Abstract
Candida infections and related mortality have become a challenge to global health. Nontoxic and natural bioactive compounds from plants are regarded as promising candidates to inhibit these multidrug resistant strains. In the present study, in vitro assays and in silico molecular docking approach was combined to evaluate the inhibitory effect of crude extracts from Allium ampeloprasum and its variety A. porrum on Candida pathogens. Phytochemical screening revealed the presence of phenolic acids and flavonoids in higher quantity. Spectral studies of the extracts support the presence of phenols, flavonoids and organosulfur compounds. Aqueous extract of A. ampeloprasum showed a total antioxidant capacity of 68 ± 1.7 mg AAE/ g and an IC50 value of 0.88 ± 2.1 mg/ml was obtained for DPPH radicals scavenging assay. C. albicans were highly susceptible (19.9 ± 1.1 mm) when treated with aqueous A. ampeloprasum extract. Minimum inhibitory concentrations were within the range of 19–40 μg/ml and the results were significant (p ≤ 0.05). In silico molecular docking studies demonstrated that bioactive phytocompounds of A. ampeloprasum and A. porrum efficiently interacted with the active site of Secreted aspartyl proteinase 2 enzyme that is responsible for the virulence of pathogenic yeasts. Rosmarinic acid and Myricetin exhibited low binding energies and higher number of hydrogen bond interactions with the protein target. Thus the study concludes that A. ampeloprasum and A. porrum that remain as underutilized vegetables in the Allium genus are potential anti-candida agents and their pharmacologically active compounds must be considered as competent candidates for drug discovery.
Keywords: A. ampeloprasum, A. porrum, Antioxidant activity, Anti-candida activity, Molecular docking, Candidapepsin 2
1. Introduction
Candida species are the most prevalent group of yeast pathogens that are capable of invading the human system. These pathogens can exist as commensals without causing complications until they are regulated by the co-existing microbiota. They occur as superficial infections in hospitalized or immunocompromised victim right after surgery (Bassetti et al. 2018). Growth and spread of opportunistic candida spp. can trigger minor to severe conditions such as oral thrush, vaginal thrush, gential infections, skin infections, urinary tract infections, and blood stream infections. Candida albicans are the leading cause of mucosal infections, but other non-albican species like C. glabrata, C. krusei, C. parapsilosis, C. tropicalis, C. auris and C. haemulonii can also asymptomatically colonize the human biota (Berkow and Lockhart 2017). Candidiasis and candidemia have become a global health crisis as more than 250,000 inhabitants are susceptible to these infections every year (Arendrup and Patterson 2017). Azole group drugs (Ketoconazole, Fluconazole, Econazole, Terconazole, Butoconazole and Miconazole) have been recognized as effective therapeutic strategy to combat candida infections but the incessant and redundant use of these drugs have led to the development of multidrug resistant strains of Candida species (Siwek et al. 2012).
The mode of action of the azole drugs generally involves the inhibition of 14α-lanosterol demethylase, a vital enzyme required for the biosynthesis of ergosterol, a specific sterol associated with the yeast membrane. Depletion of ergosterol results in the instability of the cell membrane and when combined with the intracellular accumulation of 14α methylated sterols, the membrane and organelle functions can collapse (Hoot et al., 2010). The recent finding in the mode of action suggests that azoles tend to increase the production of reactive oxygen species (ROS) in C. albicans. Inhibition of catalases and peroxidases by the drugs can aggravate the generation of ROS resulting in intracellular stress (Dbouk et al. 2019). It is possible that non-albican species can exert intrinsic resistance to the azole drugs. Moreover, due to incessant and redundant use of these drugs, Candida species have become resistant to the antifungal agents and emerged as multidrug resistant pathogens. When ERG 11 gene associated with production of ergosterol is inactivated by the antifungal agent, pathogens utilize alternative sterols for membrane functions and stability. Nevertheless inhibition of intracellular sterol production has been matched by the uptake of extracellular sterol intake. Other mechanisms involve the over expression of ERG 11 gene, MDR 1p and CDR1p/CDR 2p and activation of TAC1 gene. High level resistance is observed in Candida species that lose heterozygosity assisted by hyperactive TAC1 and mutated ERG11 (Arendrup and Patterson, 2017, Siwek et al., 2012). On the other hand the redundant consumption of azole drugs can lead to side effects such as nausea, vomiting, upset stomach, headache, dizziness, drowsiness, numbness, tingling sensation at nerve endings, rashes, discolouration of the skin, hair loss, decreased sense of taste, abdominal cramps, irritation of genital organs, impotency, adrenal insufficiency and hormone imbalance (Benitez and Carver 2019).
The harmless commensal yeast species tends to become a virulent pathogen when opportunities are offered to transverse the barriers on the account of immunosuppression. The key hydrolytic enzymes that assist in the virulence build-up of these yeast species are secreted aspartyl proteinases (SAP), phospholipase B enzymes, and lipases. Almost all Candida spp. show the presence of SAP enzymes that are encoded by ten SAP genes (Chaffin 2008). SAP is synthesized as preproenzyme which contains more than 60 amino acids and are processed and transported through the Golgi apparatus to the extracellular membrane. Increase in SAP production has been correlated with the increase in the virulence of C. albicans species. C. albicans strains isolated from heavily infected candidiasis patients are more proteolytic than the strains obtained from asymptomatic patients. Patients with advanced HIV infections have selection towards virulent proteolytic strains of C. albicans when compared to early stage patients (Mani et al. 2016). Investigation has shown that exposure to anti-candida agents such as azoles can up-regulate the expression of SAP2 gene, conferring resistance towards the agent. It is plausible that this can be a response attempted by the pathogen to overcome the inhibition of other genes (Barelle et al. 2008). Moreover SAP2 is one of the leading vaccine candidates and has a deep rooted function in candida virulence. Targeting the SAP2 enzyme is rather important because they play a crucial role in cleaving the hydrophobic amino acids in the host protein especially at the site of phenylalanines (Ghadjari et al., 2006, Naglik et al., 2004, Shukla and Rohatgi, 2020). Therefore the challenge in treating candida infections is to identify a safe, cost-effective and potent anti-fungal nil or infrequent side effects.
Plants are well known for their medicinal expertise since ancient times. Plant based medicine and traditional medicine is practised nearly by half of the population in developing and under developed nations (Al-Dhabi et al., 2015, Bin et al., 2020). Secondary metabolites are inducible in nature and are generally produced by the plants as a response to pathogen attacks. Compounds such as flavonoids, phenols, terpenoids, tannins, have significant toxicity and high molecular weight which makes them potent antifungal agents (Al-Dhabi and Arasu, 2016, Barathikannan et al., 2016, Seca and Pinto, 2019). Allium genus vegetables that belong to Amaryllidaceae family have remarkable medicinal properties and important ingredient of folklore medicine. A. ampeloprasum (wild leeks) and its variety A. porrum (cultivated leeks) are a part of a few cuisines and one of the least studied dietary allium vegetable. The well-known species of the Allium genus are onion (A. cepa) and garlic (A. sativum) which are consumed in everyday human diet and subjected to various biological studies (Bianchini and Vainio 2001). A. cepa and A. sativum are potential antimicrobial agents and a recent study reports that Silver nanoparticles synthesized from these species have shown to exert antimicrobial activity against vaginal pathogens Streptococcus pneumoniae and Pseudomonas aeruginosa (Bouqellah et al., 2018). Novel bioactive metabolites from A. sativum have been recently identified as potent anti-cancer drug targets (Padmini et al., 2020). Numerous other vegetables under the Allium genus have significant medicinal values but still remain unrecognized and underutilized. Like other Allium species, leeks are superior sources of secondary metabolites that include flavonoids, phenolic acids and their derivatives that medicinal values and health benefits (Dey and Khaled, 2015). A. ampeloprasum are regarded as low energy foods and are reported to have comparatively higher amounts of fibre and zinc than their frequently studied relatives. Additionally they are rich in linoleic acid and bioactive compounds that can revolutionize the current diet (García-Herrera et al., 2014, Ilavenil et al., 2017, Valsalam et al., 2019). Few studies show that leeks possess anti-helmintic, anti-inflammatory, antioxidant, antimicrobial and anti-cancer properties (Maidment et al., 2001, Lu et al., 2011, Sunaica et al., 2009). Moreover plant compounds are known to exert least side effects and they have the major advantage of being a part of the daily diet and a source of prevention as well as cure (Karimi et al. 2015). In the present study, combined in vitro phytochemical analysis, spectral analysis, antioxidant assays, anti-candida assays and in silico molecular docking approach has been carried out to evaluate the inhibitory effect of Allium ampeloprasum and A. porrum extracts on pathogenic Candida species.
2. Materials and methods
2.1. Reagents and plant materials
Solvents and chemicals used in the experiment were analytical grade and purchased from Hi-Media (India). Double distilled water was used throughout the experiment. The plant materials were purchased from a local producer at Kodaikanal mountainous region (Tamil Nadu, India).
2.2. Preparation and extraction of plant material
A. ampeloprasum and A. porrum are characterized by green stalks and long white stem. The procured plant material was transported to the laboratory, washed twice under running tap water and once with double distilled water. The roots and stalks (10 cm away from the white stem) were separated. The remaining portion was chopped into small sections and dried under shade for 10 days, later powdered in a laboratory grinder and stored in air tight containers at room temperature. The extracts were prepared by cold percolation method, the plant powders were weighed and added to different solvent and left for 72 h. The extracts were filtered using Whatman filter paper, grade 1 (11 μm pore size), evaporated and stored for further analysis at 4 °C.
2.3. Phytochemical analysis
2.3.1. Qualitative phytochemical analysis
Preliminary phytochemical screening was done using the procedures of Harborne, 1984, Brunton, 1995, Wagner et al., 1984. The extracts were screened for the presence of alkaloids, flavonoids, phenols, saponins, tannins and glycosides.
2.3.2. Quantitative determination of total phenolic content
Total phenolic content (TPC) was determined by Folin-Ciocalteu method according to the method of Haq et al. (2012). The absorbance was measured at 700 nm using Shimadzu UV–Visible spectrometer and the calibration curve (Supplementary Fig. 1) was plotted using Gallic acid as the standard (y = 0.0078x + 0.073; R = 0.98). TPC was expressed as Gallic Acid Equivalents (GAE)/ g weight.
2.3.3. Quantitative determination of total flavonoid content
Total flavonoid content (TFC) was determined by Aluminium chloride method as described by Chang et al. (2002). The absorbance was measured at 405 nm using Shimadzu Uv–visible spectrometer and the calibration curve (Supplementary Fig. 2) was plotted using Quercetin as standard (y = 0.0056x + 0.1939; R = 0.98). TFC was expressed as Quercetin Equivalents (QE) /g weight.
2.4. Spectral studies
2.4.1. Absorption spectroscopy
Ultra Violet-Visible (UV–Vis) spectroscopic analysis was performed to identify the presence of phytoconstituents in the plant extracts. The aqueous plant extracts (mg/ml concentration) were dissolved in double distilled water and the spectroscopic analysis was carried out in Shimadzu UV–Vis spectrophotometer UV-2600 in the range 200–800 nm (Elgubbi et al. 2019).
2.4.2. Vibrational spectroscopy
Fourier transform Infrared (FTIR) spectroscopic analysis was performed to identify the functional groups present in the plant extracts. 0.1 mg of the dry plant extracts of macerated gently with Potassium bromide and processed to obtain a transparent pellet. The pellet was analysed using Perkin Elmer spectrum 100 N in the range 4000–500 cm−1 (Elgubbi et al. 2019).
2.5. Determination of antioxidant activity
Antioxidant activity of A. ampeloprasum and A. porrum extracts were determined using different invitro antioxidant assays. DPPH (2,2-diphenyl-1-picrylhydrazyl) radical scavenging assay was done according to Manzocco et al. (1998) and the absorbance was recorded at 517 nm. The ability of the samples to scavenge H2O2 (Hydrogen peroxide) radicals was determined using the method of Ruch et al. (1989) and the absorbance was measured at 230 nm. ABTS (2, 2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)) is a commonly employed invitro assay to determine the antioxidant activity of a sample. Absorbance was measure for the samples at 750 nm according to the method of Seeram et al. (2006). FRAP (Ferric Reducing Antioxidant Power) analysis was done to evaluate the reducing power of the extracts. It was carried out according to the method of Oyaizu (1986) and the absorbance was measured at 700 nm. Phosphomolybdenum assay was done to study the total antioxidant capacity by the method described by Prieto et al. (1999). The absorbance of the samples was recorded at 695 nm. All the assays were carried out in triplicates.
2.6. In vitro determination of anticandida activity
2.6.1. Agar well diffusion method
The anti-candida activity of A. ampeloprasum and A. porrum extracts was evaluated against 5 Candida species; C. albicans -MTCC 183, C. tropicalis -MTCC 184, C. glabrata -MTCC 3019, C. parapsilosis MTCC −7043 and C. krusei -MTCC 9215. Candida strains were acquired from Microbial Type Culture Collection and Gene Bank (MTCC), Chandigarh, India. Candida cultures were preserved in Potato dextrose agar (PDA) slant at 4 °C. The culture was incubated in Potato dextrose broth (PDB), overnight in incubator cum shaker at 35 °C. The optical density of the candida culture suspensions were adjusted to 0.5 McFarland standards. Individual inoculum contained approximately 107 CFU/mL. Well diffusion assay was used to determine the anticandida activity of the extracts. PDA was sterilized and poured into autoclaved petridishes. 0.2 ml of various strains of Candida species were uniformly spread on individual petridishes. Plates were allowed to stand for 10 min to facilitate the absorption of the inoculum on the agar. Wells were then cut on the culture media and various dilutions of aqueous A. ampeloprasum extract and A. porrum extract (50 and 100 µl/ml) were added to each well. Amphotericin B was used as the standard. The plates were incubated at 37 °C for 24 h. After 24 h the inhibition zone diameters were measured and expressed in millimetres.
2.6.2. Minimum inhibitory concentration by broth macrodilution
The procured candida pathogens were allowed to grow for 24 h on sterile PDA plates at 37 °C. Sterile test tubes containing 5 ml of Potato dextrose broth were inoculated with candida pathogens that were previously grown in the same medium and adjusted to 0.5 McFarland scale. Two fold dilutions of the aqueous plant extract was added to the suspension medium, thoroughly mixed and incubated for 24 h at 37 °C. MIC of the drug was observed as the concentration at which more than 75% of the candida growth was inhibited in the broth. Amphotericin B was used as the standard antifungal agent (Balouiri et al., 2015).
2.7. In silico determination of anti-candida activity by molecular docking
Molecular docking was performed for fifteen bioactive compounds from A. ampeloprasum and A. porrum based on literature and is depicted in the Supplementary Table 1. Molecular docking was performed with AutoDockv4.2.3 in order to predict the binding and structure of the intermolecular complex between the drug targets and the identified phytocompounds. The search methods used are simulated annealing, Genetic Algorithm (GA) and the Lamarckian Genetic Algorithm (LGA). The most efficient and reliable method is LGA (Morris et al. 1998). Ligands were prepared by retrieving the structures of identified phytocompounds from PubChem database. The structure of drug target was pre-processed by removing ligands, heteroatoms and water molecules from their respective PDB crystal structures (Morris et al., 2009, Morris et al., 1998). A grid maps for all atom types of target was generated independently along with electrostatic and desolvation maps by fixing the grid box of 90 × 90 × 90 points with AutoGrid utility. The grid spacing was set to 0.375 Å and was centered based on the co-crystallized inhibitors of the respective drug target (Saravanan et al., 2012, Padmini et al., 2016).
2.8. Statistical analysis
The results are expressed as mean ± standard error. One way ANOVA was carried out to determine the significance of the tests with p ≤ 0.05 significance level. Microsoft excel 2010, SPSS Version 19.0, Origin Pro 8 packages were used for the analysis.
3. Results
3.1. Preliminary analysis
3.1.1. Qualitative phytochemical screening of crude extracts
The plant extracts were screened for various classes of phytocompounds. The results are represented in Table 1. Flavonoids were present in all the solvent extracts of A. ampeloprasum and A. porrum. Aqueous extract of A. ampeloprasum showed the presence of alkaloids, flavonoids, saponins, terpenoids, phenols and glycosides whereas alkaloids were absent in the A. porrum aqueous extract. Phytochemicals were effectively extracted in polar and mid-polar solvents rather than non-polar solvent which were evident in the results of hexane extract.
Table 1.
Phytochemical screening of A. ampeloprasum and A. porrum extracts.
| Phytochemicals |
A. ampeloprasum |
A. porrum |
||||||
|---|---|---|---|---|---|---|---|---|
| M | Aq | Ac | H | M | Aq | Ac | H | |
| Alkaloids | – | + | + | – | + | – | – | – |
| Flavonoids | + | + | + | + | + | + | + | + |
| Saponins | – | + | + | – | + | + | – | – |
| Terpenoids | + | + | – | – | – | + | + | + |
| Phenols | + | + | + | – | + | + | + | – |
| Glycosides | – | + | – | – | – | + | + | – |
M: Methanol; Aq: Aqueous; Ac: Acetone; H: Hexane.
+: presence of phytochemicals; −: absence of phytochemicals.
3.1.2. Quantification of total phenolic and flavonoid compounds
The total phenol content in the aqueous extract of A. ampeloprasum was 14.35 ± 0.01 mg GAE/g which was higher than the other extracts. The least was quantified in hexane extract of A. porrum (1.96 ± 02 mg GAE/g). Flavonoid content in the extracts of both plants was superior when compared to the total phenolics content. The highest amount of flavonoid content was quantified in aqueous extract of A. ampeloprasum (19.83 ± 0.02 mg QE/g). Methanol and acetone extracts also showed considerable amounts of total phenolics and flavonoids (Table 2).
Table 2.
Total phenol and flavonoid content in A. ampeloprasum and A. porrum.
| Extract |
Total Phenolic Content (mg GAE/g)a |
Total flavonoid Content (mg QE/g)a |
||||||
|---|---|---|---|---|---|---|---|---|
| M | Aq | Ac | H | M | Aq | Ac | H | |
| A. ampeloprasum | 8.13 ± 0.07 | 14.35 ± 0.01 | 10.56 ± 0.02 | 3.47 ± 01 | 15.26 ± 0.07 | 19.83 ± 0.02 | 9.34 ± 0.01 | 1.32 ± 0.09 |
| A. porrum | 7.16 ± 0.09 | 13.52 ± 0.07 | 10.33 ± 0.01 | 1.96 ± 02 | 11.42 ± 0.09 | 17.62 ± 01 | 11.51 ± 0.02 | 0.81 ± 0.03 |
M: Methanol; Aq: Aqueous; Ac: Acetone; H: Hexane.
Mean values (n = 3) ± standard error.
3.2. Spectroscopic analysis
3.2.1. Analysis of phytocompounds absorption spectra by UV–Visible spectroscopy
The qualitative UV–Vis spectrum of A. ampeloprasum and A. porrum aqueous extracts are represented in Fig. 1. Absorption bands were obtained at 236 nm and 275 nm for both the extracts and an additional band was observed for A. ampeloprasum extract at 316 nm.
Fig.1.
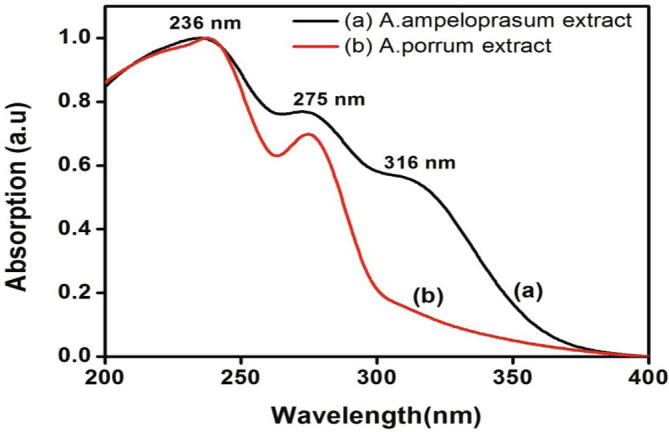
UV–Visible spectrum of A. ampeloprasum and A. porrum aqueous extracts.
3.2.2. Identification of functional groups by FT-IR analysis
FT-IR analysis was carried out to identify the functional groups of bioactive compounds present in the aqueous extracts of A. ampeloprasum and A. porrum. Peaks obtained and their assigned functional groups are given in Fig. 2. Absorption bands were obtained at 3398.10 cm−1, 2937.37 cm−1, 1644.76 cm−1, 1129.4 cm−1, 1054 cm−1, 1017.91 cm−1 and 625.04 cm−1 for aqueous A. ampeloprasum extract, whereas characteristic peaks were attained at 3418 cm−1, 2922.21 cm−1, 1615.07 cm−1, 1315.61 cm−1, 1263.01 cm−1, 1106.69 cm−1 and 678.25 cm−1. The FT-IR spectrum of both the extracts confirmed the presence of alcohols, amides, aromatics, amines, carboxylic acid and disulphide groups. Additionally, sulfoxide group was identified in A. ampeloprasum extract.
Fig. 2.
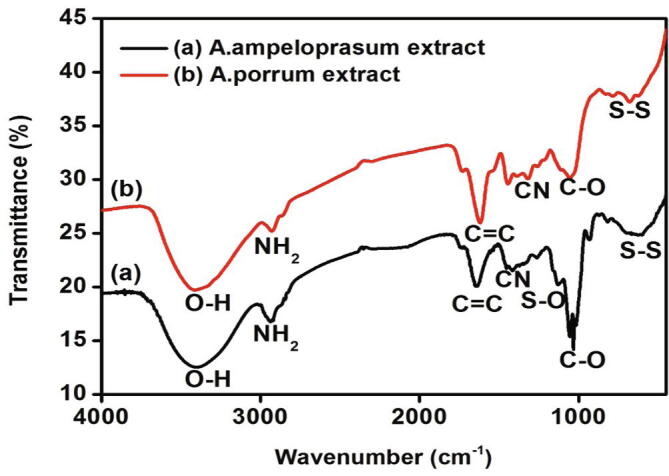
FTIR spectrum illustrating identified functional groups in A. ampeloprasum and A. porrum aqueous extracts.
3.3. In vitro free radical scavenging, reducing power and total antioxidant capacity
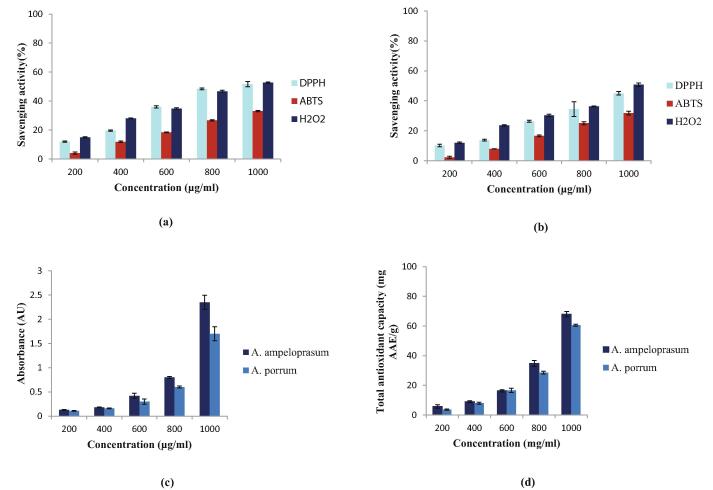
Results represented in Fig. 3a and 3b showed that A. ampeloprasum extract was effective in scavenging the DPPH, ABTS and H2O2 radicals when compared to A. porrum. IC50 values of A. ampeloprasum aqueous extracts were 0.88 ± 2.1 mg/ml (DPPH assay), 1.42 ± 0.6 mg/ml (ABTS assay) and 0.93 ± 1.2 mg/ml (H2O2 assay), whereas aqueous extract of A. porrum exhibited IC50 values of 1.12 ± 1.1 mg/ml, 1.50 ± 1.5 mg/ml and 1.05 ± 0.5 mg/ml for DPPH, ABTS and H2O2 assays respectively. The inhibition activity of the extracts increased with the increase in the concentration and thus is dose dependent. The IC50 value of Ascorbic acid was 0.33 ± 0.2 mg/ml and 0.39 ± 0.1 mg/ml for DPPH and H2O2 assay respectively, Trolox exhibited an IC50 value 0.59 ± 1.3 mg/ml for ABTS assay. Results of FRAP assay as presented in Fig. 3c revealed that the reducing power of the extracts was concentration dependent and an increase was observed in the absorbance of the samples at higher sample concentrations. The maximum absorbance recorded was 2.35 for 1000 μg/ml of A. ampeloprasum extract. 68 ± 1.78 mg AAE/ g and 60.57 ± 0.6 mg AAE/ g was found to be the total antioxidant capacity of A. ampeloprasum and A. porrum extract at g/ml concentration as estimated by Phosphomolybdenum assay (Fig. 3d).
Fig.3.
(a) Free radical scavenging activity of A. ampeloprasum extract, (b) Free radical scavenging activity of A. porrum extract, (c) Reducing power of A. ampeloprasum and A. porrum extracts (d) Total antioxidant capacity of A. ampeloprasum and A. porrum extracts.
3.4. In vitro anticandida activity
3.4.1. Zone of inhibitions determined by agar well diffusion assay
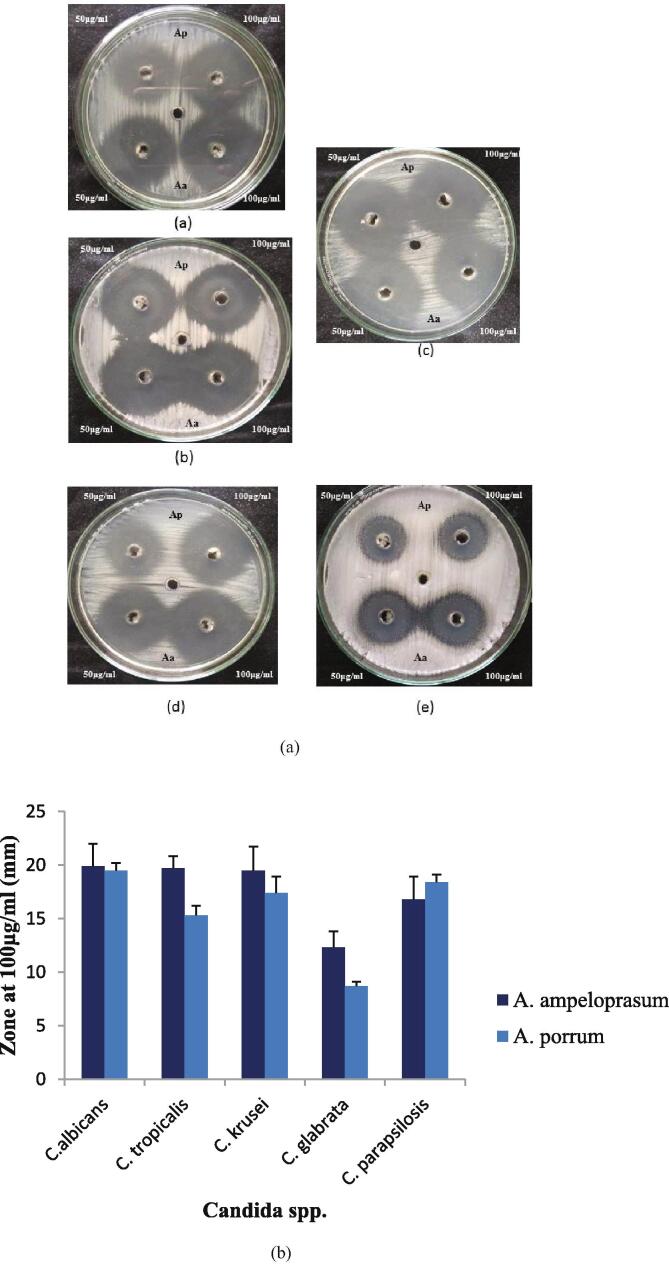
Aqueous extract of A. ampeloprasum showed significant anticandida activity against the treated pathogens. Negative control i.e., distilled water did not exhibit anticandida activity. Fig. 4a shows that the maximum zone was observed for A. ampeloprasum extract against C. albicans (19.9 ± 1.1 mm). The inhibitory activity of aqueous A. porrum extract was also high against C. albicans and a 19.5 ± 0.3 mm zone was observed. The susceptibility of C. glabrata was less when compared to the other organisms as represented in Fig. 4b. 12.3 ± 0.8 mm and 8.7 ± 0.1 mm zones were observed against C. glabrata when treated with A. ampeloprasum and A. porrum extract respectively. The zones of inhibition for C. tropicalis, C. krusei and C. parapsilosis were within the range of 15–19 mm. The results revealed that the inhibitory action of the extracts used was dose dependent. The zone of inhibition observed for Amphotericin B was within the range of 16 ± 1.2–21 ± 0.9 mm for the tested pathogens.
Fig. 4.
(a) Zone of inhibitions of A. ampeloprasum (Aa) and A. porrum (Ap) at 50 and 100 μg/ ml against (a) C. albicans, (b) C. tropicalis, (c) C. krusei, (d) C. parapsilosis and (e) C. glabrata. (b) Statistical comparison of anticandida activity of extracts with standard error.
3.4.2. Minimum inhibitory concentration determined by broth dilution method
A. ampeloprasum and A. porrum extracts were tested for their MIC in serially diluted concentrations. Analysis of Variance (One way ANOVA) revealed that the results were statistically significant (p ≤ 0.05). Results presented in Table 3 show that the lowest MIC was observed against C. albicans when treated with both extracts individually which showed that C. albicans were the most susceptible organism. As observed in anticandida activity by well diffusion method, C. glabrata was the least susceptible organism and a concentration of 33 ± 2.9 μg/ ml of A. ampeloprasum extract and 40 ± 2.9 μg/ ml of A. porrum extract were required to inhibit the organism. The MIC of Amphotericin B was observed in the range 10 ± 0.3–17 ± 0.7 μg/ ml.
Table 3.
Minimum inhibitory concentrations of A. ampeloprasum and A. porrum aqueous extracts for Candida pathogens.
| Candida Species |
Minimum Inhibitory concentration (μg/ml)a |
|
|---|---|---|
| A. ampeloprasum extract | A. porrum extract | |
| C. albicans | 19 ± 3.5 | 21 ± 2.9 |
| C. tropicalis | 24 ± 1.6 | 25 ± 1.4 |
| C. krusei | 25 ± 1.9 | 40 ± 3.1 |
| C. glabarata | 33 ± 2.9 | 40 ± 2.9 |
| C. parapsilosi | 22 ± 1.0 | 35 ± 2.4 |
Mean (n = 5) ± standard error, with significant differences at P < 0.05.
3.5. In silico molecular docking to determine the interaction of phytocompounds at the active site of SAP 2 enzyme
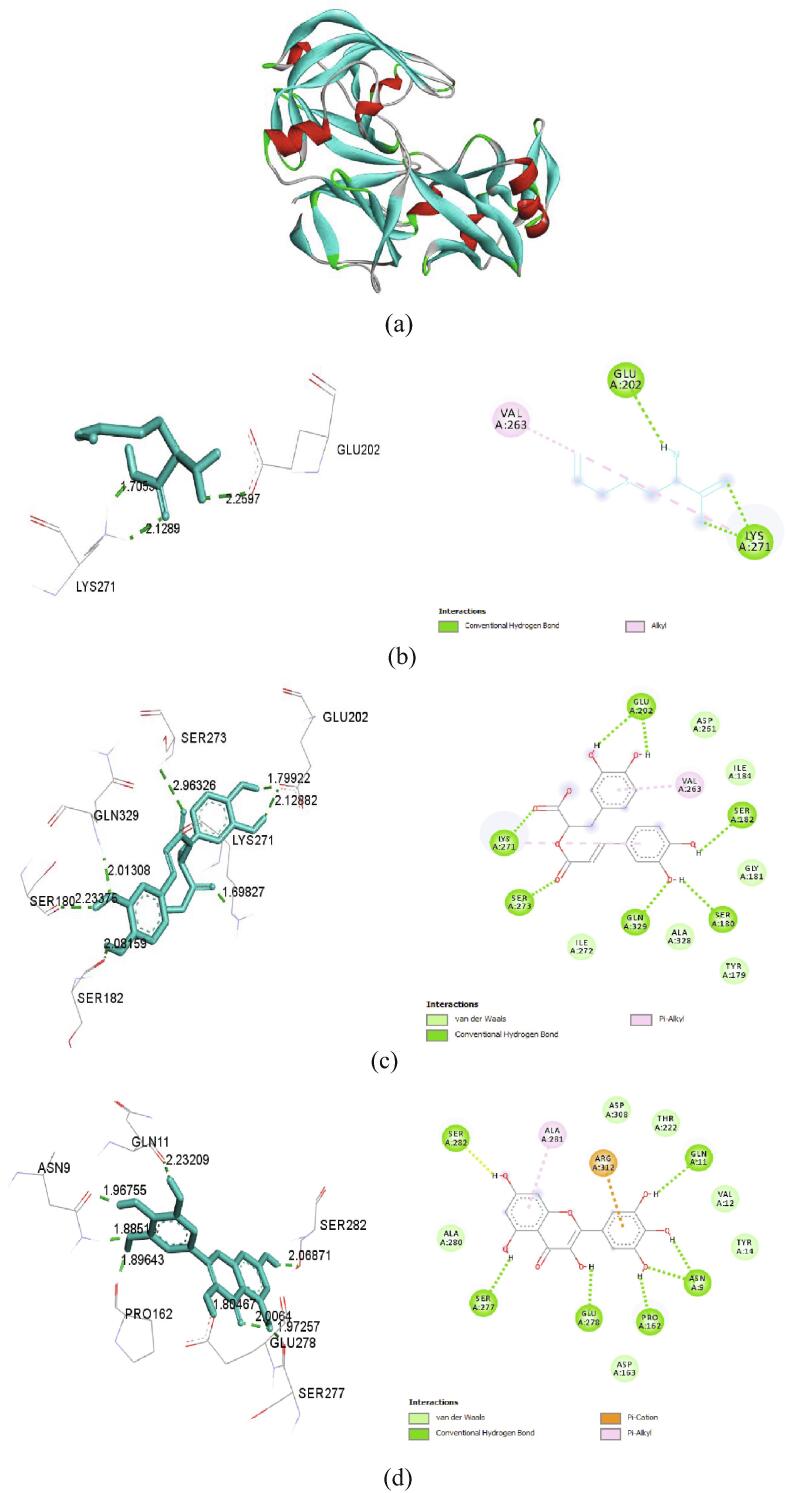
Results obtained from in silico molecular docking studies performed by Autodock software elucidated the interaction between ligands and target. Selected compounds from organo sulphur, phenol and flavonoid groups satisfied the Lipinski’s rule of five that includes criteria such as molecular weight, log P (<+5.6), hydrogen donors (<5), hydrogen acceptors (<10) and molecular refractivity within the range of 40–130 (Supplementary Table 2). The active site of SAP-2 enzyme was docked with the phytocompounds from A. ampeloprasum and A. porrum. The three dimensional structure of SAP 2 enzyme is represented in Fig. 5a. Ligands with greater affinity range indicated stronger binding of the compounds with the enzyme. Among the organosulfur compounds S allyl cysteine had the lowest binding energy of −4.27 kcal/mol and three hydrogen bond interactions with amino acids Glu202 and Lys271 (2) at the active site of SAP 2 enzyme. Rosmarinic acid showed a binding energy of −5.98 kcal/mol which was the lowest among the phenolic acids, hydrogen bond interactions were observed between amino acids such as Glu202 (2), Ser182, Ser180, Gln329, Ser273 and Lys271 present in the active site of the enzyme. Myricetin exhibited a binding energy of −6.11 kcal/mol and hydrogen bond interactions was visible between amino acids such as Ser282, Ser277, Glu278, Pro162, Asn9 (2) and Gln11. Rosmarinic acid and Myricetin formed the highest number of hydrogen bonds (7 bonds) with the active site of the enzyme (Table 4). Binding efficiency is higher in compounds that have the negative binding energy and the number of hydrogen bond determine the binding strength. The two dimensional and three dimensional interactions of S Allyl cysteine, Rosmarinic acid and Myricetin with SAP 2 enzyme are depicted in Fig. 5(b-d).
Fig. 5.
(a) Three dimensional representation of SAP protein (PDB ID:3PVK), 2D and 3D representation with 3PVK structure of (b) S allyl cysteine, (c) Rosmarinic acid (d) Myricetin.
Table 4.
In silico molecular docking study of phytocompounds and their interactions.
| S. No | Phytochemical active compounds | Binding energy (kcal/mol) | Hydrogen bond interactions | No of h-bond | Hydrogen bond interacting residues | Hydrogen bond distance (Å) | Other interactions |
|---|---|---|---|---|---|---|---|
| Diallyl disulfide | −3.54 | LEU296 |
1 |
LEU296(HN…S) |
3.05 | GLN295,LEU297,ILE223, TYR252,PHE251,ILE235, PHE298,ASP229 |
|
| Diallyl trisulfide | −3.56 | GLN329 |
1 |
GLN329(HN…S) | 2.52 | ALA270,LEU327,ILE272, VAL263,LYS271 |
|
| S allyl cysteine | −4.27 | GLU202,LYS271[2] |
3 |
GLU202(OE2…H) LYS271(HZ2…O) LYS271(HZ1…O) |
2.26 1.71 2.13 |
VAL263 | |
| Ajoene | −3.65 | ILE223,TYR225 | 2 | TYR225(HH…O) ILE223(HN…O) |
2.20 2.32 |
ILE305,ILE123,ILE30 | |
| S allyl mercapto cysteine | −3.02 | LYS81,GLN91 | 2 | LYS81(HZ1…0) GLN91(HE2…0) |
1.70 2.20 |
SER90,SER118,GLN48, THR117,SER116,SER89, GLY87 |
|
| Gallic acid | −4.8 | SER180,LYS271[2],SER182 | 4 | SER180(O…H) LYS271(O…H) LYS271(O…H) SER182(O…H) |
1.85 1.74 1.81 2.11 |
LEU183,TYR179GLY181, GLN329,ILE272,ILE184, ALA270,A269,ALA328 |
|
| Ferulic acid | −5.02 | ASP138,ASN153,ASP321, LYS145,LYS129 |
5 |
ASP138(OD1…H) ASN153(HD22…O) ASP321(OD1…H) LYS129(HZ1…O) LYS145(HZ1…O) |
1.94 1.96 1.71 2.29 2.52 |
LYS146,LEU320,VAL142 | |
|
Rosmarinic acid |
−5.98 |
GLU202[2],SER182,SER180 GLN329,SER273,LYS271 |
7 |
GLU202(OE1…H) GLU202(OE1…H) SER182(O…H) SER180(O…H) GLN329(HN…O) SER273(HN…O) LYS271(HZ2…O) |
1.79 2.12 2.08 2.23 2.01 2.96 1.69 |
ASP261,ILE184,GLY181, TYR179,ALA328,ILE272, VAL263 |
|
| Sinapinic acid | −5.29 | ASP138,LYS129,LYS145, ASP321,ASN153 |
5 | ASP138(OD…H) LYS129(HZ…O) LYS145(HZ…O) ASP321(OD…H) ASN153(HD…O) |
1.82 1.69 2.88 1.87 1.98 |
LYS146,VAL142,TYR128, LEU320 |
|
| Caffeic acid | −4.66 | LYS271[2],SER182,LEU27 | 4 | LYS271(HZ…O) LYS271(O…H) LEU327(O…H) SER182(O…H) |
1.69 1.83 2.81 1.96 |
ASN269,ALA270,ILE272, GLN329,LEU183,ALA328, GLY181,ILE184 |
|
| Naringenin |
−6.7 | ASN323,LEU320,THR130, VAL142 |
4 | ASN323(OD…H) LEU320(O…H) THR130(OG…H) VAL142(O…H) |
2.22 2.79 2.03 2.03 |
ASN153,ASP321,ARG192, ASP191,LYS129,LYS146, LYS145,TYR128 |
|
| Apigenin | −5.9 | THR6[2],ASP17,THR19 | 4 | THR6(HG…O) THR6(O…H) ASP17(HN…O) THR19(OG…H) |
2.86 1.81 2.18 2.04 |
GLY103,LEU7,ALA16, GLY102,PHE101,GLY100, ILE18,ASN24,HIS8, VAL5 |
|
| Quercetin |
−6.1 | ASP302[2],ASP211,ASN212, ASP214 |
5 | ASP302(O…H) ASP302(O…H) ASP211(OD…H) ASN212(O…H) ASP214(OD…H) |
2.35 2.23 2.20 2.60 1.78 |
ALA303,ARG195,ASN304, GLN227,LEU230,VAL213 |
|
| Kaempferol |
−6.5 | THR130[2],VAL142,LEU320, ASN323,ASP321 |
6 | THR130(NH…O) THR130(OG…H) VAL142(O…H) LEU320(O…H) ASN323(OD…H) ASP321(OD…H) |
1.84 1.93 2.19 2.84 2.27 1.93 |
ASP191,ARG192,LYS129, ASN153,LYS146,TYR128, LYS145 |
|
| Myricetin | −6.11 |
SER282,SER277,GLU278, PRO162,ASN9[2],GLN11, |
7 |
SER282(OG…H) SER277(O…H) GLU278(OE…H) PRO162(O…H) ASN9(OD…H) ASN9(HD…O) GLN11(O…H) |
2.06 1.97 1.80 1.89 1.96 1.88 2.23 |
ALA280,ASP308,THR222, VAL12,TYR14,ASP163, ARG312,ALA281 |
3.6. Discussion
Candida pathogens and related infections have become a major threat to the global health due to their multiple drug resistance traits. Bioactive compounds from higher plants have been a promising source to treat such infections since the time of early drug discovery. Presence of potential active metabolites in Allium vegetables has made them suitable candidates in the pharmaceutical industries. They also exhibit efficient anti-fungal activity against various fungal and yeast pathogens. Diba and Alizadeh 2018 have reported the anticandida activity of A. sativum and A. hirtifolium against clinical isolate of C. tropicalis, whereas Mikaili et al 2013 has reported the anticandida activity of A. sativum against C. albicans. Antifugal activity of A. cepa and A. sativum against dermatophytes has been reported by Latheef et al. 2020.
The present study focussed on combining the invitro spectral, antioxidant, anticandida studies with in silico molecular docking studies to evaluate the inhibitory potential of underutilized Allium vegetable A. ampeloprasum and its variety A. porrum. It was evident in the preliminary phytochemicals screening that aqueous extracts of both plants showed the presence of numerous phytochemicals when compared to the other extracts. According to Szychowski et al. (2018) aqueous extracts are found to be efficient in several biological activities due to their efficacy to extricate compounds. Results are in accordance with Asemani et al. (2019) who reports that allium vegetables such as A. sativum, A. cepa, A. hirtifolium, A. jesidianum and A. porrum are a rich source of phytochemicals such as flavonoids, phenolic acids, alkaloids, saponins and organo sulphur compounds. Zhang et al. (2020) has suggested A. sativum as a promising candidate for the treatment of cancer due to the similar active phytocomponents reported in our results. Results of total phenol content was consistent with the reports of Sagar et al. (2020) and total flavonoid content in the aqueous samples of the present study were parallel to the results of Albishi et al. (2013) in onion samples. The flavonoid content of A. porrum in the present study (17.62 ± 01 mg QE/g) was higher than the results obtained by Radovanovic et al. (2015) for A. porrum leaves (10.24 ± 2.84 mg QE/g).
Elgubbi et al. (2019) suggests that the bands in Uv–Vis spectrum within the range of 250 nm to 285 nm confirm the presence of flavonoid compounds which supports the results of the present study. Similarly the report also suggests that the presence of hydroxyl and carbonyl groups in the FT-IR spectrum are related to the flavone compounds of the flavonoid group. A study by Tasci et al. (2016) showed that FT-IR spectrum of alliin and allicin in A. scorodoprasum showed the presence of OH, COOH, NH2, S = O, C-O, CN and C-S groups. This finding is consistent with our results from the FT-IR spectrum and suggests that A. ampeloprasum and A. porrum can be good sources of organosulfur compounds and their precursors.
The higher phenol and flavonoid content correlated with the antioxidant activity of the plant sample. Presence of phenols, flavonoids and other phytoconstituents justifies the efficient free radical scavenging activity, reducing power and antioxidant capacity of the tested samples. The results of FRAP assay run parallel to the results of Locatelli et al. (2016) which reported that the dominant organosulfur compounds present in the allium vegetables such as allicin and ajoene have high reducing power activity. The antioxidant activity depends on the number of sulfur atoms and their ability to bond; therefore trisulfides are better scavenging agents when compared to disulphide. Allyl methyl disulphide, Methyl propyl sulphide, Dimethyl trisulfide, Dimethyl didulfide, Methyl propyl trisulfide, Propenyl propyl trisulfide are the few sulfur rich compounds reported in leeks (Monika and Abirami, 2018). Moreover, release of the gasotransmitter Hydrogen sulphide (H2S) is one of the most interesting and the least studied free radical scavenging mechanism of organosulfur compounds. Digestion of organosulfur compounds is believed to cause slow release of Hydrogen sulphide (H2S) through enzymatic and chemical reactions. H2S is reported to be produced in the gut through the digestion of leeks and is considered as an excellent endogenous reducing agent. H2S is a highly reactive molecule and can effectively scavenge free radicals such as superoxide radical anion, hydrogen peroxide, hypochlorite and peroxynitrite (Wen et al. 2018).
The findings of Diba and Alizadeh (2018) demonstrated that aqueous extracts of A. hirtifolium and A. sativum exhibited significant (p ≤ 0.05) anticandida activity against C. tropicalis, moreover the study suggests that phenols, flavonoids and organo sulphur compounds in the samples were responsible for the anti-candida activity. Similarly our results showed that A. ampeloprasum and A. porrum significantly (p ≤ 0.05) inhibited the growth of Candida pathogens and additionally the phytochemicals screening results supports the same. Agi and Azike (2019) suggest that 1 g/ml of fresh A. sativum was required to inhibit C. albicans whereas our results showed that 100 μg/ ml of A. ampeloprasum and A. porrum were efficient in inhibiting C. albicans. MICs of A. hirtifolium and A. sativum were 11.66 ± 0.36 mg/ml and 21.85 ± 0.57 mg/ml against C. tropicalis, however 24 ± 1.6 μg/ml and 25 ± 1.4 μg/ml were the determined MICs of A. ampeloprasum and A. porrum extracts required to inhibit C. tropicalis in this study. However the findings of the in vitro studies showed that wild leeks were rich in phytochemicals and biological properties when compared to the cultivated variety which implies the importance of propagating wild varieties of medicinal plants.
Structure based designing of drugs plays an important role in the development of novel drugs to target several diseases. Molecular docking analysis of selected A. ampeloprasum and A. porrum bioactive compounds from the literature against SAP 2 enzyme/Candidapesin 2 demonstrated that organo sulfur compounds, phenolic acids and flavonoids present in the samples can be developed as potential drug candidates to treat candida infections effectively. Several phytocompounds from the plant Trachyaspermum ammi were docked against Candidapepsin-1 enzyme, among which Ligustilide showed the lowest binding energy of −5.75 kcal/ mol, this was parallel to the docking results of Rosmarinic acid which showed a binding energy of −5.98 kcal/mol in our study. However the binding energy of S allyl cysteine (organo sulfur compound) was lower than the compounds reported in T. ammi (Biswal et al. 2019). The number of hydrogen bonds formed was greater as observed from our docking results of Rosmarinic acid (phenolic acid) and Myricetin (flavonoid). Flavonoids and phenolic compounds exhibited better interactions when compared to the OSCs, this might be due to the low number of hydrogen bond donors and acceptors in the OSCs. Phenolic compounds and flavonoids docked in the study showed higher number of hydrogen bond donors and hydrogen bond acceptors denoting them as potent orally active drugs with low lipophilicity. Further the phenolic compounds and flavonoids present in the allium species can support the activity of the OSCs (Maharani et al. 2020). SAP enzymes are considered as the major factors that determine the virulence of Candida spp. Ten SAP enzymes have been reported in C. albicans, four types of SAP enzyme have been reported in C. tropicalis, eight SAP enzymes have been identified in C. dubliniensis and three types of SAP are studied in C. parapsilosis. Strikingly none of the SAP enzymes are reported in C. glabrata (Parra-Ortega et al., 2009, Sikora et al., 2011, Galocha et al., 2019). The results from the in vitro studies and in silico molecular docking studies coincide in the assumption that selected phytocompounds might act as effective inhibiting agents of the SAP enzymes that are responsible for the virulence and cell membrane integrity. Additionally, the in silico studies gives us a plausible reason as to why C. glabrata (absence of SAP enzymes) was the least susceptible species in the in vitro studies.
3.7. Conclusion
Combination of in vitro and in silico studies has been carried out for the first time to evaluate the anti-candida potential of the underutilized allium vegetable A. ampeloprasum and its variety A. porrum. Phenolic acids, flavonoids and organosulfur compounds enhanced the antioxidant property and anticandidal efficacy of the plant extracts. Interaction of the pharmacologically active compounds at the active site of SAP 2/Candidapepsin 2 enzyme with low binding energy and hydrogen bond formation between amino acids provided supporting evidence to the inhibitory activity estimated in the in vitro studies. Thus A. ampeloprasum and A. porrum must be extensively studied like other allium vegetables to evaluate their biological activities and to identify their potential phytocompounds as competent drug of desired therapeutic effect.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors gratefully acknowledge the DSTSERB - Project Ref. No. SB/EMEQ-431/2014, Department of Science and Technology, New Delhi, India. The authors extend their appreciation to The Researchers Supporting Project number (RSP-2020/283), King Saud University, Riyadh, Saudi Arabia. The authors are also grateful to the research collaboration among institutes and universities.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.sjbs.2020.11.082.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Agi V., Azike C. Antifungal Action of Garlic (Allium sativum) and Ginger (Zingiber officinale) on Some Pathogenic Fungi. Asian J. Res. Biochem. 2019;4:1–6. [Google Scholar]
- Albishi T., John J.A., Al-Khalifa A.S., Shahidi F. Phenolic content and antioxidant activities of selected potato varieties and their processing by-products. J. Funct. Foods. 2013;5:590–600. [Google Scholar]
- Al-Dhabi NA, Arasu MV, Rejiniemon TS. 2015. In vitro antibacterial, antifungal, antibiofilm, antioxidant, and anticancer properties of isosteviol isolated from endangered medicinal plant Pittosporum tetraspermum. Evidence-Based Complementary and Alternative Medicine.2015. [DOI] [PMC free article] [PubMed]
- Al-Dhabi NA, Arasu MV. 2016. Quantification of phytochemicals from commercial Spirulina products and their antioxidant activities. Evidence-Based Complementary and Alternative Medicine. 2016. [DOI] [PMC free article] [PubMed]
- Arendrup M.C., Patterson T.F. Multidrug-Resistant Candida: Epidemiology, Molecular Mechanisms, and Treatment. J. Infect. Dis. 2017;216:S445–S451. doi: 10.1093/infdis/jix131. [DOI] [PubMed] [Google Scholar]
- Asemani Y., Zamani N., Bayat M., Amirghofran Z. Allium vegetables for possible future of cancer treatment. Phytother Res. 2019:1–21. doi: 10.1002/ptr.6490. [DOI] [PubMed] [Google Scholar]
- Balouiri M., Sadiki M., Saad I. Methods for In vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2015;6:71–79. doi: 10.1016/j.jpha.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barathikannan K., Venkatadri B., Khusro A., Al-Dhabi N.A., Agastian P., Arasu M.V., Choi H.S., Kim Y.O. Chemical analysis of Punica granatum fruit peel and its in vitro and in vivo biological properties. BMC Compl. Alternative Med. 2016;16:264. doi: 10.1186/s12906-016-1237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barelle C., Duncan V., Brown A., Gow N., Odds F. Azole antifungals induce up-regulation of SAP4, SAP5 and SAP6 secreted proteinase genes in filamentous Candida albicans cells In vitro and in vivo. J. Antimicrob. Chemother. 2008;61:315–322. doi: 10.1093/jac/dkm456. [DOI] [PubMed] [Google Scholar]
- Bassetti M., Righi E., Montravers P., Cornely O.A. What has changed in the treatment of invasive candidiasis? A look at the past 10 years and ahead. J. Antimicrob. Chemother. 2018;73:i14–i25. doi: 10.1093/jac/dkx445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benitez L.L., Carver P.L. Adverse Effects Associated with Long-Term Administration of Azole Antifungal Agents. Drugs. 2019;79:833–853. doi: 10.1007/s40265-019-01127-8. [DOI] [PubMed] [Google Scholar]
- Berkow E., Lockhart S. Fluconazole resistance in Candida species: a current perspective. Infect Drug Resist. 2017;10:237–245. doi: 10.2147/IDR.S118892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchini F., Vainio H. Allium vegetables and organosulfur compounds: do they help prevent cancer? Environ. Health Perspect. 2001;109:893–902. doi: 10.1289/ehp.01109893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bin C., Al-Dhabi N.A., Esmail G.A., Arokiyaraj S., Arasu M.V. Potential effect of Allium sativum bulb for the treatment of biofilm forming clinical pathogens recovered from periodontal and dental caries. Saudi J. Biol. Sci. 2020;27:1428–1434. doi: 10.1016/j.sjbs.2020.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal A., Ragunathan V., Pazhamalai V., Romauld I.S. Molecular docking of various bioactive compounds from essential oil of Trachyaspermum ammi against the fungal enzyme Candidapepsin-1. J. Appl. Pharm. Sci. 2019;9:21–32. [Google Scholar]
- Bouqellah N.A., Mohamed M.M., Ibrahim Y. Synthesis of eco-friendly silver nanoparticles using Allium sp. and their antimicrobial potential on selected vaginal bacteria. Saudi J. Biol. Sci. 2018;26:1789–1794. doi: 10.1016/j.sjbs.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunton J. Text Book of Pharmacognosy Phytochemistry and Medicinal Plants, Intercept Limited. Springer; London: 1995. pp. 592–593. [Google Scholar]
- Chaffin W. Candida albicans Cell Wall Proteins. Microbiol. Mol. Biol. reviews. 2008;72:495–544. doi: 10.1128/MMBR.00032-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C.C., Yang M.H., Wen H.M., Chern J.C. Estimation of Total Flavonoid Content in Propolis by Two Complementary Colorimetric Methods. J Food Drug Anal. 2002;10:178–182. [Google Scholar]
- Dbouk N., Covington M., Nguyen K., Chandrasekaran S. Increase of reactive oxygen species contributes to growth inhibition by fluconazole in Cryptococcus neoformans. BMC Microbiol. 2019;19:1–10. doi: 10.1186/s12866-019-1606-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey P., Khaled K.L. An Extensive Review on Allium ampeloprasum A Magical Herb. Int. J. Sci. Res. 2015;4:371–377. [Google Scholar]
- Diba A., Alizadeh F. In vitro and in vivo antifungal activity of Allium hirtifolium and Allium sativum. Avicenna J. Phytomed. 2018;8:465–474. [PMC free article] [PubMed] [Google Scholar]
- Elgubbi H., Altajtal A., Elbath A. Phytochemical Screening Study and Anti-Candida Evaluation of Acacia raddiana Leaves. EC Nutrition. 2019;14:499–507. [Google Scholar]
- Galocha M., Pais P., Cavalheiro M., Pereira D., Viana R., Teixeira M.C. Divergent Approaches to Virulence in C. albicans and C. glabrata: Two Sides of the Same Coin. Int. J. Mol. Sci. 2019;20:2345. doi: 10.3390/ijms20092345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Herrera P., Morales P., Fernández-Ruiz V., Sánchez-Mata M.C., Cámara M., Carvalho A.M., Tardio J. Nutrients, phytochemicals and antioxidant activity in wild populations of Allium ampeloprasum L., a valuable underutilized vegetable. Food Res. Int. 2014;62:272–279. [Google Scholar]
- Ghadjari A., Matthews R.C., Burnie J.P. Epitope mapping Candida albicans proteinase (SAP 2) FEMS Immunol. Med. Microbiol. 2006;19:115–123. doi: 10.1111/j.1574-695X.1997.tb01080.x. [DOI] [PubMed] [Google Scholar]
- Haq I., Ullah N., Bibi G., Kanwal S., Ahmad M.S., Mirza B. Antioxidant and Cytotoxic activities and phytochemical analysis of Euphorbia wallichii root extract and its fractions. Iran. J. Pharm. Res. 2012;11:241–249. [PMC free article] [PubMed] [Google Scholar]
- Harborne J.B. Phytochemical methods. London chapman and Hall ltd; 1984. pp. 49–188. [Google Scholar]
- Hoot S.J., Smith A.R., Brown R.P., White T.C. An A643V Amino Acid Substitution in Upc2p Contributes to Azole Resistance in Well-Characterized Clinical Isolates of Candida albicans. Antimicrob. Agents Chemother. 2010;55:940–942. doi: 10.1128/AAC.00995-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilavenil S., Kim D.H., Srigopalram S., Kuppusamy P., Arasu M.V., Lee K.D., Lee J.C., Song Y.H., Jeong Y.I., Choi K.C. Ferulic acid in Lolium multiflorum inhibits adipogenesis in 3T3-L1 cells and reduced high-fat-diet-induced obesity in Swiss albino mice via regulating p38MAPK and p44/42 signal pathways. J. Funct. Foods. 2017;37:293–302. [Google Scholar]
- Karimi A., Majlesi M., Rafieian-Kopaei M. Herbaal versus synthestic drugs; beliefs and facts. J. Neuropharmacom. 2015;4:27–30. [PMC free article] [PubMed] [Google Scholar]
- Latheef M., Raj S.M., Salam M., Chitra K. Efficacy of allium cepa and allium sativum against dermatophytes. JASC. 2020;6:2844–2855. [Google Scholar]
- Locatelli D.A., Nazareno M.A., Fusari C.M., Camargo A.B. Cooked Garlic and Antioxidant Activity: Correlation with Organosulphur Compound Compositions. Food Chem. 2016;220:219–224. doi: 10.1016/j.foodchem.2016.10.001. [DOI] [PubMed] [Google Scholar]
- Lu X., Ross C.F., Powers J.R., Aston D.E., Rasco B.A. Determination of Total Phenolic Content and Antioxidant Activity of Garlic (Allium sativum) and Elephant Garlic (Allium ampeloprasum) by Attenuated Total Reflectance-Fourier Transformed Infrared Spectroscopy. J. Agric. Food Chem. 2011;59:5215–5221. doi: 10.1021/jf201254f. [DOI] [PubMed] [Google Scholar]
- Maharani, M. G., Lestari, S. R., Lukiati, B., 2020. Molecular docking studies flavonoid (quercetin, isoquercetin, and kaempferol) of single bulb garlic (Allium sativum) to inhibit lanosterol synthase as anti-hypercholesterol therapeutic strategies. Proceedings of the 3rd international seminar on metallurgy and materials (ismm2019): exploring new innovation in metallurgy and materials.
- Maidment D.C.J., Dembny Z., Watts D.I. The anti-bacterial activity of 12 against. Nutr Food Sci. 2001;31:238–241. [Google Scholar]
- Mani P., Menakha M., Al aboody M., Alturaiki W., Rajendran V. Molecular Docking of Bioactive Compounds from Piper Plants Against Secreted Aspartyl Proteinase of Candida albicans Causing Oral Candidiasis. Int. J Pharm. Clin. Res. 2016;8:1380–1389. [Google Scholar]
- Manzocco L., Anese M., Nicoli M.C. Antioxidant properties of tea extracts as affected by processing. Lebens-mittel-Wissenschaft Und-Technologies. 1998;31:694–698. [Google Scholar]
- Mikaili P., Maadirad S., Moloudizargari M., Aghajanshakeri S., Sarahroodi S. Therapeutic Uses and Pharmacological Properties of Garlic, Shallot, and Their Biologically Active Compounds. IJBMS. 2013;16:1031–1048. [PMC free article] [PubMed] [Google Scholar]
- Monika N., Abirami S.M. Allium porrum: A review. World J. Pharm. Life sci. 2018;4:28–40. [Google Scholar]
- Morris G.M., Goodsell D.S., Halliday R.S., Huey R., Hart W.E., Belew R.K., Olson A.J. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1998;19:1639–1662. [Google Scholar]
- Morris G.M., Huey R., Lindstrom W., Sanner M.F., Belew R.K., Goodsell D.S., Olson A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009;30:2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naglik J., Albrecht A., Bader O., Hube B. Candida albicans proteinases and host/pathogen interactions. Cell Microbiol. 2004;6:915–926. doi: 10.1111/j.1462-5822.2004.00439.x. [DOI] [PubMed] [Google Scholar]
- Oyaizu M. Studies on products of browning reactions: antioxidant activities of products of browning reaction prepared from glucosamine. J Nutr. 1986;44:307–315. [Google Scholar]
- Padmini R., Maheshwari U.V., Saravanan P., Lee K.W., Razia M., Alwahibi M.S., Ravindran B., Elshikh M.S., Kim Y.O., Kim H., Kim H.-J. Identification of novel bioactive molecules from garlic bulbs: A special effort to determine the anticancer potential against lung cancer with targeted drugs. Saudi J. Biol. Sci. 2020;27(12):3274–3289. doi: 10.1016/j.sjbs.2020.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmini R., Muthusamy Razia, Roopavahini V., Aruna S.S. Molecular Docking Studies of Allylsulfur Compounds from Allium sativum against EGFR Receptor. IJCRAR. 2016;3:207–218. [Google Scholar]
- Parra-Ortega B., Cruz-Torres H., Villa-Tanaca L., Hernández-Rodríguez C. Phylogeny and evolution of the aspartyl protease family from clinically relevant Candida species. Mem. Inst. Oswaldo Cruz. 2009;104:505–512. doi: 10.1590/s0074-02762009000300018. [DOI] [PubMed] [Google Scholar]
- Prieto P., Pineda M., Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal. Biochem. 1999;269:337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- Radovanovic B., Mladenovi J., Radovanovic A., Pavlovic R., Nikolic V. Phenolic Composition, Antioxidant, Antimicrobial and Cytotoxic Activites of Allium porrum L. (Serbia) Extracts. J. Food Nutr. Res. 2015;9:564–569. [Google Scholar]
- Ruch R.J., Cheng S.J., Klaunig J.E. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogen. 1989;10:1003–1008. doi: 10.1093/carcin/10.6.1003. [DOI] [PubMed] [Google Scholar]
- Sagar N.A., Pareek S., Gonzalez-Aguilar G.A. Quantification of flavonoids, total phenols and antioxidant properties of onion skin: a comparative study of fifteen Indian cultivars. J. Food sci. Tech. 2020;57:2423–2432. doi: 10.1007/s13197-020-04277-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saravanan P., Dubey V.K., Patra S. Potential selective inhibitors against Rv0183 of Mycobacterium tuberculosis targeting host lipid metabolism. Chem. Biol. Drug Des. 2012;79:1056–1062. doi: 10.1111/j.1747-0285.2012.01373.x. [DOI] [PubMed] [Google Scholar]
- Seca A., Pinto D. Biological Potential and Medical Use of Secondary Metabolites. Medicines. 2019;6:66. doi: 10.3390/medicines6020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeram N.P., Henning S.M., Lee R., Niu Y., Scheuller H.S., Heber Catechin D. and caffeine contents of green tea dietary supplements and correlation with antioxidant activity. J. Agric. Food Chem. 2006;54:1599–1603. doi: 10.1021/jf052857r. [DOI] [PubMed] [Google Scholar]
- Shukla M., Rohatgi S. Vaccination with secreted aspartyl proteinase 2 protein from Candida parapsilosis can enhance survival of mice during Candida tropicalis mediated systemic candidiasis. Infect. Immun. 2020;88:e00312–e320. doi: 10.1128/IAI.00312-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikora M., Dabkowska M., Swoboda-Kopec E., Jarzynka S., Netsvyetayeva I., Jaworska-Zaremba M., Mlynarczyk G. Differences in proteolytic activity and gene profiles of fungal strains isolated from the total parenteral nutrition patients. Folia Microbiol. 2011;56:143–148. doi: 10.1007/s12223-011-0023-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siwek A., Stefańska J., Dzitko K., Ruszczak A. Antifungal effect of 4-arylthiosemicarbazides against Candida species. Search for molecular basis of antifungal activity of thiosemicarbazide derivatives. J Mol. Model. 2012;18:4159–4170. doi: 10.1007/s00894-012-1420-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunaica K.-T.D., Gordana P.D., Aleksandra T.N., Biserka L.V. Influence of Allium ampeloprasum L. And Allium Cepa L. essential oils on the growth of some yeasts and moulds. Proc. Nat. Sci, Matica Srpska Novi Sad. 2009;116:121–130. [Google Scholar]
- Szychowski K., Rybczyńska-Tkaczyk K., Gaweł-Bęben K., Swieca M., Karaś M., Jakubczyk A., Matysiak - Kucharek M., Binduga U., Gmiński J. Characterization of Active Compounds of Different Garlic (Allium sativum L.) Cultivars. Pol. J. Food Nutr. Sci. 2018;68:73–81. [Google Scholar]
- Tasci B., Kutuk H., Koca I. Determination of alliin and allicin in the plant of Allium scorodoprasum L. subsp. rotundum by using the infrared spectroscopy technique. Acta Hortic. 2016:133–138. [Google Scholar]
- Valsalam S., Agastian P., Esmail G.A., Ghilan A.K.M., Al-Dhabi N.A., Arasu M.V. Biosynthesis of silver and gold nanoparticles using Musa acuminata colla flower and its pharmaceutical activity against bacteria and anticancer efficacy. J. Photochem. Photobiol., B. 2019 doi: 10.1016/j.jphotobiol.2019.111670. [DOI] [PubMed] [Google Scholar]
- Wagner H.M., Bladt S., Zgainski E.M. Springer-Verlag; New York: 1984. Plant drug analysis; p. 320p. [Google Scholar]
- Wen Y.D., Wang H., Zhu Y.Z. The Drug Developments of Hydrogen Sulfide on Cardiovascular Disease. Oxid. Med. Cell. Longev. 2018;2018:1–21. doi: 10.1155/2018/4010395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Liu X., Ruan J., Zhuang X., Zhang X., Li Z. Phytochemicals of garlic: Promising candidates for cancer therapy. Biomed. Pharmacother. 2020;123:109730. doi: 10.1016/j.biopha.2019.109730. [DOI] [PubMed] [Google Scholar]
Further Reading
- El-Rehem F.A., Ali R.F. Proximate compositions, phytochemical constituents, antioxidant activities and phenolic contents of seed and leaves extracts of Egyptian leek (Allium ampeloprasum var. kurrat) Eur. J. Chem. 2013;4:185–190. [Google Scholar]
- Putnik P., Gabrić D., Roohinejad S., Barba F.J., Granato D., Mallikarjunan K., Lorenzo J.M., Bursać Kovačević D. An overview of organosulfur compounds from Allium spp. From processing and preservation to evaluation of their bioavailability, antimicrobial, and anti-inflammatory properties. Food Chem. 2019;276:680–691. doi: 10.1016/j.foodchem.2018.10.068. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.