Abstract
In the last decade, the use of nanotheranostics as emerging diagnostic and therapeutic tools for various diseases, especially cancer, is held great attention. Up to date, several approaches have been employed in order to develop smart nanotheranostics, which combine bioactive targeting on specific tissues as well as diagnostic properties. The nanotheranostics can deliver therapeutic agents by concomitantly monitor the therapy response in real-time. Consequently, the possibility of over- or under-dosing is decreased. Various non-invasive imaging techniques have been used to quantitatively monitor the drug delivery processes. Radiolabeling of nanomaterials is widely used as powerful diagnostic approach on nuclear medicine imaging. In fact, various radiolabeled nanomaterials have been designed and developed for imaging tumors and other lesions due to their efficient characteristics. Inorganic nanoparticles as gold, silver, silica based nanomaterials or organic nanoparticles as polymers, carbon based nanomaterials, liposomes have been reported as multifunctional nanotheranostics. In this review, the imaging modalities according to their use in various diseases are summarized, providing special details for radiolabeling. In further, the most current nanotheranostics categorized via the used nanomaterials are also summed up. To conclude, this review can be beneficial for medical and pharmaceutical society as well as material scientists who work in the field of nanotheranostics since they can use this research as guide for producing newer and more efficient nanotheranostics.
Keywords: Theranostics, Nanomedicine, Nanotechnology, Nanoparticles, Radiopharmaceuticals
Graphical abstract
1. Introduction
Nanomedicine is an emerging field combining nanoscience, nanoengineering, and nanotechnology with life sciences, revealing interesting results for the medical community and society [1,2]. There are several nanomedicine platforms; however nanotechnology-based drug delivery systems as well as imaging nano-agents are of the greatest interest [3].
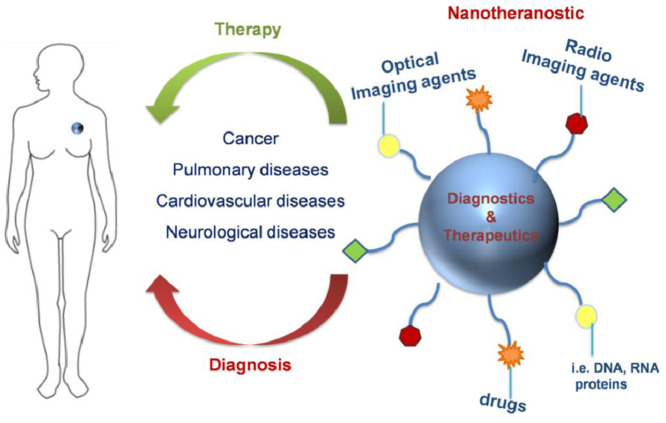
Theranostic nanomedicine involves the use of theranostics with nanosizes and multiple capabilities such as targeted delivery, sustained/controlled release, greater transport efficiency via endocytosis, stimuli responsive systems and the combination of therapeutic approaches such as multimodality diagnosis and therapy [4]. The “theranostics” word is referred to systems that can be both applied as therapeutics and imaging agents [5]. Nanotheranostics (Fig. 1) are strategies based on carriers of submicron or nano sizes [1,6]. Such nanotheranostics can be obtained from polymeric nanoparticles (NPs) [7], dendrimers [8], liposomes [9], carbon based nanomaterials [10], metal or inorganic nanocarriers [11] and systems which integrate both of such categories i.e. polymeric coated nanocarriers [12,13]. Carbon based nanomaterials, as carbon nanotubes either alone or decorated with other materials [14,15], graphene oxide [16], [17], [18], fullerenes, carbon quantum dots [19], have been employed for the detection of drugs in biological samples or their imaging ability. Prussian blue cubes are octahedral metal hexacyanoferrates which have been developed as detection tools due to their conductive and magnetic properties [20,21]. An ideal nanotheranostic should circulate for a long time in the body, present sufficient release behavior, great tissue target specificity and penetration, imaging probability and high target to background ratio [22]. Various nanotheranostics have the ability to localize diagnostic and therapeutic agents in specific sites of diseases and reduce undesired side effects. Their extended circulation time in blood is related with their nanometric size. In case of nanotheranostics applying for tumor diagnosis and therapy, the nanosized particles can easily extravagate from the blood into tumor tissues and be retained as a result of poor lymphatic drainage when compared to multifunctional small molecules or functionalized macromolecules [23], [24], [25].
Fig. 1.
A schematic representation of nanotheranostics used for simultaneous release and imaging. The functional groups of nanotheranostics can be drugs, DNA, RNA, imaging agents while their core material are polymers, inorganic and carbon based nanocarriers, dendrimers as well as liposomes.
At present, nanomedicine has provoked novel and promising applications in diagnostics and invasive therapy of various diseases. However, the development of novel tools with improved imaging characteristics, which can lead to early detection of diseases, is still of high importance. In addition, imaging agents, which can efficiently detect cancer in early stages, are beneficial for medical society. Aside from this, the nanotherapeutics are also key components for treatment of serious diseases [26]. Nonetheless, currently, most of the reported NPs are evaluated according to the results of animal models, and clinical studies are rarely performed. In the past, most of the applications based on nanotheranostics involved their use in cancer [27] but in the present, nanotheranostics for diseases such as neurological disorders [28], cardiovascular diseases (CVD) [29] have been also arised. Preclinically, theranostics have been gradually applied to CVD with encouraging findings [30].
Nuclear medicine imaging has been widely examined as advanced diagnostic tool and includes the introduction of radionuclides into the body, detection of the emitted gamma rays and generation of images which provide detailed radionuclides distribution as well as physiological characterization of organs and tissues [31]. Various nanoimaging agents have been produced presenting dual behavior as both diagnostic and therapeutic tools. However, some of these nanomaterials present low distribution and cell penetration, and thus their undesired pharmacokinetics should be improved. Many novel strategies have been employed in order to improve their pharmacokinetics and biodistribution [32].
The past years, the applications of nanotheranostics or NP based theranostics have shown an enormous advancement. By summing up the current developments on nanotheranostics could assist medical professionals on providing further progress in this field. All the more, analyzing the current application of imaging modalities and nanotheranostics could also provoke an evolution on nanomedicine field.
2. The application of imaging modalities in nanomedicine field
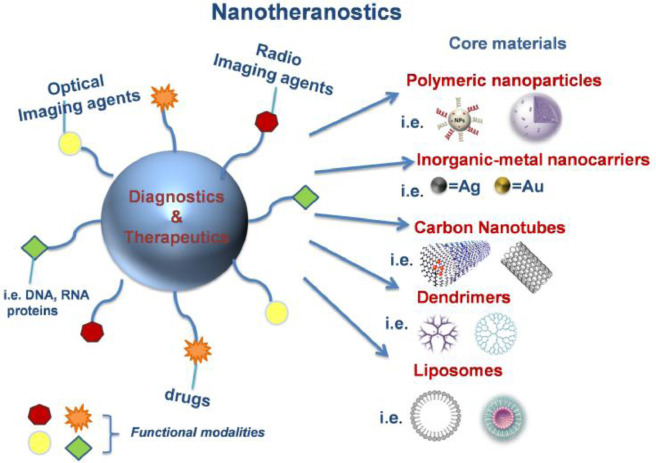
The last years, the integration of nanotools with life sciences led to the design of various diagnostic devices, contrast agents and drug delivery nanosystems. The multifunctional nanoscaled materials (Fig. 2) in most cases present promising biological activities, stability and improved biodistribution compared to larger monofunctional particles [1,2]. Besides, it has been reported that there is a correlation between the sizes and the chemical composition of nanoparticles and their internalization on cellular or intracellular surfaces. In general, NPs with sizes under 200 nm can be attached into the cells [33]. Although these nanosystems could provide complementary information arisen from different imaging agents, they don't present equivalent properties. Thus, when a new nanotheranostic system is designed, the imaging part should be chosen according to the intended administration route. For example, the targeted delivery of imaging agents should be carefully examined in order to evaluate the possible effects of the specificity of targeting and potential toxic side effects caused by off-target accumulation [1,2].
Fig. 2.
A detailed schematic illustration of multifunctional coated nanotheranostics. The imaging agents can be categorized on fluorescent or visible-infrared moieties, radionuclides whereas therapeutic target groups could be both chemical and biological agents. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
There are many imaging systems used in radiopharmacy, nuclear medicine and radiology areas. The radiographs, ultrasound, computed tomography (CT), and magnetic resonance imaging (MRI) are common imaging techniques in radiology. These methods allow the diagnosis of many diseases, mostly in cancer. Nonetheless, when the cancer cell becomes visible, it will contain about 1 billion cancer cells at about 1 cm3. Thus, the diagnosis of tumor area would be too late since the phenotypic changes begin. The detection of cancer earlier at the molecular level is very important for therapy. Because of this, the research has been focused on from radiological imaging to nuclear medicine imaging. Nuclear medicine imaging is described as the in vivo measurement of biological processes at the cellular and molecular levels, and the genetic alterations in the tissues. It also images the pathophysiologic situation noninvasively, and provides information regarding specific molecular changes, to perform early diagnosis, identify the stage of disease, fundamental information on pathological processes, and prognosis of disease and personalized medicine administration [34,35]. Nanotheranostics offer multiple and desirable advantages over conventional drug products. They can be easily prepared using standard nanotechnology procedures to provide designed functionalities and to achieve specific targeting; their surface can be conveniently modified with numerous ligands or moieties by linking, conjugation or coating [11,36,37].
Generally, NPs for imaging are classified in 4 major groups: magnetic [38], magnetofluorescent [39], Fluorescent [6] and radiolabeled [40]. They are being applied into various imaging modalities including optical, molecular, radiologic and nuclear medicine imaging [41], [42], [43]. Magnetic NPs are consisted of metals which have magnetic features. They can be prepared directly or by integrating into polymeric matrix. Recently, magnetic micro/nanoparticles have been widely used to facilitate location of cancer cells [44,45] either with the help of hyperthermia or non. Due to their magnetic features, they are handful in the diagnosis of various diseases such as treatment of cancer and neurologic disorders. In various cases, drugs are attached to magnetic NPs creating a driving force for delivery. Additionally, magnetic NPs are used as contrast agents for cancer diagnosis, molecular imaging, hyperfusion region visualization, T cell-based radiotherapy, detection of angiogenesis, apoptosis and gene expressions [46,47]. Fluorescent-magnetic NPs are multifunctional nanomedicals, composed of magnetic and fluorescent parts. The combination of these properties in single system plays a crucial role in theranostics field. They are beneficial for in vitro studies and in vivo imaging such as magnetic resonance imaging and fluorescence microscopy [48], [49], [50]
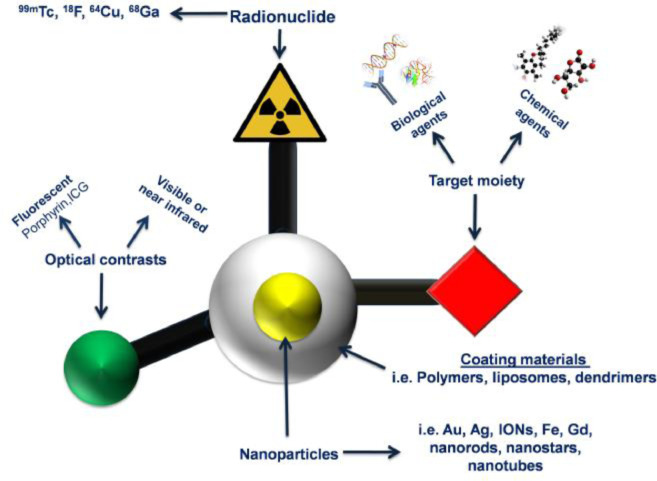
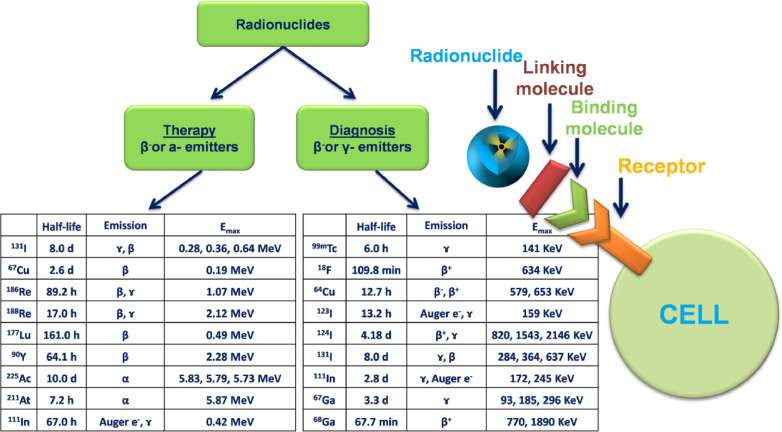
Radiopharmacy is a multidisciplinary science area and responsible for the development, formulation, preparation, quality control and administration of radiopharmaceuticals [35]. Radiopharmaceuticals are drugs containing radionuclides and pharmaceutical parts, used for the diagnosis or treatment of many diseases [51]. The pharmaceutical part is chosen on the basis of its preferential localization in the organ which is desired to be imaged or participation its in the physiological function of the organ. After its selection, the pharmaceutical part is labeled with a suitable radionuclide and after its localization in the organ it can be detected with various imaging methods [52]. Various nanotheranostics based on polymeric NPs have been developed and radiolabeled with available radionuclides using various methods [53,54] aiming the diagnosis and treatment of diseases [55]. Linking molecules such as chelator agents are binding to radionuclides and NPs (Fig. 3) in order to improve their stability [56]. The properties of short medium, and long half-lives of radionuclides, its radioactive decay and chemical structure could affect their potential application in clinical trials (Fig. 3). Hence, the radiopharmaceutical should be carefully chosen. For example, radiopharmaceuticals which emit gamma rays and beta particles have theranostic features. They serve both of diagnostic and therapeutic facilities to determine the physiological or anatomic situation in tissues. The selection of radionuclide has important role for targeting nanotheranostic to desired area. If gamma, beta and alpha emitters are used to radiolabel NPs, then the NPs obtain the theranostic property [57]. These emitters can also induce the visualization of the cellular function and the monitoring of the molecular process in the cells. Technetium-99 m is frequently applied as radionuclide to radiolabel nanosytems, presumably because of its wide availability, low cost, favorable imaging properties and a half-life (6 h) that allows imaging for up to 24 h [58]. Indium-111 is the second most-used radionuclide, followed by radioisotopes of iodine [59]. More recently, positron-emitting radionuclides such as Flour-18, Manganez-52, Zirconium-89 [60] have been increasingly used, reflecting the growing interest in positron emission tomography (PET) imaging and the increasing availability of preclinical and clinical PET scanners in radiopharmacy and nuclear medicine applications [54]. Another important parameter for the selection of radiation types are the energy levels of the emitters. The β- and γ- emitters are widely used, due to their long ranges and manageable energy levels. The α particle, or helium nucleus, is of very high mass and high energy, with very short range and thus it considered as unsuitable for radiation therapy due the extensive damage it causes to tissues [61].
Fig. 3.
The categories of radionuclides used as theranostics and diagnostic agents. According to their emmision type they can act as therapeutic or diagnostic agents. *Emax = maximum energy, half-life = the time length it takes for half of the radioactive atoms of a specific radionuclide to decay.
Another significant factor for choosing the suitable radionuclide is the effective half-life, which is the net half-life considering both physical half-life and biological half-life within the patient's body or organs. It has been reported that an acceptable half-life is within 6 h and 7 d This is because a very limited physical half-life restricts the delivery . In further, a physical long-life reserves the radiation dose and patients should be isolated for longer time while surrounding people are being exposed in radiation doses for more time. If the biological half-life is too short, the radionuclide will be discharged with a significantly high activity, leading to extensive radioactive waste management. Another feature which should be considered is decay product. More specifically, an ideal radiopharmaceutical should be able to decay into a stable daughter product [62]. While the radionuclides with short half-lives are preferred due to their diagnostic properties (e.g., technetium-99 m, gallium-68 and iodine-123) and used as imaging agents, radionuclides with medium half-lives present therapeutic properties (e.g., gallium-67, lutesium-177, yttrium-90 and iodine-131) and used in radiation therapy. The use of radionuclides containing very long half-lives in clinical research are common for the determination of absorption, distribution, metabolism and elimination parameters of new drug delivery systems [63,64].
2.1. Nanoimaging applications for cancer
Cancer is the second leading cause of death worldwide [65]; hence, early diagnosis and timely treatment of cancer are of great importance. The most common type of cancer is lung cancer followed by hepatic, colorectal, gastric and breast. In the last decade, various diagnostics and therapeutic tools for cancer have been emerged [66,67]. Laboratory diagnosis of cancer is limited to later stages of the disease and specific malignancies [68]. Consequently, early diagnostics markers should be encouraged. The classic approaches on cancer management involve the combination of surgery, radiotherapy, chemotherapy and hormonal therapy [69]. The available chemotherapy pathways as intravenous, oral [70] and topical [71].
The current cancer theranostics include the detection of novel biomarkers as advanced molecular diagnostics, new molecular imaging probes and techniques for early identification of cancer, molecular imaging guided cancer therapy and nanoplatforms incorporating both cancer imaging and therapeutic substances. The imaging techniques such as PET and single-photon emission computed tomography (SPECT) and fluorescence reflectance imaging (FRI) are widely used. Especially, PET and SPECT systems are nuclear medine imaging techniques and various radiolabeled nano systems have been administered through these methods. For these methodologies, the important fact is all the targeted molecules or the cells to become visible. Genetic agents such as photoproteins, PET [72] and magnetic resonance detectable reporter genes or radiolabeled particles [73], fluorochrome [74], magnetic molecules as labeled antibodies, small molecules or biorthogonal agents [75] are widely used.
The nanotheranostics for cancer can be categorized as conventional and biomimetics [76]. Conventional nanocarriers, such as liposomes, micelles, nanogels, and NPs present great potentional as anticancer strategies. However, their use as imaging or diagnostics is limited since they should be efficiently conjugated with fluorescent dyes or other active molecules before their application [76]. In addition, such systems should be also functionalized with agents such as poly(ethylene glycol) (PEG) which can increase the circulation time [2,77]. Biomimetics nanosystems as nanotheranostics have gained researchers’ attention since they can combine biological activities of biomimetic compounds such as proteins, phospholipids, cholesterol, cell and cell membranes, pathogens as bacteria and viruses and other pathogens, apatite and exosomes [76]. The current cancer nanotherapeutics rely on the improved permeability and retention (EPR) effect according to which NPs tend to accumulate in tumor tissue much more than they do in normal tissues. However, such a phenomenon is dependent on the tumor microenvironment and is not consistently observed in all tumor types, thereby limiting drug transport to the tumor site. In addition, it has been shown that EPR effect is not translated in the majority of humans cancers. Thus, cancer nanotheranostics should be studied in more clinically relevant cancer models should be used or in tumors which present a strong EPR effect in the clinic, such as Kaposi sarcoma, head and neck tumors [78,79].
Furthermore, cancer nanotheranostics are associated with stimuli such as light, temperature, magnetism, and sound. Photodynamic, photothermal, or phototriggered therapeutic systems are related with light or photo stimuli. The photodynamic therapy (PDT) [80] involves the administration of a tumor localizing photosensitizing agent, which may require metabolic synthesis (i.e., a prodrug), followed by activation of the agent by light of a specific wavelength. This therapy results in a sequence of photochemical and photobiologic processes that cause irreversible damage to tumor tissues. However, conventional PDT is not yet widely used for clinical cancer treatment [81]. Photothermal therapy (PTT) includes cancerous tissue irradiation with electromagnetic radiation, which induces thermal tissue damage. Finally, in phototriggered drug release, the active molecule modified with light responsive agent and the drug release in proper amount and time [82]. For example, recently a light triggered system for tumor diagnosis, combined photodynamic and hypoxia-activated prodrug therapy based on liposomes gene probe, hypoxia-activated prodrug Tirapazamine and photosensitizer Chlorin e6 was prepared [83]. Optical imaging theranostics involve phosphorescence, bioluminescence, fluorescence Raman, and photoacoustic imaging [72]. Fluorescence imaging [84] is a form of luminescence that results from the emittion of light of a certain wavelength after absorbing electromagnetic radiation [74]. Through fluorescence, images of fluorescent dyes and fluorescent proteins to mark molecular mechanisms and structures can be obtained [85]. A nanoplatform, comprised from silicon naphthalocyanine encapsulated in PEG-b-poly(ɛ-caprolactone) NPs, was developed for fluorescence image-guided surgery. The animal studies exhibited that the activatable NPs can accumulate at the tumor site following systemic administration, releasing the silicon naphthalocyanine. It was revealed that the combinatorial phototherapy mediated by the NPs could efficiently eradicate chemoresistant ovarian cancer tumors [86].
Magnetic nanotheranostics composed of magnetic substances (magnetite, iron oxide etc.) which after providing an external magnetic field can concentrate and retain the nanocarriers in specific cancerous area [87]. They are used for imaging (serving as contrast agents for MRI), therapy (combined hyperthermia-chemotherapy) as well as cell separation (cell labeling/tracking and isolation using magnetic force). Another example of magnetic nanotheranostics is their ability to be heated under a high frequency magnetic field which can induce cancer cell death [24,88]. The most common used magnetic diagnostic tool is MRI. It is a non-invasive tool, which produces images with high spatial and temporal resolution and used in radiology to form pictures of the anatomy and the physiological processes of the body. MRI scanners use strong magnetic fields, magnetic field gradients, and radio waves to generate images of the organs in the body. The majority of MRI scans don't need contrast agents, however, over 35% of clinical MR scans use contrast agents to improve their sensitivity and diagnostic accuracy. For instance, Prussian blue nanoprobes were designed as nanoplatforms for tumor imaging via PTT and MRI, due to their low toxicity and excellent in vivo performance [89]. Another example is the development of hollow manganese/cobalt oxide NPs as cancer nanotheranostics. The developed nanoplatforms can act as glutathione-responsive contrast agents for dual T1/T2-weighted MRI [90].
Ultrasound nanotheranostics are another significant category used for cancer diagnosis. Ultrasound contrast agents are mostly gas-filled microbubbles with echogenicity high degree [91]. Ultrasound as tumor diagnostic tools is present various advantages as real-time, portable, non-ionizing and deep tissue-penetrating capability [92]. Besides, it can be also be applied in cancer therapy as high intensity-focused ultrasound [93] and sonodynamic therapy [94]. Contrast-enhanced ultrasound could improve ultrasound backscatter, or reflection of the ultrasound waves which can produce unique sonogram with increased contrast due to the high echogenicity difference. More specifically, ultrasound can be used for imaging blood perfusion, blood flow as well as receptor density in tumors [95]. In summary, ultrasound molecular imaging includes photoacoustic imaging, phase-changeable imaging, multi-modality ultrasound imaging, TME-responsive ultrasound imaging, acoustic reporter genes imaging whereas ultrasound targeting therapy: sonodynamic therapy, high intensity-focused US ablation [92]. Inorganic NPs as contrast agents for ultrasound theranostics, include perfluorocarbon NPs, noble metal materials (such as Au, Ag, Pd, and Pt) carbon-based nanomaterials (such as carbon nanotubes, carbon dots, graphenes, and graphene oxides), silica-based nanomaterials [92,96]. Small molecule organic dyes with high photothermal conversion efficiency, such as indocyanine green (ICG), IR780, IR825, can absorb near infra-red (NIR) light and convert into heat energy. Most of the above diagnostic modalities can be applied for the diagnosis and treatment of other diseases.
2.2. Nanoimaging applications for pulmonary diseases
Respiratory diseases include a wide spectrum of illnesses associated with upper and down respiratory system. Nanomedicine has shown benefit for various chronic obstructive pulmonary diseases such as asthma [97] or genetic diseases as cystic fibrosis. In addition, pulmonary tuberculosis and lung cancer can be detected and treated by using nanoplatforms. It has been reported that lung can be easily targeted especially by using inhaled aerosols given that these nanosystems can be easily transferred to the airways [98]. Imaging nanoplatforms for pulmonary diseases detection are difficult to be found. However, various systems have been developed [99]. For example, Aillon et al. revealed that the iodined nanoclusters could also be an alternative option as contrast agents for lung visualization [100]. It can be said that PET and MRI [101,102] are the most frequently used techniques for NPs lung imaging [103]. Various are the nanodelivery systems used for treatment of pulmonary diseases. Nonetheless, combined imaging and therapeutic nanosystems are quite limited [104]. Lanza et al. prepared lipase labile phospholipid prodrug forms of fumagillin or docetaxel loading to lipid-based micelles for asthma management. The anti-angiogenic efficacy in asthma of αvβ3-targeted micelles -αvβ3 are expressed on lung endothelial cells- was studied via MR simultaneous dual 19F/1H neovascular molecular imaging [105]. Superparamagnetic iron oxide NPs were conjugated with a biocompatible antibody in order to examine their in vivo effect after pulmonary administration. The developed system enable specific targeting and imaging of a particular macrophage subpopulation in lipopolysaccharide-induced chronic obstructive pulmonary disease mice model [106].
2.3. Nanoimaging applications for neurological disorders
Neurodegenerative diseases as Alzheimer's (AD) [107], Parkinson's (PD) [108], Huntigton's (HD) [109] or in general neurological disorders affects millions of people worldwide. Their therapy has not been achieved yet due to the insufficient active molecules which have limited therapeutic activity, their low penetration on the targeted tissue due to blood brain barrier (BBB) and the fact that their pathophysiological mechanisms are poorly understand [110]. Thus, more and more researchers are focused on designing promising drug delivery systems or novel drug substances able to cross BBB and reach to brain [111,112]. In addition, detection of these disorders mainly focused on PET, SPECT, MRI and X-ray CT [28]. PET owing to its great sensitivity and better resolution provides enhanced imaging ability in comparison with SPECT. MRI and CT as non-invasive imaging techniques can depict morphological alterations in brain diseased tissues. It can be said that in order to achieve better penetration of drugs in brain, the nanocarriers surface can be modified with various biologically active substances which can exclusively couple expressed receptors on the brain endothelial cell [113,114]. For example, an innovative nanosystem aiming to be used as AD nanotheranostic was prepared from ceria and iron oxide nanocrystals surface coated onto the mesoporous silica NPs (MSNs) which were functionalized with amino-T807 (PET tau tracer), and methylene blue [115]. Birgitte et al. also developed manganese oxide NPs functionalized with l-DOPA able to release Mn2+ ions and l-DOPA concurrently in water due to NPs degradation. The specific nanosystem was served as MRI agent for imaging, diagnosis and therapy tool in PD [116].
2.4. Nanoimaging applications for cardiovascular disorders
CVD such as heart failure, coronary cardiac disease, myocardial infarction and inflammatory heart disease, hypertension, dyslipidemia, diabetes are the most frequent disorders.The most common problem for medications prescribed in these disorders is their low lipophilicity and thus their bioavailability [117,118]. Nanotechnology based systems are among the most useful for CVD since they present control drug delivery ability for a variety of active molecules which can be directed for the management of lipid disorders, inflammation [119] and angiogenesis within atherosclerotic plaques, and prevention of thrombosis. Herein, various imaging modalities can be applied for detection of disorder. For example, 89Zr-based PET imaging was employed to detect macrophages in atherosclerotic plaques. Macrophages in atherosclerotic lesions actively participate in lipoprotein ingestion and accumulation rising to foam cells filled with lipid droplets. Accumulation of foam cells contributes to lipid storage and atherosclerotic plaque growth. In this study, dextran NPs were functionalized with desferoxamine to enable hybrid PET-MR imaging and it was shown prominent localization in macrophages in plaques in the aortic root of atherogenic ApoE−/− mice. Dextran is a biocompatible and biodegradable molecule, and thus it can be applied in bioimaging applications [120]. Another group developed dextran-coated iron oxide based magneto-fluorescent nanoagent for near-infrared fluorescent imaging and generation of singlet oxygen. The application of nanotheranostics in an ApoE−/− mouse animal model showed desirable accumulation inside the atherosclerotic lesions and caused photo-induced apoptosis of phagocytic macrophages [121]. Gonçalves et al. developed PEGylated gold nanoparticles with 5-Aminolevulinic acid which were found to be accumulated into atheromatous plaques in atherosclerotic rabbits. It was further revealed that the acid was transformed to the active photosensitizer porphyrin IX. Thus, the nanoplatforms might be helpful in early diagnosis of atherosclerosis [122].
To summarize, nanotechnology is already playing and will play major role in developing novel and promising imaging probes and therapeutic strategies for various diseases.
3. Nanotheranostics categories according to their chemical nature
At present, an urgent need for early detection and diagnosis of diseases is needed. The main challenges for the development of efficient nanotheranostics are the use of non toxic contrast agents which can be circulate in body for more time in order to provide fast and detailed imaging of the lesions [123]. In this review, the nanobased theranostics are categorized according to their chemical nature as organic and inorganic. The most frequently found organic nanotheranostics are lipid, polymers, micelles or carbon materials whereas the most common inorganic materials are calcium phosphate, iron oxide, and metal- and silica-based NPs.
The nanotheranostics could be carefully modified in order to present small sizes which will prevent their rapid clearance from blood stream mononuclear by phagocyte system (MPS) and reticuloendothelial system (RES) [2,23,70]. Except their tiny sizes, the particles surface could be also modified with aptamers, antibodies etc. to avoid innate immunosystem recognition and to secure sufficiently long circulation to reach their targets [2,23,70]. Thus, inorganic and organic molecules could be modified or entrapped in polymer NPs cores, liposomes or dendrimers in order to be used sufficiently [1].
The clearance and toxicity of the used nanomaterials are issues, which should be carefully examined. According to numerous studies, it is believed that the toxicity and the clearance of nanomaterials are correlated with their physicochemical characteristics. For instance, injected NPs are vigorously cleared from bloodstream as a result of their sequestration by cells of the mononuclear phagocyte system preventing them reaching their target sites. Macrophages in particular are believed to be among the first and primary cell types that process NPs, mediating host inflammatory and immunological biological responses [124]. Several studies reported that the clearance of NPs is size and dose dependent [125]. A study reports that iron oxide NPs presenting large sizes (over 100 nm) are rapidly trapped in the liver and spleen through macrophage phagocytosis. On the other hand, iron oxide NPs with sizes under than 10 nm are possibly eliminated through renal clearance [126]. Tsoi et al. showed that hard NPs as quantum dots, gold and silica NPs cleared primarily in liver by Kupffer cells, endothelial cells, B cells and other cells [127]. Other studies associated the shape of NPs [128,129] and surface charge with their in vivo fate [130]. On the other hand, soft NPs resist macrophage uptake indicating higher blood circulation [131,132]. PEG-like and other hydrophilic polymers could reduce opsonin adsorption on NPs inducing them as unrecognized by the elimination mechanisms from the human body [133].
Toxicity is also a concern of NPs intended to be used in biomedical applications [134]. Similarly to clearance, the toxicity of NPs is correlated with the size [135], shape [136], the dose [137] and the surface coated [138,139]. For example, Feng et al. demonstrated that PEGylated iron oxide NPs (IONs) in BALB/c mice did not show any toxicity signs whereas poly(ethylene imine) (PEI) coated IONs exhibited dose-dependent lethal toxicity [137]. A research group showed that gold NPs with sizes lower than 6 nm effectively enter the cell nucleus, whereas larger NPs (10 or 16 nm) only penetrate through the cell membrane and are found only in the cytoplasm. This fact relates that small NPs, which enter the nucleus can present higher toxicity [135]. Similarly, the cytotoxicity of metallic is strictly reliant on the pathway of cellular internalization [140]. Furthermore, the toxicity of NPs can be associated with their chemical composition. Since many NPs can be degraded, a leakage of metal ions from the NP core can occur. Some metal ions, such as Ag and Cd, are in fact toxic causing cell damage. Others as Fe and Zn, are biologically useful which however in high concentrations could damage cellular pathways causing high toxicity [141]. In addition, it has been claimed that the toxicity of hard NPs can be decreased [141] via their incorporation on biodegradable polymeric capsules-particles [23,70], coating with biocompatible polymers [108,142] and materials or other strategies.
3.1. Inorganic-metal and carbon based nanoparticles
Until now, the use of inorganic NPs as drug carriers or diagnostic tools offered great results (Fig. 4). It thought that inorganic NPs were more stable than organic NPs; nonetheless, some evidences show that inorganic NPs may degrade individually in vivo, contributting in toxicity effects [143]. Hence, in many cases such inorganic NPs are coated with other biocompatible molecules [1,23,108,134]. MSN present solid framework of 50–300 nm particle diameter, interior porosity (pore size 2–6 nm), large pore volume, high surface area and ordered pore networks. They are relatively nontoxic with large loading capacity, great cytocompatibility and they can be easily modified [70,144]. Chen et al. prepared supramolecular photosensitizers based on MSNs as part of PDT. Tirapazamine-MSNs were further chelated with paramagnetic Gd3+ (Gadolinium). It was revealed that the nanosystem was able to be uptaken by CD44 receptor overexpressed in tumor cells whereas tumor growth was inhibited due to the combination of PDT and bioreductive chemotherapy under NIR fluorescence/MR imaging guidance. Bioreductive chemotherapy relies on the reductive activation of drugs by enzymes such as quinone oxidoreductase and P450 reductase as well as the identification of tumors abundant in such enzymes and differentiation in oxygen and pH levels within normal and tumor tissues. Subsequently, the developed system provides a new approach on nanotheranostics [145]. Another example using MSNs and quantum dots was published from Muhammad et al. Camptothecin, was loaded in quantum dots conjugated with MSNs. In further, fluorescent doxorubicin was coupled to quantum dots surface which also led to fluorescent quantum dots. The novel nanosystem showed great in vitro anticancer activity while confocal microscopy revealed the efficient imaging and release of both active molecules [146]. A very interesting study which involved functional nanocarriers of gold cluster bovine serum albumin nanogates which were engineered on MSNs, was prepared by Croissant et al. Authors loaded two anticancer agents: gemcitabine which was entrapped on positively-charged ammonium-functionalized MSN and doxorubicin which was conjugated in the negatively-charged of gold cluster bovine serum albumin nanogates and further electrostatically-attached onto ammonium-functionalized MSN. The dual delivery system was applied for targeted red nuclear staining and in vivo tumor imaging revealing its capability to act as multifunctional cancer nanotheranostic [147].
Fig. 4.
Various examples of inorganic nanoparticles used as nanotheranostics. Description: Au=Gold, Ag=Silver, MSN= Mesoporous Silica Nanoparticles, Fe3O4= Iron oxide, PB=Prussian blue.
Calcium phosphate NPs are based on nanocrystalline hydroxyapatite [108], but there is a growing interest in amorphous calcium phosphate. Indeed, calcium phosphate has been investigated for its optical ability [148]. Last years, metal NPs have been reported of the most promising candidates on biomedical field. Metal NPs can both serve as diagnostic and drug delivery tools because of their intriguing abilities such as small size, high reactive surface area, targetability to cells, functionalization capability etc. Metal or metaloid NPs as gold [149,150], silica [151] and silicon oxide [152,153], silver [154], titanium oxide [155] and iron oxide [156] are commonly used in nanotheranostics.
Magnetic NPs are based on metals (Iron-Fe, Cobalt-Co, Nickel-Ni), metal oxides (FeO, Fe2O3, Fe3O4 [157]), alloys (FePt, FePd) and ferrites (CoFe2O4, CuFe2O4). Superparamagnetic iron oxide NPs (SPIONs) are used as contrast imaging agent in magnetic particle imaging (MPI), due to their small size, good biocompatibility and the ability of surface engineering. MPI is a novel imaging modality that accomplishes a direct measurement of the magnetization of ferromagnetic NPs to calculate their local concentration. Also, they can act as therapeutic agents in hyperthermia. There are many types of MNPs products that have been developed for biomedical applications: ResovistⓇ (Fujifilm RI Pharma), EndoremTM (Guerbet S. A.), FerahemeⓇ(AMAG Pharmaceuticals), NanothermⓇ(MagForce Nanotechnologies) etc. Although some of are already commercially available on the market for clinical use, unfortunately, the majority of them is still under development [158].
Spherical gold NPs are of the most frequent gold nanoplatforms in drug delivery. Gold nanorods are used in photothermal and NIR application. Gold nanoantennas loaded with Cetuximab were characterized as an efficacious nanoprobe for in vivo tumor identification via Raman spectroscopy. Raman spectroscopy relies on the modification of frequency of light when it is inelastically scattered by molecules or atoms resulting in fingerprint information on molecular structure or environment. The nanosystem showed capability of targeting specified cancer biomarkers such as epidermal growth factor receptors. The developed nanosystem exhibits great raman signal in cancerous cells or mice tumors. According to the authors, the nanoantennas easily bind to epidermal growth factor receptors, blocking the epidermal growth factor protein from reaching the cancer cells and inhibit the signaling cascade, consequently stop proliferation and survival of targeted cells [159].
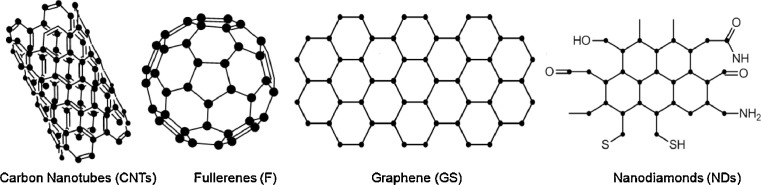
Carbon based nanomaterials (Fig. 5) such as fullerenes, carbon NPs, carbon nanotubes, graphene, and nano-diamonds have shown a tremendous development as biomedical applications especially in nanotheranostics [160]. Carbon nanotubes can be either single-walled (SWCNTs) or multi-walled (MWCNTs), and they are highly ordered, pseudo one-dimensional carbon allotropes. SWCNTs composed of rolled-up single layer of graphite tube of 0.3–2 nm diameter, while MWCNTs are multiple concentric cylindrical shells of graphite sheets. Furthermore, CNTs can penetrate into various cells and deliver drugs or other compounds to specific target tissue [161,162]. Fullerenes have been explored as tumor theranostics since they exhibit potential in cancer therapeutic approaches such as PDT, PTT, radiotherapy and chemotherapy, but they have also been used as novel contrast agents in MRI [163]. Huang et al. prepared fluorescent fullerene (C60) grafted with carboxyl groups. The developed NPs showed great water dispersibility, biocompatibility as well as fluorescence activity. Furthermore, researchers loaded cisplatin drug which released in controlled manner. The novel nanoplatform due to its properties of bioimaging and controlled drug delivery can act as promising nanotheranostic. However, in vivo studies should be further carried out in order to confirm the results [164]. Nanodiamonds are carbon NPs with a truncated octahedral architecture with 2 to 8 nm diameter. They demonstrate chemical stability, and extremely high hardness, stiffness and strength as well as small size, large surface area, and high adsorption capacity [165]. Su et al. developed fluorescent nanodiamonds in order to quantitatively track the human placenta choriodecidual membrane-derived mesenchymal stem cells in miniature pigs through magnetic modulation [166]. Graphene and graphene oxide (GO) are nanosheets of two-dimensional single monoatomic layers of sp2 hybridized carbon atoms. They are an improtant category of theranostics due to their easy synthesis and their interesting structure which can be easily conjugated with active molecules or polymers [167]. Nonetheless, their in vivo tracking is quite a challenge. Recently, a dual-element labeling method using lanthanum and cerium tagged on poly(vinylpyrrolidone) (PVP) modified GO, developed from Liang et al. The authors used the laser ablation inductively coupled plasma mass spectrometry combined with Trasmission Electron Microscope observation. It was revealed that the modified GO nanosheets, after intravenous injection, entered into lung, liver, spleen and the kidney, whereas they were slowly eliminated from the accumulated organs and some excreted via urine [125]. A previous study also exhibited that 125I-labeled nanographene sheets after intravenous administration mainly accumulate in the reticuloendothelial system including liver and spleen and can be gradually cleared by both renal and fecal excretion [168].
Fig. 5.
Carbon based nanomaterials used as detection diagnostic nanotools.
3.2. Polymer, liposomes and dendrimers as nanotheranostics
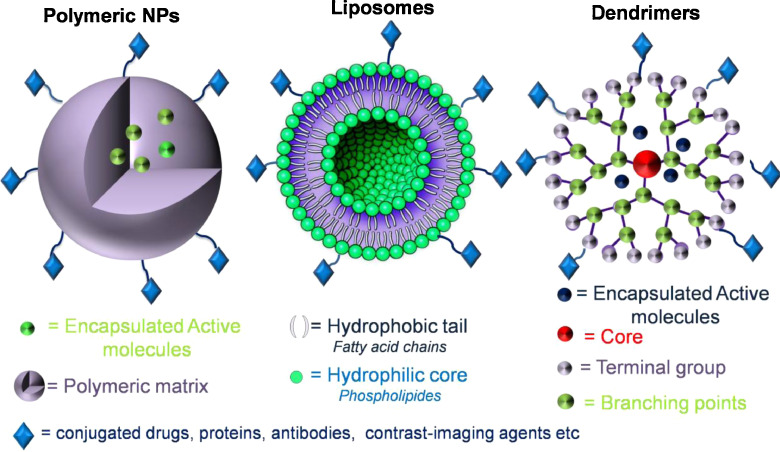
Various polymeric, liposomal and dendritic formulations have been applied as nanotheranostics (Fig. 6). As it was mentioned previously, they can coat the inorganic particles for improving their properties, avoiding their clearance and reducing their toxicity. Nonetheless, they can be also used as primary materials decorating with fluorescent molecules and drugs [1,2].
Fig. 6.
Various structures of nanotheranostics based on polymeric NPs, liposomes and dendrimers.
3.2.1. Polymeric nanoparticles
Polymers are among the most easy-handled and economical carriers due to their important characteristics such as biocompatibility, biodegradability and stability against degradation [1]. Both synthetic and natural macromolecules have been utilized as nanotheranostics. However, in most cases they should be firstly modified in order to possess imaging ability and therapeutic activity [32]. Chitosan is a fundamental linear polysaccharide with numerous properties as low toxicity, cost-effectiveness, antimicrobial activity, antioxidant properties, etc. [108,[169], [170], [171], [172]. In nanotheranostics, chitosan can be used as coating agent or polymeric core matrix. A novel multifunctional theranostic nanoplatform was fabricated via in situ growth of ultrasmall silver(I) selenide (Ag2Se) nanodots on the surface of chitosan coated-sodium yttrium tetrafluoride: ytterbium/erbium@sodium lutetium tetrafluoride: neodymium/ytterbium@ sodium lutetium tetrafluoride (NaYF4:Yb/Er@NaLuF4:Nd/Yb@NaLuF4) upconversion NPs. The theranostic agents were able of tetra-modal imaging-guided photothermal therapy of cancer for rapid and accurate delineation and elimination of tumors. The prepared nanosystems showed excellent luminescent properties, high X-ray attenuation coefficient and strong NIR absorbance [173]. Sachdev et al. developed chitosan-based hydrogel loaded with highly fluorescent carbon dots and anticancer drug, 5-fluorouracil. It was found out that the system was able to show cellular uptake as well as therapeutic effects. In addition, in vitro studies exhibited apoptosis in A549 cells. To sum up, green fluorescence of carbon dots could be used to detect apoptosis instigated by 5-fluorouracil, eliminating the need for multiplex dyes [174].
Chitosan due to its carboxyl and amino groups can be easily modified with various molecules showing remarkable properties. PEG and palmitoylated PEG-grafted chitosan were applied as polymeric shell for magnetic nanoparticles (Fe1-xMnxFe2O4). Methotrexate was encapsulated into chitosan coated NPs as anticancer drug. The prepared pH sensitive system was able to target in vitro tumor tissues, but still in vivo studies should also be performed [175]. Another derivative of chitosan, thiol chitosan was used as coating agent of gold nanoshells loaded with paclitaxel and the anti-epidermal growth factor receptor antibody. The multifunctional agents were acted as fluorescence/photoacoustic dual-modal imaging-guided chemophotothermal synergistic therapy. The nanoplatforms exhibited great biocompatibility, biosafety, broad NIR absorbance, photostability, fast and laser irradiation-controlled release as well as high targetability. It can be concluded that the prepared nanosystem can be used for tumor visualization and sufficient chemophotothermal combination cancer therapy under the guidance of photoacoustic imaging. [176]. A plasma membrane-activatable polymeric core-shell nanosystem was prepared by conjugating the photosensitizer protoporphyrin IX and PEG with glycol chitosan. The prepared nanoagents showed accumulation in tumor cells and improved in vivo fluorescence at the tumor site [177]. Chitosan and folic acid-conjugated chitosan NPs loaded with SPIONs were prepared as nanomagnetic onco-diagnostic and targeted nanomagnetic onco-diagnostic systems. In addition, uptake studies and competitive inhibition study verified the folate receptor mediated endocytosis of targeted system by MCF-7 as a folate receptor-positive cell line. The prepared novel tumor-targeting nanotheranostic agent can be applied for simultaneous MRI imaging and treatment of folate receptor-positive cancers. Nonetheless, the nanosystem lacks of in vivo studies [178]. Folate-conjugated N-palmitoyl-chitosan was formulated to novel nanobubbles combined with therapeutic ultrasound (US) to act as a safe and effective physical targeted cancer therapy. The modified nanobubbles were capable of killing cancer cells and inhibiting tumor growth. Considering their US irradiation, the innovative folate nanobubbles can be associated with promising outcomes on oncology diagnosis and therapy [179]. A nanoplatform for therapy and imaging of non-small cell lung cancer was fabricated by Zhang et al. The nanotheranostic agent composed of ICG entrapped into MSNs coupled with ZnO quantum dots. The nanosystem further coated with erlotinib-modified chitosan. The nanosystem exhibited a pH/redox dual-responsive release of ICG for precise fluorescent imaging. In vivo studies demonstrated that the nanotheranostic can provoke anticancer effect and can be an alternative option for imaging and therapy of non-small cell lung cancer [180]. Theranostic polyfunctional gold-iron oxide NPs (polyGIONs) surface coated β-cyclodextrin-chitosan and loaded with therapeutic miRNAs and the chemotherapy drug temozolomide were able to be accumulated and released in mice glioblastoma. The prepared nanosystem was administrated via intranasal route. The stable nanosystem exhibited in vivo optical fluorescence and MRI capability and thus can be applied as nanotheranostics [181].
Hyaluronic acid (HA) or hyaluronan is an anionic, nonsulfated glycosaminoglycan distributed widely throughout connective, epithelial and neural tissues. It possesses low toxicity and has been widely utilized in pharmaceutical technology. In the literature, pure HA based nanotheranostics are limited found. Nonetheless, derivatives of HA or its salts are being fabricated into nanoplatforms. A current research of Zhang et al. involved the use of gadolinium modified mesoporous silica (GD-MS) as MRI agent. Authors in order to further improve system targeting ability, they further grafted HA on GD-MS. The grafted system was further modified with iopamidol or doxorubicin so as to prepare both diagnostic and therapeutic nanomolecules against lymph cancer [182]. Zhu et al. produced bioresponsive and fluorescent HA-iodixanol nanogels aiming to be applied as targeted X-ray computed tomography imaging and chemotherapeutic agent. The anticancer drug paclitaxel was loaded into the nanogels so as to target MCF-7 human breast tumors. A high cell uptake of the nanogel and tumor inhibition was demonstrated. Moreover, an improved CT imaging was observed for MCF-7 breast tumors in nude mice while fluorescence showed that nanogels were distributed thoughout the whole tumor indicating deep tumor penetration [183]. A very recent study of Yu et al. included the use of deoxycholic acid-HA-methotrexate as carriers of ICG and doxorubicin. The developed NPs demonstrated intracellular doxorubicin uptake on CD44/folate receptors. Authors believe that the nanotheranostic present superior abilities as imaging-guided chemo-photothermal combination therapy [184]. In another study, nanoprobes from copolymers based on oxidized sodium hyaluronate and aggregation-induced emission-active dye were formulated in fluorescent NPs. It was revealed that the polymeric NPs are well dispersed in water with good biocompatibility as well as confocal imaging capability. Thus, they can be applied as alternative nanotheranostic system [185].
Cellulose is natural linear polysaccharide which is widely applied in pharmaceutical applications. Cellulose can be easily modified with active group providing derivatives with various functionalities. Carboxymethylcellulose (CMC) is among the most utilized cellulose derivatives. Leonel et al. produced core-shell nanofluids comprised from magnetic iron oxide and cobalt-doped magnetic IONs which further functionalized with CMC. The nanoconjugates showed hyperthermia ability due to iron oxide and thus can be used as anticancer nanotheranostics generated heat by magnetic hyperthermia of MION nanoconjugates [186]. In a different study, ZnS fluorescent quantum dots were modified with CMC and formulated nanocolloidal system which was further conjugated with doxorubicin. Nanocolloids with average size of 3.6 nm revealed photoluminescence emission property and biocompatibility. Thus the developed system can act as fluorescent nanoprobes and drug nanocarriers with ability to inhibit [187]. SPIONs were coated with sodium CMC and cross-linked with epichlorohydrin to improve the stability of coating. Rhodamine B was loaded as model molecule. Although the study was in preliminary stages, the characterization revealed that the nanosystem can be applied in drug magnetic targeting applications [188].
Other natural polymers were also acted as coating agents. Alginate is natural polymer which also used in drug delivery application due to its low toxicity [169]. Doxorubicin loaded modified alginate with folate-terminated PEG and rhodamine B nanogels were produced by Pei et al. Folate conjugation led to improved targeted on cancer cells while pH-responsive release behavior was also achieved. Cytotoxicity and cellular uptake studies revealed that the anticancer drug was vastly distributed in cancer cells provoking their death. Rhodamine provides fluorescent properties to the nanogels and consequently the nanosystem can be applied as real-time and noninvasive theranostic [189]. Nanogels with magnetic and dual responsive properties were developed by coupling SPIONs with a disulfide-modified alginate. The nanogels were simultaneously loaded with the anticancer drug doxorubicin. It was revealed that the dual nanosystem demonstrated magnetic-targeted ability, high drug loading content, co-triggered release behavior, high toxicity to tumor cells, low side effects to normal cells and MRI function [190]. Mohapatra et al. modified SPIONs with Carboxymethyl Assam bora rice starch-in order to evaluate its potential as magnetic drug targeting moiety. Scientists further loaded the system with the anticancer doxorubicin. It was demonstrated that the magnetic nanosystem exhibited high uptake and inhibition against HER-2 and folate receptor-α receptors over expressed in cancer cells [191]. An interesting study involved the fabrication of H2O2-activatable CO2 bubble generating ICG-loaded boronated maltodextrin NPs for imaging and therapy of peripheral arterial disease. The developed nanobubbles demonstrated improved fluorescence, US and photoacoustic signals as well as significant anti-inflammatory and proangiogenic effects in H2O2-stimulated vascular endothelial cells. Researchers revealed that the nanosystem can provide a novel insight for imaging and treatment of peripheral arterial disease [192].
Cyclodextrins are cyclic oligosaccharides which comprised from glucopyranoside units linked with (1–4) bonds and present a conical shape with an empty cavity which can host small molecules like drugs in proportion with molecules size [193]. Multifunctional nanoconjugates of magnetic Fe3O4 NPs, β-cyclodextrin and poly(N-isopropylacrylamide) were applied as drug carriers. Doxurobicin or curcumin was encapsulated and released under acidic pH conditions and elevated temperature whereas folic acid was further coupled in to the nanoconjugates to induce folate receptor targeting. Fluorophores were conjugated onto NPs to provide system fluorescence imaging ability. Moreover, nanoconjugated were able to accumulate on cancer tissues with magnetic hyperthermia. In vivo studies exhibited that after administration of nanoconjugates tumor growth was inhibited [194]. Xu et al. fabricated fluorescent organic NPs coupling red fluorescence and aggregation-induced emission dye through interactions between β-cyclodextrin and adamantine terminating aggregation-induced emission dye. The developed nanofeatures revealed excellent cytocompatibility and bioimaging ability [195].
Polypyrrole is heterocyclic conductive polymer which is used in biomedical devices or general biomedical field. Yang et al. prepared a novel nanotheranostic system based on polypyrrole which was coupled with Gd modified bovine serum albumin. The nanotheranostic presented high stability, great photothermal property and improved uptake on cancer cells. MRI revealed that the biocompatible nanoplatforms were distributed in cancer cells. In vivo studies with mice showed that polypyrrole system after exposed to 808 nm laser can hinder the tumor growth due to photothermal ablation. From the above, all the results demonstrated the well-designed nanotheranostics can utilized as tumor diagnostic and photothermal therapy [196]. In another work, tantalum oxide NPs were impregnated into polypyrrole for dual imaging guided photothermal removal of tumor. Small sized NPs of an average diameter at 45 nm were capable of reaching tumor site after systemic administration. It was revealed that the nanoplatform could improve X-ray CT and photoacoustic imaging in vivo. It should be noted that after intratumoral injection tumor was completely inhibited showing that the system can be efficiently act as targeted diagnostic and therapeutic system [197]. Except cancer applications, polypyrrole-PEI nanocomplexes were studied by Bournouf et al. as fibrinolytic therapeutics for venous or arterial thrombotic syndromes. Authors provide novel nanocomplexes exposed to near-infrared radiation as an alternative option of recent thrombolytic agents. It was demonstrated that the nanocomplexes were able of generating local hyperthermia upon NIR treatment, which appeared to produce reactive oxygen species (ROS). In vivo studies involving rats demonstrated biocompatibility, photothermal behavior and biodistribution [198].
PEG is the most frequent used hydrophilic moiety in biomedical application. PEG coating or PEGylation method onto various macromolecules is a common approach aiming to improve blood circulation of drug loaded NPs. Moreover, PEGylation can reduce toxicity of the nanosystem enhanced drug protection from degradation and numerous other properties [2]. Bovine serum albumin coated PEGylated magnetic NPs were loaded with vascular endothelial growth factor and doxorubicin as a novel cancer nanotheranostic system for improved targeting in murine breast adenocarcinoma 4T1 cell line. It was demonstrated that the NPs were capable of improving survival rate of mice bearing tumors a well as MRI imaging providing simultaneous cancer therapy and diagnostics [199]. An amphiphilic semiconducting polymer based on PEG- grafted poly(cyclopentadithiophene-alt-benzothiadiazole) (PEG-PCB) was developed by Jiang et al. The nanoplatform can be a diagnostic component for NIR fluorescence and photoacoustic imaging as well as therapeutic agent for photothermal cancer therapy [200].
Another work included the preparation of a hierarchical tumor acidity-responsive magnetic nanobomb composed from chlorin e6 (Ce6)-functionalized polypeptide ligand, methoxy poly (ethyleneglycol)-block-poly (dopamine-ethylenediamine-2,3-dimethylmaleic anhydride)-L-glutamate-Ce6 and SPIONs. The nanobombs were able to circulated in blood system for hours and provoked tumor inhibition due to their high cancer cell uptake. Both in vitro and in vivo methods demonstrated efficient tumor distribution and superior PDT [201]. Nanomicelles based on PEG-b-poly(L-leucine) entrapped doxorubicin, chemosensitizing agent XMD8-92, and superparamagnetic iron oxide NPs exhibited targeting ability, MRI imaging, controlled release behavior, improved cytotoxicity on MDR cells and sufficient tumor inhibition [202]. PEGylated GO-manganese ferrite NPs loaded with doxorubicin were developed by Qian et al. as nanotheranostic system. PEG moiety was applied for enhanced biocompatibility. The nanosystem presented MRI imaging ability whereas the system was further conjugated with radioisotope. The nanocomposites were able to reach tumor revealing high accumulation. Under the guidance of MRI/SPECT imaging, in vivo combined radioisotope therapy and chemotherapy was carried out an ihnibition of tumor growth after intravenous injection [203]. Multi-functional core-shell Fe3O4-Au NPs, conjugated with doxorubicin, methoxy PEG (mPEG), and folic acid-linked PEG were synthesized for cancer theranostic applications. The system showed saturation magnetization, improved release property and enhanced cellular distribution on cancer cells. Moreover, the NPs demonstrated high cytotoxicity under laser irradiation which led to PTT [204]. PEG was also coated magnetic gold NPs loaded with doxorubicin as anticancer nanotheranostics. In vitro and in vivo methods confirmed that the nanoplatforms were biocompatible, can release drug in controlled type, induce PTT via heat by NIR laser absorption. In addition, the multifunctional NPs can also act as MRI contrast agents [205]. Tong et al. developed PEI-PEG-reduced GO as biocompatible alternative for photothermal and chemotherapy against hepatocarcinoma. Doxorubicin was chosen as the anticancer agent. It can be concluded that the nanomaterials could effectively deliver and release drug in SMMC-7721 cells provoking cells death [206]. PEGylated black phosphorus NPs with great biocompatibility and water-solubility were prepared aiming to reduce cancer cells growth. The NPs can convert NIR light into heat, and exhibit excellent photostability. In vivo studies demonstrated the NPs accumulation in mice tumors whereas irradiation can be used for photothermal ablation of tumors [207]. A photosensitizer-assembled graphene/gold nanostar hybrid was formulated for combined cancer synergistic PDT and PTT as well as effective photothermal imaging. As it was expected, the stable PEG composite showed anticancer efficacy, tumor accumulation and inhibition and improved optical imaging [208]. Magnetic graphene nanohybrids based on GO-modified PEG and γ-Fe2O3 were prepared by Chen et al. as an encouraging option for synergistic anticancer therapy and imaging. Doxorubicin was chosen as anticancer drug. It was exhibited that the nanosystem can be mapped by MRI, photothermal and fluorescence imaging. In addition, the anticancer efficacy was revealed by both in vitro and in vivo methods which showed tumor ablation after hyperthermia and NIR light exposure [209]. Multifunctional nanotheranostics incorporating IR-780 dye, a NIR imaging probe as well as the anticancer compound known as α-tocopheryl succinate were developed by Palao-Suay et al. The core shell NPs were fabricated using PEG and poly(methacrylic α-tocopheryl) succinate. The system presented photo-inducing and fluorescence ability due to the presence of the dye. In vitro studies showed that the theranostic NPs can be effectively localized in cells revealing promising characteristics [210]. Finally, He et al. developed a peptide-based nanotheranostic based on p53-activating peptide termed PMI, functionalized PEG and fluorescent lanthanide oxyfluoride nanocrystals. It was revealed that the nanoplatform exhibit improved tumor targeting and imaging properties. In vivo studies involving a mouse model with human colon cancer showed that the nanosystem is efficacious. More specifically, the nanorods can inhibit tumor growth and be easily mapped [211]. You et al. prepared multifunctional nanosystems with sufficient cellular uptake, specific target and controlled release efficacy. The nanosystem was consisting of folate and cyclic arginine-glycine-aspartic-peptide modified NPs based on NIR light and glutathione dual stimuli-responsive release system loaded with cisplatin and ICG. The core polymer was a copolymer between poly(ε-captolactone) (PCL) and carboxylated PEG. The nanospheres were able of accumulation to tumor sites showing decreased cancer cells viability and thus it can be an alternative nanotheranostic in nanomedicine field [212].
PCL is hydrophobic polymer which is used in biomedical field especially in controlled release applications [213]. As nanotheranostics, PCL is used as coating or core material. For instance, PCL-AuNC/Fe(OH)3-PAA Janus NPs were fabricated by Zhang et al. and loaded with doxorubicin and docetaxel. The nanoplatform demonstrated greater therapeutic efficacy due to synchronous release of two drugs as well as the excellent computed X-ray tomography/magnetic resonance (CT/MR) imaging capabilities. In vivo studies using mice further depicted tumor inhibition revealing that the aforementioned system can be effective as combined cancer therapy [214].
Poly(lactic) acid (PLA) is an aliphatic polyester with high cytocompatibility which can be easily modified or formulated in various nanoforms [215]. In case of nanotheranostics, NPs of PLA were loaded with doxorubicin and further surface coated with Mn-porphyrin as potential MRI agent and pH-responsive drug delivery system. Firstly, the system exhibited that the drug was released at greater extent in acidic pH. Moreover, nanoformulations showed efficient tumor inhibition in HeLa cells and HT-29 cells. All the more, the system demonstrated that it can act as MRI contrast agent for sufficient cancer diagnosis and therapy [216].
Poly(lactic-co-glycolic acid (PLGA) is the copolymer between PLA and poly(glycolic acid) and presents high biocompatibility and extremely promising properties. It is widely used in pharmaceutical industry and technology as nanocarrier. As nanotheranostic, PLGA-based NPs impregnated with paclitaxel and superparamagnetic iron oxides to act as both therapeutic and imaging tool. The visualization of NPs accumulation was conducted using Electron Spin Resonance spectroscopy and MRI and it was depicted that the nanoplatforms exhibited improved NPs distribution, MRI contrast ability and most importantly anti-cancer activity [217]. A mPEG-PLGA-poly-l-lysine triblock copolymer was designed as photothermally triggered immunotherapeutic system loaded with SPIONs and cytosine-phosphate-guanine oligodeoxynucleotides. The under magnetic-responsive immunostimulatory nanoagents acted both as contrast agent for PA/MRI bimodal imaging and magnetic-targeting therapeutic agent. It was revealed that the NPs were accumulated in tumors and inhibit metastatic tumors simultaneously with high specificity, easy maneuverability and favorable biocompatibility [218]. Another system involved quantum dots as imaging molecule and EpCAM aptamer as target ligand which conjugated to nutlin-3a loaded PLGA NPs. The nanoplatform exhibited intriguing properties such as cell targeting and biomaging and it can be served as nanotheranostic approach on cancer therapy [219]. Castellani et al. studied CdSe/ZnS QD-loaded PLGA NPs as targeted system for metastatic liver cancer as hyperthermia triggered system. Localized MW irradiation which led to mild hyperthermia played a significant role to NPs accumulation in tumor [220]. Zhang et al. design NPs of modified PEG-PLGA with iron-gallic acid as MRI guided chemo-photothermal synergistic therapy of tumors. The drug Gallic acid was able to be released in acidic pH as tumor environment inducing apoptosis. The study revealed that the nanonetwork due to its extraordinary photostability and photothermal therapy capacity is quite promising as nanotheranostic [221]. Furthermore, paclitaxel was loaded into nanocapsules comprised from PLGA-PEG and perfluorooctyl bromide (PFOB) as nanotheranostic agents. The prepared nanocapsules showed anticancer efficacy when studied in vitro on CT-26 colon cancer cells whereas in vivo studies revealed passive accumulation of drug in CT-26 tumors in mice and tumor inhibition. Imaging capability was induced due to the presence of PFOB. Consequently, the system has great potential as cancer theranostic agents [222].
PEI is a branched cationic polymer which can be modified and act as polymer nanotheranostic. In a recent research, Shao et al. fabricated PEI-PLA NPs which simultaneously load hydrophobic antiangiogenesis agent combretastatin A4, NIR dye IR825 and heat shock protein 70 (HSP70) inhibitor (siRNA against HSP70). The nanosystems studied in vivo in a xenograft mouse tumor model, demonstrating improved photocytotoxicity and tumor inhibition and synergistic anticancer efficacy with NIR laser irradiation. The copolymer acted as drug carrier as well as self-monitor to real-time tracking of NPs biodistribution and tumor accumulation via fluorescence imaging. Finally, due to the above and fact that the nanoplatform can also be applied as photoacoustic agent for in vivo photoacoustic imaging, the nanosystem offer encouraging results as current multifunctional cancer nanotheranostic [7]. Deng et al. also used PEI as modifying agent of black phosphorus nanomaterials along with dextran. The nanoplatforms were further functionalized with folic acid and cyanine 7 depicting great stability and cell viability, near infrared optical properties for targeted imaging of tumors through photoacoustic imaging and NIR fluorescence imaging. In addition, the nanosystem is efficient as photothermal cancer therapeutic [223].
Poly(N-isopropyl acrylamide) (PNIPAM) is a pH- and temperature-responsive polymer used in variou medicine applications. Roy et al. fabricated stimuli-responsive PNIPAM-co-tyrosine modified gadolinium doped IONs as cancer theranostic agent. Methotrexate was loading in great extent and released with a stimuli dependent manner. In further, the NPs showed MRI activity as well as desirable in vitro hyperthermia response [224]. Poly(vanillin oxalate) (PVO) is antioxidant polymer which was formulated into NPs generating CO2 through H2O2-triggered oxidation of peroxalate esters and release vanillin, which exerts antioxidant and anti-inflammatory activities. PVO NPs exhibited improved ultrasound signal in the site of hepatic I/R injury and also effectively suppressed the liver damages by inhibiting inflammation and apoptosis. Consequently, the system can act as ultrasound contrast agents and therapeutic tool in H2O2-associated diseases [225].
Other polymers have also been employed as nanotheranostics. More specifically, poly (N-(2-hydroxypropyl) methacrylamide) nanocarriers of paclitaxel were further coupled with self-quenched Cy5 (SQ-Cy5). Activatable fluorescent probes as SQ-Cy5 employ a fluorescent signal which is silenced/“OFF” under physiological conditions, and is turned-ON at the designated site. Polymeric nanotheranostics could present an ‘always ON’ signal. Förster resonance energy transfer is the most common and efficient turn-on mechanism. The system was able to release the drug due to enzymic degradation in cathepsin B-overexpressing breast cancer cells. The drug releases took place simultaneously with the activation of the fluorophore to its Turn-ON state. The copolymer NPs showed better distribution and drug release in comparison with the pure drug and the probe. In addition, the marketed taxol administration system utilize the toxic chremophor EL substance for the solubilization of taxol. The developed system is water soluble and thus it can be administered in aqueous solution overcoming the use of chremophor [22]. Similarly, tumor-targeted photodynamic therapy using polymeric photosensitizers of poly (N-(2-hydroxypropyl) methacrylamide) conjugated pyropheophorbide-a was found to be a significant strategy for cancer treatment. As the majority of the nanotheranostics, the copolymeric NPs can act as encouraging therapeutic strategy in oncology field. In further, nanomicelles showed high tumor accumulation, antitumor effect under irradiation using normal xenon light source of endoscope, and clear tumor imaging profiles even in the metastatic lung cancer [226]. Shi et al. fabricated fluorescent organic NPs based on dopamine containing copolymers (poly(AC-co-PEGMA)). PEI was also mixed and the NPs demonstrated great water dispersibility, strong green fluorescence and desirable biocompatibility [227]. Polyacrylamide hydrogel NPs were surface modified and conjugated with oxygen indicator and PEG groups as photoacoustic oxygen imaging nanosystem for tumor targeting and detection. The prepared nanosystem provides an in vivo non-invasive imaging and assesement method of hypoxic tumor microenvironments. This method is crucial for the evaluation of cancer progression, metastasis and treatment [228].
PVP has been also used as pharmaceutical excipient. Herein, authors present ternary copper-based chalcogenide nanotheranostics which combine high photothermal conversion efficiency and a simultaneous ROS generation effect. Except these facts, the nanoplatforms also revealed remarkable contrast enhancement and thus they can act as multifunctional nanotheranostic agents for photoacoustic imaging, photothermal/photodynamic cancer therapy [36]. Pluronics are copolymers of polyethylene- and polypropylene oxide widely used in pharmaceutical industry. In this work, authors fabricated Pluronic coated gold NPs loaded with IR780 iodide dye as combined PDT and PTT activity with surface-enhanced resonance Raman scattering imaging facility. The nanosystem exhibited improved water-solubility, stability and NPs accumulation in Plu-IR780 by murine colon carcinoma cells (C-26). Finally, the NPs indicated that simultaneous PDT and PTT activity can be achieved [229]. Another work involved the preparation of folic acid armed polymeric core–shell iron oxide NPs as a new type of nanotheranostic agent. The NPs were further coated hyperbranched polyglycerol aiming to improve their biocompatibility. Folic acid was used to target folate receptors overexpressing on cancer cells. The results showed that NPs can be applied as cancer nanotheranstics [230].
3.2.2. Liposomes
Liposomes are structured biocompatible and biodegradable lipid carriers. Some liposomes have been approved from FDA [231]. Liposomes comprised from one or more layers of natural or synthetic lipids and an aqueous core. They have been utilized as carriers for many active molecules either hydrophilic or lipophilic [232,233]. Radio-labeled liposomes with radionuclides such as 67Ga,111In and 99mTc present diagnostic, monitoring and therapeutic functions. Besides their imaging ability due to the radio-labeling, such liposomes are an excellent tool for choosing the best therapeutic action in individual patients [234,235]. A research group radio-labeled iminothiolane-Tc-tricarbonyl complex a model liposome system. The system mimics the EpaxalⓇ and Inflexal VⓇ which are FDA approved liposomal drugs [236].
In most cases, liposomes are funtionalized or coated with active molecules such as PEG, vitamins which can induce their biocompatibility. For example, vitamin E TPGS-coated liposomes are widely prepared [237]. In further, PEG-coated and folate-PEG-coated long-circulating and pH-sensitive liposomes loaded with 159Gd and poly-l-lysine were studied as cancer nanotheranostics. The prepared liposomes provide increased animal survival and high tumor uptake [238]. Liposomes and PEGylated liposomes were functionalized with gadolinium (III) diethylenetriamine pentaacetic acid salt which acted as MRI contrast and zinc phthalocyanine as a model photosensitizer. The results showed that the liposomal formulations can serve as imagine agents [239]. A novel system comprised from liposomes containing NIR carbon dots and the anticancer drug, cinobufagin, was developed and evaluated as potential anticancer nanotheranostics. Bioimaging of the prepared system was significantly high whereas liposomes could be uptaken by cells and delivered to the tumor site. Moreover, a prolonged release behavior and high anticancer activity was recorded [240]. The theranostic liposomes were functionalized with arginine-glycine-aspartic acid-Tocopheryl succinate and loaded with docetaxel and quantum dots by Sonali et al. A prolonged drug release and biocompatibility was achieved revealing that the nanosystem can serve as brain nanotheranostic [241]. Another promising study involves the application of ultrasmall iridium nanocrystals into stealth liposomal features. The developed system showed efficient photothermal conversion ability, since the iridium nanocrystals present effective NIR responsive catalytic activity towards H2O2 decomposition. In addition, the system exhibited improved blood circulation which enhances sufficient retention in mice tumors. Thus, it could be used in enhancing cancer radiotherapy [242]. Sheng et al. prepared anticancer nanotheranostics by using the cytocombatible NIR dye, ICG. They entrapped PFOB in nanoliposomes which revealed in vivo CT contrast imaging. Moreover, the formulation after intravenous administration hindered completely the MDA-MB-231 tumor growth due to excellent oxygen carrying ability of PFOB, which effectively attenuated tumor hypoxia, improved the efficiency of collisional energy transfer between ICG and oxygen and reduced the expression of heat shock protein [243]. Stimuli-responsive liposomes were also produced featuring diagnosis and treatment features. In fact, ROS-responsive liposome were designed showing light scatter and fluorescence intensity. Authrs inserted lipid oxidation sensor, C11-BODIPY (581/591), to liposomal bilayer to provide ratiometric fluorescent nanoprobe for ROS detection. Afterwards, Mitoxantrone-chemotherapeutic substance, was loaded into C11-BODIPY (581/591) functionalized liposome. This novel system exhibited prolonged release, improved anticancer activity and imaging ability [244]. Finally, multifunctional RNA-loaded magnetic liposomes were prepared as an early biomarker of treatment response. The iron oxide loaded RNA-liposomes deliver RNA to dendritic cells, activate those dendritic cells, and enable prediction of tumor regression with MRI [245].
3.2.3. Dendrimers
Dendrimers are the most significant nanostructured materials due to their external groups, which can be modified with antibodies, peptides or proteins. Dendrimers which own their name to the Greek word “dendro-tree”, show a structure of tree-like arms or branches [246,247].
An interesting study reports the use of multifunctional polymeric dedrimers comprised from a copolymer of Boltorn H40, PCL and P(oligo(ethylene glycol) monomethyl ether methacrylate-co-3-azidopropyl methacrylate) as cancer targeted drug delivery and MRI contrast. In fact it was further modified with alkynyl-functionalized cancer cell-targeting moieties, alkynyl-folate, and T1-type MRI contrast agents, alkynyl-DOTA–Gd (DOTA is 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetrakisacetic acid). Moreover, the dendritic polymers were loaded with paclitaxel. It was revealed that the drug was released in controlled manner while in vivo MRI imaging in rats showed desirable distribution of unimolecular micelles within rat liver and kidney, prominent positive contrast enhancement, and relatively long duration of blood circulation. Thus, they can be used as alternative nanotheranostics [248]. In another study authors employed dendrimers as in vivo anti-lymphoma efficient system which also can act as diagnostic tool due to the fluorescent dye conjugation in the polymer core. Doxorubicin was used as anticancer drug, and the dendrimers were studied in murine models of malignant lymphomas including one cell line-derived xenograft and two patient-derived lymphoma xenografts (VFN-D1 and VFN-M2) [249]. Polydopamine (PDA) coated magnetite NPs were loaded in dendrimers and they were characterized as chemo- and photothermal therapeutics and imaging agents by MRI and CT-PTT of the liver cancer cells. In vivo studies exhibited that the dual therapeutics can provoke apoptosis and thus is a promising and smart system [250].
Similarly, doxorubicin and indodicarbocyanine fluorescent dye was attached to dendritic polyglycerol nanocarriers. The results exhibited that the dendritic carriers were monitored in real time in intact cancer cells revealing its potential [251]. Poly(amidoamine) (PAMAM) are the most frequently used polymers as dendrimers. Carbon quantum dots from sweet lemon peel conjugated with PAMAM dendrimers to act as nanotheranostics to triple negative breast cancer. A peptide, known as arginine–glycine–aspartic acid (RGD) peptide was further conjugated to the system to target integrin which is over expressed in the specific cancer. It was found out that the system is an alternative theranostic option since it can be sufficiently bind into cancer cells [252]. Another study included Gd-doped ferrite NPs which were further encapsulated into PAMAM dendrimers whereas doxorubicin was also added to enhance their anticancer activity. Almost 78% of doxorubicin released in the presence of a low-frequency alternating magnetic field and mildly acidic pH environment [253]. In a different study folate–PEG was attached in PAMAM dendrimers aimed to target folate receptors in cancer cells. This core was conjugated with Cadmium selenide/Zinc sulfide (CdSe/ZnS) quantum dots which are widely used as diagnostic tool. It was demonstrated that the folate coated dendritic system show high cell uptake, binding in the cell surface and doxorubicin released into the tumor cells. The developed nanosystem can act as bioimaging and therapeutic tool [254].
Multifunctional PAMAM dendrimers loaded with gold NPS which were coupled with α-tocopheryl succinate were developed as cancer targeted nanotheranostic agent for CT imaging and therapy. In addition, PAMAM dendrimers were modified with fluorescein isothiocyanate, PEG-modified α-TOS, as well as PEGylated folic acid which served as templates to synthesize Au nanoplatforms. It was found out the nanoplatfomrs exhibited sufficient targetability to cancer cells overexpressing FA receptors as well as were able to target CT imaging of the cancer cells in vitro and the xenografted tumor model in vivo [255]. Same team studied multifunctional dendrimer-entrapped gold NPs with alpha-tocopheryl succinate and arginine–glycine–aspartic acid peptide for targeted chemotherapy and CT imaging of cancer cells. Authors also used fluorescein isothiocyanate, RGD peptide coupled with PEG and PEG-linked α-TOS. The prepared nanotheranostics were stable in several conditions and can target to cancer cells overexpressing αvβ3 integrin. Moreover, they show improved growth inhibition of the cancer cells. Authors conclude that the system can be promisingly applied as nanoprobe [256].
4. Conclusions and authors’ opinion
Nanotheranostic field is an emerging research field which however has not still meet the clinical standards. This fact is arising from the complexity and the synergistic mechanisms of the used nanotheranostics. For instance, some of the systems although show great diagnostic efficacy, they lack of therapeutic ability. Other systems have shown important therapy index with low imaging ability. Nonetheless, the scientific community has conducted great efforts to translate these new nanotheranostics into clinical trials by examining various nanomaterials or modification techniques and evaluating their in vivo performance. As it is detailed in this work, most of the current studies evaluate the diagnostic and therapeutic application of nanotheranostics using in vivo animal models, showing promising results. Nonetheless, if the nanotheranostics applied on human individuals, their application mostly fails. This is correlated with the fact that other mechanisms lead the diffusion of nanotheranostics in animals and others in human. In the past, EPR effect believed as one of the leading phenomena affecting the diffusion of NPs in tumors. Hence, many researchers evaluate the passive targeting of NPs in animals. However, EPR effect is not prominent in the majority of human tumors. Thus, efficient animal models should be utilized when studying the nanotheranostics.
Another issue of nanotheranostics is their possible toxicity and safety when used in humans. Among the applied nanotheranostics, carbon nanotubes and metallic NPs have raised a concern of safety due to their slow degradation and in vivo fate. Consequently, various attempts have been performed as surface coating with biocompatible macromolecules as PEG, PLA and chitosan to enhance their suitability for future clinical use. Another idea is the use of nanotheranostics synthesized by biodegradable or biocompatible materials which have been clinically approved. Besides, several biocompatible polymers, liposomes or dendrimers have been clinically approved for other pharmaceutical applications. Hence, the modification or conjugation of already approved therapeutic formulations or materials with functional ligands which will improve their diagnostic index could be essential.
Compared to the past, more sophisticated nanotheranostics have been developed worldwide. However, a huge advancement should be done before their clinical application. As it is addressed, in this review the current nanotheranostics have been evaluated mostly for their in vitro performance. In other works, the in vivo imaging evaluation of nanotheranostics might be performed but their in vivo drug delivery was not attenuated. In further, the toxic profile of many of the developed nanotheranostics is absent. Therefore, the promising properties of nanotheranostics, which are discussed in this review, should be advanced more before their translational in clinical medicine. To conclude, although a great effort is still needed, the future of nanotheranostics utility in clinical practice is near.
Conflicts of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- 1.Siafaka P.I., Üstündağ Okur N., Karavas E., Bikiaris D.N. Surface modified multifunctional and stimuli responsive nanoparticles for drug targeting: current status and uses. Int J Mol Sci. 2016;17:1440. doi: 10.3390/ijms17091440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siafaka P.I., Betsiou M., Tsolou A., Angelou E., Agianian B., Koffa M. Synthesis of folate- pegylated polyester nanoparticles encapsulating ixabepilone for targeting folate receptor overexpressing breast cancer cells. J Mater Sci Mater Med. 2015;26:1–14. doi: 10.1007/s10856-015-5609-x. [DOI] [PubMed] [Google Scholar]
- 3.Chen X., Wang T., Le W., Huang X., Gao M., Chen Q. Smart sorting of tumor phenotype with versatile fluorescent Ag nanoclusters by sensing specific reactive oxygen species. Theranostics. 2020;10:3430–3450. doi: 10.7150/thno.38422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qi B., Crawford A.J., Wojtynek N.E., Talmon G.A., Hollingsworth M.A., Ly Q.P. Tuned near infrared fluorescent hyaluronic acid conjugates for delivery to pancreatic cancer for intraoperative imaging. Theranostics. 2020;10:3413–3429. doi: 10.7150/thno.40688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muthu M.S., Leong D.T., Mei L., Feng S.S. Nanotheranostics - application and further development of nanomedicine strategies for advanced theranostics. Theranostics. 2014;4:660–677. doi: 10.7150/thno.8698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pandey N., Menon J.U., Takahashi M., Hsieh J.T., Yang J., Nguyen K.T. Thermo-responsive fluorescent nanoparticles for multimodal imaging and treatment of cancers. Nanotheranostics. 2020;4:1–13. doi: 10.7150/ntno.39810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shao L., Li Q., Zhao C., Lu J., Li X., Chen L. Auto-fluorescent polymer nanotheranostics for self-monitoring of cancer therapy via triple-collaborative strategy. Biomaterials. 2019;194:105–116. doi: 10.1016/j.biomaterials.2018.12.021. [DOI] [PubMed] [Google Scholar]
- 8.Fu F., Wu Y., Zhu J., Wen S., Shen M., Shi X. Multifunctional lactobionic acid-modified dendrimers for targeted drug delivery to liver cancer cells: investigating the role played by PEG spacer. ACS Appl Mater Interfaces. 2014;6:16416–16425. doi: 10.1021/am504849x. [DOI] [PubMed] [Google Scholar]
- 9.Wang J.T.-W., Hodgins N.O., Al-Jamal W.T., Maher J., Sosabowski J.K., Al-Jamal K.T. Organ biodistribution of radiolabelled δγ T cells following liposomal alendronate administration in different mice tumour models. Nanotheranostics. 2020;4:71–82. doi: 10.7150/ntno.32876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Costa P.M., Wang J.T.W., Morfin J.F., Khanum T., To W., Sosabowski J. Functionalised carbon nanotubes enhance brain delivery of amyloid-targeting Pittsburgh compound B (PiB)-derived ligands. Nanotheranostics. 2018;2:168–183. doi: 10.7150/ntno.23125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mardhian D.F., Vrynas A., Storm G., Bansal R., Prakash J. FGF2 engineered SPIONs attenuate tumor stroma and potentiate the effect of chemotherapy in 3D heterospheroidal model of pancreatic tumor. Nanotheranostics. 2020;4:26–39. doi: 10.7150/ntno.38092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li G., Pei M., Liu P. pH/Reduction dual-responsive comet-shaped PEGylated CQD-DOX conjugate prodrug: synthesis and self-assembly as tumor nanotheranostics. Mater Sci Eng C. 2020;110 doi: 10.1016/j.msec.2020.110653. [DOI] [PubMed] [Google Scholar]
- 13.Guo F., Li G., Ma S., Zhou H., Chen X. Multi-Responsive Nanocarriers Based on β-CD-PNIPAM star polymer coated MSN-SS-Fc composite particles. Polymers (Basel) 2019;11:1716. doi: 10.3390/polym11101716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Govindasamy M., Manavalan S., Chen S.M., Rajaji U., Chen T.W., Al-Hemaid F.M.A. Determination of neurotransmitter in biological and drug samples using gold nanorods decorated f- MWCNTs modified electrode. J Electrochem Soc. 2018;165:B370–B377. [Google Scholar]
- 15.Muthumariappan A., Govindasamy M., Chen S.M., Sakthivel K., Mani V., Chen T.W. Determination of Non-Steroidal Anti-Inflammatory Drug (NSAID) azathioprine in human blood serum and tablet samples using Multi-Walled Carbon Nanotubes (MWCNTs) decorated manganese oxide microcubes composite film modified electrode. Int J Electrochem Sci. 2017;12:7446–7456. [Google Scholar]
- 16.Govindasamy M., Mani V., Chen S.M., Maiyalagan T., Selvaraj S., Chen T.W. Highly sensitive determination of non-steroidal anti-inflammatory drug nimesulide using electrochemically reduced graphene oxide nanoribbons. RSC Adv. 2017;7:33043–33051. [Google Scholar]
- 17.Karthik R., Govindasamy M., Chen S.M., Chen T.W., Vinoth Kumar J., Elangovan A. A facile graphene oxide based sensor for electrochemical detection of prostate anti-cancer (anti-testosterone) drug flutamide in biological samples. RSC Adv. 2017;7:25702–25709. [Google Scholar]
- 18.Keerthi M., Akilarasan M., Chen S.M., Kogularasu S., Govindasamy M., Mani V. One-pot biosynthesis of reduced graphene oxide/prussian blue microcubes composite and its sensitive detection of prophylactic drug dimetridazole. J Electrochem Soc. 2018;165:B27–B33. [Google Scholar]
- 19.Sung S.Y., Su Y.L., Cheng W., Hu P.F., Chiang C.S., Chen W.T. Graphene Quantum dots-mediated theranostic penetrative delivery of drug and photolytics in deep tumors by targeted biomimetic nanosponges. Nano Lett. 2019;19:69–81. doi: 10.1021/acs.nanolett.8b03249. [DOI] [PubMed] [Google Scholar]
- 20.Akilarasan M., Kogularasu S., Chen S.M., Govindasamy M., Chen T.W., Ali M.A. A green approach to the synthesis of well-structured prussian blue cubes for the effective electrocatalytic reduction of antiprotozoal agent coccidiostat nicarbazin. Electroanalysis. 2018;30:1661–1669. [Google Scholar]
- 21.Guan W., Ma J., Peng X., Chen K. Tailoring magnetic resonance imaging relaxivities in macroporous Prussian blue cubes. Dalt Trans. 2019;48:11882–11888. doi: 10.1039/c9dt02414j. [DOI] [PubMed] [Google Scholar]
- 22.Ferber S., Baabur-Cohen H., Blau R., Epshtein Y., Kisin-Finfer E., Redy O. Polymeric nanotheranostics for real-time non-invasive optical imaging of breast cancer progression and drug release. Cancer Lett. 2014;352:81–89. doi: 10.1016/j.canlet.2014.02.022. [DOI] [PubMed] [Google Scholar]
- 23.Filippousi M., Papadimitriou S.A., Bikiaris D.N., Pavlidou E., Angelakeris M., Zamboulis D. Novel core–shell magnetic nanoparticles for Taxol encapsulation in biodegradable and biocompatible block copolymers: preparation, characterization and release properties. Int J Pharm. 2013;448:221–230. doi: 10.1016/j.ijpharm.2013.03.025. [DOI] [PubMed] [Google Scholar]
- 24.Filippousi M., Altantzis T., Stefanou G., Betsiou M., Bikiaris D.N., Angelakeris M. Polyhedral iron oxide core–shell nanoparticles in a biodegradable polymeric matrix: preparation, characterization and application in magnetic particle hyperthermia and drug delivery. RSC Adv. 2013;3:24367. [Google Scholar]
- 25.Dasgupta A., Biancacci I., Kiessling F., Lammers T. Imaging-assisted anticancer nanotherapy. Theranostics. 2020;10:956–967. doi: 10.7150/thno.38288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pelaz B., Alexiou C., Alvarez-Puebla R.A., Alves F., Andrews A.M., Ashraf S. Diverse applications of nanomedicine. ACS Nano. 2017;11:2313–2381. doi: 10.1021/acsnano.6b06040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao Y., Fletcher N.L., Liu T., Gemmell A.C., Houston Z.H., Blakey I. In vivo therapeutic evaluation of polymeric nanomedicines: effect of different targeting peptides on therapeutic efficacy against breast cancer. Nanotheranostics. 2018;2:360–370. doi: 10.7150/ntno.27142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharma M., Dube T., Chibh S., Kour A., Mishra J., Panda J.J. Nanotheranostics, a future remedy of neurological disorders. Expert Opin Drug Deliv. 2019;16:113–128. doi: 10.1080/17425247.2019.1562443. [DOI] [PubMed] [Google Scholar]
- 29.Mog B., Asase C., Chaplin A., Gao H., Rajagopalan S., Maiseyeu A. Nano-antagonist alleviates inflammation and allows for MRI of atherosclerosis. Nanotheranostics. 2019;3:342–355. doi: 10.7150/ntno.37391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paul A., Hasan A., Kindi H.Al, Gaharwar A.K., Rao V.T.S., Nikkhah M. Injectable graphene oxide/hydrogel-based angiogenic gene delivery system for vasculogenesis and cardiac repair. ACS Nano. 2014;8:8050–8062. doi: 10.1021/nn5020787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ge J., Zhang Q., Zeng J., Gu Z., Gao M. Radiolabeling nanomaterials for multimodality imaging: new insights into nuclear medicine and cancer diagnosis. Biomaterials. 2020;228 doi: 10.1016/j.biomaterials.2019.119553. [DOI] [PubMed] [Google Scholar]
- 32.Han N., Yang Y., Wang S., Zheng S., Fan W. Polymer-based cancer nanotheranostics: retrospectives of multi-functionalities and pharmacokinetics. Curr Drug Metab. 2013;14:661–674. doi: 10.2174/1389200211314060003. [DOI] [PubMed] [Google Scholar]
- 33.Foroozandeh P., Aziz A.A. Insight into cellular uptake and intracellular trafficking of nanoparticles. Nanoscale Res Lett. 2018;13:339. doi: 10.1186/s11671-018-2728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thakor A.S., Gambhir S.S. Nanooncology: the future of cancer diagnosis and therapy. CA Cancer J Clin. 2013;63:395–418. doi: 10.3322/caac.21199. [DOI] [PubMed] [Google Scholar]
- 35.Yordanova A., Eppard E., Kürpig S., Bundschuh R., Schönberger S., Gonzalez-Carmona M. Theranostics in nuclear medicine practice. Onco Targets Ther. 2017;10:4821–4828. doi: 10.2147/OTT.S140671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hou M., Yan C., Chen Z., Zhao Q., Yuan M., Xu Y. Multifunctional NIR-responsive poly(vinylpyrrolidone)-Cu-Sb-S nanotheranostic agent for photoacoustic imaging and photothermal/photodynamic therapy. Acta Biomater. 2018;74:334–343. doi: 10.1016/j.actbio.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 37.Li M., Wu J., Ma M., Feng Z., Mi Z., Rong P. Alkyne- and Nitrile-Anchored Gold Nanoparticles for Multiplex SERS Imaging of Biomarkers in Cancer Cells and Tissues. Nanotheranostics. 2019;3:113–119. doi: 10.7150/ntno.30924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Syu W.J., Huang C.C., Hsiao J.K., Lee Y.C., Huang Y.T., Venkatesan P. Co-precipitation synthesis of near-infrared iron oxide nanocrystals on magnetically targeted imaging and photothermal cancer therapy via photoablative protein denature. Nanotheranostics. 2019;3:236–254. doi: 10.7150/ntno.24124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yao Y.Y., Gedda G., Girma W.M., Yen C.L., Ling Y.C., Chang J.Y. Magnetofluorescent carbon dots derived from crab shell for targeted dual-modality bioimaging and drug delivery. ACS Appl Mater Interfaces. 2017;9:13887–13899. doi: 10.1021/acsami.7b01599. [DOI] [PubMed] [Google Scholar]
- 40.Roobol S.J., Hartjes T.A., Slotman J.A., de Kruijff R.M., Torrelo G., Abraham T.E. Uptake and subcellular distribution of radiolabeled polymersomes for radiotherapy. Nanotheranostics. 2020;4:14–25. doi: 10.7150/ntno.37080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vithanarachchi S., Allen M. Strategies for target-specific contrast agents for magnetic resonance imaging. Curr Mol Imaginge. 2012;1:12–25. doi: 10.2174/2211555211201010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chrapko B.E., Chrapko M., Nocuń A., Stefaniak B., Zubilewicz T., Drop A. Role of 18F-FDG PET/CT in the diagnosis of inflammatory and infectious vascular disease. Nucl Med Rev. 2016;19:28–36. doi: 10.5603/NMR.2016.0006. [DOI] [PubMed] [Google Scholar]
- 43.Yen S.K., Padmanabhan P., Selvan S.T. Multifunctional iron oxide nanoparticles for diagnostics, therapy and macromolecule delivery. Theranostics. 2013;3:986–1003. doi: 10.7150/thno.4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Munir A., Wang J., Li Z., Zhou H.S. Numerical analysis of a magnetic nanoparticle-enhanced microfluidic surface-based bioassay. Microfluid Nanofluidics. 2010;8:641–652. [Google Scholar]
- 45.Gómez-Hens A., Fernández-Romero J.M., Aguilar-Caballos M.P. Nanostructures as analytical tools in bioassays. Trends Anal Chem. 2008;27:394–406. doi: 10.1016/j.trac.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ito A., Shinkai M., Honda H., Kobayashi T. Medical application of functionalized magnetic nanoparticles. J Biosci Bioeng. 2005;100:1–11. doi: 10.1263/jbb.100.1. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y., Kohler N., Zhang M. Surface modification of superparamagnetic magnetite nanoparticles and their intracellular uptake. Biomaterials. 2002;23:1553–1561. doi: 10.1016/s0142-9612(01)00267-8. [DOI] [PubMed] [Google Scholar]
- 48.Corr S.A., Rakovich Y.P., Gun'ko Y.K. Multifunctional Magnetic-fluorescent nanocomposites for biomedical applications. Nanoscale Res Lett. 2008;3:87–104. [Google Scholar]
- 49.Yan X., Song Y., Zhu C., Song J., Du D., Su X. Graphene quantum Dot–MnO2 nanosheet based optical sensing platform: a sensitive fluorescence “Turn Off–On” nanosensor for glutathione detection and intracellular imaging. ACS Appl Mater Interfaces. 2016;8:21990–21996. doi: 10.1021/acsami.6b05465. [DOI] [PubMed] [Google Scholar]
- 50.Wei J., Qiang L., Ren J., Ren X., Tang F., Meng X. Fluorescence turn-off detection of hydrogen peroxide and glucose directly using carbon nanodots as probes. Anal Methods. 2014;6:1922. [Google Scholar]
- 51.Ballinger J.R. Theranostic radiopharmaceuticals: established agents in current use. Br J Radiol. 2018;91 doi: 10.1259/bjr.20170969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Callahan R.J., Chilton H.M., Ponto J.A., Swanson D.P., Royal H.D., Bruce A.D. Procedure guideline for the use of radiopharmaceuticals 4.0. J Nucl Med Technol. 2007;35:272–275. doi: 10.2967/jnmt.107.044156. [DOI] [PubMed] [Google Scholar]
- 53.Lamb J., Holland J.P. Advanced Methods for Radiolabeling multimodality nanomedicines for SPECT/MRI and PET/MRI. J Nucl Med. 2018;59:382–389. doi: 10.2967/jnumed.116.187419. [DOI] [PubMed] [Google Scholar]
- 54.Man F., Gawne P.J., T.M. de Rosales R. Nuclear imaging of liposomal drug delivery systems: a critical review of radiolabelling methods and applications in nanomedicine. Adv Drug Deliv Rev. 2019;143:134–160. doi: 10.1016/j.addr.2019.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chinen A.B., Guan C.M., Ferrer J.R., Barnaby S.N., Merkel T.J., Mirkin C.A. Nanoparticle probes for the detection of cancer biomarkers, cells, and tissues by fluorescence. Chem Rev. 2015;115:10530–10574. doi: 10.1021/acs.chemrev.5b00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Edmonds S., Volpe A., Shmeeda H., Parente-Pereira A.C., Radia R., Baguña-Torres J. Exploiting the metal-chelating properties of the drug cargo for in vivo positron emission tomography imaging of liposomal nanomedicines. ACS Nano. 2016;10:10294–10307. doi: 10.1021/acsnano.6b05935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mody V., Siwale R. Application of nanoparticles in diagnostic imaging via ultrasonography. Internet J Med Updat - Ejournal. 2011;6 [Google Scholar]
- 58.Lipowska M., Klenc J., Taylor A.T., Marzilli L.G. fac-99mTc/Re-tricarbonyl complexes with tridentate aminocarboxyphosphonate ligands: suitability of the phosphonate group in chelate ligand design of new imaging agents. Inorganica Chim Acta. 2019;486:529–537. doi: 10.1016/j.ica.2018.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ng Q.K.T., Olariu C.I., Yaffee M., Taelman V.F., Marincek N., Krause T. Indium-111 labeled gold nanoparticles for in vivo molecular targeting. Biomaterials. 2014;35:7050–7057. doi: 10.1016/j.biomaterials.2014.04.098. [DOI] [PubMed] [Google Scholar]
- 60.Vivier D., Fung K., Rodriguez C., Adumeau P., Ulaner G.A., Lewis J.S. The influence of glycans-specific bioconjugation on the FcγRI binding and in vivo performance of 89 Zr-DFO-pertuzumab. Theranostics. 2020;10:1746–1757. doi: 10.7150/thno.39089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cuaron J.J., Hirsch J.A., Medich D.C., Rosenstein B.S., Martel C.B., Hirsch A.E. A proposed methodology to select radioisotopes for use in radionuclide therapy. Am J Neuroradiol. 2009;30:1824–1829. doi: 10.3174/ajnr.A1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yeong C.-.H., Cheng M., Ng K.-.H. Therapeutic radionuclides in nuclear medicine: current and future prospects. J Zhejiang Univ Sci B. 2014;15:845–863. doi: 10.1631/jzus.B1400131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hou X., Tanguay J., Vuckovic M., Buckley K., Schaffer P., Bénard F. Imaging study of using radiopharmaceuticals labeled with cyclotron-produced 99m Tc. Phys Med Biol. 2016;61:8199–8213. doi: 10.1088/0031-9155/61/23/8199. [DOI] [PubMed] [Google Scholar]
- 64.Ilem-Ozdemir D., Asikoglu M. Radioimaging and diagnostic applications. In: Senyigit T., Ozcan I., Ozer O., editors. Nanotechnology in progress pharmaceutical application. Research Signpost; 2012. pp. 163–176. Kerala, India. [Google Scholar]
- 65.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 66.Bor G., Mat Azmi I.D., Yaghmur A. Nanomedicines for cancer therapy: current status, challenges and future prospects. Ther Deliv. 2019;10:113–132. doi: 10.4155/tde-2018-0062. [DOI] [PubMed] [Google Scholar]
- 67.Azzawi M., Seifalian A., Ahmed W. Nanotechnology for the diagnosis and treatment of diseases. Nanomedicine. 2016;11:2025–2027. doi: 10.2217/nnm-2016-8000. [DOI] [PubMed] [Google Scholar]
- 68.Wang H.Y., Hsieh C.H., Wen C.N., Wen Y.H., Chen C.H., Lu J.J. Cancers screening in an asymptomatic population by using multiple tumour markers. PLoS ONE. 2016;11 doi: 10.1371/journal.pone.0158285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ahmed A.A., Abedalthagafi M. Cancer diagnostics: the journey from histomorphology to molecular profiling. Oncotarget. 2016;7 doi: 10.18632/oncotarget.11061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Filippousi M., Turner S., Leus K., Siafaka P.I., Tseligka E.D., Vandichel M. Biocompatible Zr-based nanoscale MOFs coated with modified poly(ε-caprolactone) as anticancer drug carriers. Int J Pharm. 2016;509:208–218. doi: 10.1016/j.ijpharm.2016.05.048. [DOI] [PubMed] [Google Scholar]
- 71.Wolinsky J.B., Colson Y.L., Grinstaff M.W. Local drug delivery strategies for cancer treatment: gels, nanoparticles, polymeric films, rods, and wafers. J Control Release. 2012;159:14–26. doi: 10.1016/j.jconrel.2011.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xu C., Chen F., Valdovinos H.F., Jiang D., Goel S., Yu B. Bacteria-like mesoporous silica-coated gold nanorods for positron emission tomography and photoacoustic imaging-guided chemo-photothermal combined therapy. Biomaterials. 2018;165:56–65. doi: 10.1016/j.biomaterials.2018.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sun X., Cai W., Chen X. Positron emission tomography imaging using radiolabeled inorganic nanomaterials. Acc Chem Res. 2015;48:286–294. doi: 10.1021/ar500362y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Coll J.-.L. Cancer optical imaging using fluorescent nanoparticles. Nanomedicine. 2011;6:7–10. doi: 10.2217/nnm.10.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee S.B., Kim H.L., Jeong H.-.J., Lim S.T., Sohn M.H., Kim D.W. Mesoporous silica nanoparticle pretargeting for pet imaging based on a rapid bioorthogonal reaction in a living body. Angew Chemie Int Ed. 2013;52:10549–10552. doi: 10.1002/anie.201304026. [DOI] [PubMed] [Google Scholar]
- 76.Li A., Zhao J., Fu J., Cai J., Zhang P. Recent advances of biomimetic nano-systems in the diagnosis and treatment of tumor. Asian J Pharm Sci. 2019 doi: 10.1016/j.ajps.2019.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mishra P., Nayak B., Dey R.K. PEGylation in anti-cancer therapy: an overview. Asian J Pharm Sci. 2016;11:337–348. [Google Scholar]
- 78.Danhier F. To exploit the tumor microenvironment: since the EPR effect fails in the clinic, what is the future of nanomedicine? J Control Release. 2016;244:108–121. doi: 10.1016/j.jconrel.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 79.Tee J.K., Yip L.X., Tan E.S., Santitewagun S., Prasath A., Ke P.C. Nanoparticles’ interactions with vasculature in diseases. Chem Soc Rev. 2019;48:5381–5407. doi: 10.1039/c9cs00309f. [DOI] [PubMed] [Google Scholar]
- 80.Zeng Q., Zhang R., Zhang T., Xing D. H2O2-responsive biodegradable nanomedicine for cancer-selective dual-modal imaging guided precise photodynamic therapy. Biomaterials. 2019;207:39–48. doi: 10.1016/j.biomaterials.2019.03.042. [DOI] [PubMed] [Google Scholar]
- 81.Cao H., Wang L., Yang Y., Li J., Qi Y., Li Y. An Assembled nanocomplex for improving both therapeutic efficiency and treatment depth in photodynamic therapy. Angew Chemie. 2018;130:7885–7889. doi: 10.1002/anie.201802497. [DOI] [PubMed] [Google Scholar]
- 82.Chen H., Zhao Y. Applications of light-responsive systems for cancer theranostics. ACS Appl Mater Interfaces. 2018;10:21021–21034. doi: 10.1021/acsami.8b01114. [DOI] [PubMed] [Google Scholar]
- 83.Zhang K., Zhang Y., Meng X., Lu H., Chang H., Dong H. Light-triggered theranostic liposomes for tumor diagnosis and combined photodynamic and hypoxia-activated prodrug therapy. Biomaterials. 2018;185:301–309. doi: 10.1016/j.biomaterials.2018.09.033. [DOI] [PubMed] [Google Scholar]
- 84.Santra S. Fluorescent silica nanoparticles for cancer imaging. Cancer Nanotechnol. 2010:151–162. doi: 10.1007/978-1-60761-609-2_10. [DOI] [PubMed] [Google Scholar]
- 85.Nagaya T., Nakamura Y.A., Choyke P.L., Kobayashi H. Fluorescence-guided surgery. Front Oncol. 2017;7:1–16. doi: 10.3389/fonc.2017.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li X., Schumann C., Albarqi H.A., Lee C.J., Alani A.W.G., Bracha S. A tumor-activatable theranostic nanomedicine platform for NIR fluorescence-guided surgery and combinatorial phototherapy. Theranostics. 2018;8:767–784. doi: 10.7150/thno.21209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gobbo O.L., Sjaastad K., Radomski M.W., Volkov Y., Prina-Mello A. Magnetic nanoparticles in cancer theranostics. Theranostics. 2015;5:1249–1263. doi: 10.7150/thno.11544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Amstad E., Textor M., Reimhult E. Stabilization and functionalization of iron oxide nanoparticles for biomedical applications. Nanoscale. 2011;3:2819–2843. doi: 10.1039/c1nr10173k. [DOI] [PubMed] [Google Scholar]
- 89.Shou P., Yu Z., Wu Y., Feng Q., Zhou B., Xing J., et al. Zn2+ doped ultrasmall prussian blue nanotheranostic agent for breast cancer photothermal therapy under mr imaging guidance. Adv Healthc Mater2019:1900948. [DOI] [PubMed]
- 90.Ren Q., Yang K., Zou R., Wan Z., Shen Z., Wu G. Biodegradable hollow manganese/cobalt oxide nanoparticles for tumor theranostics. Nanoscale. 2019;11:23021–23026. doi: 10.1039/c9nr07725a. [DOI] [PubMed] [Google Scholar]
- 91.Qin S., Caskey C.F., Ferrara K.W. Ultrasound contrast microbubbles in imaging and therapy: physical principles and engineering. Phys Med Biol. 2009;54:R27–R57. doi: 10.1088/0031-9155/54/6/R01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhou L.Q., Li P., Cui X.W., Dietrich C.F. Ultrasound nanotheranostics in fighting cancer: advances and prospects. Cancer Lett. 2019 doi: 10.1016/j.canlet.2019.11.034. [DOI] [PubMed] [Google Scholar]
- 93.Peek M.C., Wu F. High-intensity focused ultrasound in the treatment of breast tumours. Ecancermedicalscience. 2018;12:1–10. doi: 10.3332/ecancer.2018.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wan G.Y., Liu Y., Chen B.W., Liu Y.Y., Wang Y.S., Zhang N. Recent advances of sonodynamic therapy in cancer treatment. Cancer Biol Med. 2016;13:325–338. doi: 10.20892/j.issn.2095-3941.2016.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Malietzis G., Monzon L., Hand J., Wasan H., Leen E., Abel M. High-intensity focused ultrasound: advances in technology and experimental trials support enhanced utility of focused ultrasound surgery in oncology. Br J Radiol. 2013;86 doi: 10.1259/bjr.20130044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Qian X., Han X., Chen Y. Insights into the unique functionality of inorganic micro/nanoparticles for versatile ultrasound theranostics. Biomaterials. 2017;142:13–30. doi: 10.1016/j.biomaterials.2017.07.016. [DOI] [PubMed] [Google Scholar]
- 97.Durham A.L., Caramori G., Chung K.F., Adcock I.M. Targeted anti-inflammatory therapeutics in asthma and chronic obstructive lung disease. Transl Res. 2016;167:192–203. doi: 10.1016/j.trsl.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ding B., Siddiqui S., DePietro M., Petersson G., Martin U.J. Inhaler usability of a pressurized metered dose inhaler and a soft mist inhaler in patients with COPD: a simulated-use study. Chron Respir Dis. 2019;16 doi: 10.1177/1479972318787914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bahadori M., Mohammadi F. Nanomedicine for respiratory diseases. Tanaffos. 2012;11:18–22. [PMC free article] [PubMed] [Google Scholar]
- 100.Aillon K.L., El-Gendy N., Dennis C., Norenberg J.P., McDonald J., Berkland C. Iodinated nanoclusters as an inhaled computed tomography contrast agent for lung visualization. Mol Pharm. 2010;7:1274–1282. doi: 10.1021/mp1000718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Neuwelt A., Sidhu N., Hu C.A.A., Mlady G., Eberhardt S.C., Sillerud L.O. Iron-based superparamagnetic nanoparticle contrast agents for MRI of infection and inflammation. Am J Roentgenol. 2015;204:W302–W313. doi: 10.2214/AJR.14.12733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Loebinger M.R., Kyrtatos P.G., Turmaine M., Price A.N., Pankhurst Q., Lythgoe M.F. Magnetic resonance imaging of mesenchymal stem cells homing to pulmonary metastases using biocompatible magnetic nanoparticles. Cancer Res. 2009;69:8862–8867. doi: 10.1158/0008-5472.CAN-09-1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Roller J., Laschke M.W., Tschernig T., Schramm R., Veith N.T., Thorlacius H. How to detect a dwarf: in vivo imaging of nanoparticles in the lung. Nanomed Nanotechnol Biol Med. 2011;7:753–762. doi: 10.1016/j.nano.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 104.Yhee J., Im J., Nho R. Advanced therapeutic strategies for chronic lung disease using nanoparticle-based drug delivery. J Clin Med. 2016;5:82. doi: 10.3390/jcm5090082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lanza G.M., Jenkins J., Schmieder A.H., Moldobaeva A., Cui G., Zhang H. Anti-angiogenic nanotherapy inhibits airway remodeling and hyper-responsiveness of dust mite triggered asthma in the brown norway rat. Theranostics. 2017;7:377–389. doi: 10.7150/thno.16627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Al Faraj A., Sultana Shaik A., Afzal S., Al Sayed B., Halwani R. MR imaging and targeting of a specific alveolar macrophage subpopulation in LPS-induced COPD animal model using antibody-conjugated magnetic nanoparticles. Int J Nanomed. 2014;9:1491–1503. doi: 10.2147/IJN.S59394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Alzheimer Europe. Turkey 2013: the prevalence of dementia in Europe2017. https://www.alzheimer-europe.org/Policy-in-Practice2/Country-comparisons/2013-The-prevalence-of-dementia-in-Europe/Turkey.
- 108.Filippousi M., Siafaka P.I., Amanatiadou E.P., Nanaki S.G., Nerantzaki M., Bikiaris D.N. Modified chitosan coated mesoporous strontium hydroxyapatite nanorods as drug carriers. J Mater Chem B. 2015;3:5991–6000. doi: 10.1039/c5tb00827a. [DOI] [PubMed] [Google Scholar]
- 109.Cong W., Bai R., Li Y.F., Wang L., Chen C. Selenium nanoparticles as an efficient nanomedicine for the therapy of huntington's disease. ACS Appl Mater Interfaces. 2019;11:34725–34735. doi: 10.1021/acsami.9b12319. [DOI] [PubMed] [Google Scholar]
- 110.Durães F., Pinto M., Sousa E. Old drugs as new treatments for neurodegenerative diseases. Pharmaceuticals. 2018;11:44. doi: 10.3390/ph11020044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Soni S., Ruhela R.K., Medhi B. Nanomedicine in central nervous system (CNS) disorders: a present and future prospective. Adv Pharm Bull. 2016;6:319–335. doi: 10.15171/apb.2016.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sriramoju B., Kanwar R., Kanwar J. Nanomedicine based nanoparticles for neurological disorders. Curr Med Chem. 2014;21:4154–4168. doi: 10.2174/0929867321666140716095644. [DOI] [PubMed] [Google Scholar]
- 113.Yemisci M., Caban S., Fernandez-Megia E., Capan Y., Couvreur P., Dalkara T. Preparation and characterization of biocompatible chitosan nanoparticles for targeted brain delivery of peptides. In: Skaper, editor. Neurotrophic factors. Methods in molecular biology. Humana Press; New York: 2018. pp. 443–454. [DOI] [PubMed] [Google Scholar]
- 114.Sahin A., Yoyen-Ermis D., Caban-Toktas S., Horzum U., Aktas Y., Couvreur P. Evaluation of brain-targeted chitosan nanoparticles through blood–brain barrier cerebral microvessel endothelial cells. J Microencapsul. 2017;34:659–666. doi: 10.1080/02652048.2017.1375039. [DOI] [PubMed] [Google Scholar]
- 115.Chen Q., Du Y., Zhang K., Liang Z., Li J., Yu H. Tau-targeted multifunctional nanocomposite for combinational therapy of Alzheimer's disease. ACS Nano. 2018;12:1321–1338. doi: 10.1021/acsnano.7b07625. [DOI] [PubMed] [Google Scholar]
- 116.McDonagh B.H., Singh G., Hak S., Bandyopadhyay S., Augestad I.L., Peddis D. L-DOPA-coated manganese oxide nanoparticles as dual MRI contrast agents and drug-delivery vehicles. Small. 2016;12:301–306. doi: 10.1002/smll.201502545. [DOI] [PubMed] [Google Scholar]
- 117.Okur M.E., Karantas I.D., Okur N.U., Siafaka P.I. Hypertension in 2017: update in treatment and pharmaceutical innovations. Curr Pharm Des. 2017;23:6795–6814. doi: 10.2174/1381612823666170927123454. [DOI] [PubMed] [Google Scholar]
- 118.Okur M.E., Karantas I.D., Siafaka P.I. Diabetes mellitus: a review on pathophysiology, current status of oral medications and future perspectives. Acta Pharm Sci. 2017;55:61–82. [Google Scholar]
- 119.Katsuki S., Matoba T., Koga J., Nakano K., Egashira K. Anti-inflammatory nanomedicine for cardiovascular disease. Front Cardiovasc Med. 2017;4:1–13. doi: 10.3389/fcvm.2017.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Majmudar M.D., Yoo J., Keliher E.J., Truelove J.J., Iwamoto Y., Sena B. Polymeric nanoparticle PET/MR imaging allows macrophage detection in atherosclerotic plaques. Circ Res. 2013;112:755–761. doi: 10.1161/CIRCRESAHA.111.300576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.McCarthy J.R., Korngold E., Weissleder R., Jaffer F.A. A Light-activated theranostic nanoagent for targeted macrophage ablation in inflammatory atherosclerosis. Small. 2010;6:2041–2049. doi: 10.1002/smll.201000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.de Oliveira Gonçalves K., da Silva M.N., Sicchieri L.B., de Oliveira Silva F.R., de Matos R.A., Courrol L.C. Aminolevulinic acid with gold nanoparticles: a novel theranostic agent for atherosclerosis. Analyst. 2015;140:1974–1980. doi: 10.1039/c4an02166e. [DOI] [PubMed] [Google Scholar]
- 123.Han X., Xu K., Taratula O., Farsad K. Applications of nanoparticles in biomedical imaging. Nanoscale. 2019;11:799–819. doi: 10.1039/c8nr07769j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gustafson H.H., Holt-Casper D., Grainger D.W., Ghandehari H. Nanoparticle uptake: the phagocyte problem. Nano Today. 2015;10:487–510. doi: 10.1016/j.nantod.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liang S., Wang B., Li X., Chu R., Yu H., Zhou S., et al. In vivo pharmacokinetics, transfer and clearance study of graphene oxide by La/Ce dual elemental labelling method. NanoImpact2020:100213.
- 126.Kumar A., Pandey A.K., Singh S.S., Shanker R., Dhawan A. Cellular uptake and mutagenic potential of metal oxide nanoparticles in bacterial cells. Chemosphere. 2011;83:1124–1132. doi: 10.1016/j.chemosphere.2011.01.025. [DOI] [PubMed] [Google Scholar]
- 127.Tsoi K.M., MacParland S.A., Ma X.Z., Spetzler V.N., Echeverri J., Ouyang B. Mechanism of hard-nanomaterial clearance by the liver. Nat Mater. 2016;15:1212–1221. doi: 10.1038/nmat4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cao S., Tang R., Sudlow G., Wang Z., Liu K.K., Luan J. Shape-dependent biodistribution of biocompatible silk microcapsules. ACS Appl Mater Interfaces. 2019;11:5499–5508. doi: 10.1021/acsami.8b17809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li D., Zhuang J., He H., Jiang S., Banerjee A., Lu Y. Influence of particle geometry on gastrointestinal transit and absorption following oral administration. ACS Appl Mater Interfaces. 2017;9:42492–42502. doi: 10.1021/acsami.7b11821. [DOI] [PubMed] [Google Scholar]
- 130.Elci S.G., Jiang Y., Yan B., Kim S.T., Saha K., Moyano D.F. Surface charge controls the suborgan biodistributions of gold nanoparticles. ACS Nano. 2016;10:5536–5542. doi: 10.1021/acsnano.6b02086. [DOI] [PubMed] [Google Scholar]
- 131.Key J., Palange A.L., Gentile F., Aryal S., Stigliano C., Di Mascolo D. Soft discoidal polymeric nanoconstructs resist macrophage uptake and enhance vascular targeting in tumors. ACS Nano. 2015;9:11628–11641. doi: 10.1021/acsnano.5b04866. [DOI] [PubMed] [Google Scholar]
- 132.Anselmo A.C., Zhang M., Kumar S., Vogus D.R., Menegatti S., Helgeson M.E. Elasticity of nanoparticles influences their blood circulation, phagocytosis, endocytosis, and targeting. ACS Nano. 2015;9:3169–3177. doi: 10.1021/acsnano.5b00147. [DOI] [PubMed] [Google Scholar]
- 133.Zhang J., Shin M.C., David A.E., Zhou J., Lee K., He H. Long-circulating heparin-functionalized magnetic nanoparticles for potential application as a protein drug delivery platform. Mol Pharm. 2013;10:3892–3902. doi: 10.1021/mp400360q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Siafaka P.I., Okur M.E., Ayla Ş., Er S., Cağlar E.Ş., Okur N.Ü. Design and characterization of nanocarriers loaded with levofloxacin for enhanced antimicrobial activity; physicochemical properties, in vitro release and oral acute toxicity. Brazilian J Pharm Sci. 2019;55:1–13. [Google Scholar]
- 135.Huo S., Jin S., Ma X., Xue X., Yang K., Kumar A. Ultrasmall gold nanoparticles as carriers for nucleus-based Gene therapy due to size-dependent nuclear entry. ACS Nano. 2014;8:5852–5862. doi: 10.1021/nn5008572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Favi P.M., Gao M., Johana Sepúlveda Arango L., Ospina S.P., Morales M., Pavon J.J. Shape and surface effects on the cytotoxicity of nanoparticles: gold nanospheres versus gold nanostars. J Biomed Mater Res Part A. 2015;103:3449–3462. doi: 10.1002/jbm.a.35491. [DOI] [PubMed] [Google Scholar]
- 137.Feng Q., Liu Y., Huang J., Chen K., Huang J., Xiao K. Uptake, distribution, clearance, and toxicity of iron oxide nanoparticles with different sizes and coatings. Sci Rep. 2018;8:2082. doi: 10.1038/s41598-018-19628-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kim I.Y., Joachim E., Choi H., Kim K. Toxicity of silica nanoparticles depends on size, dose, and cell type. Nanomed Nanotechnol Biol Med. 2015;11:1407–1416. doi: 10.1016/j.nano.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 139.Gunduz N., Ceylan H., Guler M.O., Tekinay A.B. Intracellular accumulation of gold nanoparticles leads to inhibition of Macropinocytosis to reduce the endoplasmic reticulum stress. Sci Rep. 2017;7:40493. doi: 10.1038/srep40493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Guarnieri D., Sabella S., Muscetti O., Belli V., Malvindi M.A., Fusco S. Transport across the cell-membrane dictates nanoparticle fate and toxicity: a new paradigm in nanotoxicology. Nanoscale. 2014;6:10264. doi: 10.1039/c4nr02008a. [DOI] [PubMed] [Google Scholar]
- 141.Soenen S.J., Parak W.J., Rejman J., Manshian B. (Intra)cellular stability of inorganic nanoparticles: effects on cytotoxicity, particle functionality, and biomedical applications. Chem Rev. 2015;115:2109–2135. doi: 10.1021/cr400714j. [DOI] [PubMed] [Google Scholar]
- 142.Nguyen K.C., Seligy V.L., Massarsky A., Moon T.W., Rippstein P., Tan J. Comparison of toxicity of uncoated and coated silver nanoparticles. J Phys Conf Ser. 2013;429 [Google Scholar]
- 143.Feliu N., Docter D., Heine M., del Pino P., Ashraf S., Kolosnjaj-Tabi J. In vivo degeneration and the fate of inorganic nanoparticles. Chem Soc Rev. 2016;45:2440–2457. doi: 10.1039/c5cs00699f. [DOI] [PubMed] [Google Scholar]
- 144.Nanaki S., Siafaka P.I., Zachariadou D., Nerantzaki M., Giliopoulos D.J., Triantafyllidis K.S. PLGA/SBA-15 mesoporous silica composite microparticles loaded with paclitaxel for local chemotherapy. Eur J Pharm Sci. 2017;99:32–44. doi: 10.1016/j.ejps.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 145.Chen W.H., Luo G.F., Qiu W.X., Lei Q., Liu L.H., Wang S.B. Mesoporous silica-based versatile theranostic nanoplatform constructed by layer-by-layer assembly for excellent photodynamic/chemo therapy. Biomaterials. 2017;117:54–65. doi: 10.1016/j.biomaterials.2016.11.057. [DOI] [PubMed] [Google Scholar]
- 146.Muhammad F., Guo M., Wang A., Zhao J., Qi W., Guo Y. Responsive delivery of drug cocktail via mesoporous silica nanolamps. J Colloid Interface Sci. 2014;434:1–8. doi: 10.1016/j.jcis.2014.07.024. [DOI] [PubMed] [Google Scholar]
- 147.Croissant J.G., Zhang D., Alsaiari S., Lu J., Deng L., Tamanoi F. Protein-gold clusters-capped mesoporous silica nanoparticles for high drug loading, autonomous gemcitabine/doxorubicin co-delivery, and in vivo tumor imaging. J Control Release. 2016;229:183–191. doi: 10.1016/j.jconrel.2016.03.030. [DOI] [PubMed] [Google Scholar]
- 148.Chatzipanagis K., Iafisco M., Roncal-Herrero T., Bilton M., Tampieri A., Kröger R. Crystallization of citrate-stabilized amorphous calcium phosphate to nanocrystalline apatite: a surface-mediated transformation. CrystEngComm. 2016;18:3170–3173. [Google Scholar]
- 149.Draz M.S., Fang B.A., Zhang P., Hu Z., Gu S., Weng K.C. Nanoparticle-mediated systemic delivery of siRNA for treatment of cancers and viral infections. Theranostics. 2014;4:872–892. doi: 10.7150/thno.9404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kong F.Y., Zhang J.W., Li R.F., Wang Z.X., Wang W.J., Wang W. Unique roles of gold nanoparticles in drug delivery, targeting and imaging applications. Molecules. 2017;22:1445. doi: 10.3390/molecules22091445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Anglin E.J., Cheng L., Freeman W.R., Sailor M.J. Porous silicon in drug delivery devices and materials. Adv Drug Deliv Rev. 2008;60:1266–1277. doi: 10.1016/j.addr.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Li L., Liu T., Fu C., Liu H., Tan L., Meng X. Multifunctional silica-based nanocomposites for cancer nanotheranostics. J Biomed Nanotechnol. 2014;10:1784–1809. doi: 10.1166/jbn.2014.1886. [DOI] [PubMed] [Google Scholar]
- 153.Belz J., Castilla-Ojo N., Sridhar S., Kumar R. Radiosensitizing silica nanoparticles encapsulating docetaxel for treatment of prostate cancer. Cancer Nanotechnol. 2017:403–409. doi: 10.1007/978-1-4939-6646-2_26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kumar P., Yuvakkumar R., Vijayakumar S., Vaseeharan B. Cytotoxicity of phloroglucinol engineered silver (Ag) nanoparticles against MCF-7 breast cancer cell lines. Mater Chem Phys. 2018;220:402–408. [Google Scholar]
- 155.Thakur V., Kutty R.V. Recent advances in nanotheranostics for triple negative breast cancer treatment. J Exp Clin Cancer Res. 2019;38:430. doi: 10.1186/s13046-019-1443-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Palanisamy S., Wang Y.M. Superparamagnetic iron oxide nanoparticulate system: synthesis, targeting, drug delivery and therapy in cancer. Dalt Trans. 2019;48:9490–9515. doi: 10.1039/c9dt00459a. [DOI] [PubMed] [Google Scholar]
- 157.Zhang Q., Shan W., Ai C., Chen Z., Zhou T., Lv X. Construction of multifunctional Fe3O4-MTX@HBc nanoparticles for MR imaging and photothermal therapy/chemotherapy. Nanotheranostics. 2018;2:87–95. doi: 10.7150/ntno.21942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zarepour A., Zarrabi A., Khosravi A. Springer Singapore; Singapore: 2017. SPIONs as nano-theranostics agents. [Google Scholar]
- 159.Conde J., Bao C., Cui D., Baptista P.V., Tian F. Antibody–drug gold nanoantennas with Raman spectroscopic fingerprints for in vivo tumour theranostics. J Control Release. 2014;183:87–93. doi: 10.1016/j.jconrel.2014.03.045. [DOI] [PubMed] [Google Scholar]
- 160.Liu Z., Liang X.J. Nano-carbons as theranostics. Theranostics. 2012;2:235–237. doi: 10.7150/thno.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sanginario A., Miccoli B., Demarchi D. Carbon nanotubes as an effective opportunity for cancer diagnosis and treatment. Biosensors. 2017;7:1–23. doi: 10.3390/bios7010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Qin Y., Chen J., Bi Y., Xu X., Zhou H., Gao J. Near-infrared light remote-controlled intracellular anti-cancer drug delivery using thermo/pH sensitive nanovehicle. Acta Biomater. 2015;17:201–209. doi: 10.1016/j.actbio.2015.01.026. [DOI] [PubMed] [Google Scholar]
- 163.Chen Z., Ma L., Liu Y., Chen C. Applications of functionalized fullerenes in tumor theranostics. Theranostics. 2012;2:238–250. doi: 10.7150/thno.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Huang H., Liu M., Chen J., Mao L., Zeng G., Wen Y. Facile fabrication of carboxyl groups modified fluorescent C 60 through a one-step thiol-ene click reaction and their potential applications for biological imaging and intracellular drug delivery. J Taiwan Inst Chem Eng. 2018;86:192–198. [Google Scholar]
- 165.Zhu Y., Li J., Li W., Zhang Y., Yang X., Chen N. The biocompatibility of nanodiamonds and their application in drug delivery systems. Theranostics. 2012;2:302–312. doi: 10.7150/thno.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Su L.J., Wu M.S., Hui Y.Y., Chang B.M., Pan L., Hsu P.C. Fluorescent nanodiamonds enable quantitative tracking of human mesenchymal stem cells in miniature pigs. Sci Rep. 2017;7:45607. doi: 10.1038/srep45607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Goenka S., Sant V., Sant S. Graphene-based nanomaterials for drug delivery and tissue engineering. J Control Release. 2014;173:75–88. doi: 10.1016/j.jconrel.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 168.Yang K., Wan J., Zhang S., Zhang Y., Lee S.T., Liu Z. In vivo pharmacokinetics, long-term biodistribution, and toxicology of PEGylated graphene in mice. ACS Nano. 2011;5(1):516–522. doi: 10.1021/nn1024303. [DOI] [PubMed] [Google Scholar]
- 169.Üstündağ Okur N., Hökenek N., Okur M.E., Ayla Ş., Yoltaş A., Siafaka P.I. An alternative approach to wound healing field; new composite films from natural polymers for mupirocin dermal delivery. Saudi Pharm J. 2019;27:738–752. doi: 10.1016/j.jsps.2019.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Üstündaǧ-Okur N., Gökçe E.H., Bozbiyik D.I., Eǧrilmez S., Özer Ö., Ertan G. Preparation and in vitro-in vivo evaluation of ofloxacin loaded ophthalmic nano structured lipid carriers modified with chitosan oligosaccharide lactate for the treatment of bacterial keratitis. Eur J Pharm Sci. 2014;63:204–215. doi: 10.1016/j.ejps.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 171.Siafaka P.I., Mone M., Koliakou I.G., Kyzas G.Z., Bikiaris D.N. Synthesis and physicochemical properties of a new biocompatible chitosan grafted with 5-hydroxymethylfurfural. J Mol Liq. 2016;222:268–271. [Google Scholar]
- 172.Siafaka P.I., Titopoulou A., Koukaras E.N., Kostoglou M., Koutris E., Karavas E. Chitosan derivatives as effective nanocarriers for ocular release of timolol drug. Int J Pharm. 2015;495:249–264. doi: 10.1016/j.ijpharm.2015.08.100. [DOI] [PubMed] [Google Scholar]
- 173.Du K., Lei P., Dong L., Zhang M., Gao X., Yao S., et al. In situ decorating of ultrasmall Ag2Se on upconversion nanoparticles as novel nanotheranostic agent for multimodal imaging-guided cancer photothermal therapy. Appl Mater Today2019:100497.
- 174.Sachdev A., Matai I., Gopinath P. Carbon dots incorporated polymeric hydrogels as multifunctional platform for imaging and induction of apoptosis in lung cancer cells. Colloids Surf B Biointerfaces. 2016;141:242–252. doi: 10.1016/j.colsurfb.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 175.Karimi Z., Abbasi S., Shokrollahi H., Yousefi G., Fahham M., Karimi L. Pegylated and amphiphilic Chitosan coated manganese ferrite nanoparticles for pH-sensitive delivery of methotrexate: synthesis and characterization. Mater Sci Eng C. 2017;71:504–511. doi: 10.1016/j.msec.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 176.Manivasagan P., Nguyen V.T., Jun S.W., Hoang G., Mondal S., Kim H. Anti-EGFR antibody conjugated thiol chitosan-layered gold nanoshells for dual-modal imaging-guided cancer combination therapy. J Control Release. 2019;311–312:26–42. doi: 10.1016/j.jconrel.2019.08.007. [DOI] [PubMed] [Google Scholar]
- 177.Jia H.R., Jiang Y.W., Zhu Y.X., Li Y.H., Wang H.Y., Han X. Plasma membrane activatable polymeric nanotheranostics with self-enhanced light-triggered photosensitizer cellular influx for photodynamic cancer therapy. J Control Release. 2017;255:231–241. doi: 10.1016/j.jconrel.2017.04.030. [DOI] [PubMed] [Google Scholar]
- 178.Zarrin A., Sadighian S., Rostamizadeh K., Firuzi O., Hamidi M., Mohammadi-Samani S. Design, preparation, and in vitro characterization of a trimodally-targeted nanomagnetic onco-theranostic system for cancer diagnosis and therapy. Int J Pharm. 2016;500:62–76. doi: 10.1016/j.ijpharm.2015.12.051. [DOI] [PubMed] [Google Scholar]
- 179.Shen S., Li Y., Xiao Y., Zhao Z., Zhang C., Wang J. Folate-conjugated nanobubbles selectively target and kill cancer cells via ultrasound-triggered intracellular explosion. Biomaterials. 2018;181:293–306. doi: 10.1016/j.biomaterials.2018.07.030. [DOI] [PubMed] [Google Scholar]
- 180.Zhang Y., Zhang L., Lin X., Ke L., Li B., Xu L. Dual-responsive nanosystem for precise molecular subtyping and resistant reversal of EGFR targeted therapy. Chem Eng J. 2019;372:483–495. [Google Scholar]
- 181.Sukumar U.K., Bose R.J.C., Malhotra M., Babikir H.A., Afjei R., Robinson E. Intranasal delivery of targeted polyfunctional gold–iron oxide nanoparticles loaded with therapeutic microRNAs for combined theranostic multimodality imaging and presensitization of glioblastoma to temozolomide. Biomaterials. 2019;218 doi: 10.1016/j.biomaterials.2019.119342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zhang Y., Cheng J., Li N., Wang R., Huang G., Zhu J. A versatile theranostic nanoplatform based on mesoporous silica. Mater Sci Eng C. 2019;98:560–571. doi: 10.1016/j.msec.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 183.Zhu Y., Wang X., Chen J., Zhang J., Meng F., Deng C. Bioresponsive and fluorescent hyaluronic acid-iodixanol nanogels for targeted X-ray computed tomography imaging and chemotherapy of breast tumors. J Control Release. 2016;244:229–239. doi: 10.1016/j.jconrel.2016.08.027. [DOI] [PubMed] [Google Scholar]
- 184.Yu F., Zhu M., Li N., Ao M., Li Y., Zhong M. Imaging-guided synergistic targeting-promoted photo-chemotherapy against cancers by methotrexate-conjugated hyaluronic acid nanoparticles. Chem Eng J. 2020;380 [Google Scholar]
- 185.Wei D., Xue Y., Huang H., Liu M., Zeng G., Wan Q. Fabrication, self-assembly and biomedical applications of luminescent sodium hyaluronate with aggregation-induced emission feature. Mater Sci Eng C. 2017;81:120–126. doi: 10.1016/j.msec.2017.07.038. [DOI] [PubMed] [Google Scholar]
- 186.Leonel A.G., Mansur H.S., Mansur A.A.P., Caires A., Carvalho S.M., Krambrock K. Synthesis and characterization of iron oxide nanoparticles/carboxymethyl cellulose core-shell nanohybrids for killing cancer cells in vitro. Int J Biol Macromol. 2019;132:677–691. doi: 10.1016/j.ijbiomac.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 187.Mansur A.A.P., Caires A.J., Carvalho S.M., Capanema N.S.V., Carvalho I.C., Mansur H.S. Dual-functional supramolecular nanohybrids of quantum dot/biopolymer/chemotherapeutic drug for bioimaging and killing brain cancer cells in vitro. Colloids Surfaces B Biointerfaces. 2019;184 doi: 10.1016/j.colsurfb.2019.110507. [DOI] [PubMed] [Google Scholar]
- 188.Mallick N., Asfer M., Anwar M., Kumar A., Samim M., Talegaonkar S. Rhodamine-loaded, cross-linked, carboxymethyl cellulose sodium-coated super-paramagnetic iron oxide nanoparticles: development and in vitro localization study for magnetic drug-targeting applications. Colloids Surf A Physicochem Eng Asp. 2015;481:51–62. [Google Scholar]
- 189.Pei M., Jia X., Zhao X., Li J., Liu P. Alginate-based cancer-associated, stimuli-driven and turn-on theranostic prodrug nanogel for cancer detection and treatment. Carbohydr Polym. 2018;183:131–139. doi: 10.1016/j.carbpol.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 190.Peng N., Ding X., Wang Z., Cheng Y., Gong Z., Xu X. Novel dual responsive alginate-based magnetic nanogels for onco-theranostics. Carbohydr Polym. 2019;204:32–41. doi: 10.1016/j.carbpol.2018.09.084. [DOI] [PubMed] [Google Scholar]
- 191.Mohapatra S., Asfer M., Anwar M., Sharma K., Akhter M., Ahmad F.J. Doxorubicin loaded carboxymethyl Assam bora rice starch coated superparamagnetic iron oxide nanoparticles as potential antitumor cargo. Heliyon. 2019;5:e01955. doi: 10.1016/j.heliyon.2019.e01955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Jung E., Lee J., Jeong L., Park S., Lee M., Song C. Stimulus-activatable echogenic maltodextrin nanoparticles as nanotheranostic agents for peripheral arterial disease. Biomaterials. 2019;192:282–291. doi: 10.1016/j.biomaterials.2018.11.022. [DOI] [PubMed] [Google Scholar]
- 193.Siafaka P.I., Üstündağ Okur N., Mone M., Giannakopoulou S., Er S., Pavlidou E. Two different approaches for oral administration of voriconazole loaded formulations: electrospun fibers versus β-cyclodextrin complexes. Int J Mol Sci. 2016;17:282. doi: 10.3390/ijms17030282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Das M., Solanki A., Joshi A., Devkar R., Seshadri S., Thakore S. β-cyclodextrin based dual-responsive multifunctional nanotheranostics for cancer cell targeting and dual drug delivery. Carbohydr Polym. 2019;206:694–705. doi: 10.1016/j.carbpol.2018.11.049. [DOI] [PubMed] [Google Scholar]
- 195.Xu D., Liu M., Zou H., Huang Q., Huang H., Tian J. Fabrication of AIE-active fluorescent organic nanoparticles through one-pot supramolecular polymerization and their biological imaging. J Taiwan Inst Chem Eng. 2017;78:455–461. [Google Scholar]
- 196.Yang Z., He W., Zheng H., Wei J., Liu P., Zhu W. One-pot synthesis of albumin-gadolinium stabilized polypyrrole nanotheranostic agent for magnetic resonance imaging guided photothermal therapy. Biomaterials. 2018;161:1–10. doi: 10.1016/j.biomaterials.2018.01.026. [DOI] [PubMed] [Google Scholar]
- 197.Jin Y., Li Y., Ma X., Zha Z., Shi L., Tian J. Encapsulating tantalum oxide into polypyrrole nanoparticles for X-ray CT/photoacoustic bimodal imaging-guided photothermal ablation of cancer. Biomaterials. 2014;35:5795–5804. doi: 10.1016/j.biomaterials.2014.03.086. [DOI] [PubMed] [Google Scholar]
- 198.Burnouf T., Chen C.H., Tan S.J., Tseng C.L., Lu K.Y., Chang L.H. A bioinspired hyperthermic macrophage-based polypyrrole-polyethylenimine (Ppy-PEI) nanocomplex carrier to prevent and disrupt thrombotic fibrin clots. Acta Biomater. 2019;96:468–479. doi: 10.1016/j.actbio.2019.06.053. [DOI] [PubMed] [Google Scholar]
- 199.Semkina A.S., Abakumov M.A., Skorikov A.S., Abakumova T.O., Melnikov P.A., Grinenko N.F. Multimodal doxorubicin loaded magnetic nanoparticles for VEGF targeted theranostics of breast cancer. Nanomed Nanotechnol Biol Med. 2018;14:1733–1742. doi: 10.1016/j.nano.2018.04.019. [DOI] [PubMed] [Google Scholar]
- 200.Jiang Y., Cui D., Fang Y., Zhen X., Upputuri P.K., Pramanik M. Amphiphilic semiconducting polymer as multifunctional nanocarrier for fluorescence/photoacoustic imaging guided chemo-photothermal therapy. Biomaterials. 2017;145:168–177. doi: 10.1016/j.biomaterials.2017.08.037. [DOI] [PubMed] [Google Scholar]
- 201.Yang H.Y., Jang M.-.S., Li Y., Fu Y., Wu T.P., Lee J.H. Hierarchical tumor acidity-responsive self-assembled magnetic nanotheranostics for bimodal bioimaging and photodynamic therapy. J Control Release. 2019;301:157–165. doi: 10.1016/j.jconrel.2019.03.019. [DOI] [PubMed] [Google Scholar]
- 202.Yang C., Pang X., Chen W., Wang X., Lin G., Chu C. Environmentally responsive dual-targeting nanotheranostics for overcoming cancer multidrug resistance. Sci Bull. 2019;64:705–714. doi: 10.1016/j.scib.2019.04.019. [DOI] [PubMed] [Google Scholar]
- 203.Qian R., Maiti D., Zhong J., Xiong S., Zhou H., Zhu R. Multifunctional nano-graphene based nanocomposites for multimodal imaging guided combined radioisotope therapy and chemotherapy. Carbon N Y. 2019;149:55–62. [Google Scholar]
- 204.Rajkumar S., Prabaharan M. Multi-functional core-shell Fe3O4@Au nanoparticles for cancer diagnosis and therapy. Colloids Surfaces B Biointerfaces. 2019;174:252–259. doi: 10.1016/j.colsurfb.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 205.Elbialy N.S., Fathy M.M., AL-Wafi R., Darwesh R., Abdel-dayem U.A., Aldhahri M. Multifunctional magnetic-gold nanoparticles for efficient combined targeted drug delivery and interstitial photothermal therapy. Int J Pharm. 2019;554:256–263. doi: 10.1016/j.ijpharm.2018.11.021. [DOI] [PubMed] [Google Scholar]
- 206.Tong C., Zhang X., Fan J., Li B., Liu B., Daniyal M. PEGylated mBPEI-rGO nanocomposites facilitate hepotocarcinoma treatment combining photothermal therapy and chemotherapy. Sci Bull. 2018;63:935–946. doi: 10.1016/j.scib.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 207.Sun C., Wen L., Zeng J., Wang Y., Sun Q., Deng L. One-pot solventless preparation of PEGylated black phosphorus nanoparticles for photoacoustic imaging and photothermal therapy of cancer. Biomaterials. 2016;91:81–89. doi: 10.1016/j.biomaterials.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 208.Wu C., Li D., Wang L., Guan X., Tian Y., Yang H. Single wavelength light-mediated, synergistic bimodal cancer photoablation and amplified photothermal performance by graphene/gold nanostar/photosensitizer theranostics. Acta Biomater. 2017;53:631–642. doi: 10.1016/j.actbio.2017.01.078. [DOI] [PubMed] [Google Scholar]
- 209.Chen M.-.L., Gao Z.-.W., Chen X.-.M., Pang S.-.C., Zhang Y. Laser-assisted in situ synthesis of graphene-based magnetic-responsive hybrids for multimodal imaging-guided chemo/photothermal synergistic therapy. Talanta. 2018;182:433–442. doi: 10.1016/j.talanta.2018.02.030. [DOI] [PubMed] [Google Scholar]
- 210.Palao-Suay R., Martín-Saavedra F.M., Rosa Aguilar M., Escudero-Duch C., Martín-Saldaña S., Parra-Ruiz F.J. Photothermal and photodynamic activity of polymeric nanoparticles based on α-tocopheryl succinate-RAFT block copolymers conjugated to IR-780. Acta Biomater. 2017;57:70–84. doi: 10.1016/j.actbio.2017.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.He W., Yan J., Wang L., Lei B., Hou P., Lu W. A lanthanide-peptide-derived bacterium-like nanotheranostic with high tumor-targeting, -imaging and -killing properties. Biomaterials. 2019;206:13–24. doi: 10.1016/j.biomaterials.2019.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.You C., Wu H., Wang M., Gao Z., Sun B., Zhang X. Synthesis and biological evaluation of redox/NIR dual stimulus-responsive polymeric nanoparticles for targeted delivery of cisplatin. Mater Sci Eng C. 2018;92:453–462. doi: 10.1016/j.msec.2018.06.044. [DOI] [PubMed] [Google Scholar]
- 213.Siafaka P.I., Barmpalexis P., Lazaridou M., Papageorgiou G.Z., Koutris E., Karavas E. Controlled release formulations of risperidone antipsychotic drug in novel aliphatic polyester carriers: data analysis and modelling. Eur J Pharm Biopharm. 2015;94:473–484. doi: 10.1016/j.ejpb.2015.06.027. [DOI] [PubMed] [Google Scholar]
- 214.Zhang L., Zhang M., Zhou L., Han Q., Chen X., Li S. Dual drug delivery and sequential release by amphiphilic Janus nanoparticles for liver cancer theranostics. Biomaterials. 2018;181:113–125. doi: 10.1016/j.biomaterials.2018.07.060. [DOI] [PubMed] [Google Scholar]
- 215.Siafaka P.I., Barmbalexis P., Bikiaris D.N. Novel electrospun nanofibrous matrices prepared from poly(lactic acid)/poly(butylene adipate) blends for controlled release formulations of an anti-rheumatoid agent. Eur J Pharm Sci. 2016;88:12–25. doi: 10.1016/j.ejps.2016.03.021. [DOI] [PubMed] [Google Scholar]
- 216.Jing L., Liang X., Li X., Yang Y., Dai Z. Covalent attachment of Mn-porphyrin onto doxorubicin-loaded poly(lactic acid) nanoparticles for potential magnetic resonance imaging and pH-sensitive drug delivery. Acta Biomater. 2013;9:9434–9441. doi: 10.1016/j.actbio.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 217.Schleich N., Po C., Jacobs D., Ucakar B., Gallez B., Danhier F. Comparison of active, passive and magnetic targeting to tumors of multifunctional paclitaxel/SPIO-loaded nanoparticles for tumor imaging and therapy. J Control Release. 2014;194:82–91. doi: 10.1016/j.jconrel.2014.07.059. [DOI] [PubMed] [Google Scholar]
- 218.Guo Y., Ran Y., Wang Z., Cheng J., Cao Y., Yang C. Magnetic-responsive and targeted cancer nanotheranostics by PA/MR bimodal imaging-guided photothermally triggered immunotherapy. Biomaterials. 2019;219 doi: 10.1016/j.biomaterials.2019.119370. [DOI] [PubMed] [Google Scholar]
- 219.Das M., Duan W., Sahoo S.K. Multifunctional nanoparticle–EpCAM aptamer bioconjugates: a paradigm for targeted drug delivery and imaging in cancer therapy. Nanomed Nanotechnol Biol Med. 2015;11:379–389. doi: 10.1016/j.nano.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 220.Castellani C., Fedrigo M., Tavano R., Cappellini R., Fedeli C., Mognato M. Tumor-facing hepatocytes significantly contribute to mild hyperthermia-induced targeting of rat liver metastasis by PLGA-NPs. Int J Pharm. 2019;566:541–548. doi: 10.1016/j.ijpharm.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 221.Zhang C., Li J., Yang C., Gong S., Jiang H., Sun M. A pH-sensitive coordination polymer network-based nanoplatform for magnetic resonance imaging-guided cancer chemo-photothermal synergistic therapy. Nanomed Nanotechnol Biol Med. 2020;23 doi: 10.1016/j.nano.2019.102071. [DOI] [PubMed] [Google Scholar]
- 222.Boissenot T., Fattal E., Bordat A., Houvenagel S., Valette J., Chacun H. Paclitaxel-loaded PEGylated nanocapsules of perfluorooctyl bromide as theranostic agents. Eur J Pharm Biopharm. 2016;108:136–144. doi: 10.1016/j.ejpb.2016.08.017. [DOI] [PubMed] [Google Scholar]
- 223.Deng L., Xu Y., Sun C., Yun B., Sun Q., Zhao C. Functionalization of small black phosphorus nanoparticles for targeted imaging and photothermal therapy of cancer. Sci Bull. 2018;63:917–924. doi: 10.1016/j.scib.2018.05.022. [DOI] [PubMed] [Google Scholar]
- 224.Roy E., Patra S., Madhuri R., Sharma P.K. Stimuli-responsive poly(N-isopropyl acrylamide)-co-tyrosine@gadolinium: iron oxide nanoparticle-based nanotheranostic for cancer diagnosis and treatment. Colloids Surfaces B Biointerfaces. 2016;142:248–258. doi: 10.1016/j.colsurfb.2016.02.053. [DOI] [PubMed] [Google Scholar]
- 225.Kang C., Cho W., Park M., Kim J., Park S., Shin D. H2O2-triggered bubble generating antioxidant polymeric nanoparticles as ischemia/reperfusion targeted nanotheranostics. Biomaterials. 2016;85:195–203. doi: 10.1016/j.biomaterials.2016.01.070. [DOI] [PubMed] [Google Scholar]
- 226.Fang J., Šubr V., Islam W., Hackbarth S., Islam R., Etrych T. N-(2-hydroxypropyl) methacrylamide polymer conjugated pyropheophorbide-a, a promising tumor-targeted theranostic probe for photodynamic therapy and imaging. Eur J Pharm Biopharm. 2018;130:165–176. doi: 10.1016/j.ejpb.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 227.Shi Y., Xu D., Liu M., Fu L., Wan Q., Mao L. Facile preparation of water soluble and biocompatible fluorescent organic nanoparticles through the combination of RAFT polymerization and self-polymerization of dopamine. J Mol Liq. 2018;250:446–450. [Google Scholar]
- 228.Jo J., Lee C.H., Folz J., Tan J.W.Y., Wang X., Kopelman R. In vivo photoacoustic lifetime based oxygen imaging with tumor targeted G2 polyacrylamide nanosonophores. ACS Nano. 2019;13:14024–14032. doi: 10.1021/acsnano.9b06326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Nagy-Simon T., Potara M., Craciun A.M., Licarete E., Astilean S. IR780-dye loaded gold nanoparticles as new near infrared activatable nanotheranostic agents for simultaneous photodynamic and photothermal therapy and intracellular tracking by surface enhanced resonant Raman scattering imaging. J Colloid Interface Sci. 2018;517:239–250. doi: 10.1016/j.jcis.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 230.Mostaghasi E., Zarepour A., Zarrabi A. Folic acid armed Fe3O4-HPG nanoparticles as a safe nano vehicle for biomedical theranostics. J Taiwan Inst Chem Eng. 2018;82:33–41. [Google Scholar]
- 231.Xing H., Hwang K., Lu Y. Recent Developments of liposomes as nanocarriers for theranostic applications. Theranostics. 2016;6:1336–1352. doi: 10.7150/thno.15464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Shukla D., Chakraborty S., Singh S., Mishra B. Lipid-based oral multiparticulate formulations-advantages, technological advances and industrial applications. Expert Opin Drug Deliv. 2011;8:207–224. doi: 10.1517/17425247.2011.547469. [DOI] [PubMed] [Google Scholar]
- 233.Naseri N., Valizadeh H., Zakeri-Milani P. Solid lipid nanoparticles and nanostructured lipid carriers: structure, preparation and application. Adv Pharm Bull. 2015;5:305–313. doi: 10.15171/apb.2015.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.William T., Phillips A.B. et al. Radiolabeled liposomes as drug delivery nanotheranostics - drug delivery applications of noninvasive imaging - Wiley online library. Drug Deliv Appl Noninvasive Imaging2013.
- 235.Petersen A.L., Hansen A.E., Gabizon A., Andresen T.L. Liposome imaging agents in personalized medicine. Adv Drug Deliv Rev. 2012;64:1417–1435. doi: 10.1016/j.addr.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 236.Varga Z., Szigyártó I.C., Gyurkó I., Dóczi R., Horváth I., Máthé D. Radiolabeling and quantitative in vivo SPECT/CT imaging study of liposomes using the novel iminothiolane-99m Tc-tricarbonyl complex. Contrast Media Mol Imaging. 2017;2017:1–8. doi: 10.1155/2017/4693417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Muthu M.S., Feng S.-.S. Theranostic liposomes for cancer diagnosis and treatment: current development and pre-clinical success. Expert Opin Drug Deliv. 2013;10:151–155. doi: 10.1517/17425247.2013.729576. [DOI] [PubMed] [Google Scholar]
- 238.Soares D.C.F., De Sousa G.F., De Barros A.L.B., Cardoso V.N., De Oliveira M.C., Ramaldes G.A. Scintigraphic imaging and increment in mice survival using theranostic liposomes based on Gadolinium-159. J Drug Deliv Sci Technol. 2015;30:7–14. [Google Scholar]
- 239.Skupin-Mrugalska P., Sobotta L., Warowicka A., Wereszczynska B., Zalewski T., Gierlich P. Theranostic liposomes as a bimodal carrier for magnetic resonance imaging contrast agent and photosensitizer. J Inorg Biochem. 2018;180:1–14. doi: 10.1016/j.jinorgbio.2017.11.025. [DOI] [PubMed] [Google Scholar]
- 240.Ren W., Chen S., Liao Y., Li S., Ge J., Tao F. Near-infrared fluorescent carbon dots encapsulated liposomes as multifunctional nano-carrier and tracer of the anticancer agent cinobufagin in vivo and in vitro. Colloids Surfaces B Biointerfaces. 2019;174:384–392. doi: 10.1016/j.colsurfb.2018.11.041. [DOI] [PubMed] [Google Scholar]
- 241.Sonali Singh R.P., Sharma G., Kumari L., Koch B., Singh S. RGD-TPGS decorated theranostic liposomes for brain targeted delivery. Colloids Surfaces B Biointerfaces. 2016;147:129–141. doi: 10.1016/j.colsurfb.2016.07.058. [DOI] [PubMed] [Google Scholar]
- 242.Feng L., Dong Z., Liang C., Chen M., Tao D., Cheng L. Iridium nanocrystals encapsulated liposomes as near-infrared light controllable nanozymes for enhanced cancer radiotherapy. Biomaterials. 2018;181:81–91. doi: 10.1016/j.biomaterials.2018.07.049. [DOI] [PubMed] [Google Scholar]
- 243.Sheng D., Liu T., Deng L., Zhang L., Li X., Xu J. Perfluorooctyl bromide & indocyanine green co-loaded nanoliposomes for enhanced multimodal imaging-guided phototherapy. Biomaterials. 2018;165:1–13. doi: 10.1016/j.biomaterials.2018.02.041. [DOI] [PubMed] [Google Scholar]
- 244.Chen C., Gao K., Lian H., Chen C., Yan X. Single-particle characterization of theranostic liposomes with stimulus sensing and controlled drug release properties. Biosens Bioelectron. 2019;131:185–192. doi: 10.1016/j.bios.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 245.Grippin A.J., Wummer B., Wildes T., Dyson K., Trivedi V., Yang C. Dendritic cell-activating magnetic nanoparticles enable early prediction of antitumor response with magnetic resonance imaging. ACS Nano. 2019;13:13884–13898. doi: 10.1021/acsnano.9b05037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Nottelet B., Darcos V., Coudane J. Aliphatic polyesters for medical imaging and theranostic applications. Eur J Pharm Biopharm. 2015;97:350–370. doi: 10.1016/j.ejpb.2015.06.023. [DOI] [PubMed] [Google Scholar]
- 247.Aso E., Martinsson I., Appelhans D., Effenberg C., Benseny-Cases N., Cladera J. Poly(propylene imine) dendrimers with histidine-maltose shell as novel type of nanoparticles for synapse and memory protection. Nanomed Nanotechnol Biol Med. 2019;17:198–209. doi: 10.1016/j.nano.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 248.Li X., Qian Y., Liu T., Hu X., Zhang G., You Y. Amphiphilic multiarm star block copolymer-based multifunctional unimolecular micelles for cancer targeted drug delivery and MR imaging. Biomaterials. 2011;32:6595–6605. doi: 10.1016/j.biomaterials.2011.05.049. [DOI] [PubMed] [Google Scholar]
- 249.Etrych T., Daumová L., Pokorná E., Tušková D., Lidický O., Kolářová V. Effective doxorubicin-based nano-therapeutics for simultaneous malignant lymphoma treatment and lymphoma growth imaging. J Control Release. 2018;289:44–55. doi: 10.1016/j.jconrel.2018.09.018. [DOI] [PubMed] [Google Scholar]
- 250.Jędrzak A., Grześkowiak B.F., Coy E., Wojnarowicz J., Szutkowski K., Jurga S. Dendrimer based theranostic nanostructures for combined chemo- and photothermal therapy of liver cancer cells in vitro. Colloids Surfaces B Biointerfaces. 2019;173:698–708. doi: 10.1016/j.colsurfb.2018.10.045. [DOI] [PubMed] [Google Scholar]
- 251.Krüger H.R., Schütz I., Justies A., Licha K., Welker P., Haucke V. Imaging of doxorubicin release from theranostic macromolecular prodrugs via fluorescence resonance energy transfer. J Control Release. 2014;194:189–196. doi: 10.1016/j.jconrel.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 252.Ghosh S., Ghosal K., Mohammad S.A., Sarkar K. Dendrimer functionalized carbon quantum dot for selective detection of breast cancer and gene therapy. Chem Eng J. 2019;373:468–484. [Google Scholar]
- 253.Mekonnen T.W., Birhan Y.S., Andrgie A.T., Hanurry E.Y., Darge H.F., Chou H.Y. Encapsulation of gadolinium ferrite nanoparticle in generation 4.5 poly(amidoamine) dendrimer for cancer theranostics applications using low frequency alternating magnetic field. Colloids Surfaces B Biointerfaces. 2019;184 doi: 10.1016/j.colsurfb.2019.110531. [DOI] [PubMed] [Google Scholar]
- 254.Zhao Y., Liu S., Li Y., Jiang W., Chang Y., Pan S. Synthesis and grafting of folate–PEG–PAMAM conjugates onto quantum dots for selective targeting of folate-receptor-positive tumor cells. J Colloid Interface Sci. 2010;350:44–50. doi: 10.1016/j.jcis.2010.05.035. [DOI] [PubMed] [Google Scholar]
- 255.Zhu J., Zheng L., Wen S., Tang Y., Shen M., Zhang G. Targeted cancer theranostics using alpha-tocopheryl succinate-conjugated multifunctional dendrimer-entrapped gold nanoparticles. Biomaterials. 2014;35:7635–7646. doi: 10.1016/j.biomaterials.2014.05.046. [DOI] [PubMed] [Google Scholar]
- 256.Zhu J., Fu F., Xiong Z., Shen M., Shi X. Dendrimer-entrapped gold nanoparticles modified with RGD peptide and alpha-tocopheryl succinate enable targeted theranostics of cancer cells. Colloids Surfaces B Biointerfaces. 2015;133:36–42. doi: 10.1016/j.colsurfb.2015.05.040. [DOI] [PubMed] [Google Scholar]