Abstract
Pollution is severely threatening the wetland habitats. Heavy metals are one among of the major pollutants in wetland habitats. The cadmium (Cd), copper (Cu), chromium (Cr), cobalt (Co), lead (Pb), mercury (Hg), nickel (Ni) and zinc (Zn), were assessed in the water, sediment, benthic species (polychaetes, mollusc and crustaceans), prawn and fishes. The assessment of heavy metals was done by using double-beam Atomic Absorption Spectrophotometer (AAS). The Hg, Cr and Co were greater in sediment, Ni and Cd were higher in polychaetes and molluscs respectively. However, the Cu and Pb greater in crabs and the Zinc was greater in fishes. The concentration of metals showed significant differences among the various sources examined (P < 0.05) except Cr (P > 0.05). The inter-correlational analysis among the metals assessed from the various sources showed that the Cr and Pb not correlated among the eight metals examined. However, the Cu and Co were correlated with Hg (r = 0.307) and (r = 0.788) respectively. The nickel was correlated with Hg (r = 0.367), Cu (r = 0.362) and Co (r = 0.432). The Zinc was correlated with the Cd (r = 0.331) and Hg (r = 0.737). However, correlation of metals among the different sources shown that the metals of polychaetes correlated with sediment r = 0.637, the metals of crabs correlated with the sediment and polychaetes r = 0.630 and r = 842 respectively, the metals of molluscs was also correlated with sediment (r = 0.636), polychaetes (r = 0.889) and crabs (r = 0.894). In addition to that the metals of prawns was correlated with the polychaetes (r = 839), crabs (r = 0.628) and molluscs (r = 0.634). The metals of fishes correlated with polychaetes (r = 0.529), crabs (r = 0.710), molluscs (r = 0.493) and prawns (r = 0.593). Indeed the multiple regression model explained that the metals of sediments influence the accumulation of metals in biotic species such as polychaetes, molluscs, crustaceans, prawns and fishes with 84% (F = 21.079; p < 0.001).The order of the heavy metals in the water, sediment and biotic species was Hg > Pb > Ni > Cr > Zn > Co > Cu > Cd. The study found that the level of heavy metals at various sources in the sanctuary is showing considerable warning and the sanctuary is required intensive assessment on various aspects of pollution since the Point Calimere Wildlife Sanctuary is supporting several species of migratory and endangered shorebirds seasonally.
Keywords: Pollution, Heavy metals, Habitats, Benthic, Conservation
1. Introduction
The wetlands habitats are most productive ecosystem in the world, providing suitable habitats for various species of aquatic organisms which are depends on it. Nevertheless the coastal wetland habitats including intertidal mudflats are functioning as a proper shelter, feeding and breeding grounds for various species (Balachandran, 2012, Sivaperuman and Venkatraman, 2014). But recently several wetlands are under severe threats due to various pollutants (Agoramoorthy and Pandiyan, 2015). The heavy metals are one among the serious pollution in the aquatic habitats since these heavy metals are flexible to enter the food chain of an aquatic ecosystem (Censi et al., 2006, Pandiyan et al., 2020). The heavy metals are badly affecting the aquatic habitats and which is becomes a big threat since most of the heavy metals are severe toxic effects on several organisms (MacFarlane and Burchett, 2000). The sediments received the heavy metals through chemical and physical processing of rocks, percolating of soil and physiological process of various plants (Al-Saad et al., 1997). In addition to that urbanization, industrial and agricultural activities could also favours the contamination of soil by heavy metals (Heba et al., 2000). However these two processes i.e. man-made and natural processes are the major en-route to the heavy metal contamination in the aquatic habitats including in the coastal sediments. In addition to that the aquatic ecosystem, the heavy metals are heavily deposited into the sediments through the process of adsorption, precipitation, diffusion, chemical reactions, biological activity etc., (Ramirez et al., 2005), the heavy metals spread into the water column through the sediments (Jones and Turki, 1997).
Assessment of heavy metals in aquatic habitats is typically examined in the water, sediments and biotic species (Camusso et al., 1995). In fact the level of metals in water is relatively very low than the sediments and other biota (Namminga and Wilhm, 1976). Indeed the evaluation of heavy metals in aquatic habitats such as sediment, benthic organisms and fishes had been explored as a major environmental concern (Özmen et al., 2004, Praveena et al., 2008). Generally the metals in minerals and rocks are not harmful but it will be more toxic when they are dissolved in water.
The accumulation of heavy metals in the aquatic organisms is most probably through the dissolved phase and it also through ingestion of their food (Fisher and Reinfelder, 1995). The entry of heavy metals in polychaets, molluscs and crustaceans is predominantly through the waters, sediments and their food items (Bryan, 1971, Bryan, 1979), in prawns and fishes through the food chain and food webs (Botté et al., 2010). The assessment of heavy metals in benthic organisms and fishes are most essential since they are potential ecological indicators particularly pollution studies (Jones and Walker, 1979, Yilmaz, 2005). The benthic organisms, prawns and fishes are the major food items for various species of migrant and local migrant species of shorebirds during their migration (Pandiyan and Asokan, 2015). The shorebirds are heavily affected with heavy metals through their dietary preferences (Pandiyan et al., 2020). Therefore the current study is targeted to assess the level of heavy metals in water, sediment, benthic organisms, prawns and fishes to understand the current status of the PCWL (Ramsar site) since the sanctuary is supporting numerous species of migrant and endangered species of shorebirds annually.
2. Methods
2.1. Study area: The Point Calimere Wildlife Sanctuary
The Point Calimere Wildlife Sanctuary (PCWL), one of the Ramsar sites in India, situated 10°18‟N, 79°51‟E, Great Vedarnyam Swamp, Nagapattinam District, Tamil Nadu, India (Fig. 1). The monsoon is the period of heavy rainy season in which the PCWL receive water and it dries up during the summer season and ultimately will become a small pool of water in peak summer. The swamp land is partitioned from the Northeastern part of Indian ocean (Bay of Bengal) and Palk Strait by thin coastal area of sand banks with numerous channels such as “Manavaykal” and “Sellakkani” sea mouths. The swamp habitat is consisting of mixed aquatic ecosystem such as both fresh and seawater. In the swamp, two industrial salt units are producing edible and industrial salts, the average rainfall of PCWL is ranges 1000–1500 mm during monsoon season but the rainfall could vary on the basis of the weather conditions. The maximum temperature of the PCWL is 34 °C and minimum 24 °C during May and December respectively. Annually the PCWS swamp habitat shows strong winds during the month of June and July. Several resident and migratory waterbirds use this sanctuary as a feeding ground and some water birds using as a breeding ground (Sampath and Krishnamurthy, 1990, Balachandran et al., 2009, Pandiyan and Jagadheesan, 2016, Manikannan, 2011).
Fig. 1.
Map showing the study area of the Point Calimere Wildlife Sanctuary, Kodikkarai, (Ramsar site).
2.2. Collection of water, sediment and aquatic organisms
2.2.1. Collection of water samples
The water samples (1000 ml) were taken from the surface of each sampling sites in a clean polyprophylene containers. The collected water samples were transported to the analytical lab in the ice box. The water samples were kept in 1% HNO3 at 4 °C until the analysis of metals (Kiffney and Clements, 1993, Pandiyan et al., 2020).
2.2.2. Collection of soil sediment samples
Soil sediment samples were collected at three random sites (up to20 cm depth) (Boncompagni et al., 2003). At each site, six different soil samples were collected at a distance 500 m and the samples were mixed thoroughly to get a composite sample. The same procedure was followed for the other two sites also. The samples were collected using a core sampler (Masero et al.,1999) rinsed with HNO3 (10%), and the samples were kept in the as described by Karadede-Akin and Ünlü (2007).
2.2.3. Collection of benthic prey species
Two kilogram of mud samples (20 cm diameter) were collected at three different random sites, 20 cm depth is considered as a maximum reachable depth for foraging of most shorebirds species and where benthic or mud-dwelling invertebrates live (Masero et al., 1999). The selection of three different sites for the collection of benthic prey invertebrates is purely based on foraging and greater congregates of shorebird-used sites. At each site, six different mud samples were collected and combined to obtain a composite sample, and the same procedure was followed for the other two sites also. The mud samples were sieved in the laboratory using various sizes of sieves i.e. from 0.5 to 2.0 mm mesh sizes, and the collected benthic organisms were kept at − 20 °C for further analysis of metals.
2.2.4. Collection of fishes and prawns
Small gill net used for capturing the fishes and prawns at six different random sites. Nine samples were collected from each site and pooled into a single sample and the same procedure was employed for the other eight sites also. The fish and prawn samples were transported by using an icebox and the samples were kept at − 30 °C for further analysis of metals (Raja et al., 2009).
2.3. Digestion of water, sediment, benthic, prawn and fish samples
2.3.1. Digestion of water samples
The collected water samples were filtered by using a Whatman no.1 (0.45 mm) filter paper, in a 100 ml of water sample, in a conical flask five ml of concentrated H2SO4 was taken and it was allowed for heating about two hours at 105 °C until the volume is reached to 25 ml and the sample was transferred in a 100 ml volumetric flask. Deionised H2O was gradually added to the flask until reached 100 ml of volume of the sample. The sample solution was kept in a well cleaned analytical bottle with the label until metal analysis (Adebayo, 2017, Pandiyan et al., 2020).
2.3.2. Digestion of soil sediment
The preserved soil sediment was dried for three days to remove the moisture content. The dried soil sample was crushed to fine powder and filtered by using with a 2 mm sieve. The samples were prepared for the analysis of metals described earlier by Pandiyan et al., (2020). The filtered soil sediment sample was transferred in a 100 ml of flask (volumetric) and made the sample until 100 ml with by adding deionised H2O.
2.3.3. Digestion of benthic prey organisms
The preserved benthic organisms were thoroughly washed using double-distilled water. The whole polychaetes, tissues or soft inner body of molluscs and crabs, were used for metal analysis. The tissue samples of various species of polychaetes (5 species) were cut into many pieces and pooled for metal analysis, and the same procedure employed for the mollusc (4 species) and crabs (2 species). 25 g of collective tissue samples of each prey items (the polychaetes, molluscs and crab species) were taken into a polystyrene tube (three samples/tubes for each prey items) were dried to a constant weight at 50 °C and cooled at room temperature (25 °C) and the samples were prepared (weight) into the nearest 0.1 mg. Consequently, one ml of nitric acid was poured into each sample tube, the samples were retained for 24 h at 25 °C (room temperature) and again it was treated about 50 °C for four hours for far-reaching digestion of the benthic organisms. Additionally 100 ml of H2O2 was poured to each sample tube and it was heated at 50 °C for one hour to finish the digestion procedure for thorough digestion of the prey samples the methods followed by Newman and McIntosh, (1983). The final digested sample was transferred into well-cleaned bottles with a proper label of each prey item for further metal analysis using AAS (Pandiyan et al., 2020).
2.3.4. Digestion of prawns and fishes
The tissue samples of various fish species (6 fish species) were mixed and combined, three replicates were taken for metal analysis and the same procedure was applied for the prawn species (2 prawn species). The digestion of fish and prawn was carried out by using the method described by Pandiyan et al., (2020) using HNO3/HCL (1:3 v/v).
2.4. Quality control and analytical procedures
For quality assessment of the instrument’s stability, a quality control (QC) sample was injected for every fifteen samples. In addition to that for better accuracy blank, standard and sample were run in triplicates for each analytical course. However, the accuracy of the systematic procedure in the metal analysis the relative standard deviation (RSD) is obtained at the range of 5–10%, the RSD is calculated from the STD/mean values. For each metal (Cd, Co, Cr, Cu, Hg, Ni, Pb, and Zn), separate calibration curves were prepared at 05, 1.0, 2.0, 5.0 and 10 ppm. Nevertheless, the working solutions were also prepared for every day for the analysis of different metals by making a stock solution with the mixture of 65% (v/v) HNO3, 30% (v/v) H2O2 and H2O (v/v/v = 1:1:3) ratios. For every set of sample, the instrument was set to zero concentration by using a blank solution. The results of each metal arrived from (triplicate) samples. All glassware was rinsed with distilled water before use, soaked in nitric acid (30%) overnight, and air-dried before the analysis of metals. Analyses were performed by using ECIL-Double Beam Atomic Absorption Spectrophotometer (AAS). The results are expressed as ppm (Pandiyan et al., 2020, Ullah et al., 2013).
2.5. Statistical analysis
After carful validation of the data, the ANOVA (one way) was employed to explore the variations of metals among the various sources such as water, sediment, polychaetes, molluscs, crabs, prawns and fishes collected from the study area. To understand the relationship between the water, sediment and among the various biotic species, the Pearson correlation was applied. In addition to that simple linear regression model was also employed between the sediment and the biota to explore the influence of sediment metals on the polychaetes, molluscs, crabs, prawns and fishes. Computation of statistical analyses the SPSS 25.0 was used. The results were interpreted by using Sokal and Rohlf (2012).
3. Results
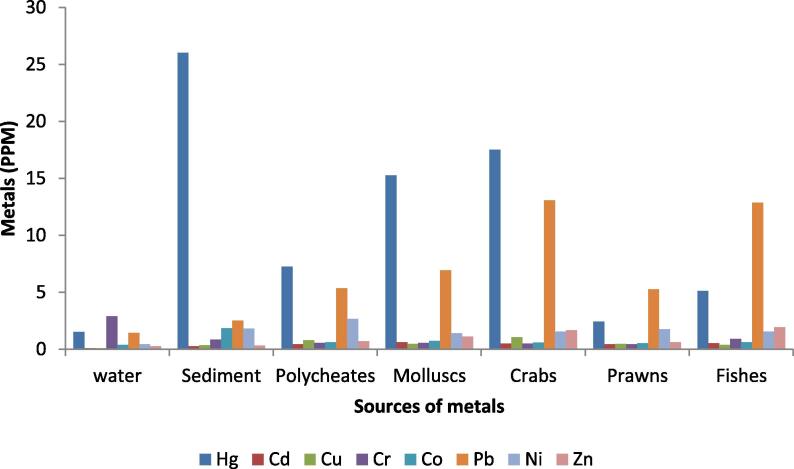
The evaluation of heavy metals was carried out in the Point Calimere Wildlife Sanctuary (PCWL), southern India. Eight different metals such as Cadmium (Cd), Cobalt (Co), Chromium (Cr), Copper (Cu), Mercury (Hg), Lead (Pb), Nickel (Ni) and Zinc (Zn) were assessed from the water, sediment, benthic organisms (polychaetes, molluscs and crustaceans), prawns and fishes. The level of metals at different sources revealed that the Hg, Cr and Co were greater in sediment, Ni and Cd were higher in polychaetes and molluscs respectively. However, the crabs showed higher level of Cu and Pb and the Zinc was greater in fishes (Table 1 and Fig. 2). The concentration of metals varied significantly among the sources examined (P < 0.05), except Cr (P > 0.05).
Table 1.
Basic descriptive statistics for the level of heavy metals in water, sediment, benthic organisms, prawns and fishes, recorded at the PCWL. (Values are mean and SE; ppm).
| Metals | water | Sediment | Polycheates | Molluscs | Crabs | Prawns | Fishes |
|---|---|---|---|---|---|---|---|
| Hg | 1.5 ± 0.57 | 26.0 ± 8.66 | 7.3 ± 1.08 | 15.3 ± 0.49 | 17.5 ± 1.31 | 2.4 ± 0.46 | 5.1 ± 0.62 |
| Cd | 0.1 ± 0.01 | 0.3 ± 0.06 | 0.4 ± 0.11 | 0.6 ± 0.13 | 0.5 ± 0.14 | 0.5 ± 0.08 | 0.5 ± 0.14 |
| Cu | 0.1 ± 0.03 | 0.4 ± 0.14 | 0.8 ± 0.21 | 0.5 ± 0.11 | 1.1 ± 0.28 | 0.5 ± 0.16 | 0.4 ± 0.13 |
| Cr | 2.9 ± 1.82 | 0.8 ± 0.16 | 0.5 ± 0.10 | 0.5 ± 0.08 | 0.5 ± 0.06 | 0.4 ± 0.09 | 0.9 ± 0.21 |
| Co | 0.4 ± 0.04 | 1.8 ± 0.44 | 0.6 ± 0.05 | 0.7 ± 0.18 | 0.6 ± 0.08 | 0.5 ± 0.01 | 0.6 ± 0.12 |
| Pb | 1.4 ± 0.07 | 2.5 ± 0.40 | 5.4 ± 2.21 | 6.9 ± 3.07 | 13.1 ± 1.55 | 5.3 ± 2.17 | 12.9 ± 0.75 |
| Ni | 0.4 ± 0.05 | 1.8 ± 0.48 | 2.6 ± 0.27 | 1.4 ± 0.36 | 1.5 ± 0.20 | 1.8 ± 0.24 | 1.6 ± 0.15 |
| Zn | 0.3 ± 0.04 | 0.3 ± 0.03 | 0.7 ± 0.07 | 1.1 ± 0.16 | 1.7 ± 0.14 | 0.6 ± 0.11 | 2.0 ± 0.24 |
Fig. 2.
Level of metals recorded in the various sources such as water, sediment, polychaetes, molluscs, crabs, prawns and fishes, Point Calimere Wildlife Sanctuary, Kodikkarai.
The correlation of metals among the sources revealed that the polychaetes correlated with sediment r = 0.637. The metals of crabs correlated with the sediment and polychaetes r = 0.630 and r = 842 respectively, the metals of molluscs was correlated with sediment (r = 0.636), polychaetes (r = 0.889) and crabs (r = 0.894). Indeed the metals of prawns was correlated with the polychaetes (r = 839), crabs (r = 0.628) and molluscs (r = 0.634). The metals of fishes correlated with polychaetes (r = 0.529), crabs (r = 0.710), molluscs (r = 0.493) and prawns (r = 0.593) (Table 2). The intercorrelational analysis among the metals examined from the different sources such as water, sediment, polychaetes, crabs, molluscs, prawns and fishes revealed that the Cr and Pb not correlated with any other eight metals examined. However, the Cu and Co were correlated with Hg (r = 0.307) and (r = 0.788) respectively. The nickel was correlated with Hg (r = 0.367), Cu (r = 0.362) and Co (r = 0.432). The Zinc correlated with the Cd (r = 0.331) and Hg (r = 0.737) (Table 3). However the multiple regression model showed that the metals of benthic organism, prawns and fishes influenced by the sediment and it was explained about 84% (F = 21.079; p < 0.001). Overall the pattern of metals at various sources was as Hg > Pb > Ni > Cr > Zn > Co > Cu > Cd.
Table 2.
Correlation of heavy metals examined among the sources such as water, sediment, benthic organisms, prawn and fishes, Point Calimere Wildlife Sanctuary, Kodikkarai (Ramsar site), India.
| Sources | Water | Sediment | Polychaetes | Crabs | Molluscs | Prawns | Fishes |
|---|---|---|---|---|---|---|---|
| Water | 1 | ||||||
| Sediment | 0.078 | 1 | |||||
| Polychaetes | 0.090 | 0.637* | 1 | ||||
| Crabs | 0.175 | 0.630* | 0.842* | 1 | |||
| Molluscs | 0.127 | 0.636* | 0.889* | 0.894* | 1 | ||
| Prawns | 0.056 | 0.243 | 0.839* | 0.628* | 0.634* | 1 | |
| Fishes | 0.119 | 0.214 | 0.529* | 0.710* | 0.493* | 0.593* | 1 |
Correlation at level P < 0.05.
Table 3.
Inter-correlational analysis among the heavy metals examined from the various sources such as water, sediment, benthic organisms, prawn and fishes, Point Calimere Wildlife Sanctuary, Kodikkarai (Ramsar site), India.
| Metals | Hg | Cd | Cu | Cr | Co | Pb | Ni | Zn |
|---|---|---|---|---|---|---|---|---|
| Hg | 1 | |||||||
| Cd | 0.092 | 1 | ||||||
| Cu | 0.307* | 0.184 | 1 | |||||
| Cr | −0.105 | −0.249 | −0.211 | 1 | ||||
| Co | 0.788** | 0.010 | 0.082 | −0.048 | 1 | |||
| Pb | 0.094 | 0.187 | 0.235 | −0.151 | −0.188 | 1 | ||
| Ni | 0.367* | 0.242 | 0.362* | −0.244 | 0.432** | 0.252 | 1 | |
| Zn | 0.043 | 0.331* | 0.301 | −0.188 | −0.152 | 0.737** | 0.094 | 1 |
Correlation at level P < 0.05
Correlation at level P < 0.001
4. Discussion
Heavy metals are almost in traces, which do not biodegrade in the habitats where released, and hence get biomagnified in the exposed organisms. Habitats such as mudflats, sandflats and other coastal wetlands are frequently used by several species including migratory shorebirds as refuel sites. These habitats are not exceptions which also get contaminated by heavy metals from various sources. In addition, Point Calimere Wildlife Sanctuary (PCWL) was upgraded as one of the Ramsar sites in 2002. The present study reveals that the PCWL are under vulnerable state because of contamination, particularly of metal concentration in water, sediment, polychaetes, molluscs, crabs, prawns and fishes. Heavy metals are most significant in relation to ecological aspects since they are not easily removed during the self-purification process of water, but they will accumulate in the biotic species and they could enter into the food chain intensively (Loska and Wiechuła, 2003). The study brings out the first hand information of the accumulation of various heavy metals at various sources and the pattern of heavy metals is as follows: Hg > Pb > Ni > Cr > Zn > Co > Cu > Cd.
The study found that Hg was greater in sediment and also in molluscs, crab, polychaetes, fishes and prawn. The Hg was higher in sediments it might be due to the surrounding areas of the wetlands is associated with agricultural lands and coastal saltpan industries, which could also easing the Hg in the wetlands. Studies are also mentioned that the Hg is a non-essential element and which is exist extensively in an any aquatic habitats and the metals are accountable for toxicity through direct or indirect processes (Johansen et al., 2004). A study reported that Hg is abundantly exists in the environment through agricultural practices such as application of fertilizers and pesticides by the farmer communities (Hashmi et al., 2013). The study found that the benthic species, prawn and fishes showed greater level of Hg, several studies stated that the Hg is higher in coastal prawns, fishes and benthic organisms (Raja et al., 2009, Kumar et al., 2012). The polychaetes, molluscs and crustaceans are accumulating greater level of metals, including mercury, as most of them are filter feeders and feed on detritus, which can increase the accumulation of metals. The Hg could enter in the benthic organisms through their normal feeding mechanisms from their surrounding environment especially sediment and water (Wyn et al., 2009). Besides the benthic species showed greater level of Hg, it could also perhaps trophic system of an coastal ecosystem, the food web is contributing to the accumulation of metals to secondary consumers such as polychaetes, molluscs and crabs, and tertiary consumers like prawn and fishes. Majority of the consumers in the coastal ecosystem are exposed to higher level of Hg and it is due to their feeding preferences particularly they are feed on detritus wastes (Eagles-Smith et al., 2008).
A study suggests that Lead (Pb) an indicator of metal pollution and anthropogenic activities contribute to it (Metcheva et al., 2006). Another study reported that Pb is distributed in the environment through agricultural practices such as the application of fertilizers and pesticides by the farmer communities (Hashmi et al., 2013). The current study found that the greater level of Pb in crabs, molluscs, polychaetes and fishes. Generally the Pb is entering into the benthic organisms through their dietary preferences, food chain and food webs (Pandiyan et al., 2020). The Pb is greater level in mud dwelling organisms which are living in coastal ecosystem or any intertidal wetlands because they are feeding on detritus (Guns et al., 1999, Mado-Filho et al., 2008). In addition to that the study found that the crabs also showed higher level of Pb and it perhaps due to the crabs are well known for scavengers and filter feeder in the coastal wetlands. Besides, the benthic organisms are consuming wide range of diets which are exist in the wetlands habitats including detritus, soil sediment, decayed plant parts and other organisms (Reichmuth et al., 2009), which are facilitate the metals in their body with higher ranges. Greater level of Pb is in fishes also indicate that they performing as a secondary predator and which is showing rich magnification of a trophic structure of an aquatic ecosystem (Kumar et al., 2012).
In fact the Nickel (Ni) is widely used in several industries, including the silver-plating, instruments manufacturing companies and heat treatment, etc., and Ni-Cd batteries. These industries use chrome, Ni, and cyanide, which are highly harmful to various taxa. In fact the study found that greater level of Ni in prawns and fishes studied. A study reported that the fishes and prawns consumed higher level of Ni in the coastal ecosystem since they are acting as a secondary and tertiary consumers of food chain of the aquatic habitats (Kumar et al., 2012). In addition to that the benthic organisms are the major reservoirs of metals since they are feed on detritus in sediments in the coastal ecosystem (Pandiyan et al.,2020). Certainly Chromium (Cr) is one of the common elements on the earth's crust (Mohanty and Patra, 2013). The Cr is released into the environment through various sewage and chemical fertilisers (Ghani, 2011). The present study revealed that the Cr was greater in water and fishes, it might be due to the wetlands receive the water not only from the back water of sea but also through the agricultural farm lands which are exists around the wetlands. In fact the fishes also showed relatively greater level of Cr, it perhaps due to the water have considerable amount of Cr and it could influence the level of Cr in fishes through their osmotic equilibrium process from their environment. Nevertheless various studies have shown that the Cr is exist with greater level in benthic organisms it perhaps due to they are feed on contaminated diets and their nature of physiology (Catsiki et al., 1994; Everaarts et al., 1989, Kumar et al., 2012).
Generally the presence of Zn in the aquatic ecosystem is facilitated from the atmosphere and it can be settled in soils and water. In addition to that the source of Zn in the PCWL could also through the existence of slat pan industries, new construction activities, national highways, solid waste burning, aquaculture industries and agricultural practices, etc. In addition, pesticides and fungicides containing Zn sulphate are an additional source of Zn at PCWL. The Zn was greater in fishes and crabs it might be due to their foraging behaviour and diet preferences. Studies also showed that the zinc is one of the metals with the greater range of accumulation in mud dwelling organisms in an coastal wetlands through their normal behaviour (Everaarts et al., 1989, Philips, 1976, Michael, 2008) and fishes (Raja et al., 2009, Kumar et al., 2012). Greater level of Zn in fishes might be also through their feeding habits and the similar kinds of inference have been stated in earlier studies (Altindag and Yigit, 2005, Viana et al., 2005). In fact the Cobalt (Co) could enter into the coastal wetlands through air and water and it will be settled in the water and sediment of a given habitat. Generally the Co not easily removed once it has settled in any aquatic environment. The current study found that the greater level of Co was found in sediment than the other sources of biotic species studied. The similar results were obtained not only sediment but also molluscs and fishes from coastal wetlands (Youssef and El-Sorogy, 2016, Youssef et al., 2017, Kumar et al., 2012). Greater level of Co in the sediments shown in intertidal mudflats and it is foreseeable because the coastal wetlands are receiving huge amount of contaminated water from various sources including agricultural practices, tanneries, industries etc., (Pandiyan et al., 2020)
The Copper (Cu) is vital and a trace nutrient for all known living organisms. Its role is vital in the physiology of cells, structure and functions of proteins of living organisms (Janssens et al., 2002). Cu is present in the environment due to severe quarrying, manufacturing of various types of equipment, and refineries as well as farm lands and waste treatment sites. The crabs and polychaetes showed greater level of Cu than the other sources including water, sediment, prawn and fishes studied. The copper in crabs and polychaetes was more in their tissues it perhaps due to both the species are filter feeders and they mainly feeding on detritus in the coastal mudflats (Pandiyan, et al., 2020). Studies showed that fishes had a higher level of Cu in coastal mudflats since they are foraging on wastes in the aquatic habitats (Everaarts et al., 1989, Kumar et al., 2012). In fact the higher level of Cd was found in the molluscs and all the other sources were also showed trace level Cd (Table 1). However the molluscs showed greater level of Cd might be due to their feeding mechanisms and the similar inferences have been made a study (McConchie et al., (1988), stated that living mechanism of molluscs responsible for the high cadmium load in their body tissues.
4.1. Inter-correlational analysis among the metals found in various sources
The Cd, Cr and Pb not correlated with any of the eight metals studied from the various sources from the PCWL (Table 3) and it indicates that these metals do not have any relationships with any other metals and their sources and their distribution are also unique. However the other metals i.e. Cu and Co were correlated with Hg, nickel was correlated with Hg, Cu and Co and Zn correlated with the Cd and Hg. It implies that these metals have considerable relationships i.e. the concentration of Cu and Co is associated with the Hg. The concentration of Ni has a close affirmation with the Hg, Cu and Co and the Zn also has a close proximity with their accumulation on the basis of Cd and Hg and it indicates that these metals are showing common origin. The relationships among the metals is purely determined by the physical and chemical factors of soil and water (Raziuddin and Khan, 1987, Soon and Abboud, 1991), in fact the present study did not assess the physical and chemical factors of soil and water but in future it will be considered to understand their origin at the PCWL.
4.2. Relationships of heavy metals among the various sources at PCWL
The correlation of heavy metals among the sources revealed that the metals of polychaetes correlated with sediment, crabs correlated with the sediment and polychaetes, molluscs was correlated with sediment, polychaetes and crabs. Indeed the metals of prawns was correlated with the polychaetes, crabs and molluscs, metals of fishes correlated with polychaetes, crabs, molluscs and prawns (Table 2). Nevertheless in a trophic level the transfer of various organic and inorganic properties from producer to consumer level is inevitable through biomagnification process. In addition to that the heavy metal pollution is vital elements in aquatic toxicology since they are considerably exist in an any wetland habitats and it will be moving ahead at various trophic levels with results of bioaccumulation and biomagnification in the aquatic ecosystem (Steroli et al 2005). In addition to that the multiple regression model also revealed that the metals of various biotic species such as polychaetes, molluscs, crabs, prawns and fishes influenced by the metals of sediment about 84% (F = 21.079; p < 0.001). Indeed the the results of the present study revealed that the sediment is ultimately facilitate the metals to various biotic species of a given habitat. Nonetheless the heavy metals are inclined to deposit in the sediments through various sources or released, and these heavy metals could moving up the various consumers by food chain and food webs (Nabawi, et al., 1987). But very few studies are exploring the bioavailability of sediment related contaminants to various biotic species (Berge and Brevik, 1996). But it is becoming increasingly important to understand metal accumulation within food webs, because, once these heavy metals reach tertiary and quaternary consumers including man, they may produce chronic effects.
4.3. Management recommendations
The study found that the level of heavy metals in sediment threatening the existence of various benthic organisms, prawns and fishes and which are principle and preferred prey items of various migratory shorebirds which are visiting to the PCWL annually. It is right time to evaluate the wetland habitats, especially Important Bird Areas (IBA) and Ramsar sites, to understand their current status so as to protect the habitat and in turn the migratory shorebirds. Assessment of heavy metals in major taxa including migratory, resident migratory and resident shorebirds should be carried out to understand their status on toxic properties especially heavy metals because the metals such as Cd, Co, Cr, Cu, Hg, Pb, Ni and Zn entered in food chain of a population ecology. Therefore intensive studies can be carried out at PCWL for proper understanding of the habitat, management and conservation of various species which are depends on the PCWL.
Acknowledgement
We thank the DST-SERB, Government of India, for funding this project (Ref No. SERB/LS-512/2013 dated-20.09.2013). The authors thank the Management of AVC College and Department of Zoology and Wildlife Biology for providing necessary facilities for the said project. The authors (SM and KAAG) would like to express their sincere appreciation to the Deanship of Scientific Research at the King University for its funding of this research through Research Group Project No. RG-1435-012.
Footnotes
Peer review under responsibility of King Saud University.
References
- Adebayo I.A. Determination of Heavy Metals in Water, Fish and Sediment from Ureje Water Reservoir. J Environ Anal Toxicol. 2017;7:486. doi: 10.4172/2161-0525.1000486. [DOI] [Google Scholar]
- Agoramoorthy G., Pandiyan J. Toxic pollution threatens migratory shorebirds in India. Environmental Science and Pollution Research. 2016;23(15):15771–15772. doi: 10.1007/s11356-016-7021-6. [DOI] [PubMed] [Google Scholar]
- Al-Saad H.T., Mostafa Y.Z., Al-Imarah F.J. Distribution of trace metals in tissues of fish from Shatt Al-Arab Estuary. Iraq. Mar. Meso. 1997;11:15–25. [Google Scholar]
- Altindag A., Yigit S. Assessment of heavy metal concentrations in the food web of lake Beysehir, Turkey. Chemosphere. 2005;60:552–556. doi: 10.1016/j.chemosphere.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Balachandran, S., Sathiyaselvam, P., Panda, S., 2009. Bird atlas of Chilka (ed. BNHS) 1–326.
- Balachandran, S., 2012. Avian diversity in coastal wetlands of India and their conservation needs. In: Proceedings of International Day for Biological Diversity May, 22, pp. 155–163.
- Berge J.A., Brevik E.M. Uptake of metals and persistent organ chlorines in crabs (Cancer pagurus) and flounder (Platichthys flesus) from contaminated sediments: Mesocosm and field experiments. Marine Pollution Bulletin. 1996;33:46–55. [Google Scholar]
- Boncompagni E., Muhammad A., Jabeen R., Orvini E., Gandini C., Sanpera C., Ruiz X., Fasola M. Egrets as monitors of trace metal contamination in wetland of Pakistan. Archives of Environmental Contamination and Toxicology. 2003;45:399–406. doi: 10.1007/s00244-003-0198-y. [DOI] [PubMed] [Google Scholar]
- Botté S.E., Freije R.H., Marcovecchio J.E. Distribution of Several Heavy Metals in Tidal Flats Sediments within Bahía Blanca Estuary (Argentina) Water Air Soil Pollut. 2010;2010(210):371–388. [Google Scholar]
- Bryan G.H. The effects of heavy metals (other than mercury) on marine and estuarine organisms. Proceedings of the Royal Society of London Series B, Biological Sciences. 1971;177:389–410. doi: 10.1098/rspb.1971.0037. [DOI] [PubMed] [Google Scholar]
- Bryan G.H. Bioaccumulation in marine organisms. Philosophical Transactions of the Royal Society of London. 1979;286:483–505. doi: 10.1098/rstb.1979.0042. [DOI] [PubMed] [Google Scholar]
- Camusso M., Vigano L., Baitstrini R. Bioaccumulation of trace metals in rainbow trout. Ecotox. Environ. Safe. 1995;31:133–141. doi: 10.1006/eesa.1995.1053. [DOI] [PubMed] [Google Scholar]
- Catsiki V.A., Katsilieri C.h., Gialamas V. Chromium distribution in benthic species from a gulf receiving tannery wastes (Gulf of Geras — Lesbos island, Greece) Science of the total environment. 1994;145(2):173–218. [Google Scholar]
- Censi P., Spoto S.E., Saiano F., Sprovieri M., Mazzola S., Nardone G., Di Geronimo S.I., Punturo R., Ottonello D. Heavy metals in coastal water systems. A case study from the north western Gulf of Thailand. Chemosphere. 2006;64:1167–1176. doi: 10.1016/j.chemosphere.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Eagles-Smith C.A., Suchanek T.H., Colwell A.E., Anderson N.L. Mercury trophic transfer in a eutrophic lake: the importance of habitat-specific foraging. Ecol Appl. 2008;18:A196–A212. doi: 10.1890/06-1476.1. [DOI] [PubMed] [Google Scholar]
- Everaarts J.M., Boon J.P., Kastro W., Fischer C.V., Razak H., Sumanta I. Copper, zinc and cadmium in benthic organisms from the Java Sea and estuarine and coastal areas around East Java. Netherland Journal of Sea Research. 1989;23(4):415–426. [Google Scholar]
- Fisher N.S., Reinfelder J.R. The trophic transfer of metals in marine systems. In: Tessier A., Turner D.R., editors. Metal Speciation and Bioavailability in Aquatic Systems. John Wiley & Sons Ltd; New York: 1995. pp. 363–406. [Google Scholar]
- Ghani A. Effect of chromium toxicity on growth, chlorophyll and some mineral nutrients of Brassica juncea L. Egyptian Acad J BiolSci. 2011;2:9–15. [Google Scholar]
- Guns M., Van Hoeyweghen P., Vyncke W., Hillewaert H. Trace Metals in Selected Benthic Invertebrates from Belgian Coastal Waters (1981–1996) Marine Pollution Bulletin. 1999;38:1184–1193. [Google Scholar]
- Hashmi H.Z., Malik R.N., Shahbaz M. Heavy metals in eggshells of cattle egret (Bubulcus ibis) and little egret (Egrettagarzetta) from the Punjab province, Pakistan. Ecotoxcol Environ Safety. 2013;89:158–165. doi: 10.1016/j.ecoenv.2012.11.029. [DOI] [PubMed] [Google Scholar]
- Heba H.M.A., Maheub A.R.S., Al-Shawafi N. Oil pollution in Gulf of Aden, Arabian Sea Coasts of Yemen. Bull. Nat. Inst Oceanogr. Fish. ARE. 2000;26:139–150. [Google Scholar]
- Janssens E., Dauwe T., Bervoets L., Eens M. Inter and intraclutch variability in heavy metals in feathers of Great tit nestlings (Parus major) along a pollution gradient. Archives of Environmental Contamination and Toxicology. 2002;43:323–329. doi: 10.1007/s00244-002-0138-2. [DOI] [PubMed] [Google Scholar]
- Jones B., Turki A. Distribution and speciation of heavy metals in surficial sediments from the Tees Estuary, north-east England. Mar. Pollut. Bull. 1997;34:768–779. [Google Scholar]
- Jones W.G., Walker K.F. Accumulation of iron, manganese, zinc and cadmium by the Australian freshwater mussel Velesunio ambiguus (Phillipi) and its potential as a biological monitor. Australian Journal of Marine and Freshwater Research. 1979;30:741–751. [Google Scholar]
- Johansen P., Asmund G., Riget F. High human exposure to lead through consumption of birds hunted with lead shot. Environ. Pollut. 2004;127:125–129. doi: 10.1016/s0269-7491(03)00255-0. [DOI] [PubMed] [Google Scholar]
- Karadede-Akin H., Ünlü E. Heavy Metal Concentrations in Water, Sediment, Fish and Some Benthic Organisms from Tigris River. Turkey. Environ Monit Assess. 2007;131:323–337. doi: 10.1007/s10661-006-9478-0. [DOI] [PubMed] [Google Scholar]
- Kiffney P.M., Clements W.H. Bioaccumulation of heavy metals by benthic invertebrates at the Arkansas River, Colorado. Environmental Toxicology and Chemistry: An International Journal. 1993;12(8):1507–1517. [Google Scholar]
- Kumar B., Sajwan K.S., Mukherjee D.P. Distribution of Heavy Metals in Valuable Coastal Fishes from North East Coast of India. Turkish Journal of Fisheries and Aquatic Sciences. 2012;12:81–88. [Google Scholar]
- Loska K., Wiechuła D. Application of principal component analysis for the estimation of source of heavy metal contamination in surface sediments from the Rybnik Reservoir. Chemosphere. 2003;51(8):723–733. doi: 10.1016/S0045-6535(03)00187-5. [DOI] [PubMed] [Google Scholar]
- Mado-Filho G.M., Salgado L.T., Rebelo M.F., Rezende C.E., Karez C.S., Pfeiffer W.C. Heavy metals in benthic organisms from Todosos Santos Bay. Brazil. Braz. J. Biol. 2008;68(1):95–100. doi: 10.1590/s1519-69842008000100013. [DOI] [PubMed] [Google Scholar]
- Manikannan R. Bharathidasan University; Tiruchirappalli, Tamil Nadu, India: 2011. Diversity of Water birds in the point Calimere wildlife sanctuary, Tamil Nadu, India; p. 264.p.. Ph.D. thesis submitted to. [Google Scholar]
- Metcheva R., Yurukova L., Teodorova S., Nikolova E. The penguin feathers as bioindicator of Antarctic environmental state. Environmental Monitoring and Assessment. 2006;362:259–265. doi: 10.1016/j.scitotenv.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Masero J.A., Pérez-González M., Basadre M., Otero-Saavedra M. Food supply for waders (Aves: Charadrii) in an estuarine area in the Bay of Cadiz (SW Iberian Peninsula) Acta oecologica. 1999;20(4):429–434. [Google Scholar]
- MacFarlane G.B., Burchettt M.D. Cellular distribution of Cu, Pb, and Zn in the Grey Mangrove Avicemnia marina (Forsk.) Vierh Aquatic Botanic. 2000;68:45–59. [Google Scholar]
- McConchie D.M., Mann A.W., Lintern M.J., Longman D., Talbot V., Gabelish A.J., Gabelish M.J. Heavy metals in marine biota, sediments, and waters from the Shark Bay area, Western Australia. J Coastal Res. 1988;4:37–58. [Google Scholar]
- Michael H. Trace Metals in the Tissues and Shells of Tympanotonus Fuscatus var. Radula from the Mangrove Swamps of the Bukuma Oil Field, Niger Delta. European Journal of Scientific Research. 2008;24:447–468. [Google Scholar]
- Mohanty M., Kumar Patra H. Effect of ionic and chelate assisted hexavalent chromium on mung bean seedlings (VignaRadiatal. Wilczek. Var k-851) during seedling growth. JSPB. 2013;9:232–241. [Google Scholar]
- Nabawi A., Heinzow B., Kruse H. As, Cd, Cu, Pb, Hg, and Zn in fish from Alexandria Region, Egypt. Bulletin of Environmental Contamination and Toxicology. 1987;39:889–897. doi: 10.1007/BF01855871. [DOI] [PubMed] [Google Scholar]
- Namminga, H. N., Wilhm, J., 1976. Effects of high discharge and an oil refinery cleanup operation bon heavy metals in water and sediments in Skeleton Creek. Proceedings of the Oklahoma Academy of Science. 56,133–138.
- Newman M.C., McIntosh A.W. Lead elimination and size effects on accumulation by two freshwater gastropods. Archives of Environmental Contamination and Toxicology. 1983;12(1):25–29. [Google Scholar]
- Özmen H., Külahçı F., Çukurovalı A., Doğru M. Concentrations of heavy metal and radioactivity in surface water and sediment of Hazar lake (Elazığ, Turkey) Chemosphere. 2004;55:401–408. doi: 10.1016/j.chemosphere.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Pandiyan J., Asokan S. Habitat use pattern of tidal mud and sand flats by shorebirds (Charadriiformes) wintering in southern India. J. Coast. Cons. 2015;20:1–11. [Google Scholar]
- Pandiyan J., Jagadheesan R. Population characteristics of migratory shorebirds in the Point Calimere Wildlife Sanctuary, Tamil Nadu India. J. Sci. Trans. Environ. Technov. 2016;10:31–36. [Google Scholar]
- Pandiyan J., Mahboob S., Jagadheesan R., Elumalai K., Krishnappa K., Al-Misned F., Kaimkhani Z.A., Govindarajan M. A novel approach to assess the heavy metal content in the feathers ofshorebirds: A perspective of environmental research. J of King Saud University, Science. 2020;32:3065–3307. [Google Scholar]
- Philips D.J.H. The common mussels Mytilus edulis as an indicator of pollution by Zinc, Cadmium, Lead, and Copper. Effects of environmental variables on uptake of metals. 1976. Mar. Biol. 1976;38:59–69. [Google Scholar]
- Praveena S.M., Radojevic M., Abdullah M.H., Aris A.Z. Application of sediment quality guidelines in the assessment of mangrove surface sediment inMengkabong lagoon, Sabah. Malaysia. Iran. J. Environ. Health. Sci. Eng. 2008;5:35–42. [Google Scholar]
- Raja P., Veerasingam S., Suresh G., Marichamy G., Venkatachalapathy R. Heavy metal concentration in four commercially valuable marine edible fish species from Parangipettai coast, south east coast of India. Journal of Animal and Veterinary Advances. 2009;1:10–14. [Google Scholar]
- Ramirez M., Serena M., Frache R., Correa J. Metal speciation and environmental impact on sandy beaches due to El Salvador copper mine. Chile Mar. Pollut. Bull. 2005;50:62–72. doi: 10.1016/j.marpolbul.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Raziuddin M.A., Khan A.U. Heavy metals in water, sediments, fish and plants of river Hindon, U.P. India. Hydrobiologia. 1987;148:151–157. [Google Scholar]
- Reichmuth J.M., Roudez R., Glover T., Weis J.S. Differences in prey capture behavior in populations of blue crab (Callinectes sapidus Rathbun) from contaminated and clean estuaries in New Jersey. Estuaries and Coasts, 32, 298–308. Raziuddin, A.M. and Khan, A.U. 1987. Heavy metals in water, sediments, fish and plants of river Hindon, U.P. India. Hydrobiologia. 2009;148:151–157. [Google Scholar]
- Sampath K., Krishnamurthy K. Shorebirds (Charadriiformes) of the Pichavaram Mangroves. Tamilnadu. India. Wader Study Group Bulletin. 1990;58:24–27. [Google Scholar]
- Sivaperuman C., Venkatraman C. Mar. Fau. Diver; India: 2014. Coastal and Marine Bird Communities of India; pp. 261–281. [Google Scholar]
- Soon Y.K., Abboud S. Can. J. Soil Sci. 1991;70:227–278. [Google Scholar]
- Sokal, R. R., Rohlf, F. I., 2012. Biometry: the principles and practice of statistics in biological research. (eds Sokal and Rohlf). 1-776.
- Ullah, K., Zaffar,M., Riffat,H., Malik, N., 2013. Heavy-Metal Levels in Feathers of Cattle Egret and Their Surrounding Environment: A Case of the Punjab Province, PakistanHeavy-Metal Levels in Feathers of Cattle Egret and Their Surrounding Environment: A Case of the Punjab Province, Pakistan. Arch Environ Contam Toxicol. DOI 10.1007/s00244-013-9939-8. [DOI] [PubMed]
- Viana F., Huertas R., Danulat E. Heavy metal levels in fish from coastal waters of Uruguay. Archives of Environmental Contamination and Toxicology. 2005;48:530–537. doi: 10.1007/s00244-004-0100-6. [DOI] [PubMed] [Google Scholar]
- Wyn B., Kidd K.A., Burgess N.M., Curry R.A. Mercury bio-magnification in the food webs of acidic lakes in Kejimkujik National Park and National Historic Site. Nova Scotia. CanJ Fish Aquat Sci. 2009;66:1532–1545. [Google Scholar]
- Yilmaz A.B. Comparison of heavy metal levels of grey mullet (Mugil cephalus L.) and sea bream (Sparusm aurata L.) caught in Iskenderun Bay (Turkey) Turkish Journal of Veterinary and Animal Sciences. 2005;29:257–262. [Google Scholar]
- Youssef M., El-Sorogy A. Environmental assessment of heavy metal contamination in bottom sediments of Al-Kharrar lagoon, Rabigh, Red Sea. Saudi Arabia. Arab. J. Geosci. 2016;9:474. [Google Scholar]
- Youssef M., Madkour H., Mansour A., Alharbi W., Atef E.T. Invertebrate shells (mollusca, foraminifera) as pollution indicators, Red Sea Coast. Egypt. Journal of African Earth Sciences. 2017;133:74–85. [Google Scholar]
Further Reading
- Fernandes C., Fontainhas-Fernandes A., Peixotoc F., Salgado M.A. Bioaccumulation of heavy metals in Liza saliens from the Esmoriz-Paramos coastal lagoon. Portugal. Ecotoxicol. Environ. Saf. 2007;66:426–431. doi: 10.1016/j.ecoenv.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Storelli M.M. Trace elements in loggerhead turtles (Caretta caretta) from the eastern Mediterranean Sea: overview and evaluation. Environ. Pollut. 2005;135:163–170. doi: 10.1016/j.envpol.2004.09.005. [DOI] [PubMed] [Google Scholar]