Abstract
Background & Aims
Despite apparent sex differences in the prevalence and incidence of nonalcoholic fatty liver disease (NAFLD), there are limited epidemiologic data regarding the associations of reproductive and hormone-related factors with NAFLD. We examined the associations of these factors and exogenous hormone use with NAFLD risk in African American, Japanese American, Latino, Native Hawaiian, and white women.
Methods
We conducted a nested case–control study (1861 cases and 17,664 controls) in the Multiethnic Cohort Study. NAFLD cases were identified using Medicare claims data; controls were selected among participants without liver disease and individually matched to cases by birth year, ethnicity, and length of Medicare enrollment. Reproductive and hormone-related factors and covariates were obtained from the baseline questionnaire. Multivariable logistic regression was used to calculate odds ratios (ORs) and 95% CIs.
Results
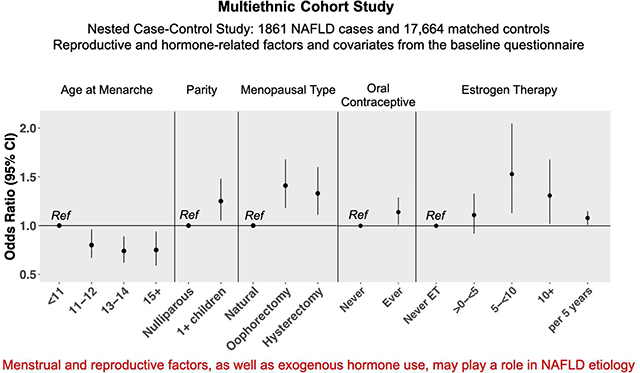
Later age at menarche was inversely associated with NAFLD (Ptrend=.01). Parity, regardless of number of children or age at first birth, was associated with increased risk of NAFLD (OR, 1.25; 95% CI, 1.05–1.48). Oral contraceptive use was also linked to increased risk of NAFLD (OR, 1.14; 95% CI, 1.01–1.29; duration of use Ptrend=.04). Compared to women with natural menopause, those with oophorectomy (OR, 1.41; 95% CI, 1.18–1.68) or hysterectomy (OR, 1.33; 95% CI, 1.11–1.60) had an increased risk of NAFLD. Longer duration of menopause hormone therapy (only estrogen therapy) was linked with increasing risk of NAFLD (OR per 5 years of use, 1.08, 95% CI, 1.01–1.15).
Conclusions
Findings from a large multiethnic study support the concept that menstrual and reproductive factors, as well as use of exogenous hormones, associate with risk of NAFLD.
Keywords: steatosis, NASH, birth control, worldwide
Graphical Abstract
Introduction
Nonalcoholic fatty liver disease (NAFLD) is a leading cause of chronic liver disease and cirrhosis and affects approximately 25% of population worldwide1, 2. Sex differences in prevalence and incidence of NAFLD have long been observed. Compared to men, women generally have lower risk of NAFLD until menopause; however, NAFLD occurs at a similar or even higher rate in postmenopausal women than age-matched men3, 4 The underlying biology driving the sex differences in NAFLD observed in population-based studies, however, remains poorly understood, largely due to limited studies that considered sex differences in study design and analysis5. There has been limited studies assessing reproductive and hormone-related factors in influencing NAFLD risk, and results from these few studies are inconsistent6–14.
Given the observed sex differences but very limited epidemiologic data on female hormonal factors on NAFLD risk, we examined the associations of menstrual, reproductive and hormone-related factors with NAFLD risk in a prospective, large ethnically diverse population. We also examined these associations by racial/ethnic groups, given the lack of epidemiologic studies in diverse ethnic groups and minority populations.
Methods
Study populations
We conducted a nested case-control study within the Multiethnic Cohort (MEC) Study. Details of the cohort design and characteristics of participants have been described15. Briefly, the MEC is a large prospective cohort study designed to investigate etiology of cancer and other chronic diseases. The cohort includes over 215,000 men and women, aged 45–75 years at cohort enrollment during 1993–1996. The MEC consists of participants primarily from five different racial/ethnic groups (African Americans, Japanese Americans, Latinos, Native Hawaiians and Whites) living in Hawaii and California, primarily Los Angeles County. At baseline, participants completed a mailed questionnaire which included information on anthropometry, lifestyle, diet, family and personal medical history, and, for women, menstrual and reproductive history and hormone use. Because NAFLD cases were identified using the Medicare fee-for-service (FFS) claim files, we restricted the study population to MEC-Medicare FFS participants (N=123,196)16. We excluded participants who were not from the five major racial/ethnic groups (N=7,511) and missing baseline information on the important relevant covariates (e.g. body mass index (BMI), diabetes, physical activity) (N=5,756). A total of 57,596 eligible women were available for nested case-control analysis of NAFLD17.
As described previously, NAFLD cases were identified from eligible participants using (ICD-9 codes 571.8 and 571.9 and ICD-10 codes K75.81, K760, K7689, K741, K769) one inpatient or two or more outpatient/carrier FFS claims on different dates between 1999 and 2016 and by excluding other etiology2, 17 (supplemental materials). Controls were selected among female Medicare FFS participants without chronic liver disease and individually matched to cases (with a ratio up to 10:1; average 9.9) on birth year, race/ethnicity, and length of FFS enrollment (i.e., total duration study participants enrolled in the FFS Medicare system; average: 10.3 years). A total of 1,861 NAFLD female cases and 18,362 matched controls were included in this study.
The Institutional Review Boards for the University of Southern California and the University of Hawaii approved this study.
Reproductive and hormone-related factors and covariates
Information regarding menstrual, reproductive factors and exogenous hormone use, including oral contraceptives (OC) and menopausal hormone therapy (MHT) was obtained from baseline questionnaire. Years of menstruation was calculated by subtracting age at menarche from age at menopause. For MHT, participants provided information on age at start and duration and type of hormones. Duration of MHT was calculated as previously described18. Menopausal status was obtained from participants’ self-reports and defined as natural or surgical (oophorectomy and/or hysterectomy). Detailed demographics and covariates information were obtained from the baseline questionnaire. The median time between baseline and the first NAFLD-related claim was 18 years.
Statistical analysis
We used multivariable conditional logistic regression to estimate odds ratio (OR) and 95% confidence interval (CI). Matched sets were used as strata in the logistic models which also accounted for factors known to be associated with NAFLD, including baseline BMI and BMI change from age 21 to baseline, alcohol intake (ethanol g/day), smoking status (never/former/current), physical activity (METs hours/day), education (high school/vocational, some college/college, higher) and diabetes. Women with alcohol consumption >24 grams/day were excluded in the analysis. Trend tests were performed by modeling the category of exposure variable (coded as 1,2,3) as a continuous variable in the multivariable models. We examined the associations of reproductive and hormone-related factors with NAFLD by race/ethnicity. To test heterogeneity in the interaction parameters for the exposure by race/ethnicity, we fit a model including all participants and interaction terms for each exposure trend variable and race/ethnicity indicators. We performed sensitivity analysis by excluding participants with a diagnosis of breast or gynecologic cancer at baseline (162 cases and 1,100 controls) and by excluding participants who used steatogenic drugs which can potentially influence lipid metabolism (98 cases and 661 controls), however, results remained very similar (data not shown). All P-values were based on two-sided tests. Analyses were performed using R software version 3.5.3.
Results
The characteristics of NAFLD cases and controls are presented in Table 1. Japanese Americans accounted for approximately 50% of the study population; the rest were Latino (21%), non-Hispanic white (15%), African American (8%) and Native Hawaiian (6%). The mean age at cohort entry was 57.5 [standard deviation (SD)=7.7] for cases and 57.5 (SD=7.7) for controls. NAFLD cases and controls were similar for the majority of the baseline characteristics, except that baseline BMI was higher in cases than controls. At enrollment, ~83% women were postmenopausal, among whom 34.7% cases and 39.9% controls never used MHT. Among postmenopausal women, over 40% experienced natural menopause, while <20% experienced oophorectomy or hysterectomy, respectively. More than 45% of the women had used OC.
Table 1.
Demographic and selected baseline characteristics of NAFLD cases and controls the Multiethnic Cohort
| Cases (N =1861) |
Controls (N =17664) |
|||
|---|---|---|---|---|
| Mean (SD) |
Mean (SD) |
|||
| Age at baseline | 57.5 (45–76) | 57.4 (45–76) | ||
| BMI at baseline | 27.4 (5.8) | 25.5 (5.2) | ||
| BMI at 21 years | 21.3 (3.3) | 21.0 (3.1) | ||
| Energy intake (Kcal/day) | 1977 (939) | 1954 (900) | ||
| N |
% |
N |
% |
|
| Race/ethnicity | ||||
| White | 292 | 15.7 | 2554 | 14.5 |
| African American | 143 | 7.7 | 1377 | 7.8 |
| Native Hawaiian | 115 | 6.2 | 1100 | 6.2 |
| Japanese | 915 | 49.2 | 8798 | 49.8 |
| Latino | 396 | 21.3 | 3835 | 21.7 |
| Age at menarche (years) | ||||
| <11 | 212 | 11.4 | 1432 | 8.1 |
| 11–12 | 828 | 44.5 | 7537 | 42.7 |
| 13–14 | 606 | 32.6 | 6483 | 36.7 |
| >15 | 200 | 10.7 | 2055 | 11.6 |
| Missing | 15 | 0.8 | 157 | 0.9 |
| Education | ||||
| < High school | 750 | 40.3 | 6929 | 39.2 |
| Vocational/some college | 534 | 28.7 | 5110 | 28.9 |
| College or higher | 560 | 30.1 | 5470 | 31.0 |
| Missing | 17 | 0.9 | 155 | 0.9 |
| Parity | ||||
| Nulliparous | 197 | 10.6 | 2351 | 13.3 |
| Parous | 1658 | 89.1 | 15273 | 86.5 |
| Missing | 6 | 0.3 | 40 | 0.2 |
| Number of children | ||||
| None | 197 | 10.6 | 2351 | 13.3 |
| 1 | 201 | 10.8 | 1911 | 10.8 |
| 2–3 | 932 | 50.1 | 8563 | 48.5 |
| 4 or more | 513 | 27.6 | 4709 | 26.7 |
| Missing | 18 | 0.9 | 133 | 0.8 |
| Age at first live birth (years) | ||||
| Nulliparous | 197 | 10.6 | 2351 | 13.3 |
| 15–20 | 443 | 23.8 | 3903 | 22.1 |
| 21–30 | 1050 | 56.4 | 9761 | 55.3 |
| > 30 | 130 | 7 | 1308 | 7.4 |
| Missing | 41 | 2.2 | 341 | 1.9 |
| Oral contraceptive use | ||||
| Never | 934 | 50.2 | 9441 | 53.4 |
| Ever | 890 | 47.8 | 7898 | 44.7 |
| Missing | 37 | 2 | 325 | 1.8 |
| Menopausal status at baseline | ||||
| Pre-Menopausal | 283 | 15.2 | 2971 | 16.8 |
| Natural Menopause | 802 | 43.1 | 8605 | 48.7 |
| Oophorectomy | 356 | 19.1 | 2413 | 13.7 |
| Simple hysterectomy | 313 | 16.8 | 2487 | 14.1 |
| Unknown reason or Missing | 107 | 5.7 | 1188 | 6.7 |
| Ever use of menopausal hormone therapy, menopausal women only | ||||
| Never use of any type of hormone therapy | 543 | 34.7 | 5812 | 39.9 |
| ET | 517 | 33.1 | 4263 | 29.3 |
| EPT | 441 | 28.2 | 3970 | 27.3 |
| Missing | 62 | 4.0 | 546 | 3.7 |
| Years of menstruation among natural menopausal women | ||||
| <30 | 133 | 16.6 | 1191 | 13.8 |
| > 30 – < 35 | 214 | 26.7 | 2414 | 28.1 |
| > 35 – < 40 | 360 | 44.9 | 3955 | 46.0 |
| > 40 | 91 | 11.3 | 1026 | 11.9 |
| Missing | 4 | 0.5 | 19 | 0.2 |
| Type 2 diabetes at baseline | ||||
| Yes | 221 | 11.9 | 1430 | 8.1 |
| No | 1640 | 88.1 | 16234 | 91.9 |
| Average METS of activity per day | ||||
| < 1.4 | 472 | 25.4 | 4100 | 23.2 |
| > 1.4 –< 1.6 | 472 | 25.4 | 4445 | 25.2 |
| > 1.6 – < 1.8 | 529 | 28.4 | 4995 | 28.3 |
| > 1.8 | 312 | 16.8 | 3397 | 19.2 |
| Missing | 76 | 4.1 | 727 | 4.1 |
| Smoking status | ||||
| Never | 1121 | 60.2 | 10943 | 62.0 |
| Past | 508 | 27.3 | 4667 | 26.4 |
| Current | 207 | 11.1 | 1812 | 10.3 |
| Missing | 25 | 1.3 | 242 | 1.4 |
| Alcohol (g/day) | ||||
| None | 1350 | 72.5 | 11815 | 66.9 |
| < 12 | 458 | 24.6 | 5103 | 28.9 |
| > 12 – <24 | 53 | 2.8 | 746 | 4.2 |
BMI=body mass index; METs=Metabolic Equivalent; SD=standard deviation
Several reproductive factors were significantly associated with NAFLD (Table 2). Later age at menarche was inversely associated with NAFLD risk (Ptrend=0.01); compared to women with age at menarche <11 years, those with age at menarche 11–12 years (OR=0.80, 95%CI=0.67–0.96), 13–14 years (OR=0.74, 95%CI=0.62–0.89) and ≥15 years (OR=0.75, 95%CI=0.59–0.94) had decreased risk. Having 1 or more children was associated with increased risk (OR=1.25, 95%CI=1.06–1.48); trend with increasing number of children was not observed (Ptrend=0.12). Compared to nulliparous women, those with age at first live birth ≤30 years had ~25% significant increased risk; no trend was observed with later age at first live birth (Ptrend=0.44). Compared to women experiencing natural menopause, those with oophorectomy (OR=1.41, 95%CI=1.18–1.68) or hysterectomy (OR=1.33, 95%CI = 1.11–1.60) had increased risk.
Table 2.
Associations between reproductive and hormone-related factors and NAFLD risk in the Multiethnic Cohort
| Cases/Controls | OR (95% CI) | |
|---|---|---|
| Age at menarche1 | ||
| <11 | 188/1290 | ref |
| 11–12 | 728/6754 | 0.80 (0.67–0.96) |
| 13–14 | 546/5698 | 0.74 (0.62–0.89) |
| >15 | 161/1745 | 0.75 (0.59–0.94) |
| T rend test P | 0.01 | |
| Parity1 | ||
| Nulliparous | 171/2095 | ref |
| 1 or more children | 1454/13428 | 1.25 (1.05–1.48) |
| Number of children1 | ||
| None | 171/2095 | ref |
| 1 | 178/1700 | 1.23 (0.98–1.54) |
| 2–3 | 845/7793 | 1.26 (1.06–1.51) |
| 4 or more | 427/3902 | 1.19 (0.97–1.45) |
| Trend test P | 0.12 | |
| Age at birth of first child1 | ||
| Nulliparous | 171/2095 | ref |
| <20 | 374/3239 | 1.28 (1.04–1.57) |
| 21–25 | 610/5590 | 1.25 (1.04–1.51) |
| 26–30 | 339/3241 | 1.22 (1.00–1.49) |
| 31–35 | 83/893 | 1.07 (0.80–1.41) |
| >35 | 26/276 | 1.20 (0.77–1.86) |
| Trend test P | 0.44 | |
| Type of menopause2 | ||
| Natural | 709/7600 | ref |
| Oophorectomy | 298/2147 | 1.41 (1.18–1.68) |
| Simple hysterectomy | 276/2136 | 1.33 (1.11–1.60) |
| Years of menstruation among natural menopausal women3 | ||
| < 30 | 112/1009 | ref |
| > 30 – < 35 | 187/2118 | 0.83 (0.65–1.07) |
| > 35 – < 40 | 331/3544 | 0.88 (0.70–1.12) |
| > 40 | 79/929 | 0.77 (0.56–1.04) |
| Trend test P | 0.22 | |
| Years of menstruation among surgical menopausal women3 | ||
| < 30 | 373/2750 | ref |
| > 30 – < 35 | 124/987 | 0.94 (0.75–1.17) |
| > 35 | 77/546 | 1.06 (0.80–1.38) |
| Trend test P | 0.89 | |
| OC use1 | ||
| Never | 813/8270 | ref |
| Ever | 812/7253 | 1.14 (1.01–1.29) |
| Years of ever use OC1 | ||
| Never | 813/8270 | ref |
| < 1 | 139/1285 | 1.06 (0.87–1.30) |
| 1–5 | 388/3359 | 1.19 (1.03–1.38) |
| 6+ | 280/2531 | 1.14 (0.97–1.34) |
| Trend test P | 0.04 | |
| Menopausal hormone therapy4 | ||
| Never use of any type of hormone therapy | 434/4639 | ref |
| Ever ET | 453/3612 | 1.18 (1.00–1.38) |
| Ever EPT | 381/3556 | 1.25 (1.07–1.46) |
| Years of ever ET use4 | ||
| Never use of any type of hormone therapy | 434/4639 | ref |
| >0 – <5 | 240/2089 | 1.11 (0.92–1.33) |
| 5 – <10 | 69/443 | 1.53 (1.13–2.05) |
| 10+ | 144/1080 | 1.31 (1.02–1.68) |
| per 5 years of use Years of ever EPT use4 | 1.08 (1.01–1.15) | |
| Never use of any type of hormone therapy | 434/4639 | ref |
| >0 – <5 | 281/2514 | 1.27 (1.07–1.51) |
| 5+ | 100/1042 | 1.20 (0.94–1.51) |
| per 5 years of use | 1.03 (0.93–1.13) | |
Multivariate logistic regression models stratified by matching set and adjusted for alcohol intake, smoking status, METs, BMI at baseline and BMI change from age 21 to baseline, education, diabetes, and mutually adjusted parity, oral contraceptive use, menopausal status and hormone use.
Among postmenopausal women only. Multivariate logistic regression models adjusted for matching factors, alcohol intake, smoking status, METs, BMI at baseline and BMI change from age 21 to baseline, education, diabetes, parity, oral contraceptive use, menopausal hormone use and years of menstruation.
Among postmenopausal women only. Multivariate logistic regression models adjusted for matching factors, alcohol intake, smoking status, METs, BMI at baseline and BMI change from age 21 to baseline, education, diabetes, parity, oral contraceptive use, menopausal hormone use.
Among postmenopausal women only for estrogen therapy use with or without progestin. Multivariate logistic regression models adjusted for matching factors, alcohol intake, smoking status, METs, BMI at baseline and BMI change from age 21 to baseline, education, diabetes, parity, oral contraceptive use, and age at and type of menopause.
Exogenous hormone use was significantly associated with NAFLD risk (Table 2). Ever use of OC was associated with higher risk (OR=1.14, 95%CI=1.01–1.29); the risk increased with longer duration of use (Ptrend=0.04). Compared to postmenopausal women who never used MHT, risk increased among those who ever used ET (OR=1.18, 95%CI=1.00–1.38) or EPT (OR=1.25, 95%CI=1.07–1.46). NAFLD risk increased with longer duration of MHT, for ET (OR per 5-year use=1.08, 95%CI=1.01–1.15) but not significant for EPT (OR per 5-year use=1.03, 95%CI=0.93–1.13). Compared to postmenopausal women who never used MHT, those who used ET for at least 10 years had over 30% increased risk (OR=1.31,95%CI=1.02–1.68). No significant difference was observed in the association with MHT in women with either natural or surgical menopause (Pinteraction=0.32) (data not shown).
The associations of age at menarche, parity, type of menopause, OC, and MHT with NAFLD did not vary significantly across race/ethnicity (Pheterogeneity>0.26) (Table 3). A significant heterogeneity by race/ethnicity was observed for years of menstruation among women with natural menopause (P=0.02), which appeared to be driven by Japanese Americans as a pattern of inverse association with increasing years of menstruation was observed in all the racial/ethnic groups except in Japanese Americans. Pair-wise comparisons in this association between Japanese Americans and the other 4 racial/ethnic groups showed significant differences (all pair-wise comparisons P<0.05).
Table 3.
Associations between reproductive and hormone-related factors and NAFLD risk by race/ethnicity in the Multiethnic Cohort
| African Americans | Native Hawaiian | Japanese American | Latino | White | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ca/Co | OR (95% CI) | Ca/Co | OR (95% CI) | Ca/Co | OR (95% CI) | Ca/Co | OR (95% CI) | Ca/Co | OR (95% CI) | |
| Age at menarche1 | ||||||||||
| <11 | 14/95 | ref | 11/115 | ref | 101/679 | ref | 31/217 | ref | 31/184 | ref |
| 11–12 | 53/482 | 0.87(0.45–1.66) | 48/450 | 1.15(0.56–2.36) | 390/3632 | 0.79(0.62–1.00) | 120/1186 | 0.79(0.51–1.23) | 117/1004 | 0.66(0.42–1.03) |
| >13 | 53/543 | 0.80(0.42–1.56) | 39/430 | 1.10(0.52–2.29) | 345/3814 | 0.72(0.56–0.92) | 147/1486 | 0.80(0.52–1.24) | 123/1170 | 0.64(0.41–0.99) |
| Trend test P | 0.52 | 0.92 | 0.02 | 0.5 | 0.13 | |||||
| Parity1 | ||||||||||
| Nulliparous | 7/140 | ref | 9/85 | ref | 104/1169 | ref | 21/302 | ref | 30/399 | ref |
| 1 or more children | 113/984 | 2.30(1.03–5.17) | 89/911 | 0.93(0.42–2.06) | 733/6979 | 1.07(0.85–1.34) | 278/2591 | 1.47(0.90–2.39) | 241/1963 | 1.50(0.99–2.27) |
| Type of menopause2 | ||||||||||
| Natural | 39/420 | ref | 38/419 | ref | 382/4116 | ref | 134/1517 | ref | 116/1128 | ref |
| Oophorectomy | 27/200 | 1.22(0.66–2.23) | 20/155 | 1.14(0.56–2.28) | 147/1130 | 1.49(1.15–1.92) | 52/326 | 1.49(1.00–2.19) | 52/336 | 1.30(0.83–2.01) |
| Simple hysterectomy Years of menstruation among | 34/272 | 0.96(0.53–1.73) | 12/142 | 0.92(0.40–2.02) | 111/803 | 1.68(1.27–2.22) | 65/550 | 1.14(0.78–1.64) | 54/369 | 1.18(0.75–1.85) |
| Years of menstruation among natural postmenopausal women3 | ||||||||||
| < 30 | 12/78 | ref | 12/80 | ref | 42/442 | ref | 29/256 | ref | 17/153 | ref |
| > 30 – < 35 | 10/124 | 0.45(0.18–1.14) | 11/122 | 0.63(0.25–1.58) | 81/1083 | 0.90(0.61–1.36) | 42/451 | 0.87(0.52–1.45) | 43/338 | 1.10(0.60–2.09) |
| > 35 | 17/218 | 0.42(0.18–1.00) | 15/217 | 0.35(0.14–0.85) | 259/2591 | 1.23(0.87–1.77) | 63/810 | 0.71(0.45–1.16) | 56/637 | 0.76(0.42–1.43) |
| Trend test P | 0.07 | 0.02 | 0.05 | 0.14 | 0.18 | |||||
| OC use1 | ||||||||||
| Never | 60/537 | ref | 47/466 | ref | 427/4668 | ref | 167/1663 | ref | 112/936 | ref |
| Ever | 60/587 | 0.82(0.51–1.33) | 51/530 | 0.85(0.52–1.39) | 410/3480 | 1.32(1.11–1.57) | 132/1230 | 1.04(0.79–1.38) | 159/1426 | 0.93(0.68–1.27) |
| Menopausal hormone therapy4 | ||||||||||
| Never use of any type of hormone therapy | 46/439 | ref | 23/325 | ref | 197/2113 | ref | 101/1207 | ref | 67/531 | ref |
| Ever ET | 35/312 | 1.04(0.62–1.72) | 27/220 | 1.34(0.68–2.65) | 208/1756 | 1.12(0.88–1.42) | 87/630 | 1.41(1.01–1.96) | 84/622 | 0.97(0.66–1.43) |
| Ever EPT | 17/130 | 1.52(0.80–2.80) | 20/161 | 1.58(0.80–3.10) | 222/2113 | 1.22(0.99–1.51) | 58/498 | 1.47(1.02–2.11) | 64/654 | 0.90(0.61–1.33) |
| Per 5 years of ever use of ET4 | 0.92(0.69–1.17) | 1.20(0.88–1.63) | 1.09(0.99–1.20) | 1.08(0.92–1.27) | 1.11(0.95–1.28) | |||||
| Per 5 years of ever use of EPT4 | 1.27(0.72–2.01) | 1.19 (0.67–1.95) | 1.03(0.90–1.18) | 1.02(0.76–1.32) | 0.94(0.73–1.19) | |||||
Multivariate logistic regression models stratified by matching set and adjusted for alcohol intake, smoking status, METs, BMI at baseline and BMI change from age 21 to baseline, education, diabetes, and mutually adjusted parity, oral contraceptive use, menopausal status and hormone use.
Among postmenopausal women only. Multivariate logistic regression models adjusted for matching factors, alcohol intake, smoking status, METs, BMI at baseline and BMI change from age 21 to baseline, education, diabetes, parity, oral contraceptive use, menopausal hormone use and years of menstruation.
Among postmenopausal women only. Multivariate logistic regression models adjusted for matching factors, alcohol intake, smoking status, METs, BMI at baseline and BMI change from age 21 to baseline, education, diabetes, parity, oral contraceptive use, menopausal hormone use.
Among postmenopausal women only for estrogen therapy use with or without progestin. Multivariate logistic regression models adjusted for matching factors, alcohol intake, smoking status, METs, BMI at baseline and BMI change from age 21 to baseline, education, diabetes, parity, oral contraceptive use, and age at and type of menopause.
Discussion
To our knowledge, this is the first study to examine the associations of menstrual, reproductive and hormone-related factors with NAFLD in ethnically diverse women in the United States. In this study, later age at menarche was associated with lower NAFLD risk, while parity, regardless of the number of children or age at first live birth, and OC use were linked to increased risk. Compared to women with natural menopause, those with oophorectomy or hysterectomy had increased risk of NAFLD. Hormone use in postmenopausal women, particularly estrogen therapy only, was associated with increased risk.
Our finding of an inverse association between age at menarche and NAFLD risk was consistent with results of four of five published studies10–13, all of which used imaging data for NAFLD diagnosis; three were statistically significant10–12 and one showed non-statistically significant inverse association13. Results were less clear in the fifth study14. Earlier age at menarche has been associated with a range of health outcomes, including adult obesity19 and metabolic risk factors for cardiovascular disease, such as insulin resistance and adverse lipid profile20. As such, adult obesity (or weight gain) or insulin resistance may potentially mediate the association of age at menarche with NAFLD risk. In our study and two previous studies10, 11, the associations were attenuated with BMI adjustment, but they remained statistically significant. Further, the association remained similar in our study after further adjustment of BMI change from age 21 to baseline. In another prospective cohort study12, the inverse association became non-significant after controlling for weight change from baseline (young adulthood) to weight at the 25th year of follow-up. On the other hand, in two studies which additionally adjusted for insulin resistance, the association was attenuated but still significant in one study10 while strengthened in another11. Our results were unchanged with additional adjustment for diabetes.
Adolescent obesity is often correlated with earlier age at menarche, and girls experiencing earlier age at menarche may have pre-existing metabolic conditions which may influence later risk of NAFLD. In our sensitivity analysis in which BMI at age 21 was adjusted, the inverse association between age at menarche and NAFLD was even stronger (Ptrend=0.0002; OR≥15 vs. <11 years=0.68, 95%CI=0.54–0.86). In a stratified analysis by median BMI at age 21 (Pinteraction=0.79), the association was observed in women with BMI<21.0 kg/m2 (Ptrend=0.02; OR≥15 vs. <11 years=0.71, 95%CI=0.52–0.97) and women with BMI≥21 kg/m2 (Ptrend=0.06; OR≥15 vs. <11 years=0.73, 95%CI=0.51–1.03). Taken together, additional mechanism(s) likely exist considering the association was also observed among women who were lean at age 21.
In the only study that examined cross-sectionally the association of live births with NAFLD risk, parity was unrelated to risk after controlling for BMI21. We found that having at least one child was associated with a 25% increased NAFLD risk, irrespective of number of children or age at first live birth. How parity modulates NAFLD development is unclear but one possible explanation could be pregnancy-related weight gain and retention. Weight gain during pregnancy and postpartum weight retention contribute to subsequent obesity, even among women whose weight gain during pregnancy is within the normal range22.
We observed an increased risk of NAFLD associated with OC use, mainly in Japanese Americans but not in other racial/ethnic groups. In the only published study on OC use and NAFLD risk (503 cases/3835 non-cases) in mainly Whites (73%), current OC use was associated with a decreased risk (OR=0.50, 95% CI=0.26–0.98), but the association was attenuated after adjusting for BMI (OR=0.55, 95% CI=0.27–1.13) or waist circumstance (OR=0.58, 95% CI=0.29–1.17), and it was observed among Whites only (OR=0.41, 95% CI=0.18–0.97) but not in African Americans or Hispanics23. The positive association with OC use in our study did not change after BMI adjustment. The inconsistent results between our study and the previous study may be partly due to differences in study population; ours consisted of mostly postmenopausal women (~83%) while the other study23 included mostly premenopausal women [mean age=33.7 (SD=0.3)]. Timing of OC use may also influence its impact on NAFLD risk, such as lowering risk during premenopausal period while the apparent protection from prior OC use goes away after reaching menopause.
In our study, risk of NAFLD was higher among women who had an oophorectomy compared to women with a natural menopause which is consistent with results from a large UK case-control study (oophorectomy OR=1.29, 95% CI=1.18–1.43)8. Another study reported that oophorectomy was linked to increased NAFLD risk (HR=1.70, 95%CI=1.01– 2.86) among endometrial cancer patients24. The increased risk related to oophorectomy may be attributed to changes in steroidal hormonal milieu. In a recent study by Stanczyk et al25 in which oophorectomized postmenopausal women served as their own controls, a 34% and 33% decrease in serum estradiol and estrone levels were seen post oophorectomy, respectively. Furthermore, the altered hormonal milieu (the declines in estradiol levels) may also affect lipid metabolism. In animal studies, ovariectomized rats given a high-fat and cholesterol diet have disrupted lipid metabolism resulting in liver fat accumulation26 and accelerated NAFLD progression27. Therefore, altered hormonal milieu and disruption in lipid metabolism among surgically menopausal women may play a role in the development of NAFLD. Interestingly, we also observed increased NAFLD risk among women who underwent simple hysterectomy in which the ovarian function is preserved. There are several possible reasons. Firstly, self-reported history of hysterectomy and/or oophorectomy without medical record validation may result in misclassification. A prior study reported less accuracy in self-reporting oophorectomy, particularly among women who underwent both hysterectomy and oophorectomy28. Women who self-reported simple hysterectomy may also have had oophorectomy but were unaware of it. Secondly, hysterectomy with ovarian conservation has been linked to long-term increased risk of metabolic conditions, including obesity and hyperlipidemia, both of which are known NAFLD risk factors29. Finally, women who had hysterectomy may be different than those experiencing natural menopause (e.g. in this study women who underwent surgical menopause were heavier compared to those experiencing natural menopause; mean BMI at baseline: 26.2 and 25.3, respectively; P<0.01).
We observed that hormone use among postmenopausal women was associated with NAFLD. Published results regarding this topic are mixed. A cross-sectional study using National Health and Nutrition Examination Survey III data showed that postmenopausal women who used hormones had a lower risk of NAFLD than postmenopausal women who did not (OR=0.69, 95%CI=0.48–0.99)6. In contrast, a study of Korean women found more than a doubling of risk associated with menopausal estrogen use7, while risk for hormone use increased non-significantly in a small study of Japanese women9. Information on duration of use was not provided in these studies7, 9. In the recent UK study, women who used MHT and had oophorectomy had 89% increased risk of NAFLD compared to women with neither; among women without oophorectomy, hormone use was associated with 64% increased risk of NAFLD8. MHT has also been linked to increased histologic severity of hepatocyte injury and inflammation among women with NAFLD30. Possible reasons for inconsistent findings include heterogeneity of study population, type and details of hormone data collection and the criteria used for NAFLD identification. The increased risk of NAFLD associated with hormone use may seem counter intuitive to the hypothesis that estrogens protect against NAFLD; however, one possible reason may relate to the increase in various hepatic proteins, notably sex hormone-binding globulin (SHBG), caused by the estrogenic component of MHT31. The increase in SHBG results in decreased free estradiol, which may influence NAFLD risk. In a sub-study of MEC Japanese-American and White women32, SHBG was one of the strongest predictors of liver fat with low SHBG levels associated with higher % liver fat. Another possible reason may be that although MHT use (e.g., oral administration of micronized estradiol) leads to substantially increased levels of estrone, it is considered to have less estrogenic effect than estradiol. Additionally, other metabolites of estradiol are sulfated and glucuronidated and thus are biologically inactive. As such, altered hormonal milieu and/or the changes in hepatic proteins, particularly SHBG, may be involved in the etiologic pathway of MHT use and NAFLD risk. Clearly, this area warrants additional research.
There are several strengths in this study, including population-based design with large sample size, an ethnically diverse study population (particularly understudied minorities at high risk for NAFLD), and well-characterized and detailed information on reproductive/hormone-related factors and other important covariates. Additionally, >99% of NAFLD cases that were diagnosed using ICD codes did not have underlying viral hepatitis based on serum testing that we performed.
There are several limitations in this study. The identification of NAFLD was based on ICD codes of CLD from Medicare claims files. This may lead to selection of NAFLD cases with more severe disease, which may explain the low prevalence of NAFLD in this study (~3.2%); however, it was consistent with other epidemiological studies that did not use imaging data (prevalence 3.1%−7.3%)33–35. Because we did not have imaging data, participants with undiagnosed NAFLD might have been inadvertently included in the control group which may lead to biased associations. However, the associations would be biased only if the diagnosis based on Medicare claims was correlated with the exposure variables. It is unlikely that the case identification based on Medicare claims would be associated with those reproductive/hormone-related factors. Furthermore, for the mostly investigated reproductive factor - age at menarche in prior studies, our results were consistent with the majority of the prior studies10–13 which used imaging data for NAFLD diagnosis. Other limitations include self-reported history of hysterectomy and oophorectomy without validation using medical records (thus potential misclassifications), lack of data on other possible risk factors related to NAFLD (e.g., abortion or polycystic ovary syndrome), and inability to incorporate known genetic factors (e.g., PNPLA3) in this analysis. Inclusion of older Medicare participants (age 65+) limits the generalizability of our results to younger populations.
In summary, among ethnically diverse women, hormone-related factors, including age at menarche and parity were associated with NAFLD risk. An interesting finding was that compared to women with natural menopause, women with surgical menopause have increased NAFLD risk. Furthermore, exogenous hormone use was associated with increased risk. Additional studies on these reproductive and hormone-related factors in other populations and investigations on the molecular mechanisms underlying these associations by incorporating genetic factors are warranted, particularly given the limited data on these factors and the increasing prevalence of NAFLD.
Supplementary Material
What You Need to Know.
Background
There are sex differences in prevalence and incidence of nonalcoholic fatty liver disease (NAFLD). However, there are limited epidemiologic data on the relationship between hormonal factors and NAFLD risk in women.
Findings
Later age at menarche was associated with lower risk of NAFLD, whereas parity, use of exogenous hormones, and type of menopause (surgical vs natural) are linked to increased risk.
Implications for patient care
Hormone and reproductive factors affect risk of NAFLD in women. Clinicians should be aware that use of contraceptives or exogenous estrogen, or surgical menopause, can alter progression of NAFLD.
Acknowledgments
Financial supports: National Cancer Institute R01CA228589 (VWS) and U01CA164973 (LRW). The views expressed in this paper are those of the authors. NCI was not involved in the study design, data collection, analysis, and data interpretation.
Abbreviations
- BMI
body mass index
- CI
confidence interval
- NAFLD
nonalcoholic fatty liver disease
- OR
odds ratio
- SD
(standard deviation)
- OC
oral contraceptive
- MHT
menopausal hormone therapy
Footnotes
Disclosures: All authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology (Baltimore, Md). 2016;64(1):73–84. Epub 2015/12/29. [DOI] [PubMed] [Google Scholar]
- 2.Setiawan VW, Stram DO, Porcel J, et al. Prevalence of chronic liver disease and cirrhosis by underlying cause in understudied ethnic groups: The multiethnic cohort. Hepatology. 2016;64(6):1969–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lonardo A, Bellentani S, Argo CK, et al. Epidemiological modifiers of non-alcoholic fatty liver disease: Focus on high-risk groups. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2015;47(12):997–1006. Epub 2015/10/12. [DOI] [PubMed] [Google Scholar]
- 4.Lonardo A, Carani C, Carulli N, et al. ‘Endocrine NAFLD’ a hormonocentric perspective of nonalcoholic fatty liver disease pathogenesis. Journal of hepatology. 2006;44(6):1196–207. Epub 2006/04/19. [DOI] [PubMed] [Google Scholar]
- 5.Lonardo A, Nascimbeni F, Ballestri S, et al. Sex Differences in NAFLD: State of the Art and Identification of Research Gaps. Hepatology (Baltimore, Md) 2019. Epub 2019/03/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark JM, Brancati FL, Diehl AM. Nonalcoholic fatty liver disease. Gastroenterology. 2002;122(6):1649–57. Epub 2002/05/23. [DOI] [PubMed] [Google Scholar]
- 7.Park SH, Jeon Wk, Kim SH, et al. Prevalence and risk factors of non-alcoholic fatty liver disease among Korean adults. Journal of gastroenterology and hepatology. 2006;21(1 Pt 1):138–43. Epub 2006/05/19. [DOI] [PubMed] [Google Scholar]
- 8.Florio AA, Graubard BI, Yang B, et al. Oophorectomy and risk of non-alcoholic fatty liver disease and primary liver cancer in the Clinical Practice Research Datalink. European journal of epidemiology. 2019;34(9):871–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamaguchi M, Kojima T, Ohbora A, et al. Aging is a risk factor of nonalcoholic fatty liver disease in premenopausal women. World journal of gastroenterology : WJG. 2012;18(3):237–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu J, Zhang J, Du R, et al. Age at menarche is associated with the prevalence of non-alcoholic fatty liver disease later in life. Journal of diabetes. 2017;9(1):53–60. Epub 2016/01/23. [DOI] [PubMed] [Google Scholar]
- 11.Ryu S, Chang Y, Choi Y, et al. Age at menarche and non-alcoholic fatty liver disease. Journal of hepatology. 2015;62(5):1164–70. Epub 2014/12/17. [DOI] [PubMed] [Google Scholar]
- 12.Mueller NT, Pereira MA, Demerath EW, et al. Earlier menarche is associated with fatty liver and abdominal ectopic fat in midlife, independent of young adult BMI: The CARDIA study. Obesity (Silver Spring, Md) 2015;23(2):468–74. Epub 2014/12/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao X, Zhou J, Yuan H, et al. Duration of reproductive lifespan and age at menarche in relation to metabolic syndrome in postmenopausal Chinese women. J Obstet Gynaecol Res. 2016;42(11):1581–7. Epub 2016/10/28. [DOI] [PubMed] [Google Scholar]
- 14.Yi KH, Hwang JS, Lim SW, et al. Early menarche is associated with non-alcoholic fatty liver disease in adulthood. Pediatrics international : official journal of the Japan Pediatric Society. 2017;59(12):1270–5. Epub 2017/09/12. [DOI] [PubMed] [Google Scholar]
- 15.Kolonel LN, Henderson BE, Hankin JH, et al. A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. American journal of epidemiology. 2000;151(4):346–57. Epub 2000/03/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Setiawan VW, Virnig BA, Porcel J, et al. Linking data from the Multiethnic Cohort Study to Medicare data: linkage results and application to chronic disease research. American journal of epidemiology. 2015;181(11):917–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noureddin M, Zelber-Sagi S, Wilkens LR, et al. Diet Associations With Nonalcoholic Fatty Liver Disease in an Ethnically Diverse Population: The Multiethnic Cohort. Hepatology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee S, Kolonel L, Wilkens L, et al. Postmenopausal hormone therapy and breast cancer risk: the Multiethnic Cohort. Int J Cancer. 2006;118(5):1285–91. [DOI] [PubMed] [Google Scholar]
- 19.Gill D, Brewer CF, Del Greco MF, et al. Age at menarche and adult body mass index: a Mendelian randomization study. Int J Obes (Lond) 2018;42(9):1574–81. Epub 2018/03/20. [DOI] [PubMed] [Google Scholar]
- 20.Feng Y, Hong X, Wilker E, et al. Effects of age at menarche, reproductive years, and menopause on metabolic risk factors for cardiovascular diseases. Atherosclerosis. 2008;196(2):590–7. Epub 2007/08/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Golabi P, Fazel S, Otgonsuren M, et al. Association of Parity in Patients with Chronic Liver Disease. Annals of hepatology. 2018;17(6):1035–41. Epub 2019/01/03. [DOI] [PubMed] [Google Scholar]
- 22.Endres LK, Straub H, McKinney C, et al. Postpartum weight retention risk factors and relationship to obesity at 1 year. Obstetrics and gynecology. 2015;125(1):144–52. Epub 2015/01/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu SH, Lazo M, Koteish A, et al. Oral contraceptive pill use is associated with reduced odds of nonalcoholic fatty liver disease in menstruating women: results from NHANES III. Journal of gastroenterology. 2013;48(10):1151–9. Epub 2012/11/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsuo K, Gualtieri MR, Cahoon SS, et al. Surgical menopause and increased risk of nonalcoholic fatty liver disease in endometrial cancer. Menopause (New York, NY) 2016;23(2):189–96. Epub 2015/07/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stanczyk FZ, Chaikittisilpa S, Sriprasert I, et al. Circulating androgen levels before and after oophorectomy in premenopausal and postmenopausal women. Climacteric. 2019;22(2):169–74. Epub 2019/01/08. [DOI] [PubMed] [Google Scholar]
- 26.Ngo Sock ET, Cote I, Mentor JS, et al. Ovariectomy stimulates hepatic fat and cholesterol accumulation in high-fat diet-fed rats. Horm Metab Res. 2013;45(4):283–90. [DOI] [PubMed] [Google Scholar]
- 27.Kamada Y, Kiso S, Yoshida Y, et al. Estrogen deficiency worsens steatohepatitis in mice fed high-fat and high-cholesterol diet. American journal of physiology Gastrointestinal and liver physiology. 2011;301(6):G1031–43. [DOI] [PubMed] [Google Scholar]
- 28.Phipps AI, Buist DS. Validation of self-reported history of hysterectomy and oophorectomy among women in an integrated group practice setting. Menopause. 2009;16(3):576–81. Epub 2009/01/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laughlin-Tommaso SK, Khan Z, Weaver AL, et al. Cardiovascular and metabolic morbidity after hysterectomy with ovarian conservation: a cohort study. Menopause. 2018;25(5):483–92. Epub 2017/12/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang JD, Abdelmalek MF, Guy CD, et al. Patient Sex, Reproductive Status, and Synthetic Hormone Use Associate With Histologic Severity of Nonalcoholic Steatohepatitis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2017;15(1):127–31 e2. Epub 2016/08/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stanczyk FZ. Parenteral versus oral treatment of postmenopausal women with estrogen. Menopause. 2007;14(6):968–70. Epub 2007/09/28. [DOI] [PubMed] [Google Scholar]
- 32.Lim U, Turner SD, Franke Aa, et al. Predicting total, abdominal, visceral and hepatic adiposity with circulating biomarkers in Caucasian and Japanese American women. PloS one 2012;7(8):e43502. Epub 2012/08/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. The American journal of gastroenterology. 2003;98(5):960–7. [DOI] [PubMed] [Google Scholar]
- 34.Ruhl CE, Everhart JE. Relation of elevated serum alanine aminotransferase activity with iron and antioxidant levels in the United States. Gastroenterology. 2003;124(7):1821–9. [DOI] [PubMed] [Google Scholar]
- 35.Ioannou GN, Boyko EJ, Lee SP. The prevalence and predictors of elevated serum aminotransferase activity in the United States in 1999–2002. The American journal of gastroenterology. 2006;101(1):76–82. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


