Abstract
One of the essential factors to be addressed in the development of aquaculture is the feeding regime. This study was investigated to assess the effects of feeding rate on growth performance, feed utilization, chemical body composition survival rate, cannibalism and morphological indices of Asian Seabass, Lates calcarifer. Intended for the trial, one hundred forty sea bass individuals with an average weight 5.47 ± 0.11 g were randomly distributed in 4 concrete tanks (914 cm × 183 cm 122 cm) each; length × width × depth) and volume 18,399 L, for total 68 days. The fishes were fed with a pelleted diet containing 46% crude protein for different feeding groups designated as (T1, T2, T3 and T4). The feed was supplied with a rate of (T1) 3%, (T2) 4%, (T3) 6%, and (T4) 9% of fish biomass per day and feeding frequency were maintained three times per day to all the groups. At the end of the trial water physicochemical parameters was in acceptable range for Asian sea bass growth. The average daily weight gain (g), weight gain (g) and specific growth rate (%) was significantly higher (p < 0.05) in T3 and T4 as compared to T1 and T2 group. The poorest feed conversion ratio was recorded in T1 group with 3% biomass per day. The cannibalism rate was significantly (p < 0.05) higher in T1 (3%) compared to T3 and T4 treatment. The morphological indices, condition factors (CF), viscerosomatic index and hepatosomatic index (HSI) was significantly higher in T4 group as compared to other treatments. The protein, moisture and ash contents of the whole biomass of the Asian sea bass were not significantly influenced by feeding rate. The fat levels in the fish bodies increased significantly (p < 0.05) with increasing feeding ratio. The phenomenal regression indicates that 6.5% feeding rate per day is optimum for best growth performance, survival and minimum cannibalism rate for Asian sea bass in captivity. The outcome of the finding will help in promotion for not only the coastal aquaculture in Pakistan abut also elsewhere.
Keywords: Asian sea bass, Growth performance, Feeding rates, Body composition, Cannibalism, Survival rate
1. Introduction
Asian sea bass, Lates calcarifer is considered a most probable applicant species appropriate for aquaculture. Also, Asian sea bass is a comparatively hardy fish which embraces a broad variety of salinity from freshwater to complete saline water with 0–56‰ salinity and has wide physiological tolerances. It has been cultured in marine ecosystems, ocean nursery, brackish estuaries and fresh waters either as monoculture or in polyculture (Sorphea et al., 2019, Ganzon-Naret, 2013). L. calcarifer is widely distributed in the Western Pacific region, Arabian Sea, Taiwan, China, and Thailand (Venkatachalam et al., 2018; Narat, 2013; Anil, et al., 2010). This fish is extremely carnivorous an opportunistic predator with cannibalistic character and protandrous hermaphrodites (Boonyaratpalin, 1997).
Currently, aquaculture actually plays a crucial role in the culture of marine species for food and nutritional reliability. It is the fastest increase in the food production industry to reduce wild stock overexploitation and keep the ecosystem from declining (Daet, 2019: Sangeeta et al., 2018). In sustainable aquaculture practice, the management of fish nutrition is necessary to minimize costs and maximize growth performance for example, an optimal feed rate, the quantity of nutrition ingested by fish per day, is needed to handle fish nutrition for the best possible growth performance and fish health (Ahmad et al., 2018). The feed and feeding is considered the main factor in production cost of aquaculture, it represent from 50 to 60% of the total production cost in aquaculture investments (El-Sayed, 1999). So, the feeding control and determination of the optimum feeding rate is essential to success the culture of marine and fresh water fish. Knowledge about feeding levels is important to achieve the best growth and feed efficiency and preventing water quality deterioration as a result of the overfeeding then the optimum feeding level contributes in tremendous savings of the feed cost (Davies et al., 2006). There are many factors influence the optimal feeding level such as fish size, species, age, feed quality rearing condition (Goddard, 1995). Numerous studies have been evaluated the effect of feeding rate on growth performance of fish species such as yellow fin sea bream Acanthopagrus arabicus (Ahmad et al., 2018), Rabbitfish Siganus rivulatus (Mohammed et al., 2017), snapper Lutjanus johnii (Abbas et al., 2015), grouper Epinephelus polyphekadion (Al Zahrani et al., 2013), black sea turbot Psetta maxima (Aydin et al., 2011) and rainbow trout Oncorhynchus mykiss (Bureau et al., 2006).
Therefore the present study was planned to determine the optimum feeding rate of L. calcarifer on the growth output body composition, feed consumption, survival rate and cannibalism in a controlled environment, taking into account the limited information available on the optimum feeding rate for Asian Sea bass cultured under closed system in Pakistan.
2. Material and methods
2.1. Design of feeding trial
The trial was conducted at the Sindh hatchery seed production unit Hawkes Bay (24 ° 86′2.08″ N, 66 ° 84′7.48″E) during October 2019 to January 2020. The Asian sea bass seeds were imported from Thailand commercial seed hatchery. A total of one hunred and forty fish individuals with initial body weight 5.47 ± 0.11 g selected and randomly distributed in four concrete tanks; the dimensions of each tank were (914 cm × 183 cm 122 cm) each; length × width × depth) and volume 18,399 L. All the groups' fish were acclimatized for 2 weeks before the start of the trial. Four feeding rates were tested in this trial [T1, 3%, T2, 4%, T3, 6% and T4, 9%, of body weight per day (BW/d-1)]. The total experimental duration was 68 days and the feed was supplied three times a day. Water was exchanged at a rate of 30 % the water volume per two days for maintaining the best water quality. After two hours of feeding, the uneaten feed was removed from the water (dry and weight).
2.2. Experimental feed preparation
The experimental feed was formulated to contain 46% protein and 20.9 KJ/g gross energy All ingredients were ground to small particles and mixed by hand. The ingredients (g/100) and a proximate analysis of feed were shown in Table 1.
Table 1.
Biochemical analysis and nutritional composition of the pelleted feed used as food for the Lates calcarifer Seabass during the experiments.
| Ingredients (%) | g 100 g-1 diet (dry) |
|---|---|
| Fish meal | 45.00 |
| Shrimp meal | 6.50 |
| Soybean meal | 24.00 |
| Octopus,Squid meal | 4.40 |
| Bread flour | 5.00 |
| Rice bran | 3.40 |
| Cod liver oil | 5.40 |
| Vitamin and mineral premix1 | 5.00 |
| Fish protein Hydrolysate | 1.30 |
| Total | 100 |
| Biochemical composition1, 2, 3, 4(%) | |
| Moisture | 10.8 ± 0.4 |
| Crude protein3 | 44.8 ± 0.5 |
| Crude fiber | 9.1 ± 0.5 |
| Crude lipid | 10.5 ± 0.06 |
| Ash | 9.3 ± 0.5 |
| NFE 4 | 26.3 ± 0.8 |
| Energy (kJg-1) | 20.93 ± 0.6 |
Vitamin and mineral composition contained the following ingredients (g 100 g-1 diet): Hexuronic acid (vit C), 16.2; thiamin HCl (vit B6)), 1.2; inositol, 38.2; calcium, 1.05; zinc, 1.1;choline chloride, 4.0; retinol (vit A), 1.2; phosphorus, 2.9;magnesium, 3.0; copper, 1.1; pyridoxine (vit B6), 1.2; α-tocopherol acetate (vit E), 4.8; phospholipids, 3.7; folic acid, 0.6;riboflavin (vit B2), 1.3; cholecalciferol (vit D3), 6.4; menadione sodium bisulfate (vit K3), 0.04; cyanocobalamin (vit B12), 0.008 manganese, 1.0; iodine, 1.5; sodium, 2.0; iron, 1.1; biotin, 0.65; nicotinic acid, 3.4;
Dry matter basis (%): mean ± SE, number of determination = 5
Measured as nitrogen × 6.25.
Nitrogen-free extract = 100 – (% protein + % fat + % ash + % fiber).
2.3. Samples collection and Biochemical analysis
The biochemical analysis of feed and fish carcass samples were conducted according to (AOAC, 2000). At the end of study four fishes were taken from each tank and then dissected to weigh the liver and viscera then determination of hepato and viscerosomatic index (HSI, VSI).
The methods of (AOAC, 2000) have been used to evaluate crude lipid (CL), moisture and crude protein (CP). Moisture was measured at 105°Celsius for 24 h using an oven (Labostar-LG122 Tabia Espec, Osaka, Japan). Chloroform/methanol (2:1v/v) extraction procedure was predicted for crude lipid (Folch, et al. 1957). Crude protein was analyzed using an automatically processed Kjeldahl (Buchi430/) using a Kjeltec method (N × 6.25) using, automatic Kjeldahl system (Buchi 430/323) model1265, Moline IL, USA) and Ash content was determined by ashing at 550° C in muffle furnace for three hours. and Gross energy (GE) was estimated for formulated diets the factors 23.62, 39.5 and 17.56 KJ/g for CP, EE and carbohydrates respectively were used (NRC, 1993).
2.4. Calculation indices
Growth parameters and morphological indices as final body weight (FBW, g) final body length (FBL, cm) length gain (LG, cm) weight gain (WG, g) and average daily weight gain, (ADWG, g/day), specific growth rate (SGR %/ day), condition factor (CF, g/cm3), hepatosomatic index (HSI) and viscerosomatic index (VSI). Feed conversion ratio (FCR), protein efficiency ratio (PER) survival rate (SR, %) and Cannibalism (C, %).
Where calculated as follow:
LG, cm = Final body length -Initial body length (IBL)
WG, g = Final body weight -initial body weight
ADWG, g/day = {(Final body weight (g)-Initial body weight)/ time (days)}
SGR, %/ day = (ln (final body weight) - ln (initial body weight) x100)/time (days)
CF, g/cm3= (Body weight(g) /Body length3 (cm)) × 100
HSI, % = liver weight/body weight × 100
VSI, % = weight of viscera and associated fat tissue/body weight × 100
FCR = Feed given (g)/Weight gain (g)
PER = wet weight gain/ protein intake
SR (%) = (No. of fish survived/ No. of fish released) × 100
Cannibalism (%) = 100 × LS-M−LC/LS
Where,LS stocked at the beginning of seabass LC is the number of seabass collected at the end of the study and M is natural mortality (Hassan et al., 2020; Szkudlarek and Zakes, 2007).
2.5. Water quality management
Water temperature (°C), salinity (‰), dissolved oxygen (ppm) and pH were recorded every day and they were measured by (Celsius glass thermometer), and Handheld Refractometer, mobile digital DO-meter (Model: HI9146) and digital pH mete) , while ammonia (mg/l), alkalinity (mg/l), nitrate (mg/l), and nitrite (mg/l) were determined every two weeks with chemical methods according to (APHA, 1995).
2.6. Statistical analysis
The experimental results were analyzed by One way analysis of variance (ANOVA) the differences among the treatments were determined by Duncan Waller at significant level (P ≤ 0.05) technique was used to test the determine biological and chemical indices of fish, between treatment means for the diverse feeding rate. The data were statistically examined by software SPSS version 20.
3. Results
3.1. Physicochemical parameters
The results of hydrological parameters are presented in (Table 2) the means (±standard error) of all the parameters did not significantly different among the treatments. The means ranged between (29.50–29.740 C°), (30.07–31.12‰), (6.62–8.03 ppm), (7.18–8.84), (0.01–0.09), (139.6–148.4) and (1.34–2.81) for temperature, salinity, DO, pH, ammonia, alkalinity, and nitrate respectively.
Table 2.
Hydrological parameters recorded at different treatment groups during experimental period.
| Hydrological parameters | Treatments Groups |
|||
|---|---|---|---|---|
| T1 (3%) | T2 (4%) | T3 (6%) | T 4 (9%) | |
| Temperature (°C) | 29.50 ± 4.25 | 29.62 ± 4.10 | 29.60 ± 4.31 | 29.74 ± 3.31 |
| Salinity (ppt) | 30.07 ± 4.17 | 31.12 ± 3.27 | 30.25 ± 3.41 | 30.25 ± 4.41 |
| D.O (mg/l) | 6.62 + 1.20 | 7.21 ± 0.08 | 7.02 ± 2.24 | 8.03 ± 1.25 |
| pH | 8.40 ± 0.24 | 8.84 ± 0.28 | 7.20 ± 1.29 | 7.18 ± 0.29 |
| Ammonia (mg/l) | 0.01 ± 0.00 | 0.04 ± 0.00 | 0.08 ± 0.12 | 0.09 ± 0.11 |
| Alkalinity (mg/l) | 142.1 ± 5.56 | 148.4 ± 5.07 | 139.6 ± 5.03 | 140.6 ± 5.03 |
| Nitrite (mg/l) | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 |
| Nitrate (mg/l) | 1.34 ± 0.37 | 1.78 ± 0.10 | 1.80 ± 0.34 | 2.81 ± 0.36 |
3.2. Growth performance
The results obtained from the feeding trial are set out in the Table 3 and indicated that, growth performance of Asian sea bass fingerlings significantly varied with difference of feeding rates. There was a significant positive correlation between increased of feeding rate from 3% to 6% of biomass and the growth indicators. But the growth curve decreased with increased feeding rate above 6% of body weight. Data of Table 3 appeared that, 6% of biomass as feeding rate (T3) had the highest FBW (84.78, g), FBL (18.86, cm), LG (13.11, cm), WG (79.68, g), SGR (4.13, %d-1), ADWG (1.17, g/d) followed by feeding rates 9% of body weight (T4) and 4% of body weight (T2) while this parameters was the least at feeding rate 3% of body weight where:- LG, WG, SGR, ADWG were 11.65 cm, 62.91 g, 3.78 %d-1, 0.92 g/d respectively.
Table 3.
Growth performance and morphological indices of Lates calcarifer at different feeding rates for 68 day trial.
| S.No. | Biotechnical parameters | Feeding rate groups (% BW d-1) |
|||
|---|---|---|---|---|---|
| T1, 3% | T2, 4% | T3, 6% | T4, 9% | ||
| 1 | IBW (g) | 5.20 ± 0.022 | 5.10 ± 0.04 | 5.10 ± 0.01 | 5.40 ± 0.02 |
| 2 | FBW (g) | 68.11 ± 0.42b | 79.86 ± 2.11b | 84.78 ± 1.66a | 81.88 ± 1.80b |
| 3 | IBL (Li,cm) | 5.20 ± 0.88b | 5.11 ± 0.44b | 6.21 ± 0.99 a | 5.4 ± 0.82b |
| 4 | FBL (Lf, cm) | 16.85 ± 0.98c | 17.88 ± 0.39b | 18.86 ± 1.98 a | 18.25 ± 1.85a |
| 5 | LG (cm) | 11.65 ± 0.10b | 11.77 ± 0.11a | 13.11 ± 0.01a | 12.49 ± 0.47a |
| 6 | WG (g) | 62.91 ± 0.20b | 74.76 ± 2.07a | 79.68 ± 1.65a | 76.48 ± 1.78a |
| 7 | SGR (%/day) | 3.78 ± 0.1b | 4.04 ± 0.2b | 4.13 ± 0.3a | 3.99 ± 0.1a |
| 8 | ADWG (g/day) | 0.92 ± 0.04c | 1.09 ± 0.02b | 1.17 ± 0.06a | 1.12 ± 0.07b |
| 9 | FCR (g) | 2.04 ± 0.6a | 1.23 ± 0.4b | 0.96 ± 0.02c | 1.36 ± 0.8b |
| 10 | PER | 1.36 ± 0.2c | 1.62 ± 0.3b | 1.70 ± 0.3a | 1.66 ± 0.1b |
| 11 | CF | 1.52 ± 0.2b | 1.53 ± 0.1b | 1.56 ± 0.1b | 2.95 ± 0.2a |
| 12 | HSI | 1.4 ± 0.1b | 1.5 ± 0.2a | 1.5 ± 0.2a | 1.5 ± 0.1a |
| 13 | VSI | 4.11 ± 0.36c | 4.30 ± 0.41c | 4.98 ± 0.52b | 5.20 ± 46a |
| 14 | Cannibalism (%) | 5.72 ± 0.00b | 0 ± 0.00a | 0 ± 0.00a | 0 ± 0.00a |
| 15 | SR (%) | 94.28 ± 0.00c | 100 ± 0.00a | 100 ± 0.00a | 97.14 ± 0.00b |
The mean ± SD of treatments (n = 35) in the same row with a number of different super-scripts differs significantly between them (P > 0.05).
3.3. Feed utilization parameters
There was a significant difference among the treatment at level 0.05 in FCR and PER.
Asian sea bass fed at 6% of biomass recorded the best FCR (0.96) and the highest PER (1.70, g). FCR and PER were insignificant between T2 and T4 but T2 was better in FCR (1.23) than T4 that had FCR (1.36). The fish fed at 3% of their biomass were the worst in FCR (2.04) and PER (1.36).
3.4. Morphological indices
The means of CF, HSI and VSI significantly increased with an increasing the feeding rate. Whereas, the highest CF value was 2.95 g/cm3 and it achieved with T4 while CF of the other treatments did not significantly differ. No significant changes were found among T2, T3 and T4 in HSI and these treatments were significantly higher than T1. There was a significant positive correlation between values of VSI and increasing of feeding rats, the highest VSI recorded with T4 (5.20%), T3 (4.98%), T2 (4.30%) and the least VSI value was 4.11% with T1.
3.5. Cannibalism and survival
It is noted that (T1) fish fed 3% body weight had the highest cannibalism in comparison with the other treatments, where there was an adverse correlation between cannibalism and a high feeding rate. Survival rate was improved with increased the feeding rate up to 6% of biomass than it decreased with increasing feeding rate to 9% of body weight. Table 3 showed significant differences in survival rate among the treatments, T3 and T2 had the best survival rate followed up T4 that had 97.14% and T1 that recorded 94.28%.
3.6. Proximate composition of fish carcass
The carcass proximate composition was calculated on basis wet weight and presented in Table 4 carcass content of moisture and protein did not significantly affected by feeding rates. Conversely, carcass content of lipid and Ash were significantly differed among the treatments. There was a positive correlation between the carcass content of lipid and increasing of feeding rate, where lipid carcass of T4 was 12.9% followed by T3 (9.88%) and no significant between T2 and T3. In relation to Ash content, there was insignificant among T4, T3 and T2 in Ash content but Ash content of T1 was 5.58% and significantly higher than the other treatments.
Table 4.
A proximate composition of final carcass composition (% wet weight) of experimental sea bass (Lates calcarifer) at different feeding levels for 68 day trial.
| Ingredients (%) |
Feeding groups (% BW d-1) |
||||
|---|---|---|---|---|---|
| T1, (3%) | T2, (4%) | T3, (6%) | T4, (9%) | ||
| Moisture | 70.4 ± 0.08 | 69.7 ± 2.1 | 70.3 ± 0.31 | 69.9 ± 0.03 | |
| Protein | 18.01 ± 0.12 | 18.02 ± 1.12 | 18.18 ± 1.20 | 18.04 ± 1.4 | |
| Lipid | 9.30 ± 1.6c | 9.32 ± 1.60c | 9.88 ± 1.8b | 12.9 ± 1.77a | |
| Ash | 5.58 ± 0.01b | 5.12 ± 0.8a | 5.10 ± 0.6a | 5.13 ± 0.8a | |
Values (mean ± SD, n = 3 and each tank consists of 35 fish per group in the same row with different superscripts are significantly different (P > 0.05).
4. Discussion
Water quality plays a crucial role as they have direct influence on the general health status of the cultured fishes (Al Zahrani, et al., 2013). The disproportionate feed increases the feeding wastage in closed aquaculture system causing degradation the water quality through its decomposition, production of ammonia and depletion of oxygen. (Puvanendran et al., 2003). Consequently, Poor water quality conditions reduced feed consumption, increased FCR value and reducing the survival rate (Santos et al., 2010, Björnsson and Ólafsdóttir, 2006).
physicochemical parameters:-like temperature, salinity, DO, pH, ammonia, alkalinity, and nitrate did insignificantly variance by the testing of feeding rates and this parameters remained within the range acceptable for Asian sea bass culture as showed in (Table 2). Whereas, it is known that Asian sea bass has an extremely wide thermal tolerance range (15–40° C) also, Asian sea bass had the best growth when were reared at 22–35C° temperature. (Anil, et al., 2010, Tucker et al., 2002). Moreover, the optimum ammonia level for Asian sea bass is from 0.001 to 0.05. The increasing of ammonia level from 0.200 to 0.500 (mg/L) fish lead to increase the mortality rate (Venkatachalam et al., 2018). In general, the results of water quality in this study is compatible to grew Asian sea bass according to (Sangeeta et al., 2018).
The fish aquaculture industry is continuously growing in worldwide and fish feed is considered as one of the greatest challenge in aquaculture that is very impressive on production costs (Ahmad et al., 2018, Silva et al., 2007). Because the feed is 50% of the cost of intensive fish farming (Tacon and Metian, 2008), therefore, feeding rate is an important variable in fish development, it has a big role to reduce the feeding wastage and preservation of water quality hence reducing the water exchange and regulation the eaten feed. The following has discussed the effects of the feeding rates variation on water quality, growth parameters, feed efficiency, morphological indices, survival and cannibalism rates and also the composition of fish carcass.
The results of the present study manifest that, the best growth performance was recorded in fingerlings of sea bass (Lates calcarifer) fed at 6.0%BWd-1 with feedings of three times daily after a 68 day trial. A 6.0% BW d-1 feeding rate showed significantly higher SGR, WG and the best FCR and PER. But increasing the feeding level till 9% BW d-1 resulted in reducing the growth performances.
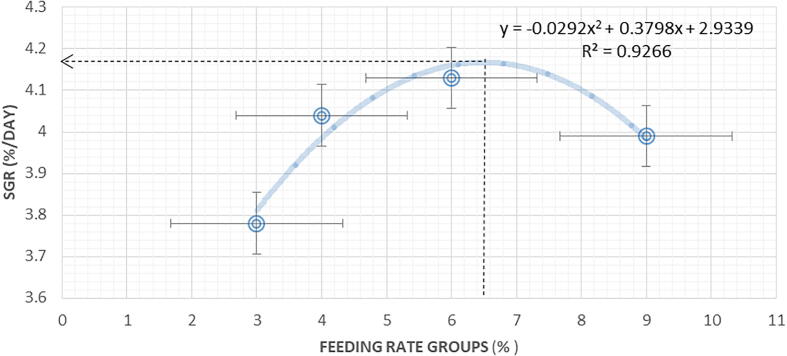
A classical of precise growth rate (SGR) plotted against the rising of feeding levels by second order line regression of sea bass growth (y = -0.0292x2 + 0.3798x – 2.9339; R2 = 0.9266) showed a reduced growth rate at high and low feeding ration. According to polynomial regression the optimum feeding rate is 6.5% BW/ day estimated in the present study for the best growth performance (SGR) in L.calcarifer (Fig. 1). These results were in agreement with Singh et al., (2003) who reported that, the enhancement of feeding level result in increasing the growth rate of fish up to a maximum rate then the growth curve decrease. Ng et al., 2000, Adebayo et al., 2000 they observed that, with increasing the feeding rate the growth rate enhancement at a higher rate than decreased at lower rate. In the same trend Wang et al. (1998) said that; feed consumption and growth curve increase with increasing the feeding level up to a given limit. Similar results were recorded with Deyab and Hussein (2015) who found the best feeding rate of Nile tilapia was 5%BW/day also Trushenski et al. (2012) noted that a maximum growth of juvenile Atlantic spadefish achieved between 5 and 7% BW/day with feeding frequency tree times daily.
Fig. 1.
The optimum feeding rate 6.5% BW d-1 of fingerling based on percent SGR as determined by the phenomenal regression.
The optimal feeding rate differ due to many factors for example fish species, whereas juvenile yellow fin sea bream (Acanthopagrus arabicus) (7% BW/d; Ahmad et al., 2018), Clarias gariepinus (8% BW/d, Marimuthu et al., 2011), tambaqui, Colossoma macropomum (10% BW/d; Silva et al., 2007), European seabass Dicentrarchus labrax(7% BW/d; Ng et al., 2000) and Clarias fuscus (6% BW/d; Anderson and Fast,1991). Age of fish species is one of the factor that effect on the optimum feeding rate, it can be noted that the small fish need a higher feeding rate than the larger fish in the same species. Definitely, Kalogeropoulos et al. (1992) found that, 4% BW/day was the best feeding rate of gilthead sea bream with an average initial weight 3 g while 6% BW/day was the best feeding rate of the same species with an average initial weight 1 g. Also, the feed quality is considered one of the impressive factors on the optimal feeding level, whereas the varying of ingredients in standard diet had a great effect on the feeding rate, it can be cleared that, fish meal replacing with plant protein from fish diets causes changing the feeding rate, also fish oil replacing with plant oil from fish diet influenced on the optimum feeding rate of Siganus rivulatus fry, whereas fry fed dietary fish oil had a maximum SGR at 9% BW/ day as feeding rate while those fed dietary linseed oil had a maximum SGR at 7% BW/day as it has been found by (Mohammed et al., 2017). the feeding frequencies per day that has an important role in regulating the operating's digestion and absorption and it has a direct impact on the optimum feeding rate, Asian sea bass gained higher SGR and growth efficiency when fed three times daily than fed two or once time a day at the same feeding rate (Biswas et al., 2010). The rearing conditions has significantly effect on the optimum feeding rate, in the same trend, recorded finding revealed that the optimum feeding rate of Juvenile Korean Rockfish was changed with the different of water temperature (Mizanur et al., 2014) moreover the photoperiod or the culture in outdoor allows to bloom the phytoplankton in the rearing ponds consequently the optimum feeding rate is lower than the culture indoor.
In regard to feed utilization parameters, the best FCR and PER was achieved with fish fed at 6% daily, consequently it can be said that the feed utilization parameters and the growth indicates agreed to T3 has the optimum feeding rate. Absolutely, an increasing the feeding rate at level higher than the optimum level result in a reduced of FCR, it could be observed that T3 had the best FCR followed by T2 and not T4, in
addition to, feed efficiency linearly reduced with an improper feeding level or overfeeding that cause an overload of fish digestion system accordingly, FCR negatively affect. this was confirmed by Mohammed et al. (2017) cleared that, 5% BW/day as the feeding rate had the best FCR followed by 7% BW/ day while the feeding rate 9% BW/day had the worst FCR.
Several works agrees with our finding and exhibited that the feeding rate significantly effect on growth characters of fish such as (Mohammed et al., 2017; El- Saied et al., 2015;Du et al., 2006). The improvement of Asian sea bass as result of increasing of feeding rate up to the optimum rate attribute to the boosting of feeding level encourage to increase the availability of resources of amino acids, fatty acids, vitamins and minerals, hence there is surplus resources of absorbed compound that will be stored as glycogen or lipid in liver or muscles. Furthermore, an increase of feeding level allow to increasing the opportunity to eat causing less aggressive and less of the lost energy as a result of crowding and competition through the feeding operation, this opinion was supported by Ashley-dejo et al. (2014) who explained that, dominant individuals are less aggressive under the optimum amount of feed whereas feed is distributed to locations occupied by subordinates.
Morphological indices as CF, HSI, and VSI are used to determinate the natural and physiological status, this indicates can provide evidence on development, health, reserves of energy and the potential of fish to survive ecological stress. In a bad, uncomfortable and critical environmental condition, fish typically liver loss more energy and is a smaller size. The statistical analysis of morphological indicates confirmed that this indicates were significantly increased with increasing the feeding rate. This observation was in agreements with (Iqbal et al., 2015, Mizanur et al., 2014, Zakęś et al., 2006) on the contrary (Mohammed et al., 2017) found that, HSI did not differ among the treatments by the changed of feeding level, in the same trend (Du et al., 2006) ascertain that HSI did not effect with increasing feeding rate.
In view of survival rate and cannibalism, the increasing feeding level up to 9% of BW/day adversely affected the survival rate but 6% BW/day and 4% BW/day had the best survival rate this may be due to the negative effects of overfeed that cause overload on the digestion system consequently a deterioration of fish health, hence increasing the mortality. On the other hand, low feeding rate contributes to reduce the growth and survival rate as a result of increasing the hunger, consequently increasing of the aggression and predation rate as it was appeared in T1. The results of survival rate in this study did not agree with Mohammed et al., 2017, Deyab and Hussein, 2015 they noted that survival rate of siganus rivulatus was insignificantly differed with the different of feeding rates.
In the present study cannibalism decreases with an increase in the feeding rate from group T2, T3 and T4 was no recorded cannibalism. Although, the survival rate was not different significantly but the highest survival was recorded in T2 and T3 group, similar findings was noted by (Sangeeta et al., 2018).
In the present research, the carcass composition (Table 4) was influenced by differences in the feeding rate. The moisture content was observed to be unchanged by the dietary level variability. Observance in terms of the impact of the feeding level of the entire body development, protein was not greatly influenced by the rate of feeding increased. However, lipid levels were significantly increased by increasing ration levels. Similar results were recorded by (Mohammed et al., 2017, Lee et al., 2016, Deyab and Hussein, 2015) they illustrated that, the whole fish body content of protein did not affected with increasing of feeding levels and body content of lipid significantly increased with increasing feeding levels. Moreover (Du et al., 2006) noted that, spadefish fed a higher level of feed had a high level of lipid ion their bodies and protein and Ash content were not affected. Conversely, Begum and Vijayaraghavan (2001) suggested that, fish content of protein increased to a high feeding level while fish content of lipid inversely related to the feeding level.
5. Conclusion
Under the prevailing environmental condition of Pakistan the results might suggest that, the optimum feeding rate for sea bass (Lates calcarifer) fingerlings with an average initial weight 5.47 g in a closed aquaculture system is 6% of biomass per day. But according to the polynomial regression the optimum feeding level for L. calcarifer is estimated as 6.5% per day for better growth performance. This rate for this species achieved a significant increase in growth rate, feed conversion ratio survival and prevention of cannibalism. Feeding regime has the most important to regular the feed offered, preserve the water quality and reduce the production cost of Asian sea bass farming.
Acknowledgements
The authors are grateful for the support received from FDB, MNFS, explicitly gratitude for Dr. Anser Chatta, Mr Junaid Wattoo, Mr. Khalid Mehmood and Mr. Mansoor for providing necessary technical guidance. We are indebted to the Department of Zoology (MRCC), University of Karachi for laboratory space.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ahmad N., Siddiqui P.J.A., Akbar N.U., Rashid M., Masroor R. The growth performance of juvenile yellow fin sea bream Acanthopagrus arabicus fed at different feeding rates while reared in floating net cages. J. Anim Plant Sci., 2018;28::1014–1020. [Google Scholar]
- Abbas G., Waryani B., Ghaffar A., Rahim A., Hafeez-ur-Rehman M., Aslam M. Effect of ration size and feeding frequency on growth, feed utilization, body composition and some haematological characteristics of juvenile snapper Lutjanus johnii. Pak. J. Zool., 2015;47::719–730. [Google Scholar]
- Adebayo O.T., Balogun A.M., Fagbenro O.A. Effects of feeding rates on growth, body composition and economic performance of juvenile clariid catfish hybrid Clarias gariepinus× Heterobranchus bidorsalis) J. Aquacult Trop., 2000;15::109–117. [Google Scholar]
- Al Zahrani, A.W., Mohamed A.H., Jr., Traifalgar, R.F.M. Effects of feeding rate and frequency on growth and feed utilization efficiency in the camouflage grouper Epinephelus polyphekadion fingerlings fed a commercial diet. Euro. J. Exp. Biol., 2013;3::596–601. [Google Scholar]
- Anderson, M., J., Fast, A.W., Temperature and feed rate effects on Chinese catfish, Clarias fuscus (Lacepede), growth. Aquac. Res., 1991;22(4):435–442. [Google Scholar]
- AOAC Assn. of Official Analytical Chemists., 2000. Coffee and tea. In: Official methods of analysis. 17th ed. Gaithersburg , Md. : AOAC.
- Anil M.K., Santhosh B., Jasmine S., Saleela K.N., George R.M., Kingsly H.J., Unnikrishnan C., Rao A.H., Rao G.S. Growth performance of the seabass Lates calcarifer in sea cage at Vizhinjam Bay along the southwest coast of India. Indian J. Fish. 2010;57::65–69. [Google Scholar]
- Ashley-dejo S.S., Olaoye O.J., Adelaja O.A., Abdulraheem I. Effects of feeding levels on growth performance feed utilization and body composition of African catfish (Clarias gariepinus, Burchell 1822) Int. J. Biol. Biolog. Sci., 2014;3::012–016. [Google Scholar]
- Aydin I., Küçük E., Sahin T., Kolotoglu L. The effect of feeding frequency and feeding rate on growth performance of juvenile black sea turbot Psetta maxima. J. Fishscicom., 2011;5::35–42. [Google Scholar]
- APHA (American Public Health Association), 1995. Standard methods for the examination of water and wastewater, 19th ed. American Public Health Association, Washington, DC.
- Begum G., Vijayaraghavan S. Carbofuran toxicity on total lipids and free fatty acids in air breathing fish during exposure and cessation of exposure–in vivo. Environ. Monit. Assess. 2001;70(3):233–239. doi: 10.1023/a:1010775224753. [DOI] [PubMed] [Google Scholar]
- Biswas G., Thirunavukkarasu A.R., Sundaray J.K., Kailasam M. Optimization of feeding frequency of Asian seabass (Lates calcarifer) fry reared in net cages under brackishwater environment. Aquacultulture. 2010;305:26–31. [Google Scholar]
- Björnsson B., Ólafsdóttir S.R. Effects of water use, water quality, blood physiology and growth rate of juvenile cod Gadus morhua. Aquaculture. 2006;256:255–263. [Google Scholar]
- Boonyaratpalin M. Nutrient requirements of marine feed fish cultured in Southeast Asia. Aquaculture. 1997;151:283–313. [Google Scholar]
- Bureau D.P., Hua K., Cho C.Y. Effect of feeding level on growth and nutrient deposition in rainbow trout Oncorhynchus mykiss Walbaum growing from 150 to 600 g. Aquac. Res. 2006;37:1090–1098. [Google Scholar]
- Daet I. Study on culture of sea bass Lates calcarifer inhapa-in-pond environment. Earth Environ. Sci. 2019;230:1755–11315. [Google Scholar]
- Davies O.A., Inko-Tariah M.B., Amachree D. Growth response and survival of Heterobranchus longifilis fingerlings fed at different feeding frequencies. Afr. J. Biotechnol., 2006;5:778–787. [Google Scholar]
- Deyab E.D.M., Hussein E.E.M. Effects of Different Feeding Rates on Growth Performance and Body Composition of Red Tilapia, Oreochromis mossambiquse x O. niloticus Fingerlings. Int. J. Aquacult., 2015;5::1–7. [Google Scholar]
- Du Z.Y., Liu Y.J., Tian L.X., He J.G., Cao J.M., Liang G.Y. The influence of feeding rate on growth, feed efficiency and body composition of juvenile grass carp (Ctenopharyngodon idella) Aquacult. Int., 2006;14::247–257. [Google Scholar]
- El-Sayed A.F.M. Alternative dietary protein sources for farmed tilapia, Oreochromisspp. Aquaculture, 1999;179:149–168. [Google Scholar]
- Goddard S. Chapman and Hall; New York: 1995. Feed Management in Intensive Aquaculture; p. 194. [Google Scholar]
- Hassan H.U., Ali Q.M., Rahman M.A., Kamal M., Tanjin S., Farooq U., Mawa Z., Badshah N., Mahmood K., Hasan M.R., Gabool K., Rima F.A., Islam M.A., Rahman O., Hossain M.Y. Growth pattern, condition and prey-predator status of 9 fish species from the Arabian Sea (Baluchistan and Sindh), Pakistan. Egyptian J. Aquatic Biol. Fisheries, 2020;24::281–292. [Google Scholar]
- Iqbal K.J., Ashraf M., Qureshi N.A., Javid A., Abbas F., Hafeez-ur-Rehman M., Abbas S. Optimizing growth potential of Labeo rohita fingerlings fed on different plant origin feeds. Pak. J. Zool., 2015;47(1):31–36. [Google Scholar]
- Kalogeropoulos N., Alexis M.N., Henderson R.J. Effects of dietary soybean and cod-liver oil levels on growth and body composition of gilthead bream Sparus aurata. Aquaculture, 1992;104:293–308. [Google Scholar]
- Lee S., Haller L.Y., Fangue N.A., Fadel J.G., Hung S.S.O. Effects of feeding rate on growth performance and nutrient partitioning of young-of-the-year white sturgeon Acipenser transmontanus. Aquac. Nutr. 2016;22(2):400–409. [Google Scholar]
- Marimuthu K., Umah R., Muralikrishnan S., Xavier R., Kathiresan S. Effect of different feed application rate on growth, survival and cannibalism of African catfish, Clarias gariepinus fingerlings. Emirates J. Food Agri. 2011;23:330–337. [Google Scholar]
- Mizanur R.M., Yun H., Moniruzzaman M., Ferreira F., Kim K.W., Bai S.C. Effects of feeding rate and water temperature on growth and body composition of juvenile Korean rockfish, Sebastes schlegeli (Hilgendorf 1880) Asian-Australasian J. Anim. Sci., 2014;27::690–699. doi: 10.5713/ajas.2013.13508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed R.A., Abdel-Aziz M.F.A., Abou-Zied R.M., Allam S.M. Effect of feeding rate and diet oil source on growth performance and feed utilization of Rabbitfish Siganus rivulatus Fry. J. Fish. Aqua. Dev. 2017;123 https://doi.org/10.29011.JFAD-123/100023. [Google Scholar]
- Ng W.K., Lu K.S., Hashim R., Ali A. Effects of feeding rate on growth, feed utilizationand body composition of a tropical bagrid catfish. Aquacult. Int., 2000;8:19–29. [Google Scholar]
- NRC . National Research Council National Academy Press, Washington D; C., USA: 1993. Nutrient requirements of fish. [Google Scholar]
- Puvanendran V., Boyce D.L., Brown J.A. Food ration requirements of yellowtail flounder Limanda ferruginea juveniles. Aquaculture, 2003;220:459–475. [Google Scholar]
- Sangeeta K., Tiwari V.K., Rani A.M.B., Rajesh K., Satya P. Effect of feeding rate on growth, survival and cannibalism in striped snakehead Channa striata fingerlings. J. Exp. Zool. India, 2018;21:205–210. [Google Scholar]
- Santos G.A., Schrama J.W., Mamauag R.E.P., Rombout J.H.W.M., Verreth J.A.J. Chronic stress impairsperformance, energy metabolism and welfare indicators in European seabass Dicentrarchus labrax: The combined effects of fish crowding and water quality deterioration. Aquaculture. 2010;299:73–80. [Google Scholar]
- Silva C.R., Gomes L.C., Brandão F.R. Effect of feeding rate and frequency on tambaqui Colossoma macropomum growth, production and feeding costs during the first growth phase in cages. Aquaculture, 2007;264:135–139. [Google Scholar]
- Singh R.K., Vartak V.R., Balange A.K., Chavan J.B. The effect of feeding te on the growth, food conversion protein efficiency of Silver dollar Metynnis schreitmulleri fry. J. Indian Fisheries Assoc., 2003;30:105–118. [Google Scholar]
- Sorphea S., Terai A., Sreyrum P., Lundh T., Barnes A.C., Da C.T., Kiessling A. Growth performance of fry and fingerling Asian Seabass Lates calcarife from Cambodian brood stock reared at different salinities. Livestock Res. Rural Dev., 2019;31:1–8. [Google Scholar]
- Szkudlarek M., Zakęś Z. Effect of stocking density on survival and growth performance of pikeperch, Sander lucioperca (L.), larvae under controlled conditions. Aquacult. Int., 2007;15::67–81. [Google Scholar]
- Tacon A.G.J., Metian M. Global overview on the use of fish meal and fish oil in industrially compounded aquafeeds: Trends and future prospects. Aquaculture, 2008;285:146–158. [Google Scholar]
- Trushenski J., Rombenso A., Schwarz M.H., Bowzer J., Gause B., Delbos B., Sampaio L.A. Feeding rate and frequency affect growth of juvenile Atlantic spadefish. North American J. Aquacult., 2012;74:107–112. [Google Scholar]
- Tucker J.W., Russell D.J., Rimmer M.A. Barramundi culture: a success story for aquaculture in Asia and Australia. World Aquacult., 2002;33:53–59. [Google Scholar]
- Venkatachalam S., Kandasamy K., Krishnamoorthy I., Narayanasamy R. Survival and growth of fish Lates calcarifer under integrated mangrove aquaculture and open-aquaculture systems. Aquaculture, 2018;9:18–24. [Google Scholar]
- Wang N., Hayward R.S., Noltie D.B. Effect of feeding frequency on food consumption, growth, size, variation, and feeding pattern of age-0 hybrid sunfish. Aquaculture. 1998;165:261–267. [Google Scholar]
- Zakęś Z., Kowalska A., Czerniak S., Demska-Zakęś K. Effect of feeding frequency on growth and size variation in juvenile pikeperch, Sander lucioperca (L.) Czech J. Animal Sci., 2006;51(2):85–91. [Google Scholar]
- Ganzon-Naret E.S. Growth response and feed intake of Lates calcarifer to four different dietary protein levels with green pea Pisum sativum under controlled laboratory condition. Anim. Biol. Anim. Husb. 2013;5(2):137–144. [Google Scholar]
Further reading
- El-Sayed A.F.M., Dickson M.W., El-Naggar, G.O. Value chain analysis of the aquaculture feed sector in Egypt. Aquaculture, 2015;437::92–101. [Google Scholar]