Abstract
Objective/Background
Insomnia is a prevalent and interfering comorbidity of HIV infection. Nearly 70% of people living with HIV/AIDS (PLWHA) experience symptoms of insomnia and associated distress. The current study examined the mechanisms of insomnia in HIV health status and health-related quality of life and explored behavioral pathways to explain relationships.
Participants
Participants (N = 103) were active patients in an HIV clinic located within a nonprofit, tertiary care hospital in a large, urban city in the Southeast United States.
Methods
Participants completed a clinical sleep interview and self-report assessments for adherence to antiretroviral medication, depression (PHQ-9), quality of life (ACTG-QOL), and relevant covariates. Viral load and CD4 were obtained via medical chart review.
Results
Insomnia affected 67% of the clinic sample. Insomnia symptoms were directly associated with poorer health-related quality of life (p<.001). Greater insomnia symptoms were also significantly associated with greater depressive symptoms [b =.495, S.E. =.061], poorer medication adherence [b = −.912, S.E. =.292], and worse health status measured by absolute CD4 count [b = −.011, S.E. =.005].
Conclusions
In this sample of PLWHA, insomnia was associated with poorer health-related quality of life and worse health status. Future research and practice should consider insomnia treatment for this population, as it could improve overall health and well-being.
Introduction
Insomnia disorder, characterized by difficulty initiating and maintaining sleep and associated daytime impairment is a prevalent, distressing, and interfering comorbidity of HIV. Disrupted sleep has been noted as a common complaint among people living with HIV/AIDS (PLWHA) since the early years of the epidemic (Darko et al., 1992); and, it has been estimated that as many as 70% of a general HIV clinic sample experience sleep problems characteristic of insomnia disorder (Rubinstein & Selwyn, 1998). In an in-depth study of HIV positive patients compared to healthy controls, HIV patients had significantly more sleep disorders than the healthy controls, and, the most common sleep disorder was insomnia (Gamaldo et al., 2013). Insomnia in this population is important because it may worsen HIV symptoms including pain, fatigue, and difficulties with cognitive functioning and affective stability (Roth, 2007; Taylor & Lichstein, 2003; Taylor et al., 2007). Yet, there has been very little progress in assessing, understanding, or treating this comorbidity (Reid & Dwyer, 2005).
In a previous study, we examined insomnia symptoms in association with depression, health-related quality of life, and life satisfaction in a sample of HIV positive patients with depressive symptoms enrolled in a CBT treatment trial for depression (Rogers et al., 2018). Our results revealed a contribution of sleep problems to both between-person and within-person effects on depression, health-related quality of life, and life satisfaction.
The current study sought to explore these relationships by testing a theoretical framework. Importantly, we believe we are the first to test such a model. In doing so, we placed insomnia symptoms prior to depressive symptoms in our model because of a robust literature demonstrating that insomnia symptoms can, and often do predate depression (Taylor, 2008). Data from longitudinal studies of insomnia and depression have shown that insomnia predates and can even be used to predict future depressive episodes (Riemann & Volderholzer, 2003). Taken together, this work suggests that insomnia symptoms may act as a prodrome for later depression. As such, it was our theoretical perspective that insomnia symptoms should be positioned as occurring at the beginning of the chain of relationships within our model.
We further hypothesized that we would find direct relationships between insomnia and viral load; and, insomnia and health-related quality of life. Specifically, it was hypothesized that there would be strong associations between insomnia and depression (Benca & Peterson, 2008; Buysse et al., 2008; Manber & Chambers, 2009a); depression and medication adherence (Gonzalez et al., 2011a; Wagner et al., 2011, p. 14); medication adherence and viral load; and, viral load and CD4 count (medication adherence directly affects viral load and viral load impacts CD4 count such that when circulating virus is present in higher levels it attacks the immune system such that higher viral load values are associated with lower CD4 counts) and poorer health-related quality of life (Balderson et al., 2013; Campsmith et al., 2003).
Methods
Participants and procedures
Participants were active patients in the HIV/AIDS clinic based in our public, nonprofit, tertiary care hospital-based clinic that provides care for underserved patients. Participants were required to be adults between the ages of 18–65 years, and able to speak and read English. Participants were excluded during the screening process if they were cognitively impaired or were otherwise unable to provide proper informed consent.
Participants (N = 107) were recruited and enrolled in the study. Four participants were excluded at the baseline visit. One participant was excluded because they were not an active patient in clinic and three were excluded for mental health concerns (n = 1 actively psychotic; n = 2 actively suicidal). Prior to beginning the study, we had determined that 100 participants would allow us to fulfill power requirements while balancing feasibility. We aimed to recruit 100 participants who attended and completed both baseline and follow up appointments. In order to achieve this level of participation, we had to enroll slightly more participants than needed, which is how we arrived at 107 attending a baseline appointment and 103 completed baseline appointments. For details about the participant characteristics, see Table 1.
Table 1.
Participant characteristics (N = 103).
| M | SD | |
|---|---|---|
| Age | 51.64 | 8.50 |
| N | % | |
| Race | ||
| Black | 84 | 81.6 |
| White | 12 | 11.7 |
| Mixed/Other | 9 | 8.7 |
| Ethnicity | ||
| Hispanic/Latino | 11 | 10.7 |
| Gender | ||
| Men | 51 | 49.51 |
| Women | 51 | 49.51 |
| Transwomen | 1 | <1.0 |
| Sexual Orientation | ||
| Gay/Lesbian/Homosexual | 12 | 11.7 |
| Bisexual | 3 | 2.8 |
| Other Sexual Minority | 3 | 2.8 |
| Men who have Sex with Men (MSM) | 15 | 28.85 |
| Women who have Sex with Women (WSW) | 14 | 27.45 |
| Education | ||
| Did not complete high school | 43 | 41.7 |
| High school graduate | 37 | 35.9 |
| Some college | 19 | 18.4 |
| College graduate | 4 | 3.9 |
Enrollment took place from May 2017 through November 2017. Participants were recruited actively via clinic staff who approached potential participants asking them if they were interested in a study about HIV and sleep. Other participants self-referred to the study via fliers they saw throughout the clinic. Potential participants were provided with additional information regarding study methods and procedures. Interested participants completed a brief screening process where they were assessed for clinic patient status, language ability, and willingness to participate.
During screening, participants were provided with information about the study including procedures, purpose, and payment schedule. If interested, participants were asked if they would like to consent to be involved in the study. Study visits were scheduled as close to the lab visits as possible to maximize the chances of obtaining contemporaneous values for viral load and CD4. Most blood test results were collected within the past month (Median = 26 days, Mean = 48.74, SD = 98.48; Range: 0–886) prior to the visit. Most of our sample (95.2%) had blood test results within the past 6 months; and, almost all (n = 90; 87.4%) were collected within 90 days or 3 months of their visit. Informed consent included a formal consent form and Health Insurance Portability and Accountability Act (HIPAA) form and permission to access medical chart data (e.g., viral load and CD4 count) from electronic medical records were obtained. Documents were signed using Samsung tablet devices through the REDCap online data collection system.
To be inclusive of participants with varying levels of formal education, all questionnaires were interviewer-administered. This reduced barriers to participation and potentially, increased data validity. Insomnia symptoms were also measured by the Insomnia Severity Index, which was completed as an interviewer-administered questionnaire. Insomnia disorder was assessed via clinical interview using the Duke Structured Clinical Interview for DSM-5 Sleep Disorder Diagnoses, which was provided with permission, from Edinger and colleagues (Edinger, 2011). This clinical interview assess diagnostic criteria for insomnia including: (1) difficulty initiating and maintaining sleep and/or early morning awakening, (2) severe distress or impairment during the day as a result of sleep disturbance, (3) sleep problems occurring at least 3 nights/week for at least 3 months, (4) inadequate sleep despite adequate opportunity for sleep and without being attributable to another sleep disorder, (5) not better explained by substance use and/or coexisting mental disorders.
Interviewers recorded participant responses on tablets connected to REDCap via a secure WiFi network. REDCap is an electronic data collection system that can be used to collect data, track users, and export data (Harris et al., 2009) and is compliant with University of Miami Internal Review Board privacy procedures as well as federal and state HIPAA guidelines. Data on REDCap are backed up daily by the university-based IT department. Interviewers were bachelors and masters level research assistants.
All clinical sleep interviews were audio-recorded using a Phillips recorder (Phillips DVT 1150). Sound files were labeled with study ID number, interviewer name, and date. Files were stored on a password-protected folder on a secure network. The study lead (B.G.R.) listened to, took notes, and provided feedback for the first interview for each of the trained interviewers, as well as any other areas of interviews, for which interviewers requested feedback. In addition, approximately 20% of all interviews were reviewed and coded by the study lead (B.G.R.) for adherence to the interview and rater reliability. A licensed clinical psychologist who is board-certified in behavioral sleep medicine (W.K.W.) provided group supervision for interviewers and guidance on differential diagnosis, as needed.
Measures
Demographics
Participants completed self-report measures of demographic information, including items regarding age, sex at birth, current gender identity, sexual orientation, race, ethnicity, spoken language, religion, educational history, and employment status.
Insomnia symptoms
The Insomnia Severity Index (ISI) (Bastien et al., 2001) is a self-administered seven-item scale assessing nighttime difficulties (e.g., falling asleep, staying asleep, and waking too early) and daytime impairments (e.g., satisfaction, distress/concern, etc.) associated with insomnia. Each item is rated on a 5-point scale and the total score ranges from 0 to 28. The ISI has strong internal consistency (Cronbach’s alpha = .91) and temporal stability (r = .80) and is a valid and reliable tool for screening insomnia within a primary care population (Gagnon et al., 2013). The following cutoff scores are used to categorize insomnia symptoms: 0–7, no clinical insomnia; 8–14, subthreshold insomnia, 15–21, moderate clinical insomnia; 22–28, severe clinical insomnia. For the purposes of our analyses, we used the ISI as a continuous measure. This measure had high internal reliability within our sample (Cronbach’s α = .86). In addition to measuring insomnia symptoms, we also administered the Duke Structured Clinical Interview for DSM-5 Sleep Disorder Diagnoses (Edinger, 2011). Insomnia diagnosis was used to quantify the number of individuals with insomnia disorder. Results categorized by insomnia disorder diagnosis (yes/no) were highly concordant with symptom severity results. Because results were the same with symptom severity and diagnosis and symptom severity offers more variability than diagnostic category for analyses the Insomnia Severity Index is used throughout as a measure of insomnia.
Obstructive sleep apnea diagnosis
For those who indicated symptoms of obstructive sleep apnea, we also assessed STOPBANG scores as part of the structured clinical interview. STOPBANG is an evaluation of the following risk factors for obstructive sleep apnea: Snoring, Tired, Observed breathing episodes, Pressure (hypertension), BMI (>35), Age (>50), Neck size (>17 inches), and Gender (men are at elevated risk). A STOPBANG score of >3 is considered at elevated risk for obstructive sleep apnea (Chung et al., 2012). Interviewers indicated a score >3 as yes/no within the interview.
Sleep quantity
To objectively assess sleep in the home environment, participants were instructed to wear an actigraph (wGT3X-BT, 2016, Actigraph Corporation) on their non-dominant wrist for 24 hours a day except when bathing. The actigraph was programmed the day of the baseline visit with height, weight, and placement and participants were provided with information about the data that would be collected and the purpose of the device. The algorithm (Cole-Kripke) utilized by the software to calculate standard sleep continuity parameters has demonstrated reliability and validity compared to itself (coefficient of variation = .66%; ICC = .91) and similar products (interunit reliability ICC>.80) (Hislop et al., 2011) and has been found to discriminate sleep from wake states even for those with 33 chronic medical conditions (Wolfe et al., 2015). Actigraph data were scored individually by a trained rater using ActiLife software version 6.13.3 (Actigraph, LLC, 2009–2015).
Sleep quality
To measure self-reported sleep quantity and quality we used the Pittsburgh Sleep Quality Index (PSQI). The PSQI (Buysse et al., 1989) is a measure of sleep that provides a general overview of recent sleep difficulties. It includes a review of different sleep difficulties (e.g., coughing, snoring, difficulty breathing) and general sleep habits (e.g., bed time, wake time). The PSQI global score ranges from 0–21 points and is comprised of other component scores including sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, and sleeping medications – measures of general sleep quantity and quality. The PSQI has a relatively high internal reliability (Cronbach’s α = 0.83) and relatively high test-retest reliability, with no significant differences noted between testing time points (r= .85) (Buysse et al., 1989).
Body mass index (BMI)
A measure of kg/m2. BMI was obtained from medical charts and was used as a covariate in regression analysis. It was also used to inform obstructive sleep apnea risk during the clinical interview.
Depressive symptoms
Depressive symptoms were measured by the Patient Health Questionnaire – 9. The PHQ-9 (Kroenke et al., 2001) is a 9-item depression scale from a larger patient health questionnaire, which was originally part of the PRIME-MD, an overall health questionnaire to be administered in primary care. The PHQ-9 assesses cognitive, behavioral, and physiological symptoms of depression, with scores ranging from 1 to 27, associated with different levels of impairment and severity. The following depression categories can be derived from the PHQ-9: 1–4 (none), 5–9 (mild), 10–14 (moderate), 15–19 (moderately severe), and 20–27 (severe). The PHQ-9 is a highly sensitive (88%) and specific (88%) measure for depression (Kroenke & Spitzer, 2002) and captures all DSM-5 criteria. It also includes an impairment question, which assesses difficulty of completing daily living activities due to depression. The PHQ-9 is frequently used as a clinical tool and in research settings. The PHQ-9 has also been found to be a reliable and valid measure in racially and ethnically diverse primary care patients (Huang et al., 2006) with Cronbach’s α ranging from 0.79–0.89. This measure had high internal reliability within our sample (Cronbach’s α = .82).
HIV medication adherence
Adherence was measured using the Wilson et al. (2016) 3-item validated measure. The validation and reliability of this scale compared medication adherence using both self-report and electronic drug monitoring for HIV medications and other medications. Strong correlations in self-report and moderate correlations for electronic drug monitoring exist for HIV and other medications. Specifically, Cronbach’s alphas for the 3-item scale for HIV is 0.83 and Pearson correlation coefficients between the calibrated 3-item scale and the electronic drug monitoring for HIV is 0.47. These questions have undergone substantial cognitive field testing and analyses in real-world settings (Wilson et al., 2014, 2016). This measure had high internal reliability within our sample (Cronbach’s α = .81).
Health-related quality of life
Health-related quality of life was measured using the AIDS Clinical Trial Group Quality of Life Measure SF-21 (ACTG-QOL SF-21). The ACTG-QOL SF-21 is a measure that assesses quality of life for PLWHA in several domains including daily functioning, cognitive functioning, social functioning, energy/vitality, and mental health. Higher scores indicate better health. The ACTG-QOL is scored by log transforming raw scores on a 0–100 scale (where 0 = worst and 100 = best quality of life). The ACTG-QOL measure has shown validity and reliability (Intraclass correlations >0.70 for all scales) in large samples of diverse gender and disease stage for PLWHA (Bozzette et al., 1995; Wu et al., 2005). Individual subscales can be examined (specific quality of life) from the scale. Each of the subscales had moderate to high internal reliability. Specifically, for the general health-related quality of life subscale used in our current analyses there was moderate internal reliability (Cronbach’s α = .65).
Log HIV RNA viral load
HIV RNA viral load data was obtained via medical chart extraction. Viral load is measured in circulating virus copies/milliliter of blood. For continuous analyses, viral load data were log-transformed prior to analyses. Log10 transformation is one way to address the wide range of values from “undetectable” (<20 copies/mL) to large values (>1,000,000 copies/mL) and is frequently used as an alternative to whole number reporting of viral load measurement (Hoffman & Rockstroh, 2011). Because values below 20 copies/mL are not able to be detected by current clinical detection methods, it is standard practice for viral load values <20 copies/mL, to be imputed with the number 20, so log transformation can be applied appropriately, and values used in analyses.
CD4
Absolute CD4 cell counts (measured as cells/mm3) were obtained via medical chart extraction. CD4 cell counts are enumerated via flow cytometry (Barnett et al., 2008). CD4 cells/mm3 is a measure of immune health used to assess HIV/AIDS. Values below 200 cells/mm3 meet the clinical definition of AIDS.
Substance use
Substance use was assessed via the Drug Abuse Screening Test – 10 (DAST-10). The DAST-10 questionnaire asks about drug use within the last 12 months and is designed to be used as a brief instrument for clinical screening and treatment evaluation research. Respondents are asked to rate 10 items about drug abuse with either a Yes-1 or No-0 answer (one item is reverse coded). Items are then summed to arrive at the total score 0–10, where 0 indicates no problems reported at this time and 10 indicates severe problems with substance use. The DAST-10 had moderate internal reliability for our sample (Cronbach’s α = .52).
Additional health conditions and medications
Information about additional health conditions and medications were obtained via medical chart extraction. These data were not essential to the analyses, however, they were used to assess and describe the patient population.
Data analytic plan
Analyses were conducted using SPSS Version 22.0 and MPlus Version 7.4. Data were examined for normality and outliers prior to analyses. Missing variables were excluded from the analyses. However, because data were collected on a tablet with verification for each field, this affected very few variables. We were missing the following: medication adherence (n = 1), Log viral load (n = 2), and CD4 cells/mm3 (n = 4). Biological values like HIV RNA copies/mL and CD4 cells/mm3 were obtained from medical charts; and, if missing in our data set it is because they were missing within the chart. One BMI value was outside the expected range and likely a typographical error (value = 2,107). As such, this value was removed prior to analyses and treated as missing. Viral load blood test result values were log-transformed prior to analyses consistent with HIV literature and analyses of these data. All variables were entered simultaneously into regression models.
A structural equation modeling (SEM) approach to path analysis was chosen as the preferred analytic technique because it allows for the evaluation of multiple direct and indirect pathways and evaluation of the complete model fit (Muthén & Muthén, 2007). MPlus was used to test the fit of the conceptual framework using SEM. Model fit was assessed using a series of fit indices that were used to indicate acceptable model fit: Bentler comparative fit index (CFI) (at or around .95) (Bentler, 1990; Browne & Cudeck, 1992); root mean square error of approximation (RMSEA) (below .06) (Browne & Cudeck, 1992); standardized root mean square residual (SRMR) (below .08) (Hu & Bentler, 1999); and, lack of significance of Chi-Square (p > .05) (Kline, 2011). Despite our small sample size, models converged, and were informative in evaluating our theoretical framework.
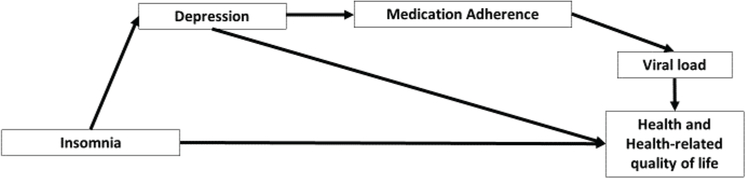
Path analysis was used to examine the relationships between variables, specifically testing the indirect relationships between insomnia and health status and insomnia and health-related quality of life via the proposed conceptual model (Figure 1).
Figure 1.
Theoretical framework: insomnia, health and health-related quality of life.
Separate models for health status (Figure 2) and health-related quality of life (Figure 3) were tested to better explicate and disentangle those factors associated with physical health and those associated with perceived health.
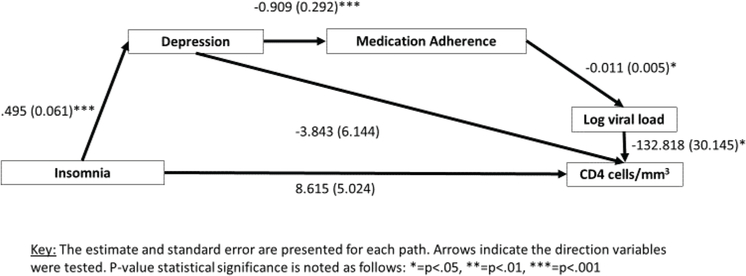
Figure 2.
Insomnia and HIV health.
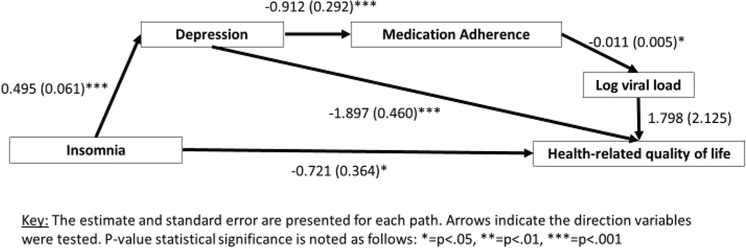
Figure 3.
Insomnia and health-related quality of life.
Results
All variables met assumptions for normality except for viral load and medication adherence. Log HIV RNA viral load was positively skewed, because of the portion (almost 70%) of the sample that was virally suppressed. Similarly, self-reported medication adherence was negatively skewed, which indicated more participants reported good medication adherence.
Sleep quantity and sleep quality
Participants reported an average bedtime of 11:45PM and an average wake time of 6:20AM. On average, participants reported that of the time they spent in bed, approximately 5.58 hours (SD = 1.70) were spent sleeping, which was relatively consistent with objective sleep measurement from actigraphy analyses (see total sleep time, above).
The average score for the PSQI was 10.32 (SD = 4.62; Range: 0–19), which is above the clinical cutoff typically used in the literature (PSQI = 7), indicating that, on average, participants had clinically significant sleep problems. Several participants reported (on the PSQI; items correspond to letters below) that at least once a week this past month, they had trouble sleeping due to the following reasons: (a) trouble falling asleep within 30 minutes (n = 76, 73.8%); (b) waking up in the middle of the night or early morning (n = 82, 79.6%); (c) having to get up to use the bathroom (n = 98, 95.1%); (d) cannot breathe comfortably (n = 26; 25.2%); (e) cough or snoring loudly (n = 35; 34.0%); (f) feel too cold (n = 37; 35.9%), (g) feel too hot (n = 63; 61.2%); (h) had bad dreams (n = 46; 44.7%); and/or (i) have pain (n = 54; 52.4%). Participants also self-reported a variety of additional factors that contributed to difficulties with initiation and maintenance of sleep including anxiety, stress, hunger, and/or bedpartners’ behaviors. Approximately half of the sample (n = 52; 50.5%) reported fairly bad or very bad sleep quality over the past month and 47.6% (n = 49) reported taking medication as sleep aids at least once a week this past month, with 32% (n = 34) taking them three or more times per week. About a quarter of participants reported daytime sleepiness that interfered with daily activities (n = 25; 24.3%) and about half (n = 49; 47.6%) reported fatigue that was impairing.
Insomnia, sleep, and health diagnoses and symptoms
Based on clinical interview, 68 (65%) individuals in the sample met criteria for insomnia disorder. Insomnia symptoms assessed by the Insomnia Severity Index ranged from 0–28. Scores less than or equal to 7 indicate no insomnia (n = 32; 31.06%) 8–14 indicate subthreshold insomnia (n = 35, 33.98%), 15–21 clinical insomnia (n = 26, 25.24%), and 22–28 clinical insomnia severe (n = 10, 9.7%). The average participant score was 11.67 (SD = 7.36). Anecdotally, occasionally during the interviewer-administered measures and clinical interviews, participants mentioned that their sleep was disrupted by poor sleep hygiene (e.g., leaving the television on to feel safe) and/or environmental stimuli including violence in their neighborhoods. These are not uncommon complaints for individuals living in low-income, urban settings; and, have been noted in other studies of sleep in PLWHA (Gutierrez et al., 2019). Participants who had sleep complaints that were only due to environmental stimuli were not included as having insomnia symptoms or disorder, as the definition of the disorder requires sleep disturbances not be due to outside causes.
Twenty-six (25.2% of total sample, 36.8% of those who met for insomnia) individuals who met diagnostic criteria for insomnia were also at elevated risk for obstructive sleep apnea based on their answers to clinical interview and their STOPBANG score (>3 considered at-risk) (Chung et al., 2012, 2008). STOPBANG scores ranged from 0–8, with the majority of individuals endorsing minimal (fewer than 3) symptoms (n = 7, 6.8%) or no symptoms of sleep apnea (n = 66, 64.1%). The remaining portion of the sample endorsed four symptoms (n = 10, 9.7%), five symptoms (n = 9, 8.7%), six symptoms (n = 7, 6.7%), seven symptoms (n = 3, 2.9%), and eight symptoms (n = 1, 1.0%), respectively. On average, individuals had a BMI of 29.57 (SD = 6.52; Range = 17.21–54.03), which is indicative of overweight status, but not obesity. Of note, the range of BMI did reach morbid obesity at the upper limit (BMI = 54.03).
Analyses were run including individuals with STOPBANG scores >3 and re-run excluding them as a sensitivity test. The direction and significance of the findings did not change. When those individuals with insomnia disorder who met criteria for OSA risk were excluded from the analyses, results were similar as insomnia not independently associated with log viral load (p= .053) or CD4 cells/mm3 (p= .233) in adjusted regression models. There was a trend in the same direction for general health outcomes, [B = −8.396 (S.E. = 5.568)]; t(96) = −1.508, p= .135, but it did not achieve significance, likely due to the smaller number of individuals in that category. Because results trended in the same direction for both insomnia overall (including those at high risk for obstructive sleep apnea based on their responses to STOPBANG during the clinical interview) and “pure insomnia disorder,” those who were “at-risk” for obstructive sleep apnea were included in the final analyses.
A small number of individuals had a sleep disorder diagnosis within their medical chart including insomnia disorder (n = 3) and obstructive sleep apnea (n = 2). A few were prescribed sleep medications (n = 4) including diphenhydramine (n = 1), hydroxyzine (n = 1), trazodone (n = 1), and zolpidem (n = 1). Several more reported use of selective serotonin reuptake inhibitors, which, on occasion are prescribed for sleep; however, this is not their intended use and so they were not counted in sleep medications.
Depressive symptoms were, on average, indicative of mild depression (M= 7.12, SD = 5.83), however, there was a large range (0–23), with 41 (39.80%) being in the “no depression” category, 24 (23.30%) in the mild category, 21 (20.38%) in the moderate category, 6 (5.82%) in the moderately severe category, and 5 (4.85%) in the severe depression category.
Sixty-one percent (n = 63) had at least one psychiatric diagnosis in their medical chart. A portion of participants had a diagnosis of depressive disorder (n = 35; 33.98%) in their medical chart. Other common psychiatric diagnoses included psychotic disorders (n = 16; 15.53%); bipolar disorder (n = 4; 3.88%); and anxiety disorders (n = 12; 11.65%). Several patients were prescribed and taking medication for their symptoms (n = 46; 44.66%) including antidepressants, mood stabilizers, and anti-anxiety medications. However, we do not have data about adherence to these medications or efficacy. Of note, we did not include anyone who was actively psychotic (n = 1) or actively suicidal (n = 2). Participants needed to be stable enough to participate.
Several had substance use (n = 11; 10.68%) or alcohol use disorders (n = 6; 5.83%) in their medical charts; however, no participants self-reported active use (many reported being in recovery). In consulting with a provider in the clinic, it is likely that these diagnoses were never removed from their charts. Thus, we do not expect that this impacted our sleep findings; however, we did control for substance use symptoms (using the DAST-10) within regression models to be overly conservative.
Seventy-one percent of participants reported that they had not missed one dose of their medicine over the past week, which is the first item on the self-report measure (Wilson et al., 2016). Weekly adherence scores, when calibrated across the three items and scored, were, on average, at 89.83% (SD = 18.07) and ranged from 25.56%−100%. Using the cutoff of 80% for the calibrated score as acceptable adherence, 79.61% (n = 82) fell in this range.
Participants reported, on average, moderate overall health with a mean of 59.79 out of 100 (SD = 25.95; Range: 8.33–100). Consistent with self-reported adherence, 71 (68.93%) of our participants had suppressed viral load. When data were log transformed, mean viral load was 1.77 (SD = 0.99; Range: 1.30–5.40). CD4 cells/mm3 was, on average, 510.98 (SD = 309.39) and had a large spread (Range: 4–1626). In our sample, 15 (14.6%) were below 200 (the cutoff for AIDS).
In addition to HIV, 92.3% of participants had several other chronic health conditions including hypertension, type II diabetes, hepatitis C, and cancer; and about a third of the sample (n = 33; 32.0%) had over five chronic health conditions in their medical chart. Participants also had a significant number of psychiatric diagnoses including histories of trauma and substance use. The majority had at least one psychiatric diagnosis (n = 61.2%; n = 63) and 43.7% (n = 45) were prescribed psychiatric medications. Overall, participants were highly complex both medically and psychiatrically. Additional descriptive statistics for key variables are presented in Table 2.
Table 2.
Sleep, physical, and mental health symptoms (N = 103).
| N | % | Range | |
|---|---|---|---|
| Clinical Interview Insomnia Diagnosis | 68 | 66.02 | |
| Obstructive Sleep Apnea Risk (STOPBANG>3) | 26 | 25.24 | |
| Suppressed viral load | 71 | 68.93 | |
| <200 (AIDS Defining CD4 at first study visit) | 15 | 14.56 | |
| Insomnia diagnosis from medical chart | 3 | 2.9% | |
| Sleep apnea diagnosis from medical chart | 2 | 1.9% | |
| Sleep medications | 8 | 7.8 | |
| Psychiatric Diagnosis | 63 | 61.2 | |
| Psychiatric Medication | 45 | 43.7 | |
| Medical comorbidities | 93 | 87.3 | |
| 5+ medical comorbidities | 33 | 32.0 | |
| Suppressed viral load | 71 | 68.93 | |
| <200 CD4 (AIDS defining) | 15 | 14.6 | |
| M | SD | ||
| Total sleep time (actigraph) | 385.53 | 72.81 | (215.50– 613.14) |
| Insomnia Severity Index | 11.67 | 7.36 | (0.00– 28.00) |
| Pittsburgh Sleep Quality Index | 10.32 | 4.62 | (0.00–19.00) |
| PHQ-9 | 7.12 | 5.83 | (0.00– 23.00) |
| Health-related quality of life | 57.79 | 25.95 | (8.33– 100.00) |
| Adherence | 89.83 | 18.07 | (25.56– 100.00) |
| Log viral load | 1.77 | .99 | (1.30– 5.40) |
| CD4 cells/mm3 | 510.98 | 309.39 | (4.00– 1626.00) |
| BMI | 29.57 | 6.52 | (17.21–54.03) |
Insomnia, health, and health-related quality of life
Direct relationships were tested using regression models. Insomnia symptoms were not associated with biomarkers of physical health in HIV (e.g., log HIV RNA viral load or CD4 count). However, insomnia symptoms were significantly associated with health-related quality of life, [(Unstd. b = − 1.65, S.E. = .310), β = − .47, t(101) = − 5.31, p < .001]. Insomnia symptoms explained 21.8% of the variance in health-related quality of life scores, R2 = .218, F(1, 101) = 28.23, p< .001. Of note, these analyses were repeated with insomnia diagnosis based on clinical interview and yielded similar results in magnitude and direction. However, insomnia severity as measured by the ISI was used in the path analysis models because it shows a range of symptomatology, which is more helpful when conducting path analysis (Kline, 2011). Additional details about these relationships are presented in Table 3.
Table 3.
Insomnia diagnosis and symptoms and health and quality of life outcomes.
| Viral load | CD4 | Health-related quality of life | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| b | S.E. | β | t | p | b | S.E. | β | t | p | b | S.E. | β | t | p | |
| Insomnia diagnosis | −.39 | .21 | −.19 | −1.87 | .064 | 129.17 | 66.11 | .20 | 1.95 | .054 | −13.84 | 5.33 | −.25 | −2.60 | .011* |
| Insomnia diagnosisa | – | – | – | – | – | – | −14.40 | 5.67 | −.26 | −2.54 | .013* | ||||
| Insomnia symptoms | .01 | .01 | .06 | .55 | .581 | 5.26 | 4.36 | .12 | 1.21 | .230 | −1.65 | .310 | −.47 | −5.31 | <.001*** |
| Insomnia symptomsa | – | – | – | – | – | – | −1.66 | .319 | −.47 | −5.20 | <.001*** | ||||
Key:
= p <.05
= p <.01
= p <.005
= adjusted model controlling for BMI, age, and current substance use severity
Mechanism model to explain insomnia and health outcomes
Health status (viral load and CD4 cells/mm3) model (Figure 2)
The model examining direct and indirect (through depression and medication adherence) relationships of insomnia on viral load and CD4 had acceptable model fit. The chi-square test of model fit indicated acceptable model fit X2 (4, N = 103) = 2.77, p= .598; The Bentler Comparative Fit Index (CFI) and Tucker-Lewis Index (TLI) both indicated acceptable fit (CFI = 1.000; TLI = 1.041). The root mean square error of approximation (RMSEA) also indicated acceptable fit (RMSEA = 0.000; 90%CI: 0.00–0.126). The standardized root mean square residual (SRMR) criteria was also consistent with acceptable model fit (SRMR = 0.029). In this model, insomnia symptoms were not directly associated with CD4 or viral load. Instead, it was associated indirectly, through associations of insomnia to depression, depression to medication adherence, medication adherence to log HIV RNA viral load, and viral load to CD4.
Specifically, insomnia symptoms were associated with depressive symptoms [β = .495, (95%CI: 0.375–0.614), S.E. = 0.061, p< .001), depressive symptoms were associated with medication adherence [β = −.909, (95%CI: −1.482–0.337), S.E. = .292, p= .001], medication adherence was associated with log HIV RNA viral load [β = −.011, (95%CI: −0.021–0.000), S.E. = 0.005, p= .048], and log HIV RNA viral load was associated with absolute CD4 count [β = −132.818, (95%CI: −191.902, −73.734), S.E. = 30.145, p< .001]. However, neither insomnia symptoms nor depressive symptoms were directly associated with absolute CD4 count.
Health-related quality of life model (Figure 3)
The model examining direct and indirect relationships of insomnia health-related quality of life also had acceptable model fit. The chi-square test of model fit indicated acceptable model fit X2 (4, N = 103) = 2.865, p= .581; The Bentler Comparative Fit Index (CFI) and Tucker-Lewis Index (TLI) both indicated acceptable fit (CFI = 1.000; TLI = 1.028). The root mean square error of approximation (RMSEA) also indicated acceptable fit (RMSEA = 0.000; 90%CI: 0.00–0.128). The standardized root mean square residual (SRMR) criteria was also consistent with acceptable model fit (SRMR = 0.029). In this model, although insomnia symptoms did have a direct association to health-related quality of life, it was also indirectly associated via a significant relationship of insomnia symptoms to depressive symptoms, and depressive symptoms to health-related quality of life was significant. Although depression was associated with adherence, and adherence to viral load, in this pathway, viral load was not associated with health-related quality of life.
Similar to the previous model, insomnia symptoms were associated with depressive symptoms [β = .495, (95%CI: 0.375–0.614), [β = ., (95%CI: 0.375–0.614), S.E. = 0.061, p< .001), depressive symptoms were associated with medication adherence [β = −.909, (95%CI: −1.482–0.337), S.E. = .292, p= .001], medication adherence was associated with log HIV RNA viral load [β = −.011, (95%CI: −0.021–0.000), S.E. = 0.005, p= .048]. In this model, insomnia syptoms [β = −.721, (95%CI:−1.435, −.008), S.E. = 0.364, p= .047] and depressive symptoms [β = −1.897, (95%CI:−2.798, −0.996), S.E. = 0.460, p< .001] were also directly associated with health-related quality of life. However, log HIV RNA viral load was not associated with health-related quality of life.
Discussion
This was the first study to our knowledge to conduct a comprehensive evaluation of insomnia, health status, and health-related quality of life in people living with HIV/AIDS. Based on clinical interview, more than half of a general, public clinic sample of people living with HIV/AIDS met diagnostic criteria for insomnia disorder. This adds to the existing literature on sleep problems within individuals with HIV/AIDS and confirms insomnia as a highly prevalent and interfering condition for this population.
We recruited a sample of participants from a public, hospital-based clinic and found that approximately 65% of participants met criteria for a DSM-5 insomnia disorder. This is consistent with prior estimates of insomnia disorder in general, clinic samples of people living with HIV/AIDS (Reid & Dwyer, 2005), and confirms our hypothesis that most of the sleep complaints in this population are, in fact, clinical insomnia disorder. Within this sample drawn from active patients in an HIV clinic it is clear that insomnia is an interfering condition that impacts quality of life. However, it is currently under identified in clinic samples, underdiagnosed, and, as a result, undertreated. Therefore, insomnia disorder represents a condition warranting more assessment within clinical samples of PLWHA.
Despite the well-established behavioral treatments for insomnia disorder including cognitive behavioral therapy for insomnia (Edinger et al., 2001; Espie et al., 2008, 1999), which has been associated with improvements in insomnia and in mental and physical health functioning in highly complex patient samples (Carney et al., 2017; Edinger et al., 2005; Manber et al., 2011, 2008; Savard et al., 2005a, 2005b; Smith et al., 2005) there has yet to be a published study testing CBT-I in a sample of people living with HIV/AIDS. With regard to clinical medical practice, there are also no published clinical guidelines for the treatment of insomnia in people living with HIV/AIDS. Our findings, in combination with previous research, serve as evidence that a need exists for identification of insomnia in PLWHA; and, that doing has the potential to improve symptoms and health outcomes.
With regard to our hypothesized relationships, we found that there was a direct relationship between insomnia and health-related quality of life. Insomnia was also indirectly associated with health-related quality of life via a significant relationship of insomnia to depression, and depression to health-related quality of life was significant. Depression was associated with adherence, and adherence to viral load, however, in this model, viral load was not associated with health-related quality of life. In contrast, there were no direct relationships between insomnia symptoms and physical health status, but strong indirect relationships between insomnia and health status operating through depression and medication adherence. This, in part, supports the direction of our model, suggesting that insomnia symptoms are linked with depressive symptoms, and in that way, are associated with HIV health outcomes. There is literature to show that psychiatric and physical health problems in PLWHA are associated with lower quality of life (Bing et al., 2000; Campsmith et al., 2003; Psaros et al., 2013) and lower health-related quality of life (Campsmith et al., 2003).
These findings are consistent with our previous research (Rogers et al., 2018). To our knowledge, we are the first to test a pathway to explain the relationship between insomnia and health status in people living with HIV/AIDS using path analysis. Our findings were significant and were based on a robust literature supporting each of these pathways. Specifically, the association between insomnia and depression (Manber & Chambers, 2009b; Riemann & Volderholzer, 2003) and depression and medication adherence (Gonzalez et al., 2011b; Wagner et al., 2011, p. 14). However, this was the first study that attempted to link these literatures to show a contribution of insomnia to this pathway, and, therefore, suggests a role for screening for insomnia in clinical samples of PLWHA.
This study is limited by cross-sectional data collection. Therefore, we are unable to make any conclusions about the temporality of the relationships observed. However, we chose to examine insomnia symptoms separately and place insomnia prior to depressive symptoms because of research that has established insomnia disorder as a separate disorder, not merely a symptom of depression, and a disorder that may even predate (Riemann & Volderholzer, 2003) and persist after treatment for depression. Perhaps the best evidence that insomnia is not better explained by co-occurring depression comes from results of the STAR*D trial (Rush et al., 2009). STAR*D examined a large, multi-site, stepped care approach to “real-world” depression treatment including multiple medication trials and cognitive therapy, showed that one of the most common residual symptoms of depression was insomnia, affecting more than half the sample (Nierenberg et al., 2010). This suggests that insomnia is unlikely to be a symptom of depression, as even with the best set of depressive treatments and remission, insomnia is often a lingering, residual symptom. Further evidence for the ordering of insomnia prior to depression in our model can be found from follow-up study of that same trial, which found that sleep onset insomnia was significantly associated with relapse in the follow-up time period of the study, suggesting that those with insomnia were more likely to have future depressive episodes (Sakurai et al., 2017).
Our study is also limited as an observational study. We did not have control over external stimuli; and, therefore, the internal validity of our findings is not as strong as it would be in a laboratory-based observational study. However, this is balanced by our external validity as we established feasibility for this type of study in our underserved, HIV-infected sample. This may have affected our ability to report on the etiology of insomnia disorder within this sample. For example, participants in our study were taking a wide array of HIV medications, and often, several at one time. To maximize generalizability of our results, we did not exclude for any medications or combinations of medications. Some medications used to treat HIV have been associated with sleep disruption (Omonuwa et al., 2009) and we cannot rule out this possibility in our sample. However, there were no notable associations between HIV medications participants reported and the occurrence of insomnia disorder in our sample. As such, we did not do further investigation into these associations.
Additionally, in the current study, blood test results for viral load and CD4 cells/mm3 were taken from existing medical chart data. A strength of our study was the effectiveness and ease of collecting data from a hard-to-reach, traditionally underserved patient population; however, that also meant that blood test results did not always align perfectly with our study visits, despite substantial effort to do so. Improving upon lab value collection would be an important next step for future research.
While it was outside the scope of this study to fully assess and diagnose obstructive sleep apnea, it is notable that so many individuals had risk factors for this sleep disorder. Insomnia disorder and obstructive sleep apnea have high co-occurrence in clinical sleep medicine settings, which has implications for assessment and treatment of both disorders, including how to determine sequencing of treatment for these sleep disorders (Cho et al., 2018; Ong et al., 2017). Thus, it is not surprising that we saw similar issues arise in our sample of PLWHA. Other studies have reported on the high number of individuals with HIV who meet criteria for obstructive sleep apnea and the impacts on fatigue and health (Goswami et al., 2015; Owens & Hicks, 2018) and this may be an important and related area of research.
Future research and clinical implications from this research include examining whether treating insomnia in a highly medically and psychiatrically complex population, like PLWHA, can positively impact health and health-related quality of life. Future work may also include more in-depth assessment of obstructive sleep apnea, as our assessments indicated this may also be a sleep disorder that is underreported and undertreated within HIV clinic settings. Finally, examining the role that inflammation may have in this model, due to the already high levels of inflammation in PLWHA due to both HIV and other chronic health conditions (Hunt, 2012; Zicari et al., 2019) and the anti-inflammatory effects of antiretroviral medications, would be another interesting area of research to pursue.
In conclusion, this study showed that insomnia was associated with HIV health via a behavioral pathway that linked insomnia with depression, medication adherence, log HIV RNA cells/mL (viral load), and CD4 in PLWHA. Insomnia was also associated with health-related quality of life through direct pathways. A unique strength of the study was the rigor with which we assessed insomnia disorder and associated sleep disorders within PLWHA, who are largely underserved and do not often have adequate access to sleep medicine professionals despite great need. Because HIV/AIDS is a manageable chronic condition, there are opportunities to address comorbidities, including sleep disorders, which affect health and health-related quality of life.
Insomnia is one comorbidity that has received little attention in the treatment literature yet is prevalent within clinical samples of PLWHA and is associated with significant health and health-related quality of life outcomes. Our findings support the importance of insomnia screening and assessment of sleep disruption more generally within HIV clinical settings as insomnia was associated with health, health-related quality of life, and overall life satisfaction. What remains to be tested is whether an intervention like CBT-I could be successfully implemented in HIV clinical settings to address insomnia and improve health and quality of life for this clinical population.
Acknowledgments
Research reported in this paper was supported by the National Institute of Mental Health of the National Institutes of Health under Award Number F31MH113481 (Rogers), 9K24DA040489 (Safren), and 5P30AI073961 (Pahwa). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This study was also supported by the Department of Psychology at the University of Miami.
Funding
This work was supported by the National Institute of Allergy and Infectious Diseases [5P30AI073961]; National Institute of Mental Health [F31MH113481]; National Institute on Drug Abuse [9K24DA040489]; University of Miami.
Footnotes
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Balderson BH, Grothaus L, Harrison RG, McCoy K, Mahoney C, & Catz S (2013). Chronic illness burden and quality of life in an aging HIV population. AIDS Care, 25(4), 451–458. 10.1080/09540121.2012.712669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett D, Walker B, Landay A, & Denny TN (2008). CD4 immunophenotyping in HIV infection. Nature Reviews. Microbiology, 6(11 Suppl), 7–15. 10.1038/nrmicro1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastien CH, Vallieres A, & Morin CM (2001). Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Medicine, 2(4), 297–307. 10.1016/S1389-9457(00)00065-4 [DOI] [PubMed] [Google Scholar]
- Benca RM, & Peterson MJ (2008). Insomnia and depression. Sleep Medicine, 9(Suppl1), S3–S9. 10.1016/S1389-9457(08)70010-8 [DOI] [PubMed] [Google Scholar]
- Bentler PM (1990). Comparative fit indexes in structural models. Psychological Bulletin, 107(2), 238–246. 10.1037/0033-2909.107.2.238 [DOI] [PubMed] [Google Scholar]
- Bing EG, Hays RD, Jacobson LP, Chen B, Gange SJ, Kass NE, Chmiel JS, & Zucconi SL (2000). Health-related quality of life among people with HIV disease: Results from the multicenter AIDS cohort study. Quality of Life Research, 9(1), 55–63. 10.1023/A:1008919227665 [DOI] [PubMed] [Google Scholar]
- Bozzette SA, Hays RD, Berry SH, Kanouse DE, & Wu AW (1995). Derivation and properties of a brief health status assessment instrument for use in HIV disease. [DOI] [PubMed]
- Browne MW, & Cudeck R (1992). Alternative ways of assessing model fit. Sociological Methods Research, 21(2), 230–258. 10.1177/0049124192021002005 [DOI] [Google Scholar]
- Buysse DJ, Angst J, Gamma A, Ajdacic V, Eich D, & Rossler W (2008). Prevalence, course, and comorbidity of insomnia and depression in young adults. Sleep-new York Then Westchester, 31(4), 473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CFI, Monk TH, Berman SR, & Kupfer DJ (1989). The Pittsburgh sleep quality index: A New instrument for psychiatric practice and research. Psychiatry Research, 28(2), 193–213. 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- Campsmith ML, Nakashima AK, & Davidson AJ (2003). Self-reported health-related quality of life in persons with HIV infection: Results from a multi-site interview project. Health and Quality of Life Outcomes, 1(1), 1. 10.1186/1477-7525-1-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney CE, Edinger JD, Kuchibhatla M, Lachowski AM, Bogouslavsky O, Krystal AD, & Shapiro CM (2017). Cognitive Behavioral insomnia therapy for those with insomnia and depression: A randomized controlled clinical trial. Sleep, 40(4). 10.1093/sleep/zsx019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung F, Subramanyam R, Liao P, Sasaki E, Shapiro C, & Sun Y (2012). High STOP-Bang score indicates a high probability of obstructive sleep apnoea. British Journal of Anaesthesia, 108(5), 768–775. 10.1093/bja/aes022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung F, Yegneswaran B, Liao P, Chung SA, Vairavanathan S, Islam S, Khajehdehi A, & Shapiro CM (2008). STOP questionnaire a tool to screen patients for obstructive sleep apnea. Anesthesiology, 108(5), 812–821. 10.1097/ALN.0b013e31816d83e4 [DOI] [PubMed] [Google Scholar]
- Darko DF, McCutchan MD, Kripke DF, Gillin JC, & Golshan S (1992). Fatigue, sleep disturbance, disability, and indices of progression of HIV infection. Am J Psychiatry, 149(4), 514–520. [DOI] [PubMed] [Google Scholar]
- Edinger JD (2011). Testing the reliability and validity of DSM-IV-TR and ICSD-2 insomnia diagnoses: Results of a multitrait-multimethod analysis. Archives of General Psychiatry, 68(10), 992. 10.1001/archgenpsychiatry.2011.64 [DOI] [PubMed] [Google Scholar]
- Edinger JD, Wohlgemuth WK, Krystal AD, & Rice JR (2005). Behavioral insomnia therapy for fibromyalgia patients: A randomized clinical trial. Archives of Internal Medicine, 165(21), 2527–2535. 10.1001/archinte.165.21.2527 [DOI] [PubMed] [Google Scholar]
- Edinger JD, Wohlgemuth WK, Radtke RA, Marsh GR, & Quillian RE (2001). Cognitive behavioral therapy for treatment of chronic primary insomnia. Journal of the American Medical Association, 285(14), 1856–1864. 10.1001/jama.285.14.1856 [DOI] [PubMed] [Google Scholar]
- Espie CA, Fleming L, Cassidy J, Samuel L, Taylor LM, White CA, Douglas NJ, Engleman HM, Kelly HL, & Paul J (2008). Randomized controlled clinical effectiveness trial of cognitive behavior therapy compared with treatment as usual for persistent insomnia in patients with cancer. Journal of Clinical Oncology, 26(28), 4651–4658. 10.1200/JCO.2007.13.9006 [DOI] [PubMed] [Google Scholar]
- Espie CA, Inglis SJ, Tessier S, & Harvey L (1999). The clinical effectiveness of cognitive behaviour therapy for chronic insomnia: Implementation and evaluation of a sleep clinic in general medical practice. Behaviour Research and Therapy, 29, 45–60. [DOI] [PubMed] [Google Scholar]
- Gagnon C, Belanger L, Ivers H, & Morin CM (2013). Validation of the insomnia severity index in primary care. The Journal of the American Board of Family Medicine, 26(6), 701–710. 10.3122/jabfm.2013.06.130064 [DOI] [PubMed] [Google Scholar]
- Gamaldo CE, Spira AP, Hock RS, Salas RE, McArthur JC, David PM, Mbeo G, & Smith MT (2013). Sleep, function and HIV: A multi-method assessment. AIDS and Behavior, 17(8), 2808–2815. 10.1007/s10461-012-0401-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez JS, Batchelder AW, Psaros C, & Safren SA (2011a). Depression and HIV/AIDS treatment nonadherence: A review and meta-analysis. JAIDS Journal of Acquired Immune Deficiency Syndromes, 1. 10.1097/QAI.0b013e31822d490a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez JS, Batchelder AW, Psaros C, & Safren SA (2011b). Depression and HIV/AIDS treatment nonadherence: A review and meta-analysis. JAIDS Journal of Acquired Immune Deficiency Syndromes, 1. 10.1097/QAI.0b013e31822d490a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami U, Baker JV, Wang Q, Khalil W, & Kunisaki KM (2015). Sleep apnea symptoms as a predictor of fatigue in an urban HIV clinic. AIDS Patient Care and STDs, 29(11), 591–596. 10.1089/apc.2015.0079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez J, Tedaldi EM, Armon C, Patel V, Hart R, & Buchacz K (2019). Sleep disturbances in HIV-infected patients associated with depression and high risk of obstructive sleep apnea. SAGE Open Medicine, 7, 2050312119842268. 10.1177/2050312119842268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, & Conde JG (2009). Research electronic data capture (REDCap) – A metadata-driven methodology and workflow process for providing translational research informatics support. 42(2), 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hislop J, Bulley C, & Mercer T (2011, May 23). Reliability and concurrent validity of GT1M and MTI actigraph Accelerometers using a mechanical set up. ICAMPAM. [Google Scholar]
- Hoffman C, & Rockstroh JK (2011). Monitoring (6.11). hivbook.com; viral load measurement. https://hivbook.com/tag/viral-load-measurement/ [Google Scholar]
- Hu L, & Bentler PM (1999). Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journa, 6(1), 1–55. 10.1080/10705519909540118 [DOI] [Google Scholar]
- Huang FY, Chung H, Kroenke K, Delucchi KL, & Spitzer RL (2006). Using the patient health questionnaire-9 to measure depression among racially and ethnically diverse primary care patients. Journal of General Internal Medicine, 21(6), 547–552. 10.1111/j.1525-1497.2006.00409.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt PW (2012). HIV and inflammation: Mechanisms and consequences. Current HIV/AIDS Reports, 9(2), 139–147. 10.1007/s11904-012-0118-8 [DOI] [PubMed] [Google Scholar]
- Kline RB (2011). Principles and practice of structural equation modeling (3rd ed.). Guilford Press. [Google Scholar]
- Kroenke K, & Spitzer RL (2002). The PHQ-9: A New diagnostic and severity measure. Psychiatric Annals, 32(9), 1–7. 10.3928/0048-5713-20020901-06 [DOI] [Google Scholar]
- Kroenke K, Spitzer RL, & Williams JB (2001). The PHQ-9 validity of a brief depression severity measure. Journal of General Internal Medicine, 16(9), 606–613. 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manber R, Bernert RA, Suh S, Nowakowski S, Siebern AT, & Ong JC (2011). CBT for insomnia in patients with high and low depressive symptom severity: Adherence and clinical outcomes. Journal of Clinical Sleep Medicine, 7(6), 645–652. 10.5664/jcsm.1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manber R, & Chambers AS (2009a). Insomnia and depression: A multifaceted interplay. Current Psychiatry Reports, 11(6), 437–442. 10.1007/s11920-009-0066-1 [DOI] [PubMed] [Google Scholar]
- Manber R, & Chambers AS (2009b). Insomnia and depression: A multifaceted interplay. Current Psychiatry Reports, 11(6), 437–442. [DOI] [PubMed] [Google Scholar]
- Manber R, Edinger JD, Gress JL, San Pedro-Salcedo MG, Kuo TF, & Kalista T (2008). Cognitive behavioral therapy for insomnia enhances depression outcome in patients with comorbid major depressive disor-der and insomnia. Sleep, 31(4), 489–495. 10.1093/sleep/31.4.489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNicholas WT (2009). Obstructive sleep apnea and inflammation. Progress in Cardiovascular Diseases, 51(5), 392–399. 10.1016/j.pcad.2008.10.005 [DOI] [PubMed] [Google Scholar]
- Morgenthaler T, Kramer M, Alessi C, Friedman L, Boehlecke B, Brown T, Coleman J, Kapur V, Lee-Chiong T, & Owens J (2006). Practice parameters for the psychological and behavioral treatment of insomnia: An update. An American academy of sleep medicine report. Sleep-new York Then Westchester-, 29(11), 1415. [PubMed] [Google Scholar]
- Muthén LK, & Muthén BO (2007). Mplus user’s guide (7th ed.). http://www.statmodel.com/download/usersguide/MplusUserGuideVer_7.pdf
- Nierenberg AA, Husain MM, Trivedi MH, Fava M, Warden D, Wisniewski SR, Miyahara S, & Rush AJ (2010). Residual symptoms after remission of major depressive disorder with citalopram and risk of relapse: A STAR*D report. Psychological Medicine, 40(1), 41–50. 10.1017/S0033291709006011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omonuwa TS, Goforth HW, Preud’homme X, & Krystal AD (2009). The pharmacologic management of insomnia in patients with HIV. Journal of Clinical Sleep Medicine: JCSM: Official Publication of the American Academy of Sleep Medicine, 5(3), 251–262. [PMC free article] [PubMed] [Google Scholar]
- Owens RL, & Hicks CB (2018). A wake-up call for human immunodeficiency virus (HIV) providers: Obstructive sleep apnea in people living with HIV. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America, 67(3), 472–476. 10.1093/cid/ciy217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psaros C, O’Cleirigh C, Bullis JR, Markowitz SM, & Safren SA (2013). The influence of psychological variables on health-related quality of life among HIV-positive individuals with a history of intravenous drug use. Journal of Psychoactive Drugs, 45(4), 304–312. 10.1080/02791072.2013.825030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid S, & Dwyer J (2005). Insomnia in HIV infection: A systematic review of prevalence, correlates, and management. Psychosomatic Medicine, 67(2), 260–269. 10.1097/01.psy.0000151771.46127.df [DOI] [PubMed] [Google Scholar]
- Riemann D, & Volderholzer U (2003). Primary insomnia: A risk factor to develop depression? Journal of Affective Disorders, 76(1–3), 255–259. 10.1016/S0165-0327(02)00072-1 [DOI] [PubMed] [Google Scholar]
- Rogers BG, Lee JS, Bainter SA, Bedoya CA, Pinkston MM, & Safren SA (2018). A multilevel examination of sleep, depression, and quality of life in people living with HIV/AIDS. Journal of Health Psychology. 10.1177/1359105318765632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth T (2007). Insomnia: Definition, prevalence, etiology, and consequences. Journal of Clinical Sleep Medicine, 3(5 Suppl), S7. 10.5664/jcsm.26929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein ML, & Selwyn PA (1998). High prevalence of insomnia in an outpatient population with HIV infection. Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology, 19(3), 260–265. 10.1097/00042560-199811010-00008 [DOI] [PubMed] [Google Scholar]
- Rush AJ, Warden D, Wisniewski SR, Fava M, Trivedi MH, Gaynes BN, & Nierenberg AA (2009). STAR*D: Revising conventional wisdom. CNS Drugs, 23(8), 627–647. 10.2165/00023210-200923080-00001 [DOI] [PubMed] [Google Scholar]
- Sakurai H, Suzuki T, Yoshimura K, Mimura M, & Uchida H (2017). Predicting relapse with individual residual symptoms in major depressive disorder: A reanalysis of the STAR*D data. Psychopharmacology, 234(16), 2453–2461. 10.1007/s00213-017-4634-5 [DOI] [PubMed] [Google Scholar]
- Savard J, Simard S, Ivers H, & Morin CM (2005a). Randomized study on the efficacy of cognitive-behavioral therapy for insomnia secondary to breast cancer, part I: Sleep and psychological effects. Journal of Clinical Oncology, 23(25), 6083–6096. 10.1200/JCO.2005.09.548 [DOI] [PubMed] [Google Scholar]
- Savard J, Simard S, Ivers H, & Morin CM (2005b). Randomized study on the efficacy of cognitive-behavioral therapy for insomnia secondary to breast cancer, part II: Immunologic effects. Journal of Clinical Oncology, 23(25), 6097–6106. 10.1200/JCO.2005.12.513 [DOI] [PubMed] [Google Scholar]
- Smith MT, Huang MI, & Manber R (2005). Cognitive behavior therapy for chronic insomnia occurring within the context of medical and psychiatric disorders. Clinical Psychology Review, 25(5), 559–592. 10.1016/j.cpr.2005.04.004 [DOI] [PubMed] [Google Scholar]
- Taylor DJ (2008). Insomnia and depression. Sleep, 31(4), 447–448. 10.1093/sleep/31.4.447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DJ, & Lichstein KL (2003). Insomnia as a health risk factor. Behavioral Sleep Medicine, 1(4), 227–247. 10.1207/S15402010BSM0104_5 [DOI] [PubMed] [Google Scholar]
- Taylor DJ, Mallory LJ, Lichstein KL, Durrence H, Riedel BW, & Bush AJ (2007). Comorbidity of chronic insomnia with medical problems. SLEEP, 30(2), 213. 10.1093/sleep/30.2.213 [DOI] [PubMed] [Google Scholar]
- Wagner GJ, Goggin K, Remien RH, Rosen MI, Simoni J, Bangsberg DR, & Liu H, & MACH14 Investigators. (2011). A closer look at depression and its relationship to HIV antiretroviral adherence. Annals of Behavioral Medicine, 42(3), 352–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson IB, Fowler FJ, Cosenza CA, Michaud J, Bentkover J, Rana A, Kogelman L, & Rogers WH (2014). Cognitive and field testing of a new set of medication adherence self-report items for HIV care. AIDS and Behavior, 18 (12), 2349–2358. 10.1007/s10461-013-0610-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson IB, Lee Y, Michaud J, Fowler FJ, & Rogers WH (2016). Validation of a new three-item self-report measure for medication adherence. AIDS and Behavior, 20(11), 2700–2708. 10.1007/s10461-016-1406-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe EM, Schneider J, Hawks EM, Pho H, Guzman MA, Schneider H, & Kirkness JP (2015). Sleep-wake transitions are detectable with actigraphy in patients with chronic obstructive pulmonary disease (COPD). In Sleep disordered breathing in pediatric and adult medical disorders. American Thoracic Society. [Google Scholar]
- Wu AW, Huang I-C, Gifford A, Spritzer L, Bozzette KL, & Hays RD (2005). Creating a crosswalk to estimate AIDS clinical trials group quality of life scores in a nationally representative sample of persons in care for HIV in the United States. 6(3), 147–157. [DOI] [PubMed] [Google Scholar]
- Zicari S, Sessa L, Cotugno N, Ruggiero A, Morrocchi E, Concato C, Rocca S, Zangari P, Manno EC, & Palma P (2019). Immune activation, inflammation, and non-AIDS co-morbidities in HIV-infected patients under long-term ART. Viruses, 11(3), 3. 10.3390/v11030200 [DOI] [PMC free article] [PubMed] [Google Scholar]