Abstract
The β-adrenergic receptors (βARs) include three subtypes, β1, β2 and β3. These receptors are widely expressed and regulate numerous physiological processes including cardiovascular and metabolic functions and airway tone. The βARs are also important targets in the treatment of many diseases including hypertension, heart failure and asthma. In some cases, the use of current βAR ligands to treat a disease is suboptimal and can lead to severe side effects. One strategy to potentially improve such treatments is the development of biased agonists that selectively regulate a subset of βAR signaling pathways and responses. Here we discuss the compounds identified to date that preferentially activate a Gs- or β-arrestin-mediated signaling pathway through βARs. Mechanistic insight on how these compounds bias signaling sheds light on the potential development of even more selective compounds that should have increased utility in treating disease.
Keywords: arrestin, G protein-coupled receptor, GRK, phosphorylation, signaling
1. Introduction
The β-adrenergic receptors (βARs) are a subfamily of G protein-coupled receptors (GPCRs) that are expressed by most cell types in humans (1). This subfamily consists of three members, β1, β2, and β3AR, and are the targets of the endogenous catecholamines epinephrine and norepinephrine (2, 3). Signaling through βARs regulates a wide variety of physiological processes including cardiac function, airway tone, metabolic function, and others (4). Due to their ubiquity and key role in human health, the βARs are cornerstone drug targets for a variety of pathologies (5), and drug discovery efforts around these receptors have generated a diverse set of pharmacological agents (6).
Canonically, β-agonists promote receptor mediated G protein activation primarily through Gs to activate the enzyme adenylyl cyclase and increase cAMP production. While there are subtype differences, activated βARs are typically phosphorylated by regulatory kinases such as GPCR kinases (GRKs), and signaling is then terminated via interaction with β-arrestins, a process called desensitization (7). In recent years, it has become widely accepted that this cycle is an incomplete description of the signaling repertoire of the βARs. An additional role of β-arrestins as signal transducers has been identified (8) and β-arrestins can also mediate receptor internalization (Fig. 1A). While regulation of β3AR signaling is less well characterized than β1AR or β2AR, the β3AR does not interact with GRKs or β-arrestins and desensitization is observed more often after hours to days rather than minutes (9). This is due in part to the lack of regulatory kinase phosphorylation sites on the C-terminal tail of the β3AR that are found in the β1AR and β2AR. Evidence suggests that regulation of β3AR involves downregulation of its mRNA and the receptor protein itself (9) (Fig. 1B). In addition, βARs have been shown to couple to multiple G proteins, and G protein and β-arrestin interaction with the receptor can be selectively promoted by ligands that stabilize distinct receptor conformations. The selective activation of these pathways is known as biased signaling (Fig. 2). In the early 2000s, the concepts of “pluridimensional efficacy” and “ligand-biased signaling” were first observed for the β2AR when compounds that were previously characterized as receptor antagonists were reported to have the ability to stimulate β-arrestin-dependent MAP kinase signaling (10) while subsequent studies pioneered the re-classification of β2AR ligands (11, 12). This review will focus on recent developments in this signaling paradigm at the βARs.
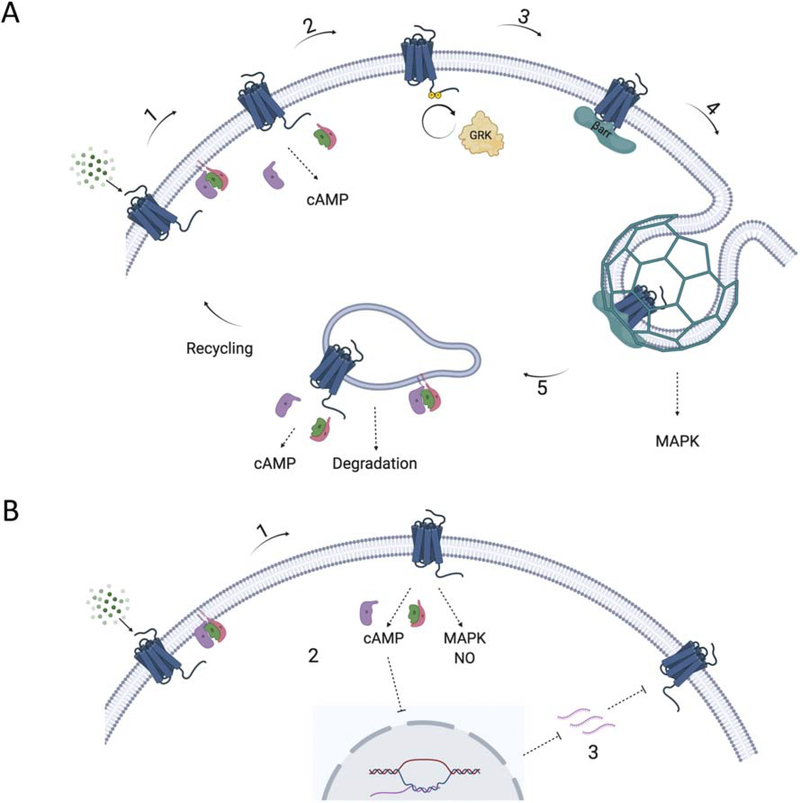
Figure 1. Schematic of βAR signaling cycle.
(A) β1AR and β2AR: 1. Activation of the βAR by a catecholamine promotes interaction with a heterotrimeric G protein (primarily Gs) which leads to GDP dissociation and subsequent GTP binding to the Gα subunit, and dissociation of the Gα GTP and Gβγ subunits from the receptor. Activated Gαs then interacts with the enzyme adenylyl cyclase to promote production of cAMP and activation of PKA. 2. Activated receptor is phosphorylated by GRKs which 3. promotes β-arrestin (βarr) interaction with the βAR. 4. β-arrestins become activated when bound to the phosphorylated receptor and the released C-terminal tail of the β-arrestin can then interact with components of the endocytic machinery including clathrin and AP2 to promote the uptake of the receptor into clathrin-coated pits for internalization. Previous studies have demonstrated that this process can promote β-arrestin-dependent activation of MAP kinases (MAPK) through both the β1AR and β2AR (79). 5. Internalized receptor can reengage with Gs to mediate additional signaling or it can be sorted for various post-endocytic fates including recycling or degradation in lysosomes. (B) β3AR: 1. Activation of the β3AR by catecholamines promotes Gs activation and subsequent cAMP production and PKA activation as described above. 2. Signaling downstream of activated β3AR can also lead to inhibition of transcription of the receptor through cAMP response elements (80). 3. Reduced transcription of the β3AR gene leads to reduced surface expression of the receptor.
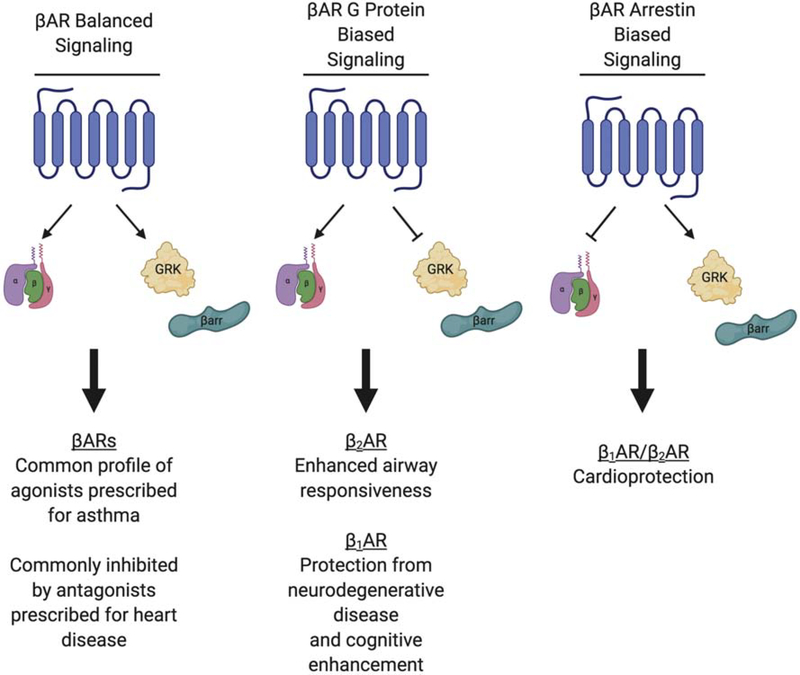
Figure 2. Overview of biased agonism at βARs.
This schematic illustrates a simplified overview of βAR biased signaling phenotypes. Balanced signaling promotes receptor interaction with G proteins, GRKs and arrestins. G protein biased signaling can occur either when G protein mediated signaling is enhanced relative to GRK and arrestin interaction, or when G protein signaling is intact and GRK and arrestin interaction is reduced. The converse of this is true for arrestin biased signaling, G protein signaling is reduced relative to GRK/arrestin signaling. This depiction is simplified in that other forms of bias are possible, but these represent the most salient phenotypes discussed in this review. Clinical applications of each signaling phenotype are listed under the representation of each signaling profile.
2. The βAR subfamily
The βAR subfamily includes the β1, β2, and β3ARs. These receptors are generally highly conserved within the transmembrane domains that mediate ligand binding and ligand-induced conformational changes while the extracellular and intracellular regions are poorly conserved (Fig. 3). These receptors coordinate physiological responses to the catecholamines epinephrine and norepinephrine, which promote activation of their cognate G protein, Gs. In recent years, an expanded view of the βAR interactome has shown evidence of activation of the non-cognate G protein Gi in addition to Gs. Gs and Gi, respectively, increase and decrease the activity of adenylyl cyclase to regulate the intracellular concentration of the second messenger cAMP to regulate downstream signaling (13). Activation of these receptors promotes receptor phosphorylation by second messenger dependent protein kinases such as protein kinase A (PKA) and by G protein-coupled receptor kinases such as GRK2, GRK5 and GRK6 (14). Interestingly, PKA has been implicated in G protein coupling specificity, promoting a switch from Gs to Gi for both the β1AR and β2ARs (15–17), while GRK phosphorylation has been shown to be important for β-arrestin binding to the βARs (14). β-arrestin1 and β-arrestin2 promote the internalization of activated phosphorylated βARs (18, 19), and are also reported to promote signaling through ERK1/2 and other pathways (10, 20). In this regard, it is worth noting that β-arrestin-mediated signaling has been reported to also require heterotrimeric G proteins (21,22), although this may be dependent on the model system being studied (23) and is certainly an area in need of further exploration.
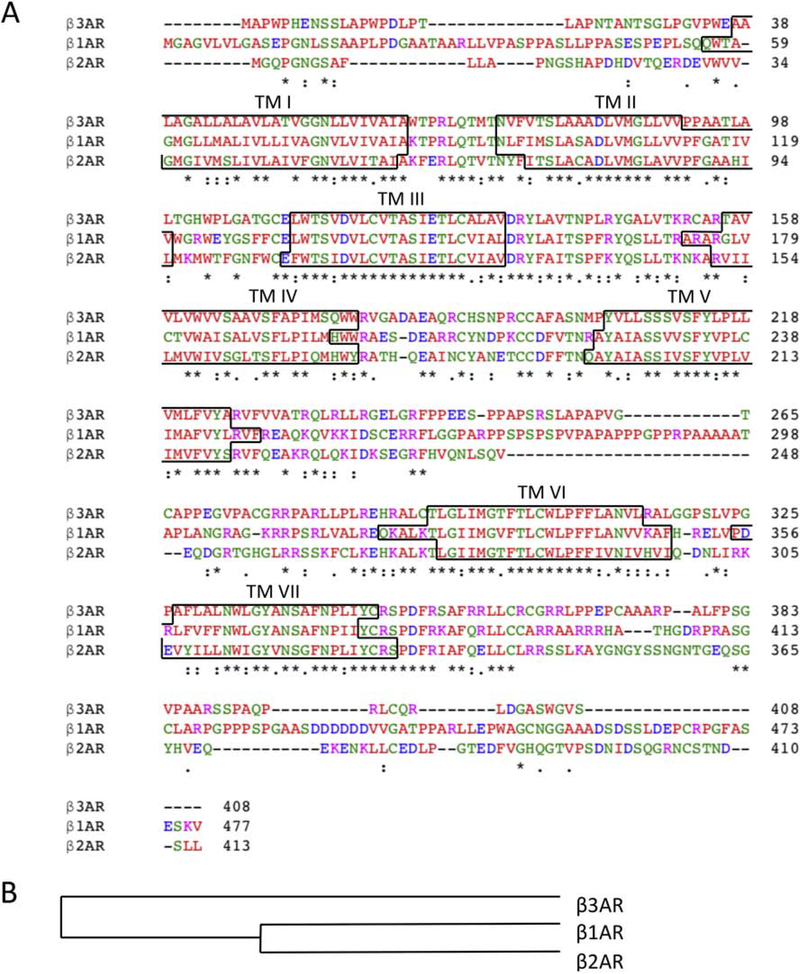
Figure 3. Protein sequence alignment of the βARs.
(A) Multiple sequence alignment of the β1, β2, and β3AR (81). Residues marked with an asterisk are uniformly conserved across all three subtypes, a colon represents conservation with strongly similar properties, and a period represents conservation with weakly similar properties. Residues in red represents small, hydrophobic side chains, blue represents acidic side chains, magenta represents basic side chains, and green represents hydroxyl, sulfhydryl, and amine side chains as well as glycine residues. Transmembrane regions are identified by the black border. (B) Cladogram representation of the βAR family members. β1 and β2AR are more closely related to each other than to the β3AR.
The βARs are ubiquitously expressed in humans, but the principal subtype varies by tissue (1). The β1AR is predominantly expressed in cardiac tissue, and is a key regulator of cardiac output. Antagonism of the β1AR is a standard of care strategy for the treatment of hypertension, cardiac arrhythmias, heart disease, and other associated pathologies. The β2AR is ubiquitously expressed with high levels in smooth muscle, especially that of the airway. Agonists of the β2AR promote airway smooth muscle relaxation and bronchodilation, and are thus a key target for treating airway diseases like asthma and chronic obstructive pulmonary disease (COPD). The β2AR has been extensively studied, and has served as an exemplar model for understanding GPCR signaling paradigms. The β3AR is predominantly expressed in adipose tissue and is involved in the regulation of lipolysis and thermogenesis. This receptor is the least well studied of the three βARs (24) and while selective β3AR agonists may have therapeutic utility for weight loss given its expression in adipose tissue, these compounds have not proven to be effective in clinical trials. Each of these receptors is a valuable drug target, and biased signaling at the βARs has the potential to enhance therapeutic effects with fewer deleterious side effects by fine-tuning the physiological response to treatment.
3. Clinical utility of biased signaling at βARs
Arrestin biased signaling at the β1AR provides additional clinical utility compared to balanced antagonists for cardiopathies. Compensatory dysregulation of the sympathetic nervous system causes an increase in circulating catecholamines in compensated, stable congestive heart failure. Blocking G protein signaling through cardiac β1AR has a reductive effect on the heart rate and ultimately leads to an improved ejection fraction. A β-arrestin biased agonist improves on this therapeutic strategy by further desensitizing β1AR at the cell membrane via internalization and also through transactivation of epidermal growth factor receptors (EGFRs) (25). EGFR is involved in the regulation of nitric oxide (NO) production, and activation of this pathway downstream of β-arrestin interaction with β1AR induces NO to promote cardioprotective effects (26). In addition to cardiopathies, β1AR is also implicated in various cognitive disorders where biased signaling through the receptor may be therapeutic (27–29).
G protein biased agonism at the β2AR could provide enhanced bronchodilation in airway smooth muscle relative to balanced β-agonists. β-agonistsare commonly prescribed for airway diseases such as asthma, but long-term use of these drugs leads to a desensitization of response to continued treatment and serious adverse effects (30). Evidence suggests that the desensitization of response to treatment and downregulation of β2AR expression are downstream of β-arrestin interaction with the receptor (31, 32). Additionally, β-arrestin2 knockout mice show improved inflammatory phenotypes when treated with β-agonists relative to wild type (33). This improved inflammatory profile may be protective from other contributing factors to worsening asthma outcomes such as airway remodeling.
Clinical interest in the β3AR is primarily focused around metabolic disorders and obesity. Stimulation of β3AR activates brown adipose tissue thermogenesis and increases mitochondrial biogenesis which leads to weight loss and selective fat decrease without reducing food intake (34). Current characterization of β3AR selective ligands suggests degrees of bias for different downstream signaling pathways, however, to date physiological evaluations of the potential benefit of this therapeutic strategy remain incomplete (35). In addition to metabolic disorders, β3AR is being explored as a therapeutic target in heart failure and is the target of marketed drugs for overactive bladder syndrome (36).
4. Arrestin biased β-agonists
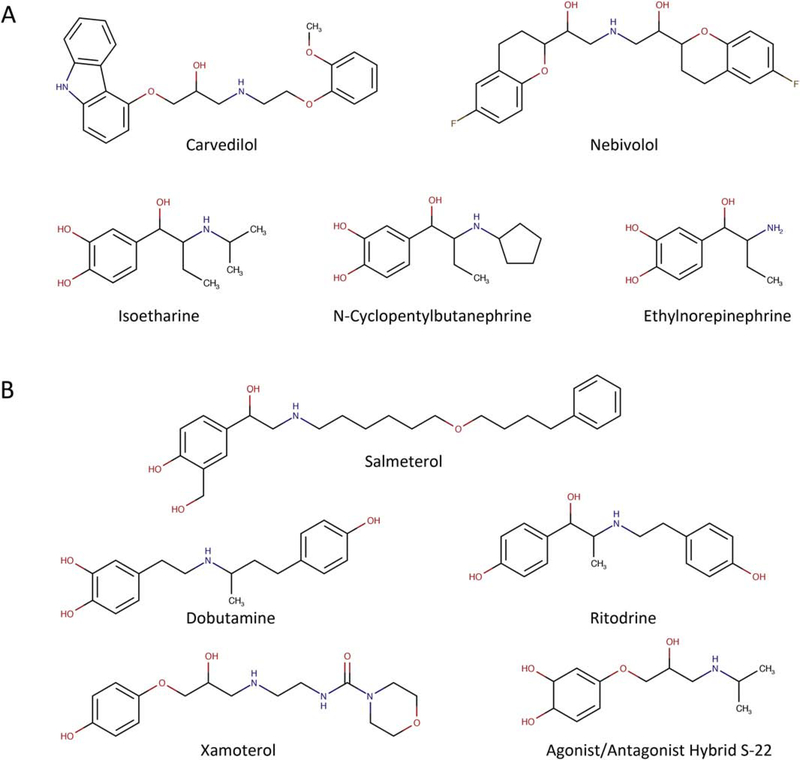
The compounds described below represent a selection of reported β-arrestin biased β-agonists. This series of compounds is not necessarily exhaustive, but is a cross-section of compounds demonstrating this signaling phenotype. The structures of the small molecules are presented in Fig. 4A, and pharmacological activity is summarized in Table 1.
Figure 4. Chemical structures of βAR biased agonists.
(A) Arrestin biased βAR ligands. (B) G protein biased βAR ligands.
Table 1.
Pharmacological profile of βAR biased agonists
| Ligand |
Receptor Specificity |
G protein* |
Arrestin* |
|
|---|---|---|---|---|
| Arrestin Biased | Carvedilol | β1, β2 | − | + |
| Isoetharine | β2 | + | ++ | |
| N-Cyclopentylbutanephrine | β2 | + | ++ | |
| Ethylnorepinephrine | β2 | + | ++ | |
| Nebivolol | β1, β2 | − | + | |
| ICL 1–9 | β2 | − | + | |
| G Protein Biased | ICL 3–9 | β2 | + | − |
| Salmeterol | β2 | ++ | + | |
| Agonist/Antagonist Hybrids | β1, β2 | − | + | |
| Dobutamine | β2 | ++ | + | |
| Ritodrine | β2 | ++ | + | |
| Xamoterol | β1 | + | − | |
− antagonism of pathway
+ agonism of pathway
++ agonism with increased activity at one pathway relative to the other
4.1. Carvedilol
Carvedilol is a widely used α1- and β-receptor blocker that was also found to function as a β-arrestin biased β-agonist (37). This compound acts as an inverse agonist at the G protein pathway, but elicits a β-arrestin dependent stimulation of other signaling pathways including ERK phosphorylation and transactivation of EGFR (25, 38). Interestingly, a recent study has shown that Gi is required for carvedilol mediated β-arrestin signaling at the β1AR, but not the β2AR (39). The authors demonstrate that carvedilol mediated ERK phosphorylation downstream of the β1AR is sensitive to pertussis toxin treatment, while ERK phosphorylation downstream of the β2AR is not. Additionally, it is shown that carvedilol selectively promotes Gi recruitment to the β1AR using an in situ proximity ligation assay with subsequent activation of the G protein. In contrast, carvedilol had no effect on Gi recruitment to the β2AR. Collectively, these data show that carvedilol mediates signaling through the β1AR that is dependent on both β-arrestin and Gi, and it highlights that signaling phenotypes for biased compounds need to be thoroughly investigated at related receptors.
4.2. Isoetharine, N-cyclopentylbutanephrine, and ethylnorepinephrine
While initial studies demonstrated a good correlation between G protein and GRK2 associated activities for various βAR ligands (40), additional efforts provided evidence for selective activation of signaling pathways through the β2AR (10, 41–43). This led Drake et al. to try to better understand pluridimensional efficacy at the β2AR (11). Here the authors noted that historical classification of receptor ligands was based on their ability to activate or inhibit a receptor (i.e. single endpoint measurements), and thus likely underestimate the diversity of GPCR signaling phenotypes. Using the β2AR as a model system, a variety of previously characterized ligands were screened using FRET-based live cell biosensors in search of β-arrestin biased agonists. This screen identified isoetharine, N-cyclopentylbutanephrine, and ethylnorepinephrine as arrestin biased agonists that had higher efficacy for promoting β-arrestin binding to the β2AR than promoting cAMP accumulation (11). This study highlighted the importance of examining the entire range of effector signaling pathways in response to ligands to determine a more accurate efficacy, and identified that ethyl substituents at the catecholamine alpha carbon confer arrestin bias in β-agonists.
4.3. Nebivolol
Nebivolol is classified as a β-blocker with partial selectivity for the β1AR (44). This compound is a unique β-blocker in that it activates endothelial nitric oxide synthase and promotes vasorelaxation (45). Interestingly, nebivolol has also been reported to be a β-arrestin biased agonist at βARs (46). In mouse embryonic fibroblasts expressing the β2AR and HL-1 cardiomyocytes expressing the β1AR and β2AR, nebivolol induced rapid internalization of the βARs without significantly altering cAMP levels. This compound also promoted ERK phosphorylation which was sensitive to β-blockers, EGFR inhibitors, and siRNA knockdown of β-arrestin1/2. In a clinical study of patients with acute myocardial infarction complicated by left ventricular dysfunction, the nebivolol treatment group experienced 12-month cardiovascular events at a lower rate than those treated with metoprolol, a β1AR selective β-blocker (47). Nebivolol is unique compared to other arrestin biased ligands like carvedilol in that it also acts as a β3AR agonist (48). This distinctive β-arrestin biased pharmacological profile may be the reason for nebivolol’s clinical efficacy and demonstrates a signaling profile that may be desirable for the treatment of other cardiopathies (49).
4.4. Pepducin ICL 1–9
Carr et al. screened a series of lipidated peptides (pepducins) derived from the β2AR and found several from the first intracellular loop (ICL1) that could promote β-arrestin binding to the β2AR without promoting cAMP production (50). One of these pepducins (ICL1-9) with the sequence palmitate-TAIAKFERLQTVTNYFIT-NH2 was further characterized and found to promote GRK-mediated receptor phosphorylation, β-arrestin recruitment, receptor internalization, ERK activation, and EGFR transactivation with comparable efficacy to carvedilol (51). Interestingly, ICL1–9 was also able to induce primary murine cardiomyocyte contraction in a β2AR and β-arrestin dependent manner, where carvedilol did not. An additional series of studies showed that intramyocardial injection of ICL1–9 into mice undergoing ischemia/reperfusion-induced injury resulted in reduced infarct size, reduced cardiomyocyte death and improved cardiac function compared to scrambled pepducin treated mice (52). Thus, ICL1–9 appears to couple contractile mechanisms and pro-survival signaling pathways through the β2AR via a unique β-arrestin biased process, a signaling phenotype that could be beneficial for the next generation of heart failure therapeutics.
5. G protein biased β-agonists
The compounds described below represent a non-exhaustive cross-section of compounds that demonstrate a G protein biased signaling phenotype through βARs. The structures of the small molecules are presented in Fig. 4B, and pharmacological activity is summarized in Table 1.
5.1. Pepducin ICL 3–9
The Carr et al. study previously mentioned also identified Gs-biased pepducins derived from the third intracellular loop of the β2AR (50). The pepducin ICL3–9 (palmitate-GRFHVQNLSQVEQDGRTIGII-NH2) promoted G protein mediated signaling in a β2AR-dependent manner, without promoting GRK-mediated phosphorylation or β-arrestin-mediated internalization of the receptor. The β2AR also had reduced desensitization in primary human airway smooth muscle cells treated with ICL3–9 compared to isoproterenol treatment. This phenotype is consistent with the role of GRKs and β-arrestins in β2AR regulation and serves as a proof of concept that a Gs-biased agonist could serve as a potentially advantageous asthma therapeutic.
5.2. Salmeterol
Salmeterol is a highly selective, long acting, partial agonist for the β2AR, and has been among the most prescribed drugs for the treatment of asthma and chronic obstructive pulmonary disease (COPD) (53, 54). Different laboratories have reported that salmeterol has a 5 to 20-fold bias towards Gs over β-arrestin interaction with the β2AR (12, 55). It is also reported that salmeterol promotes a slower rate of β2AR phosphorylation by GRKs than full agonists (11, 56), and that receptor internalization and agonist promoted desensitization are diminished (55, 57, 58). Masureel et al. published the crystal structure of salmeterol-bound β2AR in an effort to understand these pharmacological properties (59). A structural comparison between salmeterol-bound and epinephrine-bound β2AR showed differences in the hydrogen bond network involving residues Ser204 and Asn293, and subsequent mutagenesis and biophysical studies suggested that these interactions led to a distinct active state conformation that is responsible for the observed Gs bias of salmeterol.
5.3. β-agonist/antagonist hybrids
Stanek et al. (60) used a medicinal chemistry approach to develop hybrid β-agonist/antagonist compounds. Starting from prototypical adrenergic receptor ligands, catecholamine-type agonists and carbazolyl-containing β-blockers, the authors designed, synthesized and characterized three different chemotypes of agonist/antagonist hybrids (60). Ligands composed of a catechol head group and an oxypropylene spacer were found to possess significant intrinsic activity at the Gs pathway with little to no activity for the recruitment of β-arrestin to the β1AR or β2AR. Similar to the salmeterol-bound β2AR crystal structure (59), this study implicates hydrogen bonding of Ser204 and Asn293 with the aromatic head groups of the ligand as determinants of the observed bias.
5.4. Dobutamine and ritodrine
Casella et al. used resonance energy transfer to compare the differential ability of β1AR and β2AR to form a complex with Gs and β-arrestin2 in response to 45 adrenergic ligands (61). The profiles of β1 and β2AR selectivity of the ligands for the two receptor-transducer interactions were different for various ligands, highlighting that a biased agonist at one receptor may not be biased at a highly homologous receptor. Interestingly, this screen indicated that β-arrestin generally interacted with the β1AR more efficiently than with the β2AR. Among the compounds tested, dobutamine and ritodrine were both relatively efficacious for promoting receptor-G protein interaction at both the β1AR and β2AR, however, they only promoted β-arrestin interaction with the β1AR. Furthermore, dobutamine acted as a competitive antagonist of epinephrine at the β2AR for β-arrestin interaction. The authors concluded that these ligands are capable of inducing a β-arrestin favorable conformation of the receptor only for the β1AR.
5.5. Xamoterol
Xamoterol is a highly selective β1AR ligand that was initially characterized as a β-blocker for the treatment of heart failure (6, 62). This drug was found to have no benefit over placebo for patient longevity (63), and was later discovered to have significant intrinsic sympathomimetic activity (64). Xamoterol was later described as a cognitive enhancer in the Ts65Dn mouse model of Down Syndrome, identifying β1AR as a potential drug target for neurological disorders (28). A 2017 study demonstrated that xamoterol is functionally biased for cAMP production over the β-arrestin pathway, thus reclassifying it as a G protein biased β-agonist (29). In this study, the authors evaluated the effects of chronic low dose xamoterol on neuroinflammation, pathology, and behavior in the 5XFAD mouse model of Alzheimer’s disease. Data demonstrate that xamoterol treated mice had reduced neuroinflammatory markers, amyloid beta and tau pathology, and lacked behavioral deficits. These data support a role for β1AR selective G protein biased agonists as potential therapies for neurocognitive disorders. This work was also expanded by structural modification of xamoterol to enhance bioavailability and brain permeability (62). This work identified a xamoterol derivative STD-101-D1 that has an improved efficacy and PK/PD profile. Given that β1AR is highly expressed in a number of peripheral organs, modifications of this compound to improve efficacy may lead to beneficial CNS activity at lower doses with fewer effects in other tissues.
6. Mechanistic insights into βAR biased signaling
Studies with several different GPCRs suggest that transmembrane (TM) VII mediates signaling bias and receptor coupling to β-arrestin (65–68). A very recent study examined the role of TM VII of the β2AR using an in vitro single molecule fluorescence system to examine the role of conformational exchange kinetics on β-arrestin bias (69). In this study, a Cy3 fluorophore was chemically conjugated to a cysteine residue on TM VII. Using the agonist formoterol and comparatively β-arrestin biased agonist isoetharine, dwell times of inactive and active like conformers of TM VII were measured. Isoetharine prolonged the dwell time of the active conformation of TM VII relative to formoterol, providing an explanation for the observed arrestin bias of isoetharine. These results suggest that ligand-dependent changes in the kinetics of receptor conformational exchange are a contributing factor to biased signaling. Additionally, these data demonstrate that ligands are intrinsically capable of differentially modulating the conformational exchange kinetics of a receptor, and that this aspect of ligand-receptor interaction should be considered in future drug-discovery projects.
A central role for GRKs in coordinating biased agonism at the β2AR has also been demonstrated (70). Transducer binding residues were predicted through evolutionary trace analysis and mutagenesis was performed. A single point mutation of tyrosine 219 (Y219) on TM V was found to convert β2AR into a G protein biased receptor. β2AR-Y219A was modestly deficient in coupling to G protein compared to wild-type β2AR, while β-arrestin recruitment was negligible as measured by Tango and DiscoverX enzyme complementation assays. Phospho-specific antibodies were used to evaluate phosphorylation of residues known to be phosphorylated by GRK2, GRK5/6, and PKA. Compared to wild type, β2AR-Y219A showed negligible agonist promoted phosphorylation of GRK5/6 sites, reduced phosphorylation of GRK2 sites, and comparable phosphorylation of PKA sites. Using an engineered β2AR with an artificially phosphorylated C-tail, it was shown that β-arrestin is still able to sterically inhibit β2AR-Y219 engagement with G protein, suggesting that arrestin-receptor core engagement is intact. Taken together these data demonstrate that a deficiency in GRK interaction with the β2AR is sufficient to drive G protein biased signaling and highlights the importance of these kinases in regulating biased signaling.
Allosteric coupling of transducer engagement to extracellular domains of the β2AR has also been implicated as a structural driver of signal bias (71). It is well accepted that agonist binding to receptor and transducer binding to receptor can promote reciprocal allosteric changes at the intra/extracellular domains (72). Recent advances in cryo-electron microscopy have supported that distinct conformations are promoted by different transducer complexes (73). Bermudez and Bock have hypothesized that disruption of these conformational shifts via more extended GPCR ligands promote divergent pocket closure for the β2AR and other GPCRs (71). Biased agonists have been shown to extend past the orthosteric ligand-binding domain into the extracellular domains of their cognate GPCRs, thus preventing transducer induced allosteric changes at these sites. This in turn would selectively stabilize conformations for which specific transducer coupling is favorable. This proposed mechanism for bias may hold true for many class A GPCRs, and demonstrates ligand extension as a plausible starting point for identifying potential new biased ligands.
A better understanding of structural differences in GPCR complexes with G proteins, GRKs and arrestins holds significant promise for the development of compounds that can bias signaling. Of particular interest to understanding βAR function, recent studies comparing the structures of the formoterol-occupied β1AR bound to β-arrestin1 vs. a G protein mimetic nanobody Nb80 reveal considerable differences in the structures (74). For example, the β1AR-β-arrestin1 complex has an inward movement of extracellular loop 3 and the cytoplasmic ends of TM5 and TM6 as well as weakened interactions between formoterol and two serines in TM5, compared to the β1AR-Nb80 complex. The observed structural differences between these complexes suggest that small molecules could be designed to bias βAR signaling.
7. Limitations
Despite the clear therapeutic potential of biased agonism at the βARs, this class of compounds has had limited clinical success (35, 75). The difficulty in translating biased pharmacological agents from the laboratory to medical practice highlights the challenges intrinsic to this therapeutic strategy. These obstacles and strategies to mitigate them are thoroughly explored in recent reviews (76, 77). To summarize briefly, there are several explanations for why molecules that demonstrate the desired phenotypes in cell-based assays do not translate to tissue, animal models, or beyond. One such explanation is that identifying biased ligands has been reliant on characterizing multiple experimental endpoints equal to the number of potential transduction pathways and that bias cannot be observed in single endpoint measurements (75). This phenomenon is known as observation bias – selectively activating one pathway relative to another can only be determined if it is measured. This adds additional complexity to screening efforts as well as additional cost. Furthermore, the physiological consequences of activating certain pathways over others may not be well described, and therefore therapeutic value will not be obvious. To fully describe a mechanism of action for a compound, it might be necessary to determine efficacy at different transducers as well as whether the observed bias is consistent across related receptor family members. Another important factor in evaluating bias involves the specific assays that are used. For example, the measurement of second messengers such as cAMP are highly amplified and involve a series of reversible protein-protein interactions while β-arrestin complementation assays (such as Tango and DiscoveRx) often involve irreversible interactions. In this regard, it is important to utilize a reference agonist (such as isoproterenol for the β1AR and β2AR) for comparison in all assays and to validate any observed bias using multiple assays.
Another experimental hurdle is system bias. It is appreciated that signaling phenotypes that are observed in some cell types may not hold in other cell types and tissues (75). This has to do with factors such as the relative stoichiometry of receptors and transducers present in the system, relative density of receptors in different cell types, and whether or not biased signaling in animal models effectively recapitulates human physiology well enough to determine clinical relevance. These experimental difficulties can be mitigated with experimental design, but not completely eliminated. Thus, these considerations are important when considering biased drug discovery efforts (76).
Michael and Charlton illustrate these points well using the example of drug discovery for the β3AR (35).In short, β3AR discovery efforts started around the treatment for type 2 diabetes and obesity based on animal models prior to the prevalence of biased agonism as a concept. While the animal models demonstrated the ability of β3AR agonists to promote adipose tissue thermogenesis, these compounds were ineffective in humans. Thus, these compounds were repurposed for treating overactive bladder disorder. It was later found that two such compounds, selected for cAMP generation, promote different levels of agonist-induced desensitization based on cell background, and signal through multiple pathways (78). In this case, it is unclear which of these pathways is therapeutically valuable for the treatment of overactive bladder syndrome, and it is possible that the clinical development of one of these compounds may have been the result of serendipity rather than careful experimental design.
8. Conclusions
Biased signaling at βARs exemplifies the complexities of GPCR pharmacology.Our increased understanding of the molecular mechanisms of βAR signaling will allow for the development of better therapeutics with fewer side effects. Leveraging these developments will be an important aspect in facilitating the next generation of βAR ligands to generate more precise physiological responses. The popularity of the βARs as model systems for GPCR signaling paradigms has led to increases in the pharmacological tools to untangle transducer specific physiology, and understanding the experimental complexities associated with discovery of biased molecules will lead to improved translational success.
Ippolito and Benovic highlights.
G protein and β-arrestin biased agonists have been characterized for the βARs
Biased β-agonists may improve clinical outcomes compared to balanced β-agonists
TM VII / ECL movements and GRK interaction are implicated in bias mechanism
Biased drug discovery efforts and experimental design require careful consideration
Acknowledgements
This work was supported in part by National Institutes of Health grants R35 GM122541, R01 HL136219 and P01 HL114471 (to JLB) and T32 GM100836 and F31 HL139104 (to MI). Figures 1 and 2 were created with BioRender.com.
Abbreviations
- βAR
β-adrenergic receptor
- β1AR
β1-adrenergic receptor
- β2AR
β2-adrenergic receptor
- β3AR
β3-adrenergic receptor
- βarr
β-arrestin
- COPD
chronic obstructive pulmonary disease
- EGFR
epidermal growth factor receptor
- GPCR
G protein-coupled receptor
- GRK
G protein-coupled receptor kinase
- ICL
intracellular loop
- MAPK
mitogen activated protein kinase
- NO
nitric oxide
- PKA
protein kinase A or cAMP dependent protein kinase
- TM
transmembrane
Footnotes
Declaration of Competing Interest
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Velmurugan BK, Baskaran R, Huang CY, Detailed insight on β-adrenoceptors as therapeutic targets. Biomed. Pharmacother. 117, 109039 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Strosberg AD, Structure, function, and regulation of adrenergic receptors. Protein Sci. 2, 1198–1209 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Emorine LJ, et al. , The human β3-adrenergic receptor: relationship with atypical receptors. Am. J. Clin. Nutr. 55, 215–218 (1992). [DOI] [PubMed] [Google Scholar]
- 4.Insel PA, Adrenergic receptors - Evolving concepts and clinical implications. N. Engl. J. Med. 334, 580–585 (1996). [DOI] [PubMed] [Google Scholar]
- 5.Patel CB, Noor N, Rockman HA, Functional selectivity in adrenergic and angiotensin signaling systems. Mol. Pharmacol. 78, 983–992 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker JG, Hill SJ, Summers RJ, Evolution of β-blockers: From anti-anginal drugs to ligand-directed signalling. Trends Pharmacol. Sci. 32, 227–234 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hodavance SY, Gareri C, Torok RD, Rockman HA, G protein-coupled receptor biased agonism. J. Cardiovasc. Pharmacol. 67, 193–202 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lefkowitz RJ, Shenoy SK, Transduction of receptor signals by β-arrestins. Science 308, 512–517 (2007). [DOI] [PubMed] [Google Scholar]
- 9.Okeke K, Angers S, Bouvier M, Michel MC, Agonist-induced desensitisation of β3-adrenoceptors: Where, when, and how? Br. J. Pharmacol. 176, 2539–2558 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Azzi M, et al. , β-arrestin-mediated activation of MAPK by inverse agonists reveals distinct active conformations for G protein-coupled receptors. Proc. Natl. Acad. Sci. U. S. A. 100, 11406–11411 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drake MT, et al. , β-arrestin-biased agonism at the β2-adrenergic receptor. J. Biol. Chem. 283, 5669–5676 (2008). [DOI] [PubMed] [Google Scholar]
- 12.van der Westhuizen ET, Breton B, Christopoulos A, Bouvier M, Quantification of ligand bias for clinically relevant β2-adrenergic receptor ligands: implications for drug taxonomy. Mol. Pharmacol. 85, 492–509 (2014). [DOI] [PubMed] [Google Scholar]
- 13.Helper JR, Gilman AG, G proteins. Trends Biochem. Sci. 17, 383–387 (1992). [DOI] [PubMed] [Google Scholar]
- 14.Nobles KN, et al. , Distinct phosphorylation sites on the β2-adrenergic receptor establish a barcode that encodes differential functions of β-arrestin. Sci. Signal. 4, ra51 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Musa Zamah A, Delahunty M, Luttrell LM, Lefkowitz RJ, Protein kinase Amediated phosphorylation of the β2-adrenergic receptor regulates its coupling to Gs and Gi: Demonstration in a reconstituted system. J. Biol. Chem. 277, 31249–31256 (2002). [DOI] [PubMed] [Google Scholar]
- 16.Daaka Y, Luttrell LM, Lefkowitz RJ, Switching of the coupling of the β2-adrenergic receptor to different G proteins by protein kinase A. Nature 390, 88–91 (1997). [DOI] [PubMed] [Google Scholar]
- 17.Martin NP, Whalen EJ, Zamah MA, Pierce KL, Lefkowitz RJ, PKA-mediated phosphorylation of the β1-adrenergic receptor promotes Gs/Gi switching. Cell. Signal. 16, 1397–1403 (2004). [DOI] [PubMed] [Google Scholar]
- 18.Ferguson SSG, et al. , Role of β-arrestin in mediating agonist-promoted G protein-coupled receptor internalization. Science 271, 363–366 (1996). [DOI] [PubMed] [Google Scholar]
- 19.Goodman OB Jr., et al. , β-arrestin acts as a clathrin adaptor in endocytosis of the β2-adrenergic receptor. Nature 383, 447–450 (1996). [DOI] [PubMed] [Google Scholar]
- 20.DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK, Beta-arrestins and cell signaling. Annu. Rev. Physiol. 69, 483–510 (2007). [DOI] [PubMed] [Google Scholar]
- 21.O'Hayre M, et al. , Genetic evidence that β-arrestins are dispensable for the initiation of β2-adrenergic receptor signaling to ERK. Sci Signal. 10, eaal3395 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grundmann M et al. , Lack of beta-arrestin signaling in the absence of active G proteins. Nat Commun. 9, 341, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luttrell LM et al. , Manifold roles of β-arrestins in GPCR signaling elucidated with siRNA and CRISPR/Cas9. Sci Signal. 11, eaat7650, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schena G, Caplan MJ, Everything you always wanted to know about β3-AR* (*But were afraid to ask). Cells 8, 357 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim IM, et al. , β-blockers alprenolol and carvedilol stimulate β-arrestin- mediated EGFR transactivation. Proc. Natl. Acad. Sci. U. S. A. 105, 14555–14560 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Q, et al. , Carvedilol induces biased β1 adrenergic receptor-nitric oxide synthase 3-cyclic guanylyl monophosphate signaling to promote cardiac contractility. Cardiovasc. Res. (2020) Sep 21:cvaa266. doi: 10.1093/cvr/cvaa266. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu X, et al. , β-Arrestin-biased signaling mediates memory reconsolidation. Proc. Natl. Acad. Sci. U. S. A. 112, 4483–4488 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faizi M, et al. , Comprehensive behavioral phenotyping of Ts65Dn mouse model of Down Syndrome: Activation of β1-adrenergic receptor by xamoterol as a potential cognitive enhancer. Neurobiol. Dis. 43, 397–413 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ardestani PM, et al. , Modulation of neuroinflammation and pathology in the 5XFAD mouse model of Alzheimer’s disease using a biased and selective β1 adrenergic receptor partial agonist. Neuropharmacology 116, 371–386 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deshpande DA, Penn RB, Targeting G protein-coupled receptor signaling in asthma. Cell. Signal. 18, 2105–2120 (2006). [DOI] [PubMed] [Google Scholar]
- 31.Deshpande DA, Theriot BS, Penn RB, Walker JKL, β-Arrestins specifically constrain β2-adrenergic receptor signaling and function in airway smooth muscle. FASEB J. 22, 2134–2141 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gagnon AW, Kallal L, Benovic JL, Role of clathrin-mediated endocytosis in agonist-induced down-regulation of the β2-adrenergic receptor. J. Biol. Chem. 273, 6976–6981 (1998). [DOI] [PubMed] [Google Scholar]
- 33.Walker JKL, Defea KA, Role for β-arrestin in mediating paradoxical β2AR and PAR2 signaling in asthma. Curr. Opin. Pharmacol. 16, 142–147 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sleigh SH, Barton CL, Repurposing strategies for therapeutics. Pharmaceut. Med. 24, 151–159 (2010). [Google Scholar]
- 35.Michel MC, Charlton SJ, Biased agonism in drug discovery - is it too soon to choose a path? Mol. Pharmacol. 4, 259–265 (2018). [DOI] [PubMed] [Google Scholar]
- 36.Michel MC, Ochodnicky P, Summers RJ, Tissue functions mediated by β3-adrenoceptors - Findings and challenges. Naunyn. Schmiedebergs. Arch. Pharmacol. 382, 103–108 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rajagopal S, Rajagopal K, Lefkowitz RJ, Teaching old receptors new tricks: Biasing seven-transmembrane receptors. Nat. Rev. Drug Discov. 9, 373–386 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wisler JW, et al. , A unique mechanism of β-blocker action: Carvedilol stimulates β-arrestin signaling. Proc. Natl. Acad. Sci. U. S. A. 104, 16657–16662 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang J, et al. , Gαi is required for carvedilol-induced β1 adrenergic receptor β-arrestin biased signaling. Nat. Commun. 8, 1706 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benovic JL, Staniszewski C, Mayor F Jr., Caron MG, Lefkowitz RJ. beta-Adrenergic receptor kinase. Activity of partial agonists for stimulation of adenylate cyclase correlates with ability to promote receptor phosphorylation. J. Biol. Chem. 263, 3893–3897 (1988). [PubMed] [Google Scholar]
- 41.Baker JG, Hall IP, Hill SJ, Agonist and inverse agonist actions of β-blockers at the human β2-adrenoceptor provide evidence for agonist-directed signaling. Mol. Pharmacol. 64, 1357–1369 (2003). [DOI] [PubMed] [Google Scholar]
- 42.Galandrin S, Bouvier M, Distinct signaling profiles of β1 and β2 adrenergic receptor ligands toward adenylyl cyclase and mitogen-activated protein kinase reveals the pluridimensionality of efficacy. Mol. Pharmacol. 70, 1575–1584 (2006). [DOI] [PubMed] [Google Scholar]
- 43.Shenoy SK, et al. , β-arrestin-dependent, G protein-independent ERK1/2 activation by the β2 adrenergic receptor. J. Biol. Chem. 281, 1261–1273 (2006). [DOI] [PubMed] [Google Scholar]
- 44.Van de Water A, Janssens W, Van Neuten J, Xhonneux R, De Cree J, Pharmacological and hemodynamic profile of nebivolol, a chemically novel, potent, and selective β1-adrenergic antagonist. J. Cardiovasc. Pharmacol. 11, 552–563 (1988). [DOI] [PubMed] [Google Scholar]
- 45.Bowman A, Chen C, Ford G, Nitric oxide mediated venodilator effects of nebivolol. Br. J. Clin. Pharmacol. 38, 199–204 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Erickson CE, et al. , The β-blocker nebivolol is a GRK/β-arrestin biased agonist. PLoS One 8, e71980 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ozaydin M, et al. , Nebivolol versus carvedilol or metoprolol in patients presenting with acute myocardial infarction complicated by left ventricular dysfunction. Med. Princ. Pract. 25, 316–322 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Münzel T, Gori T, Nebivolol: the somewhat-different beta-adrenergic receptor blocker. J. Am. Coll. Cardiol. 54, 1491–1499 (2009). [DOI] [PubMed] [Google Scholar]
- 49.Fongemie J, Felix-Getzik E, A review of nebivolol pharmacology and clinical evidence. Drugs 75, 1349–1371 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carr III R, et al. , Development and characterization of pepducins as Gs-biased allosteric agonists. J. Biol. Chem. 289, 35668–35684 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carr III R, et al. , β-arrestin-biased signaling through the β2-adrenergic receptor promotes cardiomyocyte contraction. Proc. Natl. Acad. Sci. U. S. A. 113, E4107–E4116 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grisanti LA, et al. , Pepducin-mediated cardioprotection via β-arrestin-biased β2-adrenergic receptor-specific signaling. Theranostics 8, 4664–4678 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cazzola M, Page CP, Rogliani P, Matera MG, β2-agonist therapy in lung disease. Am. J. Respir. Crit. Care Med. 187, 690–696 (2013). [DOI] [PubMed] [Google Scholar]
- 54.Cazzola M, Donner CF, Long-acting β2 agonists in the management of stable chronic obstructive pulmonary disease. Drugs 60, 307–320 (2000). [DOI] [PubMed] [Google Scholar]
- 55.Gimenez LE, Baameur F, Vayttaden SJ, Clark RB, Salmeterol efficacy and bias in the activation and kinase-mediated desensitization of β2-adrenergic receptors. Mol. Pharmacol. 87, 954–964 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tran TM, et al. , Characterization of agonist stimulation of cAMP-dependent protein kinase and G protein-coupled receptor kinase phosphorylation of the β2-adrenergic receptor using phosphoserine-specific antibodies. Mol. Pharmacol. 65, 196–206 (2004). [DOI] [PubMed] [Google Scholar]
- 57.Carter AA, Hill SJ, Characterization of isoprenaline- and salmeterol-stimulated interactions between β2-adrenoceptors and β-arrestin 2 using β-galactosidase complementation in C2C12 cells. J. Pharmacol. Exp. Ther. 315, 839–848 (2005). [DOI] [PubMed] [Google Scholar]
- 58.Moore RH, et al. , Salmeterol stimulation dissociates β2-adrenergic receptor phosphorylation and internalization. Am. J. Respir. Cell Mol. Biol. 36, 254–261 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Masureel M, et al. , Structural insights into binding specificity, efficacy and bias of a β2AR partial agonist. Nat. Chem. Biol. 14, 1059–1066 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stanek M, et al. , Hybridization of β-adrenergic agonists and antagonists confers G protein bias. J. Med. Chem. 62, 5111–5131 (2019). [DOI] [PubMed] [Google Scholar]
- 61.Casella I, Ambrosio C, Grò MC, Molinari P, Costa T, Divergent agonist selectivity in activating β1- and β2-adrenoceptors for G-protein and arrestin coupling. Biochem. J. 438, 191–202 (2011). [DOI] [PubMed] [Google Scholar]
- 62.Yi B, et al. , Discovery of novel brain permeable and G protein-biased β1 adrenergic receptor partial agonists for the treatment of neurocognitive disorders. PLoS One. 12, e0180319 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ikram H, Crozier IG, Xamoterol in severe heart failure. Lancet 336, 517–518 (1990). [DOI] [PubMed] [Google Scholar]
- 64.Andreka P, et al. , Bucindolol displays intrinsic sympathomimetic activity in human myocardium. Circulation 105, 2429–2434 (2002). [DOI] [PubMed] [Google Scholar]
- 65.Rahmeh R, et al. , Structural insights into biased G protein-coupled receptor signaling revealed by fluorescence spectroscopy. Proc. Natl. Acad. Sci. U. S. A. 109, 6733–6738 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kang Y, et al. , Crystal structure of rhodopsin bound to arrestin by femtosecond X-ray laser. Nature 523, 561–567 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McCorvy JD, et al. , Structural determinants of 5-HT2B receptor activation and biased agonism. Nat. Struct. Mol. Biol. 25, 787–796 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Suomivuori CM, et al. , Molecular mechanism of biased signaling in a prototypical G protein-coupled receptor. Science 367, 881–887 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lamichhane R, et al. , Biased signaling of the G protein-coupled receptor β2AR is governed by conformational exchange kinetics. Structure 28, 371–377 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Choi M, et al. , G protein–coupled receptor kinases (GRKs) orchestrate biased agonism at the β2-adrenergic receptor. Sci. Signal. 11, eaar7084 (2018). [DOI] [PubMed] [Google Scholar]
- 71.Bermudez M, Bock A, Does divergent binding pocket closure drive ligand bias for Class A GPCRs? Trends Pharmacol. Sci. 40, 236–239 (2019). [DOI] [PubMed] [Google Scholar]
- 72.Strachan RT, et al. , Divergent transducer-specific molecular efficacies generate biased agonism at a G protein-coupled receptor (GPCR). J. Biol. Chem. 289, 14211–14224 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Capper MJ, Wacker D, Structural Biology: A complex story of receptor signalling. Nature 558, 529–530 (2018). [DOI] [PubMed] [Google Scholar]
- 74.Lee Y et al. , Molecular basis of beta-arrestin coupling to formoterol-bound beta1-adrenoceptor. Nature 583, 862–866, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kenakin T, Is the quest for signaling bias worth the effort?. Mol. Pharmacol. 93, 266–269 (2018). [DOI] [PubMed] [Google Scholar]
- 76.Bermudez M, Nguyen TN, Omieczynski C, Wolber G, Strategies for the discovery of biased GPCR ligands. Drug Discov. Today 24, 1031–1037 (2019). [DOI] [PubMed] [Google Scholar]
- 77.Kenakin T, Biased receptor signaling in drug discovery. Pharmacol. Rev. 71, 267–315 (2019). [DOI] [PubMed] [Google Scholar]
- 78.Hutchinson DS, Sato M, Evans BA, Christopoulos A, Summers RJ, Evidence for pleiotropic signaling at the mouse β3-adrenoceptor revealed by SR59230A [3-(2-ethylphenoxy)-1-[(1,S)-1,2,3,4- tetrahydronapth-1-ylamino]-2s-2-propanol oxalate]. J. Pharmacol. Exp. Ther. 312, 1064–1074 (2005). [DOI] [PubMed] [Google Scholar]
- 79.Eichel K, Jullié D, von Zastrow M, β-arrestin drives MAP kinase signaling from clathrin-coated structures after GPCR dissociation. Nat. Cell Biol. 18, 303–310 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thomas RF, Holt BD, Schwinn DA, Liggett S, Long‐term agonist exposure induces upregulation of β3-adrenergic receptor expression via multiple cAMP response elements. Proc. Natl. Acad. Sci. U. S. A. 89, 4490–4494 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Madeira F, et al. , The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 47, W636–W641 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]