Summary
Adult neural stem cell (NSC) generation in vertebrate brains requires thyroid hormones (THs). How THs enter the NSC population is unknown, although TH availability determines proliferation and neuronal versus glial progenitor determination in murine subventricular zone (SVZ) NSCs. Mice display neurological signs of the severely disabling human disease, Allan-Herndon-Dudley syndrome, if they lack both MCT8 and OATP1C1 transporters, or MCT8 and deiodinase type 2. We analyzed the distribution of MCT8 and OATP1C1 in adult mouse SVZ. Both are strongly expressed in NSCs and at a lower level in neuronal cell precursors but not in oligodendrocyte progenitors. Next, we analyzed Mct8/Oatp1c1 double-knockout mice, where brain uptake of THs is strongly reduced. NSC proliferation and determination to neuronal fates were severely affected, but not SVZ-oligodendroglial progenitor generation. This work highlights how tight control of TH availability determines NSC function and glial-neuron cell-fate choice in adult brains.
Key words: adult neural stem cell, subventricular zone, thyroid hormone, thyroid hormone transporters, MCT8, OATP1C1, olfaction
Highlights
-
•
MCT8 and OATP1C1 THTs are expressed in adult SVZ-NSCs and neuronal lineage
-
•
Absence of both THTs impaired NSC self-renewal and determination
-
•
Absence of THTs impaired neuroblast production while OPC generation is maintained
-
•
SVZ neurogenesis deficit leads to impairment of olfactory function
Luongo and colleagues demonstrated that the two transporters of THs, MCT8 and OATP1C1, are required for adult SVZ-NSCs proliferation and determination toward a neuronal fate. Global knockout of both THTs maintained oligodendrogenesis at the expense of neurogenesis. These results showed that both THTs act as gate-keeper of NSC behavior, underlining the crucial role of these THTs during adult NSC determination.
Introduction
In the adult mammalian brain, a pool of neural stem cells (NSCs) persists in two well-defined neurogenic regions: the subgranular zone (SGZ) of the hippocampus and the subventricular zone (SVZ) lining the lateral ventricles. Adult neurogenesis and oligodendrogenesis are complex processes that involve proliferation and differentiation of both neuronal and oligodendrocyte progenitors derived from NSCs. In rodents, newly generated SVZ-neuroblasts migrate tangentially toward the olfactory bulbs (OBs) where they differentiate into interneurons that participate in olfaction (Breton-Provencher et al., 2009; Enwere et al., 2004). SVZ-derived oligodendrocyte precursor cells (OPCs) migrate radially into the overlying white matter (e.g., the corpus callosum).
Under physiological conditions, SVZ-NSCs generate principally neuronal precursor cells (Menn et al., 2006). However, SVZ-NSCs can also generate a few oligodendrocytes in vitro and in vivo (Menn et al., 2006). The balance between NSC-derived neurons and oligodendrocytes is modulated by several physiological and pathological stimuli. For example, after a demyelinating lesion (characterized by a loss of myelinating oligodendrocytes, a hallmark of several white matter diseases such as multiple sclerosis), the generation of new SVZ-derived OPCs is favored to stimulate myelin repair (Nait-Oumesmar et al., 2007; Remaud et al., 2017; Xing et al., 2014). Thus, understanding how NSC fate decision (neurons versus glia) is modulated in the adult brain could have significant implications for neurodegenerative diseases when new neurons and/or glia are needed to promote brain repair.
T3, the biologically active form of THs, is essential for brain development in all vertebrates, including humans. In the murine brain, it has recently been established that T3 also regulates adult neurogenesis and especially NSC fate decision between neurons and glia. In the SGZ, T3 increases neuronal differentiation by enhancing proneural gene expression (Fanibunda et al., 2018; Kapoor et al., 2012, 2015). In the SVZ, T3 drives NSC fate preferentially toward a neuronal fate, while a transient lack of T3 favors the generation of new OPCs capable of functional remyelination (Remaud et al., 2017).
Local control of TH availability is crucial for spatiotemporal control of TH action, in particular within the NSC niches (Mayerl et al., 2020; Remaud et al., 2017). Numerous factors control TH availability in the brain from TH transporters (THTs) (Friesema et al., 2003, Friesema et al., 2008; Pizzagalli et al., 2002), to inactivation/activation by deiodinases (Bianco and Kim, 2006; Luongo et al., 2019), presence of receptors for TH (Flamant and Gauthier, 2013), and even co-activators/corepressors (Astapova and Hollenberg, 2013; Suzuki et al., 2007). SVZ-derived OPCs are protected from the neuralizing effects of T3 through the expression of the T3-inactivating enzyme deiodinase type 3 (Dio3) and the absence of the T3-receptor TRα1. In contrast, TRα1 was strongly expressed in neuronal progenitors, whereas Dio3 was not (Remaud et al., 2017). However, cell-membrane transporters (THTs) facilitating TH uptake represent an additional level controlling intracellular TH availability, potentially influencing NSC fate decision too, but their involvement is as yet unknown.
THTs facilitate TH uptake into different cell types, including glial and neuronal cells. The importance of THTs for brain function is illustrated by patients carrying inactivating mutations in the Slc16a2 gene encoding the monocarboxylate transporter 8 (Mct8). These patients suffer from a severe form of psychomotor retardation (Allan-Herndon-Dudley syndrome [AHDS]) combined with abnormal serum TH levels (high T3, low T4) (Dumitrescu et al., 2004; Friesema et al., 2004; Groeneweg et al., 2019). Recently, it has been shown that, in humans, mutations in the organic anion transporting polypeptide 1C1 (OATP1C1) decrease T4 uptake and are associated with developmental impairments that later evolve to neurodegenerative disease (Strømme et al., 2018). Mice deficient in MCT8 (Mct8 knockout [KO]) faithfully recapitulate the peripheral altered TH serum levels observed in humans, but do not display neurobehavioral deficits, while they suffer from mild brain hypothyroidism (Trajkovic et al., 2007a). It is well established that OATP1C1 cooperates with MCT8 to facilitate TH transport across the blood-brain barrier (BBB) and/or the blood-cerebrospinal fluid barrier into the rodent brain. Mice deficient for both MCT8 and OATP1C1 (Mct8/Oatp1c1 or double knockout [DKO]) exhibit central TH deficiency associated with locomotor abnormalities similar to those found in MCT8-deficient patients, showing that this DKO mouse is a suitable model especially for the neurological component of AHDS (Mayerl et al., 2014). To be more precise, only DKO mice display a strong decrease of both T3 and T4 contents in the brain as a consequence of a highly reduced T4 and T3 transport across brain barriers (Mayerl et al., 2014). This allowed us to compare the cellular effects underlying the absence of both transporters with our previous well-established data obtained after induction of adult-onset hypothyroidism (Gothié et al., 2017a; Remaud et al., 2017; Vancamp et al., 2019).
Here, we determined how the lack of MCT8 and/or OATP1C1 affects NSC fate in the adult mouse brain. We demonstrate that both THTs are strongly expressed in NSCs and in committed neuronal precursors but not in oligodendroglial lineage cells. Furthermore, using DKO mice, we show that THTs regulate neuronal lineage progression, and that their absence leads to impairments in olfactory function. In contrast, the generation of new OPCs derived from SVZ-NSCs is unaffected by the absence of THTs.
Results
MCT8 and OATP1C1 Are Both Expressed in NSCs and Are Retained Mainly by Committed Neuronal Precursors within the Adult SVZ
Local TH availability is tightly regulated, permitting precise temporal and cell-specific control of TH action in the central nervous system. Cell-specific TH availability is determined by (1) a balance between TH-activating (mainly Dio2 in the brain) and inactivating (Dio3) deiodinases enzymes, (2) cell-specific membrane THTs, (3) ligand (T3)-dependent nuclear receptor (TR) forms (mainly Thra1 within the adult SVZ; López-Juárez et al., 2012).
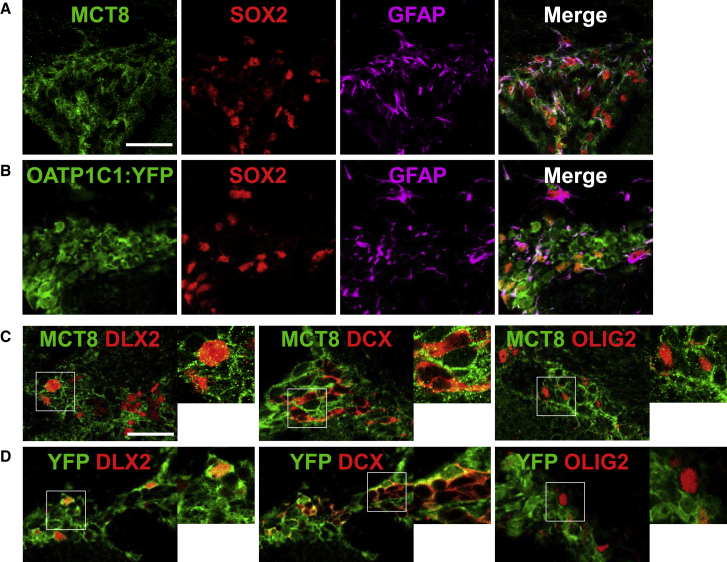
To elucidate the roles of MCT8 and OATP1C1 in adult SVZ neurogenesis, we first determined which SVZ cell types express these THTs by immunohistochemistry (IHC) (Figure 1). To detect OATP1C1 expressing cells in vivo, we used the OATP1C1:YFP reporter mouse (see Experimental Procedures), which exhibits a stronger signal (fluorescence in the cytosol) than the antibody directed against OATP1C1 (fluorescence of membrane-bound OATP1C1). Alternatively, we used this antibody to detect endogenous expression of OATP1C1 in vitro using neurosphere cultures. We observed that MCT8 (Figure 1A) and OATP1C1 (YFP+, Figure 1B) were highly expressed in NSCs co-expressing GFAP (pink) and SOX2 (red) in the adult SVZ, as was also confirmed in single cells dissociated from in vitro neurospheres (see the dotted signal in Figure S1A). MCT8 staining was absent in both the dorsal and lateral part of the SVZ in Mct8 KO mice (Figure S1B), confirming the specificity of the MCT8 antibody. The specificity of the OATP1C1 antibody was previously demonstrated (Mayerl et al., 2012). Moreover, and as expected, MCT8 and OATP1C1 were mainly expressed on the plasma membrane as shown in vitro by the colocalization with the plasma membrane marker pan-cadherin and by the 3D reconstruction (Figure S1C).
Figure 1.
MCT8 and OATP1C1 Are Strongly Expressed in NSCs and Are Maintained Principally in Committed Neuronal Cells within the Adult SVZ
(A and B) Expression of MCT8 and OATP1C1 THTs in NSCs. MCT8 (A) and OATP1C1 (B) stainings on coronal section of adult dorsal SVZ show strong expression of both THTs in SOX2+ and GFAP+ NSCs.
(C and D) Expression of THTs in neuronal (DLX2+, DCX+) and oligodendrocyte (OLIG2+) differentiation pathways. MCT8 (C) and OATP1C1-YFP (D) are maintained in the neuronal differentiation pathway but are not expressed in the oligodendrocyte differentiation pathway. Scale bar: 30 μm.
Second, we observed that both MCT8 (Figure 1C) and OATP1C1 (Figure 1D) signals were detected at a low level in cells that expressed the neuronal markers DLX2 and DCX, respectively labeling early neuronal precursors and mature neuroblasts, whereas few (5%) oligodendroglial precursors (OLIG2+ SVZ-OPCs) expressed them. Altogether, these data indicate that MCT8 and OATP1C1 transporters are strongly expressed in NSCs, and are retained by neuronal lineage cells, while largely absent from precursors of the oligodendroglial lineage.
The TH Signaling Pathway Is Dynamically Regulated during Neurogenesis
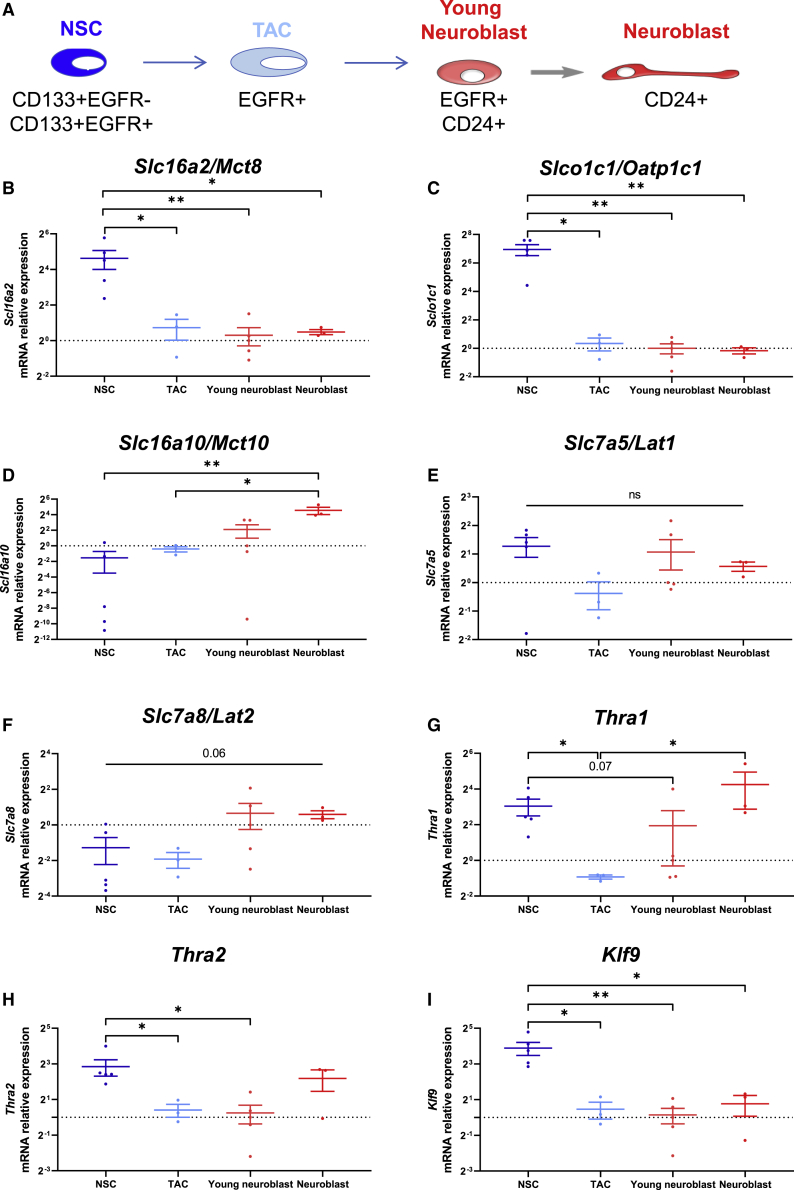
To further characterize the mRNA expression patterns of THTs and other TH signaling pathway components in the different adult SVZ cell types, we performed RT-qPCR on fluorescence-activated cell sorting (FACS)-purified SVZ populations at each stage of the neuronal lineage (Figure 2A). To this end, we purified NSCs (quiescent NSCs CD133+EGFR− and activated NSCs CD133+EGFR+), transient amplifying cells or TACs (EGFR+), immature and migrating neuroblasts (EGFR+CD24+ and EGFR-CD24+, respectively). The purity of each FACS-purified SVZ cell population was confirmed by the expression of cell-type-specific marker genes by RT-qPCR (Figure S2). Statistical results are presented in Table S1. As expected, Gfap (Figure S2A) and Nestin (Figure S2B) expressions were limited to NSCs, whereas Egfr (Figure S2C) was mainly expressed in highly proliferative TAC progenitors. Early and late neuronal lineage cell markers (Dlx2, Figure S2D, and Dcx, Figure S2E, respectively) were detected in young and mature neuroblasts. Then, we assessed the gene expression profiles of several components of the TH signaling pathway in each isolated cell population. Mct8 (Slc16a2, Figure 2B) and Oatp1c1 (Slco1c1, Figure 2C) mRNA expressions were strongly expressed in NSCs and almost undetectable in TACs and committed neuronal precursors. This discrepancy between mRNA and protein expressions can be explained by a longer half-life of both THT proteins, as previously observed in rats (Wittmann et al., 2015). Similarly, we previously demonstrated that Ttr transcripts are expressed in the SVZ of adult mice, whereas the protein is not detected by IHC (Vancamp et al., 2019).
Figure 2.
TH Signaling Components Are Dynamically Expressed in Neuronal Lineage Cells within the Adult SVZ
(A) Schematic representation of the markers used to quantify TH signaling components mRNA expression levels in quiescent and activated NSCs (CD133+EGFR), TACs (EGFR+), NPCs (EGFR+CD24+) and neuroblasts (CD24+) from FACS-sorted SVZ cells of five adult WT male mice.
(B–F) Detection of THTs mRNA expression levels in FACS-sorted SVZ cells: Slc16a2/Mct8 (B), Slco1c1/Oatp1c1 (C), Slc16a10/Mct10 (D), Slc7a5/Lat1 (E), Slc7a8/Lat2 (F).
(G and H) Detection of TH receptor mRNA expression levels in FACS-sorted SVZ cells: Thra1 (G) and Thra2 (H).
(I) Gene expression analysis of TH-responsive gene Klf9. n = 3–5 samples per cell population, Kruskal-Wallis test followed by permutation test, ∗p < 0.05, ∗∗p < 0.01. Data are presented in boxplots with medians, minimum, and maximum values.
Two other key THTs, Mct10 (Slc16a10, Figure 2D) and Lat2 (Slc7a8, Figure 2F), had the opposite profile, with a higher expression in young and mature neuroblasts compared with low expression in both NSCs and TACs. The moderate expression of Lat1 (Slc7a5, Figure 2E) was unchanged in the different SVZ cell types. Both Thra α1 (Figure 2G) and α2 (Figure 2H) isoforms were highly expressed in NSCs, significantly decreased in proliferating TACs, and then increased again in neuroblasts.
To assess in which cell type the TH signaling is preferentially activated, we quantified the expression of Klf9 (Figure 2I), a well-known TH target gene (Hu et al., 2016). We found that Klf9 was highly expressed in NSCs and decreased in more committed cell types.
Taken together, the data show a dynamic expression pattern of several TH components during neuronal lineage specification, indicating a high TH responsiveness, especially in NSCs.
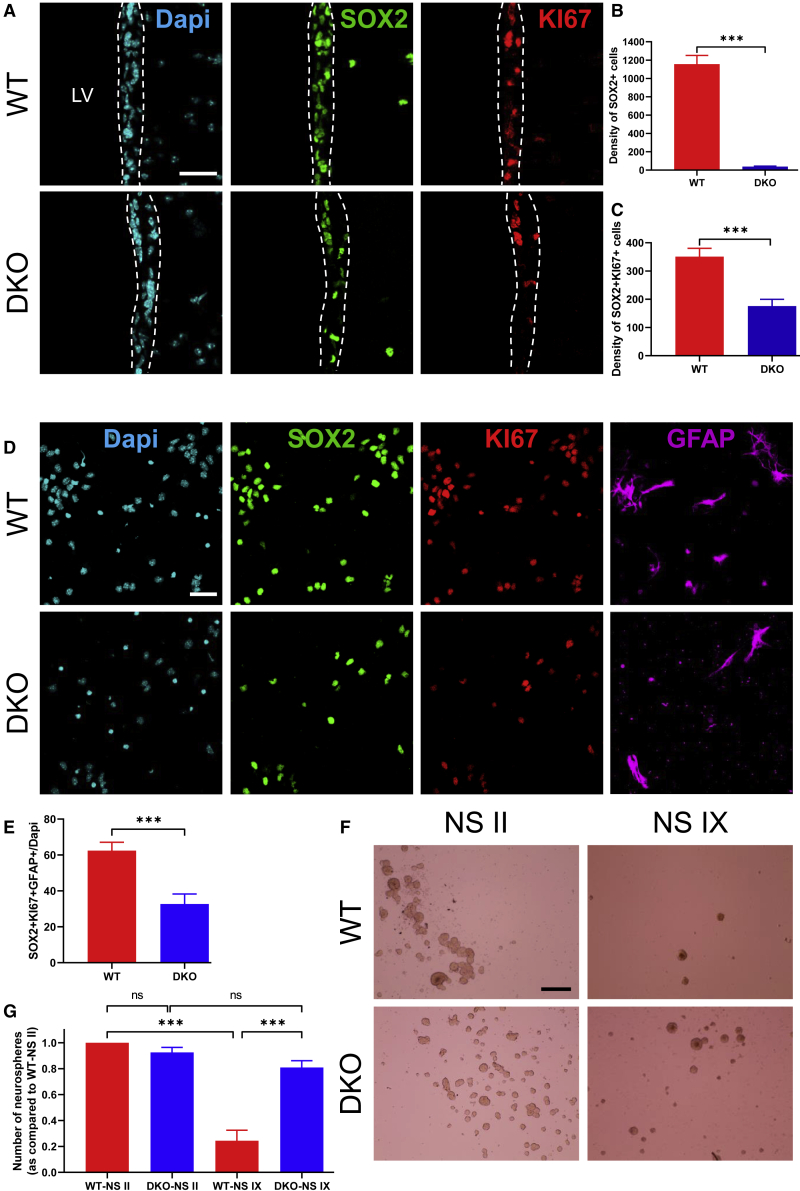
The Absence of MCT8 and OATP1C1 Impairs NSC Density and Self-Renewal Potential
As the above results suggest that both THTs are strongly expressed in adult NSCs, we next examined the functional properties of DKO-derived NSCs (Figure 3). A first observation was that the density of SOX2+ cells (Figure 3A), a pluripotency marker of both NSCs and progenitors, was reduced in the lateral SVZ of DKO mice compared with wild-type (WT) littermates (WT, 1,156 ± 95.07; DKO, 39.26 ± 4.99; t test, p < 0.0001, t = 11.73, df = 16; Figure 3B). Then, we tested if the proliferative potential of SOX2+ cells was affected in DKO mice. To this end, we performed IHC for SOX2 and KI67 (a cell proliferative associated marker, Figure 3A) to identify cycling progenitors. The density of SOX2+KI67+ cells was reduced in the SVZ of DKO mice compared with WT littermates (WT, 350.9 ± 29.5; DKO, 175.6 ± 24.32; t test, p = 0.0003, t = 4.59, df = 16; Figure 3C), suggesting that the proliferative neural stem/progenitor cell pool of the SVZ is impaired in the absence of both THTs. Accordingly, one day after plating neurosphere-dissociated cells (Figure 3D), we observed a 2-fold reduction of SOX2+KI67+ GFAP+ NSCs, showing that there were fewer cycling SVZ-NSCs in DKO mice (WT, 62.45 ± 4.68; DKO, 32.68 ± 5.59; Mann-Whitney test, p = 0.0002, U = 203.5; Figure 3E). The alteration observed in the SOX2+ population could be a consequence of a decreased self-renewal potential.
Figure 3.
DKO Adult Mice Differentially Affect NSC and Progenitor Proliferation within the Lateral SVZ
(A) Representative images of proliferative NSCs (Sox2+KI67+) within the lateral SVZ from WT and DKO adult male mice. Less proliferative NSCs are observable in DKO mice.
(B and C) Statistical analysis revealed significantly decreased SOX2+ NSCs (B) and SOX2+KI67+ proliferative NSCs (C) in the lateral SVZ of DKO mice as compared with WT mice (n = 3 mice per group and n = 2–5 sections per mouse, t test, ∗∗∗p < 0.001).
(D) Representative images of proliferative NSCs (SOX2+KI67+GFAP+) after plating primary neurospheres following a 24-h period under a low growth factor condition. DKO mice show less proliferative NSCs.
(E) DKO mice display a significant decreased SOX2+KI67+GFAP+ proliferative NSCs as compared with WT mice (n = 3–5 mice per group and n = 30 images per condition, Mann-Whitney test, ∗∗∗p < 0.001).
(F) Representative images of floating NS II and NS IX derived from lateral SVZ of adult male WT and DKO mice.
(G) The neurosphere formation in WT mice significantly decreased after nine passages (NS IX), whereas the number of neurospheres is maintained in DKO mice (n = 3 wells per condition, two-way ANOVA followed by Bonferroni's test, ∗∗∗p < 0.0001). Bars represent mean ± SEM. Scale bars: 30 μm (A and D) and 250 μm (F). LV, lateral ventricle.
To test this hypothesis, we performed a neurosphere clonal assay. We determined self-renewal capacity by counting the number of neurospheres plated at a clonal density of 10,000 cells/mL over serial clonal passaging (from passages II to IX; Figure 3F). The number of neurospheres from WT and DKO SVZ were similar at passage II (DKO-neurosphere II [NS II], 0.93 ± 0.04; Bonferroni's test following two-way ANOVA, p > 0.99, t = 1.02; Figure 3G). However, after nine passages, WT versus DKO neurospheres revealed distinct NSC behaviors (Bonferroni's test, p = 0.0003, t = 7.75). As expected (Yoshioka et al., 2015), neurosphere numbers derived from WT mice drop strongly beyond the ninth passage (WT-neurosphere IX [NS IX], 0.24 ± 0.08; Bonferroni's test, p < 0.0001, t = 10.37). In contrast, DKO mice generated a large number of neurospheres forming NSCs that could be passaged extensively (DKO-NS II, 0.93 ± 0.04; DKO-NS IX, 0.81 ± 0.05; Bonferroni's test, p = 0.89, t = 1.6; Figure 3G). Therefore, neurospheres derived from DKO mice had an enhanced self-renewal capacity, continuing to propagate beyond the ninth passage. These results show that MCT8 and/or OATP1C1 exert key roles in self-renewal and maintenance of SVZ-NSCs in adult mice.
Loss of MCT8 and OATP1C1 Impairs Regulation of NSC Lineage Determination and Olfactory Behavior
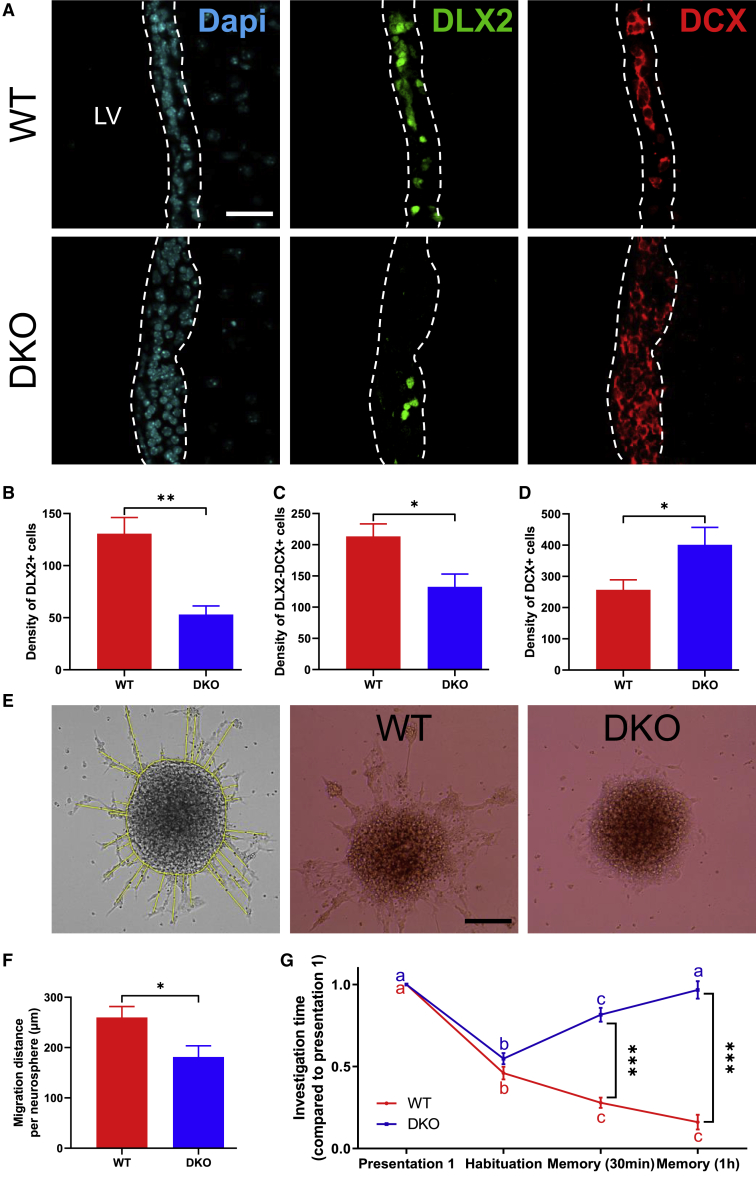
Then, we determined consequences of the combined absence of both THTs on the generation of new neuronal versus oligodendroglial precursors derived from SVZ-NSCs in vivo. First, we quantified the density of uncommitted (DLX2+, DCX−) and committed (DLX2+, DCX+) NPCs, neural progenitor cells and mature neuroblasts (DLX2−, DCX+) in the SVZ of WT and DKO mice by IHC on coronal sections (Figure 4A). In DKO mice, the density of uncommitted and committed NPCs was significantly decreased as compared with WT mice (DLX2: WT, 130.7 ± 15.55 cells/mm2; DKO, 53.03 ± 8.24 cells/mm2; t test, p = 0.002, t = 3.46, df = 30; Figure 4B. DLX2-DCX: WT, 213.4 ± 20.04 cells/mm2; DKO, 132.6 ± 20.39 cells/mm2; t test, p = 0.02, t = 2.57, df = 30; Figure 4C). Surprisingly, the density of DCX+ mature neuroblasts is higher in DKO mice in comparison with controls (WT, 256.8 ± 32.14 cells/mm2; DKO, 401 ± 55.81 cells/mm2; t test, p = 0.02, t = 2.41, df = 30; Figure 4D).
Figure 4.
DKO Adult Mice Display Reduced SVZ-Derived NPCs and Impaired Neuroblast Migration within the SVZ
(A) Representative images of NPCs (DLX2+) and neuroblasts (DCX+) within the lateral SVZ from WT and DKO adult male mice.
(B–D) Quantification of uncommitted (DLX2+ cells) and committed (DLX2+DCX+ cells) neural progenitors and mature neuroblasts (DCX+ cells) within the lateral SVZ of WT and DKO male mice. DKO mice display reduced density of uncommitted (B) and committed (C) neural progenitors as compared with WT mice. In contrast, the density of mature neuroblasts (D) increased in DKO mice as compared with WT mice (n = 3 mice per group and n = 2–5 sections per mouse, t test, ∗p < 0.05, ∗∗p < 0.01).
(E) Representative images of the migration calculation method. Migrating cells can be seen as mixed cell outgrowth constituted by both individual and chain cells that migrate from neurospheres. The migration distance is determined using a line-drawing tool under Fiji software. The lines are drawn from the outline of the neurospheres to the center of the migrating cells. All cells that migrate from the neurospheres are considered. The two other images represent plated SVZ neurospheres generated from WT and DKO mice following a 3-day period under a low growth factor condition.
(F) In vitro measurement of neural precursor migration away from plated primary neurospheres shows reduced migration distance per neurosphere in DKO mice (n = 5 neurospheres per condition, Mann-Whitney test, ∗p < 0.05).
(G) Short-term olfactory memory behavioral test. The graph represents the time spent by WT and DKO mice to investigate the odor during the first presentation, the habituation phase (2-min later), and the two-memory phase (after 30-min and 1-h rest period). The short-term olfactory memory is impaired in DKO mice, as shown by the increase of the investigation time 30 min and 1 h after the first exposure (n = 3–5 mice per group, n = 3 experiments, two-way ANOVA followed by Bonferroni's multiple comparisons test to compare the four presentations and to compared WT and DKO mice, ∗∗∗p < 0.001). Bars represent mean ± SEM. Scale bars: 30 μm (A) and 250 μm (E).
The accumulation of neuroblasts in the SVZ of DKO mice may reflect hampered or delayed migration of mature neuroblasts, and so we investigated whether the loss of MCT8 and OATP1C1 affected SVZ-NPC migration. To this end, neurospheres generated from SVZ of WT and DKO mice were plated on eight-well glass slides (Millicell) coated with poly-D-lysine (Sigma). Their migration behavior was determined after 3 days in vitro without EGF and FGF by measuring radial migration away from adherent neurospheres (Figure 4E, left box). For this, we measured the distance of each cell that migrated from the neurosphere, and averaged the distances per neurosphere. We found that, on average, cells migrated from DKO neurospheres covered a significantly shorter distance than those migrated from WT neurospheres (WT, 259.9 ± 21.79; DKO, 181.4 ± 22.21; Mann-Whitney test, p = 0.03, U = 2; Figure 4F). Thus, ablation of Mct8 and Oatp1c1 reduces the migration of neurosphere-derived NPCs.
Given that the absence of THTs strongly affects SVZ neurogenesis, we investigated potential effects on olfactory behavior by performing the short-term olfactory memory test (as described in Lazarini et al., 2009). WT versus DKO mice were exposed four times to the same non-social odor (almond, lemon, or orange blossom) at different intervals (2 min, 30 min, and 1 h). We observed that the decrease in the sniffing duration of the cotton swab after two consecutive exposures to a same odor was comparable between DKO and WT mice (habituation: WT 0.46 ± 0.04; DKO 0.55 ± 0.03. Bonferroni's test following two-way ANOVA: WT p < 0.0001, t = 10.15, DKO p < 0.0001, t = 10.95; WT versus DKO p = 0.46, t = 1.59; Figure 4G), suggesting that DKO mice are able to discriminate simple odors. In contrast, after a longer interval of 30 min and 1 h, DKO mice spent significantly more time investigating the odor compared with control animals (memory, 30 min: WT, 0.28 ± 0.03; DKO, 0.82 ± 0.04. Memory, 1 h: WT, 0.16 ± 0.05; DKO, 0.97 ± 0.05; WT versus DKO. Memory 30 min, p < 0.0001, t = 9.61; memory 1 h, p < 0.0001, t = 14.46; Figure 4G). Interestingly, the investigation time of DKO mice during the last presentation (after an interval of 1 h) was not statistically different from that of the first presentation of the odor (Bonferroni's test: WT p < 0.0001, t = 15.76; DKO p > 0.99, t = 0.81). This suggests that DKO mice are unable to memorize odors. Thus, our findings show that short-term olfactory memory is affected by the absence of THTs, associating reduced adult SVZ neurogenesis with altered olfactory memory.
The Absence of MCT8 and OATP1C1 Does Not Affect SVZ Oligodendrogenesis
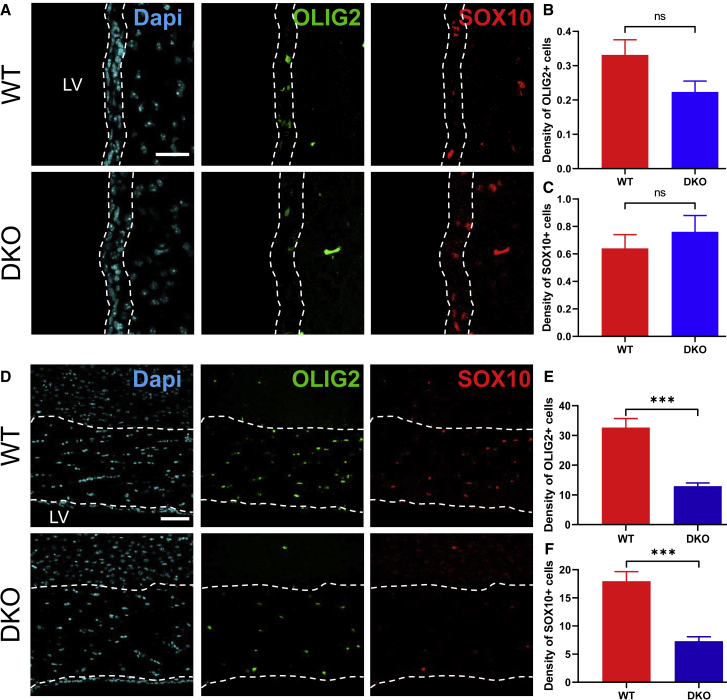
Lastly, we investigated whether the generation of new SVZ-OPCs was affected in DKO mice by performing an IHC for the oligodendroglia lineage markers OLIG2 and SOX10 (Figure 5A). We observed that neither the OLIG2+ nor the SOX10+ SVZ-OPC density was affected in DKO mice as compared with control animals (OLIG2: WT, 0.33 ± 0.04 cells/mm2; DKO, 0.22 ± 0.03 cells/mm2; Mann-Whitney test, p = 0.098, U = 24.5; Figure 5B. SOX10: WT, 0.64 ± 0.1; DKO, 0.76 ± 0.12; Mann-Whitney test, p = 0.41, U = 34.5; Figure 5C), suggesting that the generation of OPCs derived from adult SVZ-NSCs is not affected by the absence of both MCT8 and OATP1C1.
Figure 5.
DKO Adult Mice Display Reduced SVZ-Derived OPCs within the Corpus Callosum but not within the SVZ
(A) Representative images of OPCs (OLIG2+ and SOX10+) within the lateral SVZ from WT and DKO adult male mice.
(B and C) Quantification of OLIG2+ (B) and SOX10+ (C) OPCs into the lateral SVZ demonstrate no density difference between WT and DKO mice (n = 3 mice per group and n = 2–5 sections per mouse, Mann-Whitney test, p > 0.05 non-significant).
(D) Representative images of OPCs (OLIG2+ and SOX10+) within the corpus callosum from WT and DKO adult male mice.
(E and F) DKO mice display a significant decreased OLIG2+ (E) and SOX10+ (F) OPCs density in the corpus callosum as compared with WT mice (n = 3 mice per group and n = 2–5 sections per mouse, t test, ∗∗∗p < 0.001). Bars represent mean ± SEM. Scale bars: 30 μm (A) and 60 μm (D).
Two distinct populations of OPCs are present in the adult mammalian brain. Contrary to SVZ-OPCs that are continuously generated from NSCs in the adult SVZ, another source of oligodendrocytes is located in the parenchyma (pOPCs), notably in the corpus callosum. Since these parenchymal resident pOPCs are generated earlier during development and remain quiescent in the adult under steady-state conditions, we hypothesized that pOPCs were affected during development of DKO mice (Figure 5D). Although the SVZ area of DKO mice is unchanged compared with control SVZ (data not shown), the corpus callosum area is significantly thinner in DKO than in controls (619 ± 17.24 mm2 in DKO mice versus 838.01 ± 53.01 mm2 in WT), as previously observed in Mayerl et al. (2014). Using IHC, we found that the density of either OLIG2+ or SOX10+ pOPCs was strongly reduced in DKO mice as compared with WT mice (OLIG2: WT, 32.64 ± 3.05; DKO, 12.95 ± 1.05; t test, p < 0.0001, t = 5.96, df = 31; Figure 5E. SOX10: WT, 17.96 ± 1.7; DKO, 7.31 ± 0.78; t test, p < 0.0001, t = 5.57, df = 31; Figure 5F). These findings show that pOPCs are highly sensitive to the absence of MCT8 and/or OATP1C1, whereas newly generated SVZ-OPCs are unaffected.
Discussion
The SVZ niche is a well-established TH target tissue: TH preferentially drives SVZ-derived NSC fate toward a neuronal fate (Gothié et al., 2017b; López-Juárez et al., 2012; Remaud et al., 2017). In contrast, a T3-free window due to the presence of Dio3 and the absence of TRα1 is required for OPC generation. However, how intracellular TH levels are regulated within the different SVZ cell types remains unknown. In this study, we determined how the different components of the TH signaling pathway are modulated during NSC determination to a neuronal fate. In particular, we provide new insights into the functional role of two key THTs, MCT8 and OATP1C1, in NSC fate choice to a neuronal or a glial progenitor within the adult mouse brain. Our work shows that cellular uptake/efflux of TH through membrane transporters is involved in NSC function, affecting the generation of new neuronal and glial cells within the adult mouse brain.
Stem Cell TH Status
Our data support a key role for MCT8 and/or OATP1C1 in NSC function. First, we observed by RT-qPCR and IHC that both THTs MCT8 and OATP1C1 are highly expressed in NSCs within the adult SVZ. Second, in adult DKO mice, the density of SOX2+ NSCs and progenitors is decreased. This result was confirmed in vitro: the number of cycling SOX2+GFAP+ NSCs was reduced when neurospheres were grown from NSC dissected from the SVZ of DKO mice, suggesting altered stem/progenitor cell behavior in absence of THTs. Further, using the neurosphere assay, we determined that the absence of THTs does not exhaust SVZ-NSC self-renewal after IX passages, contrary to WT NSCs.
Two subpopulations of NSCs co-exist in the adult SVZ: highly proliferative stem cells (activated NSCs,aNSC) and quiescent NSCs (qNSCs) (Codega et al., 2014; Morshead et al., 1994). The mechanisms regulating the transition from a quiescent to an activated state are still poorly understood. The TH signaling pathway could be a key regulator of this cell transition, especially by controlling exit of NSCs from quiescence. The 2-fold depletion of cycling NSCs in DKO mice could indicate a return to quiescence in the absence of intracellular T3 signaling. qNSCs contribute to the generation of neurospheres when the pool of aNSCs has been experimentally depleted (Morshead et al., 1994). Hence, the challenging in vitro environment could reactivate the expected accumulation of qNSCs due to the absence of functional TH signaling in DKO mice. In the absence of intracellular T3, reactivated NSCs could proliferate indefinitely, thus maintaining the neurosphere-forming potential that we still observed after passage IX. Similarly, in the SGZ of the hippocampus, Mayerl et al. (2020) have recently demonstrated that activation of NSCs is impaired in a global Mct8 KO adult mouse model. Furthermore, Mayerl et al. (2018) have shown that the activation of muscle stem cells is reduced to about 50% in DKO mice, impairing the myogenic program. They also found that a higher expression of both Mct8 and Oatp1c1 is associated with the activation of quiescent satellite stem cells. These data support our hypothesis that the absence of both THTs and intracellular T3 induces the transition of SVZ-NSCs to a quiescent state. Altogether, our work, combined with Mayerl et al.’s studies, underlines a crucial function of MCT8 and/or OATP1C1 transporters in stem cell quiescence and reactivation. However, the relative contribution of MCT8 and/or OATP1C1 to maintain NSC identity could be further assessed by analyzing NSC behavior in simple KO mice or following inhibition using commercially available inhibitors (Mayerl et al., 2020).
Furthermore, our in vitro experiments were done in the presence of T3 (0.3 nM, present in the B27 supplement), and, in this experimental condition, we still observed a reduction in the numbers of NSCs and also impaired cell migration. Therefore, these results strongly suggest that alterations in NSCs behavior observed in vivo could be largely due to a local intracellular hypothyroidism. However, we cannot exclude an additional impact due to (1) the lack of MCT8 in both choroid plexus and endothelial cells of the BBB (Dumitrescu et al., 2006; Heuer et al., 2005; Trajkovic et al., 2007b) and (2) OATP1C1 facilitating T4 uptake across the BBB and/or the CSF (Roberts et al., 2008; Mayerl et al., 2014). Furthermore, since MCT8 and OATP1C1 are also found in radial glia cells in the prenatal human brain (López-Espíndola et al., 2019), we cannot rule out a developmental impact of the absence of both transporters contributing to the adult phenotype that we observed in the SVZ of DKO mice. Further analysis is needed to assess whether a reduction of TH availability within radial glial cells of the mouse prenatal brain has a long-lasting influence on NSC proliferation and cell fate in the adult SVZ.
A decrease of intracellular T3 could preserve/maintain the NSC pool that can be reactivated under more favorable environmental conditions. We previously demonstrated that SVZ-NSCs are blocked in G2 phase, the phase before mitosis, following an adult-onset hypothyroidism (Lemkine et al., 2005). In the Drosophila brain, G2-quiescent cells are capable of reactivating faster than G0-stem cells (Otsuki and Brand, 2018). Interestingly, the localization of NSCs in their niche (i.e., dorsal versus ventral) would determine whether they enter in phase G0 or G2: under the control of patterning factors, dorsal NSCs enter G0 quiescence while ventral NSCs undergo G2 quiescence (Otsuki and Brand, 2019). Elucidating whether SVZ-NSCs are preferentially arrested either in G0 or G2 phase following a decrease of TH availability could help to adapt therapeutic purposes so as to manipulate NSC physiology and promote generation of new neural cells.
We cannot exclude that the decreased density of cycling NSCs/progenitors might be related to a developmental issue. THs are crucial for brain development: congenital hypothyroidism and AHDS (Mct8 deficiency) are two pathological conditions where low brain TH content incurs intellectual disability (López-Espíndola et al., 2014; Schwartz and Stevenson, 2007; Vatine et al., 2017). Here, we showed that NSCs express high levels of several components of the TH signaling pathway in mice.
We speculate that high intracellular T3 levels in NSCs (high expression of both THTs, Thra1, Klf9) could be acquired during the transition from embryonic/post-natal NSCs to adult NSCs. The absence of both THTs could prevent sufficient uptake of THs and therefore an intracellular lack of TH to determine the features of adult NSCs, consequently decreasing the density of proliferating NSCs, as we observed in the adult SVZ of DKO mice. Further investigation is necessary to determine whether T3 is a key molecular signal controlling the transition from qNSC to aNSC and inversely.
Once activated, NSCs express the epidermal growth factor receptor (Egfr), a key proliferation-activating factor that also regulates NSC fate by blocking neurogenesis and promoting glial fate (Ayuso-Sacido et al., 2010). Interestingly, high Egfr expression in activated NSCs and their progeny (TACs) associates with reduced intracellular TH levels due to high expression of Dio3 and absence of TRα1 expression (Remaud et al., 2017). Similarly, we detected a low mRNA expression of both Mct8 and Oatp1c1 and other THTs such as Mct10, Lat1, and Lat2 in TACs, suggesting that absence of THTs and TRα1, combined with elevated Dio3 expression (Remaud et al., 2017), is associated with actively proliferating progenitors derived from SVZ-NSCs.
MCT8 and/or OATP1C1 Absence Impairs SVZ Neurogenesis
We demonstrated that the absence of MCT8 and/or OATP1C1 impairs SVZ neurogenesis, whereas the generation of new SVZ-OPCs is unaffected. This is consistent with our previous studies showing that TH signaling acts as a neurogenic switch in SVZ-progenitors (Gothié et al., 2017b; López-Juárez et al., 2012), whereas a T3-free window is necessary to promote SVZ oligodendrogenesis (Remaud et al., 2017). However, we did not expect to observe an accumulation of DCX+ neuroblasts along the lateral ventricles of DKO mice, suggesting a potential migration failure. Similarly, radial migration of newly generated SVZ-neural precursors was impaired in vitro.
Thus, the absence of MCT8 and/or OATP1C1 could alter the molecular and cellular machinery necessary for the phosphorylation of Doublecortin and the migration of neuroblasts, inducing the accumulation of newly immature neurons along the lateral ventricles. The role of TH signaling on neuroblast migration through the rostral migratory stream (RMS) remains unknown, although the role of TH in optimal cell migration has been shown in other brain regions, notably during development in the cerebellum (Morte et al., 2002; Portella et al., 2010) and neocortex (Pathak et al., 2010). Misregulation of two well-established TH target genes involved in neural migration, reelin (Alvarez-Dolado et al., 1999) and NCAM (Iglesias et al., 1996), blocked radial neuroblast migration, resulting in loss of cortical layering and behavioral defects. Similarly, depletion of key components of the Reelin pathway (as ApoER2/VLDLR or Dab1) resulted in accumulation of neuroblasts within the adult SVZ (Andrade et al., 2007), phenocopying the results found in our DKO mice. Thus, the interaction of TH with the Reelin signaling pathway deserves further analysis in the context of adult SVZ neurogenesis.
Using RT-qPCR analysis on isolated cell types by FACS, we observed that both Mct8 and Oatp1c1 mRNA were expressed at low levels in young and mature neuroblasts. In contrast, IHC allowed us to detect high expression of both MCT8 and OATP1C1 proteins in these cell types. To our knowledge, this is the first study highlighting a functional role of these transporters in the neuronal lineage derived from adult SVZ-NSCs. However, further work is needed to analyze the relative contribution of MCT8 and OATP1C1 during lineage progression of neural progenitors in the SVZ of adult mice. Recently, using similar approaches, Mayerl et al. (2020) detected high levels of MCT8 in neuroblasts located within the hippocampus at both the mRNA and protein levels. Furthermore, they demonstrated that the density of differentiated neuroblasts is decreased in Mct8 KO animals, showing that a global inactivation of MCT8 impaired adult neurogenesis in the mouse SGZ too. However, they found that the density of TACs and proliferating neuroblasts is unchanged in Mct8 KO, strongly suggesting that MCT8 acts at later stages during SGZ-NSC lineage progression, during cell transition from neuroblast to immature neurons. This is consistent with previous work by Kapoor et al. (2012) showing that TH signaling targets predominantly post-mitotic committed neural progenitors and that THs accelerate neuronal differentiation both in vivo and in vitro.
These studies demonstrate that TH action on adult neurogenesis is NSC niche specific and depends on the developmental window. Contrary to the adult SGZ, our study shows that MCT8 and/or OATP1C1 are involved in the proliferation of SOX2+ TACs and DLX2+ young neuroblasts, indicating that THT-dependent TH availability regulates lineage progression at multiple steps, from NSC/progenitor proliferation (Lemkine et al., 2005) to cell-fate determination to a neuronal fate (Gothié et al., 2017b; López-Juárez et al., 2012; Remaud et al., 2017) in the adult SVZ. Our findings echo a recent study showing that MCT8 knockdown reduced the pool of progenitors and impaired neurogenesis in the chicken optic tectum, thus demonstrating that MCT8-dependent TH supply is crucial for early corticogenesis (Vancamp et al., 2017).
Compared with Mct8 and Oatp1c1 mRNA, mRNA levels for other THTs (Mct10 and Lat2) are higher in the neuronal lineage. Further analysis is needed to study the function of these secondary THTs, Lat2 and Mct10, during neuronal lineage progression. In contrast to the SVZ, Mayerl et al. (2020) detected high expression of Mct10 (and Mct8, as mentioned before) in SGZ-neuroblasts, whereas Lat1 and Lat2 are expressed in NSCs and TACs, showing distinct expression patterns of these THTs in the two neurogenic niches. This recent finding is consistent with many others showing a complementary distribution pattern of both Mct8 and Mct10 in several brain areas, such as in the cerebral cortex (Müller and Heuer, 2014), strongly suggesting a compensatory function of Mct10 in the absence of Mct8. Due to the mutually exclusive distribution of Mct8 and Mct10 mRNA in the SVZ, this neurogenic region can provide a key model to assess the physiological significance of Mct10 as a specific THT in SVZ-derived neuroblasts. Moreover, the contribution of all these THTs to SVZ oligodendrogenesis should also be addressed. In particular, a high Mct10 signal has been observed in white matter regions of the adult mouse brain (Müller and Heuer, 2014), raising the question of whether Mct10 provides TH during migration of SVZ-derived oligodendrocytes toward the corpus callosum, thereby participating in axonal myelination in physiological or demyelinated conditions (Menn et al., 2006).
Thus, analyzing the function of the dynamic expression pattern of Mct8/Oatp1c1 and other THTs in the different SVZ cell populations will open new avenues on how cell-autonomous function of the TH signaling could regulate NSC commitment to a neuronal fate.
Olfactory Function Is Affected by the Absence of Both MCT8 and OATP1C1
In rodents, newly generated neuroblasts derived from NSCs migrate through the RMS to the OBs, where they differentiate into mature interneurons throughout life. This continuous integration of new neurons into the pre-existing neuronal circuits has been implicated in olfactory behavior, including odor memory and discrimination.
We showed a decreased generation of new migrating neuroblasts in DKO mice that, together with a neuroblast migration defect, may affect the recruitment of new neurons in the adult OBs. Newly generated neurons in the olfactory network play a key role in short-term olfactory memory (Breton-Provencher et al., 2009). In particular, reduced levels of SVZ neurogenesis following antimitotic drug AraC treatment caused a depletion of SVZ cells derived from proliferating cells (Doetsch et al., 1999). Moreover, a loss of olfactory memory is observed, without affecting odor discrimination. Similarly, an upregulation of SVZ neurogenesis by an olfactory-enriched environment increased olfactory memory (Rochefort et al., 2002). These data strongly suggest that olfactory memory is dependent on the rate of adult SVZ neurogenesis and that newly generated neurons play a crucial role in establishing short-term olfactory memory, but not odor discrimination (Breton-Provencher et al., 2009; Enwere et al., 2004). Finally, absence of MCT8 and/or OATP1C1 impairs (1) generation and migration of SVZ-neuroblasts, and (2) olfactory capacities, suggesting that SVZ neurogenesis in DKO mice is functionally impaired. However, the discriminative capacity of WT and DKO mice was tested using simple, easily discernable odors. Some studies have previously demonstrated that discrimination between two simple different odors is not impaired in mice with reduced SVZ neurogenesis (Breton-Provencher et al., 2009; Enwere et al., 2004). We cannot rule out that more difficult discrimination tasks using very similar odorants would have been more challenging for DKO mice, as it has been recently demonstrated in other mouse models aiming to analyze the impact of aging (Enwere et al., 2004) and consequences of a transitory increase of the NSC pool on olfactory discrimination (Bragado Alonso et al., 2019).
Myelination Status
TH deficiency is usually linked to impaired myelogenesis during development. It is well established that TH controls the timing of oligodendrocyte differentiation (Barres et al., 1994; Billon et al., 2002; Kim and Petratos, 2019). Our work showed that DKO mice have a significant lower density of resident pOPCs localized in the corpus callosum, above the lateral ventricle, corresponding to the reduced myelination observed in the corpus callosum of DKO brains with a silver staining technique (Mayerl et al., 2014). This hypomyelination state is associated with neurological and behavioral impairments described in DKO mice as well as in AHDS patients (Mayerl et al., 2014). Postmortem brain tissue analysis of an AHDS patient strongly suggest that MCT8 drives T3-dependent differentiation of OPC into mature and functional myelinating oligodendrocytes (López-Espíndola et al., 2014). These resident pOPCs, widely distributed in the parenchyma throughout the adult brain, are generated during development and can remain quiescent throughout adult life (Tokumoto et al., 2017). The significant reduction of resident parenchymal pOPCs in the corpus callosum could reflect neurodevelopmental and behavioral consequences of Mct8 and Oatp1c1 depletion in the developmental DKO mouse model. A conditional DKO mouse line would permit researchers to better distinguish effects generated early during development from those affected later during adulthood. Lastly, the crucial role of MCT8 in the differentiation of OPCs is known in humans: MCT8 downregulation in human embryonic stem cell-derived cells induces oligodendrocyte apoptosis and myelin damage (Lee et al., 2017). These data suggest that the decrease in resident OPCs in the corpus callosum of DKO mice could be due to OPCs apoptosis.
In contrast to resident pOPCs, our work shows that newly generated OPCs derived from adult SVZ-NSCs express neither MCT8 nor OATP1C1, with generation of new OPCs in the SVZ of DKO mice being unaffected. Our previous results showed that a transient hypothyroidism promotes the generation of new SVZ-OPCs and not resident pOPCs, demonstrating that both OPC populations differentially respond to a TH-free window (Gothié et al., 2017a; Remaud et al., 2017). The discrepancy observed in the number of the SVZ-OPCs compared with our previously published data could depend on the timing of when hypothyroidism was induced. In Remaud et al. (2017) and Gothié et al. (2017a), we induced a systemic transient hypothyroidism in adult mice, whereas, in the present work, brain TH deficiency already occurs during developmental stages. Thus, the absence of an increase in OPCs in the DKO mice could be due to a developmental defect. Several studies underline that SVZ-OPCs are more capable of functional repair in demyelinating lesions than pOPCs (Brousse et al., 2015; Remaud et al., 2017; Xing et al., 2014). Thus, how TH signaling preferentially regulates SVZ-OPCs, an efficient source of remyelinating cells, will provide new therapeutic options to favor myelin repair in multiple sclerosis and other demyelinating diseases. More precisely, understanding how local intracellular TH levels differentially regulate SVZ-OPCs and resident OPCs could overcome the gap of knowledge on the distinct behavior of these two kinds of OPCs after a myelin insult.
Conclusion
This work highlights, first, a new role of MCT8 and/or OATP1C1 in adult NSC physiology; second, a tight and dynamic regulation of TH signaling during neuronal lineage progression; and third, an impairment of olfactory behavior and memory. Our data could shed new light on potential therapeutic purposes for AHDS patients and more generally for demyelinating diseases where new remyelinating cells are needed. The neurological phenotype of DKO mice and AHDS patients could be due to a developmental defect worsened by an impaired neurogenesis in the adult.
Experimental Procedures
Animals
Generation and genotyping of Mct8 KO and Mct8/Oatp1c1 DKO male mice were reported elsewhere (Mayerl et al., 2014; Trajkovic et al., 2007b). For visualizing OATP1C1-expressing cells, transgenic animals were used that express YFP reporter and a tamoxifen-inducible cre-recombinase under the control of the Slco1c1 promoter (Ridder et al., 2011). Four weeks prior to immunofluorescence analysis, these OATP1C1:YFP reporter mice were treated with tamoxifen (40 μg/g intraperitoneally) for five consecutive days in order to induce YFP expression specifically in OATP1C1 expressing cells.
Only male mice on a C57BL/6 background at the age of 2 months (WT, DKO; OATP1C1:YFP) or 4 months (Mct8 KO) were used in the study. Mct8 KO, DKO, OATP1C1:YFP mice, and age-matched WT mice on the same genetic background were obtained from the Heuer lab. C57BL/6 WT mice purchased from Janvier Labs (Le Genest St. Isle, France) were used for the qRT-PCR experiment (see protocols below). Mice were left undisturbed for at least 10 days after arrival in the animal facility to limit environmental stress due to transport and to allow a better acclimatization to their new environment. Mice were housed with an enriched environment to ameliorate anxious behavior. Mice were kept under standard conditions (22°C, 50% humidity, 12 h light/dark cycle) and food and water were available ad libitum. Animals were handled and cared for in accordance with the EU directive of 22 September 2010 (2010/63/EU). All procedures were conducted according to the principles and procedures in Guidelines for Care and Use of Laboratory Animals and validated by local and national ethical committees (CEEA, comité d'ethique en expérimentation animale, Cuvier n°68; AZ 84-02.04.2017.A219 LANUV NRW Recklinghausen Germany).
Olfactory Function
The procedure is described in the Supplemental Information.
Tissue Preparation and IHC
Three WT and three DKO mice were anesthetized with pentobarbital (130 mg/kg, Centravet) and perfused rapidly through the left heart ventricle with 1× PBS followed by 4% paraformaldehyde in PBS (0.1 M, pH 7.4). Brains were harvested and post fixed at 4°C in the same fixative solution overnight. Brains were cryoprotected in 30% sucrose in 1× PBS at 4°C and embedded in OCT, optimal cutting temperature compound (Sakura) and stored at −80°C. Brain coronal sections (30 μm thick) were performed using a cryostat and were rigorously selected from bregma +0.9 mm to −0.1 mm and then collected in sterile 1× PBS. The IHC procedure is described in the Supplemental Information.
Imaging and Quantification
A Leica TCS-SP5 confocal microscope was used to acquire fluorescence images (1,024 × 1,024 pixels; 400× magnification). Images were taken on 3–5 sections per mouse. Three detailed images were taken lining the lateral ventricles corresponding to the lateral SVZ of both sides to quantify NSCs, NPCs, and OPCs. In addition, three images were taken of the corpus callosum at each side to quantify the density of pOPCs. Cell counting was performed in the freely available Fiji software. For each image, the region of interest (i.e., the lateral ventricular border or the corpus callosum) was drawn. Moreover, the immunopositive cells were quantified using the cell counter plugin. The results were expressed as the number of immunopositive cells per mm2. For MCT8 and OATP1C1 colocalization with the plasma membrane marker pan-cadherin, z stack images were acquired using 1,024 × 1,024 pixels; stack images, 0.34 μm; 630× magnification zoom, 2.5; visualizations were done on Max Intensity Z projections using Fiji software; and 3D reconstitution were processed using Imaris software.
Adult Neurosphere Assay
Three WT and five DKO animals were sacrificed by cervical dislocation and the region containing the lateral SVZ was dissected. Tissues were incubated in digestion medium (papain [Worthington], DNase [Worthington], L-cysteine [Sigma-Aldrich]) for 30 min at 37°C and mechanically dissociated to obtain a single-cell suspension. Cells were collected by centrifugation at 1,000 rcf (relative centrifugal force) for 5 min and then cultured in a tissue culture flask 25 (Techno Plastic Products, TPP) containing DMEM-F12 (Gibco), 40 mg/mL insulin (Sigma), 1/200 B-27 supplement (Gibco) containing 0.3 nM T3, 1/100 N-2 supplement (Gibco), 0.3% glucose (Sigma-Aldrich), 5 nM HEPES (Thermo Fisher), 100 U/mL penicillin/streptomycin (Thermo Fisher), 20 ng/mL EGF (Peprotech), and 20 ng/mL FGF2 (Peprotech) to obtain primary neurospheres. The cultures were maintained in a 5% CO2 environment at 37°C for 7 days and the growing medium was supplemented with EGF and FGF2 every 2 days.
Primary neurospheres were first used for cell migration (procedure described in the Supplemental Information). To analyze cell differentiation, primary neurospheres were dissociated and 50 × 103 cells were plated in eight-well glass slides (PEZGS0816, Millicell) coated with poly-D-lysine (0.1 mg/mL, Sigma-Aldrich). Differentiation was induced by EGF and FGF2 withdrawal. After 24 h of differentiation, cells were fixed with 4% paraformaldehyde in PBS (0.1 M, pH 7.4). IHC was performed as described above. Quantifications were done on maximum intensity Z projections of 4-μm stack images using Fiji software. Results are presented as the number of immunopositive cells with respect to the total number of cells.
Clonality Neurosphere Assay
Primary neurospheres (NS I), obtained as indicated above, were dissociated and then plated at 10,000 cells/mL concentration in 24-well tissue culture test plates (TPP) as indicated above (n = 5–10 wells per passage, n = 1 experimental replicate). Three wells of floating neurospheres (NS II to NS IX) were imaged with EVOS XL Core cell imaging system microscope (×10) and the number of neurospheres per well was determined using Fiji software.
Flow Cytometry and Real-Time PCR
Procedure is described in the Supplemental Information.
Statistical Analysis
Statistical analyses were performed with GraphPad Prism8 software (GraphPad Software, San Diego, CA) and StatXact8 (Cytel Inc.). A Kruskal-Wallis test followed by permutation test was used for the qPCR analyses. For in vivo and in vitro quantifications, means from two groups were compared using a two-tailed Student's t test or a Mann-Whitney U test, depending on whether data were normally distributed or not, respectively. A two-way ANOVA test followed by a Bonferroni post test was used to compare the number of neurospheres and the short-term olfactory memory in CTL, control and DKO mice. Differences were considered statistically significant for a p value <0.05. Data are presented in the histograms and expressed as mean ± SEM or in the boxplots with medians, minimum, and maximum values.
Author Contributions
C.L., L.B., A.S., and K.L. conducted the experiments. C.L., L.B., A.S., and K.L. did cell counting and analyzed data. M.S. and H.H., provided the transgenic mice. C.L., L.B., A.S., S.R., and B.D. designed the experiments and wrote the paper. All authors were involved in commenting on the paper.
Acknowledgments
The authors would like to thank S. Sosinsky and F. Uridat for the excellent animal care. We also thank the ImagoSeine platform of the Institute Jacques Monod, Université René Descartes for the imaging advice. This work was supported by the CNRS and the EU H2020 contract Thyrage (grant n°666869). H.H. and M.S. are supported by the Deutsche Forschungsgemeinschaft (DFG; TR 296/1-P01).
Published: January 14, 2021
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.stemcr.2020.12.009.
Supplemental Information
References
- Alvarez-Dolado M., Ruiz M., Del Río J.A., Alcántara S., Burgaya F., Sheldon M., Nakajima K., Bernal J., Howell B.W., Curran T. Thyroid hormone regulates reelin and dab1 expression during brain development. J. Neurosci. 1999;19:6979–6993. doi: 10.1523/JNEUROSCI.19-16-06979.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade N., Komnenovic V., Blake S.M., Jossin Y., Howell B., Goffinet A., Schneider W.J., Nimpf J. ApoER2/VLDL receptor and Dab1 in the rostral migratory stream function in postnatal neuronal migration independently of Reelin. Proc. Natl. Acad. Sci. 2007;104:8508–8513. doi: 10.1073/pnas.0611391104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astapova I., Hollenberg A.N. The in vivo role of nuclear receptor corepressors in thyroid hormone action. Biochim. Biophys. Acta - Gen. Subj. 2013;1830:3876–3881. doi: 10.1016/j.bbagen.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayuso-Sacido A., Moliterno J.A., Kratovac S., Kapoor G.S., O’Rourke D.M., Holland E.C., García-Verdugo J.M., Roy N.S., Boockvar J.A. Activated EGFR signaling increases proliferation, survival, and migration and blocks neuronal differentiation in post-natal neural stem cells. J. Neurooncol. 2010;97:323–337. doi: 10.1007/s11060-009-0035-x. [DOI] [PubMed] [Google Scholar]
- Barres B.A., Lazar M.A., Raff M.C. A novel role for thyroid hormone, glucocorticoids and retinoic acid in timing oligodendrocyte development. Development. 1994;120:1097–1108. doi: 10.1242/dev.120.5.1097. [DOI] [PubMed] [Google Scholar]
- Bianco A.C., Kim B.W. Deiodinases: Implications of the local control of thyroid hormone action. J. Clin. Invest. 2006;116:2571–2579. doi: 10.1172/JCI29812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billon N., Jolicoeur C., Tokumoto Y., Vennström B., Raff M. Normal timing of oligodendrocyte development depends on thyroid hormone receptor alpha 1 (TRα1) EMBO J. 2002;21:6452–6460. doi: 10.1093/emboj/cdf662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragado Alonso S., Reinert J.K., Marichal N., Massalini S., Berninger B., Kuner T., Calegari F. An increase in neural stem cells and olfactory bulb adult neurogenesis improves discrimination of highly similar odorants. EMBO J. 2019;38:e98791. doi: 10.15252/embj.201798791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breton-Provencher V., Lemasson M., Peralta M.R., Saghatelyan A. Interneurons produced in adulthood are required for the normal functioning of the olfactory bulb network and for the execution of selected olfactory behaviors. J. Neurosci. 2009;29:15245–15257. doi: 10.1523/JNEUROSCI.3606-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brousse B., Magalon K., Durbec P., Cayre M. Region and dynamic specificities of adult neural stem cells and oligodendrocyte precursors in myelin regeneration in the mouse brain. Biol. Open. 2015;4:980–992. doi: 10.1242/bio.012773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codega P., Silva-Vargas V., Paul A., Maldonado-Soto A.R., DeLeo A.M., Pastrana E., Doetsch F. Prospective identification and purification of quiescent adult neural stem cells from their in vivo niche. Neuron. 2014;82:545–559. doi: 10.1016/j.neuron.2014.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F., García-Verdugo J.M., Alvarez-Buylla A. Regeneration of a germinal layer in the adult mammalian brain. Proc. Natl. Acad. Sci. U. S. A. 1999;96:11619–11624. doi: 10.1073/pnas.96.20.11619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitrescu A.M., Liao X.H., Best T.B., Brockmann K., Refetoff S. A novel syndrome combining thyroid and neurological abnormalities is associated with mutations in a monocarboxylate transporter Gene. Am. J. Hum. Genet. 2004;74:168–175. doi: 10.1086/380999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitrescu A.M., Liao X.H., Weiss R.E., Millen K., Refetoff S. Tissue-specific thyroid hormone deprivation and excess in monocarboxylate transporter (Mct) 8-deficient mice. Endocrinology. 2006;147:4036–4043. doi: 10.1210/en.2006-0390. [DOI] [PubMed] [Google Scholar]
- Enwere E., Shingo T., Gregg C., Fujikawa H., Ohta S., Weiss S. Aging results in reduced epidermal growth factor receptor signaling, diminished olfactory neurogenesis, and deficits in fine olfactory discrimination. J. Neurosci. 2004;24:8354–8365. doi: 10.1523/JNEUROSCI.2751-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanibunda S.E., Desouza L.A., Kapoor R., Vaidya R.A., Vaidya V.A. In: Thyroid Hormone Regulation of Adult Neurogenesis. Vitam Horm G.B.T.-V., Litwack H., editors. Academic Press; 2018. pp. 211–251. [DOI] [PubMed] [Google Scholar]
- Flamant F., Gauthier K. Thyroid hormone receptors: The challenge of elucidating isotype-specific functions and cell-specific response. Biochim. Biophys. Acta - Gen. Subj. 2013;1830:3900–3907. doi: 10.1016/j.bbagen.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Friesema E.C.H., Ganguly S., Abdalla A., Fox J.E.M., Halestrap A.P., Visser T.J. Identification of monocarboxylate transporter 8 as a specific thyroid hormone transporter. J. Biol. Chem. 2003;278:40128–40135. doi: 10.1074/jbc.M300909200. [DOI] [PubMed] [Google Scholar]
- Friesema E.C.H., Grueters P.A., Biebermann H., Krude H., Von Moers A., Reeser M., Barrett T.G., Mancilla E.E., Svensson J., Kester M.H.A. Association between mutations in a thyroid hormone transporter and severe X-linked psychomotor retardation. Lancet. 2004;364:1435–1437. doi: 10.1016/S0140-6736(04)17226-7. [DOI] [PubMed] [Google Scholar]
- Gothié J.D., Sébillot A., Luongo C., Legendre M., Nguyen Van C., Le Blay K., Perret-Jeanneret M., Remaud S., Demeneix B.A. Adult neural stem cell fate is determined by thyroid hormone activation of mitochondrial metabolism. Mol. Metab. 2017;6:1551–1561. doi: 10.1016/j.molmet.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothié J.D., Demeneix B., Remaud S. Comparative approaches to understanding thyroid hormone regulation of neurogenesis. Mol. Cell. Endocrinol. 2017;459:1041–1115. doi: 10.1016/j.mce.2017.05.020. [DOI] [PubMed] [Google Scholar]
- Groeneweg S., van Geest F.S., Peeters R.P., Heuer H., Visser W.E. Thyroid hormone transporters. Endocr. Rev. 2019;41:146–201. doi: 10.1210/endrev/bnz008. [DOI] [PubMed] [Google Scholar]
- Heuer H., Maier M.K., Iden S., Mittag J., Friesema E.C.H., Visser T.J., Bauer K. The monocarboxylate transporter 8 linked to human psychomotor retardation is highly expressed in thyroid hormone-sensitive neuron populations. Endocrinology. 2005;146:1701–1706. doi: 10.1210/en.2004-1179. [DOI] [PubMed] [Google Scholar]
- Hu F., Knoedler J.R., Denver R.J. A mechanism to enhance cellular responsivity to hormone action: Krüppel-like factor 9 promotes thyroid hormone receptor-β autoinduction during postembryonic brain development. Endocrinology. 2016;157:1683–1693. doi: 10.1210/en.2015-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias T., Caubín J., Stunnenberg H.G., Zaballos A., Bernal J., Muñoz A. Thyroid hormone- dependent transcriptional repression of neural cell adhesion molecule during brain maturation. EMBO J. 1996;15:4307–4316. [PMC free article] [PubMed] [Google Scholar]
- Kim M.J., Petratos S. Oligodendroglial lineage cells in thyroid hormone-deprived conditions. Stem Cells Int. 2019;2019:5496891. doi: 10.1155/2019/5496891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor R., Desouza L.A., Nanavaty I.N., Kernie S.G., Vaidya V.A. Thyroid hormone accelerates the differentiation of adult hippocampal progenitors. J. Neuroendocrinol. 2012;24:1259–1271. doi: 10.1111/j.1365-2826.2012.02329.x. [DOI] [PubMed] [Google Scholar]
- Kapoor R., Fanibunda S.E., Desouza L.A., Guha S.K., Vaidya V.A. Perspectives on thyroid hormone action in adult neurogenesis. J. Neurochem. 2015;133:599–616. doi: 10.1111/jnc.13093. [DOI] [PubMed] [Google Scholar]
- Lazarini F., Mouthon M.A., Gheusi G., de Chaumont F., Olivo-Marin J.C., Lamarque S., Abrous D.N., Boussin F.D., Lledo P.M. Cellular and behavioral effects of cranial irradiation of the subventricular zone in adult mice. PLoS One. 2009;4 doi: 10.1371/journal.pone.0007017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.Y., Kim M.J., Deliyanti D., Azari M.F., Rossello F., Costin A., Ramm G., Stanley E.G., Elefanty A.G., Wilkinson-Berka J.L. Overcoming monocarboxylate transporter 8 (MCT8)-deficiency to promote human oligodendrocyte differentiation and myelination. EBioMedicine. 2017;25:122–135. doi: 10.1016/j.ebiom.2017.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemkine G.F., Raji A., Alfama G., Turque N., Hassani Z., Alegria-Prévot O., Samarut J., Levi G., Demeneix B.A. Adult neural stem cell cycling in vivo requires thyroid hormone and its alpha receptor. FASEB J. 2005;19:863–865. doi: 10.1096/fj.04-2916fje. [DOI] [PubMed] [Google Scholar]
- López-Espíndola D., Morales-Bastos C., Grijota-Martínez C., Liao X.H., Lev D., Sugo E., Verge C.F., Refetoff S., Bernal J., Guadaño-Ferraz A. Mutations of the thyroid hormone transporter MCT8 cause prenatal brain damage and persistent hypomyelination. J. Clin. Endocrinol. Metab. 2014;99:E2799–E2804. doi: 10.1210/jc.2014-2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Espíndola D., García-Aldea Á., Gómez de la Riva I., Rodríguez-García A.M., Salvatore D., Visser T.J., Bernal J., Guadaño-Ferraz A. Thyroid hormone availability in the human fetal brain: novel entry pathways and role of radial glia. Brain Struct. Funct. 2019;224:2103–2119. doi: 10.1007/s00429-019-01896-8. [DOI] [PubMed] [Google Scholar]
- López-Juárez A., Remaud S., Hassani Z., Jolivet P., Pierre Simons J., Sontag T., Yoshikawa K., Price J., Morvan-Dubois G., Demeneix B.A. Thyroid hormone signaling acts as a neurogenic switch by repressing Sox2 in the adult neural stem cell niche. Cell Stem Cell. 2012;10:531–543. doi: 10.1016/j.stem.2012.04.008. [DOI] [PubMed] [Google Scholar]
- Luongo C., Dentice M., Salvatore D. Deiodinases and their intricate role in thyroid hormone homeostasis. Nat. Rev. Endocrinol. 2019;15:479–488. doi: 10.1038/s41574-019-0218-2. [DOI] [PubMed] [Google Scholar]
- Mayerl S., Visser T.J., Darras V.M., Horn S., Heuer H. Impact of Oatp1c1 deficiency on thyroid hormone metabolism and action in the mouse brain. Endocrinology. 2012;153:1528–1537. doi: 10.1210/en.2011-1633. [DOI] [PubMed] [Google Scholar]
- Mayerl S., Müller J., Bauer R., Richert S., Kassmann C.M., Darras V.M., Buder K., Boelen A., Visser T.J., Heuer H. Transporters MCT8 and OATP1C1 maintain murine brain thyroid hormone homeostasis. J. Clin. Invest. 2014;124:1987–1999. doi: 10.1172/JCI70324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayerl S., Schmidt M., Doycheva D., Darras V.M., Hüttner S.S., Boelen A., Visser T.J., Kaether C., Heuer H., von Maltzahn J. Thyroid hormone transporters MCT8 and OATP1C1 control skeletal muscle regeneration. Stem Cell Reports. 2018;10:1959–1974. doi: 10.1016/j.stemcr.2018.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayerl S., Heuer H., Ffrench-Constant C. Hippocampal neurogenesis requires cell-autonomous thyroid hormone signaling. Stem Cell Reports. 2020;14:845–860. doi: 10.1016/j.stemcr.2020.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menn B., Garcia-Verdugo J.M., Yaschine C., Gonzalez-Perez O., Rowitch D., Alvarez-Buylla A. Origin of oligodendrocytes in the subventricular zone of the adult brain. J. Neurosci. 2006;26:7907–7918. doi: 10.1523/JNEUROSCI.1299-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morshead C.M., Reynolds B.A., Craig C.G., McBurney M.W., Staines W.A., Morassutti D., Weiss S., van der Kooy D. Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron. 1994;13:1071–1082. doi: 10.1016/0896-6273(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Morte B., Manzano J., Scanlan T., Vennström B., Bernal J. Deletion of the thyroid hormone receptor alpha 1 prevents the structural alterations of the cerebellum induced by hypothyroidism. Proc. Natl. Acad. Sci. U. S. A. 2002;99:3985–3989. doi: 10.1073/pnas.062413299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller J., Heuer H. Expression pattern of thyroid hormone transporters in the postnatal mouse brain. Front. Endocrinol. 2014;5:92. doi: 10.3389/fendo.2014.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nait-Oumesmar B., Picard-Riera N., Kerninon C., Decker L., Seilhean D., Höglinger G.U., Hirsch E.C., Reynolds R., Baron-Van Evercooren A. Activation of the subventricular zone in multiple sclerosis: Evidence for early glial progenitors. Proc. Natl. Acad. Sci. U. S. A. 2007;104:4694–4699. doi: 10.1073/pnas.0606835104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuki L., Brand A.H. Cell cycle heterogeneity directs the timing of neural stem cell activation from quiescence. Science. 2018;360:99–102. doi: 10.1126/science.aan8795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuki L., Brand A.H. Dorsal-ventral differences in neural stem cell quiescence are induced by p57KIP2/Dacapo. Dev. Cell. 2019;49:293–300.e3. doi: 10.1016/j.devcel.2019.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak A., Sinha R.A., Mohan V., Mitra K., Godbole M.M. Maternal thyroid hormone before the onset of fetal thyroid function regulates reelin and downstream signaling cascade affecting neocortical neuronal migration. Cereb. Cortex. 2010;21:11–21. doi: 10.1093/cercor/bhq052. [DOI] [PubMed] [Google Scholar]
- Pizzagalli F., Hagenbuch B., Stieger B., Klenk U., Folkers G., Meier P.J. Identification of a novel human organic anion transporting polypeptide as a high affinity thyroxine transporter. Mol. Endocrinol. 2002;16:2283–2296. doi: 10.1210/me.2001-0309. [DOI] [PubMed] [Google Scholar]
- Portella A.C., Carvalho F., Faustino L., Wondisford F.E., Ortiga-Carvalho T.M., Gomes F.C.A. Thyroid hormone receptor β mutation causes severe impairment of cerebellar development. Mol. Cell. Neurosci. 2010;44:68–77. doi: 10.1016/j.mcn.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Remaud S., Ortiz F.C., Perret-Jeanneret M., Aigrot M.S., Gothié J.D., Fekete C., Kvárta-Papp Z., Gereben B., Langui D., Lubetzki C. Transient hypothyroidism favors oligodendrocyte generation providing functional remyelination in the adult mouse brain. eLife. 2017;6:e29996. doi: 10.7554/eLife.29996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridder D.A., Lang M.-F., Salinin S., Röderer J.-P., Struss M., Maser-Gluth C., Schwaninger M. TAK1 in brain endothelial cells mediates fever and lethargy. J. Exp. Med. 2011;208:2615–2623. doi: 10.1084/jem.20110398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts L.M., Woodford K., Zhou M., Black D.S., Haggerty J.E., Tate E.H., Grindstaff K.K., Mengesha W., Raman C., Zerangue N. Expression of the thyroid hormone transporters monocarboxylate transporter-8 (SLC16A2) and organic ion transporter-14 (SLCO1C1) at the blood-brain barrier. Endocrinology. 2008;149:6251–6261. doi: 10.1210/en.2008-0378. [DOI] [PubMed] [Google Scholar]
- Rochefort C., Gheusi G., Vincent J.D., Lledo P.M. Enriched odor exposure increases the number of newborn neurons in the adult olfactory bulb and improves odor memory. J. Neurosci. 2002;22:2679–2689. doi: 10.1523/JNEUROSCI.22-07-02679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz C.E., Stevenson R.E. The MCT8 thyroid hormone transporter and Allan-Herndon-Dudley syndrome. Best Pract. Res. Clin. Endocrinol. Metab. 2007;21:307–321. doi: 10.1016/j.beem.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strømme P., Groeneweg S., Lima De Souza E.C., Zevenbergen C., Torgersbråten A., Holmgren A., Gurcan E., Meima M.E., Peeters R.P., Visser W.E. Mutated thyroid hormone transporter OATP1C1 associates with severe brain hypometabolism and juvenile neurodegeneration. Thyroid. 2018;28:1406–1415. doi: 10.1089/thy.2018.0595. [DOI] [PubMed] [Google Scholar]
- Suzuki S., Mori J., Hashizume K. μ-crystallin, a NADPH-dependent T3-binding protein in cytosol. Trends Endocrinol. Metab. 2007;18:286–289. doi: 10.1016/j.tem.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Tokumoto Y., Tamaki S., Kabe Y., Takubo K., Suematsu M. Quiescence of adult oligodendrocyte precursor cells requires thyroid hormone and hypoxia to activate Runx1. Sci. Rep. 2017;7:1019. doi: 10.1038/s41598-017-01023-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trajkovic M., Visser T.J., Mittag J., Horn S., Lukas J., Darras V.M., Raivich G., Bauer K., Heuer H. Abnormal thyroid hormone metabolism in mice lacking the monocarboxylate transporter 8. J. Clin. Invest. 2007;117:627–635. doi: 10.1172/JCI28253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trajkovic M., Visser T.J., Mittag J., Horn S., Lukas J., Darras V.M., Raivich G., Bauer K., Heuer H. Abnormal thyroid hormone metabolism in mice lacking the monocarboxylate transporter 8. J. Clin. Invest. 2007;117:627–635. doi: 10.1172/JCI28253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vancamp P., Deprez M.A., Remmerie M., Darras V.M. Deficiency of the thyroid hormone transporter monocarboxylate transporter 8 in neural progenitors impairs cellular processes crucial for early corticogenesis. J. Neurosci. 2017;37:11616–11631. doi: 10.1523/JNEUROSCI.1917-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vancamp P., Gothié J.-D., Luongo C., Sébillot A., Le Blay K., Butruille L., Pagnin M., Richardson S.J., Demeneix B.A., Remaud S. Gender-specific effects of transthyretin on neural stem cell fate in the subventricular zone of the adult mouse. Sci. Rep. 2019;9:19689. doi: 10.1038/s41598-019-56156-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatine G.D., Al-Ahmad A., Barriga B.K., Svendsen S., Salim A., Garcia L., Garcia V.J., Ho R., Yucer N., Qian T. Modeling psychomotor retardation using iPSCs from MCT8-deficient patients indicates a prominent role for the blood-brain barrier. Cell Stem Cell. 2017;20:831–843.e5. doi: 10.1016/j.stem.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann G., Szabon J., Mohácsik P., Nouriel S.S., Gereben B., Fekete C., Lechan R.M. Parallel regulation of thyroid hormone transporters OATP1c1 and MCT8 during and after endotoxemia at the blood-brain barrier of male rodents. Endocrinology. 2015;156:1552–1564. doi: 10.1210/en.2014-1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka K., Namiki K., Sudo T., Kasuya Y. p38α controls self-renewal and fate decision of neurosphere-forming cells in adult hippocampus. FEBS Open Bio. 2015;5:437–444. doi: 10.1016/j.fob.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y.L., Röth P.T., Stratton J.A.S., Chuang B.H.A., Danne J., Ellis S.L., Ng S.W., Kilpatrick T.J., Merson T.D. Adult neural precursor cells from the subventricular zone contribute significantly to oligodendrocyte regeneration and remyelination. J. Neurosci. 2014;34:14128–14146. doi: 10.1523/JNEUROSCI.3491-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.