SUMMARY
Tumor mutational burden (TMB) reflects cancer mutation quantity. Mutations are processed to neo-antigens and presented by major histocompatibility complex (MHC) proteins to T-cells. To evade immune eradication, cancers exploit checkpoints that dampen T-cell reactivity. Immune checkpoint inhibitors (ICIs) have transformed cancer treatment by enabling T-cell reactivation; however, response biomarkers are required, as most patients do not benefit. Higher TMB results in more neo-antigens, increasing chances for T-cell recognition, and clinically correlates with better ICI outcomes. Nevertheless, TMB is an imperfect response biomarker. A composite predictor that also includes critical variables, such as MHC and T-cell receptor repertoire, is needed.
Introduction
Immune checkpoint inhibitors (ICI) are profoundly altering the therapeutic landscape for many cancers. An inhibitor of the T lymphocyte-associate antigen 4 (CTLA-4) and six inhibitors of the programmed cell death protein pathway (PD1/PD-L1) have been granted Food and Drug Administration (FDA) regulatory approval in multiple malignancies.(Bellmunt et al., 2017; Brahmer et al., 2015; Hodi et al., 2010; Jardim et al., 2018; Motzer et al., 2015; Robert et al., 2015; Seiwert et al., 2016) Even so, overall response rates (RRs) with these agents as monotherapy is low (~15%-20%), but some individuals can attain durable complete remissions. (Borghaei et al., 2015; Ikeda et al., 2016; Shitara et al., 2018; Siefker-Radtke et al., 2018) Unfortunately, ICIs can also cause a number of unique immune-related toxicities (Patel and Kurzrock, 2015; Postow and Hellmann, 2018) as well as accelerated progression, termed hyperprogression, in a subset of treated individuals.(Champiat et al., 2017; Kanjanapan et al., 2019; Kato et al., 2017)
The variability of response to ICIs highlights the need for identifying predictive biomarkers. PD-L1 expression measured by immunohistochemistry (IHC) is one of the most intuitive predictive biomarkers and, in fact, can enrich the selection of candidates who may respond to ICIs. However, despite its frequent adoption in clinical practice, PD-L1 expression is associated with multiple limitations, including technical ones.(Patel and Kurzrock, 2015; Topalian et al., 2016). Another biomarker that has recently garnered significant attention is tumor mutational burden (TMB), which is a measure of the number of mutations in a cancer; the more mutations, the more neoantigens and the higher the chances that one or more of those self neo-antigens will be immunogenic and trigger a T-cell response. Since TMB was first recognized as a potential biomarker for ICIs in melanoma (Snyder et al., 2014), many studies have reported a connection between higher TMB and ICI efficacy, suggesting that TMB could be a good predictive biomarker (Tables 1 and 2).(Buttner et al., 2019; Chalmers et al., 2017; Goodman et al., 2017; Samstein et al., 2019; Turajlic et al., 2017) However, PD-L1 expression and TMB are not significantly correlated within most cancer subtypes.(Cristescu et al., 2018; Patel and Kurzrock, 2015; Yarchoan et al., 2019) Major challenges for TMB utility are the observations that TMB may not always correlate with ICI responsiveness (Paz-Ares et al., 2019) and the proper integration of TMB assessment with other ICI response/resistance biomarkers.
Table 1:
Examples of tissue TMB and response to immunotherapy (checkpoint inhibitors)
| Cancer type (N) |
TMB and response relationship | Assay type | Treatment Strategy |
Comments | References |
|---|---|---|---|---|---|
| CRPC (37 pts) |
TMB above (≥74.5 mut) vs. below (<74.5 mut) median RR: Cohort 1 (pre-chemo): 50% vs. 9.1% (p=0.03); Cohort 2 (post-chemo): 50% vs. 0% (p=0.01) PFS (both cohorts): median 7.4 vs. 2.4 months (p<0.001) |
WES | ICI+ICI | Design: exploratory analysis of phase 2 trial (CheckMate 650). (NCT02985957) Drug: ipilimumab+nivolumab Higher TMB was predictive of better RR and longer PFS |
(Sharma et al., 2019) |
| Diverse cancers (151 pts) |
TMB high (≥20 mut/Mb) vs. low to intermediate (0-19 mut/Mb) RR = 58% vs 20% (P=0.0001) Linear correlation between TMB and better outcome |
NGS Foundation Medicine | ICI | Design: retrospective analysis of single institutional data Drugs: multiple immunotherapies (mostly ICI) TMB correlated linearly with responsiveness to checkpoint inhibitors across cancers |
(Goodman et al., 2017) |
| Diverse cancers (30 pts) |
Higher TMB (≥8 mut/Mb) vs Intermediate/Low Favorable Response (stable disease or response): increased probability of a favorable response with high TMB |
NGS Foundation Medicine | ICI | Design: retrospective analysis of single institutional data Drugs: multiple ICIs |
(Bonta et al., 2017) |
| Diverse cancers (119 pts) |
TMB high (≥102 mut) vs low (<102 mut) RR: 32.3% vs 7% (p=0.0078) |
WES | ICI | Design: exploratory analysis of two prospective single arm trials (Keynote 012 and 028) (NCT01848834; NCT02054806). Drug: pembrolizumab Mutational load was only modestly correlated with interferon gene expression profile |
(Cristescu et al., 2017) |
| Diverse Cancers (77 pts) |
Responder vs no responder Higher TMB was significantly associated with responses (p=0.018) and longer PFS (p=0.051) |
WES | ICI | Design: exploratory analysis of prospective phase Ib trial (Keynote 028) (NCT02054806). Drug: pembrolizumab Correlations between TMB with PD-L1 and T-cell inflamed gene-expression profile were low |
(Ott et al., 2019) |
| Diverse cancers (755pts) |
TMB high (≥10 mut/Mb) vs. low (<10 mut/Mb) RR: 28.3% vs. 6.5% (no p-value) PFS: 2.1 (95% CI, 2.1 to 3.7 months) vs 2.1 (95% CI, 2.1 to 2.3 months) (no p-value) OS: 11.1 (95% CI, 8.1 to 16.1 months) vs 13.3 (95% CI, 11.5 to 14.8 months) (no p-value) |
NGS Foundation Medidine | ICI | Design: exploratory analysis of phase 2 trial (Keynote-158). (NCT02628067 ) Drug: pembrolizumab Led to the accelerated approval of pembrolizumab for solid tumors with TMB ≥ 10 mut/mb by the FDA |
(Marabelle et al., 2019) |
| HNSCC (261 pts) |
TMB high (≥175 mut) vs low (<175 mut) High TMB was associated with higher response rate and longer PFS; no association with OS was demonstrated |
WES | ICI | Design: exploratory analysis of prospective trials (Keynote 012 and 055). (NCT01848834 and NCT02255097). Drug: pembrolizumab TMB was not associated with PD-L1 expression and T-cell activated gene expression profile |
(Seiwert et al., 2018) |
| Melanoma (64 pts) |
TMB high (>100 mut per tumor) vs low (≤ 100 mut) OS: survival advantage for high TMB (p=0.04) |
WES | ICI | Design: retrospective analysis of multiple prospective clinical trials Drugs: ipilimumab or tremelimumab Patients with increased long-term benefit presented significantly higher mutational load |
(Snyder et al., 2014) |
| Melanoma (38 pts) |
TMB top third vs bottom third OS: improved survival (p=0.005) |
WES | ICI | Design: retrospective analysis of institutional data Drugs: pembrolizumab or nivolumab High TMB predicted better survival, but not response to anti-PD-1. BRCA2 mutations enriched in responsive patients |
(Hugo et al., 2016) |
| Melanoma (65 pts) |
TMB high (≥23.1 mut/Mb) vs medium (3.3-23.1 mut/Mb) vs low (<3.3 mut/Mb) RR (%): 85 vs 29 vs. 14 (p<0.001) PFS : not reached vs. 89 days vs. 86 days (p<0.001) OS: not reached vs. 300 days vs. 375 days (p<0.001) |
NGS Foundation Medicine | ICI | Design: retrospective analysis of multi-institutional series Drugs: nivolumab, pembrolizumab or atezolizumab Response rate, progression-free survival, and overall survival were superior in the high, compared with intermediate and low, mutation load groups. TCR clonality did not predict response |
(Johnson et al., 2016) |
| Melanoma (94 pts) | Tumor mutational load was associated with response to nivolumab followed by ipilimumab (p = 0.03), but not with response to ipilimumab followed by nivolumab | WES | ICI | Design: exploratory analysis of Checkmate 064 (NCT01783938) Drugs: nivolumab or ipilumumab |
(Weber et al., 2016) |
| Melanoma (68 pts) |
TMB high (>100 mutations) vs low (≤ 100 mutations) OS: survival advantage for high TMB (p=0.048) |
WES | ICI | Design: exploratory analysis of single arm phase II trial CheckMate 018 (NCT01621490) Drug: nivolumab Mutational burden decrease after successful therapy with checkpoint blockade |
(Riaz et al., 2017) |
| MSI-high metastatic CRC (22 pts) |
High TMB (37-41 mut) vs low TMB RR: 100% vs. 22% PFS: not reached vs. 2 months |
NGS Foundation Medicine | ICI | Design: retrospective analysis of multi institutional data Drug: PD-1/PD-L1 inhibitors TMB (as a continuous variable) showed the strongest association with an objective response (p < 0.001) |
(Fakih M et al., 2019) |
| MSI-stable solid tumors (60 pts) |
High TMB (≥20 mut/Mb) vs. Low TMB (0-19 mut/Mb) Benefit rate: 75% vs. 38% (OR: 3.29 [95%CI 0.91-10.29, p=0.07) PFS: 26.8 vs. 4.3 months, HR 0.42 (95% CI 0.22-0.77; p = 0.017) OS: median not reached, HR 0.4581 (95% CI 0.20-1.0; p=0.06) |
NGS Foundation Medicine | ICI | Design: retrospective analysis of single institutional data Drugs: PD-1/PD-L1 inhibitors MS-Stable/TMB-High characterizes a subgroup of cancers considerably larger than the MSI-High subset, normally considered for checkpoint blockade. MSI-Stable/High TMB patients were responsive to checkpoint blockade |
(Goodman et al., 2019b) |
| NSCLC (34 pts) |
TMB high (≥200 mut per tumor) vs low (<200 mu per tumor) RR: 59% vs 12% (p=0.01) PFS: not reached vs 3.4 months, HR 0.91 (95%CI 0.08-0.47, p<0.001)) |
WES | ICI | Design: exploratory analysis of prospective phase I trial Keynote 001 (NCT01295827) Drug: pembrolizumab Higher TMB had a positive correlation with RR and PFS |
(Rizvi et al., 2015) |
| NSCLC (312 pts) |
Nivolumab vs chemoterapy comparison High TMB (≥243 mut): RR 47% vs 28%; PFS HR 0.62 (95% CI 0.38-1.0); OS HR 1.10 (95% 0.64-1.88) Medium and low TMB (<243 mut): RR 23% vs. 33%; PFS HR 1.82 (95% CI 1.30-2.55); OS HR 9.99 (95% CI 0.71-1.40) |
WES | ICI | Design: exploratory analysis of randomized phase III trial CheckMate 026 (NCT02041533) Drug: nivolumab TMB was predictive of RR and PFS, but not OS |
(Carbone et al., 2017) |
| NSCLC (102 pts) |
TMB high (≥13.5 mut/Mb) vs low RR: 25% vs 20% PFS: HR 0.54 (95% CI 0.3-0.97) OS: HR 0.45 (0.17-1.16) |
NGS Foundation Medicine | ICI | Design: exploratory analysis of prospective phase II trial (NCT01846416) Drug: atezolizumab Higher TMB was associated with RR and PFS in both unselected and PD-L1-selected patients |
(Kowanetz et al., 2017) |
| NSCLC (371pts) |
TMB high (≥17.1 mut/Mb) vs low RR: 29% vs 16% PFS: HR 0.5 (95% CI 0.38-0.67) OS: HR 0.7 (0.49-1.0) |
NGS Foundation Medicine | ICI | Design: exploratory analysis of prospective phase II trial (NCT02031458) Drug: atezolizumab TMB was associated with RR, PFS and OS |
(Kowanetz et al., 2017) |
| NSCLC (92 pts) |
Atezolizumab vs docetaxel comparison TMB high (≥median = 15.8 mut/Mb): RR 20% vs 8%; OS= HR 0.5 (95%CI 0.15-1.67); PFS = HR 0.49 (95%CI 0.19-1.3) All evaluable patients: RR 13% vs 15%; OS = HR 0.65 (95% CI 0.38-1.12); PFS 0.98 (95%CI 0.63-1.53) |
NGS Foundation Medicine | ICI | Design: exploratory analysis of randomized phase II trial (NCT01903993) Drug: atezolizumab Higher TMB was an independent predictor of improved RR, PFS but not OS |
(Kowanetz et al., 2017) |
| NSCLC (444 pts) |
TMB high (≥20 mut/Mb) vs Intermediate/Low (<20 mut/Mb) Median Duration of Therapy: 7.5 vs 4.6 months (p=0.001) Median OS: not reached vs 10 months (p=0.10) |
NGS Foundation Medicine | ICI | Design: retrospective analysis of Flatiron Health Database Drug: nivolumab Higher TMB was predictive of benefit in the multivariable analysis |
(Singal et al., 2017) |
| NSCLC (75 pts) |
TMB above (>158 mutations) vs. below median (≤158 mutations) RR: 51% vs. 13% (p=0.0005) DCB: 65% vs. 34% (p=0.011) PFS: 17.1 vs 3.7 months HR 0.41, (95%CI, 0.23-0.73; p=0.0024) |
WES | ICI+ICI | Design: exploratory analysis of single phase II trial (CheckMate 012) (NCT01454102). Drug: ipilimumab plus nivolumab TMB and PD-L1 were demonstrated to present independent predictive value four outcome |
(Hellmann et al., 2018c) |
| NSCLC (299 pts) |
Ipilimumab+nivolumab vs chemotherapy comparison High TMB (≥10 mut/Mb): RR=45.3% vs 26.9%; PFS, HR 0.58 (95% CI 0.41-0.81, p<0.001) Low TMB (<10 mut/Mb): PFS, HR 1.07 (95% CI 0.84-1.15) |
NGS Foundation Medicine | ICI+ICI | Design: exploratory analysis of randomized phase III (CheckMate 227) (NCT02477826). Drug: ipilimumab plus nivolumab No correlation between TMB and PD-L1 expression level |
(Hellmann et al., 2018b) |
| NSCLC (240 pts) |
TMB above median (>7.4 mut/Mb) vs below median DCB rate: 38.6% vs. 25.1% (p=0.009) PFS: HR 1.38 p=0.024 |
MSK Impact NGS | ICI | Design: retrospective analysis of single institutional data Drugs: PD-1/PD-L1 inhibitors Estimates of TMB correlated well with NGS in 49 patients. No correlation was detected between TMB and PD-1 expression |
(Rizvi et al., 2018) |
| NSCLC (605 pts) |
Pembrolizumab+chemotherapy versus chemotherapy TMB >175 mut/exome: OS= HR 0.64 (95%CI 0.38-1.07); PFS = HR 0.32 (95%CI 0.21-0.51) TMB <175 mut/exome: OS= HR 0.64 (95%CI 0.42-0.97); PFS = HR 0.51 (95%CI 0.35-0.74) KEYNOTE-021 G (Pembro+CT) RR: 60.8% (95%CI 38.5-80.3) for tTMB <175 vs. 71.4% (95%CI 47.8-88.7) for tTMB ≥175 mut/exome |
WES | ICI+Chemo | Design: exploratory analysis of multiple prospective trials: KEYNOTE-021 C and G (NCT02039674), 189 (NCT02578680), and 407 (NCT02775435). Drug: pembrolizumab TMB was not associated with efficacy of chemotherapy plus pembrolizumab. It is plausible that giving chemotherapy with pembrolizumab could have confounded the utility of TMB, which appears predictive with immunotherapy alone. |
(Paz-Ares et al., 2019) |
| NSCLC (98 pts) |
TMB (≥10 mut/Mb) vs. (<10 mut/Mb) RR: 44% vs. 12% (no p-value) PFS: 7.1 (95% CI, 3.6 to 11.3 months) vs 2.6 (95% CI, 1.4 to 5.4 months) (no p-value) |
NGS Foundation Medicine | ICI | Design: expoloratory analysis of single arm prospective phase II trial (CheckMate 568). (NCT02659059). Drug: pembrolizumab RR was higher in TMB high patients regardless of PD-L1 expression |
(Ready et al., 2019) |
| RCC (109 pts) |
TMB High (≥64 mut) vs Low (<64/mut) Nivolumab Cohort: RR 20% vs 33% (p=0.20); PFS 1.06 (95% CI 0.64-1.75, p=0.82); OS: 0.85 (95% CI 0.43-1.67 p=0.64) Ipi+Nivo Cohort: RR 61% vs 43% (p=0.48); PFS HR 0.36 (95%CI 0.16-0.84, p=0.02); OS HR 0.71 (95%CI 0.16-3.15, p=0.65) |
WES | ICI+ICI | Design: retrospective analysis of single institutional data (MSKCC) Drugs: ipilimumab+nivolumab High TMB was predictive of longer PFS only on the ipilumumab and nivolumab cohort |
(Lee et al., 2019) |
| SCLC (211 pts) |
TMB high (≥248 mut) vs medium (143-247 mut) vs low (<143 mut) RR: Nivolumab: 21.3% vs 6.8% vs 4.8% Nivo+Ipi: 46.2% vs 16% vs 22.2% PFS (1-year): Nivolumab: 21.2% vs 3.1% vs not calculable Nivo+Ipi: 30% vs 8% vs 6.2% OS (1-year): Nivolumab: 35.2% vs 26% vs 22.1% Nivo+Ipi: 64.4% vs 19.6% vs 23.4% |
WES | ICI+ICI | Design: exploratory analysis of phase II trial (CheckMate 032) (NCT01928394). Drug: nivolumab or nivo+ipi High TMB predicted better efficacy for the combination compared.to nivolumab |
(Hellmann et al., 2018a) |
| Urothelial carcinoma (86 pts) |
TMB upper fourth quartile (≥16 mut/Mb) OS: higher compared to patients in quartiles 1-3 (p=0.004) |
NGS Foundation Medicine | ICI | Design: exploratory analysis of prospective phase II trial (Imvigor 210) (NCT02108652) Drug: atezolizumab Higher TMB predicted better survival |
(Balar et al., 2017) |
| Urothelial carcinoma (139 pts) |
TMB high (≥167 mut) vs medium (85-166 mut) vs low (<85 mut) RR (%): 31.9 vs 17.4 vs. 10.9 (p=0.0002) PFS (months): 3.02 vs. 1.87 vs. 1.91 (p=0.005) OS (months): 11.63 vs 9.66 vs 5.72 (p=0.067) |
WES | ICI | Design: exploratory analysis of single arm phase II trial (NCT02387996) Drug: nivolumab Higher TMB correlated with better RR and longer PFS |
(Galsky et al., 2017a) |
| Urothelial carcinoma (25 pts) |
Durable clinical benefit vs no durable clinical benefit TMB 3.24 mut/Mb vs 0.45 mut/mB (p=0.22) |
WES | ICI | Design: exploratory analysis of phase II trial (Imvigor210) (NCT02108652) Drug: atezolizumab Higher TMB observed in patients with durable benefit |
(Snyder et al., 2017) |
| Urothelial carcinoma (274 pts) |
Atezolizumab vs chemotherapy comparison TMB high (≥median = 9.65 mut/Mb): OS= HR 0.68 (95%CI 0.51-0.90) TMB low (<median=9.65 mut/Mb): OS = HR 1.0 (95% CI 0.75-1.32) |
NGS Foundation Medicine | ICI | Design: exploratory analysis of phase III randomized trial (Imvigor211) (NCT02302807) Drug: atezolizumab DDR mutations correlated with increased TMB |
(Powles et al., 2018) |
Abbreviations: Chemo = chemotherapy; CRC=colorectal cancer; CRPC= castration resistant prostate cancer; DCB = durable clinical benefit; DDR = DNA damage repair; HNSCC= head and neck squamous cell carcinoma; HR=hazard ratio; ICI=immune checkpoint inhibitor; ipi= ipilimumab; Mb = megabase; MSI-H= microsatellite instability-high; MSKCC= Memorial Sloan Kettering Cancer Center; mut = mutation; N = number; NGS = next generation sequencing; Nivo = nivolumab; NSCLC = non-small cell lung cancer; OS = overall survival; pts = patients; PFS = progression-free survival; RCC=renal cell carcinoma; RR = response rate; SCLC=small cell lung cancer; WES= whole exome sequencing
Table 2:
Examples of blood TMB and response to immunotherapy (checkpoint inhibitors)
| Cancer type (N) |
TMB and response relationship | Assay type | Treatment Strategy |
Comments | References |
|---|---|---|---|---|---|
| Diverse cancers (69 pts) |
VUS > 3 vs. VUS ≤ 3 SD ≥6 months/PR/CR 45% versus 15%, respectively; P = 0.014 PFS: 3.84 vs 2.07 months (HR, 0.52; 95% CI, 0.31–0.87; P=0.019) OS: NR vs 10.72 (HR, 0.39; 95% CI, 0.18– 0.83; P=0.042) |
NGS 54-70 genes (Guardant) |
ICI | Design: retrospective analysis, single institutional data Drugs: multiple checkpoint inhibitors Of the 69 patients, 20 (29%) presented >3 VUS and 49 (71%) ≤ 3 VUS |
(Khagi et al., 2017) |
| NSCLC (583 pts, validation cohort) |
Atezolizumab vs docetaxel comparison bTMB ≥16 : PFS: HR 0.65 (0.47-0.92); OS: 0.64 (0.44-0.92) bTMB <16: PFS: HR 0.98 (0.80-1.20); OS: 0.65 (0.52-0.81) |
NGS 1.1 Mb (Foundation Medicine panel) |
ICI | Design: exploratory analysis of prospective randomized phase II (POPLAR NCT01903993) and phase III trial (OAK NCT02008227_ Drug: atezolizumab Correlation with tTMB by Foundation Medicine assay was demonstrated (Spearman correlation 0.93). |
(Gandara et al., 2018) |
| NSCLC (119 pts) |
bTMB ≥16 vs. <16 ORR: 28.6% vs 4.4% (P=0.0002) PFS: 5.0 vs 3.5 months (HR 0.80 90% CI 0.54-1.18) OS: 23.9 vs. 13.4 months (HR 0.66 90% CI 0.40-1.10) |
NGS 1.1 Mb (Foundation Medicine panel) |
ICI | Design: prospective analysis of B-F1RST single arm prospective trial (NCT02848651)_ Drug: atezolizumab bTMB was predictive of higher RR; no statistical significant benefits were detected for PFS or OS |
(Kim et al., 2018; Socinski et al., 2019) |
| NSCLC (809 pts) |
Durvalumab+Tremelimuab vs. CT bTMB ≥20mut/Mb: PFS 4.2 vs 4.4 months (HR 0.53 95% CI 0.34-0.81); OS 21.9 vs 10 months (HR 0.49 [95% CI 0.32-0.74) bTMB <20mut/Mb: PFS 2.0 vs 5.0 months (HR 1.55 95% CI 1.23-1.94); OS 8.5 vs 11.6 months (HR 1.16 95% CI 0.93-1.45) |
Guardant OMNI (Guardant Health) |
ICI+ICI | Design: exploratory analysis of prospective randomized phase III trial (MYSTIC NCT02453282) Drugs: durvalumab + tremelimumab bTMB was correlated with tTMB (Spearman’s correlation = 0.6) Cut-points for dichotomization of TMB may be made to optimize positive or negative predictive value. |
(Rizvi et al., 2020) |
| NSCLC (98 pts) |
bTMB ≥6 vs. <6 PFS: NR vs 2.9 months, (HR 0.39; 95% CI, 0.18-0.84; P = 0.01) RR: 39.3% vs. 9.1% (P=0.02) |
NGS 150 genes (NCC-GP150) |
ICI | Design: retrospective analysis of multi-institutional series_ Drugs: multiple PD-1/PD-L1 inhibitors The authors demonstrated a significant correlation between bTMB vs tissue TMB in a virtual comparison to TCGA (r2=0.91) and with matched tTMB calculated by WES (Spearman correlation = 0.62) |
(Wang et al., 2019) |
| NSCLC (66 pts) |
bTMB ≥16 vs. <16 ORR: 28.6% vs 4.4% (P=0.0002) PFS: 14.1 vs 4.7 months (HR 0.30 95% CI 0.16-0.60, p<0.001) OS: not reached vs. 8.8 months (HR 0.48 90% CI 0.22-1.03; p=0.061) |
Guardant OMNI (Guardant Health) |
ICI | Design: observational prospective cohort of single institutional data (NCT03047616) Drug: pembrolizumab bTMB>16 mut/Mb is associated with improved PFS; STK11/KEAP1/PTEN and ERBB2 mutations were common in unresponsive patients |
(Aggarwal et al., 2020) |
Abbreviations: bTMB = blood tumor mutational burden; CR = complete response; CT=chemotherapy; HR=hazard ratio; ICI=immune checkpoint inhibitor; mb = megabase; N = number; NGS = next generation sequencing; NR=not reached; NSCLC=non-small cell lung cancer; ORR = objective response rate; PFS = progression-free survival; PR = partial response; pts = patients; RR = response rate; SD = stable disease, tTMB=tissue tumor mutational burden; VUS = variant of unknown significance; WES = whole exome sequencing
Definition and Experimental Determination of TMB
The conceptual definition of TMB is total number of mutations present in a tumor specimen. The actual definition of the type of genetic alterations considered for TMB has varied according to different methodologies (Figure 1). Characterization of TMB was initially performed using whole exome sequencing (WES), which by its nature considers genetic alterations restricted to exomes (coding regions). TMB calculation by WES included non-synonymous mutations in coding regions and excluded germline alterations by subtracting matched normal samples. Due to the technical complexity and high cost of WES, comprehensive gene panels using next generation sequencing (NGS) have also been used in the clinic as a substitute for WES. (Campesato et al., 2015; Johnson et al., 2016; Roszik et al., 2016).
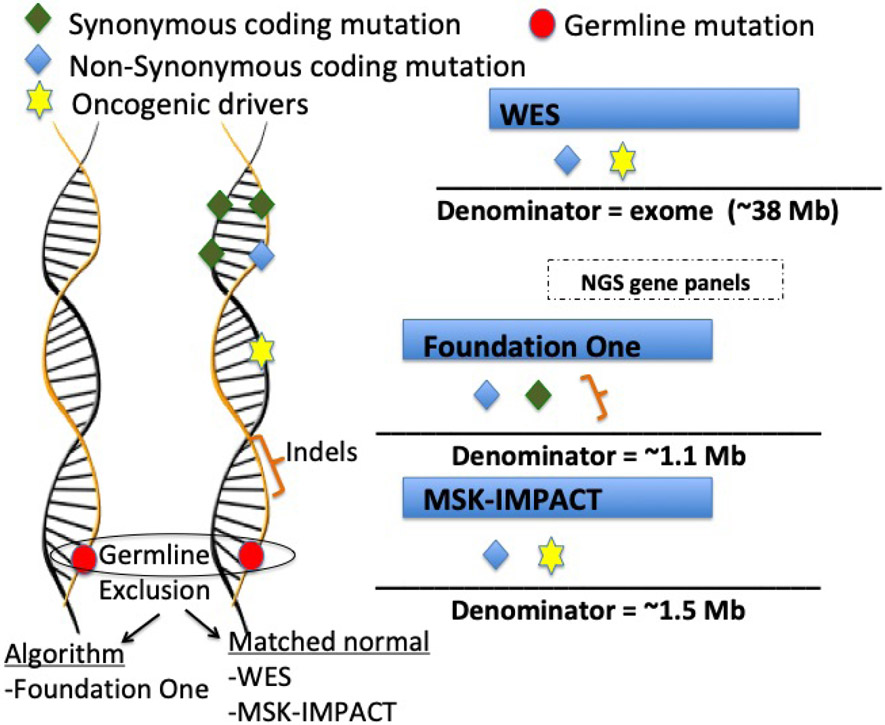
Figure 1 – Schematic representation of the main FDA-approved assays for TMB estimation as well as whole exome sequencing calculation.
Two of the NGS gene panels are FDA-approved tests (Foundation One (Frampton et al., 2013) and MSK-Impact) (Cheng et al., 2015). Symbols represent genetic alterations that are captured as mutations, while the denominator refers to the genome region that is considered for each test. The Foundation Medicine TMB assay examines a genomic region of approximately 1.1 Mb. For TMB estimation this test includes synonymous and non-synonymous mutations and short indels, while oncogenic drivers are excluded. In addition, germline alterations are excluded based on validated bioinformatics algorithms. The MSK IMPACT TMB assay examines approximately 1.5 Mb and, similar to WES, includes non-synonymous mutations in coding regions and oncogenic drivers. Germline alterations are excluded by subtracting matched normal samples.
Examples of other commercial available assays include: Illumina TruSight 500 (~2Mb exome coverage [i.e region sequenced]), Thermo Fisher Scientific Oncomine (1.7 Mb exome coverage), Caris Molecular Intelligence (1.7 Mb exome coverage); NEO New Oncology NEOplus v2 RUO (1.1 Mb exome coverage); TruSight Tumor 170 (0.5 Mb exome coverage); Tempus Plataform (2.4 Mb exome cover)
Abbreviations: Mb = megabase; WES: Whole exome sequencing
A comprehensive genomic profiling assay of 324 genes (corresponding to 1.1 Mb of coding genome) developed by Foundation Medicine (Cambridge, MA, USA) was validated as an accurate assessment of TMB estimated by WES (Table 1).(Chalmers et al., 2017) This assay was recently approved by the FDA as a companion diagnostic for pembrolizumab in patients with TMB ≥10 mutations/Mb.(FDA, 2020) The FoundationOne assay also includes in its TMB estimation synonymous mutations and short indels in intronic regions, which are excluded from WES. Indels usually create novel DNA open reading frames, leading to an entirely new stretch of peptides that perhaps have a higher chance of encoding immunogenic neo-antigens. Indeed, prior clinical observations suggested better responsiveness to ICIs in patients with higher burden of indels, especially in renal cell carcinoma (Turajlic et al., 2017), though this may not hold true for other solid tumors.(Wood et al., 2020). Another USA FDA-approved NGS assay is the MSK-IMPACT developed by Memorial Sloan Kettering. This test currently identifies somatic exonic mutations in 468 cancer-related genes (approximately 1.2 Mb).(Samstein et al., 2019)
A linear relationship between TMB and ICI responsiveness has been described. (Goodman et al., 2017) However, there is currently no consensus in the definition of TMB cut-offs for patient stratification. FoundationOne calls high TMB (TMB-H) when ≥20 mutations/Mb are detected; TMB-Intermediate, 6-19 mutations/Mb; and TMB-Low, ≤5 mutations/Mb . (Chalmers et al., 2017; Goodman et al., 2017) Nonetheless, the recent tissue-agnostic FDA approval of pembrolizumab defined elevated TMB as being ≥10 mutations/Mb. Since the absolute TMB numbers are quite variable between histologies, another potential approach would be to consider the top 20% of TMB for each histology (Samstein et al., 2019).
TMB assay harmonization
There is a need for standardization of tissue TMB calculation and reporting to ensure reproducibility.(Buttner et al., 2019; Fancello et al., 2019) Recent data generated in cohorts of patients with lung cancer suggested that harmonization of targeted panels is possible through normal transformation followed by standardization to z scores, resulting in a linear relationship.(Vokes et al., 2019) An ongoing effort to reconcile TMB methodologies is being conducted by Friends of Cancer Research (https://www.focr.org/events/tmb-harmonization-working-group-meeting).
TMB estimation using blood-based tests
Circulating tumor DNA (ctDNA) (blood biopsy) has also been used for TMB estimation.(Davis et al., 2017; Gandara et al., 2018; Khagi et al., 2017; Muller et al., 2017) The convenience of noninvasive sample collection and the possibility of repeated sampling during therapy are some of the potential advantages for this technique. Early paired comparisons for TMB estimation between tissue and ctDNA showed a lack of concordance between the techniques in some studies (Davis et al., 2017). More recently, sophisticated techniques and algorithms to evaluate blood TMB have yielded consistent promising results, and multiple studies also demonstrate the correlation between blood TMB-H and immunotherapy response (Table 2).(Gandara et al., 2018; Khagi et al., 2017; Kim et al., 2018; Peters et al., 2019; Wang et al., 2019) The use of blood-based TMB assessment may be especially important since a subgroup of patients (~15% in our experience) may not have tissue for TMB assessment, either because the tissue is unavailable or the tissue quality is not adequate.
TMB and Tumor Immune Response
Immune evasion is a hallmark of cancer. T-cells normally recognize neo-antigens produced as a result of mutations and presented by major histocompatibility complex (MHC) proteins on the surface of cancer cells, and target those cells for destruction. In order to survive, the tumor hijacks proteins that normally serve as checkpoints which attenuate immune response against healthy tissues. By blocking immune checkpoint proteins, such as PD-1, PD-L1 and CTLA-4, with ICIs, the immune system can be reawakened. However, once reactivated, the T-cells must still be able to differentiate tumor from normal cells. Recognition of cancer cells is facilitated if there are immunogenic neo-antigens presented on their surface. Since neo-antigens are produced as a result of mutations, the greater the number of mutations (i.e., the higher the TMB), the greater the chance that some of the neo-antigens presented by MHC proteins will be immunogenic, and hence enable T-cell recognition and cancer cell eradication. (Chabanon et al., 2016; Chalmers et al., 2017; Rooney et al., 2015), On this basis, it is not surprising that TMB-H correlates with better outcome after ICI therapy (Tables 1 and 2). Nonetheless, the variable attrition rates between genomic events captured by TMB and the final steps of MHC presentation and immune-mediated tumor killing can explain, in part, the inter-patient variation of TMB as a predictive factor for immunotherapy responses.(Goodman et al., 2020) Ultimately, TMB has demonstrated a reasonable prediction of responses especially to ICI, but also to other immunotherapies.(Lauss et al., 2017) However, any attempt to dichotomize the predictive ability of TMB is imperfect, as T-cell recognized neo-antigens can, in theory, originate in a low mutation setting (although with lower likelihood); conversely, large numbers of mutations do not necessarily translate to immunogenic neo-antigens.
In summary, from an immune-oncology viewpoint, it is important to highlight that TMB has important limitations as a predictive biomarker, especially when used in isolation. (Schumacher et al., 2019) These limitations may explain why the response rate in patients with tumors that have TMB-H (≥20 mutations/Mb) is only ~45% (Goodman et al., 2017). First, only a small fraction of non-synonymous mutations will result in neo-antigens that are recognized by T-cells. Second, the clonality of these neo-antigens and the specific tumor molecular signatures contribute to the ability to generate a unique and effective anti-tumor response.(Matsushita et al., 2012) Third, factors related to the host immunological microenvironment can impact T-cell mediated tumor killing: ability of T-cells to traffic to the tumor site, the balance between activating and suppressive cytokines, the type of checkpoint exploited by the tumor, and the regulation of metabolic pathways that altogether will lead to an inflamed (hot) tumor microenvironment. Host MHC and T-cell receptor (TCR) landscape also play an important part in immune responsiveness. (Blank et al., 2016) Therefore, while TMB-H correlates with better outcomes after ICI administration, the complexity of the immune response means that TMB must be considered along with multiple other factors in order to optimize prediction of ICI outcome.
Genomic Interactions Impacting TMB as a Biomarker
TMB reflects the mutagenic processes induced by environmental and intracellular factors (Figure 2). The association between TMB and mutational signatures, including those related to carcinogen exposure or endogenous mutagenic processes, such as deficient mismatch repair (dMMR) (associated with MSI-H and TMB-H), are currently the focus of many ongoing research analyses. MHC, TCR repertoire, and specific genomic alterations also play an important role in determining ICI outcome.
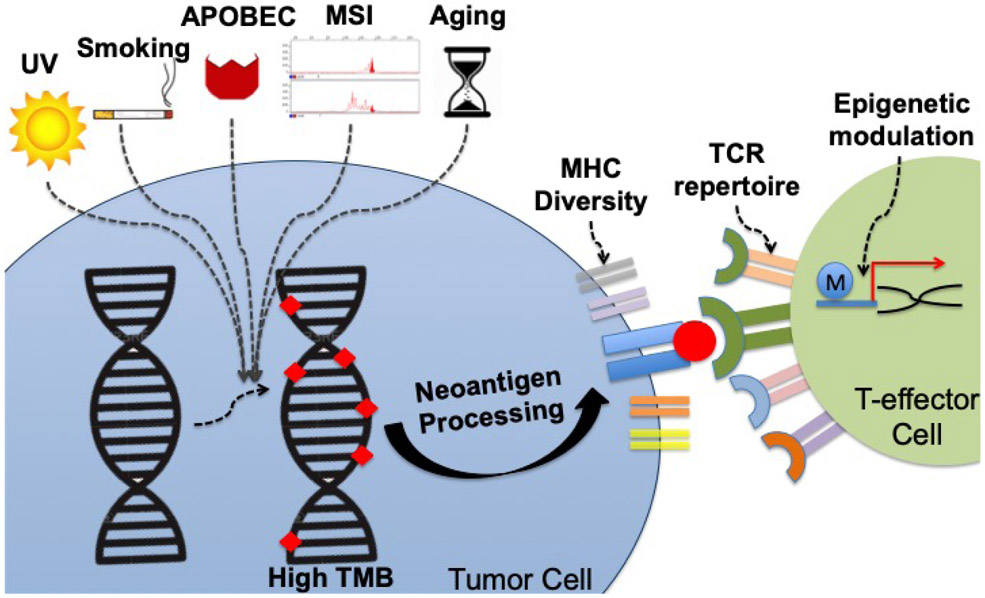
Figure 2 – Environmental and host factors that influence TMB as a biomarker for anticancer immunotherapies.
Several different processes lead to gain of genomic alterations in tumors cells, whose number can be quantified by TMB. Environmental factors (e.g. UV) and DNA editing errors (MSI) cause patterns of mutations classified under different signatures.(Zehir et al., 2017) Each signature may influence not only the number of mutations, but also the quality and immunogenicity of the neo-antigens presented as a result of the mutanome burden. Host intrinsic characteristics also impact neo-antigen presentation and recognition. For instance, MHC diversity defines how well the neo-antigens can be presented, while TCR repertoire may define neo-antigen recognition.(Chowell et al., 2018; Weber et al., 2016) Epigenetic modulation (such as by histone modifications and DNA methylation) may also influence the host ability to generate an effective immune response.(Peng et al., 2015)
Abbreviations: MHC, major histocompatibility complex; MSI, microsatellite instability; TCR, T-cell receptor; TMB: tumor mutational burden; UV, ultraviolet light
Microsatellite instability-high (MSI-H) correlates with TMB-H and with ICI response
MSI-H is an important gene signature associated with TMB-H. Tumors with deficient mismatch repair (dMMR) are characterized by a higher number of uncorrected DNA defects leading to an MSI-H phenotype. (Mardis, 2019) In fact, about 83% of MSI-H tumors also present TMB-H (≥20 mutations/Mb) (Chalmers et al., 2017). Conversely, only 16% of tumors with TMB-H are also MSI-H, demonstrating that MSI-H is only one of the possible factors that lead to TMB-H.(Chalmers et al., 2017) MSI-H is described in ~4% of cancers, most frequently colorectal, gastric, and endometrial adenocarcinomas. About 15% of MSI-H cancers are due to germline mutations in MMR genes (MLH1, MSH2, MSH6 and PMS2). MSI-H may also occur because of non-hereditary epigenetic inactivation of MMR genes in addition to somatic mutations.(Bonneville et al., 2017)
In 2017, the FDA granted accelerated approval to pembrolizumab (anti-PD-1) for both adult and pediatric patients with MSI-H or dMMR solid tumors. This was the first time the agency authorized a cancer treatment based on a genomic biomarker that was histology agnostic. RRs of MSI-H solid tumors to ICIs are ~40% in the pan-cancer setting, with a subset of responses showing long-term durability.(Marcus et al., 2019) The TMB-H that characterizes most MSI-H cancers probably accounts for the immunotherapy responsiveness.
Microsatellite-stable tumors with TMB-H are responsive to checkpoint blockade
Microsatellite-stable tumors with TMB-H also benefit from ICIs.(Goodman et al., 2019b) Median progression-free survival (PFS) for microsatellite-stable/TMB-H (≥20 mutations/Mb) versus microsatellite-stable/TMB-Low/TMB-Intermediate tumors was 26.8 vs. 4.3 months (P=0.0173). Furthermore, 2,179 of 148,803 samples (1.5%) were MSI-H, while 9,762 (6.6%) were TMB-H (7,972, microsatellite-stable/TMB-H). Therefore, microsatellite-stable/TMB-H tumors are substantially more common than MSI-H cancers. (Goodman et al., 2019b) Recent results from the TAPUR study (NCT02693535) in heavily pre-treated colorectal cancer also suggest activity of pembrolizumab in microsatellite-stable/TMB-H patients. (Meiri et al., 2020) Of interest, the FDA has recently granted tissue-agnostic accelerated approval for the pembrolizumab in TMB ≥10 mutations/Mb solid tumors.(FDA, 2020) The evidence derived from a single-arm phase II trial demonstrated RRs of ~28% in patients with TMB ≥10 mutations/Mb, with 50% of the responses being durable at two years; however, survival was not increased.(Marabelle et al., 2019) Hence, from the practical perspective, TMB is an approved biomarker for selecting patients for ICI monotherapy, albeit one with an incomplete correlation with outcome.
Genomic signatures associated with exogenous mutagens impact ICI outcome
Ultraviolet (UV) light, tobacco smoking, aflatoxin B1 and benzene exposure, as well as viruses, cause mutational signatures that influence the emergence of specific genomic alterations and TMB status as well as neo-antigen immunogenicity. As a consequence, TMB is not defined by one universal mutational signature across tumors. Signatures associated with exogenous mutagens (smoking, UV light, etc) are more frequent in melanoma and lung cancer (which are considered to be tumors that frequently have TMB-H); conversely, signatures associated with DNA repair gene (MMR, POLE) defects, also associated with TMB-H, are more predominant in endometrial, colorectal and esophagogastric cancers.(Zehir et al., 2017)
The correlation between TMB and the formation of immunogenic neo-antigens is variable and dependent on the mutational signatures (Table 3). (Alexandrov et al., 2016; Boichard et al., 2019; Kucab et al., 2019; Romualdo Barroso-Sousa, 2018). For instance, apolipoprotein B mRNA-editing cytidine-deaminase (APOBEC) signatures, characterized by C > G and C > T mutations (Alexandrov et al., 2013; Burns et al., 2013), are a result of viruses and are strongly correlated with immune activation while, for the aging process signature, the correlation is nonsignificant.(Boichard et al., 2019; Budczies et al., 2018) The APOBEC signature is also associated with the expression of PD-L1 and interferon gamma in tumor cells.(Boichard et al., 2017) APOBEC deregulation and the resulting specific mutational pattern called kataegis also correlates with the overall mutation burden(Boichard et al., 2017) and with DNA changes that mediate enhanced neo-peptide hydrophobicity, which is known to increase immunogenicity.(Boichard et al., 2019) Further, an APOBEC signature correlated with ICI responsiveness independently of TMB in a cohort of 99 patients.(Boichard et al., 2019) This relationship was established regardless of the presence of MSI-H. UV signature also correlated with increased neo-antigen hydrophobicity/immunogenicity, and was able to predict better response (P = 0.0026), PFS (P = 0.036), and survival (P = 0.052) after ICIs in patients with TMB-Low/Intermediate (<20 mutations/Mb), but not in patients with TMB-H.(Pham et al., 2020)
Table 3 –
Examples of biomarkers/genomic changes other than TMB that may influence the response to checkpoint blockade
| Gene/Protein | Comments | References |
|---|---|---|
| Possible association with increased benefit | ||
| APOBEC-related mutagenesis | APOBEC-related mutagenesis increases neo-peptide hydrophobicity, and, thus immunogenicity. APOBEC signature correlated with responses to immunotherapy independently of TMB. | (Boichard et al., 2019) |
| ARID1A | ARID1A alterations (part of the SWI/SNF chromatin remodeling complex) function as a biomarker for longer progression-free survival after anti-PD-1/PD-L1 immunotherapy | (Okamura et al., 2020) |
| CDK12 | CDK12 biallelic inactivating mutations is associated with genomic instability, including increased gene fusions. Most of the genomic alterations in these tumors are not captured by current TMB estimation, but lead to increased neo-antigen burden and T cell infiltration, suggesting benefit from check-point inhibition. | (Wu et al., 2018) |
| DDR genes* | Alterations in DDR genes are associated with increased TMB and tumor infiltration by T cells. Some series demonstrated higher response rate and longer survival during therapy with PD-1/PD-L1 in the presence of DDR alterations. DNA damage is also associated with cellular stress that may trigger immune innate mechanisms. | (Teo et al., 2018) (Chae et al., 2019) |
| KRAS | In NSCLC the presence of KRAS mutations correlates with an inflammatory microenvironment, and may indicate patients that will benefit from checkpoint-inhibitors in monotherapy. | (Liu et al., 2020a). |
| MHC1 presentation | Poor presentation of driver mutation neoantigens by MHC-I may explain why some tumors (even with a high TMB) do not respond to checkpoint blockade. | (Goodman et al., 2020) |
| MLH1, MSH2, MSH6, PMS2 | Loss of function of mismatch repair genes leads to microsatellite instability and a higher number of somatic mutations. These alterations were predictive of higher responses to checkpoint inhibitors.(Le et al., 2017) | (Le et al., 2017) |
| PBRM1 loss | PBRM1 (part of the SWI/SNF chromatin remodeling complex) loss is associated with T-cell mediated tumor killing; series of patients with renal cell carcinoma showed higher benefit from ICIs in the presence of PBRM1 loss | (Miao et al., 2018) |
| PD-1 expression | Frequency of PD-1+CD8+ T cells relative to that of PD-1+ regulatory T (Treg) cells in the tumor microenvironment can predict the clinical efficacy of anti-PD1 therapies | (Kumagai et al., 2020) |
| PD-L1 amplification | PD-L1 (CD274) amplification may be present ~0.7% of solid tumors; most PD-L1 amplified tumors demonstrated low to intermediate TMB. Clinical responses to checkpoint ICIs are frequent (66.7%) and independent of TMB in solid tumors; however, only a small number of such patients have been treated. PD-L1 amplification is also a hallmark of Hodgkin disease, which is highly responsive to checkpoint blockade. | (Goodman et al., 2018) |
| PD-L1 expression by immunohistochemistry | PD-L1 overexpression is an indicator of intra-tumoral inflammatory environment. Expression levels are independent of TMB. PD-L1 expression on tumor or immune cells may be important. | (Ott et al., 2019) (Patel and Kurzrock, 2015) (Liu et al., 2020b) (Gonzalez-Ericsson et al., 2020) |
| POLD1, POLE | Loss-of-function mutations in POLD1 and POLE correlate with increased immune infiltration and a hyper-mutated phenotype, and predict responses to anti-PD-L1 therapy. | (Budczies et al., 2018) (Wang et al., 2018) |
| SERPINB3, SERPINB4 mutations | Serpin mutations were associated with higher clinical benefit rates and longer survival in melanoma patients treated with anti-CTLA4 therapy. This possible positive predictive effect was independent of TMB. | (Riaz et al., 2016) |
| SMARCA4 | SMARCA4 (part of the SWI/SNF chromatin remodeling complex) alterations are correlated with ICI response in small cell ovarian cancer hypercalcemic type and anecdotally in other tumors | (Tischkowitz et al., 2020) |
| Tumor infiltrating lymphocytes | High tumor infiltrating lymphocytes have been associated with checkpoint inhibitor responsiveness | (Gonzalez-Ericsson et al., 2020) |
| Possible association with resistance | ||
| B2M inactivating mutations | B2M truncating mutations lead to loss of surface expression of MHC I and resistance to PD-1 blockade. | (Zaretsky et al., 2016) |
| JAK1/2 mutations | JAK1/2 loss of function mutations can lead to acquired and primary resistance to anti-PD-1 therapy, even in the setting of high TMB. | (Shin et al., 2017) |
| KEAP1 alterations | Associated with worse outcome after ICI. Not clear if these alterations are prognostic or predictive | (Chen et al., 2020; Skoulidis and Heymach, 2019) |
| STK11/LKB1 | Associated with worse outcome after ICI. Not clear if these alterations are prognostic or predictive | (Skoulidis et al., 2018) |
| Possible association with hyper-progression | ||
| MDM2/MDM4 and EGFR | MDM2/MDM4 amplifications and EGFR aberrations were associated with hyperprogression in patients treated with checkpoint inhibitors. This association was independent of TMB. | (Kato et al., 2017) (Singavi et al., 2017) |
DDR genes definitions are variable and commonly includes BRCA1, BRCA2, ATM, CHECK2, PALBB2, ATM, ERCC2, FANCA
Abbreviations: B2M: Beta-2-microglobulin, DDR: DNA damage repair; ICI= immune checkpoint inhibitor; MHC: major histocompatibility complex
Major histocompatibility complex (MHC) and the TCR repertoire mediate ICI outcome
MHC diversity determines how well neo-antigens can be presented, while TCR repertoire defines neo-antigen recognition.(Chowell et al., 2018; Weber et al., 2016) As background, the function of MHC molecules is to bind peptide fragments and display them on the cell surface for recognition by the appropriate T-cells. MHC is polygenic and the heterogeneity found in its molecules plays a significant role in shaping an individual’s immune reaction to malignancies. Specifically, MHC variation can greatly affect peptide binding during antigen presentation, which impacts the peptide repertoire presented on the cell surface by MHC class I or II proteins for T-cell recognition. While maintaining the ability to respond to foreign peptides, the T-cell population in an individual needs to avoid harmful activation by self-antigens. For instance, co-inhibitory surface receptors, such as PD-1 and CTLA-4 immune checkpoints, recognize surface-expressed ligands on self-tissues and act to dampen unwanted immune activation. Cancer hijacks these immune checkpoints in order to survive.
Important steps that are required before the somatic mutations that reflect TMB can in fact elicit T-cell destruction include (Chabanon et al., 2016): (i) somatic alterations need to be translated to neo-antigens that will bind to MHC at the surface of tumor cells; and (ii) altered neoantigens presented by the MHC need to be efficiently recognized by TCRs. Indeed, the likelihood of neo-antigen presentation by the MHC (Goodman et al., 2020) and of subsequent recognition by T cells (Luksza et al., 2017) characterize a predictive neo-antigen fitness model based on the host immune pharmacogenome.
MHC-I molecules are major players in shaping the mutational landscape of cancer (Mage et al., 2012; Marty et al., 2017) because of their ability to present potential neo-antigens derived from tumor mutations. In turn, oncogenic processes and mutations negatively correlate with MHC-I presentation, supporting MHC as having a crucial role in immune-editing. Maximal heterozygosity at MHC-I loci (leading to improved host ability to present a higher number of cancer neo-antigens) is associated with longer survival in ICI-treated patients.(Chowell et al., 2018) In addition, melanoma patients receiving ICIs demonstrated that some human leukocyte antigen (HLA) subtypes (with HLA being the human version of the MHC complex), such as HLA-B44, are able to preferentially present certain antigens enriched in the tumor, leading to improved survival.(Chowell et al., 2018) These HLA super-types enhance the positive effects of TMB-H on survival in patients with cancer. Conversely, tumors that lose HLA expression or harbor functional disruptions mediated by alterations in β2-microglobulin (a component of MHC class I) can be resistant to ICIs.(Rodig et al., 2018; Zaretsky et al., 2016) MHC class II may also be important in shaping response to the mutational processes in cancer development. Mutant peptides poorly bound to MHC-II are positively selected during tumorigenesis, more so than mutant peptides poorly bound to MHC-I.(Marty Pyke et al., 2018a) Hence, the MHC-II genotype complements MHC-I in selecting the mutational landscape during tumorigenesis, indirectly impacting the immunological potential of TMB (Marty Pyke et al., 2018b).
Another fundamental part of the immunologic response is the diverse processes that lead to an individual’s TCR repertoire, which in turn leads to different abilities to recognize MHC-presented antigens among cancer patients. Absence of a T-cell response to an MHC-presented cancer neoantigen could be driven by the lack of TCR reactivity or removal of active TCRs from the host repertoire.(Linnemann et al., 2014) In contrast, a higher clonality of TCRs could indicate the availability of proper anti-tumor reactive T-cells. In fact, during treatment with ICIs, higher TCR clonality is associated with improved survival.(Weber et al., 2016) However, the effects of TCR clonality and diversity might vary with the type of checkpoint blockade.(Hogan et al., 2019; Hopkins et al., 2018; Reuben et al., 2019; Yusko et al., 2019)
In summary, MHC and TCR repertoire are vital determinants of the immune response. Malignancies with TMB-H are more likely to respond to ICIs due to the increased chance of having an immunogenic mutated peptide/neo-antigen presented by their MHC. The MHC-I genotype and its ability to present driver neo-antigens predicts which patients with TMB-H will respond to ICIs (Goodman et al., 2020) as does increased CD8+ T-cell effector function and TCR diversity.(Hosoi et al., 2018)
Impact of specific genomic alterations on checkpoint blockade response and resistance
In addition to genomic signatures and the immune-pharmacogenome, there are a variety of genomic alterations that individually may correlate with sensitivity, resistance, and hyperprogression after immunotherapy (Table 3), sometimes independently of TMB. In the context of TMB-H, a variety of unique genomic alterations will occur, and most of them will be passenger events. But some of them can be driving the TMB-H (e.g., dMMR) or cause ICI response or resistance.
An interesting example of a genomic predictor is PD-L1 gene amplification, which is associated with high RRs to PD-1 inhibitors in Hodgkin lymphoma (Ansell et al., 2015; Roemer et al., 2016) as well as in solid tumors, surprisingly even in the absence of high PDL1 expression by IHC.(Goodman et al., 2018) Serpin genes correlate with autoimmunity, and mutations in some genes from this family were associated with positive responses to CTLA-4 inhibitors.(Riaz et al., 2016) Alterations in CDK12 (Wu et al., 2018) as well as in chromatin remodeling genes PBRM1(Miao et al., 2018), ARID1A (Okamura et al., 2020) and SMARCA4 (Tischkowitz et al., 2020) have been correlated with better ICI outcomes, though some of the data re PBRM1 remains a matter of debate, and the mechanisms require clarification.
Genomic alterations may also predict poor outcome after ICIs. Beta-2 microglobulin (B2M) mutations disrupt MHC class I and thus diminish antigen presentation to immune active cells.(Shin et al., 2017; Zaretsky et al., 2016) Consequently, inhibition of PD1/PD-L1 interaction has little effect. JAK1/2 and STK11 mutations are associated with attenuated responsiveness to immunotherapy even in a TMB-H setting, though it is unclear if STK11 alterations are a prognostic or predictive marker.(Shin et al., 2017; Skoulidis et al., 2018) Finally, MDM2 family amplification and EGFR aberrations can be associated with hyper-progression after ICIs, although the underlying mechanisms are unknown.(Kato et al., 2017).
Utility and Challenges of Using TMB as a Biomarker
Clinically, there is a substantial correlation between the TMB and objective responses to PD-1/PD-L1 inhibitors.(Chalmers et al., 2017; Goodman et al., 2017; Yarchoan et al., 2017) TMB can be responsible for 55% of the difference in response rate between cancer types (Chan et al., 2019; Yarchoan et al., 2017) and tissue TMB correlates linearly with better outcome (including RRs and PFS) to ICIs across cancers (Table 1).(Goodman et al., 2017) Series that included patients with melanoma treated with either ipilimumab (anti-CTLA-4) or a PD-1 inhibitor (Riaz et al., 2017; Snyder et al., 2014), or those with urothelial cancer treated with atezolizumab (anti-PD-L1) or nivolumab (anti-PD-1) (Balar et al., 2017; Galsky et al., 2017b; Powles et al., 2018) demonstrated improved outcome with higher TMB. In NSCLC, higher ICI RRs and longer PFS were also observed for patients with higher TMB in some studies, but not in others (Carbone et al., 2017; Hellmann et al., 2018b; Hellmann et al., 2018c; Rizvi et al., 2018; Rizvi et al., 2015), though in the latter, patients in the arm receiving ICIs also received chemotherapy, which could have confounded the results.(Paz-Ares et al., 2019). Importantly, in the studies with lung cancer, higher TMB has generally failed to show an overall survival advantage, even when PFS is improved (Table 1).
In a series of 151 patients with a variety of advanced solid tumors treated with ICIs, RRs were ~5% in patients with TMB-Low (≤5 mutations/Mb); ~25%, TMB-Intermediate (6-19 mutations/Mb); ~45%, TMB-H patients (≥20 to 49 mutations/M; and ~65% in individuals with very high TMB (≥50 mutations/Mb) (Foundation Medicine stratification)(Goodman et al., 2017). Unfortunately, TMB-H does not preclude tumor progression. In fact, one of the first patients reported to present with accelerated progression during ICI therapy had a TMB-H neoplasm.(Kato et al., 2017)
Comparing squamous cell versus other histologies is also relevant.(Goodman et al., 2019a) Squamous cell tumors have higher TMB than non-squamous cell cancers, with the highest TMB in cutaneous squamous cell tumors (with 41% demonstrating a very high TMB (≥50 mutations/Mb)). In immunotherapy-treated squamous cell cancer-bearing patients, higher TMB (≥12 mutations/Mb) correlated with significantly better outcomes; cutaneous squamous cell cancers had the highest ICI clinical benefit rate (73% versus 33% for non-cutaneous squamous cancers (p=0.008)).
The importance of TMB has been widely associated with solid tumors, but it is critical to keep in mind that the total number of mutations per coding area of genome is substantially different among tumor types (as well as among individuals within a tumor type). Hematologic cancers and astrocytoma are characterized by a low number of alterations. (Chalmers et al., 2017; Schumacher and Schreiber, 2015) However, TMB-H (≥20 mutations/Mb) may also be found in 2% of hematologic malignancies (Galanina et al., 2018a). TMB for myeloid neoplasms is generally lower than for lymphoid malignancies. Still, diffuse large B cell lymphoma (DLBCL) presents a median TMB of 10 mutations/Mb and 18.4% of cases present with TMB-H.(Chalmers et al., 2017) Although the only regulatory approval of ICI in hematological malignancies was obtained for Hodgkin lymphoma (whose hallmark is PD-L1 [CD274] amplification, another marker for ICI response (Goodman et al., 2018)), DLBCL is known to be responsive to ICIs (Hude et al., 2017) (RR, 41%)(Zinzani et al., 2017) It is plausible that TMB-H may be driving some of these responses in DLBCL. Although most studies suggest that higher TMB correlates with better outcomes after ICIs, some of the studies are limited because they are retrospective or no survival advantage is shown. Additional prospective studies with a variety of solid and hematological malignancies are needed to enhance our understanding of TMB as a tissue-agnostic biomarker.
Low TMB does not preclude responses to ICI
ICI can be effective, even in the low TMB settings, albeit in small percentages (~5%) of patients.(Goodman et al., 2017) For example, Kaposi sarcoma is a viral-related malignancy that is responsive to ICI. Indeed, six of nine patients (67%) who received anti PD-1 monotherapy achieved a complete or partial remission; all Kaposi lesions had TMB-Low and were PD-L1 negative.(Galanina et al., 2018b) Merkel cell carcinoma is another interesting example, for which ICI RRs in advanced disease are approximately 56%. Merkel cell carcinoma can be associated with a UV signature and TBM-H or with Merkel cell polyomavirus infection and TMB-Low. Responses were observed among both virus-positive and negative tumors.(Nghiem et al., 2016) It is plausible that those Merkel cell tumors with UV signature have a TMB-H leading to response, and those with Merkel cell polyomavirus have TMB-Low, but the viral antigens themselves are immunogenic.
Role of TMB as a prognostic marker in immunotherapy-naïve patients
It is plausible that higher TMB could act as a prognostic factor for better outcome, regardless of treatment type.(Ballman, 2015). In 5,371 patients with advanced cancers that never received ICI, there was no association between higher TMB (MSK-IMPACT assay) and improved survival (HR=1.12, P=0.11).(Samstein et al., 2019) In contrast, our series with 1,415 ICI-naïve patients with advanced cancers demonstrated that TMB-H is strongly associated with longer survival (Riviere et al., 2020). An important difference among these series is that the latter classified TMB-H as ≥20 mutations/Mb while the MSKCC series considered TMB-H as the top 20% for each histology. The prognostic impact of TMB-H is important because it could confound predictive attribution of increased survival after ICIs.
TMB as part of a composite biomarker to predict ICI outcome
The development of accurate predictors of ICI response will require an in-depth understanding of the complexity of immune response and resistance, and integration of multiple variables into a composite biomarker that may include TMB, expression of PD-L1, PD-1 and other checkpoints and considers the cells on which they are expressed, tumor molecular signature, neoantigen immunogenicity, the ability of the host MHC to present neo-antigens produced by the cancer mutanome, specific genes associated with ICI response or resistance, immune infiltration, microbiome, and TCR repertoire.(Anagnostou et al., 2019; Boichard et al., 2019; Boichard et al., 2017; Goodman et al., 2020; Havel et al., 2019; Hwang et al., 2020; Kato et al., 2020; Kumagai et al., 2020) A conceptual model for this integration was proposed as the “cancer immunogram”, which accounts for the several players that fit into a model to predict responses to immunotherapies. (Blank et al., 2016). One of the seven-parameter classes of cancer immunogram is tumor foreignness. Although TMB has its limitations for defining quality of neoantigens, it can be considered a proxy for foreignness. The other parameters of the cancer immunogram would include biomarkers related to immune infiltration and metabolism, absence of inhibitors, checkpoint status, and tumor sensitivity to immune effectors. One of the key aspects of this model is the assumption that cancer immunograms are not static, but evolve with the disease. Hence, in a composite platform, biomarkers could be added or removed with the evolution of disease
From a practical perspective, a recent meta-analysis suggested that multiplex immunohistochemistry has better accuracy in predicting responses to ICIs, compared to PD-L1 and TMB used in isolation. Integration of a multimodality cross-platform also significantly increased accuracy in this analysis.(Lu et al., 2019) As suggested by Ott et al, TMB integration into a combined biomarker analysis should preferentially include non-overlapping biomarkers, such as inflammatory markers (PD-L1 or T-cell gene expression profile).(Ott et al., 2019) As discussed here, it would make a lot of sense to integrate TMB into models including HLA genotype and TCR clonality, but published clinical studies testing these models are currently absent. Finally, one of the additional challenges of integration of biomarkers is avoiding excessively narrowing the group of patients that are candidates for immunotherapy.
Conclusions
Multiple lines of evidence suggest that higher TMB predicts better outcome after ICI therapy, albeit imperfectly. Although the FDA has recently granted tissue-agnostic accelerated approval for the anti-PD1 pembrolizumab in TMB ≥10 mutations/Mb solid tumors (FDA, 2020) and blood tests to assess TMB are being developed, there are still many challenges for the further development of TMB as a clinical biomarker. For instance, the predictive value of TMB for combinations of immunotherapies with targeted agents or chemotherapy is not established. Furthermore, it is critical to recognize that a subset (~5%) of patients with low TMB can respond well to ICIs and that >50% of patients with TMB-H do not respond. Reflecting immune system complexity, multiple other variables will need to be incorporated into a composite biomarker in order to make prediction of ICI outcome more accurate and fully unlock the potential benefit of immunotherapy. Moreover, broad adoption of individualized in-depth immune profiling will be necessary in order to tailor immunotherapy treatment strategies on a patient-by-patient basis. Finally, prospective randomized trials are required to establish the role of TMB and other ICI biomarkers in a variety of clinical settings.
Acknowledgements:
Funded in part by National Cancer Institute grant P30 CA023100 and the Joan and Irwin Jacobs Fund philanthropic fund. The funding source had no role in any aspect of the study.
Footnotes
Declaration of Interests
Dr. Jardim receives speaker fees from Roche, Janssen, Astellas, MSD, Bristol-Myers Squibb and Libbs, as well as consultant fees from Janssen, Bristol-Myers Squibb and Libbs.
Dr Goodman receives consult for EUSA pharma and Seattle Genetics
Dr. Gagliato receives speaker fees from Roche, Astra Zeneca, United and Libbs, as well as consultant fees from Libbs.
Dr. Kurzrock receives research funding from Genentech, Merck Serono, Pfizer, Boehringer Ingelheim, TopAlliance, Takeda, Incyte, Debiopharm, Medimmune, Sequenom, Foundation Medicine, Konica Minolta, Grifols, Omniseq, and Guardant, as well as consultant and/or speaker fees and/or advisory board for X-Biotech, Neomed, Pfizer, Actuate Therapeutics, and Roche, has an equity interest in IDbyDNA and CureMatch Inc, serves on the Board of CureMatch and CureMetrix, and is a co-founder of CureMatch.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aggarwal C, Thompson JC, Chien AL, Quinn KJ, Hwang WT, Black TA, Yee SS, Christensen TE, LaRiviere MJ, Silva BA, et al. (2020). Baseline Plasma Tumor Mutation Burden Predicts Response to Pembrolizumab-based Therapy in Patients with Metastatic Non-Small Cell Lung Cancer. Clinical cancer research : an official journal of the American Association for Cancer Research 26, 2354–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandrov LB, Ju YS, Haase K, Van Loo P, Martincorena I, Nik-Zainal S, Totoki Y, Fujimoto A, Nakagawa H, Shibata T., et al. (2016). Mutational signatures associated with tobacco smoking in human cancer. Science 354, 618–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, Bignell GR, Bolli N, Borg A, Borresen-Dale AL, et al. (2013). Signatures of mutational processes in human cancer. Nature 500, 415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostou V, Forde PM, White JR, Niknafs N, Hruban C, Naidoo J, Marrone K, Sivakumar IKA, Bruhm DC, Rosner S, et al. (2019). Dynamics of Tumor and Immune Responses during Immune Checkpoint Blockade in Non-Small Cell Lung Cancer. Cancer Res 79, 1214–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, Schuster SJ, Millenson MM, Cattry D, Freeman GJ, et al. (2015). PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. The New England journal of medicine 372, 311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balar AV, Galsky MD, Rosenberg JE, Powles T, Petrylak DP, Bellmunt J, Loriot Y, Necchi A, Hoffman-Censits J, Perez-Gracia JL, et al. (2017). Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet 389, 67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballman KV (2015). Biomarker: Predictive or Prognostic? Journal of clinical oncology : official journal of the American Society of Clinical Oncology 33, 3968–3971. [DOI] [PubMed] [Google Scholar]
- Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, Vogelzang NJ, Climent MA, Petrylak DP, Choueiri TK, et al. (2017). Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. The New England journal of medicine 376, 1015–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank CU, Haanen JB, Ribas A, and Schumacher TN (2016). CANCER IMMUNOLOGY. The "cancer immunogram". Science 352, 658–660. [DOI] [PubMed] [Google Scholar]
- Boichard A, Pham TV, Yeerna H, Goodman A, Tamayo P, Lippman S, Frampton GM, Tsigelny IF, and Kurzrock R (2019). APOBEC-related mutagenesis and neo-peptide hydrophobicity: implications for response to immunotherapy. Oncoimmunology 8, 1550341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boichard A, Tsigelny IF, and Kurzrock R (2017). High expression of PD-1 ligands is associated with kataegis mutational signature and APOBEC3 alterations. Oncoimmunology 6, e1284719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneville R, Krook MA, Kautto EA, Miya J, Wing MR, Chen HZ, Reeser JW, Yu L, and Roychowdhury S (2017). Landscape of Microsatellite Instability Across 39 Cancer Types. JCO Precis Oncol 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonta I, Isac JF, Meiri E, Bonta D, and Rich P (2017). Correlation between tumor mutation burden and response to immunotherapy. Journal of Clinical Oncology 35, e14579–e14579. [Google Scholar]
- Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E., et al. (2015). Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. The New England journal of medicine 373, 1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, et al. (2015). Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. The New England journal of medicine 373, 123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budczies J, Seidel A, Christopoulos P, Endris V, Kloor M, Gyorffy B, Seliger B, Schirmacher P, Stenzinger A, and Denkert C (2018). Integrated analysis of the immunological and genetic status in and across cancer types: impact of mutational signatures beyond tumor mutational burden. Oncoimmunology 7, e1526613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns MB, Temiz NA, and Harris RS (2013). Evidence for APOBEC3B mutagenesis in multiple human cancers. Nature genetics 45, 977–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttner R, Longshore JW, Lopez-Rios F, Merkelbach-Bruse S, Normanno N, Rouleau E, and Penault-Llorca F (2019). Implementing TMB measurement in clinical practice: considerations on assay requirements. ESMO open 4, e000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campesato LF, Barroso-Sousa R, Jimenez L, Correa BR, Sabbaga J, Hoff PM, Reis LF, Galante PA, and Camargo AA (2015). Comprehensive cancer-gene panels can be used to estimate mutational load and predict clinical benefit to PD-1 blockade in clinical practice. Oncotarget 6, 34221–34227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone DP, Reck M, Paz-Ares L, Creelan B, Horn L, Steins M, Felip E, van den Heuvel MM, Ciuleanu TE, Badin F , et al. (2017). First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. The New England journal of medicine 376, 2415–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabanon RM, Pedrero M, Lefebvre C, Marabelle A, Soria JC, and Postel-Vinay S (2016). Mutational Landscape and Sensitivity to Immune Checkpoint Blockers. Clinical cancer research : an official journal of the American Association for Cancer Research 22, 4309–4321. [DOI] [PubMed] [Google Scholar]
- Chae YK, Anker JF, Oh MS, Bais P, Namburi S, Agte S, Giles FJ, and Chuang JH (2019). Mutations in DNA repair genes are associated with increased neoantigen burden and a distinct immunophenotype in lung squamous cell carcinoma. Scientific reports 9, 3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R, Schrock A, Campbell B, Shlien A, Chmielecki J, et al. (2017). Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome medicine 9, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champiat S, Dercle L, Ammari S, Massard C, Hollebecque A, Postel-Vinay S, Chaput N, Eggermont A, Marabelle A, Soria JC, et al. (2017). Hyperprogressive Disease Is a New Pattern of Progression in Cancer Patients Treated by Anti-PD-1/PD-L1. Clinical cancer research : an official journal of the American Association for Cancer Research 23, 1920–1928. [DOI] [PubMed] [Google Scholar]
- Chan TA, Yarchoan M, Jaffee E, Swanton C, Quezada SA, Stenzinger A, and Peters S (2019). Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. Annals of oncology : official journal of the European Society for Medical Oncology 30, 44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Su C, Ren S, Zhou C, and Jiang T (2020). Pan-cancer analysis of KEAP1 mutations as biomarkers for immunotherapy outcomes. Annals of translational medicine 8, 141–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng DT, Mitchell TN, Zehir A, Shah RH, Benayed R, Syed A, Chandramohan R, Liu ZY, Won HH, Scott SN, et al. (2015). Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A Hybridization Capture-Based Next-Generation Sequencing Clinical Assay for Solid Tumor Molecular Oncology. The Journal of molecular diagnostics : JMD 17, 251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowell D, Morris LGT, Grigg CM, Weber JK, Samstein RM, Makarov V, Kuo F, Kendall SM, Requena D, Riaz N., et al. (2018). Patient HLA class I genotype influences cancer response to checkpoint blockade immunotherapy. Science 359, 582–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristescu R, Mogg R, Ayers M, Albright A, Murphy E, Yearley J, Sher X, Liu X, Nebozhyn M, Lunceford JK, et al. (2017). Mutational load (ML) and T-cell-inflamed microenvironment as predictors of response to pembrolizumab. Journal of Clinical Oncology 35, 1–1.28034063 [Google Scholar]
- Cristescu R, Mogg R, Ayers M, Albright A, Murphy E, Yearley J, Sher X, Liu XQ, Lu H, Nebozhyn M, et al. (2018). Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science 362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis AA, Chae YK, Agte S, Pan A, Simon NI, Taxter TJ, Behdad A, Carneiro BA, Cristofanilli M, and Giles FJ (2017). Comparison of tumor mutational burden (TMB) across tumor tissue and circulating tumor DNA (ctDNA). Journal of Clinical Oncology 35, e23028–e23028. [Google Scholar]
- Fakih M, Sandhu JS, Ouyang C, Sokol E., Ross SR, Miller VA, Lim D, Chao J, Catenacci DVT, Cho MT, et al. (2019). Tumor mutational burden (TMB) may be a promising predictive biomarker of response to PD-1/PD-L1 targeting in MSI-H colorectal cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 37, abstr 43 [DOI] [PubMed] [Google Scholar]
- Fancello L, Gandini S, Pelicci PG, and Mazzarella L (2019). Tumor mutational burden quantification from targeted gene panels: major advancements and challenges. Journal for immunotherapy of cancer 7, 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA (2020). FDA approves pembrolizumab for adults and children with TMB-H solid tumors News release. (FDA; ). [Google Scholar]
- Frampton GM, Fichtenholtz A, Otto GA, Wang K, Downing SR, He J, Schnall-Levin M, White J, Sanford EM, An P, et al. (2013). Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nature biotechnology 31, 1023–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanina N, Bejar R, Choi M, Goodman A, Wieduwilt M, Mulroney C, Kim L, Yeerna H, Tamayo P, Vergilio JA, et al. (2018a). Comprehensive Genomic Profiling Reveals Diverse but Actionable Molecular Portfolios across Hematologic Malignancies: Implications for Next Generation Clinical Trials. Cancers 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanina N, Goodman AM, Cohen PR, Frampton GM, and Kurzrock R (2018b). Successful Treatment of HIV-Associated Kaposi Sarcoma with Immune Checkpoint Blockade. Cancer immunology research 6, 1129–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galsky MD, Saci A, Horak C, Szabo PM, Azrilevich A, Lambert A, Siefker-Radtke A, Necchi A, and Sharma P (2017a). Impact of zumor mutation burden on nivolumab efficacy in second-line urothelial carcinoma patients: Exploratory analysis of the phase ii checkmate 275 study. Annals of Oncology 28, 0923–7534. [Google Scholar]
- Galsky MD, Saci A, Szabo PM, Azrilevich A, Horak C, Lambert A, Siefker-Radtke A, Necchi A, and Sharma P (2017b). Impact of tumormutation burden on nivolumab efficacy in secondline urothelial carcinoma patients: exploratory analysis of the phase II checkmate 275 study. Annals of Oncology 28, v295–v329. [Google Scholar]
- Gandara DR, Paul SM, Kowanetz M, Schleifman E, Zou W, Li Y, Rittmeyer A, Fehrenbacher L, Otto G, Malboeuf C, et al. (2018). Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nature medicine 24, 1441–1448. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Ericsson PI, Stovgaard ES, Sua LF, Reisenbichler E, Kos Z, Carter JM, Michiels S, Le Quesne J, Nielsen TO, Laenkholm AV, et al. (2020). The path to a better biomarker: application of a risk management framework for the implementation of PD-L1 and TILs as immuno-oncology biomarkers in breast cancer clinical trials and daily practice. The Journal of pathology 250, 667–684. [DOI] [PubMed] [Google Scholar]
- Goodman AM, Castro A, Pyke RM, Okamura R, Kato S, Riviere P, Frampton G, Sokol E, Zhang X, Ball ED, et al. (2020). MHC-I genotype and tumor mutational burden predict response to immunotherapy. Genome medicine 12, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman AM, Kato S, Bazhenova L, Patel SP, Frampton GM, Miller V, Stephens PJ, Daniels GA, and Kurzrock R (2017). Tumor Mutational Burden as an Independent Predictor of Response to Immunotherapy in Diverse Cancers. Molecular cancer therapeutics 16, 2598–2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman AM, Kato S, Chattopadhyay R, Okamura R, Saunders IM, Montesion M, Frampton GM, Miller VA, Daniels GA, and Kurzrock R (2019a). Phenotypic and Genomic Determinants of Immunotherapy Response Associated with Squamousness. Cancer immunology research 7, 866–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman AM, Piccioni D, Kato S, Boichard A, Wang HY, Frampton G, Lippman SM, Connelly C, Fabrizio D, Miller V, et al. (2018). Prevalence of PDL1 Amplification and Preliminary Response to Immune Checkpoint Blockade in Solid Tumors. JAMA oncology 4, 1237–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman AM, Sokol ES, Frampton GM, Lippman SM, and Kurzrock R (2019b). Microsatellite-Stable Tumors with High Mutational Burden Benefit from Immunotherapy. Cancer immunology research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havel JJ, Chowell D, and Chan TA (2019). The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nature reviews Cancer 19, 133–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmann MD, Callahan MK, Awad MM, Calvo E, Ascierto PA, Atmaca A, Rizvi NA, Hirsch FR, Selvaggi G, Szustakowski JD, et al. (2018a). Tumor Mutational Burden and Efficacy of Nivolumab Monotherapy and in Combination with Ipilimumab in Small-Cell Lung Cancer. Cancer cell 33, 853–861 e854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmann MD, Ciuleanu TE, Pluzanski A, Lee JS, Otterson GA, Audigier-Valette C, Minenza E, Linardou H, Burgers S, Salman P, et al. (2018b). Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. The New England journal of medicine 378, 2093–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmann MD, Nathanson T, Rizvi H, Creelan BC, Sanchez-Vega F, Ahuja A, Ni A, Novik JB, Mangarin LMB, Abu-Akeel M, et al. (2018c). Genomic Features of Response to Combination Immunotherapy in Patients with Advanced Non-Small-Cell Lung Cancer. Cancer cell 33, 843–852 e844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. (2010). Improved survival with ipilimumab in patients with metastatic melanoma. The New England journal of medicine 363, 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan SA, Courtier A, Cheng PF, Jaberg-Bentele NF, Goldinger SM, Manuel M, Perez S, Plantier N, Mouret JF, Nguyen-Kim TDL, et al. (2019). Peripheral Blood TCR Repertoire Profiling May Facilitate Patient Stratification for Immunotherapy against Melanoma. Cancer immunology research 7, 77–85. [DOI] [PubMed] [Google Scholar]
- Hopkins AC, Yarchoan M, Durham JN, Yusko EC, Rytlewski JA, Robins HS, Laheru DA, Le DT, Lutz ER, and Jaffee EM (2018). T cell receptor repertoire features associated with survival in immunotherapy-treated pancreatic ductal adenocarcinoma. JCI insight 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoi A, Takeda K, Nagaoka K, Iino T, Matsushita H, Ueha S, Aoki S, Matsushima K, Kubo M, Morikawa T, et al. (2018). Increased diversity with reduced "diversity evenness" of tumor infiltrating T-cells for the successful cancer immunotherapy. Scientific reports 8, 1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hude I, Sasse S, Engert A, and Brockelmann PJ (2017). The emerging role of immune checkpoint inhibition in malignant lymphoma. Haematologica 102, 30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugo W, Zaretsky JM, Sun L, Song C, Moreno BH, Hu-Lieskovan S, Berent-Maoz B, Pang J, Chmielowski B, Cherry G, et al. (2016). Genomic and Transcriptomic Features of Response to Anti-PD-1 Therapy in Metastatic Melanoma. Cell 165, 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S, Kwon AY, Jeong JY, Kim S, Kang H, Park J, Kim JH, Han OJ, Lim SM, and An HJ (2020). Immune gene signatures for predicting durable clinical benefit of anti-PD-1 immunotherapy in patients with non-small cell lung cancer. Scientific reports 10, 643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda S, Goodman AM, Cohen PR, Jensen TJ, Ellison CK, Frampton G, Miller V, Patel SP, and Kurzrock R (2016). Metastatic basal cell carcinoma with amplification of PD-L1: exceptional response to anti-PD1 therapy. NPJ genomic medicine 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardim DL, de Melo Gagliato D, Giles FJ, and Kurzrock R (2018). Analysis of Drug Development Paradigms for Immune Checkpoint Inhibitors. Clinical cancer research : an official journal of the American Association for Cancer Research 24, 1785–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DB, Frampton GM, Rioth MJ, Yusko E, Xu Y, Guo X, Ennis RC, Fabrizio D, Chalmers ZR, Greenbowe J, et al. (2016). Targeted Next Generation Sequencing Identifies Markers of Response to PD-1 Blockade. Cancer immunology research 4, 959–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanjanapan Y, Day D, Wang L, Al-Sawaihey H, Abbas E, Namini A, Siu LL, Hansen A, Razak AA, Spreafico A, et al. (2019). Hyperprogressive disease in early-phase immunotherapy trials: Clinical predictors and association with immune-related toxicities. Cancer. [DOI] [PubMed] [Google Scholar]
- Kato S, Goodman A, Walavalkar V, Barkauskas DA, Sharabi A, and Kurzrock R (2017). Hyperprogressors after Immunotherapy: Analysis of Genomic Alterations Associated with Accelerated Growth Rate. Clinical cancer research : an official journal of the American Association for Cancer Research 23, 4242–4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato S, Okamura R, Kumaki Y, Ikeda S, Nikanjam M, Eskander R, Goodman A, Lee S, Glenn ST, Dressman D, et al. (2020). Expression of TIM3/VISTA checkpoints and the CD68 macrophage-associated marker correlates with anti-PD1/PDL1 resistance: implications of immunogram heterogeneity. Oncoimmunology 9, 1708065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khagi Y, Goodman AM, Daniels GA, Patel SP, Sacco AG, Randall JM, Bazhenova LA, and Kurzrock R (2017). Hypermutated Circulating Tumor DNA: Correlation with Response to Checkpoint Inhibitor-Based Immunotherapy. Clinical cancer research : an official journal of the American Association for Cancer Research 23, 5729–5736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim ES, Velcheti V, Mekhail T, Leal TA, Dowell JE, Tsai ML, Dakhil CSR, Stella P, Shen V, Hu S, et al. (2018). Primary efficacy results from B-F1RST, a prospective Phase II trial evaluating blood-based tumour mutational burden (bTMB) as a predictive biomarker for atezolizumab (atezo) in 1L non-small cell lung cancer (NSCLC). Annals of oncology : official journal of the European Society for Medical Oncology 29, 0923–7534. [Google Scholar]
- Kowanetz M, Zou W, Shames D, Cummings C, Rizvi N, Spira A, Frampton G, Leveque V, Flynn S, Mocci S, et al. (2017). Tumor Mutation Burden (TMB) is Associated with Improved Efficacy of Atezolizumab in 1L and 2L+ NSCLC Patients. Journal of Thoracic Oncology 12, S321–S322. [Google Scholar]
- Kucab JE, Zou X, Morganella S, Joel M, Nanda AS, Nagy E, Gomez C, Degasperi A, Harris R, Jackson SP, et al. (2019). A Compendium of Mutational Signatures of Environmental Agents. Cell 177, 821–836 e816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai S, Togashi Y, Kamada T, Sugiyama E, Nishinakamura H, Takeuchi Y, Vitaly K, Itahashi K, Maeda Y, Matsui S, et al. (2020). The PD-1 expression balance between effector and regulatory T cells predicts the clinical efficacy of PD-1 blockade therapies. Nature Immunology. [DOI] [PubMed] [Google Scholar]
- Lauss M, Donia M, Harbst K, Andersen R, Mitra S, Rosengren F, Salim M, Vallon-Christersson J, Torngren T, Kvist A, et al. (2017). Mutational and putative neoantigen load predict clinical benefit of adoptive T cell therapy in melanoma. Nature communications 8, 1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, et al. (2017). Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 357, 409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C-H, Natale RGD, Chowell D, Makarov V, Redzematovic A, Murray SJ, Carlo MI, Voss MH, Feldman DR, Motzer RJ, et al. (2019). Genomic biomarkers of response to nivolumab/ipilimumab (nivo/ipi) and nivolumab (nivo) monotherapy in 108 patients with advanced renal cell carcinoma. Journal of Clinical Oncology 37, 641–641. [Google Scholar]
- Linnemann C, Mezzadra R, and Schumacher TN (2014). TCR repertoires of intratumoral T-cell subsets. Immunological reviews 257, 72–82. [DOI] [PubMed] [Google Scholar]
- Liu C, Zheng S, Jin R, Wang X, Wang F, Zang R, Xu H, Lu Z, Huang J, Lei Y, et al. (2020a). The superior efficacy of anti-PD-1/PD-L1 immunotherapy in KRAS-mutant non-small cell lung cancer that correlates with an inflammatory phenotype and increased immunogenicity. Cancer letters 470, 95–105. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zugazagoitia J, Ahmed FS, Henick BS, Gettinger SN, Herbst RS, Schalper KA, and Rimm DL (2020b). Immune Cell PD-L1 Colocalizes with Macrophages and Is Associated with Outcome in PD-1 Pathway Blockade Therapy. Clinical cancer research : an official journal of the American Association for Cancer Research 26, 970–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S, Stein JE, Rimm DL, Wang DW, Bell JM, Johnson DB, Sosman JA, Schalper KA, Anders RA, Wang H, et al. (2019). Comparison of Biomarker Modalities for Predicting Response to PD-1/PD-L1 Checkpoint Blockade: A Systematic Review and Meta-analysis. JAMA oncology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luksza M, Riaz N, Makarov V, Balachandran VP, Hellmann MD, Solovyov A, Rizvi NA, Merghoub T, Levine AJ, Chan TA, et al. (2017). A neoantigen fitness model predicts tumour response to checkpoint blockade immunotherapy. Nature 551, 517–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mage MG, Dolan MA, Wang R, Boyd LF, Revilleza MJ, Robinson H, Natarajan K, Myers NB, Hansen TH, and Margulies DH (2012). The peptide-receptive transition state of MHC class I molecules: insight from structure and molecular dynamics. Journal of immunology 189, 1391–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marabelle A, Fakih M, Lopez J, Shah M, shapira-frommer R, Nakagawa K, Chung H, Kindler H, Lopez-Martin J, Miller W, et al. (2019). Association of tumour mutational burden with outcomes in patients with select advanced solid tumours treated with pembrolizumab in KEYNOTE-158. Annals of Oncology Supplement 30, v477–v478. [DOI] [PubMed] [Google Scholar]
- Marcus L, Lemery SJ, Keegan P, and Pazdur R (2019). FDA Approval Summary: Pembrolizumab for the Treatment of Microsatellite Instability-High Solid Tumors. Clinical cancer research : an official journal of the American Association for Cancer Research 25, 3753–3758. [DOI] [PubMed] [Google Scholar]
- Mardis ER (2019). Neoantigens and genome instability: impact on immunogenomic phenotypes and immunotherapy response. Genome medicine 11, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty Pyke R, Thompson WK, Salem RM, Font-Burgada J, Zanetti M, and Carter H (2018a). Evolutionary Pressure against MHC Class II Binding Cancer Mutations. Cell 175, 1991. [DOI] [PubMed] [Google Scholar]
- Marty Pyke R, Thompson WK, Salem RM, Font-Burgada J, Zanetti M, and Carter H (2018b). Evolutionary Pressure against MHC Class II Binding Cancer Mutations. Cell 175, 416–428 e413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty R, Kaabinejadian S, Rossell D, Slifker MJ, van de Haar J, Engin HB, de Prisco N, Ideker T, Hildebrand WH, Font-Burgada J, et al. (2017). MHC-I Genotype Restricts the Oncogenic Mutational Landscape. Cell 171, 1272–1283 e1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita H, Vesely MD, Koboldt DC, Rickert CG, Uppaluri R, Magrini VJ, Arthur CD, White JM, Chen YS, Shea LK, et al. (2012). Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature 482, 400–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiri E, Garrett-Mayer E, Halabi S, Mangat PK, Shrestha S, Ahn ER, Osayameh O, Perla V, and Schilsky RL (2020). Pembrolizumab (P) in patients (Pts) with colorectal cancer (CRC) with high tumor mutational burden (HTMB): Results from the Targeted Agent and Profiling Utilization Registry (TAPUR) Study. Journal of Clinical Oncology 38, 133–133. [Google Scholar]
- Miao D, Margolis CA, Gao W, Voss MH, Li W, Martini DJ, Norton C, Bosse D, Wankowicz SM, Cullen D, et al. (2018). Genomic correlates of response to immune checkpoint therapies in clear cell renal cell carcinoma. Science 359, 801–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzer RJ, Rini BI, McDermott DF, Redman BG, Kuzel TM, Harrison MR, Vaishampayan UN, Drabkin HA, George S, Logan TF, et al. (2015). Nivolumab for Metastatic Renal Cell Carcinoma: Results of a Randomized Phase II Trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 33, 1430–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JN, Falk M, Talwar J, Neemann N, Mariotti E, Bertrand M, Zacherle T, Lakis S, Menon R, Gloeckner C., et al. (2017). Concordance between Comprehensive Cancer Genome Profiling in Plasma and Tumor Specimens. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 12, 1503–1511. [DOI] [PubMed] [Google Scholar]
- Nghiem PT, Bhatia S, Lipson EJ, Kudchadkar RR, Miller NJ, Annamalai L, Berry S, Chartash EK, Daud A, Fling SP, et al. (2016). PD-1 Blockade with Pembrolizumab in Advanced Merkel-Cell Carcinoma. The New England journal of medicine 374, 2542–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura R, Kato S, Lee S, Jimenez RE, Sicklick JK, and Kurzrock R (2020). ARID1A alterations function as a biomarker for longer progression-free survival after anti-PD-1/PD-L1 immunotherapy. Journal for immunotherapy of cancer 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott PA, Bang YJ, Piha-Paul SA, Razak ARA, Bennouna J, Soria JC, Rugo HS, Cohen RB, O'Neil BH, Mehnert JM, et al. (2019). T-Cell-Inflamed Gene-Expression Profile, Programmed Death Ligand 1 Expression, and Tumor Mutational Burden Predict Efficacy in Patients Treated With Pembrolizumab Across 20 Cancers: KEYNOTE-028. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 37, 318–327. [DOI] [PubMed] [Google Scholar]
- Patel SP, and Kurzrock R (2015). PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Molecular cancer therapeutics 14, 847–856. [DOI] [PubMed] [Google Scholar]
- Paz-Ares L, Langer CJ, Novello S, Halmos B, Cheng Y, Gadgeel SM, Hui R, Sugawara S, Borghaei H, Cristescu R., et al. (2019). Pembrolizumab (pembro) plus platinum-based chemotherapy (chemo) for metastatic NSCLC: Tissue TMB (tTMB) and outcomes in KEYNOTE-021, 189, and 407. Annals of Oncology 30, 0923–7534. [Google Scholar]
- Peng D, Kryczek I, Nagarsheth N, Zhao L, Wei S, Wang W, Sun Y, Zhao E, Vatan L, Szeliga W, et al. (2015). Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy. Nature 527, 249–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters S, Cho B, et, a., and Rizvi N (2019). Tumor mutational burden (TMB) as a biomarker of survival in metastatic non-small cell lung cancer (mNSCLC): Blood and tissue TMB analysis from MYSTIC, a Phase III study of first-line durvalumab ± tremelimumab vs chemotherapy. Paper presented at: AACR Annual Meeting. [Google Scholar]
- Pham TV, Boichard A, Goodman A, Riviere P, Yeerna H, Tamayo P, and Kurzrock R (2020). Role of ultraviolet mutational signature versus tumor mutation burden in predicting response to immunotherapy. Mol Oncol 14, 1680–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postow MA, and Hellmann MD (2018). Adverse Events Associated with Immune Checkpoint Blockade. The New England journal of medicine 378, 1165. [DOI] [PubMed] [Google Scholar]
- Powles T, Loriot Y, Ravaud A, Vogelzang NJ, Duran I, Retz M, Giorgi UD, Oudard S, Bamias A, Koeppen H, et al. (2018). Atezolizumab (atezo) vs. chemotherapy (chemo) in platinum-treated locally advanced or metastatic urothelial carcinoma (mUC): Immune biomarkers, tumor mutational burden (TMB), and clinical outcomes from the phase III IMvigor211 study. Journal of Clinical Oncology 36, 409–409. [Google Scholar]
- Ready N, Hellmann MD, Awad MM, Otterson GA, Gutierrez M, Gainor JF, Borghaei H, Jolivet J, Horn L, Mates M, et al. (2019). First-Line Nivolumab Plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer (CheckMate 568): Outcomes by Programmed Death Ligand 1 and Tumor Mutational Burden as Biomarkers. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 37, 992–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuben A, Zhang J, Lin HY, Little L, Gumbs C, Tran HT, Wang L, Haymaker CL, Mehran RJ, Rice DC, et al. (2019). T cell repertoire analysis of non-small cell lung cancer patients treated with neoadjuvant nivolumab alone or in combination with ipilimumab (NEOSTAR trial). Journal of Clinical Oncology 37, 8532–8532. [Google Scholar]
- Riaz N, Havel JJ, Kendall SM, Makarov V, Walsh LA, Desrichard A, Weinhold N, and Chan TA (2016). Recurrent SERPINB3 and SERPINB4 mutations in patients who respond to anti-CTLA4 immunotherapy. Nature genetics 48, 1327–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riaz N, Havel JJ, Makarov V, Desrichard A, Urba WJ, Sims JS, Hodi FS, Martin-Algarra S, Mandal R, Sharfman WH, et al. (2017). Tumor and Microenvironment Evolution during Immunotherapy with Nivolumab. Cell 171, 934–949 e916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riviere P, Goodman A, Okamura R, Barkauskas DA, Whitchurch T, Lee S, Khalid N, Collier R, Mareboina M, Frampton G, et al. (2020). High Tumor Mutational Burden Correlates with Longer Survival in Immunotherapy-Naïve Patients with Diverse Cancers . Molecular cancer therapeutics in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi H, Sanchez-Vega F, La K, Chatila W, Jonsson P, Halpenny D, Plodkowski A, Long N, Sauter JL, Rekhtman N, et al. (2018). Molecular Determinants of Response to Anti-Programmed Cell Death (PD)-1 and Anti-Programmed Death-Ligand 1 (PD-L1) Blockade in Patients With Non-Small-Cell Lung Cancer Profiled With Targeted Next-Generation Sequencing. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 36, 633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi NA, Cho BC, Reinmuth N, Lee KH, Luft A, Ahn MJ, van den Heuvel MM, Cobo M, Vicente D, Smolin A, et al. (2020). Durvalumab With or Without Tremelimumab vs Standard Chemotherapy in First-line Treatment of Metastatic Non-Small Cell Lung Cancer: The MYSTIC Phase 3 Randomized Clinical Trial. JAMA oncology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, et al. (2015). Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348, 124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E, et al. (2015). Nivolumab in previously untreated melanoma without BRAF mutation. The New England journal of medicine 372, 320–330. [DOI] [PubMed] [Google Scholar]
- Rodig SJ, Gusenleitner D, Jackson DG, Gjini E, Giobbie-Hurder A, Jin C, Chang H, Lovitch SB, Horak C, Weber JS, et al. (2018). MHC proteins confer differential sensitivity to CTLA-4 and PD-1 blockade in untreated metastatic melanoma. Science translational medicine 10. [DOI] [PubMed] [Google Scholar]
- Roemer MG, Advani RH, Ligon AH, Natkunam Y, Redd RA, Homer H, Connelly CF, Sun HH, Daadi SE, Freeman GJ, et al. (2016). PD-L1 and PD-L2 Genetic Alterations Define Classical Hodgkin Lymphoma and Predict Outcome. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 34, 2690–2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romualdo Barroso-Sousa EJ, Dewey Kim, Ann H. Partridge, Cohen Ofir, Nikhil Wagle (2018). Determinants of high tumor mutational burden (TMB) and mutational signatures in breast cancer. In ASCO Annual Meeting (Chicago, EUA). [Google Scholar]
- Rooney MS, Shukla SA, Wu CJ, Getz G, and Hacohen N (2015). Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell 160, 48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszik J, Haydu LE, Hess KR, Oba J, Joon AY, Siroy AE, Karpinets TV, Stingo FC, Baladandayuthapani V, Tetzlaff MT, et al. (2016). Novel algorithmic approach predicts tumor mutation load and correlates with immunotherapy clinical outcomes using a defined gene mutation set. BMC medicine 14, 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samstein RM, Lee CH, Shoushtari AN, Hellmann MD, Shen R, Janjigian YY, Barron DA, Zehir A, Jordan EJ, Omuro A, et al. (2019). Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nature genetics 51, 202–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher TN, Scheper W, and Kvistborg P (2019). Cancer Neoantigens. Annual review of immunology 37, 173–200. [DOI] [PubMed] [Google Scholar]
- Schumacher TN, and Schreiber RD (2015). Neoantigens in cancer immunotherapy. Science 348, 69–74. [DOI] [PubMed] [Google Scholar]
- Seiwert TY, Burtness B, Mehra R, Weiss J, Berger R, Eder JP, Heath K, McClanahan T, Lunceford J, Gause C, et al. (2016). Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. The Lancet Oncology 17, 956–965. [DOI] [PubMed] [Google Scholar]
- Seiwert TY, Haddad R, Bauml J, Weiss J, Pfister DG, Gupta S, Mehra R, Gluck I, Kang H, Worden F, et al. (2018). Biomarkers predictive of response to pembrolizumab in head and neck cancer (HNSCC). Cancer Research 78, LB-339–LB-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P, Pachynski RK, Narayan V, Flechon A, Gravis G, Galsky MD, Mahammedi H, Patnaik A, Subudhi SK, Ciprotti M, et al. (2019). Initial results from a phase II study of nivolumab (NIVO) plus ipilimumab (IPI) for the treatment of metastatic castrationresistant prostate cancer (mCRPC; CheckMate 650). Journal of Clinical Oncology 37, 142–142. [Google Scholar]
- Shin DS, Zaretsky JM, Escuin-Ordinas H, Garcia-Diaz A, Hu-Lieskovan S, Kalbasi A, Grasso CS, Hugo W, Sandoval S, Torrejon DY, et al. (2017). Primary Resistance to PD-1 Blockade Mediated by JAK1/2 Mutations. Cancer discovery 7, 188–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shitara K, Ozguroglu M, Bang YJ, Di Bartolomeo M, Mandala M, Ryu MH, Fornaro L, Olesinski T, Caglevic C, Chung HC, et al. (2018). Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet 392, 123–133. [DOI] [PubMed] [Google Scholar]
- Siefker-Radtke AO, Apolo AB, Bivalacqua TJ, Spiess PE, and Black PC (2018). Immunotherapy with Checkpoint Blockade in the Treatment of Urothelial Carcinoma. The Journal of urology 199, 1129–1142. [DOI] [PubMed] [Google Scholar]
- Singal G, Miller PG, Agarwala V, Li G, Gossai A, Albacker LA, Goldberg ME, He J, Frank S, Bourque D, et al. (2017). Analyzing biomarkers of cancer immunotherapy (CIT) response using a real-world clinico-genomic database. Annals of Oncology 28, 0923–7534. [Google Scholar]
- Singavi AK, Menon S, Kilari D, Alqwasmi A, Ritch PS, Thomas JP, Martin AL, Oxencis C, Ali S, and George B (2017). Predictive biomarkers for hyper-progression (HP) in response to immune checkpoint inhibitors (ICI) – analysis of somatic alterations (SAs). Annals of Oncology 28, 0923–7534. [Google Scholar]
- Skoulidis F, Goldberg ME, Greenawalt DM, Hellmann MD, Awad MM, Gainor JF, Schrock AB, Hartmaier RJ, Trabucco SE, Gay L, et al. (2018). STK11/LKB1 Mutations and PD-1 Inhibitor Resistance in KRAS-Mutant Lung Adenocarcinoma. Cancer discovery 8, 822–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoulidis F, and Heymach JV (2019). Co-occurring genomic alterations in non-small-cell lung cancer biology and therapy. Nature reviews Cancer 19, 495–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS, et al. (2014). Genetic basis for clinical response to CTLA-4 blockade in melanoma. The New England journal of medicine 371, 2189–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder A, Nathanson T, Funt SA, Ahuja A, Buros Novik J, Hellmann MD, Chang E, Aksoy BA, Al-Ahmadie H, Yusko E, et al. (2017). Contribution of systemic and somatic factors to clinical response and resistance to PD-L1 blockade in urothelial cancer: An exploratory multi-omic analysis. PLoS medicine 14, e1002309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socinski M, Velcheti V, Mekhail T, Chae YK, Leal TA, Dowell JE, Tsai ML, Dakhil CS, Stella P, Shen V, et al. (2019). Final efficacy results from B-F1RST, a prospective phase II trial evaluating blood-based tumour mutational burden (bTMB) as a predictive biomarker for atezolizumab (atezo) in 1L non-small cell lung cancer (NSCLC). Annals of oncology : official journal of the European Society for Medical Oncology 30, V919–V920. [Google Scholar]
- Teo MY, Seier K, Ostrovnaya I, Regazzi AM, Kania BE, Moran MM, Cipolla CK, Bluth MJ, Chaim J, Al-Ahmadie H, et al. (2018). Alterations in DNA Damage Response and Repair Genes as Potential Marker of Clinical Benefit From PD-1/PD-L1 Blockade in Advanced Urothelial Cancers. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 36, 1685–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischkowitz M, Huang S, Banerjee S, Hague J, Hendricks WPD, Huntsman DG, Lang JD, Orlando KA, Oza AM, Pautier P, et al. (2020). Small-Cell Carcinoma of the Ovary, Hypercalcemic Type-Genetics, New Treatment Targets, and Current Management Guidelines. Clinical cancer research : an official journal of the American Association for Cancer Research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian SL, Taube JM, Anders RA, and Pardoll DM (2016). Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nature reviews Cancer 16, 275–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turajlic S, Litchfield K, Xu H, Rosenthal R, McGranahan N, Reading JL, Wong YNS, Rowan A, Kanu N, Al Bakir M, et al. (2017). Insertion-and-deletion-derived tumourspecific neoantigens and the immunogenic phenotype: a pan-cancer analysis. The Lancet Oncology 18, 1009–1021. [DOI] [PubMed] [Google Scholar]
- Vokes NI, Liu D, Ricciuti B, Jimenez-Aguilar E, Rizvi H, Dietlein F, He MX, Margolis CA, Elmarakeby HA, Girshman J, et al. (2019). Harmonization of Tumor Mutational Burden Quantification and Association With Response to Immune Checkpoint Blockade in Non–Small-Cell Lung Cancer. JCO Precision Oncology, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Gong J, Tu TY, Lee PP, and Fakih M (2018). Immune profiling of microsatellite instability-high and polymerase epsilon (POLE)-mutated metastatic colorectal tumors identifies predictors of response to anti-PD-1 therapy. Journal of gastrointestinal oncology 9, 404–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Duan J, Cai S, Han M, Dong H, Zhao J, Zhu B, Wang S, Zhuo M, Sun J, et al. (2019). Assessment of Blood Tumor Mutational Burden as a Potential Biomarker for Immunotherapy in Patients With Non-Small Cell Lung Cancer With Use of a Next-Generation Sequencing Cancer Gene Panel. JAMA oncology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J, Horak C, Sanders C, Woods D, Yusko E, Hodi FS, Chang H, Robins H, and Weber J (2016). Baseline tumor T cell receptor (TcR) sequencing analysis and neo antigen load is associated with benefit in melanoma patients receiving sequential nivolumab and ipilimumab. Annals of Oncology 27, 0923–7534. [Google Scholar]
- Wood MA, Weeder BR, David JK, Nellore A, and Thompson RF (2020). Burden of tumor mutations, neoepitopes, and other variants are weak predictors of cancer immunotherapy response and overall survival. Genome medicine 12, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YM, Cieslik M, Lonigro RJ, Vats P, Reimers MA, Cao X, Ning Y, Wang L, Kunju LP, de Sarkar N , et al. (2018). Inactivation of CDK12 Delineates a Distinct Immunogenic Class of Advanced Prostate Cancer. Cell 173, 1770–1782 e1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarchoan M, Albacker LA, Hopkins AC, Montesion M, Murugesan K, Vithayathil TT, Zaidi N, Azad NS, Laheru DA, Frampton GM, et al. (2019). PD-L1 expression and tumor mutational burden are independent biomarkers in most cancers. JCI insight 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarchoan M, Hopkins A, and Jaffee EM (2017). Tumor Mutational Burden and Response Rate to PD-1 Inhibition. The New England journal of medicine 377, 2500–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusko E, Vignali M, Wilson RK, Mardis ER, Hodi FS, Horak C, Chang H, Woods DM, Robins H, and Weber J (2019). Association of Tumor Microenvironment T-cell Repertoire and Mutational Load with Clinical Outcome after Sequential Checkpoint Blockade in Melanoma. Cancer immunology research 7, 458–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaretsky JM, Garcia-Diaz A, Shin DS, Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, Torrejon DY, Abril-Rodriguez G, Sandoval S, Barthly L, et al. (2016). Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. The New England journal of medicine 375, 819–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehir A, Benayed R, Shah RH, Syed A, Middha S, Kim HR, Srinivasan P, Gao J, Chakravarty D, Devlin SM, et al. (2017). Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nature medicine 23, 703–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinzani PL, Ribrag V, Moskowitz CH, Michot JM, Kuruvilla J, Balakumaran A, Zhang Y, Chlosta S, Shipp MA, and Armand P (2017). Safety and tolerability of pembrolizumab in patients with relapsed/refractory primary mediastinal large B-cell lymphoma. Blood 130, 267–270. [DOI] [PMC free article] [PubMed] [Google Scholar]