Abstract
The importance of canonical versus non-canonical mechanisms for the generation of angiotensins remains a major challenge that, in part, is heavily swayed by the relative efficacy of therapies designed to inhibit renin, angiotensin converting enzyme or the angiotensin II receptor. Angiotensin-(1–12) is an angiotensin II forming substrate serving as a source for Ang II mediated tissue actions. This study identifies for the first time the presence of angiotensin-(1–12) in the blood of 52 normal (22 women) and 19 (13 women) hypertensive patients not receiving antihypertensive medication at the time of the study. Normal subjects of comparable ages and body habitus had similar circulating plasma angiotensin-(1–12) concentrations [women: 2.02 ± 0.62 (SD) ng/mL; men 2.05 ± 0.55 (SD) ng/mL, p > 0.05]. The higher and statistically significant values of plasma Ang-(1–12) concentrations in hypertensive men [2.51 ± 0.49 ng/mL, n = 6] and women [2.33 ± 0.63 (SD) ng/mL, n = 13] were statistically significant (p < 0.02) and correlated with elevated plasma renin activity, systolic and pulse pressure, and plasma concentrations of N-terminal-pro hormone BNP. The increased plasma angiotensin-(1–12) in hypertensive patients was not mirrored by similar changes in plasma angiotensinogen and angiotensin II concentrations. The first identification of an age-independent presence of angiotensin-(1–12) in the blood of normotensive subjects and hypertensive patients, irrespective of sex, implicates this non-renin dependent substrate as a source for Ang II production in the blood and its potential contribution to the hypertensive process.
Keywords: Blood pressure, angiotensinogen, angiotensin-(1–12), primary hypertension, natriuretic peptides, NT-proBNP, renin
Graphic Abstract.
Created with BioRender.com
Summary
Ang-(1–12) is identified as a component of the human circulating RAS at equivalent concentrations in men and women and comparatively elevated in patients with primary hypertension.
Introduction
Reminiscent of how long it took for angiotensin-(1–7) [Ang-(1–7)] to be recognized as the countervailing component within the renin angiotensin system (RAS),1 awareness of the functional relevance of the dodecapeptide angiotensin-(1–12) [Ang-(1–12)] as an endogenous substrate for direct angiotensin II (Ang II) production is limited. It is universally accepted that the catalytic activity of renal renin upon the hepatic angiotensinogen (AGT) substrate initiates the metabolic process leading to the generation of the angiotensins.2 Consensus on the importance of renin as the primary mechanism for initiating the biotransformation of the AGT substrate obscures the criticality of alternate hydrolytic pathways in which cathepsins,3 tonin,4 kallikrein,5 and elastase-26 may initiate angiotensin release from the substrate. The predominance of this thinking needs reassessment given that the clinical outcomes of using RAS’s inhibitors have been disappointing7, 8 while the result of chronic delivery of AGT small interfering RNA (siRNA) in SHR, contrary to the authors’ conclusions, resulted in modest blood pressure reductions in the absence of concomitant valsartan therapy.9 These data underscore a need to explore in which conditions non-canonical and renin-independent Ang II-forming mechanisms participate in human cardiovascular disease.
Nagata et al.10 identified proangiotensin 12 as an alternate substrate yielding Ang II in Wistar rats as evidenced by the abolition of the pressor response induced by intravenous injection of the peptide following pretreatment with captopril or candesartan. Renamed as Ang-(1–12)11 to comply with the recommendations of the AHA Council for High Blood Pressure Research12, this substrate is an endogenous component of the RAS generating Ang I in blood by the hydrolytic activity of human and rodent angiotensin converting enzyme (ACE)13 and Ang II directly through chymase in rat and human heart tissues.11, 14, 15 Ang-(1–12) causes vasoconstrictor responses that are blocked by administration of ACE inhibitors or Ang II receptor blockers (ARBs)10 while central immunoneutralization with a polyclonal Ang-(1–12) antibody yields sustained antihypertensive effects in the renin-dependent transgenic hypertensive rat.16 Ang-(1–12) functional activity as an Ang II forming substrate has been demonstrated in terms of modulation of brain stem baroreflexes17–21 and potentiation of myocyte contractility via activation of known Ang II second messenger signaling22–26. Insight into its importance in heart disease is based on reports showing increased Ang-(1–12) expression in human left atrial appendages,11, 27, 28 normal human left ventricular tissue,15 and the plasma of patients with a diagnosis of acute respiratory distress syndrome (ARDS).29 A systematic evaluation of Ang-(1–12) presence in human blood in relation to other RAS components remains to be characterized. With this in mind, we determined plasma Ang-(1–12) levels in a cohort of normotensive adults and explored its associations with other RAS components, sex, and independent markers of cardiac function via assessment of plasma natriuretic peptides. Pilot measurements of these variables in a smaller cohort of hypertensive subjects provide clues as to the discriminatory and sensitivity value of Ang-(1–12) in primary hypertension (PH).
Methods
The data for this study derives from the Mayo Clinic Biobank (Rochester MN) through the participation of co-authors (SRI and JCB). Restrictions may apply regarding data availability. Dr. JC Burnett may be contacted for questions regarding access. The study’s participants were recruited through the Mayo Clinic Biobank, a Biorepository databank supporting population-based analytic studies of disease causes and outcomes.30
Study Population and Design:
Seventy-five adults (35 women) residing in Olmsted County MN consented to participate in this study that was approved by the Mayo Clinic Institutional Review Board.
Baseline demographic, clinical, and biochemical data were abstracted from the electronic medical record. For this study, a 45 mL blood sample from a peripheral vein was collected in pre-chilled EDTA-coated tubes. Blood samples were immediately centrifuged (2,500 rpm × 10 min) and multiple plasma aliquots were frozen (−80° C) until assayed. Blood tubes to be processed for RAS components contained 1, 10-ortho-phenanthroline monohydrate (0.5 mM); p-hydroxymercuribenzoate (1 mM); pepstatin A (125 μM); and EDTA (5 mM). The efficacy of these inhibitors to prevent proteases’ activity in the collected blood has been extensively characterized and documented by us elsewhere.31, 32
Of the 75 adults incorporated in the study, 22 women and 30 men were classified as normotensive based on arterial pressure measurements of ≤ 120/80 mm Hg as defined in the most recent hypertension guidelines,33 and no reported use of antihypertensive medications. Another 19 adults (13 women) were classified as hypertensive based on the data abstracted from their medical records. Of the 19 hypertensive subjects, two men had a record of having received antihypertensive medications in the past.
Material and Methods:
A detailed description of all methods employed for measurements of RAS biomarkers including plasma concentrations of AGT, Ang-(1–12), Ang II, aldosterone, plasma renin activity (PRA) and the natriuretic system peptides is provided in the Data Supplement and documented elsewhere.34–39
Statistical Analysis:
Statistical analysis was performed using IBM SPSS Statistics for Windows software version 26.0 (IBM, New York, NY, USA). Because peptide data did not pass the Shapiro-Wilk Normality test, either log transformation or nonparametric evaluation was performed. Pearson’s correlation tests were used to determine relationships between Ang-(1–12) and other clinical and biochemical variables after log transformation. To access the association of PH to plasma renin activity, multiple linear regression was performed with all other independent variables as fixed effects. Two-way ANOVA was employed to assess sex differences among variables, and the results are presented as mean ± the standard deviation (SD). Additionally, we created a multivariate linear regression model to ultimately assess the effect size associated with each covariate on PRA. Collinearity was assessed using a variance inflation factor and condition index, with values <10 indicating acceptability. Additionally, sensitivity analyses were performed to investigate effect of covariates on range of plasma Ang-(1–12), plasma Ang II and NT-proBNP. A two-tailed P-value <0.05 was considered statistically significant.
Results
Clinical determinants of health in the subjects classified as normal are shown in Table 1. Adult normotensive men and women did not differ in terms of age, body mass index, arterial and pulse pressures while serum creatinine concentration was 21.4% higher in men compared to women (p = 0.0001). These higher serum creatinine values in men are within the normal limits based on their age.40 Sex differences in serum creatine concentrations were still present in PH men [1.03 ± 0.18 (SD) mg/dL] and women [0.83 ± 0.15 mg/dL, p 0.047].
Table 1.
Clinical Characteristics of Enrolled Normal Adults
| Variable | Women (N=22) |
Men (N=30) |
P Value |
|---|---|---|---|
| Age, years | 54 ± 8 | 55 ± 9 | 0.7477 |
| Height, cm | 164 ± 6 | 178 ± 7 | 0.0001 |
| Body weight, Kg | 75 ± 12 | 91 ± 12 | 0.0001 |
| Body mass Index, kg/m2 | 27.96 ± 5.11 | 28.69 ± 3.95 | 0.5783 |
| Systolic blood pressure, mm Hg | 117 ± 14 | 117 ± 13 | 0.9471 |
| Diastolic blood pressure, mm Hg | 72 ± 13 | 74 ± 8 | 0.5544 |
| Pulse pressure, mm Hg | 45 ± 18 | 43 ± 12 | 0.6300 |
| Serum creatinine, mg/dL | 0.84 ± 0.15 | 1.02 ± 0.12 | 0.0001 |
| Plasma aldosterone, ng/dL | 7.54 ± 5.59 | 7.17 ± 5.59 | 0.8460 |
| Plasma atrial natriuretic peptide, pg/mL | 21.27 ± 11.46 | 17.09 ± 7.16 | 0.1420 |
| Plasma brain natriuretic peptide, pg/mL | 30.55 ± 21.21 | 30.17 ± 35.91 | 0.9623 |
| Plasma NT-proBNP, pg/mL | 95.55 ± 81.54 | 36.30 ± 22.89 | 0.0030 |
| Plasma cyclic GMP, pmol/mL | 7.56 ± 2.29 | 8.60 ± 2.79 | 0.1452 |
Values are mean ± SD
Grouped graphs showing both individual points (scatter) and mean ± SD of PRA, AGT, Ang-(1–12) and Ang II for normotensive adults are documented in Figure 1. No sex differences are present in the circulating values of these variables in normotensive adults. In this first demonstration of the presence of Ang-(1–12) in human blood, the concentration of the dodecapeptide in plasma reveals a waterfall phenomenon as AGT plasma concentrations [20,555 ± 9,876 (SD) ng/mL; n = 52] are 10,000-fold higher than plasma Ang-(1–12) [2.04 ± 0.57 (SD) ng/mL; n=52]. Median ± SD of plasma Ang-(1–12) values in 22 normal women and 30 normal men are 1.95 ± 0.62 ng/mL (95% CI: 1.75 – 2.30) and 2.04 ± 0.55 ng/mL (CI: 1.85 −2.26), respectively (p > 0.05).
Figure 1.
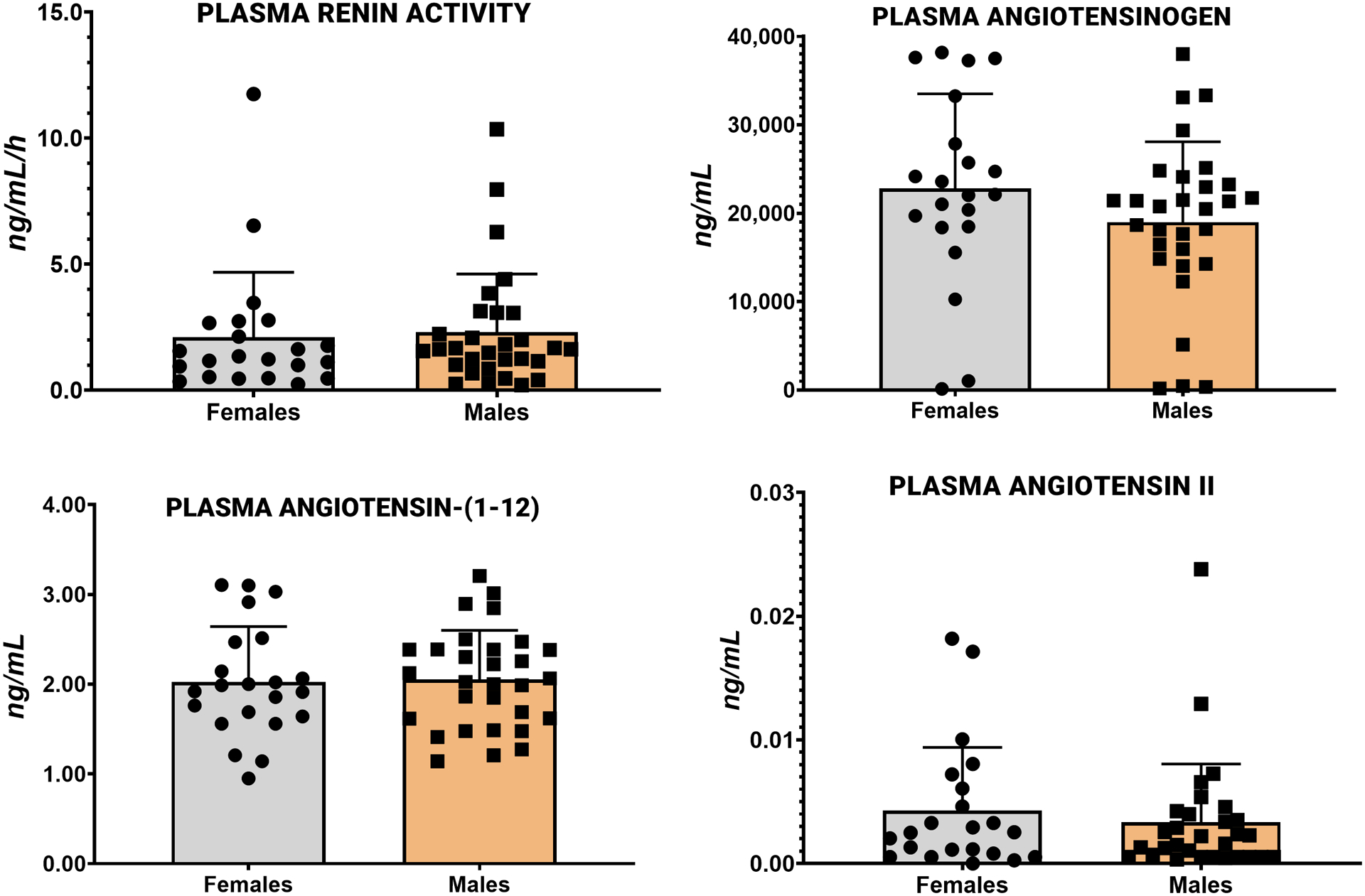
Plasma concentrations of renin angiotensin system components measured in 22 normal women and 30 normal men. Values are mean ± SD.
Plasma concentrations of ANP, BNP and cGMP were not different in the men and women of the normotensive cohort. Although, plasma NT-proBNP values are significantly higher in women compared to men (Table 1), all values fall within the normal range reported for patients’ age41, 42. Linear regression analysis of Ang-(1–12) log-transformed data revealed significant correlations with PRA (r = 0.42, p = 0.021), plasma AGT concentrations (r = 0.37, p = 0.042), and plasma aldosterone (r = 0.41, p = 0.023) in normotensive men but not women. Combining the data from normal men and women subjects resulted in the loss of statistically significant correlations among the Ang II-forming substrate with PRA and plasma aldosterone. On the other hand, the strength of the association between Ang-(1–12) and plasma AGT found in men persisted when the data from both sexes were pooled (Figure 2).
Figure 2.

Scattergram of log-transformed Ang-(1–12) versus plasma angiotensinogen concentrations in 50 of 52 normal adults.
Characterization of Ang-(1–12) as a component of the circulating RAS in normal adults at concentrations several orders of magnitude lower than AGT and higher than plasma Ang II, led us to explore whether this alternate Ang II-forming substrate may be altered in hypertensive patients. In 19 PH adults (13 women) age 62 ± 8 years (mean blood pressure of 133 ± 15/76 ± 8 mm Hg) plasma Ang-(1–12) concentrations averaged 2.39 ± 0.58 ng/mL (95% CI: 2.1 – 2.67). These Ang-(1–12) values were 12% higher than the overall mean of plasma Ang-(1–12) determined in the normal group (2.04 ± 0.57; 95% CI: 1.89 – 2.20; p = 0.027). Median, minimum, and maximal values for normal and hypertensive subjects are shown in Figure 3. The larger (13 subjects) hypertension women representation in the pilot study compared to men (6 subjects) did not influence the detection of higher plasma Ang-(1–12) values within the group. Plasma Ang-(1–12) in hypertensive women averaged 2.33 ± 0.63 (SD) ng/mL (n = 13; 95% CI: 1.95 – 2.72) compared to 2.51 ± 0.49 ng/mL (95% CI: 1.99 – 3.03) in the 6 hypertensive men (p = 0.5280).
Figure 3.

Whisker plot of comparative values of plasma Ang-(1–12) in 52 normal and 19 hypertensive adults. Fiskars are the minimal and maximal values of the median values for normal (2.00 ng/mL) and hypertensive (2.22 ng/mL) adults. Box depicts the 25th (1.62 ng/mL and 1.91 ng/mL in normal and hypertensive, respectively) and the 75th (2.39 ng/mL and 2.82 ng/mL in normal and hypertensive, respectively) percentile values.
To expand on these findings, we analyzed data specifically for age, arterial pressure, and circulating markers of RAS and natriuretic peptide system activation, after accounting for both sex and blood pressure status (Table 2). As expected, age, systolic blood pressure and pulse pressure were significantly higher in PH (BP status effects: p < 0.001 to 0.0004), irrespective of sex. Similar to the higher absolute Ang-(1–12) levels in PH, log-transformed plasma Ang-(1–12) to a normal distribution showed a significant blood pressure status effect [F(1, 67) = 6.629; p < 0.0205], with higher levels in hypertensives, regardless of sex. Marked effects of the blood pressure status also occurred with respect to PRA and plasma NT-proBNP, with significantly higher levels of these biomarkers of RAS and left ventricular strain, respectively, in PH’s when compared to normals. While PRA and plasma NT-proBNP were not influenced by sex, it is important to note the existence of an interaction effect on NT-proBNP [BP status × sex effect: F(1, 67) = 4.351; p = 0.0408], which may be due to the higher basal levels of NT-proBNP in normal women than normal men (Table 1). Unlike this indicator of an activated natriuretic system, plasma cGMP (log) was significantly altered by sex (p = 0.0011). Interestingly, while plasma cGMP levels, overall, were higher in men, the pattern between normal and hypertensive men was different from that of corresponding women [interaction effect: (F(1, 67) = 5.024, p = 0.028]; specifically, cGMP was increased by hypertension in men and unchanged or even somewhat reduced by hypertension in women, a finding consistent with the higher values of NT-proBNP.
Table 2.
Effect of Sex and Blood pressure status on Age, Hemodynamics, and Plasma Renin Angiotensin and Natriuretic Peptide Systems
| Variable | Normal (Mean ± SD) |
Hypertensive (Mean ± SD) |
Blood Pressure Status (p value) |
Sex (p value) |
Interaction (p value) |
|---|---|---|---|---|---|
| Age, (years) | |||||
| Women | 54 ± 8 | 61 ± 9 | |||
| Men | 55 ± 9 | 64 ± 8 | 0.012 | 0.4830 | 0.6989 |
| Systolic Blood Pressure, (mm Hg) | |||||
| Women | 117 ± 14 | 131 ± 16 | |||
| Men | 117 ± 13 | 136 ± 13 | 0.0002 | 0.5202 | 0.5342 |
| Pulse Pressure, (mm Hg) | |||||
| Women | 45 ± 18 | 56 ± 13 | |||
| Men | 43 ± 12 | 59 ± 10 | 0.0004 | 0.8148 | 0.5721 |
| Log Ang-(1–12), (ng/mL) | |||||
| Women | 2.02 ± 0.62 | 2.33 ± 0.63 | |||
| Men | 2.05 ± 0.55 | 2.51 ± 0.49 | 0.0216 | 0.5025 | 0.7265 |
| Plasma Renin Activity, (ng/mL/h) | |||||
| Women | 2.11 ± 2.57 | 9.17 ± 14.45 | |||
| Men | 2.30 ± 2.32 | 6.03 ± 3.22 | 0.0005 | 0.5481 | 0.7807 |
| Plasma NT-proBNP, (pg/mL) | |||||
| Women | 95.55 ± 81.54 | 141.00 ± 128.74 | |||
| Men | 36.30 ± 22.89 | 193.33 ± 146.55 | 0.0009 | 0.1682 | 0.0408 |
| Plasma cyclic GMP, (pmol/mL) | |||||
| Women | 7.56 ± 2.29 | 5.66 ± 2.31 | |||
| Men | 8.60 ± 2.79 | 10.04 ± 3.27 | 0.4722 | 0.0011 | 0.0283 |
Values are means ± SD. Lg, logarithm
Table 3 shows effect of PH to PRA after controlling for age, gender, and systolic blood pressure. Multiple regression analysis shows significant effects of PH on log-transformed plasma Ang-(1–12) (p = 0.009) and NT-proBNP (p = 0.02) when controlled for gender, age, and systolic blood pressure. Sensitivity analysis of Ang-(1–12) shows a significant effect of PH on age and blood pressure at the 3rd and 4th quartile range of Ang-(1–12) irrespective of gender. No effect of PH on age, blood pressure, and sex was found on any quartile value of plasma Ang-II while the highest (4th) quartile group value of NT-proBNP shows a significant effect of enhanced blood pressure on age and systolic blood pressure (data not shown).
Table 3.
Effect of Primary Hypertension to Plasma Renin Activity after Controlling for Age, Gender and Systolic Blood Pressure
| Dependent variable | Significance Level | 95.0% Confidence Interval | |
|---|---|---|---|
| Lower Bound | Upper Bound | ||
| Log Value of Plasma Ang-(1–12), (ng/mL) | 0.009 | −0.393 | −0.058 |
| Log Value of Plasma Ang II, (fmol/mL) | 0.337 | −1.172 | 0.407 |
| Log Value of ANP, (pg/mL) | 0.530 | −0.206 | 0.395 |
| Log Value of BNP, (pg/mL) | 0.173 | −0.662 | 0.122 |
| Log Value of NT-proBNP, (pg/mL) | 0.024 | −1.125 | −0.083 |
| Log Value of cGMP, (pmol/mL) | 0.299 | −0.107 | 0.343 |
Discussion
We document for the first time the presence of Ang-(1–12) in the blood of normal and hypertensive adults at concentrations that are several orders of magnitude lower than AGT but higher than those of blood Ang II. We further show that sex does not influence circulating Ang-(1–12) concentrations in normotensive subjects even though, for a comparatively similar age, normal women were leaner and shorter than normal men. As previously reported,43 normal women and men differed in terms of serum creatinine concentrations, a finding that relates to the body building phenotypes, caloric intake, and level of physical activity. Comparative circulating levels of plasma AGT, Ang II, aldosterone, and natriuretic peptides were found in normotensive adult men and women. On the other hand, plasma concentrations of NT-proBNP in men were less than half the values measured in the normal women. This finding agrees with the data reported by the Framingham Heart Study in which plasma NT-proBNP values were substantially higher in normal women compared with normal men at every age group.44
Statistically significant correlations were identified among circulating values of log-transformed Ang-(1–12) and PRA, plasma AGT, and plasma aldosterone in men. These associations suggest that in normal men approximately 16% (r2) of the Ang-(1–12) variance can be predicted by the dependent variables. Pooling of both normal men and women did not eliminate the correlation between Ang-(1–12) log-transformed and AGT, a finding that indicates the potential for a dependency of circulating Ang-(1–12) values on the prevailing level of plasma AGT. Prior studies showed that renin does not account for Ang-(1–12) metabolism into Ang II36, 45, 46 and that kallikrein or a kallikrein like enzyme cleaves Ang-(1–12) from AGT.1, 47 The weak correlation of PRA with Ang-(1–12) in males that is no longer found after combining the data from both sexes agrees with the more precise metabolism studies conducted in normal rats45, 46, 48 and in a humanized model of hypertension expressing the human AGT gene.36, 49 On the other hand, PRA was strongly correlated with plasma aldosterone in both men (r = 0.84, p < 0.0001) and women (r = 0.87, p < 0.0001).
The inclusion of Ang-(1–12) as a functional Ang II-forming substrate is in keeping with the demonstration of antihypertensive actions during intracerebral administration of an Ang-(1–12) polyclonal antibody to hypertensive TGR(mRen2)27,16 the normalization of arterial pressure in valsartan-treated transgenic rats expressing the human AGT gene,36 and the blockade by valsartan of the Ang-(1–12) increase of cardiac myocyte potassium current by intracellular Ang-(1–12).22 The further demonstration that the sustained blood pressure rise and high plasma aldosterone levels produced by a fortnight of continuous administration of Ang-(1–12) was reversed by losartan or perindopril agrees with the idea that Ang-(1–12) is an active member of the circulating RAS. The correlation between plasma Ang-(1–12) and AGT needs to be further investigated as it could possibly suggest a negative feedback of AGT on Ang-(1–12) in blood.
Emerging therapeutic concepts based on suppression of hepatic AGT using antisense oligonucleotides50 or small interfering RNAs (siRNAs)9 reveal that the antihypertensive actions of these interventions require greater than 90% reduction in the hepatic synthesis of the substrate. Human AGT is composed of 450 amino acids. Since the functional importance of the remaining components of the AGT protein [des-(Ang I)-AGT] are not understood,51–54 characterization of Ang-(1–12) expression in human blood may provide an alternate and more specific and safer approach to suppression of Ang II forming processes by immuno neutralization of the dodecapeptide instead of the full AGT protein. Our past demonstration that central inhibition of Ang-(1–12) activity with a polyclonal antibody resulted in a sustained blood pressure reduction in transgenic hypertensive rats is in keeping with this idea.16
The potential for Ang-(1–12) to act as an Ang II forming substrate in conditions in which the RAS is activated is underscored by the demonstration of increased cardiac Ang-(1–12) content in the heart of SHR55 while studies of Ang-(1–12) levels in rats exposed to changes in salt intake,56 bilateral nephrectomy,45 or hypertension induced by transgenic expression of human AGT,36, 49 revealed an independent regulation of the dodecapeptide in blood and tissues. The data reported here strengthen a role of Ang-(1–12) participation in the pathogenesis of PH as the increases in plasma Ang-(1–12) in this cohort showed significant associations with PRA and NT-proBNP, a selective marker of cardiac dysfunction.39
While the contribution of the RAS to the pathogenesis of PH remains undisputed, identifying one or more circulating biomarkers from this system remains a challenge.57, 58 The rise in plasma Ang-(1–12) in patients with a diagnosis of PH is a novel observation, particularly as Ang-(1–12) but not plasma AGT and plasma Ang II were altered in the same patients. On the other hand, the sex independent effect of blood pressure elevations on basal PRA and NT-proBNP levels strengthens the possible involvement of plasma Ang-(1–12) as a biomarker of altered RAS activity in hypertensive vascular disease. This conclusion will require a more rigid evaluation in a well-controlled cohort of PH as the sample included in this study contained predominantly women, no data were available regarding dietary conditions and physical activity, and the reproducibility of blood pressure measurements over several visits were not available. The bias imposed by the larger representation of women in the pilot study and the past use of antihypertensive medications in two of the 6 men did not alter the presence of higher Ang-(1–12) in these PH patients. With no attempt to mitigate the potential limitations of the present study, we can conclude that this study shows that Ang-(1–12) is a component of the circulating RAS in normal and hypertensive subjects at concentrations that are lower than circulating AGT and yet higher than those reported for Ang I59 and, as documented in this study, Ang II. The demonstration of Ang-(1–12) as a source for direct Ang II production14, 15, its higher expression in the blood of patients succumbing from Acute Respiratory Distress Syndrome (ARDS) infection29, and the visualization of Ang-(1–12) immunoreactive products in the enlarged left atria of patients undergoing heart surgery due to left heart disease27, 28 or resistant atrial fibrillation11 poses a need to rethink how primary canonical pathways are for Ang II production in the development and progression of cardiovascular and metabolic diseases.
Perspectives.
In this observational study we demonstrate the presence of Ang-(1–12) in the blood of normal and hypertensive humans and provide evidence that increased plasma levels of this Ang II-forming substrate, and not plasma AGT or plasma Ang II, associates with the presence of primary essential hypertension.
Novelty and Significance:
What Is New?
The blood of men and women contain angiotensin-(1–12) at concentrations that can easily be interpreted to serve as a source for the formation of angiotensin II in the circulation.
While plasma angiotensin-(1–12) shows no sex-differences in normotensive subjects, this Ang II-forming substrate correlated with plasma concentrations of AGT, aldosterone, and PRA only in men.
Angiotensin-(1–12) in plasma is elevated in hypertensive men and women patients in association with comparative increases in PRA and plasma NT-proBNP.
What is Relevant?
Circulating levels of Ang-(1–12) may be more predictive of increased RAS activity in human cardiovascular disease compared to plasma AGT or even Ang II.
Source(s) and Funding
This study was supported by grants HL-051952 (CMF) and HL-136340 (JCB) from the National Heart, Lung and Blood Institute of the National Institutes of Health.
Footnotes
Disclosures
No conflicts of interest, financial or otherwise, are declared by the authors.
References
- 1.Ferrario CM, Ahmad S, Nagata S, Simington SW, Varagic J, Kon N, Dell’italia LJ. An evolving story of angiotensin-ii-forming pathways in rodents and humans. Clin Sci (Lond). 2014;126:461–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferrario CM. Addressing the theoretical and clinical advantages of combination therapy with inhibitors of the renin-angiotensin-aldosterone system: Antihypertensive effects and benefits beyond bp control. Life Sci. 2010;86:289–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar R, Boim MA. Diversity of pathways for intracellular angiotensin ii synthesis. Curr Opin Nephrol Hypertens. 2009;18:33–39 [DOI] [PubMed] [Google Scholar]
- 4.Belova LA. Angiotensin ii-generating enzymes. Biochemistry (Mosc). 2000;65:1337–1345 [DOI] [PubMed] [Google Scholar]
- 5.Schmaier AH. The plasma kallikrein-kinin system counterbalances the renin-angiotensin system. J Clin Invest. 2002;109:1007–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becari C, Oliveira EB, Salgado MC. Alternative pathways for angiotensin ii generation in the cardiovascular system. Braz J Med Biol Res. 2011;44:914–919 [DOI] [PubMed] [Google Scholar]
- 7.Dusing R Mega clinical trials which have shaped the ras intervention clinical practice. Ther Adv Cardiovasc Dis. 2016;10:133–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Musini VM, Lawrence KA, Fortin PM, Bassett K, Wright JM. Blood pressure lowering efficacy of renin inhibitors for primary hypertension. Cochrane Database Syst Rev. 2017;4:CD007066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uijl E, Mirabito Colafella KM, Sun Y, Ren L, van Veghel R, Garrelds IM, de Vries R, Poglitsch M, Zlatev I, Kim JB, Hoorn EJ, Foster D, Danser AHJ. Strong and sustained antihypertensive effect of small interfering rna targeting liver angiotensinogen. Hypertension. 2019;73:1249–1257 [DOI] [PubMed] [Google Scholar]
- 10.Nagata S, Kato J, Sasaki K, Minamino N, Eto T, Kitamura K. Isolation and identification of proangiotensin-12, a possible component of the renin-angiotensin system. Biochem Biophys Res Commun. 2006;350:1026–1031 [DOI] [PubMed] [Google Scholar]
- 11.Ahmad S, Simmons T, Varagic J, Moniwa N, Chappell MC, Ferrario CM. Chymase-dependent generation of angiotensin ii from angiotensin-(1–12) in human atrial tissue. PLoS One. 2011;6:e28501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bumpus FM, Catt KJ, Chiu AT, DeGasparo M, Goodfriend T, Husain A, Peach MJ, Taylor DG Jr., Timmermans PB. Nomenclature for angiotensin receptors. A report of the nomenclature committee of the council for high blood pressure research. Hypertension. 1991;17:720–721 [DOI] [PubMed] [Google Scholar]
- 13.Moniwa N, Varagic J, Simington SW, Ahmad S, Nagata S, Voncannon JL, Ferrario CM. Primacy of angiotensin converting enzyme in angiotensin-(1–12) metabolism. Am J Physiol Heart Circ Physiol. 2013;305:H644–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmad S, Varagic J, VonCannon JL, Groban L, Collawn JF, Dell’Italia LJ, Ferrario CM. Primacy of cardiac chymase over angiotensin converting enzyme as an angiotensin-(1–12) metabolizing enzyme. Biochem Biophys Res Commun. 2016;478:559–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmad S, Wei CC, Tallaj J, Dell’Italia LJ, Moniwa N, Varagic J, Ferrario CM. Chymase mediates angiotensin-(1–12) metabolism in normal human hearts. J Am Soc Hypertens. 2013;7:128–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Isa K, Garcia-Espinosa MA, Arnold AC, Pirro NT, Tommasi EN, Ganten D, Chappell MC, Ferrario CM, Diz DI. Chronic immunoneutralization of brain angiotensin-(1–12) lowers blood pressure in transgenic (mren2)27 hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2009;297:R111–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arakawa H, Kawabe K, Sapru HN. Angiotensin-(1–12) in the rostral ventrolateral medullary pressor area of the rat elicits sympathoexcitatory responses. Exp Physiol. 2013;98:94–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arnold AC, Isa K, Shaltout HA, Nautiyal M, Ferrario CM, Chappell MC, Diz DI. Angiotensin-(1–12) requires angiotensin converting enzyme and at1 receptors for cardiovascular actions within the solitary tract nucleus. Am J Physiol Heart Circ Physiol. 2010;299:H763–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chitravanshi VC, Proddutur A, Sapru HN. Cardiovascular actions of angiotensin-(1–12) in the hypothalamic paraventricular nucleus of the rat are mediated via angiotensin ii. Exp Physiol. 2012;97:1001–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chitravanshi VC, Sapru HN. Cardiovascular responses elicited by a new endogenous angiotensin in the nucleus tractus solitarius of the rat. Am J Physiol Heart Circ Physiol. 2011;300:H230–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawabe T, Kawabe K, Sapru HN. Cardiovascular effect of angiotensin-(1–12) in the caudal ventrolateral medullary depressor area of the rat. Am J Physiol Heart Circ Physiol. 2014;306:H438–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Mello WC, Dell’Itallia LJ, Varagic J, Ferrario CM. Intracellular angiotensin-(1–12) changes the electrical properties of intact cardiac muscle. Mol Cell Biochem. 2016;422:31–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li T, Zhang X, Cheng HJ, Zhang Z, Ahmad S, Varagic J, Li W, Cheng CP, Ferrario CM. Critical role of the chymase/angiotensin-(1–12) axis in modulating cardiomyocyte contractility. Int J Cardiol. 2018;264:137–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li T, Zhang Z, Zhang X, Chen Z, Cheng HJ, Ahmad S, Ferrario CM, Cheng CP. Reversal of angiotensin-(1–12)-caused positive modulation on left ventricular contractile performance in heart failure: Assessment by pressure-volume analysis. Int J Cardiol. 2020;301:135–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prosser HC, Forster ME, Richards AM, Pemberton CJ. Cardiac chymase converts rat proangiotensin-12 (pa12) to angiotensin ii: Effects of pa12 upon cardiac haemodynamics. Cardiovasc Res. 2009;82:40–50 [DOI] [PubMed] [Google Scholar]
- 26.Reyes S, Cheng CP, Roberts DJ, Yamashita T, Ahmad S, VonCannon JL, Wright KN, Dell’Italia LJ, Varagic J, Ferrario CM. Angiotensin-(1–12)/chymase axis modulates cardiomyocyte l-type calcium currents in rats expressing human angiotensinogen. Int J Cardiol. 2019;297:104–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang H, Varagic J, Nagata S, Kon ND, Ahmad S, VonCannon JL, Wright KN, Sun X, Deal D, Groban L, Ferrario CM. Atrial angiotensin-(1–12)/chymase expression data in patient of heart diseases. Data Brief. 2020;31:105744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang H, Varagic J, Nagata S, Kon ND, Ahmad S, VonCannon JL, Wright KN, Sun X, Deal D, Groban L, Ferrario CM. Differential expression of the angiotensin-(1–12)/chymase axis in human atrial tissue. J Surg Res. 2020;253:173–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reddy R, Asante I, Liu S, Parikh P, Liebler J, Borok Z, Rodgers K, Baydur A, Louie SG. Circulating angiotensin peptides levels in acute respiratory distress syndrome correlate with clinical outcomes: A pilot study. PLoS One. 2019;14:e0213096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Melton LJ 3rd. History of the rochester epidemiology project. Mayo Clin Proc. 1996;71:266–274 [DOI] [PubMed] [Google Scholar]
- 31.Brosnihan KB, Chappell MC. Measurement of angiotensin peptides: Hplc-ria. Methods Mol Biol. 2017;1527:81–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferrario CM, Brosnihan KB, Chappell M. Measurements of angiotensin peptides. Hypertension. 1995;26:843–845 [PubMed] [Google Scholar]
- 33.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr., Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr., Williamson JD, Wright JT Jr. 2017 acc/aha/aapa/abc/acpm/ags/apha/ash/aspc/nma/pcna guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the american college of cardiology/american heart association task force on clinical practice guidelines. Hypertension. 2018;71:e13–e115 [DOI] [PubMed] [Google Scholar]
- 34.AbouEzzeddine OF, McKie PM, Scott CG, Rodeheffer RJ, Chen HH, Michael Felker G, Jaffe AS, Burnett JC, Redfield MM. Biomarker-based risk prediction in the community. Eur J Heart Fail. 2016;18:1342–1350 [DOI] [PubMed] [Google Scholar]
- 35.Burnett JC Jr., Kao PC, Hu DC, Heser DW, Heublein D, Granger JP, Opgenorth TJ, Reeder GS. Atrial natriuretic peptide elevation in congestive heart failure in the human. Science. 1986;231:1145–1147 [DOI] [PubMed] [Google Scholar]
- 36.Ferrario CM, VonCannon J, Ahmad S, Wright KN, Roberts DJ, Wang H, Yamashita T, Groban L, Cheng CP, Collawn JF, Dell’Italia LJ, Varagic J. Activation of the human angiotensin-(1–12)-chymase pathway in rats with human angiotensinogen gene transcripts. Front Cardiovasc Med. 2019;6:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Katsurada A, Hagiwara Y, Miyashita K, Satou R, Miyata K, Ohashi N, Navar LG, Kobori H. Novel sandwich elisa for human angiotensinogen. Am J Physiol Renal Physiol. 2007;293:F956–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lisy O, Redfield MM, Jovanovic S, Jougasaki M, Jovanovic A, Leskinen H, Terzic A, Burnett JC Jr. Mechanical unloading versus neurohumoral stimulation on myocardial structure and endocrine function in vivo. Circulation. 2000;102:338–343 [DOI] [PubMed] [Google Scholar]
- 39.McKie PM, Burnett JC Jr. Nt-probnp: The gold standard biomarker in heart failure. J Am Coll Cardiol. 2016;68:2437–2439 [DOI] [PubMed] [Google Scholar]
- 40.Lewis SM, Dirksen SR, Heitkemper MM, Bucher L, Harding M. Medical-surgical nursing : Assessment and management of clinical problems. St. Louis, Missouri: Elsevier/Mosby; 2014. [Google Scholar]
- 41.Januzzi JL Jr. Natriuretic peptide testing: A window into the diagnosis and prognosis of heart failure. Cleve Clin J Med. 2006;73:149–152, 155–147 [DOI] [PubMed] [Google Scholar]
- 42.Januzzi JL, van Kimmenade R, Lainchbury J, Bayes-Genis A, Ordonez-Llanos J, Santalo-Bel M, Pinto YM, Richards M. Nt-probnp testing for diagnosis and short-term prognosis in acute destabilized heart failure: An international pooled analysis of 1256 patients: The international collaborative of nt-probnp study. Eur Heart J. 2006;27:330–337 [DOI] [PubMed] [Google Scholar]
- 43.Delanaye P, Cavalier E, Pottel H. Serum creatinine: Not so simple! Nephron. 2017;136:302–308 [DOI] [PubMed] [Google Scholar]
- 44.Fradley MG, Larson MG, Cheng S, McCabe E, Coglianese E, Shah RV, Levy D, Vasan RS, Wang TJ. Reference limits for n-terminal-pro-b-type natriuretic peptide in healthy individuals (from the framingham heart study). Am J Cardiol. 2011;108:1341–1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferrario CM, Varagic J, Habibi J, Nagata S, Kato J, Chappell MC, Trask AJ, Kitamura K, Whaley-Connell A, Sowers JR. Differential regulation of angiotensin-(1–12) in plasma and cardiac tissue in response to bilateral nephrectomy. Am J Physiol Heart Circ Physiol. 2009;296:H1184–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trask AJ, Jessup JA, Chappell MC, Ferrario CM. Angiotensin-(1–12) is an alternate substrate for angiotensin peptide production in the heart. Am J Physiol Heart Circ Physiol. 2008;294:H2242–2247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wei CC, Chen Y, Powell LC, Zheng J, Shi K, Bradley WE, Powell PC, Ahmad S, Ferrario CM, Dell’Italia LJ. Cardiac kallikrein-kinin system is upregulated in chronic volume overload and mediates an inflammatory induced collagen loss. PLoS One. 2012;7:e40110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Westwood BM, Chappell MC. Divergent pathways for the angiotensin-(1–12) metabolism in the rat circulation and kidney. Peptides. 2012;35:190–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ferrario CM, VonCannon J, Jiao Y, Ahmad S, Bader M, Dell’Italia LJ, Groban L, Varagic J. Cardiac angiotensin-(1–12) expression and systemic hypertension in rats expressing the human angiotensinogen gene. Am J Physiol Heart Circ Physiol. 2016;310:H995–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mullick AE, Yeh ST, Graham MJ, Engelhardt JA, Prakash TP, Crooke RM. Blood pressure lowering and safety improvements with liver angiotensinogen inhibition in models of hypertension and kidney injury. Hypertension. 2017;70:566–576 [DOI] [PubMed] [Google Scholar]
- 51.Celerier J, Cruz A, Lamande N, Gasc JM, Corvol P. Angiotensinogen and its cleaved derivatives inhibit angiogenesis. Hypertension. 2002;39:224–228 [DOI] [PubMed] [Google Scholar]
- 52.Corvol P, Lamande N, Cruz A, Celerier J, Gasc JM. Inhibition of angiogenesis: A new function for angiotensinogen and des(angiotensin i)angiotensinogen. Curr Hypertens Rep. 2003;5:149–154 [DOI] [PubMed] [Google Scholar]
- 53.Lu H, Cassis LA, Kooi CW, Daugherty A. Structure and functions of angiotensinogen. Hypertens Res. 2016;39:492–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lu H, Wu C, Howatt DA, Balakrishnan A, Moorleghen JJ, Chen X, Zhao M, Graham MJ, Mullick AE, Crooke RM, Feldman DL, Cassis LA, Vander Kooi CW, Daugherty A. Angiotensinogen exerts effects independent of angiotensin ii. Arterioscler Thromb Vasc Biol. 2016;36:256–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jessup JA, Trask AJ, Chappell MC, Nagata S, Kato J, Kitamura K, Ferrario CM. Localization of the novel angiotensin peptide, angiotensin-(1–12), in heart and kidney of hypertensive and normotensive rats. Am J Physiol Heart Circ Physiol. 2008;294:H2614–2618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nagata S, Kato J, Kuwasako K, Kitamura K. Plasma and tissue levels of proangiotensin-12 and components of the renin-angiotensin system (ras) following low- or high-salt feeding in rats. Peptides. 2010;31:889–892 [DOI] [PubMed] [Google Scholar]
- 57.Carretero OA, Oparil S. Essential hypertension. Part i: Definition and etiology. Circulation. 2000;101:329–335 [DOI] [PubMed] [Google Scholar]
- 58.Davis J, Oparil S. Novel medical treatments for hypertension and related comorbidities. Curr Hypertens Rep. 2018;20:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ferrario CM, Jessup JA, Smith RD. Hemodynamic and hormonal patterns of untreated essential hypertension in men and women. Ther Adv Cardiovasc Dis. 2013;7:293–305 [DOI] [PMC free article] [PubMed] [Google Scholar]


