Summary
A delayed eating schedule is associated with increased risk of obesity and metabolic dysfunction in humans [1–9]. However, there are no prolonged, highly controlled experimental studies testing the effects of meal timing on weight and metabolism in adults with a body mass index (BMI) of 19-27 kg/m2 [10–18]. Twelve healthy adults (age: 26.3±3.4y; BMI: 21.9±1.7 kg/m2; 5 females) participated in a randomized crossover study in free-living conditions. Three meals and two snacks with comparable energy and macronutrient contents were provided during two, 8-week, counterbalanced conditions separated by a 2-week washout period: 1) daytime (intake limited to 0800h-1900h); and 2) delayed (intake limited to 1200h-2300h). Sleep-wake cycles and exercise levels were held constant. Weight, adiposity, energy expenditure, and circadian profiles of hormones and metabolites were assessed during four inpatient visits occurring before and after each condition. Body weight, insulin resistance (HOMA-IR), trunk-to-leg fat ratio, resting energy expenditure, respiratory quotient, and fasting glucose, insulin, total and dHDL cholesterol, and adiponectin decreased on the daytime compared to the delayed schedule. These measures, as well as triglycerides increased on the delayed compared to the daytime schedule (effect size range: d=0.397-1.019). Circadian phase and amplitude of melatonin, cortisol, ghrelin, leptin, and glucose were not differentially altered by the eating schedules. Overall, an 8-week daytime eating schedule, compared to a delayed eating schedule, promotes weight loss and improvements in energy metabolism and insulin in adults with BMI 19-27 kg/m2, underscoring the efficacy and feasibility of daytime eating as a behavioral modification for real world conditions.
eTOC Blurb
Allison et al. demonstrate that consuming meals earlier in the day promotes a healthier metabolic profile and weight than eating later in the day. This suggests that curtailing eating in the evening is a helpful strategy for managing cardiometabolic health and weight.
Results
Twenty-nine participants were screened, and 17 were enrolled. Of these 17 participants, four withdrew: two after the initial inpatient visit, citing study burden, and two during the first eating condition due to unexpected relocations. Thirteen participants completed the study, but one participant regularly did not consume most of the snacks provided on either condition in a deliberate effort to lose weight; as such, that participant’s data were excluded. This yielded a final sample of 12 participants (mean±SD age, 26.3±3.4y; BMI, 21.9±1.7 kg/m2; 5 females; 8 non-Hispanic white, 3 Asian, and 1 non-Hispanic black). The five excluded/non-completing participants (mean±SD age, 25.7±8.4y; BMI, 23.0±2.0 kg/m2; 4 females; 5 non-Hispanic white) did not differ from completers (p’s >0.16). Those randomized to the daytime versus the delayed condition first showed no sex, age, race, or BMI differences (p’s >0.19).
Compliance to Eating Conditions
Compliance to eating parameters, physical activity and sleep during each 8-week eating condition was excellent (Table 1). Only one sleep measure significantly differed between conditions: actigraphic sleep onset was 25 minutes later during the delayed than daytime condition (p=0.04). Self-reported physical activity, and actigraphic total activity counts and light, medium, and vigorous activity did not differ significantly between conditions (all p’s>0.05; data not shown for types of activity). Figure S1 shows activity counts across 24h in 10-minute intervals in the two conditions. White, blue, green, and red-light exposure, as measured by actigraphy, also did not significantly differ between conditions (all p’s>0.05; data not shown).
Table 1.
Compliance to Conditions for Timed Eating, Physical Activity and Sleep Measures
| Outcome Measures | Daytime Mean (SD) | Delayed Mean (SD) | P-value |
|---|---|---|---|
| Timed Eating Compliance | |||
| # Meals or snacks/day | 4.6 (0.6) | 4.6 (0.6) | 0.91 |
| # Eating episodes out of range* (per 8-week condition) | 5.4 (5.9) | 2.2 (2.8) | 0.052 |
| # Eating episodes with non-study food items consumed | 8.0 (5.5) | 8.8 (7.8) | 0.60 |
| Calories provided (kcals) | 2018.8 (289.1) | 2001.6 (288.3) | 0.88 |
| Calories consumed/kg (kcals) | 1998.7 (359.9) | 2005.1 (347.1) | 0.97 |
| Physical Activity Compliance | |||
| Minutes/day (self-report) | 20.0 (13.6) | 24.9 (13.9) | 0.056 |
| Total activity counts/day (actigraphy) | 250,664.8 (66,777.1) | 267,566.9 (93,149.5) | 0.17 |
| Actigraphic Sleep Compliance | |||
| Sleep duration (h) | 7.7 (0.6) | 7.5 (0.6) | 0.09 |
| Sleep onset (hh:mm; h) | 23:58 (0.7) | 24:23 (0.9) | 0.04 |
| Sleep offset (hh:mm; h) | 07:40 (0.9) | 07:54 (1.0) | 0.14 |
| Sleep midpoint (hh:mm; h) | 03:49 (0.7) | 04:09 (0.9) | 0.07 |
| Sleep onset latency (min) | 28.9 (15.0) | 25.5 (14.4) | 0.30 |
| Sleep efficiency (%) | 83.0 (3.9) | 83.2 (4.6) | 0.80 |
| Wake after sleep onset (min) | 38.7 (15.2) | 39.7 (15.2) | 0.41 |
Note: Paired t-tests compared compliance data.
A mean (SD) of 5.3 (5.8) episodes of the out of range eating during the 8-week daytime condition occurred after 1900h; a mean (SD) of 1.5 (2.0) episodes of the out of range eating during the 8-week delayed condition occurred before 1200h. See also Figure S1.
Comparison of Changes in Body Weight, Energy Expenditure, Hormones, and Other Metabolites
Body weight decreased during daytime eating, and increased during delayed eating, with a medium effect size (Table 2, Figure 1A). Resting energy expenditure (REE) and respiratory quotient (RQ; i.e., VCO2/VO2) decreased with medium and small effect sizes, respectively, during daytime eating and increased during delayed eating, the latter indicating decreased fat oxidation in the delayed condition (Table 2, Figure 1B-3C). Body composition measured by dual x-ray absorptiometry (DXA) revealed a small decrease in % total body fat in the delayed condition and a small increase in the daytime condition. Lean mass % did not change appreciably in either condition. By contrast, the % trunk fat decreased with a small effect size in the daytime condition. The trunk-to-leg fat ratio decreased during daytime compared to delayed eating, with a large effect size and significant difference (Table 2, Figure 1D). Baseline measures before each condition did not differ significantly (data not shown).
Table 2.
Comparison of Change Values in Weight, Metabolic Markers, and Fasting Hormone and Metabolites Between Conditions
| Daytime Condition | Delayed Condition | Analyses | |||||
|---|---|---|---|---|---|---|---|
| t-test | Effect Size | ||||||
| Pre- | Post- | Pre- | Post- | ||||
| OUTCOME MEASURES | Mean (SD) | Mean (SD) | t | p | d | ||
| Weight and Metabolic Markers | |||||||
| Weight (kg) | 65.4 (7.5) | 64.3 (7.6) | 64.8 (7.5) | 65.0 (8.0) | −1.976 | 0.074 | 0.571 |
| Resting Energy Expenditure (kcals) | 1570.9 (214.5) | 1477.3 (230.1) | 1563.2 (214.5) | 1587.6 (138.0) | −1.783 | 0.100 | 0.515 |
| Respiratory Quotient (CO2 eliminated/O2 consumed) | 0.84 (0.05) | 0.83 (0.04) | 0.81 (0.06) | 0.84 (0.07) | −1.571 | 0.140 | 0.454 |
| DXA Total Fat (%) | 27.6 (5.4) | 27.9 (5.0) | 28.0 (5.4) | 27.6 (5.0) | 1.647 | 0.130 | 0.476 |
| DXA Lean Mass (%) | 14.5 (2.0) | 14.7 (1.9) | 14.7 (1.9) | 15.0 (2.2) | −0.673 | 0.510 | 0.194 |
| DXA Trunk Fat (%) | 26.1 (5.3) | 25.7 (5.1) | 25.7 (5.3) | 25.6 (5.1) | −1.068 | 0.310 | 0.308 |
| DXA Trunk/Leg Fat (%) | 0.87 (0.11) | 0.85 (0.10) | 0.85 (0.11) | 0.87 (0.11) | −3.530 | 0.005 | 1.019 |
| Fasting Hormones and Metabolites* | |||||||
| Total Cholesterol (mg/dL) | 159.8 (29.1) | 155.9 (28.2) | 157.6 (38.0) | 162.0 (34.2) | −1.416 | 0.184 | 0.409 |
| dHDL Cholesterol (mg/dL) | 57.4 (12.6) | 53.8 (9.1) | 56.3 (15.5) | 60.0 (16.8) | −2.536 | 0.028 | 0.732 |
| LDL Cholesterol (mg/dL) | 87.9 (24.6) | 87.8 (25.7) | 86.2 (32.6) | 83.7 (31.2) | 1.857 | 0.090 | 0.536 |
| Triglycerides (mg/dL) | 71.8 (19.2) | 73.3 (32.7) | 78.4 (12.6) | 93.4 (46.9) | −1.895 | 0.085 | 0.547 |
| Adiponectin (ug/ml) | 17.7 (8.3) | 17.4 (6.3) | 17.4 (6.7) | 21.4 (6.6) | −1.376 | 0.196 | 0.397 |
| NEFA (mEq/L) | 0.33 (0.14) | 0.39 (0.13) | 0.40 (0.14) | 0.31 (0.19) | 1.413 | 0.185 | 0.408 |
| Glucose (mg/dL) | 95.3 (7.4) | 91.6 (11.6) | 92.1 (5.3) | 97.0 (12.7) | −1.733 | 0.111 | 0.500 |
| Insulin (ulU/ml) | 8.1 (3.0) | 7.1 (3.2) | 6.8 (2.3) | 7.8 (3.5) | −1.926 | 0.080 | 0.556 |
| HOMA-IR | 1.92 (0.72) | 1.66 (0.97) | 1.54 (0.49) | 1.84 (0.80) | −2.252 | 0.046 | 0.650 |
Note: p=p-value from paired t-tests; d=Cohen’s d;
Analyses are adjusted for weight change.
A fasting blood draw was taken at 0800h after a minimum 9h fasting period.
DXA – dual-energy x-ray absorptiometry; dHDL – direct high-density lipoprotein; LDL – low-density lipoprotein; NEFA – non-esterified fatty acid; HOMA-IR – Homeostatic Model Assessment of Insulin Resistance
Figure 1. Violin plots showing the distribution and density of changes for the daytime and delayed eating conditions.
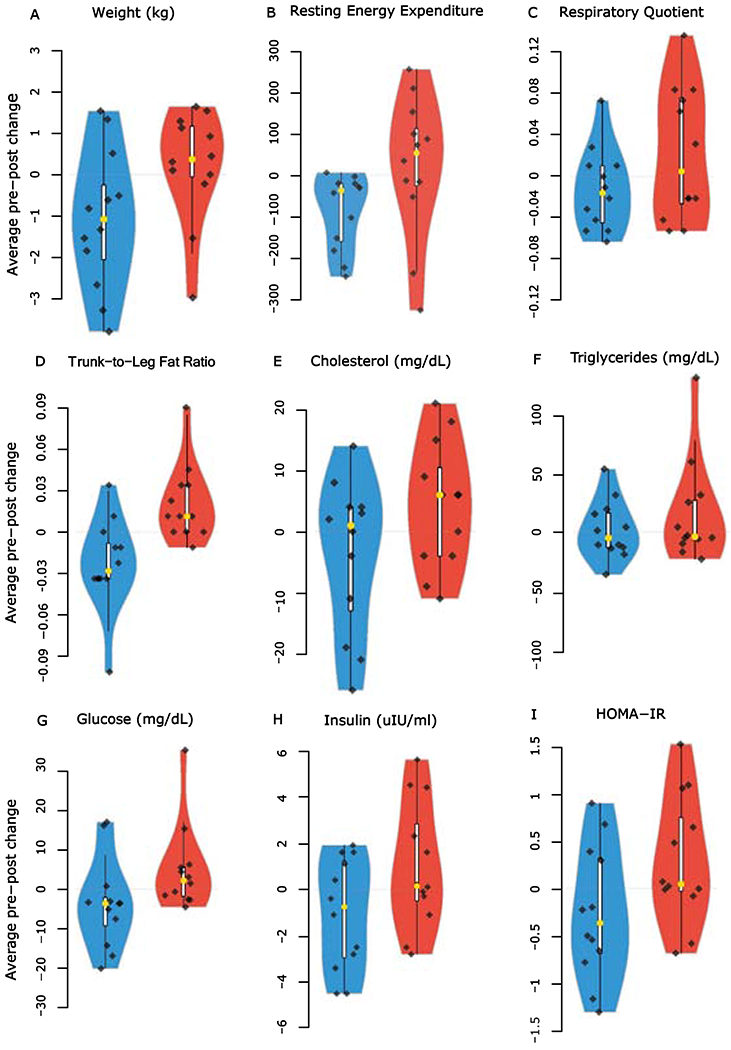
Violin plots show the following: (A) weight (kg), (B) resting energy expenditure (REE), (C) respiratory quotient (RQ), (D) trunk-to-leg fat ratio (measured by DXA), (E) total cholesterol, (F) triglycerides, (G) glucose, (H) insulin, and (I) HOMA-IR. For (E-I), a fasting blood draw was taken at 0800h after a minimum 9h fasting period. Daytime: blue, Delayed: red; Black diamonds: individual participant data points (n=12); yellow dots: median.
Regarding fasting biomarkers of metabolic health, total cholesterol and triglycerides increased on the delayed schedule with small and medium effect sizes, respectively (Table 2, Figure 1E–1F). High-density lipoprotein (dHDL) cholesterol and low-density lipoprotein (LDL) cholesterol improved on the delayed schedule, with medium effect sizes, respectively; the dHDL cholesterol difference was significant (Table 2). There was a small increase in adiponectin on the delayed schedule, suggesting slight improvement; non-esterified fatty acid (NEFA) had a small increase on the daytime schedule and a decrease on the delayed schedule (Table 2). Glucose and insulin decreased during daytime eating and increased during delayed eating, both with medium effect sizes (Table 2, Figure 1G–1H). Insulin resistance, measured by Homeostatic Model Assessment of Insulin Resistance (HOMA-IR), also decreased on the daytime schedule and increased on the delayed schedule; the HOMA-IR difference was significant with a medium effect size (Table 2, Figure 1I). Baseline measures before each condition did not differ significantly except for triglycerides, which were slightly higher in the pre-delayed than pre-daytime condition (Table 2, p=0.04).
Circadian Cosinor Analyses
Melatonin and cortisol, central clock markers, showed no significant differences in changes (for all measures in this section, daytime change values are presented first and delayed change values are presented second) in acrophase (melatonin: −0.12±1.60h vs −0.30±7.45h, p=0.94, d=0.02; cortisol: −1.14±6.68h vs −0.72±8.70h, p=0.88, d=0.05); amplitude (melatonin: −22.69±61.16 pg/ml vs 16.88±59.45 pg/ml, p=0.28, d=0.33; cortisol: 1.65±3.40 mg/dl vs 2.27±2.17 mg/dl, p=0.64, d=0.14); or mesor (melatonin: −15.27±37.63 pg/ml vs 10.15±29.58 pg/ml, p=0.21, d=0.38; cortisol: 0.23±4.29 mg/dl vs −0.31±4.89 mg/dl, p=0.76, d=0.09). The metabolic markers leptin, ghrelin, and glucose also showed no differences in changes in acrophase (leptin: 2.02±10.65h vs −3.95±7.42h, p=0.10, d=0.51; ghrelin: −4.93±6.52h vs 2.33±12.39h, p=0.12, d=0.48; glucose: −2.16±10.49h vs −6.78±12.03h, p=0.37, d=0.27); amplitude (leptin: 0.13±2.64 mg/dl vs 2.15±4.74 mg/dl, p=0.25, d=0.35; ghrelin: 19.35±90.94 pg/ml vs 7.73±157.59 pg/ml, p=0.83, d=0.07; glucose: 3.74±11.27 mg/dl vs −3.72±13.05 mg/dl, p=0.14, d=0.46); or mesor (leptin: −2.32±13.58 mg/dl vs 1.93±3.54 mg/dl, p=0.34, d=0.29; ghrelin: 29.57±86.65 pg/ml vs −107.76±321.88 pg/ml, p=0.21, d=0.39; glucose: −0.48±8.34 mg/dl vs 0.49±10.15 mg/dl, p=0.83, d=0.06).
Time Series Analyses
Melatonin did not show suppression in dim light conditions in either eating condition (Figure 2A). Comparisons of the change in mean values for melatonin, cortisol, ghrelin, leptin, and glucose were conducted at each 4-h timepoint (Figure 2A–Figure 2E). Cortisol increased at 0400h on the daytime compared to the delayed condition (3.00±6.94 mg/dl vs −2.45±5.07 mg/dl, p=0.03, d=0.72). No other comparisons were significant.
Figure 2. Mean values of blood hormones and metabolites taken every 4h from 0800h-0400h before and after the daytime and delayed eating conditions.
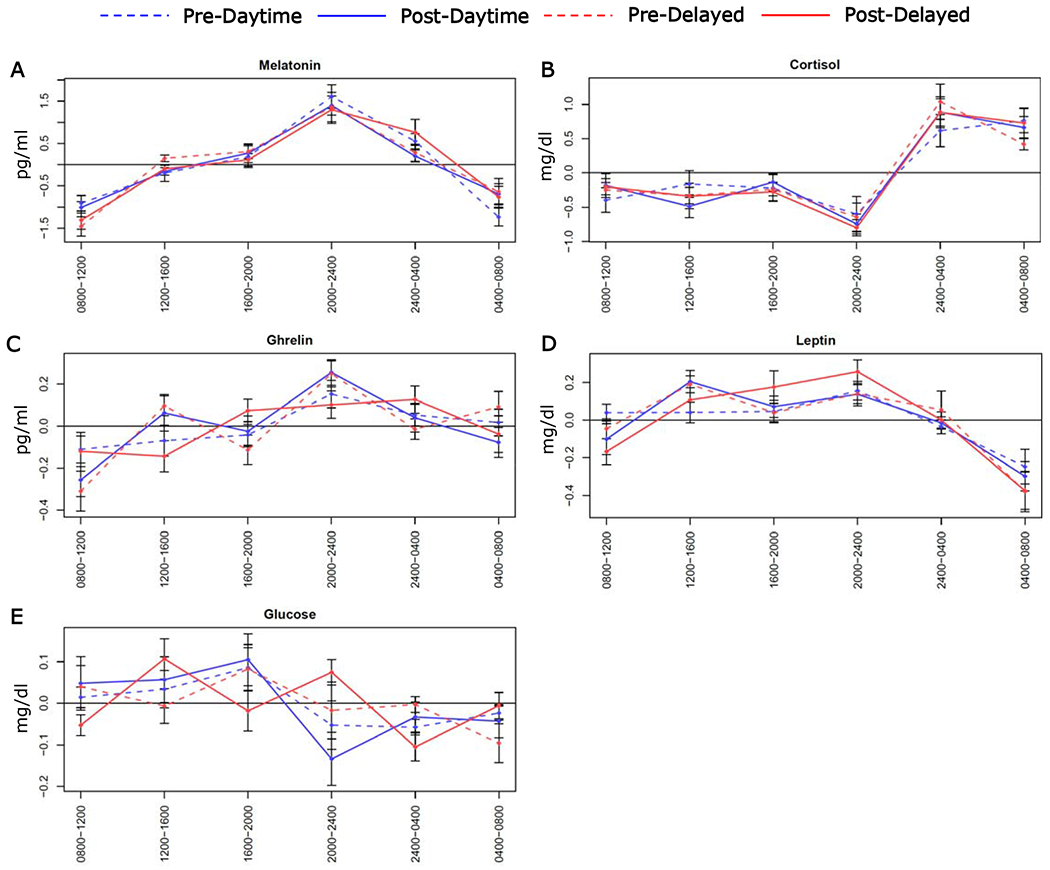
Plots show the following: (A) melatonin, (B) cortisol, (C) ghrelin, (D) leptin, and (E) glucose. For each eating schedule, data were adjusted by the daily mean. Vertical bars are standard errors.
Discussion
In this carefully controlled study of healthy participants with a BMI of 19 to 27 kg/m2, eating on a daytime as compared to a delayed schedule produced improvements in body weight, insulin sensitivity (HOMA-IR), fat oxidation (RQ), trunk-to-leg fat ratio, and fasting glucose, insulin, triglycerides and total cholesterol, while dHDL and LDL cholesterol, adiponectin and REE improved on the delayed condition, all based on effect sizes ranging from d=0.397-1.019. Notably, the circadian phase and amplitude of melatonin, cortisol, ghrelin, leptin, and glucose were not affected by the eating schedules. These findings suggest that overall, delayed eating has adverse effects on body weight and metabolic parameters independent of caloric intake, exercise or sleep; thus, adhering to a sustained daytime eating schedule shows efficacy and feasibility as a behavioral modification for promoting beneficial metabolic and weight management under real world conditions.
We found a significant reduction in HOMA-IR on the daytime compared to the delayed eating condition, suggesting increased insulin sensitivity when eating earlier. Conversely, adiponectin improved slightly with delayed eating, but to a much smaller degree than the improvement in HOMA-IR during the daytime condition. Insulin sensitivity has rarely been measured in timed eating studies in healthy populations: in the first study that measured it, timed eating failed to produce changes in insulin sensitivity [15]. The longer duration of our study compared to the previous study [15] provided more time for an effect to emerge in our healthy population, suggesting the impact of early eating may be gradual and contribute a moderate benefit over the long term by lowering glucose and insulin levels and improving insulin sensitivity [18]. Further, a cross-sectional study using a 5-h oral glucose tolerance test showed that the time by which participants typically consumed 25% of their daily calories predicted improved insulin sensitivity with the Matsuda Index and the QUICKI [19].
The reduction in the trunk-to-leg fat ratio on the daytime compared to the delayed schedule indicates a differential effect of the timing of eating on fat distribution. A higher trunk-to-leg fat ratio was associated positively with triglycerides, total cholesterol, systolic blood pressure, and C-reactive protein, and negatively with dHDL cholesterol in adolescents in the National Health and Examination Survey cohort [20], and also was positively related to diabetes risk in the Women’s Health Initiative sample [21]. Thus, eating earlier may protect against diabetes and cardiometabolic dysfunction.
RQ decreased in the daytime and increased in the delayed condition. High RQ values are indicative of low fat oxidation and high carbohydrate oxidation; thus, an increase in RQ after an overnight fast reflects a poorer ability to oxidize fatty acids and is an important contributor to weight gain and the development of metabolic syndrome and type 2 diabetes [22,23]. The decrease in RQ observed during daytime eating is consistent with a study implementing an early time restricted eating schedule [24] and may be related to our concurrent decreases in insulin. REE decreased during the daytime schedule, in contrast to the various other cardiometabolic improvements observed in this condition, likely due to weight loss, as caloric intake was similar in both conditions. In their early time restricted eating study, Ravussin and colleagues [24] measured REE in a chamber, noting variability across the day, which resulted in no differences in total daily REE; this suggests the effects of timed eating on REE are nuanced and related to differential influences of the thermal effect of food post-prandially throughout the day.
The weight increase in the delayed eating condition was modest but of significance. We note that the weight gain occurred independent of caloric intake and exercise. Because we did not directly measure all components of energy expenditure, we cannot fully account for the potential imbalance between energy intake and energy output, which could have led to weight gain in the delayed condition. Additionally, it is likely that DXA detected small changes in % total body fat but may have been less sensitive in detecting small changes in % lean mass, which could have impacted weight gain in the delayed condition.
The timing of eating did not alter the circadian phase or amplitude of central clock [suprachiasmatic nucleus, SCN] markers (plasma melatonin and cortisol), in agreement with an entrained sleep-wake cycle, and with an experimental timed eating study conducted under a constant routine [17]. Thus, our results, coupled with those of Wehrens et al. [17] indicate the observed metabolic changes occurred independently of the SCN in both inpatient and outpatient settings. We also did not detect differences in the circadian phase or amplitude of plasma leptin, ghrelin, or glucose. By contrast, Wehrens et al. [17] observed a phase delay in glucose, but not in insulin or triglycerides, of nearly 5.7h with a 5-h delay in three isocaloric daily meals under a constant routine. The delayed glucose rhythm in that study did not coincide with changes in insulin levels; thus, it is unclear how this result is related to insulin sensitivity or insulin secretion. Because a 4-h delay in eating on the delayed condition did not appreciably affect the timing or amplitude of peripheral rhythms, the weight and metabolic differences reported above are not explained by circadian changes in blood markers.
We examined only the timing of eating while holding the total number of hours of energy consumption constant at 11 h/day, which allows completion of both eating conditions within waking hours. Compliance was excellent, indicating feasibility of our long duration paradigm for outpatient use. Most adults typically eat across 14-15h/day [25], but our 11-h eating duration was realistic and sufficient for both eating schedules. Wilkinson et al. [26] showed that in persons with metabolic syndrome who typically eat at least 14 h/day, reducing eating duration to 10 h/day in a non-controlled 12-week intervention decreased caloric intake, and produced a 3.3 kg weight loss and improvements in total and LDL cholesterol, adiposity (via bioelectrical impedance), and systolic and diastolic blood pressure, but not in glucose, insulin, dHDL cholesterol, or triglycerides. Notably, timely new evidence also shows a strong positive association between both a later dinner time and a shorter interval of time between dinner and sunset and increased risk of mortality due to COVID-19 infection, possibly through increased inflammation from worsened cardiometabolic health [27]. By contrast, studies investigating Ramadan, which reduces duration and timing by restricting eating to before dawn and at night after sunset, have shown mixed effects on weight and cardiometabolic outcomes. There is some improvement in total cholesterol and triglycerides, and in increased fat oxidation, but inconsistent results for weight loss and improved insulin sensitivity [28]. Thus, during Ramadan, dividing eating duration and timing into early and late periods tempers some of the weight and cardiometabolic benefits observed in our study.
Combining an early schedule with a 6-h restricted eating window (eating from 0800h to 1400h) decreased mean ghrelin levels, improved 24-h glucose levels (measured via continuous glucose monitoring), reduced variability in self-reported hunger ratings, and tended to increase satiety and decrease the desire to eat over a 4-day period, as compared to a 12-h eating condition among adults with overweight and obesity [24,29]. In males with pre-diabetes, this same 6-h schedule produced improvements in insulin sensitivity (measured by an oral glucose tolerance test), β cell responsiveness, systolic and diastolic blood pressure, oxidative stress (8-isoprostane), and appetite [18]. While these results are interesting, eating only 6 h/day and stopping eating by 1400h daily are not feasible for most people to maintain long-term. Because our study and other evidence suggest that maintaining an early eating schedule and a 12-h or more fasting period per day benefits weight management, glucose regulation, and metabolic health, behavioral strategies that limit nighttime eating should be developed for widespread implementation.
Strengths of this study include the highly controlled conditions, elimination of other sources of variance, a realistic eating duration, and high compliance. Participants received all their food to control for caloric and macronutrient intake and were prompted daily to report any compliance issues. Compliance with the schedules and with consumption of the food provided was above 90% for both measures. Sleep-wake times were confirmed with actigraphy and showed that sleep duration, variability, and efficiency were normal and comparable between conditions, and importantly, participants were not sleep deprived. Sleep onset and offset were also close to the prescribed window for both conditions, with the delayed condition producing slightly later bedtimes. Thus, the benefits of the daytime condition can be attributed to the timing of eating and not to other factors.
The changes in body weight and several metabolic markers were medium to large in magnitude. However, the participants nearly all had normal weight and were metabolically healthy, with baseline measures in the normal range. Additionally, because we observed positive weight and metabolic changes across 8 weeks, it seems likely that adhering to daytime eating for a longer duration may result in greater benefits. The study sample size was small as this was the first test of our prolonged duration experimental paradigm. Power was optimized by the within-participants crossover design, so we relied on effect sizes to evaluate our outcomes due to this limitation. Finally, although we carefully controlled for intake by providing meals and by querying participants daily, compliance checks were self-reported and could not be independently verified, and thus this is a study limitation.
This study demonstrates that eating earlier in the day improves weight and several key metabolic outcomes in healthy individuals. Future studies should examine the long-term effects in larger populations with obesity or metabolic syndrome and probe underlying mechanisms. Identifying the metabolic consequences of late eating will provide important insights for the pathophysiology and treatment of obesity, diabetes, and related diseases.
STAR METHODS
RESOURCE AVAILABILITY
Lead Contact
Further information and requests for resources should be directed to and will be fulfilled by the Lead Contact, Kelly C. Allison, kca@pennmedicine.upenn.edu.
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability
Original/source data used for the analyses, figures and tables reported in the manuscript will be made available to qualified researchers, with minimal restrictions and in a timely manner.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Participants
Participants were recruited through a variety of advertisements. Adults of all races and ethnicities, ages 21-45 years, with a BMI of 19-27 kg/m2, and stable weight (≤4.5 kg change) over the previous 6 months were eligible. Exclusion criteria included: unstable, serious medical conditions; medications linked to weight gain or loss; oral steroid use; diabetes mellitus, cancer, or autoimmune disease; use of recreational drugs, melatonin, diuretics or hypnotics; current weight reduction program; a sleep disorder (determined by questionnaires and actigraphy); working night shifts; habitual waking after 0900h; bedtime later than 2400h; or failure to maintain a regular bedtime and wake time determined by actigraphy. Psychiatric exclusions, determined by the MINI [30], were severe major depressive disorder, and/or suicidal risk; bipolar disorder; current or past psychosis; lifetime eating disorder; or any other severe psychiatric disorder judged to interfere with long-term adherence to the proposed study. Participants were required to maintain their usual exercise levels throughout the study. The final sample size was 12 participants (5 females), with a mean±SD age of 26.3±3.4y. The CONSORT flow diagram is presented in Figure S2. Further details regarding participant characteristics and demographics are reported in the Results section.
Screening Visits
After passing an initial phone screen, participants presented for a screening visit. The Institutional Review Board of the University of Pennsylvania approved this study and all participants provided informed consent. To screen for inclusion criteria, study staff completed a clinical interview assessing typical eating and sleep patterns [Night Eating Syndrome History and Inventory, modified [31], weight history, and psychiatric status (MINI; [30]). Weight (using a calibrated Tanita WB800 digital scale), height (using a Harpenden wall-mounted stadiometer), and waist circumference were measured. Circadian preference was measured with the Composite Scale of Morningness and Eveningness [32]. Sleep patterns and potential disorders were further assessed with the Pittsburgh Sleep Quality Index (PSQI; [33]), the Epworth Sleepiness Scale (ESS; [34]), and the Multivariable Apnea Risk Index (MAP index; [35]) and mood was assessed with the Patient Health Questionnaire–9 items (PHQ-9; [36]). Participants then received logs to record food, exercise bouts, and sleep, and a wrist actigraph with light sensor (Actiwatch Spectrum, Philips Respironics) to wear on the non-dominant wrist for the next 10 days during the screening phase.
The participants were not extreme morning or evening chronotypes (Morningness and Eveningness range, 33-50, 39.08±5.00), and they did not report sleep disorders (PSQI global score=2.75±1.29; ESS score=5.33±3.37; the MAP Index score=0.07±0.06). Mood was in the normal range (PHQ-9 score=0.33±0.78). During the screening period, participants reported (mean±SD) consuming 4.67±1.03 meals or snacks/day and 2081.91±400.95 kcals/day, with their first meal at 09:13±1:06h, their last meal at 19:59h±1:10h, and an eating duration of 10:30±1:31h/day. Mean±SD actigraphic sleep variables during screening were: sleep duration (7.02±1.05h), sleep onset (23:44±0.97h), sleep offset (8:10±0.97h), sleep onset latency (21.14±17.69min), sleep efficiency (83.81±5.06%), and wake after sleep onset (43.53±15.25min).
Actigraphs were also then continuously worn for the 18 weeks of the study to determine sleep onset, offset, duration and efficiency. Actigraphy was also used to determine amounts of sedentary, light, moderate and vigorous activity, confirm periods of deliberate physical activity reported in participants’ logs [37], and determine light levels as detected at the wrist.
Once the regularity of their daily routines and their ability to comply with procedures was confirmed, participants underwent a medical history and physical. If they passed this medical screen, participants completed their baseline inpatient assessment and, afterwards, were randomized to the daytime or delayed eating schedule for their first 8-week eating condition. At the end of the 8 weeks, participants returned for their second inpatient assessment, followed by a 2-week washout during which they ate their own food on their typical schedule. They then completed a third inpatient assessment, which served as baseline for their second 8-week eating condition—either daytime or delayed, based on their randomized order—followed by the fourth and final inpatient assessment (Figure S3).
METHOD DETAILS
Inpatient Assessment Protocol
Each assessment included a 28-h stay at the Center for Human Phenomic Science (CHPS) at the Hospital of the University of Pennsylvania. The night prior to these inpatient assessments, participants fasted from 2300h until the 0800h blood draw for each condition’s baseline assessment (visits 1 and 3), and after the delayed eating condition, producing a 9-h fast, and from 1900h until the blood draw at 0800h following the daytime eating condition, producing a 13-h fast. We chose to have participants adhere to their assigned eating schedule given that the duration of the fasting period was long (≥9h) in both conditions. Previous studies found the following: 1) no significant differences in glucose or insulin levels between 7h to up to 13h of fasting [38,39], 2) fasting glucose did not differ for <8h versus ≥8h of fasting [38], and 3) glucose was stable after only 3h of fasting [40]. As such, the fasting period differences in our study conditions would not appreciably impact our fasting values for insulin, glucose, or HOMA-IR.
Participants arrived at 0700h, anthropometrics were measured, and drug and pregnancy tests (for females) were performed. They then were housed in a <20 lux room (to avoid suppression of melatonin secretion; [41]) and placed in a supine position. An indwelling intravenous line was initiated at 0730h. Blood was drawn at 4-h intervals [0800h (fasting), 1200h, 1600h, 2000h, 2400h, and 0400h] to measure amplitude and phase of circadian rhythms of glucose, leptin, ghrelin, melatonin and cortisol [42–44]. In addition, fasting levels (0800h) of insulin, adiponectin, cholesterol, triglycerides, and NEFAs were measured on morning 1. HOMA-IR was calculated using the 0800h sample and the following equation: fasting serum insulin (microU/mL) × fasting blood glucose (mg/dL)/405 [45].
On the second morning following the blood draws and overnight fast (fasting began by 2130h before the start of each eating condition and after the delayed condition, and 1900h after the daytime condition), indirect calorimetry was performed using a metabolic cart at 0800h to assess REE and fuel oxidation (RQ) (Parvo Medics TrueOne 2400). Before each measurement, the metabolic cart was calibrated with reference gas and with a flow meter calibration. After achieving a steady state (15 minutes), expired gases were collected for 30 minutes and used to calculate metabolic rate. We and other researchers have utilized this metabolic cart in prior studies—it shows validity and reliability for measurement of REE and RQ [46–48]. Finally, body composition was measured using DXA (Hologic Discovery Wi Bone Densitometer).
Eating Conditions
Prescribed meals and snacks were provided twice per week during each 8-week eating condition by participant pick-up or delivery by staff. Personalized menus accompanied the three meals and two snacks assigned for each day. Participants noted each food item consumed, the time and any modifications. The CHPS metabolic kitchen staff provided portion size training at baseline. Participants were permitted to drink non-caloric beverages (e.g., water, diet beverages, black coffee) outside of their assigned eating windows and noted all beverages consumed on their logs. For both conditions, the diet consisted of approximately 55% carbohydrate, 15% protein, and 30% fat. Foods were chosen to match participants’ preferences to maximize consumption of the provided food. Energy needs were calculated for each participant using the Harris-Benedict equation [49]. Staff also provided a new actigraph and collected the food logs during these food provisions to monitor compliance continually.
If participants were unable to consume a meal or snack as provided, they sent a picture of the meal or snack they consumed as a substitute using an electronic device. Uneaten food was returned to the metabolic kitchen, when possible. Kitchen staff pre-weighed all provided items, post-weighed any returned items, and used participant logs to enter food/drink intake into the Nutrition Data System for Research (NDSR) and compute each participant’s daily caloric and macronutrient intake.
Compliance
Frequent contact between study staff and participants allowed for feedback about the acceptability of the food items and difficulties with adherence to the eating and sleep-wake schedule (initiating sleep between 2200-2400h and waking by 0930h). To further monitor compliance, participants received the following six queries every morning via the RedCAP data capture system: 1. What time did you go to bed last night? 2. What time did you wake up this morning? 3. How many meals and snacks did you consume? 4. Did you eat any food outside of what was provided? (If yes, please provide details/send picture.) 5. Did you eat outside of the time window (0800h-1900h for daytime condition and 1200h-2300h for delayed condition)? 6. How many minutes did you exercise yesterday?
Assays
Blood was collected in pre-cooled vacutainer tubes containing EDTA and kept on ice until centrifugation at 4°C and storage at −80°C. Plasma hormones were measured by radioimmunoassay in duplicate. The precision of assays was as follows: cortisol (MP Biomedical, Solon, OH) intra-assay coefficient of variation (CV) 3.78%, inter-assay CV 11.3%; and melatonin (Tecan, Baldwin Park, CA) intra-assay CV 4.68%, inter-assay CV 26.3%. Leptin, ghrelin, adiponectin, and insulin (EMD Millipore, Billerica, MA) had CVs as follows: leptin intra-assay 3.1%, inter-assay 13.5%; total ghrelin intra-assay 4.49%, inter-assay 13.9%; adiponectin intra-assay 8.0%, inter-assay 10.4%; and insulin intra-assay 4.99%, inter-assay 11.3%. All assays had CVs within the acceptable range. Glucose was analyzed via a YSI 2900 glucometer, and NEFA were measured spectrophotometrically using Wako Life Sciences reagents (Richmond, VA). Triglycerides, total cholesterol, dHDL cholesterol and LDL cholesterol were measured using a Roche Cobas c311 Automated Clinical Chemistry Analyzer (Indianapolis, IN).
QUANTIFICATION AND STATISTICAL ANALYSIS
Descriptive statistics characterized the sample and outcome measures, including mean, standard deviation, and standard error of the mean, as indicated in the methods, results, tables, and figures. Participants were randomized based on a code generated by a random number generator. The final sample size for analyses was n=12 participants (as explained in the results, one participant’s data were excluded). Because this was the first study using this specific prolonged, rigorous protocol, Cohen’s d effect sizes were used for the primary outcomes, using the following ranges: ≥0.2 small, ≥0.5 medium, ≥0.8 large [50]. The Shapiro-Wilk test did not reject variable normality. Paired t-tests were used to compare delta values (before vs after each eating schedule) for anthropometric, compliance, and metabolic measures between the daytime and delayed schedules, with p<0.05 considered statistically significant (using two-tailed tests); for fasting hormones and metabolites, paired t-tests after adjusting for weight change were used. Cosinor analysis compared the effect of timed eating schedules on diurnal rhythm phase markers of the central clock, (melatonin and cortisol), biomarkers of appetite and metabolism (leptin, ghrelin, and glucose) and on actigraphic activity counts in 10-minute intervals (Figure S1). We used a cosinor model with a fixed 24-h period, to derive amplitude (half the peak-to-trough difference), acrophase (peak time), and mesor (mean) and compared changes in these values with paired t-tests (using two-tailed tests). Time series data were evaluated with independent t-tests for change values at each timepoint, with p<0.05 considered statistically significant (using two-tailed tests). Given this was an initial study, we did not correct for multiple comparisons in the above analyses. All statistical analyses and plotting were performed within the R statistical environment (version 3.5.1).
ADDITIONAL RESOURCES
The ClinicalTrials.gov Identifier for this trial is: NCT04414644.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Bacterial and Virus Strains | ||
| Biological Samples | ||
| Serial human blood samples | This paper | N/A |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Critical Commercial Assays | ||
| Cortisol | MP Biomedical | Cat# 07-221102 |
| Melatonin | TECAN US | Cat# RE29301 |
| Leptin | EMD Millipore | Cat# HL-81K |
| Ghrelin | EMD Millipore | Cat# GHRT-89HK |
| Adiponectin | EMD Millipore | Cat# HADP-61HK |
| Insulin | EMD Millipore | Cat# HI-14K |
| Glucose | YSI 2900 glucometer | |
| NEFA | Wako Life Sciences | Cat# 999-34691 Cat# 995-34791 Cat# 991-34891 Cat# 993-35191 |
| Triglycerides | Roche Cobas c311 Automated Clinical Chemistry Analyzer | Cat# 20767107322 |
| Total cholesterol | Roche Cobas c311 Automated Clinical Chemistry Analyzer | Cat# 03039773190 |
| dHDL cholesterol | Roche Cobas c311 Automated Clinical Chemistry Analyzer | Cat# 07528566190 |
| LDL cholesterol | Roche Cobas c311 Automated Clinical Chemistry Analyzer | Calculated by software from: Cat# 20767107322 Cat# 03039773190 Cat# 07528566190 |
| Deposited Data | ||
| Experimental Models: Cell Lines | ||
| Experimental Models: Organisms/Strains | ||
| Human subjects | This paper | N/A |
| Oligonucleotides | ||
| Recombinant DNA | ||
| Software and Algorithms | ||
| Other | ||
| Resting Energy Expenditure and Respiratory Quotient | Parvo Medics TrueOne 2400 | N/A |
| Dual-energy X-ray absorptiometry | Hologic Discovery Wi Bone Densitometer | N/A |
Highlights.
An early (0800h-2100h) versus delayed (1200h-2300h) eating schedule reduces weight
Eating early vs later improves insulin resistance, and insulin and glucose levels
Eating early vs later improves fat oxidation, cholesterol and trunk-to-leg fat ratio
An early eating schedule benefits weight and cardiometabolic health
Acknowledgments
This research was supported by NIH grants R21 DK100787 and R01 DK117488. Inpatient services at the Center for Human Phenomic Science were supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1TR000003. Additional support from National Aeronautics and Space Administration (NASA) grants NNX14AN49G and 80NSSC20K0243. R.S.A. was supported by a Bloomberg Distinguished Professorship. We thank the University of Pennsylvania Diabetes Research Center (DRC) for the use of the RIA Biomarker Core (P30-DK19525) and the Translational Core Laboratory of the Children’s Hospital of Philadelphia. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Parts of this study were previously presented in abstract form at Obesity Week 2017, SLEEP 2017 and 2019, the International Conference on Eating Disorders 2019 and Nutrition 2020.
Declaration of Interests
K.C.A. received research funding from Novo Nordisk and has served as a consultant for WW (formerly Weight Watchers, International), but these are not related to this research study. There are no other relevant disclosures among the authors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.St-Onge MP, Ard J, Baskin ML, Chiuve SE, Johnson HM, Kris-Etherton P, Varady K, American Heart Association Obesity Committee of the Council on Lifestyle and Cardiometabolic Health, Council on Cardiovascular Disease in the Young; Council on Clinical Cardiology; and Stroke Council. (2017). Meal timing and frequency: Implications for cardiovascular disease prevention: A scientific statement from the American Heart Association. Circulation 135, e96–e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maury E, Ramsey KM, and Bass J (2010). Circadian rhythms and metabolic syndrome: from experimental genetics to human disease. Circ. Res 106, 447–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waldman HS, Renteria LI, and McAllister MA (2020). Time-restricted feeding for the prevention of cardiometabolic diseases in high-stress occupations: a mechanistic review. Nutr. Rev 78, 459–464. [DOI] [PubMed] [Google Scholar]
- 4.Spaeth AM, Dinges DF, and Goel N (2015). Phenotypic vulnerability of energy balance responses to sleep loss in healthy adults. Sci. Rep 5, 14920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gallant AR, Lundgren J, and Drapeau V (2012). The night-eating syndrome and obesity. Obes. Rev 13, 528–536. [DOI] [PubMed] [Google Scholar]
- 6.Allison KC, and Goel N (2018). Timing of eating in adults across the weight spectrum: Metabolic factors and potential circadian mechanisms. Physiol. Behav 192, 158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garaulet M, Gomez-Abellan P, Alburquerque-Bejar JJ, Lee YC, Ordovas JM, and Scheer FA (2013). Timing of food intake predicts weight loss effectiveness. Int. J. Obes 37, 604–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoshida J, Eguchi E, Nagaoka K, Ito T, and Ogino K (2018). Association of night eating habits with metabolic syndrome and its components: A longitudinal study. BMC Public Health 18, 1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barrington WE, and Beresford SAA (2019). Eating occasions, obesity and related behaviors in working adults: Does it matter when you snack? Nutrients 11, E2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.LeCheminant JD, Christenson E, Bailey BW, and Tucker LA (2013). Restricting night-time eating reduces daily energy intake in healthy young men: A short-term crossover study. Brit. J. Nutr 110, 2108–2113. [DOI] [PubMed] [Google Scholar]
- 11.Bandín C, Scheer FA, Luque AJ, Ávila-Gandía V, Zamora S, Madrid JA, Gómez-Abellán P, and Garaulet M (2015). Meal timing affects glucose tolerance, substrate oxidation and circadian-related variables: A randomized, crossover trial. Int. J. Obes 39, 828–833. [DOI] [PubMed] [Google Scholar]
- 12.Hibi M, Masumoto A, Naito Y, Kiuchi K, Yoshimoto Y, Matsumoto M, Katashima M, Oka J, and Ikemoto S (2013). Nighttime snacking reduces whole body fat oxidation and increases LDL cholesterol in healthy young women. Am. J. Physiol. Regul. Integr. Comp. Physiol 304, R94–R101. [DOI] [PubMed] [Google Scholar]
- 13.Qin LQ, Li J, Wang Y, Wang J, Xu JY, and Kaneko T (2003). The effects of nocturnal life on endocrine circadian patterns in healthy adults. Life Sci. 73, 2467–2475. [DOI] [PubMed] [Google Scholar]
- 14.Singh RB, Cornelissen G, Mojto V, Fatima G, Wichansawakun S, Singh M, Kartikey K, Sharma JP, Torshin VI, Chibisov S, et al. (2020). Effects of circadian restricted feeding on parameters of metabolic syndrome among healthy subjects. Chronobiol. Int 37, 1–8. [DOI] [PubMed] [Google Scholar]
- 15.Yoshizaki T, Tada Y, Hida A, Sunami A, Yokoyama Y, Yasuda J, Nakai A, Togo F, and Kawano Y (2013). Effects of feeding schedule changes on the circadian phase of the cardiac autonomic nervous system and serum lipid levels. Eur. J. Appl. Physiol 113, 2603–2611. [DOI] [PubMed] [Google Scholar]
- 16.Bo S, Fadda M, Castiglione A, Ciccone G, De Francesco A, Fedele D, Guggino A, Parasiliti Caprino M, Ferrara S, Vezio Boggio M, et al. (2015). Is the timing of caloric intake associated with variation in diet-induced thermogenesis and in the metabolic pattern? A randomized cross-over study. Int. J. Obes 39, 1689–1695. [DOI] [PubMed] [Google Scholar]
- 17.Wehrens SMT, Christou S, Isherwood C, Middleton B, Gibbs MA, Archer SN, Skene DJ, and Johnston JD (2017). Meal timing regulates the human circadian system. Curr. Biol 27, 1768–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, and Peterson CM (2018). Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 27, 1212–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rangaraj VR, Siddula A, Burgess HJ, Pannain S, and Knutson KL (2020). Association between timing of energy intake and insulin sensitivity: A cross-sectional study. Nutrients 12, 503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cioffi CE, Alvarez JA, Welsh JA, and Vos MB (2019). Truncal-to-leg fat ratio and cardiometabolic disease risk factors in US adolescents: NHANES 2003-2006. Pediatr. Obes 14, e12509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo J, Hendryx M, Laddu D, Phillips LS, Chlebowski R, LeBlanc ES, Allison DB, Nelson DA, Li Y, Rosal MC, et al. (2019). Racial and ethnic differences in anthropometic risk factors for diabetes. Diabetes Care 42, 126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pujia A, Mazza E, Ferro Y, Gazzaruso C, Coppola A, Doldo P, Grembiale RD, Pujia R, Romeo S, and Montalcini T (2019). Lipid oxidation assessed by indirect calorimetry predicts metabolic syndrome and type 2 diabetes. Front. Endocrinol 9, 806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shook RP, Hand GA, Paluch AE, Wang X, Moran R, Hébert JR, Jakicic JM, and Blair SN (2016). High respiratory quotient is associated with increases in body weight and fat mass in young adults. Eur. J. Clin. Nutr 70, 1197–1202. [DOI] [PubMed] [Google Scholar]
- 24.Ravussin E, Beyl RA, Poggiogalle E, Hsia DS, and Peterson CM (2019). Early time-restricted feeding reducts appetite and increases fat oxidation but does not affect energy expenditure in humans. Obesity 27, 1244–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gill S, and Panda S (2015). A smartphone app reveals erratic diurnal eating patterns in humans that can be modulated for health benefits. Cell Metab. 22, 789–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilkinson MJ, Manoogian ENC, Zadourian A, Lo H, Fakhouri S, Shoghi A, Wang X, Fleischer JG, Navlakha S, Panda S, et al. (2020). Ten-hour time-restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome. Cell Metab. 31, 92–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verd S, Beiro S, Fernandez-Bernabeu M, and Ponce-Taylor J (2020). Early dinner or “dinner like a pauper”: Evidence, the habitual time of the largest meal of the day – dinner – is predisposing to severe COVID-19 outcome – death. Chronobiol. Int 37, 804–808. [DOI] [PubMed] [Google Scholar]
- 28.Hoddy KK, Marlatt KL, Çetinkaya H, and Ravussin E (2020). Intermittent fasting and metabolic health: from religious fast to time-restricted feeding. Obesity 28, S29–S37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jamshed H, Beyl RA, Della Manna DL, Yang ES, Ravussin E, and Peterson CM (2019). Early time-restricted feeding improves 24-hour glucose levels and affects markers of the circadian clock, aging and autophagy in humans. Nutrients 11, 1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, and Dunbar GC (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiat 59, 22–33. [PubMed] [Google Scholar]
- 31.Lundgren JD, Allison KC, Vinai P, and Gluck ME (2012). Assessment instruments for night eating syndrome In Night Eating Syndrome: Research, Assessment, and Treatment, Lundgren JD, Allison KC, Stunkard AJ, eds. (New York: Guilford Press; ), pp. 197–217. [Google Scholar]
- 32.Smith CS, Reilly C, and Midkiff K (1989). Evaluation of three circadian rhythm questionnaires with suggestions for an improved measure of morningness. J. Appl. Psychol. 74, 728–738. [DOI] [PubMed] [Google Scholar]
- 33.Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, and Kupfer DJ (1989). The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 28, 193–213. [DOI] [PubMed] [Google Scholar]
- 34.Johns MW (1991). A new method for measuring daytime sleepiness: The Epworth Sleepiness Scale. Sleep 14, 540–545. [DOI] [PubMed] [Google Scholar]
- 35.Maislin G, Pack AI, Kribbs NB, Smith PL, Schwartz AR, Kline LR, Schwab RJ, and Dinges DF (1995). A survey screen for prediction of apnea. Sleep 18, 158–166. [DOI] [PubMed] [Google Scholar]
- 36.Kroenke K, Spitzer RL, and Williams JB (2001). The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med 16, 606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neil-Sztramko SE, Rafn BS, Gotay CC, and Campbell KL (2017). Determining activity count cut-points for measurement of physical activity using the Actiwatch2 accelerometer. Physiol. Behav 173, 95–100. [DOI] [PubMed] [Google Scholar]
- 38.Clemmensen KKB, Quist JS, Vistisen D, Witte DR, Jonsson A, Pedersen O, Hansen T, Holst JJ, Lauritzen T, Jørgensen ME, et al. (2020). Role of fasting duration and weekday in incretin and glucose regulation. Endocr. Connect 9, 279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gu C, Brereton N, Schweitzer A, Cotter M, Duan D, Børsheim E, Wolfe RR, Pham LV, Polotsky VY, and Jun JC (2020). Metabolic effects of late dinner in healthy volunteers - A randomized crossover clinical trial. J. Clin. Endocrinol. Metab 105, 2789–2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moebus S, Göres L, Lösch C, and Jöckel K-H (2011). Impact of time since last caloric intake on blood glucose levels. Eur. J. Epidemiol 26, 719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goel N, Stunkard AJ, Rogers NL, Van Dongen HP, Allison KC, O’Reardon JP, Ahima RS, Cummings DE, Heo M, and Dinges DF (2009). Circadian rhythm profiles in women with night eating syndrome. J. Biol. Rhythms 24, 85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arnardottir ES, Nikonova EV, Shockley KR, Podtelezhnikov AA, Anafi RC, Tanis KQ, Maislin G, Stone DJ, Renger JJ, Winrow CJ, et al. (2014). Blood-gene expression reveals reduced circadian rhythmicity in individuals resistant to sleep deprivation. Sleep 37, 1589–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laing EE, Möller-Levet CS, Poh N, Santhi N, Archer SN, and Dijk DJ (2017). Blood transcriptome based biomarkers for human circadian phase. Elife 6, e20214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Archer SN, Laing EE, Möller-Levet CS, van der Veen DR, Bucca G, Lazar AS, Santhi N, Slak A, Kabiljo R, von Schantz M, et al. (2014). Mistimed sleep disrupts circadian regulation of the human transcriptome. Proc. Natl. Acad. Sci. U S A 111, E682–E691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, and Turner RC (1985). Homeostasis model assessment - insulin resistance and beta-cell function from fasting plasma-glucose and insulin concentrations in man. Diabetologia 28, 412–419. [DOI] [PubMed] [Google Scholar]
- 46.Spaeth AM, Dinges DF, and Goel N (2015). Resting metabolic rate varies by race and by sleep duration. Obesity 23, 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bassett DR Jr, Howley ET, Thompson DL, King GA, Strath SJ, McLaughlin JE, and Parr BB (2001). Validity of inspiratory and expiratory methods of measuring gas exchange with a computerized system. J. Appl. Physiol 91, 218–224. [DOI] [PubMed] [Google Scholar]
- 48.Cooper JA, Watras AC, O’Brien MJ, Luke A, Dobratz JR, Earthman CP, and Schoeller DA (2009). Assessing validity and reliability of resting metabolic rate in six gas analysis systems. J. Am. Diet. Assoc 109, 128–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harris JA, and Benedict FG (1918). A biometric study of human basal metabolism. Proc. Natl. Acad. Sci. U S A 4, 370–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cohen J (1998). Statistical Power Analysis for the Behavioral Sciences, Second Edition (Mahwah: Lawrence Erlbaum Associates; ). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Original/source data used for the analyses, figures and tables reported in the manuscript will be made available to qualified researchers, with minimal restrictions and in a timely manner.


