Abstract
Introduction:
We have demonstrated that asymptomatic cerebral small vessel disease (cSVD) measured by white matter hyperintensity volume is associated with reduced manipulative manual dexterity on the Grooved Peg Board test (GPBT) in middle-aged healthy individuals with a family history of early coronary artery disease. In this current study, we aim to identify the association of subcortical white matter microstructural impairment measured by diffusion tensor imaging (DTI), manual dexterity measured by GPBT and circulating serums ceramide; another marker for white matter injury. We hypothesize that lower regional fractional anisotropy (rFA) is associated with worse performance on GPBT and elevated serum ceramides in the same study population.
Methods:
rFA of 48 regions representing the subcortical white matters were analyzed in GeneSTAR participants in addition to serum ceramides, and GPBT scores. Unadjusted univariable analyses with Bonferroni correction for multiple comparisons were completed using Spearman correlation for testing the associations between ceramides, rFA of subcortical white matter and GPBT performance. Subsequently, sensitivity analyses were performed after excluding the participants that had any physical limitation that may influence their performance on GPBT. Finally, adjusted analysis using generalized estimating equation linear regression models were performed for the areas that met significance threshold in the unadjusted analyses.
Results:
112 subjects (age (49±11), 51% female, 39.3% African American) were included. Adjusted analyses for the significant correlations that met the Bonferroni correction threshold in the unadjusted univariable analyses identified significant negative associations between rFA of the right fornix (RF) and log-GPBT score (β=−0.497, P=0.037). In addition, rFA of RF negatively correlated with log serum ceramide levels (C18: β=−0.03, P=0.003, C20: β=−0.0002, P=0.004) and rFA of left genu of corpus callosum negatively correlated with log C18 level (β=−0.0103, P=0.027).
Conclusions:
These results demonstrate that subcortical microstructural white matter disruption is associated with elevated serum ceramides and reduced manual dexterity in a population with cSVD. These findings suggest that injury to white matter tracts undermines neural networks, with functional consequences in a middle-aged population with cardiovascular risk factors.
Keywords: ceramide, cerebral small vessel disease, diffusion tensor imaging, grooved peg board test, vascular cognitive impairment
Introduction:
Cerebral small vessel disease (cSVD) is a major cause of vascular cognitive impairment, intracerebral hemorrhage, and acute ischemic stroke[1, 2]. White matter hyperintensities (WMH) seen on the brain MRI are the most prevalent form of cSVD lesions[3]. Accumulation of these cSVD MRI lesions has been shown to result in cognitive impairment in the elderly[4]. Our group has been investigating the prevalence, characteristics and cognitive significance of cSVD features in a cohort of apparently healthy middle-aged individuals who are relatives to patients with coronary artery disease raising their predisposition to cardiovascular and cerebrovascular diseases[5, 6]. In this cohort, we have previously identified an association between subclinical impairment in manual dexterity on the Grooved Peg Board Test (GPBT) and increased WMH volume in individuals who are otherwise cognitively intact[7]. These previous findings suggest that subclinical cognitive impairment related to cSVD starts earlier in life. Additionally, in a cohort of apparently healthy middle-aged participants with Diffusion Tensor Imaging (DTI) who are subgroup of the previous study, we identified prevalent findings of microstructural impairment in the subcortical white matter that were mostly associated with a history of hypertension[8]. Diffusion Tensor Imaging (DTI) is an additional sensitive tool for detection of microstructural impairment of the white matter tracts related to axonal loss, edema or demyelination[9]. In particular, DTI offers an additional advantage compared to conventional MRI sequences by determining injury to specific white matter regions and tracts that are critical to cognitive function in cSVD[10]. This white matter tract injury disrupts the complex cortical and subcortical circuits underlying cognitive processes, such as executive function and processing speed[11]. This is particularly important in young or middle-aged populations with cardiovascular risk factors such as hypertension without signs of cognitive impairment in which detection of white matter injury in the early stages of disease will allow for the treatment of hypertension to prevent progression[12–15, 2].
In this current work, we aim to determine the association between regional subcortical white matter disruptions in areas linked to cognitive function with manipulative manual dexterity in this middle-aged population with cSVD who are relatives to patients with coronary artery disease. We use GPBT as a measure of manipulative manual dexterity. To analyze microstructural white matter injury, we utilize a detailed white matter atlas to segment the fractional anisotropy (FA) maps that were acquired using DTI. In addition, we analyze the relationship of regional fractional anisotropy (rFA) measures to a panel of circulating serum ceramides. Ceramides are highly enriched in the central nervous system particularly within the white matter tracts[16]. While, they have been implicated in the development and progression of neurodegenerative diseases such as Alzheimer’s disease[17, 18]; recent evidence links elevated ceramide to subcortical white matter injury in the cognitively normal elderly as well[16]. This suggests their utility as an additional marker for white matter injury in cSVD.
Our hypothesis is that lower rFA values of the subcortical white matter representing early injury to the white matter tracts in a population of middle-aged individuals at risk for cardiovascular disease enriched with cSVD will be associated with impaired manipulative manual dexterity as represented by worse GPBT score as well as elevation of serum ceramide, a secondary marker for white matter injury.
Materials and Methods:
Study participants:
The study cohort consisted of the participants in the Genetic Study of Atherosclerosis Risk (GeneSTAR) who had DTI sequences acquired as part of their brain MRI, GPBT performed and peripheral blood ceramide levels available. GeneSTAR is an ongoing prospective study of vascular disease risk factors, occult coronary artery disease (CAD) and cerebrovascular disease and incident CAD and strokes in 3533 initially healthy family members ascertained from probands hospitalized with documented CAD younger than 60 years of age [7]. Of these initially healthy family members, 808 were screened with cranial MRI and 112 participants had available had DTI sequences, serum ceramide levels, and GPBT. These subjects were similar in demographic configurations to the total GeneSTAR participants. This current study cohort originated from the healthy siblings, their offspring and the offspring of the probands from 92 families who were 30–72 years of age. None of these participants had a known history of coronary artery disease, stroke, or transient ischemic attacks. The study exclusion criteria included chronic use of corticosteroids, life-threatening diseases, atrial fibrillation and symptomatic cardiovascular or cerebrovascular diseases. In addition, subjects with implanted metals or known neurological disorders impairing accurate MRI interpretation were excluded. The Johns Hopkins Medicine institutional review board (NA_00002856) approved the study, and all participants provided written informed consent. All participants were examined by physicians and were screened for CAD and stroke risk factors including hypertension, diabetes, total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein cholesterol, body mass index (BMI), and cigarette smoking.
Manipulative manual dexterity test:
On the day of the MRI, manual dexterity was assessed using the Grooved Pegboard Test (GPBT) (Lafayette Instruments, Lafayette, IN). This test requires significant visual input in addition to sensory feedback and attention adjustments making it an excellent surrogate for the cognitive function of cSVD patients [19]. In addition, this test was selected because participants’ performance on it showed significant correlation with WMH volume [7]. The procedure of this test is described in detail elsewhere [7]. First, any abnormalities which may interfere with ability to perform the peg board test were noted. Subsequently, each participant was provided with 25 pegs to place into a board as quickly as possible during one trial per hand. The sum of time to compete the test (in seconds), the number of dropped pegs, and the total number of pegs placed was calculated for each hand. Since all subjects completed all 25 pegs, the scores were determined by time and the number of dropped pegs. The scores of both hands were averaged and the average was used in all analyses. A higher score indicated a worse performance [7].
Lipid extraction and liquid chromatography-electrospray ionization-tandem mass spectrometry analysis
The methods for lipid extractions and plasma ceramide and sphingomyelin level measurements have been published elsewhere [16]. In summary, crude lipid extraction from plasma was first performed using a modified Bligh and Dyer procedure with ceramide or sphingomyelin C12:0 as an internal standard (Avanti Polar Lipids, Alabaster, AL, USA). Following drying and re-suspending the plasma extracts in pure methanol, analysis was performed. First, an autosampler (LEAP technologies Inc, Carrboro, NC, USA) injected extracts into a high performance liquid chromatography (PerkinElmer, MA, USA) equipped with a reverse phase C18 column (Phenomenex, Torrance, CA, USA). The eluted sample was then injected into an electrospray ion source coupled to a triple-quadrupole mass spectrometer (API3000, AB Sciex Inc, Thornhill, Ontario, Canada). Analysis was completed using multiple reaction monitoring using an eight point calibration curves (0.1–1000 ng/ml) were constructed by plotting area under the curve separately for ceramides and sphingomyelins for each calibration standard normalized to the internal standard. Ceramide concentrations were determined by fitting the identified ceramide species to these standard curves based on acyl chain length. Instrument control and quantitation of spectral data were performed using Analyst 1.4.2 and MultiQuant software (AB Sciex Inc, Thornhill, Ontario, Canada). All sphingolipids are expressed in µg/ml for statistical analysis. A panel of 6 ceramides were selected for the subsequent statistical analysis.
MRI acquisition and DTI analysis:
MR imaging was acquired on a 3T Achieva imaging unit (Philips Healthcare, Best, Netherlands) according to standardized protocols. The following series were acquired: 1) axial T1-weighted MPRAGE-TR, 10 ms; TE, 6 ms; voxel size, 0.75 × 0.75 × 1 mm3; contiguous slices; FOV, 240 × 240 mm; matrix, 320 × 320 mm; 2) axial spin-echo T2 images- TR, 4685 ms; TE, 78 ms; voxel size, 0.47 × 0.47 × 3 mm3; contiguous slices; FOV, 240 × 240 mm; matrix, 512 × 512; 3) DTI – TR, 7043 ms, TE, 71 ms; voxel size, 0.83 × 0.83 × 2.2 mm3; contiguous slices; FOV, 212 × 212 mm; matrix, 256 × 256; 32 directions; b-value = 700 s/mm2.
DTI was preprocessed and analyzed using MRI studio package (DTIstudio, DiffeoMap, ROIEditor) publically available at www.mristudio.org through Johns Hopkins University [20]. All images were reviewed by a board-certified physician in neurology (YH) for the presence any artifact or distortion due to motion, head position or eddy current effect in the image viewer application. Tensor calculation and creation of 3D FA, mean diffusivity, and radial diffusivity maps were performed subsequently. White matter segmentation was performed in the DifeoMap and ROIEditor software applications in the subject native space. These steps were performed as described in previous work [21, 8]. In brief, following skull stripping of the FA and mean B0 maps in ROIEditor application, a dual-channel large deformation diffeomorphic mapping algorithm was used in DifeoMap to perform linear and nonlinear registration to the Eve white matter atlas (JHU-MNI-SS-SS) template in the Montreal Neurological Institute (MNI) Space. This step allowed automated segmentation of the FA subject map in the native space using the inverse registration matrix into 181 regions (supplementary table e1, Fig 1A) [21, 8]. The mean regional FA value (rFA) was calculated for each of the regions by choosing a voxel FA threshold ≥ 0.2 to exclude voxels that included CSF and grey matter. Subsequently, 48 regions representing the deep white matter tracts in regions affected by CSVD were selected for the statistical analysis similar to our previous work (supplementary table e1, Fig 1B) [8].
Figure 1A.
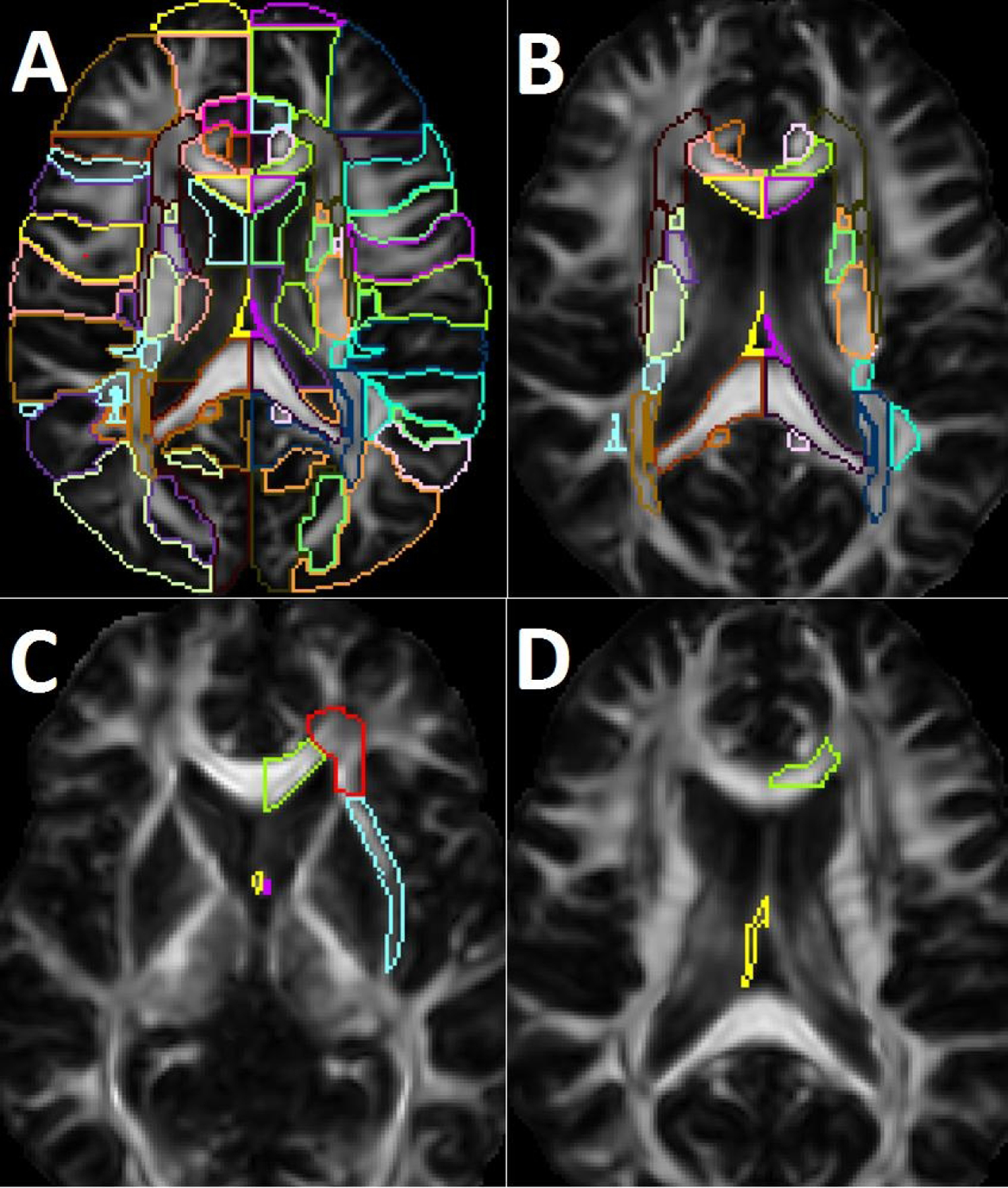
shows segmentation of the white matter regions into 181 atlas areas. Figure 1B shows the selected 48 regions representing the subcortical white matter areas of interest. Figure 1C shows white matter areas that were found to be associated with worse performance on the grooved peg board test in the whole cohort (green: left genu of the corpus callosum; red: left anterior corona radiate; blue: left external capsule; yellow: right fornix; purple: left fornix). In the sensitivity analysis, right fornix (yellow) and left external capsule (blue) were only significant. Figure 1D shows areas that were associated with elevated serum ceramide levels in the whole cohort and sensitivity analysis (yellow: right fornix; green: left genu of the corpus callosum).
Statistical analysis:
The statistical analysis was performed in SAS software (Version 9.2; SAS Institute, Cary, NC). Distributions of all continuous variables were assessed for normality and the presence of outliers. There was no outliers in the dataset so all subjects were included in the analysis. Log-transformation to normality was necessary for the ceramides and GPBT to complete the multivariable linear (adjusted) analysis. First, however, to determine the association among the variables of interest in our study, we performed unadjusted (univariable) analyses on the untransformed variables. Spearman correlations were used to test the association of: 1) rFA of subcortical white matter areas and ceramides, 2) GPBT and rFA of the subcortical white matter areas, and 3) ceramides and GPBT. The significance threshold for each of these unadjusted analyses was determined using Bonferroni correction for multiple comparisons: a p-value of 0.001 was used when assessing the relationship between rFA and GPBT considering the 48 selected white matter regions (0.05/48). The significance threshold for the relationship between rFA and ceramide was set as p< 0.00017 (0.05/48/6) considering 48 white matter regions and 6 ceramide types. Finally, the significance threshold for the relationship of peripheral ceramide with GPBT was determined to be p< 0.0083 (0.05/6) considering 6 ceramide subtypes. Subsequently, to assess the effect of any physical barriers on the performance on GPBT that were noted during examination, sensitivity analysis was performed by repeating the previously mentioned unadjusted analysis on the cohort after excluding those subjects with such physical barriers.
Finally, adjusted analyses using multivariable linear regression models controlling for age, sex, race and level of education were used to test the significant associations that were found in the unadjusted sensitivity analyses. All of these multivariable regression models in the adjusted analysis used generalized estimating equations (GEE) approach to account for family relatedness. Significant threshold of p<0.05 was selected in the adjusted models since they tested only the significant associations that were found in the unadjusted models. First, to demonstrate the association of subcortical microstructural white matter injury with peripheral ceramides, we tested the association of rFA of the subcortical white matter regions of interest (ROIs) with peripheral ceramide levels independent of age, sex, race and level of education. Then, to assess the effect of microstructural white matter injury on manual dexterity, we tested the association of GPBT with rFA of the ROIs with and without controlling for peripheral ceramides in the linear regression models.
Results:
The baseline demographics and vascular risk factors of the study cohort are shown in table 1. The study sample represented 112 patients, age 49.1±11, 50.9% females and 39.3% African American. The sample comprised overall healthy individuals with moderate load of cardiovascular risk factors (table 1). Review of the T2 sequences did not show any stroke or confluent large WMH that had predilection to specific brain area. Periventricular and deep WMH largely varied among subjects and they were mostly symmetrical. Volume of WMH in this cohort was (3436±6675)mm3 and it ranged between (0–40491)mm3 [7]. In this cohort, 5 participants were noted to have physical barriers on examination which may interfere with the performance on GPBT including 2 participants with band aids on fingers, 1 participant with chronic numbness in pinky finger, 1 with an intravenous line in the arm, and 1 participant with vision in one eye. These 5 participants were excluded in the sensitivity analyses.
Table 1.
Baseline demographics of the study cohort (N=112).
| Characteristic | Mean ± Standard Deviation or Percentage (%) |
|---|---|
| Age (years) | 49.1 ± 11.0 |
| Female sex | 50.9% |
| African American race | 39.3% |
| Education (years) | 14.4 ± 2.8 |
| Hypertension | 33.9% |
| Diabetes | 11.6% |
| Current smoker | 23.2% |
| Low density lipoprotein cholesterol (mg/dl) | 110.3 ± 38.8 |
| Blood glucose (mg/dl) | 98.7 ± 29.4 |
| Systolic blood pressure (mmHg) | 122.3 ± 15.6 |
| Diastolic blood pressure (mmHg) | 77.4 ± 8.9 |
| Body mass index (kg/m2) | 28.6 ± 5.5 |
| Grooved Pegboard Test Score | 105.2 ± 14.8 |
Relationship of rFA and Ceramide level:
The results of the unadjusted analysis of the association of rFA and peripheral ceramide levels are shown in detail in supplementary table e2. The following associations met the Bonferroni threshold for correction of multiple comparisons: 1) ceramide 18:0 inversely correlated with rFA in the left genu of the corpus callosum and right fornix (Rho=−.39, p=−2.6E-05 and −0.4, p< 1.3E-05, respectively) and 2) ceramide 20:0 also inversely correlated with rFA in the right fornix (Rho −0.41, p value <6.1E-06) (Fig 1D). Results remained the same in the sensitivity analysis after exclusion participants with physical barriers impairing their performance on GPBT (supplementary table e3 and Fig 1D). In the adjusted analysis for age, sex, race and level of education, these associations remained significant (rFA of right fornix with log ceramide 18:0 (beta=−0.03 +− 0.0099, p=0.003), rFA right fornix with log ceramide 20:0 ( beta=−0.0002, +− −0.001, p=0.004) and, rFA of the left genu of the corpus callosum and log ceramide 18:0 (beta=−0.0103, +− 0.0047, p=0.027) (table 2).
Table 2.
Associations between rFA of subcortical white matter regions and ceramide levels in the adjusted analysis.
| White matter region |
Generalized Estimating Equations Linear regression model Controlling for age, sex, race and level of education |
||
|---|---|---|---|
| β | Standard Error | P value | |
|
Genu Corpus Callosum (L) C 18:0 |
−0.0103 | 0.0047 | 0.027* |
|
Fornix (R) C18:0, and C20:0 |
C18: −0.03 | 0.0099 | 0.003* |
| C20: −0.0002 | 0.0001 | 0.004* | |
Statistically significant (p<0.05); L: left; R: right. Ceramides were log-transformed
Relationship of Grooved Pegboard Test with rFA and Ceramide level:
Spearman rank test showed correlation between a worse performance on the GPBT with lower rFA of the left anterior corona radiata, left genu of the corpus callosum, left external capsule, and bilateral fornices that met the Bonferroni threshold for multiple comparisons (Rho=−0.31,−0.31,−0.34, −0.46, p=0.00088, 0.00075, 0.0002, 0.0003, 8.28E-07, respectively) (supplementary table e4, Fig 1C). Furthermore, worse performance on GPBT was associated with elevated ceramide 18:0 (Rho=0.28, p=0.0025) in the unadjusted analysis after correction for multiple comparisons (supplementary table e5). In the unadjusted sensitivity analysis, elevated ceramide 18:0 association with worse performance on GPBT remained significant (Rho=0.271, p=0.0048) (supplementary table e5). Additionally, the association of worse performance on GPBT with rFA of the right fornix and left external capsule remained significant (Rho= −0.419, p=7.17E-06 and Rho= −0.318, p=0.00085, respectively). However, the association of rFA of the left anterior corona radiate, left genu of the corpus, and left fornix with the performance on GPBT became insignificant (p=0.002) (supplementary table e4). In the adjusted model, log-GPBT association with rFA of the right fornix was significant (Beta=−0.497, +−0.238, P = 0.037) (table 3) but it was not significant in left external capsule (p=0.407). When adding log serum ceramide 18:0 levels as a covariate to the model, the association of log-GPBT score with log-ceramide 18:0 was not significant (P=0.9) while log-GPBT and rFA of the right fornix remained significant (Beta = −0.5107 +−0.2551, p=0.045) (table 3).
Table 3.
Associations between Grooved Peg Board Test score and regional Fractional Anisotropy of the subcortical white matter regions in the adjusted analysis
| White matter region |
Generalized Estimating Equations Linear Regression model controlling for: age, sex, race and level of education * Serum C18 included in final model for R fornix only |
||
|---|---|---|---|
| β | Standard Error | P value | |
| External Capsule (L) | −0.504 | 0.608 | 0.407 |
| Fornix (R) | −0.497 | 0.238 | 0.037* |
| *Fornix (R) with Ceramide 18:0 adjustment | −0.511 | 0.255 | 0.045* |
statistically significant (p<0.05); L: left; R: right. Ceramide 18:0 and grooved peg board test score were log-transformed.
Discussion:
In this current work, we report a new association between impaired subcortical white matter bundles in the fornix and reduced manual dexterity and elevated circulating ceramides due to cSVD in middle-aged population with cardiovascular risk factors. These results extend our prior observation that asymptomatic white matter disease is associated with worse performance on the Grooved Peg Board test (GPBT) in subjects who had otherwise normal cognitive function in other tested cognitive domains [7]. Our findings suggest overall that injury to the subcortical areas undermines neural networks, and has functional consequences in a healthy high risk middle aged population with cSVD.
In our current study, worse performance on the GPBT correlated with lower rFA of right fornix. The fornix is a discrete white matter tract bundle that is critical for normal cognitive function. Its perfusion is typically maintained through perforators from proximal part of the anterior cerebral artery which makes it potentially susceptible to cSVD related injury [22]. As part of the memory circuit, it plays an important role for the formation of memory [23]. Previous study showed that rFA of the fornix was decreased in ageing in all tested components including working memory, motor speed and problem solving which suggests potential role for the fornix in executive functional decline associated with ageing [23]. The GPBT is a good measure of manipulative manual dexterity that can be used for motor speed assessment. It requires visual input as well as sensory feedback and attention adjustment [19]. Indeed, our results showed a negative association between the fornix rFA and the GPBT. Furthermore, previous literature has shown a significant relationship between performance on GPBT and white matter tract integrity on DTI pointing towards GPBT as potential measure of subclinical neurological function in otherwise cognitively intact individuals [24].
The role of DTI in detecting injury to the cerebral white matter has been affirmed in several studies previously involving patients with cSVD and Alzheimer’s disease [20, 21, 3, 13, 2]. However, the majority of this previous literature investigated patients at advanced age or with cognitive impairment. We have recently reported that impairment of white matter microstructure in patients with hypertension is even present in a middle-aged population that is similar in characteristics to this study cohort [8]. These current study results link these observed impairment of white matter microstructure to subclinical cognitive impairment. These findings suggest that our previously observed lower performance on GBPT in a larger middle-age healthy population, of which this current study population is a subset, could have been related to white matter tract injury [7].
In this current study, we have also found that serum ceramides 18:0 and 20:0 are inversely associated with rFA of the left genu of the corpus callosum and right fornix independent of the effect of age, sex, race and education in this current middle-aged population. Our group has previously shown a significant relationship of DTI of several white matter areas of cognitively intact elderly with circulating ceramides. In this previous study, elevated ceramides (C20:0, C22:0, C22:1 and C24:1) were associated with lower rFA in the anterior corona radiata and cingulum [16]. Furthermore, higher sphingomyelins (C18:1 and C20:1) have been associated with lower FA in regions such as the anterior corona radiate and body of the corpus callosum [16]. Ceramide a known potent signaling molecule that mediates key events of cellular pathophysiology including cell development, growth and apoptosis and it is the precursor of all complex sphingolipids [25]. In the central nervous system in particular, sphingolipids hold a major role in regulating development and lifespan while deregulation in sphingolipid metabolism increase the risk and progression of age-related neurodegenerative disease [26]. Specifically, ceramide 18 generation was found to result in apoptosis in cell culture model [27, 28]. Elevated brain and CSF ceramide levels have also been observed in Alzheimer’s patients [29, 17]. In our current study, we extend these prior observations to show a relationship between plasma sphingolipids and subcortical white matter microstructural integrity in a younger middle-aged population at risk for vascular disease.
Our study has several limitations inherent to its design. First, since this is a cross sectional study; causality between ceramide elevation, white matter microstructural integrity and GPBT performance cannot be established. Second, we chose a conservative approach for statistical multiple comparison adjustment using Bonferroni correction. Hence, given the widespread nature of cSVD, the brain regions associated with ceramide and GPBT performance could have been larger in number and distribution. However, our aim was to be specific in finding the brain regions affected by the disease and to avoid false positive regions. Finally, our sample size is relatively small for testing the large number of ceramides and white matter regions. To address these limitations, additional independent studies are needed to replicate our findings and determine the associations among serum ceramide levels, white matter microstructural integrity and subsequent cognitive impairment. Furthermore, our future studies aim to determine the mechanistic relationship among these white matter injury markers and their prognostic value as indicators of cognitive decline in patients with cSVD.
Conclusions:
In this exploratory study of a cohort of healthy middle-aged participants at risk for coronary artery disease, we observed white matter microstructural injury as measured by DTI to be associated with a secondary measure of WM injury, elevated serum ceramide. This disruption was associated with reduced performance on the grooved peg board test (GPBT). These findings suggest that injury to the subcortical white matter tracts may undermine complex cortical and subcortical neural networks affecting performance of complex tasks associated with manipulative manual dexterity in this healthy middle-aged population at risk for cardiovascular disease.
Supplementary Material
Acknowledgement:
Funding Sources:
This work was supported by the National Institutes of Health/National Institute of Neurological Disorders and Stroke grant NS062059, and the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, to Johns Hopkins Institute for Clinical and Translational Research, grant UL1RR025005.
Abbreviations:
- BMI
body mass index
- CAD
coronary artery disease
- cSVD
cerebral small vessel disease
- DTI
diffusion tensor imaging
- GPBT
grooved peg board test
- MRI
magnetic resonance imaging
- rFA
regional fractional anisotropy
- WM
white matter
- WMH
white matter hyperintensities
Footnotes
Statistical Analysis:
Statistical analysis was completed by Lisa Yanek and Dhananjay Vaidya.
Statement of Ethics:
This research study was conducted in accordance with the world medical association declaration of Helsinki. All participants provided written informed consent to participate in the study. The study was approved by the Johns Hopkins Institutional Review Board (IRB#NA_00002856).
Disclosure Statement:
Authors have no conflicts of interest to declare.
References:
- 1.Pantoni L Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. The Lancet Neurology. 2010. July;9(7):689–701. [DOI] [PubMed] [Google Scholar]
- 2.Wardlaw JM, Smith C, Dichgans M. Small vessel disease: mechanisms and clinical implications. The Lancet Neurology. 2019. July;18(7):684–96. [DOI] [PubMed] [Google Scholar]
- 3.Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. The Lancet Neurology. 2013. August;12(8):822–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jokinen H, Koikkalainen J, Laakso HM, Melkas S, Nieminen T, Brander A, et al. Global Burden of Small Vessel Disease-Related Brain Changes on MRI Predicts Cognitive and Functional Decline. Stroke. 2020. January;51(1):170–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nyquist PA, Bilgel MS, Gottesman R, Yanek LR, Moy TF, Becker LC, et al. Extreme deep white matter hyperintensity volumes are associated with African American race. Cerebrovascular diseases. 2014;37(4):244–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nyquist PA, Bilgel M, Gottesman R, Yanek LR, Moy TF, Becker LC, et al. Age differences in periventricular and deep white matter lesions. Neurobiology of aging. 2015. April;36(4):1653–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nyquist PA, Yanek LR, Bilgel M, Cuzzocreo JL, Becker LC, Chevalier-Davis K, et al. Effect of white matter lesions on manual dexterity in healthy middle-aged persons. Neurology. 2015. May 12;84(19):1920–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hannawi Y, Yanek LR, Kral BG, Vaidya D, Becker LC, Becker DM, et al. Hypertension Is Associated with White Matter Disruption in Apparently Healthy Middle-Aged Individuals. AJNR American journal of neuroradiology. 2018. December;39(12):2243–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2007. July;4(3):316–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Croall ID, Lohner V, Moynihan B, Khan U, Hassan A, O’Brien JT, et al. Using DTI to assess white matter microstructure in cerebral small vessel disease (SVD) in multicentre studies. Clinical science. 2017. June 1;131(12):1361–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lawrence AJ, Chung AW, Morris RG, Markus HS, Barrick TR. Structural network efficiency is associated with cognitive impairment in small-vessel disease. Neurology. 2014. July 22;83(4):304–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gottesman RF, Coresh J, Catellier DJ, Sharrett AR, Rose KM, Coker LH, et al. Blood pressure and white-matter disease progression in a biethnic cohort: Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2010. January;41(1):3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alber J, Alladi S, Bae HJ, Barton DA, Beckett LA, Bell JM, et al. White matter hyperintensities in vascular contributions to cognitive impairment and dementia (VCID): Knowledge gaps and opportunities. Alzheimer’s & dementia. 2019;5:107–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Group SMIftSR, Nasrallah IM, Pajewski NM, Auchus AP, Chelune G, Cheung AK, et al. Association of Intensive vs Standard Blood Pressure Control With Cerebral White Matter Lesions. JAMA. 2019. August 13;322(6):524–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Group SMIftSR, Williamson JD, Pajewski NM, Auchus AP, Bryan RN, Chelune G, et al. Effect of Intensive vs Standard Blood Pressure Control on Probable Dementia: A Randomized Clinical Trial. JAMA. 2019. February 12;321(6):553–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez CE, Venkatraman VK, An Y, Landman BA, Davatzikos C, Ratnam Bandaru VV, et al. Peripheral sphingolipids are associated with variation in white matter microstructure in older adults. Neurobiology of aging. 2016. July;43:156–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mielke MM, Bandaru VV, Haughey NJ, Xia J, Fried LP, Yasar S, et al. Serum ceramides increase the risk of Alzheimer disease: the Women’s Health and Aging Study II. Neurology. 2012. August 14;79(7):633–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mielke MM, Haughey NJ. Could plasma sphingolipids be diagnostic or prognostic biomarkers for Alzheimer’s disease? Clinical lipidology. 2012. October;7(5):525–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yancosek KE, Howell D. A narrative review of dexterity assessments. Journal of hand therapy : official journal of the American Society of Hand Therapists. 2009. Jul-Sep;22(3):258–69; quiz 70. [DOI] [PubMed] [Google Scholar]
- 20.Jiang H, van Zijl PC, Kim J, Pearlson GD, Mori S. DtiStudio: resource program for diffusion tensor computation and fiber bundle tracking. Computer methods and programs in biomedicine. 2006. February;81(2):106–16. [DOI] [PubMed] [Google Scholar]
- 21.Oishi K, Faria A, Jiang H, Li X, Akhter K, Zhang J, et al. Atlas-based whole brain white matter analysis using large deformation diffeomorphic metric mapping: application to normal elderly and Alzheimer’s disease participants. NeuroImage. 2009. June;46(2):486–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moudgil SS, Azzouz M, Al-Azzaz A, Haut M, Gutmann L. Amnesia due to fornix infarction. Stroke. 2000. June;31(6):1418–9. [DOI] [PubMed] [Google Scholar]
- 23.Thomas AG, Koumellis P, Dineen RA. The fornix in health and disease: an imaging review. Radiographics : a review publication of the Radiological Society of North America, Inc. 2011. Jul-Aug;31(4):1107–21. [DOI] [PubMed] [Google Scholar]
- 24.Zahr NM, Rohlfing T, Pfefferbaum A, Sullivan EV. Problem solving, working memory, and motor correlates of association and commissural fiber bundles in normal aging: a quantitative fiber tracking study. NeuroImage. 2009. February 1;44(3):1050–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mencarelli C, Martinez-Martinez P. Ceramide function in the brain: when a slight tilt is enough. Cellular and molecular life sciences : CMLS. 2013. January;70(2):181–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cutler RG, Mattson MP. Sphingomyelin and ceramide as regulators of development and lifespan. Mechanisms of ageing and development. 2001. July 15;122(9):895–908. [DOI] [PubMed] [Google Scholar]
- 27.Koybasi S, Senkal CE, Sundararaj K, Spassieva S, Bielawski J, Osta W, et al. Defects in cell growth regulation by C18:0-ceramide and longevity assurance gene 1 in human head and neck squamous cell carcinomas. The Journal of biological chemistry. 2004. October 22;279(43):44311–9. [DOI] [PubMed] [Google Scholar]
- 28.Wooten-Blanks LG, Song P, Senkal CE, Ogretmen B. Mechanisms of ceramide-mediated repression of the human telomerase reverse transcriptase promoter via deacetylation of Sp3 by histone deacetylase 1. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2007. October;21(12):3386–97. [DOI] [PubMed] [Google Scholar]
- 29.Satoi H, Tomimoto H, Ohtani R, Kitano T, Kondo T, Watanabe M, et al. Astroglial expression of ceramide in Alzheimer’s disease brains: a role during neuronal apoptosis. Neuroscience. 2005;130(3):657–66. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


