Abstract
Improving grain filling in the presernt farming systems is crucial where grain filling is a concern due to the extreme use of chemical fertilizers (CF). A field experiment was conducted at the experimental station of Guangxi University, China in 2019 to test the hypothesis that cattle manure (CM) and poultry manure (PM) combined with CF could improve rice grain filling rate, yield, biochemical and qualitative attributes. A total of six treatments, i.e., no fertilizer (T1), 100% CF (T2), 60% CM + 40% CF (T3), 30% CM + 70% CF (T4), 60% PM + 40% CF (T5), and 30% PM + 70% CF (T6) were used in this study. Results showed that the combined treatment T6increased starch metabolizing enzymes activity (SMEs), such as ADP-glucose phosphorylase (ADGPase) by 8 and 12%, soluble starch synthase (SSS) by 7 and 10%, granule bound starch synthesis (GBSS) by 7 and 9%, and starch branching enzyme (SBE) by 14 and 21% in the early and late seasons, respectively, compared with T2. Similarly, higher rice grain yield, grain filling rate, starch, and amylose content were also recorded in combined treatments. In terms of seasons, higher activity of SMEs , grain starch, and amylose content was noted in the late-season compared to the early season. The increment in these traits was mainly attributed to a lower temperature in the late season during the grain filling period. Furthermore, our results suggested that an increment in starch accumulation and grain filling rate were mainly associated with the enhanced sink capacity by regulating key enzyme activities involved in Suc-to-starch conversion. In-addition, RT-qPCR analysis showed higher expression levels of AGPS2b, SSS1, GBSS1, and GBSE11b genes, which resultantly increased the activities of SMEs during the grain filling period under combined treatments. Linear regression analysis revealed that the activity of ADGPase, SSS, GBSS, and SBE were highly positively correlated with starch and amylose accumulation. Thus, we concluded that a combination of 30% N from PM or CM with 70% N from CF is a promising option in terms of improving rice grain yield and quality. Our study provides a sustainable fertilizer management strategy to enhance rice grain yield and quality at the lowest environmental cost.
Keywords: Rice, Starch synthesis, Enzyme, Amylose content, Grain yield, Temperature
Abbreviations: N, nitrogen; CF, chemical fertilizer; PM, poultry manure; CM, cattle manure; DAA, days after anthesis; AGPase, ADP-glucose pyrophosphorylase; GBBS, granule bound starch synthase; SSS, soluble starch synthase; SBE, starch branching enzyme; DBE, starch debranching enzyme; SS, sucrose synthase; AC, amylose content
1. Introduction
Nitrogen (N) has a significant impact on plant growth and production under various environmental conditions (Leghari et al., 2016a, Leghari et al., 2016b). The application of N via CF is the most dominant source of N in crop production (Akhtar et al., 2019, Tao et al., 2015). However, the excessive use of CF adversely affects soil health, the environment, and crop production (Peng et al., 2015). Hence, there is a rising conflict among enhancing food demand and insufficient fertilizer resources; thus, recently rising crop production and quality at the lowest environmental cost is a global challenge. Alternatively, organic manure (OM) holds great promise not only to maintain crop production but also to boost soil fertility and to protect the environment (Iqbal et al., 2019, Akhtar et al., 2019). Similarly, other studies also reported that OM fertilization improves arable soil physicochemical properties (Yue et al., 2016, Khan et al., 2019). In-addition, the slow and steady release of nutrients from OM is a benefit to sole CF in achieving higher N use efficiency, cereals crop yield, and quality (Kumar et al., 2018, Zhang et al., 2018). However, OM is quite low in plant nutrients and nutrients releasing capability is also slow to meet the plant nutritional requirements in a short time. Therefore, OM combined with CF has been reported as a better approach to maintain soil health and crop productivity (Iqbal et al., 2019, Ali et al., 2020, Iqbal et al., 2020). Although the advantages of OM combined with CF are generally recognized, however, the optimal substation ratio remains unclear.
Rice (Oryza sativa L.) provides 30–60% of the calories eaten by more than 4 billion Asians. Starch in rice endosperm comprises about 75 to 90% of the dry weight, is consisting of two forms of molecules, amylopectin and amylose. Amylopectin is the major starch element in rice endosperm, usually about 75–80% and reaches almost 100% in waxy rice genotypes, as well as a highly branched polymer consisting of linear chains of α-1,4-linked glucose residues, which are connected by α-1,6-linkages (Nakamura et al., 1996, Robyt, 2008). Amylose consists of short and usually unbranched chains, consisting mostly of 500–20000 α-1, 4-D-glucose units. Rice physicochemical features and particularly nutritional quality are primarily measured by the ratio of amylose to amylopectin (Chen et al., 2019, Nakamura et al., 2002). The formation of amylose is catalyzed by ADP-glucose pyrophosphorylase (AGPase) and granule bound starch synthase (GBBS) enzymes, which is encoded by the Waxy gene (Zi et al., 2018, Fulton et al., 2002). Amylopectin is synthesized by a coordinated reaction catalyzed by AGPase, soluble starch synthase (SSS), starch branching enzyme (SBE), and starch debranching enzyme (DBE) (Myers et al., 2000, Smith and Denyer, 1997). SBE catalyzes the creation of branch points within the glucan chains by cleaving an α-1,4 linkage and re-attaching the chain to the glucan chain through α-1,6 bound (Peat et al., 1952). Sucrose synthase (SS) is the primary enzyme responsible for sucrose cleavage in sucrose-to-starch biosynthesis in rice endosperm (Beck and Ziegler 1989). Sucrose synthesis is the first step in the starch synthesis pathway and the activity of SS enzyme has been associated to sink in developing grain (Joen et al., 2010). The starch-metabolizing enzyme (SMEs), such as AGPase, GBBS, SSS, SBE, and DBE activities were found strongly associated with the starch composition and amylose content (Li et al., 2019).
It is well reported that rice grain starch structure, content, and resulting characteristics are significantly influenced by the environment, i.e., geographical location, N fertilizer, temperature, and soil fertility status (Patindol et al., 2015, Beckles and Thitisaksakul, 2014). Nitrogen N plays a key role in many physiological processes in plants, grain yields, and quality (Plett et al., 2020). The accumulation of grain starch was closely correlated with the rate and timing of N application, and the optimum level of N increased the accumulation of grain starch (Zhou et al., 2020, Yang et al., 2020). In the present farming system, the overuse of synthetic N fertilizers is not conducive to starch accumulation and does not result in optimum yields (Guo et al., 2017). Further, the activities of SMEs are also influenced by the amount of N applied to crops (Tang et al., 2019). These studies indicate that N affects starch synthesis by changing the enzyme's activity and relevant gene expression involved in starch accumulation. However, we do not yet have a full understanding of the mechanisms underlying combined organic and inorganic N fertilization. Therefore, in the present farming, improving grain-filling is very crucial in a case where grain-filling is a concern due to the excessive use of synthetic N fertilizer (Ma et al., 2019).
In-addition, grain starch accumulation, and physico-chemical properties are also affected by air temperature (Lin et al., 2020, Yao et al., 2020a, Yao et al., 2020b). In certain cases, the higher temperature during the ripening period declines grain-filling duration, however, enhances dry matter accumulation of the grain (Fujita et al., 2011). Previously, it has been noted in cereals crop particularly in rice that for every 1 °C temperature increase, the grain-filling period has been reduced by about 3 days (Fitzgerald and Resurreccion, 2009). Amylose content (AC) is considered to be a significant factor affecting the grain quality of rice. Currently, various hypotheses are reported about the impact of temperature on AC: 1) high-temperature reduces AC (Lin et al., 2020); 2) high-temperature rise AC (Zhang et al., 2013); and 3) the impact of temperature on AC is cultivar-dependent (Cheng et al., 2005a, Cheng et al., 2005b). Moreover, Deng et al. (2015) stated that the optimum daily temperature for rice grain filling ranged from 22 to 27 °C, and an increase in temperature negatively affect grain formation. The changes in starch and AC were mainly due to the impact of temperature on the activity of SMEs involved in starch biosynthesis (Ahmed et al., 2015).
Previously, many studies have been conducted on the influence of the evaluated temperature on the activity of starch biosynthesis-related enzymes, almost nothing has been reported on the difference in temperature under dual cropping systems (especially: early and late growing seasons) in southern China. Furthermore, information regarding the combined effect of CM or PM and CF on the SMEs activities and grain starch accumulation is lacking. Therefore, it was assumed for the present study that organic fertilizer combined with synthetic fertilizer could improve rice growth, grain yield, starch, and amylose content by improving SMEs a during the grain-filling period. The objectives of this study are: (1) to assess the integrated effect of organic and inorganic N fertilizers on rice growth, yield, and yield components; (2) to determine the combined effect of manure and CFs on grain starch metabolizing enzymes activity and related genes expression; (3) to evaluate the effect of combined fertilization on grain starch and amylose content.
2. Materials and methods
2.1. Experimental location
The study was conducted at the research farm of Guangxi University, China, during the early (March to July) and was repeated in the late (July to November) growing seasons in 2019. The climate is classified as a subtropical monsoon region and an average annual rainfall of 1080 mm. The average maximum and minimum temperature was 32.5 °C and 23.4 °C during the early season and 30.2 °C and 21.0 °C during the late season (Table 1) (local weather station). The soil graded as Ultisols and was slightly acidic having pH 5.94 H2O, soil organic carbon (SOC) 15.10 g kg−1, soil organic matter 25.8 g kg−1, total N (TN) 1.35 g kg−1, available N (AN) 134.7 mg kg−1, available phosphorous (AP) 23.1 mg kg−1, and available potassium (AK) 233.6 mg kg−1, with 1.36 g cm−3 soil bulk density (BD) (Table 2).
Table 1.
Mean maximum and minimum temperature, relative humidity, and the total rainfall during both growing seasons.
| Maximum | Minimum | Relative | Total | |
|---|---|---|---|---|
| Months | Temperature (°C) | Temperature (°C) | Humidity (%) | Rainfall (mm) |
| March | 24.2 | 18.6 | 83 | 72 |
| April | 29.4 | 23.5 | 76 | 92 |
| May | 33.5 | 25.6 | 83 | 176 |
| June | 35.5 | 25.7 | 81 | 211 |
| July | 36.4 | 26.6 | 82 | 231 |
| August | 35.3 | 25.4 | 80 | 151 |
| September | 33.4 | 25.3 | 79 | 115 |
| October | 30.6 | 24.4 | 82 | 98 |
| November | 27.1 | 20.2 | 87 | 110 |
Table 2.
Physical and chemical properties of soil and manure before the experimentation.
| Properties | Cattle | Poultry | |
|---|---|---|---|
| Soil | Manure | Manure | |
| Porosity (%) | 40.52 | – | – |
| Moisture content (%) | 11.93 | – | – |
| Bulk density (g cm−3) | 1.36 | 0.81 | 0.74 |
| pH (water) | 5.94 | 7.75 | 7.95 |
| SOC (g kg−1) | 14.56 | 146.33 | 164.22 |
| SOM (g kg−1) | 25.08 | 254.63 | 282.42 |
| Total N (g kg−1) | 1.41 | 9.76 | 13.58 |
| Total P (g kg−1) | 0.75 | 10.12 | 7.32 |
| Total K (g kg−1) | – | 14.22 | 9.76 |
| Available N (mg kg−1) | 134.7 | – | – |
| Available P (mg kg−1) | 23.12 | – | – |
| Available K (mg kg−1) | 233.3 | – | – |
Note: SOC—soil organic carbon, SOM—soil organic matter, N—nitrogen, P—phosphorous, K—potassium, C: N—carbon to nitrogen ratio.
2.2. Experimental setup
A field experiment was performed in a randomized complete block design (RCBD) having three replication with a plot size of 3.9 m × 6 m (23.4 m2). Organic fertilizer, such as PM and CM and inorganic fertilizer, such as urea was used in this experiment. The experiment consisted of six treatments i.e., no N fertilizer (T1); 100% CF (T2); 60% CM + 40% CF (T3); 30% CM + %70 CF (T4); 60% PM + 40% CF (T5), and 30% PM + 70% CF (T6). Noodle rice “Zhenguiai” was used as a test crop. Rice grains were sown in plastic trays and 25 days old seedlings were transplanted to the field and two seedlings were planted per hill. The recommended dose of nitrogen, phosphorus, and potassium (NPK) was 150:75:150 (kg ha−1), and every plot received 175.5 g (superphosphate), 365 g (potassium chloride), and 351 g of N from PM or CM and CF (urea). The nutrient content of organic fertilizer and the quantity of all treatments are shown in Table 2, Table 3, respectively. Nitrogen and potassium chloride were applied in three splits, i.e., one-day before transplantation (60%), tillering (20%), and the panicle stage (20%), while all superphosphate was used as a basal dose (Table 3). Organic fertilizer, such as, CM and PM were applied to the field twenty days before transplantation. No N fertilizer was applied to the T1 plot, while other recommended fertilizers such as phosphorous and potassium were applied equally to all treatment of the group. Standard flood water was provided to a depth of approximately 4 cm from transplantation to physiological maturity. All other agronomic practices were done uniformly to all treatments.
Table 3.
Nutrient content and the amount of nutrients provided to each treatment and application time.
| Treatment | N (g plot−1) | Urea (g plot−1) | CM or PM (kg plot−1) |
Basal fertilization (kg plot−1) |
Tillering (g plot−1) |
Panicle initiation (g plot−1) |
|---|---|---|---|---|---|---|
| T1: No-N | 0 | 0 | 0 | P2O2: 0.93, KCl: 0.30 | KCl: 0.30 | Urea: 0 |
| T2: 100% CF | 351 | 753 | 0 | Urea: 0.45, P2O2: 0.93,KCl: 0.30 |
Urea: 150, KCl: 0.30 |
Urea: 150 |
| T3: 60% CM + 40% CF | 351 | 301 | 21.5 | Urea: 0, CM: 21.50, P2O2: 0.93, KCl: 0.30 |
Urea: 150, KCl: 0.30 |
Urea: 150 |
| T4: 30% CM + 70% CF | 351 | 527 | 10.7 | Urea:0.22, CM: 10.70, P2O2: 0.93 s, KCl: 0.30 |
Urea: 150, KCl: 0.30 |
Urea: 150 |
| T5: 60% PM + 40% CF | 351 | 301 | 15.5 | Urea: 0, PM: 15.5, P2O2: 0.93, KCl: 0.30 |
Urea: 150, KCl: 0.30 |
Urea: 150 |
| T6: 30% PM + 70% CF | 351 | 527 | 7.7 | Urea: 0.22, PM: 7.70, P2O2: 0.93, KCl: 0.30 |
Urea: 150, KCl: 0.30 |
Urea: 150 |
Note: N—nitrogen, CF—chemical fertilizer (urea), CM—cattle manure, PM—poultry manure, P2O2—superphosphate, KCl—potassium chloride.
2.3. Sampling and analysis
2.3.1. Soil and organic manure
Subsamples of soil and organic manure were taken before the experiment to assess the physical and chemical properties. Soil organic carbon (SOC) was measured using the oxidation method. Subsoil samples (0.5 g) were digested with 5 ml of 1 M K2Cr2O7-H2SO4 and 5 ml of concentrated H2SO4 and boiled at 170 °C for 6 min, accompanied by titration of FeSO4 according to the method of Bao, (2000). For total soil N content, 200 mg soil was digested according to the salicylic acid sulfuric acid hydrogen peroxide method of Ohyama et al. (2004), and then TN was analyzed according to the micro-Kjeldahl method (Jackson and Wisconsin, 1956). Further, TP was determined by ascorbic acid as suggested by Murphy and Riley (1962), and TK was measured using an atomic absorption spectrophotometer (Z-5300; Hitachi, Tokyo, Japan) after the samples were digested at 7665 R wavelength.
2.4. Expression analysis of starch metabolism-related isoform genes
2.4.1. Total RNA extraction and RT-qPCR
Rice grains samples were collected from every treatment and immediately immersed in liquid N and placed at −80 ℃ for the extraction of RNA. Three biological replicates were employed per sample. RNA was obtained by the RNA Pure plant Kit (TIANGEN, 432, Beijing, China). The first-strand cDNA synthesis was performed by the manufacturer's instructions, with 1 μg RNA with HiScript III-RT SuperMix for qPCR (gDNA wiper) (Vazyme, R323-01, Nanjing, China). Furthermore, the RT-qPCR was conducted on Applied Biosystems QuantStudio3 real-time PCR machine on a total reaction volume of 20 μL reaction having 10 μL Cham QTM Universal SYBR qPCR Mix Master (Q711-02/03, Naning, China), 0.4 μL forward primer, 0.4 μL reverse primer, 1 μL diluted cDNA and 8.2 μL RNase-Free ddH20. The specific primer pairs for gene used in the present study are shown in Table 4. The amplification of different genes was normalized through ACTIN gene expression (used as a reference internal control gene). The RT-qPCR R R data were analyzed using the the 2-ΔΔCT method of Livak and Thomas (2001).
Table 4.
Primers design of each gene selected in this experiment.
| Genes | Accession No. |
Primers | Sequences(5′-3′) | Amplified fragment length(bp) |
|---|---|---|---|---|
| Actin | Forward | CCACTATGTTCCCTGGCATT | 178 | |
| Reverse | GTACTCAGCCTTGGCAATCC | |||
| SBEIIb | LOC-Os02g32660.1 | Forward | GCCGCAGGAGAAATCCCATA | 150 |
| Reverse | GTTGATCTTTGGCTCCGTGC | |||
| GBSSI | LOC-Os06g04200.3 | Forward | GGGGAAAGACCGGTGAGAAG | 236 |
| Reverse | GATGCCATTGGGCTGGTAGT | |||
| ISA1 | LOC-Os08g40930.1 | Forward | GCTGGTGGTTTCGCTGAATG | 203 |
| Reverse | CCACAGTTCCAGCTGAGGTT | |||
| SSSI | LOC-Os06g06560.1 | Forward | CCAGTCTTGTGCCAGTCCTT | 220 |
| Reverse | TTGACTGCCTCACCCTTGTC | |||
| AGPS2b | LOC-Os08g25734.2 | Forward | TCTTGACCGCAGTGTCGATG | 228 |
| Reverse | GCTCTTGACAGGTGACGGTT | |||
| SUS4 | LOC-Os03g22120.1 | Forward | GGCCAGTACAACGATCCGTA | 198 |
| Reverse | ATGACCCTTATGCCGATGCC |
2.5. Starch metabolizing enzyme activities
2.5.1. Preparation of enzyme extraction
The enzyme extraction solution was prepared according to the method of Nakamura et al., 1989a, Nakamura et al., 1989b. Twenty frozen grains samples were weighted and homogenized in an ice-cold mortar and pestle, that contains 5 ml of buffer solution (pH 7.5, 100 mM HEPES NaOH, 8 mM MgCL2, 2 mmol/L EDTA, 50mMl 2-mercaptoethanol, glycerol (v/v) 12.5% and insoluble polyvinylpyrrolidone-40) (w/v) 5%. Firstly, 1.8 ml of the buffer solution, 30 µL of homogenate added, which was then balanced in 2 ml of the buffer solution for GBSS activity. The rest of the homogenate solution was centrifuged at 1000g for 5 min at 0–4 °C and the resulted supernatant was further used for enzymes unless otherwise stated. The assay of AGPase, SSS, SBE, and DBE was determined by the method proposed by Nakamura et al., 1989a, Nakamura et al., 1989b.
2.5.2. Measurements of the starch-metabolizing enzyme's
Twenty rice grains were taken every seven days interval after anthesis during the grain-filling period. GBSS, AGPase, and SSS activity were measured according to the methods of Nakamura et al., 1989a, Nakamura et al., 1989b. SBE and DBE activities were assayed according to the procedures of Li et al., 1997, Nakamura et al., 1989a, Nakamura et al., 1989b, respectively. Further, sucrose synthase was measured according to Wardlaw and Willenbrink (1994).
2.5.3. Starch, amylose and amylopectin measurement
The total grain starch, amylose, and amylopectin contents were measured via the dual-wavelength iodine binding method according to Zhu et al., 2008, He, 1985. Rice grains were ground using a mortar, and the powder was then degreased two times with anhydrous ether. Further, a 100 mg fraction of each sample was used to measure AC and amylopectin content. A calibration curve was derived using pure amylose from potato (A0512; Sigma–Aldrich, St. Louis, MO, USA) and pure amylopectin from potato (A8515; Sigma–Aldrich). The sum of amylose and amylopectin contents was designated as the total starch content.
2.6. Statistical analysis
The collected data on plant growth, yield, grain qualitative and biochemical attributes of rice were analyzed according to the ANOVA techniques relevant to RCB design using Statistics 8.1. The collected data were first checked by the normality test. Data in percentage were arcsine transformed to normalize the variables before analysis. The analysis was conducted combined over the seasons, to detect differences between seasons (having different temperature) in addition to the fertilizer treatments. The experiment consisted of a single factor (i.e., fertilizer treatments were a fixed factor); however, it was repeated for the following season, thus season was also considered as a repetitive measured factor and also a fixed effect. Similarly, the interaction between fertilizer treatments and seasons was taken as a fixed effect. However, the interaction of seasons and treatments with replications was taken as a random effect. The means were separated using the least significant difference (LSD) test at p < 0.05. Linear regression analysis was performed to evaluate the relationship between starch metabolizing enzyme activities and qualitative traits.
3. Results
3.1. Meteorological data
The average monthly temperature ranged from 21.7 to 31.4 °C in the early season and from 20.7 to 30.4 °C in the late season was recorded at the experimental station Nanning, Guangxi University (Table 1). The maximum mean temperature 30.05 °C was noted in the early season during grain filling in June and July (early season), while the minimum means temperature 25.5 °C was recorded during the grain filling time in October and November (late season) as shown in Table 1. The meteorological data suggested that there was a large difference in the temperature during the grain filling period of the early and late growing seasons.
3.2. Grain starch metabolizing enzyme activities
3.2.1. Sucrose synthase (SS)
The combined organic manure and CF fertilization as well as the different seasons had a significant effect on SS activity (Fig. 1). The SS enzyme activity exhibited a single-peak trend and the SS enzyme activity increased at the early repining phase and then slowly decline in the late grain filling phase. The highest peak SS enzyme activity occurred at 14 days after anthesis (DAA). Compared to sole CF (T2), the integrated treatment T3 increased mean SS enzyme activity by 19% and 16% during the early and the late-season across the grain-filling period, respectively. However, the combined treatments, i.e., T4, T5, and T6 were statistically similar to T3. The lowest SS enzyme activity was recorded in non-N treated plots during both seasons. In the case of seasons, the SS activity was noted maximum in the late compared to the early season.
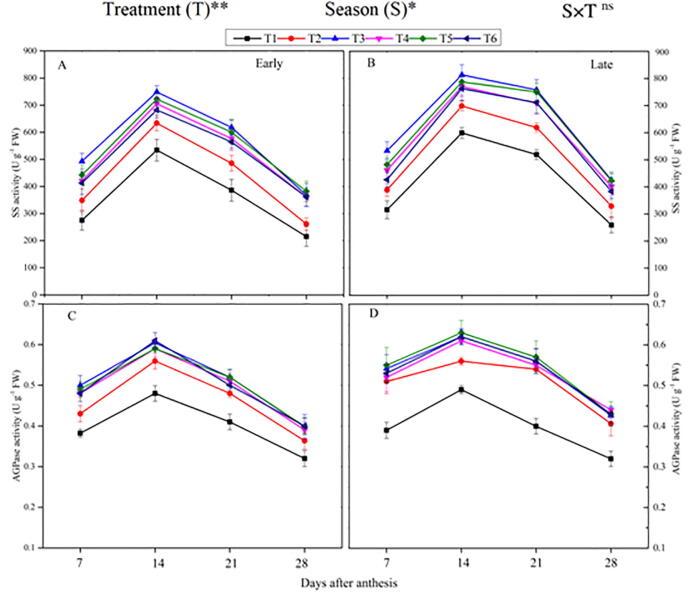
Fig. 1.
Changes in the activity of sucrose (SS) enzyme during early (A) and late-season (B) and adenosine diphosphate glucose (AGPase) during early (C) and late season (D) in rice grains under the combination organic and inorganic N fertilization. Vertical bars represent the standard error of the mean. The mean comparison was made using least significant differences (LSD) test for treatments and seasons means based on the LSD test at 5%. ns = non-significant and *,** = significant at 5% and 1%, respectively. Note: T1— no N fertilizer, T2— 100% CF, T3—60% CM + 40% CF, T4—30% CM + 70% CF, T5— 60% PM + 40% CF, T6—30% PM + 70% CF. Note: CF—chemical fertilizer, CM—cattle manure, PM—poultry manure.
3.2.2. ADP glucose phosphorylase (ADGPase) enzyme activity
The co-applied application of organic and inorganic N fertilizers and different seasons significantly affected the ADGPase activity (Fig. 1). ADGPase activity showed a single-peak curve and increased in the early grain filling and then decline in the late-ripening phase. The highest peak activity of ADGPase occurred at 14 DAA. Compared with T2, across the grain filling period, mean higher ADGPase activity by 9% and 11.3% were recorded in T3, respectively, in the early and the late seasons. However, T4, T5, and T6 were comparable (p < 0.05) to T3. The lowest ADGPase enzyme activity in both seasons was noted in non-N applied plants. In-addition, the ADPG activity was observed lower in the early-season compared to the late-season.
3.2.3. Soluble starch synthase (SSS) enzyme activity
The combination of organic and synthetic fertilizer, as well as different seasons, had significantly influenced the SSS activity (Fig. 2). The activity of the SSS enzyme in both seasons revealed a single peak trend highest at 14 DAA. Across the milking phase, the activity of the SSS enzyme considerably superior in the combined organic and inorganic N treatments than the sole CF. Relative to T2, the combined treatment T3 improved SSS enzyme activity by 7.4% and 10.3%, respectively in the early and late season. However, T3 was observed statistically non-significant with T4, T5, and T6. In-addition, the ADPG activity was found greater in the late-season as compared to the early season.
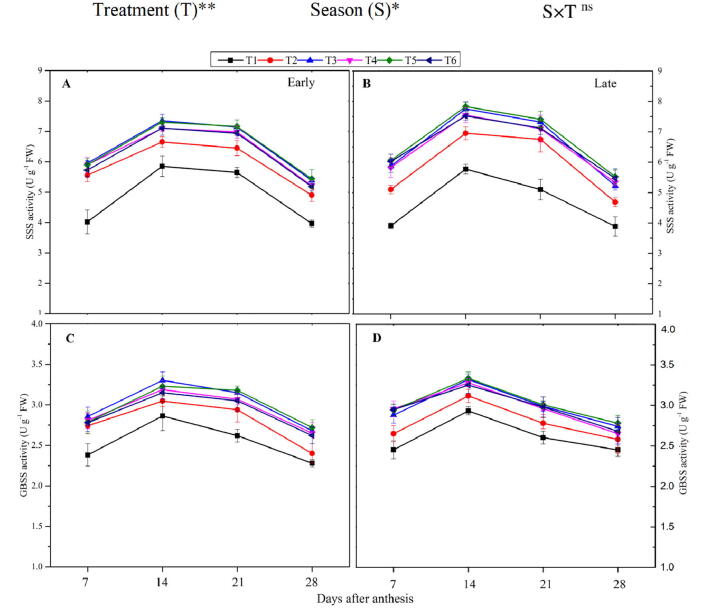
Fig. 2.
Changes in the activity of soluble starch synthesis (SSS) enzyme during the early (A) and late-season (B) and granule bound starch synthesis (GBSS) during the early (C) and late season (D) in rice grains under the combination organic and inorganic N fertilization. Vertical bars represent the standard error of the mean. The mean comparison was made using the least significant differences (LSD) test for treatments and seasons means based on the LSD test at 5%. ns = non-significant and *,** = significant at 5% and 1%, respectively.
3.2.4. Granule bound starch synthesis (GBSS) enzyme activity
The combined use of synthetic and organic N fertilizers and the different seasons had a substantial effect on GBSS activity (Fig. 2) in the present study. The GBSS enzyme showed a single-peak curve and enhanced in the early stage and decreased in the late grain filling stage. The maximum peak activity of the GBSS was noted at 14 DAA in both seasons. The averaged value of the GBSS in the grain filling, the treatment T3 increased GBSS activity by 7.3% and 9.6% in the early and late seasons, respectively, compared to the T2. However, T4, T5, and T6 were comparable (p < 0.05) to T3. The lowest activity of the GBSS was recorded in non-N treated plants. In the case of different seasons, higher GBSS activity was noted in the late season as compared to the early season.
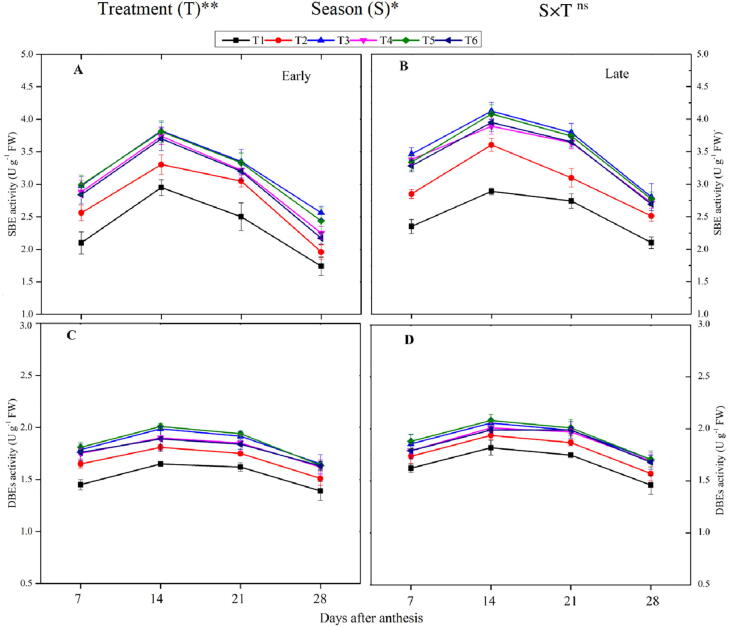
3.2.5. Starch-branching enzymeand starch debranching enzyme
The combined application of organic and inorganic fertilizer, as well as the different seasons, significantly influenced the SBE and DBE activity (Fig. 3). The activity of SBE and DBEs increased during the early ripening phase and then decreased at the late-ripening phase. The higher activity of the SBE and DBEs were noted at 14 DAA and then declined across the seasons. Across the grain filling period, T3 increased the activity of SBE by 16% and 24%, and DBEs by 6.4% and 8.6%, respectively, during the early and late season, respectively, compared to T2. However, T4, T5, and T6 were comparable (p < 0.05) to T3. The lowest activity of SBE and DBEs were recorded in non-N applied plots. Further, in the case of different seasons, maximum GBSS activity was found in the late growing season as compared to the early-season.
Fig. 3.
Changes in activities of starch branching enzyme (SBE) during the early (A) and late-season (B) and starch debranching enzyme (DBEs) during the early (C) and late season (D) in rice grains under the combination organic and inorganic N fertilization. Vertical bars represent the standard error of the mean. The mean comparison was made using least significant differences (LSD) test for treatments and seasons means based on the LSD test at 5%. ns = non-significant and *,** = significant at 5% and 1%, respectively.
3.3. Expression patterns of targeted genes related to starch metabolism
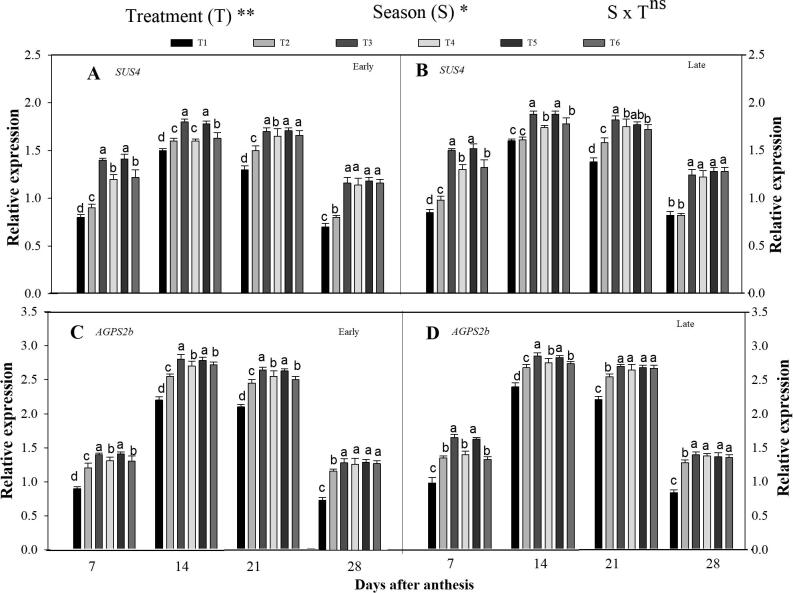
3.3.1. Expression level of SUS4 and AGPS2b
Fig. 4 shows that the SUS4 and AGPS2b were highly expressed during the grain-filling period under the combined organic and inorganic N. Further, the relative expression of genes was also noted significant among the seasons (Fig. 4). The gene expression pattern enhanced in the early ripening phase and then reduced in the late repining phase. Across the grain-filling period, in combined treatment (T3) higher relative expression of SUS4 by 23 and 15%, and AGPS2b by 14 and 12% was recorded, respectively in the early and late seasons, compared to T2. However, T3 was statistically comparable with T5. Additionally, the expression level of SUS4 and AGPS2b was also higher in T4 and T6. Moreover, in the case of seasons, SUS4 and AGPS2b genes were highly expressed in the late-season compared with the early season.
Fig. 4.
Changes in relative expression patterns of starch metabolism erelated genes [SUS4 (A ,B) and AGPS2b (C, D)] in the early and late seasons in developing rice endosperm as affected by combined organic and inorganic N fertilization. Letter a—d indicates difference is significant at 0.05 level. The mean comparison was made using the least significant differences (LSD) test for treatments and seasons means based on the LSD test at 5%. ns = non-significant and *,** = significant at 5% and 1%, respectively.
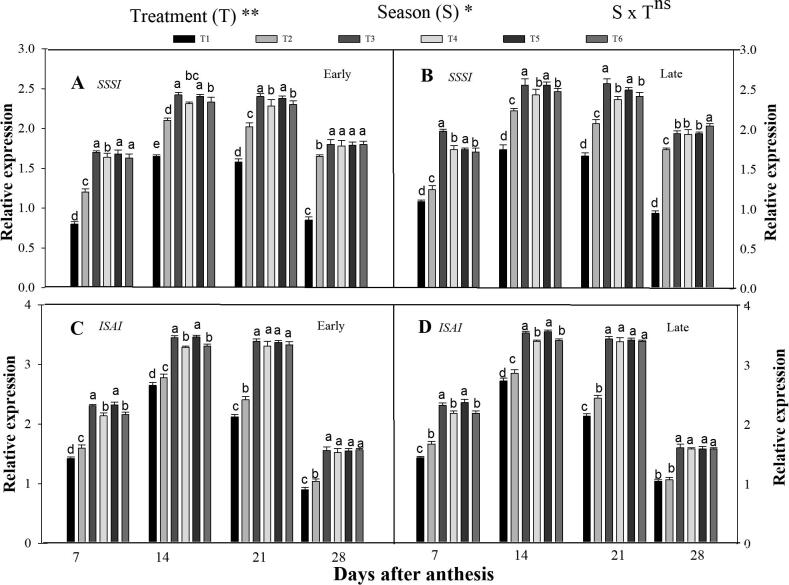
3.3.2. Expression level of SSSI and ISAI
During the grain filling period, SSSI and ISAI are strongly expressed in the combined organic and inorganic treatments (Fig. 5). Furthermore, the different seasons had also a significant effect on the genes expression level. The expression level of SSS1 and ISAI was significantly higher in the integrated treatments than in sole CF fertilization. The genes expression level displayed a single-peak curve and increased at the early filling- time and then decreased at the late-filing period. Across the grain filling phase, the T3 enhanced the expression level of SSS1 by 16% and 12%, and ISAI by 19% and 10% respectively at the early and late season, compared to T2. However, T3 was noted non-significant (p < 0.05) with T5. Moreover, the combined treatments T4 and T6 also significantly higher expression levels of SSS1 and than the sole CF fertilized plants. The expression pattern of SSS1 and ISAI genes were highly expressed in the late growing season compared with the early.
Fig. 5.
Changes in relative expression patterns of starch metabolism related genes [SSSI (A, B) and ISAI (C, D)] in the early and late seasons in developing rice endosperm as affected by combined organic and inorganic N fertilization. Letter a—d indicate difference is significant at 0.05 level. The mean comparison was made using the least significant differences (LSD) test for treatments and seasons means based on the f LSD test at 5%. ns = non-significant and *,** = significant at 5% and 1%, respectively.
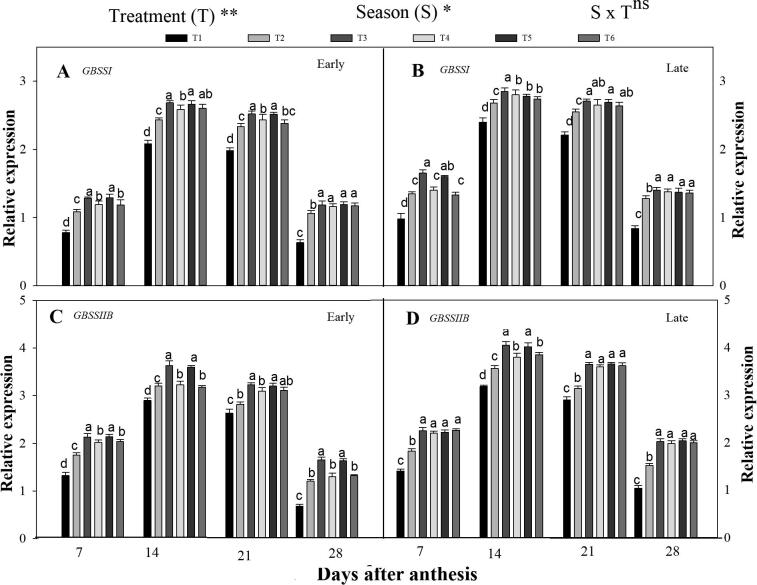
3.3.3. Expression level of GBSSI and GBSEIIb
Fig. 6 revealed that GBSSI and GBSEIIb were highly expressed during the grain-filling under the combined organic and synthetic N fertilization. The GBSSI and GBSEIIb were highly expressed at the beginning of the repining phase and then decline at the late-ripening phase. Averaged across the grain-filling period, the T3 increased the expression level of GBSSI by 19% and 13% and GBSEIIb by 29% and 20% in the early and late season, respectively, compared to T2. However, T3 was statistically similar to T5. Further, the expression level of GBSSI and GBSEIIb had also high in the integrated treatments T4 and T6 than in sole urea applied plants. In-addition, the seasons have also a significant effect on the GBSSI and GBSEIIb expression patterns. Relative to the early season, the GBSSI and GBSEIIb and ISA1 genes were highly expressed in the late season.
Fig. 6.
Changes in relative expression patterns of starch metabolism related genes [GBSSSI (A,B) and GBSSIIB (C,D)] in the early and late seasons in developing rice endosperm as affected by combined organic and inorganic N fertilization. Letter a—d indicate difference is significant at 0.05 level. The mean comparison was made using the least significant differences (LSD) test for treatments and seasons means based on the LSD test at 5%. ns = non-significant and *,** = significant at 5% and 1%, respectively.
3.4. Rice yield and yield attributes
Rice grain yield and yield traits were significantly influenced by combined organic manure and CF fertilization (Table 5). The integrated treatment T6 produced significantly higher panicles number, grain filling percentage, thousand grains weight, and grain yield by 11% and 14%, 5% and 7%, 6% and 9%, 11%, and 17%, respectively, in the early and late seasons, as compared to T2. However, T4 was found non-significant with treatment T6. Further, the combined treatments T3 and T5 had also higher yield and yield attributes as compared with non-N applied plots. Compared to the early season, rice grain yield was improved by 6% in the late seasons in combined treatment T6. The lowest yield and yield components were found in non-N treated plots.
Table 5.
Rice growth, yield and yield components under organic and inorganic N fertilizer application.
| Treatment | PN | PL | SSP | FGP | TGW | GY | |
|---|---|---|---|---|---|---|---|
| PH (cm) | (hill−1) | (cm) | (panicle-1) | (%) | (g) | (kg ha−1) | |
| Early season | 90c | 20.9d | 78.7c | 18.4d | 2942c | ||
| T1 | 7.2c | 129.2d | |||||
| T2 | 105b | 10.1a | 25.2ab | 143.9a | 84.5b | 24.2a | 5038a |
| T3 | 103ab | 9.0b | 23.8bc | 139.3b | 85.3a | 23.3bc | 4276b |
| T4 | 106a | 10.2a | 25.7a | 144.4a | 85.9a | 23.8ab | 5572a |
| T5 | 104ab | 9.6b | 23.2c | 140.7b | 85.6b | 22.8b | 4050b |
| T6 | 105a | 10.5a | 26.0a | 144.0a | 85.5a | 24.1a | 5583a |
| Late season | 94c | 21.0d | 77.5c | 18.3d | 2855d | ||
| T1 | 6.5c | 130.2d | |||||
| T2 | 107b | 9.6c | 24.6ab | 143.5ab | 84.3b | 23.8b | 4559b |
| T3 | 108ab | 10.0b | 23.9b | 142.0bc | 85.3a | 23.5b | 4197c |
| T4 | 108ab | 10.5a | 24.8a | 144.9a | 85.1a | 24.6a | 5251a |
| T5 | 109a | 10.1b | 23.4b | 142.1bc | 85.3a | 23.4b | 4168c |
| T6 | 109a | 10.6a | 25.0a | 145.4a | 85.3a | 24.8a | 5273a |
Note: T1-CF0, T2-100% CF, T3-60% CM + 40% CF, T4-30% CM + 70% CF, T5-60% PM+ 40% CF, T6-30% PM + 70% CF, CF—chemical fertilizer, CM—cattle manure, PM—poultry manure, PH—plant height, PN—panicle number, PL—panicle length, SSP—spikelet number per panicle, FGP—filled grain percent, TGW—thousand-grain weight, GY—grain yield. Values followed by the same letters, within the column, are not significantly different at p ≤ 0.05.
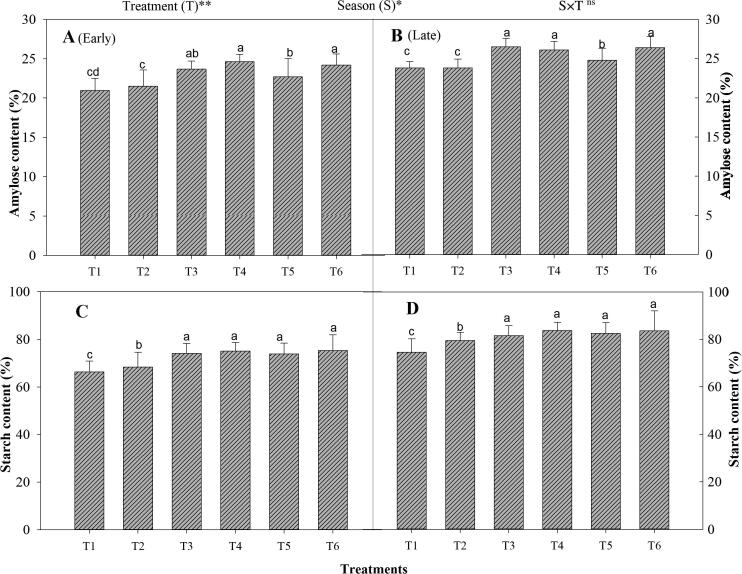
3.5. Rice grain starch and amylose content
The integrated use of organic and inorganic fertilizer had considerably improved rice grain starch and amylase content (Fig. 7). Further, significant variations were noted in scratch content and AC between the seasons. The combined treatment T3 significantly improved the starch content by 10% and 11% during the early and late seasons, compared to T2. However, the treatments T5 and T6 were found statistically similar to T3. The lowest grain starch content was noted in non-N treated plots across the seasons. Moreover, the seasons have also a significant effect on the starch content of rice grain. Relative to early seasons, higher grain starch content was noted in the late season.
Fig. 7.
Changes in rice grain amylose (A&B) and starch content (C&D) in the early and late seasons under combined organic and inorganic N fertilization. Different letters above the column indicate statistical significance at the p < 0.05. The mean comparison was made using the least significant differences (LSD) test for treatments and seasons means based on the LSD test at test at 5%. ns = non-significant and *,** = significant at 5% and 1%, respectively.
Similarly, the combined organic and inorganic treatment T6 significantly increased the AC of rice grain by 12% and 14%, respectively during the early and late seasons, relative to T2. However, T6 was observed non-significant with T3, T4, and T5. The lowest grain AC was recorded in non-N treated plots in both seasons. In the case of different seasons, higher AC was noted in the late growing season as compared to the early season as shown in Fig. 7.
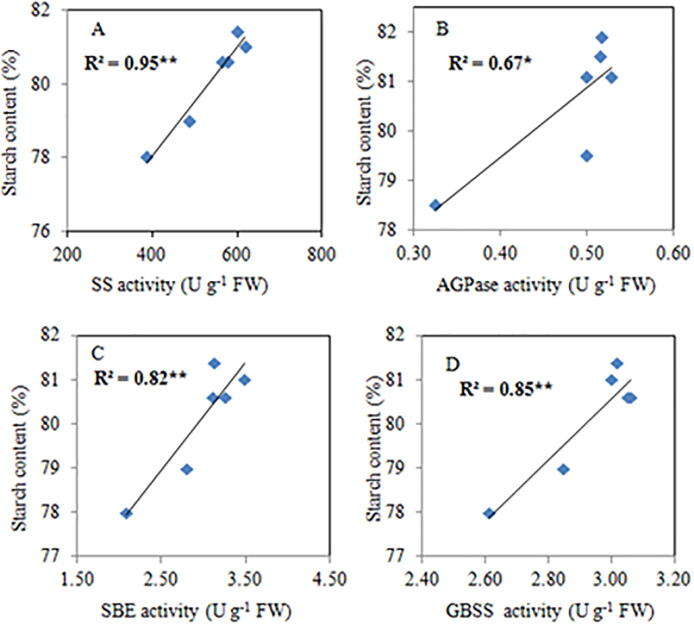
3.6. Relationship of the starch-metabolizing enzyme with grain starch and amylose content
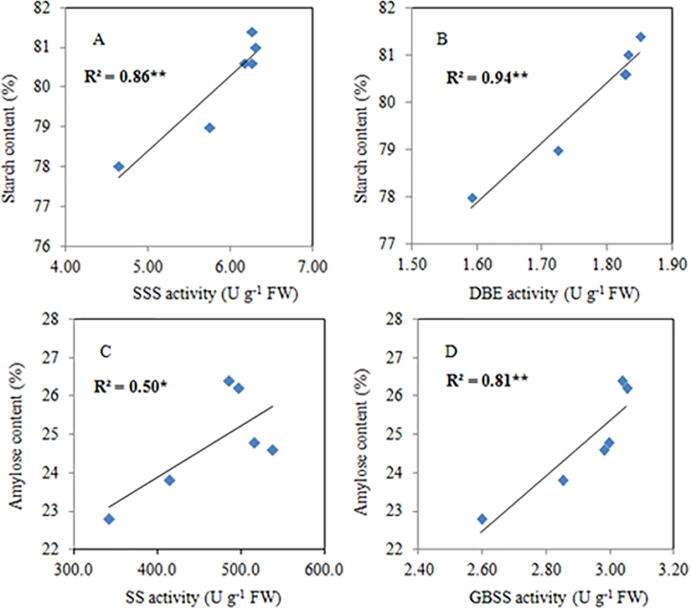
Rice grain starch and AC was strongly dependent on starch-metabolizing enzyme activity. This is confirmed in our study, the linear regression analysis showed a very strong and positive correlation between SS activity (R2 = 0.95** Fig. 8 [A]), AGPase activity (R2 = 0.67* Fig. 8 [B]), SBE activity (R2 = 0.82** Fig. 8 [C]), GBSS activity (R2 = 0.85** Fig. 8 [D], SSS activity (R2 = 0.86** Fig. 9 [A]), and DBE activity (R2 = 0.94** Fig. 9 [B]) with the rice grain starch content. Similarly, linear regression analysis also showed a close relationship to GBSS activity (R2 = 0.81** Fig. 9 D) with the AC of grains. Such findings showed that variations in starch-metabolizing enzyme activities directly influenced the starch and AC of the rice grain.
Fig. 8.
Relationship of grain starch content with SS [A], AGPase [B], SBE[C], and GBSS [D] activities * p < 0.05, ** p < 0.005.
Fig. 9.
Relationship of grain starch content with SSS [A], DBE [B], and amylose content with SS [C], and GBSS [D] activities. * p < 0.05, **p < 0.001.
4. Discussion
Environmental factors such as geographical location, climatic temperature, soil and fertility, and N fertilizers have a significant effect on rice productivity and grain quality (Siddik et al., 2019, Wu et al., 2020). Furthermore, the current farming system also affects crop production and quality due to over-fertilization of synthetic N, which adversely affects soil quality, the environment, and crop production (Gu et al., 2015). In-addition, it is also well known that the activity of SMEs is affected by the air temperature and the amount of N fertilizer (Wu et al., 2020, Zhao et al., 2020).
Carbohydrates (starch) are known to be the ultimate product of photosynthesis (Everard and Loescher, 2017). Previous studies showed that the use of CFs within a definite range can considerably enhance the photosynthetic ability of the leaf and promote the non-structured carbohydrate accumulation (NSC), but the excessive fertilization of CFs reduces the amount of NSC (Zhu et al., 2017, Zhao et al., 2020). The higher accumulation and translocation of NSC are beneficial to grain filling and it could enhance the physiological strength of grain (Zheng et al., 2010). The improved starch accumulation in rice grain in our study (Fig. 7) associated with an enhanced leaf chlorophyll content and photosynthetic rate (Data not showed). Further, the improvement in starch accumulation might be allied to the positive effect of organic manure on soil health which resulted in enhancing root area per unit soil volume, photosynthetic rate, and water use efficiency which directly influence the physiological process and carbohydrates utilization (Iqbal et al., 2019, Liu et al., 2007). Another reasonable explanation for this is that plants absorb more carbohydrates under high N condition for early growth, whereas under low N condition plants lean to lower and thus less carbohydrate are required for growth and thus accumulate in the plant body. (Peng et al., 2014).
Amylose content (AC) affects the rice noodles eating and processing quality; higher AC means the best eating quality of noodles (Zhu et al., 2020, Tao et al., 2019). The observed enhancement in AC in this study (Fig. 7) under the combined treatment plots suggests that organic and inorganic N fertilizers provide enough amounts of nutrients for plant growth and production throughout the growing period. Further, the increment in AC was mainly due to improved GBSS enzyme activity during the grain filling period (Fig. 2). Our outcomes are also in line with Kumar et al. (2017) who stated that organic manure joined with chemical fertilizer increased grain AC by 9% as relative to sole CF fertilization. Further, the incorporation of organic and inorganic fertilizers to the soil, resulting in both macro-and micronutrients, which are absorbed by plants and used for various metabolic activities to synthesize chlorophyll, proteins, and carbohydrates needed for their normal growth and development (White and Brown, 2010, Iqbal et al., 2020). Because micronutrients such as zinc and magnesium play an important role in plant physiology, e.g., activities of some enzymes related to the metabolism of carbohydrates, synthesis of nucleic acid, and auxins (Wu et al., 2018, Tripathi et al., 2015). Similarly, magnesium promotes the uptake and translocation of phosphorous (Tripathi et al., 2015).
Starch accumulation and production during the grain filling time highly dependent on the activity of SMEs, i.e., AGPase, GBSS, SSS, SBE, and DBE, which play a key role in the regulation of starch synthesis (Nakamura et al., 1989a, Nakamura et al., 1989b, and the activity of SMEs are very positively correlated with starch accumulation (Yang et al., 2004). In this study, the integrated treatments significantly increased the activity of SMEs during the grain filling period (Fig. 1, Fig. 2, Fig. 3). Therefore, improvements in starch accumulation under combined treatments were mainly due to enhance the activity of SMEs concerned in starch synthesis (Yang et al., 2004, Wu et al., 2018). This is further confirmed in our study by the linear regression analysis that the activities of (SS: R2 = 0.95**), (AGPase: R2 = 0.67*), (GBSS: R2 = 0.85**), (SSS: R2 = 0.86**), (SBE: R2 = 0.82**), and (DBE: R2 = 0.81**) were highly positively correlated with starch accumulation (Fig. 8, Fig. 9). Further, (SS: R2 = 0.50* Fig. 9C), and (GBSS: R2 = 0.81** Fig. 9D) were also strongly associated with AC. In-addition, the RT-qPCR analysis showed that the expression of SMEs-related genes, such as AGPS2b, GBSS1, SSS1, GBSEIIb, and ISA1 was highl expressed during grain-filling time under combined treatments, as compared with sole inorganic N fertilization treatments (Fig. 4, Fig. 5, Fig. 6). Therefore, the enhancement in SMEs activities was mainly due to the highly expression of genes involved in starch biosynthesis in the present study. In agreement of our study, the previous studies also showed that N fertilizers affect rice grain starch content by changes in SMEs activities due to altering their relevant gens expression (Pan et al., 2011).
Additionally, enzymes that evaluate the rate of biochemical reactions in the living organism are also influenced by air temperature (Robinson, 2015). In the present study, starch biosynthesis enzymes were significantly varied among the seasons (Fig. 1, Fig. 2, Fig. 3). Starch-metabolizing enzyme activities are strongly associated with starch accumulation (Jiang et al., 2013, Li et al., 2010). Our results showed that the reduction in starch accumulation during the early season was mainly attributed to reduced SMEs activities. Our outcomes are an agreement with Lin et al., 2020, Impa et al., 2020, they reported that the higher temperature during the grain filling period decreased the SMEs activities as compared to the lower temperature, which resulted in a decrease in the starch accumulation of the rice grain. Further, Kato et al. (2019) concluded that SBE and SSS enzymes were responsive to air temperature and the decrease in its action under high temperature may be the reason for the reduction in starch and amylose synthesis. In this study, as shown in Fig. 1, Fig. 2, Fig. 3), the activity of SSS, SBE, and GBSS in endosperm during grain filling stage at a higher temperature in the early decreased compared to the late growing season (low temperature) (Table 1). There is a steady decrease in enzyme activity with an increase in temperature in the present study. Similarly, Ahmed et al. (2015) argued that the reduction of the apparent AC attributed to the decreased GBSS enzyme activity that had been exposed to a high temperature during the grain-filling time. This was further supported by the transcriptomic study of SMEs related genes, such as AGPS2b, GBSS1, SSS1, GBSEIIb, and ISA1 were significantly varied between the seasons (Fig. 4, Fig. 5, Fig. 6). In support of our study, Mittal et al. (2012) reported that air temperature affects SMEs by altering their relevant isoform (genes) expression pattern. Similarly, Ishimaru et al., (2005) stated that the accumulation and quality of starch are highly attributed to the regulation and expression of corresponding enzyme genes. This was also confirmed in our study that high temperature during grain-filling time had a significant effect on gene expression related to starch metabolism. The possible explanation for this was the difference in air temperature during grain-filling time among the seasons (Table 1).
It is well known that air temperature during the grain-filling period highly affects rice grain starch accumulation (Yao et al., 2020a, Yao et al., 2020b). In the present study, we also noted a decrease in starch and AC in the early season compared to the late growing season. This is associated with the higher temperature during the early season as compared with the late season (Table 1). These results suggested that a high temperature in the grain-filling period has a greater effect on the accumulation and composition of starch at grain development. Our results are also in line with the finding that temperature during grain filling influences the starch and AC of grain (Cheng et al., 2005a, Cheng et al., 2005b, Akhtar et al., 2019). Differences in AC are primarily cultivar reliant; however, temperature changes have been reported to induce changes in AC rates in the same rice cultivar (Cheng et al., 2005a, Cheng et al., 2005b, Umemoto et al., 1995), and cold weather variations induced higher ACs of the same cultivar (Cheng et al., 2000, Ahmed et al., 2008).
5. Conclusion
In the current study, the combined organic and inorganic N fertilizers application increased the accumulation of starch and amylose in rice grain as well as grain yield. This was primarily associated with the enhanced activity of the starch-metabolizing enzymes during the grain-filling period. The increased enzyme activity was mainly due to the higher expression of their relevant isoforms (genes). Further, the activity of GBSS and SBE was highly positively correlated with grain amylose and starch content. In terms of seasons, higher activity of starch-metabolizing enzymes, starch, and amylose content was noted in the late-season compared with the early season. The increment in these attributes was mainly attributed to low temperature in the late season. It is suggested that PM or CM combined with CF at a ratio of 30:70 is a better plan for achieving maximum rice yields and quality with an improved grain-filling rate.
Acknowledgments
This experiment was financially sponsored by the China National Key Research and Development Project (2016YFD030050902). We would like to thank our cooperation team from the Agriculture Research Station, Guangxi University for supporting us in conducting and handling this research.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ahmed, N., Tetlow, I. J., Nawaz, S., Iqbal, A., Mubin, M., Nawaz ul Rehman, M. S., ... & Maekawa, M., 2015. Effect of high temperature on grain filling period, yield, amylose content and activity of starch biosynthesis enzymes in endosperm of basmati rice. Journal of the Science of Food and Agriculture, 95(11), 2237–2243. [DOI] [PubMed]
- Ahmed, Nisar, Maekawa, Masahiko, Tetlow, Ian J., 2008. Effects of low temperature on grain filling, amylose content, and activity of starch biosynthesis enzymes in endosperm of basmati rice. Australian Journal of Agricultural Research. [DOI] [PubMed]
- Akhtar K., Wang W., Khan A., Ren G., Afridi M.Z., Feng Y. Wheat straw mulching offset soil moisture deficient for improving physiological and growth performance of summer sown soybean. Agric. Water Manage. 2019;211:16–25. [Google Scholar]
- Bao, C., 2000. Beijing. Agro-chemical analysis of soil. Publish House of China, Beijing.
- Ali A., Ullah S., He L., Zhao Q., Iqbal A., Wei S., Jiang L. Combined application of biochar and nitrogen fertilizer improves rice yield, microbial activity and N-metabolism in a pot experiment. PeerJ. 2020;8 doi: 10.7717/peerj.10311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck E., Ziegler P. Biosynthesis and degradation of starch in higher plants. Ann. Rev. Plant Physiol. 1989;40:95–117. [Google Scholar]
- Beckles D.M., Thitisaksakul M. How environmental stress affects starch composition and functionality in cereal endosperm. Starch-Starke. 2014;66(1–2):58–71. [Google Scholar]
- Chen X., Chen M., Lin G. Structural development and physicochemical properties of starch in caryopsis of super rice with different types of panicle. BMC Plant. Biol. 2019;19:482. doi: 10.1186/s12870-019-2101-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng F.M., Ding Y.S., Zhu B.Y. The formation of amylose content in rice grain and its relation with field temperature. Acta Ecol. Sinica (in Chinese) 2000;20:644–652. [Google Scholar]
- Cheng F., Lianjin Z., Ningchun Z., Yi L., Goping Z. Temperature induced changes in the starch components and biosynthetic enzymes of two rice varieties. Plant Growth Regul. 2005;46(1):87–95. [Google Scholar]
- Cheng F., Zhong L., Zhao N., Liu Y., Zhang G. Temperature induced changes in the starch components and biosynthetic enzymes of two rice varieties. Plant Growth Regul. 2005;46(1):87–95. [Google Scholar]
- Everard, J.D., Loescher, W.H., 2017. Primary Products of Photosynthesis, Sucrose and Other Soluble Carbohydrates.
- Deng N., Ling X., Sun Y., Zhang C., Fahad S., Peng S., Huang J. Influence of temperature and solar radiation on grain yield and quality in irrigated rice system. Eur. J. Agron. 2015;64:37–46. [Google Scholar]
- Fitzgerald M.A., Resurreccion A.P. Maintaining the yield of edible rice in a warming worl. Funct. Plant Biol. 2009;36:1037–1045. doi: 10.1071/FP09055. [DOI] [PubMed] [Google Scholar]
- Fujita N., Satoh R., Hayashi A., Kodama M., Itoh R., Aihara S. Starch biosynthesis in rice endosperm requires the presence of either starch synthase I or IIIa. J. Exp. Bot. 2011;62:4819–4831. doi: 10.1093/jxb/err125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton D.C., Edwards A., Pillings E. Role of granule-bound starch synthesis in determination of amylopectin structure and starch granule morphology in potato. J. Biol. Chem. 2002;277:10834–10841. doi: 10.1074/jbc.M111579200. [DOI] [PubMed] [Google Scholar]
- Gu J., Chen J., Chen L., Wang Z., Zhang H., Yang J. Grain quality changes and responses to nitrogen fertilizer of japonica rice cultivars released in the Yangtze River Basin from the 1950s to 2000s. Crop J. 2015;3(4):285–297. [Google Scholar]
- Guo J., Gao L., Xie K., Ling N., Shen Q., Guo S. rice production practices of high yield and high nitrogen use efficiency in Jiangsu, China. Scientific Rep. 2017;7(1):1–10. doi: 10.1038/s41598-017-02338-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z. Agriculture press; Beijing: 1985. Grain Quality and Its Analysis Technology. [Google Scholar]
- Impa S.M., Vennapusa A.R., Bheemanahalli R., Sabela D., Boyle D., Walia H., Jagadish S.K. High night temperature induced changes in grain starch metabolism alters starch, protein, and lipid accumulation in winter wheat. Plant Cell Environ. 2020;43(2):431–447. doi: 10.1111/pce.13671. [DOI] [PubMed] [Google Scholar]
- Iqbal A., Liang H., Aziz K., Shangqin W., Kashif A., Izhar A., Ullah Saif, Fazal M., Quan Z., Ligeng J. Organic manure coupled with inorganic fertilizer: an approach for the sustainable production of rice by improving soil properties and nitrogen use efficiency. Agronomy. 2019;9(10):651. [Google Scholar]
- Iqbal A., He L., Ali I., Ullah S., Khan A., Khan A., Akhtar K., Wei S., Zhao Q., Zhang J., Jiang L. Manure combined with chemical fertilizer increases rice productivity by improving soil health, post-anthesis biomass yield, and nitrogen metabolism. Plos one. 2020;15(10) doi: 10.1371/journal.pone.0238934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimaru T., Tatsuro H., Toshiaki M., Akitoshi G., Kazunari T., Haruto S., Tomio T., Ishii Ryu-ichi, Ryu O., Tohru Y. Expression patterns of genes encoding carbohydrate-metabolizing enzymes and their relationship to grain filling in rice (Oryza sativa L.): comparison of caryopses located at different positions in a panicle. Plant Cell Physiol. 2005;46(4):620–628. doi: 10.1093/pcp/pci066. [DOI] [PubMed] [Google Scholar]
- Jackson, M.J.M., Wisconsin. 1956. Soil chemical analysis—advanced course: Published by the author. CRC Press. 895.
- Jiang, F.U, Xu, Y.J., Lu, C.H.E.N., Yuan, L.M., Wang, Z.Q., Yang, J.C., 2013. Changes in enzyme activities involved in starch synthesis and hormone concentrations in superior and inferior spikelets and their association with grain filling of super rice. Rice Sci. 20(2), 120–128.
- Jeon, J.S., Ryoo, N., Hahn, T.R., Walia, H., Nakamura, Y., 2010. Starch Biosynthesis in Cereal Endosperm. Plant Physiology and Biochemistry 48(6), 383–392. [DOI] [PubMed]
- Kato K., Suzuki Y., Hosaka Y., Takahashi R., Kodama I., Sato K., Fujita N. Effect of high temperature on starch biosynthetic enzymes and starch structure in japonica rice cultivar ‘Akitakomachi’(Oryza sativa L.) endosperm and palatability of cooked rice. J. Cereal Sci. 2019;87:209–214. [Google Scholar]
- Khan A., Fahad S., Khan A., Saud S., Adnan M., Wahid F. Managing tillage operation and manure to restore soil carbon stocks in wheat-maize cropping system. Agron. J. 2019;111(5):2600–2609. [Google Scholar]
- Kumar A., Joseph S., Tsechansky L., Privat K., Schreiter I.J., Schüth C., Graber E.R. Biochar aging in contaminated soil promotes Zn immobilization due to changes in biochar surface structural and chemical properties. Total Environ. 2018;626:953–961. doi: 10.1016/j.scitotenv.2018.01.157. [DOI] [PubMed] [Google Scholar]
- Kumar U., Shahid D.M., Tripathi R., Mohanty S., Kumar A., Bhattacharyya P., Lal B., Gautam P., Raja R., Panda B.B. Variation of functional diversity of soil microbial community in sub-humid tropical rice-rice cropping system under long-term organic and inorganic fertilization. Ecol. Indic. 2017;73:536–543. [Google Scholar]
- Leghari S.J., Wahocho N.A., Laghari G.M., HafeezLaghari A., MustafaBhabhan G., HussainTalpur K., Lashari A.A. Role of nitrogen for plant growth and development: a review. Adv. Environ. Biol. 2016;10(9):209–219. [Google Scholar]
- Leghari S., Jahan N., Ahmed W., Ghulam M.L., Abdul H., Ghulam M.B., Khalid H.T., Tofique A.B., Safdar A.W., Ayaz A.L. Role of nitrogen for plant growth and development: a review. Adv. Environ. Biol. 2016;10(9):209–219. [Google Scholar]
- Li, X.G, Liu, H.Y., Jin, Z.X., Liu, H.L., Huang, X., Xu, M.L., Zhang, F.Z, 2010. Changes in activities of key enzymes for starch synthesis and glutamine synthetase in grains of progenies from a rice cross during grain filling. Rice Sci., 17(3), 243–246.
- Li H., Yu W., Dhital S., Gidley M.J., Gilbert R.G. Starch branching enzymes contributing to amylose and amylopectin fine structure in wheat. Carbohydr. Polym. 2019;224 doi: 10.1016/j.carbpol.2019.115185. [DOI] [PubMed] [Google Scholar]
- Li T., Shen B., Chen N., Luo Y. Effect of Q-enzyme on the chalkiness formation of rice grain. Acta Agronomica Sinica. 1997;23:338–344. [Google Scholar]
- Lin G., Yang Y., Chen X., Yu X., Wu Y., Xiong F. Effects of high temperature during two growth stages on caryopsis development and physicochemical properties of starch in rice. Int. J. Biol. Macromol. 2020;145:301–310. doi: 10.1016/j.ijbiomac.2019.12.190. [DOI] [PubMed] [Google Scholar]
- Liu S.P., Nie X.T., Dai Q.G., Huo Z.Y., Ke X.U. Effect of interplanting with zero tillage and straw manure on rice growth and rice quality. Rice Sci. 2007;14(3):204–210. [Google Scholar]
- Livak K.J., Thomas D.S. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method.“. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Ma G., Liu W., Li S., Zhang P., Wang C., Lu H., Kang G. Determining the optimal N input to improve grain yield and quality in winter wheat with reduced apparent N loss in the North China Plain. Front. Plant Sci. 2019;10:181. doi: 10.3389/fpls.2019.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal D., Dinesh A., Anil G. Gene expression analysis in response to low and high temperature and oxidative stresses in rice: combination of stresses evokes different transcriptional changes as against stresses applied individually. Plant Sci. 2012;197:102–113. doi: 10.1016/j.plantsci.2012.09.008. [DOI] [PubMed] [Google Scholar]
- Murphy J., Riley J.P.J. Aca. A modified single solution method for the determination of phosphate in natural waters. Analytica chimica acta. 1962;27:31–36. [Google Scholar]
- Myers A.M., Morell M.K., James M.G. Recent progress toward understanding biosynthesis of the amylopectin crystal. Plant Physiol. 2000;122:989–997. doi: 10.1104/pp.122.4.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y., Sakurai A., Inaba Y. The fine structure of amylopectin in endosperm from Asian cultivated rice can be largely classified into two classes. Starch. 2002;54:117–131. [Google Scholar]
- Nakamura Y., Umemoto T., Takahata Y. Changes in structure of starch and enzyme activities affected by sugary mutations in developing rice endosperm. Possible role of starch debranching enzyme (R-enzyme) in amylopectin biosynthesis. Physiol. Plant. 1996;97:491–498. [Google Scholar]
- Nakamura Y., Yuki K., Park S. Carbohydrate metabolism in the developing endosperm of rice grain. Plant. Cell Physiol. 1989;30(6):833–839. [Google Scholar]
- Nakamura Y., Yuki K., Park S.-Y., Ohya T. Carbohydrate metabolism in the developing endosperm of rice grains. Plant Cell Physiol. 1989;30:833–839. [Google Scholar]
- Ohyama T., Tewari K., Abdel-Latif S., Ruamrungsri S., Komiyama S., Ito S. Direct analysis of 15N abundance of Kjeldahl digested solution by emission spectrometry. Bull. Facul. Agric. Niigata Univ. 2004;57(1):33–40. [Google Scholar]
- Pan J., Cui K., Wei D., Huang J., Xiang J., Nie L. Relationships of non-structural carbohydrates accumulation and translocation with yield formation in rice recombinant inbred lines under two nitrogen levels. Physiol. Plant. 2011;141(4):321–331. doi: 10.1111/j.1399-3054.2010.01441.x. [DOI] [PubMed] [Google Scholar]
- Patindol J.A., Siebenmorgen T.J., Wang Y.J. Impact of environmental factors on rice starch structure: a review. Starch-Stärke. 2015;67(1–2):42–54. [Google Scholar]
- Peat S., Whelan W.J., Thomas G.J. Evidence of multiple branching in waxy maize starch. J. Chem.Soc. 1952:4546–4548. [Google Scholar]
- Peng X., Yang Y., Yu C., Chen L., Zhang M., Liu Z. Crop management for increasing rice yield and nitrogen use efficiency in Northeast China. Agron. J. 2015;107(5):1682–1690. [Google Scholar]
- Peng Y., Li C., Fritschi F.B. Diurnal dynamics of maize leaf photosynthesis and carbohydrate concentrations in response to differential N availability. Environ. Exp. Bot. 2014;99:18–27. [Google Scholar]
- Plett D.C., Ranathunge K., Melino V.J., Kuya N., Uga Y., Kronzucker H.J. The intersection of nitrogen nutrition and water use in plants: new paths toward improved crop productivity. J. Exp. Bot. 2020 doi: 10.1093/jxb/eraa049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson P.K. Enzymes: principles and biotechnological applications. Essays Biochem. 2015;59:1–41. doi: 10.1042/bse0590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robyt J.F. Starch: structure, properties, chemistry, and enzymology. In: Fraser-Reid B.O., Tatsuta K., Thiem J., editors. Glycoscience. Springer; Berlin, Heidelberg: 2008. pp. 1437–1472. [Google Scholar]
- Siddik, M.A., Zhang, J., Chen, J., Qian, H., Jiang, Y., kareem Raheem, A., Deng, A., Song, Z., Zheng, C. and Zhang, W., 2019. Responses of indica rice yield and quality to extreme high and low temperatures during the reproductive period. European Journal of Agronomy, 106, pp.30-38.
- Smith A.M., Denyer K. The synthesis of starch granule. Ann. Rev. Plant Physiol. Plant. Mol. Biol. 1997;48:67–87. doi: 10.1146/annurev.arplant.48.1.67. [DOI] [PubMed] [Google Scholar]
- Tang She, Haixiang Z., Wenzhe L., Zhi D., Qinyang Z., Wenzhu C., Shaohua W., Yanfeng D. Nitrogen fertilizer at heading stage effectively compensates for the deterioration of rice quality by affecting the starch-related properties under elevated temperatures. Food Chem. 2019;277(2019):455–462. doi: 10.1016/j.foodchem.2018.10.137. [DOI] [PubMed] [Google Scholar]
- Tao, Keyu., Wenwen Yu., Sangeeta P., Robert G., 2019. Gilbert. “High-amylose rice: Starch molecular structural features controlling cooked rice texture and preference. Carbohydrate Polym 219: 251–260. [DOI] [PubMed]
- Tao, Rui., Yongchao, L., Steven, A., 2015. Wakelin, and Guixin Chu. “Supplementing chemical fertilizer with an organic component increases soilbiological function and quality. Applied Soil Ecology, 96 : 42-51.
- Tripathi D.K., Singh S., Singh S., Mishra S., Chauhan D.K., Dubey N.K. Micronutrients and their diverse role in agricultural crops: advances and future prospective. Acta Physiologiae Plantarum. 2015;37(7):139. [Google Scholar]
- Umemoto T., Nakamura Y., Ishikur N. Activity of starch synthase and the amylose content in rice endosperm. Phytochemistry. 1995;40:1613–1616. [Google Scholar]
- Wardlaw I.F., Willenbrink J. Carbohydrate storage and mobilization by the culm of wheat between heading and grain maturity: the relation to sucrose synthase and sucrose-phosphate synthase. Austra. J. Plant Physiol. 1994;21:255–271. [Google Scholar]
- White P.J., Brown P.H. Plant nutrition for sustainable development and global health. Ann. Bot. 2010;105(7):1073–1080. doi: 10.1093/aob/mcq085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q., Wang Y., Chen T., Zheng J., Sun Y., Chi D. Soil nitrogen regulation using clinoptilolite for grain filling and grain quality improvements in rice. Soil Tillage Res. 2020;199 [Google Scholar]
- Wu T.Y., Gruissem W., Bhullar N.K. Facilitated citrate-dependent iron translocation increases rice endosperm iron and zinc concentrations. Plant Sci. 2018;270:13–22. doi: 10.1016/j.plantsci.2018.02.002. [DOI] [PubMed] [Google Scholar]
- Yang J., Zhang J., Wang Z., Xu G., Zhu Q. Activities of key enzymes in sucrose-to-starch conversion in wheat grains subjected to water deficit during grain filling. Plant Physiol. 2004;135(3):1621–1629. doi: 10.1104/pp.104.041038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Lin G., Yu X., Wu Y., Xiong F. Rice starch accumulation at different endosperm regions and physical properties under nitrogen treatment at panicle initiation stage. Int. J. Biol. Macromol. 2020 doi: 10.1016/j.ijbiomac.2020.05.210. [DOI] [PubMed] [Google Scholar]
- Yao D., Wu J., Luo Q., Li J., Zhuang W., Xiao G., Bai B. Influence of high natural field temperature during grain filling stage on the morphological structure and physicochemical properties of rice (Oryza sativa L.) starch. Food Chem. 2020;310 doi: 10.1016/j.foodchem.2019.125817. [DOI] [PubMed] [Google Scholar]
- Yao D., Jun W., Qiuhong L., Jianwu L., Wen Z., Gui X., Qiyun D., Dongyang L., Bin Bai. Influence of high natural field temperature during grain filling stage on the morphological structure and physicochemical properties of rice (Oryza sativa L.) starch. Food Chem. 2020;310 doi: 10.1016/j.foodchem.2019.125817. [DOI] [PubMed] [Google Scholar]
- Yue X., Zhang J., Shi A., Yao S., Zhang B. Manure substitution of mineral fertilizers increased functional stability through changing structure and physiology of microbial communities. Eur. J. Soil Biol. 2016;77:34–43. [Google Scholar]
- Zhang, F., Chen, X., Vitousek, P., 2013. An experiment for the world. 497:33–35. Doi: 10.1038/497033a PMID: 23636381. [DOI] [PubMed]
- Zhang M., Yuanlin Y., Yuhua T., Ke C., Meng Z., Miao Z., Bin Yin. Increasing yield and N use efficiency with organic fertilizer in Chinese intensive rice cropping systems. Field Crops Res. 2018;227:102–109. [Google Scholar]
- Zhao Q., Yu Ye, Zhanyu H., Lujian Z., Xianyue G., Gang P., Fangmin C. SSIIIa-RNAi suppression associated changes in rice grain quality and starch biosynthesis metabolism in response to high temperature. Plant Sci. 2020;294 doi: 10.1016/j.plantsci.2020.110443. [DOI] [PubMed] [Google Scholar]
- Zheng Y.M., Ding Y.F., Liu Z.H., Wang S.H. Effects of panicle nitrogen fertilization on non-structural carbohydrate and grain filling in indica rice. Agric. Sci.China. 2010;9(11):1630–1640. [Google Scholar]
- Zhou T., Zhou Q., Li E., Yuan L., Wang W., Zhang H., Gu J. Effects of nitrogen fertilizer on structure and physicochemical properties of ‘super’rice starch. Carbohydr. Polym. 2020;116237 doi: 10.1016/j.carbpol.2020.116237. [DOI] [PubMed] [Google Scholar]
- Zhu D., Fang C., Qian Z., Guo B., Huo Z. Differences in starch structure, physicochemical properties and texture characteristics in superior and inferior grains of rice varieties with different amylose contents. Food Hydrocolloids. 2020;106170 [Google Scholar]
- Zhu D., Hong-cheng Z., Bao-wei G., Xu Ke, Qi-gen D., Hai-yan W., Gao H., Ya-jie H., Pei-yuan C., Zhong-yang H. Effects of nitrogen level on yield and quality of japonica soft super rice. J. Integrat. Agric. 2017;16(5):1018–1027. [Google Scholar]
- Zhu T., Jackson D.S., Wehling R.L., Geera B. Comparison of amylose determination methods and the development of a dual wavelength iodine binding technique. Cereal Chem. 2008;85:51–58. [Google Scholar]
- Zi Y., Ding J., Song J., Humphreys G., Peng Y., Li C., Guo W. Grain yield, starch content and activities of key enzymes of waxy and non-waxy wheat (Triticum aestivum L.) Sci. Rep. 2018;8(1):1–12. doi: 10.1038/s41598-018-22587-0. [DOI] [PMC free article] [PubMed] [Google Scholar]