Abstract
Background:
Sézary syndrome (SS) and mycosis fungoides (MF), types of cutaneous T-cell lymphomas (CTCL), cause significant morbidity and adversely affect quality of life (QoL). This study assessed QoL measurement changes in patients receiving mogamulizumab versus vorinostat.
Patients and Methods:
A multicenter phase 3 trial was conducted in stage IB-IV MF/SS patients with ≥1 failed systemic therapy. QoL measurements included Skindex-29 and Functional Assessment of Cancer Therapy-General (FACT-G). Symptoms, function, and QoL subdomains were longitudinally modeled using mixed models with prespecified covariates. Meaningful change thresholds (MCTs) were defined using distribution-based methods. Categorical change by group over time and time to clinically meaningful worsening were analyzed.
Results:
Among 372 randomized patients, mogamulizumab demonstrated improvement in Skindex-29 symptoms (Cycles 3,5,7; P<.05) and functional (Cycles 3,5; P<.05) scales. A significantly greater proportion of mogamulizumab-treated patients improved by ≥MCT from baseline on Skindex-29 symptoms domain (Cycles 3,5,7,11) and functioning domain (Cycle 5). Significant differences in FACT-G physical well-being (Cycles 1,3,5; P<.05) were observed in favor of mogamulizumab, and a greater proportion of patients declined by ≥MCT at Cycles 1, 3, 5, and 7 with vorinostat treatment. Median time to symptom worsening on Skindex-29 was 27.4 months for mogamulizumab versus 6.6 for vorinostat. In SS patients, time to worsening favored mogamulizumab (P<.005) on all Skindex-29 domains; time to worsening was similar between MF treatment arms.
Conclusion:
MF/SS patients’ symptoms, function, and overall QoL favored mogamulizumab over vorinostat across time points. Patients with highest symptom burden and functional impairment derived the most QoL benefit from mogamulizumab.
Keywords: Mycosis fungoides, Sézary syndrome, patient-reported outcome, Skindex-29, FACT-G
MICROABSTRACT
Cutaneous T-cell lymphomas (CTCL) including mycosis fungoides (MF) and Sézary syndrome (SS) cause itching and other symptoms that can impair quality of life (QoL). This pre-specified analysis examined in detail how monoclonal antibody mogamulizumab treatment improved MF/SS patients’ symptoms, function, and overall QoL across time points relative to vorinostat, providing a health-related QoL benchmark for these patients.
INTRODUCTION
Cutaneous T-cell lymphomas (CTCL) are rare, often indolent non-Hodgkin’s lymphomas that primarily affect the skin and are often associated with severe pruritus and other morbidities that can profoundly impact quality of life (QoL).1-5 Physical symptoms of CTCL at all stages of disease have been shown to represent a significant burden on not only patients’ physical but also their psychological and social well-being, such as employment, leisure, and relationships.6-9 Compared with earlier, less advanced stages, advanced-stage disease causes a more severe and pervasive negative impact on QoL. Given the chronic, symptomatic course of CTCL, lack of curative treatments, and current patterns of care, which often include life-long therapy, it is essential to assess the clinical benefit of any emerging treatment regimens by including QoL outcomes.
The phase 3 MAVORIC (mogamulizumab versus vorinostat in previously treated cutaneous T-cell lymphoma) trial, to our knowledge the largest prospective trial of systemic therapy in CTCL, compared the efficacy and safety of mogamulizumab, a novel anti-CCR4 monoclonal antibody, for the treatment of adult patients with the two most common subtypes of CTCL, mycosis fungoides (MF) and Sézary syndrome (SS), after one prior systemic therapy, versus vorinostat (NCT01728805).10 A total of 372 patients, 186 in each arm, were enrolled. Mogamulizumab resulted in significantly greater progression-free survival (PFS) compared with vorinostat (median PFS of 7.7 months versus 3.1 months [P<.0001]). Treatment-emergent adverse events (TEAEs) that occurred more frequently in the mogamulizumab arm included infusion-related reactions (33.2% versus 0.5%) and drug-related rash (24% versus 0.5%). Common TEAEs reported more often with vorinostat included diarrhea (23% in mogamulizumab versus 62% in vorinostat), nausea (15% versus 42%), thrombocytopenia (11% versus 31%), dysgeusia (3.3% versus 29%), and increased blood creatinine (3.3% versus 28%).10
Key secondary endpoints of the MAVORIC study included measurement of patient-reported symptom experience and QoL using the Skindex-29, Functional Assessment of Cancer Therapy-General (FACT-G), ItchyQoL, and EQ-5D-3L scales. In the primary analysis of the MAVORIC study, the Skindex-29, FACT-G, ItchyQoL, and EQ-5D-3L measurements were reported to have improved in mogamulizumab-treated patients at the 6-month assessment compared with vorinostat-treated patients.10,11 The primary objective of this patient-reported outcome (PRO) analysis was to longitudinally assess the results of Skindex-29 and FACT-G since the literature and the investigators’ experience suggest that these scales are the most accurate instruments deemed fit-for-purpose for characterizing the symptomatic burden of this disease.11 Although EQ-5D-3L is a less optimal measure for this patient population because it is not a cancer-specific scale, longitudinal results are also reported for this instrument for completeness.
PATIENTS AND METHODS
Study Design
MAVORIC was an open-label, international, phase 3, randomized controlled trial of mogamulizumab versus vorinostat in patients with previously treated MF and SS. Study design details have been previously published, including that this trial was conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonisation consolidated Good Clinical Practice guideline, and any applicable national and local laws and regulations and that the protocol and all subsequent amendments were reviewed and approved by institutional review boards or independent ethics committees at each site.10 Adult patients with stage IB to IVB histologically confirmed MF or SS who had ≥1 failed systemic therapy were enrolled. Patients were randomized 1:1 to mogamulizumab (1 mg/kg by intravenous infusion on Days 1, 8, 15, and 22 of the first 28-day cycle and Days 1 and 15 of subsequent cycles) or oral vorinostat (400 mg daily) and treated until disease progression or unacceptable toxicity. Vorinostat patients were allowed to cross over to mogamulizumab following documented disease progression or unacceptable toxicity. The primary endpoint was investigator-assessed PFS. Pre-specified key secondary endpoints included global composite response rate, duration of response, and HRQoL as measured by the validated instruments Skindex-29, FACT-G, and EQ-5D-3L (administered at baseline and Day 1 of every other treatment cycle).
Key Secondary QoL Assessments
Skindex-29
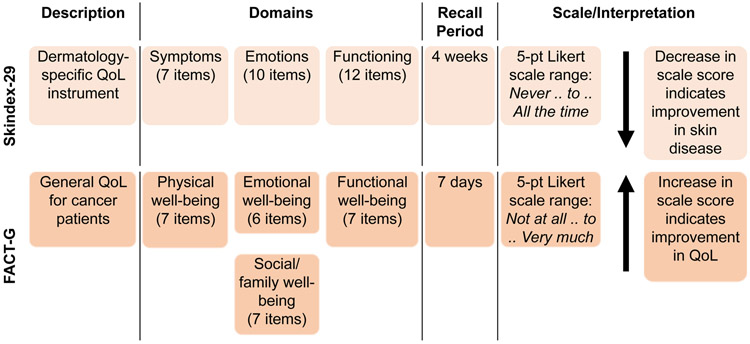
Skindex-29 is a validated instrument for measuring the effect of skin disease on health-related QoL (HRQoL) (Figure 1).5,8,12 Responses are transformed into a linear scale of 100 (0=“never,” 25=“rarely,” 50=“sometimes,” 75=“often,” and 100=“all the time”) for scale score calculation. Scale score is the mean of a subject’s responses to the items in a given scale, and the composite Skindex-29 score is calculated as the average of the three scale scores to measure the overall impact on QoL. Lower scores indicate less impact of skin disease on QoL.
Figure 1. Key QoL Instruments in MAVORIC5,12.
Abbreviations: FACT-G = Functional Assessment of Cancer Therapy-General; QoL = quality of life.
FACT-G
FACT-G is a validated instrument for assessing HRQoL in subjects with cancer (Figure 1).8 Total FACT-G score is obtained by summing individual subscale scores. Response scores on negatively phrased questions are reversed before summing. Higher scores for scales and subscales indicate better QoL.
EQ-5D-3L
EQ-5D-3L is a standardized measure of QoL used to provide a general measure of overall health status. To derive health state utilities for economic modeling, the questionnaire includes a descriptive health utility system assessing the following dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression, as well as the visual analog scale (VAS; 0-100). Higher scores indicate better QoL.
Statistical Analyses
Longitudinal Mixed Models of Treatment Effect
Longitudinal modeling of the continuous subdomains was performed to estimate the effect difference on repeated responses over a selected period and between the arms. Mixed models were conducted on a set of covariates for a fully exhaustive model, including arm, baseline value of outcome, region (US, Japan, Rest of World), age group (<65 or ≥65 years), gender, race category, CCR4 expression status, disease subtypes (MF or SS), disease stage (IB and II versus III or IV), and blood involvement (yes or no).
A parsimonious model that included arm, age, and region was also used. For each evaluated model, a covariate was retained in the model only if the significance associated with the main effect of the variable was ≤0.10 in a univariate analysis.
For each subdomain score, the mixed model considered the following: (1) change scores from baseline to subsequent visits were used as the primary repeated dependent variables; (2) treatment arm was included as a fixed effect; (3) region was treated as a fixed effect; (4) visit number; (5) subject was treated as a random effect; and (6) treatment-by-time effect was derived by including an interaction term of treatment arm by visit. Maximum likelihood with the Satterthwaite method for determining denominator degrees of freedom was used, and the residual plots for model fit evaluation were considered. Additionally, level of study completion may have been included based on a trend analysis to evaluate whether specific patterns of completion impacted HRQoL improvement.
Mixed models cited least-squares (LS) mean, difference in LS mean between treatment arms, and 95% confidence intervals (CIs) for the differences. Standard error (SE) was also calculated for each LS mean. A P value that tested the difference in LS mean change from baseline between adjacent treatment arms was presented. A two-sided test with P value ≤.05 (unadjusted for multiplicity) was considered statistically significant.
Meaningful Change Thresholds (MCTs) and Responder Proportions
MCTs were defined as the smallest difference in score that subjects perceived as beneficial and were evaluated using distribution-based methods. In this case, using the blinded clinical trial data, an effect size of one SE of measurement was used (SE of measurement = standard deviation [SD] × [1 – reliability]1/2, where reliability was measured by Cronbach’s alpha).13,14 This meaningful change was then used to discriminate between arms. Evidence to support the level of meaningful change for a particular endpoint requires a set of data analytic procedures that could assist in the interpretation of test scores beyond that provided by inferential or statistically significant results. A patient was considered a responder if their change from baseline meets or exceeds the MCT. Tests of proportions of responders for each domain or summary score by cycle were assessed using Chi-square or Fisher’s exact test when the expected cell size was ≤5. Two-sided P values from these tests are reported.
Time to Clinically Meaningful Worsening
Time to deterioration was defined as time from the day of randomization until the PRO domain score had worsened in magnitude of the MCT. Patients whose PRO domain score improved, remained the same, or did not worsen to the magnitude of the MCT were censored at the last dose date of the treatment or the last date of the PRO domain assessment, whichever was first. Patients without a baseline and/or post-baseline PRO domain assessment were censored at the date of randomization. Median event times and two-sided 95% CIs for each median were determined. A Cox proportional hazard model with treatment, disease type, disease stage, and region as covariates was used to assess the magnitude of the treatment difference of the PRO domains. The hazard ratio along with the 95% CI obtained from the Cox proportional hazard model is presented.
RESULTS
Study Population
Baseline characteristics were generally similar across arms (Table 1). The majority of patients were white (69.9%) and male (58.1%). Patients were equally distributed between age groups (50.5% of patients were <65 years, and 49.5% of patients were ≥65 years). Nearly half (45.2%) of the patients enrolled in the MAVORIC study had SS.
Table 1.
Baseline Characteristics in MAVORIC
| Demographic or Clinical Item | Mogamulizumab (n=186) |
Vorinostat (n=186) |
Total (N=372) |
|---|---|---|---|
| Age group | |||
| <65 years | 99 (53.2%) | 89 (47.8%) | 188 (50.5%) |
| ≥65 years | 87 (46.8%) | 97 (52.2%) | 184 (49.5%) |
| Gender | |||
| Female | 77 (41.4%) | 79 (42.5%) | 156 (41.9%) |
| Male | 109 (58.6%) | 107 (57.5%) | 216 (58.1%) |
| Race | |||
| Black/African American | 24 (12.9%) | 13 (7.0%) | 37 (9.9%) |
| White | 125 (67.2%) | 135 (72.6%) | 260 (69.9%) |
| Other | 37 (19.9%) | 38 (20.4%) | 75 (20.2%) |
| Disease type | |||
| MF | 105 (56.5%) | 99 (53.2%) | 204 (54.8%) |
| SS | 81 (43.5%) | 87 (46.8%) | 168 (45.2%) |
| Disease stage | |||
| IB or II | 68 (36.6%) | 72 (38.7%) | 140 (37.6%) |
| III or IV | 118 (63.4%) | 114 (61.3%) | 232 (62.4%) |
| Blood involvement | |||
| Yes | 123 (66.1%) | 122 (66.3%) | 245 (66.2%) |
| No | 63 (33.9%) | 62 (33.7%) | 125 (33.8%) |
| Missing/no response | 0 | 2 | 2 |
| Region | |||
| US | 98 (52.7%) | 103 (55.4%) | 201 (54.0%) |
| Japan | 9 (4.8%) | 6 (3.2%) | 15 (4.0%) |
| EU/Australiaa | 79 (42.5%) | 77 (41.4%) | 156 (41.9%) |
Data are presented as n (%).
16 patients were enrolled in Australia: 9 mogamulizumab, 7 vorinostat.
Quality of Completion
Overall study compliance was high (>92%) and consistent for both Skindex-29 and FACT-G throughout the study. Regardless of arm and scale, at each prespecified time point, the percentage of patients missing all data was low (range from 0.3% to 7.6%).
Effects and Impact of Treatments on Skindex-29 and FACT-G
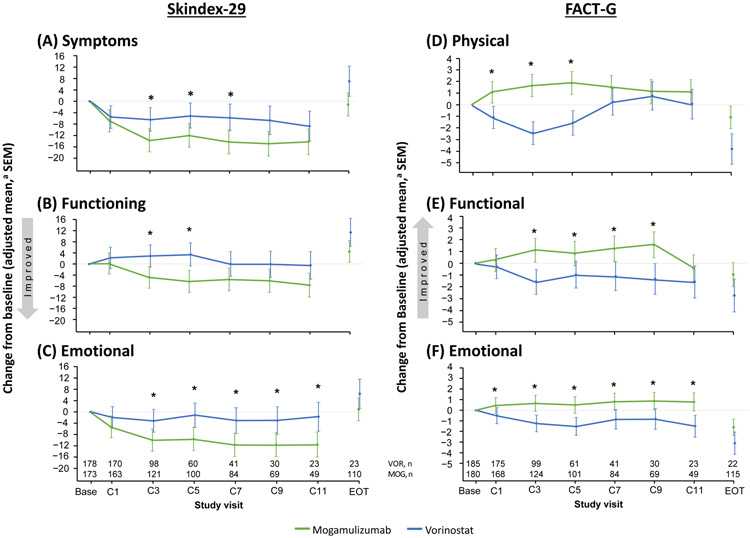
Longitudinal analysis of the effects of treatment on HRQoL favored mogamulizumab over vorinostat in all Skindex-29 and FACT-G domains (Figure 2). In the Skindex-29 symptoms domain, vorinostat-treated patients reported worsening symptoms at the beginning of the study (based on questions such as how frequent their skin itched, burned or stung, hurt, bled, etc.). Differences were observed starting at the first cycle and were statistically significant in favor of mogamulizumab at Cycles 3, 5, and 7 (P≤.03) (Figure 2A). For the Skindex-29 functioning domain, vorinostat patients experienced worsening ability to function at the beginning of the study (based on questions including their interaction with others, the desire to be with other people, difficulty in showing affection, effect on social life, and difficulty doing work or hobbies, etc.). Significantly lower scores in favor of mogamulizumab were observed at Cycles 3 and 5 (P≤.01) (Figure 2B). The Skindex-29 emotions domain (including questions on worry, frustration, embarrassment, annoyance, or depression about their skin condition, etc.) had significantly lower scores in favor of mogamulizumab at Cycles 3-11 (P≤.04) (Figure 2C).
Figure 2. Treatment Effects on Skindex-29 and FACT-G.
Abbreviations: C = cycle; FACT-G = Functional Assessment of Cancer Therapy-General; MOG = mogamulizumab; SEM = standard error of the mean; VOR = vorinostat.
*P<.05.
aAdjusted mixed model contained arm, visits, treatment visit interaction, baseline value of outcome, age group (<65 or ≥65 years), gender, region, race category (Black or African American, White, Other), CCR4 expression status, disease subtypes (mycosis fungoides or Sézary syndrome), disease stage (IB and II versus III or IV), and compartment involvement (blood involvement or no blood involvement).
In the FACT-G physical well-being domain, vorinostat-treated patients reported an overall worsening in physical well-being (cancer-specific) at the beginning of the study (based on lack of energy, nausea, pain, feeling bothered by their side effects of treatment, feeling ill, etc.). Similar results were observed in FACT-G functional well-being (based on their ability to work, enjoy life, sleep well, etc.). Significant improvements in favor of mogamulizumab were reported on the physical well-being scale at Cycles 1-5 (P≤.0002) and on the functional well-being scale at Cycles 3-9 (P≤.04) (Figures 2D-2E).
In the FACT-G emotional well-being domain, vorinostat patients reported worsening of their overall emotional well-being (cancer-specific) at the beginning of the study (based on questions that included sadness, satisfaction with how they were coping with illness, feeling nervous, worrying about dying, etc.). Significant improvements in favor of mogamulizumab were reported at Cycles 1-11 (P≤.04) (Figure 2F). Vorinostat patients also reported worsening of their overall social well-being (cancer-specific) at the beginning of the study (based on questions around emotional support from family, acceptance of their illness from family, feeling satisfied with family communication regarding their illness, etc.). Significant improvements in favor of mogamulizumab were reported at most time points, including Cycle 3 (0.4 versus −0.9 for mogamulizumab and vorinostat; P=.02) and Cycle 5 (0.5 versus −0.9; P=.04).
Subgroup analyses were conducted on the SS (n=168; 45.2%) and MF (n=204; 54.8%) patients. These analyses suggested variability in proximal skin-related symptoms and function for these patient subtypes. Specifically, for the symptoms, functioning, and emotions domains of the skin-specific Skindex-29 measure, mogamulizumab-treated patients with SS experienced large, significant improvements compared with vorinostat-treated patients at all post-Cycle 1 cycles (P<.05), whereas results were statistically equivalent for patients with MF.
Clinically Meaningful Changes in Skindex-29 and FACT-G
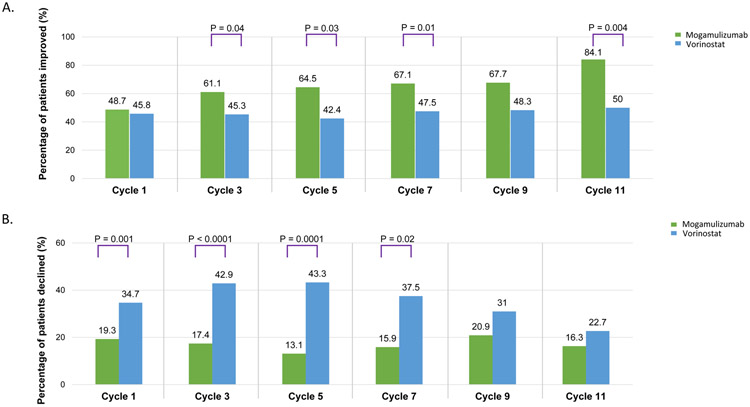
Overall, the percentage of mogamulizumab-treated patients who were categorized as improved compared with patients treated with vorinostat and the percentage of vorinostat-treated patients reported as declined versus mogamulizumab-treated patients were significantly higher at most time points for the Skindex-29 symptoms scale domain (Figure 3A). At least 60% of patients randomized to mogamulizumab reported clinically meaningful improvements in symptoms from Cycle 3 through Cycle 11 (Figure 3A). A significant difference was observed for the Skindex-29 summary score domain at Cycle 5 (64.5% of mogamulizumab patients versus 44.1% of vorinostat patients were categorized as improved; P=.0449) and the functioning scale domain at Cycle 5 (54.3% versus 28.8%; P=.007). No significant differences were observed for the emotional scale domain.
Figure 3.
Clinically Meaningful Changes in MAVORIC. (A) Clinically Meaningful Improvements in Patient-Reported Symptoms on Skindex-29. (B) Clinically Meaningful Declines in Patient-Reported Physical Well-Being on FACT-G
On the FACT-G physical well-being domain, significantly more patients treated with vorinostat were categorized as declined compared with mogamulizumab at most time points (Figure 3B). Specifically, significant differences were observed at Cycles 1-7 (P≤.03).
Similar results were observed for the FACT-G Total Score domain, with a statistically significant difference observed at Cycle 1 (9.1% mogamulizumab versus 17.4% vorinostat patients categorized as declined; P=.01), Cycle 3 (8.3% versus 30.9%; P<.0001) and Cycle 5 (10.1% versus 30.5%; P=.001), and the emotional well-being domain (Cycle 3: 11.6% versus 24.5% [P=.01]; Cycle 5: 14.1% versus 28.3% [P=.05]). Significant differences were also observed for the FACT-G functional well-being (Cycle 3: 20.0% of mogamulizumab versus 38.8% of vorinostat patients were categorized as declined; P=.003) and FACT-G social well-being domains (Cycle 3: 27.3% of mogamulizumab versus 13.3% of vorinostat patients categorized as improved; P=.04), although these results were not considered clinically meaningful.
Time to Clinically Meaningful Worsening
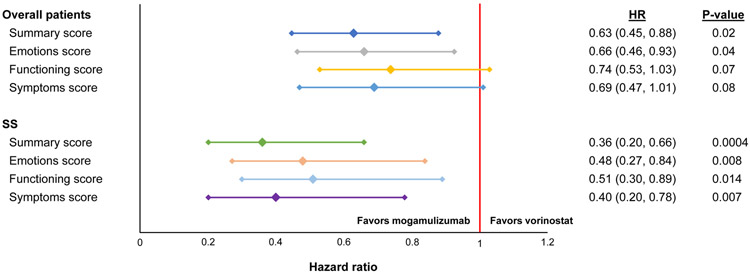
Time to clinically meaningful worsening was greater for patients in the mogamulizumab arm versus vorinostat for most domains of Skindex-29 and FACT-G. Specifically, significant differences favoring mogamulizumab were observed for the Skindex-29 summary score (not estimable versus 5.0 months in mogamulizumab and vorinostat; P=0.02) and the emotions domain (13.9 versus 4.6 months; P=.04). Statistically significant differences favoring mogamulizumab were observed in the FACT-G emotional well-being (10.5 versus 6.4 months; P=.05), functional well-being (8.3 versus 3.8 months; P=.0076), and physical well-being (7.1 versus 2.8 months; P=.002) domains and the total score (17.6 versus 6.0 months; P=.002); all other domains were statistically equivalent between arms.
The median months to clinically meaningful worsening for the Skindex-29 symptoms domain was 27.4 months (range 27.4 – not estimable) versus 6.6 months in mogamulizumab and vorinostat (P>.05), and the Skindex-29 Functioning domain was 9.5 versus 4.6 median months (P>.05). Time to worsening was delayed with mogamulizumab overall and on all three Skindex-29 scales; it was most pronounced in patients with SS (Figure 4).
Figure 4. Time to Clinically Meaningful Worsening on Skindex-29.
Abbreviation: SS = Sézary syndrome.
EQ-5D-3L
Change from baseline EQ-5D-3L score was significantly higher in the mogamulizumab group relative to the vorinostat group at Cycle 3 (LS mean scores of −0.006 versus −0.068; P=.0127). Statistically significant differences in favor of the mogamulizumab group were also observed for VAS score at Cycle 3 (LS mean scores of 1.4 versus −5.0; P=.0215) and Cycle 5 (2.6 versus −4.2; P=.0397).
DISCUSSION
Although QoL plays a very important role in the routine management of patients with MF/SS-type CTCL, a bespoke MF/SS-validated, disease-specific instrument for measuring QoL had not been developed at the time of this trial.11 Several tools have been used in clinical trials to assess the impact of CTCL on HRQoL; however, there is no clear consensus on the best instrument.15-22 Notably, Skindex-29 has been qualitatively tested in a CTCL population alongside ItchyQoL, which demonstrated acceptable content validity when paired with a general cancer-specific PRO measure.11 The MAVORIC trial is the largest clinical trial of systemic therapy in MF and SS to date. The clinical trial design of MAVORIC included a robust set of multiscale-assessed QoL endpoints aimed at capturing and comparing the effects of treatment on PROs. A preplanned primary analysis of changes from baseline to 6 months demonstrated that mogamulizumab-treated patients experienced greater improvements on all QoL scales administered (Skindex-29, FACT-G, EQ-5D-3L, and ItchyQoL), as reported previously.10 This report focuses on secondary analyses of the key HRQoL. This includes Skindex-29 to assess skin-related function and emotional impact as well as symptoms and FACT-G to assess cancer-specific HRQoL.
Longitudinal analyses found a statistically significant benefit for mogamulizumab versus vorinostat on symptom, emotional, and functional scales, with improvements observed as early as Cycle 3 for all domains of Skindex-29 and FACT-G. In addition, a higher percentage of patients treated with mogamulizumab were categorized as improved (based on defined meaningful thresholds) for symptoms associated with their skin condition compared with vorinostat-treated patients. Moreover, the percentage of patients who had worsened functional ability was higher in the vorinostat group compared with the mogamulizumab group. Mogamulizumab patients also had delayed clinically meaningful deterioration for all domains compared with vorinostat.
Similar results were observed with patients’ cancer-specific physical, functional, emotional, social, and overall well-being (based on FACT-G assessments). Specifically, the cross-sectional and longitudinal analyses demonstrated significant improvements favoring mogamulizumab over vorinostat. In addition, emotional, physical, and overall well-being were improved in patients treated with mogamulizumab and worsened in patients treated with vorinostat. Patients also had a significant delay in clinically meaningful deterioration for all domains in the mogamulizumab group compared with the vorinostat group, with significant differences in the emotional, functional, physical, and overall well-being scores.
Limitations of this analysis include the fact that MAVORIC was open-label and there was significant missing QoL data at later cycles due to treatment discontinuation. In addition, there are inherent drawbacks of employing QoL instruments not specifically validated in the disease studied. For example, the content of FACT-G physical functional well-being is not well-defined and may be misleading, as it combines disease symptoms and treatment symptoms. Furthermore, Skindex-29 is susceptible to recall error after 4 weeks,23 and in MAVORIC, it was assessed every 8 weeks following the first cycle. Skindex-29 has been extensively studied in different populations who have skin diseases, including CTCL.8,22 Therefore, in the absence of an MF- or SS-specific measure of QoL, Skindex-29 is the most meaningful and clinically relevant instrument available.
Patients with advanced-stage MF and SS have more severe and pervasive symptoms and greater disease-specific impact on QoL, specifically in the physical and symptoms domains. Of the MAVORIC trial population, 44.5% had SS, and 24% had stage III/IV MF. Thus, more than two-thirds of the patients enrolled had the type and degree of disease burden that most severely affects QoL. The high level of improvement observed here is consistent with the baseline level of QoL impairment in the advanced patients enrolled in MAVORIC. The ALCANZA trial of brentuximab vedotin in CD30+ CTCL, in contrast, excluded SS, but 67% of enrolled patients had advanced-stage MF. In ALCANZA, brentuximab vedotin resulted in improvements in the Skindex symptoms domain but did not result in improvement on the Skindex emotional or functional domains or any domains of FACT-G.22
CONCLUSION
The symptoms, emotions, function, and overall QoL impacts on patients treated with mogamulizumab were generally more improved compared with patients treated with vorinostat across the majority of function and symptom areas. Overall, these results suggest that patients on mogamulizumab had improved QoL associated with their disease and cancer-specific conditions and overall QoL, with a statistically significant decreased risk of experiencing a more rapid deterioration in their QoL compared with vorinostat.
Clinical Practice Points.
Given the chronic, symptomatic course of MF/SS-type CTCL, the lack of both curative treatments and treatments able to control the cutaneous symptoms of MF/SS, and the need for prolonged exposure to therapy, any assessment of the clinical benefit of a treatment regimen for patients with MF/SS should include measurements of QoL.
In MAVORIC, one of the largest phase 3 trials of systemic treatment in MF/SS-type CTCL to date (N=372), patients with previously treated MF/SS received the anti-CCR4 monoclonal antibody mogamulizumab (n=186) or standard-of-care vorinostat (n=186). The pre-specified analysis reported here included an in-depth assessment of patient-reported QoL outcomes using Skindex-29 and FACT-G instruments.
Mogamulizumab showed a significant benefit compared with vorinostat on symptom, emotional, and functional scales as early as treatment cycle 3 for all domains of Skindex-29 and FACT-G.
Mogamulizumab delayed time to worsening compared with vorinostat on all three Skindex-29 scales, and this effect was more pronounced in patients with SS.
Because of the overall impact of QoL symptoms in general on patients with MF/SS, demonstration of improvements with mogamulizumab in clinically meaningful patient-reported symptoms provides important information for practitioners when considering treatment options for these patients.
Acknowledgments
Kyowa Kirin Pharmaceutical Development, Inc., (Princeton, NJ, USA) consulted with advisors and study investigators on the design of this study, provided financial and material support for the study, and, with the assistance of study investigators, monitored the conduct of the study, collected data from the investigative centers, and analyzed the data.
On behalf of all, we thank the patients and investigators who participated in the MAVORIC study. Medical writing assistance was provided by Chad Green, PharmD, MBA, of Clinical Outcomes Solutions, and additional editorial assistance was provided by MedVal Scientific Information Services, LLC (Princeton, NJ). Writing and editorial support was funded by Kyowa Kirin, Inc. (Bedminster, NJ, USA). This research was funded in part through NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
PP served in a consulting/advisory role with Celgene and Innate Pharma and received research funding from Celgene, Infinity, Millennium, OncoMed, and Seattle Genetics.
SHudgens is an employee of Clinical Outcomes Solutions.
SHorwitz served in a consulting/advisory role with Affimed, Astex, Celgene, Kyowa Hakko Kirin, Millennium/Takeda, Trillium, and Verastem and received research funding from ADC Therapeutics, Aileron, Celgene, Corvus, Daiichi Sankyo, Forty Seven, Millennium/Takeda, Portola, Seattle Genetics, Trillium, and Verastem.
PQ served in a consulting/advisory role with 4SC, Actelion, Kyowa, Innate Pharma, Takeda, and Therakos.
RC received honoraria from, served in a consulting/advisory role with, and provided expert testimony for Kyowa Kirin.
LG served in a consulting/advisory role with Actelion, Kyowa Kirin, Mallinkrodt, and Soligenix; received research funding from Helsinn, Kyowa Kirin, Merck, and Mallinkrodt; and received personal fees for travel, accommodations, and expenses from Kyowa Kirin.
MB-B served in consulting/advisory role with and received personal fees for travel, accommodations, and expenses from Kyowa Kirin.
LF is an employee of Clinical Outcomes Solutions.
MB holds stock or other ownership with Innate Pharma; served in a consulting/advisory role with Innate Pharma, Kyowa Kirin, and Takeda; and received personal fees for travel, accommodations, and expenses from Innate Pharma, Janssen, Kyowa Kirin, and Novartis.
AT received honoraria from, served in a consulting/advisory role with, and received personal fees for travel, accommodations, and expenses from Kyowa Kirin.
AM served in a consulting/advisory role with ADC Therapeutics, Bristol-Myers Squibb, Cell Medica, Erytech, Kyowa Hakko Kirin, MiRagen, Seattle Genetics, and Takeda and received research funding from Bristol-Myers Squibb, Incyte, Merck, and Seattle Genetics.
AH served in a consulting/advisory role with Kyowa Kirin and Seattle Genetics; received research funding from Galderma, Kyowa Kirin, MiRagen, Rhizen, and Seattle Genetics; and received personal fees for travel, accommodations, and expenses from Galderma.
BD has no conflicts of interest to disclose.
SDalle received research funding from Kyowa Kirin.
DC has no conflicts of interest to disclose.
ML, SDale, and FH are employees of Kyowa Kirin.
MD served in a consulting/advisory role with Guide Point; served on a speakers’ bureau for Jonathan Woods; received research funding from Eisai, Kyowa Kiran, MiRagen, Seattle Genetics, and Trillium; and received personal fees for travel, accommodations, and expenses from Soligenix.
Previous Presentations
Presented at the American Society of Clinical Oncology Annual Meeting; June 1–5 2018; Chicago, IL, US.
Presented at the 23rd Congress of the European Hematology Association; June 14–17 2018; Stockholm, Sweden.
Presented at the Society of Hematologic Oncology Sixth Annual Meeting; September 12–15, 2018; Houston, TX, USA.
Presented at EORTC Cutaneous Lymphoma Task Force (EORTC-CLTF); Sept 27–29, 2018; St Gallen, Switzerland.
Presented at Deutsche Gesellschaft fur Hamatologie und Medizinische Onkologie (DGHO); Sept 28–Oct 2, 2018; Vienna, Austria.
Presented at Spanish Society of Hematology and Hemotherapy (SEHH); Oct 11–13, 2018; Granada, Spain.
Presented at Journées Dermatologiques de Paris; December 11, 2018; Paris, France.
REFERENCES
- 1.Korgavkar K, Xiong M, Weinstock M. Changing incidence trends of cutaneous T-cell lymphoma. JAMA Dermatol. 2013;149(11):1295–1299. [DOI] [PubMed] [Google Scholar]
- 2.Horwitz SM, Olsen EA, Duvic M, Porcu P, Kim YH. Review of the treatment of mycosis fungoides and Sézary syndrome: a stage-based approach. J Natl Compr Canc Netw. 2008;6(4):436–442. [DOI] [PubMed] [Google Scholar]
- 3.Meyer N, Paul C, Misery L. Pruritus in cutaneous T-cell lymphomas: frequent, often severe and difficult to treat. Acta Derm Venereol. 2010;90(1):12–17. [DOI] [PubMed] [Google Scholar]
- 4.Kim YH, Liu HL, Mraz-Gernhard S, Varghese A, Hoppe RT. Long-term outcome of 525 patients with mycosis fungoides and Sézary syndrome: clinical prognostic factors and risk for disease progression. Arch Dermatol. 2003;139(7):857–866. [DOI] [PubMed] [Google Scholar]
- 5.Chren MM, Lasek RJ, Flocke SA, Zyzanski SJ. Improved discriminative and evaluative capability of a refined version of Skindex, a quality-of-life instrument for patients with skin diseases. Arch Dermatol. 1997;133(11): 1433–1440. [PubMed] [Google Scholar]
- 6.Demierre M, Whittaker S, Kim Y, et al. Pooled analyses of two international, multicenter clinical studies of romidepsin in 167 patients with cutaneous T-cell lymphoma (CTCL) [Abstract]. J Clin Oncol. 2009;27(155):8546. [Google Scholar]
- 7.Demierre MF, Gan S, Jones J, Miller DR. Significant impact of cutaneous T-cell lymphoma on patients' quality of life: results of a 2005 National Cutaneous Lymphoma Foundation Survey. Cancer. 2006;107(10):2504–2511. [DOI] [PubMed] [Google Scholar]
- 8.Demierre MF, Tien A, Miller D. Health-related quality-of-life assessment in patients with cutaneous T-cell lymphoma. Arch Dermatol. 2005;141(3):325–330. [DOI] [PubMed] [Google Scholar]
- 9.Beynon T, Selman L, Radcliffe E, et al. 'We had to change to single beds because I itch in the night': a qualitative study of the experiences, attitudes and approaches to coping of patients with cutaneous T-cell lymphoma. Br J Dermatol. 2015;173(1):83–92. [DOI] [PubMed] [Google Scholar]
- 10.Kim YH, Bagot M, Pinter-Brown L, et al. Mogamulizumab versus vorinostat in previously treated cutaneous T-cell lymphoma (MAVORIC): an international, open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2018;19(9):1192–1204. [DOI] [PubMed] [Google Scholar]
- 11.Linos E, Kim YH, PSR S, Sutherland K, Chen SC. Development of a quality of life instrument specific for cutaneous lymphoma [abstract]. Blood. 2011;118(21):3158. [Google Scholar]
- 12.Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11(3):570–579. [DOI] [PubMed] [Google Scholar]
- 13.Litwin MS. How to Measure Survey Reliability and Validity. Thousand Oaks, CA: Sage Publications, Inc.; 1995. [Google Scholar]
- 14.Wyrwich KW, Nienaber NA, Tierney WM, Wolinsky FD. Linking clinical relevance and statistical significance in evaluating intra-individual changes in health-related quality of life. Med Care. 1999;37(5):469–478. [DOI] [PubMed] [Google Scholar]
- 15.Duvic M, Kuzel TM, Olsen EA, et al. Quality-of-life improvements in cutaneous T-cell lymphoma patients treated with denileukin diftitox (ONTAK). Clin Lymphoma. 2002;2(4):222–228. [DOI] [PubMed] [Google Scholar]
- 16.Duvic M, Hymes K, Heald P, et al. Bexarotene is effective and safe for treatment of refractory advanced-stage cutaneous T-cell lymphoma: multinational phase II-III trial results. J Clin Oncol. 2001;19(9):2456–2471. [DOI] [PubMed] [Google Scholar]
- 17.Heald P, Mehlmauer M, Martin AG, et al. Topical bexarotene therapy for patients with refractory or persistent early-stage cutaneous T-cell lymphoma: results of the phase III clinical trial. J Am Acad Dermatol. 2003;49(5):801–815. [DOI] [PubMed] [Google Scholar]
- 18.Olsen EA, Kim YH, Kuzel TM, et al. Phase IIb multicenter trial of vorinostat in patients with persistent, progressive, or treatment refractory cutaneous T-cell lymphoma. J Clin Oncol. 2007;25(21):3109–3115. [DOI] [PubMed] [Google Scholar]
- 19.Whittaker SJ, Demierre MF, Kim EJ, et al. Final results from a multicenter, international, pivotal study of romidepsin in refractory cutaneous T-cell lymphoma. J Clin Oncol. 2010;28(29):4485–4491. [DOI] [PubMed] [Google Scholar]
- 20.Bisaccia E, Gonzalez J, Palangio M, Schwartz J, Klainer AS. Extracorporeal photochemotherapy alone or with adjuvant therapy in the treatment of cutaneous T-cell lymphoma: a 9-year retrospective study at a single institution. J Am Acad Dermatol. 2000;43(2 pt 1):263–271. [DOI] [PubMed] [Google Scholar]
- 21.Yu JB, Khan AM, Jones GW, Reavely MM, Wilson LD. Patient perspectives regarding the value of total skin electron beam therapy for cutaneous T-cell lymphoma/mycosis fungoides: a pilot study. Am J Clin Oncol. 2009;32(2):142–144. [DOI] [PubMed] [Google Scholar]
- 22.Dummer R, Prince HM, Whittaker S, et al. Patient-reported quality of life in patients with relapsed/refractory cutaneous T-cell lymphoma: results from the randomised phase III ALCANZA study. Eur J Cancer. 2020;133:120–130. [DOI] [PubMed] [Google Scholar]
- 23.Center for Drug Evaluation and Research. Poteligeo (mogamulizumab-kpkc). Multi-disciplinary review and evaluation. Application number: 761051. 2018. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/761051Orig1s000MultidisciplineR.pdf. Accessed August 13, 2020.