Abstract
Purpose
Our aim was to develop a novel approach for lung cancer screening among a diverse population that integrates the Centers for Medicare and Medicaid Services (CMS) recommended components including shared decision making (SDM), low-dose CT (LDCT), reporting of results in a standardized format, smoking cessation, and arrangement of follow-up care.
Methods
Between October of 2015 and March of 2018, we enrolled patients, gathered data on demographics, delivery of SDM, reporting of LDCT results using Lung-RADS, discussion of results, and smoking cessation counseling. We measured adherence to follow-up care, cancer diagnosis, cancer treatment, and smoking cessation at 2 years after initial LDCT.
Results
We enrolled 505 patients who were 57% African American, 30% Caucasian, 13% Hispanic, < 1% Asian, and 61% were active smokers. All participants participated in SDM, 88.1% used a decision aid, and 96.1% proceeded with LDCT. Of 496 completing LDCT, all received a discussion about results and follow-up recommendations. Overall, 12.9% had Lung-RADS 3 or 4, and 3.2% were diagnosed with lung cancer resulting in a false-positive rate of 10.7%. All 48 patients with positive screens but no cancer diagnosis adhered to follow-up care at 1 year, but only 35.4% adhered to recommended follow-up care at 2 years. The annual follow-up for patients with negative lung cancer screening results (Lung-RADS 1 and 2) was only 23.7% after one year and 2.8% after 2 years. All active smokers received smoking cessation counseling, but only 11% quit smoking.
Conclusion
The findings show that an integrated lung cancer screening program can be safely implemented in a diverse population, but adherence to annual screening is poor.
Keywords: Lung cancer screening, Low-dose CT scan, Diverse population, African American, Adherence to lung cancer screening
Introduction
Lung cancer is the most common cause of cancer death in the United States, with estimated 228,000 new cases of lung cancer and 143,000 lung cancer deaths occurring annually [1]. In 2011, the National Lung Screening Trial (NLST) demonstrated a 20% reduction in lung cancer mortality using low-dose computed tomography (LDCT) [2]. The overall benefits of lung cancer screening can be attributed to the 95% adherence to screening protocols. A review by Bach et al. showed reduced compliance in cohort studies, particularly studies involving screening protocols with unstructured implementation [3]. The United States Preventive Services Task Force Services (USPSTF) and Centers for Medicare and Medicaid (CMS) issued guidelines for lung cancer screening implementation [4, 5]. These requirements include a shared decision-making (SDM) visit discussing potential benefits and harms of screening, use of a decision aid, LDCT scan with specific parameters for lung cancer screening, discussion of results with the patient, multidisciplinary follow-up of screening results, and smoking cessation counseling. It is unknown if all of these required elements of lung cancer screening can be implemented, especially in diverse populations. Uptake of lung cancer screening in the United States is less than 4% among those eligible [6]. Furthermore, racial disparities in knowledge and utilization of lung cancer screening continue to exist, with lower levels of knowledge and use noted in particular among African Americans when compared to their Caucasian counterparts [7, 8]. For ethnic minorities, individuals with low socioeconomic status, and persons with poor access to health care, the multiple required steps of lung cancer screening may pose a barrier and ultimately result in a disparity of lung cancer care [9–11]. We hypothesized that by integrating SDM, LDCT, reporting of results, smoking cessation counseling, and coordination of follow-up care, we could implement lung cancer screening according to CMS standards. We sought to test this in a predominantly African-American, urban population of low socioeconomic status in Philadelphia.
Methods
We conducted a prospective single-institution feasibility study of an integrated approach to lung cancer screening. All protocols and procedures were approved by the Temple University Institutional Review Board.
Pilot study infrastructure
We developed a community-based engagement strategy to enhance awareness and uptake of lung cancer screening. We visited churches, community centers, and health fairs to discuss lung cancer risk and lung cancer screening. We handed out pamphlets with instructions on how to proceed with screening if eligible. We also engaged and educated a pre-existing system of “block captains” in Philadelphia who are appointed representatives of neighborhoods. We developed a separate engagement strategy directed at referring physicians. We created printed and web-based information about lung cancer screening criteria, the risks and benefits of screening, and essential components of screening including SDM, use of a decision aid, standardized reporting, follow-up care, and smoking cessation. We encouraged health-care providers to engage people at high risk for lung cancer in a discussion about lung cancer screening and refer eligible patients to our integrated lung cancer screening protocol. We visited annual practice meetings, faculty meetings, and individual clinics in the surrounding community to help physicians overcome the barriers of implementing lung cancer screening. We also created continuing medical education (CME) seminars to disseminate detailed information about screening from the perspective of radiologists, interventional radiologists, pulmonologists, oncologists, radiation oncologists, and specialists. At last, we streamlined the process of lung cancer screening by creating a single order, either within the electronic medical record or paper form that can be mailed or faxed to the lung cancer screening program. A lung cancer screening nurse then contacted the patient to confirm eligibility for screening, arrange an integrated lung cancer screening visit, and arrange insurance payment or payment plan (Fig. 1).
Fig. 1.
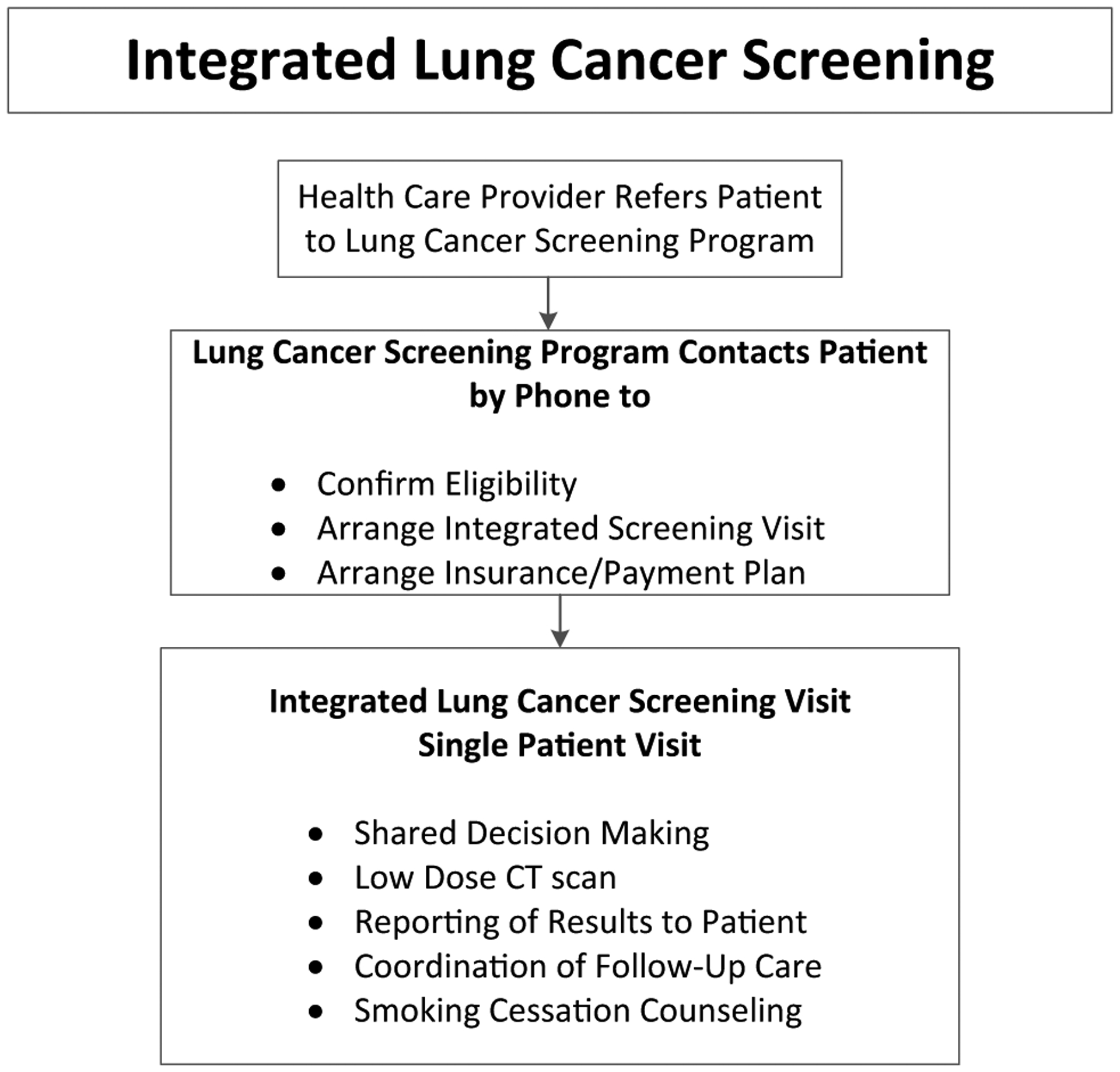
In the integrated lung cancer screening process, patients receive confirmation of eligibility and arrange for a screening visit. Screening participants receive shared decision making, low-dose CT (LDCT) scanning, LDCT results, smoking cessation counseling, and arrangement of follow-up care in a single visit
Our multidisciplinary lung cancer screening team consisted of thoracic surgeons, pulmonologists, thoracic radiologists, physician assistants, nurse practitioners, primary care physicians, and nurse coordinators. We created an integrated lung cancer screening (Fig. 1) that aimed to deliver SDM, LDCT, explanation of results to the patient, smoking cessation counseling, and discussion about follow-up care, including information on annual screening. During the lung cancer screening visit, a lung cancer screening specialist (physician, nurse practitioner, or physician assistant in the Department of Thoracic Medicine and Surgery) and patients participated in an SDM discussion using a decision aid [12, 13]. LDCT was performed according to guidelines outlined by the American College of Radiology [14]. An official radiology report was provided within 24 h by a chest radiologist using the Lung-RADS reporting system [15]. The same lung cancer screening specialist discussed LDCT results with each patient and arranged for appropriate follow-up. If the official chest radiologist’s interpretation was not available, patients received preliminary results from the lung cancer screening specialist and later received a phone call if there were any changes to the interpretation or recommended follow-up. Patients with negative lung cancer screens, i.e., those with Lung-RADS 1 or 2 interpretations, received recommendations for annual screening. Patients with Lung-RADS 0, meaning inadequate screen, received a recommendation for additional imaging. Patients with Lung-RADS 3 and 4 received personalized recommendation for appropriate diagnostic imaging, consultations with specialists (interventional radiologist, pulmonologist, thoracic surgeon, oncologists), and diagnostic procedures. Patients diagnosed with cancer received recommendations for treatment options based on their stage and their complete clinical presentation. Patients chose a multidisciplinary plan that best suited their goals of care. Patients with positive screens, but no evidence of cancer, on follow-up imaging and diagnostic procedures, were counseled to repeat imaging annually after the initial, baseline LDCT.
Former smokers and the lung cancer screening specialist discussed importance of staying smoke free, and active smokers received counseling consisting of the five major steps to intervention (5 A’s: Ask, Advise, Assess, Assist, and Arrange) [16]. The treatment options discussed included counseling and counseling plus pharmacotherapy (nicotine replacement using nicotine patches, nicotine lozenges, nicotine gum, and/or oral medications including bupropion or varenicline). We measured treatment plan chosen by the patient. Measurement of time to progress through the integrated visit was beyond the scope of this study, but a typical integrated visit lasted about 3 h.
Participants
Between 1 October 2015 and 1 March 2018, patients with orders for lung cancer screening from their physician were enrolled in a single-visit lung cancer screening. Participants who were eligible for lung cancer screening (between ages 55 and 77 had a smoking history of at least 30 pack-years and were either active smokers or had quit smoking within the past 15 years) were eligible for this study. We chose eligibility criteria were based on guidelines established by CMS [5] because traditionally 84% of patients seeking care at our institution are covered by Medicare or Medicaid. Each participant provided written informed consent for lung cancer screening and a separate consent for participation in our integrated protocol for lung cancer screening study. Information on patient demographics and smoking history was collected. Estimated household income was determined by median household income of the zip code of the participant.
Assessment of lung cancer screening implementation
We measured lung cancer screening referrals, number of patients eligible for lung cancer screening, and patients entering into our study. Of patients participating in the integrated lung cancer screening, we measured the number of patients who participated in an SDM visit and number who used a decision aid as documented in each patient’s electronic medical record. We measured the number of patients who declined screening and the number of patients who proceeded with LDCT. Of patients participating in LDCT, we measured the number who received Lung-RADS interpretation and discussion of results and recommended follow-up. We measured the number of smokers who received any smoking cessation counseling and the number who received greater than 10 min of counseling. We measured patient engagement in smoking cessation by measuring acceptance of treatment options and adherence to counseling appointments for smoking cessation. We also measured patient-reported smoking status at 2 years either by telephone, in person during follow-up visits within the health system or query of the electronic medical record. Information was collected on follow-up participation in annual screening, follow-up imaging and procedures, and subsequent lung cancer diagnoses, treatment, stage, and complications. For patients who did not follow-up with recommended care (annual screening or diagnostic work-up), the electronic medical record was surveyed for any lung cancer diagnosis, treatment, or complications from diagnostic or treatment procedures resulting from lung cancer screening, smoking status, and death. Social security death records were queried for mortality of all participants. Data were stored in a secure database. Descriptive analyses were reported as mean, median, or percentage, as appropriate.
Results
Participants of lung cancer screening
During the study period, 532 patients were referred to the integrated lung cancer screening program. Of these, 26 did not meet criteria for lung cancer screening, and one declined participation in the study. Therefore, 505 patients participated in our lung cancer screening study. The patient characteristics are described in Table 1. The participants were 51% women, 49% male with an average age of 63.8 (standard deviation (SD) 5.6; interquartile range (IQR) 8.4). Race and ethnicity of patients were 56.8% African American, 30.1% Caucasian, 12.7% Hispanic, and 0.4% Asian. The average household income was $33,726 (SD12348.5; IQR 16,281); the median household income was $28,516. The mean number of pack-years was 51 (SD 25.6, IQR 22), median of 45, range of 30–275. Active smokers comprised 61% of participants.
Table 1.
Demographics of patients undergoing integrative lung cancer screening
| Patient characteristics | Number | Percentage (%) |
|---|---|---|
| Total enrolled in study | 505 | (100) |
| Gender | ||
| Male | 250 | (49.5) |
| Female | 255 | (50.5) |
| Age (mean 63.8 y; SD 5.6; IQR 8.4) | ||
| 55–59 | 160 | (31.7) |
| 60–69 | 274 | (54.3) |
| > 70 | 71 | (14.0) |
| Race | ||
| African American | 287 | (56.8) |
| Caucasian | 152 | (30.1) |
| Hispanic | 64 | (12.7) |
| Asian | 2 | (0.4) |
| Household income | ||
| Average (SD) | $33,726 (12,348.5) | |
| Median | $28,516 | |
| IQR | 16,281 | |
| Education level | ||
| ≤ 8th grade | 36 | (7.1) |
| 9–11th grade | 127 | (25.1) |
| High school | 175 | (34.7) |
| Some college | 26 | (5.1) |
| Associate’s degree | 70 | (13.9) |
| Bachelor’s degree | 29 | (5.7) |
| Graduate degree | 23 | (4.6) |
| Unknown/declined to answer | 19 | (3.7) |
| Smoking history | ||
| Mean pack-years (SD) | 51 (25.6) | |
| Median pack-years | 45 | |
| Range | 30–275 | |
| IQR | 22 | |
| Active smokers | 308 | (61.0) |
| Former smokers | 197 | (39.0) |
SD standard deviation, IQR interquartile range
Lung cancer screening implementation
All 505 patients who enrolled in our study participated in an SDM visit, and 445 (88.1%) used a decision aid (Table 2). Nine patients (1.8%) declined LDCT after SDM. Of the 496 patients receiving LDCT, all received Lung-RADS interpretation and a discussion of these results and discussion regarding recommended follow-up. Most patients (427, 86.1%) participated in follow-up discussion on the same day as the LDCT. The remainder preferred to return for another visit or receive a phone call to discuss the results and recommended follow-up. Of 308 active smokers, all received smoking cessation counseling; 281 (91.2%) received greater than 10 min of counseling.
Table 2.
Implementation of integrated lung cancer screening
| Number | Percent (%) | |
|---|---|---|
| Total | 505 | |
| Participation in shared decision making | 505 | (100) |
| Use of a shared decision-making tool | 445 | (88.1) |
| Declined lung cancer screening | 9 | (1.8) |
| Received low-dose CT scan | 496 | (96.1) |
| Active smokers | 308 | (60.1) |
| Smoking cessation counseling | 308 | (100) |
| > 10 min of counseling | 281 | (91.2) |
| Total participants receiving low-dose CT scan | 496 | |
| Received lung-RADS report | 496 | (100) |
| Discussion of results and follow-up care | 496 | (100) |
LDCT results and follow-up
Results of the 496 patients undergoing LDCT are reported in Table 3. Follow-up was recorded at 2 years. Only 1.2% of patients had a nondiagnostic Lung-RADS 0 interpretation, and repeat scanning resulted in a conversion to Lung-RADS 1 or 2 in all patients. In our study, the percentage of patients with Lung-RADS 1, 2, 3, or 4 was 44.1%, 41.7%, 4.6%, and 8.3%, respectively. All 64 participants with positive screens (23 patients with Lung-RADS 3 and 41 patients with Lung-RADS 4) participated in follow-up care after the positive screen. All patients with Lung-RADS 3 or 4 had additional imaging in the form of a CT scan or CT/PET scan. Overall, 16 patients were diagnosed with lung cancer; one had a Lung-RADS 3 result on LDCT and 14 had a Lung-RADS 4 result. (Table 3). One patient had a Lung-RADS 1 interpretation on LDCT, a nodule detected 12 months after the initial LDCT, and eventual diagnosis of stage III adenocarcinoma. Altogether, diagnostic work-up for patients with lung cancer included interval CT scans (4), CT-guided biopsy (3), endobronchial ultrasound-guided biopsy (3), and surgical resection (6). Three patients (0.6%) underwent biopsy (two CT-guided and one endobronchial ultrasound-guided biopsy) with no evidence of cancer. Of those diagnosed with lung cancer, 12 were stage I (75%). One patient (6.2%) had stage II, two had stage III (10.2%), and one had stage IV (6.2%) lung cancer. All 16 patients diagnosed with lung cancer proceeded with treatment (Table 4). Three patients had segmentectomy, 7 had lobectomy, three had stereotactic body radiation therapy (SBRT), one had chemotherapy and radiation therapy, one had chemotherapy, radiation therapy, and lobectomy, and one received chemotherapy alone for stage IV disease (Table 4). One person with Lung-RADS 4 received a diagnosis of stage IV laryngeal cancer with laryngoscopic biopsy. There were no mortalities or major complications of any patients undergoing diagnostic procedures or treatment for lung cancer.
Table 3.
Results of LDCT scan for lung cancer screening
| Lung-RADS category | Number | (%) | Number with lung cancer | % of lung-RADS category with lung cancer |
|---|---|---|---|---|
| 0 | 6 | (1.2) | 0 | (0) |
| 1 | 219 | (44.1) | 1 | (4.3) |
| 2 | 207 | (41.7) | 0 | (0) |
| 3 | 23 | (4.6) | 1 | (4.3) |
| 4 | 41 | (8.3) | 14 | (43.5) |
| Total | 496 | (100) | 16 | (3.2) |
Table 4.
Patients diagnosed with malignancy
| Patient | Lung-RADS | Diagnostic procedure | Histology | Stage | Treatment |
|---|---|---|---|---|---|
| 1 | 3 | Wedge | Adenocarcinoma | IA | Lobectomy |
| 2 | 4A | Interval CT with change | — | 1 | SBRT |
| 3 | 4A | Segmentectomy | Squamous Cell | IA | Segmentectomy |
| 4 | 4A | CT-guided biopsy | Squamous Cell | IA | Segmentectomy |
| 5 | 4A | Interval CT with change | Squamous Cell | IA | Lobectomy |
| 6 | 4x | Wedge | Adenocarcinoma | IA | Lobectomy |
| 7 | 4x | Lobectomy | Adenocarcinoma | IA | Lobectomy |
| 8 | 4x | CT-guided biopsy | Adenocarcinoma | IA | SBRT |
| 9 | 4x | CT-guided biopsy | Adenocarcinoma | IA | SBRT |
| 10 | 4x | Segmentectomy | Adenocarcinoma | IA | Segmentectomy |
| 11 | 4x | Interval CT with change | Adenocarcinoma | IA | Lobectomy |
| 12 | 4A | Wedge | Squamous Cell | 1B | Lobectomy |
| 13 | 4B | Interval CT with change | Adenocarcinoma | II | Lobectomy |
| 14 | 1a | EBUS | Adenocarcinoma | IIIA | Chemo, XRT, Lobectomy |
| 15 | 4A | EBUS | Squamous Cell | IIIB | Chemo, XRT |
| 16 | 4B | EBUS | Adenocarcinoma | IV | Chemo |
SBRT stereotactic body radiation therapy, EBUS endobronchial ultrasound, XRT radiation therapy, Chemo chemotherapy
One patient had Lung-RADS 1 at baseline, but 12 months later had a nodule detected and eventual diagnosis of stage III lung cancer
Adherence to annual screening and surveillance
All of the patients diagnosed with cancer (16 with lung cancer, 1 with laryngeal cancer) adhered to surveillance CT scanning at 1 and 2 years following treatment except one who died from stage III lung cancer, one who died from IV lung cancer, and one who died from stage IV head and neck cancer. All of the remaining 48 patients with either Lung-RADS 3 (22) or 4 (26) and no cancer diagnoses adhered to the recommended diagnostic work-up and continued care 1 year after their initial, baseline LDCT. However, only 35.4% of these people with a positive screen, but no diagnosis of cancer, adhered to follow-up imaging 2 years after the initial LDCT (6 (27.2%) with Lung-RADS 3 and 11 (42.3%) with Lung-RADS 4) (Table 5). Adherence to annual screening in people with negative screens was low with only 23.2% of patients with Lung-RADS 1, and only 24.2% of patients with Lung-RADS 2 results obtaining LDCT one year after their baseline scan. Overall, only 23.7% of those with negative screens adhered to recommended annual screening at 1 year. Only 3% of those with negative screens adhered to recommended annual screening at 2 years after the initial LDCT (2.8% of those with Lung-RADS 1 interpretation and 3.4% of those with Lung-RADS 2).
Table 5.
Adherence to recommended follow-up for people without cancer diagnoses
| Lung-RADS category | Annual CT recommended | Adherent at 1 year | Adherent at 2 years |
|---|---|---|---|
| 0 | 6 | 0 (0%) | 0 (0%) |
| 1 | 219 | 51 (23.3%) | 6 (2.8%)c |
| 2 | 207 | 50 (24.2%) | 7 (3.4%) |
| 3 | 22a | 22 (100%) | 6 (27.2%) |
| 4 | 26b | 26 (100%) | 11 (42.3%) |
| Total | 480 | 149 (31%) | 62 (12.9%)c |
Of the 23 patients with Lung-RADS 3, 1 was diagnosed with cancer. The other 22 received recommendations for at least annual CT scan
Of the 41 patients with Lung-RADS 4, 14 were diagnosed with lung cancer, 1 with laryngeal cancer. The other 26 received recommendations for at least annual CT scan
One patient with a baseline Lung-RADS 1 died of lung cancer between year 1–2, so only 218 had the recommendation of annual CT
Performance of LDCT for lung cancer screening
At 2 years follow-up, 22 out of 23 patients with Lung-RADS 3 results had no evidence of lung cancer (95.7%). Of the 41 patients with Lung-RADS 4 results, 26 had no evidence of cancer (63.4%). Altogether, 64 patients (12.9%) received a positive lung cancer screening, meaning Lung-RADS 3 or 4. Of these, 48 did not have cancer, resulting in a false-positive rate of 10.7%. Three patients (0.6%) underwent biopsy (two CT-guided and one endobronchial ultrasound-guided biopsy) as a result of a false-positive lung cancer screening. No patients underwent surgery as a result of a false-positive screen, and no patients suffered mortality or morbidity as a result of a false-positive lung cancer screening. The positive predictive value for any diagnosis of lung cancer within 2 years of the initial, baseline LDCT among this population was 23.4%. The negative predictive value for any diagnosis of lung cancer within 2 years of the initial, baseline LDCT was 89.7%.
Results of smoking cessation counseling
Of the 308 smokers, 18 (5.8%) declined any further intervention. Two hundred ninety smokers (94.2%) accepted additional intervention including 210 (68.2%) accepting pharmacotherapy with additional counseling. Of these, 93 (30.2%) chose nicotine replacement (lozenges, gum, inhaled, spray, patches), 39 (12.7%) chose oral medication, and 78 (25.3%) chose both oral medication and nicotine replacement. Eighty people (26.0%) declined pharmacotherapy but agreed to additional counseling for smoking cessation. Only 74 of the 290 (25.5%) people agreeing to additional counseling actually attended the counseling visits. Of the 308 smokers, only 34 (11%) quit smoking. Three (1%) succeeded outside of our program’s interventions (2 used no intervention, 1 used hypnosis), 14 (4.5%) used nicotine replacement, 6 (1.9%) used oral medication, 9 (2.9%) used nicotine replacement plus oral medication, and 2 (0.6%) used counseling with our program without pharmacotherapy.
Discussion
Data from NLST demonstrated a 20% reduction in lung cancer mortality using LDCT as the primary screening modality [2]. Based on this result, lung cancer screening could potentially avert 12,000 deaths annually in the United States [17]. However, it is unknown if lung cancer screening can be implemented with fidelity to CMS guidelines which include an SDM visit with use of a decision aid, standardized reporting of LDCT results, multidisciplinary follow-up, and smoking cessation, especially in diverse and underserved populations [10]. Our study is the first to prospectively assess the implementation of these critical components of lung cancer screening with 2-year follow-up in a diverse population.
Implementation of shared decision making
USPSTF, CMS, the National Cancer Institute (NCI), and several professional societies recommend a SDM discussion between a knowledgeable health-care provider and the lung cancer screening candidate [4, 5]. Patients using decision aids not only have increased knowledge about lung cancer screening but also find them useful in the decision-making process. However, few studies demonstrate feasibility of SDM for lung cancer screening. By coordinating the SDM visit on the same day as LDCT, we were able to engage 100% of our patients in a SDM discussion, and 86.7% of patients used a decision aid.
Although we were able to implement SDM, it is unknown what affects integrating SDM shortly before LDCT has on decision making. It is possible that patients who have already committed to an integrated visit may have had a bias to proceed with screening. In our study, 96% of participants chose to proceed with LDCT. Our data are similar to another study demonstrating a high rate of LDCT uptake (94.6%) when SDM was offered on the same day [18]. This is in contrast to the 58% uptake rate reported when SDM was performed over the phone prior to scheduling an LDCT visit [18]. According to the NCI’s Health Information National Trends Survey (HINTS), only 4.3% of smokers report having a discussion about lung cancer screening with their health-care provider [19]. To increase participation in screening, SDM should ideally begin before an integrated lung cancer screening visit. However, an additional SDM discussion, formalized with a decision aid and taking place prior to LDCT, likely fosters a provider–patient relationship that facilitates subsequent care.
Implementation of standardized reporting of LDCT results and discussion of follow‑up care
Our findings indicate that integrated lung cancer screening facilitates reporting of LDCT results. All patients received Lung-RADS interpretation of LDCT, discussion of results, and recommendations for follow-up care. Thirteen percent of patients chose to receive results on a separate day from when they underwent LDCT. These patients bring attention to shortcomings of the integrated approach, namely the challenge of participating in SDM, LDCT, and reporting on the same visit. We did not formally study the time required to complete an integrated visit, but it generally required three hours. In the future, we intend to study the investment of time from patients and providers and their perceptions of the integrated approach.
Implementation of smoking cessation counseling
The findings further indicated that an integrated approach to lung cancer screening facilitates the delivery of smoking cessation in active smokers. Smoking cessation counseling in the context of lung cancer screening can potentially avert 90,000 deaths [20] and improve the cost-effectiveness of screening programs [21]. Our study demonstrates that many smokers are receptive to behavioral change, with 91.2% of individuals participating in greater than 10 min of smoking cessation counseling during the integrated visit and 94.2% accepting additional smoking cessation intervention. Nicotine replacement and counseling without pharmacotherapy were the most popular choices for intervention. Unfortunately, only 25.5% of people agreeing to counseling actually attended the appointment. Despite integration of a rigorous smoking cessation protocol into the process of lung cancer screening, only 11% of patients actually quit smoking. Further study regarding the barriers to smoking cessation in our population is needed. The Smoking Cessation within the Context of Lung Cancer Screening (SCALE) collaborative is addressing these issues on a national level [22].
Results of lung cancer screening with LDCT
Of 496 patients screened, 12.9% received an interpretation of Lung-RADS 3 or 4, and 3.1% were diagnosed with cancer. The false-positive rate was 10.7%, as determined through 2 year follow-up. Although 0.6% of patients underwent a biopsy as a result of a false-positive screen, there were no mortalities or morbidities from diagnosis or treatment of any participants. These results are comparable to other lung cancer screening experiences [2, 24–26]. Our integrated, multidisciplinary approach, particularly a timely discussion of positive screening results, likely led to adherence to recommended diagnostic plans. Of the 12.9% of patients with positive screens, all adhered to the recommended diagnostic work-up and follow-up imaging at 1 year. All patients diagnosed with cancer (3.1%) proceeded with cancer treatment. Proceeding with treatment after screening is critical to the success of a screening program [27]. If people do not adhere to treatment recommendations, they fail to realize the main benefit of screening, namely averting of lung cancer death. Screening programs should have multidisciplinary teams capable of treating detected lung cancer. Our integrated screening approach shows that it is possible to safely implement lung cancer screening and subsequent treatment of detected lung cancer among low-income and ethnically diverse populations.
Unfortunately, our integrated approach to lung cancer screening had little impact on individuals with negative screening results. Only 23.7% of people with negative screens followed-up with annual screening. Furthermore, only 3.0% of people with negative screens followed up with two annual screens. Even patients with positive screens had decreased adherence after diagnostic work-up did not reveal cancer. Only 35.4% of individuals with initial false-positive screens adhered to the recommended annual LDCT screening recommendation. These results are alarming considering that reduction of lung cancer mortality depends on annual screening, not a single LDCT [23]. Between 32 and 59% of lung cancers are found on annual screening after at least one negative screen [2]. Indeed, one of our patients was diagnosed with lung cancer greater than 1 year after his initial negative screen. A negative screen or negative work-up may be falsely reassuring to people at high risk of cancer. Therefore, health-care providers and patients should be aware of the importance of annual screening. Our results show that greater effort should be placed to adherence to annual screening. The poor adherence to annual screening in our study is far below both the 95% compliance with annual screening demonstrated in the NLST dataset [2] and the 85% compliance reported by Alshora et al. [28]. Our screening population differed from these studies in being diverse and predominantly African American. In addition, 32.2% of participants had less than a high school education. Our participants were also from underserved neighborhoods with a household income of $32,000. While our study has identified low annual lung cancer screening rates in low-income, diverse populations, further study is needed to identify the barriers. Through understanding barriers to annual lung cancer screening, it may be possible to identify interventions to improve adherence. Additional study is also needed to understand the impact of lengthening the interval of lung cancer screening [29], which may decrease the burden of screening on patients and providers.
Our study has important limitations. It is possible that follow-up care occurred outside our integrated lung cancer screening program. Thus, follow-up and false-negative results of screening may be underreported. Our follow-up was a minimum of 2 years; longer-term studies are needed to fully understand lung cancer mortality and false-positive and false-negative rates. At last, our study population consisted of patients who had been referred by primary care physicians. Despite beyond the scope of this study, barriers to engaging all people at high-risk of lung cancer, especially those reluctant to see or discuss risk with physicians, will be crucial to widespread implementation of lung cancer screening.
Conclusion
Our study shows, in a diverse and underserved population, which essential components of lung cancer screening can be delivered in an integrated visit. However, adherence to annual screening was poor. Further research needs to investigate the patient characteristics and contextual factors associated with poor adherence to annual lung cancer screening.
Acknowledgments
This project was partially supported by TUFCCC/ HC Regional Comprehensive Cancer Health Disparity Partnership, Award Number U54 CA221704(5) from the National Cancer Institute of National Institutes of Health (NCI/NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCI/NIH.
References
- 1.Siegel RL, Miller KD, Jemal A (2019) Cancer statistics. CA Cancer J Clin 69(1):7–34 [DOI] [PubMed] [Google Scholar]
- 2.Aberle DR, Adams AM et al. (2012) National Lung Screening Trial Research Team, Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 365(5):395–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bach PB, Mirkin JN, Oliver TK et al. (2012) Benefits and harms of CT screening for lung cancer: a systematic review. JAMA 307(22):2418–2429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.U.S. Preventive Services Task Force (2013) Lung cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/lung-cancer-screening#Pod. Accessed 18 June 2020
- 5.Centers for Medicare and Medicaid Services (n.d.) Decision memo for screening for lung cancer with low dose computed tomography (LDCT) (CAG-00439N). https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=274. Accessed 18 June 2020
- 6.Doria-Rose VP, White MC, Klabunde CN et al. (2012) Use of lung cancer screening tests in the United States: results from the 2010 National Health Interview Survey. Cancer Epidemiol Biomark Prev 21(7):1049–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Japuntich SJ, Krieger NH, Salvas AL, Carey MP (2018) Racial disparities in lung cancer screening: an exploratory investigation. J Natl Med Assoc 110(5):424–427 [DOI] [PubMed] [Google Scholar]
- 8.Carter-Harris L, Slaven JE Jr, Monahan PO, Shedd-Steele R, Hanna N, Rawl SM (2018) Understanding lung cancer screening behavior: racial, gender, and geographic differences among Indiana long-term smokers. Prev Med Rep 10:49–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tangka FK, Subramanian S, Mobley LR et al. (2017) Racial and ethnic disparities among state Medicaid programs for breast cancer screening. Prev Med 102:59–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanner NT, Gebregziabher M, Hughes Halbert C, Payne E, Egede LE, Silvestri GA (2015) Racial differences in outcomes within the national lung screening trial. Implications for widespread implementation. Am J Respir Crit Care Med 192(2):200–208 [DOI] [PubMed] [Google Scholar]
- 11.Wools A, Dapper EA, Leeuw JRJ (2015) Colorectal cancer screening participation: a systematic review. Eur J Public Health 26(1):158–168 [DOI] [PubMed] [Google Scholar]
- 12.EBSCO Health (n.d.) Shared decision making, patient decision aids, patient education, evidence-based patent information. https://health.ebsco.com/products/option-grid. Accessed 18 June 2020
- 13.Lau YK, Caverly TJ, Cao P et al. (2015) Evaluation of a personalized, web-based decision aid for lung cancer screening. Am J Prev Med 49(6):e125–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kazerooni EA, Austin JHM, Black WC et al. (2014) ACR–STR practice parameter for the performance and reporting of lung cancer screening thoracic computed tomography (CT): 2014 (Resolution 4)*. J Thorac Imaging 29(5):310–316 [DOI] [PubMed] [Google Scholar]
- 15.American College of Radiology (n.d.) Lung-RADS. https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/Lung-Rads. Accessed 18 June 2020
- 16.Shaik SS, Doshi D, Bandari SR, Madupu PR, Kulkarni S (2016) Tobacco use cessation and prevention - a review. J Clin Diagn Res 10(5):13–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma J, Ward EM, Smith R, Jemal A (2013) Annual number of lung cancer deaths potentially avertable by screening in the United States. Cancer 119(7):1381–1385 [DOI] [PubMed] [Google Scholar]
- 18.Mazzone PJ, Tenenbaum A, Seeley M, Petersen H, Lyon C, Han X, Wang XF (2017) Impact of a lung cancer screening counseling and shared decision-making visit. Chest 151(3):572–578 [DOI] [PubMed] [Google Scholar]
- 19.Kinsinger LS, Anderson C, Kim J, Larson M et al. (2017) Implementation of lung cancer screening in the Veterans Health Administration. JAMA Intern Med 177(3):399–406 [DOI] [PubMed] [Google Scholar]
- 20.Huo J, Hong YR, Bian J, Guo Y, Wilkie DJ, Mainous AG (2019) Low rates of patient-reported physician-patient discussion about lung cancer screening among current smokers: data from health information national trends survey. Cancer Epidemiol Biomark Prev 28(5):963–973 [DOI] [PubMed] [Google Scholar]
- 21.Moolgavkar SH, Holford TR, Levy DT, Kong CY et al. (2012) Impact of reduced tobacco smoking on lung cancer mortality in the United States during 1975–2000. J Natl Cancer Inst 104(7):541–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Villanti AC, Jiang Y, Abrams DB, Pyenson BS (2013) A costutility analysis of lung cancer screening and the additional benefits of incorporating smoking cessation interventions. PLoS ONE 8(8):e71379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joseph AM, Rothman AJ, Almirall D et al. (2018) Lung cancer screening and smoking cessation clinical trials. SCALE (smoking cessation within the context of lung cancer screening) collaboration. Am J Respir Crit Care Med 197(2):172–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pinsky PF, Gierada DS, Black W et al. (2015) Performance of Lung-RADS in the national lung screening trial: a retrospective assessment. Ann Intern Med 162(7):485–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aberle DR (2017) Implementing lung cancer screening: the US experience. Clin Radiol 72(5):401–406 [DOI] [PubMed] [Google Scholar]
- 26.Muñoz-Largacha JA, Steiling KA, Kathuria H et al. (2018) Initial surgical experience following implementation of lung cancer screening at an urban safety net hospital. J Thorac Cardiovasc Surg 155(6):2674–2681 [DOI] [PubMed] [Google Scholar]
- 27.Erkmen CP, Mitchell M, Randhawa S et al. (2018) An enhanced shared decision making model to address willingness and ability to undergo lung cancer screening and follow-up treatment in minority underserved populations. J Community Health 43(1):27–32 [DOI] [PubMed] [Google Scholar]
- 28.Alshora S, McKee BJ, Regis SM, Borondy Kitts AK, Bolus CC, McKee AB, French RJ, Flacke S, Wald C (2018) Adherence to radiology recommendations in a clinical CT lung screening program. J Am Coll Radiol 15(2):282–286 [DOI] [PubMed] [Google Scholar]
- 29.Robbins HA, Berg CD, Cheung LC, Chaturvedi AK, Katki HA (2019) Identification of candidates for longer lung cancer screening intervals following a negative low-dose computed tomography result. J Natl Cancer Inst 111(9):996–999 [DOI] [PMC free article] [PubMed] [Google Scholar]


