Abstract
Visit-to-visit blood pressure variability (BPV) is associated with cardiovascular events in the general population. Data are scarce in chronic kidney disease (CKD). We hypothesized that BPV would be associated with cardiovascular outcomes, death, and end-stage kidney disease (ESKD) and that diuretics would modify these associations in patients with CKD.
We studied U.S. Veterans with non-dialysis CKD stages 1-5 and hypertension on non-diuretic antihypertensive monotherapy. At the time of second antihypertensive agent prescription, we propensity-matched for exposure to a loop or thiazide diuretic vs. any other antihypertensive. BPV was defined as the coefficient of variation of systolic blood pressure over 6 months after second agent prescription. Cox proportional hazards regression measured associations of BPV with a primary cardiovascular event composite (fatal or non-fatal myocardial infarction or ischemic stroke; heart failure hospitalization). Secondary outcomes included all-cause death, each primary outcome component, ESKD, and cardiovascular death.
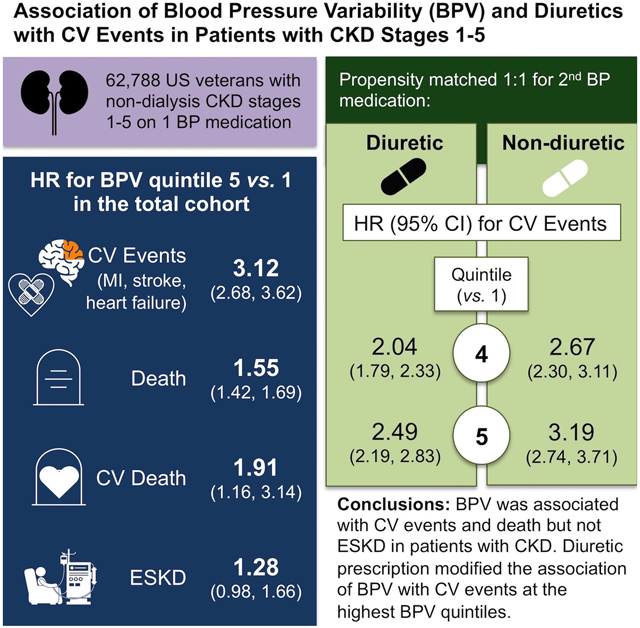
There were 31,394 participants in each group. BPV was associated with composite cardiovascular events, hazard ratio (95% confidence interval) at second, third, fourth, and fifth vs. first quintile: 1.79 (1.53-2.11), 2.32 (1.99-2.71), 2.60 (2.24-3.02), and 3.12 (2.68-3.62). Diuretics attenuated associations between the fourth and fifth BPV quintiles with composite events (Pinteraction=0.03 and 0.04, respectively). BPV was associated with all secondary outcomes except ESKD, with no diuretic interactions.
BPV was associated with cardiovascular events and death but not ESKD in patients with CKD, with attenuated associations with CV events in the diuretic-treated group at high BPV quintiles. Future studies should investigate whether other antihypertensive classes modify these risks.
Keywords: chronic kidney disease, blood pressure variability, diuretics, cardiovascular events, death
Graphical Abstract
INTRODUCTION
Individuals with chronic kidney disease (CKD) are at disproportionately high cardiovascular (CV) risk, and traditional CV risk factors such as diabetes and hypertension, although more common in individuals with CKD, do not predict risk as well in these patients as in the general population.1, 2 This highlights the need to identify novel factors to improve CV risk stratification in patients with CKD. Outpatient visit-to-visit blood pressure variability (BPV) has been shown to be independently associated with poor CV outcomes in the general population.3, 4 However, data in patients with CKD are scarce and restricted to advanced stage CKD, when interventions may not be as effective late in the course of disease.5-7
Furthermore, no studies investigated whether antihypertensive medication class may affect BPV and its association with outcomes. Secondary analyses from clinical trials suggest that treatment with diuretics, as compared to other antihypertensives, may be associated with decreased BPV in the general population,8-10 and one observational study in patients with advanced CKD noted lower BPV in those treated with diuretics compared to other drug classes.7 The potential ameliorating effect of diuretics on BPV could be particularly pronounced in patients with CKD given that volume overload is common and contributes to hypertension in this population.
Given that extracellular volume plays a significant role in hypertension in patients with CKD and may affect BPV, we hypothesized that loop and thiazide diuretics would mitigate BPV and its association with long-term CV outcomes and end-stage kidney disease (ESKD) compared to non-diuretic antihypertensive agents among patients with prevalent non-dialysis CKD stages 1-5. Our specific aims were to 1) determine if thiazide or loop diuretic prescription was associated with decreased BPV compared to non-diuretic antihypertensive medications; 2) determine whether BPV was associated with CV outcomes, death, and ESKD in patients with prevalent CKD stages 1-5; and 3) determine whether diuretic prescription modified the association of BPV with CV events, death, and ESKD.
MATERIALS AND METHODS
Data Sources
Applications to access the dataset from qualified researchers trained in human subject confidentiality protocols may be submitted through the Veterans Affairs (VA) Data Access Request Tracker. Real-world national data were obtained from January 1, 2010 to December 31, 2016 from inpatient and outpatient demographic, comorbidity, laboratory, and pharmacy datasets from the VA Corporate Data Warehouse and accessed via the VA Informatics and Computing Infrastructure. Dates of diagnosis of incident ESKD during follow up were obtained from United States Renal Data System (USRDS) data.
Study Design and Participants
We conducted an observational cohort study using real-world clinical data from a national sample of United States veterans with prevalent non-dialysis CKD stages 1-5.11 The study was approved by the Institutional Review Board at the VA North Texas Health Care System (protocol number 17-107) and a waiver of informed consent was granted.
We identified adult individuals ≥18 years of age from January 1, 2010 through December 31, 2016 with prevalent CKD using laboratory values from routine care. CKD was defined as 2 outpatient instances ≥3 months apart of either an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 or the presence of proteinuria or albuminuria, defined as a spot urine albumin-to-creatinine ratio ≥30 mg/g, a spot urine protein-to-creatinine ratio >0.15 g/g, 24-hour urine albumin ≥30 mg/day, 24-hour urine protein >150 mg/day, or a dipstick urinalysis positive for protein ≥30 mg/dL.12, 13 CKD stages 1-5 were defined by Kidney Disease Outcomes Quality Initiative criteria.12 Individuals with prevalent ESKD at the index date were identified by date of incident ESKD diagnosis data from the USRDS and were excluded. Inclusion in the study required prescription of a non-diuretic medication as initial monotherapy for hypertension, with the prescription of a second antihypertensive agent during the observation period (Figure 1A). Those who were prescribed diuretic antihypertensive monotherapy, 2 initial medications simultaneously for the treatment of hypertension, or were already prescribed ≥2 antihypertensive agents when inclusion criteria were met were excluded. Individuals with any exposure to loop or thiazide diuretics within 3 months prior to the index date were also excluded to ensure washout of any previously prescribed diuretic medications and inclusion of truly new users of diuretics.14 The index date was defined as the time of prescription of the second antihypertensive medication (Figure 1A).
Figure 1.
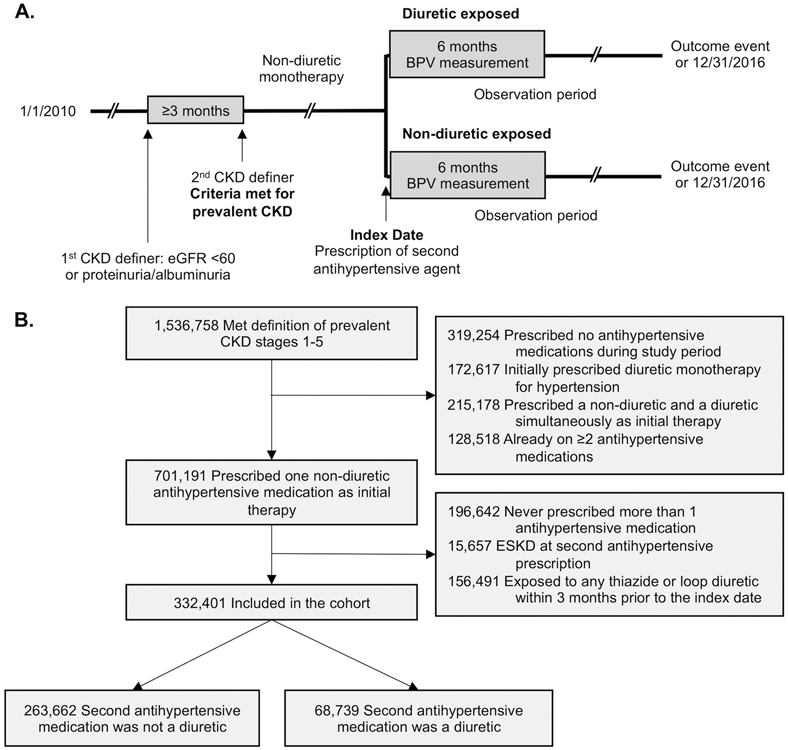
Study design (A) and diagram of inclusion in the cohort (B)
Exposure Variable
The exposure variable was defined by the prescription of a second antihypertensive medication after initial monotherapy with a non-diuretic antihypertensive agent. Participants exposed to a loop or thiazide diuretic as the second agent were compared to those whose second medication was a non-diuretic, including angiotensin converting enzyme inhibitors (ACEi), angiotensin receptor blockers (ARB), mineralocorticoid receptor antagonists, calcium channel blockers, beta blockers, alpha blockers, hydralazine, clonidine, aliskiren, minoxidil, or amiloride (a complete list of anti-hypertensive agents in each category can be found at http://hyper.ahajournals.org).
Clinical Variables
Comorbid medical conditions were defined using International Classifications of Disease, Clinical Modification, revisions 9 and 10 (ICD-9, ICD-10) codes (please see http://hyper.ahajournals.org). Baseline characteristics, including demographics, comorbidities, and laboratory values, were captured within 1 year prior to the index date. Quantitative measures of albuminuria and proteinuria were divided into deciles to account for the degree of albuminuria or proteinuria at baseline, with an additional category added to represent missing values (please see http://hyper.ahajournals.org). Body mass index (BMI) was calculated from baseline weight and height prior to the index date. Baseline systolic and diastolic blood pressure values were defined as the most recent outpatient blood pressure measurement recorded on or prior to the index date.
Blood Pressure Variability
For the primary analysis, BPV was defined as the coefficient of variation of outpatient systolic blood pressure values for 6 months after the index date. Blood pressures obtained in emergency department or urgent care settings were excluded. When multiple blood pressures were measured on a single date, only the first measurement from that date was used in the BPV calculation. The coefficient of variation was calculated by dividing the standard deviation by the mean of the systolic blood pressure values. In sensitivity analyses, BPV was additionally calculated by other common definitions, including the average real variability and the standard deviation of the systolic blood pressure, to account for different blood pressure patterns that can be uncovered by these alternative measures.15
Outcome Variables
Participants were followed for up to 5 years for outcome events. The primary composite outcome was CV events, defined as fatal or non-fatal myocardial infarction, fatal or non-fatal ischemic stroke, or hospitalization for heart failure. Secondary outcomes included all-cause death (identified by the National Death Index, VA death data, and the Centers for Medicare and Medicaid Services), each component of the primary composite outcome, ESKD, and CV death, defined as death within 31 days of a myocardial infarction, ischemic stroke, or hospitalization for heart failure. Definitions of outcome events by ICD codes can be found at http://hyper.ahajournals.org.
Statistical Analysis
Logistic regression models were used to create the propensity score for diuretic exposure, which modeled the probability of exposure use given 38 study covariates at baseline, including demographic characteristics, comorbidities, medications, laboratory values, and clinical data (complete list of variables included in the propensity model can be found at http://hyper.ahajournals.org). Participants who received diuretics as their second antihypertensive medication were propensity-matched 1:1 without replacement with individuals who received non-diuretic antihypertensive medications. Nearest-neighbor matching was performed with a caliper of 0.0001. Baseline characteristics between matched groups were compared using standardized differences.16 BPV before and after the index date and the change in BPV were compared between groups using standardized differences. In the absence of data to support clinically useful cutoffs, BPV was divided into quintiles in the entire cohort for analysis, consistent with prior similar studies.7, 10, 17 Associations of BPV with outcomes were calculated using Cox proportional hazards regression, including diuretic treatment x BPV interaction terms. Outcomes models were adjusted for the number of blood pressure measurements included in BPV calculations and the number of visits in 1 year prior to the index date to capture factors important to the calculation of BPV and the overall medical complexity of the participants.18 Other covariates included age, sex, race, eGFR, systolic blood pressure, BMI, proteinuria, albuminuria, smoking, diabetes mellitus, congestive heart failure, vascular disease, malignancy, and any exposure in 1 year prior to the index date to ACEi, ARB, spironolactone, beta blockers, calcium channel blockers, clonidine, hydralazine, or statins. Participants were censored at the date of last follow up or death. Pre-specified sub-group analyses were performed by CKD stage, race, sex, diabetes mellitus, congestive heart failure, and prior exposure to ACEi or ARB. Statistical analysis was conducted using STATA 15 (StataCorp, College Station, TX).
Sensitivity Analyses
Several sensitivity analyses were conducted to test the robustness of the findings. First, we examined BPV as a continuous variable rather than by quintile. Then we selected for individuals who had been exposed to no more than 1 or 2 antihypertensive agents in 1 year prior to the index date, which identified a sample of participants whose antihypertensive agents had remained unmodified or minimally modified in 1 year prior to prescription of the second agent. Next, we selected only those who had 4-15 blood pressure measurements included in the calculation of BPV to exclude those who had too few measurements to calculate a true variability and to exclude the very ill who presented to the outpatient clinic setting as often as several times per month. Finally, we conducted survival analysis censoring individuals at the time of prescription of a third antihypertensive agent and treating death as a competing risk.
RESULTS
Baseline Characteristics
There were 1,536,758 participants who met criteria for prevalent non-dialysis CKD stages 1-5. A total of 332,401 met criteria for inclusion in the cohort. Of those, we identified 68,739 individuals on non-diuretic monotherapy for hypertension whose second antihypertensive medication was a diuretic and 263,662 whose second agent was a non-diuretic (Figure 1B). The 1:1 propensity matched cohort included 62,788 participants, with 31,394 participants in each group. Baseline characteristics were similar in the matched cohort (Table 1). Baseline characteristics of the unmatched cohort are available at http://hyper.ahajournals.org.
Table 1.
Baseline characteristics of the 1:1 propensity matched cohort
| Variable | Non-Diuretic Exposed N=31,394 |
Diuretic Exposed N=31,394 |
Standardized Difference |
|---|---|---|---|
| Age, years, mean (SD) | 72.2 (10.9) | 72.2 (11.1) | 0.008 |
| Female Sex, N (%) | 1,197 (3.8) | 1,185 (3.8) | 0.002 |
| Race, N (%) | 0.01 | ||
| White | 23,798 (75.8) | 24,027 (76.5) | |
| Black | 4,527 (14.4) | 4,361 (13.9) | |
| Native Hawaiian or Pacific Islander | 331 (1.1) | 310 (1.0) | |
| American Indian or Alaska Native | 173 (0.6) | 203 (0.7) | |
| Asian | 158 (0.5) | 162 (0.5) | |
| Unknown or Multi-race | 2,407 (7.7) | 2,331 (7.4) | |
| Comorbidities*, N (%) | |||
| COPD | 6,746 (21.5) | 6,750 (21.5) | 0.0003 |
| Diabetes mellitus | 14,896 (47.5) | 14,851 (47.3) | 0.003 |
| HIV | 201 (0.6) | 205 (0.7) | 0.002 |
| Peripheral vascular disease | 3,666 (11.7) | 3,750 (11.9) | 0.008 |
| Liver disease | 780 (2.5) | 782 (2.5) | 0.0004 |
| Malignancy | 5,447 (17.4) | 5,436 (17.3) | 0.0009 |
| Vascular disease | 6,589 (21.0) | 6,607 (21.1) | 0.001 |
| Hemiplegia or paraplegia | 111 (0.4) | 107 (0.3) | 0.002 |
| Myocardial infarction | 1,063 (3.4) | 1,086 (3.5) | 0.004 |
| Congestive heart failure | 3,833 (12.2) | 3,900 (12.4) | 0.006 |
| Dementia | 490 (1.6) | 488 (1.6) | 0.0005 |
| Rheumatic disease | 596 (1.9) | 602 (1.9) | 0.001 |
| Peptic ulcer disease | 433 (1.4) | 430 (1.4) | 0.0008 |
| Smoking, N (%) | 611 (2.0) | 585 (1.9) | 0.006 |
| Medications†, N (%) | |||
| Nitrates | 3,267 (10.4) | 3,213 (10.2) | 0.006 |
| Statins | 13,401 (42.7) | 13,841 (44.1) | 0.03 |
| Aspirin | 5,663 (18.0) | 5,672 (18.1) | 0.0008 |
| ACE inhibitors | 13,329 (42.5) | 14,093 (44.9) | 0.05 |
| ARBs | 4,469 (14.2) | 4,372 (13.9) | 0.009 |
| Beta blockers | 15,922 (50.7) | 16,237 (51.7) | 0.02 |
| Calcium channel blockers | 10,499 (33.4) | 10,169 (32.4) | 0.02 |
| Spironolactone | 1,612 (5.1) | 1,383 (4.4) | 0.03 |
| Hydralazine | 1,129 (3.6) | 810 (2.6) | 0.06 |
| Clonidine | 844 (2.7) | 610 (1.9) | 0.05 |
| Minoxidil | 74 (0.2) | 59 (0.2) | 0.01 |
| Amiloride | 30 (0.1) | 16 (0.1) | 0.02 |
| Laboratory and clinical variables | |||
| Estimated glomerular filtration rate, mL/min/1.73 m2, median (IQR) | 51.9 (42.7, 59.3) | 51.2 (41.5, 58.5) | 0.07 |
| Albuminuria, mg/g, median (IQR) | 29.0 (8.8, 103.4) | 31.5 (9.7, 122.4) | 0.03 |
| Proteinuria, g/g, median (IQR) | 0.2 (0.1, 0.7) | 0.3 (0.1, 0.8) | 0.005 |
| Serum potassium, mmol/L, mean (SD) | 4.4 (0.5) | 4.4 (0.5) | 0.03 |
| Serum albumin, g/dL, mean (SD) | 3.9 (0.4) | 3.9 (0.5) | 0.006 |
| Systolic blood pressure, mmHg, mean (SD) | 138.1 (22.1) | 137.5 (22.3) | 0.03 |
| Diastolic blood pressure, mmHg, mean (SD) | 75.9 (13.2) | 75.5 (13.8) | 0.02 |
| Body mass index, kg/m2, mean (SD) | 29.9 (6.2) | 29.8 (6.1) | 0.02 |
| Number of visits in 1 year prior to the index date, mean (SD) | 12.8 (11.2) | 12.7 (11.6) | 0.003 |
| Number of specialists in 1 year prior to the index date, mean (SD) | 2.2 (2.1) | 2.2 (2.2) | 0.008 |
Abbreviations: ACE inhibitors, angiotensin converting enzyme inhibitors; ARBs, angiotensin receptor blockers; COPD, chronic obstructive pulmonary disease; HIV, human immunodeficiency virus
Defined by International Classification of Diseases codes
Defined as exposure in pharmacy records within 1 year prior to the index date
Blood Pressure Variability
After the index date, median (IQR) systolic BPV was 10.4 (7.4, 13.7) in the non-diuretic group and 10.5 (7.4, 13.8) in the diuretic group (please see http://hyper.ahajournals.org). Higher BPV was seen with more advanced CKD stages, in those prescribed hydralazine or clonidine, and in those with more frequent medical visits or specialists in the 12 months prior to the index date (please see http://hyper.ahajournals.org).
Outcomes
Increasing quintiles of BPV were associated with composite CV events, all-cause death, CV death, myocardial infarction, heart failure hospitalization, and stroke, but not ESKD (Figure 2). Similar associations with the primary outcome were seen when BPV was measured as the average real variability or standard deviation of the systolic blood pressure, and when evaluating quintiles of BPV prior to the index date (please see http://hyper.ahajournals.org). Those with the greatest decrease in BPV had a lower risk of composite CV events than those whose BPV increased from the 6 months before to the 6 months after the index date (please see http://hyper.ahajournals.org). There was a significant interaction of treatment group on the association of the fourth and fifth quintiles of BPV with the primary outcome, interaction P=0.03 and 0.04, respectively, indicating that diuretic treatment attenuated the association of BPV with composite CV events (Table 2).
Figure 2. Outcome events by quintile of BPV in the entire matched cohort.
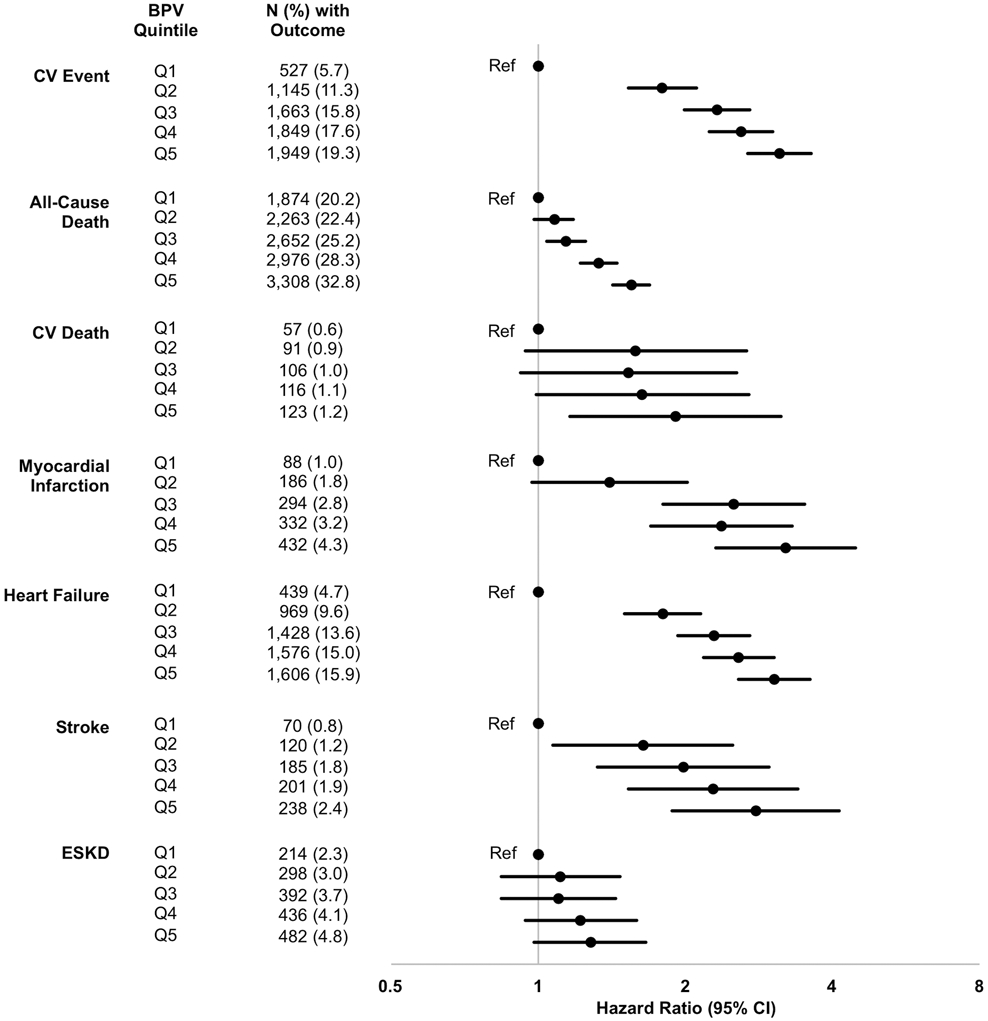
Models were adjusted for the total number of blood pressure measurements in the BPV calculation, the total number of clinic stops in 1 year prior to the index date, age, sex, race, eGFR, systolic blood pressure, BMI, albuminuria, proteinuria, smoking, diabetes mellitus, congestive heart failure, vascular disease, and malignancy, treatment in 1 year prior to the index date with an ACEi or ARB, spironolactone, a beta blocker, a calcium channel blocker, clonidine or hydralazine, or a statin
Table 2.
Association of quintile of systolic BPV with time to outcome event by diuretic exposure
| Model | Non-Diuretic Exposed HR (95% CI) N=31,394 |
Diuretic Exposed HR (95% CI) N=31,394 |
Treatment group x BPV interaction P value |
|---|---|---|---|
| CV Event | |||
| Q1 | Ref | Ref | |
| Q2 | 1.83 (1.56, 2.15) | 1.59 (1.39, 1.83) | 0.31 |
| Q3 | 2.37 (2.03, 2.76) | 1.92 (1.69, 2.19) | 0.10 |
| Q4 | 2.67 (2.30, 3.11) | 2.04 (1.79, 2.33) | 0.03 |
| Q5 | 3.19 (2.74, 3.71) | 2.49 (2.19, 2.83) | 0.04 |
| All-Cause Death | |||
| Q1 | Ref | Ref | |
| Q2 | 1.07 (0.98, 1.18) | 1.07 (0.99, 1.16) | 0.86 |
| Q3 | 1.13 (1.03, 1.24) | 1.09 (1.00, 1.18) | 0.35 |
| Q4 | 1.31 (1.20, 1.43) | 1.18 (1.09, 1.28) | 0.03 |
| Q5 | 1.53 (1.40, 1.67) | 1.49 (1.37, 1.61) | 0.37 |
| CV Death | |||
| Q1 | Ref | Ref | |
| Q2 | 1.54 (0.91, 2.61) | 1.28 (0.83, 1.98) | 0.49 |
| Q3 | 1.49 (0.89, 2.51) | 1.26 (0.82, 1.94) | 0.49 |
| Q4 | 1.57 (0.94, 2.61) | 1.36 (0.89, 2.09) | 0.51 |
| Q5 | 1.86 (1.12, 3.08) | 1.55 (1.02, 2.36) | 0.46 |
| Myocardial Infarction | |||
| Q1 | Ref | Ref | |
| Q2 | 1.37 (0.95, 1.99) | 1.86 (1.30, 2.65) | 0.30 |
| Q3 | 2.48 (1.77, 3.46) | 1.83 (1.29, 2.59) | 0.18 |
| Q4 | 2.34 (1.67, 3.27) | 2.31 (1.64, 3.24) | 0.87 |
| Q5 | 3.19 (2.29, 4.43) | 3.52 (2.53, 4.90) | 0.71 |
| Heart Failure | |||
| Q1 | Ref | Ref | |
| Q2 | 1.83 (1.53, 2.18) | 1.61 (1.39, 1.87) | 0.40 |
| Q3 | 2.32 (1.96, 2.76) | 1.95 (1.70, 2.25) | 0.22 |
| Q4 | 2.64 (2.23, 3.13) | 2.05 (1.78, 2.36) | 0.06 |
| Q5 | 3.11 (2.63, 3.69) | 2.40 (2.08, 2.76) | 0.05 |
| Stroke | |||
| Q1 | Ref | Ref | |
| Q2 | 1.65 (1.08, 2.53) | 1.11 (0.73, 1.70) | 0.21 |
| Q3 | 1.98 (1.31, 2.97) | 1.77 (1.21, 2.60) | 0.73 |
| Q4 | 2.28 (1.53, 3.40) | 1.68 (1.14, 2.48) | 0.28 |
| Q5 | 2.74 (1.84, 4.08) | 2.26 (1.55, 3.29) | 0.46 |
| ESKD | |||
| Q1 | Ref | Ref | |
| Q2 | 1.18 (0.88, 1.57) | 1.04 (0.82, 1.32) | 0.83 |
| Q3 | 1.15 (0.87, 1.51) | 1.06 (0.84, 1.33) | 0.99 |
| Q4 | 1.34 (1.02, 1.77) | 1.18 (0.94, 1.48) | 0.98 |
| Q5 | 1.33 (1.01, 1.75) | 1.30 (1.04, 1.62) | 0.68 |
Abbreviations: BPV, blood pressure variability; CV, cardiovascular; ESKD, end-stage kidney disease; Q1, first quintile; Q2, second quintile; Q3, third quintile; Q4, fourth quintile; Q5, fifth quintile
Adjusted for the total number of blood pressure measurements in the BPV calculation, the total number of clinic stops in 1 year prior to the index date, age, sex, race, eGFR, systolic blood pressure, BMI, albuminuria, proteinuria, smoking, diabetes mellitus, congestive heart failure, vascular disease, and malignancy, treatment in 1 year prior to the index date with an ACEi or ARB, spironolactone, a beta blocker, a calcium channel blocker, clonidine or hydralazine, or a statin
Over 163,591 person-years of follow up, there were 3,391 (10.8%) composite CV events in the non-diuretic group and 3,935 (12.5%) in the diuretic group (P<0.0001), with the corresponding event rates being 42.29/1,000 person-years and 47.18/1,000 person years. More participants in the diuretic group than non-diuretic group reached all-cause death (9,123 [29.1%] vs. 7,444 [23.7%], P<0.0001), CV death (295 [0.9%] vs. 237 [0.8%], P=0.01), heart failure hospitalization (3,408 [10.9%] vs. 2,765 [8.8%], P<0.0001), and ESKD (1,163 [3.7%] vs. 866 [2.8%], P<0.0001). There was no difference in the number experiencing myocardial infarction (655 [2.1%] vs. 710 [2.3%], P=0.13) or stroke (398 [1.3%] vs. 438 [1.4%], P=0.16) between groups. Treatment group did not modify the relationship between quintiles of BPV with any of the secondary outcomes (Table 2). Sensitivity analyses showed similar relationships between quintile of BPV with CV events as the primary analysis (Table 3).
Table 3.
Sensitivity analyses of association of systolic BPV with CV events
| Quintile | Outcome Events N (%) |
Main Effects HR (95% CI) |
Non-Diuretic Exposed HR (95% CI) |
Diuretic Exposed HR (95% CI) |
Treatment group x BPV interaction P value |
|---|---|---|---|---|---|
| Takes BPV as a continuous variable* | |||||
| 7,326 (11.7) | 1.05 (1.05, 1.06) | 1.05 (1.04, 1.06) | 1.05 (1.04, 1.06) | 0.32 | |
| Excludes individuals exposed to more than 1 anti-hypertensive agent in 1 year prior to the index date | |||||
| Q1 | 213 (4.1) | Ref | Ref | Ref | |
| Q2 | 525 (9.0) | 2.14 (1.63, 2.79) | 2.16 (1.65, 2.83) | 1.72 (1.41, 2.10) | 0.21 |
| Q3 | 710 (12.3) | 2.89 (2.23, 3.74) | 2.88 (2.22, 3.73) | 2.23 (1.83, 2.70) | 0.12 |
| Q4 | 776 (13.7) | 3.26 (2.53, 4.21) | 3.26 (2.53, 4.21) | 2.29 (1.88, 2.78) | 0.03 |
| Q5 | 834 (15.7) | 4.34 (3.38, 5.58) | 4.33 (3.37, 5.58) | 2.88 (2.37, 3.49) | 0.009 |
| Excludes individuals exposed to more than 2 anti-hypertensive agents in 1 year prior to the index date | |||||
| Q1 | 468 (5.4) | Ref | Ref | Ref | |
| Q2 | 1,035 (11.0) | 1.89 (1.59, 2.24) | 1.92 (1.62, 2.28) | 1.61 (1.40, 1.86) | 0.20 |
| Q3 | 1,473 (15.1) | 2.40 (2.04, 2.83) | 2.45 (2.08, 2.88) | 1.92 (1.68, 2.21) | 0.08 |
| Q4 | 1,606 (16.6) | 2.64 (2.25, 3.10) | 2.73 (2.32, 3.21) | 1.98 (1.73, 2.28) | 0.02 |
| Q5 | 1,715 (18.6) | 3.25 (2.77, 3.81) | 3.34 (2.84, 3.92) | 2.50 (2.18, 2.87) | 0.03 |
| Excludes individuals who had <4 or >15 blood pressure measurements in 6 months after the index date | |||||
| Q1 | 325 (8.7) | Ref | Ref | Ref | |
| Q2 | 829 (12.1) | 1.35 (1.11, 1.65) | 1.36 (1.11, 1.66) | 1.00 (0.84, 1.19) | 0.02 |
| Q3 | 1,109 (15.6) | 1.52 (1.25, 1.85) | 1.53 (1.26, 1.86) | 1.25 (1.06, 1.47) | 0.12 |
| Q4 | 1,167 (16.5) | 1.75 (1.45, 2.12) | 1.76 (1.45, 2.14) | 1.20 (1.02, 1.42) | 0.004 |
| Q5 | 1,270 (20.2) | 2.07 (1.71, 2.52) | 2.08 (1.71, 2.53) | 1.72 (1.46, 2.02) | 0.14 |
| Censors participants at the time of prescription of the third antihypertensive agent | |||||
| Q1 | 248 (2.7) | Ref | Ref | Ref | |
| Q2 | 533 (5.3) | 1.96 (1.50, 2.56) | 2.07 (1.59, 2.71) | 1.65 (1.38, 1.99) | 0.38 |
| Q3 | 787 (7.5) | 2.73 (2.12, 3.52) | 2.85 (2.21, 3.68) | 1.97 (1.65, 2.35) | 0.07 |
| Q4 | 878 (8.3) | 3.22 (2.51, 4.14) | 3.42 (2.66, 4.39) | 2.22 (1.86, 2.65) | 0.04 |
| Q5 | 944 (9.4) | 4.19 (3.27, 5.37) | 4.35 (3.39, 5.57) | 2.60 (2.18, 3.10) | 0.004 |
| Treats non-CV death as a competing risk | |||||
| Q1 | 527 (5.7) | Ref | Ref | Ref | |
| Q2 | 1,145 (11.3) | 1.79 (1.52, 2.11) | 1.83 (1.56, 2.15) | 1.61 (1.40, 1.84) | 0.36 |
| Q3 | 1,663 (15.8) | 2.31 (1.99, 2.69) | 2.36 (2.02, 2.76) | 1.94 (1.70, 2.21) | 0.13 |
| Q4 | 1,849 (17.6) | 2.52 (2.16, 2.94) | 2.59 (2.22, 3.02) | 2.05 (1.79, 2.34) | 0.07 |
| Q5 | 1,949 (19.3) | 2.95 (2.54, 3.43) | 3.01 (2.58, 3.51) | 2.40 (2.11, 2.74) | 0.07 |
Abbreviations: CV, cardiovascular; Q1, first quintile; Q2, second quintile; Q3, third quintile; Q4, fourth quintile; Q5, fifth quintile
Adjusted for the total number of blood pressure measurements in the BPV calculation, the total number of clinic stops in 1 year prior to the index date, age, sex, race, eGFR, systolic blood pressure, BMI, albuminuria, proteinuria, smoking, diabetes mellitus, congestive heart failure, vascular disease, and malignancy, treatment in 1 year prior to the index date with an ACEi or ARB, spironolactone, a beta blocker, a calcium channel blocker, clonidine or hydralazine, or a statin
Hazard ratios are per 1% increase in coefficient of variation of systolic blood pressure
Subgroup Analysis
The relationship between quintiles of BPV and CV events did not differ based on subgroups by CKD stage, race, sex, or exposure to renin-angiotensin-aldosterone system (RAAS) blockade with an ACEi or ARB (Figure 3). The second, third, and fourth quintiles of BPV were more strongly associated with CV events in individuals without diabetes mellitus (interaction P=0.03, 0.04, and 0.002, respectively), and the fifth quintile was more strongly associated in those without heart failure (interaction P=0.02). Significant interactions of diuretic treatment were seen at the fourth and fifth quintiles of BPV for men and patients with diabetes and the fifth quintile in those with heart failure or not on RAAS blockade (Figure 3). BPV taken continuously was associated with CV events in all subgroups, with no treatment group x BPV interactions (please see http://hyper.ahajournals.org).
Figure 3. Association of quintile of BPV with composite CV events by subgroups.
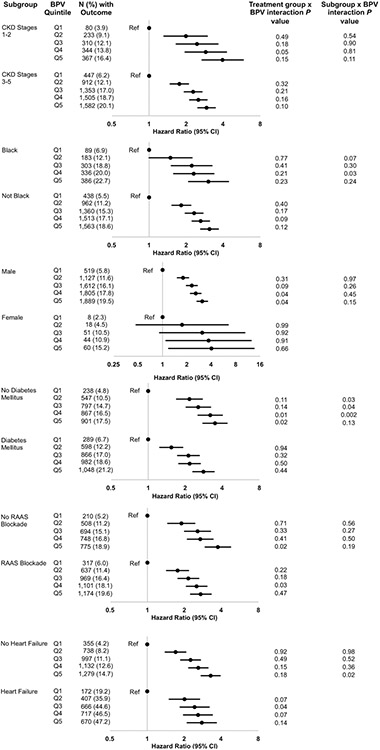
Models were adjusted for the total number of blood pressure measurements in the BPV calculation, the total number of clinic stops in 1 year prior to the index date, age, sex, race, eGFR, systolic blood pressure, BMI, albuminuria, proteinuria, smoking, diabetes mellitus, congestive heart failure, vascular disease, and malignancy, treatment in 1 year prior to the index date with an ACEi or ARB, spironolactone, a beta blocker, a calcium channel blocker, clonidine or hydralazine, or a statin
DISCUSSION
We demonstrated that among veterans with prevalent non-dialysis CKD stages 1-5, BPV was strongly and independently associated with CV events, all-cause death, CV death, myocardial infarction, heart failure hospitalization, and ischemic stroke, but not progression of CKD to ESKD. We further showed that the association of BPV with CV events was diminished in those with diabetes compared to those without diabetes, but there was no difference in this association based on CKD stage. Finally, thiazide or loop diuretic-based antihypertensive regimens were not associated with decreased BPV compared to non-diuretic regimens but did modify the association of BPV with CV events at the highest BPV quintiles.
Prior studies in the general population mostly showed associations between visit-to-visit BPV and CV outcomes.19 In the general population and in individuals without CKD, BPV was associated with all-cause death, CV death, incident coronary heart disease, stroke, major adverse CV events, and incident atrial fibrillation.3, 4, 19-23 In a secondary analysis of clinical trials of patients with diabetes, higher BPV was associated with death and a composite of death, CV events, and kidney events.24 However, among 7,879 participants in the Systolic Blood Pressure Intervention Trial (SPRINT), BPV, defined as the coefficient of variation of systolic blood pressure, was associated with all-cause death but not with CV outcomes.10
Similarly, the few studies in patients with CKD showed direct associations between BPV and adverse CV outcomes and death. A secondary analysis of SPRINT revealed that among participants without diabetes and with non-dialysis CKD stages 3-5, diastolic BPV was associated with the composite of acute coronary syndrome, acute heart failure, and CV death.5 Another study of 402 patients with CKD stages 1-5 (35% with diabetes) reported that systolic BPV was associated with the composite of death or CV event.6 A secondary analysis of the African American Study of Kidney Diseases (which included only individuals without diabetes) with GFR 20-65 mL/min/1.73 m2, revealed that systolic BPV was strongly associated with all-cause and CV death.25 One real-world study of 114,900 patients with CKD stages 3-4, higher quintiles of systolic BPV were separately associated with outcomes of all-cause death, heart failure, and hemorrhagic stroke, but not with acute coronary syndrome, or ischemic stroke.7 Recently, a study of 470 participants with CKD stages 3-5 showed that systolic BPV was associated with the composite of non-fatal stroke, non-fatal MI, and all-cause death.26 In contrast to these studies, one combined analysis of patients with diabetes and proteinuria enrolled in the Irbesartan Diabetic Nephropathy Trial (IDNT) and Reduction of End Points in Non-Insulin-Dependent Diabetes with Angiotensin II Antagonist Losartan (RENAAL) clinical trials showed that higher tertiles of systolic BPV were associated with death but not with CV death or CV events.27 In sum, most but not all studies of patients with CKD showed associations between BPV and CV events or all-cause death. However, these studies predominantly included patients with advanced stages of CKD.
Our results are consistent with these prior studies but, importantly, extend these findings to include a large cohort of patients with earlier stages of CKD from a national health care system. In our study, higher quintiles of BPV were strongly associated with CV outcomes. The consistent association of BPV with CV death, myocardial infarction, heart failure, and ischemic stroke but not with ESKD suggests that BPV is likely associated specifically with poor CV health. Furthermore, quintile of BPV was associated with composite CV events across all evaluated subgroups, including by CKD stage, race, sex, diabetes mellitus status, heart failure, or exposure to an ACEi or an ARB, supporting that the relationship of BPV with outcomes was true of all-comers, rather than driven by particular subgroups. Our study adds to the literature inclusion of non-dialysis CKD stages 1-5 in a population of patients that has more chronic illness and higher BPV than populations previously studied. We further add a sufficiently sized cohort to robustly study even rare outcomes, as well as various subgroups with adequate power to adjust for important confounders.
The few prior studies of associations of BPV with kidney outcomes in patients with CKD are mixed. The study of participants enrolled in IDNT and RENAAL showed associations of higher tertile of BPV with incident ESKD, doubling of creatinine, and the composite of both,27 and a prospective cohort study (N=470) showed that high systolic BPV was independently associated with eGFR decline >3 mL/min/1.73 m2 per year.26 However, in the large retrospective real-world observational study of patients with CKD stages 3-4, there was no association observed between BPV and incident ESKD,7 similar to the results of our analysis. Further studies will be required to elucidate the relationships between BPV and progression of kidney disease to clinically meaningful outcomes such as ESKD in patients with non-dialysis CKD.
In addition to this, we evaluated the interaction of diuretics to test whether these commonly prescribed and widely available medications may modify BPV and its association with CV events. Despite the association of BPV with clinically important events as detailed above, few studies have investigated potential interventions to mitigate BPV and possibly improve outcomes. Although the mechanisms of BPV are poorly understood, it is possible that extracellular volume, which is considered to have a key pathophysiologic role in hypertension in patients with CKD, may contribute to BPV. Natriuretic peptides, clinically used to measure fluid overload in patients with heart failure, are associated with BPV, independent of other important clinical factors such as left ventricular hypertrophy and diastolic dysfunction.28 This may explain prior secondary analyses of clinical trials showing that diuretic therapy may lower BPV compared to other classes of antihypertensive agents.7-10 In our analysis, although we observed no difference in BPV between those whose second antihypertensive agent was a diuretic vs. a non-diuretic, there were significant interactions of diuretic treatment on the association of the fourth and fifth quintiles of BPV with the primary composite CV outcome. This could be because extracellular volume may play a weightier role in the elevated CV risk among those with more severe BPV. Alternatively, BPV may have other complex underlying mechanisms, such that decreasing extracellular volume with diuretics may not fully account for other contributing causes of BPV. These may include vascular stiffness, sympathetic nervous system activation, medication nonadherence, stress and anxiety, physical activity, and endothelial dysfunction.29, 30 This could also explain why we observed a diminished association of BPV with CV events in those with diabetes mellitus, who have high baseline CV risk likely due to mechanisms unrelated to BPV and extracellular volume. In addition, because calcium channel blockers have also been associated with decreased BPV compared to other antihypertensive medication classes, their inclusion in the non-diuretic group may have biased our results toward the null.7, 9, 10, 31, 32
Our study has several strengths. We identified participants with prevalent CKD using laboratory values, which allowed us to use more sensitive guideline-based definitions rather than diagnosis codes. The large sample drew from a national health care system with near universal health care coverage and had high numbers of outcome events, including CV death, allowing adequate power to evaluate these outcomes when adjusting for relevant covariates. This study also has important limitations. CV outcomes were drawn only from VA data, which may miss outcome events occurring outside the VA system and decrease the power of the study. However, given the large sample size, our study was still adequately powered to test our hypothesis. In an observational study with such a large sample size, the high precision of point estimates, multiple comparisons, and residual confounding could impact interpretation of these results, such that marginally statistically significant findings may not represent clinically meaningful relationships. However, we showed a strong and consistent dose-response relationships across quintiles of BPV with each outcome except ESKD, and the point estimates of associations with composite CV events were strong, supporting the robustness of these findings. Furthermore, the relationship between BPV and CV events was consistent across all evaluated subgroups, indicating that the association of BPV with adverse CV outcomes is not driven by specific sub-populations of particular risk. The lack of observed association between BPV and ESKD further supports a meaningful relationship between BPV and CV events, as it is less likely that individuals with higher BPV were simply more ill overall. We did not evaluate BPV over a longer period of time such as 12 months, but the timeframe of 6 months we used is consistent with other real-world studies.7 A limitation of the subgroup analysis was the lower number of outcome events in women, although other subgroups had sufficient outcome events to conduct rigorous analysis. We do not know of any data to indicate that the observed associations would be different in women as compared with men. Finally, because the association of BPV with CV events was seen for each individual CV outcome and across each studied subgroup, it is possible that BPV is a marker of poor CV health rather than an intervenable risk factor.
In conclusion, we demonstrated that BPV was strongly associated with composite CV events, all-cause death, CV death, myocardial infarction, hospitalization for heart failure, and ischemic stroke in patients with prevalent non-dialysis CKD stages 1-5. There was no association between BPV and ESKD. The association of BPV with CV events was seen across all subgroups, but was attenuated in individuals with diabetes. Diuretic prescription modified the association of BPV with CV events at the highest BPV quintiles. BPV may be a promising potentially intervenable target to reduce CV events in patients with CKD.
Supplementary Material
Table S1. List of included anti-hypertensive medications by class
Table S2. Propensity score model variable definitions
Table S3. Outcome variable definitions
Table S4. Definitions of proteinuria and albuminuria variables
Table S5. Baseline characteristics of the unmatched cohort
Table S6. Systolic blood pressure coefficient of variation after second anti-hypertensive medication by subgroups
Table S7 Association of quintile of systolic blood pressure ARV and SD with time to CV event
Table S8. Association of quintile of systolic BPV prior to the index date and change in BPV with time to CV events
Table S9. Subgroup analyses of the association of the coefficient of variation of systolic blood pressure, taken continuously, with time to CV events
Figure S1. BPV before and after the index date by diuretic treatment group
PERSPECTIVES.
These results suggest that although outpatient visit-to-visit BPV is strongly associated with CV events among patients with CKD, treatment with diuretics may only decrease the association of BPV with outcomes in those with the highest BPV. It is possible that BPV has a complex underlying pathophysiology mediated by factors other than extracellular volume, or that high BPV is a marker of overall poor CV health. Nonetheless, BPV remains a promising target to improve outcomes in patients with CKD, so further studies should investigate whether other classes of antihypertensive agents impact BPV and its associations with outcomes.
NOVELTY AND SIGNIFICANCE.
What Is New?
Little is known about the associations of BPV with adverse outcomes patients with CKD, particularly in the earliest stages.
Although prior studies showed that diuretics may decrease BPV, none have investigated this as a potential strategy for reducing BPV and its association with adverse CV and kidney outcomes in patients with CKD.
What Is Relevant?
BPV may be a promising potentially intervenable target to reduce CV events in patients with CKD.
Diuretics may be effective to reduce the associations of high BPV with CV events.
Summary
BPV was associated with CV events and death but not ESKD in patients with non-dialysis CKD stages 1-5. Among individuals with high BPV, a decreased association of BPV with CV events was seen in those prescribed diuretics vs. non-diuretics.
Acknowledgments
Sources of Funding
This study was supported by a grant from the Texas Health Resources Foundation (L. P. Gregg). C. A. Alvarez received support from the National Institutes of Health (K08DK101602) and Agency for Healthcare Research and Quality (R24HS022418).
Footnotes
Disclosures
Outside the submitted work, Dr. Navaneethan has served on an independent event adjudication committee for clinical trials sponsored by Bayer and Boehringer Ingelheim, served as a consultant to Tricida and Reata pharmaceuticals and received investigator-initiated research support from Keryx Biopharmaceuticals. Dr. Virani reports receiving funding from the Department of Veterans Affairs, World Heart Federation, Tahir and Jooma Family; Honorarium: American College of Cardiology (Associate Editor for Innovations, acc.org). Dr. Winkelmayer reports having served on advisory boards to Akebia, AstraZeneca, Bayer, Daichii-Sankyo, Janssen, Merck, Relypsa, and Vifor-Fresenius Medical Care Renal Pharma. The remaining authors have no relevant conflict of interest to disclose.
REFERENCES
- 1.Gregg LP, Hedayati SS. Management of traditional cardiovascular risk factors in ckd: What are the data? American journal of kidney diseases : the official journal of the National Kidney Foundation. 2018;72:728–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. The New England journal of medicine. 2004;351:1296–1305 [DOI] [PubMed] [Google Scholar]
- 3.Gosmanova EO, Mikkelsen MK, Molnar MZ, Lu JL, Yessayan LT, Kalantar-Zadeh K, Kovesdy CP. Association of systolic blood pressure variability with mortality, coronary heart disease, stroke, and renal disease. Journal of the American College of Cardiology. 2016;68:1375–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kostis JB, Sedjro JE, Cabrera J, Cosgrove NM, Pantazopoulos JS, Kostis WJ, Pressel SL, Davis BR. Visit-to-visit blood pressure variability and cardiovascular death in the systolic hypertension in the elderly program. J Clin Hypertens (Greenwich). 2014;16:34–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mezue K, Goyal A, Pressman GS, Horrow JC, Rangaswami J. Blood pressure variability predicts adverse events and cardiovascular outcomes in chronic kidney disease: A post-hoc analysis of the sprint trial. Am J Hypertens. 2017;31:48–52 [DOI] [PubMed] [Google Scholar]
- 6.Mallamaci F, Tripepi G, D'Arrigo G, Borrelli S, Garofalo C, Stanzione G, Provenzano M, De Nicola L, Conte G, Minutolo R, Zoccali C. Blood pressure variability, mortality, and cardiovascular outcomes in chronic kidney disease patients. Clinical journal of the American Society of Nephrology : CJASN. 2019;14:233–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang TI, Tabada GH, Yang J, Tan TC, Go AS. Visit-to-visit variability of blood pressure and death, end-stage renal disease, and cardiovascular events in patients with chronic kidney disease. Journal of hypertension. 2016;34:244–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang JG, Yan P, Jeffers BW. Effects of amlodipine and other classes of antihypertensive drugs on long-term blood pressure variability: Evidence from randomized controlled trials. J Am Soc Hypertens. 2014;8:340–349 [DOI] [PubMed] [Google Scholar]
- 9.Muntner P, Levitan EB, Lynch AI, Simpson LM, Whittle J, Davis BR, Kostis JB, Whelton PK, Oparil S. Effect of chlorthalidone, amlodipine, and lisinopril on visit-to-visit variability of blood pressure: Results from the antihypertensive and lipid-lowering treatment to prevent heart attack trial. J Clin Hypertens (Greenwich). 2014;16:323–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang TI, Reboussin DM, Chertow GM, Cheung AK, Cushman WC, Kostis WJ, Parati G, Raj D, Riessen E, Shapiro B, Stergiou GS, Townsend RR, Tsioufis K, Whelton PK, Whittle J, Wright JT, Papademetriou V, Group* SR. Visit-to-visit office blood pressure variability and cardiovascular outcomes in sprint (systolic blood pressure intervention trial). Hypertension. 2017;70:751–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suissa S, Moodie EE, Dell'Aniello S. Prevalent new-user cohort designs for comparative drug effect studies by time-conditional propensity scores. Pharmacoepidemiol Drug Saf. 2017;26:459–468 [DOI] [PubMed] [Google Scholar]
- 12.National Kidney F. K/doqi clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2002;39:S1–266 [PubMed] [Google Scholar]
- 13.Norton JM, Ali K, Jurkovitz CT, Kiryluk K, Park M, Kawamoto K, Shang N, Navaneethan SD, Narva AS, Drawz P. Development and validation of a pragmatic electronic phenotype for ckd. Clinical journal of the American Society of Nephrology : CJASN. 2019;14:1306–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ray WA. Evaluating medication effects outside of clinical trials: New-user designs. Am J Epidemiol. 2003;158:915–920 [DOI] [PubMed] [Google Scholar]
- 15.Hussein WF, Chang TI. Visit-to-visit variability of systolic blood pressure and cardiovascular disease. Curr Hypertens Rep. 2015;17:14. [DOI] [PubMed] [Google Scholar]
- 16.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–3107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang TI, Flythe JE, Brunelli SM, Muntner P, Greene T, Cheung AK, Chertow GM. Visit-to-visit systolic blood pressure variability and outcomes in hemodialysis. J Hum Hypertens. 2014;28:18–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levitan EB, Kaciroti N, Oparil S, Julius S, Muntner P. Blood pressure measurement device, number and timing of visits, and intra-individual visit-to-visit variability of blood pressure. J Clin Hypertens (Greenwich). 2012;14:744–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diaz KM, Tanner RM, Falzon L, Levitan EB, Reynolds K, Shimbo D, Muntner P. Visit-to-visit variability of blood pressure and cardiovascular disease and all-cause mortality: A systematic review and meta-analysis. Hypertension. 2014;64:965–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muntner P, Shimbo D, Tonelli M, Reynolds K, Arnett DK, Oparil S. The relationship between visit-to-visit variability in systolic blood pressure and all-cause mortality in the general population: Findings from nhanes iii, 1988 to 1994. Hypertension. 2011;57:160–166 [DOI] [PubMed] [Google Scholar]
- 21.Muntner P, Whittle J, Lynch AI, Colantonio LD, Simpson LM, Einhorn PT, Levitan EB, Whelton PK, Cushman WC, Louis GT, Davis BR, Oparil S. Visit-to-visit variability of blood pressure and coronary heart disease, stroke, heart failure, and mortality: A cohort study. Annals of internal medicine. 2015;163:329–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee SR, Choi YJ, Choi EK, Han KD, Lee E, Cha MJ, Oh S, Lip GYH. Blood pressure variability and incidence of new-onset atrial fibrillation: A nationwide population-based study. Hypertension. 2020;75:309–315 [DOI] [PubMed] [Google Scholar]
- 23.Clark D 3rd, Nicholls SJ, St John J, Elshazly MB, Ahmed HM, Khraishah H, Nissen SE, Puri R. Visit-to-visit blood pressure variability, coronary atheroma progression, and clinical outcomes. JAMA Cardiol. 2019;4:437–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohkuma T, Woodward M, Jun M, Muntner P, Hata J, Colagiuri S, Harrap S, Mancia G, Poulter N, Williams B, Rothwell P, Chalmers J, Group AC. Prognostic value of variability in systolic blood pressure related to vascular events and premature death in type 2 diabetes mellitus: The advance-on study. Hypertension. 2017;70:461–468 [DOI] [PubMed] [Google Scholar]
- 25.McMullan CJ, Bakris GL, Phillips RA, Forman JP. Association of bp variability with mortality among african americans with ckd. Clinical journal of the American Society of Nephrology : CJASN. 2013;8:731–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jhee JH, Seo J, Lee CJ, Park JT, Han SH, Kang SW, Park S, Yoo TH. Ambulatory blood pressure variability and risk of cardiovascular events, all-cause mortality, and progression of kidney disease. Journal of hypertension. 2020;38:1712–1721 [DOI] [PubMed] [Google Scholar]
- 27.McMullan CJ, Lambers Heerspink HJ, Parving HH, Dwyer JP, Forman JP, de Zeeuw D. Visit-to-visit variability in blood pressure and kidney and cardiovascular outcomes in patients with type 2 diabetes and nephropathy: A post hoc analysis from the renaal study and the irbesartan diabetic nephropathy trial. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2014;64:714–722 [DOI] [PubMed] [Google Scholar]
- 28.Takahari K, Nagai M. Higher visit-to-visit blood pressure variability and n-terminal pro-brain natriuretic peptide elevation: Influence of left ventricular hypertrophy and left ventricular diastolic function. Blood Press Monit. 2020;25:126–130 [DOI] [PubMed] [Google Scholar]
- 29.Mancia G, Grassi G. Mechanisms and clinical implications of blood pressure variability. J Cardiovasc Pharmacol. 2000;35:S15–19 [DOI] [PubMed] [Google Scholar]
- 30.Parati G, Ochoa JE, Lombardi C, Bilo G. Assessment and management of blood-pressure variability. Nat Rev Cardiol. 2013;10:143–155 [DOI] [PubMed] [Google Scholar]
- 31.Park S, Yan P, Cerezo C, Jeffers BW. Effect of visit-to-visit blood pressure variability on cardiovascular events in patients with coronary artery disease and well-controlled blood pressure. J Am Soc Hypertens. 2016;10:799–810 [DOI] [PubMed] [Google Scholar]
- 32.Zhang L, Yang J, Li L, Liu D, Xie X, Dong P, Lin Y. Comparison of amlodipine versus other calcium channel blockers on blood pressure variability in hypertensive patients in china: A retrospective propensity score-matched analysis. J Comp Eff Res. 2018;7:651–660 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. List of included anti-hypertensive medications by class
Table S2. Propensity score model variable definitions
Table S3. Outcome variable definitions
Table S4. Definitions of proteinuria and albuminuria variables
Table S5. Baseline characteristics of the unmatched cohort
Table S6. Systolic blood pressure coefficient of variation after second anti-hypertensive medication by subgroups
Table S7 Association of quintile of systolic blood pressure ARV and SD with time to CV event
Table S8. Association of quintile of systolic BPV prior to the index date and change in BPV with time to CV events
Table S9. Subgroup analyses of the association of the coefficient of variation of systolic blood pressure, taken continuously, with time to CV events
Figure S1. BPV before and after the index date by diuretic treatment group


