Abstract
Traumatic brain injury (TBI), a growing public health problem, is a leading cause of death and disability worldwide, although its prevention measures and clinical cares are substantially improved. Increasing evidence shows that TBI may increase the risk of mood disorders and neurodegenerative diseases, including Alzheimer’s disease (AD). However, the complex relationship between TBI and AD remains elusive. Metabolic dysfunction has been the common pathology in both TBI and AD. On the one hand, TBI perturbs the glucose metabolism of the brain, and causes energy crisis and subsequent hyperglycolysis. On the other hand, glucose deprivation promotes amyloidogenesis via β-site APP cleaving enzyme-1 dependent mechanism, and triggers tau pathology and synaptic function. Recent findings suggest that TBI might facilitate Alzheimer’s pathogenesis by altering metabolism, which provides clues to metabolic link between TBI and AD. In this review, we will explore how TBI-induced metabolic changes contribute to the development of AD.
Keywords: Alzheimer’s disease, Traumatic brain injury, Glucose metabolism, Neurological disorder
Introduction
Traumatic brain injury (TBI) is a devastating public health problem, with 50 million new cases each year worldwide. TBI has a high mortality rate, representing 30%–40% of all injury-related deaths across all ages.1 The majority of survivors suffer from chronic neurobehavioral sequelae, including psychiatric and cognitive deficits, emotional and personality changes, etc., which pose a huge burden on patients, their families and the society.2 TBI pathogenesis is a complex process that results from primary and secondary insults.3 Primary injury is caused by mechanical force and occurs at the moment of injury, followed by delayed and protracted secondary injury.4 Secondary injuries occur as a consequence of diverse pathological mechanisms, such as excitotoxicity,5,6 oxidative stress,7, 8, 9 cerebral metabolic dysfunction,10,11 cerebrovascular pathology,12,13 chronic inflammatory events,14, 15, 16 and mitochondrial dysfunction.17,18
Alzheimer’s disease (AD) is the most common form of dementia, characterized by progressive memory loss, cognitive impairment and personality changes. However, currently, no effective treatments are available to prevent, halt or reverse AD.19 The two prominent neuropathological hallmarks of AD include extracellular deposits of amyloid-β (Aβ) in the form of senile plaques and intracellular accumulation of neurofibrillary tangles composed of hyperphosphorylated tau, both of which comprise highly insoluble, densely packed filaments.20 The Aβ peptide, consisting of about 40 amino acids, is derived from the sequential enzymatic cleavages of the amyloid precursor protein (APP) by β- and γ-secretase.21 β-secretase (β-site APP cleaving enzyme, BACE), the rate-limiting enzyme for Aβ production, was identified as the transmembrane aspartic protease responsible for initial cleavage of APP to form a membrane bound C-terminal fragment, which is in turn rapidly cleaved by γ-secretase to generate Aβ.22,23 γ-secretase is a promiscuous protease, resulting in the heterogeneity of Aβ peptide.24 Among all kinds of Aβ isoforms, Aβ40 and more hydrophobic Aβ42 are major components of accumulated Aβ in senile plaques.25,26 On the other hand, the phosphoprotein tau is a principal neuronal microtubule-associated protein with a preferential axonal localization.27,28 Tau promotes assembly and stabilizes microtubules, thereby contributing to the regulation of intracellular trafficking. The microtubule assembly activity of tau is tightly regulated by its degree of phosphorylation.29 Hyperphosphorylated tau undergoes conformational changes and is polymerized into paired helical filaments and straight filaments, leading to the formation of intracellular neurofibrillary tangles in the brain.30, 31, 32
TBI and AD are debilitating neurological diseases,33 and share common pathophysiology, including neuronal loss,34, 35, 36, 37 cytoskeletal disruption,38, 39, 40 metabolic perturbation41, 42, 43, 44 and neuroinflammation.45, 46, 47, 48 And, a growing body of epidemiological and molecular evidence suggests TBI as a principle epigenetic risk factor for AD,49, 50, 51, 52, 53 especially the identification of AD-like pathologies in the brains of both TBI patients and animal models.33,54, 55, 56 However, despite the well-established clinical association between TBI and the development and progression of AD, the precise mechanisms linking TBI to AD have not yet been completely elucidated. On the one hand, the brain is an energy metabolism sensitive organ,57 On the other hand, compelling data from neurological studies demonstrate that metabolism perturbation is closely involved in the pathology of TBI and AD, implying that metabolic dysfunction may be the missing link between TBI and AD. Here, the review intends to explore the metabolic link between TBI and AD, focusing on metabolism of glucose, which could lead to a better understanding of correlations between TBI and AD.
Glucose metabolism in brain
Glucose is the obligatory fuel source of the adult brain.58 To maintain and restore ion gradients used for synaptic transmission, action potential and the recycling of neurotransmitters, the brain, 2% of the total body mass, utilizes approximately 20% the oxygen and 25% of the glucose consumed by the resting body.57 Glucose is transported across the cell membranes by specific sodium-independent facilitated glucose transporters (GLUTs), which contains 14 members and comprises 12 membrane-spanning regions with intracellularly located animo- and carboxyl-ternini.59,60 Among them, GLUT1 and GLUT3 are the major brain glucose transporters. GLUT1 is predominantly located both in the luminal and the abluminal membranes of the endothelial cells of blood-brain barrier, and in astrocytes, whereas GLUT3 is highly expressed in neurons.61, 62, 63
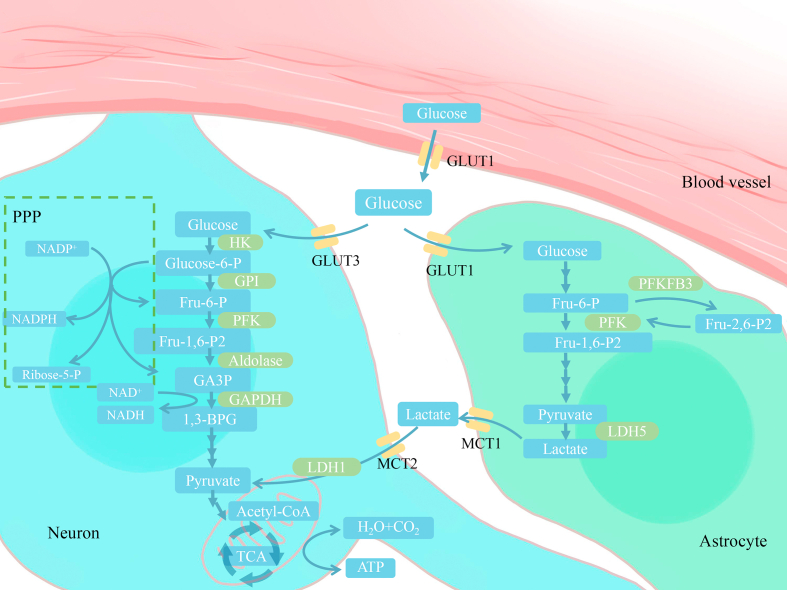
Upon entry into the cell, glucose is phosphorylated by hexokinase, a key enzyme for glucose utilization, to produce glucose-6-phosphate.64 Glucose-6-phosphate constitutes the metabolic crossroad of different pathways (Fig. 1),57 including (1) glycolysis with pyruvate or lactate as the end product; (2) the tricarboxylic acid (TCA) cycle and oxidative phosphorylation in mitochondria for energy production in the form of ATP; (3) the pentose phosphate pathway (PPP) which generates reducing equivalents in the form of NADPH, used for reductive biosynthesis such as fatty acid synthesis and against oxidative stress, and provides ribose-5-phosphate for nucleotide biosynthesis; (4) glycogenesis exclusively in astrocytes. Highly coordinated interactions among these processes guarantee cerebral glucose utilization and energy requirement, and either abnormality of glucose transportation or intracellular catabolism perturbation would influence cerebral glucose metabolism, which possibly results in metabolic disorders of the brain, such as AD.
Fig. 1.
Schematic diagram of glucose metabolism in the brain. Glucose is transported via glucose transporter 1 (GLUT1) across the endothelial cells of the blood-brain barrier, and then enters neurons and astrocytes through GLUT3 and GLUT1. In neurons, HK catalyzes the conversion of glucose to glucose-6-P, which is the irreversible process and ATP-driven phosphorylation. Glucose-6-P, the crossroad-metabolite, connects different pathways of intracellular glucose metabolism. It can be catabolized by glycolysis in cytoplasm to produce pyruvate. Pyruvate can then enter mitochondria and be subsequently utilized to generate ATP via TCA cycle and oxidative phosphorylation. Additionally, glucose-6-P can also enter the pentose phosphate pathway (PPP), which is the main source of reducing equivalents (NADPH) and provides the precursors for biomacromolecules synthesis such as ribose-5-P. In astrocytes, glutamatergic neurotransmission can induce the astrocyte-neuron lactate shuttle (ANLS). Increased lactate in astrocytes is shuttled to neurons by monocarboxylate transporters, which can be used by neurons to generate ATP.
HK: hexokinase; GPI: glucose-6-phosphate isomerase; Fru-6-P: fructose-6-phosphate; PFK: phosphofructokinase-1; GAPDH: glyceraldehydes-3-phophate dehydrogenase; GA3P: glyceraldehydes-3-phosphate; 1,3-BPG: 1,3-bisphosphoglycerate; TCA: tricarboxylic acid cycle; Fru-1,6-P2: fructose-1,6-bisphosphate; Fru-2,6-P2: fructose-2,6-bisphosph ate; PFKFB3: 6-phosphofructose-2-Kinase/fructose-2,6-bisphophatase-3; LDH: lactate dehydrogenase.
Glucose metabolism in TBI
Metabolic perturbation of glucose is the predominant cellular process accompanying TBI, which mainly results from imbalance of demand and supply of cerebral energy requirements induced by primary and secondary insults. Initially, the primary mechanical damage occurring at the moment of impact causes cell membrane disruption, which leads to significant excitatory neurotransmitter dependent efflux of K+ and alters the membrane potential.65,66 To restore ionic equilibrium and maintain normal synaptic transmission, cerebral neurons and glias uptake more glucose to obtain cellular energy in the form of ATP.67 Besides, TBI triggers DNA damage by both oxidative and nitrosative stresses, which in turn activates poly (ADP-ribose) polymerase-1 (PARP-1).68 PARP-1, a nuclear NAD+-consuming enzyme responsible for post-translational modification of proteins by poly ADP-ribosylation, is involved in DNA repair and genomic stability.69,70 However, excessive PARP-1 activation leads to energetic depletion due to consumption of ATP for NAD+ synthesis.71 Taken together, increased ATP demand for maintenance of ionic equilibrium and DNA repair partly contributes to the post-TBI energy crisis.
The facilitative hexose GLUTs mediate glucose transport from the circulation across the endothelial cells of the BBB into neurons and glia.72 Abnormality of the expression and activity of GLUT1 and GLUT3 could affect glucose uptake. Cornford and colleagues showed that GLUT1 was complete loss in areas of microvessel endothelial cells adjacent to small vessel injury in the resected tissues of two patients 7–8 h after acute TBI.73 Accordingly, in the rat model of moderate closed head injury,74 the expression of GLUT1 was significantly decreased in both impacted brain sections and isolated microvessels, and the changes were sustained until 24 h post-injury. Altered expression of GLUT1 could result in impairment of cerebral glucose utilization and metabolism. In contrast, Hamlin et al.75 demonstrated that severe diffuse traumatic brain in rats induced rapid upregulation of neuronal specific GLUT3 in cortex and cerebellum, while glial specific GLUT1 underwent no significant change. Increased expression of GLUT3 were likely the consequence of excessive energy demands for neuronal repair after TBI, whereas paradoxical change of GLUT1 in endothelial cell and GLUT3 in neuron possibly pointed to imbalance of demand and supply of glucose in injured brain.
Pyruvate generated by glycolysis can subsequently be oxidized to CO2 and water in mitochondria for energy production, or converted to lactate by lactate dehydrogenase.76 Following TBI, it is well-known that the extracellular lactate concentration is dramatically elevated.77 Hyperglycolysis-derived lactate could result from compromised cerebral blood flow, disruption of TCA cycle, and the activity of glycolytic enzyme such as glyceraldehyde-3-phosphate dehydrogenase (GAPDH).67 GAPDH, a key enzyme in glycolysis, catalyzes the conversion of glyceraldehyde-3-phosphate to 1,3-bisphosphoglycerate, which takes NAD+ as cofactor.78 As already mentioned, NAD+ could be massively consumed by PARP-1, which limits the activity of GAPDH. However, the conversion of pyruvate to lactate regenerates the NAD+, thereby maintaining the high glycolytic cascades for ATP production. Besides, increasing evidence demonstrates that glutamatergic transmission induced activation of astrocytes can convert glucose to lactate for neurons to generate ATP, which is the central point of the astrocyte-neuron lactate shuttle.57,79 Therefore, glutamate release after TBI, on the one hand, causes neuronal excitotoxicity in brain80; on the other hand, possibly stimulates lactate production from glucose in astrocytes and leads to cerebral extracellular lactate accumulation.
Decreased ATP production, even significantly increased cerebral glucose uptake during the acute period, together with injury induced self-protection and repair biological processes, suggest that glucose could be directed to different metabolic pathway in TBI. Accumulating evidence demonstrates that PPP is crucial for prevention of secondary injury or recovery, including defense against oxidative stress and repair and synthesis of biomacromolecules, through providing reducing equivalents NADPH and precursor of biomacromolecules such as ribose-5-phosphate.81 Using ex vivo 13C NMR spectroscopy to determine the metabolic fate of [1,2–13C2] glucose, Bartnik et al.82 showed that PPP activity was significantly enhanced in the cortex of controlled cortical impact injured rats at 3.5 h and 24 h after impact, which was further corroborated by data from patients with severe TBI. Based on intravenous infusion of [1,2–13C2] glucose, Dusick et al.81 found that PPP flux was significantly higher in six severe TBI patients than in six healthy controls, on average 19.6% versus 6.9% within 7 days of injury. Consistently, Jalloh et al.83 demonstrated that several TBI patients exhibited elevated PPP activity by adopting microdialysis catheter mediated infusion of [1,2 13C2] glucose.
Glucose metabolism in AD
Disturbance of cerebral glucose metabolism is a prominent pathological feature of AD, and precedes the manifestation of clinical symptoms even decades.43,84,85 Impaired cerebral glucose metabolism in AD could result from several ways. Among them, the earliest change in glucose metabolism is the decreased glucose transport.86,87 And the marked reduction in expression of cerebral glucose transporters has been established in the brains of AD patients and rodent AD models.86,88 Two major brain glucose transporters GLUT1 and GLUT3 were remarkably decreased in AD patients, which correlated with O-GlcNAcylation reduction, thereby contributing to abnormal hyperphophorylation of tau and neurofibrillary degeneration.89 Moreover, GLUT1 deficiency in endothelium of mice overexpressing Aβ could lead to BBB breakdown and related cerebrovascular degenerative changes, and induce Aβ pathology and progressive neuronal neurodegeneration.87 Besides, genetic overexpression of GLUT1 in an adult-onset Drosophila model of AD attenuated neuronal degeneration and prolonged lifespan, which was associated with downregulation of the unfolded protein response (UPR) negative master regulator Grp78 and enhanced UPR.90,91 Overall, these findings present an intimate relationship between glucose transporters and AD pathology, and causative role of glucose transporters in AD.
The mitochondrial pyruvate dehydrogenase complex (PDC) catalyzes the oxidative decarboxylation of pyruvate and controls the irreversible conversion of pyruvate into acetyl-CoA. PDC plays a vital role in the metabolism of pyruvate to maintain glucose homeostasis.92,93 The protein level and activity of PDC were significantly decreased in AD, which had the highest correlation with clinical state.94,95 Furthermore, the activity of cytochrome c oxidase, the major regulation site for oxidative phosphorylation,96 was significantly diminished in both platelets and temporal cortex and hippocampus of AD patients,97,98 which was also confirmed by AD animal model.95 The decreased activity of cytochrome c oxidase contributed to impaired glucose metabolism and energy generation. Altogether, these data indicate that mitochondrial dysfunction induced abnormality of glucose metabolism likely evokes neuronal perturbation in AD.
Possible cascades linking TBI and AD
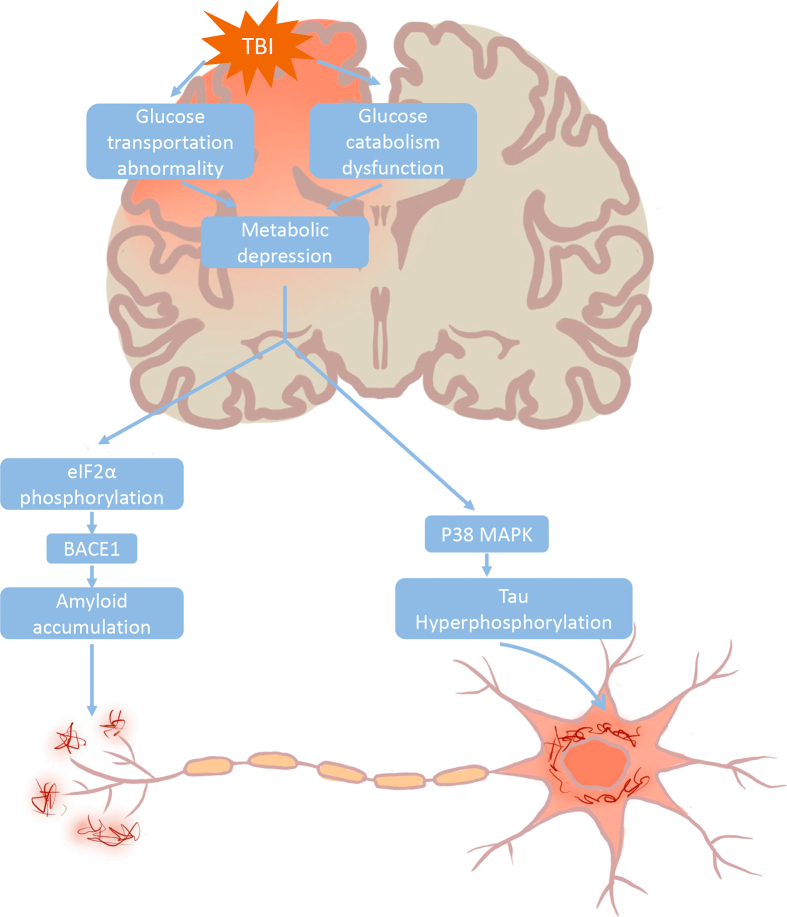
TBI evokes the prolonged glucose metabolic depression and consequent energy crisis, which reflects that glucose uptake into brain could not meet the demand of neuronal function.67 Impaired cerebral glucose metabolism could induce diverse biological cascades, leading to AD-like pathology. Energy deprivation in vitro or in vivo could trigger phosphorylation of the translation initiation factor eIF2α, which directly enhances the translation of BACE1, thereby promoting amyloidogenesis.99,100 Furthermore, through activating the p38 mitogen-activated protein kinase (MAPK) cascade, impaired glucose metabolism and utilization could induce tau phosphorylation and neuronal apoptosis, which would, in turn, cause the defect of memory and synaptic function (Fig. 2).101 Taken together, impaired glucose may build a bridge between TBI and AD.
Fig. 2.
Hypothetical pathogenic cascades linking TBI and Alzheimer’s disease. TBI perturbs cerebral glucose metabolism by affecting glucose transportation and intracellular glucose catabolism, thereby resulting in metabolic depression. Reduced energy availability triggers eIF2α phosphorylation and in turn enhances the translation of BACE1, which ultimately leads to amyloidogenesis. On other hand, energy deprivation could induce activation of p38 MAPK cascade and consequent hyperphosphorylated tau protein, which eventually no longer binds microtubules and aggregates into intracellular neurofibrillary tangles.
TBI: traumatic brain injury; BACE: β-site APP cleaving enzyme; MAPK: mitogen-activated protein kinase.
Besides, TBI could directly regulate p38 MAPK and eIF2α through post-translational modifications.102,103 MAPK pathway was dramatically activated post-TBI. And the phosphorylation of p38 MAPK was significantly elevated, thereby exacerbating the secondary injury after TBI. p38 MAPK signaling could elicit the chronic microglia activation after diffuse and focal TBI injury and cause motor deficits and synaptic protein loss, whereas knockout of p38α attenuated multiple pro-inflammatory responses and improved outcome.104,105 Additionally, TBI mediated p38 activation evoked mitochondrial damage and mitochondrial apoptosis as well as astrocyte activation while overexpression of SIRT1, the NAD+-dependent protein deacetylases, mitigated the activity of p38 MAPK and improved the neurobehavioral function, which implied that TBI might influence the activity of p38 MAPK through phosphorylation and acetylation.106,107
Phosphorylation of eIF2α compromises general translation, and concurrently selectively triggers the translation of a subset of mRNAs.108 Emerging evidence shows that increased phosphorylation of eIF2α impairs long-term memory formation.108, 109, 110, 111 TBI could induce integrated stress response mediated eIF2α phosphorylation and result in cognitive dysfunction, whereas integrated stress response inhibition attenuated TBI associated memory deficits.111 Furthermore, TBI triggered endoplasmic reticulum stress and subsequent phosphorylation of eIF2α, which consequently led to increased expression of APP and phosphorylated tau in the frontal cortex.112 The data summarized above imply that TBI may contribute to the development of AD through regulation of p38 MAPK and eIF2α by glucose metabolism and post-translational modification.
Conclusion
The correlation between TBI and AD is enormously complex, especially metabolic connection. The direct metabolic link between them is unavailable, although they share common impaired energy metabolism. However, the existing data imply that glucose perturbation may be the point that TBI aggravates the risk of developing AD, particularly in severe TBI patients. So, clarifying the metabolic link with TBI and AD would assist with drug development and therapies for neurodegenerative disease.
Funding
This work was supported by grants from National Natural Science Foundation of China (81471238, 81771327), Construction of Central Nervous System Injury Basic Science and Clinical Translational Research Platform, Budget of Beijing Municipal Health Commission 2020 (No. PXM2020_026280_000002).
Ethical statement
This is a review and ethical requirement was inapplicable.
Declaration of competing interest
All authors declared no conflicts of interest.
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Maas A.I.R., Menon D.K., Adelson P.D. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017;16:987–1048. doi: 10.1016/S1474-4422(17)30371-X. [DOI] [PubMed] [Google Scholar]
- 2.McAllister T.W. Neurobehavioral sequelae of traumatic brain injury: evaluation and management. World Psychiatr. 2008;7:3–10. doi: 10.1002/j.2051-5545.2008.tb00139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galgano M., Toshkezi G., Qiu X. Traumatic brain injury: current treatment strategies and future endeavors. Cell Transplant. 2017;26:1118–1130. doi: 10.1177/0963689717714102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bae Y.H., Joo H., Bae J. Brain injury induces HIF-1α-dependent transcriptional activation of LRRK2 that exacerbates brain damage. Cell Death Dis. 2018;9:1125. doi: 10.1038/s41419-018-1180-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tehse J., Taghibiglou C. The overlooked aspect of excitotoxicity:Glutamate-independent excitotoxicity in traumatic brain injuries. Eur J Neurosci. 2019;49:1157–1170. doi: 10.1111/ejn.14307. [DOI] [PubMed] [Google Scholar]
- 6.Fujikawa D.G. The role of excitotoxic programmed necrosis in acute brain injury. Comput Struct Biotechnol J. 2015;13:212–221. doi: 10.1016/j.csbj.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cornelius C., Crupi R., Calabrese V. Traumatic brain injury:oxidative stress and neuroprotection. Antioxidants Redox Signal. 2013;19:836–853. doi: 10.1089/ars.2012.4981. [DOI] [PubMed] [Google Scholar]
- 8.Chen W., Guo Y., Yang W. Connexin40 correlates with oxidative stress in brains of traumatic brain injury rats. Restor Neurol Neurosci. 2017;35:217–224. doi: 10.3233/RNN-160705. [DOI] [PubMed] [Google Scholar]
- 9.Greco T., Glenn T.C., Hovda D.A. Ketogenic diet decreases oxidative stress and improves mitochondrial respiratory complex activity. J Cerebr Blood Flow Metabol. 2016;36:1603–1613. doi: 10.1177/0271678X15610584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glenn T.C., Kelly D.F., Boscardin W.J. Energy dysfunction as a predictor of outcome after moderate or severe head injury: indices of oxygen, glucose, and lactate metabolism. J Cerebr Blood Flow Metabol. 2003;23:1239–1250. doi: 10.1097/01.WCB.0000089833.23606.7F. [DOI] [PubMed] [Google Scholar]
- 11.Soustiel J.F., Glenn T.C., Shik V. Monitoring of cerebral blood flow and metabolism in traumatic brain injury. J Neurotrauma. 2005;22:955–965. doi: 10.1089/neu.2005.22.955. [DOI] [PubMed] [Google Scholar]
- 12.Ramos-Cejudo J., Wisniewski T., Marmar C. Traumatic brain injury and Alzheimer’s disease: the cerebrovascular link. EBioMedicine. 2018;28:21–30. doi: 10.1016/j.ebiom.2018.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Len T.K., Neary J.P. Cerebrovascular pathophysiology following mild traumatic brain injury. Clin Physiol Funct Imag. 2011;31:85–93. doi: 10.1111/j.1475-097X.2010.00990.x. [DOI] [PubMed] [Google Scholar]
- 14.Kumar R.G., Boles J.A., Wagner A.K. Chronic inflammation after severe traumatic brain injury: characterization and associations with outcome at 6 and 12 months postinjury. J Head Trauma Rehabil. 2015;30:369–381. doi: 10.1097/HTR.0000000000000067. [DOI] [PubMed] [Google Scholar]
- 15.Corrigan F., Mander K.A., Leonard A.V. Neurogenic inflammation after traumatic brain injury and its potentiation of classical inflammation. J Neuroinflammation. 2016;13:264. doi: 10.1186/s12974-016-0738-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clark D.P.Q., Perreau V.M., Shultz S.R. Inflammation in traumatic brain injury: roles for toxic A1 astrocytes and microglial-astrocytic crosstalk. Neurochem Res. 2019;44:1410–1424. doi: 10.1007/s11064-019-02721-8. [DOI] [PubMed] [Google Scholar]
- 17.Hiebert J.B., Shen Q., Thimmesch A.R. Traumatic brain injury and mitochondrial dysfunction. Am J Med Sci. 2015;350:132–138. doi: 10.1097/MAJ.0000000000000506. [DOI] [PubMed] [Google Scholar]
- 18.Pandya J.D., Leung L.Y., Yang X. Comprehensive profile of acute mitochondrial dysfunction in a preclinical model of severe penetrating TBI. Front Neurol. 2019;10:605. doi: 10.3389/fneur.2019.00605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang Y., Mucke L. Alzheimer mechanisms and therapeutic strategies. Cell. 2012;148:1204–1222. doi: 10.1016/j.cell.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baker S.K., Chen Z.L., Norris E.H. Blood-derived plasminogen drives brain inflammation and plaque deposition in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA. 2018;115(41):E9687–E9696. doi: 10.1073/pnas.1811172115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kametani F., Hasegawa M. Reconsideration of amyloid hypothesis and tau hypothesis in Alzheimer’s disease. Front Neurosci. 2018;12:25. doi: 10.3389/fnins.2018.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vassar R., Bennett B.D., Babu-Khan S. Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286:735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- 23.Coimbra J.R.M., Marques D.F.F., Baptista S.J. Highlights in BACE1 inhibitors for Alzheimer’s disease treatment. Front Chem. 2018;6:178. doi: 10.3389/fchem.2018.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gertsik N., Chiu D., Li Y.M. Complex regulation of gamma-secretase:from obligatory to modulatory subunits. Front Aging Neurosci. 2014;6:342. doi: 10.3389/fnagi.2014.00342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tran J., Chang D., Hsu F. Cross-seeding between Abeta40 and Abeta42 in Alzheimer’s disease. FEBS Lett. 2017;591(1):177–185. doi: 10.1002/1873-3468.12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stewart K.L., Radford S.E. Amyloid plaques beyond Abeta: a survey of the diverse modulators of amyloid aggregation. Biophys Rev. 2017;9:405–419. doi: 10.1007/s12551-017-0271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iqbal K., Liu F., Gong C.X. Tau in Alzheimer disease and related tauopathies. Curr Alzheimer Res. 2010;7:656–664. doi: 10.2174/156720510793611592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laurent C., Buee L., Blum D. Tau and neuroinflammation: what impact for Alzheimer’s disease and tauopathies? Biomed J. 2018;41:21–33. doi: 10.1016/j.bj.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kimura T., Sharma G., Ishiguro K. Phospho-tau bar code: analysis of phosphoisotypes of tau and its application to tauopathy. Front Neurosci. 2018;12:44. doi: 10.3389/fnins.2018.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alonso A., Zaidi T., Novak M. Hyperphosphorylation induces self-assembly of tau into tangles of paired helical filaments/straight filaments. Proc Natl Acad Sci USA. 2001;98:6923–6928. doi: 10.1073/pnas.121119298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simic G., Babic Leko M., Wray S. Tau protein hyperphosphorylation and aggregation in Alzheimer’s disease and other tauopathies, and possible neuroprotective strategies. Biomolecules. 2016;6(1):6. doi: 10.3390/biom6010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fitzpatrick A.W.P., Falcon B., He S. Cryo-EM structures of tau filaments from Alzheimer’s disease. Nature. 2017;547:185–190. doi: 10.1038/nature23002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson V.E., Stewart W., Smith D.H. Traumatic brain injury and amyloid-beta pathology:a link to Alzheimer’s disease? Nat Rev Neurosci. 2010;11:361–370. doi: 10.1038/nrn2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sato M., Chang E., Igarashi T. Neuronal injury and loss after traumatic brain injury: time course and regional variability. Brain Res. 2001;917:45–54. doi: 10.1016/s0006-8993(01)02905-5. [DOI] [PubMed] [Google Scholar]
- 35.Hemphill M.A., Dauth S., Yu C.J. Traumatic brain injury and the neuronal microenvironment:a potential role for neuropathological mechanotransduction. Neuron. 2015;85:1177–1192. doi: 10.1016/j.neuron.2015.02.041. [DOI] [PubMed] [Google Scholar]
- 36.Niikura T., Tajima H., Kita Y. Neuronal cell death in Alzheimer’s disease and a neuroprotective factor, humanin. Curr Neuropharmacol. 2006;4:139–147. doi: 10.2174/157015906776359577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nobili A., Latagliata E.C., Viscomi M.T. Dopamine neuronal loss contributes to memory and reward dysfunction in a model of Alzheimer’s disease. Nat Commun. 2017;8:14727. doi: 10.1038/ncomms14727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hill C.S., Coleman M.P., Menon D.K. Traumatic axonal injury: mechanisms and translational opportunities. Trends Neurosci. 2016;39:311–324. doi: 10.1016/j.tins.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sassin I., Schultz C., Thal D.R. Evolution of Alzheimer’s disease-related cytoskeletal changes in the basal nucleus of Meynert. Acta Neuropathol. 2000;100:259–269. doi: 10.1007/s004019900178. [DOI] [PubMed] [Google Scholar]
- 40.Geddes J.F., Vowles G.H., Nicoll J.A. Neuronal cytoskeletal changes are an early consequence of repetitive head injury. Acta Neuropathol. 1999;98:171–178. doi: 10.1007/s004010051066. [DOI] [PubMed] [Google Scholar]
- 41.Bowman C.E., Scafidi J., Scafidi S. Metabolic perturbations after pediatric TBI: It’s not just about glucose. Exp Neurol. 2019;316:74–84. doi: 10.1016/j.expneurol.2019.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DeVience S.J., Lu X., Proctor J. Metabolic imaging of energy metabolism in traumatic brain injury using hyperpolarized [1-13C]pyruvate. Sci Rep. 2017;7:1907. doi: 10.1038/s41598-017-01736-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen Z., Zhong C. Decoding Alzheimer’s disease from perturbed cerebral glucose metabolism:implications for diagnostic and therapeutic strategies. Prog Neurobiol. 2013;108:21–43. doi: 10.1016/j.pneurobio.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 44.An Y., Varma V.R., Varma S. Evidence for brain glucose dysregulation in Alzheimer’s disease. Alzheimers Dement. 2018;14:318–329. doi: 10.1016/j.jalz.2017.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simon D.W., McGeachy M.J., Bayir H. The far-reaching scope of neuroinflammation after traumatic brain injury. Nat Rev Neurol. 2017;13:171–191. doi: 10.1038/nrneurol.2017.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alawieh A., Langley E.F., Weber S. Identifying the role of complement in triggering neuroinflammation after traumatic brain injury. J Neurosci. 2018;38:2519–2532. doi: 10.1523/jneurosci.2197-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heneka M.T., Carson M.J., El Khoury J. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14:388–405. doi: 10.1016/s1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Terada T., Yokokura M., Obi T. In vivo direct relation of tau pathology with neuroinflammation in early Alzheimer’s disease. J Neurol. 2019;266:2186–2196. doi: 10.1007/s00415-019-09400-2. [DOI] [PubMed] [Google Scholar]
- 49.Hicks A., James A., Spitz G. Traumatic brain injury as a risk factor for dementia and Alzheimer’s disease: critical review of study methodologies. J Neurotrauma. 2019;36:3191–3219. doi: 10.1089/neu.2018.6346. [DOI] [PubMed] [Google Scholar]
- 50.Fleminger S., Oliver D.L., Lovestone S. Head injury as a risk factor for Alzheimer’s disease: the evidence 10 years on; a partial replication. J Neurol Neurosurg Psychiatry. 2003;74:857–862. doi: 10.1136/jnnp.74.7.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dams-O’Connor K., Guetta G., Hahn-Ketter A.E. Traumatic brain injury as a risk factor for Alzheimer’s disease:current knowledge and future directions. Neurodegener Dis Manag. 2016;6:417–429. doi: 10.2217/nmt-2016-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Julien J., Joubert S., Ferland M.C. Association of traumatic brain injury and Alzheimer disease onset: a systematic review. Ann Phys Rehabil Med. 2017;60:347–356. doi: 10.1016/j.rehab.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 53.Djordjevic J., Sabbir M.G., Albensi B.C. Traumatic brain injury as a risk factor for Alzheimer’s disease: is inflammatory signaling a key player? Curr Alzheimer Res. 2016;13:730–738. doi: 10.2174/1567205013666160222110320. [DOI] [PubMed] [Google Scholar]
- 54.Huber B.R., Meabon J.S., Martin T.J. Blast exposure causes early and persistent aberrant phospho- and cleaved-tau expression in a murine model of mild blast-induced traumatic brain injury. J Alzheimers Dis. 2013;37:309–323. doi: 10.3233/jad-130182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tran H.T., LaFerla F.M., Holtzman D.M. Controlled cortical impact traumatic brain injury in 3xTg-AD mice causes acute intra-axonal amyloid-beta accumulation and independently accelerates the development of tau abnormalities. J Neurosci. 2011;31:9513–9525. doi: 10.1523/jneurosci.0858-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sayed N., Culver C., Dams-O’Connor K. Clinical phenotype of dementia after traumatic brain injury. J Neurotrauma. 2013;30:1117–1122. doi: 10.1089/neu.2012.2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Belanger M., Allaman I., Magistretti P.J. Brain energy metabolism:focus on astrocyte-neuron metabolic cooperation. Cell Metabol. 2011;14:724–738. doi: 10.1016/j.cmet.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 58.Mergenthaler P., Lindauer U., Dienel G.A. Sugar for the brain: the role of glucose in physiological and pathological brain function. Trends Neurosci. 2013;36:587–597. doi: 10.1016/j.tins.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Navale A.M., Paranjape A.N. Glucose transporters: physiological and pathological roles. Biophys Rev. 2016;8:5–9. doi: 10.1007/s12551-015-0186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scheepers A., Joost H.G., Schurmann A. The glucose transporter families SGLT and GLUT: molecular basis of normal and aberrant function. JPEN - J Parenter Enter Nutr. 2004;28:364–371. doi: 10.1177/0148607104028005364. [DOI] [PubMed] [Google Scholar]
- 61.Benarroch E.E. Brain glucose transporters:implications for neurologic disease. Neurology. 2014;82:1374–1379. doi: 10.1212/wnl.0000000000000328. [DOI] [PubMed] [Google Scholar]
- 62.Duelli R., Kuschinsky W. Brain glucose transporters:relationship to local energy demand. News Physiol Sci. 2001;16:71–76. doi: 10.1152/physiologyonline.2001.16.2.71. [DOI] [PubMed] [Google Scholar]
- 63.Patching S.G. Glucose transporters at the blood-brain barrier: function, regulation and gateways for drug delivery. Mol Neurobiol. 2017;54:1046–1077. doi: 10.1007/s12035-015-9672-6. [DOI] [PubMed] [Google Scholar]
- 64.John S., Weiss J.N., Ribalet B. Subcellular localization of hexokinases I and II directs the metabolic fate of glucose. PloS One. 2011;6 doi: 10.1371/journal.pone.0017674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Katayama Y., Becker D.P., Tamura T. Massive increases in extracellular potassium and the indiscriminate release of glutamate following concussive brain injury. J Neurosurg. 1990;73:889–900. doi: 10.3171/jns.1990.73.6.0889. [DOI] [PubMed] [Google Scholar]
- 66.Reinert M., Khaldi A., Zauner A. High level of extracellular potassium and its correlates after severe head injury:relationship to high intracranial pressure. J Neurosurg. 2000;93:800–807. doi: 10.3171/jns.2000.93.5.0800. [DOI] [PubMed] [Google Scholar]
- 67.Prins M., Greco T., Alexander D. The pathophysiology of traumatic brain injury at a glance. Dis Model Mech. 2013;6:1307–1315. doi: 10.1242/dmm.011585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Besson V.C. Drug targets for traumatic brain injury from poly(ADP-ribose)polymerase pathway modulation. Br J Pharmacol. 2009;157:695–704. doi: 10.1111/j.1476-5381.2009.00229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Piao L., Fujioka K., Nakakido M. Regulation of poly(ADP-Ribose) polymerase 1 functions by post-translational modifications. Front Biosci. 2018;23:13–26. doi: 10.2741/4578. [DOI] [PubMed] [Google Scholar]
- 70.Ray Chaudhuri A., Nussenzweig A. The multifaceted roles of PARP1 in DNA repair and chromatin remodelling. Nat Rev Mol Cell Biol. 2017;18:610–621. doi: 10.1038/nrm.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sarnaik A.A., Conley Y.P., Okonkwo D.O. Influence of PARP-1 polymorphisms in patients after traumatic brain injury. J Neurotrauma. 2010;27:465–471. doi: 10.1089/neu.2009.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jha M.K., Morrison B.M. Glia-neuron energy metabolism in health and diseases: new insights into the role of nervous system metabolic transporters. Exp Neurol. 2018;309:23–31. doi: 10.1016/j.expneurol.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cornford E.M., Hyman S., Cornford M.E. Glut1 glucose transporter activity in human brain injury. J Neurotrauma. 1996;13:523–536. doi: 10.1089/neu.1996.13.523. [DOI] [PubMed] [Google Scholar]
- 74.Balabanov R., Goldman H., Murphy S. Endothelial cell activation following moderate traumatic brain injury. Neurol Res. 2011;23:175–182. doi: 10.1179/016164101101198514. [DOI] [PubMed] [Google Scholar]
- 75.Hamlin G.P., Cernak I., Wixey J.A. Increased expression of neuronal glucose transporter 3 but not glial glucose transporter 1 following severe diffuse traumatic brain injury in rats. J Neurotrauma. 2001;18:1011–1018. doi: 10.1089/08977150152693700. [DOI] [PubMed] [Google Scholar]
- 76.Gray L.R., Tompkins S.C., Taylor E.B. Regulation of pyruvate metabolism and human disease. Cell Mol Life Sci. 2014;71:2577–2604. doi: 10.1007/s00018-013-1539-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Carpenter K.L., Jalloh I., Hutchinson P.J. Glycolysis and the significance of lactate in traumatic brain injury. Front Neurosci. 2015;9:112. doi: 10.3389/fnins.2015.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Seki S.M., Gaultier A. Exploring non-metabolic functions of glycolytic enzymes in immunity. Front Immunol. 2017;8:1549. doi: 10.3389/fimmu.2017.01549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Barros L.F., Weber B. CrossTalk proposal: an important astrocyte-to-neuron lactate shuttle couples neuronal activity to glucose utilisation in the brain. J Physiol. 2018;596:347–350. doi: 10.1113/jp274944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guerriero R.M., Giza C.C., Rotenberg A. Glutamate and GABA imbalance following traumatic brain injury. Curr Neurol Neurosci Rep. 2015;15:27. doi: 10.1007/s11910-015-0545-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dusick J.R., Glenn T.C., Lee W.N. Increased pentose phosphate pathway flux after clinical traumatic brain injury:a [1,2-13C2]glucose labeling study in humans. J Cerebr Blood Flow Metabol. 2007;27:1593–1602. doi: 10.1038/sj.jcbfm.9600458. [DOI] [PubMed] [Google Scholar]
- 82.Bartnik B.L., Sutton R.L., Fukushima M. Upregulation of pentose phosphate pathway and preservation of tricarboxylic acid cycle flux after experimental brain injury. J Neurotrauma. 2005;22:1052–1065. doi: 10.1089/neu.2005.22.1052. [DOI] [PubMed] [Google Scholar]
- 83.Jalloh I., Carpenter K.L., Grice P. Glycolysis and the pentose phosphate pathway after human traumatic brain injury: microdialysis studies using 1,2-(13)C2 glucose. J Cerebr Blood Flow Metabol. 2015;35:111–120. doi: 10.1038/jcbfm.2014.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mullins R., Reiter D., Kapogiannis D. Magnetic resonance spectroscopy reveals abnormalities of glucose metabolism in the Alzheimer’s brain. Ann Clin Transl Neurol. 2018;5:262–272. doi: 10.1002/acn3.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mosconi L. Glucose metabolism in normal aging and Alzheimer’s disease: methodological and physiological considerations for PET studies. Clin Transl Imaging. 2013;1 doi: 10.1007/s40336-013-0026-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ding F., Yao J., Rettberg J.R. Early decline in glucose transport and metabolism precedes shift to ketogenic system in female aging and Alzheimer’s mouse brain: implication for bioenergetic intervention. PloS One. 2013;8 doi: 10.1371/journal.pone.0079977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Winkler E.A., Nishida Y., Sagare A.P. GLUT1 reductions exacerbate Alzheimer’s disease vasculo-neuronal dysfunction and degeneration. Nat Neurosci. 2015;18:521–530. doi: 10.1038/nn.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hooijmans C.R., Graven C., Dederen P.J. Amyloid beta deposition is related to decreased glucose transporter-1 levels and hippocampal atrophy in brains of aged APP/PS1 mice. Brain Res. 2007;1181:93–103. doi: 10.1016/j.brainres.2007.08.063. [DOI] [PubMed] [Google Scholar]
- 89.Liu F., Iqbal K., Grundke-Iqbal I. O-GlcNAcylation regulates phosphorylation of tau:a mechanism involved in Alzheimer’s disease. Proc Natl Acad Sci USA. 2004;101:10804–10809. doi: 10.1073/pnas.0400348101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Niccoli T., Cabecinha M., Tillmann A. Increased glucose transport into neurons rescues Aβ toxicity in drosophila. Curr Biol. 2016;26(17):2291–2300. doi: 10.1016/j.cub.2016.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Duran-Aniotz C., Hetz C. Glucose metabolism: a sweet relief of Alzheimer’s disease. Curr Biol. 2016;26:R806–R809. doi: 10.1016/j.cub.2016.07.060. [DOI] [PubMed] [Google Scholar]
- 92.Patel M.S., Nemeria N.S., Furey W. The pyruvate dehydrogenase complexes: structure-based function and regulation. J Biol Chem. 2014;289:16615–16623. doi: 10.1074/jbc.r114.563148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang S., Hulver M.W., McMillan R.P. The pivotal role of pyruvate dehydrogenase kinases in metabolic flexibility. Nutr Metab. 2014;11:10. doi: 10.1186/1743-7075-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bubber P., Haroutunian V., Fisch G. Mitochondrial abnormalities in Alzheimer brain: mechanistic implications. Ann Neurol. 2005;57:695–703. doi: 10.1002/ana.20474. [DOI] [PubMed] [Google Scholar]
- 95.Yao J., Irwin R.W., Zhao L. Mitochondrial bioenergetic deficit precedes Alzheimer’s pathology in female mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA. 2009;106:14670–14675. doi: 10.1073/pnas.0903563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li Y., Park J.S., Deng J.H. Cytochrome c oxidase subunit IV is essential for assembly and respiratory function of the enzyme complex. J Bioenerg Biomembr. 2006;38:283–291. doi: 10.1007/s10863-006-9052-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cardoso S.M., Proenca M.T., Santos S. Cytochrome c oxidase is decreased in Alzheimer’s disease platelets. Neurobiol Aging. 2004;25:105–110. doi: 10.1016/s0197-4580(03)00033-2. [DOI] [PubMed] [Google Scholar]
- 98.Maurer I., Zierz S., Moller H.J. A selective defect of cytochrome c oxidase is present in brain of Alzheimer disease patients. Neurobiol Aging. 2000;21:455–462. doi: 10.1016/s0197-4580(00)00112-3. [DOI] [PubMed] [Google Scholar]
- 99.O’Connor T., Sadleir K.R., Maus E. Phosphorylation of the translation initiation factor eIF2alpha increases BACE1 levels and promotes amyloidogenesis. Neuron. 2008;60:988–1009. doi: 10.1016/j.neuron.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wong P.C. Translational control of BACE1 may go awry in Alzheimer’s disease. Neuron. 2008;60:941–943. doi: 10.1016/j.neuron.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 101.Lauretti E., Li J.G., Di Meco A. Glucose deficit triggers tau pathology and synaptic dysfunction in a tauopathy mouse model. Transl Psychiatry. 2017;7 doi: 10.1038/tp.2016.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mori T., Wang X.Y., Jung J.C. Mitogen-activated protein kinase inhibition in traumatic brain injury: in vitro and in vivo effects. J Cerebr Blood Flow Metabol. 2002;22:444–452. doi: 10.1097/00004647-200204000-00008. [DOI] [PubMed] [Google Scholar]
- 103.Morris A. Neuroendocrinology: integrated stress response linked to TBI. Nat Rev Endocrinol. 2017;13(9):501. doi: 10.1038/nrendo.2017.102. [DOI] [PubMed] [Google Scholar]
- 104.Bachstetter A., Rowe R.K., Kaneko M. The p38α MAPK regulates microglial responsiveness to diffuse traumatic brain injury. J Neurosci. 2013;33:6143–6153. doi: 10.1523/jneurosci.5399-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Morganti J.M., Goulding D.S., Van Eldik L.J. Deletion of p38α MAPK in microglia blunts trauma-induced inflammatory responses in mice. J Neuroinflammation. 2019;16:98. doi: 10.1186/s12974-019-1493-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yang H., Gu Z.T., Li L. SIRT1 plays a neuroprotective role in traumatic brain injury in rats via inhibiting the p38 MAPK pathway. Acta Pharmacol Sin. 2017;38:168–181. doi: 10.1038/aps.2016.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li D., Liu N., Zhao H.H. Interactions between Sirt1 and MAPKs regulate astrocyte activation induced by brain injury in vitro and in vivo. J Neuroinflammation. 2017;14:67. doi: 10.1186/s12974-017-0841-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Costa-Mattioli M., Sossin W.S., Klann E. Translational control of long-lasting synaptic plasticity and memory. Neuron. 2009;61:10–26. doi: 10.1016/j.neuron.2008.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Costa-Mattioli M., Gobert D., Stern E. eIF2alpha phosphorylation bidirectionally regulates the switch from short- to long-term synaptic plasticity and memory. Cell. 2007;129:195–206. doi: 10.1016/j.cell.2007.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jiang Z.H., Belforte J.E., Lu Y. eIF2alpha phosphorylation-dependent translation in CA1 pyramidal cells impairs hippocampal memory consolidation without affecting general translation. J Neurosci. 2010;30:2582–2594. doi: 10.1523/jneurosci.3971-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chou A., Krukowski K., Jopson T. Inhibition of the integrated stress response reverses cognitive deficits after traumatic brain injury. Proc Natl Acad Sci U S A. 2017;114:E6420–E6426. doi: 10.1073/pnas.1707661114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Begum G., Yan H.Q., Li L.L. Docosahexaenoic acid reduces ER stress and abnormal protein accumulation and improves neuronal function following traumatic brain injury. J Neurosci. 2014;34:3743–3755. doi: 10.1523/jneurosci.2872-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]