Abstract
Background:
Despite the advances in treatment of differentiated thyroid cancer (DTC), predicting prognosis remains a challenge. Immune cells in the tumor microenvironment may provide an insight to predicting recurrence. Therefore, the objective of this study was to investigate the association of tumor-associated macrophages (TAMs) and tumor-associated neutrophils (TANs) with recurrence in DTC and to identify serum cytokines that correlate with the presence of these immune cells in the tumor.
Materials and Methods:
Forty-two DTC tissues from our institutional neoplasia repository were stained for immunohistochemistry markers for TAMs and TANs. Additionally, cytokine levels were analyzed from these patients from preoperative blood samples. TAM and TAN staining were compared to clinical data and serum cytokine levels.
Results:
Neither TAM nor TAN scores alone correlated with tumor size, presence of lymph node metastases, multifocal tumors, lymphovascular or capsular invasion, or presence of BRAFV600E mutation (all p>0.05). There was no association with recurrence-free survival (RFS) in TAN density (mean RFS 169.1 vs 148.1 months, p=0.23) or TAM density alone (mean RFS 121.3 vs 205.2 months, p= 0.54). However, when scoring from both markers were combined, patients with high TAM density and TAN negative scores had significantly lower RFS (mean RFS 50.7 vs 187.3 months, p=0.04) compared to the remaining cohort. Patients with high TAM/negative TAN tumors had significantly lower serum levels of IL-12p70, IL8, TNF-alpha and TNF-beta.
Conclusion:
In differentiated thyroid cancers, high density of TAMs in the absence of TANs is associated with worse outcome. Assessment of multiple immune cell types and serum cytokines may predict outcomes in DTC.
Keywords: tumor associated macrophages, tumor associated neutrophils, thyroid cancer, cytokines
Introduction
Thyroid cancer is the most common endocrine cancer, with an increasing incidence in the past decade, now accounting for approximately 3.8% of all new cancer cases in the United States (1). By 2030, thyroid cancer is projected to be the 2nd most common cancer in women and 4th most common cancer overall (2). Some investigators have suggested that the introduction and the subsequent widespread use of ultrasonography and fine-needle aspiration biopsy, along with the increased use of diagnostic imaging modalities, such as CT, MRI and PET, has led to increased detection of small thyroid nodules and diagnosis of thyroid cancer at an early stage (3-5). Despite favorable long-term survival in differentiated thyroid cancers, recurrence remains a major concern with 40-yr recurrence rates reported as high as 35%, two thirds of which occurred within the first decade after initial therapy (6). Some of the prognostic factors currently used in predicting recurrence include age at diagnosis, size of primary tumor, presence of soft tissue invasion or distant metastasis, American Thyroid Association (ATA) risk stratification guideline as well other information such as mutational analysis (7-10). With current prognostic factors and surveillance practices, attempts to predict tumor recurrence often produces inaccurate results. As such, novel markers are needed to more precisely predict disease progression. Recently, in many cancer types, attention has been directed towards the tumor microenvironment as a source for predictors of recurrence.
The tumor microenvironment (TME) is a complex milieu of cell types that communicate with one another to assist in the growth and spread of cancer. A few examples of cells found in the TME include tumor associated macrophages (TAM), tumor associated neutrophils (TAN), cancer-associated fibroblasts, pericytes and regulatory T-cells. In previous studies, increased TAM density in poorly differentiated thyroid cancer was associated with poor histologic grade, tumor invasiveness and decreased cancer-related survival (11). However, there is a paucity of data on the prognostic role of TANs, as well as TAMs in conventional DTC. As such, the objective of this study was to determine the prognostic role of both TAMs and TANs in conventional DTC. Furthermore, we sought to identify how tumor immune cell composition correlates with cytokine markers in serum from patients with DTC.
MATERIALS AND METHODS
Forty-two paraffin embedded samples of DTC were collected from the endocrine neoplasia repository of the Ohio State University Wexner Medical Center. Samples were stained via immunohistochemistry (IHC) for macrophage marker CD163 (rabbit anti-CD163, 1:900, cat. ab87099, Abcam, Cambridge, UK) and neutrophil marker CD66b (rabbit anti-CD66b, 1:100, cat. ab197678, Abcam, Cambridge, UK) according to the following protocol. Paraffin embedded tissue was cut at 4 microns and placed on positively charged slides. Slides with specimens were then placed in a 60 °C oven for 1 hour and cooled. Slides were then placed on the Leica Bond III Autostainer. All slides are deparaffinized and rehydrated with Bond Dewax Solution (product code AR9222) and 100% alcohol. All slides were stained with in-house created protocol. Slides were quenched for 5 minutes in a 3% hydrogen peroxide solution to block for endogenous peroxidase. Antigen retrieval was performed using Leica’s Bond Epitope Retrieval Solution 1 (ER1 low pH, product code AR9961) for 20 minutes. Primary antibody was incubated for 15 mins (for CD66b) or 30 mins (for CD163) at room temperature. The antibodies were detected using Leica’s Bond Polymer Refine Detection system (product code DS9800). Lastly, sections were incubated with DAB mixed on-line for 10 minutes. Slides were then counterstained using Leica hematoxylin for 3 minutes then removed and dehydrated through graded ethanol solutions and coverslipped.
Expression levels were scored with the assistance of a clinical pathologist, L.S similarly to the method published by Fagin et al (11). Briefly, TAM density was scored numerically from 0 to 3 with 0 representing absence of macrophages per 10x high-power field (HPF) and 3 representing presence of densely populated macrophages per 10x HPF. The scoring was further collapsed into two major categories: low TAM density for samples with scores that range from 0 to 1 and high TAM density for those with scores of 2 to 3. Expression levels of TAN density was scored similarly to the methods used by Galdiero et al (12). CD66+ cells were counted and scored by a clinical pathologist blinded to patient outcomes. The median value of CD66+ counts (9 neutrophils per 10x high-powerfield) was used as a cutoff. TAN density was grouped into 2 major categories: a negative group for samples with less than 9 neutrophils per 10x HPF and a positive group for samples with greater than 9 neutrophils per 10x HPF.
Clinicopathologic data collected included gender, age at diagnosis, cancer histology, tumor size, capsular invasion, lymph node metastasis, lymphovascular invasion, multifocal tumor and BRAFV600E mutation, obtained through a database maintained within the institution’s endocrine neoplasia repository. Per protocol, patient serum was collected at a preoperative office visit or on the day of surgery. A commercially available comprehensive human 30-plex cytokine panel that contained known cytokines associated with tumor immune cells including TAMs and TANs was used in this study. Patient serum was then analyzed for expression levels using Luminex ® Multiplex Assay. A waiver of consent was obtained as this was a retrospective study. The study was approved under the Ohio State University Wexner Medical Center Institutional Review Board.
Demographics and disease characteristics are reported as mean or median. Cytokine expression levels were reported as logarithm transformed values with interquartile range (IQR). Categorical variables were compared using Pearson’s chi-square test. Recurrence-free survival was evaluated using Kaplan-Meier estimate, with comparisons between groups made using logrank test. Univariable and multivariable Cox regression analysis were performed to assess the effect of clinicopathologic features on recurrence. All tests of statistical significance were twosided with level of statistical significance established at p<0.05. Statistical analysis was performed using SPSS v 25 (SPSS, Inc., Chicago, IL, USA).
RESULTS
Clinicopathologic characteristics
Forty-two patients were included in the cohort. Clinicopathologic characteristics are included in Table 1. The median age at diagnosis was 50 years (range 20-86). The majority of patients (80.5%) had papillary thyroid cancer as their final pathologic diagnosis while 19.5% had follicular thyroid cancer. The overall recurrence rate was 23.8% and the mean follow-up period was 61 months (IQR 11.3-92.1 months).
Table 1:
Clinicopathologic characteristics of cohort
| Patient Characteristics | N (%) | |
|---|---|---|
| Male | 14(33.3) | |
| Female | 28(66.7) | |
| Age at Diagnosis | ||
| median (range) years | 50 (20-86) | |
| Age (Years) | ≤ 55 Years | 25(59.5) |
| > 55 Years | 17(40.5) | |
| Cancer Histology | Follicular | 8(19.5) |
| Papillary | 34 (80.9) | |
| (Classic variant) | 25(73.5) | |
| (Follicular variant) | 8(23.5) | |
| (Oncocytic variant) | 1(4.0) | |
| Tumor Size | < 4 cm | 33(78.6) |
| > 4 cm | 8(19.0) | |
| Unknown | 1(2.0) | |
| Capsular Invasion | Yes | 9(21.4) |
| No | 0(0) | |
| Unknown/NA | 33(78.6) | |
| Encapsulated | Follicular | |
| Yes | 2(25) | |
| No | 6(75) | |
| Papillary | ||
| Yes | 7(20.6) | |
| No | 27(79.4) | |
| Lymph Node Status at Thyroidectomy (PTC) | Positive | 11(26.2) |
| Negative | 22(52.4) | |
| Unknown | 1(2.0) | |
| Lymphovascular Invasion | Yes | 11(26.2) |
| No | 30(71.4) | |
| Unknown | 1(2.0) | |
| Multifocal tumor | Yes | 17(40.5) |
| No | 24(57.1) | |
| Unknown | 1(2.0) | |
| BRAFV600E positive | Yes | 22(52.4) |
| No | 19(45.2) | |
| Unknown | 1(2.0) | |
The univariable and multivariable Cox regression analyses of recurrence-free survival (RFS) calculated from the date of initial operation are summarized in Table 2. Age >55, and T stage IV disease were all associated with worse survival on univariable analysis while female gender was associated with improved recurrence-free survival. Multivariable analysis showed that only T stage IV disease (hazard ratio, 5.183; 95% CI, 1.225-21.924, p= 0.025) was independently associated with RFS.
Table 2:
Recurrence-free survival univariable and multivariable analyses
| Characteristic | Univariable analysis Hazard ratio (95% confidence interval) |
P value | Multivariable analysis Hazard ratio (95% confidence interval) |
P value |
|---|---|---|---|---|
| Age>55 | 4.771 (1.225-18.580) | 0.02 | 1.668 (0.357-7.781) | 0.52 |
| Female | 0.196 (0.050-0.761) | 0.02 | 0.281 (0.065-1.205) | 0.09 |
| T stage IV | 6.922 (1.852-25.875) | <0.01 | 5.183 (1.225-21.924) | 0.03 |
| Lymph node metastasis | 1.874 (0.468-7.501) | 0.38 | NA | NA |
|
Multifocal tumors |
1.781 (0.478-6.634) | 0.39 | NA | NA |
|
Lymphovascular invasion |
2.823 (0.756-10.537) | 0.12 | NA | NA |
|
Capsular invasion |
1.625 (0.388-6.813) | 0.51 | NA | NA |
| BRAFV600E | 1.481 (0.299-7.348) | 0.63 | NA | NA |
| TAN density | 0.302 (0.038-2.417) | 0.23 | NA | NA |
| TAM density | 1.891 (0.239-14.946) | 0.54 | NA | NA |
Correlation of TAMs with clinicopathologic characteristics and serum cytokines
There was no significant correlation between tumor associated macrophage density and clinicopathologic factors collected (Table 3). Next, RFS based on TAM density was analyzed (Figure 1). There was no significant difference in RFS between patients with high TAM density tumors compared to low TAM density tumors (mean RFS 121.3 months vs 205.2 months, p=0.539).
Table 3:
Correlating tumor associated macrophages with clinicopathologic characteristics
| Clinicopathologic Characteristics | Low TAM | High TAM | p-value |
|---|---|---|---|
| Age >55 | 16.7% | 44.4% | 0.20 |
| Female gender | 66.7% | 61.1% | 0.80 |
| T stage IV | 0% | 25% | 0.23 |
| Lymph node metastasis present | 50% | 48.6% | 0.95 |
| Multifocal tumors present | 33.3% | 42.9% | 0.66 |
| Lymphovascular invasion present | 33.3% | 25.7% | 0.70 |
| Capsular invasion present | 50% | 21.2% | 0.21 |
| BRAFV600E positive | 50% | 69% | 0.45 |
Figure 1:
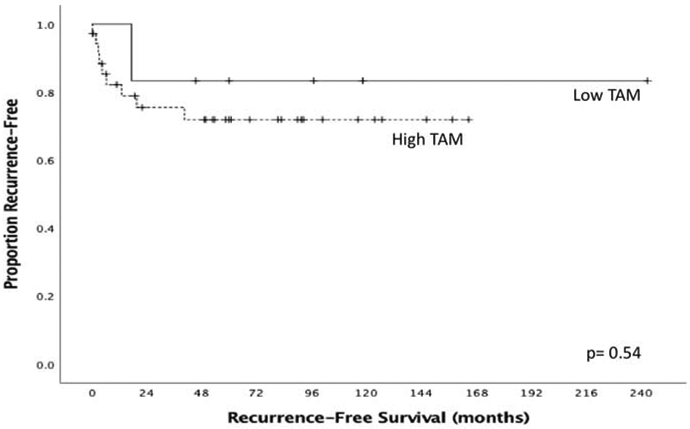
Recurrence-free survival by TAM density
Key immunoregulatory cytokines present in the patients’ serum were compared to TAM tumor density (Table 4). Patients with high TAM density tumors had higher serum levels of the cytokines Mip1-alpha (p= 0.03) and IL-8 (p= 0.012). Patients with low TAM density tumors had significantly higher serum levels of MCP-1 (p=0.014).
Table 4:
Comparison of serum cytokine levels with tumor associated macrophage expression
| Low TAM N=24 |
High TAM N=18 |
p-value | |
|---|---|---|---|
| Eotaxin | 4.17 (3.95-4.34) | 4.08 (3.86-4.23) | 0.50 |
| MIP1a | 2.57 (2.52-2.70) | 2.69 (2.63-2.83) | 0.03 |
| IL16 | 4.19 (4.09-4.48) | 4.24 (4.08-4.59) | 0.99 |
| VEGF | 3.60 (3.48-3.85) | 3.55 (3.39-3.83) | 0.79 |
| IL2 | 2.67 (2.63-2.69) | 2.66 (2.64-2.69) | 0.85 |
| Eotaxin3 | 2.51 (2.39-2.58) | 2.52 (2.42-2.56) | 0.96 |
| MIP1b | 4.08 (3.92-4.15) | 4.08 (4.00-4.20) | 0.54 |
| IL17 | 2.59 (2.50-2.68) | 2.54 (2.50-2.59) | 0.52 |
| IFNgamma | 3.02 (2.92-3.14) | 2.94 (2.86-3.16) | 0.50 |
| IL4 | 2.35 (2.32-2.38) | 2.37 (2.32-2.46) | 0.26 |
| IL8 | 2.21 (2.19-2.26) | 2.26 (2.23-2.28) | 0.01 |
| TARC | 4.04 (3.91-4.23) | 4.00 (3.83-4.12) | 0.29 |
| IL1a | 2.59 (2.54-2.64) | 2.56 (2.50-2.63) | 0.29 |
| IL10 | 2.74 (2.71-2.86) | 2.74 (2.72-2.76) | 0.81 |
| IL6 | 2.69 (2.56-2.88) | 2.70 (2.51-2.96) | 0.93 |
| IP10 | 4.87 (4.79-5.02) | 4.80 (4.64-4.92) | 0.16 |
| GMCSF | 2.32 (2.30-2.37) | 2.34 (2.30-2.36) | 0.88 |
| IL5 | 2.73 (2.70-2.78) | 2.75 (2.69-2.84) | 0.31 |
| IL12p70 | 2.47 (2.43-2.53) | 2.47 (2.44-2.56) | 0.56 |
| AE | 3.38 (3.30-3.64) | 3.55 (3.38-3.93) | 0.14 |
| MCP1 | 4.75 (4.66-4.98) | 4.64 (4.55-4.79) | 0.01 |
| IL12 | 4.18 (4.03-4.32) | 4.16 (4.09-4.41) | 0.77 |
| IL7 | 2.62 (2.56-2.93) | 2.62 (2.55-2.77) | 0.38 |
| IL13 | 2.30 (2.28-2.32) | 2.34 (2.29-2.44) | 0.08 |
| TNFalpha | 3.14 (3.07-3.26) | 3.14 (3.06-3.31) | 0.89 |
| MDC | 4.33 (4.22-4.40) | 4.33 (4.25-4.46) | 0.77 |
| IL15 | 2.91 (2.89-2.99) | 2.91 (2.84-2.94) | 0.36 |
| TNFbeta | 2.59 (2.55-2.67) | 2.61 (2.56-2.64) | 0.74 |
| IL1b | 2.93 (2.85-3.19) | 2.92 (2.83-3.63) | 0.79 |
| RANTES | 4.22 (3.99-4.98) | 4.15 (4.01-4.42) | 0.57 |
Data are presented as log transformed values. Median (interquartile range)
Correlation of TANs with clinicopathologic characteristics and serum cytokines
Age >55 and female gender were significantly associated with TAN negative tumors (Table 5). There was no significant difference in RFS between patients with TAN negative tumors compared to TAN positive tumors (mean RFS 169.1 months vs 148.1 months, p=0.231, Figure 2).
Table 5:
Correlating tumor associated neutrophils with clinicopathologic characteristics
| Clinicopathologic Characteristics | TAN positive | TAN negative | p-value |
|---|---|---|---|
| Age >55 | 10% | 50% | 0.03 |
| Female gender | 50% | 90% | 0.03 |
| T stage IV | 0% | 18.5% | 0.14 |
| Lymph node metastasis present | 30% | 51.9% | 0.24 |
| Multifocal tumors present | 30% | 44.4% | 0.43 |
| Lymphovascular invasion present | 20% | 33.3% | 0.43 |
| Capsular invasion present | 33.3% | 16.7% | 0.30 |
| BRAFV600E positive | 70% | 63.2% | 0.71 |
Figure 2:
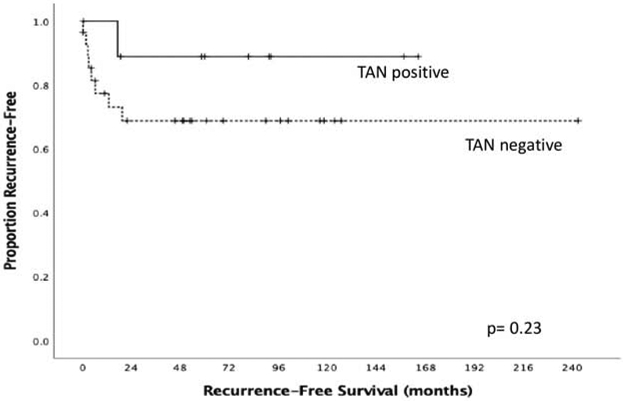
Recurrence-free survival by TAN density
Key immunoregulatory cytokines present in the patients’ serum were compared to presence of TANs in the tumor (Table 6), with patients having TAN negative tumors expressing higher serum levels of IL-12p70 compared to patients with TAN positive tumors (p=0.008).
Table 6:
Comparison of serum cytokine levels with tumor associated neutrophil expression
| TAN Negative N=28 |
TAN positive N=10 |
p-value | |
|---|---|---|---|
| Eotaxin | 4.16 (3.97-4.34) | 4.20 (3.76-4.34) | 0.89 |
| MIP1a | 2.68 (2.52-2.80) | 2.64 (2.56-2.77) | 0.86 |
| IL16 | 4.24 (4.09-4.59) | 4.14 (4.06-4.56) | 0.65 |
| VEGF | 3.62 (3.39-3.85) | 3.60 (3.47-3.72) | 0.93 |
| IL2 | 2.67 (2.63-2.70) | 2.66 (2.65-2.69) | 0.87 |
| Eotaxin3 | 2.52 (2.42-2.59) | 2.49 (2.40-2.55) | 0.51 |
| MIP1b | 4.08 (3.92-4.20) | 4.06 (4.00-4.13) | 0.82 |
| IL17 | 2.55 (2.49-2.62) | 2.57 (2.54-2.67) | 0.50 |
| IFNgamma | 3.02 (2.92-3.14) | 3.00 (2.84-3.18) | 0.72 |
| IL4 | 2.37 (2.32-2.39) | 2.36 (2.34-2.42) | 0.65 |
| IL8 | 2.26 (2.21-2.26) | 2.22 (2.18-2.26) | 0.26 |
| TARC | 4.01 (3.91-4.22) | 4.01 (3.76-4.39) | 0.93 |
| IL1a | 2.59 (2.54-2.64) | 2.56 (2.51-2.63) | 0.57 |
| IL10 | 2.74 (2.72-2.86) | 2.74 (2.72-2.76) | 0.33 |
| IL6 | 2.73 (2.54-2.90) | 2.64 (2.55-2.81) | 0.50 |
| IP10 | 4.86 (4.79-5.05) | 4.83 (4.71-4.95) | 0.47 |
| GMCSF | 2.33 (2.30-2.37) | 2.33 (2.31-2.35) | 0.50 |
| IL5 | 2.75 (2.71-2.79) | 2.72 (2.70-2.81) | 0.68 |
| IL12p70 | 2.50 (2.45-2.56) | 2.42 (2.41-2.47) | 0.01 |
| MCP1 | 4.75 (4.64-4.82) | 4.76 (4.60-4.92) | 0.89 |
| IL12 | 4.18 (4.03-4.35) | 4.15 (4.04-4.36) | 0.93 |
| IL7 | 2.61 (2.56-2.78) | 2.66 (2.49-2.91) | 0.93 |
| IL13 | 2.30 (2.29-2.35) | 2.29 (2.28-2.32) | 0.47 |
| TNFalpha | 3.14 (3.07-3.31) | 3.12 (3.08-3.16) | 0.16 |
| MDC | 4.33 (4.22-4.36) | 4.35 (4.29-4.45) | 0.37 |
| IL15 | 2.91 (2.85-2.99) | 2.90 (2.82-2.94) | 0.38 |
| TNFbeta | 2.62 (2.57-2.69) | 2.59 (2.54-2.60) | 0.10 |
| IL1b | 2.91 (2.85-3.34) | 2.94 (2.86-3.24) | 0.89 |
| RANTES | 4.09 (3.98-4.58) | 4.28 (4.11-4.73) | 0.39 |
Data are presented as log transformed values. Median (interquartile range)
Outcome for combination of immune markers
Combined levels of these immune cells in the microenvironment were assessed because their interaction in vivo may play a role in the biology of the disease. Although this has not been directly shown in a tumor model, there are published data on the interaction of neutrophils and macrophages in models of tissue inflammation (13-15). Interestingly, when scores from both markers were combined, patients with tumors that had high TAM density and were TANnegative had a significantly lower RFS compared to the rest of the cohort (mean RFS 50.7 months vs 187.3 months, p=0.04, Figure 3). We then assessed the difference in serum cytokine expression between this group and the rest of the cohort (Table 7). Patients with high TAM density/TAN negative tumors expressed lower serum levels of cytokines IL-12p70, IL-8, TNF-alpha and TNF-beta compared to the remainder of the cohort.
Figure 3:
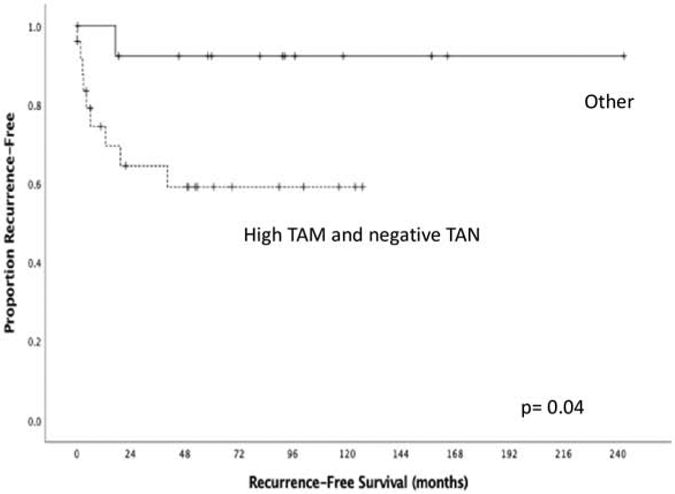
Patients with high TAM density and negative TANs have significantly lower RFS
Table 7:
Comparison of combined High TAM/Negative TAN with serum cytokines.
| Other N=20 |
High TAM Negative TAN N=11 |
p-value | |
|---|---|---|---|
| IP10 | 4.89 (4.80-5.08) | 4.80 (4.68-4.92) | 0.17 |
| MIP1beta | 4.10 (3.93-4.24) | 4.07 (4.00-4.13) | 0.51 |
| IL15 | 2.91 (2.85-2.99) | 2.90 (2.85-2.94) | 0.26 |
| IL5 | 2.75 (2.72-2.79) | 2.72 (2.69-2.79) | 0.48 |
| IFNgamma | 3.02 (2.92-3.15) | 2.93 (2.85-3.11) | 0.41 |
| IL1beta | 2.90 (2.85-3.34) | 2.95 (2.85-3.34) | 1.00 |
| IL8 | 3.42 (3.21-3.70) | 3.38 (3.38-3.44) | 0.92 |
| Eotaxin | 4.12 (3.97-4.39) | 4.17 (3.90-4.29) | 0.97 |
| MCP1 | 4.76 (4.64-4.83) | 4.74 (4.61-4.83) | 0.74 |
| TARC | 4.02 (3.87-4.19) | 3.99 (3.79-4.33) | 0.84 |
| IL16 | 4.24 (4.11-4.60) | 4.15 (4.05-4.43) | 0.56 |
| IL7 | 2.61 (2.56-2.77) | 2.62 (2.49-3.00) | 0.84 |
| IL10 | 2.76 (2.73-2.86) | 2.73 (2.70-2.75) | 0.06 |
| IL2 | 2.67 (2.63-2.70) | 2.67 (2.65-2.68) | 0.98 |
| TNFalpha | 3.17 (3.13-3.34) | 3.10 (3.06-3.14) | 0.01 |
| Eotaxin3 | 2.53 (2.41-2.61) | 2.51 (2.41-2.56) | 0.69 |
| MDC | 4.33 (4.25-4.38) | 4.34 (4.23-4.43) | 0.93 |
| GMCSF | 2.34 (2.30-2.38) | 2.32 (2.29-2.34) | 0.13 |
| IL17 | 2.57 (2.50-2.65) | 2.54 (2.50-2.66) | 0.54 |
| TNFbeta | 2.63 (2.58-2.69) | 2.58 (2.53-2.60) | 0.01 |
| IL12p70 | 2.51 (2.47-2.56) | 2.43 (2.41-2.50) | 0.01 |
| IL4 | 2.37 (2.32-2.40) | 2.36 (2.34-2.38) | 0.90 |
| RANTES | 4.10 (3.97-4.50) | 4.23 (4.05-5.04) | 0.34 |
| IL8 | 2.26 (2.21-2.28) | 2.21 (2.19-2.25) | 0.02 |
| MIP1alpha | 2.70 (2.55-2.81) | 2.58 (2.49-2.68) | 0.16 |
| IL12 | 4.22 (4.04-4.41) | 4.12 (3.97-4.32) | 0.25 |
| IL1alpha | 2.58 (2.54-2.64) | 2.56 (2.51-2.63) | 0.71 |
| VEGF | 3.58 (3.43-3.83) | 3.60 (3.39-3.85) | 0.90 |
| IL13 | 2.30 (2.29-2.36) | 2.29 (2.28-2.34) | 0.30 |
| IL6 | 2.80 (2.64-2.95) | 2.59 (2.53-2.69) | 0.15 |
Data are presented as log transformed values. Median (IQR).
Discussion
Multiple studies have shown that immune cell composition of the tumor microenvironment impacts tumor development, progression and clinical outcome (11, 16-18). Tumor-associated inflammatory cells are not uncommon in thyroid cancer and have been shown to correlate with outcomes (19, 20). In our cohort, scores of TAMs or TANs alone did not correlate with aggressive histologic features or recurrence-free survival. However, when both markers were combined, we found that the presence of high TAM density in the absence of TANs is associated with worse outcomes. Furthermore, in this cohort, we found patients with high TAM density in addition to absent TAN had lower serum levels of the cytokines IL-12p70, Il-8, TNF-alpha and TNF-beta.
Previous studies have demonstrated that an increased density of TAMs is associated with tumor progression in advanced thyroid cancer (11, 21, 22). Ryder et al found that increased TAMs in poorly differentiated thyroid cancer was associated with capsular invasion, extrathyroidal extension and decreased cancer-related survival (11). In our cohort, we found that there was no significant difference in RFS between patients with high TAM density tumors compared to low TAM density tumors. Our study differs from the work of Ryder et al given that our cohort consisted of well differenced thyroid cancer. One reason why this difference potentially did not reach statistical significance in our study is secondary to relatively low sample size (n=42), as our power calculation shows that 740 patients will be needed to adequately detect a difference between the two groups.
Investigations of the clinical relevance and prognostic value of TAN density reveals differences based on cancer types. The presence of TANs in hepatocellular carcinoma, Stage I/II melanoma, and head and neck cancers have been shown to be independently associated with poor overall survival(OS), RFS, and disease-specific survival outcomes(23-25).In contrast, high levels of TANs in stage II colorectal cancer has been associated with improved OS(26). Prior to our study, there has not been a link between tumor associated neutrophil density and recurrence in DTC. Other studies have looked at the peripheral blood neutrophil to lymphocyte ratio (NLR) and found a positive correlation with tumor size among patients with DTC (27-29). Additionally, patients with high American Thyroid Association (ATA) risk for recurrence presented with higher baseline NLR, however, blood NLR was not compared to lymphocytic infiltration within and surrounding the thyroid tumor (27).
There has been debate on the optimal strategy to develop biomarkers for use in early detection, diagnosis, prognosis and assessment of response in cancer (30-33). Tumor infiltrating immune cells and tumor cells in the microenvironment secrete cytokines and chemokines into circulation to regulate tumor growth. Numerous serum cytokine levels have been studied to understand their role in predicting benign versus malignant thyroid disease (30, 34, 35). Indeed, serum cytokine levels have been previously investigated to see if they correlate with intra-tumor cytokine expression and tumor infiltrating immune cells. In breast cancer, Jabeen et al found that serum cytokine interferon gamma-induced protein 10 (IP10) correlated with mRNA expression in the breast tumor and serum platelet derived growth factor (PDGF) correlated with the tumor immune cells pro-B and natural killer T (NKT) cells (36). Serum Il-2, IL-2R and IL-10 levels have been implicated in distinguishing disease-free patients from those with active disease in thyroid cancer (37). Provatopoulou et al evaluated serum levels of interleukins (IL-1B, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12 and IL-13) in a cohort that consisted of 20 papillary thyroid cancer patients, 38 patients with benign nodules and 50 healthy controls using a multiplex assay(35). Peripheral venous blood samples were collected from participants preoperatively. They observed that serum IL-6 and IL-10 concentrations were high in benign and malignant thyroid nodules compared to serum obtained from healthy controls(35). However, none of the individual cytokines were sufficient to discriminate between benign and malignant thyroid nodule. In our study, we investigated the correlation between serum cytokines and tumor infiltrating immune cells in thyroid cancer. We found that tumor with high TAM/low TAN correlated with lower serum levels of the cytokines IL-12p70, Il-8, TNF-alpha and TNF-beta. These cytokines are responsible for a range of signaling events within immune cells (macrophages, neutrophils, T and NK cells) which lead to cellular destruction of tumor cells through processes such as necrosis or apoptosis. We would expect that patients with poor outcomes depicted by lower RFS (high TAM/low TAN tumors) would have associated low levels of these cytokines, produced by the immune cells, in circulation which is what we saw in our study. In our study, patients with low TAM density tumors had significantly higher serum levels of MCP-1, a potent chemoattract that greatly contributes to the recruitment of blood monocytes/macrophage to infiltrate sites of inflammation or tumors. Given this knowledge, we would have expected to see a strong correlation between low TAM density and lower serum levels of MCP-1 and vice versa but this was not the case in our study. It is plausible that we did not see this correlation in our study because the cytokines in circulation may not accurately reflect the intra-tumor cytokines. We acknowledge that this cytokine part of our study is hypothesis-generating, and further work is needed to confirm if circulating cytokines are a true reflection of tumor cytokines in DTC and the potential mechanism for elevation of these cytokines in circulation.
The current study had several limitations that need to be considered when interpreting the results. A major limitation was the relatively low sample size in this analysis. Differentiated thyroid cancer has generally good prognosis and, as such, a larger number of patients with long term follow-up may be necessary to achieve enough power to draw more definitive conclusions regarding the role of tumor immune cell component in predicting outcomes. Cytokine expression in blood was not correlated with intra-tumor cytokine expression and may not completely reflect the changes in the tumor microenvironment. We did not account for previous episodes of fine needle aspiration prior to definitive resection and it is possible that this may affect the density of immune cells present in the resected specimen. Also, we did not have information on background Hashiomoto’s disease in our cohort and as such cannot conclude if autoimmune disease was a contributing factor to TAM and TAN density. Our finding in the association between high TAM/TAN negative density with lower recurrence-free survival should be interpreted with caution as this is a single institution, observational study. These results should be viewed as hypothesis generating, requiring further validation.
In summary, while scores of TAMs or TANs alone did not predict aggressive histologic features or worse outcomes, when combined, the presence of high TAM density in the absence of TANs is associated with worse outcomes. Additionally, cytokine levels in serum varied by macrophage or neutrophil density present in the tumor microenvironment. Furthermore, assessment of multiple immune cell types in the tumor microenvironment could more accurately predict outcomes in differentiated thyroid cancer. Finally, serum cytokine markers may reflect immune cell density in the tumor microenvironment however further work is needed to confirm this finding.
Highlights.
Assessment of multiple immune cell types may predict outcomes in thyroid cancer
High tumor macrophages in low neutrophil density is associated with poor outcome
Serum cytokine levels may correlate with tumor immune cell composition
Lower serum levels of IL-12p70, IL8, TNF-α and TNF-β correlated with poor outcome
Acknowledgments
Financial Support: Funding support for this paper is through National Cancer Institute award number grants P50 CA168505 (Thyroid SPORE); P01 CA124570 from the National Cancer Institute; award number grant KL2TR002734 from the National Center for Advancing Translational Sciences; award number grant 1T32AI106704–01A1 (Advanced Research Training in Immunology for Surgery Trainees) from the National Institute of Allergy and Infectious Diseases; and AASF/AAS Trainee Research Fellowship Award. The research reported in this publication and the content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Declaration of Interest
The authors declare no conflict of interest. Additionally, the authors maintain full control of all primary data included in this article and will make it available for review if requested.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference:
- 1.Nguyen QT, Lee EJ, Huang MG, Park YI, Khullar A, Plodkowski RA. Diagnosis and treatment of patients with thyroid cancer. Am Health Drug Benefits. 2015;8(1):30–40. [PMC free article] [PubMed] [Google Scholar]
- 2.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913–21. [DOI] [PubMed] [Google Scholar]
- 3.Kent WD, Hall SF, Isotalo PA, Houlden RL, George RL, Groome PA. Increased incidence of differentiated thyroid carcinoma and detection of subclinical disease. CMAJ. 2007;177(11):1357–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA. 2006;295(18):2164–7. [DOI] [PubMed] [Google Scholar]
- 5.Sosa JA, Hanna JW, Robinson KA, Lanman RB. Increases in thyroid nodule fine-needle aspirations, operations, and diagnoses of thyroid cancer in the United States. Surgery. 2013;154(6):1420–6; discussion 6-7. [DOI] [PubMed] [Google Scholar]
- 6.Mazzaferri EL, Kloos RT. Clinical review 128: Current approaches to primary therapy for papillary and follicular thyroid cancer. J Clin Endocrinol Metab. 2001;86(4):1447–63. [DOI] [PubMed] [Google Scholar]
- 7.Cady B, Rossi R. An expanded view of risk-group definition in differentiated thyroid carcinoma. Surgery. 1988;104(6):947–53. [PubMed] [Google Scholar]
- 8.Hay ID, Bergstralh EJ, Goellner JR, Ebersold JR, Grant CS. Predicting outcome in papillary thyroid carcinoma: development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery. 1993;114(6):1050–7; discussion 7-8. [PubMed] [Google Scholar]
- 9.Hay ID. Papillary thyroid carcinoma. Endocrinol Metab Clin North Am. 1990;19(3):545–76. [PubMed] [Google Scholar]
- 10.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryder M, Ghossein RA, Ricarte-Filho JC, Knauf JA, Fagin JA. Increased density of tumor-associated macrophages is associated with decreased survival in advanced thyroid cancer. Endocr Relat Cancer. 2008;15(4):1069–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galdiero MR, Bianchi P, Grizzi F, Di Caro G, Basso G, Ponzetta A, et al. Occurrence and significance of tumor-associated neutrophils in patients with colorectal cancer. Int J Cancer. 2016;139(2):446–56. [DOI] [PubMed] [Google Scholar]
- 13.Kasama T, Strieter RM, Standiford TJ, Burdick MD, Kunkel SL. Expression and regulation of human neutrophil-derived macrophage inflammatory protein 1 alpha. J Exp Med. 1993;178(1):63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamashiro S, Kamohara H, Wang JM, Yang D, Gong WH, Yoshimura T. Phenotypic and functional change of cytokine-activated neutrophils: inflammatory neutrophils are heterogeneous and enhance adaptive immune responses. J Leukoc Biol. 2001;69(5):698–704. [PubMed] [Google Scholar]
- 15.Takano T, Azuma N, Satoh M, Toda A, Hashida Y, Satoh R, et al. Neutrophil survival factors (TNF-alpha, GM-CSF, and G-CSF) produced by macrophages in cats infected with feline infectious peritonitis virus contribute to the pathogenesis of granulomatous lesions. Arch Virol. 2009;154(5):775–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–44. [DOI] [PubMed] [Google Scholar]
- 17.Pagès F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353(25):2654–66. [DOI] [PubMed] [Google Scholar]
- 18.Cunha LL, Morari EC, Guihen AC, Razolli D, Gerhard R, Nonogaki S, et al. Infiltration of a mixture of immune cells may be related to good prognosis in patients with differentiated thyroid carcinoma. Clin Endocrinol (Oxf). 2012;77(6):918–25. [DOI] [PubMed] [Google Scholar]
- 19.Pusztaszeri MP, Faquin WC, Sadow PM. Tumor-Associated Inflammatory Cells in Thyroid Carcinomas. Surg Pathol Clin. 2014;7(4):501–14. [DOI] [PubMed] [Google Scholar]
- 20.Gentles AJ, Newman AM, Liu CL, Bratman SV, Feng W, Kim D, et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med. 2015;21(8):938–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ivanova K, Manolova I, Ignatova MM, Gulubova M. Immunohistochemical Expression of TGF-B1, SMAD4, SMAD7, TGFβRII and CD68-Positive TAM Densities in Papillary Thyroid Cancer. Open Access Maced J Med Sci. 2018;6(3):435–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jung KY, Cho SW, Kim YA, Kim D, Oh BC, Park DJ, et al. Cancers with Higher Density of Tumor-Associated Macrophages Were Associated with Poor Survival Rates. J Pathol Transl Med. 2015;49(4):318–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li YW, Qiu SJ, Fan J, Zhou J, Gao Q, Xiao YS, et al. Intratumoral neutrophils: a poor prognostic factor for hepatocellular carcinoma following resection. J Hepatol. 2011;54(3):497–505. [DOI] [PubMed] [Google Scholar]
- 24.Jensen TO, Schmidt H, Møller HJ, Donskov F, Høyer M, Sjoegren P, et al. Intratumoral neutrophils and plasmacytoid dendritic cells indicate poor prognosis and are associated with pSTAT3 expression in AJCC stage I/II melanoma. Cancer. 2012;118(9):2476–85. [DOI] [PubMed] [Google Scholar]
- 25.Trellakis S, Bruderek K, Dumitru CA, Gholaman H, Gu X, Bankfalvi A, et al. Polymorphonuclear granulocytes in human head and neck cancer: enhanced inflammatory activity, modulation by cancer cells and expansion in advanced disease. Int J Cancer. 2011;129(9):2183–93. [DOI] [PubMed] [Google Scholar]
- 26.Berry RS, Xiong MJ, Greenbaum A, Mortaji P, Nofchissey RA, Schultz F, et al. High levels of tumor-associated neutrophils are associated with improved overall survival in patients with stage II colorectal cancer. PLoS One. 2017;12(12):e0188799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu CL, Lee JJ, Liu TP, Chang YC, Hsu YC, Cheng SP. Blood neutrophil-to-lymphocyte ratio correlates with tumor size in patients with differentiated thyroid cancer. J Surg Oncol. 2013;107(5):493–7. [DOI] [PubMed] [Google Scholar]
- 28.Lang BH, Ng CP, Au KB, Wong KP, Wong KK, Wan KY. Does preoperative neutrophil lymphocyte ratio predict risk of recurrence and occult central nodal metastasis in papillary thyroid carcinoma? World J Surg. 2014;38(10):2605–12. [DOI] [PubMed] [Google Scholar]
- 29.Kim JY, Park T, Jeong SH, Jeong CY, Ju YT, Lee YJ, et al. Prognostic importance of baseline neutrophil to lymphocyte ratio in patients with advanced papillary thyroid carcinomas. Endocrine. 2014;46(3):526–31. [DOI] [PubMed] [Google Scholar]
- 30.Linkov F, Ferris RL, Yurkovetsky Z, Marrangoni A, Velikokhatnaya L, Gooding W, et al. Multiplex analysis of cytokines as biomarkers that differentiate benign and malignant thyroid diseases. Proteomics Clin Appl. 2008;2(12):1575–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kattan MW. Judging new markers by their ability to improve predictive accuracy. J Natl Cancer Inst. 2003;95(9):634–5. [DOI] [PubMed] [Google Scholar]
- 32.Maruvada P, Srivastava S. Joint National Cancer Institute-Food and Drug Administration workshop on research strategies, study designs, and statistical approaches to biomarker validation for cancer diagnosis and detection. Cancer Epidemiol Biomarkers Prev. 2006;15(6):1078–82. [DOI] [PubMed] [Google Scholar]
- 33.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM, et al. Reporting recommendations for tumor marker prognostic studies. J Clin Oncol. 2005;23(36):9067–72. [DOI] [PubMed] [Google Scholar]
- 34.Kobawala TP, Patel GH, Gajjar DR, Patel KN, Thakor PB, Parekh UB, et al. Clinical utility of serum interleukin-8 and interferon-alpha in thyroid diseases. J Thyroid Res. 2011;2011:270149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Provatopoulou X, Georgiadou D, Sergentanis TN, Kalogera E, Spyridakis J, Gounaris A, et al. Interleukins as markers of inflammation in malignant and benign thyroid disease. Inflamm Res. 2014;63(8):667–74. [DOI] [PubMed] [Google Scholar]
- 36.Jabeen S, Espinoza JA, Torland LA, Zucknick M, Kumar S, Haakensen VD, et al. Noninvasive profiling of serum cytokines in breast cancer patients and clinicopathological characteristics. Oncoimmunology. 2019;8(2):e1537691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martins MB, Marcello MA, Batista FA, Peres KC, Meneghetti M, Ward MAL, et al. Serum interleukin measurement may help identify thyroid cancer patients with active disease. Clin Biochem. 2018;52:1–7. [DOI] [PubMed] [Google Scholar]


