Abstract
Primary aldosteronism is an underdiagnosed cause of hypertension. Although inadequate screening is one reason for underdiagnosis, another important contributor is that clinicians may inappropriately exclude the diagnosis when screening aldosterone concentrations fall below traditionally established thresholds. We evaluated the intra-individual variability in screening aldosterone concentrations and aldosterone-to-renin ratios, and how this variability could impact case detection, among 51 patients with confirmed primary aldosteronism who had two or more screening measurements of renin and aldosterone on different days. There were a total of 137 screening measurements with a mean of 3 (range 2–6) per patient. The mean intra-individual variability, expressed as coefficients of variation, was 31% for aldosterone and 45% for the aldosterone-to-renin ratio. Aldosterone concentrations ranged from 4.9 to 51 ng/dL; 49% of patients had at least one aldosterone measurement below 15 ng/dL, 29% had at least two aldosterone measurements below 15 ng/dL, and 29% had at least one measurement below 10 ng/dL. Individual aldosterone-to-renin ratios ranged from 8.2 to 427 ng/dL per ng/mL/h; 57% had at least one ratio below 30 ng/dL per ng/mL/h, 27% had at least two ratios below 30 ng/dL per ng/mL/h, and 24% had at least one ratio below 20 ng/dL per ng/mL/h. Aldosterone concentrations and aldosterone-to-renin ratios are highly variable in patients with primary aldosteronism, with many screening values falling below conventionally accepted diagnostic thresholds. The diagnostic yield for primary aldosteronism may be substantially increased by re-calibrating the definition of a ‘positive screen’ to include more liberal thresholds for aldosterone and the aldosterone-to-renin ratio.
Keywords: Aldosterone, renin, primary aldosteronism, hypertension
Graphical Abstract

Introduction:
Primary aldosteronism is common but largely underdiagnosed. The prevalence ranges from 5–20% in patients with hypertension, and can exceed 30% in patients with resistant hypertension.1–11 Correct identification and treatment of primary aldosteronism is critical, as patients with this syndrome have excess cardiovascular morbidity and mortality compared to patients with essential hypertension, independent of blood pressure.12–16
One major reason for primary aldosteronism underdiagnosis is that less than 3% of patients who meet criteria for primary aldosteronism screening actually undergo testing for the disorder in the United States,17,18 and less than 7–8% of eligible patients are screened in Europe.19,20 However, beyond insufficient screening for primary aldosteronism, a second major contributor to underdiagnosis is the misinterpretation of screening tests, which leads to inappropriate exclusion of the diagnosis. Conventionally, the first step in making a diagnosis is to demonstrate a ‘positive screen’ based on the aldosterone-to-renin ratio (ARR). Commonly accepted guidelines define a positive screen for primary aldosteronism as an ARR of ≥ 30 ng/dL per ng/mL/h, with a suppressed renin, and plasma aldosterone concentration (PAC) of at least 15 ng/dL.21,22 However, these thresholds are the focus of considerable debate.
Plasma aldosterone concentrations exhibit substantial variability, both in normal subjects and in patients with primary aldosteronism, which can create misleading results for primary aldosteronism screening when the PAC falls below conventional thresholds required for a ‘positive screen’.23–26 For example, nearly a quarter of patients with resistant hypertension and primary aldosteronism may have at least one PAC below 10 ng/dL.27,28 To account for this increasingly recognized aldosterone variability and to diminish the likelihood of a false negative test, more permissive screening recommendations for primary aldosteronism have been proposed which consider an ARR ≥ 20 ng/dL per ng/mL/h with a PAC of at least 10 ng/dL to constitute a positive screen and warrant a dynamic confirmatory test.21,29 Some have proposed even more permissive criteria wherein any aldosterone greater than 5 or 6 ng/dL in the context of a suppressed renin may represent a positive screen worthy of further evaluation.16,21,30–32 This shift from relying only on the ARR to considering lower thresholds of aldosterone in the context of a suppressed renin parallels the use of newer LC-MS/MS aldosterone assays that report substantially lower aldosterone values than immunoassays,33 and reflects the increasing awareness that primary aldosteronism exists on a spectrum of severity where PAC and ARR can vary considerably to include levels below the traditional thresholds for diagnosis.8,16,23–26
Herein, we investigated the intra-individual variability of aldosterone and ARR values among patients with confirmed primary aldosteronism to characterize its potential impact on using traditionally accepted diagnostic thresholds for diagnosis.
Methods:
The data that support the findings of this study are available and can be requested from the corresponding author.
Study Objectives
The purpose of this study was to characterize the intra-individual variability in aldosterone concentrations and aldosterone-to-renin ratios (ARRs) over multiple measurements in patients with confirmed primary aldosteronism to assess the implications for real-life ambulatory diagnostic evaluations for primary aldosteronism.
Study design and participants
This was a retrospective study design that only included patients if:
They met conventional Endocrine Society criteria for the confirmation of primary aldosteronism,21 either via a recommended dynamic confirmatory test or via a very high aldosterone (i.e. aldosterone concentration ≥ 20 ng/dL) in the context of a suppressed renin, hypertension, and hypokalemia.
They had two or more paired measurements of aldosterone and renin, obtained on separate days. The underlying reason for obtaining multiple measurements of aldosterone and renin varied for each patient, and included diagnostic uncertainty after the first measurement and/or referral from a primary care physician to a specialist who then repeated the measurements. All samples were obtained in the seated position, on an ad libitum diet, at the time of the ambulatory clinic visit which could have occurred at any time during the day.
All renin measurements were suppressed, defined as a plasma renin activity less than or equal to 1.0 ng/mL/h.
Patients were excluded from this study if:
They did not have confirmed primary aldosteronism based on Endocrine Society criteria.
They had fewer than two serial aldosterone and renin measurements.
They had any renin values that were greater than 1.0 ng/mL/h.
They were taking a mineralocorticoid receptor antagonist or epithelial sodium channel inhibitor at the time of any aldosterone or renin measurements. The use of all other anti-hypertensive medication classes was permitted since measurement and interpretation of screening aldosterone and renin while on these medications is convention in most of the United States. However, to eliminate the introduction of new pharmacologic variability on aldosterone levels, if a patient initiated an ACE inhibitor or angiotensin receptor blocker in between measurements, all subsequent measurements were excluded.
A total of 51 patients, seen between 2005–2019, met all of these inclusion criteria and represented the study population. This study was approved by our institutional ethics and human research committees.
Measurements
Paired aldosterone and renin measurements were obtained using one of three pairs of assays, listed in the order of decreasing frequency below:
LC-MS/MS assay for aldosterone and LC-MS/MS assay for plasma renin activity from Mayo Clinic (Rochester, MN). A total of 31 (61%) patients had values measured with these assays. The coefficient of variation (CV) for the aldosterone assay ranged from 8.7% at lower concentrations to 3.9% at higher concentrations; the CV for the renin assay was 18.8% at low concentrations.
Quantitative chemiluminescent immunoassay for aldosterone concentration and enzyme-linked immunosorbent assay for plasma renin activity from ARUP Laboratories (Salt Lake City, UT). Fourteen patients (27%) had values measured with these assays. The CV for the aldosterone assay ranged from 10% at lower concentrations to 6% at higher concentrations; the CV for the renin assay was 15%.
LC-MS/MS assay for aldosterone and LC-MS/MS assay for plasma renin activity from Quest Diagnostics (Chantilly, VA). Six patients (12%) had values measured with these assays. The CV for the aldosterone assay ranged from 2.7% to 4.4%; the CV for the renin assay was 11% at low concentrations.
Analysis Plan and Statistical Methods
We first demonstrated the intra-individual variability in serial aldosterone and ARR measurements by graphically displaying the data. Intra-individual coefficients of variation (defined as the standard deviation divided by the mean value) and the percent difference (the highest value in an individual minus the lowest value, the result of which was divided by the mean value) were calculated for each patient’s aldosterone and ARR measurements as metrics of intra-individual variability. Subsequently, we quantified the percentage of patients with single and mean aldosterone levels and single and mean ARR measurements generally considered to be low for the diagnosis of primary aldosteronism using a variety of well-established and traditional thresholds (< 10, 15, and 20 ng/dL for aldosterone; < 20, 25, and 30 ng/dL per ng/mL/h for the ARR). We then divided patients into tertiles based on their aldosterone and ARR values and assessed for any demographic or diagnostic differences that could potentially predict underlying reasons for these differences.
We conducted several sensitivity analyses to assess the reproducibility and robustness of the findings. Most plasma renin activity assays report only to a lower limit of 0.6 ng/mL/h; however, a minority report to 0.1 ng/mL/h. To mitigate any effect that the lower limit of the plasma renin activity assays could have had on the perceived variability of the ARR, we reanalyzed data in which the minimum plasma renin activity was set at 0.6 ng/mL/h. Additionally, since it is known that aldosterone LC-MS/MS assays tend to be more precise than immunoassays and report lower values,33 we performed a sensitivity analysis including only patients who had LC-MS/MS measurements of aldosterone. Finally, low serum potassium levels are known to suppress aldosterone secretion and a minority of patients did not have a concomitant serum potassium measured. Thus, we performed sensitivity analyses using only paired aldosterone and renin measurements obtained with a concomitant potassium level and also analyzed the data after eliminating all aldosterone and renin measurements obtained at a time when the serum potassium was less than 3.5 MEq/L, as recommended by the Endocrine Society.21
For continuous variables, differences between two groups were analyzed via student’s t-test if data were parametric and the Wilcoxon rank-sum test if data were non-parametric; differences between tertiles were analyzed using ANOVA with parametric data and the Kruskal-Wallis test with non-parametric data. The chi-squared test was used to compare categorical variables between two or more groups. Analyses were conducted using STATA version 15 (College Station, TX).
Results:
Study Population
Basic demographic and diagnostic data of the 51 patients with confirmed primary aldosteronism are displayed in Table 1. As expected, the study population was hypertensive despite the average use of three antihypertensive medications, and on average had a low serum potassium at the time of the first aldosterone and renin blood draw. The mean number of paired aldosterone and renin measurements was three (range 2–6 measurements). The mean aldosterone and median ARR values across all patients were significantly elevated and consistent with the diagnosis of primary aldosteronism. The majority of patients had a unilateral adrenal nodule on imaging and many underwent adrenal venous sampling (AVS), in which adrenal vein measurements of cortisol and aldosterone were obtained in triplicate both before and after cosyntropin; unstimulated measurements were used to calculate the lateralization index and stimulated measurements were used to enhance selectivity, as previously described.34–38 At AVS, the majority of patients lateralized to one side regardless of the unstimulated lateralization index threshold used, and most were treated with a unilateral intervention rather than medical therapy. Post-treatment outcomes with either medical therapy or unilateral intervention (i.e. surgical adrenalectomy or radiofrequency ablation) are reported in Table S1 and were no different between treatment groups; clinical outcomes and biochemical outcomes in the unilateral intervention group were defined according to the Primary Aldosteronism Surgical Outcome (PASO) study.39
Table 1.
Patient demographic and diagnostic characteristics.
| Characteristic | Mean (SD) or Median [IQR] |
|---|---|
| Age – years | 52 (10) |
| Male sex – % | 71% |
| Race – % | |
| White | 57% |
| Black | 31% |
| Asian | 4% |
| Unknown/other | 8% |
| BMI – kg/m2 | 32 (6) |
| Systolic blood pressure at diagnosis – mmHg | 146 (21) |
| Diastolic blood pressure at diagnosis – mmHg | 88 (11) |
| Potassium at diagnosis – MEq/L | 3.5 (0.4) |
| # of blood pressure medications at 1st aldosterone/renin measurement | 3 (1) |
| # of aldosterone measurements | 3 (1) |
| Mean aldosterone – ng/dL | 20.3 (7.8) |
| Median plasma renin activity – ng/mL/h | 0.6 [0.3–0.6] |
| Median ARR – [(ng/dL)/(ng/mL/h)] | 47.5 [31.6–77.3] |
| Imaging findings – % | |
| Normal adrenal glands | 26% |
| Unilateral left adrenal nodule | 38% |
| Unilateral right adrenal nodule | 28% |
| Bilateral adrenal nodules | 8% |
| Lateralization at AVS (unstimulated, lateralization index ≥ 2) – % | |
| Bilateral disease | 25% |
| Unilateral disease | 75% |
| Lateralization at AVS (unstimulated, lateralization index ≥ 4) – % | |
| Bilateral disease | 39% |
| Unilateral disease | 61% |
| Treatment – % | |
| Medical therapy | 41% |
| Unilateral adrenalectomy or ablation | 59% |
Intra-Individual Variability of Aldosterone and ARR
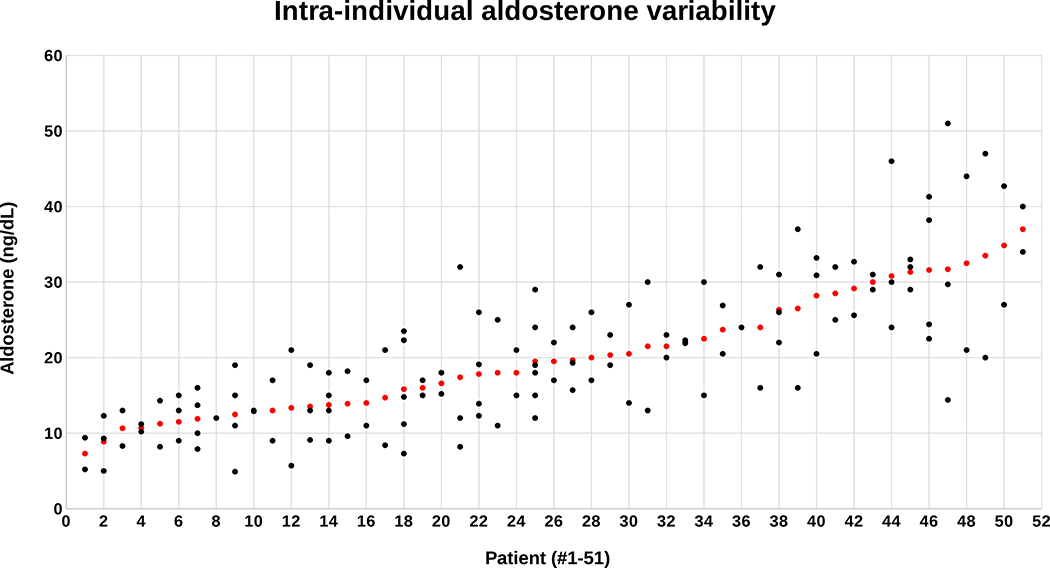
There were a total of 137 paired renin and aldosterone measurements performed on the 51 patients. Although all patients had a confirmed diagnosis of primary aldosteronism, individual aldosterone measurements ranged from a low of 4.9 ng/dL to a high of 51 ng/dL (Figure 1). The mean aldosterone for each individual patient also varied considerably, from a low of 7.3 ng/dL to a high of 37 ng/dL (Figure 1). The mean intra-individual coefficient of variation for aldosterone was 31% (range 0%–81%), at least 3–6-fold greater than the variability of the aldosterone assays, and the mean intra-individual percent difference for aldosterone was 52% (range 0–137%). There was no association between imaging findings or lateralization at adrenal venous sampling and aldosterone variability. Nearly half (49%) of all patients had a single aldosterone level < 15 ng/dL, nearly one-third (29%) had at least two aldosterone levels < 15 ng/dL, and precisely one-third (33%) had the average of all their aldosterone levels < 15 ng/dL (Table 2). More striking was the observation that nearly 30% of patients had at least one aldosterone concentration < 10 ng/dL and 4% of patients had at least two aldosterone levels below this permissive threshold (Table 2).
Figure 1: Scatterplot of screening aldosterone concentrations.
Individual aldosterone levels (black dots) are displayed directly above each patient on the x axis and arranged in order of the mean aldosterone concentration for each patient (red dot). All patients had at least two, or more, aldosterone measurements.
Table 2.
Intra-individual variability of aldosterone concentrations in relation to conventional diagnostic thresholds.
| At least 1 aldosterone (ng/dL) | % of patients | At least 2 aldosterone levels (ng/dL) | % of patients | Mean aldosterone (ng/dL) | % of patients |
|---|---|---|---|---|---|
| < 10 | 29% | < 10 | 4% | < 10 | 4% |
| < 15 | 49% | < 15 | 29% | < 15 | 33% |
| < 20 | 69% | < 20 | 43% | < 20 | 53% |
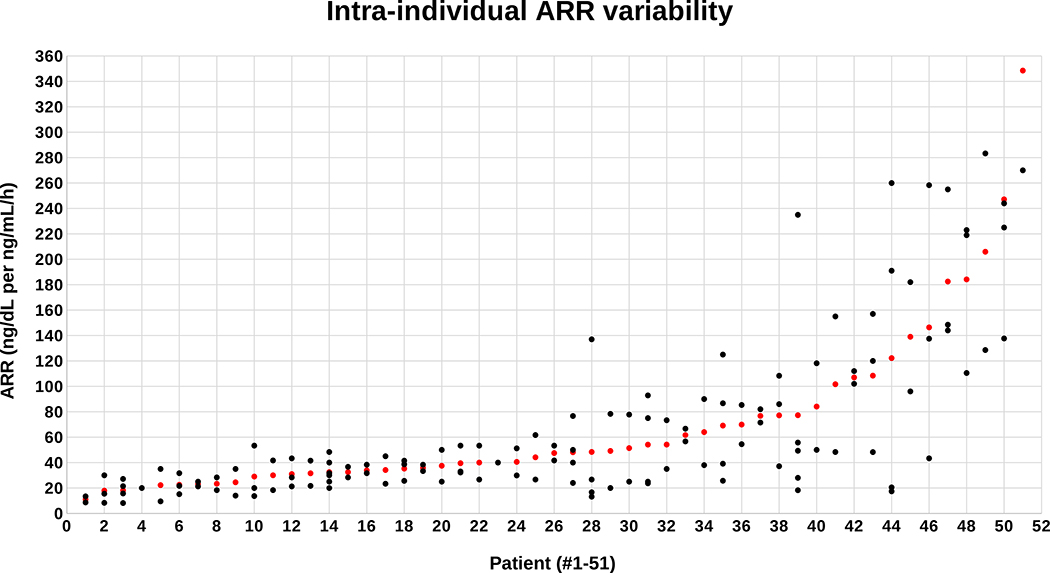
ARR values also varied considerably from patient-to-patient, with the 137 single ARR values ranging from a low of 8.2 ng/dL per ng/mL/h to a high of 427 ng/dL per ng/mL/h (Figure 2). The mean ARR for each patient ranged from a low of 11 ng/dL per ng/mL/h to a high of 349 ng/dL per ng/mL/h (Figure 2). The mean intra-individual coefficient of variation for the ARR was 45% (range 0%–123%) and the mean intra-individual percent difference for the ARR was 79% (range 0%–281%). ARR variability was also not associated with imaging findings or lateralization at adrenal venous sampling. Well over half of patients (57%) had at least one ARR < 30 ng/dL per ng/mL/h and over one-quarter (27%) had at least two values below this threshold (Table 3). Strikingly, 24% of patients had at least one ARR value, and 8% of patients had at least two ARR values, that were < 20 ng/dL per ng/mL/h, which is the most permissive threshold typically recommended (Table 3).40
Figure 2: Scatterplot of screening aldosterone-to-renin ratios (ARR’s).
Individual ARR values (black dots) are displayed directly above each patient on the x axis and arranged in order of the mean ARR for each patient (red dot). All patients had at least two, or more, ARR measurements.
Table 3.
Intra-individual variability of ARR in relation to conventional diagnostic thresholds.
| At least 1 ARR (ng/dL per ng/mL/h) | % of patients | At least 2 ARR’s (ng/dL per ng/mL/h) | % of patients | Mean ARR (ng/dL per ng/mL/h) | % of patients |
|---|---|---|---|---|---|
| < 20 | 24% | < 20 | 8% | < 20 | 6% |
| < 25 | 39% | < 25 | 16% | < 25 | 18% |
| < 30 | 57% | < 30 | 27% | < 30 | 20% |
Predictors of Variability
When divided into tertiles based on the average aldosterone and ARR levels, and the single lowest aldosterone and ARR levels, there were no clinically significant attributes that were significantly associated with the variability in aldosterone or ARR (Tables S2A, S2B, S3A, and S3B). Although samples were obtained from early morning until late afternoon, there was no association between the time of day at which the measurements were obtained and either the aldosterone levels (r = −0.04, p=0.66) or ARR values (r = 0.08, p=0.40) (Figures S1A and S1B).
Sensitivity Analyses for Robustness and Reproducibility
When ARR values were re-calculated after fixing the lower limit of PRA at 0.6 ng/mL/h, the percentage of patients with ARR values below the predetermined thresholds substantially increased and the overall ARR variability remained high, although it was predictably lower than in the original analysis (Table S4). When restricting analyses to only those measurements that were obtained using LC-MS/MS assays, the study population declined from 51 to 37 and the number of measurements decreased from 137 to 97. Despite this, the aldosterone and ARR levels below the pre-specified diagnostic thresholds were similar to the original analyses, as was the overall aldosterone and ARR variability (Tables S5A and S5B). When we eliminated all paired aldosterone and renin measurements obtained without a concomitant potassium, the number of patients declined from 51 to 41 and the total number of measurements decreased to 106, but the findings did not differ from the original analysis (Tables S6A and S6B). When we further eliminated all potassium values less than 3.5 MEq/L, the number of patients decreased from 41 to 19, the number of individual measurements fell to 45, and the mean potassium was 3.8 MEq/L. In this setting of normokalemia, the intra-individual variability of both aldosterone and the ARR increased, while the number of patients below the pre-specified thresholds was higher for aldosterone and similar for the ARR compared to the original analysis (Tables S7A and S7B).
Discussion:
In this study, we characterized the intra-individual variability of screening diagnostics for primary aldosteronism by demonstrating that a relatively high percentage of patients with bona fide primary aldosteronism can have one or more aldosterone and/or ARR values below commonly accepted diagnostic thresholds. Nearly 30% of patients with primary aldosteronism had at least one aldosterone between 5–10 ng/dL and 24% of patients had at least one ARR < 20 ng/dL per ng/mL/h. Like many similar studies, this study has some inherent bias since it could only retrospectively include those patients who were ultimately confirmed to have primary aldosteronism (rather than the many patients whose diagnosis was missed due to lack of screening or misinterpretation of screening); thus, it may be that our observations represent only a conservative estimate of the true variability that exists, as has been shown in prior studies that conducted unbiased confirmatory testing rather than relying on screening aldosterone and ARR.41–43 From a clinical perspective, these findings highlight the fact that many of these patients with confirmed primary aldosteronism may have not been diagnosed if their clinicians had relied on just a single, rather than repeated, screening assessment.
This study extends the findings of several prior studies demonstrating the limitations of the screening aldosterone-to-renin ratio due to aldosterone variability, and provides impetus for re-calibrating the interpretation of screening diagnostics. Siragy et al. showed that beyond diurnal variation, aldosterone concentrations in primary aldosteronism exhibit a burst-like pulsatility throughout the day, resulting in a range of aldosterone values that span across a 4-fold magnitude.24 Tanabe et al. previously showed that among patients with pathologically confirmed primary aldosteronism from an adrenal adenoma, aldosterone and ARR measured by radioimmunoassay were highly variable, such that 63% of their patients studied did not have consistently “typical” profiles for primary aldosteronism (the typical profile was defined as an ARR > 35 ng/dL per ng/mL/h, PAC > 15 ng/dL, and PRA < 0.5 ng/mL/h).25 Kline et al. and Yozamp et al. recently demonstrated that patients with confirmed primary aldosteronism can frequently have aldosterone concentrations at the time of adrenal venous sampling that are below 5 ng/dL,26,38 and that there exists large variability in adrenal venous aldosterone concentrations (reflecting acutely variable aldosterone production), with coefficients of variation for repeated adrenal vein measurements ranging from 30–39%.38 Finally, Brown et al. found that nearly 25% of patients with resistant hypertension who had confirmed primary aldosteronism had at least one aldosterone concentration less than 10 ng/dL.27,44 Collectively, these studies highlight the fallibility of single aldosterone measurements and illustrate a need to re-calibrate the thresholds for a ‘positive screen’ for primary aldosteronism, particularly since newer LC-MS/MS aldosterone assays report aldosterone concentrations that can be over 20% lower than immunoassays.33 These studies also underscore the point that a single “normal” or “low” screening aldosterone concentration should not exclude the possibility of a primary aldosteronism diagnosis.16
It is often advised that screening for primary aldosteronism be conducted after accounting for important factors that may lower aldosterone and the ARR, including hypokalemia, the time of day, characteristics of the assays (the type of assay for aldosterone and the lower limit of the renin assay), and anti-hypertensive medication classes.21 However, our findings suggest that there is substantial, and clinically-relevant, intra-individual variability in aldosterone and ARR values regardless of serum potassium, time of day, type of assay, and even after the potential effect of major medication interference is accounted for. In other words, complex and laborious efforts to minimize confounders may not be necessary, and a more practical and simplified screening approach can be recommended for front-line clinicians who are responsible for the majority of screening efforts. In this regard, if public health efforts to increase screening rates for primary aldosteronism above 3–8%17–20 are to be implemented, one approach may be to encourage screening for primary aldosteronism regardless of the conditions, but with modifications of the interpretation of the results to reflect more liberalized criteria for a positive screen.
For every chosen threshold for a positive screen for primary aldosteronism, and approach to conducting screening, there is a trade-off in the risk for false positive versus false negative results. From a public health perspective, the risk of a false positive result from an overly sensitive test is an increase in healthcare expenditures because of needless or potentially harmful testing. The risk of a false negative result from a poorly sensitive test is untreated morbidity and mortality from the undiagnosed condition, which in the case of primary aldosteronism has targeted therapies. Although further studies are needed to quantify the healthcare economics associated with increased screening, it is likely that the cardiometabolic morbidity and mortality associated with unrecognized primary aldosteronism is far more costly than the relatively inexpensive changes to screening interpretation that might identify more cases. Our results suggest that a re-calibration of screening thresholds could substantially improve the detection of primary aldosteronism cases by minimizing false negative testing. Emphasis on inappropriate aldosterone production in the context of suppressed renin, rather than solely on an arbitrarily elevated ARR, is likely to increase the number of positive screening tests. Our results suggest that a single aldosterone or ARR may not be reliable; rather, at least two, or repeated measurements provide greater sensitivity. Further, liberalizing the definition of an inappropriate aldosterone concentration to 5 or 6 ng/dL (measured by modern LC-MS/MS assays), when renin is suppressed, should be strongly considered to maximize detection of true cases, especially when the pre-test probability for the diagnosis is high. Although it is advised that blood draws be obtained in the morning,21 the time of day should not preclude testing, and reliable interpretations can still be made with the aforementioned approach. Although hypokalemia can suppress the secretion of aldosterone and theoretically lead to a false negative screening test,30 we found that aldosterone levels were just as variable, if not more variable, when potassium was replete, with even more patients falling below conventional thresholds (Tables S7A and S7B). Finally, in resource-poor settings where screening tests are prohibitively expensive or difficult to perform but the pretest probability of primary aldosteronism is high, empiric treatment with a mineralocorticoid receptor antagonist is reasonable.16
One potential limitation of this study is the relatively small sample size, although it is doubtful that the percentage of patients with aldosterone and ARR values below our pre-specified cut-offs would drastically change if the study population were larger. Another limitation is that we retroactively searched for patients with confirmed primary aldosteronism who had multiple measurements of aldosterone and renin. This is likely to have introduced a bias favoring inclusion of patients with the most severe disease, since many patients with milder primary aldosteronism were likely never diagnosed. A third limitation is that three different assays were used for aldosterone and renin (two LC-MS/MS assays each and one immunoassay apiece), each with a different intrinsic coefficient of variation. Nonetheless, the results did not substantively change when only one type of assay (the LC-MS/MS) was analyzed and the intra-individual coefficients of variation for aldosterone we observed were substantially higher than the intrinsic variability of the aldosterone assays, suggesting an inherent in vivo biological contribution to the variability we observed. The presence of multiple assays is also reflective of the real-life experience of clinicians, in which patients are frequently referred from other institutions with a different clinical laboratory, and where results are reported using assays the clinicians are not always familiar with or knowledgeable about. A fourth limitation is that most patients were on antihypertensive medications, as is the practical standard for screening in most parts of the United States; certain medications, such as RAAS inhibitors and calcium channel blockers, have been implicated in lowering aldosterone production and may have thus contributed to the aldosterone and ARR variability observed in this study. However, we minimized the impact of this clinical reality by excluding patients on mineralocorticoid receptor antagonists or potassium-sparing diuretics and excluding any values obtained after a patient started an ACE inhibitor or angiotensin receptor blocker, after which one would expect a decrease in aldosterone and increase in renin. A fifth limitation is that we did not directly measure all the many possible intrinsic factors that may have influenced aldosterone variability, including posture-responsiveness,45,46 ACTH-dependence associated with diurnal rhythm or stress,47–49 and the presence of certain mutations in genes, such as KCNJ5.50 Yet, it should be noted that all patients were seated at the time of measurement (thus controlling for posture), and aldosterone and ARR variability were no different regardless of the time of day. Finally, a number of patient-specific factors including dietary sodium/potassium intake, race,51 and sex may have contributed to our findings, although it is unlikely that any patients were on a sodium-restricted diet and no meaningful differences in aldosterone and ARR levels by race and sex were observed.
Perspectives:
There is substantial intra-individual variability in plasma aldosterone concentrations and ARRs in patients with primary aldosteronism. Single, and even multiple, aldosterone and ARR values are frequently lower than the traditionally recommended screening diagnostic thresholds for primary aldosteronism and may incorrectly exclude the diagnosis. Given the high prevalence of primary aldosteronism, the low rates of diagnosis, and the cardiometabolic morbidity and mortality associated with untreated disease, these findings suggest that a re-calibration towards more permissive screening criteria, and reliance on more than one screening assessment, could improve detection of more true cases of primary aldosteronism and decrease false negative screening interpretations.
Supplementary Material
Novelty and Significance:
What is New?
We analyzed the intra-individual variability of screening aldosterone concentrations and aldosterone-to-renin ratios (ARR’s) in patients with confirmed primary aldosteronism to evaluate the potential impact of this variability on conventional diagnostic approaches.
What is Relevant?
Aldosterone concentrations in primary aldosteronism patients ranged from 4.9 to 51 ng/dL, with 49% of patients having at least one aldosterone measurement below 15 ng/dL and 29% having at least one measurement below 10 ng/dL.
Individual ARR values ranged from 8.2 to 427 ng/dL per ng/mL/h, with 57% having at least one ARR below 30 ng/dL per ng/mL/h and 24% having at least one ARR below 20 ng/dL per ng/mL/h
Summary:
Patients with primary aldosteronism exhibit marked intra-individual variability of aldosterone concentrations and aldosterone-to-renin ratios, such that many patients had values below conventionally-accepted screening thresholds. A re-calibration of diagnostic thresholds, and reliance on more than one screening assessment, could improve the diagnostic yield of primary aldosteronism case detection.
Acknowledgements:
We would like to thank the clinical research center staff that assisted with specimen processing and storage.
Sources of funding: We thank our funding sources for supporting this work: R01 DK115392 (AV), R01 HL153004 (AV), R01 DK16618 (AV), and 2T32 HL007609-32 (NY).
Footnotes
Disclosures: AV reports consulting fees unrelated to the contents of this work from Corcept Therapeutics, CatalysPacific, HRA Pharma.
References:
- 1.Markou A, Pappa T, Kaltsas G, Gouli A, Mitsakis K, Tsounas P, Prevoli A, Tsiavos V, Papanastasiou L, Zografos G, Chrousos GP, Piaditis GP. Evidence of Primary Aldosteronism in a Predominantly Female Cohort of Normotensive Individuals: A Very High Odds Ratio for Progression into Arterial Hypertension. J Clin Endocrinol Metab. 2013;98(4):1409–1416. doi: 10.1210/jc.2012-3353 [DOI] [PubMed] [Google Scholar]
- 2.Monticone S, Burrello J, Tizzani D, Bertello C, Viola A, Buffolo F, Gabetti L, Mengozzi G, Williams TA, Rabbia F, Veglio F, Mulatero P. Prevalence and Clinical Manifestations of Primary Aldosteronism Encountered in Primary Care Practice. J Am Coll Cardiol. 2017;69(14):1811–1820. doi: 10.1016/j.jacc.2017.01.052 [DOI] [PubMed] [Google Scholar]
- 3.Lorena Mosso, Cristian Carvajal, Alexis González, Adolfo Barraza, Fernando Avila, Montero Joaquín Huete Alvaro, Alessandra Gederlini, Fardella Carlos E. Primary Aldosteronism and Hypertensive Disease. Hypertension. 2003;42(2):161–165. doi: 10.1161/01.HYP.0000079505.25750.11 [DOI] [PubMed] [Google Scholar]
- 4.Mulatero P, Stowasser M, Loh K-C, Fardella CE, Gordon RD, Mosso L, Gomez-Sanchez CE, Veglio F, Young WF. Increased Diagnosis of Primary Aldosteronism, Including Surgically Correctable Forms, in Centers from Five Continents. J Clin Endocrinol Metab. 2004;89(3):1045–1050. doi: 10.1210/jc.2003-031337 [DOI] [PubMed] [Google Scholar]
- 5.Omura M, Saito J, Yamaguchi K, Kakuta Y, Nishikawa T. Prospective Study on the Prevalence of Secondary Hypertension among Hypertensive Patients Visiting a General Outpatient Clinic in Japan. Hypertens Res. 2004;27(3):193–202. doi: 10.1291/hypres.27.193 [DOI] [PubMed] [Google Scholar]
- 6.Rossi E, Regolisti G, Negro A, Sani C, Davoli S, Perazzoli F. High prevalence of primary aldosteronism using postcaptopril plasma aldosterone to renin ratio as a screening test among Italian hypertensives. Am J Hypertens. 2002;15(10):896–902. doi: 10.1016/S0895-7061(02)02969-2 [DOI] [PubMed] [Google Scholar]
- 7.Rossi GP, Bernini G, Caliumi C, Desideri G, Fabris B, Ferri C, Ganzaroli C, Giacchetti G, Letizia C, Maccario M, Mallamaci F, Mannelli M, Mattarello M-J, Moretti A, Palumbo G, Parenti G, Porteri E, Semplicini A, Rizzoni D, Rossi E, Boscaro M, Pessina AC, Mantero F. A Prospective Study of the Prevalence of Primary Aldosteronism in 1,125 Hypertensive Patients. J Am Coll Cardiol. 2006;48(11):2293–2300. doi: 10.1016/j.jacc.2006.07.059 [DOI] [PubMed] [Google Scholar]
- 8.Vaidya A, Mulatero P, Baudrand R, Adler GK. The Expanding Spectrum of Primary Aldosteronism: Implications for Diagnosis, Pathogenesis, and Treatment. Endocr Rev. 2018;39(6):1057–1088. doi: 10.1210/er.2018-00139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yozamp N, Vaidya A. The prevalence of primary aldosteronism and evolving approaches for treatment. Curr Opin Endocr Metab Res. 2019;8:30–39. doi: 10.1016/j.coemr.2019.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calhoun DA, Nishizaka MK, Zaman MA, Thakkar RB, Weissmann P. Hyperaldosteronism Among Black and White Subjects With Resistant Hypertension. Hypertension. 2002;40(6):892–896. doi: 10.1161/01.HYP.0000040261.30455.B6 [DOI] [PubMed] [Google Scholar]
- 11.Calhoun DA, Nishizaka MK, Zaman MA, Harding SM. Aldosterone Excretion Among Subjects With Resistant Hypertension and Symptoms of Sleep Apnea. CHEST. 2004;125(1):112–117. doi: 10.1378/chest.125.1.112 [DOI] [PubMed] [Google Scholar]
- 12.Milliez P, Girerd X, Plouin P-F, Blacher J, Safar ME, Mourad J-J. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol. 2005;45(8):1243–1248. doi: 10.1016/j.jacc.2005.01.015 [DOI] [PubMed] [Google Scholar]
- 13.Monticone S, D’Ascenzo F, Moretti C, Williams TA, Veglio F, Gaita F, Mulatero P. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2018;6(1):41–50. doi: 10.1016/S2213-8587(17)30319-4 [DOI] [PubMed] [Google Scholar]
- 14.Martin Reincke, Evelyn Fischer, Sabine Gerum, Katrin Merkle, Sebastian Schulz, Anna Pallauf, Marcus Quinkler, Gregor Hanslik, Katharina Lang, Stefanie Hahner, Bruno Allolio, Christa Meisinger, Rolf Holle, Felix Beuschlein, Martin Bidlingmaier, Stephan Endres. Observational Study Mortality in Treated Primary Aldosteronism. Hypertension. 2012;60(3):618–624. doi: 10.1161/HYPERTENSIONAHA.112.197111 [DOI] [PubMed] [Google Scholar]
- 15.Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Cardiometabolic Outcomes and Mortality in Medically Treated Primary Aldosteronism: A Retrospective Cohort Study. Lancet Diabetes Endocrinol. 2018;6(1):51–59. doi: 10.1016/S2213-8587(17)30367-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaidya A, Carey RM. The Evolution of the Primary Aldosteronism Syndrome: Updating the Approach. J Clin Endocrinol Metab. Published online August 31, 2020. doi: 10.1210/clinem/dgaa606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruhle BC, White MG, Alsafran S, Kaplan EL, Angelos P, Grogan RH. Keeping primary aldosteronism in mind: Deficiencies in screening at-risk hypertensives. Surgery. 2019;165(1):221–227. doi: 10.1016/j.surg.2018.05.085 [DOI] [PubMed] [Google Scholar]
- 18.Jaffe G, Gray Z, Krishnan G, Stedman M, Zheng Y, Han J, Chertow GM, Leppert JT, Bhalla V. Screening Rates for Primary Aldosteronism in Resistant Hypertension: A Cohort Study. Hypertens Dallas Tex 1979. 2020;75(3):650–659. doi: 10.1161/HYPERTENSIONAHA.119.14359 [DOI] [PubMed] [Google Scholar]
- 19.Mulatero P, Monticone S, Burrello J, Veglio F, Williams TA, Funder J. Guidelines for primary aldosteronism: uptake by primary care physicians in Europe. J Hypertens. 2016;34(11):2253–2257. doi: 10.1097/HJH.0000000000001088 [DOI] [PubMed] [Google Scholar]
- 20.Reincke M, Beuschlein F, Williams TA. Progress in Primary Aldosteronism 2019: New Players on the Block? Horm Metab Res Horm Stoffwechselforschung Horm Metab. 2020;52(6):345–346. doi: 10.1055/a-1156-9926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, Stowasser M, Young WF. The Management of Primary Aldosteronism: Case Detection, Diagnosis, and Treatment: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2016;101(5):1889–1916. doi: 10.1210/jc.2015-4061 [DOI] [PubMed] [Google Scholar]
- 22.Young WF. Primary aldosteronism: renaissance of a syndrome. Clin Endocrinol (Oxf). 2007;66(5):607–618. doi: 10.1111/j.1365-2265.2007.02775.x [DOI] [PubMed] [Google Scholar]
- 23.Vieweg WV, Veldhuis JD, Carey RM. Temporal pattern of renin and aldosterone secretion in men: effects of sodium balance. Am J Physiol. 1992;262(5 Pt 2):F871–877. doi: 10.1152/ajprenal.1992.262.5.F871 [DOI] [PubMed] [Google Scholar]
- 24.Siragy HM, Vieweg WV, Pincus S, Veldhuis JD. Increased disorderliness and amplified basal and pulsatile aldosterone secretion in patients with primary aldosteronism. J Clin Endocrinol Metab. 1995;80(1):28–33. doi: 10.1210/jcem.80.1.7829626 [DOI] [PubMed] [Google Scholar]
- 25.Tanabe A, Naruse M, Takagi S, Tsuchiya K, Imaki T, Takano K. Variability in the renin/aldosterone profile under random and standardized sampling conditions in primary aldosteronism. J Clin Endocrinol Metab. 2003;88(6):2489–2494. doi: 10.1210/jc.2002-021476 [DOI] [PubMed] [Google Scholar]
- 26.Kline GA, Darras P, Leung AA, So B, Chin A, Holmes DT. Surprisingly low aldosterone levels in peripheral veins following intravenous sedation during adrenal vein sampling: implications for the concept of nonsuppressibility in primary aldosteronism. J Hypertens. 2019;37(3):596–602. doi: 10.1097/HJH.0000000000001905 [DOI] [PubMed] [Google Scholar]
- 27.Brown JM, Siddiqui M, Calhoun DA, Carey RM, Hopkins PN, Williams GH, Vaidya A. The Unrecognized Prevalence of Primary Aldosteronism: A Cross-sectional Study. Ann Intern Med. 2020;173(1):10–20. doi: 10.7326/M20-0065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Funder JW. Primary Aldosteronism: At the Tipping Point. Ann Intern Med. 2020;173(1):65–66. doi: 10.7326/M20-1758 [DOI] [PubMed] [Google Scholar]
- 29.Byrd JB, Turcu AF, Auchus RJ. Primary Aldosteronism: Practical Approach to Diagnosis and Management. Circulation. 2018;138(8):823–835. doi: 10.1161/CIRCULATIONAHA.118.033597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stowasser M, Ahmed AH, Pimenta E, Taylor PJ, Gordon RD. Factors Affecting the Aldosterone/Renin Ratio. Horm Metab Res. 2012;44(3):170–176. doi: 10.1055/s-0031-1295460 [DOI] [PubMed] [Google Scholar]
- 31.Gordon RD, Stowasser M, Klemm SA, Tunny TJ. Primary aldosteronism and other forms of mineralocorticoid hypertension In: Swales JD, ed. Textbook of Hypertension. London, UK: Blackwell Scientific; 1994:865–892. [Google Scholar]
- 32.Rossi GP, Gioco F, Fassina A, Gomez-Sanchez CE. Normoaldosteronemic aldosterone-producing adenoma: immunochemical characterization and diagnostic implications. J Hypertens. 2015;33(12):2546–2549. doi: 10.1097/HJH.0000000000000748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thuzar M, Young K, Ahmed AH, Ward G, Wolley M, Guo Z, Gordon RD, McWhinney BC, Ungerer JP, Stowasser M. Diagnosis of Primary Aldosteronism by Seated Saline Suppression Test-Variability Between Immunoassay and HPLC-MS/MS. J Clin Endocrinol Metab. 2020;105(3). doi: 10.1210/clinem/dgz150 [DOI] [PubMed] [Google Scholar]
- 34.Yatabe M, Bokuda K, Yamashita K, Morimoto S, Yatabe J, Seki Y, Watanabe D, Morita S, Sakai S, Ichihara A. Cosyntropin stimulation in adrenal vein sampling improves the judgment of successful adrenal vein catheterization and outcome prediction for primary aldosteronism. Hypertens Res Off J Jpn Soc Hypertens. 2020;43(10):1105–1112. doi: 10.1038/s41440-020-0445-x [DOI] [PubMed] [Google Scholar]
- 35.Rossitto G, Amar L, Azizi M, Riester A, Reincke M, Degenhart C, Widimsky J, Naruse M, Deinum J, Schultzekool L, Kocjan T, Negro A, Rossi E, Kline G, Tanabe A, Satoh F, Rump LC, Vonend O, Willenberg HS, Fuller P, Yang J, Nian Chee NY, Magill SB, Shafigullina Z, Quinkler M, Oliveras A, Chang C-C, Wu VC, Somloova Z, Maiolino G, Barbiero G, Battistel M, Lenzini L, Quaia E, Pessina AC, Rossi GP. Subtyping of Primary Aldosteronism in the AVIS-2 Study: Assessment of Selectivity and Lateralization. J Clin Endocrinol Metab. 2020;105(6). doi: 10.1210/clinem/dgz017 [DOI] [PubMed] [Google Scholar]
- 36.St-Jean M, Bourdeau I, Therasse É, Lacroix A. Use of peripheral plasma aldosterone concentration and response to ACTH during simultaneous bilateral adrenal veins sampling to predict the source of aldosterone secretion in primary aldosteronism. Clin Endocrinol (Oxf). 2020;92(3):187–195. doi: 10.1111/cen.14137 [DOI] [PubMed] [Google Scholar]
- 37.Wannachalee T, Zhao L, Nanba K, Nanba AT, Shields JJ, Rainey WE, Auchus RJ, Turcu AF. Three Discrete Patterns of Primary Aldosteronism Lateralization in Response to Cosyntropin During Adrenal Vein Sampling. J Clin Endocrinol Metab. 2019;104(12):5867–5876. doi: 10.1210/jc.2019-01182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yozamp N, Hundemer GL, Moussa M, Underhill J, Fudim T, Sacks B, Vaidya A. Variability of Aldosterone Measurements During Adrenal Venous Sampling for Primary Aldosteronism. Am J Hypertens. 2020; In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams TA, Lenders JW, Mulatero P, Burrello J, Rottenkolber M, Adolf C, Satoh F, Amar L, Quinkler M, Deinum J, Beuschlein F, Kitamoto KK, Pham U, Morimoto R, Umakoshi H, Prejbisz A, Kocjan T, Naruse M, Stowasser M, Nishikawa T, Young WF, Gomez-Sanchez CE, Funder JW, Reincke M. Outcome of adrenalectomy for unilateral primary aldosteronism: International consensus and remission rates. Lancet Diabetes Endocrinol. 2017;5(9):689–699. doi: 10.1016/S2213-8587(17)30135-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baudrand R, Guarda FJ, Torrey J, Williams G, Vaidya A. Dietary Sodium Restriction Increases the Risk of Misinterpreting Mild Cases of Primary Aldosteronism. J Clin Endocrinol Metab. 2016;101(11):3989–3996. doi: 10.1210/jc.2016-1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brown JM, Siddiqui M, Calhoun DA, Carey RM, Hopkins PN, Williams GH, Vaidya A. Web Exclusive. The Unrecognized Prevalence of Primary Aldosteronism. Ann Intern Med. Published online 26 2020. doi: 10.7326/M20-0065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parasiliti-Caprino M, Lopez C, Prencipe N, Lucatello B, Settanni F, Giraudo G, Rossato D, Mengozzi G, Ghigo E, Benso A, Maccario M. Prevalence of primary aldosteronism and association with cardiovascular complications in patients with resistant and refractory hypertension. J Hypertens. 2020;38(9):1841–1848. doi: 10.1097/HJH.0000000000002441 [DOI] [PubMed] [Google Scholar]
- 43.Tsiavos V, Markou A, Papanastasiou L, Kounadi T, Androulakis II, Voulgaris N, Zachaki A, Kassi E, Kaltsas G, Chrousos GP, Piaditis GP. A new highly sensitive and specific overnight combined screening and diagnostic test for primary aldosteronism. Eur J Endocrinol. 2016;175(1):21–28. doi: 10.1530/EJE-16-0003 [DOI] [PubMed] [Google Scholar]
- 44.Funder JW. Primary Aldosteronism: At the Tipping Point. Ann Intern Med. Published online May 26, 2020. doi: 10.7326/M20-1758 [DOI] [PubMed] [Google Scholar]
- 45.Tunny TJ, Klemm SA, Stowasser M, Gordon RD. Angiotensin-responsive aldosterone-producing adenomas: postoperative disappearance of aldosterone response to angiotensin. Clin Exp Pharmacol Physiol. 1993;20(5):306–309. doi: 10.1111/j.1440-1681.1993.tb01690.x [DOI] [PubMed] [Google Scholar]
- 46.Guo Z, Nanba K, Udager A, McWhinney BC, Ungerer JPJ, Wolley M, Thuzar M, Gordon RD, Rainey WE, Stowasser M. Biochemical, Histopathological and Genetic Characterization of Posture Responsive and Unresponsive APAs. J Clin Endocrinol Metab. Published online June 9, 2020. doi: 10.1210/clinem/dgaa367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Daimon M, Kamba A, Murakami H, Takahashi K, Otaka H, Makita K, Yanagimachi M, Terui K, Kageyama K, Nigawara T, Sawada K, Takahashi I, Nakaji S. Association Between Pituitary-Adrenal Axis Dominance Over the Renin-Angiotensin-Aldosterone System and Hypertension. J Clin Endocrinol Metab. 2016;101(3):889–897. doi: 10.1210/jc.2015-3568 [DOI] [PubMed] [Google Scholar]
- 48.El Ghorayeb N, Bourdeau I, Lacroix A. Role of ACTH and Other Hormones in the Regulation of Aldosterone Production in Primary Aldosteronism. Front Endocrinol. 2016;7. doi: 10.3389/fendo.2016.00072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Markou A, Sertedaki A, Kaltsas G, Androulakis II, Marakaki C, Pappa T, Gouli A, Papanastasiou L, Fountoulakis S, Zacharoulis A, Karavidas A, Ragkou D, Charmandari E, Chrousos GP, Piaditis GP. Stress-induced Aldosterone Hyper-Secretion in a Substantial Subset of Patients With Essential Hypertension. J Clin Endocrinol Metab. 2015;100(8):2857–2864. doi: 10.1210/jc.2015-1268 [DOI] [PubMed] [Google Scholar]
- 50.Eisenhofer G, Durán C, Cannistraci CV, Peitzsch M, Williams TA, Riester A, Burrello J, Buffolo F, Prejbisz A, Beuschlein F, Januszewicz A, Mulatero P, Lenders JWM, Reincke M. Use of Steroid Profiling Combined With Machine Learning for Identification and Subtype Classification in Primary Aldosteronism. JAMA Netw Open. 2020;3(9):e2016209. doi: 10.1001/jamanetworkopen.2020.16209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tu W, Eckert GJ, Hannon TS, Liu H, Pratt LM, Wagner MA, Dimeglio LA, Jung J, Pratt JH. Racial differences in sensitivity of blood pressure to aldosterone. Hypertens Dallas Tex 1979. 2014;63(6):1212–1218. doi: 10.1161/HYPERTENSIONAHA.113.02989 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.