Abstract
Background:
Sapovirus is increasingly recognized as an important cause of acute gastroenteritis (AGE) in children. We identified risk factors and characterized the clinical profile of sapovirus AGE in a birth cohort in León, Nicaragua.
Methods:
We conducted a case-control study nested within a birth cohort (n=444). Fieldworkers conducted weekly household AGE surveillance. AGE stools were tested for sapovirus by RT-qPCR. For each first sapovirus episode, we selected two healthy age-matched controls and estimated independent risk factors of sapovirus AGE using conditional logistic regression. We compared clinical characteristics of sapovirus AGE episodes with episodes associated with other etiologies, and identified co-infections with other enteric pathogens.
Results:
From June 2017 to July 2019 we identified 63 first sapovirus AGE episodes and selected 126 controls. Having contact with an individual with AGE symptoms and vaginal delivery were independent risk factors for sapovirus AGE. All cases experienced diarrhea, lasting a median 6 days; 23% experienced vomiting. Compared to children with AGE due to another etiology, sapovirus AGE was similar in severity, with less reported fever. Most cases experienced co-infections, and were more likely than controls to be infected with diarrheagenic E. coli or astrovirus.
Conclusions:
Sapovirus was a commonly-identified AGE etiology in this Central American setting, and symptoms were similar to AGE associated with other etiologies. The association between vaginal delivery and sapovirus is a novel finding. Gut microbiome composition might mediate this relationship, or vaginal delivery might be a proxy for other risk factors. Further investigation into more specific biological mechanisms is warranted.
Keywords: sapovirus, diarrhea, gastroenteritis, children, Nicaragua
Introduction
Sapovirus, a genus in the Caliciviridae family, is increasingly recognized as an important cause of childhood acute gastroenteritis (AGE). Studies in high- and low-income countries have detected sapovirus in 3-17% of childhood AGE episodes [1–4]. Notably, the Malnutrition and Enteric Disease Study (MAL-ED), a cohort study with eight sites in sub-Saharan Africa, Asia, and South America, identified sapovirus as the second greatest contributor to the diarrhea burden in children under two years of age [5]. Furthermore, while earlier reports describe sapovirus AGE as less severe than norovirus and rotavirus AGE [6,7]. recent studies have shown that sapovirus infections can result in hospitalizations and severe dehydration [3,8].
Despite this high disease burden, little is known about risk factors for sapovirus AGE to guide prevention efforts. Unlike norovirus infection, sapovirus infection is not associated with host histo-blood group antigen phenotypes [3,9,10]. However, like norovirus, sapovirus is transmitted via the fecal-oral route, including foodborne transmission [11]. Household crowding is another reported risk factor for sapovirus AGE [2]. Thus, sapovirus is likely transmitted directly via close contact and indirectly via food, water, contaminated objects, or environmental surfaces [12]. One study reported an increased odds of disease following contact with a person with gastroenteritis inside (OR=4.4) or outside the household (OR=2.8) in the week prior to symptom onset [13].
The primary goal of this study was to identify risk factors for sapovirus AGE using a large population-based cohort of children. As no therapeutic agents or vaccine for sapovirus exist, identifying risk factors for sapovirus AGE may inform control efforts to reduce disease burden. Another goal of this study was to characterize the clinical profile of sapovirus AGE as compared to AGE associated with other etiologies. We can best represent the true spectrum of disease using active household AGE surveillance, avoiding biases associated with analysis of health care utilization data.
Methods
Study Design
The Sapovirus-Associated Gastro-Enteritis (SAGE) study is a population-based birth cohort study in León, Nicaragua with a nested case-control component. The recruitment period spanned from June 12, 2017 to July 31, 2018, during which mothers of all live-born singleton infants living in 14 contiguous health sectors in the Perla Maria Norori Health District (Perla) were offered study participation. Exclusion criteria included estimated gestational age <36 weeks, birthweight <2,000g, known chronic health condition, plans to move during the study period, known immune disorder or blood transfusion in the infant or mother within the past 9 months, or another household member already enrolled in the birth cohort. The study population included high-income families in the city center and low-income families in peri-urban neighborhoods, creating a scientifically-informative gradient to evaluate socioeconomic and environmental risk factors for sapovirus AGE. Informed consent for study participation was required of the child’s mother or legal guardian. The study was approved by the Institutional Review Boards of the National Autonomous University of Nicaragua, León (UNAN-León, acta No. 45, 2017) and the University of North Carolina at Chapel Hill (Study #: 16-2079).
During the initial household visit at 10-14 days since birth, trained female fieldworkers collected information on infant characteristics (e.g. sex, birth history, nutritional status), family characteristics (e.g. age, education, and employment of household members), and household characteristics (e.g. water sources, sanitation system, floor type). Subsequently, fieldworkers visited children in their households every seven days to assess for AGE, defined as the onset of diarrhea and/or vomiting, following at least three symptom-free days. Diarrhea was defined as an increase in stool frequency of at least three stools per 24-hour period or a substantial change in stool consistency (bloody, very loose, watery). Stool samples were requested from children for each AGE episode that occurred during the study. Detailed clinical characteristics were collected for each episode from the child’s caregiver, including maximum number of stools within 24 hours, fever, vomiting, blood in stool, and health care utilization for the episode. Additionally, information on recent potential risks or protective factors (e.g. breastfeeding, consumption of uncooked produce, consumption of seafood, eating outside the home, caregiver handwashing practices, attending social gatherings, contact with an individual inside or outside the household experiencing AGE during the past week) were collected at the time of the episode. Each month, field workers collected routine stool samples from each child, measured and weighed each child, and collected more extensive risk factor data, including factors that were unlikely to change on a weekly basis (e.g. water treatment measures, water storage, presence of animals in the household).
Stool samples were tested for sapovirus within 24 hours of collection using reverse transcription quantitative PCR (RT-qPCR). Within 48 hours, field workers returned to the households of any sapovirus-positive children and collected stools from 1) each parent, 2) the child’s caregiver (if different than the parent), 3) anyone who prepares food in the household, 4) any child age 12 years or under, and 5) anyone reporting AGE. For each first sapovirus case, we randomly selected two controls from within the SAGE cohort with no history of laboratory-confirmed sapovirus AGE, matched on age (+/− three months of the age of the case). We collected 1) AGE risk factor data from household contacts of the control children, and 2) stool samples from household contacts for one of the two control households. Stool samples from household contacts were analyzed for evidence of enteric infection. For control children, we did not collect stools at the time of onset of the case; rather, the most recent routine stool collected during monthly SAGE study visits was analyzed for evidence of asymptomatic infections (See Figure Supplemental Digital Content 1).
Specimen Collection and Laboratory Methods
Stool specimens were collected in the household within two hours of defecation, and transported in a sterile plastic container or in a soiled diaper at 4° C to the Microbiology Department of UNAN-León for analysis. A 10% (wt/vol) suspension of stool was prepared using phosphate-buffered saline (pH=7.2) and viral RNA extraction was performed using the QIAamp Viral RNA Mini Kit (Qiagen, Hilden, Germany), according to manufacturer’s instructions. Extracted viral RNA from stool suspensions was analyzed by reverse transcriptase quantitative polymerase chain reaction (RT-qPCR) for sapovirus as previously described [14]. Briefly, RT-qPCR was performed with the AgPath-ID OneStep RT-PCR Kit (Thermo Fisher Scientific, Waltham, MA, USA) using a 7500 Real-Time PCR System. A sample was considered sapovirus-positive if the Ct value was ≤ 35. To control for RT-PCR inhibitors, each stool sample was spiked with an internal control (MS2 phage, cat. ATCC 15597-B1). The expected Ct value ranged from 26 to 29. If inhibitors were detected, the samples were re-examined by diluting the RNA (1:10, 1:100 and 1:1000).
All case samples and half of the control samples were tested by qPCR for 12 other common enteric pathogens using oligonucleotide primers described by Liu. et 2014 in the multiplex qPCR platform [15]. These included adenovirus, astrovirus, norovirus GI/GII, rotavirus, Campylobacter jejuni/C. coli, Salmonella spp., Shigella spp./enteroinvasive Escherichia coli (EIEC), enteroaggregative E. coli (EAEC), enterotoxigenic E. coli (ETEC), enteropathogenic E. coli (EPEC), Cryptosporidium spp., and Giardia lamblia.
Statistical Analysis
This analysis covered the observation period from June 12, 2017 to July 31, 2019. First, we compared the clinical presentation of sapovirus AGE episodes to episodes in which sapovirus was not detected from the full cohort. We estimated the relative odds of experiencing clinical symptoms with binary responses (yes/no, present/absent), and differences in group means for clinical symptoms with continuous or count responses. The clinical severity of sapovirus AGE episodes was described using a scale of 0-15, in which points were assigned based on symptom severity (Diarrhea lasting 1-2 days = 1 point; 3-4 days = 2 points; 5+ days = 3 points. Vomiting lasting 1-2 days = 1 point; 3-4 days = 2 points; 5+ days = 3 points. Maximum of 4-5 stools per day = 1 point; 6-7 stools = 2 points; 8+ stools = 3 points. Presence of fever = 3 points. Received intravenous fluid for dehydration = 3 points). We used generalized estimating equations models to account for clustering within children who experienced multiple AGE episodes, and adjusted for the number of prior episodes reported.
Next, we investigated risk factors for sapovirus AGE episodes by comparing characteristics of cases and control children. We restricted this analysis to the first sapovirus AGE episode experienced by each child; the seven secondary episodes were analyzed separately. For each of the 63 first cases of sapovirus AGE, we selected two controls (n=126), for a final analytic sample of 189. In 177 case and control households, a total of 690 child contacts provided data on their history of sapovirus risk factors in the past week. Among those, 244 were contacts of a case child, and 446 were contacts of a control child. RT-qPCR analysis was performed on 160 stool samples from contacts of cases, and 125 from contacts of controls. Weight-for-age Z-scores (WAZ) and length-for-age Z-scores were calculated using WHO standards [16]. For categorical predictors, we used the Cochran-Mantel-Haenszel Chi-squared test and the Fisher’s exact test for cell sizes <5. For continuous predictors, we used the Student’s T-test to compare means between independent samples. Characteristics associated with sapovirus AGE below the α=0.1 level were included in a conditional logistic regression model to estimate odds ratios (OR) and 95% confidence intervals (CI) for individual characteristics, conditioning on the case-control matching structure.
Finally, we described the co-infections that were detected in the symptomatic stools of sapovirus cases and in the routinely-collected asymptomatic stools of half of the controls. We compared the proportions of cases and controls infected with other viruses, bacteria, and parasites using the Mantel-Haenszel chi-squared test. All analyses were conducted using SAS 9.4 (SAS Institute, Cary, North Carolina) software.
Results
Staff at local public health posts identified 991 women residing in Perla who were expected to give birth during the recruitment period, of whom 742 were successfully contacted in the home by SAGE fieldworkers. Of these, 20 reported spontaneous abortion or stillbirth; 137 met exclusion criteria; and 141 declined to participate. In total, 444 children were enrolled in the birth cohort.
During the observation period from June 12, 2017 to July 31, 2019, 358 children experienced 1,122 AGE episodes over 561.5 child-years. Stool samples were collected and analyzed for 971 (87%) of these episodes. Seventy (7.2%) of the stool samples among 63 children tested positive for sapovirus by RT-qPCR, for a crude incidence rate of 12.2 sapovirus AGE episodes/100 child-years. After performing a multiple imputation procedure for the missing stool samples, we estimated an adjusted incidence rate of 13.3 sapovirus AGE episodes/100 child-years (95% CI: 10.6, 16.8).
Sapovirus AGE cases had a median of six days of diarrhea and a maximum of six stools per day. One-quarter of cases reported vomiting (Table 1). Compared to AGE episodes associated with other known etiologies among all cohort participants (n=896), duration of symptoms, severity of episodes, and receipt of treatment for the episodes was similar between sapovirus and non-sapovirus episodes. Notably, fever was 48% less common in sapovirus AGE episodes compared to non-sapovirus episodes (OR: 0.52, 95% CI: 0.31, 0.91) (Table 1). Bloody stool was less common among sapovirus cases, while care-seeking in the emergency department was more common among sapovirus cases; however, the low prevalence of these outcomes led to imprecise estimates. Approximately one-third of children received zinc in accordance with local and international AGE treatment guidelines [17,18]. The most frequently-prescribed treatments for episodes of sapovirus and non-sapovirus etiologies were antibiotics, probiotics, and symptom-relieving agents. Sapovirus infections were less likely to be treated with antibiotics (OR: 0.76, 95% CI: 0.38, 1.55), and more likely to be treated with probiotics (OR: 1.49, 95% CI: 0.80, 2.80) than non-sapovirus infections, but it is unknown how treatment decisions were made at the point of care.
Table 1.
Clinical characteristics of sapovirus gastroenteritis episodes compared to episodes caused by other etiologies in a birth cohort of children in León, Nicaragua
| Sapovirus (n=70) | Other Etiologiesa (n=896) | Effect Measure (95% Confidence Interval)b | |
|---|---|---|---|
| Clinical characteristic | N (%) or median (IQR) | ||
| Diarrhea | 70 (100.0) | 861 (96.1) | - |
| Median duration in days | 6 (3, 9) | 5 (3, 8) | 1.22 (0.96, 1.55) |
| Median number of stools in a 24-hour period | 6 (4, 7) | 5 (4, 7) | 0.98 (0.90, 1.07) |
| Vomiting | 16 (22.9) | 223 (25.0) | 0.88 (0.50, 1.56) |
| Median duration in days | 2 (1, 3) | 1 (1, 3) | 1.02 (0.70, 1.48) |
| Fever (1 missing) | 19 (27.1) | 370 (41.3) | 0.52 (0.31, 0.91) |
| Blood in stool (1 missing) | 1 (1.4) | 38 (4.3) | 0.35 (0.05, 2.49) |
| Received care at primary care clinic (2 missing) | 22 (31.4) | 342 (38.3) | 0.76 (0.46, 1.27) |
| Received care at emergency department (2 missing) | 11 (15.7) | 112 (12.5) | 1.35 (0.71, 2.59) |
| Gastroenteritis severity score (scale 0-15)c | 5 (4, 6) | 5 (4, 7) | 0.98 (0.88, 1.10) |
| Received zinc (2 missing) | 26 (37.1) | 296 (33.1) | 1.17 (0.71, 1.94) |
| Received intravenous fluid (2 missing) | 1 (1.4) | 32 (3.6) | 0.41 (0.06, 3.01) |
| Received other medications in the last 7 days (4 missing) | 34 (48.6) | 460 (51.6) | 0.88 (0.54, 1.45) |
| Medications received | n=460 | ||
| Antibiotics | 9 (26.5) | 140 (30.4) | 0.76 (0.38, 1.55) |
| Probiotics | 11 (32.4) | 103 (22.4) | 1.49 (0.80, 2.80) |
| Symptom-relieving agent | 8 (23.5) | 120 (26.1) | 0.83 (0.94, 1.17) |
| Anti-helminthic | 0 (0.0) | 3 (0.65) | - |
| Unknown | 1 (2.9) | 5 (1.1) | - |
Includes astrovirus, norovirus GI/GII, and rotavirus.
Generalized estimating equations model comparing sapovirus AGE to non-sapovirus AGE. Adjusted for number of prior episodes and clustering of multiple episodes within children. Binary outcomes analyzed using logistic models to estimate odds ratios, and continuous outcomes were analyzed using log-linear models to estimate differences in group means between cases and controls.
Severity score calculated based on duration of diarrhea and or vomiting symptoms, the maximum number if stools reported per day, presence of fever, and receipt of intravenous fluid for dehydration. Adapted from the scale developed by Lee, et al.
Seven children experienced two sapovirus AGE episodes. Relative to first sapovirus AGE episodes, second episodes lasted half as long and a lower proportion experienced vomiting (14.3%); none reported fever or bloody stool, and none required emergency treatment. The overall severity score was lower for the second sapovirus AGE episode compared to the first (see Table, Supplemental Digital Content 2).
The first sapovirus AGE episode occurred at a mean age of 12.5 months (median: 10.7 months).
Sapovirus cases were more likely than controls to have been delivered vaginally versus by Cesarean delivery (p=<0.0001); to have a mother who completed primary education or less (p=0.04); to have a pig (p=0.06) and/or “other” animals (i.e. horses and ducks, p=0.01) in the household; and were twice as likely to have had contact with an person with AGE symptoms in the past week (p=0.06) (Table 2). Among 57 cases and 115 controls for which stool samples were collected from household contacts, cases had five contacts who tested positive for sapovirus whereas controls had none (p=0.002) (Table 2). Characteristics pertaining to socioeconomic status, household sanitation, personal hygiene, nutrition, interpersonal contact, and AGE risk factors were comparable between cases and controls.
Table 2.
Characteristics of sapovirus cases relative to age-matched controls in a birth cohort of children in León, Nicaraguaa.
| Characteristic n (%) or mean (STD) | Cases (n=63) | Controls (n=126) | p |
|---|---|---|---|
| Mean age at the time of sapovirus case (in months) | 12.5 (6.1) | 13.1 (5.7) | 0.6 |
| Birth Characteristics | |||
| Sex (% female) | 28 (44.4) | 67 (53.2) | 0.3 |
| Mode of delivery (% vaginal delivery) | 48 (76.2) | 58 (46.0) | <0.0001 |
| Gestational age at birth (in completed weeks) | 38.7 (1.1) | 38.7 (1.2) | 0.8 |
| Mean birthweight (in grams) | 3135.6 (394) | 3126.9 (420) | 0.9 |
| Mean age of mother (years) at child’s birth | 23.6 (5.1) | 23.1 (5.0) | 0.5 |
| Socioeconomic Indicators | |||
| Maternal educational attainment (Missing: 2 cases) | |||
| Completed primary education or less | 21 (34.4) | 26 (20.6) | 0.04 |
| Completed any secondary education | 40 (65.6) | 100 (79.4) | |
| Mother employed at time of child’s birth | 11 (17.5) | 23 (18.3) | 0.9 |
| Poverty index (% poor or extremely poor) (Missing: 3 cases, 5 controls) | 33 (55.0) | 70 (57.9) | 0.7 |
| Crowding index (>2.5 people/bedroom) (Missing: 3 cases, 5 controls) | 17 (28.3) | 34 (28.1) | 1.0 |
| Floor type (% non-dirt) | 39 (61.9) | 85 (67.5) | 0.5 |
| Household Sanitation | |||
| Water source (% municipal in home) | 50 (79.4) | 101 (80.2) | 0.9 |
| Sanitation type (% indoor toilet) | 40 (63.5) | 89 (70.6) | 0.3 |
| Shares sanitation (toilet/latrine) with another home | 3 (4.8) | 8 (6.4) | 0.7 |
| Any water storage in the home | 33 (52.4) | 58 (46.0) | 0.4 |
| Always uses ≥1 effective means of water purificationb | 8 (12.7) | 12 (9.5) | 0.5 |
| Water source interruption in the past week | 8 (12.7) | 9 (7.1) | 0.2 |
| Animals in the home, any | |||
| Dog | 34 (54.0) | 74 (58.7) | 0.8 |
| Cat | 20 (31.7) | 28 (22.2) | 0.2 |
| Chickens | 16 (25.4) | 24 (19.1) | 0.3 |
| Pig | 7 (11.1) | 5 (4.0) | 0.06 |
| Rabbit | 3 (4.8) | 2 (1.6) | 0.3 |
| Bird | 6 (9.5) | 15 (11.9) | 0.6 |
| Turtle | 0 (0.0) | 1 (0.8) | 1.0 |
| Mice | 7 (11.1) | 7 (5.6) | 0.2 |
| Other, including horses and/or ducks | 7 (11.1) | 2 (1.6) | 0.01 |
| Personal Hygiene | |||
| Mother practices handwashing at appropriate momentsc | 46 (73.0) | 96 (76.2) | 0.6 |
| Use of alcohol hand sanitizer in home (% mothers using at least sometimes) | 10 (15.9) | 26 (20.6) | 0.4 |
| Nutrition | |||
| Ever breastfed | 63 (100.0) | 126 (100.0) | - |
| Currently breastfeeding | 46 (73.0) | 84 (66.7) | 0.4 |
| Mean age of introduction of any supplementary foods/drink (in weeks) | 3.5 (3.0) | 3.1 (1.4) | 0.3 |
| Mean Weight-for-Age Z-score | 0.1 (1.0) | 0.4 (1.0) | 0.1 |
| Mean Length-for-Age Z-score | 0.003 (1.6) | 0.3 (1.4) | 0.1 |
| Mean BMI-for-Age Z score | 0.2 (1.2) | 0.2 (1.3) | 0.8 |
| Ate uncooked fruit/vegetable in the past week | 54 (85.7) | 107 (84.9) | 0.9 |
| Ate seafood in the past week | 11 (17.5) | 24 (19.1) | 0.8 |
| Ate outside the home in the past week (Missing: 1 case) | 20 (32.3) | 45 (35.7) | 0.6 |
| Shared a bottle or cup with another person in the past week | 29 (46.0) | 46 (36.5) | 0.2 |
| Interpersonal Contact | |||
| Other child in home in diapers | 6 (9.5) | 17 (13.5) | 0.4 |
| Attended a social event in the past week | 16 (25.4) | 37 (29.4) | 0.6 |
| Met/played with a child outside the home in the past week (Missing: 2 cases, 8 controls) | 20 (32.8) | 53 (44.9) | 0.1 |
| Used public transportation in the past week | 21 (33.3) | 40 (31.8) | 0.8 |
| Went swimming in the past week | 1 (1.6) | 2 (1.6) | - |
| Had contact with anyone with diarrhea and/or vomiting in the past week | 12 (19.1) | 12 (9.5) | 0.06 |
| Gastroenteritis risk factors | |||
| Completed rotavirus vaccination | 63 (100.0) | 124 (98.4) | 0.6 |
| Prior episode of gastroenteritis (of any cause) | 45 (71.4) | 97 (77.0) | 0.4 |
| Child had contact with someone with sapovirusd (n=57 cases, 115 controls) | 5 (8.1) | 0 (0.0) | 0.002 |
Cases were matched 1:2 with controls ± 3 months of age. Infants with a history of sapovirus gastroenteritis by RT-qPCR were not eligible to be controls. Controls who were selected more than once were included multiple times in the predictors analysis, and controls who later became cases were included in both case and control columns. P-values have not been adjusted for multiple comparisons, or for individuals who contributed data multiple times.
Water treatment options include: using a water filter (including sand and mud/ceramic filters); boiling water; adding bleach or chlorine; solar disinfection; straining through a cloth; letting water settle; purchasing purified water.
Appropriate moments include: after caring for a sick person; before eating; before preparing food; after using the bathroom; after changing diapers.
Stool samples were requested from contacts of all cases and contacts of half of the controls. Analysis is restricted to case and control children for whom at least one contact provided a stool sample for sapovirus testing.
The conditional logistic regression model adjusted for all bivariate predictors below the α=0.1 level and residual age difference between cases and controls after age-matching. Because none of the control children had a sapovirus-positive household contact, we could not include this variable in the adjusted model. While presence of “other” animals was higher among cases than controls, we deemed this category too heterogeneous to be informative in the multivariable analysis. In the adjusted model, vaginal birth (adjusted OR: 5.03 [95% CI: 2.14, 11.80]) and having had contact with a person with AGE symptoms in the past week (adjusted OR: 3.23 [95% CI: 1.12, 9.28]), remained associated with increased odds of sapovirus AGE at α<0.05 (Table 3).
Table 3.
Associations between selected predictors and sapovirus case status in a birth cohort of children in León, Nicaragua
| Crude OR (95% CI) | Adjusted OR (95% CI)a | |
|---|---|---|
| Vaginal vs. Cesarean birth | 4.34 (2.03, 9.27) | 5.03 (2.14, 11.80) |
| Mother completed any secondary education vs. less | 0.51 (0.26, 0.98) | 0.57 (0.27, 1.24) |
| Pig in the home vs. no pig | 3.18 (0.79, 15.0) | 3.11 (0.80, 12.16) |
| Had contact with anyone with diarrhea and/or vomiting in the past week | 2.16 (0.92, 5.05) | 3.23 (1.12, 9.28) |
| Child had contact with someone with sapovirusb (n=57 cases, 115 controls) | 13.5 (2.44, ∞) | - |
Model adjusted for all other variables in the column and age difference between cases and controls. Bivariate predictors at p<0.1 from Table 2 were included in the adjusted model, except for presence of “other” animals in the home.
Stool samples were requested from contacts of all cases and contacts of half of the controls. Analysis is restricted to case (n=58) and control (n=117) children for whom at least one contact provided a stool sample for sapovirus testing.
Most sapovirus AGE cases (n=58, 92%) were co-infected with another enteric pathogen, including 21 (33%) with viral co-infections and 54 (86%) with bacterial co-infections (Figure 1, see Table, Supplemental Digital Content 3). The most common co-infections were observed with diarrheagenic E. coli (n=49), including EAEC (n=29), EPEC (n=22), and ETEC (n=21), and EIEC (n=4) (see Table, Supplemental Digital Content 3). Infections with these pathogens were less prevalent among asymptomatic children, affecting 36 (60.0%) of the controls. Prevalence of sapovirus (100.0% vs. 8.3%, p<0.0001), astrovirus (19.1% vs. 5.2%, p=0.03), and diarrheagenic E. coli (77.8% vs. 53.3%, p=0.004) were higher among cases than controls (see Table, Supplemental Digital Content 3).
Figure 1.
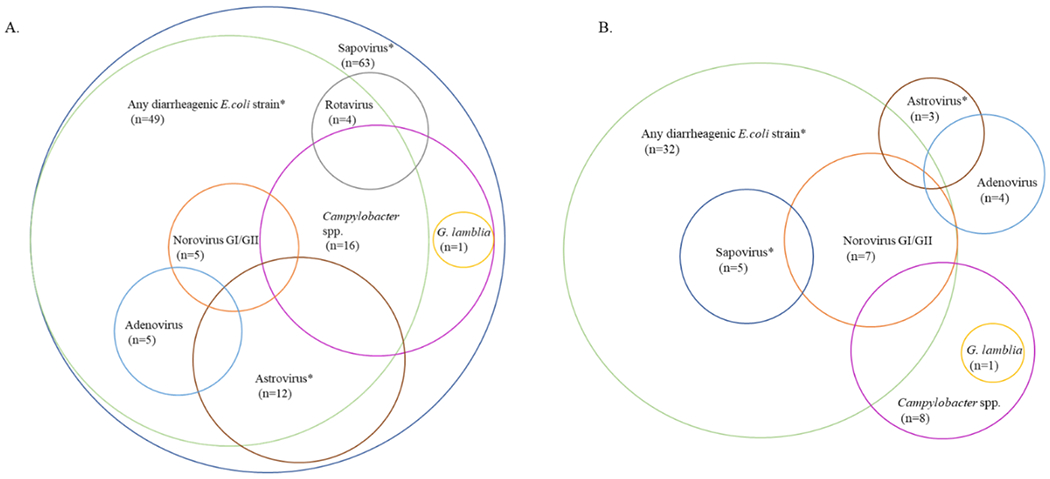
Viral, bacterial and parasitic co-infections among sapovirus cases and controls in a birth cohort of children on León, Nicaragua.
The Venn diagrams depict the combinations of enteric pathogens that were detected in the stools of A) sapovirus cases (n=63), and B) age-matched asymptomatic controls (n=60; complete RT-qPCR results were missing for 3 controls). Escherichia coli (E. coli) species include Shigella spp./enteroinvasive E. coli, enteroaggregative E. coli, enterotoxigenic E. coli, and enteropathogenic E. coli. Pathogens marked with an asterisk (*) are more prevalent in cases compared with controls at α=0.05 (sapovirus: p<0.0001; astrovirus: p=0.03; any E. coli strain: p=0.004).
Discussion
We found a high burden of sapovirus AGE in this population-based birth cohort of young children. Approximately one in seven children experienced an AGE episode associated with sapovirus over one year. Also, the clinical severity of the sapovirus AGE episodes was similar to AGE episodes in which sapovirus was not detected. This study adds to the growing body of literature describing the clinical presentation and risk factors for sapovirus gastroenteritis to aid in treatment and prevention efforts.
Relative to studies of childhood sapovirus AGE in Japan and Peru [6,19], the duration of symptoms was slightly longer in our study, which may reflect the longer duration of the first episodes of sapovirus AGE that we included in our analyses. Our findings concur with those from South Africa demonstrating 7.7% detection of sapovirus in diarrheal stools of hospitalizing children [2], but one study from Burkina Faso detected sapovirus in 18% of children under the age of five in a community-based study [3]. In our study and others, sapovirus more commonly detected in children older than six months of age [2–5], and feature diarrhea in all episodes and vomiting in a minority of episodes [6]. Due to a small number of sapovirus reinfections (n=7), the current analysis was restricted to understanding risk factors for the first sapovirus episode. Extended follow-up through 36 months of age is ongoing for children enrolled in the SAGE cohort, allowing us to capture a larger number of reinfections. A future publication will describe the molecular epidemiology of sapovirus infections, and elucidate the potential for type-specific reinfection.
Most children with sapovirus AGE were co-infected with another enteric pathogen, and other studies of gastroenteritis etiologies conducted in low- and middle-income countries have made similar findings. The MAL-ED study identified an average of 3.4 pathogens (SD 2.0) in diarrheal stools and 2.5 pathogens (SD 1.8) in non-diarrheal stools [5]. We found that sapovirus was rarely detected in control stools, supporting the association of sapovirus infection with clinical symptoms. However, several of the co-infections, such as EAEC, EPEC, and ETEC, have been previously found to be equally present in diarrheal and non-diarrheal stools in this setting, raising questions about the relative importance of each pathogen in causing disease [20]. Poorly-understood synergistic interactions between pathogens might be associated with clinical manifestations [21,22].
We identified two independent risk factors for infection. Not surprisingly, having contact with an individual with diarrhea or vomiting in the past week was associated with increased odds of sapovirus AGE. The authors further hypothesize that sapovirus transmission occurs frequently within households, and that the detection of sapovirus in the stools of household members would be strongly positively associated with the odds of sapovirus in the children under observation. However, incomplete stool collection from household members (160/244 cases and 125/446 controls) precluded the assessment of this variable as a predictor of sapovirus risk. We were most surprised to find that being born by vaginal delivery was associated with an increased odds sapovirus AGE. We conducted sensitivity analyses to understand if vaginal delivery may be a marker of increased socioeconomic status or of having contact with multiple children in the household, factors that might be associated with sapovirus AGE risk. In these models, vaginal delivery remained an independent risk factor for sapovirus AGE. It is well-established that the gut microbiome differs in infants born by vaginal vs. Cesarean delivery, with vaginal bacteria predominating in infants born vaginally, and skin bacteria predominating in infants born by Cesarean delivery [23,24]. Furthermore, maternal defecation that occurs during vaginal delivery may introduce other bacterial species to the newborn, leading to perturbations in the gut microbiome and causing the newborn to have gut microbiome composition similar to that of the mother [25,26]. The influence of delivery mode on the infant’s gut microbiome composition may persist for several years after birth [27]. The differences in the gut microbiome may result in differences in the development of infant’s immune system, that may alter an infant’s susceptibility to viral enteric infections [28,29]. Furthermore, in animal models there is evidence that norovirus infection is enhanced in the presence the human gut microbiome, suggesting that interactions between norovirus and gut microbiota facilitate infection [30]. Further research is needed to elucidate biological mechanisms that mediate sapovirus AGE risk, including the potential role of the infant gut microbiome.
This study is the first to suggest that presence of pigs was associated with increased odds of sapovirus AGE; however, the estimate from the conditional logistic regression model was imprecise, and the data are also compatible with a null association. It is possible that the presence of pigs may be an indicator of decreased hygiene in the household. Study participants were more frequently recruited from peri-urban households than from urban households, and cases were more frequent in peri-urban households. Consequently, our study might be underpowered to assess risk factors associated with urbanicity. Analyses of geographical factors associated with sapovirus AGE risk are ongoing.
Our study demonstrated the burden and severity of sapovirus AGE in this setting. Efforts to further reduce the burden of childhood AGE after the global roll-out of rotavirus vaccines should, in addition to norovirus, target sapovirus. Our risk factor analysis suggested the importance of limiting contact with symptomatic individuals both inside and outside the household to avoid disease transmission, and suggests future investigation to understand a possible role of household animals in mediating risk. While we found an association between vaginal delivery and sapovirus AGE risk, avoidance of vaginal delivery is not recommended because of to the overwhelming benefits of vaginal delivery to the mother and newborn [35]. However, this finding might indicate future studies to understand sapovirus pathogenesis and, potentially, interventions to modify gut microbiome composition that may prevent sapovirus AGE in children.
Supplementary Material
Supplemental Digital Content 2. Clinical characteristics of first and second sapovirus gastroenteritis episodes in a birth cohort of children in León, Nicaragua (table)
Supplemental Digital Content 3. Enteric pathogens detected in case and control stools in a birth cohort of children in León, Nicaragua (n=125) (table)
Supplemental Digital Content 1. Data and biospecimen collection procedures for case-control study (figure)
Acknowledgements
We greatly appreciate the participation of the parents of the children contributing to the SAGE cohort, and recognize the immense efforts of the laboratory and fieldwork team: Merling Balmaceda, Vanessa Bolaños, Nancy Corea, Jhosselyng Delgado, Marvel Fuentes, Yadira Hernandez, Llurvin Madriz, Patricia Mendez, Yuvielka Martinez, Maria Mendoza, Ruth Neira, Xiomara Obando, Veronica Pravia, Yorling Picado, Aura Scott, and Mileydis Soto. The authors are grateful for support from the local Ministry of Health office in León, and we thank the personnel from the Perla Maria Norori Health Center for their administrative support in recruitment of study participants. The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Funding: This work was supported by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health [R01AI127845 and K24AI141744 to S.B.D. and S.V.] and the Fogarty International Center [D43TW010923 to L.G., F.G., and Y.R.].
Funding: This study was supported by award R01AI127845 from the National Institute of Allergy and Infectious Diseases (NIAID). FG, YR, and OZ are supported by an international research capacity-building award from the Fogarty International Center D43TW010923. SBD and SV are supported by K24AI141744 from the NIAID.
References:
- [1].Chhabra P, Payne DC, Szilagyi PG, et al. Etiology of viral gastroenteritis in children <5 years of age in the United States, 2008-2009. J. Infect. Dis 2013;208:790–800. [DOI] [PubMed] [Google Scholar]
- [2].Page N, Groome MJ, Murray T, et al. Sapovirus prevalence in children less than five years of age hospitalised for diarrhoeal disease in South Africa, 2009-2013. J. Clin. Virol 2016;78:82–88. [DOI] [PubMed] [Google Scholar]
- [3].Matussek A, Dienus O, Djeneba O, et al. Molecular characterization and genetic susceptibility of sapovirus in children with diarrhea in Burkina Faso. Infect. Genet. Evol [Internet]. 2015;32:396–400. Available from: 10.1016/j.meegid.2015.03.039. [DOI] [PubMed] [Google Scholar]
- [4].Phan TG, Trinh QD, Yagyu F, et al. Outbreak of sapovirus infection among infants and children with acute gastroenteritis in Osaka City, Japan during 2004-2005. J. Med. Virol 2006; [DOI] [PubMed] [Google Scholar]
- [5].Platts-Mills JA, Liu J, Rogawski ET, et al. Use of quantitative molecular diagnostic methods to assess the aetiology, burden, and clinical characteristics of diarrhoea in children in low-resource settings: a reanalysis of the MAL-ED cohort study. Lancet Glob. Heal 2018;6:e1309–e1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sakai Y, Nakata S, Honma S, et al. Clinical severity of Norwalk virus and Sapporo virus gastroenteritis in children in Hokkaido, Japan. Pediatr. Infect. Dis. J 2001;20:849–853. [DOI] [PubMed] [Google Scholar]
- [7].Pang X, Honma S, Nakata S, et al. Human Caliciviruses in Acute Gastroenteritis of Young Children in the Community. J. Infect. Dis 2000;181:S288–S294. [DOI] [PubMed] [Google Scholar]
- [8].Bucardo F, Reyes Y, Svensson L, et al. Predominance of norovirus and sapovirus in nicaragua after implementation of universal rotavirus vaccination. PLoS One. 2014;9:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bucardo F, Carlsson B, Nordgren J, et al. Susceptibility of children to sapovirus infections Nicaragua, 2005-2006. Emerg. Infect. Dis 2012;18:1875–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Shirato-Horikoshi H, Ogawa S, Wakita T, et al. Binding activity of norovirus and sapovirus to histo-blood group antigens. Arch. Virol 2007;152:457–461. [DOI] [PubMed] [Google Scholar]
- [11].Oka T, Wang Q, Katayama K, et al. Comprehensive review of human sapoviruses. Clin. Microbiol. Rev 2015;28:32–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Nenonen NP, Hannoun C, Svensson L, et al. Norovirus GII.4 detection in environmental samples from patient rooms during nosocomial outbreaks. J. Clin. Microbiol 2014;52:2352–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].de Wit MA, Koopmans MP, van Duynhoven YT. Risk Factors for Norovirus, Sapporo-like Virus, and Group A Rotavirus Gastroenteritis. Emerg. Infect. Dis 2003;9:1563–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Oka T, Katayama K, Hansman G, et al. Detection of human sapovirus by real-time reverse transcription-polymerase chain reaction. J. Med. Virol 2006;78:1347–1353. [DOI] [PubMed] [Google Scholar]
- [15].Liu J, Kabir F, Manneh J, et al. Development and assessment of molecular diagnostic tests for 15 enteropathogens causing childhood diarrhoea: A multicentre study. Lancet Infect. Dis 2014; [DOI] [PubMed] [Google Scholar]
- [16].World Health Organization. The WHO Child Growth Standards. [Google Scholar]
- [17].Khan WU, Sellen DW. Zinc supplementation in the management of diarrhoea [Internet]. e-Library Evid. Nutr. Actions Geneva, Switzerland: World Health Organization; 2011. [cited 2020 Apr 2]. Available from: https://www.who.int/elena/titles/bbc/zinc_diarrhoea/en/. [Google Scholar]
- [18].Ministerio de Salud. Normativa No. 017: Guía para la Atención Clínica de las Enfermedades y Accidentes más comunes de la Infancia para niños y niñas de 1 mes a 5 años de edad. Managua, Nicaragua; 2018. p. 177–194. [Google Scholar]
- [19].Liu X, Jahuira H, Gilman RH, et al. Etiological role and repeated infections of sapovirus among children aged less than 2 years in a cohort study in a peri-urban community of Peru. J. Clin. Microbiol 2016;54:1598–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Becker-Dreps S, Bucardo F, Vilchez S, et al. Etiology of childhood diarrhea after rotavirus vaccine introduction: A prospective, population-based study in Nicaragua. Pediatr. Infect. Dis. J 2014;33:1156–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lindsay B, Ramamurthy T, Gupta S Sen, et al. Diarrheagenic pathogens in Polymicrobial infections. Emerg. Infect. Dis 2011;17:606–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Azevedo M, Mullis Li, Agnihothram S. Viral and bacterial co-infection and its implications. SciFed Virol Res J. 2017;1: 10.23959/sfjv-1000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Dominguez-Bello MG, Costello EK, Contreras M, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. U. S. A 2010;107:11971–11975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hill CJ, Lynch DB, Murphy K, et al. Evolution of gut microbiota composition from birth to 24 weeks in the INFANTMET Cohort. Microbiome. 2017;5:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Reyman M, van Houten MA, van Baarle D, et al. Impact of delivery mode-associated gut microbiota dynamics on health in the first year of life. Nat. Commun [Internet]. 2019;10:1–12. Available from: 10.1038/s41467-019-13014-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bäckhed F, Roswall J, Peng Y, et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe. 2015;17:690–703. [DOI] [PubMed] [Google Scholar]
- [27].Jakobsson HE, Abrahamsson TR, Jenmalm MC, et al. Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by Caesarean section. Gut. 2014;63:559–566. [DOI] [PubMed] [Google Scholar]
- [28].Mazmanian SK, Cui HL, Tzianabos AO, et al. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–118. [DOI] [PubMed] [Google Scholar]
- [29].Olszak T, An D, Zeissig S, et al. Microbial Exposure During Early Life Has Persistent Effects on Natural Killer T Cell Function. Science (80-. ). 2012;336:489–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lei S, Twitchell EL, Ramesh AK, et al. Enhanced GII.4 human norovirus infection in gnotobiotic pigs transplanted with a human gut microbiota. J. Gen. Virolory 2019;100:1530–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Li J, Zhang W, Cui L, et al. Metagenomic identification, genetic characterization and genotyping of porcine sapoviruses. Infect. Genet. Evol [Internet]. 2018;62:244–252. Available from: 10.1016/j.meegid.2018.04.034. [DOI] [PubMed] [Google Scholar]
- [32].Wang L, Marthaler D, Fredrickson R, et al. Genetically divergent porcine sapovirus identified in pigs, United States. Transbound. Emerg. Dis 2020;67:18–28. [DOI] [PubMed] [Google Scholar]
- [33].Blandon P Detección y Caracterización Molecular de Sapovirus en niños con gastroenteritis y en cerdos asintomáticos de la ciudad de León [Internet]. Universidad Nacional Autónoma de Nicaragua; 2011. Available from: http://riul.unanleon.edu.ni:8080/jspui/bitstream/123456789/6020/1/223016.pdf. [Google Scholar]
- [34].Yang B, Yang B, Shan X, et al. Short communication: Immune responses in sows induced by porcine sapovirus virus-like particles reduce viral shedding in suckled piglets. Res. Vet. Sci [Internet]. 2018;117:196–199. Available from: 10.1016/j.rvsc.2017.12.016. [DOI] [PubMed] [Google Scholar]
- [35].Sandall J, Tribe RM, Avery L, et al. Short-term and long-term effects of caesarean section on the health of women and children. Lancet [Internet]. 2018;392:1349–1357. Available from: 10.1016/S0140-6736(18)31930-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 2. Clinical characteristics of first and second sapovirus gastroenteritis episodes in a birth cohort of children in León, Nicaragua (table)
Supplemental Digital Content 3. Enteric pathogens detected in case and control stools in a birth cohort of children in León, Nicaragua (n=125) (table)
Supplemental Digital Content 1. Data and biospecimen collection procedures for case-control study (figure)


