Abstract
Aims:
To identify when smoking cessation treatments affect craving, negative affect and anhedonia, and how these symptoms relate to abstinence, to help evaluate the effects of particular intervention components in multicomponent treatments and accelerate treatment refinement.
Design:
Secondary analysis of data from a 2-arm randomized controlled trial.
Setting:
Seven primary care clinics in Wisconsin, USA.
Participants:
Adult primary care patients who smoked daily (N=574).
Intervention and comparator:
Intervention was Abstinence-Optimized Treatment (A-OT, n=276) comprising 3 weeks of nicotine mini-lozenges pre-target quit day (TQD), 26 weeks of combination nicotine patch and mini-lozenges post-TQD, and extensive psychosocial support. Comparator was Recommended Usual Care (RUC, n=298) comprising brief counseling and 8 weeks of nicotine patch post-TQD.
Measurements:
Time-varying effect models examined dynamic effects of A-OT (versus RUC) on the primary outcomes of nightly cigarette craving, negative affect, and anhedonia from 1 week pre- to 2 weeks post-TQD. Exploratory models examined within-person relations between nicotine medication use and same-day symptom ratings. Secondary logistic regression analyses examined associations between post-TQD craving, negative affect and anhedonia and 1-month post-TQD abstinence.
Findings:
A-OT significantly suppressed pre- and post-TQD craving (β=−0.27 to −0.46 across days) and post-TQD anhedonia (β=−0.24 to −0.38 across days), relative to RUC. Within persons, using patches was associated with lower negative affect in RUC (β=−0.42 to −0.52), but not in A-OT. Using more mini-lozenges was associated with greater craving (β=0.04 to 0.07) and negative affect (β=0.03 to 0.05) early, and with lower anhedonia (β=−0.06 to −0.12) later. Greater post-TQD craving (OR=0.68) and anhedonia (OR= 0.85) predicted lower odds of abstinence 1 month post-TQD.
Conclusion:
Time-varying effect models showed that a multicomponent treatment intervention for smoking cessation suppressed significant withdrawal symptoms better than recommended usual care among daily adult smokers motivated to quit. The intervention reduced craving pre- and post-target quit day (TQD) and anhedonia post-TQD.
Keywords: Smoking cessation, withdrawal, time-varying effect modeling, multiphase optimization strategy, nicotine replacement therapy
Even with intensive smoking cessation treatment, long-term abstinence rates rarely exceed 30% [1, 2]. In addition, treatments often have modest effects on the symptoms that prompt relapses [3–5]. Thus, there is still substantial room for improvement in smoking cessation therapy. Better understanding of how and when treatments achieve their effects on particular targets predictive of abstinence may accelerate treatment refinement.
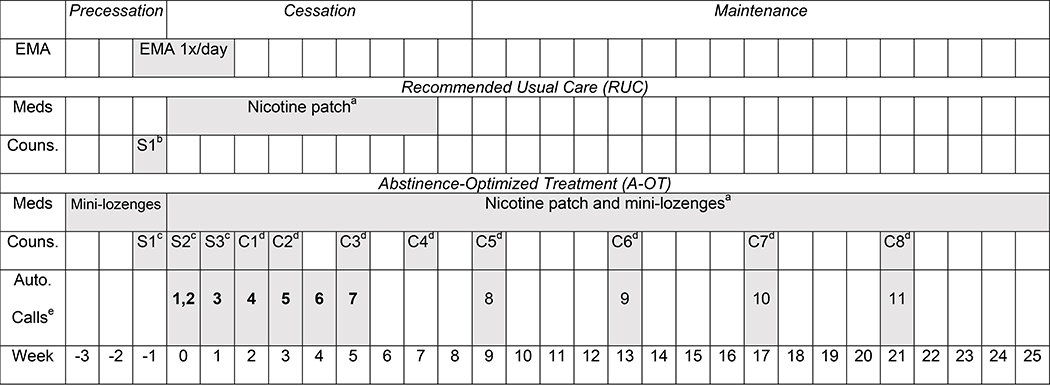
Baker and colleagues [6] have proposed using engineering principles [7] to design treatments that effectively target phase-specific challenges in the multi-phase smoking cessation process. The Phase-Based Model of Smoking Intervention [6] holds that different phases of treatment (i.e., motivation, precessation, cessation, and maintenance) may require different interventions. Informed by this model and the Multiphase Optimization Strategy (MOST) for intervention development [7, 8], factorial screening experiments identified effective phase-specific intervention components [9–11] that were then tested in a randomized controlled trial of a multicomponent treatment (RCT) [12]. The RCT compared an Abstinence-Optimized Treatment (A-OT) comprising promising precessation, cessation, and maintenance intervention components with lower-intensity Recommended Usual Care (RUC) [12]. In A-OT, participants received 3 weeks of precessation treatment involving fast-acting nicotine replacement therapy (NRT: nicotine mini-lozenges) and in-person counseling (Figure 1). Cessation-phase treatment comprised combination NRT (C-NRT: patch and mini-lozenge), 6 counseling sessions, and 7 automated calls through 8 weeks post-target-quit-day (TQD). Maintenance-phase treatment included C-NRT through 26 weeks post-TQD, 4 maintenance counseling calls, and 4 automated adherence reminders (for those still smoking 8 weeks post-TQD). A-OT was compared with RUC comprising brief counseling (a single clinic visit and referral to the state quit line and a stop-smoking app) and 8 weeks of nicotine patch therapy (Figure 1). Six-month post-TQD biochemically confirmed point-prevalence abstinence rates were 16% in A-OT and 6% in RUC [12]. Thus, A-OT is relatively effective, but needs improvement.
Figure 1. Timeline of study phases, ecological momentary assessment, and treatments by treatment condition.
a Participants were encouraged to use the full-course of medication, regardless of whether they had returned to smoking (unless they experienced intolerable side effects).
b In RUC, Session 1 (S1) involved a face-to-face counseling session of 10 minutes, fax referral to the Wisconsin Tobacco Quit Line, and instruction in downloading the QuitNow smartphone app.
c In A-OT, Session 1–3 (S1-S3) were 20-minute face-to-face counseling sessions.
d In A-OT, Calls 1–8 (C1-C8) were 15-minute counseling calls.
e In A-OT, all participants received the first 7 automated calls to promote medication adherence (shown in bold); only those who were still smoking at week 8 received automated calls 8–11.
The current study aimed to generate hypotheses about ways to refine A-OT by examining the time course of its effects on distinct symptoms thought to motivate smoking [13]. The goal of this approach is to identify how and when A-OT affects cravings to smoke, negative affect, and anhedonia (i.e., reduced responsivity to reward) in the critical pericessation period. Although craving, negative affect, and anhedonia do not occur only in the context of nicotine withdrawal [14–19], these symptoms are sensitive to nicotine deprivation [14, 20–22], predictive of difficulty quitting smoking [4, 23–25], and mediate pharmacotherapy effects on abstinence [4, 26, 27]. In addition, drug motivation theory identifies negative affect (from withdrawal or other sources) as the dominant driver of smoking motivation [28], and identifies craving as subjective awareness of high levels of such motivation [28, 29]. As such, these constructs are identified by theory as key predictors of smoking. Anhedonia, on the other hand, was recently identified as a nicotine withdrawal symptom and predictor of smoking [23, 25, 30–32] and has not been adequately assessed as a mediator of smoking cessation interventions. The current study seeks to address this gap by exploring the responsivity of anhedonia to pericessation treatment [33–35].
Treatment relations with abstinence are strongest during the pericessation period, as lapse and relapse survival curves show maximal differentiation by treatment immediately after the TQD [36]. We therefore examined treatment-symptom relations in the week preceding and two weeks following the TQD, and symptom relations with abstinence 1-month post-TQD (undiluted by distal stressors or other relapse precipitants). Although these effects will be imperfectly related to longer-term (e.g., 6-month) abstinence outcomes of public health importance, understanding which symptoms are sensitive to treatment during the pericessation period may generate hypotheses about ways to refine treatment packages such as A-OT by adjusting the dose, targets, or timing of particular intervention components. Clinically, this knowledge could help determine when to evaluate treatment responsivity and consider alternative or adaptive treatments.
Time-varying effect modeling (TVEM) is an analytical tool that can identify the periods of greatest treatment effect through graphical displays of relations between treatment and outcomes over time [37]. These models showed that the magnitude of NRT effects (versus placebo) on smoking declines after the first week post-TQD, and that craving and negative affect have different relations with smoking over the first two weeks of quitting [38]. The current study applied TVEM to data from the RCT of A-OT versus RUC [12] to examine the time course of treatment effects on pericessation craving, negative affect, and anhedonia, assessed nightly via interactive voice response (IVR) system. We also examined relations between these ratings and later (1-month post-TQD) point-prevalence abstinence via logistic regression analyses. Finally, we examined within-person relations between deviations in medication use from each individual’s average use [39] and craving, negative affect, and anhedonia severity to explore temporal patterns in within-person medication effects and identify when treatment use was most tightly associated with symptom severity.
Method
Data came from an RCT [12] in which 623 adult daily smokers motivated to quit smoking and recruited from primary care clinics were randomized to either A-OT (n=308) or RUC (n=315). Participants in both conditions completed 4 clinic visits and 6 phone follow-up assessments through 12-months post-TQD; those in A-OT completed 3 additional visits for cessation counseling.
Participants
Participants were recruited from 7 primary care clinics in two Wisconsin healthcare systems. During clinic visits, clinic staff were prompted by the electronic health record (EHR) to invite patients who smoked to participate in a smoking treatment study. Patients who accepted this offer were referred to study staff who called patients to screen them for eligibility. Inclusion criteria required participants be: over age 18, English-literate, smoking at least 5 cigarettes per day in the previous 6 months, motivated to quit smoking in the next 30 days, willing to refrain from using non-study medication during the study, and reachable by telephone. Study exclusion criteria included: current use of bupropion; history of stroke, heart attack, transient ischemic attack, or an abnormal electrocardiogram in the past 4 weeks; diagnosis or treatment of serious mental illness (schizophrenia, a psychotic disorder, bipolar disorder) in the past 10 years; and pregnancy or unwillingness to use an approved method of birth control during treatment. After the phone screen, individuals were invited to their clinic to confirm eligibility and provide written informed consent. Study procedures were approved by the University of Wisconsin Health Sciences Institutional Review Board.
Treatment
As shown in Figure 1, the experimental treatment, A-OT, comprised 3 weeks of pre-TQD mini-lozenges; 26 weeks of post-TQD C-NRT with nicotine patches and mini-lozenges; 3 in-person and 8 phone counseling sessions; and 7–11 automated calls to promote medication use (7 calls for those who reported abstinence by week 8, and 11 for those still smoking at week 8). As recommended by the 2008 PHS Guideline [2], 15–20 minute A-OT counseling sessions were designed to help participants prepare to quit, develop knowledge and skills to cope with craving and withdrawal, identify and avoid or mitigate triggers to smoke, and provide social support. Counseling protocols for both conditions are included in the supplementary material online.
RUC participants received less intensive treatment comprising: 8 weeks of nicotine patch starting on the TQD; a single, 10-min face-to-face counseling session; faxed referral to the Wisconsin Tobacco Quit Line (WTQL) for phone counseling; and instructions for installing a free smoking cessation mobile app (QUITNOW).
Measures
At an initial research visit, participant demographics (gender, ethnicity, age, marital status, education, and employment), smoking history, and exhaled carbon monoxide (CO) were assessed. Tobacco dependence was assessed with the Fagerström Test of Cigarette Dependence (FTCD; [40, 41]). Additional baseline assessments will not be discussed further.
Daily craving, negative affect and anhedonia.
Participants provided ecological momentary assessment (EMA) data nightly via IVR [42]. Participants were prompted to complete a report every night an hour before going to bed from 1 week pre-TQD through 2 weeks post-TQD to assess daily smoking, medication use (patch and mini-lozenge use), and how they felt in general over the day in terms of craving, negative affect, and anhedonia.
Craving was assessed by taking the average of 2 craving items, “Wanting to smoke” and “Bothered by urges to smoke” (r =.70) adapted from the Wisconsin Smoking Withdrawal Scale (WSWS; [43]) and rated on a scale from 1 (not at all) to 7 (extremely). Negative affect was assessed by taking the average of 3 adapted WSWS items, “Feeling anxious, worried or stressed,” “Feeling angry or irritated,” and “Feeling sad or unhappy” (Cronbach’s α=.81) rated on the same 7-point scale. Craving and negative affect scores were moderately correlated both pre-TQD (r=.40) and post-TQD (r=.52). Anhedonia was assessed with 3 EMA items [32] assessing pleasure experienced that day in 3 domains (social contact, school/work, and recreation) (α=.84) used in validated anhedonia scales [44, 45]. These items were rated on a scale ranging from 1 (no pleasure) to 7 (extreme pleasure), reverse coded so that higher scores indicated greater anhedonia. Anhedonia scores were weakly correlated with craving (r=−.02 pre-TQD, r=.10 post-TQD) and negative affect (r=.18 pre-TQD, r=.19 post-TQD).
Patch and mini-lozenge use.
Patches were available for at least 8 weeks post-TQD in both treatment conditions. Patch use was assessed nightly as a binary variable (1=used a patch, 0=no patch) from the TQD through 2 weeks post-TQD. The number of mini-lozenges used (range 0–25) was assessed nightly in the A-OT condition from 1 week pre- to 2 weeks post-TQD.
Cigarette counts.
The number of cigarettes smoked each day was assessed nightly from 1 week pre- to 2 weeks post-TQD in both conditions.
7-day point-prevalence abstinence 1-month post-TQD.
Abstinence was coded as binary (1= abstinent, 0=smoking or missing) based on self-reported smoking over the past 7 days collected via timeline follow-back interview [46].
Data analyses
Time-varying effects models were fit using SAS macro suite %TVEM, version 3.1.1 [47]. Parameter coefficients were estimated using maximum likelihood estimation and the p-spline method, which selects optimal regression coefficient functions with an optimal number of knots [48]. We examined time-varying effects of randomly assigned treatment condition (A-OT versus RUC) separately on craving, negative affect, and anhedonia from 1 week pre-TQD (when mini-lozenges and psychosocial treatment were available in A-OT) to 2 weeks post-TQD. Next, we explored within-person time-varying relations between patch use and symptoms in the combined sample, and the interaction between treatment condition and within-person patch effects post-TQD (patch use started on the TQD). In the A-OT condition only, we explored within-person relations between mini-lozenge use and symptoms, and the interaction between mini-lozenge and patch use over 2 weeks post-TQD (to see if mini-lozenge-symptom relations differed depending on patch use). To disaggregate within- and between-person effects, we person-mean-centered patch and mini-lozenge use variables and controlled for person-level averages of patch or mini-lozenge use in models. Exploratory models examining interactions between within-person patch or mini-lozenge use and smoking status in TVEM models are presented in supplementary material online.
Models were conducted both with and without the following covariates: baseline FTCD total score, clinical site (0=Milwaukee, 1=Madison), a binary indicator of assessment epoch (pre- vs. post-quit), a time-varying binary indicator of EMA-assessed daily smoking (1=smoked, 0=abstinent), and a post-quit indicator by daily smoking status interaction term. Model results are displayed graphically with 95% confidence intervals (CI) around daily average intercepts or coefficients (slopes). Slopes for a particular day are significantly different from 0 at α=0.05 if the 95% CI does not include 0. Models with and without covariates yielded similar results. We present models adjusted for FTCD and daily smoking status in the results and note where the adjusted and unadjusted models differed.
Logistic regression models separately tested relations between mean craving, negative affect, anhedonia, patch use, and mini-lozenge use rates in the first 2 weeks post-TQD and later intent-to-treat (with missing abstinence imputed as smoking), 7-day point prevalence abstinence 1-month post-TQD. Participants who provided at EMA data on at least one of the 21 days of interest were included in analyses to make use of all of available observations [48].
Results
Participant characteristics
A total of 11,038 EMA reports (90.4% of those scheduled) from the 574 participants with at least one EMA report (92.1% of the 623 randomized) were included in TVEM analyses. Table 1 presents demographics and baseline smoking history variables for the study sample, by treatment condition. The analyzed sample differed from those without sufficient data (n=49) in gender composition and age. More men (n=28, 10.5% of 266 enrollees) than women (n=21, 5.9% of 357 enrollees) failed to provide EMA data (χ2(1, N=623)=4.54, p=.03). Those retained were significantly older (M=50.1, SD=12.7 years) than those who attrited (M=45.2, SD=12.4 years, t(620)=2.58, p=.01).
Table 1.
Sample characteristics by treatment condition
| Total sample (n=574) | Recommended Usual Care (n=298) | Abstinence-Optimized Treatment (n=276) | |
|---|---|---|---|
| n (%) | |||
| Gender (Female) | 336 (58.5) | 179 (60.1) | 157 (56.9) |
| Race | |||
| White | 396 (69.1) | 200 (67.1) | 196 (71.3) |
| Minority group | 177 (30.9) | 98 (32.9) | 79 (28.7) |
| Education | |||
| Less than college | 315 (55.0) | 176 (59.3) | 139 (50.4) |
| College or more | 258 (45.0) | 121 (40.7) | 137 (49.6) |
| Annual household income | |||
| Less than $24,999 | 275 (53.2) | 139 (51.3) | 136 (55.3) |
| $25,000 or more | 242 (46.8) | 132 (48.7) | 110 (44.7) |
| Mean (SD) | |||
| Age | 50.1 (12.7) | 49.6 (12.9) | 50.7 (12.5) |
| Cigarettes per day | 16.8 (9.3) | 17.1 (9.8) | 16.4 (8.7) |
| FTCD score | 4.8 (2.2) | 4.9 (2.2) | 4.7 (2.2) |
| Quitting motivation (1–7) | 6.4 (1.0) | 6.4 (1.0) | 6.4 (1.0) |
| Quitting confidence (1–7) | 5.6 (1.4) | 5.5 (1.5) | 5.7 (1.4) |
FTCD: Fagerström Test of Cigarette Dependence.
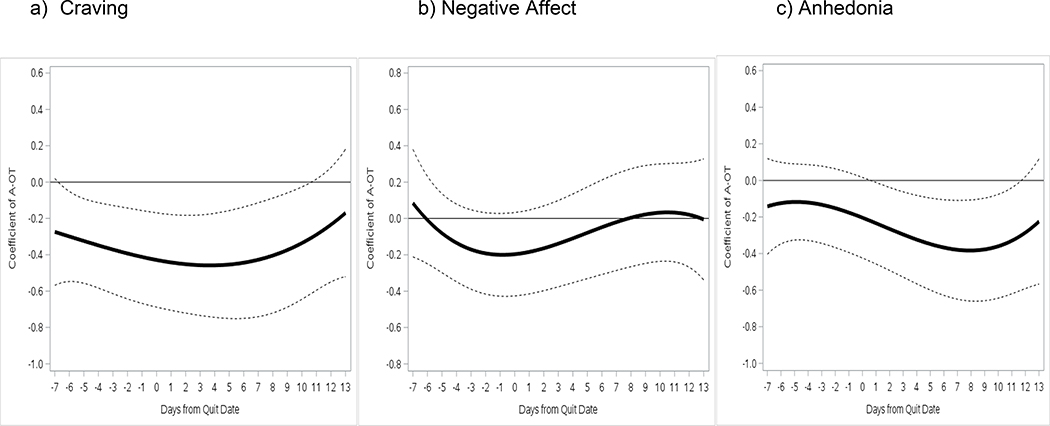
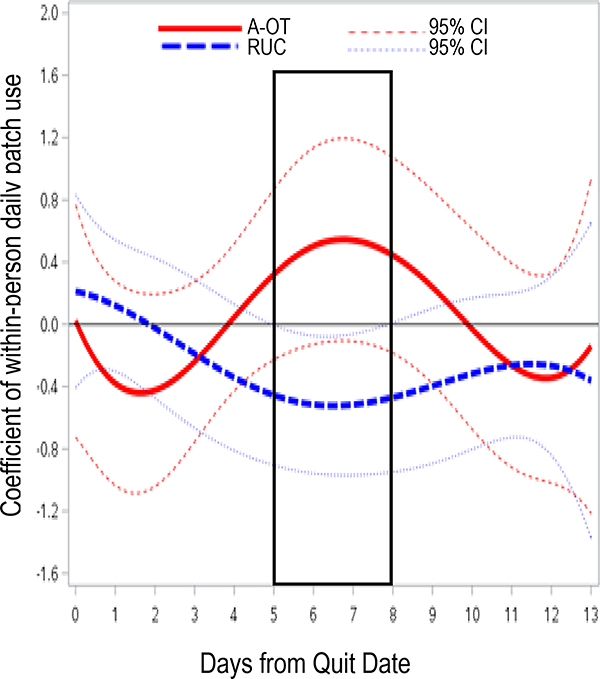
Time varying effects of A-OT versus RUC Time-varying effects of A-OT on symptoms, with baseline FTCD and daily smoking covariates, are shown in Figure 2. Relative to RUC, A-OT significantly suppressed craving from 6 days pre-TQD through 10 days post-TQD (β=−0.27 to −0.46). A-OT treatment did not significantly affect negative affect in models with covariates, but suppressed negative affect from 2 days pre- to 3 days post-TQD in unconditional models (not shown). A-OT significantly suppressed anhedonia on days 1–12 post-TQD (β=−0.24 to −0.38) relative to RUC. Site was associated with symptoms, such that Madison participants reported greater post-TQD craving and negative affect (not shown), but including site did not change the pattern of treatment effects. The indicators of site, assessment epoch (pre- vs. post-TQD), the non-significant interaction between epoch and daily smoking status were pruned from this and all subsequent models without changing the pattern of results.
Figure 2.
Time-varying associations between Abstinence-Optimized Treatment (A-OT) vs. Recommended Usual Care (RUC) and (a) craving, (b) negative affect, and (c) anhedonia, with corresponding 95% confidence intervals (dotted lines) from 1 week pre-TQD to 2 weeks post-TQD.
Time varying effects of within-person daily patch use
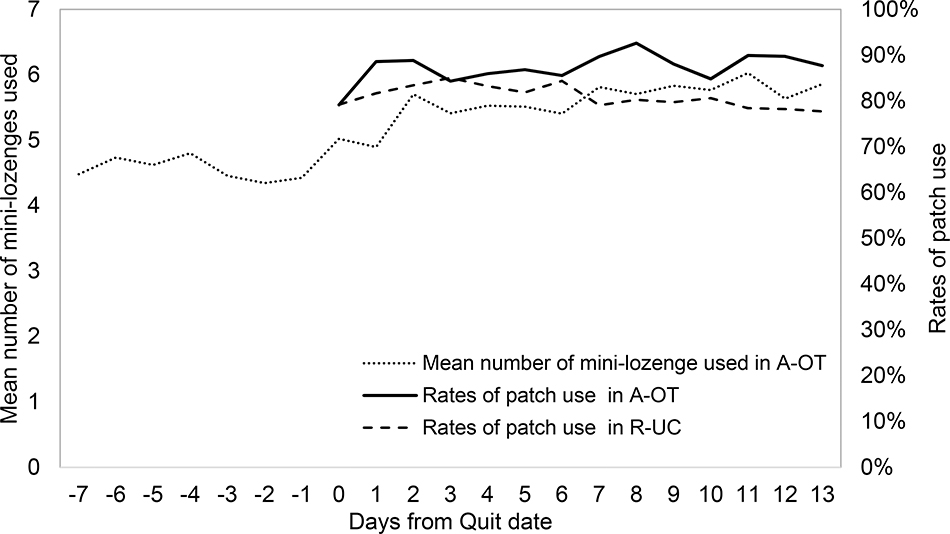
Figure 3 shows the rates of patch use by day through 2 weeks post-TQD in each treatment condition. Patch use rates exceeded 70% every day in both conditions, but were higher in A-OT than in RUC. Greater patch use predicted abstinence 1-month post-TQD in logistic regression (B=2.21, SE=0.42, Wald=28.21, OR=9.13, 95% CI=4.04–20.66, p<.001), and the relation between patch use and abstinence did not differ by condition (B=−0.06, SE=0.85, Wald=0.01, OR=0.93, 95% CI=0.18–4.89, p=0.93).
Figure 3.
Mean number of mini-lozenge used in A-OT during 1 week pre-TQD and 2 weeks post-TQD and rates of patch use in A-OT and RUC during 2 weeks post-TQD.
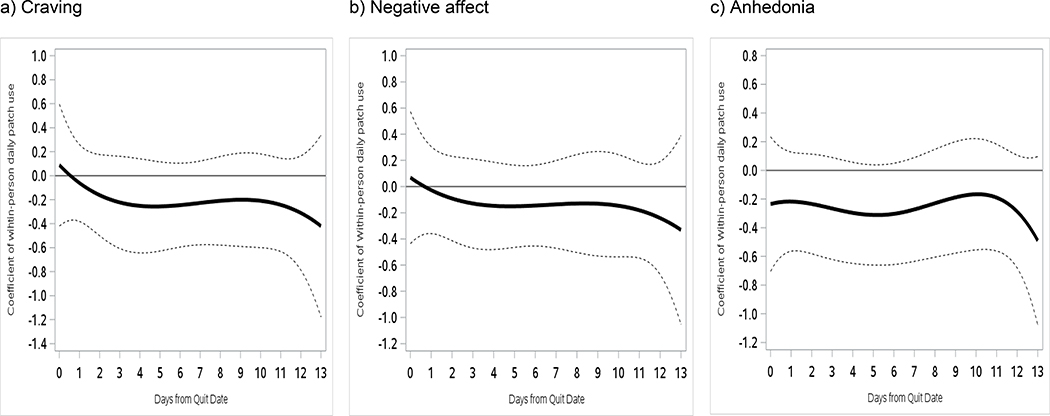
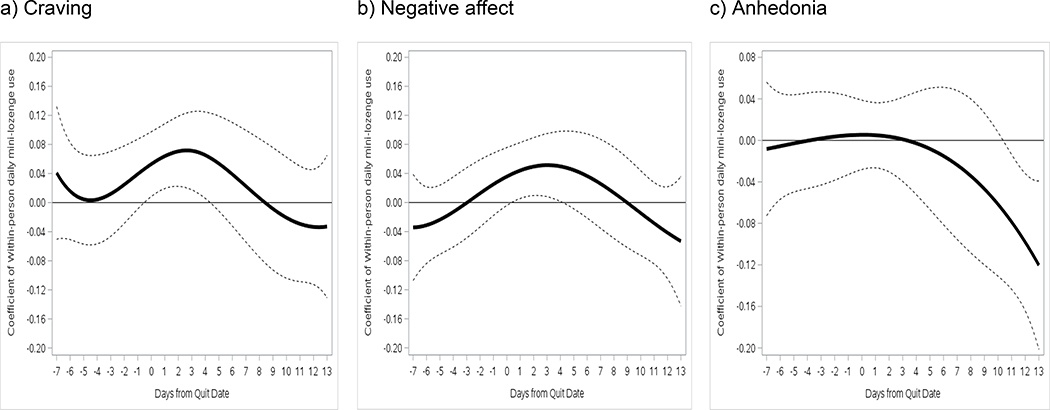
Figure 4 illustrates time-varying relations between within-person daily patch use and craving, negative affect, and anhedonia over 2 weeks post-TQD in the combined sample, controlling for baseline FTCD and daily smoking status. Within-person daily patch use was not significantly related to craving, negative affect, or anhedonia, meaning that symptom levels did not differ on days participants deviated from their usual patch use behavior (e.g., wearing one when this was rare for them). Patch use interacted significantly with treatment condition (on days 5–8), however (Figure 5). Patch use was associated with significantly lower negative affect 5–8 days post-TQD in RUC (β=−0.42 to −0.52), but not in A-OT (β=0.30 to 0.58).
Figure 4.
Time-varying main effects of within-person daily patch use on (a) craving, (b) negative affect, and (c) anhedonia from the TQD to 2 weeks post-TQD. 95% confidence intervals are indicated by dotted lines in all panels.
Figure 5.
Simple time-varying main effects of patch use on negative affect by treatment condition. Days on which there were significant interaction effects are the days within the black rectangle. 95% confidence intervals are indicated by dotted lines.
Time varying effects of within-person mini-lozenge use in A-OT
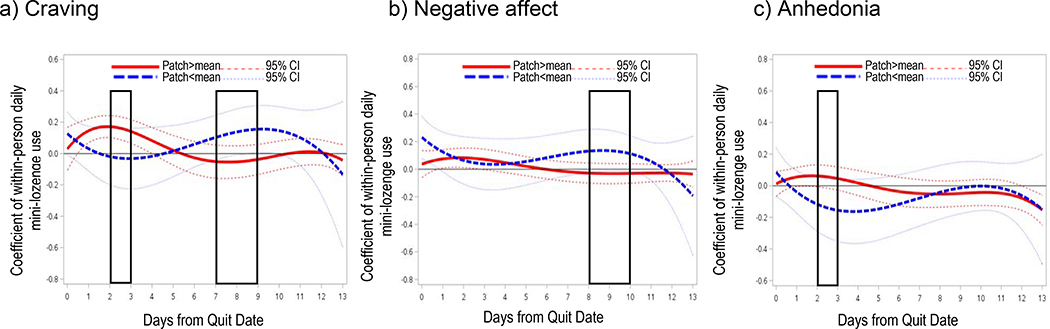
Daily means of self-reported number of mini-lozenges used are shown by day in Figure 3. Mini-lozenge count averages were below recommended levels (9–20 lozenges per day) throughout the assessment period, but increased post-TQD. Using more mini-lozenges on average did not significantly predict abstinence 1-month post-TQD in a bivariate logistic regression analysis (B=0.06, SE=0.04, Wald=2.47, OR=1.07, 95% CI=0.99–1.15, p=0.12). Within-persons (Figure 6), using more mini-lozenges than average was associated with greater craving from 1 day pre-TQD to day 5 post-TQD (β=0.04 to 0.07), greater negative affect in the first 4 days post-TQD (β=0.03 to 0.05), but reduced anhedonia on days 10–13 post-TQD (β=−0.06 to −0.12). Wearing a patch moderated relations between mini-lozenge use and symptoms at times. Spikes in mini-lozenge use were more strongly associated with worse craving (β=0.12 to 0.17) and anhedonia (β=0.03 to 0.06) on days 2–3 post-TQD if patch use was above average (i.e., was worn that day, but not all others), and more weakly associated with negative affect 8–10 days post-TQD (β=0.12 to 0.15) if patch use was below average (Figure 7).
Figure 6.
Time-varying main effects of within-person daily mini-lozenges use on (a) craving, (b) negative affect, and (c) anhedonia from 1 week pre-TQD to 2 weeks post-TQD. 95% confidence intervals are indicated by dotted lines in all panels.
Figure 7.
Simple time-varying main effects of within-person daily mini-lozenge use in A-OT on (a) craving, (b) negative affect, and (c) anhedonia by patch use over 2 weeks post-TQD. Days on which there were significant interaction effects are the days within the black rectangles. 95% confidence intervals are indicated by dotted lines.
Craving, negative affect, and anhedonia relations with abstinence
In multiple logistic regression analyses, mean post-TQD craving (B=−0.39, SE=0.08, Wald=26.59, OR=0.68, 95% CI=0.58–0.79, p<.001) and anhedonia (B=−0.16, SE=0.07, Wald=5.02, OR=0.85, 95% CI=0.74–0.98, p=.03) significantly predicted lower odds of abstinence 1-month post-TQD, but negative affect did not (B=−0.05, SE=0.09, Wald=0.32, OR=0.95, 95% CI=0.80–1.14, p=.57), despite a significant bivariate relation with abstinence (B=−0.30, SE=0.07, Wald=17.44, OR=0.74, 95% CI=0.64–0.85, p<.001). Abstinence odds were significantly higher in Madison than in Milwaukee (B=0.51, SE=.19, Wald=6.93, OR=1.66, 95% CI=1.14–2.42, p=.009).
Discussion
This secondary analysis of pericessation craving, negative affect, and anhedonia detected significant benefits of an effective multi-component smoking cessation treatment [12] in terms of craving and anhedonia suppression, in comparison with usual care. Craving suppression was evident in A-OT versus RUC in the week preceding the TQD, when psychosocial treatment was available in both conditions but mini-lozenges were available only in A-OT. A-OT suppression of craving continued through 10 days post-TQD, a period when A-OT received intensive psychosocial support and C-NRT, while RUC received patch monotherapy and much less psychosocial support. Anhedonia was significantly lower for the first 12 days of the quit attempt in A-OT versus RUC, as well. Negative affect was not significantly improved by A-OT, and an interaction showed that within-person patch use was associated with reduced negative affect only in RUC (not A-OT), and only when smoking (not abstinent). Mini-lozenge use was generally positively related to symptom severity, particularly if abstinent before the TQD. These results suggest that precessation medication may enhance craving control and that C-NRT and/or intensive psychosocial support may improve control of pericessation craving and anhedonia, motivationally significant symptoms that predict later abstinence. Although formal mediation analyses were not conducted, these results are consistent with previous research [4, 27, 32, 35, 49, 50].
Improved management of craving and anhedonia may be attributable to psychosocial components in A-OT, nicotine mini-lozenges, and/or higher rates of patch use in A-OT than in RUC. Between persons, higher rates of nicotine patch use, but not nicotine mini-lozenge use, predicted abstinence at 1-month post-TQD. Within participants, however, nicotine patch use was not associated with lower symptom ratings in A-OT. In RUC, using a patch when this was rare was associated with lower negative affect on days 5–8 post-TQD, but this was not true in A-OT, where daily patch use rates were high. In the first few days of the quit attempt, patch use was associated with lower symptoms levels only if a person was smoking; patch use was associated with higher symptoms on abstinent days. As such, differences in within-person acute patch use relations with symptoms do not seem to account for greater reductions in craving and anhedonia in A-OT than RUC.
Within persons in A-OT, use of more mini-lozenges was associated with greater craving and negative affect early in the quit attempt if abstinent, and with greater symptoms later in the quit attempt if not using a patch. This pattern of results indicates that spikes in mini-lozenge use might occur in response to more severe withdrawal. More mini-lozenge use was associated with reduced anhedonia during the end of the second week post-TQD, at least among those smoking or using patches. This may reflect a benefit of intensifying rather than tapering mini-lozenge use, but the clinical significance of this delayed effect is unclear. More use of ad lib NRT may sometimes quell symptoms or may sometimes reflect greater abstinence motivation or distress.
The following limitations should be considered when interpreting these results. First, we are not able to attribute the effects of A-OT on craving and anhedonia to specific components of A-OT, as this was a comparison of multicomponent treatments rather than a factorial or dismantling study that permits isolation of the effects of specific intervention components. The psychosocial components of A-OT treatment may have cumulative effects that cannot be pinpointed to specific days, particularly given that the dynamic effects of such components on mediators were not assessed. Medication use is also self-selected and likely related to smoking status [51], which also limits inferences that can be drawn from within-person medication use analyses. Second, we did not conduct formal mediation analyses or analyses of treatment effects on prolonged abstinence of more clinical significance (e.g., 6 months post-TQD) due to the complexity of examining treatment-mediator effects that vary by day. Third, the clinical significance of the roughly 0.2–0.4-point reductions observed in craving and anhedonia is difficult to state, although lower mean levels of craving and anhedonia were predictive of abstinence. Fourth, we did not account for random effects of clinics or site in computing standard errors [52]. However, treatment randomization and treatment delivery by research staff rather than clinic personnel possibly mitigate the confounding effects of clinics. Despite site differences in craving, negative affect, and abstinence, controlling for site did not change the pattern of results. Finally, the %TVEM macro does not yet support integration across multiple imputed datasets. Instead, maximum likelihood estimation was used to handle missing EMA data.
Despite these limitations, results demonstrate benefits of A-OT (versus RUC) in reduced craving and anhedonia. These results suggest that craving and anhedonia are sensitive to intensive treatment at both the precessation and cessation phases of treatment [6] and predictive of abstinence. More broadly, the time-varying effects observed highlight the value of a phase-based approach to smoking treatment, and of examining time as a moderator of treatment effects. Treatments may have different functional relations with smoking motivation at different points in time; understanding this may facilitate development of treatment packages aimed at varying targets (e.g., craving, anhedonia) to maximize benefits.
Supplementary Material
Acknowledgments
Funding: This research was supported by a grant 5P01CA180945-05 from the National Cancer Institute to the University of Wisconsin Center for Tobacco Research and Intervention and by the Wisconsin Partnership Program.
Footnotes
Declaration of interests: None
Compliance with Ethical Standards: The study was approved by the University of Wisconsin Health Sciences Institutional Review Board, and all participants gave written informed consent.
Clinical trial registration: ClinicalTrials.gov NCT02301403
References
- 1.Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev 2013(5):CD009329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz N, Curry SJ, et al. Treating tobacco use and dependence: 2008 update. Rockville, MD: U.S. Department of Health and Human Services, U.S. Public Health Service; 2008. [Google Scholar]
- 3.Baker TB, Collins LM, Mermelstein R, Piper ME, Schlam TR, Cook JW, et al. Enhancing the effectiveness of smoking treatment research: conceptual bases and progress. Addiction. 2016;111(1):107–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCarthy DE, Piasecki TM, Lawrence DL, Jorenby DE, Shiffman S, Baker TB. Psychological mediators of bupropion sustained-release treatment for smoking cessation. Addiction. 2008;103(9):1521–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCarthy DE, Piasecki TM, Jorenby DE, Lawrence DL, Shiffman S, Baker TB. A multi-level analysis of non-significant counseling effects in a randomized smoking cessation trial. Addiction. 2010;105(12):2195–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker TB, Mermelstein R, Collins LM, Piper ME, Jorenby DE, Smith SS, et al. New methods for tobacco dependence treatment research. Ann Behav Med 2011;41(2):192–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collins LM, Baker TB, Mermelstein RJ, Piper ME, Jorenby DE, Smith SS, et al. The Multiphase Optimization Strategy for engineering effective tobacco use interventions. Ann Behav Med 2011;41(2):208–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins LM, Murphy SA, Nair VN, Strecher VJ. A strategy for optimizing and evaluating behavioral interventions. Ann Behav Med 2005;30(1):65–73. [DOI] [PubMed] [Google Scholar]
- 9.Cook JW, Collins LM, Fiore MC, Smith SS, Fraser D, Bolt DM, et al. Comparative effectiveness of motivation phase intervention components for use with smokers unwilling to quit: a factorial screening experiment. Addiction. 2016;111(1):117–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piper ME, Fiore MC, Smith SS, Fraser D, Bolt DM, Collins LM, et al. Identifying effective intervention components for smoking cessation: a factorial screening experiment. Addiction. 2016;111(1):129–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schlam TR, Fiore MC, Smith SS, Fraser D, Bolt DM, Collins LM, et al. Comparative effectiveness of intervention components for producing long-term abstinence from smoking: a factorial screening experiment. Addiction. 2016;111(1):142–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piper ME, Cook JW, Schlam TR, Jorenby DE, Smith SS, Collins LM, et al. A randomized controlled trial of an optimized smoking treatment delivered in primary care. Ann Behav Med 2018;52(10):854–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baker TB, Brandon TH, Chassin L. Motivational influences on cigarette smoking. Annu Rev Psychol 2004;55:463–91. [DOI] [PubMed] [Google Scholar]
- 14.Leventhal AM, Trujillo M, Ameringer KJ, Tidey JW, Sussman S, Kahler CW. Anhedonia and the relative reward value of drug and nondrug reinforcers in cigarette smokers. J Abnorm Psychol 2014;123(2):375–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stone MD, Audrain-McGovern J, Leventhal AM. Association of anhedonia with adolescent smoking susceptibility and initiation. Nicotine Tob Res 2017;19(6):738–42. [DOI] [PubMed] [Google Scholar]
- 16.Kassel JD, Stroud LR, Paronis CA. Smoking, stress, and negative affect: Correlation, causation, and context across stages of smoking. Psychol Bull. 2003;129(2):270–304. [DOI] [PubMed] [Google Scholar]
- 17.Heinz AJ, Kassel JD, Berbaum M, Mermelstein R. Adolescents’ expectancies for smoking to regulate affect predict smoking behavior and nicotine dependence over time. Drug Alcohol Depend 2010;111(1–2):128–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hughes JR. Nicotine withdrawal, dependence and abuse In: Widiger TA, Frances AJ, Pincus HA, First MB, Ross R, Davis W, editors. DSM-IV sourcebook. Washington, D.C.: American Psychiatric Association; 1994. [Google Scholar]
- 19.Dar R, Rosen-Korakin N, Shapira O, Gottlieb Y, Frenk H. The craving to smoke in flight attendants: relations with smoking deprivation, anticipation of smoking, and actual smoking. J Abnorm Psychol 2010;119(1):248–53. [DOI] [PubMed] [Google Scholar]
- 20.West R, Hajek P. Evaluation of the mood and physical symptoms scale (MPSS) to assess cigarette withdrawal. Psychopharmacology (Berl). 2004;177(1–2):195–9. [DOI] [PubMed] [Google Scholar]
- 21.Hughes JR, Gust SW, Skoog K, Keenan RM, Fenwick JW. Symptoms of tobacco withdrawal. A replication and extension. Arch Gen Psychiatry 1991;48(1):52–9. [DOI] [PubMed] [Google Scholar]
- 22.Shiffman S Effect of nicotine lozenges on affective smoking withdrawal symptoms: secondary analysis of a randomized, double-blind, placebo-controlled clinical trial. Clin Ther 2008;30(8):1461–75. [DOI] [PubMed] [Google Scholar]
- 23.Leventhal AM, Piper ME, Japuntich SJ, Baker TB, Cook JW. Anhedonia, depressed mood, and smoking cessation outcome. J Consult Clin Psychol 2014;82(1):122–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cook J, Spring B, McChargue D, Doran N. Effects of anhedonia on days to relapse among smokers with a history of depression: a brief report. Nicotine Tob Res 2010;12(9):978–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leventhal AM, Waters AJ, Kahler CW, Ray LA, Sussman S. Relations between anhedonia and smoking motivation. Nicotine Tob Res 2009;11(9):1047–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lerman C, Roth D, Kaufmann V, Audrain J, Hawk L, Liu A, et al. Mediating mechanisms for the impact of bupropion in smoking cessation treatment. Drug Alcohol Depend 2002;67(2):219–23. [DOI] [PubMed] [Google Scholar]
- 27.Bolt DM, Piper ME, Theobald WE, Baker TB. Why two smoking cessation agents work better than one: role of craving suppression. J Consult Clin Psychol 2012;80(1):54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol Rev 2004;111(1):33–51. [DOI] [PubMed] [Google Scholar]
- 29.Curtin JJ, McCarthy DE, Piper ME, Baker TB. Implicit and explicit drug motivational processes: A model of boundary conditions In: Weirs RW, Stacy AW, editors. Handbook of implicit cognition and addiction. Thousand Oaks, CA: Sage; 2006. p. 233–50. [Google Scholar]
- 30.Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393(6680):76–9. [DOI] [PubMed] [Google Scholar]
- 31.Cook JW, Lanza ST, Chu W, Baker TB, Piper ME. Anhedonia: its dynamic relations with craving, negative affect, and treatment during a quit smoking attempt. Nicotine Tob Res 2017;19(6):703–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cook JW, Piper ME, Leventhal AM, Schlam TR, Fiore MC, Baker TB. Anhedonia as a component of the tobacco withdrawal syndrome. J Abnorm Psychol 2015;124(1):215–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hughes JR. Effects of abstinence from tobacco: etiology, animal models, epidemiology, and significance: a subjective review. Nicotine Tob Res 2007;9(3):329–39. [DOI] [PubMed] [Google Scholar]
- 34.Javitz HS, Lerman C, Swan GE. Comparative dynamics of four smoking withdrawal symptom scales. Addiction. 2012;107(8):1501–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCarthy DE, Piasecki TM, Fiore MC, Baker TB. Life before and after quitting smoking: an electronic diary study. J Abnorm Psychol 2006;115(3):454–66. [DOI] [PubMed] [Google Scholar]
- 36.Piasecki TM, Fiore MC, McCarthy DE, Baker TB. Have we lost our way? The need for dynamic formulations of smoking relapse proneness. Addiction. 2002;97(9):1093–108. [DOI] [PubMed] [Google Scholar]
- 37.Shiyko MP, Lanza ST, Tan X, Li R, Shiffman S. Using the time-varying effect model (TVEM) to examine dynamic associations between negative affect and self confidence on smoking urges: differences between successful quitters and relapsers. Prev Sci 2012;13(3):288–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vasilenko SA, Piper ME, Lanza ST, Liu X, Yang J, Li R. Time-varying processes involved in smoking lapse in a randomized trial of smoking cessation therapies. Nicotine Tob Res 2014;16 Suppl 2:S135–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoffman L, Stawski RS. Persons as contexts: evaluating between-person and within-person effects in longitudinal analysis. Res Hum Dev 2009;6:97–120. [Google Scholar]
- 40.Fagerstrom K Determinants of tobacco use and renaming the FTND to the Fagerstrom Test for Cigarette Dependence. Nicotine Tob Res 2012;14(1):75–8. [DOI] [PubMed] [Google Scholar]
- 41.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict 1991;86(9):1119–27. [DOI] [PubMed] [Google Scholar]
- 42.Stone AA, Shiffman S. Ecological momentary assessment (EMA) in behavorial medicine. Ann Behav Med 1994;16(3):199–202. [Google Scholar]
- 43.Welsch SK, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB. Development and validation of the Wisconsin Smoking Withdrawal Scale. Exp Clin Psychopharmacol 1999;7(4):354–61. [DOI] [PubMed] [Google Scholar]
- 44.Fawcett J, Clark DC, Scheftner WA, Gibbons RD. Assessing anhedonia in psychiatric patients. Arch Gen Psychiatry 1983;40(1):79–84. [DOI] [PubMed] [Google Scholar]
- 45.Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. Br J Psychiatry 1995;167(1):99–103. [DOI] [PubMed] [Google Scholar]
- 46.Robinson SM, Sobell LC, Sobell MB, Leo GI. Reliability of the Timeline Followback for cocaine, cannabis, and cigarette use. Psychol Addict Behav 2014;28(1):154–62. [DOI] [PubMed] [Google Scholar]
- 47.PennState Methodology Center. TVEM SAS Macro Suite (Version 3.1.1) [Software] 2018. Available from: http://methodology.psu.edu. [Google Scholar]
- 48.Tan X, Shiyko MP, Li R, Li Y, Dierker L. A time-varying effect model for intensive longitudinal data. Psychol Methods. 2012;17(1):61–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Piper ME, Schlam TR, Cook JW, Sheffer MA, Smith SS, Loh WY, et al. Tobacco withdrawal components and their relations with cessation success. Psychopharmacology (Berl). 2011;216(4):569–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Magee JC, Lewis DF, Winhusen T. Evaluating nicotine craving, withdrawal, and substance use as mediators of smoking cessation in cocaine- and methamphetamine-dependent patients. Nicotine Tob Res 2016;18(5):1196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shiffman S, Sweeney CT, Ferguson SG, Sembower MA, Gitchell JG. Relationship between adherence to daily nicotine patch use and treatment efficacy: secondary analysis of a 10-week randomized, double-blind, placebo-controlled clinical trial simulating over-the-counter use in adult smokers. Clin Ther 2008;30(10):1852–8. [DOI] [PubMed] [Google Scholar]
- 52.Luo W, Kwok OM. The impacts of ignoring a crossed factor in analyzing cross-classified data. Multivariate Behav Res 2009;44(2):182–212. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.