Abstract
Background
Failure of effective transitions of care following hospitalization can lead to excess days in the hospital, readmissions, and adverse events. Evidence identifies both patient and system factors that influence poor care transitions, yet health systems struggle to translate evidence into complex interventions that have a meaningful impact on care transitions.
Objective
We report on our experience developing, pilot testing, and evaluating a complex intervention (Addressing Complex Transitions program, or ACT program) that aims to improve care transitions for complex patients.
Design
Following the Medical Research Council (MRC) framework, we engaged in iterative, stakeholder-driven work to develop a complex care intervention, assess feasibility and pilot methods, evaluate the intervention in practice, and facilitate ongoing implementation monitoring and dissemination.
Participants
Patients receiving care from UW Medicine’s health system including 4 hospitals and 20-site Post-Acute Care network.
Intervention
Literature review and prospective data collection activities informed ACT program design. ACT program components include a tailored risk calculator that provides real-time scoring of transitions of care risk factors, a multidisciplinary team with the capacity to address complex barriers to safe transitions, and enhanced discharge workflows to improve care transitions for complex patients.
Key Measures
Program evaluation metrics included estimated hospital days saved and program acceptance by care team members.
Key Results
During the 6-month pilot, 565 patients were screened and 97 enrolled in the ACT program. An estimated 664 hospital days were saved for the index admission of ACT program participants. Analysis of pre/post-hospital utilization for ACT program participants showed an estimated 3227 fewer hospital days after ACT program enrollment.
Conclusions
Health systems need to address increasingly difficult challenges in care delivery. The use of evidence-based frameworks, such as the MRC framework, can guide systems to design complex interventions that respond to their local context and stakeholder needs.
KEY WORDS: complex intervention, care transitions, post-acute care, long length of stay
INTRODUCTION
Healthcare systems are under increasing pressure to improve quality of care while decreasing potentially preventable readmissions and prolonged hospital length of stay (LOS), in part, to contain healthcare costs. Multiple factors are associated with potentially preventable readmission and excess hospital days including patient-level factors (e.g., health literacy), clinical factors (e.g., medical complexity), social factors (e.g., homelessness), and health system factors (e.g., discharge communication).1–4 Excess days in the hospital exposes patients to hospital-acquired conditions, misdiagnoses, and inappropriate treatments and/or medications.5, 6 It also leads to higher costs and may delay access to the health system for other patients in need of specialized care if beds are limited.7 Social vulnerabilities are often identified late during the course of hospitalization and are not comprehensively screened for in most health systems.8–12 Furthermore, hospital-based teams are often lacking in specialized knowledge of post-acute care and community resources that may facilitate safe discharge for patients.4, 13 Due to the complexity of care transitions, interventions aimed at effective hospital discharge should utilize a complex intervention approach that includes (1) multiple interacting components, (2) changing behavior around how care is delivered, and (3) flexible implementation design with the ability to adapt to a local setting.14–16
The use of frameworks to support the design of complex interventions can better identify potential barriers to intervention effectiveness, adoption, and scalability. The Medical Research Council (MRC) framework was developed in 2000 and updated in 2008 to incorporate learnings across its diverse application in healthcare.16–18 The MRC framework guides intervention development from problem recognition through design, pilot, and evaluation, and it emphasizes both the iterative aspect of intervention design as well as the critical influence of context on the effectiveness of the intervention and its outcomes. In this paper, we describe process of developing a complex health system intervention (Addressing Complex Transitions program, or ACT program) aiming to improve care transitions for the UW Medicine health system, guided by the MRC framework. UW Medicine includes 4 hospitals: a quaternary referral center (the hospital site for this study) and a level-one trauma hospital serving the five-state WWAMI (Washington, Wyoming, Alaska, Montana, Idaho) region and two community hospitals. In addition, UW Medicine has formal partnerships with 20 post-acute care (PAC) facilities including skilled nursing facilities, home health agencies, home care, and adult day health facilities across Washington State which acts as a learning health system to track and evaluate cross-continuum outcomes and engage in quality improvement.19
The ACT program was designed to address challenges in care transitions and evaluate outcomes related to resource utilization and efficiency of discharge from the hospital, care team satisfaction, and improved patient care. The development of the ACT program was supported by Centers for Medicare & Medicaid Innovation funding. We report here on the development of the intervention, pilot evaluation, and recommendations for broader translation.
METHODS
We used the MRC framework to guide the design, development, pilot testing, and evaluation of a complex intervention over the course of 24 months from 2017 to 2018. We engaged in multiple activities for each of the MRC framework steps, as outlined in Table 1, often evolving in a non-linear, iterative way as new learnings added insights into our understanding of the problem and potential intervention components.17 All activities were reviewed and approved by our setting’s Institutional Review Board. Some study data was collected and managed using Research Electronic Data Capture (REDCap) electronic data capture tools. REDCap is a secure, web-based application designed to support data capture for research studies, providing (1) an intuitive interface for validated data entry; (2) audit trails for tracking data manipulation and export procedures; (3) automated export procedures for seamless data downloads to common statistical packages; and (4) procedures for importing data from external sources.20 The datasets generated during this study are not publicly available but are available from the corresponding author on reasonable request.
Table 1.
Mapping Team Activities to MRC Framework for Complex Interventions
| Phase 1: Developing complex intervention | |
| Identifying existing evidence | • Reviewed published literature related to readmission risk, best practices for transitions of care, and optimizing post-acute care resources utilization |
| Identifying and developing theory |
• Evaluated internal health system readmission rates, potential preventable readmissions, and comparisons with state data and national data trends • Analyzed length of stay data, factors impacting excess hospital days, and barriers to discharge • Engaged with stakeholders throughout the healthcare continuum (e.g., nursing, social work, hospitalists, primary care, SNF, home health) via the PAC stakeholder network to identify and prioritize contextual factors affecting care transitions • Conducted prospective data collection (e.g., short survey, in-depth root cause analysis) on care transition processes and outcomes |
| Modeling process and outcomes | • Modeled potential impact of utilizing readmission risk prediction tools with data from our health system’s patient population to assess fidelity, accuracy, and usability. |
| Phase 2: Assessing feasibility and piloting methods | |
| Testing procedures for acceptability, compliance, and intervention delivery |
• Mapped proposed intervention components to workflow to estimate impacts on feasibility and usability and to identify most acceptable design • Conducted iterative testing of intervention components in pre-pilot phase • Sought regular user feedback on feasibility and acceptability of intervention |
| Estimating recruitment and retention |
• Evaluated and prioritized patient populations that would most benefit from intervention activities • Estimated ACT team caseload capacity and hospital team readiness for implementation |
| Determining sample size |
• Pre-launch testing to assess eligible patient population volume • Real-time implementation monitoring during pilot (e.g., assessing patient load, average time per patient needed, etc.) to align intervention activities with team capacity |
| Phase 3: Evaluating complex intervention | |
| Assessing effectiveness |
• Estimated impact on reduced excess hospital days (i.e., “days saved”) during hospital stay • Conducted pre/post-evaluation of hospital utilization (180 days pre and post-ACT program intervention) • Assessed post-acute care service utilization (e.g., appropriate use, expanded use) |
| Understanding processes |
• Evaluated intervention acceptability by patients and care teams • Assessed intervention fidelity and implementation strategies that aided or hindered success • Documented intervention adaptations |
| Assessing cost-effectiveness | • Conducted assessment of estimated cost savings (e.g., estimated hospital days saved, intervention resources needed) |
| Implementation and beyond | |
| Dissemination |
• Conducted local dissemination through practice improvement channels, clinical and administrative leadership, and relevant department teams • Engaged in national dissemination via conferences and peer-reviewed publications |
| Surveillance and monitoring |
• Ongoing program evaluation to assess continued modifications • Scale and spread of intervention to additional patient populations |
| Long-term follow-up | • Assessed impact on patient outcomes beyond 30 days |
RESULTS
Phase 1: Developing Complex Intervention (Identifying Existing Evidence, Identifying and Developing Theory, Modeling Process and Outcomes)
Existing evidence was evaluated from peer-reviewed journals related to determinants and intervention components that demonstrated efficacy for improving care transitions. Findings from articles were cataloged and organized around key determinants of prolonged LOS and preventable readmission, including clinical factors, patient factors, social factors, and health system factors. Review of the evidence confirmed the relationship between clinical, patient, social, and health system domains, and it further clarified that interventions to improve care transitions should target (1) communication across healthcare teams early in admission, (2) alignment of PAC service needs after discharge with patient and family expectations, and (3) patient readiness for discharge. The evidence review, however, also illuminated that interventions often fail to affect patients with risk factors across multiple domains.21, 22
Our evidence review informed prospective data gathering with hospital and PAC stakeholders to explore how potential risk factors impacted care transitions across our 4 hospitals and 20-site PAC network. Data collection processes included a three-question short survey and targeted in-depth root cause analysis (RCA) modeled after a previously published approach used in transitions of care.1, 23–25 First, to understand clinician perspectives on potential contributors to poor transitions, we deployed a short electronic survey to the discharging clinician for all 30-day readmissions across all services over a 12-month period. Clinicians completed surveys for 287 (response rate 15.6%) readmissions. Clinicians who completed the surveys indicated that nearly half of readmissions were potentially preventable. The most significant contributing factors for readmissions were symptom control and patient inability to manage care, followed by lack of social support and medication management challenges. We completed in-depth RCAs on 40 selected readmissions. Sampling approaches for the RCA were purposive (targeting cardiology, general medicine, and surgery services) and guided by literature findings around risk factors for poor transitions. RCA data collection included EMR data abstraction, brief surveys for hospital and PAC care team members, and patient interviews (n = 26).23, 26 Not all patients of the 40 RCA samples were able to be interviewed due to clinical status, patient refusal to participate, or staff availability.
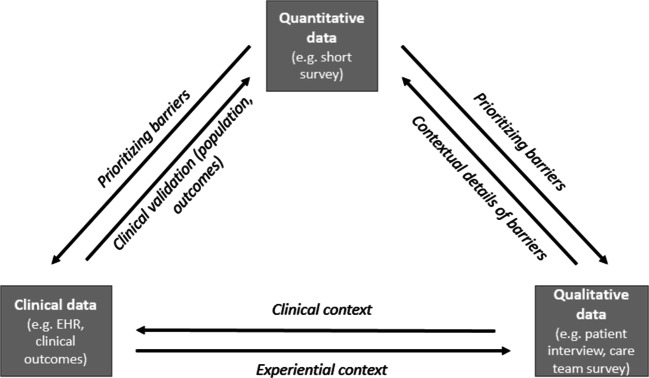
Rapid, iterative analysis was used to identify and triangulate the RCA data (EMR, survey, interview) with the short survey data to contextualize contributing factors for poor care transitions within our setting (see Fig. 1).27, 28 We engaged our PAC stakeholders (inclusive of hospitalists, SNF and home healthcare team members, administrators, nurses, social workers, and health services researchers) on a monthly basis to reflect on and characterize the factors that contribute to poor care transitions within our system. RCA data provided greater clarity on how the combination of clinical, patient, social, and health system factors contributed to potentially preventable readmission and excess hospital days.
Figure 1.
Data triangulation to inform program theory and design.
Feedback from these initial stakeholder engagement activities informed the development of our intervention components. First, stakeholders indicated that applying evidence-based strategies to patients needed to be highly individualized and customizable to each patient; therefore, attempting to “protocolize” processes for managing high-risk transitions might decrease the effectiveness of the intervention. Second, cross-disciplinary problem-solving, particularly with roles representing the full care continuum, was needed to address the level of complexity reached when patients had multiple competing risk factors in play that impeded their ability to transition to the next care setting. As a result, the core component of our intervention was the creation of a dedicated team, the Addressing Complex Transitions (ACT) team consisting of (1) a nurse care coordinator with experience managing complex patients and knowledge of local community-based resources; (2) a social worker with bandwidth to provide coordination across the continuum of care, including post-discharge follow-up; (3) a post-acute care clinician with experience managing medically and social complex patients in the inpatient, outpatient, and PAC setting; and (4) a health system administrator who can liaise with administrative stakeholders, navigate regulatory barriers, and authorize critical resources.
All ACT team members worked collaboratively to address the barriers to complex care transitions, leveraging their individual training, expertise, and capacity. For example, the post-acute care clinician served as an inpatient consultant to help identify appropriate patients, partner with inpatient teams to adjust medications to optimize patients for the post-acute care setting, and liaise with PAC facilities to facilitate safe transitions of care. This position was filled by a physician who proactively met with service line leaders and individual attendings to build trust and identify service-specific priorities, values, and practice patterns. In this way, ACT team roles were less prescriptive than traditional hospital team role structures (see Table 2).
Table 2.
Examples of ACT Program Response for Different Areas of Impact
| Area of impact | Example of contributing factors | Impact on care transition | Examples of ACT program response |
|---|---|---|---|
| Clinical acuity |
• High-acuity care • High-risk medications (e.g., anticoagulants, opioids) • Multiple comorbidities |
• Conflicting discharge processes and documentation from multiple services • Complex post-discharge care needs |
• Establishment of complex care plan • Reconciliation of discharge plans across services • Training of PAC care team on high-acuity wound care protocols |
| Patient factors |
• High utilization patterns • Behavioral dementia • Limited English proficiency |
• Potential unmet care needs • Difficulty understanding discharge care plans |
• Coaching and/or motivational interviewing to set patient and family expectations for care transition • Standardized warm handoff to next care team, including patient preferences and concerns |
| Social supports/social determinants of health |
• Homelessness • Limited social supports • Legal issues • Complex family dynamics |
• Barriers to implementing discharge care plans • Preventable readmissions |
• Connect patients to PAC services and/or community resources (e.g., food, housing, social supports) • Post-discharge follow-up (phone calls, home visits) |
| System-level factors |
• Hospital capacity barriers • Insurance coverage barriers • PAC placement barriers |
• Prolonged LOS • Failure to transition patient to most appropriate PAC setting |
• Modification of care plans to address PAC care setting resources and needs • Medication adjustments to reduce cost burden • Interdisciplinary problem-solving (hospital and PAC) |
The key functions of the ACT team were to (1) engage in cross-disciplinary problem-solving and (2) identify and implement evidence-based strategies that were tailored to the specific interplay of risk factors each patient experienced. Changing the behavior of hospital clinical teams during complex discharges was a pivotal facilitator of the ACT program functions. Michie et al. (2011) present a framework for understanding behavior in complex contexts, emphasizing the role of an individual’s capabilities, opportunities, and motivation, and considering influencing factors that are both internal and external to the individual.29 Following Michie’s COM-B framework, Table 3 provides examples of how ACT program activities influenced the behavior of clinical teams during transitions for complex patients.
Table 3.
Relationship Between ACT Program Activities and COM-B Behavior Change Constructs
| COM-B construct29 | Example of behavioral barrier for complex transitions | Example of ACT program impact |
|---|---|---|
| Capability (e.g., knowledge and skills to conduct behavior) | Hospital clinical team member does not have the knowledge of funding options for a complex patient | Align hospital clinical team with ACT team member with high knowledge of funding options (i.e., coaching, leveraging experts) |
| Opportunity (e.g., external physical or social prompts that enable behavior) | Hospital clinical team member does not have workflow to conduct additional follow-up for PAC placement for a complex patient | ACT team participation in discharge rounds to prompt hospital clinical team behavior and provide support for completion (i.e., facilitation, task transfer) |
| Motivation (e.g., attitudes and cognitive processing around behavior decision) | Hospital care team member does not feel confident in their ability to solve placement problems for a complex patient | Interdisciplinary problem-solving with ACT team members enhances available support and expertise to solve problem (i.e., changing structure of team) |
Feedback from the design phase identified the need for a screening tool that the ACT team could use to evaluate patients who would be most likely to benefit from the ACT program. Using retrospective EMR data from our health system, we modeled several existing risk tools to identify patients at high risk for poor care transitions.30 We then presented samples of these score outputs to representative physicians, social workers, nurses, and readmission reduction quality improvement programs across the hospital system and sought feedback to validate their accuracy (e.g., outcomes) and applicability to practice (e.g., process). Generic risk scores such as LACE captured too wide of a population to provide meaningful insights into clinical teams.31, 32 Condition-specific risk scores provided more accurate information about risk for specific outcomes (e.g., death or readmission); however, the implementation of these tools in practice was greatly complicated by clinical complexity and comorbid conditions in our patient population.33–36 Most importantly, clinical stakeholders struggled with the actionability and feasibility of these tools. Patients with complex clinical and administrative factors required significantly more time to manage, as well as more specialized knowledge about how to navigate individualized financial, social work, and patient education-related needs. Therefore, drawing from learnings of the literature review, data gathering activities, and limitations of existing risk scores, we built a tailored risk tool that weighted non-clinical factors for which a potential intervention or accommodation was possible, such as placement barriers or availability of appropriate support at home following discharge.
Phase 2: Assessing Feasibility Within the Workforce and Piloting Methods (Testing Procedures for Acceptability, Compliance, and Intervention Delivery; Estimating Recruitment and Retention; Determining Sample Size)
The screening tool was developed over a 2-month period to assess (1) the ability to capture the patients at highest risk for prolonged LOS and readmission and (2) the feasibility for early intervention during the hospital stay. The screening tool aggregated clinical and administrative data for inpatient populations and assigned a risk score (0–40) based on criteria and weights established in the first phase of this work, primarily factors related to medical complexity, psychosocial assessment, and need for additional social support. During the following 6-month testing phase, ACT team members used the risk score daily and reviewed outputs and provided feedback on variables that needed to be added, changed, or removed based on data quality issues with underlying EMR data. They also incorporated data about patients identified as “high risk” in real time by the clinical teams that did not rise to the top of the risk score using the tool. The ACT team reviewed outputs of the screening tool weekly to provide feedback on tool design and assess accuracy of risk scoring.
Once the screening tool was tested, the ACT team launched a pilot phase to understand patient volume, reach, and necessary implementation supports. The team reviewed risk score outputs daily to evaluate the potential caseload for their team. A goal of the program was to maintain intentionally small caseloads of only the most complex patients, in order to maximize the impact of the ACT team’s specialized training and to afford care teams the capacity to better serve less complex patient populations.37 Through this work, the team determined an estimate of the risk score cut point for ACT team support versus usual care with existing care teams and social workers.
Lastly, as the ACT team prepared for launch of a formal pilot, they sought feedback from care teams across the system about key implementation approaches that would support integration with existing care delivery. Care teams emphasized the need for regular communication and the integration of status updates into existing care team processes. In response to this feedback, ACT team members ensured that hospital clinical team daily discharge rounds included updates on ACT program patients by either attending discharge rounds in person or meeting directly with hospital team liaisons prior to rounds to ensure information was communicated to support care delivery. Additional feedback from care teams was integrated into implementation process and implementation monitoring plans.
Phase 3: Evaluating Complex Intervention (Assessing Effectiveness, Understanding Processes, Assessing Outcomes: Readmission, LOS, Cost-effectiveness)
The ACT program was piloted over a 6-month period in 2018. We evaluated a variety of implementation and service outcomes for the pilot that were informed by both Proctor’s framework for implementation outcomes and input from our stakeholders (see Table 4), with a focus on impacts for the health system.38 Client outcomes, including patient satisfaction and functional status, will be assessed in future work as this initial pilot expands and will warrant additional stakeholder input to inform appropriate outcome selection given the target population’s variability in clinical status.
Table 4.
Summary of Pilot Evaluation Metrics
| Outcome domain38 | Target metrics | Data sources |
|---|---|---|
| Implementation outcomes |
• Acceptability (e.g., hospital and ACT team perspectives) • Uptake (e.g., ACT team patient load capacity) • Costs (e.g., estimated cost benefit of program) |
• Hospital clinical team evaluation survey (n = 27) • ACT team pilot debriefings (weekly, and at pilot conclusion) • ACT team caseload documentation • Estimated vs. actual LOS prospective comparison |
| Service outcomes |
• Efficiency (e.g., LOS) • Patient-centeredness (e.g., hospital team perspectives of care intensity and appropriateness) • Effectiveness (e.g., reduction of post-discharge hospital utilization) |
• Administrative data (LOS, readmission, ER visit) • Hospital clinical team evaluation survey (n = 27) |
During the pilot, 565 patients were screened using the risk tool, and 97 patients were triaged into the ACT program based on their risk score, and on average 65% of patients enrolled required some form of PAC services. ACT team members had a weekly caseload of 8–11 patients and spent an average of 12 h per patient case. Upon a patient’s enrollment to the ACT program, clinical teams identified an anticipated discharge date based on current barriers to discharge. The anticipated discharge date was then compared to the actual discharge date that occurred after ACT program intervention, and the difference resulted in estimated hospital days saved. For the 97 patients enrolled in the ACT program, an estimated 664 total hospital days were saved on their index admission.
Since the ACT team worked, by design, with the highest risk patients, event rates for this population were not comparable to the population overall. Therefore, we compared event rates for the study cohort in the 6 months prior to and post-intervention. Considerable differences were seen in utilization before and after ACT program involvement (see Fig. 2). ACT program participants had an average of 129 fewer ER and hospital encounters (278 vs. 149 encounters) and 3227 fewer inpatient days (4067 vs. 840 days) in the 6 months following ACT intervention as compared with the 6 months prior. When considering the subset of patients that were readmitted to the hospital following ACT program enrollment (n = 36), ACT program patients had an average of 2.5 admissions in the 6 months following ACT intervention, as compared with 4.9 in the 6 months prior. An assessment of our health system’s financial impact of the ACT program compared the direct program costs (e.g., costs of care, labor, screening tool development) to estimated cost savings (e.g., hospital days saved) and increased revenue (e.g., increased bed availability and hospital capacity). The average financial impact per patient enrolled in the intervention was estimated to be $26,659 in cost savings.
Figure 2.
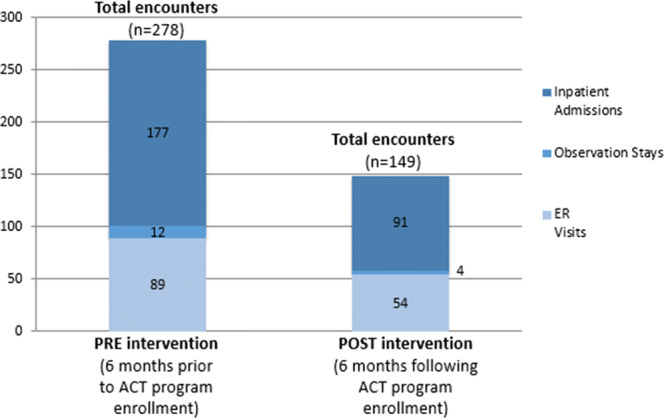
Comparison of pre- and post-hospital utilization encounter counts for ACT program participants.
At the conclusion of the pilot, 27 hospital clinical team members (n = 13 social workers and/or discharge care coordinators, and n = 14 clinicians) who had patients enrolled in the ACT program were surveyed via electronic survey to assess their perspectives on program acceptability and impact on care delivery. Uniformly, clinical teams indicated that the ACT team addressed complex barriers to discharge more thoroughly and holistically than traditional approaches by providing a timely, intensive focus on individual patient discharge barriers (86%), accessing new funding resources for patient services (85.7%), and more effectively engaging patients and families in discharge planning (71.4%).
DISCUSSION
For safe and timely discharge, health systems strive for “discharge planning to begin on admission.” This requires (1) timely identification of key risk factors and (2) a team with capacity and expertise to engage community resources during the transition from hospitalization to PAC. Best practices that target elements of transitions of care exist, however, assuring these initiatives are implemented in a meaningful way while still meeting the needs and staffing constraints of the local health system is an ongoing challenge.39 Standardized protocols are considered successful when they address the needs of 80% of patients. Patient populations in the remaining 20% may have higher acuity clinical issues and greater vulnerability related to psychosocial needs. Using a complex intervention framework, we applied transitional care best practice recommendations to the gaps identified by key stakeholders within our local health system to develop a multicomponent intervention targeting key performance metrics that were important to our health system.
The MRC framework was instrumental in guiding our development of the ACT program. While the core components of the ACT program (i.e., a comprehensive screening tool and complex discharge team) could be implemented in many health systems, the contextual features of how these components are built and implemented should be adapted to the local environment of each health system. The ACT team worked across the hospital (e.g., medicine, transplant, cardiology) successfully adapting to the culture and resources within individual clinical teams. The ACT team’s work also spanned the continuum of care by providing patient-specific insights and continued engagement with clinical teams after discharge. The composition of the ACT team (e.g., role function, expertise) was informed by our local needs assessment and required that the team be adaptable and resourceful given the changing and sometimes limited resources available in PAC settings. The greatest function of this team was the capacity to share knowledge, think creatively, and solve problems collaboratively to overcome interdisciplinary barriers to safe transitions of care. Examples of interdisciplinary work by the ACT team included coordination of care with addiction medicine, anesthesia pain services, and outpatient social services to tailor discharge care plans for patients with opioid use disorder and complex pain management to the SNF environment. Additionally, the ACT program allowed our health system to better engage with community-based resources that could address vulnerable gaps in our ability to support critical patient needs (e.g., day rest beds, respite centers, low-barrier medication-assisted treatment for opioid use disorder).
There are some limitations to consider for this work. First, the risk tool included key social determinants of health that were not consistently recorded in the medical record (e.g., high risk for homelessness, poor health literacy). As noted above, the risk tool was validated for the patient population within our setting, but it is anticipated that the tool will need further modifications to reflect other patient populations when implemented elsewhere. Next, we used LOS and readmission as proxies for safe transitions of care, recognizing that they are imperfect measures of patient safety and hospital resource utilization. Readmissions may be life-saving for a patient who clinically worsens after discharge, and prolonged days in the hospital may support a safer transitional plan. Estimated hospital days saved was used to evaluate cost-related outcomes; however, there are limited evidence-based methods for prospectively estimating LOS, especially across clinical populations. Prior research has focused on provider assessments of estimated LOS (often finding that providers underestimate LOS).40 We use a multidisciplinary assessment for our estimates, but recognize the limitation in this methodology. Furthermore, we did not collect data on patients who left the health system (e.g., moved out of state) or were admitted outside the UW Medicine health system. Lastly, while we assessed cost savings using hospital days saved, it is recognized that there are many methods for estimating the value of a hospital bed day, and health systems may value the opportunity costs of available beds more than the direct cost.41, 42 While we have theorized core components based on program evaluation and stakeholder feedback, more detailed cost-benefit analysis of individual program components would be valuable for future implementation.
By using the MRC framework, we developed a theory- and evidence-informed intervention that aligned with real-world care delivery. The ACT program was effective for our health system due to the continuous engagement with stakeholders and our unique collaboration with a network of PAC settings. Future work will explore in-depth measures of clinical status in the PAC period, patient satisfaction, and quality of transitional care measures. Health systems should use an approach that elucidates the complexity of care transitions for their most vulnerable patients and highest utilizers to address safer care transitions and reduce unnecessary hospital resource utilization.
Acknowledgments
We would like to acknowledge the contributions of the following individuals to this work: Carol Charles, Brian C. Giddens, Elicia Hawken-Dennis, Ashley McLoud, Jocelyn Nelson, Hanh Pan, Caroline Shevrin, and members of the University of Washington Medicine Post-Acute Care Committee.
Funding Information
This project was supported by Funding Opportunity Number CMS-331-44-501 from the US Department of Health & Human Services, Centers for Medicare & Medicaid Services (PI: Flum).
Compliance with Ethical Standards
All activities were reviewed and approved by our setting’s Institutional Review Board.
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Disclaimer
The contents provided are solely the responsibility of the authors and do not necessarily represent the official views of HHS or any of its agencies
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Elizabeth J. Austin, Email: austie@uw.edu.
Jen Neukirch, Email: jneuk@uw.edu.
Thuan D. Ong, Email: thuano@uw.edu.
Louise Simpson, Email: ljws@uw.edu.
Gabrielle N. Berger, Email: gberger@uw.edu.
Carolyn Sy Keller, Email: cdsy@uw.edu.
David R Flum, Email: daveflum@uw.edu.
Elaine Giusti, Email: ganoulis@uw.edu.
Jennifer Azen, Email: jazen@uwpn.org.
Giana H. Davidson, Email: ghd@uw.edu.
References
- 1.Auerbach AD, Kripalani S, Vasilevskis EE, et al. Preventability and causes of readmissions in a national cohort of general medicine patients. JAMA Intern Med. 2016;176(4):484. doi: 10.1001/jamainternmed.2015.7863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Walraven C, Forster AJ. When projecting required effectiveness of interventions for hospital readmission reduction, the percentage that is potentially avoidable must be considered. J Clin Epidemiol. 2013;66(6):688–690. doi: 10.1016/j.jclinepi.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Vest JR, Gamm LD, Oxford BA, Gonzalez MI, Slawson KM. Determinants of preventable readmissions in the United States: a systematic review. Implement Sci. 2010;5:88. doi: 10.1186/1748-5908-5-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Health policy brief: improving care transitions | health affairs. https://www.healthaffairs.org/do/10.1377/hblog20120921.023379/full/. Accessed September 3, 2019.
- 5.Tess BH, Glenister HM, Rodrigues LC, Wagner MB. Incidence of hospital-acquired infection and length of hospital stay. Eur J Clin Microbiol Infect Dis. 1993;12(2):81–86. doi: 10.1007/bf01967579. [DOI] [PubMed] [Google Scholar]
- 6.Mathew PJ, Jehan F, Kulvatunyou N, et al. The burden of excess length of stay in trauma patients. Am J Surg. 2018;216(5):881–885. doi: 10.1016/j.amjsurg.2018.07.044. [DOI] [PubMed] [Google Scholar]
- 7.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418–28. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 8.Arozullah AM, Lee S-YD, Khan T, et al. The roles of low literacy and social support in predicting the preventability of hospital admission. J Gen Intern Med. 2006;21(2):140–145. doi: 10.1007/s11606-006-0248-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flythe JE, Hilbert J, Kshirsagar AV, Gilet CA. Psychosocial factors and 30-day hospital readmission among individuals receiving maintenance dialysis: a prospective study. Am J Nephrol. 2017;45(5):400–408. doi: 10.1159/000470917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vaida B. For super-utilizers, integrated care offers a new path. Health Affairs. 2017;36(3):394–397. doi: 10.1377/hlthaff.2017.0112. [DOI] [PubMed] [Google Scholar]
- 11.Berkowitz SA, Hulberg AC, Standish S, Reznor G , Atlas SJ. Addressing unmet basic resource needs as part of chronic cardiometabolic disease management. JAMA Intern Med. 2017;177(2):244-252. doi:10.1001/jamainternmed.2016.7691. [DOI] [PMC free article] [PubMed]
- 12.Seligman HK, Schillinger D. Hunger and socioeconomic disparities in chronic disease. N Engl J Med. 2010;363(1):6–9. doi: 10.1056/NEJMp1000072. [DOI] [PubMed] [Google Scholar]
- 13.Bailey JE, Surbhi S, Wan JY, et al. Effect of intensive interdisciplinary transitional care for high-need, high-cost patients on quality, outcomes, and costs: a quasi-experimental study. J Gen Intern Med. 2019;34(9):1815–1824. doi: 10.1007/s11606-019-05082-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berry JG, Blaine K, Rogers J, et al. A framework of pediatric hospital discharge care informed by legislation, research, and practice [published online August 25, 2014]. JAMA Pediatr. doi:10.1001/jamapediatrics.2014.891. [DOI] [PMC free article] [PubMed]
- 15.Apkon M, Friedman JN. Planning for effective hospital discharge. JAMA Pediatr. 2014;168(10):890–891. doi: 10.1001/jamapediatrics.2014.1028. [DOI] [PubMed] [Google Scholar]
- 16.Medical Research Council. Developing and evaluating complex interventions. 2019. Accessed at: https://mrc.ukri.org/documents/pdf/complex-interventions-guidance/. Accessed August 30, 2019
- 17.Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ. September 2008:a1655. doi:10.1136/bmj.a1655 [DOI] [PMC free article] [PubMed]
- 18.Bobrow K, Farmer A, Cishe N, et al. Using the Medical Research Council framework for development and evaluation of complex interventions in a low resource setting to develop a theory-based treatment support intervention delivered via SMS text message to improve blood pressure control. BMC Health Serv Res. 2018;18(1):33. doi: 10.1186/s12913-017-2808-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davidson GH, Austin E, Thornblade L, Simpson L, Ong TD, Pan H, Flum DR. Improving transitions of care across the spectrum of healthcare delivery: A multidisciplinary approach to understanding variability in outcomes across hospitals and skilled nursing facilities. Am J Surg. 2017;213(5):910–914. doi: 10.1016/j.amjsurg.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Preyde M, Brassard K. Evidence-based risk factors for adverse health outcomes in older patients after discharge home and assessment tools: a systematic review. J Evid Based Soc Work. 2011;8(5):445–468. doi: 10.1080/15433714.2011.542330. [DOI] [PubMed] [Google Scholar]
- 22.Lin FOY, Luk JKH, Chan TC, Mok WWY, Chan FHW. Effectiveness of a discharge planning and community support programme in preventing readmission of high-risk older patients. Hong Kong Med J. 2015;21(3):208–216. doi: 10.12809/hkmj144304. [DOI] [PubMed] [Google Scholar]
- 23.Auerbach AD, Patel MS, Metlay JP, et al. The hospital medicine reengineering network (Homerun): a learning organization focused on improving hospital care. Academic Medicine. 2014;89(3):415–420. doi: 10.1097/ACM.0000000000000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Austin, E; Neukirch, J; Berger, G; Ong, T; Simpson, L; Nelson, J; McLoud, A; Flum, D; Sy Keller, C; Davidson, G. Implementation of the Addressing Complex Transitions (ACT) program to address long length of hospital stay. Presentation at: Academy Health Annual Research Meeting; 2019 Jun 1-3, Washington DC.
- 25.Austin, E; Neukirch, J; Lavallee, D; Simpson, L; Ong, T; Hawken-Dennis, E; Pan, H; Flum, D; Davidson, G. Engaging cross-setting stakeholders to improve the quality of post-acute care transitions: Results of the Transfer Alerts and Communication (TAC) transition tool. Poster at: Academy Health Annual Research Meeting; 2018 Jun 23-26, Seattle WA.
- 26.Palinkas LA, Horwitz SM, Green CA, Wisdom JP, Duan N, Hoagwood K. Purposeful sampling for qualitative data collection and analysis in mixed method implementation research. Adm Policy Ment Health. 2015;42(5):533–544. doi: 10.1007/s10488-013-0528-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsieh H-F, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277–1288. doi: 10.1177/1049732305276687. [DOI] [PubMed] [Google Scholar]
- 28.Gale RC, Wu J, Erhardt T, et al. Comparison of rapid vs in-depth qualitative analytic methods from a process evaluation of academic detailing in the Veterans Health Administration. Implementation Sci. 2019;14(1):11. doi: 10.1186/s13012-019-0853-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michie S, van Stralen MM, West R. The behaviour change wheel: A new method for characterising and designing behaviour change interventions. Implementation Sci. 2011;6(1):42. doi: 10.1186/1748-5908-6-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Austin, E; Neukirch, J; Liao, J; Pan, H; Simpson, L; Flum, D; Davidson, G. A health systems framework for evaluating different approaches to predicting readmission risk. Poster at: Academy Health Annual Research Meeting; 2018 Jun 23-26, Seattle WA.
- 31.Damery S, Combes G. Evaluating the predictive strength of the LACE index in identifying patients at high risk of hospital readmission following an inpatient episode: a retrospective cohort study. BMJ Open. 2017;7(7):e016921. doi: 10.1136/bmjopen-2017-016921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robinson R, Hudali T. The HOSPITAL score and LACE index as predictors of 30 day readmission in a retrospective study at a university-affiliated community hospital. PeerJ. 2017;5. doi:10.7717/peerj.3137 [DOI] [PMC free article] [PubMed]
- 33.Hakim MA, Garden FL, Jennings MD, Dobler CC. Performance of the LACE index to predict 30-day hospital readmissions in patients with chronic obstructive pulmonary disease. Clin Epidemiol. 2017;10:51–59. doi: 10.2147/CLEP.S149574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Diepen S, Graham MM, Nagendran J, Norris CM. Predicting cardiovascular intensive care unit readmission after cardiac surgery: derivation and validation of the Alberta Provincial Project for Outcomes Assessment in Coronary Heart Disease (Approach) cardiovascular intensive care unit clinical prediction model from a registry cohort of 10,799 surgical cases. Crit Care. 2014;18(6). doi:10.1186/s13054-014-0651-5 [DOI] [PMC free article] [PubMed]
- 35.Valero V, Grimm JC, Kilic A, et al. A novel risk scoring system reliably predicts readmission following pancreatectomy. J Am Coll Surg. 2015;220(4):701–713. doi: 10.1016/j.jamcollsurg.2014.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen SY, Stem M, Cerullo M, et al. Predicting the risk of readmission from dehydration after ileostomy formation: the dehydration readmission after ileostomy prediction score. Dis Colon Rectum. 2018;61(12):1410–1417. doi: 10.1097/DCR.0000000000001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choi J, Choi JE, Fucile JM. Power up your staffing model with patient acuity. Nurs Manage. 2011;42(9):40–43. doi: 10.1097/01.NUMA.0000403278.96754.fb. [DOI] [PubMed] [Google Scholar]
- 38.Proctor E, Silmere H, Raghavan R, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health. 2011;38(2):65–76. doi: 10.1007/s10488-010-0319-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woolf SH, Grol R, Hutchinson A, Eccles M, Grimshaw J. Potential benefits, limitations, and harms of clinical guidelines. BMJ. 1999;318(7182):527–530. doi: 10.1136/bmj.318.7182.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kimmel SE, Praestgaard A, Saybolt MD, Durstenfeld MS, Kimmel SE. Physician predictions of length of stay of patients admitted with heart failure. Journal of Hospital Medicine. 2016;11(9). 10.1002/jhm.2605 [DOI] [PubMed]
- 41.Page K, Barnett AG, Graves N. What is a hospital bed day worth? A contingent valuation study of hospital Chief Executive Officers. BMC Health Serv Res. 2017;17(1):137. doi: 10.1186/s12913-017-2079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Calculating the cost of length of stay | newsroom – Vizient. https://newsroom.vizientinc.com/blog/clinical-performance/calculating-cost-length-stay. Accessed September 3, 2019.