accumbens impairs behavioral inhibition. Current biology. 26:2834–2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McEwen BS (2001): Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Ann N Y Acad Sci. 933:265–277. [DOI] [PubMed] [Google Scholar]
- 18.Raison CL, Miller AH (2003): When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry. 160:1554–1565. [DOI] [PubMed] [Google Scholar]
- 19.Sapolsky RM, Krey LC, McEwen BS (1986): The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocr Rev. 7:284–301. [DOI] [PubMed] [Google Scholar]
- 20.Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, et al. (2006): Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 26:7870–7874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McEwen BS, Morrison JH (2013): The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron. 79:16–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Radley JJ, Rocher AB, Miller M, Janssen WG, Liston C, Hof PR, et al. (2006): Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex. 16:313–320. [DOI] [PubMed] [Google Scholar]
- 23.Conrad CD, LeDoux JE, Magarinos AM, McEwen BS (1999): Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci. 113:902–913. [DOI] [PubMed] [Google Scholar]
- 24.Wood GE, Norris EH, Waters E, Stoldt JT, McEwen BS (2008): Chronic immobilization stress alters aspects of emotionality and associative learning in the rat. Behav Neurosci. 122:282–292. [DOI] [PubMed] [Google Scholar]
- 25.Chugani HT, Behen ME, Muzik O, Juhász C, Nagy F, Chugani DC (2001): Local brain functional activity following early deprivation: a study of postinstitutionalized Romanian orphans. Neuroimage. 14:1290–1301. [DOI] [PubMed] [Google Scholar]
- 26.De Bellis MD, Keshavan MS, Clark DB, Casey BJ, Giedd JN, Boring AM, et al. (1999): A.E. Bennett Research Award. Developmental traumatology. Part II: Brain development. Biol Psychiatry. 45:1271–1284. [DOI] [PubMed] [Google Scholar]
- 27.De Bellis MD, Hall J, Boring AM, Frustaci K, Moritz G (2001): A pilot longitudinal study of hippocampal volumes in pediatric maltreatment-related posttraumatic stress disorder. Biol Psychiatry. 50:305–309. [DOI] [PubMed] [Google Scholar]
- 28.Hanson JL, Chung MK, Avants BB, Shirtcliff EA, Gee JC, Davidson RJ, et al. (2010): Early stress is associated with alterations in the orbitofrontal cortex: a tensor-based morphometry investigation of brain structure and behavioral risk. Journal of Neuroscience. 30:7466–7472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Brito SA, Viding E, Sebastian CL, Kelly PA, Mechelli A, Maris H, et al. (2013): Reduced orbitofrontal and temporal grey matter in a community sample of maltreated children. Journal of child psychology and psychiatry, and allied disciplines. 54:105–112. [DOI] [PubMed] [Google Scholar]
- 30.Carrion VG, Weems CF, Eliez S, Patwardhan A, Brown W, Ray RD, et al. (2001): Attenuation of frontal asymmetry in pediatric posttraumatic stress disorder. Biol Psychiatry. 50:943–951. [DOI] [PubMed] [Google Scholar]
- 31.Sheridan MA, Fox NA, Zeanah CH, McLaughlin KA, Nelson CA 3rd (2012): Variation in neural development as a result of exposure to institutionalization early in childhood. Proc Natl Acad Sci U S A. 109:12927–12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Bellis MD, Keshavan MS, Shifflett H, Iyengar S, Beers SR, Hall J, et al. (2002): Brain structures in pediatric maltreatment-related posttraumatic stress disorder: a sociodemographically matched study. Biol Psychiatry. 52:1066–1078. [DOI] [PubMed] [Google Scholar]
- 33.Sapolsky RM (1987): Glucocorticoids and hippocampal damage. Trends in Neurosciences. 10:346–349. [Google Scholar]
- 34.Brunson KL, Eghbal-Ahmadi M, Bender R, Chen Y, Baram TZ (2001): Long-term, progressive hippocampal cell loss and dysfunction induced by early-life administration of corticotropin-releasing hormone reproduce the effects of early-life stress. Proceedings of the National Academy of Sciences. 98:8856–8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen Y, Bender RA, Brunson KL, Pomper JK, Grigoriadis DE, Wurst W, et al. (2004): Modulation of dendritic differentiation by corticotropin-releasing factor in the developing hippocampus. Proceedings of the National Academy of Sciences. 101:15782–15787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heckers S (2001): Neuroimaging studies of the hippocampus in schizophrenia. Hippocampus. 11:520–528. [DOI] [PubMed] [Google Scholar]
- 37.Nelson MD, Saykin AJ, Flashman LA, Riordan HJ (1998): Hippocampal volume reduction in schizophrenia as assessed by magnetic resonance imaging: a meta-analytic study. Arch Gen Psychiatry. 55:433–440. [DOI] [PubMed] [Google Scholar]
- 38.Szeszko PR, Goldberg E, Gunduz-Bruce H, Ashtari M, Robinson D, Malhotra AK, et al. (2003): Smaller anterior hippocampal formation volume in antipsychotic-naive patients with first-episode schizophrenia. Am J Psychiatry. 160:2190–2197. [DOI] [PubMed] [Google Scholar]
- 39.Krishnan KR, Doraiswamy PM, Figiel GS, Husain MM, Shah SA, Na C, et al. (1991): Hippocampal abnormalities in depression. J Neuropsychiatry Clin Neurosci. 3:387–391. [DOI] [PubMed] [Google Scholar]
- 40.Sheline YI, Liston C, McEwen BS (2019): Parsing the Hippocampus in Depression: Chronic Stress, Hippocampal Volume, and Major Depressive Disorder. Biol Psychiatry. 85:436–438. [DOI] [PubMed] [Google Scholar]
- 41.Sheline YI, Mittler BL, Mintun MA (2002): The hippocampus and depression. Eur Psychiatry. 17 Suppl 3:300–305. [DOI] [PubMed] [Google Scholar]
- 42.Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW (1996): Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci U S A. 93:3908–3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vythilingam M, Vermetten E, Anderson GM, Luckenbaugh D, Anderson ER, Snow J, et al. (2004): Hippocampal volume, memory, and cortisol status in major depressive disorder: effects of treatment. Biol Psychiatry. 56:101–112. [DOI] [PubMed] [Google Scholar]
- 44.Yehuda R (1999): Linking the neuroendocrinology of post-traumatic stress disorder with recent neuroanatomic findings. Semin Clin Neuropsychiatry. 4:256–265. [DOI] [PubMed] [Google Scholar]
- 45.Bremner JD, Vythilingam M, Vermetten E, Adil J, Khan S, Nazeer A, et al. (2003): Cortisol response to a cognitive stress challenge in posttraumatic stress disorder (PTSD) related to childhood abuse. Psychoneuroendocrinology. 28:733–750. [DOI] [PubMed] [Google Scholar]
- 46.Vythilingam M, Luckenbaugh DA, Lam T, Morgan CA 3rd, Lipschitz D, Charney DS, et al. (2005): Smaller head of the hippocampus in Gulf War-related posttraumatic stress disorder. Psychiatry Res. 139:89–99. [DOI] [PubMed] [Google Scholar]
- 47.Teicher MH, Anderson CM, Polcari A (2012): Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proc Natl Acad Sci U S A. 109:E563–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D, et al. (2012): Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol Psychiatry. 71:286–293. [DOI] [PubMed] [Google Scholar]
- 49.Samplin E, Ikuta T, Malhotra AK, Szeszko PR, Derosse P (2013): Sex differences in resilience to childhood maltreatment: effects of trauma history on hippocampal volume, general cognition and subclinical psychosis in healthy adults. J Psychiatr Res. 47:1174–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Woon FL, Hedges DW (2008): Hippocampal and amygdala volumes in children and adults with childhood maltreatment-related posttraumatic stress disorder: a meta-analysis. Hippocampus. 18:729–736. [DOI] [PubMed] [Google Scholar]
- 51.Carrion VG, Weems CF, Watson C, Eliez S, Menon V, Reiss AL (2009): Converging evidence for abnormalities of the prefrontal cortex and evaluation of midsagittal structures in pediatric posttraumatic stress disorder: an MRI study. Psychiatry Res. 172:226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carrion VG, Weems CF, Reiss AL (2007): Stress predicts brain changes in children: a pilot longitudinal study on youth stress, posttraumatic stress disorder, and the hippocampus. Pediatrics. 119:509–516. [DOI] [PubMed] [Google Scholar]
- 53.Tottenham N, Hare TA, Quinn BT, McCarry TW, Nurse M, Gilhooly T, et al. (2010): Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Developmental science. 13:46–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pechtel P, Lyons-Ruth K, Anderson CM, Teicher MH (2014): Sensitive periods of amygdala development: the role of maltreatment in preadolescence. Neuroimage. 97:236–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lupien SJ, Parent S, Evans AC, Tremblay RE, Zelazo PD, Corbo V, et al. (2011): Larger amygdala but no change in hippocampal volume in 10-year-old children exposed to maternal depressive symptomatology since birth. Proceedings of the National Academy of Sciences. 108:14324–14329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moreno-Lopez L, Ioannidis K, Askelund AD, Smith AJ, Schueler K, van Harmelen AL (2020): The Resilient Emotional Brain: A Scoping Review of the Medial Prefrontal Cortex and Limbic Structure and Function in Resilient Adults With a History of Childhood Maltreatment. Biological psychiatry Cognitive neuroscience and neuroimaging. 5:392–402. [DOI] [PubMed] [Google Scholar]
- 57.van Erp TG, Hibar DP, Rasmussen JM, Glahn DC, Pearlson GD, Andreassen OA, et al. (2016): Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol Psychiatry. 21:547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ho TC, Gutman B, Pozzi E, Grabe HJ, Hosten N, Wittfeld K, et al. (2020): Subcortical shape alterations in major depressive disorder: Findings from the ENIGMA major depressive disorder working group. Human brain mapping. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Logue MW, van Rooij SJH, Dennis EL, Davis SL, Hayes JP, Stevens JS, et al. (2018): Smaller Hippocampal Volume in Posttraumatic Stress Disorder: A Multisite ENIGMA-PGC Study: Subcortical Volumetry Results From Posttraumatic Stress Disorder Consortia. Biol Psychiatry. 83:244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Teicher Samson JA, Anderson CM, Ohashi K (2016): The effects of childhood maltreatment on brain structure, function and connectivity. Nature Reviews Neuroscience. 17:652. [DOI] [PubMed] [Google Scholar]
- 61.Arnsten AF, Raskind MA, Taylor FB, Connor DF (2015): The effects of stress exposure on prefrontal cortex: Translating basic research into successful treatments for post-traumatic stress disorder. Neurobiology of stress. 1:89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arnsten AF (2009): Stress signalling pathways that impair prefrontal cortex structure and function. Nature reviews neuroscience. 10:410–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lupien SJ, McEwen BS, Gunnar MR, Heim C (2009): Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature reviews neuroscience. 10:434–445. [DOI] [PubMed] [Google Scholar]
- 64.Andersen SL, Tomada A, Vincow ES, Valente E, Polcari A, Teicher MH (2008): Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. The Journal of neuropsychiatry and clinical neurosciences. 20:292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cohen RA, Grieve S, Hoth KF, Paul RH, Sweet L, Tate D, et al. (2006): Early life stress and morphometry of the adult anterior cingulate cortex and caudate nuclei. Biological psychiatry. 59:975–982. [DOI] [PubMed] [Google Scholar]
- 66.Tomoda A, Suzuki H, Rabi K, Sheu Y-S, Polcari A, Teicher MH (2009): Reduced prefrontal cortical gray matter volume in young adults exposed to harsh corporal punishment. Neuroimage. 47:T66–T71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Frodl T, Reinhold E, Koutsouleris N, Reiser M, Meisenzahl EM (2010): Interaction of childhood stress with hippocampus and prefrontal cortex volume reduction in major depression. Journal of psychiatric research. 44:799–807. [DOI] [PubMed] [Google Scholar]
- 68.Arnsten AF (2007): Catecholamine and second messenger influences on prefrontal cortical networks of "representational knowledge": a rational bridge between genetics and the symptoms of mental illness. Cerebral cortex (New York, NY: 1991). 17 Suppl 1:i6–15. [DOI] [PubMed] [Google Scholar]
- 69.Goodkind M, Eickhoff SB, Oathes DJ, Jiang Y, Chang A, Jones-Hagata LB, et al. (2015): Identification of a common neurobiological substrate for mental illness. JAMA psychiatry. 72:305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thomaes K, Dorrepaal E, Draijer N, de Ruiter MB, van Balkom AJ, Smit JH, et al. (2010): Reduced anterior cingulate and orbitofrontal volumes in child abuse-related complex PTSD. Journal of Clinical Psychiatry. 71:1636. [DOI] [PubMed] [Google Scholar]
- 71.Lim L, Hart H, Mehta M, Worker A, Simmons A, Mirza K, et al. (2018): Grey matter volume and thickness abnormalities in young people with a history of childhood abuse. Psychol Med. 48:1034–1046. [DOI] [PubMed] [Google Scholar]
- 72.Kelly PA, Viding E, Wallace GL, Schaer M, De Brito SA, Robustelli B, et al. (2013): Cortical thickness, surface area, and gyrification abnormalities in children exposed to maltreatment: neural markers of vulnerability? Biol Psychiatry. 74:845–852. [DOI] [PubMed] [Google Scholar]
- 73.McLaughlin KA, Sheridan MA, Winter W, Fox NA, Zeanah CH, Nelson CA (2014): Widespread reductions in cortical thickness following severe early-life deprivation: a neurodevelopmental pathway to attention-deficit/hyperactivity disorder. Biol Psychiatry. 76:629–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kring AM, Barch DM (2014): The motivation and pleasure dimension of negative symptoms: neural substrates and behavioral outputs. Eur Neuropsychopharmacol. 24:725–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fettes P, Schulze L, Downar J (2017): Cortico-Striatal-Thalamic Loop Circuits of the Orbitofrontal Cortex: Promising Therapeutic Targets in Psychiatric Illness. Frontiers in systems neuroscience. 11:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McCrory EJ, Viding E (2015): The theory of latent vulnerability: Reconceptualizing the link between childhood maltreatment and psychiatric disorder. Development and psychopathology. 27:493–505. [DOI] [PubMed] [Google Scholar]
- 77.McCrory EJ, Gerin MI, Viding E (2017): Annual research review: childhood maltreatment, latent vulnerability and the shift to preventative psychiatry–the contribution of functional brain imaging. Journal of Child Psychology and Psychiatry. 58:338–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.De Bellis MD, Hooper SR, Spratt EG, Woolley DP (2009): Neuropsychological findings in childhood neglect and their relationships to pediatric PTSD. Journal of the International Neuropsychological Society. 15:868–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cohen NJ, Lojkasek M, Zadeh ZY, Pugliese M, Kiefer H (2008): Children adopted from China: A prospective study of their growth and development. Journal of Child Psychology and Psychiatry. 49:458–468. [DOI] [PubMed] [Google Scholar]
- 80.Van Den Dries L, Juffer F, Van Ijzendoorn MH, Bakermans-Kranenburg MJ (2010): Infants' physical and cognitive development after international adoption from foster care or institutions in China. Journal of Developmental & Behavioral Pediatrics. 31:144–150. [DOI] [PubMed] [Google Scholar]
- 81.Rutter M, O'Connor TG (2004): Are there biological programming effects for psychological development? Findings from a study of Romanian adoptees. Developmental psychology. 40:81. [DOI] [PubMed] [Google Scholar]
- 82.Van Ijzendoorn MH, Luijk MP, Juffer F (2008): IQ of children growing up in children’s homes: A meta-analysis on IQ delays in orphanages. Merrill-Palmer Quarterly (1982-).341–366. [Google Scholar]
- 83.Burdick KE, Gunawardane N, Woodberry K, Malhotra AK (2009): The role of general intelligence as an intermediate phenotype for neuropsychiatric disorders. Cognitive neuropsychiatry. 14:299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.LaBar KS, Cabeza R (2006): Cognitive neuroscience of emotional memory. Nat Rev Neurosci. 7:54–64. [DOI] [PubMed] [Google Scholar]
- 85.Gruhn MA, Compas BE (2020): Effects of maltreatment on coping and emotion regulation in childhood and adolescence: A meta-analytic review. Child Abuse Negl. 103:104446. [DOI] [PubMed] [Google Scholar]
- 86.Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD (2008): Functional grouping and cortical–subcortical interactions in emotion: a meta-analysis of neuroimaging studies. Neuroimage. 42:998–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tottenham N, Galván A (2016): Stress and the adolescent brain: Amygdala-prefrontal cortex circuitry and ventral striatum as developmental targets. Neurosci Biobehav Rev. 70:217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Birn RM, Roeber BJ, Pollak SD (2017): Early childhood stress exposure, reward pathways, and adult decision making. Proceedings of the National Academy of Sciences. 114:201708791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pechtel P, Pizzagalli DA (2013): Disrupted reinforcement learning and maladaptive behavior in women with a history of childhood sexual abuse: a high-density event-related potential study. JAMA psychiatry. 70:499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Richards JM, Plate RC, Ernst M (2013): A systematic review of fMRI reward paradigms used in studies of adolescents vs. adults: the impact of task design and implications for understanding neurodevelopment. Neurosci Biobehav Rev. 37:976–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nestler EJ, Carlezon WA Jr. (2006): The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 59:1151–1159. [DOI] [PubMed] [Google Scholar]
- 92.Edmiston EE, Wang F, Mazure CM, Guiney J, Sinha R, Mayes LC, et al. (2011): Corticostriatallimbic gray matter morphology in adolescents with self-reported exposure to childhood maltreatment. Archives of pediatrics & adolescent medicine. 165:1069–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Baker LM, Williams LM, Korgaonkar MS, Cohen RA, Heaps JM, Paul RH (2013): Impact of early vs. late childhood early life stress on brain morphometrics. Brain imaging and behavior. 7:196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Whittle S, Vijayakumar N, Dennison M, Schwartz O, Simmons JG, Sheeber L, et al. (2016): Observed measures of negative parenting predict brain development during adolescence. PloS one. 11:e0147774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zald DH, Treadway MT (2017): Reward processing, neuroeconomics, and psychopathology. Annual review of clinical psychology. 13:471–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Collishaw S, Pickles A, Messer J, Rutter M, Shearer C, Maughan B (2007): Resilience to adult psychopathology following childhood maltreatment: evidence from a community sample. Child Abuse Negl. 31:211–229. [DOI] [PubMed] [Google Scholar]
- 97.Linscott RJ, van Os J (2013): An updated and conservative systematic review and meta-analysis of epidemiological evidence on psychotic experiences in children and adults: on the pathway from proneness to persistence to dimensional expression across mental disorders. Psychological Medicine. 43:1133–1149. [DOI] [PubMed] [Google Scholar]
- 98.Beer MD (1996): The dichotomies: psychosis/neurosis and functional/organic: a historical perspective. History of psychiatry. 7:231–255. [DOI] [PubMed] [Google Scholar]
- 99.DeRosse P, Karlsgodt KH (2015): Examining the Psychosis Continuum. Curr Behav Neurosci Rep. 2:80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wood SJ, Reniers RL, Heinze K (2013): Neuroimaging findings in the at-risk mental state: a review of recent literature. The Canadian Journal of Psychiatry. 58:13–18. [DOI] [PubMed] [Google Scholar]
- 101.Garner B, Pariante CM, Wood SJ, Velakoulis D, Phillips L, Soulsby B, et al. (2005): Pituitary volume predicts future transition to psychosis in individuals at ultra-high risk of developing psychosis. Biol Psychiatry. 58:417–423. [DOI] [PubMed] [Google Scholar]
- 102.Buschlen J, Berger GE, Borgwardt SJ, Aston J, Gschwandtner U, Pflueger MO, et al. (2011): Pituitary volume increase during emerging psychosis. Schizophr Res. 125:41–48. [DOI] [PubMed] [Google Scholar]
- 103.Karanikas E, Garyfallos G (2015): Role of cortisol in patients at risk for psychosis mental state and psychopathological correlates: a systematic review. Psychiatry and clinical neurosciences. 69:268–282. [DOI] [PubMed] [Google Scholar]
- 104.Aiello G, Horowitz M, Hepgul N, Pariante CM, Mondelli V (2012): Stress abnormalities in individuals at risk for psychosis: a review of studies in subjects with familial risk or with “at risk” mental state. Psychoneuroendocrinology. 37:1600–1613. [DOI] [PubMed] [Google Scholar]
- 105.O'Driscoll C, Laing J, Mason O (2014): Cognitive emotion regulation strategies, alexithymia and dissociation in schizophrenia, a review and meta-analysis. Clinical psychology review. 34:482–495. [DOI] [PubMed] [Google Scholar]
- 106.Liu J, Chua JJX, Chong SA, Subramaniam M, Mahendran R (2020): The impact of emotion dysregulation on positive and negative symptoms in schizophrenia spectrum disorders: A systematic review. Journal of clinical psychology. [DOI] [PubMed] [Google Scholar]
- 107.Chapman HC, Visser KF, Mittal VA, Gibb BE, Coles ME, Strauss GP (2020): Emotion regulation across the psychosis continuum. Development and psychopathology. 32:219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nittel CM, Lincoln TM, Lamster F, Leube D, Rief W, Kircher T, et al. (2018): Expressive suppression is associated with state paranoia in psychosis: An experience sampling study on the association between adaptive and maladaptive emotion regulation strategies and paranoia. British Journal of Clinical Psychology. 57:291–312. [DOI] [PubMed] [Google Scholar]
- 109.Liu J, Chan TC, Chong SA, Subramaniam M, Mahendran R (2019): Impact of emotion dysregulation and cognitive insight on psychotic and depressive symptoms during the early course of schizophrenia spectrum disorders. Early intervention in psychiatry. [DOI] [PubMed] [Google Scholar]
- 110.Kimhy D, Gill K, Brucato G, Vakhrusheva J, Arndt L, Gross J, et al. (2016): The impact of emotion awareness and regulation on social functioning in individuals at clinical high risk for psychosis. Psychological medicine. 46:2907–2918. [DOI] [PubMed] [Google Scholar]
- 111.Hoid D, Pan D-n, Wang Y, Li X (2020): Implicit emotion regulation deficits in individuals with high schizotypal traits: an ERP study. Scientific Reports. 10:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Henry JD, Rendell PG, Green MJ, McDonald S, O'Donnell M (2008): Emotion regulation in schizophrenia: Affective, social, and clinical correlates of suppression and reappraisal. Journal of abnormal psychology. 117:473. [DOI] [PubMed] [Google Scholar]
- 113.Green MF, Horan WP, Lee J (2015): Social cognition in schizophrenia. Nature reviews Neuroscience. 16:620–631. [DOI] [PubMed] [Google Scholar]
- 114.Modinos G, Kempton MJ, Tognin S, Calem M, Porffy L, Antoniades M, et al. (2020): Association of Adverse Outcomes With Emotion Processing and Its Neural Substrate in Individuals at Clinical High Risk for Psychosis. JAMA Psychiatry. 77:190–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Waltz JA, Gold JM (2007): Probabilistic reversal learning impairments in schizophrenia: further evidence of orbitofrontal dysfunction. Schizophr Res. 93:296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Waltz JA, Frank MJ, Robinson BM, Gold JM (2007): Selective reinforcement learning deficits in schizophrenia support predictions from computational models of striatal-cortical dysfunction. Biol Psychiatry. 62:756–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Strauss GP, Frank MJ, Waltz JA, Kasanova Z, Herbener ES, Gold JM (2011): Deficits in positive reinforcement learning and uncertainty-driven exploration are associated with distinct aspects of negative symptoms in schizophrenia. Biol Psychiatry. 69:424–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Strauss GP, Robinson BM, Waltz JA, Frank MJ, Kasanova Z, Herbener ES, et al. (2011): Patients with schizophrenia demonstrate inconsistent preference judgments for affective and nonaffective stimuli. Schizophr Bull. 37:1295–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Heerey EA, Robinson BM, McMahon RP, Gold JM (2007): Delay discounting in schizophrenia. Cognitive neuropsychiatry. 12:213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Heerey EA, Matveeva TM, Gold JM (2011): Imagining the future: degraded representations of future rewards and events in schizophrenia. J Abnorm Psychol. 120:483–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Treadway MT, Peterman JS, Zald DH, Park S (2015): Impaired effort allocation in patients with schizophrenia. Schizophr Res. 161:382–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Treadway MT, Buckholtz JW, Schwartzman AN, Lambert WE, Zald DH (2009): Worth the ‘EEfRT’? The effort expenditure for rewards task as an objective measure of motivation and anhedonia. PLoS One. 4:e6598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Barch DM, Treadway MT, Schoen N (2014): Effort, anhedonia, and function in schizophrenia: reduced effort allocation predicts amotivation and functional impairment. J Abnorm Psychol. 123:387–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gold JM, Strauss GP, Waltz JA, Robinson BM, Brown JK, Frank MJ (2013): Negative symptoms of schizophrenia are associated with abnormal effort-cost computations. Biol Psychiatry. 74:130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Barch DM, Carter CS, Gold JM, Johnson SL, Kring AM, MacDonald AW, et al. (2017): Explicit and implicit reinforcement learning across the psychosis spectrum. J Abnorm Psychol. 126:694–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Da Silva S, Saperia S, Siddiqui I, Fervaha G, Agid O, Daskalakis ZJ, et al. (2017): Investigating consummatory and anticipatory pleasure across motivation deficits in schizophrenia and healthy controls. Psychiatry Res. 254:112–117. [DOI] [PubMed] [Google Scholar]
- 127.Fervaha G, Foussias G, Agid O, Remington G (2015): Motivational deficits in early schizophrenia: prevalent, persistent, and key determinants of functional outcome. Schizophr Res. 166:9–16. [DOI] [PubMed] [Google Scholar]
- 128.Fervaha G, Foussias G, Agid O, Remington G (2014): Motivational and neurocognitive deficits are central to the prediction of longitudinal functional outcome in schizophrenia. Acta Psychiatr Scand. 130:290–299. [DOI] [PubMed] [Google Scholar]
- 129.Wotruba D, Heekeren K, Michels L, Buechler R, Simon JJ, Theodoridou A, et al. (2014): Symptom dimensions are associated with reward processing in unmedicated persons at risk for psychosis. Frontiers in behavioral neuroscience. 8:382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Schlosser DA, Fisher M, Gard D, Fulford D, Loewy RL, Vinogradov S (2014): Motivational deficits in individuals at-risk for psychosis and across the course of schizophrenia. Schizophr Res. 158:52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Maia TV, Frank MJ (2017): An Integrative Perspective on the Role of Dopamine in Schizophrenia. Biol Psychiatry. 81:52–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Howes O, Bose S, Turkheimer F, Valli I, Egerton A, Stahl D, et al. (2011): Progressive increase in striatal dopamine synthesis capacity as patients develop psychosis: a PET study. Molecular psychiatry. 16:885–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Charney Dennis S., M.D. (2004): Psychobiological Mechanisms of Resilience and Vulnerability: Implications for Successful Adaptation to Extreme Stress. American Journal of Psychiatry. 161:195–216. [DOI] [PubMed] [Google Scholar]
- 134.Feder A, Nestler EJ, Charney DS (2009): Psychobiology and molecular genetics of resilience. Nature reviews Neuroscience. 10:446–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Richter A, Kramer B, Diekhof EK, Gruber O (2019): Resilience to adversity is associated with increased activity and connectivity in the VTA and hippocampus. NeuroImage Clinical. 23:101920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pantelis C, Velakoulis D, McGorry PD, Wood SJ, Suckling J, Phillips LJ, et al. (2003): Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet (London, England). 361:281–288. [DOI] [PubMed] [Google Scholar]
- 137.Tognin S, Pettersson-Yeo W, Valli I, Hutton C, Woolley J, Allen P, et al. (2014): Using structural neuroimaging to make quantitative predictions of symptom progression in individuals at ultra-high risk for psychosis. Frontiers in psychiatry. 4:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Blaizot X, Mansilla F, Insausti AM, Constans JM, Salinas-Alaman A, Pro-Sistiaga P, et al. (2010): The human parahippocampal region: I. Temporal pole cytoarchitectonic and MRI correlation. Cerebral cortex (New York, NY : 1991). 20:2198–2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Borgwardt SJ, McGuire PK, Aston J, Gschwandtner U, Pfluger MO, Stieglitz RD, et al. (2008): Reductions in frontal, temporal and parietal volume associated with the onset of psychosis. Schizophr Res. 106:108–114. [DOI] [PubMed] [Google Scholar]
- 140.Takahashi T, Wood SJ, Yung AR, Phillips LJ, Soulsby B, McGorry PD, et al. (2009): Insular cortex gray matter changes in individuals at ultra-high-risk of developing psychosis. Schizophr Res. 111:94–102. [DOI] [PubMed] [Google Scholar]
- 141.Katagiri N, Pantelis C, Nemoto T, Tsujino N, Saito J, Hori M, et al. (2018): Symptom recovery and relationship to structure of corpus callosum in individuals with an ‘at risk mental state’. Psychiatry Research: Neuroimaging. 272:1–6. [DOI] [PubMed] [Google Scholar]
- 142.Katagiri N, Pantelis C, Nemoto T, Zalesky A, Hori M, Shimoji K, et al. (2015): A longitudinal study investigating sub-threshold symptoms and white matter changes in individuals with an ‘at risk mental state’(ARMS). Schizophrenia research. 162:7–13. [DOI] [PubMed] [Google Scholar]
- 143.Stip E, Lungu O (2012): Agenesis of corpus callosum and emotional information processing in schizophrenia. Frontiers in psychiatry. 3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cropley VL, Lin A, Nelson B, Reniers R, Yung AR, Bartholomeusz CF, et al. (2016): Baseline grey matter volume of non-transitioned "ultra high risk" for psychosis individuals with and without attenuated psychotic symptoms at long-term follow-up. Schizophr Res. 173:152–158. [DOI] [PubMed] [Google Scholar]
- 145.de Wit S, Wierenga LM, Oranje B, Ziermans TB, Schothorst PF, van Engeland H, et al. (2016): Brain development in adolescents at ultra-high risk for psychosis: Longitudinal changes related to resilience. NeuroImage: Clinical. 12:542–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.de Wit S, Ziermans TB, Nieuwenhuis M, Schothorst PF, van Engeland H, Kahn RS, et al. (2017): Individual prediction of long-term outcome in adolescents at ultra-high risk for psychosis: Applying machine learning techniques to brain imaging data. Human brain mapping. 38:704–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.DeRosse P, Ikuta T, Peters BD, Karlsgodt KH, Szeszko PR, Malhotra AK (2014): Adding insult to injury: childhood and adolescent risk factors for psychosis predict lower fractional anisotropy in the superior longitudinal fasciculus in healthy adults. Psychiatry Res. 224:296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.McLaughlin KA, DeCross SN, Jovanovic T, Tottenham N (2019): Mechanisms linking childhood adversity with psychopathology: Learning as an intervention target. Behaviour research and therapy. 118:101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Callaghan BL, Tottenham N (2016): The Stress Acceleration Hypothesis: Effects of early-life adversity on emotion circuits and behavior. Current opinion in behavioral sciences. 7:76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kopala-Sibley DC, Cyr M, Finsaas MC, Orawe J, Huang A, Tottenham N, et al. (2020): Early Childhood Parenting Predicts Late Childhood Brain Functional Connectivity During Emotion Perception and Reward Processing. Child Dev. 91:110–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.McGorry PD (2015): Early intervention in psychosis: obvious, effective, overdue. The Journal of nervous and mental disease. 203:310. [DOI] [PMC free article] [PubMed] [Google Scholar]