Abstract
Background & Aims:
Mast cells are believed to contribute to development of eosinophilic gastrointestinal disorders (EGIDs). We quantified mast cells and eosinophils in biopsies from patients with EGIDs and without known esophageal or gastrointestinal disease to investigate associations between these cell types and EGID and its features.
Methods:
We conducted a retrospective study of patients with EGID (n=52) and of children and adults who underwent upper endoscopy and were found to have no evidence of gastrointestinal or systemic conditions (n=123). We re-reviewed archived gastric and duodenal biopsies to quantify mast cells (by tryptase immunohistochemistry) and eosinophils. We calculated the specificity of cell count thresholds for identification of patients with EGIDs and evaluated the correlation between mast cell and eosinophil counts and clinical and endoscopic features.
Results:
In the gastric biopsies from patients without esophageal or gastrointestinal diseases, the mean mast cell count was 18.1±7.2 cells/higher-power field (hpf) and the peak mast cell count was 21.9±8.2 cells/hpf. In the duodenal biopsies from patients without esophageal or gastrointestinal diseases, the mean mast cell count was 23.6±8.1 cells/hpf and the peak mast cell count was 28.1±9.3 cells/hpf. Mean and peak eosinophil counts in gastric biopsies from patients without disease were 3.8±3.6 eosinophils/hpf and 5.8±5.0 eosinophils/hpf; mean and peak eosinophil counts in duodenal biopsies were 14.6±8.9 eosinophils/hpf and 19.5±11.0 eosinophils/hpf. A mean count of 20 eosinophils/hpf in gastric biopsies or 30 eosinophils/hpf in duodenal biopsies identified patients with EGIDs with high specificity. Gastric and duodenal biopsies from patients with EGIDs had significant increases in mean mast cell counts compared to biopsies from patients without EGIDs. There was a correlation between mean mast cell and eosinophil counts in duodenal biopsies (R=0.47; P=.01). Mean mast cell and eosinophil counts did not correlate with symptoms or endoscopic features of EGIDs.
Conclusions:
We identified thresholds for each cell type that identify patients with EGIDs with 100% specificity. The increased numbers of mast cells and eosinophils in gastric and duodenal tissues from patients with EGIDs supports the concept that these cell types are involved in pathogenesis. However, cell counts are not associated with symptoms or endoscopic features of EGIDs.
Keywords: diagnosis, immune cells, abdominal pain, inflammation
Introduction
Gastrointestinal dysfunction and eosinophilic-predominant inflammation characterize eosinophilic gastrointestinal disorders (EGIDs) following the exclusion of alternative etiologies of gastrointestinal eosinophilia.1–4 Though eosinophilic esophagitis (EoE) represents a major contributor to gastrointestinal morbidity,5,6 pathologic eosinophilia occurs throughout the gastrointestinal tract, and non-EoE EGIDs are poorly understood.7,8 Controversy exists over normal gastrointestinal eosinophil counts,9,10,19,11–18 diagnostic eosinophil count thresholds,7,20 number of biopsies to obtain,21 and pathogenesis.22–25
Mast cell over-accumulation and inappropriate activation suggest a role in EGID pathogenesis.22–27 Though mast cell levels correlate with some EoE disease features,28 associations between mast cells and non-EoE EGID disease features are poorly described.29 As with eosinophil counts, limited data describing normal mast cell counts and diagnostic thresholds for increased mast cell levels further complicate this knowledge gap.11,29–36
This study aimed to determine levels of gastric and duodenal mast cells and eosinophils in a cohort of adults and children without known gastrointestinal or systemic disease and to explore diagnostic thresholds for increased cell levels. We also aimed to quantify mast cells and eosinophils in patients with eosinophilic gastroenteritis with stomach and/or duodenal involvement and assess for associations between these cells and clinical disease features.
Methods
We conducted a retrospective cohort study at the University of North Carolina (UNC). The UNC IRB approved this study. There were two main study aspects. The first was to establish the normal level of gastric and duodenal mast cells and eosinophils in a non-diseased cohort. To this end, we utilized the UNC endoscopy database to select a purposive sample of approximately 100 gastric and 100 duodenal tissue samples from children and adults undergoing outpatient upper endoscopy between January and June 2018. Eligible patients had a normal gastric and duodenal endoscopic appearance; normal biopsy results on clinical interpretation and on re-review for this study (see below); no known pre-existing gastrointestinal or serious systemic condition; and no subsequently diagnosed gastrointestinal or serious systemic conditions after our data review. Patients with abdominal pain as the indication for endoscopy and/or receipt of corticosteroids or mast cell stabilizers were excluded. Gastric biopsy samples were restricted to patients seen with an esophageal (e.g. heartburn) or small bowel indication (e.g. diarrhea). Duodenal biopsy samples were restricted to patients with an esophageal indication. Helicobacter pylori was excluded routinely on gastric biopsy samples.
Following non-diseased cohort assembly, the patients’ archived paraffin-embedded tissue blocks were obtained and a set of slides was prepared for hematoxylin and eosin staining and mast cell tryptase immunohistochemistry staining. Immunohistochemistry was performed using an automated slide stainer (Biocare intelliPATH). Sections were de-paraffinized, and heat induced epitope retrieval was performed. Endogenous peroxidase activity was blocked with a 3% hydrogen peroxide solution, and a protein block was applied. Slides were incubated with mouse anti-human Mast Cell Tryptase primary antibody (Clone AA1; Dako M7052, Cambridge, UK; 1:1000 dilution) at room temperature for 1 hour. Negative controls were treated as above except the primary antibody was replaced with the same concentration of a mouse IgG1 antibody (Dako X0931). The primary antibody was detected using an anti-mouse polymer and visualized with DAB (Vector Labs, SK-4100, Burlingame, CA). Slides were counterstained with Mayer’s Hematoxylin.
Biopsies were re-read by a single expert pathologist and any patients with newly identified pathology were excluded (Supplementary table 7). Immunoreactive mast cells were counted using an Olympus BX43 microscope equipped with a 40X lens and 22-mm diameter oculars with a resulting higher-power field [hpf] area measuring 0.237 mm2 (i.e. multiply cells/hpf by 4.22 for cells/mm2). A total of 5 hpfs were counted and averaged to yield a mean count per subject. When more than one fragment of tissue was present on one or more slides from the same biopsy set, at least one sample field from each fragment was utilized. For duodenal biopsies, the portion of the duodenal mucosa comprised between Brunner’s glands and the base of villi was used for counting. Eosinophil counts were similarly quantified. Specifically, each slide was surveyed at low and medium power to determine areas with highest cell density. A total of 5 hpfs were counted from biopsies of the stomach and duodenum, and the counts averaged. Peak counts for eosinophil and mast cell counts were reported as the highest single hpf.
In the second study component, we assessed for associations between mast cell counts and clinical EGID features, utilizing the UNC EGID clinicopathologic database.37 Patients within this database demonstrated ≥20 peak eosinophils/hpf (eos/hpf) on gastric (eosinophilic gastritis patients) and/or duodenal biopsy (eosinophilic gastroenteritis), GI symptoms, and no secondary cause of eosinophilia. Similar to the first cohort, patients’ archived paraffin-embedded tissue blocks were sectioned for new hematoxylin and eosin and mast cell immunohistochemistry tryptase staining. Staining procedures and cell quantification for the first and second study components were identical.
Clinical data were extracted from electronic medical records on standardized case report forms and entered into a study database for each included patient, either at the time of referral for upper endoscopy in our non-diseased cohort or at the time of EGID diagnosis for our EGID cohort. Extracted data included demographics, medications, endoscopic findings, treatment, and symptoms.
Summary statistics described the cohort. Student’s t-test compared mast cell counts and demographic features of our non-diseased cohort. Simple linear regression assessed for associations between tissue eosinophil and mast cell counts and clinical EGID features and mast cell levels. Analysis of variance assessed for an association between study indication and mast cells. Student’s t-test compared mast cell counts between the non-diseased and EGID cohort. To assess the diagnostic specificity for a range of cell count thresholds, we determined the proportion of patients under a chosen threshold count and with a range of hpfs required. Statistical analyses were performed using Stata 14.2 (Stata Corp, College Station, TX).
Results
Baseline characteristics of non-diseased cohort
Of 123 patients (mean age 27.7 years, 39% male, 64% white), 92 contributed gastric biopsies (mean age 23.9; 41% male; 62% white; 40% on proton pump inhibitor [PPI]) and 94 contributed duodenal biopsies (mean age 24.8; 37% male; 68% white; 39% on PPI) (Table 1). The most common diagnoses were ultimately gastroesophageal reflux disease (25%) and functional or chronic abdominal pain without any other structural or inflammatory diagnosis (17%).
Table 1.
Demographic data for non-diseased cohort
| Gastric biopsy patients (n = 92) | Duodenal biopsy patients (n = 94) | |
|---|---|---|
| Age (mean ± SD1; range) | 23.9 ± 18.3; 3 – 77 | 24.8 ± 21.0 (2–83) |
| Children <18 (n, %) | 56 (61) | 61 (65) |
| Male (n, %) | 38 (41) | 35 (37) |
| Race (n, %) | ||
| White | 57 (62) | 64 (68) |
| African-American | 11 (12) | 10 (11) |
| Asian | 1 (1) | 0 (0) |
| Pacific Islander | 1 (1) | 1 (1) |
| Other | 13 (14) | 10 (11) |
| Unknown | 9 (10) | 10 (11) |
| Ethnicity (n, %) | ||
| Hispanic/Latino | 12 (13) | 8 (9) |
| Non-Hispanic/Latino | 70 (76) | 77 (82) |
| Unknown | 10 (11) | 9 (10) |
| On a PPI2 (n, %) | 37 (40) | 37 (39) |
| Procedure indication (n, %) | ||
| Heartburn/reflux | 40 (43) | 42 (44) |
| Dysphagia | 18 (20) | 22 (23) |
| Diarrhea | 14 (15) | 5 (5) |
| Anemia3 | 12 (13) | 17 (18) |
| Weight loss/feeding difficulties | 5 (5) | 7 (7) |
| Nausea | 2 (2) | 2 (2) |
| Chest pain | 1 (1) | 1 (1) |
SD: standard deviation
PPI: proton pump inhibitor
Non-GI or iron deficiency-related
Normal gastric and duodenal mast cell counts and diagnostic threshold considerations
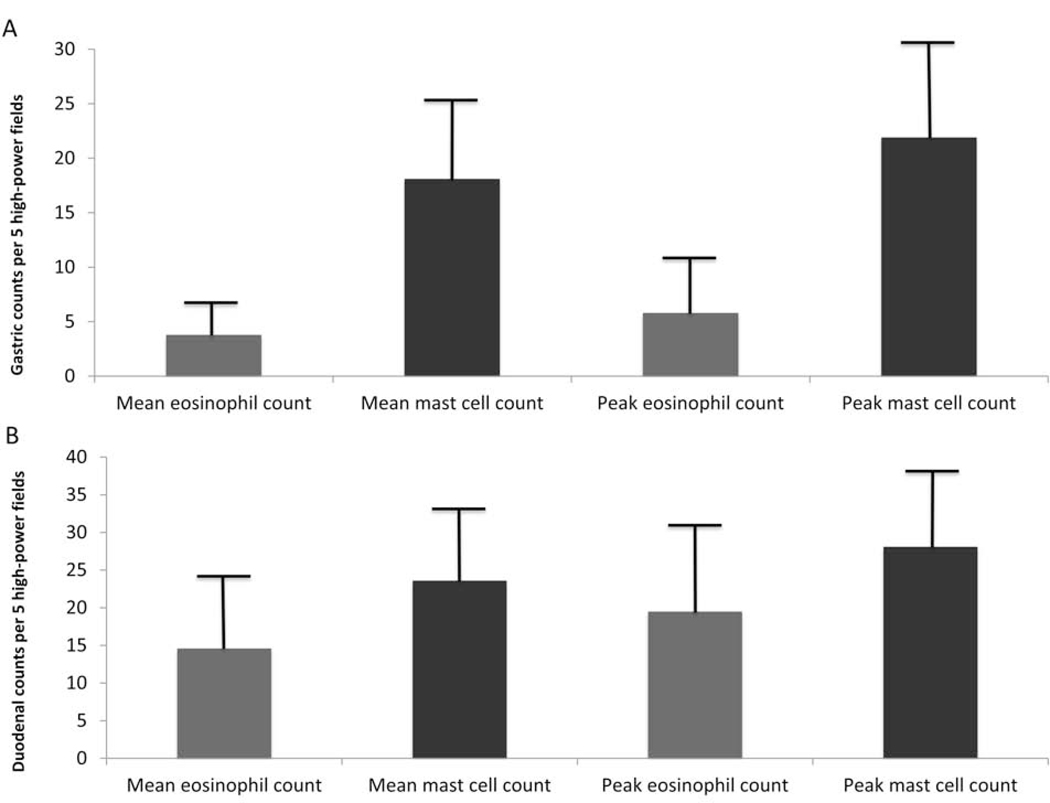
Most gastric biopsies were of oxyntic and antrum origin (45%) or oxyntic origin alone (37%), 13% were from antrum, and 5% were from transitional mucosa. The mean gastric mast cell count was 18.1± 7.2 mast cells/hpf, and the mean peak gastric mast cell count was 21.9 ± 8.2. For mean and peak duodenal counts, the values were 23.6 ± 8.1 and 28.1 ± 9.3 mast cells/hpf, respectively (Figures 1A/B).
Figure 1.
Mean and peak gastric (A) and duodenal (B) eosinophil and mast cell counts in patients without known gastrointestinal or systemic disease per 5 high-power fields; error bars represent a standard deviation.
Peak and mean gastric and duodenal mast cell counts were lower in patients receiving PPI (gastric peak: 23.4 ± 8.2 vs 19.7 ± 7.6 mast cells/hpf; p=0.03) and differed by origin (antrum peak: 15.2 ± 7.1 mast cells/hpf vs. oxyntic: 24.1 ± 8.5; p=0.002). No differences in mast cell counts were found by age or sex (Table 2) or procedure indication. All patients underwent endoscopy in winter or spring. No differences were seen for any cell counts by season (e.g. gastric peak eosinophil in winther vs. spring: 6.6 vs. 6.2; p = 0.74).
Table 2.
Comparison of peak and mean gastric and duodenal mast cell and eosinophil counts by factors of interest in non-diseased cohort
| PPI | No PPI | P value | |
|---|---|---|---|
|
| |||
| Gastric counts1 | N = 37 | N = 55 | |
| Mast cells | |||
| Peak | 19.7 ± 7.6 | 23.4 ± 8.2 | 0.03 |
| Mean | 16.3 ± 6.8 | 19.2 ± 7.3 | 0.06 |
| Eosinophils | |||
| Peak | 6.0 ± 5.0 | 5.7 ± 5.0 | 0.77 |
| Mean | 3.8 ± 3.3 | 3.8 ± 3.8 | 0.94 |
| Duodenal counts | N = 37 | N = 58 | |
| Mast cells | |||
| Peak | 25.6 ± 9.1 | 29.7 ± 9.2 | 0.04 |
| Mean | 21.2 ± 7.4 | 25.1 ± 8.3 | 0.02 |
| Eosinophils | |||
| Peak | 18.7 ± 12.3 | 20.0 ± 10.2 | 0.60 |
| Mean | 14.0 ± 9.8 | 15.0 ± 8.2 | 0.57 |
|
| |||
| Child | Adult | P value | |
|
| |||
| Gastric counts | N = 56 | N = 36 | |
| Mast cells | |||
| Peak | 22.6 ± 7.7 | 20.8 ± 8.8 | 0.3 |
| Mean | 18.9 ± 6.7 | 16.8 ± 7.9 | 0.2 |
| Eosinophils | |||
| Peak | 4.7 ± 4.5 | 7.5 ± 5.2 | 0.007 |
| Mean | 2.9 ± 2.9 | 5.2 ± 4.1 | 0.003 |
| Duodenal counts | N = 61 | N = 34 | |
| Mast cells | |||
| Peak | 28.8 ± 9.3 | 26.7 ± 11.7 | 0.3 |
| Mean | 23.9 ± 6.6 | 22.9 ± 10.5 | 0.6 |
| Eosinophils | |||
| Peak | 18.8 ± 10.6 | 20.7 ± 11.8 | 0.43 |
| Mean | 13.9 ± 8.2 | 16.0 ± 9.9 | 0.26 |
|
| |||
| Male | Female | P value | |
|
| |||
| Gastric counts | N = 38 | N = 54 | |
| Mast cells | |||
| Peak | 21.2 ± 7.4 | 22.4 ± 8.6 | 0.5 |
| Mean | 17.3 ± 6.5 | 18.6 ± 7.7 | 0.4 |
| Eosinophils | |||
| Peak | 5.7 ± 5.0 | 5.9 ± 5.0 | 0.87 |
| Mean | 3.5 ± 3.1 | 4.0 ± 4.0 | 0.49 |
| Duodenal counts | N = 35 | N = 60 | |
| Mast cells | |||
| Peak | 29.3 ± 8.0 | 27.4 ± 10.0 | 0.3 |
| Mean | 24.1 ± 7.0 | 23.3 ± 8.8 | 0.7 |
| Eosinophils | |||
| Peak | 21.4 ± 12.3 | 18.3 ± 10.2 | 0.19 |
| Mean | 15.8 ± 9.5 | 13.9 ± 8.4 | 0.31 |
|
| |||
| White | Non-white | P value | |
|
| |||
| Gastric counts | N = 57 | N = 35 | |
| Mast cells | |||
| Peak | 22.4 ± 7.7 | 21.1 ± 9.0 | 0.5 |
| Mean | 18.6 ± 6.8 | 17.2 ± 7.9 | 0.4 |
| Eosinophils | |||
| Peak | 4.9 ± 4.8 | 7.3 ± 5.0 | 0.03 |
| Mean | 5.1 ± 4.2 | 3.0 ± 2.9 | 0.005 |
| Duodenal counts | N = 63 | N = 31 | |
| Mast cells | |||
| Peak | 28.4 ± 9.6 | 27.4 ± 8.8 | 0.6 |
| Mean | 23.8 ± 8.4 | 23.1 ± 7.7 | 0.7 |
| Eosinophils | |||
| Peak | 20.2 ± 11.6 | 18.0 ± 9.6 | 0.38 |
| Mean | 15.1 ± 9.2 | 13.6 ± 8.0 | 0.43 |
|
| |||
| Antral cells | Oxyntic cells | P value | |
|
| |||
| Gastric counts | N = 12 | N = 37 | |
| Mast cells | |||
| Peak | 15.2 ± 7.1 | 24.1 ± 8.5 | 0.002 |
| Mean | 12.4 ± 6.0 | 20.0 ± 7.5 | 0.003 |
| Eosinophils | |||
| Peak | 5.4 ± 4.8 | 4.9 ± 4.3 | 0.75 |
| Mean | 3.2 ± 3.0 | 3.5 ± 3.4 | 0.73 |
Peak count per 5 high-power fields and mean per 5 high-power fields
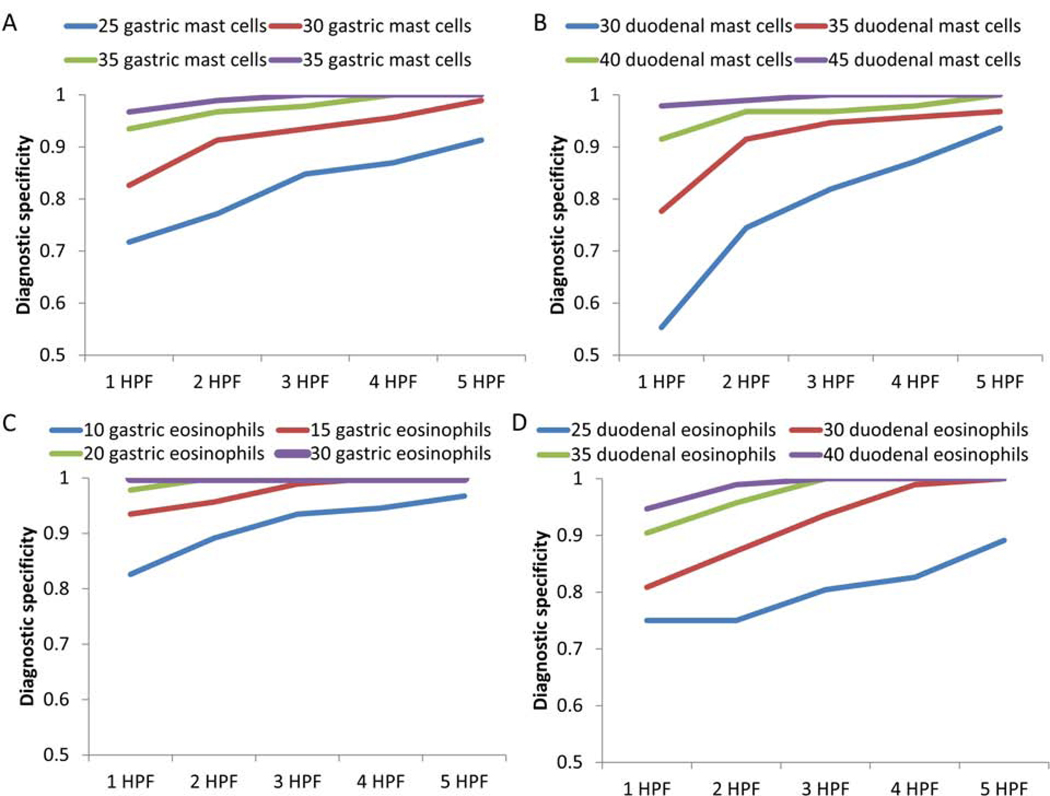
Diagnostic thresholds were calculated for peak and mean gastric and duodenal mast cell counts. For patients with a mean gastric mast cell count >20, 25, 30, or 35 mast cells/hpf, diagnostic specificities were 0.67, 0.79, 0.92, and 0.98, respectively. For the number of hpfs exceeding the threshold, the diagnostic specificity for 1 hpf exceeding 25 mast cells was 0.71, the diagnostic specificity for 3 hpfs exceeding 30 mast cells was 0.93, and the diagnostic specificity for 4 hpfs exceeding 35 mast cells was 1.0 (Figure 2A) (Supplementary table 1).
Figure 2.
Diagnostic specificities for elevated gastric mast cells (A) gastric mast cells, (B) duodenal mast cells, (C) gastric eosinophils, (D) duodenal eosinophils at both designated cutpoint thresholds per high-power field (HPF) and number of HPFs required for counting.
A mean duodenal mast cell count >30, 35, or 40 mast cells/hpf had diagnostic specificities of 0.79, 0.94, and 0.97, respectively. The diagnostic specificity of 1 hpf with greater than 30 mast cells/hpf was 0.55; the diagnostic specificity of 3 hpfs with greater than 35 mast cells/hpf was 0.95; the diagnostic specificity of 5 hpfs with greater than 40 mast cells/hpf was 1.0 (Figure 2B) (Supplementary table 1).
Normal gastric and duodenal eosinophil cell counts and diagnostic threshold considerations
The mean and peak gastric eosinophil counts were 3.8 ± 3.6 and 5.8 ± 5.0 eos/hpf. The mean and peak duodenal eosinophil counts were 14.6 ± 8.9 and 19.5 ± 11.0 eos/hpf.
Among the 92 patients contributing gastric biopsies, diagnostic specificities of 0.75, 0.92, and 0.99 were found for patients with a mean eosinophil count > 5, 10, and 15 eosinophils per 5 hpfs, respectively. The diagnostic specificity for 1 hpf > 10 eosinophils was 0.83; the diagnostic specificity for 2 hpfs with > 10 eosinophils was 0.89; the diagnostic specificity for 3 hpfs > 10 eosinophils was 0.93 (Figure 2C) (Supplementary table 1).
For duodenal eosinophil counts, diagnostic specificities for mean eosinophil counts > 20, 25, and 30 were 0.74, 0.84, and 0.90 per 5 hpfs. A single hpf with >30 eosinophils had a specificity of 0.81; diagnostic specificity of two hpfs with >30 eosinophils >30 was 0.87; diagnostic specificity of three hpfs >30 was 0.94; diagnostic specificity of 2 hpfs with >35 eosinophils was 0.96 (Figure 2D) (Supplementary table 1).
Eosinophilic gastroenteritis eosinophil and mast cell counts and associations with clinical disease features
There were 52 patients with eosinophilic gastroenteritis of the stomach and/or duodenum (mean age 20.6; 52% male; 63% white; 46% concurrent EoE). The most frequent presenting symptoms were regurgitation or vomiting (71%), abdominal pain (58%), and dysphagia (27%) (Supplementary table 2). The mean and mean peak gastric eosinophil counts were 63.5 ± 64.0 and 84.2 ± 75.9 eos/hpf, respectively. Mean and mean peak duodenal eosinophil counts were 62.5 ± 38.8 and 81.5 ± 52.3 eos/hpf. Mean peak and mean mast cell counts of the stomach were 44.2 ± 19.6 and 37.6 ± 17.1. For duodenal counts, mean and mean peak mast cell counts were 40.4 ± 13.3 and 47.5 ± 14.8. Counts were all significantly higher than in the non-disease cohort (p < 0.001 for all comparisons).
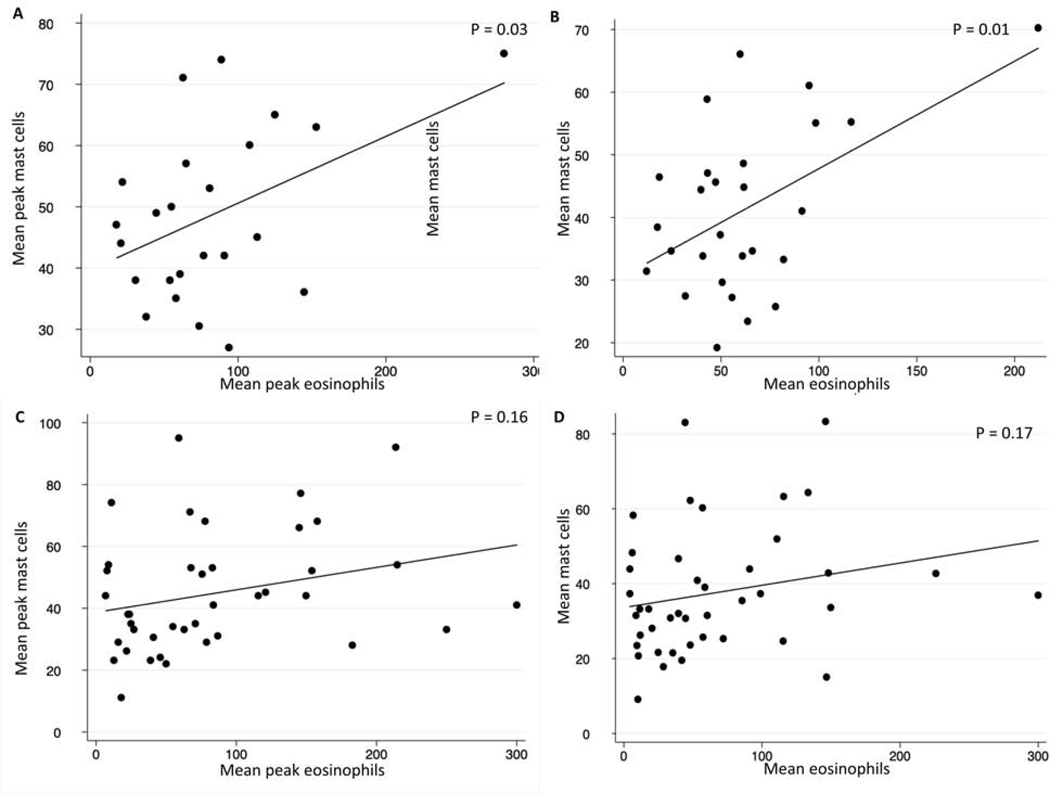
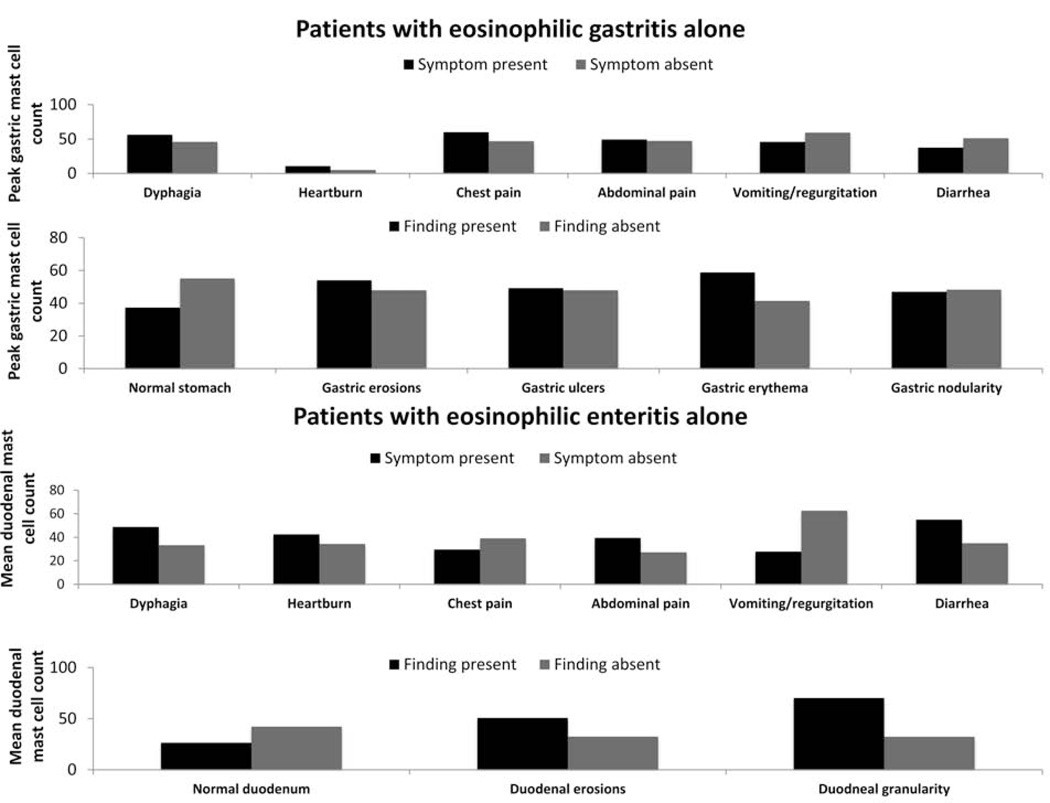
A weak association between mean peak gastric eosinophil and mast cells counts was found, with gastric peak eosinophil and peak masts cell counts slightly correlating (R=0.22; p=0.16). Similarly, the association between mean gastric and duodenal eosinophil and mast cell counts was weak (R=0.21; p = 0.17). However, a moderate association was for mean peak (R=0.41; p=0.03) and mean (R=0.47; p=0.01) duodenal eosinophil and mast cell counts (Figure 3). No consistent associations were found between mast cell and eosinophil levels and clinical disease features of eosinophilic gastroenteritis patients (Figure 4, data not shown).
Figure 3.
Associations in EGID cohort. between (A) duodenal eosinophil and mast cell peak counts; (B) duodenal eosinophil and mast cell mean counts; (C) gastric eosinophil and mast cell mean peak counts; (D) gastric eosinophil and mast cell mean counts.
Figure 4.
Associations between clinical symptoms and endoscopic findings, and gastric and duodenal mast cell counts in eosinophilic gastrointestinal disease patients.
Discussion
Non-EoE EGIDs are rare38,397,8 and uncertainties remain including normal levels of gastrointestinal eosinophilia,9–18 eosinophil thresholds for diagnosis,7,20 and number of biopsies to maximize sensitivity.21 The understanding of EGID pathogenesis also remains incomplete. However, the association between mast cell levels and EoE disease features,28 and the inappropriate activation of mast cells and associated chronic mucosal tissue remodeling22,26,27,40 in EGID patients implicate a role for mast cells in pathogenesis.22–25 Here, we established normal gastric and duodenal mast cell and eosinophil levels in patients without known GI diseases and described thresholds providing high diagnostic specificity. We also quantified mast cells and eosinophils in patients with EGIDs and assessed for associations between mast cells and disease features.
In a non-diseased cohort, the mean and peak gastric mast cell counts were 18 and 22 mast cells/hpf. For mean and peak duodenal counts, values were 24 and 28, respectively. Levels did not substantially vary by clinical factors, but were significantly lower than those of the EGID cohort. While mast cell levels did not closely associate with clinical features in our EGID cohort, high levels and increased activation state40 suggest a role in EGID pathogenesis. Interestingly, a moderate correlation existed between duodenal eosinophils and mast cells, though correlation was weak in the stomach. A poor correlation between these cell types has also been described in EoE.41,42 PPI reduced gastric and duodenal mast cell counts, though not to a clinically meaningful extent. The mechanism remains unclear, though based on data from the esophagus, it may derive from either an anti-inflammatory/anti-eosinophilic effect or improvement of mucosal integrity.
We assessed gastroduodenal mast cell and eosinophil thresholds for diagnosis (Supplementary table 1). For average gastric mast cell levels over 20 and 35 cells per 5 hpfs, specificity ranged from 0.67 to 0.97. Similarly, specificity ranged from 0.71 for one hpf exceeding 25 to 1.0 for four hpfs exceeding 35 mast cells. Diagnostic specificity increases when raising the threshold and/or the number of hpfs required. When maximizing specificity, the work of the interpreting pathologist and proportion of false negatives rise (i.e. lower sensitivity). For example, a patient with more than 35 gastric mast cells in 4 hpfs is unlikely to have anything but an EGID.
Prior studies assessing mast cells and associations with non-EoE EGIDs are limited; a study of 12 pediatric lower EGID patients did not find a correlation between eosinophil and mast cell counts (r = −0.11; p = 0.59).29 Unlike our study, mast cell levels of the non-diseased cohort and EGID patients were not appreciably different. More is known about esophageal mast cells and their association with EoE. A study documented an elevation of total esophageal mast cell counts in EoE patients with a histologic but poor clinical response.28 Conversely, another study did not find an association between EoE clinical symptoms and esophageal mast cell levels.42
Normal gastric and duodenal eosinophil and mast cell counts have been debated in the literature, though quantified in several non-diseased cohorts (Supplemental tables 3-4). Study limitations make interpreting and implementing results challenging. Studies may be limited to children;9–11,16,17,29,30,32 do not describe the size of a hpf;15,17,18,30,33,35 and others included small numbers,30,31,35 estimated rather than fully enumerating cell counts,15,17,30 or did not present full clinical details.31 Th findings stress the heterogeneity by which gastrointestinal mast and eosinophils have been visualized, quantified, and stained, and no prior study proposed specificities or sensitivities for diagnostic thresholds. The inclusion of functional dyspepsia may overestimate eosinophil counts within a ‘non-diseased’ cohort. However, these patients only constituted 17% of this group, and their inclusion would bias differences to the null. An important study aspect is the provision of diagnostic thresholds for eosinophil counts in eosinophilic gastroenteritis based on count specificities. A mean gastric eosinophil count > 20 in five hpfs provides a specificity of 100%, as does as peak count of >20 in two hpfs. Similarly, a peak duodenal eosinophil count > 30 in 3 hpfs provides a specificity of 94%. Interestingly, these thresholds are lower than what some studies have used based on expert opinion,43,44 which suggests that a less stringent cut-point that is easier to clinically implement is feasible.
Limitations to our study exist. We were unable to use validated measures and standardized instruments for symptoms or endoscopic findings. We collected a purposive sample for our control cohort that may limit generalizability, but the sample size is large covering pertinent clinical features. Mast cell and eosinophil assessment only highlights the mast cell presence and not activation status. However, fresh tissue was not available for such analyses. We were also not able to discern retrospectively where in the duodenum duodenal biopsies derived. There are study strengths. Pathology slides were re-interpreted by a single expert pathologist with a strict protocol. It is the only study to assess mast cells and clinical features of non-EoE EGIDs and to assess diagnostic thresholds for eosinophilic gastroenteritis and increased levels of gastroduodenal mast cells. This is crucial in both clinical trials and clinical practice.
These data provide a baseline to define the threshold for pathologic accumulation of gastroduodenal mast cells and eosinophils in EGIDs. Though mast cells and eosinophils from gastric and duodenal biopsies of EGID patients were significantly higher than in a non-diseased cohort, they were not closely associated with disease features. Future studies are underway to determine associations between mast cells and EGID clinical features and to elucidate the role of mast cells in EGID pathogenesis.
Supplementary Material
What you need to know.
Background
Mast cells are believed to contribute to the pathogenesis of eosinophilic gastrointestinal disorders (EGIDs), but it is not clear whether numbers of these cells are increased in gastric or intestinal tissues from patients with EGIDs.
Findings
Gastric and duodenal biopsies from patients with EGIDs have increased mean and peak mast cell and eosinophil counts, compared to patients without EGIDs, supporting the concept that mast cells are involved in pathogenesis.
Implications for Patient Care
These data provide a baseline to define the threshold for pathologic accumulation of gastroduodenal mast cells and eosinophils.
Acknowledgments
Grant Support: This research was supported by NIH Award R01 DK101856 (ESD), used resources from UNC Center for GI Biology and Disease (NIH P30 DK034987) and the UNC Translational Pathology Lab, which is supported in part by grants from the NCI (2-P30-CA016086–40), NIEHS (2-P30ES010126–15A1), UCRF, and NCBT (2015-IDG-1007), and received support from an educational grant from Allakos.
Potential competing interests: Dr. Dellon is a consultant for Abbott, Adare, Aimmune, Allakos, Arena, AstraZeneca, Biorasi, Calypso, Eli Lilly, EsoCap, Gossamer Bio, GSK, Receptos/Celegene, Regeneron, Robarts, Salix, and Shire/Takeda, receives research funding from Adare, Allakos, GSK, Meritage, Miraca, Nutricia, Receptos/Celgene, Regeneron, and Shire/Takeda, and has received an educational grant from Allakos, Banner, and Holoclara. Dr. Wechsler is a consultant for Allakos. Dr. Youngblood is an employee of Allakos. None of the other authors report and potential conflicts of interest with this study.
Abbreviations:
- (EoE)
Eosinophilic esophagitis
- (EGIDs)
eosinophilic gastrointestinal disorders
- (eos/hpf)
eosinophils/high-power field
- (UNC)
University of North Carolina
Footnotes
Writing assistance: No writing assistance was utilized.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dellon ES, Gonsalves N, Hirano I, et al. ACG clinical guideline: Evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE). Am J Gastroenterol. 2013;108(5):679–692; quiz 693. [DOI] [PubMed] [Google Scholar]
- 2.Lucendo AJ, Molina-Infante J, Arias Á, et al. Guidelines on eosinophilic esophagitis: evidence-based statements and recommendations for diagnosis and management in children and adults. United Eur Gastroenterol J. 2017;5(3):335–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prussin C. Eosinophilic gastroenteritis and related eosinophilic disorders. Gastroenterol Clin North Am. 2014;43(2):317–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walker MM, Potter M, Talley NJ. Eosinophilic gastroenteritis and other eosinophilic gut diseases distal to the oesophagus. Lancet Gastroenterol Hepatol. 2018;3(4):271–280. [DOI] [PubMed] [Google Scholar]
- 5.Dellon ES, Kim HP, Sperry SLW, et al. A phenotypic analysis shows that eosinophilic esophagitis is a progressive fibrostenotic disease. Gastrointest Endosc. 2014;79(4):577–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jensen ET, Kappelman MD, Martin CF, Dellon ES. Health-care utilization, costs, and the burden of disease related to eosinophilic esophagitis in the United States. Am J Gastroenterol. 2015;110(5):626–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pesek RD, Reed CC, Muir AB, et al. Increasing Rates of Diagnosis, Substantial Co-Occurrence, and Variable Treatment Patterns of Eosinophilic Gastritis, Gastroenteritis, and Colitis Based on 10-Year Data Across a Multicenter Consortium. Am J Gastroenterol. 2019;114(6):984–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pesek RD, Reed CC, Collins MH, et al. Association Between Endoscopic and Histologic Findings in a Multicenter Retrospective Cohort of Patients with Non-esophageal Eosinophilic Gastrointestinal Disorders. Dig Dis Sci. 2019. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silva J, Canão P, Espinheira MC, Trindade E, Carneiro F, Dias JA. Eosinophils in the gastrointestinal tract: how much is normal? Virchows Arch. 2018;473(3):313–320. [DOI] [PubMed] [Google Scholar]
- 10.Lee EH, Yang HR, Lee HS. Quantitative analysis of distribution of the gastrointestinal tract eosinophils in childhood functional abdominal pain disorders. J Neurogastroenterol Motil. 2018;24(4):614–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chernetsova E, Sullivan K, De Nanassy J, et al. Histologic analysis of eosinophils and mast cells of the gastrointestinal tract in healthy Canadian children. Hum Pathol. 2016;54:55–63. [DOI] [PubMed] [Google Scholar]
- 12.Matsushita T, Maruyama R, Ishikawa N, et al. The number and distribution of eosinophils in the adult human gastrointestinal tract : A study and comparison of racial and environmental factors. Am J Surg Pathol. 2015;39(4):521–7. [DOI] [PubMed] [Google Scholar]
- 13.Lwin T, Melton SD, Genta RM. Eosinophilic gastritis: Histopathological characterization and quantification of the normal gastric eosinophil content. Mod Pathol. 2011;24(4):556–63. [DOI] [PubMed] [Google Scholar]
- 14.Hurrell JM, Genta RM, Melton SD. Histopathologic diagnosis of eosinophilic conditions in the gastrointestinal tract. Adv Anat Pathol. 2011;18(5):335–48. [DOI] [PubMed] [Google Scholar]
- 15.Talley NJ, Walker MM, Aro P, et al. Non-ulcer Dyspepsia and Duodenal Eosinophilia: An Adult Endoscopic Population-Based Case-Control Study. Clin Gastroenterol Hepatol. 2007;5(10):1175–83. [DOI] [PubMed] [Google Scholar]
- 16.DeBrosse CW, Case JW, Putnam PE, et al. Quantity and distribution of eosinophils in the gastrointestinal tract of children. Pediatr Dev Pathol. 2006. [DOI] [PubMed] [Google Scholar]
- 17.Lowichik A, Weinberg AG. A quantitative evaluation of mucosal eosinophils in the pediatric gastrointestinal tract. Mod Pathol. 1996;9(3):210–8. [PubMed] [Google Scholar]
- 18.Jenkins D, Goodall A, Gillet FR, Scott BB. Defining duodenitis: Quantitative histological study of mucosal responses and their correlations. J Clin Pathol.1985;38(10):119–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Genta RM, Sonnenberg A, Turner K. Quantification of the duodenal eosinophil content in adults: a necessary step for an evidence-based diagnosis of duodenal eosinophilia. Aliment Pharmacol Ther. 2018;47(8):1143–1150. [DOI] [PubMed] [Google Scholar]
- 20.Collins MH. Histopathologic features of eosinophilic esophagitis and eosinophilic gastrointestinal diseases. Gastroenterol Clin North Am. 2014;43(2):257–268. [DOI] [PubMed] [Google Scholar]
- 21.Dellon ES, Collins MH, Bonis PA, et al. Substantial Variability in Biopsy Practice Patterns Among Gastroenterologists for Suspected Eosinophilic Gastrointestinal Disorders. Clin Gastroenterol Hepatol. 2016;14(12):1842–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lucendo AJ, Bellón T, Lucendo B. The role of mast cells in eosinophilic esophagitis. Pediatr Allergy Immunol. 2009;20(6):512–8. [DOI] [PubMed] [Google Scholar]
- 23.Aceves SS, Chen D, Newbury RO, et al. Mast cells infiltrate the esophageal smooth muscle in patients with eosinophilic esophagitis, express TGF-β1, and increase esophageal smooth muscle contraction. J Allergy Clin Immunol. 2010;126(6):1198–204. [DOI] [PubMed] [Google Scholar]
- 24.Straumann A, Bauer M, Fischer B, et al. Idiopathic eosinophilic esophagitis is associated with a TH2-type allergic inflammatory response. J Allergy Clin Immunol. 2001;108(6):954–61. [DOI] [PubMed] [Google Scholar]
- 25.O’Shea KM, Aceves SS, Dellon ES, et al. Pathophysiology of Eosinophilic Esophagitis. Gastroenterology. 2018;154(2):333–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niranjan R, Mavi P, Rayapudi M, et al. Pathogenic role of mast cells in experimental eosinophilic esophagitis. Am J Physiol Gastrointest Liver Physiol. 2013;304:G1087–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berton A, Levi-Schaffer F, Emonard H, et al. Activation of fibroblasts in collagen lattices by mast cell extract: A model of fibrosis. Clin Exp Allergy. 2000;30(4):485–92. [DOI] [PubMed] [Google Scholar]
- 28.Bolton SM, Kagalwalla AF, Arva NC, et al. Mast Cell Infiltration Is Associated With Persistent Symptoms and Endoscopic Abnormalities Despite Resolution of Eosinophilia in Pediatric Eosinophilic Esophagitis. Am J Gastroenterol. 2020;115(2):224–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mir SAV, Schady D, Olive AP, et al. Mucosal mast cell counts in pediatric eosinophilic gastrointestinal disease. Pediatr Allergy Immunol. 2014;25(1):94–5. [DOI] [PubMed] [Google Scholar]
- 30.Kosnai I, Kuitunen P, Savilahti E, Sipponen P. Mast cells and eosinophils in the jejunal mucosa of patients with intestinal cow’s milk allergy and celiac disease of childhood. J Pediatr Gastroenterol Nutr. 1984;3(3):368–72. [DOI] [PubMed] [Google Scholar]
- 31.Hahn HP, Hornick JL. Immunoreactivity for CD25 in gastrointestinal mucosal mast cells is specific for systemic mastocytosis. Am J Surg Pathol. 2007;31(11):1669–76. [DOI] [PubMed] [Google Scholar]
- 32.Tison B, Debrosse C, Rainey H, et al. Number and distribution of mast cells in the pediatric gastrointestinal tract. J Allergy Clin Immunol. 2010;125(2):AB182. [Google Scholar]
- 33.Jakate S, Demeo M, John R, et al. Mastocytic enterocolitis: Increased mucosal mast cells in chronic intractable diarrhea. Arch Pathol Lab Med. 2006;130(3):362–7. [DOI] [PubMed] [Google Scholar]
- 34.Walker MM, Talley NJ, Prabhakar M, et al. Duodenal mastocytosis, eosinophilia and intraepithelial lymphocytosis as possible disease markers in the irritable bowel syndrome and functional dyspepsia. Aliment Pharmacol Ther. 2009;29(7):765–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martínez C, Lobo B, Pigrau M, et al. Diarrhoea-predominant irritable bowel syndrome: An organic disorder with structural abnormalities in the jejunal epithelial barrier. Gut. 2013;62(8):1160–8. [DOI] [PubMed] [Google Scholar]
- 36.Doyle LA, Sepehr GJ, Hamilton MJ, et al. A clinicopathologic study of 24 cases of systemic mastocytosis involving the gastrointestinal tract and assessment of mucosal mast cell density in irritable bowel syndrome and asymptomatic patients. Am J Surg Pathol. 2014;38(6):832–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reed C, Woosley JT, Dellon ES. Clinical characteristics, treatment outcomes, and resource utilization in children and adults with eosinophilic gastroenteritis. Dig Liver Dis. 2015;47(3):197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jensen ET, Martin CF, Kappelman MD, Dellon ES. Prevalence of eosinophilic gastritis, gastroenteritis, and colitis: Estimates from a national administrative database. J Pediatr Gastroenterol Nutr. 2016;62(1):36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mansoor E, Saleh MA, Cooper GS. Prevalence of Eosinophilic Gastroenteritis and Colitis in a Population-Based Study, From 2012 to 2017. Clin Gastroenterol Hepatol. 2017;15(11):1733–1741. [DOI] [PubMed] [Google Scholar]
- 40.Youngblood BA, Brock EC, Leung J, et al. Siglec-8 antibody reduces eosinophils and mast cells in a transgenic mouse model of eosinophilic gastroenteritis. JCI Insight. 2019;4(19). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dellon ES, Chen X, Miller CR, et al. Tryptase staining of mast cells may differentiate eosinophilic esophagitis from gastroesophageal reflux disease. Am J Gastroenterol. 2011;106(2):264–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tappata M, Eluri S, Perjar I, et al. Association of mast cells with clinical, endoscopic, and histologic findings in adults with eosinophilic esophagitis. Allergy Eur J Allergy Clin Immunol. 201873(10):2088–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dellon E, KA P, JA M, et al. Efficacy and Safety of AK002 in Adult Patients with Active Eosinophilic Gastritis and Eosinophilic Enteritis: Primary Results from a Randomized, Double-Blind Placebo-Controlled Phase 2 Trial (ENIGMA Study). Am J Gastroenterol. 2019;44 (Suppl)(36). [Google Scholar]
- 44.Ko HM, Morotti RA, Yershov O, Chehade M. Eosinophilic gastritis in children: Clinicopathological correlation, disease course, and response to therapy. Am J Gastroenterol. 2014;109(8):1277–85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.