Abstract
There are several therapeutic approaches in type 2 diabetes mellitus (T2DM). When diet and exercise fail to control hyperglycemia, patients are forced to start therapy with antidiabetic agents. However, these drugs present several drawbacks that can affect the course of treatment. The major disadvantages of current oral modalities for the treatment of T2DM are mainly depicted in the low bioavailability and the immediate release of the drug, generating the need for an increase in frequency of dosing. In conjugation with the manifestation of adverse side effects, patient compliance to therapy is reduced. Over the past few years nanotechnology has found fertile ground in the development of novel delivery modalities that can potentially enhance anti-diabetic regimes efficacy. All efforts have been targeted towards two main vital steps: (a) to protect the drug by encapsulating it into a nano-carrier system and (b) efficiently release the drug in a gradual as well as controllable manner. However, only a limited number of studies published in the literature used in vivo techniques in order to support findings. Here we discuss the current disadvantages of modern T2DM marketed drugs, and the nanotechnology advances supported by in vivo in mouse/rat models of glucose homeostasis. The generation of drug nanocarriers may increase bioavailability, prolong release and therefore reduce dosing and thus, improve patient compliance. This novel approach might substantially improve quality of life for diabetics. Application of metal nanoformulations as indirect hypoglycemic agents is also discussed.
Keywords: Type 2 diabetes mellitus, Nanotechnology, Hyperglycemia, Controlled release, In vivo
Graphical abstract
1. Introduction
According to the Centers of Disease Control and Prevention (CDC, USA) the rise of the diabetic patients between 1990 and 2010 almost tripled [1]. Recently it was shown that diabetes mellitus is the third leading cause of death in the United States accountable for 12% of deaths in 2010. Moreover, pre-diabetes as a risk factor raises by 2% the overall deaths attributable to diabetes [2]. Although this constitutes a small increase, it reflects the greater danger underlying diabetes mellitus mortality. International Diabetes Federation estimates that almost 415 million adults (aged 20–79 years) worldwide have diabetes mellitus [3]. In the next 20 years this estimate is expected to rise to 642 million [4]. If no further action is to be taken, the mortality related to diabetes will continue to increase in the following decades.
Diabetes is “roughly” classified into type 1 and type 2. Type 1 (T1DM) is characterized by the inability of pancreatic β-cells to produce insulin (presence of autoantibodies against pancreatic β-cells) while type 2 (T2DM) by the reduced insulin production by pancreas or the defective insulin action (insulin resistance) in the tissues (absence of autoantibodies against pancreatic β-cells). This is only a simplified classification. In fact, T2DM is a heterogeneous disease. It was recently proposed that diabetic patients can be stratified into 5 subgroups with differing disease progression and risk of diabetic complications and specifically to patients with: (a) severe autoimmune diabetes (SAID), (b) severe insulin-deficient diabetes (SIDD), (c) severe insulin-resistant diabetes (SIRD), (d) mild-obesity related diabetes (MORD) and (e) mild-age related diabetes (MARD) [5]. A more detailed stratification of diabetic patients could potentially prove a significant step in the development of precision medicine in diabetes.
The common pharmacological management of T2DM consists of metformin (first-line treatment), sylfonylureas that can replace metformin as a first-line treatment (for example if metformin is not tolerated by the patient, or if the patient has normal weight) but are most commonly used as second-line treatment, and thiazolidiediones-a third-line therapy that can alternative be used as second-line. Newer therapies include incretin mimetics/analogs and dipeptidylpeptidase-4 (DPP4) inhibitors that target the incretin axon (second-line treatment) [6] and sodium glucose cotransporters inhibitors 2 (SGLT2) that target the reabsoption of glucose by the kidneys and can be used as monotherapy (if metformin is not tolerated or indicated by the patient) or as second-line and third-line drugs [7]. The characteristics of these drugs are described in Table 1.
Table 1.
Current anti-diabetic treatment for T2DM (excluding insulin therapy).
| Drug class | Drugs | Treatment | Action | Side effects | Reference |
|---|---|---|---|---|---|
| Biguanide | Metformin | 1st line | Increase hepatic insulin sensitivity Increase uptake of glucose into peripheral cells | lactic acidosis, gastric discomfort, chest pain, allergic reactions | [8] |
| Reduce hepatic glucose production | |||||
| Sulfonylureas | 1st generation: acetohexamide, carbutamide, chlorpropamide, glycyclamide, metahexamide, tolazamide, tolbutamide | 1st/2nd line | Induce glucose independent insulin release from pancreatic beta-cells | Hypoglycemia, hyponatremia, water retention | [9] |
| 2nd generation: glibemclamide, glibornuride, gliclazide, glipizide, gliquidone, glisoxepide, glyclopyramide | |||||
| 3rd generation: glimepiride | |||||
| Thiazolidiediones | Pioglitazone, Rosiglitazone, Lobeglitazone | 2nd/3rd line | Activate PPARs – decrease insulin resistance | Water retention, heart failure | [10] |
| Incretin mimetics/ analogs | Exetatide, Lixisenatide, Dulaglutide, Liraglutide | 2nd line | Activate GLP-1 receptors on pancreatic beta-cells Enhance insulin secretion and synthesis |
Mild to moderate transient nausea and vomiting, headache, upper respiratory infection | [11] |
| DDP4 inhibitors | Sitagliptin, Saxagliptin, Vildagliptin, Linagliptin, Algogliptin | 2nd/3rd line | Stimulate insulin release | Nausea, diarrhoea, stomach pain, headache, sore throat, runny nose, skin reactions | [12] |
| SGLT2 | Canagliflozin, Dapagliflozin, Empagliflozin | 1st/2nd/3rd line | Increase glucose excretion | Diabetic ketoacidosis, genital and urinary tract infection, cancer, bone fracture and foot and leg amputation | [13] |
2. Nanotechnology in medicine
The term “Nanotechnology” is used to describe the manipulation of matter on an atomic, molecular and supramolecular scale where unique quantum mechanical effects take place. Thus, the reduction of at least one dimension at the nanoscopic scale (1–100 nm) involves the design, production, characterization and application of various nanoscale materials in different potential areas providing novel technological advances [14]. Nanoparticles (NPs) possess a series of excellent properties compared to their bulk structures as nano-materials become more dependent on its shape and size and interfaces are easier to be accessed [15]. As an example, metallic nanoparticles (NPs) exhibit characteristic colors depending on their nano-size and shape which can be extensively exploited in bioimaging applications [16].
The use of nanomaterials and nanodevices in the field of health and medicine, has open the door to the establishment of a new nanoscience area, this of nanomedicine. The advancements of nanotechnology in medicine can be summarised into three categories:
-
A.
Drug delivery/therapeutics: The development of novel nanomaterial-based carrier systems aims to the controllable and targeted release and bio-distribution of a pharmaceutical compound [17]. Nanotechnology is also applied in drug design in order to increase absorbability. For example, many drugs are mildly-water soluble while others are absorbed quickly and then removed from the body as waste before the active substances reach their optical concentration thus treatment can be ineffective. Furthermore, nanotechnology have aroused the attention due to its ability to generate particles that are attracted to specific types of cells (specifically to the diseased cells for a direct treatment, e.g. cancer cells). As an example of targeted delivery, researchers from the University of California have developed modular-multifunctional micelles containing a fluorophore or a fluorophore and a drug, that selectively attach to the atherosclerotic plaques in ApoE null mice [18]. Recently, stem cells nanovesicles were constructed that are attracted to an injury in order to increase the amount of stem cells delivered to the injured tissue [19]. Some NPs have unique properties that enables them to be directly used in therapy; magnetic NPs can induce heating of malignant cells without affecting the surrounding normal tissue [20] and silver and zinc-oxide NPs show effective antimicrobial activity and could potentially become alternatives to antibiotics [21].
-
B.
Diagnosis/imaging: Through nanomedicine, early detection, diagnosis and prevention of diseases can be improved by using certain NPs as labels for diagnostic tools and high-resolution imaging or substrates for the development of biosensors [22]. The application of nano-sensors will eventually lead to the production of highly sensitive biomedical devices for the fast and high throughput detection of disease biomarkers [23]. For example, the continuous glucose monitoring from sweat is feasible by extremely sensitive metal oxide nano-sensors [24]. Nanotechnology offers advantages in the area of diagnosis considering that the unique properties of some nanomaterials (biological, physical, optical, magnetic, chemical, structural properties) render them suitable for diagnostic imaging (tumor detection, atherosclerotic plaque imaging etc.) [25]. Modification of quantum mechanics at the nanoscale makes the NPs more adaptable to optical and magnetic feature than larger imaging materials. The size of the NP affects the color produced and thus labeling materials with differential color coding can be extremely useful during diagnostic tests. Moreover, the application of nanoscale magnetic materials generates enhanced MRI images with more details [26].
-
C.
Tissue repairing/biomaterials: Nanomaterials are used for the design of artificial cell and implants for the repair or reproduction of damaged tissues [27]. Nanotechnology allows the development of biocompatible scaffolds, mimicking extracellular matrix (ECM) complexity and functionality, which are used for tissue regeneration [28]. Moreover, nano-featured scaffolds are designed to encapsulate and control the spatiotemporal release of drugs (e.g. growth factors). Nanotechnology-based biomaterials (nano-coatings or nano-structured surfaces) are also used to overcome several issues of implant materials, such as bacterial adhesion or corrosion resistance, for example in orthopaedics [29].
Breakthroughs in Nanotechnology have strongly affected the scientific world mainly in the field of medicine since the size of NPs is similar to that of most biological molecules. Nanomedicine, in the form of nano-therapeutics, could be defined as an application of nanotechnology were NPs load the drug improving its therapeutic properties and reducing morbidity, while the drug can be delivered to targeted tissue with high efficacy. The most employed NPs for drug delivery are presented in Fig. 1.
Fig. 1.
An overview of the different nanocarriers used for the delivery of anti-diabetic drugs. In brief, liposomes are small spherical vesicles created from cholesterol and non-toxic phospholipids. Niosomes are multilameller vesicular structures of non-ionic surfactants. Solid lipids are made of solid lipids or lipid blends. Metallic NPs are nanosized metals that can easily conjugate with various biological agents. Nanospheres are matricial nanostructures of spherical shapes (usually polymeric); Polymeric micelles are core/shell structures formed by amphiphilic block copolymers. Chitosan NPs are NPs formed by the incorporation of a polyanion (e.g. such as tripolyphosphate) with chitosan. Porous silicon NPs are hollow NPs made of porous silicon.
Nanotechnology has been applied to a wide range of medical pathological conditions, such as cancer, Parkinson's disease, Alzheimer's disease, tuberculosis and diabetes mellitus [30]. In the case of diabetes, one of the most significant contributions of nanotechnology is the development of novel nano-sensors for the facile, accurate and sensitive blood glucose measurement [31]. Nanotechnology has enabled the design of robust insulin delivery vehicles which facilitate the direct transfer of insulin molecules into the bloodstream, bypassing the gastric acidic environment and thus offering an alternative to daily subcutaneous injections [32]. Moreover, nanotechnology is applied to the design of nanodrugs or bio-functional foods for prediabetes treatment [33].
Classification of type 2 diabetic patients into 5 subgroups magnifies the diversity of the disease that is generally characterised as a state of severe insulin deficiency or resistance. In fact, the 5-subgroup classification reveals that in more than 60% of the patients T2DM is not related to insulin resistance or deficiency but mainly to obesity. Almost 24% of the patients suffers from insulin deficiency (SAID and SIDD) and 15% from insulin resistance (SIRD) [5]. Nanomedicine can be applied for the management of T2DM subgroups. Specifically:
-
A.
Drug delivery: the past few years nanotechnology has found fertile ground in the development of novel delivery modalities that can potentially enhance anti-diabetic regimes efficacy [34,35]. Various smart material formulations were generated that targeted towards two main vital steps: (a) to protect the drug by encapsulating it into a nano-carrier system and (b) efficiently release the drug in a gradual as well controllable manner. Thus, antidiabetic regimens that enhance insulin production or decrease insulin release can be administered to patients with SIRD and nano-formulations of insulin in patients with insulin deficiency (SAID and SIDD).
-
B.
Diagnosis (detection and drug delivery): Today therapy of diabetes is relying on “open-loop” delivery methods, where the patient administers the drug to his or herself at different times of the day. A most advanced approach is the “closed-loop” therapy, where the involvement of the patient in maintaining glucose control is minimal. A “closed-loop” system determines insulin or drug requirement in real time and delivers the proper dosage (for example the development of “synthetic pancreas” an external device that uses glucose sensors and pumps) [36]. The development of highly sensitive nano-sensors as well as nanomaterials that improve glucose sensor function will eventually improve the lives of patients living with diabetes (T1 and T2DM).
Drug administration has been associated with various anatomical, physiological and chemical barriers [37]. These barriers can be overcome by NP-drug delivery systems that offer improved targeting of specific cells by utilizing different approaches: active, passive and stimuli-responsive targeting [38]. A typical example of passive targeting by nanocarriers is seen in cancer, where targeted drug delivery is of the essence in order to avoid damage to healthy tissues. Solid tumours are characterised by leaky blood vessels, a feature that is exploited by nanomedicines which, due to their small size, accumulate in tissues through leakage in the blood vessels. The nanocarrier cannot escape from the cancerous tumour due to the absence of effective lymphatic drainage. This mechanism is called enhanced permeability and retention (EPR) and drug concentration inside tumours can be increased even up to 100 times compared to free drug [39]. EPR is also seen in inflammatory diseases that are also characterised by blood vessel leakage of the inflamed tissues [40]. However, not all types of NPs take advantage of the EPR effect with micelles, polymers and liposomes representing the most potent candidates [41]. Active cell targeting requires the attachment of an appropriate ligand (antibody, peptide, aptamer etc.) on the surface of the nanocarrier which is recognised by receptors on the cells’ membranes. Ligand binding to the receptors facilitates the cellular uptake of the nanocarrier through endocytosis [42]. This strategy, however, relies on the overexpression of specific biomarkers on the cell membranes, usually seen in cancer cells. Until now there is a lack of clinical application mainly due to the limitations regarding the drug loss caused by lysosomal digestion following receptor-mediated endocytosis and the immunogenicity of the targeting ligand which can lead to accelerated blood clearance [43]. Finally, drug molecules can be incorporated within specific NPs formulations (such as lipid bilayers, polymers, etc.) and released at a particular time and location after exposure to a specific external stimulus (pH changes, heat, magnetism, ultrasound etc.). The external stimuli cause NP coating to undergo physicochemical structural changes and thus targeted drug release can be achieved. Controlled release formulations (CRF) (or sustained-release/action, prolonged action or long-acting) are designed to release the drug in a controlled manner over a prolonged period, ideally with a predetermined rate and at a specific location [44]. For the controlled release formulations, the concentration of the drug in the blood rises slowly allowing a broader concentration curve over time. An example of a CRF in diabetes is NPH insulin (also known as isophane insulin) that provides a sustained release of insulin over an extended period.
According to a recent review by Kalaydina et al. in the clinical setting smart NPs offer a wider therapeutic window, overcome multiple drug resistance and minimize off-target unwanted side-effects. They represent also a switchable pH-regulated system. On the other hand, CRF can improve compliance of patients by minimizing frequent drug intake. Controlled release of the drug can also extent symptoms relief. Common characteristics of smart NPs and CRF are their superior efficacy and decreased toxicity [41].
Nowadays, the majority of approved nano-systems (FDA and EMA) relate to cancer therapy [45]. Up until 2017, FDA has approved 50 nano-pharmaceuticals. Reporting the progress in nanomedicine, Vantola listed the FDA-approved nanodrugs available in clinical use as well as their clinical benefits [46]. Compared to the conventional formulations, the nanodrugs show reduced toxicity but fail to demonstrate improved efficacy. However, NPs are increasingly tested in the clinical setting in order to improve the efficiency of the treatment. A quick review in the major literature databases reveals that only in the last 5 years drugs’ nanocarriers and CRF have been tested also in diabetes, acne, psoriasis, pityriasis, osteoporosis, multiple sclerosis, pulmonary hypertension, atopic dermatitis, glaucoma, vaccination and infections. More than 50% of the NPs types in investigational drugs are liposomes and almost 20% polymers [46]. The current ongoing clinical trials are promising; many new nanomedicines present improved efficacy and should eventually come to market [47]. The growing application of nanotechnology in medicine should eventually improve the therapeutic and diagnostic arsenal in clinical practice.
In this article, applications of nanotechnology that aim in the treatment of T1DM along with nanoformulations of insulin will not be discussed. Indeed, current pharmacological nano-approach on diabetes mellitus focuses mostly on the development of “carriers” that regulate insulin release according to glucose blood level. Although insulin replacement therapy will be eventually applied in most type 2 diabetic patients, this review will only examine the current research on remedies used at the initial stages of T2DM. We focus on the drawbacks of the modern marketed drugs that hinder therapy that can potentially be overcome by the application of nanotechnology. We will also report the advantages and disadvantages in using experimental NPs as indirect hypoglycemic agents.
3. Aims of nanotechnology in prediabetes/T2DM treatment
Nanotechnology is applied to resolve major drawbacks of the modern marketed drugs that hinder therapy such as the limited bioavailability and the quick drug release into blood stream that consequently cause unwanted side effects (Fig. 2). To this purpose nanostructured-biomolecules and nanomaterials are synthesized to 1. Increase bioavailability by protecting oral drugs and ensuring safe reach to blood circulation from initial absorption in the gastrointestinal tract e.g. protection of GLP1 from enzymatic digestion (DPP4 enzymes) 2. Prolong drug release: (a) Maintain constant drug concentration; (b) Reduce frequency of dosing and therefore; (c) Improve patient compliance and 3. Reduce drug's potential side effects (combination of 1 + 2) such as hyperglycemia, weight gain, increase in insulin resistance, β-cells destruction, renal and cardiovascular complications.
Fig. 2.
Nanotechnology approach in T2DM treatment.
Zhang et al. praised the interplay of nanotechnology with pharmacology and (patho)-physiology for the development of high efficiency innovative drugs for cancer treatment [48]. The in-depth understanding of the pharmacological mechanisms in conjunction with the physiological characteristics of normal and disease states should also guide the design for nanodrugs in diabetes treatment. This conjunction (pharmacology, patho-physiology and nanotechnology) will potentially produce nanotechnology-based drug formulations with the desired functionalities for precision treatment in T2DM.
4. Biomolecule-based nanomaterials
The glucagon-like peptide (GLP-1), an incretin hormone that leads to glucose dependent insulin release and reduced glucagon release, is a particularly nano-attractive biomolecule that can be integrated with nanomaterials to produce hybrid systems. As mentioned above, GLP-1 belongs to the newer therapies targeting the incretin axis, presenting a potent insulinotropic effect. Nevertheless, GLP-1 antidiabetic activity is compromised by its instability in the gastrointestinal tract, its poor absorption efficiency and the rapid degradation by the DPP4 enzyme and therefore oral bioavailability remains a challenge for the pharmaceutical industry.
4.1. (GLP-1)-based nanomaterials
Kaasalainen et al. studied the absorption and desorption of GLP-1 peptide into porous silicon (PSi) NPs and pointed out the crucial role of synthesis conditions in maximizing the peptides release [49]. Araujo et al. loaded the GLP-1 peptide into three nanosystems composed by different biomaterials [(poly(lactide-co-glycolide) polymer (PLGA), Witepsol E85 lipid (solid lipid NPs, SLN) and PSi] and studied their permeability in vitro [50]. These nanosystems could be used for the oral delivery of the peptide, actively protecting it from degradation. Moreover, in order to prolong the peptide release, the authors coated their nanosystems with chitosan (CS). The size of the NPs varied significantly ranging from 183 ± 5 nm for the PLGA (w/o CS coating) to 363 ± 8 nm for the PSi+CS. The PSi±CS GLP-1 loading degree was multiple times higher than the other NPs (estimated to 95–120 higher). CS-coating sustained GLP-1 release and improved nanosystems interaction with the intestinal cells (Caco-2, HT29-MTX and Raji B monolayers). In vitro release tests mimicking the conditions in the gastrointestinal tract (stomach and small intestine; pH 1.2 for 2 h and pH 6.5 for 4 h, respectively) verified that coating of the NPs with CS sustained the release of GLP-1, even though total percentage of released GLP-1 was only 35% after 6 h.
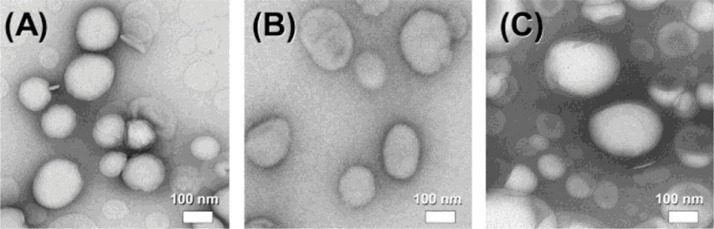
Several researchers have produced (GLP-1)-based nanomaterials and studied their hypoglycemic activity using animal models. Almost 10 year ago the GLP-1 peptide was integrated into liposomes [51]. Three different types of liposomal formulations were prepared (anionic, nonionic and cationic that were composed from DSPE-PG8G (distearoyl phosphatidyl ethanolamine-polyglyceline), DPPC (dipalmitoyl phosphatidylchorine), DPPG (dipalmitoyl phosphatidylglycerol), cholesterol or stearyl amine, respectively) that were spherical with mean diameters of 100–200 nm (Fig. 3). The liposomes where administered to healthy Wistar rats 15 min before the injection with a glucose solution (2 g/kg) (intraperitoneal glucose tolerance test, IPGTT). Amongst these formulations the anionic liposomes had the higher GLP-1 encapsulation efficiency (80.2% vs. 40.3% for the nonionic and 27.8% for the cationic liposomes) and led to almost a 50% reduction in the glucose ΔAUC0-120 compared with control. Anionic liposomal formulation advantage over the GLP-1 solution is attributed to its ability to induce higher serum GLP-1 level (by 260%) and higher insulin secretion (by 70%) [45]. Recently, Li et al. used self-assembling peptides (a class of peptides that spontaneously assembly and form nanostructures) to prevent degradation of GLP1 by DPP4 enzyme and renal clearance. Specifically, the authors modified GLP1 to produce self-assembling GLP1 derivatives. Two of these derivates (cadyglp1e and cadyglp1m that showed similar binding affinity compared to native-GLP1and improved stabilization and glucoregulatory properties) were tested in a series of in vitro and in vivo trials against native GLP1. It was shown that both cadyglp1e and cadyglp1m improve pharmacokinetic and pharmacodynamic profiles and exert a long-acting glucoregulatory activity by prolonging the presence of high insulin level and thus, reducing glucose concentration in blood [52].
Fig. 3.
Representative transmission electron microscopy images of the (A) anionic, (B) nonionic and (C) cationic CLP-1 formulations. Reprinted with permission from [51]. Copyright 2009 Elsevier B.V.
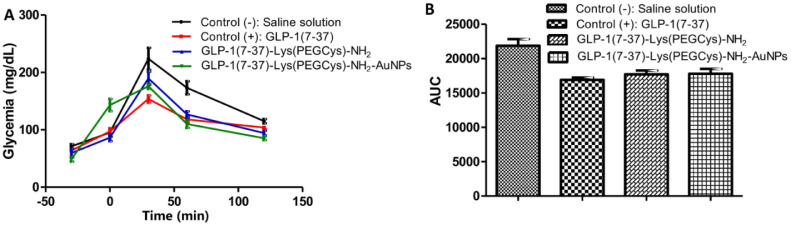
Gold NPs (AuNPs) are considered as suitable agents for drug delivery, since they are characterized as chemically inert and exert minimal toxicity [53,54]. Because the surface of colloid gold does not require modification for the chemisorption of ligands, AuNPs were used by Perez-Ortiz et al. to functionalize three new analogues of incretin GLP-1(7–37) [GLP-1(7–37)-Lys(Ac)-NH2, GLP-1(7–37)-Lys(Cys)-NH2 and GLP-1(7–37)-Lys(PEGCys)-NH2]. Moreover, to improve biocompatibility and increase absorption, the AuNPs were coated with polyethylene glycol (PEG). The conjugate GLP-1(7–37)-Lys(PEGCys)-NH2] was able to cross Caco-2 cells biological barriers and deliver the peptide at higher proportions than the other two so it was selected to examine the in vivo hypoglycemic activity in rats. The authors showed that both conjugates [GLP-1(7–37)- Lys (PEGCys)- NH2 and GLP-1(7–37)- Lys (PEGCys)- NH2-AuNPs] were able to produce a similar to the control [GLP-1(7–37)] decrease of AUC in normo-glycemic rats treated with 2 g/kg glucose. However, compared to the native and the analogue GLP-1, the conjugated GLP-1 analogue to AuNPs achieved a greater reduction in glucose level after 2 h of administration (Fig. 4). Thus, AuNPs manage to maintain a longer insulinotropic activity of the incretin [55].
Fig. 4.
(A) Blood glucose response after loading with 2 g/kg glucose and treatment with saline solution, GLP-1(7–37), GLP-1(7–37)-Lys(PEGCys)-NH2 and GLP-1(7–37)-Lys(PEGCys)-NH2 conjugated to AuNPs and (B) AUC obtained after data analysis from Fig. 5. Reprinted with permission from [55]. Copyright 2016 Elsevier B.V.
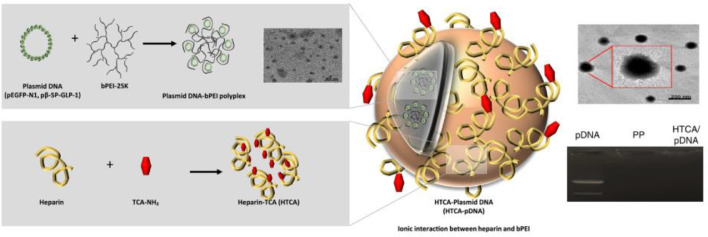
Another therapeutic approach for treating T2DM is the non-viral delivery of GLP-1 plasmid DNA (pDNA) complex through intravenous or oral routes [56,57]. In gene therapy the drug is a pDNA that codes a specific protein. Thus, the protein is manufactured by the cells of the small intestine and is delivered into the bloodstream [58]. Nurunnabi et al. synthesized a nano-sized gene complex [Heparin-Taurocholic acid (HTCA)-GLP1] that enabled them to prevent gastrointestinal degradation (Fig. 5). Moreover, the engineering of the complex allowed them to take advantage of the biological bile acid-based dietary lipid transport system to achieve active transport which, according to the authors, excels in terms of therapeutic efficacy passive transport [59]. Indeed, the peptide managed to normalize glucose level in a high-fat diet induced diabetic mouse model and a genetically engineered T2DM rat model.
Fig. 5.
Synthesis of the Heparin-Taurocholic acid (HTCA))-GLP1. Reprinted with permission from [59]. Copyright 2017 Elsevier B.V.
4.2. (GLP-1)-based nanomaterials/nano-DPP4 inhibitor carriers
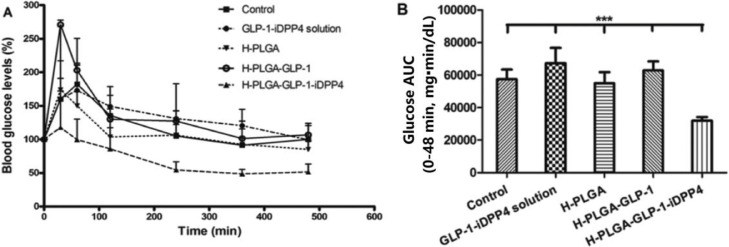
Since GLP-1 is rapidly degraded by the DPP4 enzyme, the incorporation of both GLP-1 and DPP4-inhibitors into a single delivery nanosystem would potentially present a higher efficacy than just the GLP-1 alone. In this regard Araujo et al. and Shrestha et al. produced two different dual-drug delivery nanosystems that were tested in the same animal model for T2DM. Specifically, the former used polymeric PLGA NPs functionalized with CS and a cell penetrating peptide (to facilitate cellular uptake) (Fig. 6) [60] while the later used chitosan-modified porous silicon (CSUn) NPs, which were coated by an gastro-resistant polymer (hydroxylpropyl methylcellulose acetate succinate, MF) [61]. The experimental T2DM model was induced by the administration of streptozotocin (STZ) (60 mg/kg) and nicotinamide (120 mg/kg) at 7 weeks Wistar rats. Shrestha et al. administered a higher amount of GLP-1 than Araujo et al. (250 µg/kg vs. 200 µg/kg, both were given as oral gavage) and showed that at 4 h the H-CSUn NPs reduces blood glucose level by 45% (∼80% vs. 125% of initial) and enhances pancreatic insulin content (6 × fold increase). In contrast, Araujo et al. showed a higher reduction (75%, 55% vs. 130% of initial) (Fig. 7) but no difference in pancreatic insulin content, when compared to the GLP-1 + iDPP4 solution.
Fig. 6.
Scanning electron microscopy image of the H-PGLA particles. Reprinted with permission from [60]. Copyright 2016 The Royal Society of Chemistry.
Fig. 7.
(A) Glycemic profile of T2DM-induced rats after oral administration of phthalate buffer solution (control), GLP-1 iDPP4 solution, H-PGLA particles, H-PGLA-GLP-1 particles and H-PGLA-GLP-1 iDPP4 particles and (B) AUC for a period of 6 h after oral administration. **P < 0.001, as compared with the H-PGLA-GLP-1 iDPP4. Reprinted with permission from [60]. Copyright 2016 The Royal Society of Chemistry.
4.3. Pituitary adenylate cyclase activating peptide
Pituitary adenylate cyclase activating peptide (PACAP) is a neuroendocrine peptide that belongs to the vasoactive intestinal peptide (VIP) family and exerts a significant role in carbohydrate and lipid metabolism [62,63]. It activates VPAC1 and 2 receptors that mediate the glucagon production and glucose-dependent insulin secretion, respectively. Thus, it represents a potential target in T2DM treatment. As a peptide it has a short half-life because it is susceptible to DPP4 degradation. In order to overcome these shortcomings, Zhao et al. produced novel peptide-conjugated selenium NPs specifically designed to bind to VPAC2 encapsulated with chitosan (named as SCD) to prolong release. Pharmacokinetic studies in mice relieved a remarkable prolongation of SCD circulating half-life (168.4 fold longer than PACAP). In db/db mice with type 2 diabetes SCD exerts a sustained hypoglycemic effect, comprising enhanced insulin secretion and low blood glucose level, which outmatches Exendin-4 (GLP-1 receptor agonist) [64].
5. Nano-drug carriers
Encapsulation of drugs into NPs aims to prolong drug release and presence into systemic circulation. Moreover, gradual (slow) release may improve drug uptake from target tissues and reduce toxicity.
Sulfonylureas are a class of antidiabetic drugs that act by enhancing insulin release from the beta cells in the pancreas. One of the most frequently used sulfonylureas is gliclazide which requires twice daily administration. Entrapment of gliclazide into a biodegradable and biocompatible carrier could offer a sustained release of the drug into circulation. In view of the above, Rathi et al. used niosomes to develop a delivery system that would prolong release of gliclazide and maximise its hypoglycemic activity. As mentioned above, niosomes are made up of spherical lipid bilayers (Fig. 1). Niosomes containing gliclazide and gliclazide pure drug were given orally to healthy Wistar rats after overnight fasting. Gliclazide produced a rapid reduction in glucose level (45%, 2 h post-administration) whereas gliclazide niosomes effect on blood glucose was slow and maximum reduction was achieved after 6 h (∼48%). Glucose concentration returned to normal 8–10 h after gliclazide administration. Gliclazide niosomes sustained low glucose level for more than 12 h and restoration to initial level was not achieved even 24 h post-administration (glucose level was 25% lower than normal) [65]. Glipizide, a second-class sulfonylurea that stimulates the release of insulin from pancreas, has a biological half-life of almost 3.5 h and is administered 2 or 3 times daily [66]. To extent its release and reduce dose frequency it was encapsulated into the biodegradable and biocompatible polymer, poly-E-caprolactone (PCL) [67]. When administered to STZ-induced diabetic rats glipizide loaded PCL NPs significantly lower glucose level compared to glipizide; although this difference tends to wear off by time (from 50 mg/dl 1 d post-treatment to less than 30 mg/dl on the 7th d). The dose of glipizide was 800 µg/kg (three times per day), for 7 d. In vitro release kinetic evaluation showed that the formulation's dissolution was slow; almost 25% cumulative release in the first 24 h to 65% at 168 h.
Niosomes were also used by two more studies to encapsulate metformin, a first-class drug that acts by decreasing glucose production by the liver and enhancing insulin sensitivity by the peripheral tissues [68,69]. Metformin hydrochloride (MH) oral bioavailability is estimated at 50% with a plasma elimination half-life of 6.2 h [8]. Sankhyan and Pawar showed that entrapment of MH into niosomes is achievable at a high degree and drug release is extended over a longer period of time [69]. The MH niosomes generated by Hasan et al., were smaller than of Sankhyan and Pawar (mean particle size ranged from 224 to 385 nm vs. 388 to 645 nm) but with equally high entrapment efficiency (ranged from 83% to 93% vs. 84% to 87%). Positively charged niosomes were selected over negatively or neutral charged niosomes for oral administration to STZ-nicotinamide induced type 2 diabetic rats due to their mild release of MH in the initial period. Maximum reduction in blood glucose level was reached 4 h later after oral administration of MH niosomes (for free MH was 1 h), was higher than of free MH (45.89% vs. 25.21%) and was sustained for a longer period; blood glucose returned to initial level 6–8 h post-administration compared to 2–4 h with the free drug solution (Fig. 8) [68].
Fig. 8.
Effects of the free MH solution or MH loaded niosomes on blood glucose levels of STZ-induced diabetic rats. Reprinted with permission from [68]. Copyright 2013 Taylor and Francis.
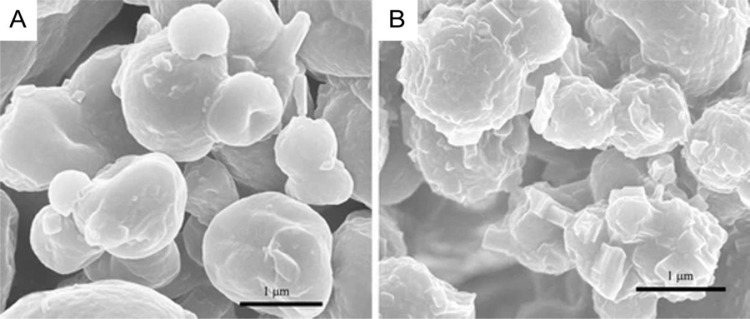
Manconi et al. loaded metformin into liposomes coated with chitosan cross linked with the biocompatible β-glycerolphosphate in order to increase the biomaterial stability in the gastrointestinal tract (Fig. 9). Their size was larger (4.730 ± 1.600 nm) than that of MH niosomes reported previously. MH liposomes presented enhanced oral bioavailability in Wistar rats almost twice as high as MH aqueous solution (38% vs. 20%). MH dosage range was 200–300 mg/kg body weight [70]. The study of MH loaded polymeric NPs has been reported in the past by Lekshmi and Reddy [71]. Unfortunately, neither Manconi et al. nor Lekshmi et al. included results from glucose homeostasis experiments during MH NP treatment in diabetic rats or from a glucose tolerance test in normoglycemic rats.
Fig. 9.
Scanning electron microscopy images of (A) CS-coated liposomes and (B) β-glycerolphosphate /CS microcomplexes. Reprinted with permission from [70]. Copyright 2013 Springer Nature.
Thiazolidinediones are a class of antidiabetic drug that reduce insulin resistance and thus increase glucose uptake in the peripheral tissues (skeletal muscle and adipose tissue) and suppress glucose release from the liver. Pioglitazone, a peroxisome proliferator-activated receptor (PPAR-γ agonist, increases transcription of insulin responsive genes and thus increases insulin sensitivity) falls into this class [72]. Haider et al. applied the response surface methodology (RSM) to optimize particle size, entrapment efficiency and release of pioglitazone nano-formulations [73]. Formulations size varied from 145 to 500 nm, entrapment efficiency from 67% to 84% and cumulative release from 70% to 95%. The hypoglycemic potential of the formulation with the lowest particle size (145 nm), highest entrapment efficiency (84%) and cumulative release (95%) was compared with pure pioglitazone (10 mg/kg) in STZ-induced diabetic rats. It was shown that pioglitazone niosomes achieves a greater reduction in glucose level than pure pioglitazone 24 h after oral administration. Although the potential antidiabetic effect of pioglitazone formulation is clear, the lack of continuous monitoring glucose level (instead for only one measurement at 24 h) conceals its precise biological effect.
Repaglinide is a hypoglycemic drug that belongs to the class of meglitinides. It is an antidiabetic agent that stimulates the release of insulin from pancreatic beta cells. It is rapidly metabolised by the liver resulting in poor oral bioavailability while its half-life in systemic blood circulation is about 1 h. Attempts have been made to generate carriers to sustain oral delivery. In 2010 Lekshmi et al. used a biodegradable polymer, poly (methyl methacrylate) (PMMA), to formulate repaglinide loaded PMMA NPs [74]. These NPs prolonged the in vitro release of the drug with no toxic effects in vivo. However, the authors did not present any data regarding the hypoglycaemic properties of the repaglinide loaded PMMA NPs. Another toxicological study for repaglinide-loaded SLNs showed that the surfactant type used during the nanocarrier preparation procedure effects the physicochemical properties of the SLNs that in terms affects the drug release profile [75]. The different surfactant formulations generated NPs which particle size ranged from 83 to 91 nm, their release rate from 1.12% to 1.63%/h and their maximum percent of drug depletion from 47% to 86%. Nevertheless, the ability of SLNs to reduce glucose level is not included in this study.
Efforts to prepare ripaglinide-loaded niosomes were also published [76]. Different surfactants and preparation methods were applied to generated carriers with the least particle size and high entrapment efficiency. This approach led to the formulation of nine different batches whose particle size ranged from 144 to 497 nm and their entrapment efficiency from 54% to 88%. The second batch (particle size 144 nm and entrapment efficiency 82%) was administered to STZ -induced diabetic rats to study the antihyperglycemic activity. Free repaglinide (2 mg/kg per oral) achieved maximum reduction in mean blood glucose level 4 h post-administration (by 70%) whereas niosomes formulation (2 mg/kg per oral) 8 h post-administration (by 68%). Both drugs led to blood glucose that was 30% lower than mean level in rats with normoglycemia. Hyperglycemia was restored in diabetic rats treated with free repaglinide after 8 h; no data were provided for the post 8 h effect of the niosomes formulation on glucose level [76].
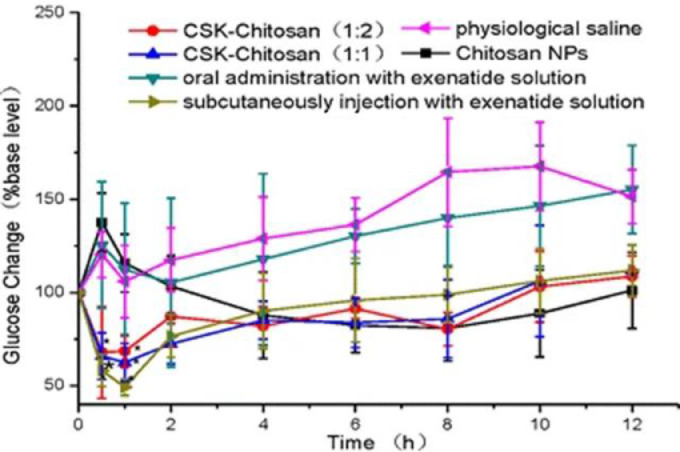
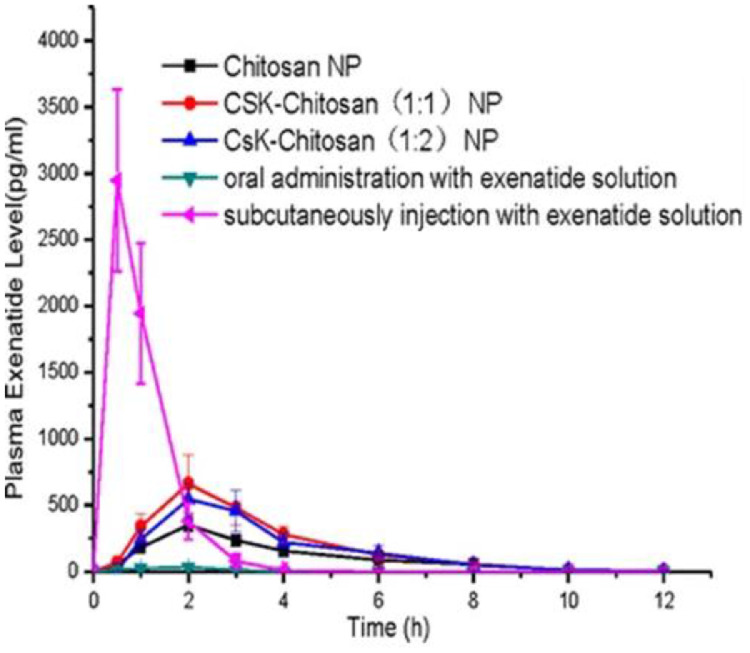
Exenatide is a synthetic version of exendin-4, an incretin analog found in lizard saliva. It is only administrated only by subcutaneous injection thus causing inconvenience and pain to the patients. Li et al. proposed that exenatide could be given orally if encapsulated into modified CS NPs conjugated with a goblet cell target peptide in order to increase effectiveness [77]. Loading into CS NPs a 6 times higher dose of exenatide than of that injected subcutaneously (30 µg/kg vs. 5.0 µg/kg) leads to a similar change in glucose blood level over a period of 12 h (Fig. 10). CS-NPs generated a gradual release of exenatide into the blood circulation (maximum level concentration 2 h post-administration) whereas subcutaneous injection produces an immediate acute rise at 0.5 h post-injection (Fig. 11) [77].
Fig. 10.
Glycemic profile of diabetic rats following the oral administration of CS-NPs, CSK-CS (1:1) NPs, CSK-CS(1:2) NPs (30.0 µg/kg exenatide), exenatide solution (7.5 µg/kg), physiological saline and SC injection with exenatide solution (5.0 µg/kg). Reprinted with permission from [77]. Copyright 2015 Springer Nature.
Fig. 11.
Plasma exenatide level in diabetic rats following the oral administration of CS-NPs, CSK-CS (1:1) NPs, CSK-CS(1:2) NPs (30.0 µg/kg exenatide), exenatide solution (50 IU/kg), with SC injection with exenatide solution (5.0 IU/kg) used as positive control. Reprinted with permission from [77]. Copyright 2015 Springer Nature.
Recent studies used different techniques to encapsulate vildagliptin, a DPP4 inhibitor, into polymer microspheres [78] or into DNA nanospheres [79]. The former orally treated STZ-induced diabetic rats with 2.5 mg/kg body weight pure or microsphere containing vildagliptin. Both drugs induced a similar hypoglycemic effect (up to 70% reduction in blood glucose level); however, they were achieved at different times (at 4 h and 8 h for pure vildagliptin and for vildagliptin containing microsphere, respectively). Furthermore, the vildagliptin containing microspheres maintained lower blood glucose concentration for a longer period compared to pure vildagliptin [78]. Baig et al. (2018) used Db/Db genetically altered mice, an in vivo model that closely resemble to human T2DM. Postprandial concentration of GLP-1 and blood glucose level were measured in mice were orally administered with Eudragit (a cationic polymer)-DNA nanospheres with (1 mg/kg/d) or without vildagliptin. Vildagliptin nanospheres preserved stable blood glucose level for up to 4 h, In the meantime and specifically at 3 h GLP-1 concertation increased more than 6-fold attributed to the Eudragit-DNA nanospheres properties: their small size, high vildagliptin loading efficiency and ability to bypass the acidic pH of the stomach and inhibit the degradation of GLP-1. The absence of a positive control (pure vildagliptin) however limits the range of the conclusions that can be drawn [79].
6. Metal NPs as indirect antidiabetic drugs
Various trace elements have been linked with glucose homeostasis including zinc, vanadium, chromium, selenium and lithium. These metals are involved as cofactors in many biochemical enzymatic reactions and several studies have highlighted their biological effects in glucometabolic disorders [80].
Alkaladi et al. studied the hypoglycemic effects of zinc oxide (ZnONPs) and silver NPs (SNPs) compared with insulin treatment on STZ-induced diabetic rats [81]. Animals received a single intraperitoneal dose of 100 mg/kg STZ for induction of diabetes and were treated with 10 mg/kg ZnONPs or SNPs for 30 d or 0.6 units/50 g insulin. Biochemical analysis showed that although zinc and silver NPs reduce blood glucose, they were unable to completely restore normoglycemia. Both NPs act by enhancing insulin secretion (ZnONPs effect was more profound than SNPs), the expression of insulin receptor-α gene and the activity of glucokinase in the liver of the diabetic animals. In a previous study Umrani et al. administered orally for 4 weeks three different doses of ZnONPS (1, 3 and 10 mg/kg) to STZ-induced diabetic rats (90 mg/kg). All three dosages were equally effective in reducing fasting blood glucose level but insulin level increased significantly only at the higher dose of 10 mg/kg [82]. Moreover, ZnONPs supplementation restores function and structure of beta cells as well as several other diabetic dysfunction indices, including the silencing of microRNA-103 and 143 that results in improved glucose homeostasis [83]. ZnONPs in combination with vildagliptin (DPP4 inhibitor) provide a synergistic effect that enhances the positive treatment outcome.
7. Limitations of existing literature and future perspectives
Generally, a drug's effects are evaluated in terms of potency (the amount of drug that produces an effect), efficacy (the capacity of drug to produce an effect) and effectiveness. Effectiveness differs from efficacy since a drug might have high efficacy but low effectiveness because it causes many side effects. When considering these features, nanoformulations of antidiabetic drugs (in experimental models of T2DM) transcend conventional drugs: treatment at similar doses (potency) has higher efficacy (prolongation of low glucose concentration) and effectiveness (less or no side effects) (Table 2). In short, the safe and controlled delivery of antidiabetic drugs to the specific site is the solution offered by the nano-delivery systems that translates into a better control of T2DM. However, this does not mean that nanodrugs lack of limitations which are mainly related with the stability of the carriers and the reproducibility of their characteristics.
Table 2.
In vivo effects of antidiabetic NP formulations discussed in the current review.
| NPs | Drug | Animal model | Healthy/ Diabetes | Outcome |
Reference | |
|---|---|---|---|---|---|---|
| BG | Insulin | |||||
| (GLP-1)-based nanomaterials | ||||||
| Liposomes | GLP1 | Rat | Healthy | ↓ vs. control nd vs. drug |
↑ vs. control ↑ vs. drug |
[51] |
| HTCA-pDNA complex | GLP1 | Mice | HFD | ↓ vs. control | ↑ vs. control | [59] |
| Self-assembled peptides | GLP1 | Rat | ZDF | ↓ vs. drug | ↑ vs. drug | [52] |
| Gold | GLP1 analogs | Rat | Healthy | nd vs. control | na | [55] |
| (GLP-1)-based nanomaterials / nano-DPP4 inhibitors | ||||||
| Polymeric | Liraglutide/ Exenatide/ iDPP4 |
Rat | Diabetic/STZ induced | ↓ vs. control ↓ vs. drug |
↑ vs. control nd vs. drug |
[60] |
| Chitosan, Porous silicon | GLP1/i DPP4 | Rat | Diabetic/STZ induced | ↓ vs. drug | ↑ vs. drug | [61] |
| Pituitary adenylate cyclase activating peptide (PACAP) | ||||||
| Peptide-conjugated selenium | VPAC2 agonist | Mice | Db/Db | ↓ vs. control ↓ vs. drug |
↓ vs. control ↓ vs. drug |
[64] |
| Nano-drug carriers | ||||||
| Niosomes | Repaglinide | Rat |
Diabetic/STZ induced | ↓ vs. control ↓ vs. drug |
na | [76] |
| Niosomes | Metformin | Rat | Diabetic/STZ induced | ↓ vs. drug | na | [68] |
| Niosomes | Pioglitazone | Rat | Diabetic/STZ induced | ↓ vs. control ↓ vs. drug |
na | [73] |
| Polymeric | Viglagliptin | Rat | Diabetic/STZ induced | ↓ vs. control nd vs. drug |
na | [78] |
| Nanospheres | Viglagliptin | Mice | Db/Db | ↓ vs. control | na | [79] |
| Chitosan | Exenatide | Mice | Db/Db | ↓ vs. control nd vs. drug |
na | [77] |
| Niosomes | Gliclazide | Rat | Healthy | ↓ vs. drug | na | [65] |
| Polymeric | Glipizide | Rat | Diabetic/STZ induced | ↓ vs. control ↓ vs. drug |
na | [67] |
| Metal NPs | ||||||
| Zinc oxide | Rat | Diabetic/STZ induced | ↓ vs. control ↑ vs. drug |
↑ vs. control ↓ vs. drug |
[81] | |
| Silver | Rat | Diabetic/STZ induced | ↓ vs. control ↑ vs. drug |
nd vs. control ↓ vs. drug |
[81] | |
| Zinc oxide | Viglagliptin | Rat | HFD Diabetic/STZ induced | ↓ vs. control nd vs. drug |
↑ vs. control nd vs. drug |
[83] |
| Zinc oxide | Rat | Diabetic/STZ induced | ↓ vs. control | ↑ vs. control | [82] | |
BG, blood glucose; HFD, high fat diet; STZ, streptozotocin; nd, not different; na, not available.
We must emphasize that most of the in vivo studies presented in Table 2 took only snapshots to evaluate the hypoglymecic properties of their formulations; short one-time monitoring ranging from 8 up to 48 h. Only a small number of trials applied long term therapeutics protocols. Thus, the need for comparative studies especially in slow T2DM progression animal models (such as rat or mice strains that develop mild hyperglycemia, insulin resistant, hyperinsulinemia and finally T2DM) is imperative to fully exploit the pros and cons of the nanoformulations over pure drugs.
8. Conclusions
Year by year it is becoming more urgent to implement strategies for the management of diabetes mellitus. The economic cost of diabetes burdens the patients, their family, the society and eventually the public health system.
Most of the current antidiabetic drugs act by enhancing either insulin release or glucose uptake in certain peripheral tissues. Often, these drugs fail to control or even delay hyperglycemia and eventually insulin therapy is imperative. Antidiabetic treatment for T2DM usually comes in the form of tablets or capsules and most of the times is accompanied with unwanted effects as summarized in Table 1. The development of side effects in T2DM treatment depicts the limited effectiveness of current drugs and specifically their inability to access the site of action at desired concentrations in combination with a narrow therapeutic window as they fail to sustain prolonged release. Thus, complex dosing schedules (to maintain constant drug concentration) are implemented.
The therapy goals of diabetes are to improve quality of the patients’ life, to prevent or delay the onset of disease complications and to ultimately decrease mortality [84]. The aim of the antidiabetic treatment is to achieve optimal glycemic control that can be summarised as target values for FPG 70–130 mg/dl, 2-h postprandial glucose < 180 mg/dl and bedtime glucose 90–150 mg/dl. Control of glycemia can be imperative for the prevention of microvascular complications such as neuropathy, nephropathy and retinopathy; however, glycemic control alone cannot be effective for the prevention of macrovascular complications (ischemic heart disease, peripheral arteriopathy etc.) [85].
An optimal therapeutic profile of a nanodrug should aim to maintain glucose levels as close to normal as possible for an extended period. Thus, a steep decrease with an analogous increase in short period of time is not desirable. The ability of the drug to maintain a progressive increase of blood glucose over time is of essence, although this task is extremely difficult in diabetes due to the clearance of the drug from the circulation. Thus, the ideal profile of a nanocarrier for diabetes should encompass specific characteristics such as: (a) respond to blood glucose concentration and accelerate or decelerate drug release, (b) remain in blood circulation for prolonged periods in order to maintain glucose levels as low as possible and (c) release drug gradually and minimize drug burst. The first one is a characteristic of smart nanocarriers while the next two characterise extended release nano-formulations. Moreover, in the direction of improving health quality of diabetic patients, the contribution of biomedical devices (such as closed-loop delivery systems) and novel glucose-responsive microneedle patches (incorporating smart NPs) might prove an asset for the tight control of blood glucose control.
The rapid expansion of experimental nano-drugs for the treatment of various pathological conditions (mainly cancer) and T1DM (nano-formulations of insulin) should also flourish against T2DM; not only in the form of evolving current regimens by eliminating their potential side-effects but also by applying nanotechnology for the development of novel antidiabetic drugs. To this extend it is anticipated that novel approaches, which will fully exploit the progress of nanotechnology in the fight against the increasing prevalence of T2DM, will emerge in the near future.
Conflicts of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ajps.2020.05.001.
Appendix. Supplementary materials
References
- 1.Centers for Disease Control and Prevention Diabetes report card 2014. 2015 [Google Scholar]
- 2.Stokes A., Preston S.H. Deaths attributable to diabetes in the United States: comparison of data sources and estimation approaches. PLoS ONE. 2017;12(1):1–12. doi: 10.1371/journal.pone.0170219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ogurtsova K., Rocha Fernandes JD da, Huang Y., Linnenkamp U., Guariguata L., Cho N.H. IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40–50. doi: 10.1016/j.diabres.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 4.Zheng Y., Ley S.H., Hu F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14(2):88–98. doi: 10.1038/nrendo.2017.151. [DOI] [PubMed] [Google Scholar]
- 5.Ahlqvist E., Storm P., Käräjämäki A., Martinell M., Dorkhan M., Carlsson A. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 2018;8587(18):1–9. doi: 10.1016/S2213-8587(18)30051-2. [DOI] [PubMed] [Google Scholar]
- 6.Tasyurek H.M., Altunbas H.A., Balci M.K., Sanlioglu S. Incretins: their physiology and application in the treatment of diabetes mellitus. Diabetes Metab Res Rev. 2014;30(5):354–371. doi: 10.1002/dmrr.2501. [DOI] [PubMed] [Google Scholar]
- 7.Wang K., Zhang Y., Zhao C., Jiang M. SGLT-2 Inhibitors and DPP-4 inhibitors as second-line drugs in patients with type 2 diabetes: a meta-analysis of randomized clinical trials. Horm Metab Res. 2018;50(10):768–777. doi: 10.1055/a-0733-7919. [DOI] [PubMed] [Google Scholar]
- 8.Turner R. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) Lancet. 1998;352(9139):854–865. [PubMed] [Google Scholar]
- 9.Thulé P.M., Umpierrez G. Sulfonylureas: a new look at old therapy. Curr Diab Rep. 2014;14(4):473. doi: 10.1007/s11892-014-0473-5. [DOI] [PubMed] [Google Scholar]
- 10.Soccio R.E., Chen E.R., Lazar M.A. Thiazolidinediones and the promise of insulin sensitization in type 2 diabetes. Cell Metab. 2014;20(4):573–591. doi: 10.1016/j.cmet.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansen K.B., Vilsboll T., Knop F.K. Incretin mimetics: a novel therapeutic option for patients with type 2 diabetes - a review. Diabetes Metab Syndr Obes. 2010;3:155–163. [PMC free article] [PubMed] [Google Scholar]
- 12.Dicker D. DPP-4 Inhibitors: impact on glycemic control and cardiovascular risk factors. Diabetes Care. 2011;34:S276–S278. doi: 10.2337/dc11-s229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh M., Kumar A. Risks associated with SGLT2 inhibitors: an overview. Curr Drug Saf. 2018;13(2):84–91. doi: 10.2174/1574886313666180226103408. [DOI] [PubMed] [Google Scholar]
- 14.Bhatia S. Natural polymer drug delivery systems. Springer; Cham: 2016. Nanoparticles types, classification, characterization, fabrication methods and drug delivery applications; pp. 33–93. [Google Scholar]
- 15.Wicki A., Witzigmann D., Balasubramanian V., Huwyler J. Nanomedicine in cancer therapy: challenges, opportunities, and clinical applications. J Control Release. 2015;200:138–157. doi: 10.1016/j.jconrel.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 16.Jain P.K., Huang X., El-Sayed I.H., El-Sayed M. Noble metals on the nanoscale: optical and photothermal properties and some applications in imaging, sensing, biology, and medicine. Acc Chem Res. 2008;41(12):1578–1586. doi: 10.1021/ar7002804. [DOI] [PubMed] [Google Scholar]
- 17.Boisseau P., Loubaton .B. Nanomedicine, nanotechnology in medicine. Comptes Rendus Phys. 2011;12(7):620–636. [Google Scholar]
- 18.Peters D., Kastantin M., Kotamraju V.R., Karmali P.P., Gujraty K., Tirrell M. Targeting atherosclerosis by using modular, multifunctional micelles. Proc Natl Acad Sci USA. 2009;106(24):9815–9819. doi: 10.1073/pnas.0903369106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang J., Su T., Huang K., Dinh P.U., Wang Z., Vandergriff A. Targeted repair of heart injury by stem cells fused with platelet nanovesicles. Nat Biomed Eng. 2018;2(1):17–26. doi: 10.1038/s41551-017-0182-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang D., Lim M., Goos J.A.C.M., Qiao R., Ng Y.Y., Mansfeld F.M. Biologically targeted magnetic hyperthermia: potential and limitations. Front Pharmacol. 2018;(9):831. doi: 10.3389/fphar.2018.00831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang L., Hu C., Shao L. The-antimicrobial-activity-of-nanoparticles–present-situati. Int J Nanomed. 2017;12:1227–1249. doi: 10.2147/IJN.S121956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Róz AL Da, Ferreira M., Leite F.D.L. Nanoscience and its applications. In: Cancino-Bernardi J., Marangoni V.S., editors. Nanoscience and its applications. Elsevier Inc; 2017. p. 228. VZ. [Google Scholar]
- 23.Munawar A., Ong Y., Schirhagl R., Tahir M.A., Khan W.S., Bajwa S.Z. Nanosensors for diagnosis with optical, electric and mechanical transducers. RSC Adv. 2019:6793–6803. doi: 10.1039/c8ra10144b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strakosas X., Selberg J., Pansodtee P., Yonas N., Manapongpun P., Teodorescu M. A non-enzymatic glucose sensor enabled by bioelectronic pH control. Sci Rep. 2019;9(1):1–7. doi: 10.1038/s41598-019-46302-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ventola C.L., Bharali D.J., Mousa S.A. The nanomedicine revolution: part 1: emerging concepts. Pharmacy and therapeutics. Pharmacol Ther. 2010;128(9):512–525. [Google Scholar]
- 26.Gul S., Khan S.B., Rehman I.U., Khan M.A., Khan M.I. A comprehensive review of magnetic nanomaterials modern day theranostics. Front Mater. 2019;6:1–15. [Google Scholar]
- 27.Dahman Y. Nanotechnology and functional materials for engineers. Elsevier Inc; 2017. Nanomedicine; pp. 229–249. [Google Scholar]
- 28.Goldberg M., Langer R., Jia X. Nanostructured materials for applications in drug delivery and tissue engineering. J Biomater Sci Polym Ed. 2007;18(3):241–268. doi: 10.1163/156856207779996931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar S., Nehra M., Kedia D., Dilbaghi N., Tankeshwar K., Kim K.H. Nanotechnology-based biomaterials for orthopaedic applications: recent advances and future prospects. Mater Sci Eng C Mater Biol Appl. 2020;106 doi: 10.1016/j.msec.2019.110154. [DOI] [PubMed] [Google Scholar]
- 30.Nikalje A.P. Medicinal chemistry Nanotechnology and its applications in medicine. Med Chem Los Angel. 2015;5(2):81–89. [Google Scholar]
- 31.Disanto R.M., Subramanian V., Gu Z. Recent advances in nanotechnology for diabetes treatment. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015;7(4):548–564. doi: 10.1002/wnan.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gupta R. Diabetes treatment by nanotechnology. J Biotechnol Biomater. 2017;7(3):268. [Google Scholar]
- 33.Miñon-Hernández D., Villalobos-Espinosa J., Santiago-Roque I., Gonzalez-Herrera S.L., Herrera-Meza S., Meza-Alvarado E. Biofunctionality of native and nano-structured blue corn starch in prediabetic Wistar rats. CyTA J Food. 2018;16(1):477–483. [Google Scholar]
- 34.Sharma G., Sharma A.R., Nam J.S., Doss G.P.C., Lee S.S., Chakraborty C. Nanoparticle based insulin delivery system: the next generation efficient therapy for type 1 diabetes. J Nanobiotechnol. 2015;13(1):74. doi: 10.1186/s12951-015-0136-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alai M.S., Lin W.J., Pingale S.S. Application of polymeric nanoparticles and micelles in insulin oral delivery. J Food Drug Anal. 2015;23(3):351–358. doi: 10.1016/j.jfda.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Russell S.J., El-Khatib F.H., Sinha M., Magyar K.L., McKeon K., Goergen L.G. Outpatient glycemic control with a bionic pancreas in type 1 diabetes. N Engl J Med. 2014;371(4):313–325. doi: 10.1056/NEJMoa1314474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lammers T. Improving the efficacy of combined modality anticancer therapy using HPMA copolymer-based nanomedicine formulations. Adv Drug Deliv Rev. 2010;62(2):203–230. doi: 10.1016/j.addr.2009.11.028. [DOI] [PubMed] [Google Scholar]
- 38.Li X., Szewczuk M.R., Malardier-Jugroot C. Folic acid-conjugated amphiphilic alternating copolymer as a new active tumor targeting drug delivery platform. Drug Des Devel Ther. 2016;10:4101–4110. doi: 10.2147/DDDT.S123386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kalyane D., Raval N., Maheshwari R., Tamble V., Kalia K., Tekade R.K. Employment of enhanced permeability and retention effect (EPR): nanoparticle-based precision tools for targeting of therapeutic and diagnostic agent in cancer. Mater Sci Eng C Mater Biol Appl. 2019;98:1252–1276. doi: 10.1016/j.msec.2019.01.066. [DOI] [PubMed] [Google Scholar]
- 40.Nehoff H., Parayath N.N., Domanovitch L., Taurin S., Greish K. Nanomedicine for drug targeting: strategies beyond the enhanced permeability and retention effect. Int J Nanomedicine. 2014;9(1):2539–2555. doi: 10.2147/IJN.S47129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kalaydina R.V., Bajwa K., Qorri B., Decarlo A., Szewczuk M.R. Recent advances in “smart” delivery systems for extended drug release in cancer therapy. Int J Nanomed. 2018;13:4727–4745. doi: 10.2147/IJN.S168053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoo J., Park C., Yi G., Lee D., Koo H. Active targeting strategies using biological ligands for nanoparticle drug delivery systems. Cancers Basel. 2019;11(5):640. doi: 10.3390/cancers11050640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen W.C., Zhang A.X., Li S. Limitations and niches of the active targeting approach for nanoparticle drug delivery. Eur J Nanomed. 2012;4(2–4):89–93. [Google Scholar]
- 44.Heng P.W.S. Controlled release drug delivery systems. Pharm Dev Technol. 2018;23(9):833. doi: 10.1080/10837450.2018.1534376. [DOI] [PubMed] [Google Scholar]
- 45.Martinelli C., Pucci C., Ciofani G. Nanostructured carriers as innovative tools for cancer diagnosis and therapy. APL Bioeng. 2019;3(1) doi: 10.1063/1.5079943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ventola C.L. Progress in nanomedicine: approved and investigational nanodrugs. P T. 2017;42(12):742–755. [PMC free article] [PubMed] [Google Scholar]
- 47.Caster J.M., Patel A.N., Zhang T., Wang A. Investigational nanomedicines in 2016: a review of nanotherapeutics currently undergoing clinical trials. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2017;9(1):e1416. doi: 10.1002/wnan.1416. [DOI] [PubMed] [Google Scholar]
- 48.Zhang R.X., Li J., Zhang T., Amini M.A., He C., Lu B. Importance of integrating nanotechnology with pharmacology and physiology for innovative drug delivery and therapy-an illustration with firsthand examples. Acta Pharmacol Sin. 2018;39(5):825–844. doi: 10.1038/aps.2018.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaasalainen M., Rytkonen J., Makila E., Narvanen A., Salonen J. Electrostatic interaction on loading of therapeutic peptide GLP-1 into porous silicon nanoparticles. Langmuir. 2015;31(5):1722–1729. doi: 10.1021/la5047047. [DOI] [PubMed] [Google Scholar]
- 50.Araújo F., Shrestha N., Shahbazi M.A., Fonte P., Makila E.M., Salonen J.J. The impact of nanoparticles on the mucosal translocation and transport of GLP-1 across the intestinal epithelium. Biomaterials. 2014;35(33):9199–9207. doi: 10.1016/j.biomaterials.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 51.Hanato J., Kuriyama K., Mizumoto T., Debari K., Hatanaka J., Onoue S. Liposomal formulations of glucagon-like peptide-1: improved bioavailability and anti-diabetic effect. Int J Pharm. 2009;382(1–2):111–116. doi: 10.1016/j.ijpharm.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 52.Li Y., Cui T., Kong X., Yi X., Kong D., Zhang J. Nanoparticles induced by embedding self-assembling cassette into glucagon-like peptide 1 for improving in vivo stability. FASEB J. 2018;32(6):2992–3004. doi: 10.1096/fj.201701033RRR. [DOI] [PubMed] [Google Scholar]
- 53.Connor E.E., Mwamuka J., Gole A., Murphy C.J., Wyatt M.D. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small. 2005;1(3):325–327. doi: 10.1002/smll.200400093. [DOI] [PubMed] [Google Scholar]
- 54.Villiers C.L., Freitas H., Couderc R., Villiers M.B., Marche P. Analysis of the toxicity of gold nano particles on the immune system: effect on dendritic cell functions. J Nanoparticle Res. 2010;12(1):55–60. doi: 10.1007/s11051-009-9692-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pérez-Ortiz M., Zapata-Urzúa C., Acosta G.A., Alvarez-Lueje A., Albericio F., Kogan M.J. Gold nanoparticles as an efficient drug delivery system for GLP-1 peptides. Colloids Surf B Biointerfaces. 2017;158:25–32. doi: 10.1016/j.colsurfb.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 56.Tonne J.M., Sakuma T., Deeds M.C., Munoz-Gomez M., Barry M.A., Kudva Y.C. Global gene expression profiling of pancreatic islets in mice during streptozotocin-induced - cell damage and pancreatic Glp-1 gene therapy. Dis Model Mech. 2013;6(5):1236–1245. doi: 10.1242/dmm.012591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oh S., Lee M., Ko K.S., Choi S., Kim S.W. GLP-1 gene delivery for the treatment of type 2 diabetes. Mol Ther. 2003;7(4):478–483. doi: 10.1016/s1525-0016(03)00036-4. [DOI] [PubMed] [Google Scholar]
- 58.Rothman S., Tseng H., Goldfine I. Oral gene therapy: a novel method for the manufacture and delivery of protein drugs. Diabetes Technol Ther. 2005;7(3):549–557. doi: 10.1089/dia.2005.7.549. [DOI] [PubMed] [Google Scholar]
- 59.Nurunnabi M., Lee S.A., Revuri V., Hwang Y.H., Kang S.H., Lee M. Oral delivery of a therapeutic gene encoding glucagon-like peptide 1 to treat high fat diet-induced diabetes. J Control Release. 2017;268:305–313. doi: 10.1016/j.jconrel.2017.08.035. [DOI] [PubMed] [Google Scholar]
- 60.Araújo F., Shrestha N., Gomes M.J., Herranz-Blanco B., Liu D., Hirvonen J.J. In vivo dual-delivery of glucagon like peptide-1 (GLP-1) and dipeptidyl peptidase-4 (DPP4) inhibitor through composites prepared by microfluidics for diabetes therapy. Nanoscale. 2016;8(20):10706–10713. doi: 10.1039/c6nr00294c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shrestha N., Araújo F., Shahbazi M.A., Makila E., Gomes M.J., Airavaara M. Oral hypoglycaemic effect of GLP-1 and DPP4 inhibitor based nanocomposites in a diabetic animal model. J Control Release. 2016;232:113–119. doi: 10.1016/j.jconrel.2016.04.024. [DOI] [PubMed] [Google Scholar]
- 62.Vaudry D., Falluel-Morel A., Bourgault S., Basille M., Burel D., Wurtz O. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev. 2009;61(3):283–357. doi: 10.1124/pr.109.001370. [DOI] [PubMed] [Google Scholar]
- 63.Gray S.L., Cummings K.J., Jirik F.R., Sherwood N.M. Targeted disruption of the pituitary adenylate cyclase-activating polypeptide gene results in early postnatal death associated with dysfunction of lipid and carbohydrate metabolism. Mol Endocrinol. 2001;15(10):1739–1747. doi: 10.1210/mend.15.10.0705. [DOI] [PubMed] [Google Scholar]
- 64.Zhao S.J., Wang D.H., Li Y.W., Han L., Xiao X., Ma M. A novel selective VPACAC2 agonist peptide-conjugated chitosan modified selenium nanoparticles with enhanced anti-type 2 diabetes synergy effects. Int J Nanomed. 2017;12:2143–2160. doi: 10.2147/IJN.S130566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tamizharasi S., Dubey A., Rathi V., Rathi J.C. Development and characterization of niosomal drug delivery of gliclazide. J Young Pharm. 2009;1(3):205. [Google Scholar]
- 66.Goyal S., Rai J.K., Narang R.K., Rajesh K.S. Sulfonyl ureas for antidiabetic therapy, an overview for glipizide. Int J Pharm Pharm Sci. 2010;2:1–6. [Google Scholar]
- 67.Lokhande A., Mishra S., Kulkarni R., Naik J. Formulation and evaluation of glipizide loaded nanoparticles. Int J Pharm Pharm Sci. 2013;5(4):147–151. [Google Scholar]
- 68.Hasan A.A., Madkor H., Wageh S. Formulation and evaluation of metformin hydrochloride-loaded niosomes as controlled release drug delivery system. Drug Deliv. 2013;20(3–4):120–126. doi: 10.3109/10717544.2013.779332. [DOI] [PubMed] [Google Scholar]
- 69.Sankhyan A., Pawar P.K. Metformin loaded non-ionic surfactant vesicles: optimization of formulation, effect of process variables and characterization. DARU. 2013;21(1):7. doi: 10.1186/2008-2231-21-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Manconi M., Nácher A., Merino V., Merino-Sanjuan M., Manca M.L., Mura C. Improving oral bioavailability and pharmacokinetics of liposomal metformin by glycerolphosphate–chitosan microcomplexation. AAPS PharmSciTech. 2013;14(2):485–496. doi: 10.1208/s12249-013-9926-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lekshmi U.M.D., Reddy P.N. Preliminary toxicological report of metformin hydrochloride loaded polymeric nanoparticles. Toxicol Int. 2012;19(3):267. doi: 10.4103/0971-6580.103667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Satheeshkumar N., Shantikumar S., Srinivas R. Pioglitazone: a review of analytical methods. J Pharm Anal. 2014;4(5):295–302. doi: 10.1016/j.jpha.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Haider M., Kanoujia J., Tripathi C.B., Arya M., Kaithwas G., Saraf S.A. Pioglitazone loaded vesicular carriers for anti-diabetic activity: development and optimization as per central composite design. J Pharm Sci Pharmacol. 2015;2(1):11–20. [Google Scholar]
- 74.Dhana lekshmi U.M., Poovi G., Kishore N., Reddy P.N. In vitro characterization and in vivo toxicity study of repaglinide loaded poly (methyl methacrylate) nanoparticles. Int J Pharm. 2010;396(1–2):194–203. doi: 10.1016/j.ijpharm.2010.06.023. [DOI] [PubMed] [Google Scholar]
- 75.Ebrahimi H.A., Javadzadeh Y., Hamidi M., Jalali M.B. Repaglinide-loaded solid lipid nanoparticles: effect of using different surfactants/stabilizers on physicochemical properties of nanoparticles. DARU. 2015;23(1):46. doi: 10.1186/s40199-015-0128-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Namdev S., Gujar K., Mandlik S., Jamkar P. Preparation and in vivo characterization of niosomal carriers of the antidiabetic drug repaglinide. Int J Pharm Sci Nanotechnol. 2015;8(1):2756–2767. [Google Scholar]
- 77.Li X., Wang C., Liang R., Sun F., Shi Y., Wang A. The glucose-lowering potential of exenatide delivered orally via goblet cell-targeting nanoparticles. Pharm Res. 2015;32(3):1017–1027. doi: 10.1007/s11095-014-1513-1. [DOI] [PubMed] [Google Scholar]
- 78.Dewan I., Islam S., Rana M.S. Characterization and compatibility studies of different rate retardant polymer loaded microspheres by solvent evaporation technique: in vitro-in vivo study of vildagliptin as a model drug. J Drug Deliv. 2015;2015 doi: 10.1155/2015/496807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Baig M.M.F.A., Khan S., Naeem M.A., Khan G.J., Ansari M.T. Vildagliptin loaded triangular DNA nanospheres coated with eudragit for oral delivery and better glycemic control in type 2 diabetes mellitus. Biomed Pharmacother. 2018;97(24):1250–1258. doi: 10.1016/j.biopha.2017.11.059. [DOI] [PubMed] [Google Scholar]
- 80.Wiernsperger N., Rapin J. Trace elements in glucometabolic disorders: an update. Diabetol Metab Syndr. 2010;2:70. doi: 10.1186/1758-5996-2-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Alkaladi A., Abdelazim A.M., Afifi M. Antidiabetic activity of zinc oxide and silver nanoparticles on streptozotocin-induced diabetic rats. Int J Mol Sci. 2014;15(2):2015–2023. doi: 10.3390/ijms15022015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Umrani R.D., Paknikar K.M. Zinc oxide nanoparticles show antidiabetic activity in streptozotocin- induced Type 1 and 2 diabetic rats. Nanomedicine. 2014;9(1):89–104. doi: 10.2217/nnm.12.205. [DOI] [PubMed] [Google Scholar]
- 83.El-Gharbawy R.M., Emara A.M., Abu-Risha S.E.S. Zinc oxide nanoparticles and a standard antidiabetic drug restore the function and structure of beta cells in type-2 diabetes. Biomed Pharmacother. 2016;84:810–820. doi: 10.1016/j.biopha.2016.09.068. [DOI] [PubMed] [Google Scholar]
- 84.Simó R., Hernández C. Treatment of diabetes mellitus: general goals, and clinical practice management. Rev Esp Cardiol. 2002;55(8):845–860. doi: 10.1016/s0300-8932(02)76714-6. [DOI] [PubMed] [Google Scholar]
- 85.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulfonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes. Endocrinologist. 1999;9(2):149. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.